Abstract
Immunotherapy has been recently considered as a promising alternative for cancer treatment. Indeed, targeting of immune checkpoint (ICP) strategies have shown significant success in human malignancies. However, despite remarkable success of cancer immunotherapy in pancreatic cancer (PCa), many of the developed immunotherapy methods show poor therapeutic outcomes in PCa with no or few effective treatment options thus far. In this process, immunosuppression in the tumor microenvironment (TME) is found to be the main obstacle to the effectiveness of antitumor immune response induced by an immunotherapy method. In this paper, the latest findings on the ICPs, which mediate immunosuppression in the TME have been reviewed. In addition, different approaches for targeting ICPs in the TME of PCa have been discussed. This review has also synopsized the cutting-edge advances in the latest studies to clinical applications of ICP-targeted therapy in PCa.
KEY WORDS: Immune checkpoint, Pancreatic cancer, Tumor microenvironment, Immunotherapy, Clinical development
Graphical abstract
The latest findings on the immune checkpoints (ICPs) mediating immunosuppression in tumor microenvironment (TME) are reviewed. Different approaches for targeting ICPs in TME of pancreatic cancer (PCa) and the latest clinical applications are discussed.
1. Introduction
In recent years, researchers have highlighted the importance of pancreatic cancer (PCa) in its severity and generalizability. In the past few decades, the concept of PCa has been faced with many arguments in many aspects, however the general view toward the PCa has been kept consistent, and PCa is still one of the most fatal cancers in the world. Accordingly, PCa is defined as the disease in which malignant cancer cells appear in the tissues of the pancreas1. Pancreatic tumors are classified into exocrine or neuroendocrine based on the cell from which they originate. This classification is critical as it provides distinct functional characteristics and treatment strategies between these two types. In the United States, PCa is the 9th and 10th most frequently diagnosed cancer in females and males, respectively2. Approximately 93% of PCa patients are exocrine tumors. According to the American Cancer Society, PCa patients account for approximately 3% of all adult cancer cases in the United States, with only about 22% of exocrine PCa patients still living one year after surgery. Above 56,000 Americans are expected to be diagnosed with PCa in 2019, with an average of above 150 diagnoses per day3. Recently, significant developments have been made in cognizing the molecular biology, diagnosis, staging, and treatment of PCa in patients4. As a turning point, cancer immunotherapy has emerged through monoclonal antibodies (mAbs) that obstructs inhibitory receptors on immune-effector cells or their ligands on tumor cells and antigen-presenting cells (APCs) alleged ‘immune checkpoints’ (ICPs). The main ICPs that are expressed on immune cells are programmed death 1 (PD-1), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), and lymphocyte-activation gene 3 (LAG-3). The ligands of PD-1 (PD-L1 and PD-L2), CTLA-4 (CD80 and CD86), and LAG-3 (MHC-II) could bind to ICPs and trigger immunosuppression in the tumor microenvironment (TME)5. This review is an attempt to focus on recent advances as well as an outlook on targeting ICPs for attenuation or elimination of PCa cells.
ICPs are regulatory molecules that maintain immune homeostasis; however, they are overexpressed to suppress the anti-tumoral immune response in the TME6. The immune system plays a critical role in the elimination of tumor cells and fight against cancer. It was demonstrated that the immune system unremittingly checks cells recognize any inappropriate and foreign antigens such as tumor antigens. This process of inspection of the immune system over the cells is called immune surveillance7. One of the strategies used by tumors such as pancreatic ductal adenocarcinoma (PDA) is to bypass the immune surveillance by the misusage of ICPs to escape immune recognition8. It is in view of this particular phenomenon that immunotherapy against ICPs is expounded as a powerful strategy in the field of anti-cancer therapy9, in which mAbs against PD-L1 and CTLA-4 were established to be effective9, 10, 11. An immunosuppressive hallmark of elevated expression of the ICP is the reduction of T cells activity12. B7-H1 or PD-L1 (CD274), as well as B7-DC and PD-L2 (CD273), are ligands of PD-1 (CD279). PD-L1, PD-L2, and PD-1 are transmembrane glycoprotein type I belong to the immunoglobulin (Ig) superfamily B7 and CD28, respectively13. The interaction between PD-1 on T cells and PD-L1 on APCs of the TME and PCa cells promotes the suppression and exhaustion of the T cells. Exhausted T cells have a significant role in leading to a defective T cell reaction which weakens the tumor-specific responses14. In PDA, CTLA-4, PD-1, and PD-L1 are three major inhibitory checkpoints which are expressed in the TME15. CTLA-4 (CD152) is a member of the Ig superfamily that binds to CD28 on T cells and transmits an inhibitory signal to T cells16. CTLA-4 has been first demonstrated as an inhibitory ICP molecule, which can suppress T cells, as well as autoreactive T cells, ordinary in lymph nodes16, 17, 18. Despite the mode of action of CTLA-4, PD-1 influences T cells with its inhibitory role at late stages of a T cell activity where PD-1 ligands are expressed. The expression of PD-L1 on tumor cells and also myeloid-derived suppressor cells (MDSC) in the TME could inhibit the activation of T cells5. A study showed that PD-L1 expression in PDA cells enhanced cancer progression19. In addition to the direct suppressive effects of the PD-1/PD-L1 axis on effector T cells, studies have shown the role of this pathway in the induction of regulatory T cells (Treg)20. In vitro studies have indicated that the presence of PD-L1 induces Treg activity21,22. Furthermore, PD-1 inhibitors could hamper the induction of transforming growth factor-β (TGF-β) and retinoic acid (RA)-induced Treg23. It was shown the elevated proportion of Treg for T CD8+ cells led to poor prognosis in cancers, suggesting that the induction of Treg is one of the major approaches used by tumor cells to escape immune surveillance24, 25, 26.
In PCa, the TME and the immune system show a vital role in tumor growth. PCa features an extremely immunosuppressive microenvironment, described by a dense desmoplastic stroma that inhibits blood flow to the area, prevents delivery of drug, and stops the antitumor immune reaction27. This supports cancer development and metastasis through protecting pancreatic tumors from immune surveillance. Moreover, the hypoxic milieu, acidic extracellular pH, and high interstitial fluid pressure in the TME also have a role in augmenting tumorigenesis28. Furthermore, the PDA microenvironment is generally composed of Treg, MQs and MDSCs, which stop the anti-tumoral function of effector CD4+ and CD8+ T cells. Treg has been shown to play critical role in PDA tumor development29. Tumor-associated macrophages (TAMs) are also responsible for inflammation, development and metastasis of PCa30. Upregulation of negative T cell co-stimulatory molecules is another reason of stimulation of PDA immunosuppression31. PD-L1 and PD-L2 are overexpressed in PDA patients and correlate with decreased tumor-infiltrating leukocytes (TILs) and a poor prognosis. Therefore, PD-L1 downregulation prevents cell proliferation in PCa32,33. In addition, an augmented expression of inhibitory molecules on inactivated T cells has been presented as another strategy to stimulate immunosuppression of PCa34.
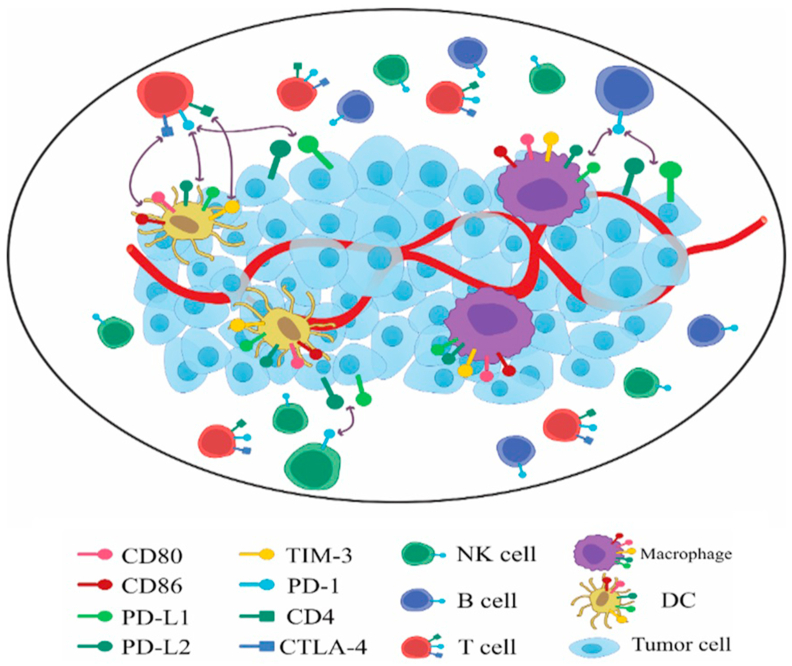
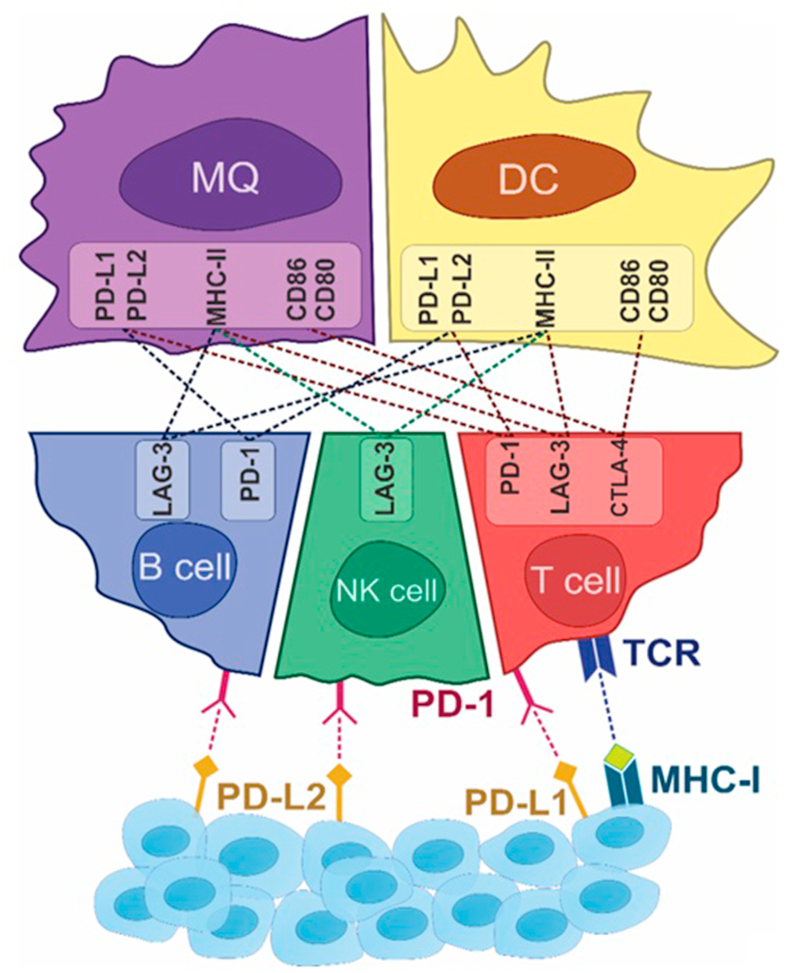
High CD8+ TIL correlates with a better prognosis due to their cytotoxic functions in several types of solid tumors. The acquisition of effector T cells (CD4+ and CD8+) in human PCa can improve overall survival (OS). Likewise, CD8+ effector T cells reduce whereas suppressive Treg contain a higher CD4 T cell level, which leads to a low number of TIL and a high number of immunosuppressive cells35. PCa is poorly immune-responsive, mainly due to its complex and suppressive TME. Therefore, PCa requires combinational therapy to provoke an immune response, for instance by employing vaccines to enhance the accumulation of lymphoid aggregates36. Thus, TME, on account of high levels of immunosuppression and poor immunogenicity, provides a unique challenge in PCa immunotherapy36. The significant milestones for the expression profile of immune cell and ICPs in the TME are pointed out in Fig. 1.
Figure 1.
The expression profile of immune cells and ICPs in the TME.
2. Immune checkpoint pathways
2.1. CTLA-4 pathway
Activation of T cells is a complex process and requires more than one activating signal. Binding T cell receptor (TCR) to major histocompatibility complex (MHC) is vital for T cell activation, and other required costimulatory signals. In order to activate the stimulatory signal in T cell, B7-2 (CD86) or B7-1 (CD80) molecules bind to CD28 molecules on the APCs. Adequate concentrations of CD28 for binding to B7 1/2 (CD80/CD86) leads to generation of T cell with improved endurance, differentiation in the enhanced cytokines structure like interleukin-2 (IL-2), adjustment of durability of cell for genes and improved energy metabolism. CTLA-4 delivers a negative regulatory signal to the T cell through binding to B7 1/2 molecules. CTLA-4, a CD28 homologue, shows higher binding affinity to B737,38; however, the binding of CTLA-4 to B7 withholds a stimulating signal through CD28 reverse. The competition binding can block the stimulatory signal generated by CD28–B7 binding38, 39, 40. Furthermore, the corresponding quantity of CD28–B7 versus CTLA-4–B7 restricts the activation or energy of a T cell16. In addition, some data suggest that CTLA-4–B7 can truly provide inhibitory signals which prevent CD28–B7 and TCR:MHC binding stimulating signals41,42. CTLA-4 location within the cell is under a controlled mechanism and initially located in the intracellular segment of resting naive T cells40,41,43. Consequently, the stimulatory signals, including both TCR and CD28–B7 binding, trigger upregulation of CTLA-4 through CTLA-4-included vesicles exocytosis on the cell surface44, which explains how TCR signals extract high translocation of CTLA-4 in a categorized feedback loop to the cell surface. Whereas, inhibition of IL-2 production as well as stimulation of the cell cycle progression has limited CTLA-4–B7 binding to fully activate of T cells45. Tregs regulate the effector T cells' activity and are indispensable to maintain peripheral tolerance46,47. Tregs constitutively express CTLA-4 unlike effector T cell, thus, this is deemed crucial for their suppressive capabilities46. In the animals, the absence of CTLA-4 on Treg impaired their suppressive ability46,48. The downregulation of CD80 and CD86 on APCs is one of Treg's mechanisms to regulate the function of effector T cells48,49.
2.2. PD-1 pathway
PD-1 as a part of the B7/CD28 costimulatory family regulates T cell activation by binding to their ligands, PD-L1 and PD-L2. Unlike CTLA-4, binding to PD-1 blocks the generation of stimulating cytokines, such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and IL-2, which lead to decrease in the activity of T cells50. Where T cell activation coincides with TCR–PD-1 binding, the phosphorylation of intermediate TCR signals is inhibited by PD-1 generated signals, which with terminating TCR signals result in decrease T cell activation41,51. Expression of PD-1 indicates “exhausted” T cells that practice extreme rates of stimulation or reduced CD4+ T cell support52. Dysfunction of T cell which is happening in the suboptimal administration of tumors and some diseases, identifies this stage of fatigue that occurs when chronic infections and cancer occur. Both CTLA-4 and PD-1 receptors have proven negative effects on T cell activation; however, the timing of downregulation, efficiency of negative signaling pathway and the anatomical regions of inhibition are different17,50. While CTLA-4 utilizes T cell activation in the priming state, PD-1 operates at the point of the effector, predominantly within peripheral tissues50. For CTLA-4, the combination of PD-1 ligands varies too. The B7 ligands for CTLA-4 are expressed by special APCs that usually stay in the lymph nodes or spleen, although PD-1 and PD-L2 are expressed in T cell and, APC and more widely cancer cells, respectively17,39,53,54. PD-L1 is expressed on leukocytes, non-hematopoietic cells, non-lymphoid tissues as well as in parenchymal cells through tumorigenic signals or inflammatory cytokines55. Expression of PD-L1 is differently identified in several tumor types and is incorporated with the increase and decrease of the amount of TILs and prognosis, respectively56, 57, 58. The expression of PD-L2 is considerable in DCs and monocytes and can be triggered depending on the microenvironment by a broad range of particular immune and non-immune cells59. Binding affinity of PD-1 for PD-L2 compared to PD-L1 showed unique tendency, which can be efficient for the differential immune response involvement of the ligands60. The interactions of PD-1 with PD-L1 and PD-L2 are developed to make tolerance within infiltrated tissue around due to PD-1 ligands are expressed in peripheral tissues. One type of contradictory roles of PD-L1 and PD-L2 on natural killer (NK) T cells activation includes improved Th2 activity through restriction of PD-L2 binding points, although CD80 binding to PD-L1 was provided to hinder T cell responses61, 62, 63. The multiple biologic effects lead to differences in the toxicity and activity for PD-1-directed antibodies which prevent to bind both ligands as compared to interaction of PD-1 with PD-L1. Despite Treg expression on both PD-1 and CTLA-4, the purpose of PD-1 expression remains dubious. PD-L1 not only provides Treg with the exchange of naive CD4+ T cells, but also prevents T cell responses by enhancing Treg induction and maintenance22. Similar to these results, the blockade of PD-1 is capable of inverting the Treg-mediated defeat of the effector T cells in vivo64. The immune response is reduced by PD-1 binding to its ligands on T cells, which are busied on the effector T cell responses. This equal restriction of T cell activation, which is linked to CTLA-4 blockade, may describe immune related adverse events through the potentially inferior occurrence of PD-1, equivalent to a CTLA-4 blockade52,65. Comparisons between CTLA-4 and PD-1 demonstrate that they are both B7 receptor family components66, expressed by activated T cells44,50, expression affected by the strength and/or continuity of TCR signaling50,52,67, decreased T cell proliferation, glucose metabolism, cytokine manufacturing, durability and also arranging an extended T cell proliferation17,39,50.
However, there are such differences include CTLA-4 expressed through T cells, whereas PD-1 expressed through T cells and other immune cells and further CTLA-4 first restricts T cell responses, originally on lymphoid tissues, whereas PD-1 limits T cell response anywhere usually. In peripheral tissues, PD-1 action clashes with more T cell signaling mechanisms than CTLA-4 ligands expressed by professional antigen-presenting neurons. PD-L1/2 expressed by APCs and other immune cells mostly, although the ligands can be expressed on non-immune cells such tumor cells. On the other hand, CTLA-4 inducing Treg functioning, thus, the function of PD-1 on Treg is uncertain17,39,48,50.
3. Potential predictive biomarkers
3.1. CTLA-4
The structural similarity of CTLA-4 to CD28, let this inhibitory checkpoint the ability to interact with CD80 and CD86 but in a stronger affinity than CD2868. CTLA-4 can mediate the suppression of T cells through several mechanisms. First, CTLA-4 plays an efficient role to reduce CD28 co-stimulation16,69, and its interaction with CD80/86 of APCs can decrease APCs stimulatory role in the T cells activation70. Second, expression of CTLA-4 on Treg which gives them the ability to inhibit T cells function71. Third, inhibition of TCR genes and CD28-induced genes72,73. Fourth, the expression of CTLA-4 in dendritic cells (DCs) that inhibits T cells18.
In the case of PDA, it was shown that anti-CTLA-4 approach did not provide an optimal clinical benefit and anti-tumor function neither in human nor in cancer models74,75. However, a study stated that there was an induction of the infiltration of CD4+ T cells in the TME, as a result of anti-CTLA-4 approach, which has no remarkable changes in the migration of CD8+ T cells75. This is in total contrast with the approach of anti-CTLA-4 therapy for melanoma patients, which showed a significant increase in infiltration of CD8+ T cells76. Thus, it can be suggested that CTLA-4 implicates the recruitment of CD4+ T cell in PDA patients. Like these findings, Bengsch et al.75 exhibited that anti-CTLA-4 therapy promotes T cell infiltration into the TME as well as T cell activation. However, this approach was not clinically useful in PDA patients. The interesting observation was the influence of CD80 inhibition in the induction of T cell recruitment into the TME. Consequently, the interaction of CTLA-4 and costimulatory molecules (CD80/86) can regulate the migration of T cells to the TME75. Basso et al.77 demonstrated that despite expression of PD-L1 and CTLA-4 in splenic DCs, no PDA patients showed increased levels of both PD-L1 and CTLA-4 expressions. They observed that, for the first time, the S100A8/A9, also known as calprotectin, could downregulate CTLA-4. Intriguingly, CTLA-4 negative DCs diminished T cell proliferation. DCs were also shown to augment the inhibitory role of Treg in the inhibition of allogeneic T cell responses through their CD8078. This is while CD86 showed opposing function on the Treg activity78.
3.2. PD-1
Activated monocytes, DC, NK, T and B cells express another important ICP called PD-179. Like to CTLA-4, the responsibility of PD-1 is to regulate T cell responses80. Domain-containing phosphatase-1 and 2 (SHP-1&2) affiliated with immunoreceptor tyrosine-based switch motif (ITSM) of PD-1 for stimulation human T cell, whereas ligation of PD-1 inhibit T cell activation81,82. It was shown that CTLA-4 acts as an inhibitory molecule in the primary phases of activation of T cells83 while the interaction of PD-1 and its ligands mainly happens on activated effector T cells in the periphery5. In the TME of PDA, the PD-1 expression has predominantly happened in TILs, which leads to immune escape by tumor cells13,84. However, recent research presented PD-1 expression in PDA tumor cells85. It was demonstrated that the amount of PD-1, which was expressed on CD8+ T cells elevated in tumor tissue where this expression was significantly correlated with the clinical stage of PDA patients. Indeed, higher expression of PD-1 in PDA tumor tissue was considered as a poor prognostic factor86. However, another investigation disclosed elevated stromal PD-1+TILs as a decisive prognostic factor. In fact, they suggested a correlation between intraepithelial and peripheral compartment of PD-1+TILs infiltration with better progression-free survival (PFS) and distant metastases-free survival (DMFS)87. Nevertheless, investigations on a diversity of cancers such as renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), and melanoma demonstrated an enhanced PD-1 expression, which was affiliated with disease with poor prognosis88, 89, 90. Thus, there is a controversy about the role of PD-1 as a satisfactory target for the targeted ICP therapy87. It was demonstrated that PDA patients with elevated PD-1 expression and dense stroma were associated with better OS91. Interestingly, the stroma of PDA, is observed to have a role in the upsurge of immune cell migration and accumulation, which led to a boosted inflammatory response92. The expression of PD-1 in peripheral blood stays stable even after surgical treatment and this stable PD-1 level of peripheral CD8+ T cells was shown to be associated with higher PDA recurrence86.
3.3. PD-L1
As conferred, PD-L1 is one of the inhibitory molecules expressed in solid tumors, DCs and MQs of the TME93,94. It was shown that PD-L1 from T γδ cells of PDA could inhibit tumor-specific cytotoxic T lymphocytes and Th1 cells95. Interesting data revealed that not all but some the PD-L1 producing cells have the ability to suppress T cells via binding to PD-114. In general, the B7 family consists of PD-L1 (B7-H1) and PD-L2 (B7-H2)93. Although there is still a need for further studies to understand the differences between the practical function of PD-L1 and PD-L214, it was shown that PD-L1 is the primary molecule that is presented in solid tumors13. PD-L1 can bind to PD-1, CD80 of T cells, and APCs63. This binding leads to apoptosis, energy exhaustion, and finally inhibition of effector T cells13,96,97. In the case of PDA, PD-L1 was demonstrated to have the ability to upregulate Treg infiltration and to induce immune suppression20. The expression of PD-L1 in PDA was shown to be associated with tumor growth, drug resistance, and finally to high tumor invasion98. Another study in human PDA showed that PD-L1 and PD-L2 were expressed in cancerous pancreatic tissue. There was also a negative correlation between PD-L1 expression and CD8+ T cells. Furthermore, 20 out of 51 PDA patients who were PD-L1 positive showed poor prognosis99. The inhibition of blocking colony-stimulating factor 1 (CSF-1) and its receptor resulted in an increase in CD4+/CD8+ T cells infiltration and the expression of PD-L1100. Similarly, high expression of HLA class I with PD-L1–PDA was indicated to be associated with high CD8+ T cells infiltration and good prognosis. It was suggested that the presence of HLA-I probability possess the potential to promote immunostimulatory condition, whereas PD-L1 could induce the immunosuppressive one. Thus, the immunological response of PDA pertains to the balance of the TME between the two discussed conditions101. Intriguingly, there was an association between the expression of PD-L1, low tumor differentiation, and diminished advanced tumor stage102. However, PD-L1 expression correlates with the existence of immunosuppressive cells101.
It was shown that the remarkable function of PD-L1 happened in the early stages of the disease99, while it was previously shown that both PD-L1 and PD-L2 had their strong effect in the advanced stages of esophageal cancer. This is while a correlation exists between the expression of PD-L1 at high levels in PCa and the metastasis of lymph node103. In another study, researchers reported that there might be an association between miss match repair (MMR) system deficient-cancer and the anti-PD-L1 treatment. This is a result of the fact that this deficiency promotes the production of multiple neo-antigens, which are further targeted by the augmented immune system91.
4. Targeting immune checkpoints
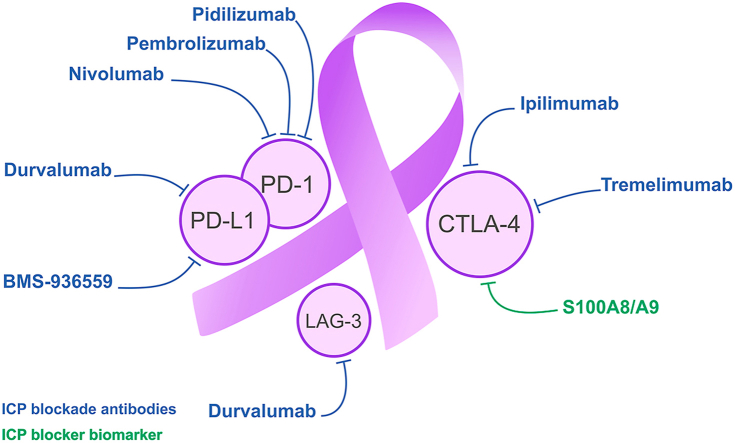
Tumor cells can grow fast and spread in part by targeting the immune system of the host. During the past decade, immunotherapy has emerged as an effective and standard method of treating different cancers104. Even though, different treatment strategies, such as surgery and conventional chemotherapies, have prolonged patient survival and they do not inhibit restorative. Consequently, new treatment strategies are indispensable105,106. Over the past decade, cancer immunotherapy has paved the path from a promising preclinical use to a clinical reality107. Manipulation of ICPs is one of the most current promising strategies for cancer therapy35, therefore clinical studies confirm that inhibition of ICPs disarranges adverse immune regulations and induces immune system as well as anti-tumor activities108. For this purpose, mAbs target inhibitory ICPs and show significant results in different cancers109. Since they are accepted by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), ICP blockade drugs have been developed for application to treat different cancers109, 110, 111, 112, 113, 114. Accordingly, it could also be a highly effective therapy against PCa15. In this part, the latest advances and future challenges of ICP inhibitors application in PCa are being summarized. Pivotal ICPs in PCa and their interplay with their specific ligands have been shown in Fig. 2.
Figure 2.
Pivotal ICPs in PCa and their interaction with specific ligand. The interaction of negative ICPs, including PD-1, CTLA-4, LAG-3, with their specific ligand were shown in detail. Interplay of PD-L2 on the TME with B cells and NK cells and PD-L1 and CTLA-4 on the TME with T cell through their specific ligand activates the immunosuppression and causes tumor immune evasion. Overexpressed LAG-3 on all of B cells, NK cells and T cell deactivates DC and MQ functions which result in tumor-induced immunosuppression.
4.1. CTLA-4 immune checkpoint blockade
Recently, CTLA-4, the first clinically targeted ICP receptor, has been studied extensively in cancer immunotherapy11,39,115. According to studies conducted in the late 90s, the opposing effect of CD28 and CTLA-4 had demonstrated on T cell activation in vitro, whereas blocking CD28 and CTLA-4 have declined and intensified anti-tumor responses, respectively116. Indeed, monotherapy-based mice treated with anti-CTLA-4 antibodies led to the complete tumor elimination and durable immunity. Subsequently, mechanistic studies showed that this anti-tumor function was related to the augmented ratio of both CD4 and CD8 effector cells to FoxP3+ (forkhead box P3) Treg117. Ipilimumab (Ipi) and tremelimumab (Tre), two human anti-CTLA-4-antibodies, have been applied clinically in PCa.
4.1.1. Ipilimumab
Ipi, a fully humanized IgG1 kappa mAb, was the first drug developed against CTLA-4118. In 2011, Ipi was approved by the FDA to treat melanoma and is now under clinical evaluation in various tumors119. Royal et al.120 investigated the effect of Ipi in the regression of advanced pancreatic adenocarcinoma (APA) during the phase II study. Twenty-seven people with APA received 3.0 mg intravenous Ipi, following which response evaluation criteria in solid tumors (RECIST) and toxicity were measured. This study found that the above mentioned dose-scheme of Ipi is not effective in PCa and suggested the use of higher dosages of Ipi at earlier stages of the disease, possibly with combinatorial agents120. Granulocyte-macrophage colony-stimulating factor (GM-CSF) of cell-based vaccines have been discovered to provide a synergistic activity to anti-CTLA-4 antibodies. Therefore, in a phase Ib of a study, Ipi has effectively been combined with GM-CSF cell-based vaccines GVAX (a cancer vaccine containing whole tumor cells which modified to secrete the immune stimulatory agents). In this study, thirty patients with PCa were listed and Ipi (arm 1) and Ipi + GVAX (arm 2) were examined121. Following the administration, fifth patients indicated stable disease, and seven patients indicated CA19-9 reduction. An enhancement in OS was detected that was related to the clinical activity in the combination arm. In conclusion, this study showed that checkpoint blockade plus GVAX is a potential candidate for checkpoint immunotherapy, and requires assessment in larger studies121. Kamath et al.122 tested a phase Ib clinical trial of CTLA-4 inhibition with Ipi in combination with gemcitabine (Gem) in patients with advanced PCa. This study established a maximum tolerated dose for Gem and Ipi with a good overall safety profile. Although the observed response rate was similar to Gem alone, the durability of responses suggests a component of immune activation that may warrant further investigation122. Moreover, Parikh et al.123 conducted a phase II study of Ipi and Niv with radiation in metastatic PCa. Dual blockade of CTLA-4 and PD-1 with radiation is feasible and reveals hopeful action in these patients.
4.1.2. Tremelimumab
Tre is an anti-CTLA-4 mAb, which is assessed in melanoma, colon cancer, gastric cancer, and mesothelioma in clinical trials124 possesses a greater affinity toward CTLA-4 than CD28. Ex vivo studies of patient samples with expanded and metastatic melanoma disclosed that application of Tre decreases tumor development via considerable stimulation of cytotoxic T cell activity125. A phase I study in PCa patients, analyzed the tolerability, safety, and maximum tolerated dose of Tre plus Gem. This combination indicated a manageable safety and tolerability profile and prolonged OS126. A recent study investigated durvalumab (Dur) with or without Tre for patients with metastatic PDA. Treatment was well tolerated, and the efficiency of Dur plus Tre therapy and Dur monotherapy presented a population of patients with mPDAC who had poor prognoses and quickly developing disease127. Moreover, a phase II study is testing Dur alone or Dur plus Tre in metastatic PCa128. Furthermore, a study of hypofractionated radiotherapy in combination with Dur and Tre in metastatic PCa patients is underway129.
4.2. PD-1/PD-L1 immune checkpoint blockade
The second ICP receptor is PD-1, which appeared as a marker for successful cancer immunotherapy39. It interacts with the PD-L1 and PD-L2 ligands and stops T cell induction130. PD-1/PD-L1 axis enables tumor evasion from the immune responses, and this interaction blockade with anti-PD-1 as well as anti-PD-L1 can augment the tumor immunity. Moreover, the proliferation of CD8+ T cell and production of cytokine can be induced by this blockade131. Numerous PCa-related anti-PD-1 and anti-PD-L1 antibodies have been assessed in clinical trials. Nivolumab (Niv), pembrolizumab (Pem), and pidilizumab (Pid) are the antibodies that block PD-1. On the other hand, Dur and BMS-936559 are PD-L1 targeted antibodies132.
4.2.1. Pembrolizumab
Pem (MK-3475) is a mAb of the IgG4κ isotype considered to stop PD-1 and PD-L1/2 interaction133. Recently, a phase I/IIa study is analyzing the effect of colony-stimulating factor 1 receptor (CSF1R) inhibitor (pexidartinib/PLX3397) and Pem combination in PDA134. NCT02305186, another phase Ib/II study, assesses safety and immunological effect of chemoradiation therapy (CRT) plus Pem and CRT monotherapy in PCa patients135. Also, NCT02362048 is ongoing to investigate the Bruton tyrosine kinase (BTK) inhibitor, acalabrutinib, alone or with Pem for metastatic PCa136. Furthermore, Mahalingam et al.137 reported that reolysin (pelareorep), in combination with chemotherapy and Pem, can be clinically successful and demonstrated convenient safety profiles and anti-tumor function in metastatic PCa patients. Furthermore, a study investigated Pem in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced PCa. Pelareorep in combination with chemotherapy and Pem in these patients was well-tolerated and presented durable efficacy. Further examination of pelareorep and anti-PD-1 therapy is ongoing in follow-up studies138. A phase II study of PEGPH20 in combination with Pem for patients with hyaluronan (HA)-high refractory metastatic PCa is ongoing. This research will examine the efficacy, safety and translational biomarkers of PEGPH20 plus Pem in these patients139. Moreover, Halama et al.140 tested pharmacodynamic impact and safety of monotherapy with CXCL12 inhibitor NOX-A12 plus Pem in PCa patients. These patients with impaired immune systems and a high tumor load had several unsuccessful prior lines of treatment. NOX-A12 plus Pem showed induction of immune response, stabilize disease in 25% of patients, and prolonged time on treatment versus prior therapy for 35% of patients140.
4.2.2. Nivolumab
Niv is another therapeutic IgG4 antibody against PD-1 for improving anti-tumor responses. In vitro assays showed that Niv enhances T cell induction and cytokine production. Similar to Pem, Niv stops PD-1 and its ligands interaction141. To evaluate the effect of CY/GVAX and CRS-207 alone or in combination with Niv, a phase II study is being directed in PDA patients who have unsuccessful chemotherapy for metastatic disease142. Correspondingly, an ongoing study by Firdaus et al.143 is studying nab-paclitaxel and Niv plus Gem in advanced PCa patients. Presently, Niv is being tested for safety and efficacy in phases I and II trials for resectable (NCT02451982) and metastatic (NCT02423954) PDA144. Wang-Gillam et al.145 are testing the effect of cabiralizumab (Cab) given with Niv with and without chemotherapy in PCa patients. Also, a recent research is evaluating SD-101 (toll-like receptor 9 agonist), Niv, and radiation therapy in treating patients with chemotherapy-refractory metastatic PCa146.
4.2.3. Pidilizumab
In recent years, Pid as another anti-PD-1 ICP inhibitor has received consideration. It is a humanized IgG4κ mAb. In PDA, two clinical trials, were administered imperfectly, and stopped thenceforth147,148.
4.2.4. Durvalumab
Dur is also another mAb of the IgG4κ isotype which targets PD-L1, stops the binding of PD-L1 to the PD-1 and CD80 molecules. In a recent study, Duffy et al.149 evaluated the impact of combination therapy of radiation together with Tre and/or Dur in PCa patients. This study showed that radiation could intensify the impact of ICP stopping in these patients. Dur with or without Tre in metastatic PCa patients was performed in phase II study129. Additionally, a phase Ib/II study assesses the safety and efficacy of Dur plus ibrutinib (BTK inhibitor) in PCa patients150. Currently, a phase Ib study examined the combination of galunisertib (Gal) and Dur in recurrent or refractory metastatic PCa. The combination of Gal plus Dur had a suitable tolerability and safety profile. The effect of this combination in second and third line PCa patients warrants additional attention151. The study of Dur and stereotactic radiotherapy in locally advanced PCa was safe, well tolerated and appears to be clinically active with high rates of margin-negative resection152. Cassier et al.153 performed a phase I dose escalation research to assess the safety and clinical function of a combined treatment relating an anti-CSF1R (pexidartinib) with Dur in patients with advanced/metastatic PCa. Toxicity was consistent with the expected profiles of the individual drugs and no unpredicted findings were observed with the combination.
4.2.5. Status of dMMR/MSI and PD1/PD-L1 and CTLA-4 antibodies
The mismatch repair (MMR) system plays an important role in repair of DNA sequence during replication. Deficiency in the MMR system (dMMR) or lack of performance of one of the MMR proteins, including MLH1, MSH2, MSH6 and PMS2, lead to an accumulation of somatic mutations, resulting in microsatellite instability (MSI) and a higher neoantigen load that enhances proinflammatory cytokines and activation of T cells154. Given the recent tissue-agnostic approval of Pem, MSI testing is now recommended by the National Comprehensive Cancer Network (NCCN) for locally advanced or metastatic PDA. A recent study of MMR status in PDA using next generation sequencing (NGS) revealed that dMMR occurs at a low frequency of 0.8% in PDA, and all these cases also have Lynch syndrome155. Furthermore, Yamamoto et al.156 and Macherla et al.157 examined the prognostic impact of MSI in PCa cases and found that MSI positives patients had longer survival time than negative ones.
PD-L1 overexpression and dMMR/MSI status could indeed be useful predictive biomarkers for the response to immunotherapy. For this purpose, Kim et al.158 analyzed both PD-L1 and MLH1/MSH2 expressions and showed a remarkable association between PD-L1 expression and MLH1/MSH2 loss. Moreover, Salem et al.159 studied the correlation between tumor mutational load (TML), dMMR, and PD-L1 and found a lower frequency of TML-high in PDA, and a positive PD-L1 expression in MSI-H and MSS PDA cases at about 11.1% and 9%, respectively157,159. The majority of pancreatic adenocarcinoma patients with either MSI-H or MSI-L showed low TML level. Future studies are needed to indicate the suitability of PD-L1, MSI, and TML as appropriate predictive markers of response to immunotherapy in PDAC157,160. Pem was recently evaluated in clinical trials in heavily pretreated patients with MSI-H tumors161,162. Interestingly, these studies demonstrated the benefit ICB in patients with MSI-H tumors regardless of their PD-L1 status. Furthermore, Niv, as a PD-1 inhibitor, which is approved for progressed MSI-H/dMMR metastatic colorectal cancer (CRC) following first-line treatment, indicated that dMMR plays a robust predictive of response to ICB in comparison to PD-L1157,163. Furthermore, the activated V-domain immunoglobulin suppressor of T cell activation (VISTA) which is expressed on the MQs pathway, decreases T cell responses in the tumor at a greater rate compared to PD-L1 blockade. Therefore, PD-1/PD-L1 blockade might breakdown PDA treatment due to suppression of the immune response through an untreated VISTA pathway. Enhancement of T cell infiltration, using anti-CTLA-4 mAb with anti-VISTA antibody to target MQs as combination therapy, holds a promising new strategy for PDA treatment164,165.
4.2.6. BMS-936559
BMS-936559, a humanized IgG4 and PD-L1-specific mAb, can prevent the interaction of PD-L1 with both PD-1 and CD80. In NSCLC, RCC and melanoma patients, durable tumor regression and long-term disease stabilization were observed by PD-L1 blockade; however, in PCa, no objective response was detected9,166.
4.3. LAG-3 immune checkpoint blockade
LAG-3 or CD223, a homolog of CD4, was cloned over 25 years ago167. The negative regulatory role for LAG3–MHC-II interaction is the most prominent characteristic of LAG-3, and this fact represents LAG-3 as a potential treatment target168. In 2006, targeted immunotherapy through LAG with a soluble LAG-3Ig fusion protein (IMP321) was introduced. While, IMP321 was used for three clinical trials in RCC, metastatic breast carcinoma, and melanoma previously with average success. It is still being tested in other novel clinical trials, where it may reveal additional therapeutic advantages169, 170, 171. LAG-3 was shown to synergize with PD-1 in downregulating T cell activities and stimulating immune evasion by cancer cells. Even though, targeting of LAG-3 alone showed little effect, and blockade of LAG-3 has been revealed to synergize with PD-L1 and augment its anti-tumor effect172. These trials are investigating the application of combination anti-LAG-3 and anti-PD-1 versus anti-LAG-3 alone in diverse solid tumors173. These results have received certain interest in perspective, hence LAG-3 will be considered as the promising targeted immunotherapy and predictive biomarker174. Table 19,120, 121, 122, 123,127,129,134, 135, 136, 137, 138, 139,142, 143, 144, 145, 146,149, 150, 151, 152, 153,166,175,176 summarizes the clinical trials of targeted ICPs in PCa.
Table 1.
Clinical trials of targeted ICP in Pca.
| Target | Drug | Phase | Status | Clinical Trials identifier | Ref. |
|---|---|---|---|---|---|
| CTLA-4 | Ipilimumab | II | Completed | NCT00112580 | 120 |
| Ipilimumab ± GVAX | I | Completed | NCT00836407 | 121 | |
| Ipilimumab + GVAX | II | Recruiting | NCT01896869 | 175 | |
| Ipilimumab + gemcitabine | Ib | Completed | NCT01473940 | 122 | |
| Ipilimumab + nivolumab with radiation | II | Recruiting | NCT03104439 | 123 | |
| Tremelimumab (CP-675,206) + gemcitabine | Ib | Completed | NCT00556023 | 128 | |
| Tremelimumab + durvalumab | II | Completed | NCT02558894 | 127 | |
| PD-1 | Pembrolizumab + REOLYSIN + chemotherapy | II | Recruiting | NCT02620423 | 137 |
| Pembrolizumab + ACP-196 | II | Active but not recruiting | NCT02362048 | 136 | |
| Pembrolizumab (MK3475) | I/II | Recruiting | NCT02305186 | 135 | |
| Pembrolizumab + PLX3397 | I | Recruiting | NCT02452424 | 134 | |
| Pembrolizumab + oncolytic virus pelareorep | Ib | Recruiting | NCT02620423 | 138 | |
| Pembrolizumab + PEGPH20 | II | Recruiting | NCT03634332 | 139 | |
| Pembrolizumab + NOX-A12 | II | Completed | NCT03168139 | 176 | |
| Nivolumab + GVAX + cyclophosphamide | I/II | Active but not recruiting | NCT02451982 | 144 | |
| Nivolumab + Nab-paclitaxel + gemcitabine | I | Recruiting | NCT02309177 | 143 | |
| Nivolumab + GVAX + CRS-207 | II | Recruiting | NCT02243371 | 142 | |
| Nivolumab + cabiralizumab + chemotherapy | II | Active but not recruiting | NCT03336216 | 145 | |
| Nivolumab + SD-101 | I | Recruiting | NCT04050085 | 146 | |
| PD-L1 | BMS-936559 | I | Completed | NCT00729664 | 9,166 |
| Durvalumab + ibrutinib mesylate | Ib/II | Recruiting | NCT02403271 | 150 | |
| Durvalumab + galunisertib | 1 b | Completed | NCT02734160 | 151 | |
| Durvalumab + stereotactic radiotherapy | I/II | Recruiting | NCT03245541 | 152 | |
| Durvalumab + pexidartinib | I | Completed | NCT02777710 | 153 | |
| PD-L1, CTLA-4 | Tremelimumab + MEDI4736 | I | Recruiting | NCT02311361 | 149 |
| Durvalumab + tremelimumab | II | Recruiting | NCT02558894 | 129 |
5. In vitro studies of immune checkpoint blockade
The expression of PD-L1 protein is typically limited to MQ lineage in human and it has the capacity to be induced in B cells, tumor cells such as PCa, as well as other hematological cells, and non-lymphatic tissues13,177. Furthermore, PD-L2 expression is inducible for MQs and DCs178. A study was conducted in Panc-02 cells, where they were directly injected into the pancreas. It was shown that blocking antibodies against B7–H1 could suppress tumor growth179. Studies in the MiaPaCa-2 and Su86.80 cell lines showed that the anti-inflammatory cytokines such as IL-10 resulted in a modest decrease in mRNA level of PD-L1 in PCa cells. In contrast, treatment with IFN-γ upregulated mRNA expression of PD-L120. Following hemi-spleen implantation of tumor Panc-02 cells, which are treated with cyclophosphamide (Cy) and GVAX or αPD-1/αPD-L1 therapy, it showed elevated amounts of IFN-γ secreted from CD8+ T cells and tumor-specific CD8+ T cells in the TME. CD8+ T cells isolated from spleen and TILs with tumor Panc-02 cells as antigenic targets were treated with anti-PD-1, Cy + GVAX + IgG, and Cy + GVAX + anti-PD-1, showing enhanced amounts of IFN-γ secreted from CD8+ T cells in incremental order compared to the untreated cells180. A syngeneic orthotopic tumor Panc-02 cells poorly responded for blockage of PD-L1 and CTLA-4 as well as Gem, while CD40 agonist antibody (αCD40) treatment significantly delayed tumor growth and increased survival. It also drove maturation of myeloid cells and expansion of memory T cell in spleen and upregulated the PD-L1 mRNA expression in Pan-02 tumors. The combination of αCD40 with αPD-L1 resulted in a significant upsurge of OS rate compared to either agent of the alone treatment trials181. Many of the cancer studies have observed tumor cells via types I and type II IFN signaling pathways upregulated PD-L1 expression in human and murine PCa cell lines. In many studies, nivolumab on PANC-1 cell182, ruxolitinib (JAK–STAT pathway inhibitor) on PANC-02-H7 cell183 and 5-fluorouracil, Gem or paclitaxel (upregulate cell surface PD-L1 expression) on AsPC-1, MIA PaCa-2 and Pan-02 cells184 have a significant influence in downregulation of PD-1/PD-L1 protein levels on the surface of tumor cells. The combined effect of these drugs and IFNs indicated that PD-L1 is activated through the JAK–STAT1 pathway by both type I and II IFNs. It also uncovered that JAK–STAT pathway inhibition increases the effectiveness of anti-PD-1 immunotherapy to suppress the growth of PCa185. Both type I and II interferon cytokines engage JAK–STAT signaling pathways. The expression of PD-L1 is a primary limiting factor for CTL activities in gastric cancers and is significantly upregulated by IFN-γ exposure184,186,187. Taken together, these findings suggest that PD-1/PD-L1 might be a critical target for controlling the growth of PCa.
CTLA-4 is homologous to the T-cell costimulatory protein called CD28 and is constitutively expressed in Treg but is only upregulated after activation in conventional T cells. It appears to be particularly notable in cancers and acts as an “off” switch on the surface of APCs once bounded to CD80 (B7-1) or CD86 (B7-2)16,188,189.
LAG-3 which belongs to immunoglobulin (Ig) superfamily is expressed on activated T cells, NK cells, B cells, and plasmacytoid DCs. Although LAG-3 acts as target of different drug development programs, it has diverse biological effects on T cell functions167,190, 191, 192, 193. The binding of LAG-3 to MHC class II (its ligand) has a higher affinity than CD4 (immune cell surface glycoprotein)194. LAG-3 helps to maintain the tolerogenicity of CD8+ T cells and CD8 exhaustion during chronic infection, contributing to the maturation as well as activation of DCs, and like CTLA-4 and PD-1, it negatively regulates proliferation, activation, and homeostasis of T cells12,161,162,167,195, therefore plays a vital role in the suppression of Treg function168. Co-inhibitory molecules, including IL-12 and TGF-β, which play the role of induction and suppression of LAG-3 and PD-1, respectively, block ICPs to reverse pathogenic Treg. In addition, the effects of co-inhibitors on NK cells indicate different expression in response to cytokine stimulations of IL-15 at least, demonstrating the regulatory role of the co-inhibitors on human NK cells. Furthermore, IL-15 can promote NK cell-mediated killing in pancreatic stellate cells (PSC)196,197.
6. siRNA as immune checkpoints inhibitors
siRNAs, a group of regulatory therapeutic factors, were rabidly designed to combat various diseases, including cancer, neurodegenerative disorders, and infectious diseases198,199. The application of siRNA in PCa to target immune checkpoints was studied in some investigations. A recent study used a PD-L1-specific siRNA conjugated to a magnetic nanocarrier (MN-siPDL1) in combination with gemcitabine as a therapeutic method in murine PCa models. The strategy reduced the tumor growth and elevated the survival ratio significantly. At 2 weeks from the beginning of the treatment, a 90% tumor volume reduction was attained. This is while 100% of the control group animals were observed with a tumor by 6 weeks after starting the treatment200.
Although the failure of anticancer vaccines in PCa patients, a multilateral effort to develop new vaccines demonstrates an efficient efficacy in case survival. Several strategies have been developed to improve the efficacy and safety of tumor vaccines. These include single immunotherapy approaches such as breaking immunosuppression within the tumor microenvironment and overcoming tolerance to TAAs, as well as combinatorial immunotherapy approaches such as the combination of anticancer vaccines with ICP inhibitors and chemotherapy, radiation therapy or even surgery201,202. Recent findings suggest a multipronged approach for therapeutic efficacy, including various types of agents such as vaccines or oncolytic viruses and additional agents to prime the immune microenvironment, followed by ICP inhibitors127. Despite the lack of an effective tumor vaccine, a dozen more clinical trials are ongoing. For instance, use of heterologous prime-boost followed by a low dose of Cy/GM-CSF gene-transfected tumor cell (GVAX) and live-attenuated Listeria monocytogenes-expressing mesothelin (CRS-207) has extended patient survival with minimal toxicity through enhancement of innate and adaptive immunity203.
7. Conclusions and perspectives
The adverse effects caused by immunotherapy in the order of their importance include neutropenia, nausea and vomiting, alopecia, fatigue, and thrombocytopenia204. Despite of the adverse effects, high levels of immunosuppression in the TME and poor immunogenicity provide significant challenges to prosperous immunotherapy of human PCa. On the other hand, ICPs have been discovered to perform an axial role in the establishment of tumor-induced immunosuppression in the TME. Therefore, targeting of ICPs is suggested to be a hopeful strategy for improving the therapeutic efficacy of PCa immunotherapy. CTLA-4 and PD1/PD-L1 are the most well-known ICPs that have been targeted for overcoming immunosuppression in the TME and induction of effective anticancer immune responses in PCa. The results of clinical studies in patients showed the safety and superior therapeutic efficacy of combination therapy with anti-CTLA-4 and anti-PD1 or anti-PD-L1 mAbs in PCa. Despite the encouraging results, several clinical studies showed poor response to checkpoint blockade with anti-CTLA-4 and anti-PD-1 or anti-PD-L1 immunotherapies in PCa patients due to resistance to CTLA-4 and PD-1/PD-L1-targeted therapies. The lack of significant clinical response to PD-1 or CTLA-4-targeted therapy in PCa is possibly due to the presence of unknown and new emerging ICPs that may be involved in tumor-induced immunosuppression and our incomplete understanding of the function of immune cells and molecules in the TME. Our continued progress toward understanding the immunobiology and targeting of ICPs in this type of human malignancy, as discussed in the present article, promises a new hope in the treatment of PCa in the future.
Author contributions
Seyed Hossein Kiaie and Behzad Baradaran designed the work. Seyed Hossein Kiaie, Mohammad Javad Sanaei, Masoud Heshmati, Zahra Asadzadeh, and Saleh Hadidi collected data and wrote the manuscript. Seyed Hossein Kiaie and Mohammad Javad Sanaei designed and regenerated the conceptual pictures. Seyed Hossein Kiaie, Iman Azimi and Reza Jafari checked and revised the article. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Khorana A.A., Mangu P.B., Berlin J., Engebretson A., Hong T.S., Maitra A. Potentially curable pancreatic cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:2324–2328. doi: 10.1200/JCO.2017.72.4948. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.De La Cruz M.S.D., Young A.P., Ruffin M.T. Diagnosis and management of pancreatic cancer. Am Fam Physician. 2014;89:626–632. [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Topalian S.L., Drake C.G., Pardoll D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGranahan N., Furness A.J.S., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.Ryschich E., Nötzel T., Hinz U., Autschbach F., Ferguson J., Simon I. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res. 2005;11:498–504. [PubMed] [Google Scholar]
- 9.Brahmer J.R., Tykodi S.S., Chow L.Q.M., Hwu W.J., Topalian S.L., Hwu P. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerkar S.P., Restifo N.P. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–3130. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn S.D., Shin H., Haining W.N., Zou T., Workman C.J., Polley A. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 14.Dyck L., Mills K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 15.Johansson H., Andersson R., Bauden M., Hammes S., Holdenrieder S., Ansari D. Immune checkpoint therapy for pancreatic cancer. World J Gastroenterol. 2016;22:9457–9476. doi: 10.3748/wjg.v22.i43.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alegre M.L., Frauwirth K.A., Thompson C.B. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 17.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y., Yang W., Huang Y., Cui R., Li X., Li B. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell Physiol Biochem. 2018;47:721–734. doi: 10.1159/000490025. [DOI] [PubMed] [Google Scholar]
- 19.Curiel T.J., Wei S., Dong H., Alvarez X., Cheng P., Mottram P. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 20.Loos M., Giese N.A., Kleeff J., Giese T., Gaida M.M., Bergmann F. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L., Pino-Lagos K., de Vries V.C., Guleria I., Sayegh M.H., Noelle R.J. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyck L., Wilk M.M., Raverdeau M., Misiak A., Boon L., Mills K.H.G. Anti-PD-1 inhibits Foxp3+ Treg cell conversion and unleashes intratumoural effector T cells thereby enhancing the efficacy of a cancer vaccine in a mouse model. Cancer Immunol Immunother. 2016;65:1491–1498. doi: 10.1007/s00262-016-1906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato E., Olson S.H., Ahn J., Bundy B., Nishikawa H., Qian F. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Q., Qiu S.J., Fan J., Zhou J., Wang X.Y., Xiao Y.S. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 26.Angelova M., Charoentong P., Hackl H., Fischer M.L., Snajder R., Krogsdam A.M. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol. 2015;16:64. doi: 10.1186/s13059-015-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feig C., Gopinathan A., Neesse A., Chan D.S., Cook N., Tuveson D.A. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waghray M., Yalamanchili M., di Magliano M.P., Simeone D.M. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol. 2013;29:537–543. doi: 10.1097/MOG.0b013e328363affe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ino Y., Yamazaki-Itoh R., Shimada K., Iwasaki M., Kosuge T., Kanai Y. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchem J.B., Brennan D.J., Knolhoff B.L., Belt B.A., Zhu Y., Sanford D.E. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song X., Liu J., Lu Y., Jin H., Huang D. Overexpression of B7-H1 correlates with malignant cell proliferation in pancreatic cancer. Oncol Rep. 2014;31:1191–1198. doi: 10.3892/or.2013.2955. [DOI] [PubMed] [Google Scholar]
- 33.Razzaque S., Ashraf N., Chavez J.C., Malafa M.P., Coppola D., Springett G.M. Expression of programmed death ligand 1 (PD-L1) in malignant and nonmalignant pancreatic tissue. J Clin Oncol. 2013;31:215. [Google Scholar]
- 34.Looi C.K., Chung F.F., Leong C.O., Wong S.F., Rosli R., Mai C.W. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38:162. doi: 10.1186/s13046-019-1153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topalian S.L., Taube J.M., Anders R.A., Pardoll D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson BA 3rd, Yarchoan M., Lee V., Laheru D.A., Jaffee E.M. Strategies for increasing pancreatic tumor immunogenicity. Clin Cancer Res. 2017;23:1656–1669. doi: 10.1158/1078-0432.CCR-16-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins A.V., Brodie D.W., Gilbert R.J.C., Iaboni A., Manso-Sancho R., Walse B. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 38.Chambers C.A., Kuhns M.S., Egen J.G., Allison J.P. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 39.Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egen J.G., Kuhns M.S., Allison J.P. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 41.Schönfeld D., Matschiner G., Chatwell L., Trentmann S., Gille H., Hülsmeyer M. An engineered lipocalin specific for CTLA-4 reveals a combining site with structural and conformational features similar to antibodies. Proc Natl Acad Sci U S A. 2009;106:8198–8203. doi: 10.1073/pnas.0813399106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haspot F., Villemain F., Laflamme G., Coulon F., Olive D., Tiollier J. Differential effect of CD28 versus B7 blockade on direct pathway of allorecognition and self-restricted responses. Blood. 2002;99:2228–2234. doi: 10.1182/blood.v99.6.2228. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y., Schneider H., Rudd C.E. Murine regulatory T cells differ from conventional T cells in resisting the CTLA-4 reversal of TCR stop-signal. Blood. 2012;120:4560–4570. doi: 10.1182/blood-2012-04-421420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valk E., Rudd C.E., Schneider H. CTLA-4 trafficking and surface expression. Trends Immunol. 2008;29:272–279. doi: 10.1016/j.it.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunner-Weinzierl M.C., Hoff H., Burmester G.R. Multiple functions for CD28 and cytotoxic T lymphocyte antigen-4 during different phases of T cell responses: implications for arthritis and autoimmune diseases. Arthritis Res Ther. 2004;6:45–54. doi: 10.1186/ar1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piccirillo C.A., Shevach E.M. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Noh M.Y., Lee W.M., Lee S.J., Kim H.Y., Kim S.H., Kim Y.S. Regulatory T cells increase after treatment with poly (ADP-ribose) polymerase-1 inhibitor in ischemic stroke patients. Int Immunopharm. 2018;60:104–110. doi: 10.1016/j.intimp.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 49.Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunner-Weinzierl M.C., Rudd C.E. CTLA-4 and PD-1 control of T-cell motility and migration: implications for tumor immunotherapy. Front Immunol. 2018;9:2737. doi: 10.3389/fimmu.2018.02737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu H., Boudova S., Mvula G., Divala T.H., Mungwira R.G., Harman C. Prolonged PD1 expression on neonatal Vδ2 lymphocytes dampens proinflammatory responses: role of epigenetic regulation. J Immunol. 2016;197:1884–1892. doi: 10.4049/jimmunol.1600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan N., Vidyarthi A., Amir M., Mushtaq K., Agrewala J.N. T-cell exhaustion in tuberculosis: pitfalls and prospects. Crit Rev Microbiol. 2017;43:133–141. doi: 10.1080/1040841X.2016.1185603. [DOI] [PubMed] [Google Scholar]
- 53.Chen D.S., Irving B.A., Hodi F.S. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 54.Umezu D., Okada N., Sakoda Y., Adachi K., Ojima T., Yamaue H. Inhibitory functions of PD-L1 and PD-L2 in the regulation of anti-tumor immunity in murine tumor microenvironment. Cancer Immunol Immunother. 2019;68:201–211. doi: 10.1007/s00262-018-2263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Podojil J.R., Miller S.D. Targeting the B7 family of co-stimulatory molecules: successes and challenges. BioDrugs. 2013;27:1–13. doi: 10.1007/s40259-012-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Q., Hu F., Xiao Z., Li M., Wu X., Zhao Y. Comprehensive molecular profiling of the B7 family in gastrointestinal cancer. Cell Prolif. 2018;51:e12468. doi: 10.1111/cpr.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taube J.M., Klein A., Brahmer J.R., Xu H., Pan X., Kim J.H. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drakes M.L., Mehrotra S., Aldulescu M., Potkul R.K., Liu Y., Grisoli A. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand-1 (PD-L1) in ovarian cancer. J Ovarian Res. 2018;11:43. doi: 10.1186/s13048-018-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rozali E.N., Hato S.V., Robinson B.W., Lake R.A., Lesterhuis W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340. doi: 10.1155/2012/656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ritprajak P., Hashiguchi M., Akiba H., Yagita H., Okumura K., Azuma M. Antibodies against B7-DC with differential binding properties exert opposite effects. Hybridoma. 2005;31:40–47. doi: 10.1089/hyb.2011.0087. 2012. [DOI] [PubMed] [Google Scholar]
- 61.Huber S., Hoffmann R., Muskens F., Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–3320. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- 62.Akbari O., Stock P., Singh A.K., Lombardi V., Lee W.L., Freeman G.J. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butte M.J., Keir M.E., Phamduy T.B., Sharpe A.H., Freeman G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart R., Morrow M., Hammond S.A., Mulgrew K., Marcus D., Poon E. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 65.Ott P.A., Hodi F.S., Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 66.Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 67.Krishnadas D.K., Wang Y., Sundaram K., Bai F., Lucas K.G. Expansion of cancer germline antigen-specific cytotoxic T lymphocytes for immunotherapy. Tumour Biol. 2017;39 doi: 10.1177/1010428317701309. :1010428317701309. [DOI] [PubMed] [Google Scholar]
- 68.Pentcheva-Hoang T., Egen J.G., Wojnoonski K., Allison J.P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 69.Masteller E.L., Chuang E., Mullen A.C., Reiner S.L., Thompson C.B. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 70.Onishi Y., Fehervari Z., Yamaguchi T., Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jain N., Nguyen H., Chambers C., Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riley J.L., Mao M., Kobayashi S., Biery M., Burchard J., Cavet G. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schneider H., Downey J., Smith A., Zinselmeyer B.H., Rush C., Brewer J.M. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 74.Feig C., Jones J.O., Kraman M., Wells R.J.B., Deonarine A., Chan D.S. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bengsch F., Knoblock D.M., Liu A., McAllister F., Beatty G.L. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol Immunother. 2017;66:1609–1617. doi: 10.1007/s00262-017-2053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang R.R., Jalil J., Economou J.S., Chmielowski B., Koya R.C., Mok S. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res. 2011;17:4101–4109. doi: 10.1158/1078-0432.CCR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Basso D., Fogar P., Falconi M., Fadi E., Sperti C., Frasson C. Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One. 2013;8:54824. doi: 10.1371/journal.pone.0054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng Y., Manzotti C.N., Liu M., Burke F., Mead K.I., Sansom D.M. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172:2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 79.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 81.Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chemnitz J.M., Parry R.V., Nichols K.E., June C.H., Riley J.L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 83.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Komura T., Sakai Y., Harada K., Kawaguchi K., Takabatake H., Kitagawa H. Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+ T cells with clinical impact. Cancer Sci. 2015;106:672–686. doi: 10.1111/cas.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao M., Lin M., Moffitt R.A., Salazar M.A., Park J., Vacirca J. Direct therapeutic targeting of immune checkpoint PD-1 in pancreatic cancer. Br J Cancer. 2019;120:88–96. doi: 10.1038/s41416-018-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen T., Zhou L., Shen H., Shi C., Jia S., Ding G.P. Prognostic value of programmed cell death protein 1 expression on CD8+ T lymphocytes in pancreatic cancer. Sci Rep. 2017;7:7848. doi: 10.1038/s41598-017-08479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diana A., Wang L.M., D'Costa Z., Allen P., Azad A., Silva M.A. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:40992–41004. doi: 10.18632/oncotarget.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson R.H., Dong H., Lohse C.M., Leibovich B.C., Blute M.L., Cheville J.C. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 89.Waki K., Yamada T., Yoshiyama K., Terazaki Y., Sakamoto S., Matsueda S. PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Sci. 2014;105:1229–1235. doi: 10.1111/cas.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krönig H., Julia Falchner K., Odendahl M., Brackertz B., Conrad H., Muck D. PD-1 expression on Melan-A-reactive T cells increases during progression to metastatic disease. Int J Cancer. 2012;130:2327–2336. doi: 10.1002/ijc.26272. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y., Lin J., Cui J., Han T., Jiao F., Meng Z. Prognostic value and clinicopathological features of PD-1/PD-L1 expression with mismatch repair status and desmoplastic stroma in Chinese patients with pancreatic cancer. Oncotarget. 2017;8:9354–9365. doi: 10.18632/oncotarget.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tjomsland V., Niklasson L., Sandström P., Borch K., Druid H., Bratthäll C. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clin Dev Immunol. 2011;2011:212810. doi: 10.1155/2011/212810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 94.Wang L., Ma Q., Chen X., Guo K., Li J., Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059–1065. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- 95.Daley D., Zambirinis C.P., Seifert L., Akkad N., Mohan N., Werba G. γδ T cells support pancreatic oncogenesis by restraining αβ T cell activation. Cell. 2016;166:1485–1499. doi: 10.1016/j.cell.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 97.Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song X., Liu J., Lu Y., Jin H., Huang D. Overexpression of B7-H1 correlates with malignant cell proliferation in pancreatic cancer. Oncol Rep. 2014;31:1191–1198. doi: 10.3892/or.2013.2955. [DOI] [PubMed] [Google Scholar]
- 99.Nomi T., Sho M., Akahori T., Hamada K., Kubo A., Kanehiro H. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 100.Zhu Y., Knolhoff B.L., Meyer M.A., Nywening T.M., West B.L., Luo J. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Imai D., Yoshizumi T., Okano S., Uchiyama H., Ikegami T., Harimoto N. The prognostic impact of programmed cell death ligand 1 and human leukocyte antigen class I in pancreatic cancer. Cancer Med. 2017;6:1614–1626. doi: 10.1002/cam4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geng L., Huang D., Liu J., Qian Y., Deng J., Li D. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol. 2008;134:1021–1027. doi: 10.1007/s00432-008-0364-8. [DOI] [PubMed] [Google Scholar]
- 103.Ohigashi Y., Sho M., Yamada Y., Tsurui Y., Hamada K., Ikeda N. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 104.Marin-Acevedo J.A., Soyano A.E., Dholaria B., Knutson K.L., Lou Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018;11:8. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brunet L.R., Hagemann T., Andrew G., Mudan S., Marabelle A. Have lessons from past failures brought us closer to the success of immunotherapy in metastatic pancreatic cancer?. Oncoimmunology. 2015;5:1112942. doi: 10.1080/2162402X.2015.1112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 107.Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Solinas C., Gombos A., Latifyan S., Piccart-Gebhart M., Kok M., Buisseret L. Targeting immune checkpoints in breast cancer: an update of early results. ESMO Open. 2017;2 doi: 10.1136/esmoopen-2017-000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jelinek T., Mihalyova J., Kascak M., Duras J., Hajek R. PD-1/PD-L1 inhibitors in haematological malignancies: update 2017. Immunology. 2017;152:357–371. doi: 10.1111/imm.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hodi F.S., Mihm M.C., Soiffer R.J., Haluska F.G., Butler M., Seiden M.V. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grosso J.F., Jure-Kunkel M.N. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 117.Quezada S.A., Peggs K.S., Curran M.A., Allison J.P. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morse M.A. Technology evaluation: ipilimumab, medarex/bristol-myers squibb. Curr Opin Mol Therapeut. 2005;7:588–597. [PubMed] [Google Scholar]
- 119.Le Mercier I., Lines J.L., Noelle R.J. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol. 2015;6:418. doi: 10.3389/fimmu.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Le D.T., Lutz E., Uram J.N., Sugar E.A., Onners B., Solt S. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother Appl. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kamath S.D., Kalyan A., Kircher S., Nimeiri H., Fought A.J., Benson A., III Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncology. 2019;25:e808–e815. doi: 10.1634/theoncologist.2019-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Murphy J.E., Wo J.Y., Ryan D.P., Clark J.W., Jiang W. Yeap BY, et al. Total neoadjuvant therapy with folfirinox in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–1027. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung K.Y., Gore I., Fong L., Venook A., Beck S.B., Dorazio P. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 125.Ribas A., Hanson D.C., Noe D.A., Millham R., Guyot D.J., Bernstein S.H. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncology. 2007;12:873–883. doi: 10.1634/theoncologist.12-7-873. [DOI] [PubMed] [Google Scholar]
- 126.Aglietta M., Barone C., Sawyer M.B., Moore M.J., Miller W.H., Jr., Bagalà C. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750–1755. doi: 10.1093/annonc/mdu205. [DOI] [PubMed] [Google Scholar]
- 127.O'Reilly E.M., Oh D.Y., Dhani N., Renouf D.J., Lee M.A., Sun W. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1431–1438. doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O'Reilly E.M., Oh D.Y., Dhani N., Renouf D.J., Lee M.A., Sun W. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019:191588. doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pedoeem A., Azoulay-Alfaguter I., Strazza M., Silverman G.J., Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153:145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 130.Fife B.T., Pauken K.E., Eagar T.N., Obu T., Wu J., Tang Q. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sasidharan Nair V., Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. 2018;96:21–33. doi: 10.1111/imcb.1003. [DOI] [PubMed] [Google Scholar]
- 132.Infante J.R., Powderly J.D., Burris H.A., Kittaneh M., Grice J.H., Smothers J.F. Clinical and pharmacodynamic (PD) results of a phase I trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. J Clin Oncol. 2013;31:3044. [Google Scholar]
- 133.Schepisi G., Farolfi A., Conteduca V., Martignano F., De Lisi D., Ravaglia G. Immunotherapy for prostate cancer: where we are headed. Int J Mol Sci. 2017;18:2627. doi: 10.3390/ijms18122627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sachdev J.C., Hu-Lieskovan S., Patnaik A., Eisenberg P.D., Weise A., Hutchinson M.A. Phase 1/2a study of double immune suppression blockade by combining a CSF1R inhibitor (pexidartinib/PLX3397) with an anti PD-1 antibody (pembrolizumab) to treat advanced melanoma and other solid tumors. Gynecol Oncol. 2016;141:147–148. [Google Scholar]
- 135.Katz M.H., Bauer T.W., Varadhachary G.R., Petroni G.R., Bullock T., Slingluff C.L. A randomized multicenter phase Ib/II study to assess the safety and the immunological effect of chemoradiation therapy (CRT) in combination with pembrolizumab (anti-PD1) to CRT alone in patients with resectable or borderline resectable pancreatic cancer. J Clin Oncol. 2015;3:P167. [Google Scholar]
- 136.Overman M.J., Lopez C.D., Benson A.B., Neelapu S.S., Mettu N.B., Ko A.H. A randomized phase 2 study of the Bruton tyrosine kinase (Btk) inhibitor acalabrutinib alone or with pembrolizumab for metastatic pancreatic cancer (mPC) J Clin Oncol. 2016;34:4130. [Google Scholar]
- 137.Mahalingam D., Fountzilas C., Moseley J.L., Noronha N., Cheetham K., Dzugalo A. A study of REOLYSIN in combination with pembrolizumab and chemotherapy in patients (pts) with relapsed metastatic adenocarcinoma of the pancreas (MAP) J Clin Oncol. 2017;35 [Google Scholar]
- 138.Mahalingam D., Wilkinson G.A., Eng K.H., Fields P., Raber P., Moseley J.L. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: a phase Ib study. Clin Cancer Res. 2020;26:71–81. doi: 10.1158/1078-0432.CCR-19-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chiorean E.G., Ritch P.S., Zhen D.B., Poplin E., George B., Hendifar A.E. PCRT16-001: phase II study of PEGPH20 plus pembrolizumab for patients (pts) with hyaluronan (HA)-high refractory metastatic pancreatic ductal adenocarcinoma (mPDA) J Clin Oncol. 2020;38:TPS785. [Google Scholar]
- 140.Halama N., Pruefer U., Frömming A., Beyer D., Eulberg D., Ju Jungnelius. Evaluation of tumor biomarkers in patients with microsatellite-stable, metastatic colorectal or pancreatic cancer treated with the CXCL12 inhibitor NOX-A12 and preliminary safety in combination with PD-1 checkpoint inhibitor pembrolizumab. J Clin Oncol. 2018;36 [Google Scholar]
- 141.Wang C., Thudium K.B., Han M., Wang X.T., Huang H., Feingersh D. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 142.Le D.T., Crocenzi T.S., Uram J.N., Lutz E.R., Laheru D.A., Sugar E.A. Randomized phase II study of the safety, efficacy, and immune response of GVAX pancreas vaccine (with cyclophosphamide) and CRS-207 with or without nivolumab in patients with previously treated metastatic pancreatic adenocarcinoma (STELLAR) J Clin Oncol. 2015;3:P155. [Google Scholar]
- 143.Firdaus I., Waterhouse D.M., Gutierrez M., Wainberg Z.A., George B., Kelly K. Nab-paclitaxel (nab-P)+ nivolumab (Nivo)±gemcitabine (Gem) in patients (pts) with advanced pancreatic cancer (PC) J Clin Oncol. 2016;34:TPS475. [Google Scholar]
- 144.Zheng L. National Library of Medicine (US); ClinicalTrialsgov Bethesda (MD): 2015. Neoadjuvant/adjuvant GVAX pancreas vaccine (with CY) with or without nivolumab trial for surgically resectable pancreatic cancer.https://clinicaltrials.gov/ct2/show/NCT02451982 Available from: [Google Scholar]
- 145.Wang-Gillam A., O'Reilly E.M., Bendell J.C., Wainberg Z.A., Borazanci E.H., Bahary N. A randomized phase II study of cabiralizumab (cabira) + nivolumab (nivo)± chemotherapy (chemo) in advanced pancreatic ductal adenocarcinoma (PDAC) J Clin Oncol. 2019;37:4. [Google Scholar]
- 146.Chen J., Huynh J., Monjazeb A., Cho M.T., Kim E.J.H. A pilot study of intratumoral SD-101 (toll-like receptor 9 agonist), nivolumab, and radiotherapy for treatment of chemotherapy-refractory metastatic pancreatic adenocarcinoma. J Clin Oncol. 2020;38:TPS782. [Google Scholar]
- 147.CT-011 and p53 genetic vaccine for advanced solid tumors. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01386502.
- 148.Khleif SN. Gemcitabine and CT-011 for resected pancreatic cancer. Available from: https://ClinicalTrials.gov/show/NCT01313416.
- 149.Duffy A.G., Makarova-Rusher O.V., Pratt D., Kleiner D.E., Alewine C., Fioravanti S. A pilot study of immune checkpoint inhibition (tremelimumab and/or MEDI4736) in combination with radiation therapy in patients with unresectable pancreatic cancer. J Clin Oncol. 2016;34:TPS470. [Google Scholar]
- 150.Borazanci E.H., Hong D.S., Gutierrez M., Rasco D.W., Reid T.R., Veeder M.H. Ibrutinib + durvalumab (MEDI4736) in patients (pts) with relapsed or refractory (R/R) pancreatic adenocarcinoma (PAC): a phase Ib/II multicenter study. J Clin Oncol. 2016;34:TPS484. [Google Scholar]
- 151.Melisi D., Hollebecque A., Oh D.Y., Calvo E., Varghese A.M., Borazanci E.H. A phase Ib dose-escalation and cohort-expansion study of safety and activity of the transforming growth factor (TGF) β receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Clin Oncol. 2019;37:4124. [Google Scholar]
- 152.Tuli R., Nissen N., Lo S., Tighiouart M., Placencio V., Hendifar A. AACR special conference on pancreatic cancer: advances in science and clinical care. 2019. Abstract B58: a phase I/II study of durvalumab and stereotactic radiotherapy in locally advanced pancreatic cancer.https://cancerres.aacrjournals.org/content/79/24_Supplement/B58 Boston, MA, USA. September 6–9, Available from: [Google Scholar]
- 153.Cassier P.A., Garin G., Eberst L., Delord J.-P., Chabaud S., Terret C. MEDIPLEX: a phase 1 study of durvalumab (D) combined with pexidartinib (P) in patients (pts) with advanced pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC) J Clin Oncol. 2019;37:2579. [Google Scholar]
- 154.Fraune C., Burandt E., Simon R., Hube-Magg C., Makrypidi-Fraune G., Kluth M. MMR deficiency is homogeneous in pancreatic carcinoma and associated with high density of CD8-positive lymphocytes. Ann Surg Oncol. 2020;27:1–10. doi: 10.1245/s10434-020-08209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hu Z.I., Shia J., Stadler Z.K., Varghese A.M., Capanu M., Salo-Mullen E. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24:1326–1336. doi: 10.1158/1078-0432.CCR-17-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamamoto H., Itoh F., Nakamura H., Fukushima H., Sasaki S., Perucho M. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res. 2001;61:3139–3144. [PubMed] [Google Scholar]
- 157.Macherla S., Laks S., Naqash A.R., Bulumulle A., Zervos E., Muzaffar M. Emerging role of immune checkpoint blockade in pancreatic cancer. Int J Mol Sci. 2018;19:3505. doi: 10.3390/ijms19113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim S.T., Klempner S.J., Park S.H., Park J.O., Park Y.S., Lim H.Y. Correlating programmed death ligand 1 (PD-L1) expression, mismatch repair deficiency, and outcomes across tumor types: implications for immunotherapy. Oncotarget. 2017;8:77415–77423. doi: 10.18632/oncotarget.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Salem M.E., Puccini A., Grothey A., Raghavan D., Goldberg R.M., Xiu J. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018;16:805–812. doi: 10.1158/1541-7786.MCR-17-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oliveira A.F., Bretes L., Furtado I. Review of PD-1/PD-L1 inhibitors in metastatic dMMR/MSI-H colorectal cancer. Front Oncol. 2019;9:396. doi: 10.3389/fonc.2019.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Andreae S., Piras F., Burdin N., Triebel F. Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223) J Immunol. 2002;168:3874–3880. doi: 10.4049/jimmunol.168.8.3874. [DOI] [PubMed] [Google Scholar]
- 162.Grosso J.F., Kelleher C.C., Harris T.J., Maris C.H., Hipkiss E.L., De Marzo A. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sohal D.P.S., Kennedy E.B., Khorana A., Copur M.S., Crane C.H., Garrido-Laguna I. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:2545–2556. doi: 10.1200/JCO.2018.78.9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eso Y., Shimizu T., Takeda H., Takai A., Marusawa H. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol. 2020;55:15–26. doi: 10.1007/s00535-019-01620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee H.T., Lee S.H., Heo Y.S. Molecular interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in immuno-oncology. Molecules. 2019;24:1190. doi: 10.3390/molecules24061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goldberg M.V., Drake C.G. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang C.T., Workman C.J., Flies D., Pan X., Marson A.L., Zhou G. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 169.Brignone C., Escudier B., Grygar C., Marcu M., Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res. 2009;15:6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 170.Brignone C., Gutierrez M., Mefti F., Brain E., Jarcau R., Cvitkovic F. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med. 2010;8:71. doi: 10.1186/1479-5876-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Romano E., Michielin O., Voelter V., Laurent J., Bichat H., Stravodimou A. MART-1 peptide vaccination plus IMP321 (LAG-3Ig fusion protein) in patients receiving autologous PBMCs after lymphodepletion: results of a phase I trial. J Transl Med. 2014;12:97. doi: 10.1186/1479-5876-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Woo S.-R., Turnis M.E., Goldberg M.V., Bankoti J., Selby M., Nirschl C.J. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nguyen L.T., Ohashi P.S. Clinical blockade of PD1 and LAG3—potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 174.Ascierto P.A., McArthur G.A. Checkpoint inhibitors in melanoma and early phase development in solid tumors: what's the future?. J Transl Med. 2017;15:173. doi: 10.1186/s12967-017-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Patel R.K., Ko A.H., Onners B., Uram J.N., Parkinson R., Sugar E. A phase 2, multicenter study of FOLFIRINOX followed by ipilimumab in combination with allogeneic GM-CSF transfected pancreatic tumor vaccine in the treatment of metastatic pancreatic cancer. J Clin Oncol. 2014;32:4160. [Google Scholar]
- 176.Halama N., Prüfer U., Frömming A., Beyer D., Eulberg D., Jungnelius J. 613P—phase I/II study with CXCL12 inhibitor NOX-A12 and pembrolizumab in patients with microsatellite-stable, metastatic colorectal or pancreatic cancer. Ann Oncol. 2019;30 :v231. [Google Scholar]
- 177.Loke Pn, Allison J.P. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Latchman Y., Wood C.R., Chernova T., Chaudhary D., Borde M., Chernova I. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 179.Okudaira K., Hokari R., Tsuzuki Y., Okada Y., Komoto S., Watanabe C. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741–749. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- 180.Soares K.C., Rucki A.A., Wu A.A., Olino K., Xiao Q., Chai Y. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother Appl. 2015;38:1–11. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Luheshi N.M., Coates-Ulrichsen J., Harper J., Mullins S., Sulikowski M.G., Martin P. Transformation of the tumour microenvironment by a CD40 agonist antibody correlates with improved responses to PD-L1 blockade in a mouse orthotopic pancreatic tumour model. Oncotarget. 2016;7:18508–18520. doi: 10.18632/oncotarget.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ding G., Shen T., Yan C., Zhang M., Wu Z., Cao L. IFN-γ down-regulates the PD-1 expression and assist nivolumab in PD-1-blockade effect on CD8+ T-lymphocytes in pancreatic cancer. BMC Cancer. 2019;19:1053. doi: 10.1186/s12885-019-6145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lu C., Talukder A., Savage N.M., Singh N., Liu K. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology. 2017;6:1291106. doi: 10.1080/2162402X.2017.1291106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Doi T., Ishikawa T., Okayama T., Oka K., Mizushima K., Yasuda T. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol Rep. 2017;37:1545–1554. doi: 10.3892/or.2017.5399. [DOI] [PubMed] [Google Scholar]
- 185.Jiang J., Zhou H., Ni C., Hu X., Mou Y., Huang D. Immunotherapy in pancreatic cancer: new hope or mission impossible?. Cancer Lett. 2019;445:57–64. doi: 10.1016/j.canlet.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 186.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mimura K., Teh J.L., Okayama H., Shiraishi K., Kua L.F., Koh V. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Syn N.L., Teng M.W.L., Mok T.S.K., Soo R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:731–741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 189.Engelhardt J.J., Sullivan T.J., Allison J.P. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006;177:1052–1061. doi: 10.4049/jimmunol.177.2.1052. [DOI] [PubMed] [Google Scholar]
- 190.Kisielow M., Kisielow J., Capoferri-Sollami G., Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005;35:2081–2088. doi: 10.1002/eji.200526090. [DOI] [PubMed] [Google Scholar]
- 191.Mason D., André P., Bensussan A., Buckley C., Civin C., Clark E. CD antigens 2001. Immunology. 2001;103:401–406. doi: 10.1046/j.1365-2567.2001.01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Workman C.J., Wang Y., El Kasmi K.C., Pardoll D.M., Murray P.J., Drake C.G. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Workman C.J., Vignali D.A. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol. 2003;33:970–979. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- 194.Sierro S., Romero P., Speiser D.E. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin Ther Targets. 2011;15:91–101. doi: 10.1517/14712598.2011.540563. [DOI] [PubMed] [Google Scholar]
- 195.Workman C.J., Cauley L.S., Kim I.J., Blackman M.A., Woodland D.L., Vignali D.A.A. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 196.Van Audenaerde J.R.M., De Waele J., Marcq E., Van Loenhout J., Lion E., Van den Bergh J.M.J. Interleukin-15 stimulates natural killer cell-mediated killing of both human pancreatic cancer and stellate cells. Oncotarget. 2017;8:56968–56979. doi: 10.18632/oncotarget.18185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sun H., Sun C., Xiao W. Expression regulation of co-inhibitory molecules on human natural killer cells in response to cytokine stimulations. Cytokine. 2014;65:33–41. doi: 10.1016/j.cyto.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 198.Davis M.E., Zuckerman J.E., Choi C.H.J., Seligson D., Tolcher A., Alabi C.A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hannon G.J., Rossi J.J. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 200.Yoo B., Jordan V.C., Sheedy P., Billig A.M., Ross A., Pantazopoulos P. RNAi-mediated PD-L1 inhibition for pancreatic cancer immunotherapy. Sci Rep. 2019;9:4712. doi: 10.1038/s41598-019-41251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Salman B., Zhou D., Jaffee E.M., Edil B.H., Zheng L. Vaccine therapy for pancreatic cancer. Oncoimmunology. 2013;2:26662. doi: 10.4161/onci.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Soares K.C., Zheng L., Edil B., Jaffee E.M. Vaccines for pancreatic cancer. Cancer J. 2012;18:642–652. doi: 10.1097/PPO.0b013e3182756903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Le D.T., Wang-Gillam A., Picozzi V., Greten T.F., Crocenzi T., Springett G. Safety and survival with GVAX pancreas prime and listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun D., Ma J., Wang J., Zhang F., Wang L., Zhang S. Clinical observation of immune checkpoint inhibitors in the treatment of advanced pancreatic cancer: a real-world study in Chinese cohort. Therapeut Clin Risk Manag. 2018;14:1691–1700. doi: 10.2147/TCRM.S173041. [DOI] [PMC free article] [PubMed] [Google Scholar]