Abstract
Increasing understanding of the pathogenesis of rheumatoid arthritis (RA) has remarkably promoted the development of effective therapeutic regimens of RA. Nevertheless, the inadequate response to current therapies in a proportion of patients, the systemic toxicity accompanied by long-term administration or distribution in non-targeted sites and the comprised efficacy caused by undesirable bioavailability, are still unsettled problems lying across the full remission of RA. So far, these existing limitations have inspired comprehensive academic researches on nanomedicines for RA treatment. A variety of versatile nanocarriers with controllable physicochemical properties, tailorable drug release pattern or active targeting ability were fabricated to enhance the drug delivery efficiency in RA treatment. This review aims to provide an up-to-date progress regarding to RA treatment using nanomedicines in the last 5 years and concisely discuss the potential application of several newly emerged therapeutic strategies such as inducing the antigen-specific tolerance, pro-resolving therapy or regulating the immunometabolism for RA treatments.
KEY WORDS: Nanomedicines, Rheumatoid arthritis, Targeted drug delivery, Liposome, Micelle, Stimulus-responsive delivery systems, Immune tolerance, Inflammation resolution
Graphical abstract
This review summarizes the characteristics of physiological microenvironment of the inflamed joint in the development of rheumatoid arthritis (RA) and discusses the current therapeutic strategies for RA.
1. Introduction
As a complicated autoimmune disease, rheumatoid arthritis (RA) often causes invasive inflammatory infiltration and serious articular destruction, as well as extensive comorbidities in the cardiovascular system, gastrointestinal and pulmonary tissues, severely reducing the life quality and lifetime of RA patients1. RA affects approximately 1% of the global population and 0.28% of population in China2. It is generally considered that RA is principally induced by the cross-talking of environmental factors and genetic predisposition3. Genome-wide studies identify that genes encoding major histocompatibility complex II (MHC II) molecules which are involved in the T cell recognition of autoreactive peptides are closely related with RA onset and development1,4. The interaction of aberrant T cells and B cells, as the prelude of this autoimmune disorder, activates both innate and adaptive immune response and breaks the balance between host tolerance and immune homeostasis5. Along with the altered immune reactivity, environmental stimulus such as smoking, dust inhalation and microbiota infection might also contribute to the progression of RA4. Although RA is a systemic disease affecting a variety of organs and lymphoid tissues, the synovium in inflamed joints is the center of inflammation (Fig. 1). Owing to the rapid proliferation and activation of inflammatory cells such as T cells, B cells, macrophages, vascular endothelial cells (VEC), fibroblast-like synoviocyte (FLS), etc., the synovium tissue abnormally expands with neovascularization, hyperproliferative intimal lining and a prominent amount of inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), prostaglandins etc., invasive proteases such as matrix metalloproteinases (MMPs), and autoantibody such as rheumatoid factors (RF) and anti-citrullinated protein antibodies (ACPA). In addition to massive inflammation infiltration, the destructive FLS and osteoclast, as well as MMPs would lead to the damage of cartilage and bone tissues3,6.
Figure 1.
The comparison of physiological microenvironment between normal synovium and inflamed sites. Compared with normal synovial tissues, massive inflammatory cells are recruited to inflamed joints. Afterwards, rapid proliferation and activation of cells involved in inflammation lead to the formation of pannus and the thickening of the synovial lining. Antigen-presenting cells (APCs) including DCs, activated B cells and macrophages present antigens to T cells, leading to the activation of autoreactive T cells. Other than antigen presentation, B cells would also secrete autoantibodies such as ACPA and RF, which can attack our own tissues. On the other hand, large number of inflammatory cytokines (TNF-α, IL-6 and IL-1) and invasive proteases would be produced, mediating the expansion of inflammatory network and the destruction of cartilage and bone tissue. FLS, fibroblast-like synoviocyte; DC, dendritic cell; VEC, vascular endothelial cells; MMPs, matrix metalloproteinase; Autoantibodies including rheumatoid factors (RFs) and anti-citrullinated protein antibodies (ACPAs); Inflammatory cytokines including TNF-α, IL-1β, IL-6, etc.
Current clinical treatments, including non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), glucocorticoids (GCs) and biological agents, mainly focus on alleviating the symptoms of RA via immunosuppression or inhibition of certain inflammatory mediators7. NSAIDs such as ibuprofen could effectively relieve pain and swelling by the inhibition of cyclooxygenase (COX). However, they cannot modify the underlying disease process and are associated with serious gastrointestinal damage8. GCs such as dexamethasone (Dex) exhibit powerful an-inflammatory efficacy and can rapidly decrease the inflammatory infiltration, but their adverse effects limit the long-term or high-dose usage9. DMARDs including methotrexate (MTX) are considered to be effective in slowing down the RA progress. It is highly recommended to use the combination with multiple DMARDs or GCs in clinical applications to achieve better anti-inflammatory effect and avoid the side effects caused by dose escalation10,11. Biological agents including cytokine antagonists, B cell depleting agents, T cell co-stimulation modulators, and kinase inhibitors show great improvement in controlling RA activity due to their specificity and selectivity12. Humira (monoclonal antibody against TNF-α) is one of the world's top10 best selling drugs during the past 3 years, which shows the high efficacy in the treatment of rheumatic diseases13. However, a significant number of patients show reduced responsiveness after multiple administrations14. The traditional Chinese medicines extracted from natural plants, including curcumin, resveratrol, ferulic acid and triptolide have also been demonstrated effective for the treatment of inflammatory diseases including arthritis15. The advantages and disadvantages of clinical drugs have been summarized in Table 1.
Table 1.
The advantages and disadvantages of clinical drugs in RA treatment.
| Classification | Drug | Advantage | Disadvantage |
|---|---|---|---|
| NSAIDs | Indomethacin | Rapidly reduce pain and inflammation by inhibiting COX | Inability to stop joint damage, gastrointestinal bleeding and kidney dysfunction |
| Ibuprofen | |||
| Diclofenac sodium | |||
| DMARDs | Methotrexate | Effective in controlling the disease progression | Take effect slowly and hepatic cirrhosis, kidney failure |
| Sulfasalazine | |||
| GCs | Dexamethasone | Powerful inhibition of inflammation | Hypertension, osteoporosis and immunosuppression |
| Prednisolone | |||
| Betamethasone | |||
| Biological agents | Adalimumab | High specificity and efficacy | High cost, low responsiveness and increased risk of infection |
| Rituximab | |||
| Anakinra |
Except the abovementioned conventional medications for RA therapy, gene therapy is gradually entering clinical practice. In 2017, the South Korean Ministry of Food and Drug Safety approved the first gene therapeutic Invossa, which is taking the advantage of retrovirus to deliver transforming growth factor-β (TGF-β) gene into affected joints for arthritis therapy16. Meanwhile, a phase I clinical trial of recombinant Adeno-associated virus (AAV) to deliver interferon-β to the joints of RA patients is taking place in the Netherlands. Although notable strides have been made towards genetic medicine in arthritis management, this field is still in its infancy. Thus, we expect that encouraging achievements in genetic medicines for RA therapy will become widely available in the near future.
Before the therapeutic agents reach the inflamed sites and take effect, multiple physiological barriers in vivo must be overcome to achieve the desirable therapeutic outcome. Once the drug formulations enter the blood circulation, they will be faced with the phagocytose of reticuloendothelial-system (RES) and the destruction of proteases. In addition, the drug formulations would distribute throughout the body indistinguishably when administrated in vivo, resulting in the impaired therapeutic efficacy and elevated risk of side effects. Most importantly, even if the drug reaches the inflamed joints, it will still be cleared rapidly from the articular space. In general, the ideal drug delivery system should overcome the abovementioned barriers in vivo and ultimately maximize the therapeutic efficacy of encapsulated agents.
Nanoscale drug delivery systems provide a promising approach to overcome the drawbacks in current treatments. It has been shown that nanocarriers can increase the solubility of drugs, extend the circulation time of drugs, reduce the clearance of drugs, and deliver drugs to disease sites in a controlled way17,18. More recently, the emerging multifunctional nanocarriers with sophisticated targeted delivery or transformable properties are designed to achieve intelligent drug delivery and promote therapeutic efficacy.
Generally, the integration of efficacious therapeutic agents with effective delivery strategies is an attractive attempt for RA therapy. In this review, we will highlight the recent progress of advanced drug delivery for RA therapy in the last 5 years. In addition, the emerging promising therapeutic strategies and future perspectives of both academic research and clinical translation in terms of RA therapy are also discussed. Our review will probably bring new thoughts for further investigations of effective RA therapy.
2. Delivery strategies for different types of agents
The synovial in inflamed joints is abnormally expanded, accompanied by angiogenesis and inflammatory cell infiltration. Thus, the emergence of endothelial gaps in microenvironment of RA allows for the leakage of colloidal nanoparticles into the affected joints, subsequently followed by the sequestration of exogenous nanoparticles mediated by various inflammatory cells6. This passive targeting mechanism is similar to the famous enhanced permeability and retention (EPR) effect occurred in tumor tissues due to the analogous leaky vessels presented in disease sites. Unlike EPR in tumors, the retention mechanism in RA mainly relies on the sequestration of local inflammatory cells.
In order to improve the efficacy of current available agents in RA therapy, researchers have developed diverse drug carriers by taking advantages of this passive targeting mechanism. The designing and construction of favorable drug delivery systems should be based on the physicochemical properties of therapeutic agents, as well as their dilemma in vivo. In this section, various drug vehicles for encapsulating and delivering different types of drugs will be summarized and classified in Table 219, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, which aims to provide general principles and inspiring insights for further explorations.
Table 2.
Delivery strategies for different types of agents.
| Category of agents | Agent | Formulation | Ref. |
|---|---|---|---|
| Small molecule | Dexamethasone | PEG‒Dex conjugates | 19 |
| Methotrexate | Dextran sulfate‒MTX conjugates | 20 | |
| Methotrexate | Albumin‒MTX conjugates | 21 | |
| Methylprednisolone | Cyclodextrin‒α-methylprednisolone conjugates | 22 | |
| Dexamethasone | PEGylation liposomes | 23 | |
| Dexamethasone | DC8,9PC and DSPE-PEG liposomes | 24 | |
| Dexamethasone | PCL‒PEG micelles | 25 | |
| Methotrexate | SA-Dex-OA/MTX micelles | 26 | |
| Curcumin | HA-Cur micelles | 27 | |
| Methotrexate | MTX-loaded albumin nanoparticles | 28 | |
| Methotrexate | Au/Fe half-shell PLGA nanoparticles | 29 | |
| Nucleic acid | IL-1β siRNA | Lipidoid‒polymer hybrid nanoparticles | 30 |
| p65 siRNA | PCL‒PEI and PCL‒PEG micelles | 31 | |
| siRNA against BTK | PEG-b-PLGA nanoparticles | 32 | |
| TNF-α siRNA | PEGylated solid-lipid nanoparticles | 33 | |
| Peptide/protein | Core peptide | PEGylated liposomes | 34 |
| TNF-α antibody | Carboxymethyl cellulose microneedle | 35 | |
| Etanercept | HA crosslinked microneedle | 36 | |
| TRAIL | HSA‒TRAIL conjugates | 37 | |
| Tocilizumab | Gold nanoparticles | 38 |
PEG, polyethylene glycol; DC8,9PC, 1,2-bis (10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine; PCL‒PEG, poly(ethylene glycol)‒poly(ε-caprolactone); SA, sialic acid; OA, octadecanoic acid; PCL‒PEI, poly(ε-caprolactone)-polyetherimide; HA, hyaluronic acid; Cur, curcumin; PLGA, poly(lactic-co-glycolic acid); TRAIL, tumor necrosis factor (TNF)-related apoptosis-inducing ligand; HSA, human serum albumin.
2.1. Small molecule drug-based delivery systems
At present, the vast majority of drugs used frequently in clinic for RA treatment is small molecule drugs such as DMARDs and GCs, which are the cornerstone of RA management. However, the low solubility, poor pharmacokinetics behavior and non-targeted distribution of small molecule drugs, not only hamper their efficacy, but also give rise to multiple adverse effects. The suitable nanocarriers designed for delivering small molecule drugs should encapsulate the drugs with high drug loading and avoid drug leakage and burst release before reaching the inflamed sites. So far, multifarious delivery strategies aiming at solving these problems principally fall into two parts: conjugation of drugs with macromolecules and encapsulation of drugs within nanocarriers.
2.1.1. Conjugation of drugs with macromolecules
Drug conjugated with macromolecule such as synthetic polymers, natural polysaccharides or proteins via covalent bonding offers a direct and efficient approach to modify the pharmacokinetics performance, promote drug stability and avoid drug leakage or burst release in circulation39. Polyethylene glycol (PEG) is one of the widely investigated polymer used for drug conjugation due to its good biocompatibility, long circulation in vivo and controllable properties. For example, Wang and coworkers19 synthesized various PEG–Dex conjugates with different densities of Dex. The introduction of the acid-cleavable hydrazone bond between PEG and Dex significantly avoided the burse release of Dex in circulation and ensured the selective release in acidic disease sites. The optimized PEG-Dex formulation showed superior therapeutic efficacy. MTX is a first-line treatment for RA patients. Nevertheless, side effects often occurred as MTX is often required for long-term administration. To improve the efficacy of MTX, Chen et al.20 designed a dextran sulfate–MTX conjugate for targeting the activated macrophages in inflamed sites. This dextran sulfate–MTX conjugates exhibited enhanced cellular uptake and accumulation in arthritic joints owing to the high affinity of dextran sulfate to the scavenger receptors overexpressed on activated macrophages. RA often causes the degradation of joint cartilage, leading to the high friction between articular cartilages. To improve joint lubrication during the treatment of RA, hyaluronic acid (HA), an important component of lubricant synovial fluids, was conjugated with natural anti-inflammatory agent curcumin (Cur). After being injected into arthritic rats, the HA–Cur conjugates significantly lowered their edema degrees and protected the cartilage caused by joint friction27.
Other drug conjugates such as albumin-MTX21, cyclodextrin-α-methylprednisolone22 reported earlier also displayed the successful improvement of anti-inflammatory effects. Despite many benefits of drug conjugates, several issues existed in drug conjugates should also be taken into consideration in further investigations such as complicated chemical synthesis and limited drug loading yields.
2.1.2. Encapsulation of drugs within nanocarriers
The encapsulation of drugs within nanocarriers is a direct and useful approach to provide the protection of drugs from environments and tailor their in vivo performance by manipulating the size, shape, and surface properties of nanocarriers.
Liposomes are a well-known nanocarrier in improving the bioavailability of drugs for RA treatment40. Liposomes have sparked great interests in drug delivery for a long time, for it can incorporate both hydrophilic and hydrophobic agents simultaneously. In order to systematically explore how the physical and chemical properties of liposomes affect their in vivo fate, Ren et al.23 prepared a series of liposomes with different sizes, surface charges, PEG lengths. They found that liposomes with the diameter of ∼100 nm, a slightly negative charge, and the 10% incorporation of 5 kDa PEG had superior in vivo circulation time and inflamed joint targeting. PEG or other functional ligand modified liposomes also showed the significant improvement of the delivery efficiency in RA therapy41,42. Nevertheless, these conventional liposomes still face the challenges such as poor stability and drug leakage in vivo43. Recently, Wang et al.24 designed a polymerized liposome composed of 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine (DC8,9PC) and DSPE–PEG, in which DC8,9PC molecules were cross-linked in the bilayer of the liposomes upon UV irradiation and the PEG present at the surface of the liposome provided a stealth layer. The polymerized stealth liposome was highly stable and showed long circulation time in vivo. After being administrated into arthritic rats, the Dex-loaded polymerized stealth liposomes significantly suppressed the proinflammatory level in joint tissues and reduced the swelling of inflamed joints.
Most of small molecule drugs used in RA therapy are hydrophobic. Thus, micelles with hydrophobic core are frequently adopted for solubilizing and delivering hydrophobic drugs. In addition to solubilizing hydrophobic drugs in aqueous solution, self-assembled micelles could keep stable as long as the concentration of micelles is higher than the critical micellization concentration (CMC). Wang et al.25 demonstrated the Dex-loaded poly(ethylene glycol)-poly(ε-caprolactone) (PCL–PEG) micelles could potently reduce the joint swelling and bone erosion of arthritic rats at a low Dex dose without causing any side effect. In order to achieve anti-inflammatory effect and bone repair simultaneously, Xu et al.26 developed a sialic acid-dextran-octadecanoic acid (SA-Dex-OA/MTX) micelle to deliver MTX. This MTX-loaded micelle greatly improved the drug accumulation in arthritic joints via interaction between SA and vascular endothelial cells, finally displaying favorable therapeutic effects and minor side effects.
Albumin is the most abundant plasma protein in vivo. It is the major protein responsible for the colloid osmotic pressure in blood44. Nanoparticles made from albumin have attracted considerable attention as a drug carrier due to their excellent in vivo circulation. More recently, Liu et al.28 found that the expression of secreted protein acidic and rich in cysteine (SPARC) significantly increased in RA synovium. Considering the intrinsic high affinity of SPARC to albumin, they investigated the therapeutic effect of MTX-loaded albumin nanoparticles in a collagen-induced arthritis model (CIA) and confirmed the large accumulation and long joint retention of MTX-loaded albumin nanoparticles in inflamed joints, as well as a better therapeutic index compared with free MTX. Besides abovementioned soft nanocarriers, inorganic nanoparticles such as silicon nanoparticles and metallic nanoparticles were also used for versatile drug delivery due to their unique physicochemical properties such as exceptional optical absorption or controllable physicochemical properties29,38,45, 46, 47. For example, Kim and co-workers29 fabricated an Au/Fe half-shell poly(lactic-co-glycolic acid) (PLGA) nanoparticle modified by arginine-glycine-aspartic acid (RGD), which could be used for multimodal imaging and magnetic targeted chemo-photothermal treatment at the same time in RA therapy.
2.2. Nucleic acid-based delivery systems
Gene therapy has hold great potential in the treatment of inflammatory arthritis. After many ups and downs, great development of gene therapy in clinical trials have been made. Although a number of clinical trials of gene therapeutics are ongoing, there are very limited clinically available genetic agents16. Due to the unique characteristics of nucleic acid such as high molecular weight, negative charge and instability, the application of gene therapeutics such as siRNA, mRNA, microRNA and plasmid DNA has been hindered by a series of biological barriers, including enzymatic degradation, inability to transport across cell membrane, elicitation of immune response, as well as endosomal degradation48. It is nearly infeasible to directly use naked nucleic acids for clinical applications. As a result, there has been great interest in developing delivery vehicles to overcome these biological barriers. In order to efficiently transport gene therapeutics to the sites of diseases, the gene delivery must not only be able to complex with nucleic acid, but also must meet the following requirements: 1) protect the nucleic acid from the degradation of nuclease; 2) facilitate the sufficient cellular uptake of nucleic acid; 3) release nucleic acid in cytoplasm in time from endosome. Currently, the most used non-viral delivery vehicles for nucleic acid delivery are cationic lipids and polymers. Song et al.30 designed a polymer-lipid hybrid nanoparticle (FS14-NP) composed of F127 and spermidine-based lipidoid. The intravenous administration of FS14-NP/IL-1β siRNA complexes resulted in the enhanced siRNA accumulation within the arthritic joints pathology. Furthermore, FS14-NP/IL-1β siRNA complexes significantly down-regulated the level of pro-inflammatory cytokines and obviously improved joint. Nuclear factor kappa-B (NF-κB) is a major transcription factor of inflammatory genes and plays a key role in the pathogenesis of RA. In an attempt to inhibit NF-κB signaling efficiently, Wang et al.31 fabricated a polymeric hybrid micelle consisted of poly(ε-caprolactone)–polyetherimide (PCL–PEI) and poly(ε-caprolactone)–poly(ethylene glycol) (PCL–PEG) to co-deliver Dex and p65 siRNA which could silence the expression of p65 subunit in NF-κB family. The dual drug-loaded hybrid micelle was able to switch macrophages from the M1 to M2 state and effectively reduce the inflammatory level synergistically. It was reported that Bruton's tyrosine kinase (BTK) is a critical mediator in facilitating B cell activation and macrophages polarization. Thus, it is considered as a potential therapeutic target for RA. Zhao and co-workers32 employed cationic lipid-assisted PEG-b-PLGA nanoparticles (CLANs) to encapsulate siRNA against BTK. In a CIA model, the intravenous administration of CLANs/siRNA dramatically reduced joint inflammation without causing any toxicity. Aldayel et al.33 reported a pH-induced sheddable PEGylated nanocarrier by incorporating TNF-α siRNA into solid-lipid nanoparticles. The lipid nanoparticles showed a high siRNA encapsulation efficiency (>90%) and a minimum burst release of siRNA (<5%). The systemic injection of this formulation significantly reduced the paw thickness and bone damage in a CIA mice model.
Generally, gene therapeutics provide considerable potential because of their capability of inducing the specific knockdown or expression of a broad range of genetic targets. However, the undesirable delivery efficiency of gene therapeutics remains to be a challenging issue. Hence, safe and effective delivery approaches are still in demand for the application of gene therapeutics in RA therapy.
2.3. Peptide/protein agent-based delivery systems
Peptide/protein therapeutics have drawn extensive attention due to their advantages over small molecule drugs such as high efficacy, low toxicity, and specificity49. Despite their unique properties, the clinical application of these macromolecules agents has been greatly hampered by their instability, low bioavailability and immunogenicity50. Therefore, seeking for new strategies to enable the effective delivery of peptide/proteins is full of thorns. Peptide/protein agents often have a relatively large molecular weight and unique spatial structure. The drug vehicles used for delivering peptide/protein agents should possess enough space to accommodate such kind of agents. And peptide/protein agents are easily degraded by the widespread proteases in vivo. Therefore, the drug carriers should be responsible for offering the proper protection for the peptide/protein agents.
Bioactive peptides, which were used for the treatment of chronic inflammatory diseases, are very limited. Among them, melittin, derived from bee venom, shows good anti-inflammatory effect on AIA rat model via the inhibition of NF-κB pathway51. However, no further investigation aiming at improving the bioavailability of melittin using drug delivery systems has been reported. Besides, Vanniasinghe et al.34 found that immunosuppressive peptide (core peptide, CP) loaded liposomes were effective in ameliorating arthritis. The suppression of inflammation might be attributed to the peptide-induced inhibition of T-cell activation, which consequently resulted in a more sustained long-term effect compared to prednisolone-loaded liposomes.
Therapeutic proteins such as monoclonal antibodies or fusion proteins have become a dominant therapeutic modality in autoimmune diseases in the worldwide scale52. In 2018, the top-10 best-selling drugs have a total sale of 79.7 billion dollars. Among them, three protein drugs were used in combating RA in clinical applications. In an attempt to achieve efficient and biologically effective TNF-α antibody delivery, Korkmaz et al.35 applied a dissolvable microneedle array for the localized delivery of TNF-α antibody. When administrated intradermally, the microneedle array efficiently delivered TNF-α antibodies to the dermis of skin, leading to the reduction of the local inflammation. In another similar study, Cao and co-workers36 fabricated a HA crosslinked microneedle system to encapsulate and deliver etanercept. This microneedle system could avoid the low compliance caused by the parenteral administration usually adopted in clinical use. In vivo evaluation on arthritic mice showed a good bioequivalence to the conventional administration. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a type II transmembrane protein in TNF family. Once binding to death receptors, TRAIL would lead to the programmed death of abnormal cells. Due to its apoptotic and anti-proliferative effect on inflammatory cells, TRAIL has been considered as a potential candidate in RA treatment. Human serum albumin (HSA–TRAIL) conjugates with the diameter of ∼15.4 nm displayed enhanced therapeutic efficacy compared to free TRAIL. The enhanced therapeutic efficacy might be attributed to the extended systemic circulation and efficient targeting delivery in inflamed sites endowed by HSA37. Tocilizumab (TCZ), a monoclonal antibody against IL-6 signaling, could rapidly suppress inflammation and prevent the joint destruction in RA patients. Lee et al.38 developed HA modified gold nanoparticles to encapsulate TCZ for improving the therapeutic efficacy of TCZ. The TCZ-loaded gold nanoparticles exerted the synergistic therapeutic effect in CIA mice model by simultaneously targeting vascular endothelial growth factor (VEGF) and IL-6R.
So far, only a few nanomedicines entered the clinical trials. A prednisone-loaded liposomal formulation with improved safety and efficacy has been confirmed in a phase II clinical study53. Apart from that, several clinical studies using adeno-associated virus or retrovirus to deliver gene therapeutics for RA therapy are undergoing16.
Although significant achievements have been made, the translation of nanomedicines in clinic and marketplace is still challenging. Several problems lying in the way of nanomedicines translation should be addressed before novel nanomedicines being investigated in clinical trials. For example, inappropriate use of nanomaterials with potential toxicity or immunogenicity, low reproducibility of formulation with complicated preparation technique and insufficient study of in vivo safety, hinder the current nanomedicines from bench to besides. Therefore, we propose that investigators try to use the FDA-approved pharmaceutic excipients to fabricate the drug formulations and avoid the usage of too much toxic chemical reagents. And detailed pre-clinical evaluation in terms of in vivo pharmacokinetics and underlying safety risk should be systemically investigated before beginning the clinical trial.
3. Functional delivery strategies in RA treatment
As outlined above, the drug-loaded nanocarriers exhibited obvious advantages such as enhanced therapeutic efficacy and reduced side effects in RA therapy compared with conventional treatments. Meanwhile, researchers are continuously seeking for superior approaches to further improve the delivery efficacy and decrease the risk of side effects. Now, more intelligent drug delivery systems with multifunctionalities such as selective accumulation, intelligent drug release and active targeting to a certain cell type are under intense investigation according to the special pathological characteristics in RA.
3.1. Actively targeted delivery systems
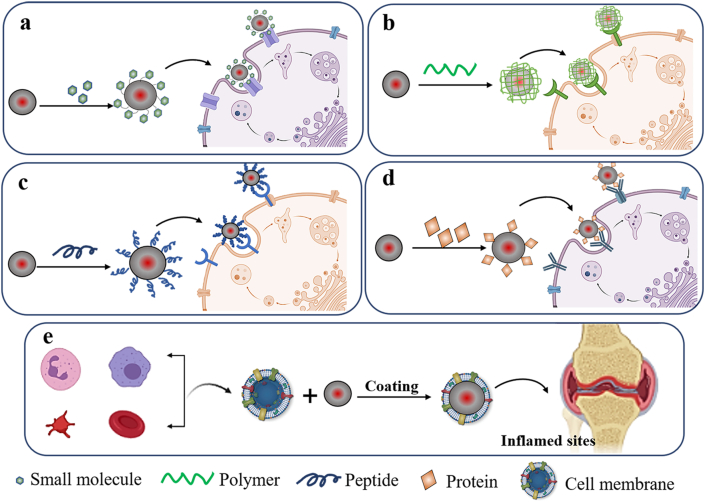
In RA pathology, cells involved in RA onset and development would go through a series of alterations such as the up-regulation of specific surface receptors or transformation of phenotype54. As a result, nanocarriers with the special binding affinity to these inflammation-related cells would consequently further promote the targeted drug delivery in inflamed sites. In the past five years, enormous progress has been made in the actively targeted drug delivery in various inflammatory disorders. Herein, nanomedicines engineered with small molecules, polymers, peptides, or proteins to achieve the active targeting are comprehensively summarized in Fig. 2. In the following sections, several representatives of actively targeted strategies in RA therapy were showed and discussed (Table 3)55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84.
Figure 2.
Schematic illustration of active targeting delivery strategies.
Table 3.
Active targeting strategies.
| Type | Receptor | Delivery strategy | Drug | Ref. |
|---|---|---|---|---|
| Small molecule | ||||
| FA | Folate receptor β | BSA nanoparticles | Etoricoxib | 55 |
| Dendrimer G5 | Methotrexate | 56 | ||
| FA-PEG-DSPE liposomes | Prednisolone and methotrexate | 57 | ||
| DOPE/CH/DSPE-mPEG liposomes | Methotrexate | 58 | ||
| Nanogold-core dendrimer | Methotrexate | 59 | ||
| FA-PSA-CC micelles | Dexamethasone | 60 | ||
| FA-PLGA nanoparticles | Dexamethasone | 61 | ||
| FA-PEG-CH-DEAE15 nanoparticles | siRNA | 62 | ||
| Mannose | Mannose receptor | DSPC/Chol/Man liposomes | Withaferin-A | 63 |
| DSPC/Chol/F-DHPE/Man liposomes | Morin | 64 | ||
| SA | E-selectin | SA-Dex-OA micelles | Methotrexate | 26 |
| L-selectin | SA-cholesterol conjugated liposomes | Dexamethasone palmitate | 65 | |
| Galactose | Galactose-specific C-type lectin | GDR-TPT nanoparticles | Triptolide | 66 |
| Polymer | ||||
| DS | Scavenger receptor | DS-b-PCL nanoparticles | Methotrexate | 67 |
| DS-g-MTX micelles | Methotrexate | 20 | ||
| HA | CD44 | HA–MTX conjugate | Methotrexate | 68 |
| HA‒DPPE liposomes | Prednisolone | 69 | ||
| HA/Cur micelles | Curcumin | 27 | ||
| HA polymeric nanoparticles | Dexamethasone | 70 | ||
| HA‒phospholipid micelles | Triamcinolone | 71 | ||
| HA‒gold nanoparticles | Tocilizumab | 38 | ||
| Protein | ||||
| Anti-CD64 antibody | CD64 | Anti-CD64-SPIONs-PLGA nanoparticles | Methotrexate | 72 |
| Albumin | SPARC | MTX@HSA nanoparticles | Methotrexate | 28 |
| TAC‒HSA nanoparticles | Tacrolimus | 73 | ||
| CD163 monoclonal antibody | Haemoglobin scavenger receptor CD163 | Anti-CD163-dexamethasone conjugate | Dexamethasone | 74 |
| Peptide | ||||
| RGD | αvβ3 integrins | RGD-Lip-PRE liposomes | Prednisone | 34 |
| Au/Fe/Au PLGA nanoparticles | Methotrexate | 29 | ||
| Au PLGA nanoparticles | Methotrexate | 75 | ||
| Perfluorocarbon nanoparticles | Fumagillin prodrug | 76 | ||
| RGD-PEG-PLA micelles | Methotrexate and nimesulide | 77 | ||
| VIP | VIP receptor | DSPE-PEG3400-VIP micelles | Camptothecin | 78 |
| Tuftsin | Fc and neuropilin-1 receptors | Alginate nanoparticles | IL-10 DNA | 79 |
| HAP-1 | Synovial | HAP-1 liposomes | Prednisolone | 34 |
| CBP | Collagens | CBP–α-TNF conjugates | TNF-α antibody | 80 |
| ADK | Endothelial cells | ART-1-IL-27 liposomes | IL-27 | 41 |
| Membrane | ||||
| Platelet membrane | P-selectin and GVPI | PLGA nanoparticles | Tacrolimus | 81 |
| Neutrophil membrane | LFA-1 | PLGA nanoparticles | None | 82 |
| Macrophage membrane | Mac-1 | PLGA nanoparticles | Tacrolimus | 83 |
| TRAIL-expressing vein endothelial cell membrane | TRAIL ligands | PLGA nanoparticles | Hydroxychloroquine | 84 |
FA, folic acid; BSA, bovine serum albumin; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, DSPE-mPEG, N-(carbonyl methoxypolyethylene glycol)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine; PSA, polysialic acid; PLGA, poly(lactic-co-glycolic acid); CH, chitosan; DEAE, diethylethylamine; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; Chol, cholesterol; Man, mannose; F-DHPE, N-(fluorescein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-phosphoethanolamine, triethylammonium salt; SA, sialic acid; Dex, dexamethasone; OA, octadecanoic acid; GDR, galactosyl-dextran-retinal; TPT, triptolide; DS, dextran sulfate; PCL, poly(ε-caprolactone); MTX, methotrexate; HA, hyaluronic acid; DPPE, dipalmitoyl-sn-glycero-3-phosphoethanolamine; Cur, curcumin; SPIONs, superparamagnetic iron oxide nanoparticles; SPARC, secreted protein acidic and rich in cysteine; HSA, human serum albumin; TAC, tacrolimus; RGD, arginine-glycine-aspartic acid; PRE, prednisone; PLA, polylactic acid; VIP, vasoactive intestinal peptide; CBP, collagen-binding peptide; ART-1, CRNADKFPC; TRAIL, tumor necrosis factor (TNF)-related apoptosis-inducing ligand.
3.1.1. Small molecule modification mediated targeted delivery
Folate acid (FA) is known to show high affinity to FA receptors, which are broadly expressed on tumor cells and activated synovial macrophages in RA85,86. In a study, a folate-modified dextran-methotrexate conjugate was synthesized to self-assemble into micelles. The MTX-loaded micelles exhibited the improved macrophage uptake mediated by the FA modification and possessed the improved biodistribution in inflammatory regions87. In CIA mice, these FA-modified micelles showed stronger remission of arthritis in comparison with free MTX and unmodified micelles. More recently, Sun et al.88 constructed an FA-conjugated PEG-PLGA nanoparticle for delivering Mcl-1 siRNA to inflamed joints in RA, in which a pH-sensitive linker was introduced into the nanoparticles to facilitate the intracellular release of Mcl-1 siRNA. These nanoparticles treatment led to a significant improvement in the clinical outcomes of paws and reduced the level of pro-inflammatory cytokines.
Sialic acid (SA), a natural monosaccharide, is mainly located on the surface of cell membranes. As the binding ligand of E-selectin receptors, SA has been used to improve the efficiency of targeted delivery in a recent research89. Xu et al.26 fabricated an MTX encapsulated SA-dextran-octadecanoic acid micelle (SA-Dex-OA/MTX) to inhibit the inflammatory response and enhance the bone repair function in the treatment of RA. In vivo studies showed increased accumulation of micelles in inflamed paws due to the presence of SA.
Galactosyl, also known as a ligand binding to selectins, shows good macrophages targeting in CIA mice model. In this study, an amphiphilic galactosyl dextran-retinal (GDR) nanoparticle has been constructed, in which dextran was conjugated with all-trans retinal via an acid-sensitive hydrazone linker, followed by the modification of galactosyl on the hydrophilic part to finally acquire macrophages-targeting ability66. Results showed that GDR nanoparticles preferentially accumulated in inflammatory tissues. When anti-inflammatory compound triptolide was incorporated, treatments using the nanoparticles resulted in a remarkable decrease in inflammation infiltration. While toxic effect caused by triptolide was attenuated owing to this targeting strategy. In addition to these abovementioned molecules, other small molecular ligands such as mannose90 and sialyl lewis are also emerging as promising targeting ligands in arthritis treatments previously42,91. The receptors of these ligands are highly expressed on the monocytes or monocyte-derived cells in the synovial tissues of arthritic joints.
3.1.2. Polymer-based targeted delivery
HA is one of frequently used polymers for inflammation-targeting. Due to the high affinity between HA and CD44 receptors, nanocarriers constituted or modified by HA are capable of targeting the cells with highly-expressed CD44 molecules such as activated lymphocytes and macrophages in inflamed sites of RA92,93. Alam et al.94 prepared a pH-responsive nanoparticle by incorporating mineralized calcium phosphate onto PEGylated HA-based nanoparticles. They showed that the presence of calcium phosphate ensured the controlled release of encapsulated MTX in acidic conditions. The HA segments on the surface of the nanoparticles ensured the targeted delivery in inflammatory lesions, consequently improving arthritis pathology with remarkable safety even at high drug dose. To selectively deliver prednisolone to the inflamed tissues, Gouveia et al.69 formed HA-conjugated pH-sensitive liposomes and demonstrated that they were more effectively taken up by activated macrophages and fibroblasts. Dextran sulfate (DS) is a specific ligand for scavenger receptor class A, which is overexpressed in the activated macrophages in inflamed joints95. Due to its superior biodegradability, biocompatibility and specific targeting, DS has been widely employed in targeted delivery for RA treatment in the past decades. Kim et al.67 conjugated DS to a hydrophobic block PCL to form an amphipathic copolymer (DS-b-PCL), which would self-assemble into micelles. Biodistribution studies showed that accumulation of DS-b-PCL micelles in the affected joints of RA was approximately 10 times higher than that in control groups, indicating good active targeting efficiency of DS modification.
3.1.3. Peptide-based targeted delivery
Arginine-glycine-aspartic acid (RGD) and its derivates have been widely used for enhancing the delivery efficiency of therapeutic agents77,96,97. They are recognized as a specific ligand for αvβ3 integrin receptors, which is associated with the occurrence of angiogenesis in tumor or inflammation. Nanocarriers coupling with RGD or its derivates displayed the remarkable improvement in therapeutic outcomes. Apart from RGD, other peptides used for targeted delivery in RA previously including vasoactive intestinal peptide (VIP)78, synovial fibroblast-homing peptide HAP-134, macrophage-targeted peptide tuftsin79, also showed favorable efficiency of drug delivery. Taking advantages of the phage display technique, Yang et al.98 identified a 9-amino acid peptide ligand (ADK) that homes selectively to an inflamed joint. In a subsequent study, they found the ADK-modified liposomes revealed higher affinity to endothelial cells and better in vivo accumulation in the inflamed joints compared to unmodified liposomes. It was also found that IL-27-loaded liposomes coupled with ADK were more effective in suppressing RA symptoms in contrast to free IL-27 or unmodified formulation when intravenously administrated after the disease onset41. More recently, Cook et al.99 described the exploration of a series of cystine-dense peptides (CDPs) which could rapidly accumulate in cartilage of rats after systemic administration. These CDPs are usually existed in the venom of spiders, snakes, and scorpions. One of the CDPs, CDP-11R, exhibited the optimal half-life and accumulation in cartilage. When linked to a steroid payload, a peptide-steroid conjugate was formed, which alleviated joint inflammation and avoided the toxicities occurred in steroid treatment. Unlike the regular targeting mechanism, Katsumata et al.80 discovered a collagen-binding peptide (CBP) derived from decorin, which could bind to the collagens on the surface of cartilages. The harvested complexes formed by conjugating CBP with TGF-β showed the enhanced drug retention at inflamed tissues and thereby improved inflammatory conditions after systemic injections.
3.1.4. Protein-based targeted delivery
Protein-targeted delivery in the treatment of RA mainly focuses on the utilization of antibodies, which accurately guide therapeutic payloads to cells by specifically binding to the antigens at the surface of cell membranes100. For example, the CD134 antibody has been modified on the surface of a liposome formulation to precisely target to activated T cells in RA101. To specifically targeted payloads to the hemoglobin scavenger receptor (CD163) in macrophages, a CD163 antibody was conjugated to GCs to facilitate the interaction between macrophages and drug vehicles74. Previous researches found the cell metabolism of affected joints in active RA is dramatically increased thereby have high demand for albumin consumption. A recent research revealed that albumin possessed intrinsic high affinity to secreted protein acidic and rich in cysteine (SPARC), which was up-regulated in the synovial tissues from RA patients and CIA mice28. Therefore, albumin has potential for the targeted delivery in inflammatory diseases.
3.1.5. Biomimetic targeted delivery
Biomimetic delivery systems based on cell membrane-camouflaged nanoparticles have gained increasing attention and emerged as a novel strategy to achieve natural targeting to disease sites45,102. A large number of inflammation-related cells would be recruited to inflamed sites by specific chemokines and inflammatory infiltrates in the synovium during the development of RA. A quite high proportion of them are monocytes-derived cells such as macrophages and neutrophils, which adhere to the inflamed endothelium and then extravasate into the affected synovium. Hence, there has been interest in coating nanoparticles with cell membranes to achieve the homing profile of the source cells and the active targeting to arthritic joints. For example, Li et al.83 coated PLGA nanoparticles with macrophage membranes by using cytochalasin B to relax the binding between the cytoskeleton and the membrane. Both in vitro and in vivo evaluations showed the improved targeting efficiency in desired sites. After incorporating tacrolimus, the biomimetic nanoparticles significantly suppressed the progression of RA in mice. It was reported that neutrophil would secrete micro-vesicles that are able to bind and neutralize inflammatory cytokines, leading to the protection of the arthritic synovium103. Based on this finding, Zhang et al.82 fabricated a neutrophil membrane-coated nanoparticle to inhibit synovial inflammation in arthritic mice. They showed that these nanoparticles could permeate deep into the cartilage of RA and provide protection for the cartilage against inflammation damage. Ultimately, treatment using these membrane-coated nanoparticles without extra drug loading achieved inflammation resolution and tissues repair.
Inspired by the intrinsic interactions between platelets and inflammatory tissues via P-selectin, a platelet-mimic nanoparticle was constructed for targeted delivery in RA treatment. The platelet-mimic nanoparticles showed the prolonged circulation time in vivo and increased accumulation in inflamed joints81. Furthermore, this biomimetic nanoparticle loaded with FK506 significantly improved the joint inflammation in CIA mice. The direct modification of the cell membrane with specific ligands would customize the targeted delivery. However, this approach might disrupt the structure or function of proteins in the cell membrane. Shi et al.84 used a genetic engineering technique to collect TRAIL-expressing cell membranes from human umbilical vein endothelial cell line (HUVEC). The TRAIL-anchored membranes were fused onto hydroxychloroquine-loaded PLGA nanoparticles to block the inflammatory cytokines and actively deliver therapeutic payloads to RA joints. Upon intravenous injection, the high accumulation and long retention of the biomimetic nanocarriers in inflamed joints were observed, which lead to good therapeutic outcomes.
3.2. Stimulus-responsive delivery systems
The change of physiological parameters is a pivotal indication between normal and arthritis tissues, providing promising targets in designing bio-responsive drug delivery systems104. There has been great interest in designing stimuli-responsive drug vehicles, which is able to release drugs in response to the change of specific physiological signals or environmental factors. In the development of active RA, there are massive inflammatory cell infiltration and aggressive proliferation accompanied by the rapid increase of cellular metabolism in the joint tissues, consequently leading to the local acidosis and accumulation of oxidative intermedia. In addition, there are excess MMPs produced by inflammatory cells in the inflamed joints of RA. Various stimuli-responsive nanocarriers were fabricated for achieving the controlled release of drugs in response to the physiological changes in the inflamed joints of RA92,105,106. We have summarized the details of the inflammatory stimulus used for the development of bio-responsive delivery system in Table 4107, 108, 109, 110, 111, 112. This section highlights recent advances in the application of stimulus-responsive drug delivery systems in RA treatments (Table 5)19,94,113, 114, 115, 116, 117, 118, 119, 120, 121, 122.
Table 4.
Summary of typical inflammatory stimulus in vivo.
| Stimulus | Type | Detail | Ref. |
|---|---|---|---|
| pH | – | Acidic pH range: 7.2–5.0 | 107,108 |
| Enzymes | MMP-1 | Produced by chondrocytes, osteoblasts and synovial cells | 109 |
| MMP-3 | Produced by fibroblasts | 110 | |
| MMP-9 | Produced by monocytes and macrophages | 110 | |
| PLA2 | Produced by activated monocytes, macrophages and neutrophils | 111 | |
| ROS | O2‒ Hydroxyl radical |
Produced by granulocytes | 112 |
– Not applicable.
MMPs, matrix metalloproteinase; PLA2, phospholipase A2; ROS, reactive oxygen species.
Table 5.
Stimulus-responsive drug delivery systems.
| Stimulus | Responsive unit | Delivery strategy | Drug | Ref. |
|---|---|---|---|---|
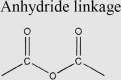
 |
 |
PEG-based micelles | Dexamethasone | 19 |
 |
mPEG-PPF micelles | Ibuprofen | 113 | |
 |
AKP-Dex nanoparticles | Dexamethasone | 114 | |
| Calcium phosphate | MP-HA nanoparticles | Methotrexate | 94 | |
 |
Triglycerol monostearate | DSPE-PEG/TGMS nanoparticles | Dexamethasone | 115 |
| βIG-H3 derivatives | – | YH18 peptide/dhfas-1 | 116 | |
 |
Manganese ferrite and ceria | MFC-MSNs nanoparticles | Methotrexate | 117 |
 |
TPP@PMM micelles | Prednisolone | 118 | |
| TPP@PMM micelles | Prednisolone | 118 | ||
| -Se-Se- | VES-PLGA-Se-Se-mPEG micelles | Berberine | 119 | |
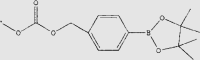 |
Oxi-αCD nanoparticles | Dexamethasone | 120 | |
 |
Fe | Fe-EC nanoparticles | Diclofenac sodium | 121 |
| Fe | PSS-doped CaCO3 microcapsules | Prednisolone | 122 |
PPF, polypropylene fumarate; AKP, acetone-based ketal-linked prodrugs; MP, mineralization of PEGylated; TGMS, triglycerol monostearate; MFC-MSNs, manganese ferrite and ceria nanoparticle-anchored mesoporous silica nanoparticles; TPP, two-photon prednisolone; PMM, PMPC (2-methacryloyloxyethyl phosphorylcholine)−PMEMA (2-(methylthio) ethanol methacrylate); VES, vitamin E succinate; Oxi-αCD, oxygen species α-cyclodextrin; EC, ethylcellulose; PSS, poly(sodium 4-styrenesulfonate).
3.2.1. pH
The pH value in acidic inflamed joints is usually close to 6.0, sometimes even as low as 5.0 when in the active phase of RA107. According to the slightly acidic microenvironment, a wide variety of pH-sensitive vehicles have been rationally designed, in which hydrazone bonds or acetal bonds are frequently investigated acid-cleavable linkers. For example, Wang et al.19 synthesized pH-responsive micelles by conjugating hydrophobic Dex to the side chains of hydrophilic PEG derivates via hydrazone linkers. The introduction of hydrazone groups effectively avoided the burst release of Dex in vitro and drug leakage in circulation, finally facilitating the selective drug release in acidic arthritic joints. Compared with hydrazone bond, acetal-based bonds are more sensitive to acidic environment. In a recent research, Xu et al.114 designed an acetal-based prodrug of Dex, which was then linked with DSPE-PEG to form pH-sensitive Dex-loaded nanoparticles. The pH-sensitive Dex-loaded nanoparticles preferentially accumulated in the inflamed joints and tracelessly released Dex in the acidic joint tissues, leading to an improved efficacy against RA. Additionally, pH-responsive biomineralized nanoparticles assembled by ionic interactions were also developed. For example, Alam et al.94 prepared nanoparticles with the calcium phosphate shell containing PEGylated HA and 5β-cholanic acid. The nanoparticles showed the biodistribution in arthritic paws after systemic injections and released MTX in response to the acidic joints of RA. A significant therapeutic index was thereafter observed. Apart from the acid-triggered drug release, the acidic environment of the inflamed joints of RA was used to induce the shape transformation of nanocarriers from vesicles to fibers, which led to the extended retention in diseases sites123.
3.2.2. Redox
The excessive accumulation of oxidative intermedia and high oxidative stress would often associate with inflammatory signaling cascades and tissues damage124. Due to the higher level of reactive oxygen species (ROS) in inflamed tissues than that in normal tissues, ROS-sensitive groups such as thioether, selenium-containing compounds, thioketal and phenylboronic ester have been considered as potential means to manipulate the release behavior of drugs108,125. Recently, Xu and co-workers114 established FA-decorated ROS-responsive nanoparticles by incorporating a 4-phenylboronic acid (4-PBA) pinacol ester linker. The nanoparticles showed the ROS-responsive drug release profile and the efficient elimination of H2O2 in activated macrophages. After being loaded with Dex, the treatment with ROS-responsive nanoparticles significantly reduced histological damage and alleviate inflammation in CIA mice. Fan et al.119 formed berberine-loaded micelles by the self-assembly of a selenocystamine-based polymer, which is cleavable by ROS. The ROS-responsive micelles released the encapsulated therapeutic cargo at the inflammatory microenvironment in response to ROS. The treatment of berberine-loaded micelles exhibited 10-fold higher efficacy than that of free berberine. Chen et al.126 synthesized an FA-modified liposome co-encapsulating MTX and catalase to actively target to arthritic joints. The encapsulated catalase is able to catalyze the production of oxygen in the presence of ROS. They found that the elevated ROS level within cytoplasm would trigger the generation of oxygen, which could destabilize and destroy the liposomes, eventually facilitating MTX release.
3.2.3. Enzyme
Disease-associated enzymes have also served as specific stimulus for designing bio-responsive nanomedicines. The upregulated expression of MMPs within the inflamed joints is a distinguishing feature in RA. Therefore, stimuli-responsive nanocarriers based on the aberrant secretion of MMPs have been extensively explored for the controlled drug delivery in RA. Previously, MMP substrate peptides, which would be recognized and cleaved by MMPs, were frequently incorporated into the drug delivery systems for manipulating drug release or deshielding of PEG shell to improve cellular uptake or tissue penetration116,127. More recently, He et al.115 reported a facile method to prepare MMPs-responsive nanoparticles by the co-assembling of triglycerol monostearate (TGMS) and DSPE-PEG. The ester bond within TGMS is cleavable by MMPs, ensuring the rapid release of Dex in response to highly-expressed MMPs in inflamed sites. After being systemically injected to AIA rats, the Dex-loaded MMP-responsive nanoparticles significantly reduced the degree of joint swelling and inhibited the inflammatory level. Other than MMPs, the expression of phospholipase A2 (PLA2) is also closely related to the severity of RA and cancer. PLA2, usually produced by activated macrophages, neutrophils and monocytes in inflammatory regions, is able to catalyze the hydrolysis of phospholipids128. It has been utilized as a trigger for lipid-based drug vehicles in the treatment of RA. Recently, Wang et al.129 fabricated a lipid tubule self-assembled by DC8,9PC molecules. After subcutaneous administration to the arthritic rats, the overexpressed PLA2 at the inflamed sites facilitate the drug release from DC8,9PC lipid tubules on-demand, providing a sustained drug supply over a long time.
3.2.4. Others
Apart from abovementioned biological environment-responsive drug delivery systems, external stimulus such as temperature, sonication and magnetic field have been utilized as exogenous triggers to regulate drug release and in vivo performance. Among them, temperature is one of the widely explored stimulus in RA treatment. For example, Costa Lima et al.130 integrated MTX and gold nanoparticles into PLGA nanospheres through emulsion–diffusion evaporation technique. Upon near-infrared irradiation, the heat generated by gold nanoparticles accelerated the release of MTX at inflammation region, improving the therapeutic effect. In addition to the temperature rise-caused drug release based on light irradiation, some researchers also take advantages of thermo-responsive polymers to control the behavior of drug delivery. Jung et al.131 developed a temperature-responsive nanocarrier with a temperature-sensitive amphiphilic polyelectrolyte and positively charged etanercept. At the physiological temperature (37.5 °C), which was higher than the clouding temperature of the polyelectrolyte, the long-term stability of etanercept was improved, which was beneficial for the in vivo application of the nanocarrier. As the temperature in normal tissues and inflamed sites in vivo is very close, this formulation is only suitable for local administration such as intra-articular injection. The magnetism is originally employed in magnetic resonance imaging (MRI) for in vivo imaging and diagnosis. Recently, magnetic nanocarriers have been frequently used for manipulating the drug release and in vivo distribution in cancer treatments. While in the treatment of RA, only several in vitro explorations using magnetic field controlling drug release has been reported. Thus, how to regulate the drug behaviors in vivo in response to magnetic field remains to be further confirmed. Ultrasound is a clinically common technique for disease diagnosis and treatment. Recently, ultrasound is also emerging as an external trigger in the development of stimulus-responsive nanocarriers. Zhu et al.132 prepared ultrasound-responsive nanocarriers, termed as “nanobombs” consisted of a perfluorocarbon (PFP) core surrounded by the shell of an FA-modified PEG-phospholipid. Under the 1 MHz ultrasound stimulation, the explosion of “nanobombs” promoted the targeted drug release from the inner core. The ultrasound-triggered drug release significantly reduced the synovial inflammation and cartilage damages in a CIA rat model. Compared to the internal stimulus-based strategies, the external stimulus-based approach is more convenient to precisely control the drug behaviors by adjusting the parameters of the external stimulus. To achieve desirable drug delivery efficiency using external stimulus-based delivery systems, the researchers should make sure that the stable drug loading before applying external stimulation to the inflamed sites and adequate drug release in response to the external stimulus.
4. Newly emerged promising therapeutic approaches
Efficient RA remission still remains to be a challenge. Exploiting new therapeutic regimens for effective RA treatment is in great demand. As more and more aspects of RA pathogenesis have been discovered, a variety of potential targets or therapeutic insights have emerged increasingly (Fig. 3). Alternative anti-inflammatory approaches which can overcome the multiple physiological barriers in inflammatory disorders are highly desirable. In the following section, most recent advances in the newly emerged therapeutic strategies in RA therapy would be summarized and illustrated.
Figure 3.
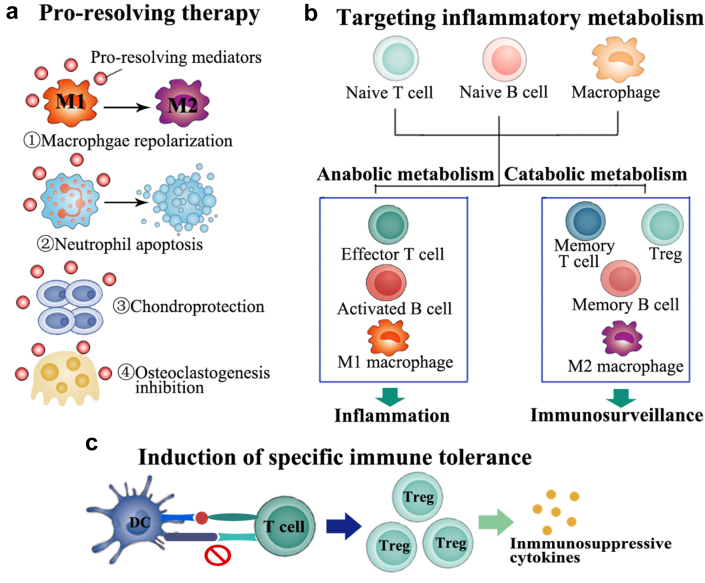
The newly emerged promising therapeutic approaches for RA treatment. (a) Endogenous pro-resolving mediators initiate resolution function by acting on specific cell types, including promoting macrophages repolarization from pro-inflammatory M1 type to anti-inflammatory M2 type, facilitating neutrophils apoptosis and play a key role in chondroprotection and osteoclastgenesis inhibition. (b) Cells involved in inflammation development would undergo a metabolic reprogramming, displaying a highly increased metabolic demand. Increased anabolic metabolism would promote inflammation and motivate immune response. Therefore, facilitating the balance of cell metabolism would be a possible therapeutic intervention option. (c) Induction of specific immune tolerance during the interaction between DCs and T cells would produce increased level of Treg and decrease the level of autoreactive immune cells, which might provide a long-term, sustained disease remission.
4.1. Induction of specific immune tolerance
As an autoimmune disease, the development of RA is mainly driven by the undue activation of immune systems. Massive autoreactive T cells and B cells produce autoantibodies and attack own tissues, eventually resulting in inflammation networks and tissue damages5,133,134. In view of the overactivated immune response in the RA pathogenesis, inducing specific immune tolerance can reprogram autoreactive cells, resulting in long-term immune homeostasis. Tengvall et al.135 demonstrated the successful induction of specific immune tolerance via lentiviral-mediated gene therapy in a CIA model. They showed that only 5% of the mice suffered arthritis onset after the treatment and the percentage of Tregs increased at day 3 in the CII-tolerized mice. Adoptively transferring of B cells or T cells from tolerized mice to naïve mice mediated a certain degree of tolerance, demonstrating the sustainable tolerance to CIA was induced during the course of arthritis. In RA patients, citrullinated protein is a major target of disease-specific autoantibodies and often regarded as a specific biomarker for RA diagnose. Gertel et al.136 constructed a multiepitope citrullinated peptide constituted by citrullinated filaggrin, fibrinogen, vimentin, and collagen type II. Arthritic rats treated with the multiepitope peptides via subcutaneous injection displayed a reduced disease severity. Further investigation indicated this treatment resulted in the increased Treg cell subset and decreased Th17 cells, ultimately leading to the amelioration of RA symptoms. In order to generate the expansion of RA-specific Treg cells to combat this autoimmune diseases, Clemente-Casares et al.137 fabricated a nanoparticle coated with RA-relevant peptides bound to major histocompatibility complex class II molecules. The systemic injection of the nanoparticles produced the expansion of antigen-specific Treg cell subset in CIA mice models, finally resulting in the remission of established arthritis. The occurring of nonspecific immunosuppression in conventional anti-inflammatory treatments is often associated with the high risk of infections. Therefore, the induction of specific immune tolerance to modulate the aberrant immune responses in RA would be a feasible strategy. Specifically, the efficacy of this therapeutic approach is definitively dependent on the discovery of the autoantigens that dominantly generate the autoimmune response138. As the leading self-antigens in RA have not been completely identified, the therapeutic options might merely remain on fundamental research for the moment.
4.2. Pro-resolving therapy
Chronic inflammation is commonly characterized by a pro-inflammatory phase and subsequent resolution phase139. In the resolution phase, endogenous pro-resolving mediators would engage in inflammation recession and tissue repair. As a result, the persistence of chronic inflammation might be not just due to the excessive proinflammatory mediators but also due to the absence of resolution process. Therefore, the exploitation of pro-resolving mediators for the treatment of RA could promote the cessation of inflammation and homeostasis of the immune response, providing a safe and effective solution to correct inflammatory arthritis140, 141, 142. It was reported that neutrophil-derived extracellular vesicles are enriched with pro-resolving mediators143,144. In an experimental inflammatory arthritis, the extracellular vesicles were able to accumulate in inflamed cartilage and protect it from damage143. An important mediator of resolution is the glucocorticoid-regulated protein AnxA1, which can modulate neutrophil trafficking and macrophage repolarization145. Nanoparticles encapsulating AnxA1-derived peptides substantially achieved the inflammation resolution and showed the protective effect in various inflammatory models146,147. The inflammation resolution-mediated therapy would activate patient's own protective and reparative processes, leading to favorable anti-inflammatory efficacy and the lower burden of adverse effects. The further exploration and clinical trials by mimicking endogenous resolution mediators are ongoing currently.
4.3. Targeting inflammatory metabolism in RA
One of the fundamental characteristics of RA is the increased metabolic demand of inflammatory resident cells. After undergoing metabolic reprogramming, the cell phenotype would change from a resting state to a highly active subset, further leading to the exacerbation of inflammatory conditions. Therefore, the regulation of the metabolic pathways involved in inflammation developments offers a promising avenue for RA treatment148, 149, 150, 151, 152. Mammalian target of rapamycin (mTOR) is a key player in the metabolism of T cells during RA process. The activation of mTOR signaling is often associated with the differentiation of T cells towards to autoreactive phenotypes151,153. In a CIA model of RA, metformin treatments restored the immune balance and alleviated arthritis by inhibiting transducer and activator of transcription 3 (STAT3) and mTOR activity154. Inflammatory resident cells with elevated metabolism would upregulate the level of aerobic glycolysis. 2-deoxy-d-glucose, a glycolysis inhibitor, could block the aerobic glycolysis by interacting with glucose transporter type 1 (GLUT1), resulting in the normalization of activated inflammatory cells and the mitigation of arthritis155. Targeting metabolism in inflamed sites could selectively alter the abnormal function and phenotype of inflammatory cells and have few impacts on other cells with the normal metabolic level151. Thus, this approach affords attractive therapeutic insights by regulating inappropriately activated cells in inflamed sites, leading to the cell-specific blockade. When combined with targeted delivery systems, this therapeutic strategy would potentially bring in new hope for RA therapy.
4.4. Others
In recent years, a variety of new attempts has emerged for the management of RA. For instance, the phototherapy including photothermal therapy (PTT) and photodynamic therapy (PDT), which is commonly applied in tumor therapy, has been investigated for RA treatment currently. Inspired by the fact that Cu-based nanoparticles can induce PTT and PDT at the same time, Lu et al.156 fabricated Cu7.2S4 nanoparticles for the phototherapy of RA. The treatment of Cu7.2S4 nanoparticle under the NIR irradiation inhibited the inflammatory level and improved the bone mineral density of arthritic rats. However, some evidences indicated that phototherapy might also generate inflammatory response and tissue damages157, which could exacerbate the RA condition. As a result, the detailed studies on the underlying impact of phototherapy in the treatment of RA are needed.
Nitric oxide (NO) is a bioactive gas molecule secreted by immune cells and monocyte-derived cells. Yeo et al.158 suggested the overproduced NO would be highly correlated with the pathogenesis of RA. They developed a NO-responsive nanogel by introducing a NO-cleavable group, which acts as an endogenous NO-scavenger to eliminate the NO in local inflamed sites. The intra-articular injection of the nanogel successfully suppressed the onset of RA. However, other studies reported a conflicting result. The nanoparticles was found to be capable of inducing endogenous NO production159. The elevated NO inhibited the activity of inflammatory signaling pathway and reduced the levels of inflammatory cytokines. The contradictory results might be due to the different judgement on NO concentrations in RA. Practically, it is still not clear how the level of NO affects the evolution of RA.
Recent studies suggested that cell-free DNA (cfDNA), originated from the degradation of DNA, played a critical role in RA development160. Elevated cfDNA level was observed in the serum and synovial fluids of RA patients and also involved in eliciting immunity response and inflammatory reaction. In order to scavenge the cfDNA in arthritic rats, Wu et al.161 developed cationic nanoparticles composed of PLGA and poly(2-(diethylamino)ethyl methacrylate) (PDMA) with a high DNA-binding affinity. The intravenous administration of the cationic nanoparticles mitigated the joint swelling and cartilage damage of RA. Despite the successful anti-rheumatic effects of these newly appeared approaches, their safety issues and dose effects are still needed to be further validated.
5. Perspectives
There has been great interest in the development of effective nanocarriers for RA therapy in the past years. The therapeutic outcomes and in vivo safety of a variety of nanocarriers have been validated. Despite of the great progresses, which have been achieved for these developed therapeutic strategies, there are still challenges remained. It is broadly accepted that these developed strategies are incapable of providing the “full recovery”. In view of the percentages of patients showing low responsiveness to current therapies, the high incidence of adverse effects and the insufficient public awareness of the disease as well as a low rate of remission, RA creates a huge social and economic burden worldwide, making the development of effective and safe therapeutic approaches in great demand. Some rheumatologists believe that reversal of disease progression might become possible by taking advantages of preventative therapies. In other words, interfering the RA development in the pre-arthritic process before the disease has manifested clinically would probably prevent the deterioration of RA and achieve total remission.
Although established animal models mimicking RA are helpful, they do not always accurately replicate the human disease phenotype. The pathogenetic of RA in patients is much more complicated than that of animal models. Therefore, the success achieved in animal model sometimes might be impracticable in RA patients. On the other hand, despite the functions and constitution of nanocarriers are becoming more and more diversified, they are actually far away from clinical applications due to the complicated synthesis, poor biocompatibility, unpredictable in vivo behaviors and difficulty in scale-up production.
The original aspiration of pharmaceutical researchers is pushing the finding of drug formulations from bench to besides. Moving forward, further efforts are still in need to advance the development of potential drug delivery systems in clinical trials and marketing in the field of RA therapy. We expect the rapid progresses for the treatment of RA or other autoimmune diseases are in near future.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 82003661).
Author contributions
Qin Wang wrote the manuscript. Xianyan Qin drew the tables and figures. Jiyu Fang polished the language. Xun Sun supervised the work. The final version of the manuscript has been approved by all authors.
Conflicts of interest
All authors declare no competing interests.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Qin Wang, Email: wangqin666@swjtu.edu.cn.
Xun Sun, Email: sunxun@scu.edu.cn.
References
- 1.McInnes I.B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 2.Li Z.G. A new look at rheumatology in China—opportunities and challenges. Nat Rev Rheumatol. 2015;11:313–317. doi: 10.1038/nrrheum.2014.218. [DOI] [PubMed] [Google Scholar]
- 3.Smolen J.S., Aletaha D., Barton A., Burmester G.R., Emery P., Firestein G.S. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 4.Kim K., Bang S.Y., Lee H.S., Bae S.C. Update on the genetic architecture of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:13–24. doi: 10.1038/nrrheum.2016.176. [DOI] [PubMed] [Google Scholar]
- 5.Weyand C.M., Fujii H., Shao L., Goronzy J.J. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–588. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q., Sun X. Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater sci. 2017;5:1407–1420. doi: 10.1039/c7bm00254h. [DOI] [PubMed] [Google Scholar]
- 7.Koenders M.I., van den Berg W.B. Novel therapeutic targets in rheumatoid arthritis. Trends Pharmacol Sci. 2015;36:189–195. doi: 10.1016/j.tips.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Yang M., Feng X., Ding J., Chang F., Chen X. Nanotherapeutics relieve rheumatoid arthritis. J Control Release. 2017;252:108–124. doi: 10.1016/j.jconrel.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Baschant U., Lane N.E., Tuckermann J. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:645. doi: 10.1038/nrrheum.2012.166. [DOI] [PubMed] [Google Scholar]
- 10.Emery P. Treatment of rheumatoid arthritis. BMJ. 2006;332:152–155. doi: 10.1136/bmj.332.7534.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wailoo A., Hernández Alava M., Scott I.C., Ibrahim F., Scott D.L. Cost-effectiveness of treatment strategies using combination disease-modifying anti-rheumatic drugs and glucocorticoids in early rheumatoid arthritis. Rheumatology. 2014;53:1773–1777. doi: 10.1093/rheumatology/keu039. [DOI] [PubMed] [Google Scholar]
- 12.Timlin H., Bingham C.O. Efficacy and safety implications of molecular constructs of biological agents for rheumatoid arthritis. Expet Opin Biol Ther. 2014;14:893–904. doi: 10.1517/14712598.2014.900536. [DOI] [PubMed] [Google Scholar]
- 13.Sinha S., Ghosh B., Bandyopadhyay S., Fatima F., Bandi V.K., Thakur P. Comparative evaluation of efficacy, pharmacodynamics, and safety of Hetero's adalimumab (Mabura®, Hetero Biopharma Ltd.) and reference adalimumab (Humira®, Abbvie Inc.) in patients with active rheumatoid arthritis on concomitant methotrexate therapy. BMC Rheumatol. 2020;4:24. doi: 10.1186/s41927-020-00124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolen J.S., Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11:276. doi: 10.1038/nrrheum.2015.8. [DOI] [PubMed] [Google Scholar]
- 15.Recio M.C., Andujar I., Rios J.L. Anti-inflammatory agents from plants: progress and potential. Curr Med Chem. 2012;19:2088–2103. doi: 10.2174/092986712800229069. [DOI] [PubMed] [Google Scholar]
- 16.Evans C.H., Ghivizzani S.C., Robbins P.D. Arthritis gene therapy is becoming a reality. Nat Rev Rheumatol. 2018;14:381–382. doi: 10.1038/s41584-018-0009-5. [DOI] [PubMed] [Google Scholar]
- 17.Xiao S., Tang Y., Lv Z., Lin Y., Chen L. Nanomedicine—advantages for their use in rheumatoid arthritis theranostics. J Control Release. 2019;316:302–316. doi: 10.1016/j.jconrel.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Wang S., Lv J., Meng S., Tang J., Nie L. Recent advances in nanotheranostics for treat-to-target of rheumatoid arthritis. Adv Healthc Mater. 2020;9:1901541. doi: 10.1002/adhm.201901541. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q., Li Y., Chen X., Jiang H., Zhang Z., Sun X. Optimized in vivo performance of acid-liable micelles for the treatment of rheumatoid arthritis by one single injection. Nano Research. 2019;12:421–428. [Google Scholar]
- 20.Yang M., Ding J., Feng X., Chang F., Wang Y., Gao Z. Scavenger receptor-mediated targeted treatment of collagen-induced arthritis by dextran sulfate-methotrexate prodrug. Theranostics. 2017;7:97–105. doi: 10.7150/thno.16844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiehn C., Kratz F., Sass G., Müller-Ladner U., Neumann E. Targeted drug delivery by in vivo coupling to endogenous albumin: an albumin-binding prodrug of methotrexate (MTX) is better than MTX in the treatment of murine collagen-induced arthritis. Ann Rheum Dis. 2008;67:1188–1191. doi: 10.1136/ard.2007.086843. [DOI] [PubMed] [Google Scholar]
- 22.Hwang J., Rodgers K., Oliver J.C., Schluep T. Alpha-methylprednisolone conjugated cyclodextrin polymer-based nanoparticles for rheumatoid arthritis therapy. Int J Nanomed. 2008;3:359–371. doi: 10.2147/ijn.s3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren H., He Y., Liang J., Cheng Z., Zhang M., Zhu Y. Role of liposome size, surface charge, and pegylation on rheumatoid arthritis targeting therapy. ACS Appl Mater Interfaces. 2019;11:20304–20315. doi: 10.1021/acsami.8b22693. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q., He L., Fan D., Liang W., Fang J. Improving the anti-inflammatory efficacy of dexamethasone in the treatment of rheumatoid arthritis with polymerized stealth liposomes as a delivery vehicle. J Mater Chem B. 2020;8:1841–1851. doi: 10.1039/c9tb02538c. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q., Jiang J., Chen W., Jiang H., Zhang Z., Sun X. Targeted delivery of low-dose dexamethasone using PCL–PEG micelles for effective treatment of rheumatoid arthritis. J Control Release. 2016;230:64–72. doi: 10.1016/j.jconrel.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Xu X.L., Li W.-S., Wang X.J., Du Y.L., Kang X.Q., Hu J.B. Endogenous sialic acid-engineered micelles: a multifunctional platform for on-demand methotrexate delivery and bone repair of rheumatoid arthritis. Nanoscale. 2018;10:2923–2935. doi: 10.1039/c7nr08430g. [DOI] [PubMed] [Google Scholar]
- 27.Fan Z., Li J., Liu J., Jiao H., Liu B. Anti-inflammation and joint lubrication dual effects of a novel hyaluronic acid/curcumin nanomicelle improve the efficacy of rheumatoid arthritis therapy. ACS Appl Mater Interfaces. 2018;10:23595–23604. doi: 10.1021/acsami.8b06236. [DOI] [PubMed] [Google Scholar]
- 28.Liu L., Hu F., Wang H., Wu X., Eltahan A.S., Stanford S. Secreted protein acidic and rich in cysteine mediated biomimetic delivery of methotrexate by albumin-based nanomedicines for rheumatoid arthritis therapy. ACS Nano. 2019;13:5036–5048. doi: 10.1021/acsnano.9b01710. [DOI] [PubMed] [Google Scholar]
- 29.Kim H.J., Lee S.M., Park K.H., Mun C.H., Park Y.B., Yoo K.H. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials. 2015;61:95–102. doi: 10.1016/j.biomaterials.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Song P., Yang C., Thomsen J.S., Dagnæs-Hansen F., Jakobsen M., Brüel A. Lipidoid-siRNA nanoparticle-mediated IL-1β gene silencing for systemic arthritis therapy in a mouse model. Mol Ther. 2019;27:1424–1435. doi: 10.1016/j.ymthe.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q., Jiang H., Li Y., Chen W., Li H., Peng K. Targeting NF-κB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials. 2017;122:10–22. doi: 10.1016/j.biomaterials.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Zhao G., Liu A., Zhang Y., Zuo Z.Q., Cao Z.T., Zhang H.B. Nanoparticle-delivered siRNA targeting Bruton's tyrosine kinase for rheumatoid arthritis therapy. Biomater Sci. 2019;7:4698–4707. doi: 10.1039/c9bm01025d. [DOI] [PubMed] [Google Scholar]
- 33.Aldayel A.M., O'Mary H.L., Valdes S.A., Li X., Thakkar S.G., Mustafa B.E. Lipid nanoparticles with minimum burst release of TNF-α siRNA show strong activity against rheumatoid arthritis unresponsive to methotrexate. J Control Release. 2018;283:280–289. doi: 10.1016/j.jconrel.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanniasinghe A., Manolios N., Schibeci S., Lakhiani C., Kamali-Sarvestani E., Sharma R. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin Immunol. 2014;151:43–54. doi: 10.1016/j.clim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Korkmaz E., Friedrich E.E., Ramadan M.H., Erdos G., Mathers A.R., Burak Ozdoganlar O. Therapeutic intradermal delivery of tumor necrosis factor-alpha antibodies using tip-loaded dissolvable microneedle arrays. Acta Biomater. 2015;24:96–105. doi: 10.1016/j.actbio.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao J., Zhang N., Wang Z., Su J., Yang J., Han J. Microneedle-assisted transdermal delivery of etanercept for rheumatoid arthritis treatment. Pharmaceutics. 2019;11:235. doi: 10.3390/pharmaceutics11050235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byeon H.J., Min S.Y., Kim I., Lee E.S., Oh K.T., Shin B.S. Human serum albumin-TRAIL conjugate for the treatment of rheumatoid arthritis. Bioconjugate Chem. 2014;25:2212–2221. doi: 10.1021/bc500427g. [DOI] [PubMed] [Google Scholar]
- 38.Lee H., Lee M.-Y., Bhang S.H., Kim B.-S., Kim Y.S., Ju J.H. Hyaluronate–gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano. 2014;8:4790–4798. doi: 10.1021/nn500685h. [DOI] [PubMed] [Google Scholar]
- 39.Ekladious I., Colson Y.L., Grinstaff M.W. Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov. 2019;18:273–294. doi: 10.1038/s41573-018-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chuang S.Y., Lin C.H., Huang T.H., Fang J.Y. Lipid-based nanoparticles as a potential delivery approach in the treatment of rheumatoid arthritis. Nanomaterials (Basel) 2018;8:42. doi: 10.3390/nano8010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meka R.R., Venkatesha S.H., Moudgil K.D. Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis. J Control Release. 2018;286:279–288. doi: 10.1016/j.jconrel.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirai M., Minematsu H., Kondo N., Oie K., Igarashi K., Yamazaki N. Accumulation of liposome with Sialyl Lewis X to inflammation and tumor region: application to in vivo bio-imaging. Biochem Biophys Res Commun. 2007;353:553–558. doi: 10.1016/j.bbrc.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 43.Briuglia M.-L., Rotella C., McFarlane A., Lamprou D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5:231–242. doi: 10.1007/s13346-015-0220-8. [DOI] [PubMed] [Google Scholar]
- 44.Fanali G., di Masi A., Trezza V., Marino M., Fasano M., Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspect Med. 2012;33:209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Fontana F., Albertini S., Correia A., Kemell M., Lindgren R., Mäkilä E. Bioengineered porous silicon nanoparticles@macrophages cell membrane as composite platforms for rheumatoid arthritis. Adv Funct Mater. 2018;28:1801355. [Google Scholar]
- 46.Gramoun A., Crowe L.A., Maurizi L., Wirth W., Tobalem F., Grosdemange K. Monitoring the effects of dexamethasone treatment by MRI using in vivo iron oxide nanoparticle-labeled macrophages. Arthritis Res Ther. 2014;16:R131. doi: 10.1186/ar4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C.L., Siow T.Y., Chou C.H., Lin C.H., Lin M.H., Chen Y.C. Targeted superparamagnetic iron oxide nanoparticles for in vivo magnetic resonance imaging of T-cells in rheumatoid arthritis. Mol Imag Biol. 2017;19:233–244. doi: 10.1007/s11307-016-1001-6. [DOI] [PubMed] [Google Scholar]
- 48.Lostalé-Seijo I., Montenegro J. Synthetic materials at the forefront of gene delivery. Nat Rev Chem. 2018;2:258–277. [Google Scholar]
- 49.Janakiraman K., Krishnaswami V., Rajendran V., Natesan S., Kandasamy R. Novel nano therapeutic materials for the effective treatment of rheumatoid arthritis-recent insights. Mater Today Commun. 2018;17:200–213. doi: 10.1016/j.mtcomm.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruno B.J., Miller G.D., Lim C.S. Basics and recent advances in peptide and protein drug delivery. Ther Deliv. 2013;4:1443–1467. doi: 10.4155/tde.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S.K., Park K.Y., Yoon W.C., Park S.H., Park K.K., Yoo D.H. Melittin enhances apoptosis through suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3 activation and Bcl-2 expression for human fibroblast-like synoviocytes in rheumatoid arthritis. Joint Bone Spine. 2011;78:471–477. doi: 10.1016/j.jbspin.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Nikiphorou E., Buch M.H., Hyrich K.L. Biologics registers in RA: methodological aspects, current role and future applications. Nat Rev Rheumatol. 2017;13:503–510. doi: 10.1038/nrrheum.2017.81. [DOI] [PubMed] [Google Scholar]
- 53.Prasad L.K., O'Mary H., Cui Z. Nanomedicine delivers promising treatments for rheumatoid arthritis. Nanomedicine. 2015;10:2063–2074. doi: 10.2217/nnm.15.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M., Daddy J.C.K., Xiao Y., Ping Q., Zong L. Advanced nanomedicine for rheumatoid arthritis treatment: focus on active targeting. Expet Opin Drug Deliv. 2017;14:1141–1144. doi: 10.1080/17425247.2017.1372746. [DOI] [PubMed] [Google Scholar]
- 55.Bilthariya U., Jain N., Rajoriya V., Jain A.K. Folate-conjugated albumin nanoparticles for rheumatoid arthritis-targeted delivery of etoricoxib. Drug Dev Ind Pharm. 2015;41:95–104. doi: 10.3109/03639045.2013.850705. [DOI] [PubMed] [Google Scholar]
- 56.Qi R., Majoros I., Misra A.C., Koch A.E., Campbell P., Marotte H. Folate receptor-targeted dendrimer-methotrexate conjugate for inflammatory arthritis. J Biomed Nanotechnol. 2015;11:1431–1441. doi: 10.1166/jbn.2015.2077. [DOI] [PubMed] [Google Scholar]
- 57.Verma A., Jain A., Tiwari A., Saraf S., Panda P.K., Agrawal G.P. Folate conjugated double liposomes bearing prednisolone and methotrexate for targeting rheumatoid arthritis. Pharm Res (N Y) 2019;36:123. doi: 10.1007/s11095-019-2653-0. [DOI] [PubMed] [Google Scholar]
- 58.Nogueira E., Lager F., Le Roux D., Nogueira P., Freitas J., Charvet C. Enhancing methotrexate tolerance with folate tagged liposomes in arthritic mice. J Biomed Nanotechnol. 2015;11:2243–2252. doi: 10.1166/jbn.2015.2170. [DOI] [PubMed] [Google Scholar]
- 59.Pandey P.K., Maheshwari R., Raval N., Gondaliya P., Kalia K., Tekade R.K. Nanogold-core multifunctional dendrimer for pulsatile chemo-, photothermal- and photodynamic-therapy of rheumatoid arthritis. J Colloid Interface Sci. 2019;544:61–77. doi: 10.1016/j.jcis.2019.02.073. [DOI] [PubMed] [Google Scholar]
- 60.Zhang N., Xu C., Li N., Zhang S., Fu L., Chu X. Folate receptor-targeted mixed polysialic acid micelles for combating rheumatoid arthritis: in vitro and in vivo evaluation. Drug Deliv. 2018;25:1182–1191. doi: 10.1080/10717544.2018.1472677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao J., Naeem M., Noh J.K., Lee E.H., Yoo J.W. Dexamethasone phosphate-loaded folate-conjugated polymeric nanoparticles for selective delivery to activated macrophages and suppression of inflammatory responses. Macromol Res. 2015;23:485–492. [Google Scholar]
- 62.Shi Q., Rondon-Cavanzo E.P., Dalla Picola I.P., Tiera M.J., Zhang X., Dai K. In vivo therapeutic efficacy of TNFα silencing by folate-PEG-chitosan-DEAE/siRNA nanoparticles in arthritic mice. Int J Nanomed. 2018;13:387–402. doi: 10.2147/IJN.S146942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sultana F., Neog M.K., Rasool M. Withaferin-A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-induced arthritic rats. Colloids Surf B Biointerfaces. 2017;155:349–365. doi: 10.1016/j.colsurfb.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 64.Sultana F., Neog M.K., Rasool M. Targeted delivery of morin, a dietary bioflavanol encapsulated mannosylated liposomes to the macrophages of adjuvant-induced arthritis rats inhibits inflammatory immune response and osteoclastogenesis. Eur J Pharm Biopharm. 2017;115:229–242. doi: 10.1016/j.ejpb.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Hu L., Luo X., Zhou S., Zhu J., Xiao M., Li C. Neutrophil-mediated delivery of dexamethasone palmitate-loaded liposomes decorated with a sialic acid conjugate for rheumatoid arthritis treatment. Pharm Res (N Y) 2019;36:97. doi: 10.1007/s11095-019-2609-4. [DOI] [PubMed] [Google Scholar]
- 66.Li P., Yang X., Yang Y., He H., Chou C.K., Chen F. Synergistic effect of all-trans-retinal and triptolide encapsulated in an inflammation-targeted nanoparticle on collagen-induced arthritis in mice. J Control Release. 2020;319:87–103. doi: 10.1016/j.jconrel.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 67.Kim S.H., Kim J.H., You D.G., Saravanakumar G., Yoon H.Y., Choi K.Y. Self-assembled dextran sulphate nanoparticles for targeting rheumatoid arthritis. Chem Commun (Camb) 2013;49:10349–10351. doi: 10.1039/c3cc44260h. [DOI] [PubMed] [Google Scholar]
- 68.Shin J.M., Kim S.H., Thambi T., You D.G., Jeon J., Lee J.O. A hyaluronic acid-methotrexate conjugate for targeted therapy of rheumatoid arthritis. Chem Commun (Camb) 2014;50:7632–7635. doi: 10.1039/c4cc02595d. [DOI] [PubMed] [Google Scholar]
- 69.Gouveia V.M., Lopes-de-Araújo J., Lima S.A.C., Nunes C., Reis S. Hyaluronic acid-conjugated pH-sensitive liposomes for targeted delivery of prednisolone on rheumatoid arthritis therapy. Nanomedicine. 2018;13:1037–1049. doi: 10.2217/nnm-2017-0377. [DOI] [PubMed] [Google Scholar]
- 70.Yu C., Li X., Hou Y., Meng X., Wang D., Liu J. Hyaluronic acid coated acid-sensitive nanoparticles for targeted therapy of adjuvant-induced arthritis in rats. Molecules. 2019;24:146. doi: 10.3390/molecules24010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saadat E., Shakor N., Gholami M., Dorkoosh F.A. Hyaluronic acid based micelle for articular delivery of triamcinolone, preparation, in vitro and in vivo evaluation. Int J Pharm. 2015;489:218–225. doi: 10.1016/j.ijpharm.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Moura C.C., Segundo M.A., Neves J., Reis S., Sarmento B. Co-association of methotrexate and SPIONs into anti-CD64 antibody-conjugated PLGA nanoparticles for theranostic application. Int J Nanomed. 2014;9:4911–4922. doi: 10.2147/IJN.S68440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thao le Q., Byeon H.J., Lee C., Lee S., Lee E.S., Choi H.G. Pharmaceutical potential of tacrolimus-loaded albumin nanoparticles having targetability to rheumatoid arthritis tissues. Int J Pharm. 2016;497:268–276. doi: 10.1016/j.ijpharm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Graversen J.H., Svendsen P., Dagnæs-Hansen F., Dal J., Anton G., Etzerodt A. Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol Ther. 2012;20:1550–1558. doi: 10.1038/mt.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee S.M., Kim H.J., Ha Y.J., Park Y.N., Lee S.K., Park Y.B. Targeted chemo-photothermal treatments of rheumatoid arthritis using gold half-shell multifunctional nanoparticles. ACS Nano. 2013;7:50–57. doi: 10.1021/nn301215q. [DOI] [PubMed] [Google Scholar]
- 76.Zhou H.F., Chan H.W., Wickline S.A., Lanza G.M., Pham C.T. Alphavbeta3-targeted nanotherapy suppresses inflammatory arthritis in mice. Faseb J. 2009;23:2978–2985. doi: 10.1096/fj.09-129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Liu Z., Li T., Chen L., Lyu J., Li C. Enhanced therapeutic effect of RGD-modified polymeric micelles loaded with low-dose methotrexate and nimesulide on rheumatoid arthritis. Theranostics. 2019;9:708–720. doi: 10.7150/thno.30418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koo O.M., Rubinstein I., Onyüksel H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm Res (N Y) 2011;28:776–787. doi: 10.1007/s11095-010-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jain S., Tran T.H., Amiji M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials. 2015;61:162–177. doi: 10.1016/j.biomaterials.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katsumata K., Ishihara J., Mansurov A., Ishihara A., Raczy M.M., Yuba E. Targeting inflammatory sites through collagen affinity enhances the therapeutic efficacy of anti-inflammatory antibodies. Sci Adv. 2019;5 doi: 10.1126/sciadv.aay1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He Y., Li R., Liang J., Zhu Y., Zhang S., Zheng Z. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Research. 2018;11:6086–6101. [Google Scholar]
- 82.Zhang Q., Dehaini D., Zhang Y., Zhou J., Chen X., Zhang L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13:1182. doi: 10.1038/s41565-018-0254-4. [DOI] [PubMed] [Google Scholar]
- 83.Li R., He Y., Zhu Y., Jiang L., Zhang S., Qin J. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19:124–134. doi: 10.1021/acs.nanolett.8b03439. [DOI] [PubMed] [Google Scholar]
- 84.Shi Y., Xie F., Rao P., Qian H., Chen R., Chen H. TRAIL-expressing cell membrane nanovesicles as an anti-inflammatory platform for rheumatoid arthritis therapy. J Control Release. 2020;320:304–313. doi: 10.1016/j.jconrel.2020.01.054. [DOI] [PubMed] [Google Scholar]
- 85.Chen W.-T., Mahmood U., Weissleder R., Tung C.-H. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res Ther. 2005;7:R310. doi: 10.1186/ar1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu J., He J., Cao D., Zhang M., Ni P. Core cross-linked polyphosphoester micelles with folate-targeted and acid-cleavable features for pH-triggered drug delivery. Polym Chem. 2015;6:3205–3216. [Google Scholar]
- 87.Yang M., Ding J., Zhang Y., Chang F., Wang J., Gao Z. Activated macrophage-targeted dextran–methotrexate/folate conjugate prevents deterioration of collagen-induced arthritis in mice. J Mater Chem B. 2016;4:2102–2113. doi: 10.1039/c5tb02479j. [DOI] [PubMed] [Google Scholar]
- 88.Sun X., Dong S., Li X., Yu K., Sun F., Lee R.J. Delivery of siRNA using folate receptor-targeted pH-sensitive polymeric nanoparticles for rheumatoid arthritis therapy. Nanomed Nanotechnol Biol Med. 2019;20:102017. doi: 10.1016/j.nano.2019.102017. [DOI] [PubMed] [Google Scholar]
- 89.Hu J.B., Liu D., Qi J., Lu K.J., Jin F.Y., Ying X.Y. An E-selectin targeting and MMP-2-responsive dextran-curcumin polymeric prodrug for targeted therapy of acute kidney injury. Biomater Sci. 2018;6:3397–3409. doi: 10.1039/c8bm00813b. [DOI] [PubMed] [Google Scholar]
- 90.Pei Y., Yeo Y. Drug delivery to macrophages: challenges and opportunities. J Control Release. 2016;240:202–211. doi: 10.1016/j.jconrel.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 91.Jin K., Luo Z., Zhang B., Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8:23–33. doi: 10.1016/j.apsb.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sung S., Alam M.M., Han H.S., Kang J.H., Hasan F.A., Jung M.J. FRI0362mineralized, pegylated hyaluronic acid nanoparticles for pH-responsive delivery of methotrexate in inflammatory arthritis. Ann Rheum Dis. 2014;73:518. [Google Scholar]
- 93.Trujillo-Nolasco R.M., Morales-Avila E., Ocampo-García B.E., Ferro-Flores G., Gibbens-Bandala B.V., Escudero-Castellanos A. Preparation and in vitro evaluation of radiolabeled HA-PLGA nanoparticles as novel MTX delivery system for local treatment of rheumatoid arthritis. Mater Sci Eng C. 2019;103:109766. doi: 10.1016/j.msec.2019.109766. [DOI] [PubMed] [Google Scholar]
- 94.Alam M.M., Han H.S., Sung S., Kang J.H., Sa K.H., Al Faruque H. Endogenous inspired biomineral-installed hyaluronan nanoparticles as pH-responsive carrier of methotrexate for rheumatoid arthritis. J Control Release. 2017;252:62–72. doi: 10.1016/j.jconrel.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 95.Heo R., You D.G., Um W., Choi K.Y., Jeon S., Park J.S. Dextran sulfate nanoparticles as a theranostic nanomedicine for rheumatoid arthritis. Biomaterials. 2017;131:15–26. doi: 10.1016/j.biomaterials.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 96.Koning G.A., Schiffelers R.M., Wauben M.H.M., Kok R.J., Mastrobattista E., Molema G. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54:1198–1208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- 97.Nam E.J., Kang J.H., Sa K.H., Sung S., Park J.Y., Jo D.G. Robust therapeutic efficacy of matrix metalloproteinase-2-cleavable Fas-1-RGD peptide complex in chronic inflammatory arthritis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang Y.H., Rajaiah R., Ruoslahti E., Moudgil K.D. Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis. Proc Natl Acad Sci U S A. 2011;108:12857–12862. doi: 10.1073/pnas.1103569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cook Sangar M.L., Girard E.J., Hopping G., Yin C., Pakiam F., Brusniak M.Y. A potent peptide-steroid conjugate accumulates in cartilage and reverses arthritis without evidence of systemic corticosteroid exposure. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aay1041. [DOI] [PubMed] [Google Scholar]
- 100.Ferrari M., Onuoha S.C., Pitzalis C. Trojan horses and guided missiles: targeted therapies in the war on arthritis. Nat Rev Rheumatol. 2015;11:328. doi: 10.1038/nrrheum.2015.17. [DOI] [PubMed] [Google Scholar]
- 101.Boot E.P., Koning G.A., Storm G., Wagenaar-Hilbers J.P., van Eden W., Everse L.A. CD134 as target for specific drug delivery to auto-aggressive CD4+ T cells in adjuvant arthritis. Arthritis Res Ther. 2005;7:R604–R615. doi: 10.1186/ar1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan H., Shao D., Lao Y.H., Li M., Hu H., Leong K.W. Engineering cell membrane-based nanotherapeutics to target inflammation. Adv Sci. 2019;6:1900605. doi: 10.1002/advs.201900605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malda J., Boere J., van de Lest C.H.A., van Weeren P.R., Wauben M.H.M. Extracellular vesicles—new tool for joint repair and regeneration. Nat Rev Rheumatol. 2016;12:243–249. doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu X.L., Lu K.J., Yao X.Q., Ying X.Y., Du Y.Z. Stimuli-responsive drug delivery systems as an emerging platform for treatment of rheumatoid arthritis. Curr Pharmaceut Des. 2019;25:155–165. doi: 10.2174/1381612825666190321104424. [DOI] [PubMed] [Google Scholar]
- 105.Joshi N., Yan J., Levy S., Bhagchandani S., Slaughter K.V., Sherman N.E. Towards an arthritis flare-responsive drug delivery system. Nat Commun. 2018;9:1–11. doi: 10.1038/s41467-018-03691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hitchon C.A., El-Gabalawy H.S. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuan F., Quan L.D., Cui L., Goldring S.R., Wang D. Development of macromolecular prodrug for rheumatoid arthritis. Adv Drug Deliv Rev. 2012;64:1205–1219. doi: 10.1016/j.addr.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu Y., Aimetti A.A., Langer R., Gu Z. Bioresponsive materials. Nat Rev Mater. 2016;2:16075. [Google Scholar]
- 109.Burrage P., Mix K., Brinckerhoff C. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 110.Murphy G., Knäuper V., Atkinson S., Butler G., English W., Hutton M. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002;4(Suppl 3):S39–S49. doi: 10.1186/ar572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pruzanski W., Vadas P., Stefanski E., Urowitz M. Phospholipase A2 activity in sera and synovial fluids in rheumatoid arthritis and osteoarthritis. Its possible role as a proinflammatory enzyme. J Rheumatol. 1985;12:211–216. [PubMed] [Google Scholar]
- 112.Mirshafiey A., Mohsenzadegan M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy, Asthma Immunol. 2008;7:195–202. [PubMed] [Google Scholar]
- 113.Seetharaman G., Kallar A.R., Vijayan V.M., Muthu J., Selvam S. Design, preparation and characterization of pH-responsive prodrug micelles with hydrolyzable anhydride linkages for controlled drug delivery. J Colloid Interface Sci. 2017;492:61–72. doi: 10.1016/j.jcis.2016.12.070. [DOI] [PubMed] [Google Scholar]
- 114.Xu Y., Mu J., Xu Z., Zhong H., Chen Z., Ni Q. Modular acid-activatable acetone-based ketal-linked nanomedicine by dexamethasone prodrugs for enhanced anti-rheumatoid arthritis with low side effects. Nano Lett. 2020;20:2558–2568. doi: 10.1021/acs.nanolett.9b05340. [DOI] [PubMed] [Google Scholar]
- 115.He L., Fan D., Liang W., Wang Q., Fang J. Matrix metalloproteinase-responsive pegylated lipid nanoparticles for controlled drug delivery in the treatment of rheumatoid arthritis. ACS Appl Bio Mater. 2020;3:3276–3284. doi: 10.1021/acsabm.0c00242. [DOI] [PubMed] [Google Scholar]
- 116.Nam E.J., Kang J.H., Sung S., Sa K.H., Kim K.H., Seo J.S. A matrix metalloproteinase 1-cleavable composite peptide derived from transforming growth factor β-inducible gene h3 potently inhibits collagen-induced arthritis. Arthritis Rheum. 2013;65:1753–1763. doi: 10.1002/art.37932. [DOI] [PubMed] [Google Scholar]
- 117.Kim J., Kim H.Y., Song S.Y., Go S.H., Sohn H.S., Baik S. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano. 2019;13:3206–3217. doi: 10.1021/acsnano.8b08785. [DOI] [PubMed] [Google Scholar]
- 118.Ma B., Xu H., Zhuang W., Wang Y., Li G., Wang Y. Reactive oxygen species responsive theranostic nanoplatform for two-photon aggregation-induced emission imaging and therapy of acute and chronic inflammation. ACS Nano. 2020;14:5862–5873. doi: 10.1021/acsnano.0c01012. [DOI] [PubMed] [Google Scholar]
- 119.Fan X.X., Xu M.Z., Leung E.L.H., Jun C., Yuan Z., Liu L. ROS-responsive berberine polymeric micelles effectively suppressed the inflammation of rheumatoid arthritis by targeting mitochondria. Nano-Micro Lett. 2020;12:76. doi: 10.1007/s40820-020-0410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni R., Song G., Fu X., Song R., Li L., Pu W. Reactive oxygen species-responsive dexamethasone-loaded nanoparticles for targeted treatment of rheumatoid arthritis via suppressing the iRhom2/TNF-α/BAFF signaling pathway. Biomaterials. 2020;232:119730. doi: 10.1016/j.biomaterials.2019.119730. [DOI] [PubMed] [Google Scholar]
- 121.Arias J.L., López-Viota M., López-Viota J., Delgado A.V. Development of iron/ethylcellulose (core/shell) nanoparticles loaded with diclofenac sodium for arthritis treatment. Int J Pharm. 2009;382:270–276. doi: 10.1016/j.ijpharm.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 122.Prabu C., Latha S., Selvamani P., Ahrentorp F., Johansson C., Takeda R. Layer-by-layer assembled magnetic prednisolone microcapsules (MPC) for controlled and targeted drug release at rheumatoid arthritic joints. J Magn Magn Mater. 2017;427:258–267. [Google Scholar]
- 123.Liang P., Zheng J., Dai S., Wang J., Zhang Z., Kang T. pH triggered re-assembly of nanosphere to nanofiber: the role of peptide conformational change for enhanced cancer therapy. J Control Release. 2017;260:22–31. doi: 10.1016/j.jconrel.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 124.Varga G., Gattorno M., Foell D., Rubartelli A. Redox distress and genetic defects conspire in systemic autoinflammatory diseases. Nat Rev Rheumatol. 2015;11:670–680. doi: 10.1038/nrrheum.2015.105. [DOI] [PubMed] [Google Scholar]
- 125.Lee S., Stubelius A., Hamelmann N., Tran V., Almutairi A. Inflammation-responsive drug-conjugated dextran nanoparticles enhance anti-inflammatory drug efficacy. ACS Appl Mater Interfaces. 2018;10:40378–40387. doi: 10.1021/acsami.8b08254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen M., Amerigos J.C.K., Su Z., Guissi N.E.I., Xiao Y., Zong L. Folate receptor-targeting and reactive oxygen species-responsive liposomal formulation of methotrexate for treatment of rheumatoid arthritis. Pharmaceutics. 2019;11:582. doi: 10.3390/pharmaceutics11110582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li J., Ge Z., Liu S. PEG-sheddable polyplex micelles as smart gene carriers based on MMP-cleavable peptide-linked block copolymers. Chem Commun. 2013;49:6974–6976. doi: 10.1039/c3cc43576h. [DOI] [PubMed] [Google Scholar]
- 128.Mellal D., Zumbuehl A. Exit-strategies—smart ways to release phospholipid vesicle cargo. J Mater Chem B. 2014;2:247–252. doi: 10.1039/c3tb21086c. [DOI] [PubMed] [Google Scholar]
- 129.Wang Q., He L., Fan D., Liang W., Wang X., Fang J. PLA2-triggered release of drugs from self-assembled lipid tubules for arthritis treatments. ACS Appl Bio Mater. 2020;3:6488–6496. doi: 10.1021/acsabm.0c00883. [DOI] [PubMed] [Google Scholar]
- 130.Costa Lima S.A., Reis S. Temperature-responsive polymeric nanospheres containing methotrexate and gold nanoparticles: a multi-drug system for theranostic in rheumatoid arthritis. Colloids Surf B Biointerfaces. 2015;133:378–387. doi: 10.1016/j.colsurfb.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 131.Jung Y.S., Park W., Na K. Temperature-modulated noncovalent interaction controllable complex for the long-term delivery of etanercept to treat rheumatoid arthritis. J Control Release. 2013;171:143–151. doi: 10.1016/j.jconrel.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 132.Zhu B., Wang L., Huang J., Xiang X., Tang Y., Cheng C. Ultrasound-triggered perfluorocarbon-derived nanobombs for targeted therapies of rheumatoid arthritis. J Mater Chem B. 2019;7:4581–4591. [Google Scholar]
- 133.Mosanya C.H., Isaacs J.D. Tolerising cellular therapies: what is their promise for autoimmune disease?. Ann Rheum Dis. 2019;78:297–310. doi: 10.1136/annrheumdis-2018-214024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Colmegna I., Ohata B.R., Menard H.A. Current understanding of rheumatoid arthritis therapy. Clin Pharmacol Ther. 2012;91:607–620. doi: 10.1038/clpt.2011.325. [DOI] [PubMed] [Google Scholar]
- 135.Tengvall S., Eneljung T., Jirholt P., Turesson O., Wing K., Holmdahl R. Gene therapy induces antigen-specific tolerance in experimental collagen-induced arthritis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gertel S., Serre G., Shoenfeld Y., Amital H. Immune tolerance induction with multiepitope peptide derived from citrullinated autoantigens attenuates arthritis manifestations in adjuvant arthritis rats. J Immunol. 2015;194:5674–5680. doi: 10.4049/jimmunol.1402457. [DOI] [PubMed] [Google Scholar]
- 137.Clemente-Casares X., Blanco J., Ambalavanan P., Yamanouchi J., Singha S., Fandos C. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 138.Cho J.H., Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat Med. 2015;21:730–738. doi: 10.1038/nm.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perretti M., Cooper D., Dalli J., Norling L.V. Immune resolution mechanisms in inflammatory arthritis. Nat Rev Rheumatol. 2017;13:87. doi: 10.1038/nrrheum.2016.193. [DOI] [PubMed] [Google Scholar]
- 140.Gilroy D.W., Lawrence T., Perretti M., Rossi A.G. Inflammatory Resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 141.Fullerton J.N., Gilroy D.W. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 142.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Headland S.E., Jones H.R., Norling L.V., Kim A., Souza P.R., Corsiero E. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7:315ra190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wright H.L., Moots R.J., Edwards S.W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:593–601. doi: 10.1038/nrrheum.2014.80. [DOI] [PubMed] [Google Scholar]
- 145.Perretti M., D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 146.Kamaly N., Fredman G., Subramanian M., Gadde S., Pesic A., Cheung L. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci Unit States Am. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fredman G., Kamaly N., Spolitu S., Milton J., Ghorpade D., Chiasson R. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa1065. 275ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fearon U., Hanlon M.M., Wade S.M., Fletcher J.M. Altered metabolic pathways regulate synovial inflammation in rheumatoid arthritis. Clin Exp Immunol. 2019;197:170–180. doi: 10.1111/cei.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rhoads J.P., Major A.S., Rathmell J.C. Fine tuning of immunometabolism for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13:313–320. doi: 10.1038/nrrheum.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pålsson-McDermott E.M., O'Neill L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020;30:300–314. doi: 10.1038/s41422-020-0291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patel C.H., Leone R.D., Horton M.R., Powell J.D. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat Rev Drug Discov. 2019;18:669–688. doi: 10.1038/s41573-019-0032-5. [DOI] [PubMed] [Google Scholar]
- 152.Weyand C.M., Goronzy J.J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:291. doi: 10.1038/nrrheum.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pierdominici M., Vacirca D., Delunardo F., Ortona E. mTOR signaling and metabolic regulation of T cells: new potential therapeutic targets in autoimmune diseases. Curr Pharmaceut Des. 2011;17:3888–3897. doi: 10.2174/138161211798357809. [DOI] [PubMed] [Google Scholar]
- 154.Son H.J., Lee J., Lee S.Y., Kim E.K., Park M.J., Kim K.W. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediat Inflamm. 2014;2014:973986. doi: 10.1155/2014/973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Abboud G., Choi S.C., Kanda N., Zeumer-Spataro L., Roopenian D.C., Morel L. Inhibition of glycolysis reduces disease severity in an autoimmune model of rheumatoid arthritis. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu Y., Li L., Lin Z., Wang L., Lin L., Li M. A new treatment modality for rheumatoid arthritis: combined photothermal and photodynamic therapy using Cu7.2S4 nanoparticles. Adv Healthc Mater. 2018;7:1800013. doi: 10.1002/adhm.201800013. [DOI] [PubMed] [Google Scholar]
- 157.Melamed J.R., Edelstein R.S., Day E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano. 2015;9:6–11. doi: 10.1021/acsnano.5b00021. [DOI] [PubMed] [Google Scholar]
- 158.Yeo J., Lee Y.M., Lee J., Park D., Kim K., Kim J. Nitric oxide-scavenging nanogel for treating rheumatoid arthritis. Nano Lett. 2019;19:6716–6724. doi: 10.1021/acs.nanolett.9b00496. [DOI] [PubMed] [Google Scholar]
- 159.Liu Y., Ma L., Zhou H., Zhu X., Yu Q., Chen X. Polypeptide nano-Se targeting inflammation and theranostic rheumatoid arthritis by anti-angiogenic and NO activating AMPKα signaling pathway. J Mater Chem B. 2018;6:3497–3514. doi: 10.1039/c8tb00080h. [DOI] [PubMed] [Google Scholar]
- 160.Liang H., Peng B., Dong C., Liu L., Mao J., Wei S. Cationic nanoparticle as an inhibitor of cell-free DNA-induced inflammation. Nat Commun. 2018;9:4291. doi: 10.1038/s41467-018-06603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu J., Liang H., Li Y., Shi Y., Bottini M., Chen Y. Cationic block copolymer nanoparticles with tunable dna affinity for treating rheumatoid arthritis. Adv Funct Mater. 2020;30:2000391. [Google Scholar]