Abstract
Background
Intraventricular hemorrhage (IVH) is a serious complication of premature (<32 weeks) deliveries, especially in very-low-birth-weight (VLBW; <1500 g) neonates. Infants developing severe IVH are more prone to long-term developmental disabilities. Although 62%–79% of women in Saudi Arabia receive antenatal steroids, IVH incidence remains high. We analyzed the risk factors for IVH in preterm VLBW neonates in the central region of Saudi Arabia.
Methods
We included premature infants with IVH (n = 108) and gestational age- and birth weight-matched control group infants (n = 108) admitted to our neonatal intensive care unit. Cases were divided into mild (grades I and II; n = 56) and severe (grades III and IV; n = 52) IVH groups. Association of IVH with risk factors in the first week of life was investigated.
Results
The following risk factors were associated with severe IVH: lack of antenatal steroid administration (P < .001), pulmonary hemorrhage (P = .023), inotrope use (P = .032), neonatal hydrocortisone administration (P = .001), and patent ductus arteriosus (PDA) (P = .005). Multivariable logistic regression analysis revealed the following to be significant: lack of antenatal dexamethasone (adjusted odds ratio [aOR]: 0.219, 95% confidence interval [95% CI] 0.087–0.546), neonatal hydrocortisone administration (aOR: 3.519, 95% CI 1.204–10.281), and PDA (aOR: 2.718, 95% CI 1.024–7.210). Low hematocrit in the first 3 days of life was significantly associated with severe IVH (all P < .01).
Conclusions
Failure to receive antenatal dexamethasone, PDA, hydrocortisone administration for neonatal hypotension, and low hematocrit in the first 3 days of life was associated with severe IVH in VLBW neonates. Clinicians and healthcare policy makers should consider these factors during decision-making.
1. Introduction
The World Health Organization defines preterm birth as any birth before 37 completed weeks of gestation (or 259 days from the mother’s last menstrual period). Preterm births are further subdivided into low birth weight (LBW; 1500–2500 g), very low birth weight (VLBW; 1000–1499 g), and extremely low birth weight (ELBW; <1000 g) [1]. Intraventricular hemorrhage (IVH) is the most serious complication of premature deliveries, especially in neonates with birth weight <1500 g and gestational age <32 weeks, as it leads to short- and long-term morbidities [2,3]. Infants who develop a severe grade of IVH (grades III and IV) are more prone to develop significant long-term developmental disabilities including cerebral palsy and posthemorrhagic hydrocephalus [4]. Approximately 50% of IVH occurs in the first 72 h of life, with <10% occurring after day 5 [[4], [5], [6], [7]]; however, its severity can increase during the following days. Therefore, head ultrasound (HUS) is performed between days 5 and 7 to catch attention of the last severity grade of IVH. The incidence increases with decreased gestational age and birth weight [8,9].
In the United States, approximately 12,000 premature infants develop IVH annually [3,10], and more than a million deaths occur annually due to the complications of preterm birth [1]. In addition, the reported rates of antenatal steroid administration vary across regions worldwide. In Saudi Arabia, in 2010, there were more than 35,000 preterm deliveries, with 8% mortality due to the complications of preterm birth [8]. IVH incidence in premature infants born across Saudi Arabia ranges from 13% to 27% [10,11]. Antenatal steroids have been shown to reduce IVH risk in preterm neonates [[12], [13], [14]]. Although 62%–79% women in Saudi Arabia receive antenatal steroids, IVH incidence continues to be high [9,11]. Thus, it is crucial to identify additional risk factors for IVH in preterm neonates and develop effective prevention strategies.
Known risk factors for IVH in premature infants include intrauterine infection, prolonged labor, male sex, premature rupture of membranes, metabolic acidosis, postnatal resuscitation, early onset of neonatal sepsis, and respiratory distress syndrome [10,15,16].
Several studies worldwide, including those conducted in Saudi Arabia, have evaluated risk factors for IVH in premature infants [10,11,14,17]. Our center, King Saud Medical City, receives both booked and unbooked labor patients; therefore, the percentage of mothers who have received antenatal steroids is lower in our center (50%) than in other centers across Saudi Arabia (62%–79%) [9,11].
Our study evaluated the risk factors for IVH in premature infants in the central region of Saudi Arabia. A secondary aim of this study was to predict the time of IVH by detecting a sudden, significant drop in hematocrit.
2. Methods
2.1. Study design and patient details
Our matched retrospective case–control study was conducted at the neonatal intensive care unit (NICU) of the King Saud Medical City (KSMC) between January 2015 and June 2018, specifically at the Hospital of Paediatrics at KSMC. Neonates born at ≤32 weeks of gestation, with birth weight <1500 g, and admitted to the level 3 NICU were eligible for inclusion in our study. We excluded premature neonates who died before undergoing HUS, were born outside the hospital, had asphyxia, were transferred from the NICU, or died within the first 72 h of life and those who had congenital anomalies. Among the included patients, all premature infants with IVH were divided into two subgroups depending on the IVH grade: mild IVH (grades I and II) and severe IVH (grades III and IV). Then each group was matched 1:1 with the control group comprising patients who did not have IVH. Each group was matched for gestational age (1 week) and birth weight (50 g). The ethical committee of the KSMC approved this study (H1RI-21-Dec17-01). Because this was a retrospective case–control study, the need for informed consent from patients’ guardians was waived.
Our NICU protocol for routine HUS is to perform the first HUS between days 5 and 7 of life. If the findings are abnormal, then HUS is repeated the next week, and if the findings are normal, it is repeated after 1 month. Additional HUS is performed if required. HUS was interpreted by a radiologist who was not involved in the study and was blinded to its objective.
A secondary aim of this study was to predict the time of IVH by detecting a sudden, significant drop in hematocrit. Hematocrit values in the first 7 days of life were compared between the cases and controls, and the drop was considered significant if it was significantly more in the case group than in the control group.
2.2. Definitions
IVH was classified into grades I to IV according to Papile et al.‘s IVH classification [12].
IVH was diagnosed on the basis of the findings of HUS performed between days 5 and 7 of life after birth [18,19]. All the ultrasound scans were performed by one expert radiologist and checked by another expert radiologist. Transducers of 7.5 and 10 MHz (LOGIQ e; GE Medical Systems Co., Ltd., Jiangsu, China) were used to perform ultrasound in sagittal and coronal planes.
2.3. Data collection and follow-up
Most data were collected prospectively by the NICU team. Maternal data included demographic information, antenatal history, delivery mode, Apgar score, fetal growth restriction, birth weight, gestational age, maternal hypertension, and premature delivery cause (maternal or fetal indications). Neonatal data included respiratory support, surfactant use, inotrope and hydrocortisone use for treating hypotension in the first week of life, and PDA presence (treated or not treated). If missing, data were extracted from the medical records by our study team, and NICU charts were reviewed to confirm the accuracy of information. All patients whose relevant study data were unavailable were excluded.
Our study evaluated the following risk factors for IVH in premature infants in the central region of Saudi Arabia: antenatal steroid administration, cesarean section, pregnancy-induced hypertension, gestational diabetes mellitus, male sex, respiratory distress syndrome, surfactant use, invasive respiratory support, pneumothorax, pulmonary hemorrhage, inotrope and hydrocortisone use, PDA presence, and nitric oxide use.
For women between 24 and 33 ± 6/7 weeks of gestational age who are at risk of preterm birth, the American College of Obstetricians and Gynecologists (ACOG) guidelines recommend two doses of 12 mg intramuscular betamethasone every 24 h or four doses of 6 mg dexamethasone every 12 h [20]. KSMC follows a slightly different treatment regimen: two doses of 12 mg intramuscular dexamethasone every 12 h to pregnant women between 24 and 33 ± 6/7 weeks of gestational age in the presence of the following risk factors: risk of preterm delivery within 1 week, multiple gestations, or premature rupture of membrane. Because most pregnant women who arrived in labor were unbooked, they had not received the aforementioned dexamethasone therapy. Unbooked pregnant women meeting the criteria who arrived in labor were given single-dose 12 mg intramuscular dexamethasone immediately after an obstetrician received them, as recommended by several guidelines [20,21].
2.4. Statistical analysis
Descriptive statistics including means, medians, and standard deviations were used to describe the study population and to know whether the data were normally distributed. Chi-square test and Fisher’s exact test were used for categorical variables and unpaired Student’s t-test for continuous variables. If the data were not normally distributed and/or when the variances were significantly different, Mann–Whitney U test was performed.
A sample size of 216 premature infants (108 cases and 108 controls) was analyzed to detect a difference in morbidity, at a significance level of 0.05 with 80% power.
Analysis of the primary outcome was performed in two steps. Initially, demographic, maternal, and neonatal variables were plotted in a univariate logistic regression model to identify unadjusted factors significantly associated with IVH. Subsequently, only significant factors were plotted in a multivariate logistic regression model for the dichotomous outcome, using enter method with P values < .1. The model was tested for goodness of fit using Hosmer–Lemeshow test (considered well fitted if P > .05).
All statistical tests were performed and graphs plotted using Statistical Package for the Social Sciences, version 25.0 (SSPS Inc., Chicago, IL, USA). All statistical tests were two-tailed and considered significant at P < .05; however, there was no correction for multiple testing.
3. Results
3.1. Demographics and patient features
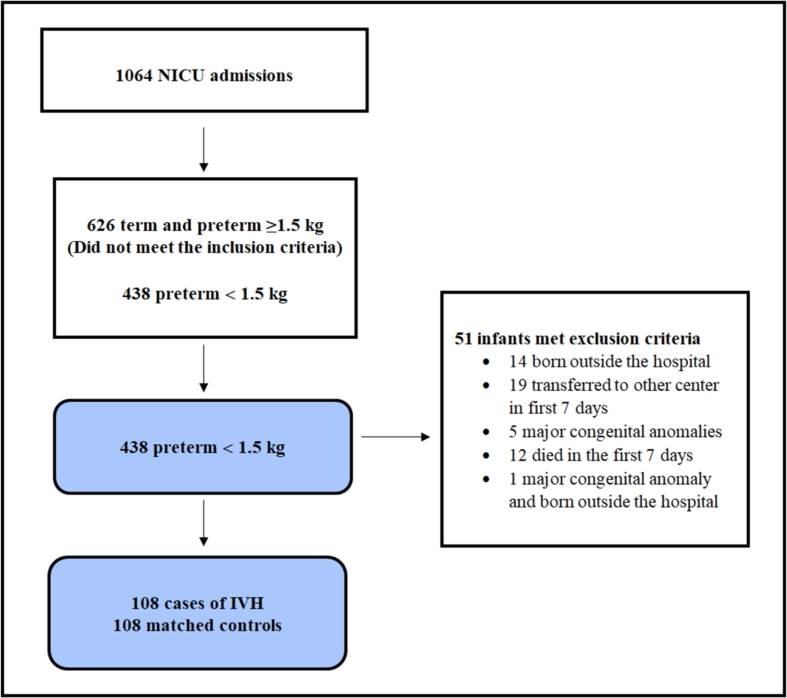
Fig. 1 presents the flowchart of patient selection. In total, 1064 infants were admitted to the NICU in our hospital between January 2015 and June 2018. Of them, 438 had <32 weeks of gestational age and birth weight ≤1500 g.
Fig. 1.
Flowchart of patient section. The control group included 1:1 gestational age- and birth weight-matched patients. IVH: Intraventricular hemorrhage; NICU: Neonatal intensive care unit.
We excluded 14 neonates born outside our hospital, 19 transferred to other centers in the first 7 days of life, 5 with major congenital anomalies, 12 who died in the first 7 days, and 1 who had a major congenital anomaly and was born outside the hospital. Thus, the remaining 387 neonates were eligible for 1:1 matching for birth weight and gestational age. Eventually, the case group included 108 IVH cases and the control group included 108 infants with no IVH.
There were no significant demographic differences between the case and control groups (Table 1), and as expected because of matching, mean gestational age (P = .372) and mean birth weight (P = .919) were comparable.
Table 1.
Clinicodemographic data of preterm very-low-birth-weight infants with IVH (cases) and without IVH (controls).
| Parameters | Cases (n = 108) | Controls (n = 108) | P value |
|---|---|---|---|
| Booked | 15 (14.4) | 18 (17.3) | .57 |
| Cesarean section | 53 (49.1) | 60 (55.6) | .341 |
| Gestational age, weeks | 27.8 (2.4) | 28.1 (2.3) | .372 |
| Birth weight, g | 1014 (250) | 1018 (246) | .919 |
| 1-min Apgar | 5 (0, 8) | 5 (0, 8) | .240 |
| 5-min Apgar | 7 (3, 9) | 7 (1, 9) | .376 |
| PH | 7.28 (0.16) | 7.31 (0.15) | .453 |
| Base excess | −7.35 (4.48) | −5.01 (4.23) | .241 |
Data are represented as median (range) or mean ± standard deviation as appropriate.
IVH: Intraventricular hemorrhage.
3.2. Risk factors for IVH
Table 2 shows a comparison of each variable contributing to the risk of any IVH, mild IVH, and severe IVH.
Table 2.
Univariate analysis comparing neonates with any IVH, mild IVH, and severe IVH and controls (no IVH).
| Parameters | Any IVH group (n = 108) | Control group (n = 108) | P value | Mild IVH group (n = 56) | Control group (n = 56) | P value | Severe IVH group (n = 52) | Control group (n = 52) | P value |
|---|---|---|---|---|---|---|---|---|---|
| Antenatal steroid (≥1 dose) | 49 (45.4) | 69 (63.9) | .007* | 34 (60.7) | 33 (58.9) | .847 | 15 (28.8) | 36 (69.2) | <.001* |
| Cesarean section | 53 (49.1) | 60 (55.6) | .341 | 30 (53.6) | 34 (60.7) | .445 | 23 (44.2) | 26 (50) | .556 |
| PIH | 21 (19.4) | 27 (25) | .327 | 14 (25) | 16 (28.6) | .670 | 7 (13.5) | 11 (21.2) | .303 |
| GDM | 8 (7.4) | 9 (8.3) | .163 | 7 (5.4) | 7 (12.5) | .197 | 1 (1.9) | 2 (3.8) | .566 |
| Male | 58 (53.7) | 53 (49.1) | .496 | 32 (57.1) | 27 (48.2) | .345 | 26 (50) | 26 (50) | 1 |
| RDS surfactant use | 80 (74.1) | 71 (65.7) | .183 | 36 (64.3) | 31 (55.4) | .336 | 44 (84.6) | 40 (76.9) | .322 |
| Invasive respiratory support | 89 (75) | 79 (84.3) | .104 | 43 (76.8) | 36 (64.3) | .149 | 46 (88.5) | 43 (82.7) | .405 |
| Pneumothorax | 11 (10.2) | 3 (2.8) | .038* | 6 (10.7) | 0 (0) | .999 | 5 (9.6) | 3 (5.8) | .360 |
| Pulmonary hemorrhage | 17 (15.7) | 5 (4.6) | .011* | 4 (7.1) | 1 (1.8) | .204 | 13 (25) | 4 (7.7) | .023* |
| Inotropes | 49 (45.4) | 32 (29.6) | .018* | 17 (30.4) | 11 (19.6) | .193 | 32 (61.5) | 21 (40.4) | .032* |
| Hydrocortisone | 34 (31.5) | 19 (17.6) | .019* | 7 (12.5) | 9 (16.1) | 0.590 | 27 (51.9) | 10 (19.2) | .001* |
| PDA | 69 (63.9) | 53 (49.1) | .029* | 29 (51.8) | 27 (48.2) | .706 | 40 (76.9) | 26 (50 | .005* |
| PDA (treated with medication) | 15 (13.9) | 14 (13) | .842 | 4 (7.1) | 6 (10.7) | .510 | 11 (21.2) | 8 (15.4) | .448 |
| Nitric oxide | 1 (0.93) | 0 | 1 | 0 | 0 | .849 | 1 (1.9) | 0 | 1 |
*P < .05.
IVH: Intraventricular hemorrhage; PIH: Pregnancy-induced hypertension; GDM: Gestational diabetes mellitus; RDS: Respiratory distress syndrome; PDA: Patent ductus arteriosus.
In premature infants with any IVH, significant risk factors were lack of antenatal steroid administration (P = .007), pneumothorax (P = .038), pulmonary hemorrhage (P = .011), use of inotropes (P = .018), neonatal treatment with hydrocortisone (P = .019), and presence of PDA (P = .029).
In premature infants with severe IVH, significant risk factors were lack of antenatal steroid administration (P < .001), pulmonary hemorrhage (P = .023), use of inotropes (P = .032), neonatal treatment with hydrocortisone (P = .001), and presence of PDA (P = .005).
Multivariable analysis for any IVH revealed no significant difference, whereas regarding severe IVH, infants of mothers who did not receive dexamethasone had a higher risk of severe IVH (adjusted odds ratio [aOR]: 0.219, 95% confidence interval [95% CI] 0.087–0.546). Other significant risk factors for severe IVH were neonatal administration of hydrocortisone (aOR: 3.519, 95% CI 1.204–10.281) and presence of PDA (aOR: 2.718, 95% CI 1.024–7.210) (Table 3).
Table 3.
Multivariate analysis of any IVH and severe IVH.
| Parameters | Odds ratio of any IVH (95% CI) | Odds ratio of severe IVH (95% CI) |
|---|---|---|
| Antenatal steroid | 0.576 (0.32–1.04) | 0.219 (0.087–0.546)a |
| Pneumothorax | 4.284 (0.958–19.158) | – |
| Pulmonary hemorrhage | 2.468 (0.814–7.479) | 2.076 (0.504–8.543) |
| Inotropes | 0.984 (0.471–2.054) | 0.818 (0.287–2.334) |
| Hydrocortisone | 1.225 (0.558–2.692) | 3.519 (1.204–10.281)a |
| PDA | 1.425 (0.774–2.625) | 2.718 (1.024–7.210)a |
CI; confidence interval; IVH: Intraventricular hemorrhage; PDA: Patent ductus arteriosus.
Indicates significant differences.
Infants of mothers who received at least one dose of dexamethasone were less likely to develop severe IVH (P < .001) (Table 2).
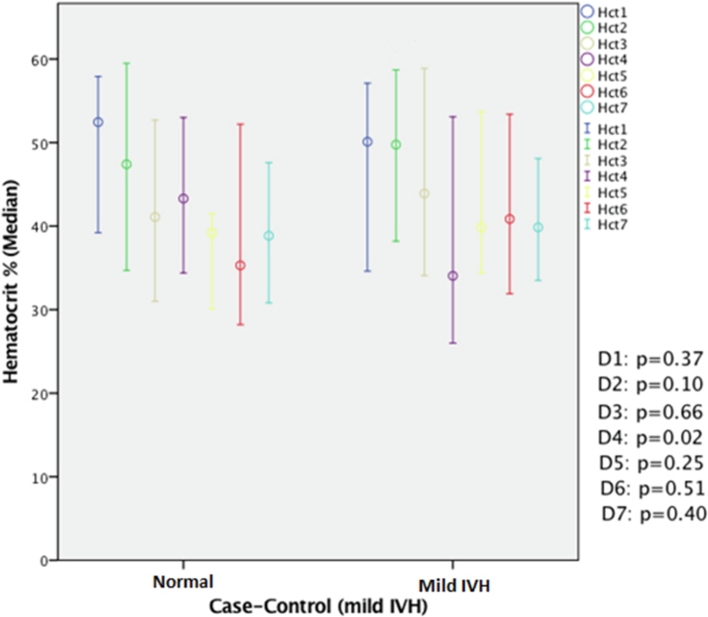
We found a significant difference in the drop in hematocrit patterns between premature infants with severe IVH and those with mild IVH. Low hematocrit in the first 3 days of life was observed in severe IVH cases (Fig. 2); a significant drop in hematocrit on day 4 of life was observed in mild IVH cases (Fig. 3).
Fig. 2.
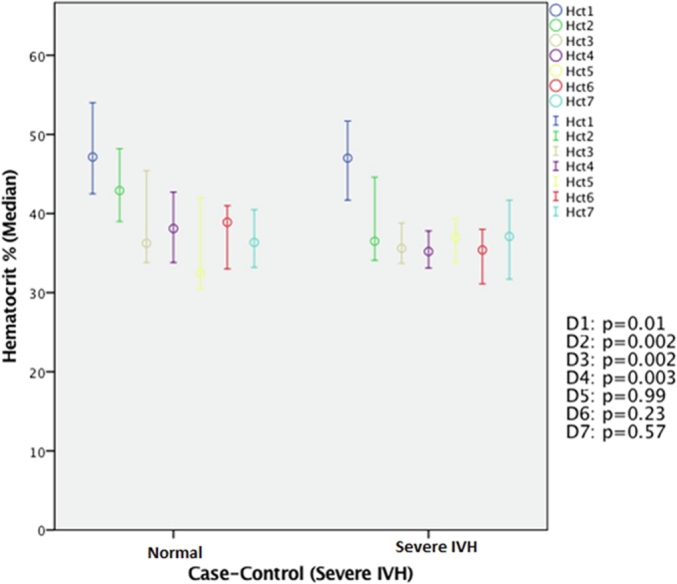
Comparison of hematocrit between cases of severe IVH and controls in the first 7 days of life. Significantly low hematocrit was observed in cases of severe IVH compared with controls on days 1, 2, and 3 of life (all P < .01). IVH: Intraventricular hemorrhage; Hct: hematocrit.
Fig. 3.
Comparison of hematocrit between cases of mild IVH and controls in the first 7 days of life. A significant drop in hematocrit was observed in cases of mild IVH (P = .02) compared with controls on day 4 of life. IVH: Intraventricular hemorrhage; Hct: hematocrit.
4. Discussion
We aimed to identify risk factors for IVH in premature infants in the central region of Saudi Arabia. We found that lack of antenatal steroid administration and neonatal hydrocortisone administration was significantly associated with severe IVH; this finding is consistent with previous studies [22]. Our data revealed that antenatal steroids significantly reduced the percentage of severe IVH, but not mild IVH, in VLBW infants. These results point to the importance of antenatal care, particularly antenatal steroids, in preventing severe IVH. Most cases of mild IVH occur between days 3 and 4 of life, indicating that neonatal care and management in NICU are directly related to the occurrence of mild IVH.
Antenatal steroids do not have a major role in preventing mild IVH. A recent Cochrane review has shown that a single course of antenatal steroids significantly reduces the rate of IVH among premature infants (relative risk 0.55, 95% CI 0.40–0.76) [13].
However, it did not discuss the relationship between the timing of IVH and the antenatal steroids. Wei et al. [14] have found that antenatal steroids reduce any-grade IVH in premature infants; however, their study is a cross-sectional study and has many missing data (such as timing and number of antenatal steroid doses regardless of whether partial or full course was given; lack of imaging data for a good number of patients).
Most cases of severe IVH occur on the first day of life, corresponding to a sharp drop in hematocrit between days 1 and 2. The low hematocrit on the first day of life in infants with severe IVH can be due to bleeding; however, a low hematocrit can also accelerate the cerebral blood flow and contribute to further bleeding [15,23]. It remains uncertain whether low hematocrit is the cause of hemorrhage or occurs secondary to it. In contrast, patients with mild IVH developed bleeding beyond the first day—between days 3 and 4. As most severe IVH cases occur on day 1 of life, they reflect events during antenatal care, maternal health problems, history of delivery, and neonatal resuscitation process.
In our study, multivariate analysis showed that PDA was a risk factor for severe IVH in premature infants, consistent with many studies [24,25]. PDA causes fluctuation in cerebral blood flow; prophylactic treatment of PDA with indomethacin on the first day of life in extremely premature infants can reduce severe IVH incidence by enhancing cerebral autoregulation and preventing pulmonary hemorrhage, an identified risk factor for severe IVH in our study and previous studies [26]. Despite this strong evidence of the efficacy of prophylactic treatment of PDA in decreasing severe IVH incidence, we do not routinely give indomethacin on the first day of life for many reasons: (1) it does not improve the survival rate and neurosensory impairment at 18 months of corrected age [27]; (2) possibility of thrombocytopenia or platelet dysfunction on the first day of life; (3) presence of oliguria or acute kidney injury on the first day of life; and (4) ibuprofen or acetaminophen has the same efficacy as indomethacin in treating significant PDA with fewer side effects [28].
We also found hydrocortisone administration to be a risk factor for severe IVH among VLBW infants. We administer intravenous hydrocortisone 3 mg/kg/day divided every 8 h for treating premature infants with refractory hypotension in the first days of life [29]. Although fluid volume bolus is the first-line treatment for neonatal hypotension, nonresponders are given inotropes to improve cardiac contractility and cardiac output. Hydrocortisone is then used as the third-line treatment [29]. Both saline boluses and inotropes are well known to be associated with severe brain injury [30,31]. Because we usually administer hydrocortisone after initiating volume boluses and vasopressors, it was found to be associated with IVH in our study.
We used HUS to diagnose neonatal IVH. Researchers have tried to detect the time of bleeding by measuring the blood pressure reading and respiratory pattern [17,19,[32], [33], [34], [35]]. However, available evidence remains subjective based on very small sample size. Many studies have been conducted to predict and prevent IVH using new modalities such as positron emission tomography and near-infrared spectroscopy. These modalities are not available at our NICU and have questionable validity and safety for premature infants in the first days of their life.
The strengths of our study are as follows: (1) 1:1 matching for gestational age (1 week) and birth weight (50 g) between both cases of severe and mild IVH; (2) Measuring and adjusting for multiple potential confounders such as age and sex; (3) Assessing the efficacy of the modified version of the ACOG guidelines for antenatal steroids that our institution follows; and (4) finally, performing diagnosis based on HUS findings in a blinded manner.
Our study has many limitations. First, this was a retrospective case–control study. Second, we could not measure all the potential confounding variables because most pregnant women were not followed up at our maternity hospital. Thus, it was not feasible to perform HUS on the first day of life—but this point is not exclusive to our NICU, as we follow the same protocol as many other NICUs worldwide [36]. Finally, the association of severe IVH with drop in hematocrit value observed in our study requires further investigation for the confirmation of possible underlying reasons.
5. Conclusion
Severe IVH in VLBW neonates is associated with failure to give antenatal steroids, a sharp drop in hematocrit in the first day of life, presence of PDA, and hydrocortisone given for neonatal hypotension. Clinicians and healthcare policy makers should consider these factors during decision-making; for example, coverage of (complete) antenatal steroid therapy should be expanded to reduce the incidence and severity of neonatal IVH. Further studies in a larger population of neonates of Middle Eastern origin are required to evaluate all the possible risk factors that may be peculiar to this population.
Disclosure
The authors declare no conflict of interest.
Ethical approval
This study was approved (Reference number: H1RI-21-Dec17-01) by the institutional review board of the study setting. Because this was a retrospective case–control study, the need for informed consent from patients’ guardians was waived.
Ethical statement
I testify on behalf of all co-authors that our article submitted to International Journal Of Pediatric and Adolescent Medicine.
Acknowledgments
We did not receive any funding or financial grant for this study.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Howson C.P., Kinney M.V., Lawn J.E. World Health Organization; Geneva: 2012. Born too soon: the global action report on preterm birth; pp. 1–26. [Google Scholar]
- 2.Fanaroff A.A., Stoll B.J., Wright L.L., Carlo W.A., Ehrenkranz R.A., Stark A.R. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196 doi: 10.1016/j.ajog.2006.09.014. 147.e1-8. [DOI] [PubMed] [Google Scholar]
- 3.Kochanek K.D., Kirmeyer S.E., Martin J.A., Strobino D.M., Guyer B. Annual summary of vital statistics: 2009. Pediatrics. 2012;129:338–348. doi: 10.1542/peds.2011-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwer A., Groenendaal F., van Haastert I.L., Rademaker K., Hanlo P., de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152:648–654. doi: 10.1016/j.jpeds.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Patra K., Wilson-Costello D., Taylor H.G., Mercuri-Minich N., Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149:169–173. doi: 10.1016/j.jpeds.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Mukerji A., Shah V., Shah P.S. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics. 2015;136:1132–1143. doi: 10.1542/peds.2015-0944. [DOI] [PubMed] [Google Scholar]
- 7.Pascal A., Govaert P., Oostra A., Naulaers G., Ortibus E., Van den Broeck C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol. 2018;60:342–355. doi: 10.1111/dmcn.13675. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Country data and rankings for preterm birth data. 2016. https://www.who.int/pmnch/media/news/2012/201204_borntoosoon_countryranking.pdf [Retrieved August 10 (2012)]. Available from:
- 9.Al-Alaiyan S., Al-Abdi S., Alallah J., Al-Hazzani F., AlFaleh K. Pre-viable newborns in Saudi Arabia: where are we now and what the future may hold? Curr Pediatr Rev. 2013;9:4–8. [Google Scholar]
- 10.Heuchan A.M., Evans N., Smart D.H., Simpson J.M. Perinatal risk factors for major intraventricular hemorrhage in the Australian and New Zealand Neonatal Network, 1995-97. Arch Dis Child-Fetal. 2002;86:F86–F90. doi: 10.1136/fn.86.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abolfotouh M.A., Al Saif S., Altwaijri W.A., Al Rowaily M.A. Prospective study of early and late outcomes of extremely low birthweight in Central Saudi Arabia. BMC Pediatr. 2018;18:280. doi: 10.1186/s12887-018-1248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papile L.A., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 13.Roberts D., Brown J., Medley N., Dalziel S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Db Syst Rev. 2017;3 doi: 10.1002/14651858.CD004454.pub3. CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J.C., Catalano R., Profit J., Gould J.B., Lee H.C. Impact of antenatal steroids on intraventricular hemorrhage in very-low-birth weight infants. J Perinatol. 2016;36:352–356. doi: 10.1038/jp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linder N., Haskin O., Levit O., Klinger G., Prince T., Naor N. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics. 2003;111:e590–e595. doi: 10.1542/peds.111.5.e590. [DOI] [PubMed] [Google Scholar]
- 16.Jain N.J., Kruse L.K., Demissie K., Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern Fetal Neonatal Med. 2009;22:491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- 17.Osborn D.A., Evans N., Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics. 2003;112:33–39. doi: 10.1542/peds.112.1.33. [DOI] [PubMed] [Google Scholar]
- 18.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huvanandana J., Nguyen C., Thamrin C., Tracy M., Hinder M., McEwan A.L. Prediction of intraventricular hemorrhage in preterm infants using time series analysis of blood pressure and respiratory signals. Sci Rep UK. 2017;7 doi: 10.1038/srep46538. 46538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics Practice bulletin No. 171: management of preterm labor. Obstet Gynecol. 2016;128:e155–e164. doi: 10.1097/AOG.0000000000001711. [DOI] [PubMed] [Google Scholar]
- 21.Crane J., Armson A., Brunner M., De La Ronde S., Farine D., Keenan-Lindsay L. Antenatal corticosteroid therapy for fetal maturation. J Obstet Gynaecol Can. 2003;25:45–52. doi: 10.1016/s1701-2163(16)31081-7. [DOI] [PubMed] [Google Scholar]
- 22.Szpecht D., Szymankiewicz M., Nowak I., Gadzinowski J. Intraventricular hemorrhage in neonates born before 32 weeks of gestation—retrospective analysis of risk factors. Child Nerv Syst. 2016;32:1399–1404. doi: 10.1007/s00381-016-3127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaver D.C., Bada H.S., Korones S.B., Anderson G.D., Wong S.P., Arheart K.L. Early and late intraventricular hemorrhage: the role of obstetric factors. Obstet Gynecol. 1992;80:831–837. [PubMed] [Google Scholar]
- 24.Mullaart R.A., Hopman J.C., Rotteveel J.J., Stoelinga G.B., De Haan A.F., Daniëls O. Cerebral blood flow velocity and pulsation in neonatal respiratory distress syndrome and periventricular hemorrhage. Pediatr Neurol. 1997;16:118–125. doi: 10.1016/s0887-8994(96)00291-3. [DOI] [PubMed] [Google Scholar]
- 25.Jim W.T., Chiu N.C., Chen M.R., Hung H.Y., Kao H.A., Hsu C.H. Cerebral hemodynamic change and intraventricular hemorrhage in very low birth weight infants with patent ductus arteriosus. Ultrasound Med Biol. 2005;31:197–202. doi: 10.1016/j.ultrasmedbio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Fowlie P.W., Davis P.G., McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Db Syst Rev. 2010;(7) doi: 10.1002/14651858.CD000174.pub2. CD000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt B., Davis P., Moddemann D., Ohlsson A., Roberts R.S., Saigal S. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 28.Mitra S., Florez I.D., Tamayo M.E., Mbuagbaw L., Vanniyasingam T., Veroniki A.A. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA, J Am Med Assoc. 2018;319:1221–1238. doi: 10.1001/jama.2018.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson P.J. Hydrocortisone for treatment of hypotension in the newborn. Neonatal Netw. 2015;34:46–51. doi: 10.1891/0730-0832.34.1.46. [DOI] [PubMed] [Google Scholar]
- 30.Eriksen V.R., Hahn G.H., Greisen G. Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatr. 2014;103:1221–1226. doi: 10.1111/apa.12817. [DOI] [PubMed] [Google Scholar]
- 31.Sankaran J., Brandsma E., Kushnir A. Effect of administration of normal saline bolus on intraventricular hemorrhage in preterm neonates. Pediatrics. 2018;141(1 MeetingAbstract):517. [Google Scholar]
- 32.Lin P.Y., Hagan K., Fenoglio A., Grant P.E., Franceschini M.A. Reduced cerebral blood flow and oxygen metabolism in extremely preterm neonates with low-grade germinal matrix-intraventricular hemorrhage. Sci Rep UK. 2016;6 doi: 10.1038/srep25903. 25903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhagen E.A., ter Horst H.J., Keating P., Martijn A., Van Braeckel K.N., Bos A.F. Cerebral oxygenation in preterm infants with germinal matrix–intraventricular hemorrhages. Stroke. 2010;41:2901–2907. doi: 10.1161/STROKEAHA.110.597229. [DOI] [PubMed] [Google Scholar]
- 34.Noori S., McCoy M., Anderson M.P., Ramji F., Seri I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J Pediatr. 2014;164:264–270. doi: 10.1016/j.jpeds.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 35.Alderliesten T., Lemmers P.M., Smarius J.J., van de Vosse R.E., Baerts W., van Bel F. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr. 2013;162:698–704. doi: 10.1016/j.jpeds.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Perlman J.M., Rollins N. Surveillance protocol for the detection of intracranial abnormalities in premature neonates. Arch Pediatr Adolesc Med. 2000;154:822–826. doi: 10.1001/archpedi.154.8.822. [DOI] [PubMed] [Google Scholar]