Abstract
Hypertension is the largest risk factor for cardiovascular disease, the leading cause of mortality worldwide. As blood pressure regulation is influenced by multiple physiological systems, hypertension cannot be attributed to a single identifiable etiology. Three decades of research into Mendelian forms of hypertension implicated alterations in the renal tubular sodium handling, particularly the distal convoluted tubule (DCT)-native, thiazide-sensitive Na–Cl cotransporter (NCC). Altered functions of the NCC have shown to have profound effects on blood pressure regulation as illustrated by the over activation and inactivation of the NCC in Gordon's and Gitelman syndromes respectively. Substantial progress has uncovered multiple factors that affect the expression and activity of the NCC. In particular, NCC activity is controlled by phosphorylation/dephosphorylation, and NCC expression is facilitated by glycosylation and negatively regulated by ubiquitination. Studies have even found parvalbumin to be an unexpected regulator of the NCC. In recent years, there have been considerable advances in our understanding of NCC control mechanisms, particularly via the pathway containing the with-no-lysine [K] (WNK) and its downstream target kinases, SPS/Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress responsive 1 (OSR1), which has led to the discovery of novel inhibitory molecules. This review summarizes the currently reported regulatory mechanisms of the NCC and discusses their potential as therapeutic targets for treating hypertension.
KEY WORDS: NaCl-cotransporter NCC, Cardiovascular disease, CUL3/KLHL3-WNK-SPAK/OSR1, Blood pressure regulation, Kinase inhibitors, Membrane trafficking, Therapeutic targets, Hypertension
Abbreviations: ATP, adenosine triphosphate; Ca2+, calcium ion; CCC, cation-coupled chloride cotransporters; CCT, conserved carboxy-terminal; CNI, calcineurin inhibitors; CUL3, cullin 3; DAG, diacylglycerol; DCT, distal convoluted tubule; DUSP, dual specificity phosphatases; ECF, extracellular fluid; ELISA, enzyme-bound immunosorbent analysis; EnaC, epithelial sodium channels; ERK, extracellular signal-regulated kinases; GABA, gamma-aminobutyric acid; HEK293, human embryonic kidney 293; I1, inhibitor 1; K+, potassium ion; KCC, potassium-chloride-cotransporters; KLHL3, kelch-like 3; KS-WNK1, kidney specific-WNK1; MAPK, mitogen-activated protein kinase; mDCT, mammalian DCT; MO25, mouse protein-25; mRNA, messenger RNA; Na+, sodium ion; NaCl, sodium chloride; NCC, sodium–chloride cotransporters; NKCC, sodium–potassium–chloride-cotransporter; OSR1, oxidative stress-responsive gene 1; PCT, proximal convoluted tubule; PHAII, pseudohypoaldosteronism type II; PP, protein phosphatase; PV, parvalbumin; RasGRP1, RAS guanyl-releasing protein 1; ROMK, renal outer medullary potassium; SLC12, solute carrier 12; SPAK, Ste20-related proline-alanine-rich-kinase; TAL, thick ascending limb; WNK, with-no-lysine kinases
Graphical abstract
This review proposes integrated model of NCC regulation, and summarizes therapeutic strategies to target the NCC via its regulatory pathway.
1. Renal sodium handling and hypertension
The renal system plays a critical role in the homeostasis of blood pressure. One of the ways this role is accomplished is through the maintenance of electrolyte balance in the extracellular fluid (ECF) whereby electrolyte intake is equalized with electrolyte excretion by the kidneys. As sodium (Na+) is the main ionic constituent of the ECF, Na+ reabsorption in the kidney is tightly coupled to obligatory water reabsorption. Thus, a disturbance in Na+ reabsorption provokes abnormal water retention or loss. Water retention in particular, increases blood pressure due to ECF volume expansion which places considerable strain on the blood vessels leaving the kidneys1. Consistent high blood pressure or hypertension is a major risk factor for many cardiovascular diseases2.
The functional unit of the kidney, the nephron, is divided into two segments: (i) the renal corpuscle for which blood plasma is filtered and (ii) the renal tubule where substances are reabsorbed. Of the 99% of Na+ reabsorbed along various parts of the renal tubule, 50%–60% is reabsorbed by the proximal convoluted tubule (PCT), 20%–30% is reabsorbed by the thick ascending limb (TAL), and 5%–10% by the distal convoluted tubule (DCT). Although a huge portion of Na+ is reabsorbed in the PCT, high salt (NaCl) intake is usually offset by decreased Na+ reabsorption in the DCT as it is uniquely capable of adapting to changes in hormonal stimuli3. The importance of the DCT is further supported by insights gained from genetic disorders of hypertension that revealed a close association between Na+ handling in the DCT and blood pressure regulation.
2. Na–Cl cotransporter (NCC) and the SLC12 gene family
The solute carrier 12 (SLC12) gene family encodes for the electroneutral cation-coupled chloride cotransporters (CCCs) family of membrane proteins. Genes within the family are highly homologous and are further divided into subfamilies of chloride translocation either with Na+ or potassium (K+) in a 1:1 stoichiometry4. The Na+ driven family consists of NCC and two isoforms of sodium–potassium–chloride cotransporter (NKCCs): NKCC1 and NKCC2. The K+ driven family of potassium–chloride cotransporters (KCCs) is made up of KCC1–KCC4. Two additional proteins, CCC6 and CCC9, are uncharacterized thus far. Although all members of the SLC12 family are regulated by kinase-induced phosphorylation and phosphatase-induced dephosphorylation at key serine/threonine residues, phosphorylation has the opposite effects on the two subfamilies5. Phosphorylation activates the Na+-dependent branches and inactivates the Na+-independent branch.
Genetic mutations in genes that encode for the Na+ driven family revealed the importance of this subfamily in blood pressure regulation. NKCC2 is kidney-specific and is the major salt transport pathway in the TAL (Fig. 1). Inhibition of NKCC2 by loop diuretics impairs Na+ reabsorption, ultimately decreasing blood pressure6. Loss-of-function mutations in the SLC12A1 gene that encodes for NKCC2, such as c.1833 deletion, cause type 1 Bartter syndrome, a disorder characterised by hypokalaemia, alkalosis along with normal-to hypotension7. Contrarily, although NKCC1 is widely distributed, its blood pressure regulating effects remain poorly understood. NCC is exclusively expressed in the DCT and is the site of one of the most effective antihypertensive, thiazide diuretics8,9. As the NCC is important for fine-tuning of salt homeostasis, altered functions of the NCC have profound effects on blood pressure regulation. Gitelman syndrome, a loss of NCC function, is characterised by salt loss and hypotension. Gain-of-function mutations in genes encoding for the regulators of NCC lead to the clinical inverse of Gitelman, Gordon's syndrome [also known as pseudohypoaldosteronism type II (PHAII)], a disorder characterised by salt retention and hypertension10.
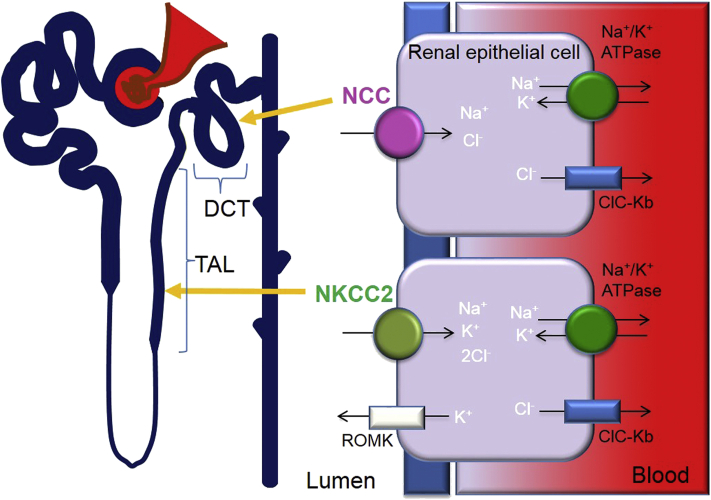
Figure 1.
Sodium handling in the distal nephron. The thick ascending limb (TAL) is a region responsible for 20%–30% of sodium (Na+) reabsorption. The predominant mechanism of transport in the TAL is the Na–K–Cl cotransporter 2 (NKCC2). The distal convoluted tubule (DCT) is responsible for 5%–10% of Na+ reabsorption. The major Na+ transport in the DCT is the Na–Cl co-transporter (NCC). Other ion transport mechanisms include the renal outer medullary potassium channel (ROMK), the sodium potassium pump (Na+/K+ ATPase) and the chloride channel Kb (CLC-Kb).
3. Regulation of the NCC
The distal nephron has a central role in blood pressure regulation1. This role is accomplished through maintenance of Na+ balance in the ECF. Although the majority of Na+ is reabsorbed in the proximal nephron, the distinctive capability of the DCT to respond to changes in hormonal stimuli means that it is responsible for fine-tuning of Na+ homeostasis in the ECF3. Amongst the Na+ transporters in the distal nephron, the effects of mutations in NCC, a salt transporter exclusively expressed in the DCT, and its regulators on the ECF, illustrates the importance of the NCC in blood pressure regulation.
NCC is functional in a homodimeric form and is glycosylated for efficient function and surface expression11. Like all the other SCL12 family of cotransporters, NCC activity is regulated by phosphorylation/dephosphorylation at key serine/threonine residues. However, unlike the K+ driven family of SLC12 cotransporters, phosphorylation activates the NCC and dephosphorylation halts their activity. Ubiquitination and consequent endocytosis of NCC downregulates NCC surface expression12.
3.1. Phosphorylation by WNK-SPAK/OSR1 kinases: master regulator of the NCC
Phosphorylation at key serine/threonine sites (Thr46, Thr55 and Thr60) triggers the activation of the NCC and inhibits the ubiqutination and subsequent endocytosis of NCC. With-no-lysine kinases (WNKs) are serine–threonine kinases that are characterized by the atypical placement of their catalytic lysine residue13. WNKs modulate the SLC12 family of CCCs for transport between cells. Their primary target is the NCC (Fig. 2). Two isoforms of the WNK family (WNK1–4), WNK1 and WNK4, are expressed in the mammalian DCT. However, the predominant isoform in the DCT is a kidney-specific short isoform of WNK1, kidney specific-WNK1 (KS-WNK1), which lacks a kinase domain. Although the physiological role of KS-WNK1 remains elusive, recent evidence suggests it has a positive effect on the WNK signaling14. In 2001, mutations in the genes that encode for WNK1 and WNK4 were discovered to cause Gordon's syndrome10,15,16. Mutations in the WNK1 gene are intronic deletions that lead to the ectopic expression of full-length WNK1, an isoform that is expressed at low levels in the DCT. Mouse models overexpressing WNK1 displayed enhanced phosphorylation of NCC10,17. Increased phosphorylation and thus activation of NCC allows for more Na+ reabsorption, consequently raising blood pressure. The link was observed in animal models that carry mutated WNK4, which demonstrated increased phosphorylated NCC levels and produced Gordon's-like phenotype. Wnk4 deficient mice displayed reduced NCC phosphorylation and hypotension similar to that of Gitelman phenotype18,19. These studies provided insight into the pathological mechanisms underlying hypertensive-causing WNK mutations, demonstrating an essential role for WNK in the kidney.
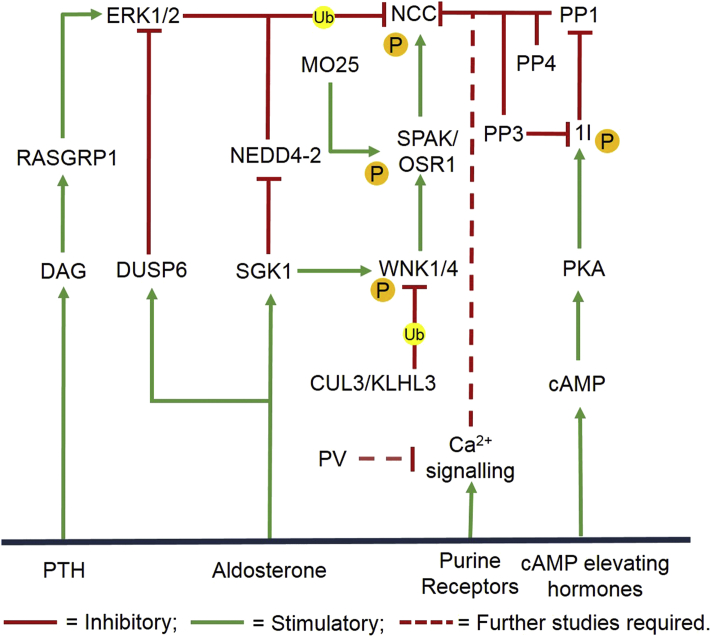
Figure 2.
Proposed integrated model of NCC regulation. The Na+–Cl− co-transporter (NCC) is the principal salt absorptive pathway in the distal convoluted tubule (DCT). The NCC is activated by kinase-induced phosphorylation (P) via the with-no-lysine [K] (WNK) and its downstream target kinases, SPS/Ste20-related proline–alanine-rich kinase (SPAK) and oxidative stress responsive (OSR) and deactivated by phosphatase-induced dephosphorylation via protein phosphatase 1-3-4 (PP1-3-4). Activation of SPAK is enhanced via attachment of the mouse protein-25 (MO25). The expression of NCC is directly regulated via ubiquitination and subsequent endocytosis of NCC via the RAS guanyl nucleotide-releasing protein (RasGRP) and its downstream target extracellular signal-regulated protein kinase (ERK) 1/2 and NEDD4-2 or indirectly via ubiquitination and suppression of WNK by the cullin3 (CUL3)/kelch-like-3 (KLHL3) ubiquitin ligase complex. ERK1/2 is inhibited by DUSP6 and the phosphatases are inhibited by an endogenous inhibitor 1 (I1). Further studies are required to evaluate the NCC regulatory effects of parvalbumin (PV) via modulations of the ATP-induced Ca2+ signaling. The modulators of these regulatory events include parathyroid hormone (PTH), aldosterone, purine receptors and cAMP elevating hormones.
The molecular mechanism by which WNKs regulate blood pressure was discovered when it was reported that WNKs bind and phosphorylate both Ste20-related proline–alanine-rich kinase (SPAK) and oxidative stress-responsive gene 1 (OSR1) thus inducing their activation20, 21, 22, 23. Furthermore, mice with defected SPAK and OSR1 had reduced baseline blood pressure significantly24, 25, 26, 27, 28. Increased phosphorylated NCC was observed in cells overexpressing SPAK29. Based on their interactions with WNKs and NCCs, it is believed that WNK regulates the NCC through SPAK/OSR1 (Fig. 2). Further analyses found that upon activation, SPAK and OSR1 subsequently bind mouse protein-25 (MO25), a scaffolding protein which significantly enhances their basal activity by 80- to 100-fold, respectively30. Active SPAK and OSR1 in complex with MO25 phosphorylate a selection of cotransporters including NCC at different residues: Thr45/46/50/55/60 and Ser71/73/91. Such phosphorylation influences movement of salt through the NCC, ensuing changes in electrolyte balance which ultimately translate into changes in blood pressure. Phosphorylation of NCC enhances transport activity at the plasma membrane and also prevents NCC ubiquitination and consequent endocytosis31,32. Polymorphisms in the SPAK33,34, WNK135, 36, 37, 38 and WNK439 have been linked to human hypertension.
More recent investigations reveal multiple physiological regulators of NCC such as dietary K+ intake, insulin and more. However, these electrolyte and hormonal stimuli are increasingly associated with the WNK–SPAK/OSR1 signaling pathway40, 41, 42, 43, 44. Notably, although aldosterone was previously thought to directly upregulate NCC, aldosterone has been shown to indirectly regulate the WNK–SPAK/OSR1 pathway and by extension, the NCC via modulation of plasma potassium levels42,45,46. Less extensively studied but recognized WNK–SPAK/OSR1-dependent hormonal manipulators of NCC are insulin, norepinephrine and angiotensin II47. A more detailed account of the modulators of the WNK–SPAK pathway can be found in recent reviews by Wu et al.12 and Furusho et al48. Taken together, these investigations support the role of WNK–SPAK/OSR1 as the master activator of the NCC.
3.2. Dephosphorylation by PP3/PP4: the counter-regulatory system
Although much work has been focused on phosphorylation induced activation of NCC, it has been reported that dephosphorylation of NCC involving the serine–threonine protein phosphatase (PP)-3 or calcineurin, along with PP1 and PP4, can counterbalance the kinases acting on the NCC (Fig. 2). Studies by Glover et al.49 and Gamba et al.50 using the Xenopus oocyte system, observed PP4 inhibition of NCC activity in a phosphatase-dependent manner, suggesting that phosphatases may inhibit NCC. Similarly, experiments with pharmacological inhibitors further support the counter-regulatory mechanisms of phosphatases on NCC. Calcineurin inhibitors (CNI) are used as anti-rejection drugs in transplant patients51. The common side effects of CNIs resemble the cardinal features of Gordon's syndrome, potentially due to its effect on the NCC. Indeed, administration of two CNIs, tacrolimus52 and cyclosporine53, has been shown to increase phosphorylation and activation of NCC. Inhibition of the phosphatase calcineurin and the consequent inhibition of NCC dephosphorylation is a likely mechanism to counterbalance the phosphorylation activity of the WNK kinases. This is supported by recent studies that observed a rise in blood pressure in wild-type mice treated with tacrolimus in comparison to NCC-knockout mice, emphasized by exaggerated effects in mice overexpressing NCC54. The addition of hydrochlorothiazide, reversed the tacrolimus-induced hypertension in the mice54. These findings are consistent in patients as immunohistochemistry of transplant biopsies from kidney donor recipients revealed a pronounced increase in total and phosphorylated NCCs of those treated with tacrolimus when compared to the control group54.
Consistent with the regulatory role of phosphatase in NCC activity, treatment with the pharmacological inhibitor of PP1, calyculin A has been observed to enhance NCC phosphorylation55. Coincidentally, the endogenous PP1 inhibitor, inhibitor 1 (I1), is expressed in the DCT and has been demonstrated to promote NCC activity55. Observations of phosphorylation and activation of I1 by cAMP-dependent PKA, coupled with increased phosphorylated NCC observed with cAMP elevating hormones (PTH and β-adrenergic agonists), suggests a link between PP1 phosphatase activity and NCC (Fig. 2). I1 knockout mice exhibited decreased levels of phosphorylated NCC56. Studies on the regulation of the NKCC1 found that PP1 binds directly to the N-terminal tail of NKCC1 in direct proximity to SPAK and that direct dephosphorylation is only 1 of 3 PP1 activities on NKCC1 regulation. Other inhibitory activities of PP1 on NKCC1 activity include dephosphorylation of SPAK and another undefined mechanism independent of its catalytic activity57. Although NCC lacks the acidic motif of the facilitated NKCC1 binding to PP1, in vitro assays revealed that PP1 directly interacts with and dephosphorylates NCC55. However, recent studies link PP1 to the WNK–SPAK/OSR1 pathway through modulation of WNK4 and SPAK phosphorylation despite the lack of a significant involvement of I1 in SPAK/OSR1 regulation58. These findings do not preclude the indirect control of NCC by phosphates via the WNK–SPAK/OSR1 pathway. However, as the study specifically investigated the role of PP1 on WNK4 inhibition of the renal outer medullary potassium (ROMK) channel, a channel that is inversely regulated by WNK4 in comparison to NCC, further research is needed to explore possible indirect effect of PP1 through WNK4. Although additional work will be necessary to determine the mechanism of phosphatase action, the current findings suggest that phosphatases may inhibit NCC activity directly through dephosphorylation and inactivation of the NCC.
3.3. Ubiquitination by RasGRP1/NEDD4-2/KLHL3/CUL3: negative regulation of NCC
Early studies reported that functional NCC is expressed in a glycosylated homodimeric form on the plasma membrane or in sub-apical vesicles59,60. However, very little is known of the regulatory mechanisms that govern the membrane expression of NCC61. Extracellular signal-regulated kinases (ERK1/2) mitogen-activated protein kinase (MAPK) is an established modulator of other ion transporters expression such as ROMK and epithelial sodium channels (ENaC) through ubiquitination and subsequent degradation62,63. A study was conducted by Ko and colleagues64 to assess if ubiquitination of NCC is regulated by ERK1/2 MAPK signaling pathway, or if there is a potential role of ERK1/2 MAPK in NCC surface expression. Utilizing heterologous mammalian expression, the study reported a reduction in cells and NCC surface expression via ubiquitination by ERK1/2. Ubiquitination by ERK1/2 led to endocytosis and decreased NCC activity (Fig. 2). This process could be disabled by inhibition of ubiquitin-activating enzyme E1 with UBEI-4164. Further studies revealed that ERK1/2 MAPK activation is dependent on phosphorylation by RAS guanyl-releasing protein 1 (RasGRP1). RasGRP1 is directly activated by diacylglycerol (DAG). Treatment with phorbol ester, an analog of DAG, showed reduced NCC membrane activation and a rise in internalized NCC65. Concurrently, studies using the Xenopus laevis oocytes as expression systems revealed that WNK4 reduces NCC abundance on the plasma membrane66. Overexpression of WNK4 in COS-7 cells also demonstrated reduced NCC surface protein expression via enhanced degradation through a lysosomal pathway67, 68, 69. Although SPAK/OSR1 is downstream effectors of WNKs, WNK4 modulation of NCC expression has been revealed to occur via activation of the ERK1/2 MAPK signaling pathway68, 69, 70. A study by Zhou and colleagues70 demonstrated that WNK4 enhanced phosphorylation of ERK1/2 signaling in a dose-dependent manner and that knockdown of WNK4 reduced ERK1/2 phosphorylation and raised the total endogenous expression of NCC in mDCT cells. Other modulators confirmed to regulate NCC via the RasGRP1–ERK1/2 MAPK pathway are the parathyroid hormones71,72. Dual specificity phosphatases (DUSP), inhibitor of ERK1/2, decrease ubiquitination of NCC, consequently increasing NCC abundance73. As mentioned in previous sections and noted by Rosanbaek and other teams31, the levels of NCC phosphorylation and ubiquitination are linked despite their contrasting roles in NCC plasma membrane level modulation. A greater plasma level of NCC is seen with increased levels of phosphorylated NCC and reduced plasma membrane levels of ubiquitinated NCC correlated to decreased NCC endocytosis31,74,75. The ubiquitin–protein ligase, NEDD4-2, is implicated in NCC ubiquitination and consequent suppression. Modulation of NEDD4-2 in vitro and in vivo reduces NCC abundance and expression on the membrane76, 77, 78. NEDD4-2 knockout mice possessed features akin to Gordon's syndrome and an amplification of NCC phosphorylation. Although NCC co-immunoprecipitated with NEDD4-278, the effect of NEDD4-2 was eliminated by WNK379. Thus further research is needed to further elucidate the influence or absence of WNK kinases on the NCC endocytic pathway.
The WNK–SPAK/OSR1–NCC pathway has been shown to be downregulated via degradation by the cullin 3 (CUL3)–kelch-like 3 (KLHL3) E3 ubiquitin ligase complex (Fig. 2). KLHL3 and CUL3 make up the E3 ubiquitin ligase complex that targets their substrate proteins for proteasome degradation via attachment of ubiquitous moieties80. WNKs are substrates of the KLHL3–CUL3 ligase complex81, 82, 83. Upon binding to the ligase complex, WNKs are ubiquitinated. The ubiquitinated WNK is then targeted for degradation via the ubiquitin–proteasome system. In 2012, mutations in CUL3 and KLHL3 were identified in families with Gordon's syndrome. Mutations of KLHL3 impair its bindings to WNK and CUL3 and mutations of CUL3 lead to enhanced ubiquitin ligase activity and the subsequent degradation of KLHL384,85. These mutations consequently reduce WNK degradation leading to the accumulation of NCC. Overall, these studies establish a compelling rationale for the importance of ubiquitination for the direct regulation of NCC via the RasGRP1–ERK1/2 pathway and NEDD4-2 or indirect regulation of NCC via the ubiquitination and suppression of WNK.
3.4. Parvalbumin (PV): an unexpected regulator of NCC and diuretic response
PV is a calcium (Ca2+) binding protein. In neurons and skeletal cell muscles, PV is a calcium buffer capable of modulating calcium currents induced by purinergic agonists such as adenosine triphosphate (ATP, Fig. 2). These calcium currents can cause a decrease in transport systems including those that are involved in Na+ reabsorption. PV is selectively expressed in the early part of the mammalian DCT (mDCT) where it co-localizes with the NCC86,87. Studies using mDCT cells reveal a decrease in endogenous expression of NCC, WNK1 and WNK4, following PV knockdown88,89. The role of PV regulation on the NCC is further emphasized by phenotypic analysis of PV knockout mice that presented mild salt wasting, kaliuresis and enhanced Ca2+ reabsorption, a phenotype similar to Gitelman syndrome88. In addition, the mice exhibited a significant decrease in NCC expression which is reflected by impaired response to diuretics, suggesting a functional link between PV and the NCC. It should be noted that reduced messenger RNA (mRNA) levels of WNK4 and KS-WNK1 were also observed in the PV deficient mice and that the sample size was low88.
Further studies demonstrated that PV control of endogenous NCC expression is via the ATP-induced Ca2+ current in mDCT cells. PV was revealed to modulate the shape and the duration of intracellular Ca2+ signaling by effectively reducing the amplitude of the ATP-induced cytoplasmic Ca2+ elevation. This finding is consistent with previous work which supported ATP induced inhibition of Na+ reabsorption in the DCT via purinergic receptors90. A study in 201491 demonstrated that purinergic receptor activation led to decreased expression of NCC and that the silencing of these receptors reduced ATP-induced downregulation of NCC expression. However, it is important to note that the mice without NCC displayed slightly different pathologies to the mice without PV. The mice without NCC presented with alterations of the DCT, which was not observed in the mice without PV. This suggests that PV is not a significant regulator of NCC and that there may be other variables and pathways that are more influential. This is further supported by transcriptional analysis of Gitelman syndrome patients (n = 79; P < 0.05) who lack the inactivating mutations in the SLC12A3 gene that encodes for the NCC but did not reveal mutations in their PV genes92,93. As PV expression has been repeatedly confirmed to be critical for sodium handling and responses to diuretics, the authors of the conflicting study speculate that the negative results could be due to inter-species differences. Although a link between PV expression and a Mendelian disease has not been established, the confirmed phenotype suggests that NCC expression could be indirectly regulated by PV via modulations of the ATP-induced Ca2+ signaling. However, further investigation is needed to evaluate the significance of PV to the regulation of the NCC and tubulopathies in the DCT.
4. Potential therapeutic targets
To date, antihypertensives are insufficient as monotherapy and often provoke multiple off-target side effects94,95. The use of thiazides, an antihypertensive that reduces blood pressure by inhibiting the NCC, comes with an increased risk of type II diabetes. Prolonged usage of thiazide diuretics has been shown to increase the membrane density of NCC but not enhanced reabsorption of Na+96,97. The prevalence of resistant hypertension, defined as uncontrollable blood pressure despite treatment with 3 different antihypertensives, and refractory hypertension, defined as high blood pressure despite maximal therapy, both of which substantially increase the risk of heart attack and stroke, presents a pressing global challenge in treating hypertension98. Therefore, new therapeutics targets are urgently needed.
The importance of the NCC in blood pressure regulation is suggested by the monogenic disorders that present with either high or low blood pressure as a result of mutations in the NCC and its regulators. NCC over-activation in particular results in a form of hypertension termed Gordon's syndrome. Gordon's syndrome is caused by mutations in WNKs and in the ubiquitin ligase component that regulates them, CUL3/KLHL3. These genetic defects inappropriately activate the NCC leading to enhanced Na+ reabsorption, consequently raising blood pressure. As the WNK–SPAK/OSR1 pathway is also the master regulator of NCC, research is focused on identifying novel targets within the pathway for use in Gordon's syndrome and non-Mendelian forms of hypertension.
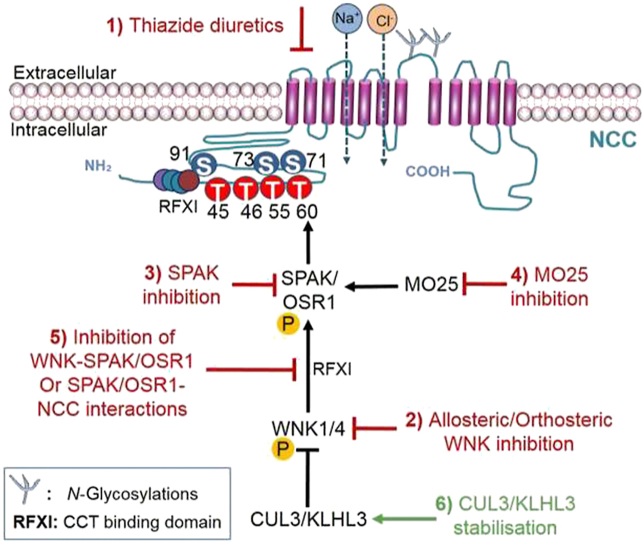
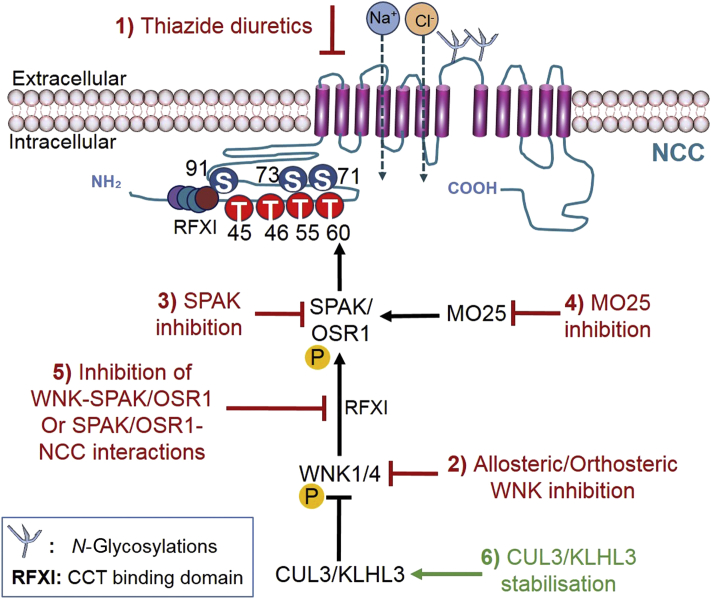
The WNK–SPAK/OSR1 signaling pathway provides 6 points of intervention. NCC over activation could be attenuated by (1) inhibition of NCC by thiazide diuretics, (2) allosteric or orthosteric inhibition of WNK kinases, (3) direct inhibition of SPAK/OSR1, (4) inhibition of MO25, (5) inhibition of WNK–SPAK/OSR1 interaction, and (6) stabilization of CUL3/KLHL3 interaction. An alternative strategy outside of the WNK–SPAK/OSR1 regulatory pathway is (7) the impairment of glycosylation. A summary of the therapeutic interventions of regulatory mechanisms of NCC is depicted in Fig. 3. As NCC inhibition of thiazide diuretics is a strategy currently used to treat hypertensive patients, this review will focus on the other targets that are not in clinical use.
Figure 3.
Summary of therapeutic strategies to target the NCC via its regulatory pathway. Over activation of the Na+–Cl– co-transporter (NCC) leads to salt retention and hypertension. The CUL3/KLHL3–WNK–SPAK/OSR1 regulatory pathway of NCC (black arrows) presents 6 points of interventions. Red arrows represent the therapeutic inhibition of 1) NCC, 2) WNK, 3) SPAK, 4) MO25, and 5) WNK–SPAK/OSR1 interactions which would all suppress NCC activation. The green arrow represents 6) the stabilisation of CUL3/KLHL3 interactions which would increase degradation of WNK and thus suppress NCC activation by the WNK–SPAK/OSR1 pathway. Glycosylation of the NCC is critical for the function and trafficking of NCC to the plasma membrane and thus (7) impairment of glycosylation could also suppress NCC activity and potentially be therapeutic.
4.1. Allosteric or orthosteric inhibition of WNK kinases
Despite the overwhelming evidence of WNK–SPAK/OSR1 signaling pathway in blood pressure regulation, there are currently no clinically-approved WNK–SPAK/OSR1 targeting drugs that are used to treat hypertension. Targeting NCC by inhibiting WNK is to date the strategy furthest along the drug discovery pipeline. This is due to the high level of selectivity provided by the irregular placement of the catalytic lysine residue of WNK that creates a WNK-specific back pocket. ATP-competitive molecules inhibits WNKs orthosterically by exploiting this abnormal structural configuration of WNK99. Multiple screenings by various groups identified several inhibitors100, 101, 102. Notably, a Novartis group screened compounds and identified WNK463, the first orally bioavailable WNK kinase inhibitor. WNK463 prevented WNK-mediated phosphorylation of OSR1 in human embryonic kidney 293 (HEK293) cells expressing OSR1 and produced a dose-dependent decrease in blood pressure in hypertensive rats103. However, as the ATP binding site is conserved in all 4 isoforms, WNK463 was found to potently inhibit all isoforms of WNKs (WNK1 IC50 = 5 nmol/L, WNK2 IC50 = 1 nmol/L, WNK3 IC50 = 6 nmol/L, WNK4 IC50 = 9 nmol/L). This posed a challenge as WNKs are ubiquitously expressed and are participants in various physiological processes, thus further development of WNK463 was discontinued due to an unacceptable safety profile. Recent molecular modeling and docking simulations on the binding of WNK463 across all isoforms confirmed the lack of specificity but found that despite the high sequence similarity (>80%) among WNK kinases, the composition of residues in the ATP binding region that produced the marginal differences in selectivity could be exploited104. Further screening by the Novartis group found an allosteric binding pocket of WNK1 that co-crystallized with multiple compounds105. Inhibitors of allosteric targets provide better selectivity as the region is less conserved in relation to the ATP binding pocket. The list was filtered and compounds were optimized structurally due to selectivity or inadequate pharmacokinetics profile until the discovery of compound 11. Oral dosing of compound 11 led to reduction in systolic blood pressure, regulated blood fluid, and electrolyte homeostasis in normotensive and hypertensive rodent models in a dose-dependent manner106. However, when administered at higher doses, unspecified events beyond those reported in the cardiovascular and renal systems, such as induced ataxia and breathing difficulties, were observed in mice at 1–10 mg/kg doses107. These adverse effects may be due to the lack of specificity of WNK463, thus further development of kidney-specific WNK inhibitors are needed in the field for hypertension treatment. Despite the discovery of small molecule inhibitors that are able to inhibit WNKs, a major challenge remains identifying reagents that are able to better differentiate WNK isoforms.
4.2. Inhibition of SPAK/OSR1
Various SPAK mouse models have indicated that inhibition of SPAK and OSR1 may reduce blood pressure. To validate SPAK as a target, researchers have confirmed a reduction in the blood pressure of either SPAK or OSR1 or both in knockout mice24,108. The first SPAK and OSR1 kinase inhibitor was reported in 2015 when the Uchida's group109 developed an enzyme-bound immunosorbent analysis (ELISA) assay that was utilized to screen >20,000 small-molecule compounds in addition to 840 compounds of FDA-approved drugs. This study led to the identification of two structurally related SPAK inhibitors: Stock 1S-14279 (IC50 = 0.26 μmol/L) and closantel (IC50 = 0.7 μmol/L). Promising in vitro studies led to acute administration of both compounds in mice. Despite a rapid drop in blood pressure and heart rate within 30 min and a significant decrease in phosphorylated NCC, the effect of both compounds was transient, lasting only 120 min. Although both compounds have passed critical stages of clinical development and testing, thus are great candidates for hypertensive treatment, repeated injections of Stock 1S-14279 were lethal and prolonged administration of closantel failed to reduce blood pressure by Day 7109. Recent molecular studies report that both compounds inhibit SPAK independent of ATP, revealing a highly conserved secondary pocket on the conserved carboxyl-terminal (CCT) domain of SPAK/OSR1110. Following the discovery of the secondary pocket, an in silico screening was completed by Mehellou and colleagues111 to identify inhibitory compounds. Rafoxanide, a compound structurally similar to closantel, was identified to inhibit OSR1 in an ATP-dependent manner (IC50 = 8.18 μmol/L). However, rafoxanide was only able to inhibit endogenous SPAK and OSR1 in cells at concentrations <15 μmol/L110. Alternatively, recent reports by a group using high-throughput screening of 1200 U.S. Food and Drug Administration (FDA)-approved compounds at 20 μmol/L yielded verteporfin, an inhibitor of SPAK and OSR1 in vitro in an ATP-independent manner111. Verteporfin binds to an allosteric site adjacent to the kinase domain. Although in vivo studies have not been completed, this finding is consistent with the observation of reduced blood pressure in animals treated with verteporfin112. However, further screening revealed verteporfin potently inhibits (>70%) 8 other kinases at 1 μmol/L. Therefore, structural optimization of verteporfin will be needed to ensure its selectivity for SPAK/OSR1 to prevent undesirable side effects. Although inhibition of SPAK/OSR1 proves to be a promising strategy, targeting SPAK/OSR1 may disturb the reverse regulation of gamma-aminobutyric acid (GABA) signaling mediated by SPAK in the brain5. Additionally, even if the kidney is selectively targeted, adaptive mechanisms to chronic usage of thiazide have been identified in SPAK knockout mice97,113, 114, 115, 116, 117. These compensatory changes include DCT remodeling and activation of a paracrine signaling system to induce salt reabsorption pathways in other parts of the nephrons. No data on compensatory action due to OSR1 inhibition could be provided as knockout of OSR1 is embryonic lethal118. Nonetheless, inhibitors of SPAK increases therapeutic options, ultimately increasing the number of drugs in trials.
4.3. Inhibition of MO25
As the kinase activity of SPAK and OSR1 is significantly enhanced (80- to 100-fold, respectively) by binding to the scaffolding protein, MO25, a fluorescence polarization assay was used to screen a library of ∼4000 compounds for inhibitors of SPAK/OSR1–MO25 interaction. The screen uncovered HK01 (IC50 = 78 ± 4 μmol/L)119. Binding assays confirmed that HK01 binds directly to MO25 and inhibited phosphorylation of NKCC1 in a concentration-dependent manner. Although this approach would only produce limited inhibitory effects on the SPAK/OSR1 kinase activity, this may be desirable as mild reduction could prevent extreme phenotypic effects.
4.4. Inhibition of WNK-SPAK/OSR1 interactions
Alternatively, the observation of reduced phosphorylation of NCC in mice with homozygous mutations in the SPAK CCT domain, a domain which recognizes WNK and NCC, supports for the inhibition of SPAK CCT domain which interferes with SPAK/OSR1 binding to WNK kinases. Screening of 17,000 compounds identified two compounds, STOCK1S-50699 and STOCK2S-2601 which bind to the SPAK/OSR1 CCT domain20,120. Although both compounds exhibited inhibition of SPAK phosphorylation and its downstream targets NCC and NKCC2 in cultured cell lines, STOCK2S-26016 did not suppress SPAK/OSR1 phosphorylation in vitro and STOCK1S-50699 displayed undesirable pharmacokinetics in vivo. More recently, a potent and selective SPAK inhibitor, ZT-1a, was developed by Zhang et al.107. ZT-1a, an amalgamation of pharmacophores, inhibited less than 1% of kinases that were tested and reduced NCC phosphorylation in vivo. However, it is not clear whether ZT-1a administration will lead to blood pressure reduction. As research on ZT-1a in the kidney is limited, further studies are needed to investigate its effects in normotensive and hypertensive rodent models.
4.5. Stabilization of CUL3/KLHL3 interaction
The central and multifaceted roles of ubiquitination in modulating NCC activity and expression justify the relevance in identifying modulators of ubiquitination that could be targeted to avoid pathological consequences. Mutations in the E3 ubiquitin ligase complex, KLHL3 and CUL3, prevent the degradation of WNK through disruptions in the binding of the ubiquitin ligase complex121,122. Therefore, rather than inhibition, stabilization of CUL3 and KLHL3 interactions may be an approach to lower blood pressure in NCC-dependent hypertension. There are currently no stabilizing molecules that have been reported. As the most severe causative mutations in Gordon's is CUL3>recessive KLHL3>dominant KLHL3>WNK4>WNK1, CUL3/KLHL3 may be mistaken as the most attractive target in the pathway. However, the lack of pharmacological agents or compounds has proven CUL3 to be the most challenging target due to the nature of CUL3 mutations. Mutations in CUL3 produce a modified form of CUL3 that has an enhanced ability to ubiquitinated itself or substrates, one of which is KLHL3123. The gain-of-function mutant is also more susceptible to enhanced activation by Nedd8 through a process termed neddylation. Neddylation is the covalent attachment of Nedd8 to CUL3, leading to CUL3 activation and deneddylation is the removal of Nedd8 modifications from CUL3 by the COP9 signalosome. Hence, the inhibition of deneddylation or the promotion of neddylation to increase CUL3 activation and consequently promote WNK degradation could represent viable therapeutic strategies82. However, CUL3 over activation may promote off-target self-ubiquitination. Moreover, KLHL3 is highly expressed in the DCT, thus targeting KLHL3 could possibly lead to compensatory adaptations by the kidney as seen in some patients on thiazide diuretics124. Accordingly, there is no doubt that significant progress towards understanding the physiological and pathological function of CUL3/KLHL3 is desirable to guide the development of novel antihypertensive drugs. More specifically, the studies discussed suggest that additional chemistry work is needed to identify a specific Nedd8 inhibitor that will allow for the evaluation of Nedd8 as a potential target for the treatment of hypertension.
4.6. Targeting glycosylation
As mentioned previously, NCC functions in a glycosylated state. There are two glycosylation sites in humans (N406 and N426) and rats (N404 and N424). Mutations of both glycosylation sites result in symptoms resembling Gitelman syndrome. Although impaired glycosylation obstructs folding and processing of the NCC to the plasma membrane, and reduces NCC activity remarkably, elimination of the glycosylation sites in rats increases the affinity to metolazone, a thiazide-like diuretic125. There have also been suggestions that the variable glycosylation of NCC accounts for the irregular sensitivity to metolazone among Gitelman disease NCC mutants126. Studies using the X. oocyte system expressing NCC mutations identified in patients with Gitelman syndrome, exhibited increased metolazone sensitivity127. Further studies into novel NCC mutants of Gitelman disease, uncovered a Thr392I1e mutation that displayed severely reduced Na+ uptake. Due to the close proximity of the Thr392I1e mutation to the glycosylation residues, the mutation may have therefore impaired glycosylation. Studies using Western blot revealed an absence of glycosylated NCC in Thr329I1e mutation128. Thus, impaired glycosylation likely obstructs processing of NCC to the plasma membrane, consequently diminishes NCC transport activity and could be a therapeutic strategy in hypertension. Further studies are needed to confirm increased glycosylation in the pathogenesis of hypertension before screening could be done to identify specific compounds targeting glycosylation.
5. Conclusions and future directions
The discovery of genetic mutations in monogenic forms of hypertension, coupled with recent molecular and pre-clinical researches, has further elucidated the regulatory mechanisms of NCC activity and expression in blood pressure regulation. NCC is activated by phosphorylation via the WNK–SPAK/OSR1 pathway and deactivated by phosphatase-induced dephosphorylation. The expression of NCC is regulated through ubiquitination by ERK1/2, NEDD4-2 and glycosylation. Recent investigations revealed PV as a regulator of NCC through modulation of ATP-induced Ca2+ signaling. Collectively, these insights illustrate a pharmacological potential to treat hypertension. Indeed, this has already led to the identification and confirmation of molecular targets particularly within the WNK–SPAK/OSR1 pathway. Although there is overwhelming evidence of WNK–SPAK/OSR1 potential as a therapeutic strategy, the drug discovery process is impeded by a lack of selectivity across WNK and SPAK isoforms and the threat of resistance mechanisms by the kidneys. Further studies and screening concerning the inhibition of the ubiquitination and glycosylation of NCC are important and could be beneficial in identifying alternative therapeutic strategies for hypertension.
Acknowledgment
Maarten P. Koeners is supported by the University of Exeter Medical School (UK). Jinwei Zhang is supported by the University of Exeter Medical School (UK) and NIH Grants R01 NS109358 (USA).
Author contributions
Nur Farah Meor Azlan, and Jinwei Zhang were responsible for writing the whole passage. Nur Farah Meor Azlan, Maarten P. Koeners, and Jinwei Zhang were in charge of checking and revision. All the figures in the article were made by Nur Farah Meor Azlan and Jinwei Zhang.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Strazzullo P., Galletti F., Barba G. Altered renal handling of sodium in human hypertension: Short review of the evidence. Hypertension. 2003;41:1000–1005. doi: 10.1161/01.HYP.0000066844.63035.3A. [DOI] [PubMed] [Google Scholar]
- 2.He J., Whelton P.K. Elevated systolic blood pressure and risk of cardiovascular and renal disease: Overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138:211–219. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 3.Matsubara M. Renal sodium handling for body fluid maintenance and blood pressure regulation. Yakugaku Zasshi. 2004;124:301–309. doi: 10.1248/yakushi.124.301. [DOI] [PubMed] [Google Scholar]
- 4.Subramanya A.R., Ellison D.H. Distal convoluted tubule. Clin J Am Soc Nephrol. 2014;9:2147–2163. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Los Heros P., Alessi D.R., Gourlay R., Campbell D.G., Deak M., Macartney T.J. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+–Cl– co-transporters. Biochem J. 2014;458:559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castrop H., Schiessl I.M. Physiology and pathophysiology of the renal Na–K–2Cl cotransporter (NKCC2) Am J Physiol Ren Physiol. 2014;307:F991–F1002. doi: 10.1152/ajprenal.00432.2014. [DOI] [PubMed] [Google Scholar]
- 7.Sun M., Ning J., Xu W., Zhang H., Zhao K., Li W. Genetic heterogeneity in patients with Bartter syndrome type 1. Mol Med Rep. 2017;15:581–590. doi: 10.3892/mmr.2016.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer L.G., Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10:676–687. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright J.M., Musini V.M., Gill R. First-line drugs for hypertension. Cochrane Database Syst Rev. 2018;4:CD001841. doi: 10.1002/14651858.CD001841.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson F.H., Disse-Nicodeme S., Choate K.A., Ishikawa K., Nelson-Williams C., Desitter I. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 11.de Jong J.C., Willems P.H., Mooren F.J., van den Heuvel L.P., Knoers N.V., Bindels R.J. The structural unit of the thiazide-sensitive NaCl cotransporter is a homodimer. J Biol Chem. 2003;278:24302–24307. doi: 10.1074/jbc.M303101200. [DOI] [PubMed] [Google Scholar]
- 12.Wu A., Wolley M., Stowasser M. The interplay of renal potassium and sodium handling in blood pressure regulation: Critical role of the WNK–SPAK–NCC pathway. J Hum Hypertens. 2019;33:508–523. doi: 10.1038/s41371-019-0170-6. [DOI] [PubMed] [Google Scholar]
- 13.Xu B., English J.M., Wilsbacher J.L., Stippec S., Goldsmith E.J., Cobb M.H. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 14.Argaiz E.R., Chavez-Canales M., Ostrosky-Frid M., Rodriguez-Gama A., Vazquez N., Gonzalez-Rodriguez X. Kidney-specific WNK1 isoform (KS-WNK1) is a potent activator of WNK4 and NCC. Am J Physiol Ren Physiol. 2018;315:F734–F745. doi: 10.1152/ajprenal.00145.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong H., Tang Z., Yang Y., Sun L., Zhang W., Wang W. A patient with pseudohypoaldosteronism type II caused by a novel mutation in WNK4 gene. Endocrine. 2008;33:230–234. doi: 10.1007/s12020-008-9084-8. [DOI] [PubMed] [Google Scholar]
- 16.Golbang A.P., Murthy M., Hamad A., Liu C.H., Cope G., Van't Hoff W. A new kindred with pseudohypoaldosteronism type II and a novel mutation (564D>H) in the acidic motif of the WNK4 gene. Hypertension. 2005;46:295–300. doi: 10.1161/01.HYP.0000174326.96918.d6. [DOI] [PubMed] [Google Scholar]
- 17.Vidal-Petiot E., Elvira-Matelot E., Mutig K., Soukaseum C., Baudrie V., Wu S. WNK1-related familial hyperkalemic hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci U S A. 2013;110:14366–14371. doi: 10.1073/pnas.1304230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi D., Mori T., Nomura N., Khan M.Z., Araki Y., Zeniya M. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep. 2014;34 doi: 10.1042/BSR20140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castaneda-Bueno M., Cervantes-Perez L.G., Vazquez N., Uribe N., Kantesaria S., Morla L. Activation of the renal Na+:Cl– cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A. 2012;109:7929–7934. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitari A.C., Thastrup J., Rafiqi F.H., Deak M., Morrice N.A., Karlsson H.K. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397:223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitari A.C., Deak M., Morrice N.A., Alessi D.R. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. [Google Scholar]
- 22.Piechotta K., Lu J., Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline–alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 23.Moriguchi T., Urushiyama S., Hisamoto N., Iemura S., Uchida S., Natsume T. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 24.Yang S.S., Lo Y.F., Wu C.C., Lin S.W., Yeh C.J., Chu P. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21:1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick J.A., Mutig K., Nelson J.H., Saritas T., Hoorn E.J., Yang C.L. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metabol. 2011;14:352–364. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm P.R., Taneja T.K., Liu J., Coleman R., Chen Y.Y., Delpire E. SPAK isoforms and OSR1 regulate sodium–chloride co-transporters in a nephron-specific manner. J Biol Chem. 2012;287:37673–37690. doi: 10.1074/jbc.M112.402800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Siew K., Macartney T., O'Shaughnessy K.M., Alessi D.R. Critical role of the SPAK protein kinase CCT domain in controlling blood pressure. Hum Mol Genet. 2015;24:4545–4558. doi: 10.1093/hmg/ddv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess K., Jovanovic S., Sudhir R., Jovanovic A. Area under the curve analysis of blood pressure reveals increased spontaneous locomotor activity in SPAK knock-in mice: Relevance for hypotension induced by SPAK inhibition?. Phys Rep. 2019;7 doi: 10.14814/phy2.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandai S., Mori T., Sohara E., Rai T., Uchida S. Generation of hypertension-associated STK39 polymorphism knockin cell lines with the clustered regularly interspaced short palindromic repeats/Cas9 system. Hypertension. 2015;66:1199–1206. doi: 10.1161/HYPERTENSIONAHA.115.05872. [DOI] [PubMed] [Google Scholar]
- 30.Filippi B.M., de los Heros P., Mehellou Y., Navratilova I., Gourlay R., Deak M. MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J. 2011;30:1730–1741. doi: 10.1038/emboj.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaek L.L., Kortenoeven M.L., Aroankins T.S., Fenton R.A. Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem. 2014;289:13347–13361. doi: 10.1074/jbc.M113.543710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacheco-Alvarez D., Cristobal P.S., Meade P., Moreno E., Vazquez N., Munoz E. The Na+:Cl– cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem. 2006;281:28755–28763. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., O'Connell J.R., McArdle P.F., Wade J.B., Dorff S.E., Shah S.J. From the cover: Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci U S A. 2009;106:226–231. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fava C., Danese E., Montagnana M., Sjogren M., Almgren P., Engstrom G. Serine/threonine kinase 39 is a candidate gene for primary hypertension especially in women: Results from two cohort studies in Swedes. J Hypertens. 2011;29:484–491. doi: 10.1097/HJH.0b013e328342b2c1. [DOI] [PubMed] [Google Scholar]
- 35.Tobin M.D., Raleigh S.M., Newhouse S., Braund P., Bodycote C., Ogleby J. Association of WNK1 gene polymorphisms and haplotypes with ambulatory blood pressure in the general population. Circulation. 2005;112:3423–3429. doi: 10.1161/CIRCULATIONAHA.105.555474. [DOI] [PubMed] [Google Scholar]
- 36.Putku M., Kepp K., Org E., Sober S., Comas D., Viigimaa M. Novel polymorphic AluYb8 insertion in the WNK1 gene is associated with blood pressure variation in Europeans. Hum Mutat. 2011;32:806–814. doi: 10.1002/humu.21508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newhouse S.J., Wallace C., Dobson R., Mein C., Pembroke J., Farrall M. Haplotypes of the WNK1 gene associate with blood pressure variation in a severely hypertensive population from the British genetics of hypertension study. Hum Mol Genet. 2005;14:1805–1814. doi: 10.1093/hmg/ddi187. [DOI] [PubMed] [Google Scholar]
- 38.Newhouse S., Farrall M., Wallace C., Hoti M., Burke B., Howard P. Polymorphisms in the WNK1 gene are associated with blood pressure variation and urinary potassium excretion. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo X.G., Ding J., Xu H., Xuan T.M., Jin W.Q., Yin X. Comprehensive assessment of the association of WNK4 polymorphisms with hypertension: Evidence from a meta-analysis. Sci Rep. 2014;4:6507. doi: 10.1038/srep06507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas-Vega L., Gamba G. Mini-review: Regulation of the renal NaCl cotransporter by hormones. Am J Physiol Ren Physiol. 2016;310:F10–F14. doi: 10.1152/ajprenal.00354.2015. [DOI] [PubMed] [Google Scholar]
- 41.Vitzthum H., Seniuk A., Schulte L.H., Muller M.L., Hetz H., Ehmke H. Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J Physiol. 2014;592:1139–1157. doi: 10.1113/jphysiol.2013.266924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terker A.S., Zhang C., McCormick J.A., Lazelle R.A., Zhang C., Meermeier N.P. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metabol. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotchen T.A., Cowley A.W., Jr., Frohlich E.D. Salt in health and disease—a delicate balance. N Engl J Med. 2013;368:2531–2532. doi: 10.1056/NEJMc1305326. [DOI] [PubMed] [Google Scholar]
- 44.Chiga M., Rai T., Yang S.S., Ohta A., Takizawa T., Sasaki S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 2008;74:1403–1409. doi: 10.1038/ki.2008.451. [DOI] [PubMed] [Google Scholar]
- 45.Terker A.S., Zhang C., Erspamer K.J., Gamba G., Yang C.L., Ellison D.H. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016;89:127–134. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terker A.S., Yarbrough B., Ferdaus M.Z., Lazelle R.A., Erspamer K.J., Meermeier N.P. Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol. 2016;27:2436–2445. doi: 10.1681/ASN.2015070815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishida H., Sohara E., Nomura N., Chiga M., Alessi D.R., Rai T. Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK–OSR1/SPAK–NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension. 2012;60:981–990. doi: 10.1161/HYPERTENSIONAHA.112.201509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furusho T., Uchida S., Sohara E. The WNK signaling pathway and salt-sensitive hypertension. Hypertens Res. 2020;43:733–743. doi: 10.1038/s41440-020-0437-x. [DOI] [PubMed] [Google Scholar]
- 49.Glover M., Mercier Zuber A., Figg N., O'Shaughnessy K.M. The activity of the thiazide-sensitive Na+–Cl– cotransporter is regulated by protein phosphatase PP4. Can J Physiol Pharmacol. 2010;88:986–995. doi: 10.1139/y10-080. [DOI] [PubMed] [Google Scholar]
- 50.Gamba G. Regulation of the renal Na+–Cl– cotransporter by phosphorylation and ubiquitylation. Am J Physiol Ren Physiol. 2012;303:F1573–F1583. doi: 10.1152/ajprenal.00508.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zununi Vahed S., Ardalan M., Samadi N., Omidi Y. Pharmacogenetics and drug-induced nephrotoxicity in renal transplant recipients. Bioimpacts. 2015;5:45–54. doi: 10.15171/bi.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoda W., Nomura N., Ando F., Mori Y., Mori T., Sohara E. Calcineurin inhibitors block sodium–chloride cotransporter dephosphorylation in response to high potassium intake. Kidney Int. 2017;91:402–411. doi: 10.1016/j.kint.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Melnikov S., Mayan H., Uchida S., Holtzman E.J., Farfel Z. Cyclosporine metabolic side effects: Association with the WNK4 system. Eur J Clin Invest. 2011;41:1113–1120. doi: 10.1111/j.1365-2362.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- 54.Hoorn E.J., Walsh S.B., McCormick J.A., Furstenberg A., Yang C.L., Roeschel T. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011;17:1304–1309. doi: 10.1038/nm.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penton D., Moser S., Wengi A., Czogalla J., Rosenbaek L.L., Rigendinger F. Protein phosphatase 1 inhibitor-1 mediates the cAMP-dependent stimulation of the renal NaCl cotransporter. J Am Soc Nephrol. 2019;30:737–750. doi: 10.1681/ASN.2018050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Picard N., Trompf K., Yang C.L., Miller R.L., Carrel M., Loffing-Cueni D. Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol. 2014;25:511–522. doi: 10.1681/ASN.2012121202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gagnon K.B., Delpire E. Multiple pathways for protein phosphatase 1 (PP1) regulation of Na–K–2Cl cotransporter (NKCC1) function: The N-terminal tail of the Na–K–2Cl cotransporter serves as a regulatory scaffold for Ste20-related proline/alanine-rich kinase (SPAK) AND PP1. J Biol Chem. 2010;285:14115–14121. doi: 10.1074/jbc.M110.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murillo-de-Ozores A.R., Rodriguez-Gama A., Bazua-Valenti S., Leyva-Rios K., Vazquez N., Pacheco-Alvarez D. C-terminally truncated, kidney-specific variants of the WNK4 kinase lack several sites that regulate its activity. J Biol Chem. 2018;293:12209–12221. doi: 10.1074/jbc.RA118.003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plotkin M.D., Kaplan M.R., Verlander J.W., Lee W.S., Brown D., Poch E. Localization of the thiazide sensitive Na–Cl cotransporter, rTSC1 in the rat kidney. Kidney Int. 1996;50:174–183. doi: 10.1038/ki.1996.300. [DOI] [PubMed] [Google Scholar]
- 60.Gamba G., Miyanoshita A., Lombardi M., Lytton J., Lee W.S., Hediger M.A. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium–(potassium)–chloride cotransporter family expressed in kidney. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 61.Mount D.B. Regulated endocytosis of NCC. Am J Physiol Ren Physiol. 2010;299:F297–F299. doi: 10.1152/ajprenal.00280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soundararajan R., Zhang T.T., Wang J., Vandewalle A., Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem. 2005;280:39970–39981. doi: 10.1074/jbc.M508658200. [DOI] [PubMed] [Google Scholar]
- 63.Babilonia E., Li D., Wang Z., Sun P., Lin D.H., Jin Y. Mitogen-activated protein kinases inhibit the ROMK (Kir 1.1)-like small conductance K channels in the cortical collecting duct. J Am Soc Nephrol. 2006;17:2687–2696. doi: 10.1681/ASN.2006050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ko B., Kamsteeg E.J., Cooke L.L., Moddes L.N., Deen P.M., Hoover R.S. RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium–chloride cotransporter. Am J Physiol Ren Physiol. 2010;299:F300–F309. doi: 10.1152/ajprenal.00441.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ko B., Joshi L.M., Cooke L.L., Vazquez N., Musch M.W., Hebert S.C. Phorbol ester stimulation of RasGRP1 regulates the sodium–chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci U S A. 2007;104:20120–20125. doi: 10.1073/pnas.0709506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang C.L., Angell J., Mitchell R., Ellison D.H. WNK kinases regulate thiazide-sensitive Na–Cl cotransport. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou B., Zhuang J., Gu D., Wang H., Cebotaru L., Guggino W.B. WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol. 2010;21:82–92. doi: 10.1681/ASN.2008121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subramanya A.R., Liu J., Ellison D.H., Wade J.B., Welling P.A. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem. 2009;284:18471–18480. doi: 10.1074/jbc.M109.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai H., Cebotaru V., Wang Y.H., Zhang X.M., Cebotaru L., Guggino S.E. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int. 2006;69:2162–2170. doi: 10.1038/sj.ki.5000333. [DOI] [PubMed] [Google Scholar]
- 70.Zhou B., Wang D., Feng X., Zhang Y., Wang Y., Zhuang J. WNK4 inhibits NCC protein expression through MAPK ERK1/2 signaling pathway. Am J Physiol Ren Physiol. 2012;302:F533–F539. doi: 10.1152/ajprenal.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lupp A., Klenk C., Rocken C., Evert M., Mawrin C., Schulz S. Immunohistochemical identification of the PTHR1 parathyroid hormone receptor in normal and neoplastic human tissues. Eur J Endocrinol. 2010;162:979–986. doi: 10.1530/EJE-09-0821. [DOI] [PubMed] [Google Scholar]
- 72.Ko B., Cooke L.L., Hoover R.S. Parathyroid hormone (PTH) regulates the sodium chloride cotransporter via Ras guanyl releasing protein 1 (Ras-GRP1) and extracellular signal-regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) pathway. Transl Res. 2011;158:282–289. doi: 10.1016/j.trsl.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng X., Zhang Y., Shao N., Wang Y., Zhuang Z., Wu P. Aldosterone modulates thiazide-sensitive sodium chloride cotransporter abundance via DUSP6-mediated ERK1/2 signaling pathway. Am J Physiol Ren Physiol. 2015;308:F1119–F1127. doi: 10.1152/ajprenal.00543.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenbaek L.L., Rizzo F., Wu Q., Rojas-Vega L., Gamba G., MacAulay N. The thiazide sensitive sodium chloride co-transporter NCC is modulated by site-specific ubiquitylation. Sci Rep. 2017;7:12981. doi: 10.1038/s41598-017-12819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hossain Khan M.Z., Sohara E., Ohta A., Chiga M., Inoue Y., Isobe K. Phosphorylation of Na–Cl cotransporter by OSR1 and SPAK kinases regulates its ubiquitination. Biochem Biophys Res Commun. 2012;425:456–461. doi: 10.1016/j.bbrc.2012.07.124. [DOI] [PubMed] [Google Scholar]
- 76.Rotin D., Staub O. Nedd4-2 and the regulation of epithelial sodium transport. Front Physiol. 2012;3:212. doi: 10.3389/fphys.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ronzaud C., Loffing-Cueni D., Hausel P., Debonneville A., Malsure S.R., Fowler-Jaeger N. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest. 2013;123:657–665. doi: 10.1172/JCI61110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arroyo J.P., Lagnaz D., Ronzaud C., Vazquez N., Ko B.S., Moddes L. Nedd4-2 modulates renal Na+–Cl– cotransporter via the aldosterone–SGK1–Nedd4-2 pathway. J Am Soc Nephrol. 2011;22:1707–1719. doi: 10.1681/ASN.2011020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lagnaz D., Arroyo J.P., Chavez-Canales M., Vazquez N., Rizzo F., Spirli A. WNK3 abrogates the NEDD4-2-mediated inhibition of the renal Na+–Cl– cotransporter. Am J Physiol Ren Physiol. 2014;307:F275–F286. doi: 10.1152/ajprenal.00574.2013. [DOI] [PubMed] [Google Scholar]
- 80.Lai F., Orelli B.J., Till B.G., Godley L.A., Fernald A.A., Pamintuan L. Molecular characterization of KLHL3, a human homologue of the Drosophila kelch gene. Genomics. 2000;66:65–75. doi: 10.1006/geno.2000.6181. [DOI] [PubMed] [Google Scholar]
- 81.Wu G., Peng J.B. Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation. FEBS Lett. 2013;587:1717–1722. doi: 10.1016/j.febslet.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shibata S., Zhang J., Puthumana J., Stone K.L., Lifton R.P. Kelch-like 3 and cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A. 2013;110:7838–7843. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohta A., Schumacher F.R., Mehellou Y., Johnson C., Knebel A., Macartney T.J. The CUL3–KLHL3 E3 ligase complex mutated in Gordon's hypertension syndrome interacts with and ubiquitylates WNK isoforms: Disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013;451:111–122. doi: 10.1042/BJ20121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Susa K., Sohara E., Rai T., Zeniya M., Mori Y., Mori T. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. 2014;23:5052–5060. doi: 10.1093/hmg/ddu217. [DOI] [PubMed] [Google Scholar]
- 85.Mori Y., Wakabayashi M., Mori T., Araki Y., Sohara E., Rai T. Decrease of WNK4 ubiquitination by disease-causing mutations of KLHL3 through different molecular mechanisms. Biochem Biophys Res Commun. 2013;439:30–34. doi: 10.1016/j.bbrc.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 86.Loffing J., Loffing-Cueni D., Valderrabano V., Klausli L., Hebert S.C., Rossier B.C. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Ren Physiol. 2001;281:F1021–F1027. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- 87.Bindels R.J., Timmermans J.A., Hartog A., Coers W., van Os C.H. Calbindin-D9k and parvalbumin are exclusively located along basolateral membranes in rat distal nephron. J Am Soc Nephrol. 1991;2:1122–1129. doi: 10.1681/ASN.V261122. [DOI] [PubMed] [Google Scholar]
- 88.Belge H., Gailly P., Schwaller B., Loffing J., Debaix H., Riveira-Munoz E. Renal expression of parvalbumin is critical for NaCl handling and response to diuretics. Proc Natl Acad Sci U S A. 2007;104:14849–14854. doi: 10.1073/pnas.0702810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zacchia M., Capasso G. Parvalbumin: A key protein in early distal tubule NaCl reabsorption. Nephrol Dial Transplant. 2008;23:1109–1111. doi: 10.1093/ndt/gfm886. [DOI] [PubMed] [Google Scholar]
- 90.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Ren Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 91.Gailly P., Szutkowska M., Olinger E., Debaix H., Seghers F., Janas S. P2Y2 receptor activation inhibits the expression of the sodium–chloride cotransporter NCC in distal convoluted tubule cells. Pflügers Archiv. 2014;466:2035–2047. doi: 10.1007/s00424-013-1438-2. [DOI] [PubMed] [Google Scholar]
- 92.Riveira-Munoz E., Devuyst O., Belge H., Jeck N., Strompf L., Vargas-Poussou R. Evaluating PVALB as a candidate gene for SLC12A3-negative cases of Gitelman's syndrome. Nephrol Dial Transplant. 2008;23:3120–3125. doi: 10.1093/ndt/gfn229. [DOI] [PubMed] [Google Scholar]
- 93.Riveira-Munoz E., Chang Q., Godefroid N., Hoenderop J.G., Bindels R.J., Dahan K. Transcriptional and functional analyses of SLC12A3 mutations: New clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol. 2007;18:1271–1283. doi: 10.1681/ASN.2006101095. [DOI] [PubMed] [Google Scholar]
- 94.Sica D.A. Diuretic-related side effects: Development and treatment. J Clin Hypertens (Greenwich) 2004;6:532–540. doi: 10.1111/j.1524-6175.2004.03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maitland-van der Zee A.H., Turner S.T., Schwartz G.L., Chapman A.B., Klungel O.H., Boerwinkle E. Demographic, environmental, and genetic predictors of metabolic side effects of hydrochlorothiazide treatment in hypertensive subjects. Am J Hypertens. 2005;18:1077–1083. doi: 10.1016/j.amjhyper.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Morsing P., Velazquez H., Wright F.S., Ellison D.H. Adaptation of distal convoluted tubule of rats. II. Effects of chronic thiazide infusion. Am J Physiol. 1991;261:F137–F143. doi: 10.1152/ajprenal.1991.261.1.F137. [DOI] [PubMed] [Google Scholar]
- 97.Kim G.H. Long-term adaptation of renal ion transporters to chronic diuretic treatment. Am J Nephrol. 2004;24:595–605. doi: 10.1159/000082314. [DOI] [PubMed] [Google Scholar]
- 98.Kamel K.S., Ethier J.H., Quaggin S., Levin A., Albert S., Carlisle E.J. Studies to determine the basis for hyperkalemia in recipients of a renal transplant who are treated with cyclosporine. J Am Soc Nephrol. 1992;2:1279–1284. doi: 10.1681/ASN.V281279. [DOI] [PubMed] [Google Scholar]
- 99.Knighton D.R., Zheng J.H., Ten Eyck L.F., Ashford V.A., Xuong N.H., Taylor S.S. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- 100.Yagi Y.I., Abe K., Ikebukuro K., Sode K. Kinetic mechanism and inhibitor characterization of WNK1 kinase. Biochemistry. 2009;48:10255–10266. doi: 10.1021/bi900666n. [DOI] [PubMed] [Google Scholar]
- 101.Elkins J.M., Fedele V., Szklarz M., Abdul Azeez K.R., Salah E., Mikolajczyk J. Comprehensive characterization of the published kinase inhibitor set. Nat Biotechnol. 2016;34:95–103. doi: 10.1038/nbt.3374. [DOI] [PubMed] [Google Scholar]
- 102.Apsel B., Blair J.A., Gonzalez B., Nazif T.M., Feldman M.E., Aizenstein B. Targeted polypharmacology: Discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4:691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamada K., Park H.M., Rigel D.F., DiPetrillo K., Whalen E.J., Anisowicz A. Small-molecule WNK inhibition regulates cardiovascular and renal function. Nat Chem Biol. 2016;12:896–898. doi: 10.1038/nchembio.2168. [DOI] [PubMed] [Google Scholar]
- 104.Jonniya N.A., Kar P. Investigating specificity of the anti-hypertensive inhibitor WNK463 against with-no-lysine kinase family isoforms via multiscale simulations. J Biomol Struct Dyn. 2020;38:1306–1321. doi: 10.1080/07391102.2019.1602079. [DOI] [PubMed] [Google Scholar]
- 105.Yamada K., Zhang J.H., Xie X., Reinhardt J., Xie A.Q., LaSala D. Discovery and characterization of allosteric WNK kinase inhibitors. ACS Chem Biol. 2016;11:3338–3346. doi: 10.1021/acschembio.6b00511. [DOI] [PubMed] [Google Scholar]
- 106.Yamada K., Levell J., Yoon T., Kohls D., Yowe D., Rigel D.F. Optimization of allosteric with-no-lysine (WNK) kinase inhibitors and efficacy in rodent hypertension models. J Med Chem. 2017;60:7099–7107. doi: 10.1021/acs.jmedchem.7b00708. [DOI] [PubMed] [Google Scholar]
- 107.Zhang J., Bhuiyan M.I.H., Zhang T., Karimy J.K., Wu Z., Fiesler V.M. Modulation of brain cation–Cl– cotransport via the SPAK kinase inhibitor ZT-1a. Nat Commun. 2020;11:78. doi: 10.1038/s41467-019-13851-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin S.H., Yu I.S., Jiang S.T., Lin S.W., Chu P., Chen A. Impaired phosphorylation of Na+–K+–2Cl– cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci U S A. 2011;108:17538–17543. doi: 10.1073/pnas.1107452108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kikuchi E., Mori T., Zeniya M., Isobe K., Ishigami-Yuasa M., Fujii S. Discovery of novel SPAK inhibitors that block WNK kinase signaling to cation chloride transporters. J Am Soc Nephrol. 2015;26:1525–1536. doi: 10.1681/ASN.2014060560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.AlAmri M.A., Kadri H., Alderwick L.J., Simpkins N.S., Mehellou Y. Rafoxanide and closantel inhibit SPAK and OSR1 kinases by binding to a highly conserved allosteric site on their C-terminal domains. ChemMedChem. 2017;12:639–645. doi: 10.1002/cmdc.201700077. [DOI] [PubMed] [Google Scholar]
- 111.AlAmri M.A., Kadri H., Alderwick L.J., Jeeves M., Mehellou Y. The photosensitising clinical agent verteporfin is an inhibitor of SPAK and OSR1 kinases. Chembiochem. 2018;19:2072–2080. doi: 10.1002/cbic.201800272. [DOI] [PubMed] [Google Scholar]
- 112.Charisis S.K., Naoumidi, Ginis H.S., Detorakis E.T., Tsilimbaris M.K. Contact transcleral ciliary body photodynamic therapy with verteporfin in pigmented rabbits: Effect of repeated treatments. Photochem Photobiol. 2010;86:194–199. doi: 10.1111/j.1751-1097.2009.00638.x. [DOI] [PubMed] [Google Scholar]
- 113.Yang S.S., Huang C.L., Chen H.E., Tung C.S., Shih H.P., Liu Y.P. Effects of SPAK knockout on sensorimotor gating, novelty exploration, and brain area-dependent expressions of NKCC1 and KCC2 in a mouse model of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;61:30–36. doi: 10.1016/j.pnpbp.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Loffing J., Vallon V., Loffing-Cueni D., Aregger F., Richter K., Pietri L. Altered renal distal tubule structure and renal Na+ and Ca2+ handling in a mouse model for Gitelman's syndrome. J Am Soc Nephrol. 2004;15:2276–2288. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 115.Grimm P.R., Lazo-Fernandez Y., Delpire E., Wall S.M., Dorsey S.G., Weinman E.J. Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest. 2015;125:2136–2150. doi: 10.1172/JCI78558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Geng Y., Byun N., Delpire E. Behavioral analysis of Ste20 kinase SPAK knockout mice. Behav Brain Res. 2010;208:377–382. doi: 10.1016/j.bbr.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brooks H.L., Sorensen A.M., Terris J., Schultheis P.J., Lorenz J.N., Shull G.E. Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC null mice using targeted proteomics. J Physiol. 2001;530:359–366. doi: 10.1111/j.1469-7793.2001.0359k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Delpire E., Gagnon K.B. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 119.Kadri H., Alamri M.A., Navratilova I.H., Alderwick L.J., Simpkins N.S., Mehellou Y. Towards the development of small-molecule MO25 binders as potential indirect SPAK/OSR1 kinase inhibitors. Chembiochem. 2017;18:460–465. doi: 10.1002/cbic.201600620. [DOI] [PubMed] [Google Scholar]
- 120.Mori T., Kikuchi E., Watanabe Y., Fujii S., Ishigami-Yuasa M., Kagechika H. Chemical library screening for WNK signalling inhibitors using fluorescence correlation spectroscopy. Biochem J. 2013;455:339–345. doi: 10.1042/BJ20130597. [DOI] [PubMed] [Google Scholar]
- 121.Louis-Dit-Picard H., Barc J., Trujillano D., Miserey-Lenkei S., Bouatia-Naji N., Pylypenko O. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet. 2012;44:456–460. doi: 10.1038/ng.2218. S1-3. [DOI] [PubMed] [Google Scholar]
- 122.Boyden L.M., Choi M., Choate K.A., Nelson-Williams C.J., Farhi A., Toka H.R. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCormick J.A., Yang C.L., Zhang C., Davidge B., Blankenstein K.I., Terker A.S. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest. 2014;124:4723–4736. doi: 10.1172/JCI76126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sasaki E., Susa K., Mori T., Isobe K., Araki Y., Inoue Y. KLHL3 knockout mice reveal the physiological role of KLHL3 and the pathophysiology of pseudohypoaldosteronism type II caused by mutant KLHL3. Mol Cell Biol. 2017;37:e00508–e00516. doi: 10.1128/MCB.00508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoover R.S., Poch E., Monroy A., Vazquez N., Nishio T., Gamba G. N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na+:Cl– cotransporter. J Am Soc Nephrol. 2003;14:271–282. doi: 10.1097/01.asn.0000043903.93452.d0. [DOI] [PubMed] [Google Scholar]
- 126.Sabath E., Meade P., Berkman J., de los Heros P., Moreno E., Bobadilla N.A. Pathophysiology of functional mutations of the thiazide-sensitive Na–Cl cotransporter in Gitelman disease. Am J Physiol Ren Physiol. 2004;287:F195–F203. doi: 10.1152/ajprenal.00044.2004. [DOI] [PubMed] [Google Scholar]
- 127.De Jong J.C., Van Der Vliet W.A., Van Den Heuvel L.P., Willems P.H., Knoers N.V., Bindels R.J. Functional expression of mutations in the human NaCl cotransporter: Evidence for impaired routing mechanisms in Gitelman's syndrome. J Am Soc Nephrol. 2002;13:1442–1448. doi: 10.1097/01.asn.0000017904.77985.03. [DOI] [PubMed] [Google Scholar]
- 128.Glaudemans B., Yntema H.G., San-Cristobal P., Schoots J., Pfundt R., Kamsteeg E.J. Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome. Eur J Hum Genet. 2012;20:263–270. doi: 10.1038/ejhg.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]