Abstract
Two series of functionalized imidazothiazolotriazine derivatives were synthesized via the condensation of imidazo[4,5-e]-1,2,4-triazine-3-thiones with acetylenedicarboxylic acid dimethyl and diethyl esters (DMAD and DEAD) and subsequent base-catalyzed rearrangement of the obtained imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines into regioisomeric imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine derivatives.
Keywords: amidine rearrangement; cyclocondensation; heterocycles; thiazolidine-4-one; 1,2,4-triazine
Introduction
The thiazolidin-4-one heterocyclic system is a well-known, accessible and, as a consequence, a widely used pharmacophore in the chemistry of biologically active compounds possessing antimicrobial [1], antituberculosis [2], anti-inflammatory [3–4], anticancer [5], antidiabetic [6–7], and antiviral activities [8].
A significant number of biologically active thiazolidines amount to their heteroannelated derivatives, namely, condensed thiazolo[3,2-a]pyrimidines [9] and thiazolo[3,2-b]-1,2,4-triazoles [10], as well as related thiazolo[3,2-b]-1,2,4-triazines and thiazolo[2,3-c]-1,2,4-triazines possessing antimicrobial, antidepressant, anti-HIV, and anticancer activities [10–15]. Modifications of the position 5 of the thiazolidine cycle often lead to an enhancement of the pharmacological properties of the resulting products, which have received considerable attention in reviews [16–17]. The structures of some of the thiazolidine derivatives and their biological properties are specified in Figure 1.
Figure 1.
Biologically active compounds having thiazolidin-4-one and thiazolo-1,2,4-triazine units.
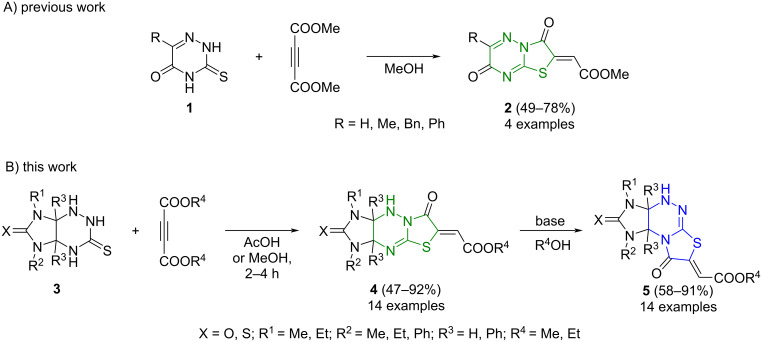
One of the effective approaches to the preparation of heteroannelated thiazolidin-4-one derivatives consists in the condensation of acetylenedicarboxylic acid esters with heterocyclic compounds containing a thiourea fragment, e.g., pyrimidinethiones [18–19], 1,2,4-triazolethiols [20], and 1,2,4-triazinethiones [21–22]. An important feature of the reactions of dialkyl acetylenedicarboxylates with asymmetric substrates is the high regioselectivity of the cyclizations of intermediate Michael adducts at one of several reactive nitrogen atoms. For example, reported by Giannola et al. [21], the reaction of 3-thioxo-1,2,4-triazin-5-ones 1 with dimethyl acetylenedicarboxylate (DMAD) leads to the only products, namely, thiazolo[3,2-b]-1,2,4-triazines 2 (Scheme 1) while the regioisomeric thiazolo[2,3-с]-1,2,4-triazine derivatives remain unavailable.
Scheme 1.
Regioselectivity of the cyclization of 3-thioxo-1,2,4-triazin-5-ones 1 with DMAD (A) and general concept of this work (B).
The present work is devoted to the development of methods for the regiodirected synthesis of two series of functionalized imidazothiazolotriazines based on the sequential condensation of imidazo[4,5-e]-1,2,4-triazine-3-thiones 3 with DMAD or DEAD and skeletal rearrangement of linear imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines 4 into isomeric imidazo[4,5-e]thiazolo[2,3-с]-1,2,4-triazines 5 having an angular structure.
Results and Discussion
We started by examining the regioselectivity of the condensation of imidazo[4,5-e]triazine-3-thiones 3 with DEAD. The effects of temperature, the nature of the solvent, and the structure of the starting substrates on the total yields and ratios of isomeric products 4 and 5 were investigated (Table 1, see also Supporting Information File 1 for details).
Table 1.
Results for the screening of the reaction conditionsa.
 | ||||||
| entry | compound 3 | X, R1, R2, R3 | conditions | ratio of 4 and 5 |
total yield of 4 + 5 [%] |
yield of 4 [%]b |
| 1 | 3a | X = O, R1 = R2 = Me, R3 = H | MeOH, reflux, 2 h | 38:62 | 80 | –c |
| 2 | 95% EtOH, reflux, 2 h | 30:70 | 72 | –c | ||
| 3 | Anh. EtOH, reflux, 2 h | 32:68 | 71 | –c | ||
| 4 | AcOH, 50 °C, 2 h | 83:17 | 72 | –c | ||
| 5 | AcOH, 40 °C, 2 h | 84:16 | 79 | 45 | ||
| 6 | AcOH, 20 °C, 2 h | 91:9 | 80 | 53 | ||
| 7 | 3b | X = O, R1 = R2 = Et, R3 = H | MeOH, reflux, 2 h | 54:46 | 68 | –c |
| 8 | AcOH, 20 °C, 2 h | 66:34 | 71 | 47 | ||
| 9 | 3c | X = O, R1 = R2 = Me, R3 = Ph | MeOH, reflux, 2 h | 97:3 | 81 | 79 |
| 10 | 95% EtOH, reflux, 2 h | 100:0 | 68 | 68 | ||
| 11 | 3d | X = O, R1 = Me, R2 = Ph, R3 = H | MeOH, reflux, 2 h | 77:23 | 48 | –c |
| 12 | AcOH, 20 °C, 2 h | 100:0 | 64 | 64d | ||
| 13 | AcOH, 20 °C, 4 h | 100:0 | 86 | 86 | ||
| 14 | 3f | X = S, R1 = Me, R2 = Ph, R3 = H | MeOH, reflux, 4 h | 100:0 | 52 | 52 |
| 15 | AcOH, 20 °C, 4 h | – | 0 | 0 | ||
aReaction conditions: stirring the mixture of imidazo[4,5-e][1,2,4]triazin-3-thione 3 (2.0 mmol) and DEAD (2.1 mmol) in solvent (4 mL). bIsolated yield. cPure product 4 was not isolated. dProduct 4k was isolated as a mixture with 3d.
The reaction of 5,7-dimethylimidazo[4,5-e]triazine-3-thione (3a) with DEAD proceeded in alcohols with moderate regioselectivity and isomer 5 predominated in the filtered precipitates (Table 1, entries 1–3). The use of absolute or 95% ethanol as a solvent did not significantly affect the total yields of isomers 4 and 5 and their ratio, while carrying out the reaction in acetic acid, as expected [13,23–24], led to a change in regioselectivity and the formation of linear isomer 4 as the main product (Table 1, entries 4–6).
5,7-Diethylimidazo[4,5-e]triazine-3-thione (3b) reacted with DEAD in a similar manner (Table 1, entries 7 and 8), but with less selectivity. Substrates 3 bearing phenyl substituents at the different positions, vice versa, reacted with DEAD with high selectivity to form imidazo[4,5-e]thiazolo[3,2-b]triazines 4 as main products (Table 1, entries 9–14). The corresponding isomeric imidazo[4,5-e]thiazolo[2,3-c]triazines 5 were formed in trace amounts and were detected only in the 1H NMR spectra of the evaporated reaction mixtures. The reactions of bicyclic structures 3a–g with dimethyl acetylenedicarboxylate proceeded with similar selectivity. However, the total yields of the corresponding methyl esters were often higher.
The optimized conditions found for each group of starting substrates 3a–g were applied to prepare a series of imidazo[4,5-e]thiazolo[3,2-b]triazines 4a–n with a linear structure (Scheme 2). Precipitates of compounds 4c–g,j–n bearing phenyl substituents contained no impurities of the corresponding isomers 5, while the structures 4a,b,h,i were isolated in individual form only during fractional crystallization from the reaction mixtures.
Scheme 2.
Synthesis of imidazo[4,5-e]thiazolo[3,2-b]triazine derivatives 4a–n by the reaction of imidazo[4,5-e]triazines 3a–g and DMAD or DEAD.
It was previously shown [24–26] that the products of aldol condensation of imidazo[4,5-e]thiazolo[3,2-b]triazines with carbonyl compounds, namely, aromatic aldehydes and isatins, are capable of skeletal rearrangement of the thiazolotriazine system proceeding in methanol upon treatment with KOH and resulting in the corresponding isomeric imidazo[4,5-e]thiazolo[2,3-c]triazine derivatives. In this regard, the possibility of prepearing esters 5 with an angular structure on the basis of directed isomerization of linear imidazo[4,5-e]thiazolo[3,2-b]triazines 4a–n in basic media has been studied.
Indeed, boiling ethyl ester 4h in methanol in the presence of 0.5 equiv of a 40% KOH aqueous solution resulted in a skeletal rearrangement of the tricyclic system, which, however, was accompanied by reesterification with methanol and partial hydrolysis of the ester group. As a result, the methyl ester 5a was obtained in 66% yield (Scheme 3).
Scheme 3.
Reaction of imidazo[4,5-e]thiazolo[3,2-b]triazine 4h with aqueous KOH.
Rearrangement of 1,3-dimethyl- and 1,3-diethylimidazo[4,5-e]thiazolo[3,2-b]triazines 4a,b,h,i upon treatment with an equivalent amount of triethylamine in corresponding alcohols proceeded without hydrolysis of ester groups and led to the formation of the corresponding regioisomeric derivatives 5a,b,h,i (Scheme 4). The isomerization of imidazo[4,5-e]thiazolo[3,2-b]triazines 4c–g,j–n bearing phenyl groups occurred only upon refluxing the starting compounds in corresponding alcohols in the presence of sodium alcoholates. Imidazo[4,5-e]thiazolo[2,3-c]triazines 5a–n were synthesized in yields of 58–91%.
Scheme 4.
Rearrangement of imidazo[4,5-e]thiazolo[3,2-b]triazines 4a–n into imidazo[4,5-e]thiazolo[2,3-c]triazines 5a–n.
Because the reaction of imidazo[4,5-e]triazines 3a,b with DMAD or DEAD led always to the formation of compounds 4a,b,h,i along with their isomeric structures 5a,b,h,i, an attempt was made to sequentially obtain and convert the resulting mixtures of compounds 4 and 5 into the individual isomers 5 in a one-pot mode (Scheme 5).
Scheme 5.
One-pot synthesis of compounds 5a,b,h,i from imidazo[4,5-e]triazines 3a,b.
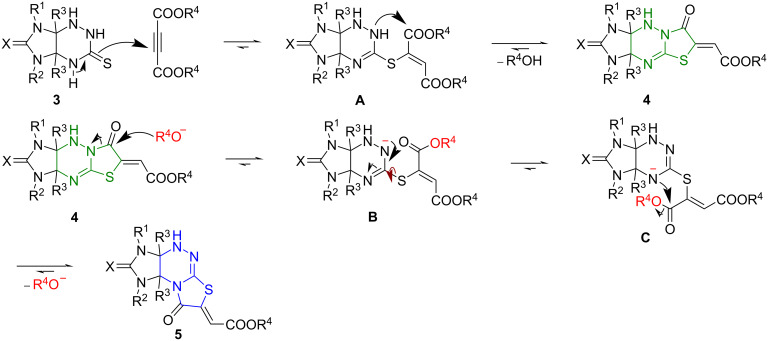
The plausible mechanism of the formation of compounds 4 and 5 is detailed in Scheme 6. The reaction of imidazothiazolotriazines 3 with esters of acetylenedicarboxylic acid affords the Michael addition product A, which undergoes cyclization involving the nitrogen atom N(2) to give compound 4. Rearrangement of the latter is, probably, a result of a transamidation reaction upon the treatment with a base, for example, alkoxide anion. The nucleophilic attack of the alkoxide anion leads to the cleavage of the C(7)–N(8) bond to form intermediates B and C followed by the recyclization of the thiazolidine ring involving the nitrogen atom N(4) [25] to afford the product 5.
Scheme 6.
Plausible mechanism of the formation and the rearrangement of compounds 4 into isomers 5.
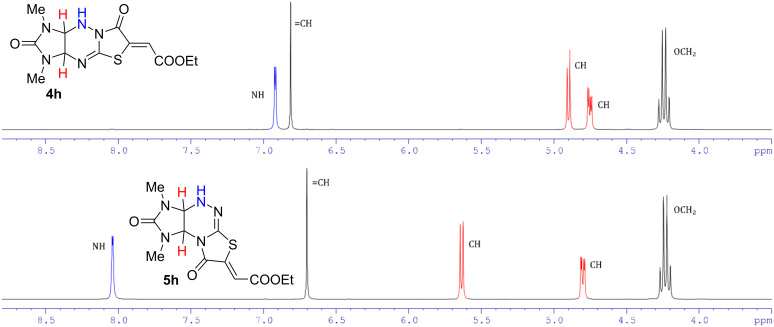
The structures of compounds 4a–n and 5a–n were elucidated by IR, 1H and 13C NMR, and HRMS spectral data. There are downfield shifts of the NH group proton signal from 6.9–7.2 to 8.0–8.4 ppm in the 1H NMR spectra of angular structures 5 in comparison to the spectra of the linear isomers 4. Downfield shifts from 4.9–5.0 for 4a,b,h,i to 5.6–5.7 ppm for 5a,b,h,i and from 5.5–5.7 for 4d–g,k–n to 6.3–6.5 ppm for 5d–g,k–n are also observed for the doublet of one of the bridging protons 3a-H in compounds 4 (9a-H in compounds 5, Figure 2).
Figure 2.
1H NMR spectra of compounds 4h and 5h in DMSO-d6 in the region of 3.5–8.8 ppm.
The structure of compound 5i was additionally confirmed by X-ray diffraction analysis (Figure 3).
Figure 3.
X-ray crystal structure of compound 5i.
Conclusion
Thus, the conditions for the regioselective preparation of each of the regioisomeric functionalized derivatives of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines 4 and imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazines 5 in an individual form were found. The methodology proved to be effective for the synthesis of a wide range of target compounds with various substituents in the tricyclic fragment. Investigations of the antiproliferative activity of the synthesized products 4 and 5, as well as possible ways of their further transformations in basic media, are continuing.
Supporting Information
Experimental and analytical data.
CIF file for compound 5i.
Acknowledgments
Crystal structure determination was performed in the Department of Structural Studies of the N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences.
References
- 1.Pansare D N, Mulla N A, Pawar C D, Shende V R, Shinde D B. Bioorg Med Chem Lett. 2014;24:3569–3573. doi: 10.1016/j.bmcl.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 2.Subhedar D D, Shaikh M H, Arkile M A, Yeware A, Sarkar D, Shingate B B. Bioorg Med Chem Lett. 2016;26:1704–1708. doi: 10.1016/j.bmcl.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 3.Chougala B M, Samundeeswari S, Holiyachi M, Shastri L A, Dodamani S, Jalalpure S, Dixit S R, Joshi S D, Sunagar V A. Eur J Med Chem. 2017;125:101–116. doi: 10.1016/j.ejmech.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Koppireddi S, Komsani J R, Avula S, Pombala S, Vasamsetti S, Kotamraju S, Yadla R. Eur J Med Chem. 2013;66:305–313. doi: 10.1016/j.ejmech.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Rashid M, Husain A, Shaharyar M, Mishra R, Hussain A, Afzal O. Eur J Med Chem. 2014;83:630–645. doi: 10.1016/j.ejmech.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed Iqbal A K, Khan A Y, Kalashetti M B, Belavagi N S, Gong Y-D, Khazi I A M. Eur J Med Chem. 2012;53:308–315. doi: 10.1016/j.ejmech.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Raza S, Srivastava S P, Srivastava D S, Srivastava A K, Haq W, Katti S B. Eur J Med Chem. 2013;63:611–620. doi: 10.1016/j.ejmech.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 8.Barreca M L, Chimirri A, De Luca L, Monforte A-M, Monforte P, Rao A, Zappalà M, Balzarini J, De Clercq E, Pannecouque C, et al. Bioorg Med Chem Lett. 2001;11(13):1793–1796. doi: 10.1016/s0960-894x(01)00304-3. [DOI] [PubMed] [Google Scholar]
- 9.Tozkoparan B, Ertan M, Kelicen P, Demirdamar R. Farmaco. 1999;54:588–593. doi: 10.1016/s0014-827x(99)00068-3. [DOI] [PubMed] [Google Scholar]
- 10.Uzgören-Baran A, Tel B C, Sarıgöl D, Öztürk E İ, Kazkayası İ, Okay G, Ertan M, Tozkoparan B. Eur J Med Chem. 2012;57:398–406. doi: 10.1016/j.ejmech.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Cai D, Li T, Xie Q, Yu X, Xu W, Chen Y, Jin Z, Hu C. Molecules. 2020;25:1307. doi: 10.3390/molecules25061307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Shehry M F, El-Hag F A A, Ewies E F. Russ J Org Chem. 2020;56:129–136. doi: 10.1134/s1070428020010200. [DOI] [Google Scholar]
- 13.Gazieva G A, Izmest’ev A N, Anikina L V, Pukhov S A, Meshchaneva M E, Khakimov D V, Kolotyrkina N G, Kravchenko A N. Mol Diversity. 2018;22:585–599. doi: 10.1007/s11030-018-9813-8. [DOI] [PubMed] [Google Scholar]
- 14.Izmest’ev A N, Gazieva G A, Kulikov A S, Anikina L V, Kolotyrkina N G, Kravchenko A N. Russ J Org Chem. 2017;53:753–763. doi: 10.1134/s1070428017050177. [DOI] [Google Scholar]
- 15.Trepanier D L, Krieger P E, inventors. Thiazolo-as-triazines. US3,641,019. U.S. Patent. 1972 Feb 8;
- 16.Tomašić T, Mašič L P. Curr Med Chem. 2009;16:1596–1629. doi: 10.2174/092986709788186200. [DOI] [PubMed] [Google Scholar]
- 17.Gazieva G A, Izmest’ev A N. Chem Heterocycl Compd. 2015;50(11):1515–1527. doi: 10.1007/s10593-014-1619-8. [DOI] [Google Scholar]
- 18.Ren D, Hu X, Li X. Chem Heterocycl Compd. 2019;55:275–279. doi: 10.1007/s10593-019-02453-1. [DOI] [Google Scholar]
- 19.Georgiev V S, Bennett G A, Radov L A, Kamp D K, Trusso L A. J Heterocycl Chem. 1986;23:1359–1362. doi: 10.1002/jhet.5570230519. [DOI] [Google Scholar]
- 20.Fotouhi L, Hekmatshoar R, Heravi M M, Sadjadi S, Rasmi V. Tetrahedron Lett. 2008;49:6628–6630. doi: 10.1016/j.tetlet.2008.09.023. [DOI] [Google Scholar]
- 21.Giannola L I, Giammona G, Palazzo S, Lamartina L. J Chem Soc, Perkin Trans 1. 1984:2707–2710. doi: 10.1039/p19840002707. [DOI] [Google Scholar]
- 22.Heravi M M, Rahimizadeh M, Iravani E, Ghassemzadeh M. Phosphorus, Sulfur Silicon Relat Elem. 2003;178:797–802. doi: 10.1080/10426500307786. [DOI] [Google Scholar]
- 23.Gazieva G A, Poluboyarov P A, Nelyubina Y V, Struchkova M I, Kravchenko A N. Chem Heterocycl Compd. 2012;48:1382–1389. doi: 10.1007/s10593-012-1147-3. [DOI] [Google Scholar]
- 24.Gazieva G A, Izmest'ev A N, Nelyubina Y V, Kolotyrkina N G, Zanin I E, Kravchenko A N. RSC Adv. 2015;5(55):43990–44002. doi: 10.1039/c5ra07669b. [DOI] [Google Scholar]
- 25.Izmest’ev A N, Vasileva D A, Melnikova E K, Kolotyrkina N G, Borisova I A, Kravchenko A N, Gazieva G A. New J Chem. 2019;43:1038–1052. doi: 10.1039/c8nj05058a. [DOI] [Google Scholar]
- 26.Izmest'ev A N, Kim N A, Karnoukhova V A, Kolotyrkina N G, Kravchenko A N, Gazieva G A. ChemistrySelect. 2019;4(35):10483–10487. doi: 10.1002/slct.201902461. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental and analytical data.
CIF file for compound 5i.