Abstract
Heart failure (HF) is a global public health problem with high morbidity and mortality. A large number of studies have shown that HF is caused by severe energy metabolism disorders, which result in an insufficient heart energy supply. This deficiency causes cardiac pump dysfunction and systemic energy metabolism failure, which determine the development of HF and recovery of heart. Current HF therapy acts by reducing heart rate and cardiac preload and afterload, treating the HF symptomatically or delaying development of the disease. Drugs aimed at cardiac energy metabolism have not yet been developed. In this review, we outline the main characteristics of cardiac energy metabolism in healthy hearts, changes in metabolism during HF, and related pathways and targets of energy metabolism. Finally, we discuss drugs that improve cardiac function via energy metabolism to provide new research ideas for the development and application of drugs for treating HF.
KEY WORDS: Heart failure, Energy deficit, Cardiac dysfunction, Energy metabolism, Substrate metabolism, Hormones, Natural products, Synthetic drugs
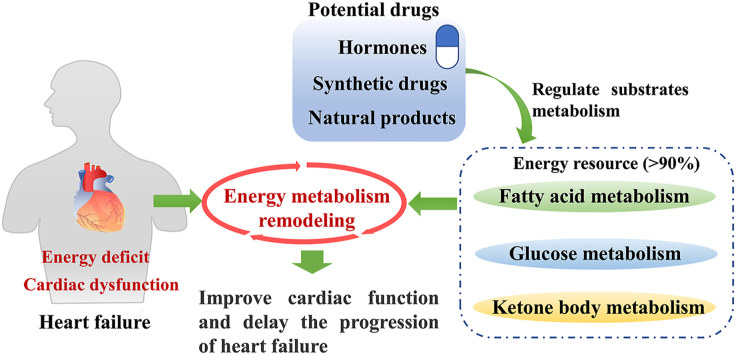
Graphical abstract
Energy metabolism regulation is a potential way to treat heart failure. In this review, the changes of energy metabolism and some potential therapeutic drugs in heart failure are extensively summarized.
1. Introduction
Heart failure (HF), with high morbidity and mortality, is the final outcome of many end-stage heart diseases1. In recent decades, the treatment of HF has seen great innovations in medical treatment and equipment, but its incidence is still increasing, and the quality of life, function, and life expectancy of patients with HF declined to varying degrees of damage2. Studies have found that patients with HF will experience severe energy metabolism disorders, including disturbances in substrates absorption and utilization, oxidative phosphorylation, and the adenosine triphosphate (ATP) shuttle, resulting in an inadequate cardiac energy supply. This deficiency causes cardiac pump dysfunction and systemic energy metabolism failure, which determine the development and recovery from HF3,4. Therefore, identifying the changes in energy metabolism that occur during HF is of great significance for clarifying the pathophysiology of HF and promoting the development of HF treatment. Existing HF therapies reduce heart load by reducing heart rate, preload, and afterload to treat symptoms and delay disease progression2. There are many basic studies on drugs that affect cardiac energy metabolism. Unfortunately, few drugs are available for clinical treatment. In this review, we outline the main characteristics of cardiac energy metabolism in healthy hearts, the transformation of metabolism during HF, pathways and targets related to energy metabolism, and for the first time we discuss potential drugs and natural ingredients that improve heart function through energy metabolism. We hope to provide new research ideas for the development and application of drugs to HF.
2. Normal myocardial energy metabolism
The heart uses various substrates such as glucose, lipids, amino acids, and ketone bodies to provide energy to maintain a normal heart beat. Its preference for substrate change with life cycle physiology, pathology, and external environment5. The human fetus gestates in an environment of hypoxia and low fatty acids, relies mainly on glucose and lactate metabolism6. After birth, the cardiac hemodynamic load and oxygen tension of the newborn increase, promoting the conversion of energy metabolism. Simultaneously, a rapid increase in the number of mitochondria increases the heart's oxidative capacity7. The heart's dependence on glucose decreases, and blood lactic acid level begins to decline. As triacylglycerol content increases, fatty acid oxidation becomes the main source of heart energy8. From birth to adulthood, myocardial cells gradually mature, and oxidative capacity increases significantly6,9. In the adult heart, fatty acids are the main energy source, accounting for 60%–90%, and the remaining 10%–40% comes from glucose, amino acids, pyruvate, lactic acid, ketone bodies, and other sources9.
2.1. Fatty acid energy metabolism
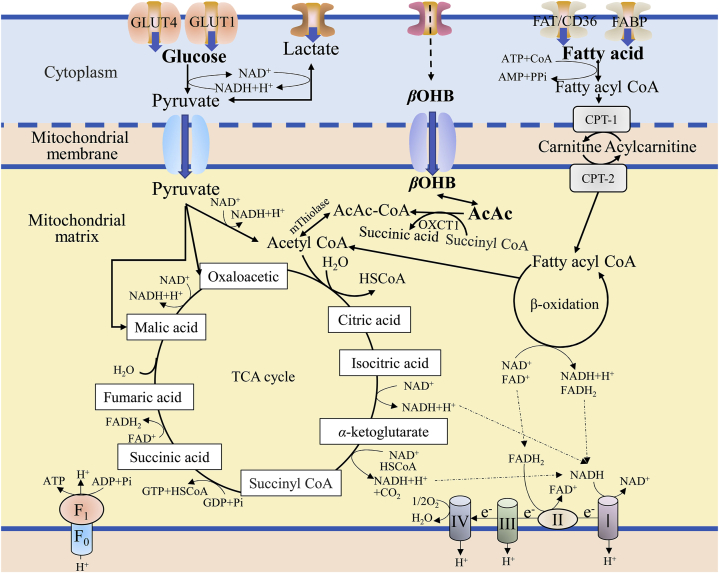
Fatty acids are the main energy-supplying substance for the adult heart and provide many necessary coenzyme factors for oxidative phosphorylation by the mitochondria. Fatty acids are transported into cells via fatty acid transporters (FAT/CD36) and fatty acid binding proteins (FABP) on the cell membrane and are converted into long-chain fatty acyl-CoA by fatty acyl-CoA synthetase in the cytoplasm; long-chain fatty acyl-CoA is converted to acylcarnitine by carnitine palmitoyl transferase (CPT)-1 on the outer membrane of mitochondria; acylcarnitine is transported to the mitochondrial inner membrane by carnitine transposase, and CPT-2 is used to cut carnitine to reduce the acylcarnitine to long-chain fatty acyl-CoA; and finally, long-chain fatty acyl-CoA undergoes β-oxidation in the mitochondrial matrix10,11 (Fig. 1). β-Oxidation of fatty acid produces products such as acetyl-CoA, reduced nicotinamide adenine (NADH), and reduced flavin dinucleotide (FADH2). These products are used by the electron transfer chain to generate large amounts of ATP, which provides the heart energy10.
Figure 1.
The energy metabolism process of fatty acid, glucose and ketone body. Various metabolic substrates are transported to the cytoplasm to form corresponding intermediate product, such as pyruvate, fatty acyl CoA, etc. These intermediates enter the mitochondrial matrix through a specific transport system and generate NADH, FADH2, GTP through the TCA cycle, fatty acid oxidation and other pathways. Then NADH and FADH2 produce ATP through the electron transfer chain, which provides energy for cardiomyocytes.
2.2. Glucose energy metabolism
The glucose used in the heart is derived from exogenous glucose or glycogenolysis, and a lesser amount of glycogen is stored in the heart. Exogenous glucose enters myocardial cells mainly through glucose transporter (GLUT-4), followed by GLUT-112. After entering myocardial cells, glucose is phosphorylated by hexokinase to form glucose-6-phosphate (G-6-P), which is then converted to pyruvate by glycolysis12. In the presence of oxygen, pyruvate is converted to acetyl-CoA by pyruvate dehydrogenase in the cell matrix; acetyl-CoA enters the tricarboxylic acid (TCA) cycle, producing ATP to provide energy (Fig. 1). Under hypoxia, pyruvate can produce lactic acid through anaerobic oxidation in the cell matrix, which generates a small amount of ATP for energy10,11. In addition, pyruvate can be carboxylated to oxaloacetate or malic acid as an anaplerosis of the citric acid cycle13.
2.3. Ketone body energy metabolism
Ketone bodies consist of acetoacetic acid (AcAc), β-hydroxybutyric acid (βOHB) and acetone, which are intermediate products of oxidative decomposition of fatty acids in the liver. Ketone body oxidation is the main source of energy metabolism in extrahepatic tissues in many physiological states, such as neonatal period, fasting, and exercise. Ketone bodies are also substrates for the synthesis of fats and sterols in the brain, liver, and breast14. Ketone bodies may become one of the high-energy fuels that replace glucose during HF. βOHB is oxidized to AcAc by d-β-hydroxybutyrate dehydrogenase in mitochondria; 3-ketoacyl-CoA transferase 1 (OXCT1) is also known as succinyl-CoA ketoacyl-CoA transferase. OXCT1 catalyzes the exchange of CoA between succinyl-CoA and AcAc to produce AcAc-CoA and succinic acid15. This step is the rate-limiting phase of ketone body utilization, because OXCT1 exists in all tissues except the liver16. Subsequently, AcAc-CoA is reversibly converted into acetyl CoA under the action of acetoacetyl-CoA thiolase (mThiolase), and enters the TCA cycle to produce ATP in mitochondria. However, this reaction is conducive to the reverse reaction, so continuous consumption of ketone bodies is necessary to promote the conversion of AcAc-CoA to acetyl-CoA14.
2.4. Molecular mechanism of cardiac substrate metabolism
Cardiac substrate metabolism is mainly determined by the catalytic activity of rate-limiting enzymes and the expression of some enzymes and transporters in the cell3.
2.4.1. Relationship between substrate metabolism and rate limiting enzymes
The catalytic activity of rate-limiting enzymes is mainly regulated by the allosteric regulation of enzymes and transporters, as well as complex pathways between substrates and products. For example, malonyl-CoA is one of the key enzymes that regulate the oxidation of cardiac fatty acids. It can inhibit CPT-1 activity by binding to CPT-1. At the same time, malonyl-CoA is regulated by acetyl-CoA carboxylase (ACC) and malonyl-CoA decarboxylase (MCD)17. Phosphofructose kinase-1 (PFK-1) is a key regulatory enzyme in the glycolysis pathway, catalyzing the production of fructose 1,6-diphosphate, which in turn can inhibit the activity of PFK-1. In addition, PFK-1 is also regulated by adenosine diphosphate, adenosine monophosphate, phosphate group activation, ATP, fructose 2,6-diphosphate, and citric acid18. Pyruvate dehydrogenase (PDH) complex E1 subunit, the glucose oxidation rate-limiting enzyme, can be phosphorylated and inactivated by PDH kinase (PDK), and can be activated by PDH phosphatase dephosphorylation17. When fatty acid oxidation increases, PDK expression can increase and inhibit glucose oxidation17.
2.4.2. Relationship between substrate metabolism and expression of enzymes and transporters
The expression of enzymes and transporters in cells is regulated by transcription and translation processes. For example, the adenosine 5′-monophosphate-activated protein kinase (AMPK) signaling pathway can mediate GLUT4 gene expression and increase glucose uptake19. It can also activate peroxidase proliferator-activated receptor alpha (PPARα), increase CD36 translocation, inhibit ACC activity, and increase fatty acid oxidation20. PPARs are a transcription factor for lipid metabolism, which can upregulate the related proteins involved in fatty acid uptake and metabolism, and can activate a variety of genes related to oxidative phosphorylation, thereby regulating mitochondrial oxidative phosphorylation metabolism and other functions21. Peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1α) can upregulate the expression of several genes in the TCA cycle and mitochondrial fatty acid oxidation pathway, as well as various genes related to oxidative phosphorylation, regulate fatty acid metabolism, glucose metabolism and mitochondrial oxidative phosphorylation metabolic function22. In short, various transcription factors and their co-activators interact to form a complex signal network that regulates long-term energy requirements to produce ATP. At the same time, the expression of these enzymes or transporters can also be a target for regulating heart energy metabolism.
3. Changes in energy metabolism during in HF
3.1. Changes in the metabolic substrate
The heart is a highly energy-consuming organ and needs a continuous supply of energy to keep it functioning normally. In normal conditions, fatty acids and glucose can be fully utilized, and they depend on the concentration of metabolic substrates and oxygen. Fig. 2 shows that the amount of ATP produced by the complete oxidation of 1 mol 20-carbon fatty acid (about 134 mol) is much larger than that produced by 1 mol glucose (about 30 mol). Research also shows that when they produce the same amount of ATP, fatty acid oxidation requires more oxygen than glucose oxidation; in the absence of oxygen, the productivity efficiency of fatty acid oxidation is significantly lower than that of glucose oxidation23. Studies have shown that the hypoxic environment after HF reverses the heart's energy metabolism toward that of the fetal period, in which glucose metabolism is the main energy source24. During HF, glucose intake and glycolysis rates increase significantly to compensate for fatty acid oxidation to provide energy. However, Umbarawan et al.25 believed that the increase in glucose uptake and glycolysis is used for biosynthesis rather than ATP production, and fatty acid oxidation is still the main source of heart energy. Sung et al.26 stated that the transformation of metabolic substrate from fatty acid to glucose may be caused by the faster and earlier decrease in fatty acid oxidation rate, rather than the enhancement of glucose oxidation pathway. In addition, because of the failure of fatty acid intake during HF, the utilization of free fatty acids in cardiomyocytes is significantly reduced, and the concentration of free fatty acids in plasma is increased, which may further increase energy metabolism disorders and myocardial damage27. Whether the conversion of metabolic substrates from fatty acids to glucose occurs during HF is still controversial, but it is certain that fatty acid oxidation efficiency is significantly reduced in end-stage HF23.
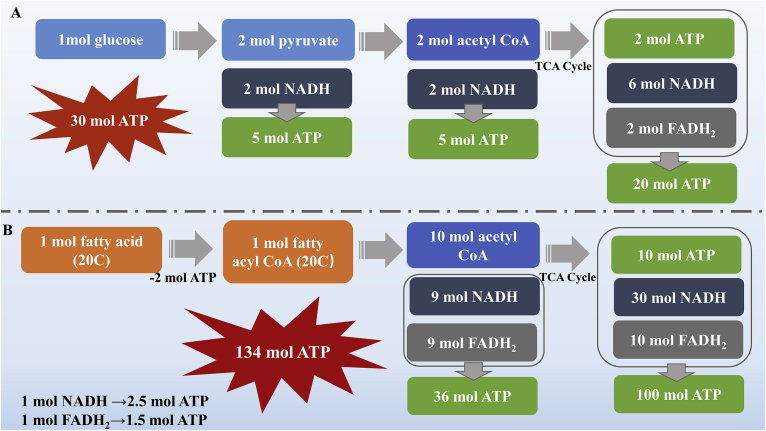
Figure 2.
Energy produce of glucose (A) and fatty acid (B) oxidation. In an ideal state, 1 mol glucose is converted into 2 mol pyruvic acid and 2 mol NADH. 1 mol pyruvic acid can produce 1 mol acetyl CoA and 1 mol NADH. 1 mol acetyl CoA can generate 1 mol ATP, 3 mol NADH and 1 mol FADH2 after one TCA cycle. 1 mol NADH and FADH2 can generate corresponding 2.5 mol ATP and 1.5 mol ATP through electron chain transfer respectively. 1 mol glucose is completely oxidized to produce 30 mol ATP. 1 mol fatty acid (20C) consumes 2 ATP can produce 1 mol fatty acyl CoA (20C). After β oxidation, 10 mol of acetyl-CoA can be generated by 1 mol fatty acyl CoA (20C), and then enter the TCA cycle. Therefore, 1 mol of fatty acid can be completely oxidized to generate 134 mol of ATP.
As the substrate of cardiac metabolism, ketone bodies have received widespread attention in recent years. Compared with fatty acids and glucose, ketone bodies are more easily converted to acetyl-CoA and more easily enter the TCA cycle28. They could reduce oxygen consumption and increase energy output to ensure heart efficiency, especially under hypoxia and high energy requirements29. A clinical study showed a significant increase of ketone body intake in HF patients with left ventricular hypertrophy due to reduced ejection fraction, and increase of fatty acid intake in HF patients with left ventricular hypertrophy due to aortic stenosis30. It is worth noting that energy metabolism is more complex in HF patients with diabetes. Insulin resistance significantly reduces glucose oxidation, increases dependence on fatty acid oxidation, activates β-oxidation, and increases ketone body production31. At the same time, the presence of ketone bodies will competitively inhibit the uptake and oxidation of fatty acids, thereby improving the utilization of ketone bodies29,32. A study showed that ketone bodies uptake in HF patients with diabetes was higher than that of non-diabetic patients with HF33. A recent study showed that an increase of the ketone body oxidation rate increased overall energy production without affecting glucose or fatty acid metabolism, but it did not increase the efficiency of the heart34. It is necessary to study whether ketone bodies can be used as the source of energy metabolism substrates and whether their utilization is adaptive in HF.
It is unclear whether the conversion of substrate metabolism after HF is a favorable compensatory response or maladaptive pathological changes, as well as should promote the use of fatty acids or glucose in the heart. Therefore, it is not advisable to simply regulate the metabolism of a certain substrate. In the study of energy metabolism of HF, we should pay attention to the balance between the metabolism of various substrates and increase the total energy supply, which is also the direction and difficulty of the development of HF drugs.
3.2. Mitochondrial changes
Ninety percent of the energy in the normal adult heart is provided by oxidative phosphorylation of the mitochondria, which plays an important role in providing energy for cell life activities. In recent years, many studies have confirmed that cardiovascular diseases, such as arrhythmia, cardiomyopathy and HF, are related to changes in mitochondrial function and structure35,36. For example, the number of mitochondria in the cardiomyocytes of patients with congestive HF is 78% lower than that of normal cardiomyocytes37. Changes in mitochondrial quantity, morphology, and function have also been observed in patients with hypertrophic cardiomyopathy38. What's more, mitochondrial dysfunction could cause a large amount of reactive oxygen species (ROS) to be produced39. Excessive accumulation of ROS will attack and damage mitochondrial DNA and mitochondrial proteins, cause mitochondrial dysfunction, and trigger cardiomyocyte apoptosis induced by mitochondria, forming a vicious cycle40. Studies have shown that the mutation rate of mitochondrial genes is significantly increased in HF myocardial cells, causing mitochondrial distortion, affecting the activity of mitochondrial oxidative respiratory chain related enzyme complexes and mitochondrial protein synthesis41. These changes lead to mitochondrial dysfunction, affect mitochondrial energy metabolism, deplete the energy in myocardial cells, and promote HF. In addition, the oxidative damage caused by the large amounts of ROS impairs the calcium ion transport mechanism, allowing a large amount of calcium ions to enter the cardiac muscle cells42. Excessive calcium ions damage the inner membrane and structure of the mitochondria43.
4. Related pathways and targets of energy metabolism
4.1. Sirtuin protein family
Sirtuin (SIRT) is a highly conserved deacetylase present in life forms ranging from bacteria to humans. There are seven recognized members of the human sirtuin family, SIRT1–SIRT7. They can affect many metabolic functions, such as the TCA cycle, the urea cycle, amino acid metabolism, and fatty acid oxidation. Furthermore, they are involved in stress response, gene expression, DNA damage repair, body aging, and autophagy44. Research reported that sirtuin proteins play an important role in delaying the progression of cardiovascular disease45. For example, all of SIRT1, SIRT2, SIRT6, and SIRT7 reduce myocardial hypertrophy and maintain cardiac function46, 47, 48, 49. In addition, SIRT1 regulates cardiac electrophysiology by acetylating sodium channels50. SIRT3, SIRT5, and SIRT6 regulate cardiac remodeling and reduce myocardial ischemia reperfusion injury51, 52, 53. SIRT7 increases the resistance of cardiomyocytes, prevents apoptosis and prevents the occurrence of cardiomyopathy54. The effect of SIRT4 on the heart is unclear. A study reported that SIRT4 can increase ROS levels in the heart, promote myocardial hypertrophic growth, and produce fibrosis and cardiac dysfunction55. Table 156, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 summarizes their biological functions related to energy metabolism.
Table 1.
Biological functions of sirtuin proteins related to energy metabolism.
| Protein | Location | Enzyme activity | Biological functions related to energy metabolism |
|---|---|---|---|
| SIRT1 | Nucleus | Deacetylase |
|
| SIRT2 | Cytoplasm Nucleus |
Deacetylase | |
| SIRT3 | Mitochondria | Deacetylase |
|
| SIRT4 | Mitochondria | ADP-ribosyltransferase |
|
| SIRT5 | Mitochondria | Deacetylase | |
| SIRT6 | Nucleus | Deacetylase/ADP-ribosyltransferase |
|
| SIRT7 | Nucleolus | Deacetylase |
|
4.2. AMPK signaling pathway
AMPK is an energy sensor in the body. When energy supply is insufficient, AMPK can regulate the balance between energy supply and consumption by regulating mitochondrial synthesis, glucose metabolism, fatty acid absorption and oxidation, and activating the mammalian target of rapamycin (mTOR) pathway (Fig. 3)70, 71, 72. Research shows that AMPK could increase energy supply, reduce stress response and delay HF progression73.
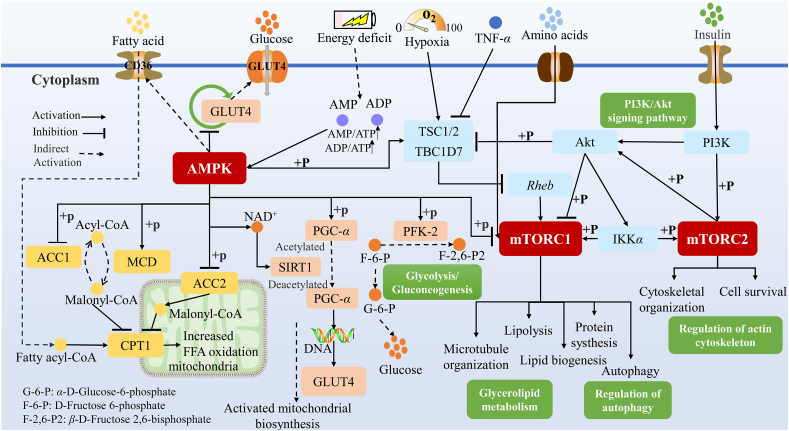
Figure 3.
AMPK and mTOR signaling pathway. When energy is insufficient, AMPK can be activated to increase the intake of fatty acids and glucose, inhibit glycogen synthesis, promote fatty acid oxidation and increase energy production. At the same time, it can promote the synthesis of mitochondria by regulating PGC-α. AMPK activation, hypoxia conditions and insulin can also inhibit mTORC1 activation, so as to regulate fatty acid metabolism, protein synthesis, autophagy, etc. Insulin can also directly activate mTORC2, regulate cell survival and cytoskeleton formation.
AMPK has important regulatory effects on mitochondrial gene expression and mitochondrial occurrence. On the one hand, it directly regulates the function of PGC-1α, activates mitochondrial DNA replication and transcription, and promotes mitochondrial synthesis73. On the other hand, AMPK also indirectly regulates PGC-1α. When the AMP/ATP ratio is increased, AMPK is activated to promote lipid oxidation in the mitochondria and promote the expression of nicotinamide phosphate ribose transferase. These signals increase the level of nicotinamide adenine dinucleotide (NAD+) in the cell, which in turn activates SIRT1 and eventually catalyzes the deacetylation of PGC-1α to activate it, thereby promoting mitochondrial synthesis73.
AMPK also regulates glucose metabolism and fatty acid metabolism. AMPK activation promotes intracellular transport of GLUT4 from the vesicles to the sarcolemma, increase glucose uptake by the cells, inhibits the GLUT4 endocytic cycle, and increases GLU4 content19,74. At the same time, its activation promotes the phosphorylation of phosphofructokinase-2 (PFK-2), upregulates glycolysis, inhibits glycogen synthesis, and reduces energy consumption75,76. In addition, AMPK upregulates the fatty acid transporter CD36, promoting fatty acids into cells20,77. AMPK phosphorylation inhibits ACC, activates MCD, and increases the conversion of malondialdehyde-CoA to acetyl-CoA, reducing CPT-1 inhibition and promoting fatty acid oxidation78.
4.3. mTOR signaling pathway
mTOR is a serine/threonine protein kinase with a molecular weight of 289 kDa. It belongs to the phosphoinositide 3-kinase (PI3K) protein family79. It plays a role in regulating cell functions mainly through two different protein complexes, mTORC1 and mTORC2. mTORC1 plays a key regulatory role in protein synthesis, cell proliferation and differentiation, ribosome and mitochondrial origin, autophagy and metabolism80,81. mTORC2 is related to cell survival and cytoskeleton formation82,83. mTOR senses the nutrition, oxygen, energy and other states of cells, and then regulates cell survival and metabolism to respond to environmental changes (Fig. 3). Nutrients such as amino acids are transported into cells by binding to corresponding receptors, which directly activates mTORC84. When energy is consumed, AMPK is activated and phosphorylates tuberous sclerosis 2 (TSC2) to inhibit mTORC1 activation. Hypoxia also promotes TSC1/2 activation and inhibits mTOR1 signaling85. Furthermore, AMPK also directly phosphorylates to inhibit mTORC1 activation, regulating lipid metabolism, protein synthesis, cell autophagy and other functions80.
Insulin affects mTOR in two ways. One is to influence mTORC1 and mTORC2 through PI3K-AKT signaling, and the other is to activate mTORC2 directly through PI3K phosphorylation, regulating cell activity82. Modulation of the mTOR pathway may be a potential therapeutic intervention for cardiovascular disease. A study showed that deletion of the mTORC1 gene under normal physiological conditions can lead to strong induction of atrial and brain natriuretic peptides and β-myosin heavy chains, and changes in multiple genes involved in regulating energy metabolism86. Under stress overload, mTORC1 gene-deficient mice developed dilated cardiomyopathy and accelerated HF, which may be related to apoptosis, autophagy, and increased mitochondrial dysfunction87,88. There are fewer reports about cardiovascular effects of mTORC2 than of mTORC1, but it is essential for normal heart development and the maintenance of heart structure and function after birth. Deletion of mTORC2 results in significant activation of mammalian sterile 20-like kinase 1, leading to cardiac dysfunction and dilation, affecting cardiac growth and adaption to stress overload89,90.
4.4. Insulin signal
The heart is an organ of insulin dependence and high energy consumption, which is rich in insulin receptors. Insulin can bind to insulin receptor in cardiomyocytes and mediate its signal transduction. Insulin combines with insulin receptor (such as insulin-like-1 receptor, IGF-1R) in cardiac myocytes, which makes insulin receptor self-phosphorylate, thus increasing the activity of insulin substrate (IRS) protein, and activating intracellular signal pathways including PI3K-AKT, mitogen-activated protein kinases (MAPK), mTOR, etc., so as to regulate the uptake of glucose, fatty acid oxidation and mitochondrial metabolism in the heart91,92. When the insulin receptor is missing, the insulin signaling is interrupted, which will cause the heart to become smaller and mitochondrial dysfunction after birth93. IRS protein activation is an important initial step of insulin and IGF receptor signaling, and also necessary for the next level of signaling. Studies have shown that the absence of IRS-1 and IRS-2 genes can lead to unconstrained autophagy, cardiac energy deficiency, mitochondrial dysfunction, myocardial structural damage and loss of contractile function, leading to myocardial cell loss, and premature death94,95. In fact, glucose metabolism and fatty acid metabolism in the heart are strictly controlled by insulin signal. Impaired insulin signal or insulin resistance play a decisive role in the occurrence and development of HF96. Therefore, the correction of insulin resistance and the elimination of insulin signal-mediated interference factors in patients with HF are of positive significance to prevent heart disease and improve the prognosis of patients with HF.
4.5. Purine signal
Purine signaling is mediated by purine receptors, which are expressed in most of the heart and blood vessels. It is divided into four P1G protein coupled receptor subtypes (A1, A2A, A2B and A3), seven P2X(1–7) ion channel subtypes and eight P2Y protein coupled receptor subtypes (P2Y1/2/4/6/11/12/13/14). Among them, A1, A2A, A2B, A3, P2Y4, P2Y6, P2Y12, P2Y11, P2X3, P2X4, and P2X7 have all been studied in the treatment of heart disease, but the therapeutic potential of other receptors has not been confirmed except of A1 receptor97. As early as the end of the 20th century, adenosine (A1 receptor agonist) has been shown to protect the heart of patients with chronic HF through A1 and A3 receptors98. In recent years, some adenosine A1 agonists have been proved to be effective drugs in the treatment of HF, with the potential to enhance cardiac metabolism, calcium homeostasis, cardiac structure and function, and prognosis of patients99. Purine signal protection of the heart may be related to energy metabolism. For example, adenosine can improve the transmission efficiency of mitochondrial electron chain, and has the potential to improve the efficiency of ATP synthesis and reduce the generation of excessive reactive oxygen species100. Animal studies have shown that some A1 receptor agonists can increase the expression of GLUT1 and GLUT4 glucose transporters to near normal levels101. In addition, the treatment of some A1R agonists is also related to the normalization of protein levels that mediate fatty acid oxidation and reduced plasma levels of free fatty acids102.
4.6. PPARs
PPARs are a nuclear receptor superfamily of transcription factors (including three subtypes of PPARα, PPARβ/δ, and PPARγ) and play an important central role in the fatty acid and glucose metabolism of the heart21,103. PPARα affects left ventricular function and fatty acid oxidation efficiency in HF mice. The left ventricular function and fatty acid oxidation rate of HF model mice with transverse aortic constriction decrease with a decrease in PPARα expression; when PPARα is activated, fatty acid metabolism rate significantly increases and left ventricular function is retained104. Deletion of PPARβ/δ inhibits fatty acid oxidation in the heart, and a healthy heart will develop lipotoxic cardiomyopathy hypertrophy and HF105. Overexpression of PPARβ/δ does not affect fatty acid oxidation, but increases oxidative phosphorylation of glucose, GLUT4 expression, and insulin sensitivity106. PPARγ agonists reduce the concentration of triglycerides in the plasma by increasing the accumulation of lipids in adipose tissue, thereby reducing the absorption and oxidation of fatty acids by the heart, increasing oxidative phosphorylation of glucose and lactate, and increasing cardiac contractility107. These research results indicate that PPARα, PPARβ/δ, and PPARγ may be potential targets for treating HF and improving myocardial function and energy metabolism.
4.7. PGC-1α
PGC-1α is a 91 kDa transcription factor that regulates lipid metabolism and long-chain fatty acid oxidation by upregulating the expression of several genes in the TCA cycle and the mitochondrial fatty acid oxidation pathway22,108. In addition, PGC-1α activates a variety of genes related to oxidative phosphorylation, such as PPARα and nuclear respiration factors, and regulates the mitochondrial oxidative phosphorylation metabolism function, thereby regulating systemic energy balance22. PGC-1α may be an important therapeutic target for the reducing morbidity and mortality of HF. PGC-1α deletion results in impaired mitochondrial respiratory function and reduced expression of genes involved in multiple mitochondrial metabolic pathways109. Mitochondrial enzyme activity and ATP levels are decreased in the heart of PGC-1α knockout mice110.
4.8. Creatine kinase
Creatine kinase (CK) is a mobile energy store in the cytoplasm. It can catalyze the reversible reaction of creatine phosphate (PCr)+ADP+H+→creatine+ATP111. A study showed that the rate of ATP resynthesis by CK in normal myocardium is faster than that of ATP produced by oxidative phosphorylation112. At high energy requirements, CK can generate creatine phosphate through oxidative phosphorylation, which produces a large amount of ATP112. CK may be an important target for the treatment of myocardial ischemia. Its moderate overexpression does not adversely affect cell metabolism, mitochondria or cardiac function, but it changes the opening of mitochondrial permeability transition pores, reduces ischemia–reperfusion injury, and promotes cardiac function recovery113. Animal study has found that when heart function is normal, CK overexpression increases ATP content without affecting systolic function, and it improves cardiac systolic function in HF114. In the early stages of HF, CK overexpression does not improve left ventricular remodeling and function, but it maintains cardiac energy changes, which is of great significance for increasing the survival rate of HF during the acute compensation115.
4.9. SET and MYND domain-containing protein 1 (SMYD1)
SMYD1 is a muscle-specific histone methyltransferase whose role in bone and myocardial development is well known. The absence of SMYD1 will cause the hypertrophic growth of mouse heart, and then develop into HF116. In the recent study, SMYD1 has been found to regulate the energy metabolism of the heart117. In SMYD1 deficient mice, the mitochondrial protein involved in oxidative phosphorylation was down regulated and the respiratory capacity of mitochondria was also reduced117. This study demonstrated the role of SMYD1 as the main regulator of heart energy.
5. Potential drugs for reducing HF by regulating energy metabolism
5.1. Hormones
Hormones are essential substances, playing important roles in regulating metabolism, growth, development, reproduction and other processes. In recent years, some hormonal drugs, including thyroxine, relaxin and estrogen, have been used to treat cardiovascular and cerebrovascular diseases. Thyroxine is an indispensable growth hormone in the body and is related to growth, apoptosis and energy metabolism. A recent study has shown that thyroxine reduces myocardial cell apoptosis, reduces energy loss, and prevents heart damage caused by doxorubicin via the LKB1/AMPK/mTOR pathway118. It is expected to become a new drug for clinical prevention of heart damage caused by doxorubicin chemotherapy. Relaxin is a polypeptide hormone that has a relaxing effect on the birth canal before delivery in mammals. It is produced mainly during pregnancy and is present at very low levels in normal humans119. A large number of clinical studies and translational studies on relaxin have shown that it may be effective in treating cardiovascular diseases, especially HF120,121. A clinical study has shown that relaxin-2, administered in the same amounts as produced during pregnancy, can prolong survival in patients with acute HF122. The molecular mechanism of relaxin for HF is currently unknown, and it may be related to the synthesis of endogenous long-chain polyunsaturated fatty acids, energy metabolism in modification of amino acid, and cardiovascular structural regulation123. Estrogen is the primary female sex hormone. It promotes the maturation of female accessory sexual organs and the appearance of secondary sexual characteristics, and it maintains normal sexual desire and reproductive function. Research has shown that estrogen protects the right ventricular function of pulmonary hypertension model mice by preserving mitochondrial content and oxidative capacity124.
5.2. Synthetic drugs
5.2.1. Metformin
Metformin is the first choice in the treatment of type 2 diabetes. Clinical observation shows that metformin increases the myocardial efficiency, reduces oxygen consumption and improves cardiac function in patients with non-diabetes HF125. Studies show that metformin restores left ventricular diastolic function and weakens immune and inflammatory response to a certain extent126. It also increases the contractile function of mice with postinfarction HF and reduces apoptosis of myocardial cells127. The mechanism of metformin improving cardiac function may be related to regulating glucose absorption, mitochondrial function, and oxidative stress. Metformin increases glucose uptake by stimulating the PI3K–PKB/AKT pathway and AMPK activation, and this positive effect has been observed in both insulin-resistant cardiomyocytes and normal insulin-sensitive cardiomyocytes128. Metformin also improves the heart function in mice by regulating the level of PGC-1α acetylation that is reduced by SIRT3, reducing the damaged mitochondrial membrane potential and increasing mitochondrial respiratory function127. Furthermore, it reduces the cardiotoxicity caused by doxorubicin by inhibiting oxidative stress and reducing HSCoA consumption in the mitochondria, increasing cardiac glutathione, HSCoA, ATP levels, and mRNA expression levels of catalase and NAD(P)H quinone dehydrogenase 1129.
5.2.2. Sodium-glucose transporter 2 inhibitor
Sodium-glucose transporter 2 (SGLT2) inhibitors are recently developed class of antidiabetic drugs, such as dapagliflozin, empagliflozin, canagliflozin, and ipragliflozin. Interestingly, SGLT2 inhibitors have been found to be potential cardiovascular protecting drugs and can be used to treat HF when clinically evaluated for cardiovascular safety130. Clinical studies have found that dapagliflozin reduces the risk of diabetic cardiomyopathy and improves left ventricular function in patients with type 2 diabetes and HF131. In addition, dapagliflozin can also reduce symptoms, improve physical function and quality of life for HF patients, and reduce the mortality rate from cardiovascular disease and the progression of HF132. It has also been observed that empagliflozin and canagliflozin improve the condition of patients with HF133,134. The mechanism of SGLT2 in the treatment of HF is not clear. According to existing research, it may be related to the following pathways:
-
•
Increasing ketone bodies. SGLT2 inhibitors reduce the blood glucose levels, thus reducing insulin levels and increasing glucagon. Glucagon promotes the degradation of adipose tissue and releases free fatty acids, which is conducive to the increase in ketone bodies29. Kim and Lee135 found that SGLT-2 inhibitors can increase β-hydroxybutyrate levels throughout the body and by upregulating ketogenic-related enzymes and transporters in the liver, kidney, and intestine. The latest literature reported that englitazine can also reduce the activation of NLRP3 inflammatory body through ketone body and insulin, thus playing a role in heart protection136.
-
•
Regulating of mitochondrial function. Ipragliflozin reduces mitochondrial dysfunction caused by a high-fat diet by restoring optic atrophy factor 1 (OPA1) and mitofusion 2 to normal levels in the body137. Empagliflozin restores the mitochondrial morphology and quantity in the hearts of diabetic rats by upregulating superoxide dismutase 2 and catalase, and protects against the mitochondrial morphological changes of diabetic-induced myocardial infarction in rats by inhibiting ROS and promoting autophagy138. In another study, empagliflozin inhibited mitochondrial division in an AMPK-dependent manner, thereby inhibiting oxidative stress and maintaining the barrier function of cardiac microvascular endothelial cells139.
-
•
Changing intracellular sodium homeostasis and promoting sodium-mediated cardiac protection. Dapagliflozin, empagliflozin and canagliflozin, can directly inhibit the cardiac Na+/H+ exchanger flux by binding to the Na+ binding site of Na+/H+ exchanger-1, reducing the Na+ concentration in the heart muscle, and slow heart rate for cardiac protection140.
5.2.3. Pioglitazone
Pioglitazone is an insulin sensitized thiazolidinedione and a PPARγ agonist. Study has shown that it reduces cardiovascular events and diabetes in patients with insulin resistance after a recent apoplexia or transient ischemic attack141. A study found that pioglitazone can reverse severe pulmonary hypertension and vascular remodeling and prevent right ventricular HF, which is related to the regulation of cardiac hypertrophy, fibrosis, myocardial contractility, fatty acid transport/oxidation and transforming growth factor signal transduction through miRNA/mRNA network142.
5.2.4. Simvastatin
Pressure overload induced by angiotensin II is one cause of the HF pathogenesis143. Simvastatin is an angiotensin II receptor inhibitor and is commonly used to treat hyperlipidemia and hypertension. Clinical observation found that taking simvastatin for 20 days significantly reduces the levels of proinflammatory markers interleukin-6 and C-reactive protein in patients with chronic HF144. It also induces lipid droplet accumulation, provides energy for maintaining mitochondrial function, promotes mitochondrial autophagy and phagocytosis, and inhibits mitochondrial damage and cardiomyocyte apoptosis145. In addition, simvastatin also reduces oxidative stress, endothelial thrombosis and atrial fibrillation in rats with ischemic HF by reducing atrial induced nitric oxide synthase, sodium calcium exchanger and Rac1 expression activities146.
5.2.5. Fenofibrate
Fenofibrate is a fibrate lipid-lowering drug. It is a highly selective and efficient PPARα ligand, that reduces levels of low-density lipoprotein, total cholesterol, and triglycerides and increases high-density lipoprotein147. Labinskyy et al.148 found that fenofibrate prevents the transformation of cardiac metabolic substrates in HF model dogs and moderately improves cardiac function. A study showed that fenofibrate promotes fatty acid oxidation in the mitochondria of isoproterenol-induced HF rats, increases myocardial energy metabolism and oxidative stress, and protects heart function149. In addition, fenofibrate also reducing aldosterone-induced myocardial hypertrophy, but it may not be related to fatty acid oxidation150.
5.2.6. Trimetazidine
Trimetazidine is commonly used to treat angina pectoris and is one of the most widely studied drugs in metabolic therapy. Clinical studies have found that it improves cardiac function classification, exercise tolerance, quality of life, left ventricular ejection fraction, and heart volume in patients with HF151. It may inhibit fatty acid oxidation by inhibiting long-chain mitochondrial 3-ketoacyl CoA thiolase, promote glucose metabolism, and maintain the PCr/ATP ratio in cells152.
5.2.7. Carvedilol
Carvedilol is an α1 and β receptor blocker and has a vasodilating effect. It is often used to treat patients with mild or moderate hypertension, or with renal insufficiency and diabetes. A clinic study showed that long-term use of carvedilol can increase survival in patients with non-ischemic HF and higher dosages (7.5 mg/day) of carvedilol increase survival for HF patients with low heart rate and low ejection fraction153. But carvedilol is not effective for patients with ejection fraction retention153. Carvedilol reduces myocardial fatty acid utilization in patients with congestive HF but has no effect on glucose utilization154. It can also increase ejection fraction, reduce New York Heart Association heart grade, increase PCr/ATP ratio and metabolic equivalent, and maintain myocardial high-energy phosphate levels155. In addition, it can increase the mitochondrial energy charge and reduce the lag of phosphorylation in myocardial ischemia model rats, and improve mitochondrial function during myocardial ischemia156.
These synthetic drugs to treat HF have been found to improve energy metabolism and protect the heart muscle in the treatment of other diseases, rather than specifically developed to improve energy metabolism of HF. This suggests that we can find new or best therapeutic targets for HF through the mechanism of these drugs in vivo and it provides a possibility to develop drugs to treat HF from clinical drugs. Meanwhile, it is necessary to further study the specificity and safety of these synthetic drugs and their derivatives for treating HF caused by different factors, providing more references for their clinical application.
5.3. Natural products
5.3.1. Polyphenols
Polyphenols are a large group of phytochemicals that are present in variety of foods, such as fruits, vegetables, beans, cereals, cocoa or chocolate, and beverages including red wine, coffee, and tea. According to their structural characteristics, they can be roughly divided into flavonoids, phenolic acids, lignans and stilbene. Polyphenols, such as resveratrol, quercetin, curcumin and epigallocatechin gallate, have obvious protective effects against atherosclerosis, hypertension, myocardial infarction, cardiomyopathy caused by anthracyclines, angiogenesis, and HF157. Table 2158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172 summarizes the polyphenols to treat HF via energy metabolism mechanisms. A large number of polyphenols have been proven to be effective by reducing cardiovascular disease through energy metabolism, but only resveratrol has entered a systematic and standardized clinical trial173. In addition, some polyphenol-rich extracts deserve attention. For example, long-term use of green tea extract can improve the cellular mechanical properties and intracellular calcium dynamics of normal cardiomyocytes, increase energy utilization, and eliminate the inhibitory effect of phospholamban on sarcoplasmic reticulum Ca2+-dependent ATPase 2a174. Amalaki rasayana is a traditional herbal from India. It is rich in polyphenols and can reverse the remodeling changes of left ventricular myocardial hypertrophy and age-related cardiac dysfunction by improving myocardial energy metabolism, muscle contraction function, and exercise endurance175.
Table 2.
Related mechanisms of polyphenols in HF treatment.
| Compound | Source | HF model | Dosage | Result |
|---|---|---|---|---|
| Resveratrol | Wine | Pressure-overload | 450 mg/kg/day, 2 weeks |
Resveratrol increase exercise capacity of HF mice, increase skeletal muscle insulin sensitivity, and increase systemic glucose utilization and basal metabolic rate158. |
| Tesaglitazar induction | 100 mg/kg/day, 6weeks |
Resveratrol reduces cardiac dysfunction and corrects myocardial mitochondrial respiration by mediating SIRT1 activation in tesaglitazar-induced C57BL/6 mice and diabetic mice, but it has no effect on myocardium in SIRT1 deficient mice159. | ||
| Myocardial infarction | 200 mg/kg/day, 2 weeks |
Resveratrol significantly increase ejection fraction and physical activity of myocardial infarction rats, restore myocardial fatty acid oxidation levels, significantly increases myocardial energy metabolism, and reduces left ventricular and atrial remodeling caused by myocardial infarction160. | ||
| Ischemia–reperfusion (I/R) injury | 50 mg/kg/day, 2 weeks |
Inhibit stromal interaction molecule1 -induced intracellular Ca2+ accumulation, reduce the mortality of myocardial I/R injured mice, reduce the area of myocardial infarction, and improve cardiac function161. | ||
| Epigallocatechin gallate (EGCG) | Green tea | Knockout manganese superoxide dismutase | 20 mg/kg/day, 8weeks |
EGCG reduces oxidative stress and free fatty acid levels. At the same time, it also delays the progression of HF by preventing the increased expression of nitric oxide synthase 2, nitrotyrosine, fatty acid synthase, Toll-like receptor 4 and SIRT1162. |
| Pressure-overload | 50 mg/kg/day, 12 weeks |
EGCG prevents left ventricular dilatation, increase ejection fraction and left ventricular short axis shortening rate, maintain cardiac function and upregulation of sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) via the modification of histone acetylation to prevent HF caused by stress overload163. | ||
| Pressure-overload | 10 mg/kg/day, 4 weeks |
EGCG reverses changes in left ventricular diastolic diameter and systolic diameter of HF mice, increase ejection fraction; inhibits myocardial fibrosis; reduces oxidative stress, inflammation, and myocardial cell apoptosis; and reduces collagen I and III expression levels, thereby inhibiting myocardial fibrosis and reducing ventricular collagen remodeling, delaying the occurrence and progression of HF. It may work by inhibiting the transforming TGF-β1/SMAD3 signaling pathway164. | ||
| Pressure-overload | 25,50 or 100 mg/kg/day, 4 weeks |
EGCG can reduce the desensitization of β1 receptor by inhibiting G protein-coupled receptor kinase 2 (GRK2) transfer membrane. It can also regulate left ventricular end-diastolic pressure, mean blood pressure, heart weight and posterior wall thickness, left ventricular systolic pressure, left ventricular maximum pressure rise rate (+dP/dtmax), and left ventricular maximum pressure drop rate (–dP/dtmax) and other constants to improve cardiomyocyte morphology165. | ||
| Puerarin | Puerariae Radix | Pressure-overload | 60 mg/kg/day, 4 weeks |
Puerarin reduces the activity of lactate dehydrogenase and succinate dehydrogenase, increase the expression level of GLUT4, and reduces the expression level of CD36. It also reduces the levels of inflammatory factors in myocardial tissue and regulate PPARα and its downstream target genes166. |
| Myocardial infarction with diabetes | 100 mg/kg/day, 4 weeks |
Puerarin increases AKT phosphorylation, decreases PPARα expression, increases GLUT4 expression and translocation, and reduces CD36 expression and translocation, thereby regulating cardiac energy metabolism, increasing heart function and improving survival rate in mice with HF167. | ||
| Tanshinone IIA | Salvia miltiorrhiza Bunge | Pressure-overload | 1.5 mg/kg/day, 4 weeks |
Tanshinone IIA mediates the expression of related molecules by upregulating AMPK and downregulating mTOR to increase autophagy and inhibit apoptosis168. |
| Pressure-overload | 20 mg/kg/day, 8 weeks |
Tanshinone IIA reduce inflammatory response and cardiomyocyte apoptosis in HF rats by regulating serum B-type brain natriuretic peptide, interleukin 6 and C-reactive protein levels and myocardial B-cell lymphoma-2 associated X protein levels169. | ||
| 7,8-Dihydroxyflavone | / | Doxorubicin-induction | 5 mg/kg/day, 2 weeks |
7,8-Dihydroxyflavone increase cell viability in vitro and reduce doxorubicin-induced cell death. At the same time, it improves the heart function of HF mice, reduces heart injury, and restores AMPK and signal transducing activator of transcription 3 (STAT3) expression by increasing mitochondrial respiration, membrane potential and OPA1 protein expression in vivo170. |
| Isoquercetin | / | Lipopolysaccharide induction | 50 mg/kg/day, 5 days |
Isoquercetin significantly reduces the inflammatory response and reduces the energy deficiency caused by lipopolysaccharide. It acts by increasing the expression of PGC1β and PPARα, activating AMPKα, and increasing fatty acid oxidation, thereby increasing cardiac and cellular ATP levels171. |
| Naringenin | / | I/R injury | 50 mg/kg/day, 1 week |
Naringenin reduces infarct size and myocardial cell index and reduces ischemia–reperfusion injury by inhibiting mitochondrial oxidative stress and increasing mitochondrial biogenesis through AMPK–SIRT3 signing172. |
Note: “/” means that its resource is rich.
5.3.2. Saponins
At present, ginsenosides are the main saponins used to HF. Ginseng total saponins increase cardiac energy metabolism by activating specific proteins in the TCA cycle176. Ginsenoside Rb1 restores heart and mitochondrial function, increases glucose uptake and prevents cardiac remodeling through TGF-β1/SMAD ERK and AKT signaling pathways177. Ginsenoside Rb3 protects the heart by regulating PPARα receptors to regulate fatty acid oxidation, protect the integrity of the mitochondrial membrane, and exert antiapoptotic effects178. Arjunolic acid, a naturally occurring chiral triterpenoid saponin, significantly inhibits the phosphorylation of P47phox and ERK in neutrophils from myocardial infarction, and reduces oxidative phosphorylation activity, reactive oxygen levels and oxidative stress in cardiomyocytes to reduce mitochondrial dysfunction and increase glycolysis rate179. Furthermore, it upregulates PPARα, reduces the phosphorylation level of TAK1, decreases p38 MAPK and NF-κB P65 activation, reduces excessive collagen synthesis and cardiac hypertrophy, and protects the heart180. Astragaloside IV is an extract from Astragali Radix. It increases the expression of PPARα, medium-chain acyl-CoA dehydrogenase (MCAD) and muscle carnitine palmitoyl transferase-1 (MCP1) and increases utilization of free fatty acids in chronic HF rats to improve cardiac function and inhibit ventricular remodeling181. It also stimulates fatty acid beta oxidation and improves mitochondrial function in HF animals, and is a potential drug to inhibit the progress of HF182.
5.3.3. Polysaccharides
Many polysaccharides, extracted from plants, have therapeutic effects on cardiovascular diseases, which are related to their anti-inflammatory and antioxidant effects183, 184, 185. There are limited reports on whether polysaccharides regulate cardiac energy metabolism. Only Ophiopogon polysaccharides, Lycium barbarum polysaccharides and Astragalus polysaccharides have been reported. Ophiopogon polysaccharides significantly reduce the levels of aminotransferase, lactate dehydrogenase, CK and CK-MB, increase ATPase activity, and exert protective effects on ischemia-induced myocardial injury186. The extract of Lycium barbarum promotes muscle differentiation and energy metabolism by upregulating mitochondrial biological gene regulatory factors187. Lycium barbarum polysaccharides exert a cardioprotective effect by reducing inflammatory cytokines and lipid peroxidation in HF mice188. Astragalus polysaccharides regulate the energy biosynthesis mediated by TNF-α/PGC-1 signaling pathway, decrease the mRNA and protein expression of ANP, increase the ratios of ATP/ADP and ATP/AMP, and reduce the free fatty acids content and cardiomyocyte hypertrophy189.
5.3.4. Alkaloids
Salsolinol is an alkaloid from the Aconitum plants. It reduces doxorubicin-induced chronic HF, reduces serum myocardial injury marker levels, decreases tissue damage to the heart, and increases the relative mRNA expression levels of key enzymes downstream of the TCA cycle to increase cardiac energy metabolism190. Higenamine, a typical β2-adrenergic selective receptor activator, is also an alkaloid in Aconitum plants that significantly increases myocardial contractility191. Combined with 6-gingerol, it upregulates PPARα/PGC-1α/SIRT3 pathway, increases cell viability, ameliorates doxorubicin-induced mitochondrial dysfunction, improves mitochondrial oxygen consumption and extracellular acidification rates, promotes mitochondrial energy metabolism and prevents HF192. Ligustrazine, also known as tetramethylpyrazine, is an alkaloid isolated from Ligusticum chuanxiong. It promotes the transfer of BCL-2 to mitochondria and improves mitochondrial function of by upregulating the expression of 14-3-3γ, thereby preventing myocardial damage caused by lipopolysaccharide193. It also alleviates H9c2 apoptosis induced by hypoxia by downregulating miR-499a, upregulating SIRT1, and activating the PI3K/AKT pathway194.
5.3.5. Traditional Chinese medicine patent prescription
Different natural products have different mechanisms for increasing cardiac energy metabolism. In traditional Chinese medicine, natural products of different mechanisms or even several herbs are combined according to certain rules so as to maximize their efficacy and reduce toxicity and side effects. This therapy is used as in clinical complementary and alternative therapies. Shengmai injection consists of Panax ginseng C.A. Mey., Ophiopogon japonicus (Thunb.) Ker Gawl., and Schisandra chinensis (Turcz.) Baill. extracts. It inhibits myocardial cell hypertrophy and apoptosis by activating the AMPK energy-dependent signaling pathway195. Shenfu granules are composed of Panax ginseng C.A. Mey. and Radix aconiti lateralis preparata. Clinical studies have found that oral Shenfu granules significantly improve the quality of life of patients with chronic HF196. Shenfu injection reduces the area of myocardial infarction and protects the myocardium by stimulating antioxidant and changing phospholipid levels, distribution, and levels of taurine, glutathione and phospholipid197. In addition, clinical observations have found that Qili Qiangxin capsule, consisting of 11 herbal extracts, improves the quality of life of patients with HF and reduces the incidence of cardiovascular disease and rehospitalization198. It has not been demonstrated whether the effect of Qili Qiangxin capsule is related to increasing energy metabolism. However, some of its components, such as astragaloside181, ginsenoside176, salsolinol192, and higenamine190, have been reported to be related to improving energy metabolism. At present, the combined use and safety of some natural ingredients and herbs are still being studied, but they provide possibilities for replacement and complementary therapy of HF and the development of HF drugs.
In recent years, natural products have received widespread attention for their unique safety and therapeutic effects. Natural products, such as polyphenols, saponins, polysaccharides, and alkaloids, have found components that can alleviate HF by regulating energy metabolism. It provides an idea for complementary and alternative therapy to treat HF. However, the content of natural products is low, and the technical requirements for its separation, extraction and purification are high. In addition, clinical researches of natural products are relatively insufficiency, and their mechanism and safety in the combine use are still in the exploration and research stage. These may be the reasons that limit their development into HF drugs.
5.4. Integration analysis of drug, target and metabolic pathway
We have sorted out and summarized the hormones, synthetic drugs and natural products mentioned above (Table 3). It is found that most of the drugs or components can act on multiple pathways or targets, and regulate multiple substrate metabolism. This suggests that we can consider the combination of different drugs, especially some drugs with large dosage and narrow safety window, which can reduce dosage, drug resistance and side effects. At present, some components, such as relaxin, estrogen, salsolinol and Chinese patent medicine, have been found to regulate cardiac energy metabolism, but their molecular is unclear. These components should be further study and provide reliable data for its development and application. In addition, we should also focus on utilizing the in vivo mechanisms of these drugs to find new or optimal therapeutic targets for HF. Then, we should apply the theoretical results of the study to clinical practice and develop more effective drugs and reasonable treatment strategies for treating HF.
Table 3.
Summary of known pathways, targets and metabolic pathways of drugs.
| Drug | Known pathway or target | Known metabolic pathway |
|---|---|---|
| Metformin | PI3k-PKB/AKT, AMPK, PGC-1α | Glucose metabolism, mitochondrial function, oxidative stress |
| Pioglitazone | PPARγ | Fatty acid oxidation, mitochondrial function |
| SGLT2 inhibitors | AMPK, Na+/H+ exchanger-1, NLRP3 inflammatory corpuscle | Ketone metabolism, mitochondrial function, intracellular sodium homeostasis, insulin signaling, oxidative stress |
| Simvastatin | iNOSitol, INCX, Rac1 | Mitochondrial function, oxidative stress |
| Fenofibrate | PPARα | Fatty acid metabolism |
| Trimetazidine | Long-chain mitochondrial 3-ketoacyl CoA thiolase | Glucose metabolism, fatty acid metabolism |
| Carvedilol | α1, β receptor | Fatty acid metabolism, mitochondrial function |
| Resveratrol | SIRT1, CYP1B2, stromal interaction molecule1 | Glucose metabolism, fatty acid metabolism, mitochondrial function, insulin signal, Ca2+ concentration |
| EGCG | TGF-β1/SMAD3, Toll-like receptor 4, SIRT1, SERCA2a, GRK2 transfer membrane, nitric oxide synthase 2, nitrotyrosine, fatty acid synthase, | Fatty acid metabolism, oxidative stress, cell function |
| Puerarin | GLU4, CD36, PPARα | Glucose metabolism, fatty acid metabolism |
| Tanshinone IIA | AMPK, mTOR | Apoptosis |
| 7,8-Dihydroxyflavone | AMPK, STAT3 | Mitochondrial function |
| Isoquercetin | PGC1β, PPARα, AMPKα | Fatty acid metabolism |
| Naringenin | AMPK-SIRT3 | Mitochondrial function |
| Ginsenoside Rb1 | TGF-β1/SMAD ERK, AKT | Glucose metabolism |
| Ginsenoside Rb3 | PPARα | Fatty acid metabolism, mitochondrial function |
| Arjunolic acid | P47phox, ERK, PPARα, TAK1, p38 MAPK, NF-κB P65 | Glucose metabolism, mitochondrial function, oxidative stress |
| Astragaloside IV | PPARα, MCAD, MCP1 | Fatty acid metabolism, mitochondrial function |
| Ophiopogon polysaccharide | AST, LDH, CK, CK-MB, ATPase | Mitochondrial function |
| Lycium barbarum polysaccharide | Mitochondrial biological gene regulatory factors | Fatty acid metabolism, mitochondrial function |
| Astragalus polysaccharide | TNF-α/PGC-1 | Fatty acid metabolism |
| Higenamine | PPARα/PGC-1α/SIRT3 | Mitochondrial function |
| Tetramethylpyrazine | 14-3-3γ, miR-499a, SIRT1, PI3K/AKT | Mitochondrial function |
| Thyroxine | LKB1/AMPK/mTOR | Fatty acid metabolism |
| Salsolinol | – | Mitochondrial function |
| Relaxin | – | Fatty acid synthesis, amino acid metabolism |
| Estrogen | – | Mitochondrial function |
–Unclear.
6. Conclusions and prospects
Much evidence shows that optimizing myocardial energy metabolism, especially regulating substrate metabolism, preserves or improves myocardial mechanical function, delays the progression of HF, and improves cardiac function classification, exercise tolerance, quality of life, left ventricular ejection scores and even survival rates. However, it is a research direction, as well as an urgent problem for HF drugs how to select appropriate substrate for adequate metabolism, promote the activity of myocardial metabolizer and increase the overall energy supply according to different pathological conditions. In addition, the development of most drugs to treat HF via energy metabolism is still in the basic research stage, and the changes in efficacy and application of these drugs also lack clinical data support. Researchers should further explore the association between the pathogenesis of HF and changes in myocardial energy metabolism, elucidate reveal the signaling pathways and key regulatory factors that affect HF energy metabolism, and develop effective new methods, including natural medicines, for preventing the occurrence and development of HF.
Acknowledgments
We are grateful to the support of National Key Research and Development Program (2018YFC1707205, China), and Sichuan Provincial Administration of Traditional Chinese Medicine Research Project (2018NQ008, China).
Author contributions
Li Han, Ming Yang, Dingkun Zhang and Yanan He designed the study; Lumeng Cheng, Runchun Xu, Nan Li, and Fang Wang supplied materials and analytic tools; Yanan He, Chen Zhang, Dingkun Zhang and Wei Huang wrote the paper.
Conflicts of interest
There are no conflicts to declare.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Li Han, Email: 2900480797@qq.com.
Ming Yang, Email: yangming16@126.com.
Dingkun Zhang, Email: 465790643@qq.com.
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R. Heart disease and stroke statistics—2017 update: a report from the american heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 3.Bertero E., Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. 2018;15:457–470. doi: 10.1038/s41569-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A., Houston B. A comprehensive review of the bioenergetics of fatty acid and glucose metabolism in the healthy and failing heart in nondiabetic condition. Heart Fail Rev. 2017;22:825–842. doi: 10.1007/s10741-017-9623-6. [DOI] [PubMed] [Google Scholar]
- 5.Ritterhoff J., Tian R. Metabolism in cardiomyopathy: every substrate matters. Cardiovasc Res. 2017;113:411–421. doi: 10.1093/cvr/cvx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sano H.I., Toki T., Naito Y., Tomita M. Developmental changes in the balance of glycolytic ATP production and oxidative phosphorylation in ventricular cells: a simulation study. J Theor Biol. 2017;419:269–277. doi: 10.1016/j.jtbi.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Lopaschuk G.D., Jaswal J.S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56:130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 8.de Carvalho A., Bassaneze V., Forni M.F., Keusseyan A.A., Kowaltowski A.J., Krieger J.E. Early postnatal cardiomyocyte proliferation requires high oxidative energy metabolism. Sci Rep. 2017;7:15434. doi: 10.1038/s41598-017-15656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heggermont W.A., Papageorgiou A.P., Heymans S., van Bilsen M. Metabolic support for the heart: complementary therapy for heart failure?. Eur J Heart Fail. 2016;18:1420–1429. doi: 10.1002/ejhf.678. [DOI] [PubMed] [Google Scholar]
- 10.Doenst T., Nguyen T.D., Abel E.D. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterzan M.A., Lygate C.A., Neubauer S., Rider O.J. Metabolic remodeling in hypertrophied and failing myocardium: a review. Am J Physiol Heart Circ Physiol. 2017;313:H597–H616. doi: 10.1152/ajpheart.00731.2016. [DOI] [PubMed] [Google Scholar]
- 12.Aerni-Flessner L., Abi-Jaoude M., Koenig A., Payne M., Hruz P.W. GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle. Cardiovasc Diabetol. 2012;11:63. doi: 10.1186/1475-2840-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Des Rosiers C., Labarthe F., Lloyd S.G., Chatham J.C. Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res. 2011;90:210–219. doi: 10.1093/cvr/cvr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotter D.G., Schugar R.C., Crawford P.A. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H1060–H1076. doi: 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puchalska P., Crawford P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–284. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S., Xie C. The role of OXCT1 in the pathogenesis of cancer as a rate-limiting enzyme of ketone body metabolism. Life Sci. 2017;183:110–115. doi: 10.1016/j.lfs.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Noordali H., Loudon B.L., Frenneaux M.P., Madhani M. Cardiac metabolism—a promising therapeutic target for heart failure. Pharmacol Ther. 2018;182:95–114. doi: 10.1016/j.pharmthera.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Stanley W.C., Recchia F.A., Lopaschuk G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 19.Yang J., Holman G.D. Insulin and contraction stimulate exocytosis, but increased AMP-activated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of GLUT4 in cardiomyocytes. J Biol Chem. 2005;280:4070–4078. doi: 10.1074/jbc.M410213200. [DOI] [PubMed] [Google Scholar]
- 20.Samovski D., Sun J., Pietka T., Gross R.W., Eckel R.H., Su X. Regulation of AMPK activation by CD36 links fatty acid uptake to beta-oxidation. Diabetes. 2015;64:353–359. doi: 10.2337/db14-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abushouk A.I., El-Husseny M.W.A., Bahbah E.I., Elmaraezy A., Ali A.A., Ashraf A. Peroxisome proliferator-activated receptors as therapeutic targets for heart failure. Biomed Pharmacother. 2017;95:692–700. doi: 10.1016/j.biopha.2017.08.083. [DOI] [PubMed] [Google Scholar]
- 22.Cheng C.F., Ku H.C. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 2018;19:3447. doi: 10.3390/ijms19113447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karwi Q.G., Uddin G.M., Ho K.L., Lopaschuk G.D. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med. 2018;5:68. doi: 10.3389/fcvm.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kundu B.K., Zhong M., Sen S., Davogustto G., Keller S.R., Taegtmeyer H. Remodeling of glucose metabolism precedes pressure overload-induced left ventricular hypertrophy: review of a hypothesis. Cardiology. 2015;130:211–220. doi: 10.1159/000369782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umbarawan Y., Syamsunarno M., Koitabashi N., Yamaguchi A., Hanaoka H., Hishiki T. Glucose is preferentially utilized for biomass synthesis in pressure-overloaded hearts: evidence from fatty acid-binding protein-4 and -5 knockout mice. Cardiovasc Res. 2018;114:1132–1144. doi: 10.1093/cvr/cvy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung M.M., Das S.K., Levasseur J., Byrne N.J., Fung D., Kim T.T. Resveratrol treatment of mice with pressure-overload-induced heart failure improves diastolic function and cardiac energy metabolism. Circ Heart Fail. 2015;8:128–137. doi: 10.1161/CIRCHEARTFAILURE.114.001677. [DOI] [PubMed] [Google Scholar]
- 27.Gruzdeva O., Uchasova E., Dyleva Y., Belik E., Kashtalap V., Barbarash O. Relationship between free fatty acids, insulin resistance markers, and oxidized lipoproteins in myocardial infarction and acute left ventricular failure. Diabetes Metab Syndr Obes. 2013;6:103–111. doi: 10.2147/DMSO.S37830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian N., Wang Y. Ketone body metabolism in diabetic and non-diabetic heart failure. Heart Fail Rev. 2020;25:817–822. doi: 10.1007/s10741-019-09857-3. [DOI] [PubMed] [Google Scholar]
- 29.Ferrannini E., Mark M., Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a "thrifty substrate" hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 30.Voros G., Ector J., Garweg C., Droogne W., Van Cleemput J., Peersman N. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.004953. [DOI] [PubMed] [Google Scholar]
- 31.Chong C.R., Clarke K., Levelt E. Metabolic remodeling in diabetic cardiomyopathy. Cardiovasc Res. 2017;113:422–430. doi: 10.1093/cvr/cvx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley W.C., Meadows S.R., Kivilo K.M., Roth B.A., Lopaschuk G.D. beta-Hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Am J Physiol Heart Circ Physiol. 2003;285:H1626–H1631. doi: 10.1152/ajpheart.00332.2003. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno Y., Harada E., Nakagawa H., Morikawa Y., Shono M., Kugimiya F. The diabetic heart utilizes ketone bodies as an energy source. Metabolism. 2017;77:65–72. doi: 10.1016/j.metabol.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Ho K.L., Zhang L., Wagg C., Al Batran R., Gopal K., Levasseur J. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res. 2019;115:1606–1616. doi: 10.1093/cvr/cvz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Zhou H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia–reperfusion injury. Acta Pharm Sin B. 2020;10:1866–1879. doi: 10.1016/j.apsb.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tai Y., Li L., Peng X., Zhu J., Mao X., Qin N. Mitochondrial uncoupler BAM15 inhibits artery constriction and potently activates AMPK in vascular smooth muscle cells. Acta Pharm Sin B. 2018;8:909–918. doi: 10.1016/j.apsb.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzman Mentesana G., Baez A., Cordoba R., Dominguez R., Lo Presti S., Rivarola W. Role of mitochondria and reactive oxygen species in the progression of heart failure. Rev Fac Cien Med Univ Nac Cordoba. 2010;67:150–158. [PubMed] [Google Scholar]
- 38.Unno K., Isobe S., Izawa H., Cheng X.W., Kobayashi M., Hirashiki A. Relation of functional and morphological changes in mitochondria to myocardial contractile and relaxation reserves in asymptomatic to mildly symptomatic patients with hypertrophic cardiomyopathy. Eur Heart J. 2009;30:1853–1862. doi: 10.1093/eurheartj/ehp184. [DOI] [PubMed] [Google Scholar]
- 39.Battogtokh G., Choi Y.S., Kang D.S., Park S.J., Shim M.S., Huh K.M. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: current strategies and future perspectives. Acta Pharm Sin B. 2018;8:862–880. doi: 10.1016/j.apsb.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maack C., Bohm M. Targeting mitochondrial oxidative stress in heart failure throttling the afterburner. J Am Coll Cardiol. 2011;58:83–86. doi: 10.1016/j.jacc.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 41.Sabbah H.N. Targeting the mitochondria in heart failure: a translational perspective. JACC Basic Transl Sci. 2020;5:88–106. doi: 10.1016/j.jacbts.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orrenius S., Gogvadze V., Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun. 2015;460:72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 43.Zhou B., Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carafa V., Rotili D., Forgione M., Cuomo F., Serretiello E., Hailu G.S. Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenet. 2016;8:61. doi: 10.1186/s13148-016-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cencioni C., Spallotta F., Mai A., Martelli F., Farsetti A., Zeiher A.M. Sirtuin function in aging heart and vessels. J Mol Cell Cardiol. 2015;83:55–61. doi: 10.1016/j.yjmcc.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Lu J., Sun D., Liu Z., Li M., Hong H., Liu C. SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl Res. 2016;172:96–112. doi: 10.1016/j.trsl.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Ryu D., Jo Y.S., Lo Sasso G., Stein S., Zhang H., Perino A. A SIRT7-dependent acetylation switch of GABPbeta1 controls mitochondrial function. Cell Metab. 2014;20:856–869. doi: 10.1016/j.cmet.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Sanz M.N., Grimbert L., Moulin M., Gressette M., Rucker-Martin C. Inducible cardiac-specific deletion of Sirt1 in male mice reveals progressive cardiac dysfunction and sensitization of the heart to pressure overload. Int J Mol Sci. 2019;20:5005. doi: 10.3390/ijms20205005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang X., Chen X.F., Wang N.Y., Wang X.M., Liang S.T., Zheng W. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation. 2017;136:2051–2067. doi: 10.1161/CIRCULATIONAHA.117.028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vikram A., Lewarchik C.M., Yoon J.Y., Naqvi A., Kumar S., Morgan G.M. Sirtuin 1 regulates cardiac electrical activity by deacetylating the cardiac sodium channel. Nat Med. 2017;23:361–367. doi: 10.1038/nm.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X.X., Wang X.L., Tong M.M., Gan L., Chen H., Wu S.S. SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3alpha-dependent antioxidant defense mechanisms. Basic Res Cardiol. 2016;111:13. doi: 10.1007/s00395-016-0531-z. [DOI] [PubMed] [Google Scholar]
- 52.Zou R., Shi W., Tao J., Li H., Lin X., Yang S. SIRT5 and post-translational protein modifications: a potential therapeutic target for myocardial ischemia–reperfusion injury with regard to mitochondrial dynamics and oxidative metabolism. Eur J Pharmacol. 2018;818:410–418. doi: 10.1016/j.ejphar.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Dikalova A.E., Pandey A., Xiao L., Arslanbaeva L., Sidorova T., Lopez M.G. Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ Res. 2020;126:439–452. doi: 10.1161/CIRCRESAHA.119.315767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vakhrusheva O., Smolka C., Gajawada P., Kostin S., Boettger T., Kubin T. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 55.Luo Y.X., Tang X., An X.Z., Xie X.M., Chen X.F., Zhao X. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. 2017;38:1389–1398. doi: 10.1093/eurheartj/ehw138. [DOI] [PubMed] [Google Scholar]
- 56.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 57.Khan S.A., Sathyanarayan A., Mashek M.T., Ong K.T., Wollaston-Hayden E.E., Mashek D.G. ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1alpha/PPAR-alpha signaling. Diabetes. 2015;64:418–426. doi: 10.2337/db14-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bordone L., Motta M.C., Picard F., Robinson A., Jhala U.S., Apfeld J. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040031. e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang F., Tong Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1's repressive interaction with PPARgamma. Mol Biol Cell. 2009;20:801–808. doi: 10.1091/mbc.E08-06-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomes P., Fleming Outeiro T., Cavadas C. Emerging role of sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol Sci. 2015;36:756–768. doi: 10.1016/j.tips.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 61.He X., Zeng H., Chen S.T., Roman R.J., Aschner J.L., Didion S. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J Mol Cell Cardiol. 2017;112:104–113. doi: 10.1016/j.yjmcc.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Y., Gao W.N., Xue Y.N., Zhang L.C., Zhang J.J., Lu S.Y. SIRT3 aggravates metformin-induced energy stress and apoptosis in ovarian cancer cells. Exp Cell Res. 2018;367:137–149. doi: 10.1016/j.yexcr.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 63.Haigis M.C., Mostoslavsky R., Haigis K.M., Fahie K., Christodoulou D.C., Murphy A.J. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 64.Nasrin N., Wu X., Fortier E., Feng Y., Bare O.C., Chen S. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285:31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao J., Zhang J., Ling Y., McCall C.E., Liu T.F. Mitochondrial sirtuin 4 resolves immune tolerance in monocytes by rebalancing glycolysis and glucose oxidation homeostasis. Front Immunol. 2018;9:419. doi: 10.3389/fimmu.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rardin M.J., He W., Nishida Y., Newman J.C., Carrico C., Danielson S.R. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhong L., D'Urso A., Toiber D., Sebastian C., Henry R.E., Vadysirisack D.D. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong X., Tao R., DePinho R.A., Dong X.C. Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanfi Y., Peshti V., Gil R., Naiman S., Nahum L., Levin E. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9:162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 70.Moussa A., Li J. AMPK in myocardial infarction and diabetes: the yin/yang effect. Acta Pharm Sin B. 2012;2:368–378. doi: 10.1016/j.apsb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qi D., Young L.H. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015;26:422–429. doi: 10.1016/j.tem.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J., Zhong L., Wang F., Zhu H. Dissecting the role of AMP-activated protein kinase in human diseases. Acta Pharm Sin B. 2017;7:249–259. doi: 10.1016/j.apsb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X., Liu J., Lu Q., Ren D., Sun X., Rousselle T. AMPK: a therapeutic target of heart failure-not only metabolism regulation. Biosci Rep. 2019;39 doi: 10.1042/BSR20181767. BSR20181767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell R.R., 3rd, Li J., Coven D.L., Pypaert M., Zechner C., Palmeri M. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halse R., Fryer L.G., McCormack J.G., Carling D., Yeaman S.J. Regulation of glycogen synthase by glucose and glycogen: a possible role for AMP-activated protein kinase. Diabetes. 2003;52:9–15. doi: 10.2337/diabetes.52.1.9. [DOI] [PubMed] [Google Scholar]
- 76.Magnoni L.J., Vraskou Y., Palstra A.P., Planas J.V. AMP-activated protein kinase plays an important evolutionary conserved role in the regulation of glucose metabolism in fish skeletal muscle cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Samovski D., Su X., Xu Y., Abumrad N.A., Stahl P.D. Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase. J Lipid Res. 2012;53:709–717. doi: 10.1194/jlr.M023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zordoky B.N., Nagendran J., Pulinilkunnil T., Kienesberger P.C., Masson G., Waller T.J. AMPK-dependent inhibitory phosphorylation of ACC is not essential for maintaining myocardial fatty acid oxidation. Circ Res. 2014;115:518–524. doi: 10.1161/CIRCRESAHA.115.304538. [DOI] [PubMed] [Google Scholar]
- 79.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 80.Ben-Sahra I., Manning B.D. mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol. 2017;45:72–82. doi: 10.1016/j.ceb.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rabanal-Ruiz Y., Otten E.G., Korolchuk V.I. mTORC1 as the main gateway to autophagy. Essays Biochem. 2017;61:565–584. doi: 10.1042/EBC20170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Linke M., Fritsch S.D., Sukhbaatar N., Hengstschlager M., Weichhart T. mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett. 2017;591:3089–3103. doi: 10.1002/1873-3468.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Senoo H., Kamimura Y., Kimura R., Nakajima A. Phosphorylated Rho-GDP directly activates mTORC2 kinase towards AKT through dimerization with Ras-GTP to regulate cell migration. Nat Cell Biol. 2019;21:867–878. doi: 10.1038/s41556-019-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolfson R.L., Sabatini D.M. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 2017;26:301–309. doi: 10.1016/j.cmet.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolff N.C., Vega-Rubin-de-Celis S., Xie X.J., Castrillon D.H., Kabbani W., Brugarolas J. Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Mol Cell Biol. 2011;31:1870–1884. doi: 10.1128/MCB.01393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shende P., Plaisance I., Morandi C., Pellieux C., Berthonneche C., Zorzato F. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 87.Sciarretta S., Forte M., Frati G., Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang D., Contu R., Latronico M.V., Zhang J., Rizzi R., Catalucci D. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sciarretta S., Zhai P., Maejima Y., Del Re D.P., Nagarajan N., Yee D. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep. 2015;11:125–136. doi: 10.1016/j.celrep.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shende P., Xu L., Morandi C., Pentassuglia L., Heim P., Lebboukh S. Cardiac mTOR complex 2 preserves ventricular function in pressure-overload hypertrophy. Cardiovasc Res. 2016;109:103–114. doi: 10.1093/cvr/cvv252. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J., Yu Z., You G. Insulin-like growth factor 1 modulates the phosphorylation, expression, and activity of organic anion transporter 3 through protein kinase A signaling pathway. Acta Pharm Sin B. 2020;10:186–194. doi: 10.1016/j.apsb.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riehle C., Abel E.D. Insulin signaling and heart failure. Circ Res. 2016;118:1151–1169. doi: 10.1161/CIRCRESAHA.116.306206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo C.A., Guo S. Insulin receptor substrate signaling controls cardiac energy metabolism and heart failure. J Endocrinol. 2017;233 doi: 10.1530/JOE-16-0679. R131-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Riehle C., Wende A.R., Sena S., Pires K.M., Pereira R.O., Zhu Y. Insulin receptor substrate signaling suppresses neonatal autophagy in the heart. J Clin Invest. 2013;123:5319–5333. doi: 10.1172/JCI71171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qi Y., Xu Z., Zhu Q., Thomas C., Kumar R., Feng H. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38α MAPK during insulin resistance. Diabetes. 2013;62:3887–3900. doi: 10.2337/db13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saotome M., Ikoma T., Hasan P., Maekawa Y. Cardiac insulin resistance in heart failure: the role of mitochondrial dynamics. Int J Mol Sci. 2019;20:3552. doi: 10.3390/ijms20143552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burnstock G. The therapeutic potential of purinergic signalling. Biochem Pharmacol. 2018;151:157–165. doi: 10.1016/j.bcp.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 98.Birkenfeld A.L., Jordan J., Dworak M., Merkel T., Burnstock G. Myocardial metabolism in heart failure: purinergic signalling and other metabolic concepts. Pharmacol Ther. 2019;194:132–144. doi: 10.1016/j.pharmthera.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 99.Greene S.J., Sabbah H.N., Butler J., Voors A.A., Albrecht-Küpper B.E., Düngen H.D. Partial adenosine A1 receptor agonism: a potential new therapeutic strategy for heart failure. Heart Fail Rev. 2016;21:95–102. doi: 10.1007/s10741-015-9522-7. [DOI] [PubMed] [Google Scholar]
- 100.Dinh W., Albrecht-Küpper B., Gheorghiade M., Voors A.A., van der Laan M., Sabbah H.N. Partial adenosine A1 agonist in heart failure. Handb Exp Pharmacol. 2017;243:177–203. doi: 10.1007/164_2016_83. [DOI] [PubMed] [Google Scholar]
- 101.Sabbah H.N., Gupta R.C., Kohli S., Wang M., Rastogi S., Zhang K. Chronic therapy with a partial adenosine A1-receptor agonist improves left ventricular function and remodeling in dogs with advanced heart failure. Circ Heart Fail. 2013;6:563–571. doi: 10.1161/CIRCHEARTFAILURE.112.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Staehr P.M., Dhalla A.K., Zack J., Wang X., Ho Y.L., Bingham J. Reduction of free fatty acids, safety, and pharmacokinetics of oral GS-9667, an A1 adenosine receptor partial agonist. J Clin Pharmacol. 2013;53:385–392. doi: 10.1002/jcph.9. [DOI] [PubMed] [Google Scholar]
- 103.Liu J., Wang Y., Lin L. Small molecules for fat combustion: targeting obesity. Acta Pharm Sin B. 2019;9:220–236. doi: 10.1016/j.apsb.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaimoto S., Hoshino A., Ariyoshi M., Okawa Y., Tateishi S., Ono K. Activation of PPAR-alpha in the early stage of heart failure maintained myocardial function and energetics in pressure-overload heart failure. Am J Physiol Heart Circ Physiol. 2017;312:H305–H313. doi: 10.1152/ajpheart.00553.2016. [DOI] [PubMed] [Google Scholar]
- 105.Cheng L., Ding G., Qin Q., Huang Y., Lewis W., He N. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 106.Burkart E.M., Sambandam N., Han X., Gross R.W., Courtois M., Gierasch C.M. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jaswal J.S., Keung W., Wang W., Ussher J.R., Lopaschuk G.D. Targeting fatty acid and carbohydrate oxidation—a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 108.Rigano D., Sirignano C., Taglialatela-Scafati O. The potential of natural products for targeting PPARα. Acta Pharm Sin B. 2017;7:427–438. doi: 10.1016/j.apsb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leone T.C., Lehman J.J., Finck B.N., Schaeffer P.J., Wende A.R., Boudina S. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3 doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metabol. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 111.Schlattner U., Tokarska-Schlattner M., Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762:164–180. doi: 10.1016/j.bbadis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 112.Smith C.S., Bottomley P.A., Schulman S.P., Gerstenblith G., Weiss R.G. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114:1151–1158. doi: 10.1161/CIRCULATIONAHA.106.613646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Whittington H.J., Ostrowski P.J., McAndrew D.J., Cao F., Shaw A., Eykyn T.R. Over-expression of mitochondrial creatine kinase in the murine heart improves functional recovery and protects against injury following ischaemia–reperfusion. Cardiovasc Res. 2018;114:858–869. doi: 10.1093/cvr/cvy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gupta A., Akki A., Wang Y., Leppo M.K., Chacko V.P., Foster D.B. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122:291–302. doi: 10.1172/JCI57426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cao F., Maguire M.L., McAndrew D.J., Lake H.A., Neubauer S., Zervou S. Overexpression of mitochondrial creatine kinase preserves cardiac energetics without ameliorating murine chronic heart failure. Basic Res Cardiol. 2020;115:12. doi: 10.1007/s00395-020-0777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Franklin S., Kimball T., Rasmussen T.L., Rosa-Garrido M., Chen H., Tran T. The chromatin-binding protein Smyd1 restricts adult mammalian heart growth. Am J Physiol Heart Circ Physiol. 2016;311:H1234–H1247. doi: 10.1152/ajpheart.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Warren J.S., Tracy C.M., Miller M.R., Makaju A., Szulik M.W., Oka S.I. Histone methyltransferase Smyd1 regulates mitochondrial energetics in the heart. Proc Natl Acad Sci U S A. 2018;115 doi: 10.1073/pnas.1800680115. E7871-e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y., Zhu S., Liu H., Wei W., Tu Y., Chen C. Thyroxine alleviates energy failure, prevents myocardial cell apoptosis, and protects against doxorubicin-induced cardiac injury and cardiac dysfunction via the LKB1/AMPK/mTOR axis in mice. Dis Markers. 2019;2019:7420196. doi: 10.1155/2019/7420196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nonhoff J., Ricke-Hoch M., Mueller M., Stapel B., Pfeffer T., Kasten M. Serelaxin treatment promotes adaptive hypertrophy but does not prevent heart failure in experimental peripartum cardiomyopathy. Cardiovasc Res. 2017;113:598–608. doi: 10.1093/cvr/cvw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martins R.C., Pintalhao M., Leite-Moreira A., Castro-Chaves P. Relaxin and the cardiovascular system: from basic science to clinical practice. Curr Mol Med. 2020;20:167–184. doi: 10.2174/1566524019666191023121607. [DOI] [PubMed] [Google Scholar]
- 121.Sarwar M., Du X.J., Dschietzig T.B., Summers R.J. The actions of relaxin on the human cardiovascular system. Br J Pharmacol. 2017;174:933–949. doi: 10.1111/bph.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miro O., Herrero-Puente P., Prieto B., Garcia-Garcia M., Garcia-Hernandez P., Martin-Sanchez F.J. The subset of patients with acute heart failure able to secrete relaxin-2 at pregnancy concentrations could have a longer survival: a pilot study. Biomarkers. 2018;23:573–579. doi: 10.1080/1354750X.2018.1463564. [DOI] [PubMed] [Google Scholar]
- 123.Aragon-Herrera A., Feijoo-Bandin S., Abella V., Alvarez L., Rosello-Lleti E., Portoles M. Serelaxin (recombinant human relaxin-2) treatment affects the endogenous synthesis of long chain poly-unsaturated fatty acids and induces substantial alterations of lipidome and metabolome profiles in rat cardiac tissue. Pharmacol Res. 2019;144:51–65. doi: 10.1016/j.phrs.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 124.Liu A., Philip J., Vinnakota K.C., Van den Bergh F., Tabima D.M., Hacker T. Estrogen maintains mitochondrial content and function in the right ventricle of rats with pulmonary hypertension. Physiol Rep. 2017;5 doi: 10.14814/phy2.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larsen A.H., Jessen N., Norrelund H., Tolbod L.P., Harms H.J., Feddersen S. A randomised, double-blind, placebo-controlled trial of metformin on myocardial efficiency in insulin-resistant chronic heart failure patients without diabetes. Eur J Heart Fail. 2020;22:1628–1637. doi: 10.1002/ejhf.1656. [DOI] [PubMed] [Google Scholar]
- 126.Jo W., Kang K.K. Metformin alleviates left ventricular diastolic dysfunction in a rat myocardial ischemia reperfusion injury model. Int J Mol Sci. 2020;21:1489. doi: 10.3390/ijms21041489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun D., Yang F. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem Biophys Res Commun. 2017;486:329–335. doi: 10.1016/j.bbrc.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 128.Bertrand L., Ginion A., Beauloye C., Hebert A.D., Guigas B., Hue L. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol. 2006;291:H239–H250. doi: 10.1152/ajpheart.01269.2005. [DOI] [PubMed] [Google Scholar]
- 129.Ashour A.E., Sayed-Ahmed M.M., Abd-Allah A.R., Korashy H.M., Maayah Z.H., Alkhalidi H. Metformin rescues the myocardium from doxorubicin-induced energy starvation and mitochondrial damage in rats. Oxid Med Cell Longev. 2012;2012:434195. doi: 10.1155/2012/434195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Custodio J.S., Jr., Duraes A.R., Abreu M., Albuquerque Rocha N., Roever L. SGLT2 inhibition and heart failure-current concepts. Heart Fail Rev. 2018;23:409–418. doi: 10.1007/s10741-018-9703-2. [DOI] [PubMed] [Google Scholar]
- 131.Tanaka H., Soga F., Tatsumi K., Mochizuki Y., Sano H., Toki H. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc Diabetol. 2020;19:6. doi: 10.1186/s12933-019-0985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kosiborod M.N., Jhund P.S., Docherty K.F., Diez M., Petrie M.C., Verma S. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141:90–99. doi: 10.1161/CIRCULATIONAHA.119.044138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Boer R.A., Nunez J., Kozlovski P., Wang Y., Proot P. Effects of the dual sodium-glucose linked transporter inhibitor, licogliflozin versus placebo or empagliflozin in patients with type 2 diabetes and heart failure. Br J Clin Pharmacol. 2020;86:1346–1356. doi: 10.1111/bcp.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Udell J.A., Yuan Z. Cardiovascular outcomes and mortality after initiation of canagliflozin: analyses from the EASEL Study. Endocrinol Diabetes Metab. 2020;3 doi: 10.1002/edm2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim J.H., Lee M. Sodium-glucose cotransporter 2 inhibitors regulate ketone body metabolism via inter-organ crosstalk. Diabetes Obes Metab. 2019;21:801. doi: 10.1111/dom.13577. [DOI] [PubMed] [Google Scholar]
- 136.Kim S.R., Lee S.G., Kim S.H., Kim J.H., Choi E., Cho W. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11:2127. doi: 10.1038/s41467-020-15983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takagi S., Li J., Takagaki Y., Kitada M., Nitta K., Takasu T. Ipragliflozin improves mitochondrial abnormalities in renal tubules induced by a high-fat diet. J Diabetes Investig. 2018;9:1025–1032. doi: 10.1111/jdi.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mizuno M., Kuno A., Yano T., Miki T., Oshima H., Sato T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6 doi: 10.14814/phy2.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou H., Wang S., Zhu P., Hu S., Chen Y., Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. doi: 10.1016/j.redox.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Uthman L., Baartscheer A., Bleijlevens B., Schumacher C.A., Fiolet J.W.T., Koeman A. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia. 2018;61:722–726. doi: 10.1007/s00125-017-4509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kernan W.N., Viscoli C.M., Furie K.L., Young L.H., Inzucchi S.E., Gorman M. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Legchenko E., Chouvarine P., Borchert P., Fernandez-Gonzalez A., Snay E. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aao0303. [DOI] [PubMed] [Google Scholar]
- 143.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 144.Pinchuk T.V., Fedulaev Y.N., Khairetdinova G.A., Denisova N.N., Chura O.V., Logunova I.Y. Anti-inflammatory effects of simvastatin in patients with chronic heart failure. Bull Exp Biol Med. 2014;157:552–554. doi: 10.1007/s10517-014-2612-z. [DOI] [PubMed] [Google Scholar]
- 145.Hsieh C.C., Li C.Y. Mitochondrial protection by simvastatin against angiotensin II-mediated heart failure. Br J Pharmacol. 2019;176:3791–3804. doi: 10.1111/bph.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cho K.I., Koo S.H., Cha T.J., Heo J.H., Kim H.S., Jo G.B. Simvastatin attenuates the oxidative stress, endothelial thrombogenicity and the inducibility of atrial fibrillation in a rat model of ischemic heart failure. Int J Mol Sci. 2014;15:14803–14818. doi: 10.3390/ijms150814803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li P., Luo S., Pan C., Cheng X. Modulation of fatty acid metabolism is involved in the alleviation of isoproterenol-induced rat heart failure by fenofibrate. Mol Med Rep. 2015;12:7899–7906. doi: 10.3892/mmr.2015.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Labinskyy V., Bellomo M., Chandler M.P., Young M.E., Lionetti V., Qanud K. Chronic activation of peroxisome proliferator-activated receptor-alpha with fenofibrate prevents alterations in cardiac metabolic phenotype without changing the onset of decompensation in pacing-induced heart failure. J Pharmacol Exp Ther. 2007;321:165–171. doi: 10.1124/jpet.106.116871. [DOI] [PubMed] [Google Scholar]
- 149.Dhyani N., Saidullah B., Fahim M., Omanwar S. Fenofibrate ameliorates neural, mechanical, chemical, and electrical alterations in the murine model of heart failure. Hum Exp Toxicol. 2019;38:1183–1194. doi: 10.1177/0960327119860173. [DOI] [PubMed] [Google Scholar]
- 150.Lebrasseur N.K., Duhaney T.A., De Silva D.S., Cui L., Ip P.C., Joseph L. Effects of fenofibrate on cardiac remodeling in aldosterone-induced hypertension. Hypertension. 2007;50:489–496. doi: 10.1161/HYPERTENSIONAHA.107.092403. [DOI] [PubMed] [Google Scholar]
- 151.Milinkovic I., Rosano G., Lopatin Y., Seferovic P.M. The role of ivabradine and trimetazidine in the new ESC HF guidelines. Card Fail Rev. 2016;2:123–129. doi: 10.15420/cfr.2016:13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dalal J.J., Mishra S. Modulation of myocardial energetics: an important category of agents in the multimodal treatment of coronary artery disease and heart failure. Indian Heart J. 2017;69:393–401. doi: 10.1016/j.ihj.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nagara K., Suzuki A., Shiga T. Long-term outcome of carvedilol therapy in Japanese patients with nonischemic heart failure. Heart Ves. 2020;35:957–966. doi: 10.1007/s00380-020-01560-w. [DOI] [PubMed] [Google Scholar]
- 154.Podbregar M., Voga G. Effect of selective and nonselective beta-blockers on resting energy production rate and total body substrate utilization in chronic heart failure. J Card Fail. 2002;8:369–378. doi: 10.1054/jcaf.2002.130238. [DOI] [PubMed] [Google Scholar]
- 155.Spoladore R., Fragasso G., Perseghin G., De Cobelli F., Esposito A., Maranta F. Beneficial effects of beta-blockers on left ventricular function and cellular energy reserve in patients with heart failure. Fundam Clin Pharmacol. 2013;27:455–464. doi: 10.1111/j.1472-8206.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 156.Monteiro P., Duarte A.I., Moreno A., Goncalves L.M., Providencia L.A. Carvedilol improves energy production during acute global myocardial ischaemia. Eur J Pharmacol. 2003;482:245–253. doi: 10.1016/j.ejphar.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 157.Santos C.N., Gomes A., Oudot C., Dias-Pedroso D., Rodriguez-Mateos A., Vieira H.L. Pure polyphenols applications for cardiac health and disease. Curr Pharm Des. 2018;24:2137–2156. doi: 10.2174/1381612824666180608102344. [DOI] [PubMed] [Google Scholar]
- 158.Sung M.M., Byrne N.J., Robertson I.M., Kim T.T., Samokhvalov V., Levasseur J. Resveratrol improves exercise performance and skeletal muscle oxidative capacity in heart failure. Am J Physiol Heart Circ Physiol. 2017;312:H842–H853. doi: 10.1152/ajpheart.00455.2016. [DOI] [PubMed] [Google Scholar]
- 159.Kalliora C., Kyriazis I.D., Oka S.I., Lieu M.J., Yue Y., Area-Gomez E. Dual peroxisome-proliferator-activated-receptor-alpha/gamma activation inhibits SIRT1-PGC1alpha axis and causes cardiac dysfunction. JCI Insight. 2019;5 doi: 10.1172/jci.insight.129556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matsumura N., Takahara S., Maayah Z.H., Parajuli N., Byrne N.J., Shoieb S.M. Resveratrol improves cardiac function and exercise performance in MI-induced heart failure through the inhibition of cardiotoxic HETE metabolites. J Mol Cell Cardiol. 2018;125:162–173. doi: 10.1016/j.yjmcc.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 161.Xu H., Cheng J., Wang X., Liu H., Wang S., Wu J. Resveratrol pretreatment alleviates myocardial ischemia/reperfusion injury by inhibiting STIM1-mediated intracellular calcium accumulation. J Physiol Biochem. 2019;75:607–618. doi: 10.1007/s13105-019-00704-5. [DOI] [PubMed] [Google Scholar]
- 162.Oyama J.I., Shiraki A., Nishikido T., Maeda T., Komoda H., Shimizu T. EGCG, a green tea catechin, attenuates the progression of heart failure induced by the heart/muscle-specific deletion of MnSOD in mice. J Cardiol. 2017;69:417–427. doi: 10.1016/j.jjcc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 163.Liu L., Zhao W., Liu J., Gan Y., Liu L., Tian J. Epigallocatechin-3 gallate prevents pressure overload-induced heart failure by up-regulating SERCA2a via histone acetylation modification in mice. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen K., Chen W., Liu S.L., Wu T.S., Yu K.F., Qi J. Epigallocatechin gallate attenuates myocardial injury in a mouse model of heart failure through TGFbeta1/Smad3 signaling pathway. Mol Med Rep. 2018;17:7652–7660. doi: 10.3892/mmr.2018.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Q., Hu L., Chen L., Li H., Wu J., Liu W. (–)-Epigallocatechin-3-gallate, the major green tea catechin, regulates the desensitization of beta1 adrenoceptor via GRK2 in experimental heart failure. Inflammopharmacology. 2018;26:1081–1091. doi: 10.1007/s10787-017-0429-x. [DOI] [PubMed] [Google Scholar]
- 166.He L., Wang T., Chen B.W., Lu F.M., Xu J. Puerarin inhibits apoptosis and inflammation in myocardial cells via PPARalpha expression in rats with chronic heart failure. Exp Ther Med. 2019;18:3347–3356. doi: 10.3892/etm.2019.7984. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Cheng W., Wu P., Du Y., Wang Y., Zhou N., Ge Y. Puerarin improves cardiac function through regulation of energy metabolism in streptozotocin-nicotinamide induced diabetic mice after myocardial infarction. Biochem Biophys Res Commun. 2015;463:1108–1114. doi: 10.1016/j.bbrc.2015.06.067. [DOI] [PubMed] [Google Scholar]
- 168.Zhang X., Wang Q., Wang X., Chen X., Shao M., Zhang Q. Tanshinone IIA protects against heart failure post-myocardial infarction via AMPKs/mTOR-dependent autophagy pathway. Biomed Pharmacother. 2019;112:108599. doi: 10.1016/j.biopha.2019.108599. [DOI] [PubMed] [Google Scholar]
- 169.Li X., Xiang D. Mitigating effect of tanshinone IIA on ventricular remodeling in rats with pressure overload-induced heart failure. Acta Cir Bras. 2019;34 doi: 10.1590/s0102-865020190080000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhao J., Du J., Pan Y., Chen T., Zhao L., Zhu Y. Activation of cardiac TrkB receptor by its small molecular agonist 7,8-dihydroxyflavone inhibits doxorubicin-induced cardiotoxicity via enhancing mitochondrial oxidative phosphorylation. Free Radic Biol Med. 2019;130:557–567. doi: 10.1016/j.freeradbiomed.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 171.Huang S.H., Xu M., Wu H.M., Wan C.X., Wang H.B., Wu Q.Q. Isoquercitrin attenuated cardiac dysfunction via AMPKalpha-dependent pathways in LPS-treated mice. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201800955. [DOI] [PubMed] [Google Scholar]
- 172.Yu L.M., Dong X., Xue X.D., Zhang J., Li Z., Wu H.J. Naringenin improves mitochondrial function and reduces cardiac damage following ischemia–reperfusion injury: the role of the AMPK–SIRT3 signaling pathway. Food Funct. 2019;10:2752–2765. doi: 10.1039/c9fo00001a. [DOI] [PubMed] [Google Scholar]
- 173.Dyck G.J.B., Raj P., Zieroth S., Dyck J.R.B., Ezekowitz J.A. The effects of resveratrol in patients with cardiovascular disease and heart failure: a narrative review. Int J Mol Sci. 2019;20:904. doi: 10.3390/ijms20040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bocchi L., Savi M., Naponelli V., Vilella R., Sgarbi G., Baracca A. Long-term oral administration of theaphenon-E improves cardiomyocyte mechanics and calcium dynamics by affecting phospholamban phosphorylation and ATP production. Cell Physiol Biochem. 2018;47:1230–1243. doi: 10.1159/000490219. [DOI] [PubMed] [Google Scholar]
- 175.Kumar V., Aneesh K.A., Kshemada K., Ajith K.G.S., Binil R.S.S., Deora N. Amalaki rasayana, a traditional Indian drug enhances cardiac mitochondrial and contractile functions and improves cardiac function in rats with hypertrophy. Sci Rep. 2017;7:8588. doi: 10.1038/s41598-017-09225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang J.R., Zhou H., Yi X.Q., Jiang Z.H., Liu L. Total ginsenosides of Radix Ginseng modulates tricarboxylic acid cycle protein expression to enhance cardiac energy metabolism in ischemic rat heart tissues. Molecules. 2012;17:12746–12757. doi: 10.3390/molecules171112746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zheng X., Wang S., Zou X., Jing Y., Yang R., Li S. Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp Anim. 2017;66:217–228. doi: 10.1538/expanim.16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen X., Wang Q., Shao M., Ma L., Guo D., Wu Y. Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARalpha pathway. Biomed Pharmacother. 2019;120:109487. doi: 10.1016/j.biopha.2019.109487. [DOI] [PubMed] [Google Scholar]
- 179.Miriyala S., Chandra M., Maxey B., Day A., St Clair D.K., Panchatcharam M. Arjunolic acid ameliorates reactive oxygen species via inhibition of p47(phox)-serine phosphorylation and mitochondrial dysfunction. Int J Biochem Cell Biol. 2015;68:70–77. doi: 10.1016/j.biocel.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bansal T., Chatterjee E., Singh J., Ray A., Kundu B., Thankamani V. Arjunolic acid, a peroxisome proliferator-activated receptor alpha agonist, regresses cardiac fibrosis by inhibiting non-canonical TGF-beta signaling. J Biol Chem. 2017;292:16440–16462. doi: 10.1074/jbc.M117.788299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tang B., Zhang J.G., Tan H.Y., Wei X.Q. Astragaloside IV inhibits ventricular remodeling and improves fatty acid utilization in rats with chronic heart failure. Biosci Rep. 2018;38 doi: 10.1042/BSR20171036. BSR20171036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dong Z., Zhao P., Xu M., Zhang C., Guo W., Chen H. Astragaloside IV alleviates heart failure via activating PPARalpha to switch glycolysis to fatty acid beta-oxidation. Sci Rep. 2017;7:2691. doi: 10.1038/s41598-017-02360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rao S.V., Zeymer U., Douglas P.S., Al-Khalidi H., Liu J., Gibson C.M. A randomized, double-blind, placebo-controlled trial to evaluate the safety and effectiveness of intracoronary application of a novel bioabsorbable cardiac matrix for the prevention of ventricular remodeling after large ST-segment elevation myocardial infarction: rationale and design of the PRESERVATION I trial. Am Heart J. 2015;170:929–937. doi: 10.1016/j.ahj.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 184.Dai H., Jia G., Liu X., Liu Z., Wang H. Astragalus polysaccharide inhibits isoprenaline-induced cardiac hypertrophy via suppressing Ca2+-mediated calcineurin/NFATc3 and CaMKII signaling cascades. Environ Toxicol Pharmacol. 2014;38:263–271. doi: 10.1016/j.etap.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 185.Zhang R., Xu Y., Niu H., Tao T., Ban T., Zheng L. Lycium barbarum polysaccharides restore adverse structural remodelling and cardiac contractile dysfunction induced by overexpression of microRNA-1. J Cell Mol Med. 2018;22:4830–4839. doi: 10.1111/jcmm.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fan S., Zhang J., Xiao Q., Liu P., Zhang Y., Yao E. Cardioprotective effect of the polysaccharide from Ophiopogon japonicus on isoproterenol-induced myocardial ischemia in rats. Int J Biol Macromol. 2020;147:233–240. doi: 10.1016/j.ijbiomac.2020.01.068. [DOI] [PubMed] [Google Scholar]
- 187.Ma J., Meng X., Kang S.Y., Zhang J., Jung H.W., Park Y.K. Regulatory effects of the fruit extract of Lycium chinense and its active compound, betaine, on muscle differentiation and mitochondrial biogenesis in C2C12 cells. Biomed Pharmacother. 2019;118:109297. doi: 10.1016/j.biopha.2019.109297. [DOI] [PubMed] [Google Scholar]
- 188.Pop C., Berce C., Ghibu S., Scurtu I., Soritau O., Login C. Effects of Lycium barbarum L. polysaccharides on inflammation and oxidative stress markers in a pressure overload-induced heart failure rat model. Molecules. 2020;25:466. doi: 10.3390/molecules25030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Luan A., Tang F., Yang Y., Lu M., Wang H., Zhang Y. Astragalus polysaccharide attenuates isoproterenol-induced cardiac hypertrophy by regulating TNF-alpha/PGC-1alpha signaling mediated energy biosynthesis. Environ Toxicol Pharmacol. 2015;39:1081–1090. doi: 10.1016/j.etap.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 190.Wen J., Zhang L., Liu H., Wang J., Li J., Yang Y. Salsolinol attenuates doxorubicin-induced chronic heart failure in rats and improves mitochondrial function in H9c2 cardiomyocytes. Front Pharmacol. 2019;10:1135. doi: 10.3389/fphar.2019.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang N., Lian Z., Peng X., Li Z., Zhu H. Applications of higenamine in pharmacology and medicine. J Ethnopharmacol. 2017;196:242–252. doi: 10.1016/j.jep.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 192.Wen J., Wang J., Li P., Wang R., Wang J., Zhou X. Protective effects of higenamine combined with [6]-gingerol against doxorubicin-induced mitochondrial dysfunction and toxicity in H9c2 cells and potential mechanisms. Biomed Pharmacother. 2019;115:108881. doi: 10.1016/j.biopha.2019.108881. [DOI] [PubMed] [Google Scholar]
- 193.Huang B., You J., Qiao Y., Wu Z., Liu D., Yin D. Tetramethylpyrazine attenuates lipopolysaccharide-induced cardiomyocyte injury via improving mitochondrial function mediated by 14-3-3gamma. Eur J Pharmacol. 2018;832:67–74. doi: 10.1016/j.ejphar.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 194.Zhang X., Dong H., Liu Y., Han J., Tang S., Si J. Tetramethylpyrazine partially relieves hypoxia-caused damage of cardiomyocytes H9c2 by downregulation of miR-449a. J Cell Physiol. 2019;234:15098–15107. doi: 10.1002/jcp.28151. [DOI] [PubMed] [Google Scholar]
- 195.Li Y., Ruan X., Xu X., Li C., Qiang T., Zhou H. Shengmai Injection suppresses angiotensin II-induced cardiomyocyte hypertrophy and apoptosis via activation of the AMPK signaling pathway through energy-dependent mechanisms. Front Pharmacol. 2019;10:1095. doi: 10.3389/fphar.2019.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wei H., Wu H., Yu W., Yan X., Zhang X. Shenfu decoction as adjuvant therapy for improving quality of life and hepatic dysfunction in patients with symptomatic chronic heart failure. J Ethnopharmacol. 2015;169:347–355. doi: 10.1016/j.jep.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 197.Wu H., Dai Z., Liu X., Lin M., Gao Z., Tian F. Pharmacodynamic evaluation of shenfu injection in rats with ischemic heart failure and its effect on small molecules using matrix-assisted laser desorption/ionization-mass spectrometry imaging. Front Pharmacol. 2019;10:1424. doi: 10.3389/fphar.2019.01424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sun J., Zhang K., Xiong W.J., Yang G.Y., Zhang Y.J., Wang C.C. Clinical effects of a standardized Chinese herbal remedy, Qili Qiangxin, as an adjuvant treatment in heart failure: systematic review and meta-analysis. BMC Complement Altern Med. 2016;16:201. doi: 10.1186/s12906-016-1174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]