Abstract
Background
Idiopathic hypersomnia is a disorder of excessive daytime sleepiness, often accompanied by long sleep times or pronounced difficulty in awakening, in the absence of a known cause. The optimal treatment strategy for idiopathic hypersomnia is currently unknown.
Objectives
To assess the effects of medications for daytime sleepiness and related symptoms in individuals with idiopathic hypersomnia and, in particular, whether medications may: 1. reduce subjective measures of sleepiness; 2. reduce objective measures of sleepiness; 3. reduce symptoms of cognitive dysfunction; 4. improve quality of life; and 5. be associated with adverse events.
Search methods
We searched the following databases on 4 February 2021: Cochrane Register of Studies (CRS Web), MEDLINE (Ovid, 1946 to 1 February 2021), and reference lists of articles. CRS Web includes randomized or quasi‐randomized controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialized registers of Cochrane Review Groups, including the Cochrane Epilepsy Group. We previously searched the WHO ICTRP separately when loading of ICTRP records into CRS Web was temporarily suspended.
Selection criteria
Randomized studies comparing any medication to placebo, another medication, or a behavioral intervention.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality. We contacted study authors for additional data. We collected data on adverse events from the included trials.
Main results
We included three trials, with a total of 112 participants. Risk of bias was low for the included studies. Two pharmaceutical company‐sponsored trials compared modafinil with placebo, involving 102 participants, nearly all of whom had idiopathic hypersomnia without long sleep time. Modafinil significantly improved self‐reported sleepiness on the Epworth Sleepiness Scale by 5.08 points more than placebo (95% confidence interval (CI) 3.01 to 7.16; 2 studies, 101 participants; high‐certainty evidence). Modafinil also significantly improved disease severity on the Clinical Global Impression of Severity scale by 1.02 points (95% CI 0.11 to 1.93; 1 study, 30 participants; moderate‐certainty evidence) and resulted in a greater proportion of participants who were "much improved" or "very much improved" on the Clinical Global Impression of Change (odds ratio (OR) for improvement 5.14, 95% CI 1.76 to 15.00; 1 study, 70 participants; moderate‐certainty evidence). Ability to remain awake on the Maintenance of Wakefulness Test was significantly improved with modafinil, by 4.74 minutes more than with placebo (95% CI 2.46 to 7.01; 2 studies, 99 participants; high‐certainty evidence). Ratings of exhaustion and effectiveness/performance were improved with modafinil compared to placebo in one study. Number of naps per week was no different between modafinil and placebo across two studies. Participants receiving modafinil experienced more side effects, although the difference did not reach statistical significance (OR 1.68, 95% CI 0.28 to 9.94; 2 studies, 102 participants; low‐certainty evidence).
One trial studying 20 participants with different disorders of sleepiness included 10 participants with idiopathic hypersomnia, with or without long sleep time, and compared clarithromycin to placebo. We only included the subset of trial data for those participants with idiopathic hypersomnia, per our protocol. There were no significant differences between clarithromycin and placebo for the Epworth Sleepiness Scale, psychomotor vigilance testing, sleep inertia, other subjective ratings, or side effects.
Authors' conclusions
Modafinil is effective for the treatment of several aspects of idiopathic hypersomnia symptomatology, based on studies predominantly including participants with idiopathic hypersomnia without long sleep times, with low risk of bias, and evidence certainty ranging from high to low. There is insufficient evidence to conclude whether clarithromycin is effective for the treatment of idiopathic hypersomnia. There is a clear need for additional studies testing interventions for the treatment of idiopathic hypersomnia.
Plain language summary
Medications for daytime sleepiness in individuals with idiopathic hypersomnia
Review question We reviewed the evidence for the effects of different medications on daytime sleepiness in people with idiopathic hypersomnia.
Background We wanted to know which medications are helpful for treating people with idiopathic hypersomnia, a disease that causes severe daytime sleepiness, and can sometimes cause people to sleep for very long amounts of time and have difficulty waking up and thinking or concentrating. It is called 'idiopathic' hypersomnia because the cause or causes of the disease are currently unknown. We wanted to find out which medications help with daytime sleepiness and other symptoms of the disease. We looked for studies testing any medication for idiopathic hypersomnia compared to a placebo (dummy pill) or another treatment.
Search date The evidence is current to February 2021.
Study characteristics We identified three studies. Two studies tested modafinil, and one study tested clarithromycin.
The two studies testing modafinil included a total of 102 people with idiopathic hypersomnia. Most of these people slept fewer than 10 hours per night. Both studies included people from multiple sleep clinics. One study took place in Germany, and the other in Japan. Both studies compared modafinil to placebo, lasted for three weeks, and were funded by pharmaceutical companies with commercial interest in the results of the studies.
The study testing clarithromycin included a total of 20 participants, from a single sleep clinic in the USA, 10 of whom had idiopathic hypersomnia. We only included information for the 10 people with idiopathic hypersomnia in the review. This study compared clarithromycin to placebo. Although the study lasted five weeks, we only included information from the first two weeks. This study was funded by the American Academy of Sleep Medicine Foundation, a charitable organization.
Key results Modafinil improves sleepiness and the ability to stay awake during testing in a sleep laboratory. It probably improves the overall severity of idiopathic hypersomnia, feelings of exhaustion, and daytime performance. Modafinil may cause headaches and stomach symptoms such as nausea.
It is uncertain whether or not clarithromycin helps with daytime sleepiness or other symptoms of idiopathic hypersomnia. It is uncertain whether there is a difference in side effects between clarithromycin and placebo, based on the information included in this review.
Quality of the evidence We judged the overall quality of the evidence in the three studies as ranging from high to low, depending on the outcome and drug being studied. All three studies were well‐conducted. The studies were small. We found very few studies testing medication treatments for idiopathic hypersomnia. More studies are needed before we can be certain which medications are best for treating people with idiopathic hypersomnia.
Summary of findings
Background
Description of the condition
Idiopathic hypersomnia is a chronic neurologic sleep disorder that manifests as excessive daytime sleepiness occurring despite normal or prolonged sleep times (i.e. exceeding 11 hours of sleep per 24‐hour period), in the absence of other disorders that impair sleep quality, such as sleep apnea. Symptoms and signs of idiopathic hypersomnia also include marked sleep inertia (severe and prolonged difficulties with awakening), long and unrefreshing naps, and high measured sleep efficiency in the sleep laboratory, although some people meeting the diagnostic criteria for idiopathic hypersomnia do not experience these classic ancillary symptoms (Anderson 2007; Bassetti 1997; ICSD‐3 2014). Difficulties with concentration and attention are frequently present in people with idiopathic hypersomnia, and quality of life is reduced (Ozaki 2012; Vernet 2009; Vernet 2010).
The cause of idiopathic hypersomnia remains unknown, although abnormalities of sleep macro‐ and microstructure have been noted (Pizza 2013; Vernet 2009). Idiopathic hypersomnia, especially that without long nocturnal sleep times, shares many clinical similarities with narcolepsy without cataplexy (narcolepsy type 2), and the two disorders may be indistinguishable except for findings on the Multiple Sleep Latency Test (MSLT) (Šonka 2015). At least two major diagnostic classification schema, the International Classification of Sleep Disorders (ICSD) and the Diagnostic and Statistical Manual of Mental Disorders (DSM), have published diagnostic guidelines for this entity, although the diagnostic criteria in the two systems are not identical. The currently preferred terminology is 'idiopathic hypersomnia' in the ICSD, third edition, and 'hypersomnolence disorder' in the DSM‐5. Other previously used terms include idiopathic central nervous system hypersomnolence, non‐rapid eye movement (NREM) narcolepsy, NREM hypersomnia, primary hypersomnia, and hypersomnia with sleep drunkenness. Hypersomnia disorder (DSM‐IV) has a population prevalence of 0.5% in the USA (Ohayon 2013).
Description of the intervention
Multiple medications have been used in clinical practice for the treatment of idiopathic hypersomnia. Many of these medications are known or suspected to be effective in treating excessive daytime sleepiness in other disorders, such as narcolepsy or obstructive sleep apnea, and are then extended for use in idiopathic hypersomnia with or without specific testing in this population. Clinical series suggest that this strategy results in satisfactory symptom control in the majority of people, with a poorer response to treatment in up to one‐third of individuals (Ali 2009; Anderson 2007; Bassetti 1997). Medication classes that have been used in the treatment of idiopathic hypersomnia include amphetamines, non‐amphetamine wake‐promoting agents (e.g. modafinil, mazindol), histamine H3 antagonists/inverse agonists, gamma‐Aminobutyric acid (GABA)‐B/gamma hydroxybutyrate agonists, levothyroxine, alerting antidepressants such as bupropion, melatonin (for sleep inertia), and GABA‐A antagonists (Leu‐Semenescu 2014; Leu‐Semenescu 2016; Montplaisir 2001; Morgenthaler 2007; Nittur 2013; Rye 2012; Shinno 2011; Trotti 2015; Trotti 2016).
How the intervention might work
Amphetamines both increase the release of and block the reuptake of monoaminergic neurotransmitters (i.e. dopamine, norepinephrine, and serotonin). Their effects on dopaminergic neurotransmission are particularly important for promoting wakefulness (Banerjee 2004; Gowda 2014). Multiple potential mechanisms of action have been proposed for modafinil, including effects on dopaminergic neurotransmission (Banerjee 2004; Gowda 2014). The alerting potential of mazindol and bupropion may also be related to their actions as dopamine reuptake inhibitors (Heal 2014). Histamine H3 antagonists/inverse agonists promote wakefulness by increasing central nervous system histamine levels (Leu‐Semenescu 2014). The mechanism by which agonists of GABA‐B/gamma hydroxybutyrate reduce sleepiness is not known, although deep sleep is increased (Gowda 2014). Levothyroxine might improve alertness via hormonal effects, or by increasing central nervous system histamine levels (Shinno 2011). Because people with idiopathic hypersomnia tend to have a circadian phase delay, evening use of melatonin has been proposed as a method of advancing the circadian rhythm and improving sleep inertia (Vernet 2009). GABA‐A antagonists have been used in people with idiopathic hypersomnia, based on the finding of a positive allosteric modulator of GABA‐A receptors within the cerebrospinal fluid of people who have hypersomnolence (Rye 2012), and the known sleep‐promoting effects of the GABA‐A system (Lu 2006).
Why it is important to do this review
There are currently no medications approved for the treatment of idiopathic hypersomnia by the US Food and Drug Administration, thus all pharmacologic treatment of people with idiopathic hypersomnia in the USA is 'off‐label.' The European Medicines Agency (EMA) has explicitly stated that modafinil should only be used in people with narcolepsy, not in those with idiopathic hypersomnia (EMA Modafinil 2010), although modafinil is one of the medications recommended for the treatment of idiopathic hypersomnia in expert consensus guidelines (Morgenthaler 2007). As a result of this disagreement and lack of regulatory approval, clinical decision‐making regarding treatment of idiopathic hypersomnia can be challenging. Furthermore, medications used for the treatment of idiopathic hypersomnia carry the risk of serious side effects or medication interactions, including, but not limited to, addiction (amphetamines), respiratory depression or coma (sodium oxybate), superinfection and antibiotic resistance (the GABA‐A receptor antagonist clarithromycin), and reduced efficacy of hormonal birth control (modafinil, armodafinil) (Krahn 2015; Trotti 2013). People with idiopathic hypersomnia and prescribers must carefully weigh the trade‐offs of risk and benefit when choosing a treatment option.
Objectives
To assess the effects of medications for daytime sleepiness and related symptoms in individuals with idiopathic hypersomnia and, in particular, whether medications may:
reduce subjective measures of sleepiness;
reduce objective measures of sleepiness;
reduce symptoms of cognitive dysfunction;
improve quality of life; and
be associated with adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel or cross‐over randomized controlled trials (RCTs). Double, single, and unblinded trials were eligible for inclusion. In the case of cross‐over studies, we only used the first period.
Types of participants
We included studies evaluating treatment effects in people with a diagnosis of idiopathic hypersomnia, of any age and gender. Idiopathic hypersomnia could be diagnosed using the ICSD (any edition) or the equivalent diagnosis within the DSM (any edition; diagnoses of primary hypersomnia or hypersomnolence disorder). Studies involving diagnoses of idiopathic hypersomnia based on clinical assessment, in the absence of diagnostic MSLT findings, were eligible for inclusion if they provided sufficient detail about how the diagnosis was made.
Studies that included both people with idiopathic hypersomnia and those with another disorder (e.g. narcolepsy) were eligible for inclusion if data for the subgroup with idiopathic hypersomnia were obtainable. In such cases, we used only the data from the subgroup with idiopathic hypersomnia.
Types of interventions
We included any medication (at any dose), either prescription or over‐the‐counter, that is hypothesized to help with symptoms of idiopathic hypersomnia or is used to treat sleepiness in other conditions, compared to placebo, another medication, or a behavioral intervention.
Studies that allowed the use of other wake‐promoting or psychoactive medications during the trial were eligible for inclusion, if access to such medications was equal in all groups.
Types of outcome measures
Primary outcomes
Subjective measure of daytime sleepiness using the Epworth Sleepiness Scale (ESS).
Secondary outcomes
Other subjective measures of hypersomnia, including other scales quantifying subjective sleepiness, sleep logs or other participant‐completed reports of sleep times, subjective rating scales of sleep drunkenness/sleep inertia.
Objective measures of sleepiness, including MSLT mean sleep latency, Maintenance of Wakefulness Test (MWT) mean sleep latency, psychomotor vigilance test (PVT) measures of reaction times, pupillometry, driving simulators or real‐life measured driving performance, actigraphy, 24‐hour ad‐lib polysomnography.
Subjective reports of cognitive dysfunction or objective measures of cognitive performance, assessed by standardized questionnaire or testing instruments.
Quality of life scales or measures of ability to function in work, school, or other important activities.
-
Adverse events, as measured by:
rate of adverse events;
dropout or withdrawal due to adverse events;
dropout or withdrawal due to lack of efficacy.
Search methods for identification of studies
Electronic searches
We searched the following databases on 4 February 2021:
Cochrane Register of Studies (CRS Web), using the search strategy shown in Appendix 1;
MEDLINE (Ovid) 1946 to 1 February 2021, using the search strategy shown in Appendix 2.
CRS Web includes randomized or quasi‐randomized controlled trials from PubMed, Embase, the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialized registers of Cochrane Review Groups, including the Cochrane Epilepsy Group. We previously searched WHO ICTRP (apps.who.int/trialsearch/) separately, using the search strategy shown in Appendix 3, when loading of WHO ICTRP records into CRS Web was temporarily suspended.
Searching other resources
We reviewed relevant conference proceedings (e.g. the abstract book from the Associated Professional Sleep Societies' annual SLEEP meeting) and systematic reviews, as well as the reference lists of included studies, for additional relevant studies.
Data collection and analysis
Selection of studies
Two review authors independently screened all titles and abstracts of publications identified by the search. We excluded any publications that clearly did not meet the inclusion criteria, as judged independently by both review authors, and obtained the full texts of the remaining publications to identify studies for inclusion in the review. In the case where one of the review authors was an investigator on an identified study, that review author did not take part in decision‐making about that study. Instead, two review authors not involved in the study decided on its eligibility. Any disagreements over study inclusion were resolved by consensus.
Data extraction and management
We used a data extraction form within Covidence. Two review authors independently extracted characteristics of each included trial from the published reports, resolving any disagreements by consensus. Review authors who were investigators on an included study did not participate in extraction of data for that study. Where a published report did not contain sufficient information, we contacted the study investigators to request additional data, including cases where review authors were also investigators on an included study.
We extracted the following data items.
Participant factors
Age.
Sex.
Baseline MSLT results.
Baseline sleep length (by actigraphy, sleep log, 24‐hour polysomnography, or other measure).
Baseline subjective sleepiness severity.
Trial design
Study design (randomization, blinding, parallel versus cross‐over).
Study duration, start date, and end date.
Country of first author.
Where the study was conducted (both country and care setting, e.g. tertiary referral center for idiopathic hypersomnia versus general sleep clinic).
Multi‐ or single‐center trial.
Study inclusion and exclusion criteria.
Criteria used to define idiopathic hypersomnia.
People with idiopathic hypersomnia only, or people from multiple diagnostic categories included.
Number of participants enrolled and number completing in each intervention group.
Number and type of wake‐promoting or psychoactive medications allowed as co‐interventions during the trial.
Funding source.
Intervention and control
Active and control interventions (e.g. type, dosing, duration, etc.).
Outcomes measured.
Adverse events.
Any subgroup analyses performed.
Assessment of risk of bias in included studies
Two review authors assessed risk of bias of the included studies using the Cochrane 'Risk of bias' tool, according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where a review author was an investigator on an included study, two review authors not involved in the trial undertook the 'Risk of bias' assessment.
Risk of bias was assessed as low risk, high risk, or unclear risk, for each of the following characteristics.
Sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessors
Incomplete outcome data
Selective outcome reporting
Other potential threats to validity
Measures of treatment effect
We performed statistical analyses in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). For continuous data available as means and standard deviations, we calculated the mean difference (MD) with 95% confidence interval (CI) to measure treatment effect. For dichotomous data and adverse event data, we used odds ratio (OR) with 95% CI.
Unit of analysis issues
The unit of analysis was the individual trial participant. In the case of cross‐over trials, we only considered data from the first treatment period.
Dealing with missing data
Studies that included any outcome of interest were eligible for inclusion, even if they did not measure the ESS, the primary outcome of this review. However, all included studies used the ESS. We intended to use intention‐to‐treat analyses whenever possible to deal with missing data, but the included studies did not have substantial missing data.
Assessment of heterogeneity
For each intervention effect with sufficient data, we tested heterogeneity using the standard Chi2 and I2 statistics, considering a P value of less than 0.10 as suggestive of heterogeneity. We had planned to assess possible sources of heterogeneity via subgroup analyses, if sufficient numbers of trials were available. However, our largest meta‐analysis included only two studies, limiting our ability to assess for sources of heterogeneity.
Assessment of reporting biases
We compared published protocols to study reports to assess incomplete outcome reporting.
Data synthesis
We generated effect estimates using random‐effects meta‐analyses included in RevMan Web (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We prespecified subgroup analyses for those with long sleep versus normal sleep duration, and by method of diagnosis of idiopathic hypersomnia (e.g. comparing those meeting MSLT criteria versus those with a clinical diagnosis lacking MSLT criteria; ICSD versus DSM criteria; MSLT versus measured total sleep time of > 660 minutes). However, there were insufficient data to perform these analyses.
Sensitivity analysis
We prespecified sensitivity analyses, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), to evaluate the effect of including versus excluding those studies at high risk of bias. We did not identify any studies at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to interpret findings (Schünemann 2021). We used GRADEpro GDT software to create 'Summary of findings' tables for each comparison included in the review for the primary outcome and the adverse events outcome (GRADEpro GDT). The 'Summary of findings' table for each comparison includes information on overall certainty of the evidence from the trials and information of importance for healthcare decision‐making. The GRADE approach determines the certainty of evidence on the basis of an evaluation of eight criteria (risk of bias, inconsistency, indirectness, imprecision, publication bias, effect size, presence of plausible confounding that will change effect, and dose‐response gradient). We used the GRADE criteria for evaluating RCTs to guide our conclusions.
Results
Description of studies
Results of the search
We identified 510 references from the literature search and other sources, 78 of which were duplicates (see Figure 1). We judged 409 of these references to be irrelevant based on title and abstract screening. We assessed the remaining 23 studies for eligibility via review of full text, published abstract, clinical trial registration, or a combination of these, depending on availability. Five of these studies were ongoing (NCT03542851; NCT03597555; NCT03772314; NCT04026958; NCT04091438), and three were recently completed but unpublished, and therefore awaiting classification (EUCTR2017‐002127‐16; NCT02512588; NCT03533114). We excluded seven publications that were additional publications referring to a study already assessed as irrelevant, ongoing, or excluded. We excluded five additional studies for methodological reasons (see Excluded studies). We included three studies in the review.
1.
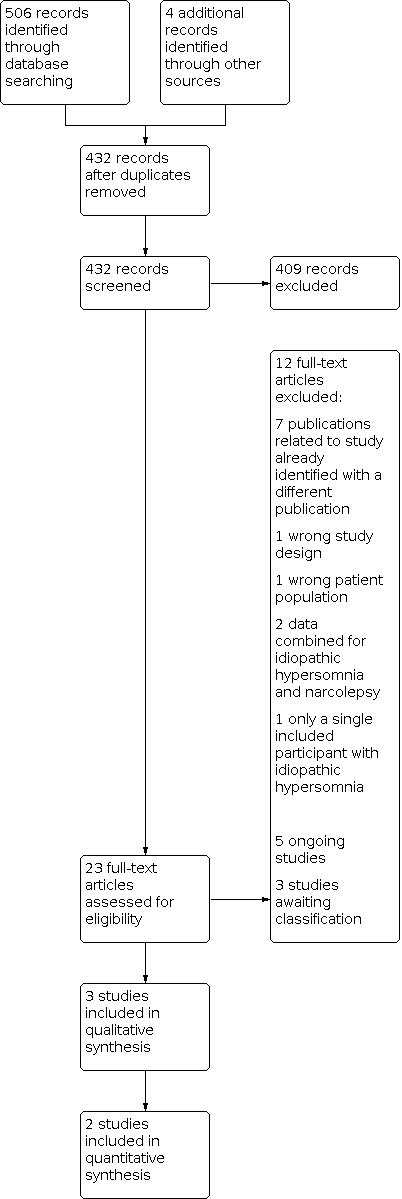
Study flow diagram.
Included studies
Two of the three included studies compared modafinil versus placebo, and one study compared clarithromycin versus placebo.
Mayer 2015 was a placebo‐controlled, double‐blind, randomized, parallel‐group study of modafinil, 100 mg in the morning and 100 mg at noon for three weeks. The trial enrolled 33 participants, and included 31 participants in the analyses who had received either modafinil or placebo. The trial recruited participants from three sleep centers in Germany. Participants were diagnosed with idiopathic hypersomnia without long sleep time, based on the ICSD‐2. Outcomes included ESS, MWT, Clinical Global Impression of Severity (CGI‐S), participant‐reported weekly nap frequency, participant‐reported daytime sleep per week, and participant‐reported effectiveness/performance (rated on a 1‐to‐6‐point scale every evening, where lower numbers indicated better performance). When the study presented data as means and standard deviations, we used the published values for week 3 measures. For outcomes not reported in the publication, we used data provided by Professor Mayer to calculate the mean and standard deviation. This study was funded by Cephalon.
Inoue 2021 was a placebo‐controlled, double‐blind, randomized, parallel‐group study of modafinil, 200 mg in the morning for three weeks. The study included 71 participants across multiple sites in Japan, 70 of whom completed the study. Participants were diagnosed with idiopathic hypersomnia based on the ICSD‐2. The majority (69/71) had idiopathic hypersomnia without long sleep time; two participants had idiopathic hypersomnia with long sleep time. The outcomes included ESS (using the Japanese translation), MWT, CGI of Change (CGI‐C), participant‐reported weekly nap frequency, and nocturnal polysomnography. This study was funded by Alfresa Pharmaceuticals.
Trotti 2015 was a placebo‐controlled, double‐blind, randomized, cross‐over trial of clarithromycin, 500 mg in the morning and 500 mg at noon for two weeks. The trial randomly assigned 23 people from a single sleep center in the USA, and included 20 participants in the analyses, of which 10 had idiopathic hypersomnia (diagnosed by the ICSD‐2, five with long sleep time and five without long sleep time). In accordance with our protocol, we only included data from these 10 participants in the review. Outcomes included ESS, a 10‐point single‐item assessment of sleep inertia, sleep log, PVT, Functional Outcomes of Sleep Questionnaire (FOSQ), the 36‐item Short Form Health Survey (SF‐36), Stanford Sleepiness Scale (SSS), and Pittsburgh Sleep Quality Index (PSQI). This study was funded by the American Academy of Sleep Medicine Foundation.
Excluded studies
Two RCTs of modafinil for excessive daytime sleepiness included participants with idiopathic hypersomnia and evaluated several of our outcomes of interest (Philip 2014; Sagaspe 2019). However, these trials only provided data in aggregate for participants with idiopathic hypersomnia and those with narcolepsy, and were therefore excluded based on our inclusion criteria.
One randomized controlled trial of flumazenil for excessive daytime sleepiness included participants with idiopathic hypersomnia and evaluated outcomes of interest to this review (NCT01183312). However, only a single participant had idiopathic hypersomnia, therefore we excluded this study.
We excluded one study that did not use the current terminology for hypersomnia syndromes, included a mixture of different diagnoses, and was not a controlled trial (Nodine 1960).
The excluded JapicCTI‐142615 study was an open‐label extension of an included controlled trial (Inoue 2021).
Risk of bias in included studies
We judged the included studies to be at low risk of bias (Figure 2; Figure 3)
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies had a low risk of selection bias, considering both random sequence generation and allocation concealment (Inoue 2021; Mayer 2015; Trotti 2015).
Blinding
All three studies were reported as double‐blind trials, and where therefore judged to be at low risk for performance and detection bias (Inoue 2021; Mayer 2015; Trotti 2015). All of the included studies provided additional data about blinding by the pharmacy or third‐party.
Incomplete outcome data
All three trials analyzed data for the majority of enrolled participants with idiopathic hypersomnia, and were therefore judged to be low risk of attrition bias (Inoue 2021; Mayer 2015; Trotti 2015). The Mayer 2015 study analyzed 31 of 33 participants; one participant was a screen‐fail, and a second dropped out at baseline. The Inoue 2021 study analyzed data for adverse events in 71 of 71 enrolled participants, and other outcomes in the 70 participants who completed the study. Regarding the Trotti 2015 study, we only included data from the 10 participants with idiopathic hypersomnia in this review, and all 10 enrolled participants with idiopathic hypersomnia completed the study.
Selective reporting
We judged the risk of reporting bias to be low across all three included studies (Inoue 2021; Mayer 2015; Trotti 2015). All three studies were registered at clinical trials registries, and all three reported data on their primary outcomes and other outcomes listed in registration documents. All three studies reported results for the ESS, which is a very commonly used self‐report measure of sleepiness severity and the primary outcome measure of this review. All studies also reported an objective measure of sleepiness or vigilance, two with the MWT and one with the PVT.
Other potential sources of bias
We judged all of the included studies to be at low risk for other sources of bias.
Effects of interventions
Summary of findings 1. Modafinil compared to placebo for idiopathic hypersomnia.
| Modafinil compared to placebo for idiopathic hypersomnia | ||||||
| Patient or population: idiopathic hypersomnia Setting: outpatient Intervention: modafinil Comparison: placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects (95% CI) | Certainty of the evidence (GRADE) | What occurs | ||
| Without modafinil | With modafinil | Difference | ||||
| Epworth Sleepiness Scale (ESS), week 3 № of participants: 101 (2 RCTs) | ‐ | Mean ESS at week 3 without modafinil was 15.0. | ‐ | MD 5.08 points lower (3.01 lower to 7.16 lower) | ⊕⊕⊕⊕ HIGH | Modafinil reduces sleepiness as measured by ESS scores. |
| Number of naps/week, week 3 № of participants: 99 (2 RCTs) | ‐ | Mean number of naps/week, week 3 without modafinil was 7.0 naps. | ‐ | MD 2.0 fewer naps (5.5 fewer to 1.5 more) | ⊕⊕⊝⊝ LOW1 | Modafinil may reduce the number of naps per week. |
| Clinical Global Impression ‐ Severity (CGI‐S), change from baseline № of participants: 30 (1 RCT) | ‐ | Mean CGI‐S change from baseline without modafinil was −0.36 points. | ‐ | MD 1.02 points lower (1.93 lower to 0.11 lower) | ⊕⊕⊕⊝ MODERATE2 | Modafinil probably improves CGI‐S scores. |
| Clinical Global Impression ‐ Change (CGI‐C), proportion much improved or very much improved at week 3 № of participants: 70 (1 RCT) |
OR 5.14 (1.76 to 15.00) | 18.9% | 54.5% | 35.6% more | ⊕⊕⊕⊝ MODERATE2 | Modafinil probably increases the proportion of people who are much improved or very much improved on the CGI‐C. |
| Maintenance of Wakefulness Test (MWT), week 3 № of participants: 99 (2 RCTs) | ‐ | Mean MWT at week 3 without modafinil was 8.9 minutes. | ‐ | MD 4.74 minutes higher (2.46 higher to 7.01 higher) | ⊕⊕⊕⊕ HIGH | Modafinil improves the amount of time that participants are able to remain awake during the MWT. |
| Effectiveness/performance, week 3 № of participants: 31 (1 RCT) | ‐ | Mean effectiveness/performance, week 3 without modafinil was 3.28 points. | ‐ | MD 0.68 points lower (1.26 lower to 0.1 lower) | ⊕⊕⊕⊝ MODERATE2 | Modafinil probably improves ratings of effectiveness/performance. |
| Number of participants with any adverse events № of participants: 102 (2 RCTs) | OR 1.68 (0.28 to 9.94) | Study population | ⊕⊕⊝⊝ LOW1 | Modafinil may result in more adverse events. | ||
| 37.3% | 56.9% | 19.6% more | ||||
| CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by two levels for very serious imprecision: confidence interval includes the possibility of harm. 2Downgraded by one level for imprecision: wide confidence interval.
Summary of findings 2. Clarithromycin compared to placebo for idiopathic hypersomnia.
| Clarithromycin compared to placebo for idiopathic hypersomnia | ||||||
| Patient or population: idiopathic hypersomnia Setting: outpatient Intervention: clarithromycin Comparison: placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What occurs | ||
| Without clarithromycin | With clarithromycin | Difference | ||||
| Epworth Sleepiness Scale (ESS), change from baseline № of participants: 10 (1 RCT) | ‐ | Mean ESS change from baseline without clarithromycin was −1.75 points. | ‐ | MD 0.5 points lower (5.04 lower to 4.04 higher) | ⊕⊕⊝⊝ LOW1 | There might be little or no reduction in sleepiness as measured by ESS scores. |
| Stanford Sleepiness Scale (SSS), change from baseline № of participants: 10 (1 RCT) | ‐ | Mean SSS change from baseline without clarithromycin was −1.25 points. | ‐ | MD 0.33 points lower (2 lower to 1.34 higher) | ⊕⊕⊝⊝ LOW1 | There might be little or no reduction in sleepiness as measured by SSS scores. |
| Average nocturnal sleep time from sleep diary, weeks 1 and 2 № of participants: 10 (1 RCT) | ‐ | Mean average nocturnal sleep time from sleep diary weeks 1 and 2 without clarithromycin was 462.0 minutes. | ‐ | MD 79.7 minutes lower (171.7 lower to 12.4 higher) | ⊕⊕⊝⊝ LOW1 | There might be little or no reduction in nocturnal sleep time as measured by sleep diary. |
| Difficulty waking in the morning, average of weeks 1 and 2 № of participants: 10 (1 RCT) | ‐ | Mean difficulty waking in the morning average of weeks 1 and 2 without clarithromycin was 5.67 points. | ‐ | MD 0.31 points higher (2 lower to 2.62 higher) | ⊕⊕⊝⊝ LOW1 | There might be little or no improvement difficulty waking in the morning as measured by sleep diary. |
| Psychomotor vigilance task (PVT) reciprocal of reaction time, change from baseline № of participants: 10 (1 RCT) | ‐ | Mean PVT reciprocal of reaction time change from baseline without clarithromycin was 0.32. | ‐ | MD 0.51 lower (1.19 lower to 0.17 higher) | ⊕⊕⊝⊝ LOW1 | There might be little or no change in simple reaction times as measured by PVT reciprocal of reaction time. |
| Functional Outcomes of Sleep Questionnaire (FOSQ), change from baseline № of participants: 10 (1 RCT) | ‐ | Mean FOSQ change from baseline without clarithromycin was 1.62 points. | ‐ | MD 0.79 points higher (3.02 lower to 4.6 higher) | ⊕⊕⊝⊝ LOW1 | There might be little or no change in functional impairment due to sleepiness. |
| Number of participants with any adverse events № of participants: 10 (1 RCT) | OR 0.41 (0.01 to 12.64) | Study population | ⊕⊕⊝⊝ LOW1 | There might be little difference in adverse events between clarithromycin and placebo. | ||
| 100.0% | 83.3% | 16.7% fewer (46.5% fewer to 13.2% more) | ||||
| CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by two levels for imprecision: confidence interval includes the possibility of harm.
Modafinil versus placebo
Subjective sleepiness as measured by the Epworth Sleepiness Scale (ESS)
Two studies evaluated the effect of modafinil versus placebo on our primary outcome, subjective sleepiness measured by the ESS (Inoue 2021; Mayer 2015). Participants taking modafinil had significantly lower ESS scores at week 3 than did those on placebo, by 5.08 points (95% confidence interval (CI) 7.16 to 3.01 points lower with modafinil, I2 = 15%, 2 studies, 101 participants; Analysis 1.1; Figure 4; Table 1).
1.1. Analysis.

Comparison 1: Modafinil versus placebo, Outcome 1: Subjective sleepiness, using ESS, week 3
4.

Modafinil versus placebo, Analysis 1.1: Subjective sleepiness, using Epworth Sleepiness Scale, week 3
Other subjective measures of sleepiness or hypersomnia
We considered other subjective measures of hypersomnia as secondary outcomes for this review. Two studies included reported weekly nap frequency (Inoue 2021; Mayer 2015), and one study reported total minutes of daytime sleep per week (Mayer 2015). There was no difference in weekly nap frequency at week 3 between the modafinil and placebo groups (mean difference (MD) 2.01 fewer naps/week in the modafinil group, 95% CI 5.50 fewer to 1.48 more naps, I2 = 81%, 2 studies, 99 participants; Analysis 1.2 (1.2.1)). There was no difference in total minutes of daytime sleep at week 3 between modafinil and placebo groups in the Mayer 2015 study (MD 7.00 minutes more in the modafinil group, 95% CI 81.59 less to 95.59 minutes more; Analysis 1.2 (1.2.2)).
1.2. Analysis.

Comparison 1: Modafinil versus placebo, Outcome 2: Other subjective measures of sleepiness or hypersomnia
The Mayer 2015 study reported several sleep diary measures that were assessed daily and then averaged across a one‐week period. These measures included: exhaustion (4‐point scale, lower numbers signify less exhaustion), feeling of being refreshed (measurement unspecified), and nocturnal sleep duration. Use of modafinil reduced ratings of exhaustion at week 3 to a greater extent than did placebo (MD 0.67 points lower with modafinil than placebo, 95% CI 1.13 points lower to 0.21 points lower; Analysis 1.2 (1.2.3)). Change from baseline to week 3 in nocturnal sleep time from sleep logs did not differ between treatments (MD 0.25 hours less in the modafinil group, 95% CI 0.98 less to 0.48 hours more sleep in modafinil group; Analysis 1.2 (1.2.4)). The study did not provide data for feelings of being refreshed, although the study authors noted that the modafinil group became more refreshed with treatment compared to baseline, whereas the placebo group had no change on this measure.
Two studies assessed CGI, using different measures. The Mayer 2015 study evaluated change from baseline in Clinical Global Impression of Severity (CGI‐S). Modafinil resulted in a greater improvement from baseline to week 3 on the CGI‐S than did placebo, by 1.02 points (95% CI improved by 1.93 to 0.11 points; Analysis 1.3). The Inoue 2021 study evaluated the percentage of participants who were "much improved" or "very much improved" on the CGI of Change. Modafinil resulted in a significantly greater proportion of participants who were much or very much improved (odds ratio (OR) 5.14, 95% CI 1.76 to 15.00; Analysis 1.4).
1.3. Analysis.

Comparison 1: Modafinil versus placebo, Outcome 3: CGI‐S, change from baseline to week 3
1.4. Analysis.

Comparison 1: Modafinil versus placebo, Outcome 4: CGI‐C, "much" or "very much" improved at week 3
Neither study included measures of sleep drunkenness/sleep inertia.
Objective measures of hypersomnia
Both modafinil studies used Maintenance of Wakefulness Test (MWT) mean sleep latency as the objective measure of sleepiness (Inoue 2021; Mayer 2015). Modafinil resulted in a significantly longer mean sleep latency at week 3 than did placebo, by 4.74 minutes (95% CI 2.46 to 7.01 minutes longer; I2 = 0%; 2 studies, 99 participants; Analysis 1.5; Figure 5).
1.5. Analysis.

Comparison 1: Modafinil versus placebo, Outcome 5: Objective sleepiness, using MWT, week 3
5.

Modafinil versus placebo, Analysis 1.5: Objective sleepiness, using Maintenance of Wakefulness Test, week 3.
The Inoue 2021 study also included nocturnal polysomnographic measures, including sleep latency, total sleep time, and percentage of sleep stages. These results were not detailed, but it was noted that changes in these parameters from baseline to week 3 did not differ between the modafinil and placebo groups.
Cognitive performance
Neither study of modafinil used subjective reports of cognitive dysfunction or objective measures of cognitive performance.
Quality of life or ability to function
The Mayer 2015 study used a subjective report of ability to function via a single, 6‐point measure of "effectiveness/performance" reported every evening and then averaged across each week. At week 3, this measure was better in the modafinil group than in the placebo group, by 0.68 points (95% CI 1.26 to 0.1 points better in the modafinil group; Analysis 1.6).
1.6. Analysis.

Comparison 1: Modafinil versus placebo, Outcome 6: Quality of life or ability to function
Adverse events
Both studies reported adverse events (Inoue 2021; Mayer 2015). Participants receiving modafinil experienced more side effects than those in the placebo group, although the difference did not reach statistical significance (OR 1.68, 95% CI 0.28 to 9.94; I2 = 76%; 2 studies, 102 participants; Analysis 1.7). In the Inoue 2021 study, there was no significant difference between groups in dropout due to adverse events (OR 3.36, 95% CI 0.13 to 85.26). In the Mayer 2015 study, there were no dropouts due to adverse events in either group. There were no dropouts due to lack of efficacy in either study.
1.7. Analysis.

Comparison 1: Modafinil versus placebo, Outcome 7: Adverse events
In the Mayer 2015 study, individual adverse events of headache and gastrointestinal symptoms were more common in the modafinil group. Headache occurred in 26.4% of the modafinil group and 3.3% of the placebo group, whereas gastrointestinal symptoms occurred in 19.8% and 6.6% of participants in the modafinil and placebo groups, respectively. In the Inoue 2021 study, headache occurred in 17.6% of the modafinil group and 8.1% of the placebo group. Gastrointestinal symptoms were listed individually in this study, with nausea occurring in 8.8% of participants in the modafinil group and none in the placebo group; diarrhea in 5.9% of participants in the modafinil group and none in the placebo group; and loss of appetite in 5.9% of participants in the modafinil group and none in the placebo group.
Clarithromycin versus placebo
Subjective sleepiness as measured by the Epworth Sleepiness Scale (ESS)
One study evaluated the effect of clarithromycin versus placebo on our primary outcome, subjective sleepiness measured by the ESS (Trotti 2015). As stated in our protocol (Trotti 2017), we only used data for those participants with idiopathic hypersomnia, and only from the first period of this cross‐over study. The change in ESS score from baseline to on‐treatment (an average of week 1 and week 2 scores) did not differ between participants with idiopathic hypersomnia initially randomized to clarithromycin and those randomized to placebo (MD 0.50 points more improvement in the clarithromycin group, 95% CI 5.04 points more to 4.04 points less to improvement in the clarithromycin group; Analysis 2.1; Table 2).
2.1. Analysis.

Comparison 2: Clarithromycin versus placebo, Outcome 1: Subjective sleepiness, using the ESS
Other subjective measures of sleepiness or hypersomnia
The Trotti 2015 study also included other subjective measures of hypersomnia, including the SSS, sleep log, and a 10‐point scale assessing difficulty in awakening every morning. Change from baseline to on‐treatment (average of weeks 1 and 2) for the SSS did not differ between clarithromycin and placebo groups (MD 0.33 points more improvement in the clarithromycin group, 95% CI 2.00 points better in the clarithromycin group to 1.34 better in the placebo group; Analysis 2.2 (2.2.1)). Sleep time was calculated from sleep diaries and averaged across each two‐week treatment period. Sleep duration did not differ between the clarithromycin and placebo groups (MD 79.67 minutes less sleep in the clarithromycin group, 95% CI 171.71 minutes less to 12.37 minutes more in the clarithromycin group; Analysis 2.2 (2.2.2)). Difficulty waking in the morning (a measure of sleep inertia) did not differ between groups (MD 0.31 points harder in the clarithromycin group, 95% CI 2.00 points easier to 2.62 points harder in the clarithromycin group; Analysis 2.2 (2.2.3)).
2.2. Analysis.

Comparison 2: Clarithromycin versus placebo, Outcome 2: Other subjective measures of sleepiness or hypersomnia
Objective measures of hypersomnia
The Trotti 2015 study evaluated performance on the psychomotor vigilance test (PVT) as an objective measure of sleepiness using the reciprocal of reaction time, a measure of the speed of participants' reaction to stimuli. For this measure, the change from baseline did not differ between clarithromycin and placebo groups (MD improved by 0.51 more in the placebo group, 95% CI improved by 1.19 more to 0.17 less in the placebo group; Analysis 2.3).
2.3. Analysis.

Comparison 2: Clarithromycin versus placebo, Outcome 3: Objective sleepiness using the PVT
Cognitive performance
Trotti 2015 did not include any measures of cognitive function.
Quality of life or ability to function
The Trotti 2015 study included two measures of quality of life or functional impairment. The trialists measured quality of life using the SF‐36 subscales. There were no differences between clarithromycin and placebo groups on any of the SF‐36 subscales (data not shown). Functional impairment due to sleepiness was assessed using the FOSQ, but this did not differ between clarithromycin and placebo groups (MD 0.79 points more improved in the clarithromycin group, 95% CI 3.02 less improved to 4.60 points more improved in the clarithromycin group; Analysis 2.4).
2.4. Analysis.

Comparison 2: Clarithromycin versus placebo, Outcome 4: Quality of life or ability to function
Adverse events
The overall rate of any adverse event did not differ between clarithromycin and placebo groups in the Trotti 2015 study (OR 0.41 for any adverse event in the clarithromycin group, 95% CI 0.01 to 12.64; Analysis 2.5). There were no dropouts in either group due to adverse events or lack of efficacy.
2.5. Analysis.

Comparison 2: Clarithromycin versus placebo, Outcome 5: Adverse events
Discussion
Summary of main results
Modafinil is effective for the treatment of several aspects of idiopathic hypersomnia symptoms. Two RCTs showed reductions in Epworth Sleepiness Scale (ESS) scores, our primary measure of daytime sleepiness. Objective sleepiness, as measured by the Maintenance of Wakefulness Test (MWT) sleep latency, also significantly improved with modafinil in these two studies. Clinical Global Impression (CGI) improved in both studies, measured as change in severity in one study, and proportion of participants who were much or very much improved in the other study. Exhaustion and effectiveness/performance both improved in a single study. Weekly nap frequency did not significantly improve in the two studies. The increase in adverse events in the modafinil group did not reach statistical significance. Dropout due to adverse event was not significantly different between modafinil and placebo groups.
The available data were insufficient to determine whether clarithromycin is superior to placebo for the treatment of idiopathic hypersomnia. Further studies of this intervention would better clarify its effects.
Overall completeness and applicability of evidence
The currently available evidence in support of the optimal treatment for people with idiopathic hypersomnia is limited. The intervention studied the most in RCTs is modafinil, for which the total sample size in the included studies was 102 participants. One of these trials had a target sample size of 40 participants based on power calculations (Mayer 2015), but only 31 participants completed the study, which may have reduced the ability of the study to find beneficial or harmful effects of modafinil. Two excluded modafinil RCTs included participants with idiopathic hypersomnia, but disease‐specific data were not available from these trials. Nearly all participants in the included studies had idiopathic hypersomnia without long sleep time (per International Classification of Sleep Disorders (ICSD‐2) diagnostic criteria), therefore it is currently unknown whether modafinil would be similarly effective in people with ICSD‐2‐defined idiopathic hypersomnia with long sleep time. Idiopathic hypersomnia is no longer subdivided into with and without long sleep time in the ICSD‐3.
Clarithromycin is the only other intervention for which RCT data have been published as of the writing of this review, despite the use of a broader range of medications in clinical practice. Several recently completed or ongoing studies will add to the evidence base for idiopathic hypersomnia once data become available.
Certainty of the evidence
The certainty of the evidence for the outcomes of interest ranged from high to low for modafinil and low for clarithromycin. Imprecision influenced our assessment of the certainty of the evidence.
Potential biases in the review process
Two review authors (LMT, RH) have served or currently serve as investigators on RCTs evaluating treatments for idiopathic hypersomnia. In order to minimize the risk of bias related to their involvement in these trials, we prespecified that data extraction and quality assessment would be performed by investigators not involved in a given trial. This was required for the clarithromycin study (Trotti 2015), but was not necessary for one trial for which data are not yet available (NCT02512588).
Our use of only subsets of data may have restricted our ability to identify medication benefits. The clarithromycin study analyzed data from 20 participants, consistent with that study's pre‐study power calculation (Trotti 2015). However, in our review we restricted analysis to only those with idiopathic hypersomnia (50% of the original study population), and then performed parallel‐group rather than cross‐over analyses; both of these prespecified methods reduced our power to detect beneficial or harmful effects of clarithromycin.
All of the included studies were relatively small, which limited our ability to identify rare but serious adverse events. Small sample sizes may also have resulted in the finding that neither modafinil nor clarithromycin was more likely than placebo to cause side effects, a finding that is inconsistent with data from larger studies of other conditions.
Agreements and disagreements with other studies or reviews
The American Academy of Sleep Medicine's (AASM) 2021 guideline on the treatment of idiopathic hypersomnia gives a strong recommendation for modafinil (Maski 2021). The strong recommendation for modafinil was based on one of the RCTs included here (Mayer 2015) as well as non‐RCT evidence, and is in agreement with this review that modafinil is effective for the treatment of idiopathic hypersomnia.
The AASM guideline gives a conditional recommendation for the use of clarithromycin (Maski 2021), based on the RCT included here (Trotti 2015) as well as non‐RCT evidence. This is somewhat different than our conclusion that data are insufficient to determine whether clarithromycin is effective for idiopathic hypersomnia, likely reflecting difference in methodology. For this review, we pre‐specified that we would only analyze the first period of cross‐over studies, limited to only those participants with idiopathic hypersomnia. In applying this to the Trotti 2015 study, we found no significant difference between clarithromycin and placebo groups, but the reduced sample size and use of only between‐subjects comparisons reduced power to see an effect. In the AASM guideline, only data from participants with idiopathic hypersomnia were included, but data from both cross‐over periods were considered, allowing within‐subjects comparisons. The AASM guideline also incorporated non‐RCT evidence.
The original publication of the clarithromycin study reported statistically significant, positive results for our primary outcome, the ESS, with clarithromycin compared to placebo (Trotti 2015). In the overall cohort of 20 participants, the average reduction in ESS with clarithromycin compared to placebo was four points. In contrast, in our analysis of only the first period of the cross‐over, limited to those 10 participants with idiopathic hypersomnia, we found no significant difference between clarithromycin and placebo groups. Although this might reflect a true difference between participants with idiopathic hypersomnia and those with other hypersomnolence disorders, there was no significant difference in treatment response to ESS based on diagnosis when analyzed in the Trotti 2015 study, making this less likely. The 95% CI in this review includes the possibilities of meaningful improvement or worsening of ESS with clarithromycin use.
Authors' conclusions
Implications for practice.
There are limited data to guide treatment decisions for people with idiopathic hypersomnia. The existing data are strongest in support of modafinil, which is beneficial for key symptoms. In cases in which modafinil is insufficient to control symptoms or results in problematic side effects, the current data do not define which treatment should be considered as second line, therefore a number of different options may be reasonable based on clinical judgement.
Implications for research.
There is a large unmet need for data to guide treatment decisions in people with idiopathic hypersomnia, and additional randomized controlled trials of a variety of interventions are needed. There are currently more ongoing than published trials for this disorder, so it is likely that clinical practice for this disorder may change in the coming years, as these additional trials are completed.
History
Protocol first published: Issue 7, 2017
Acknowledgements
We gratefully acknowledge Professor Mayer for providing study data beyond those reported in the published manuscript. We extend thanks to Graham Chan for his assistance with drafting the search strategy and running the search, and to the Cochrane Epilepsy Group for administrative and technical assistance.
We, and the Cochrane Epilepsy Group, are grateful to the following peer reviewers for their time and comments: Nimisha Kumar, Maria Sudell, and a content referee who has chosen to remain anonymous.
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Epilepsy Group, and the National Institutes of Health (NIH), via grant funding to Dr Trotti (K23 NS083748 and R01 NS11280). Dr. Trotti is a member of the Board of Directors of the American Academy of Sleep Medicine. However, the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, the Department of Health and Social Care, the NIH, or the American Academy of Sleep Medicine.
Appendices
Appendix 1. CRS Web search strategy
1. MeSH DESCRIPTOR Idiopathic Hypersomnia EXPLODE ALL AND CENTRAL:TARGET
2. hypersomn* AND CENTRAL:TARGET
3. ("non‐rapid eye movement" or NREM) and narcolep* AND CENTRAL:TARGET
4. #1 OR #2 OR #3
Appendix 2. MEDLINE (Ovid) search strategy
This strategy includes a modification of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (Lefebvre 2021).
1. exp Idiopathic Hypersomnia/
2. hypersomn$.tw.
3. (("non‐rapid eye movement" or NREM) and narcolep$).tw.
4. 1 or 2 or 3
5. exp controlled clinical trial/ or (randomi?ed or placebo or randomly).ab.
6. clinical trials as topic.sh.
7. trial.ti.
8. 5 or 6 or 7
9. exp animals/ not humans.sh.
10. 8 not 9
11. 4 and 10
12. remove duplicates from 11
Appendix 3. WHO ICTRP search strategy
sleepiness AND Idiopathic Hypersomnia
Data and analyses
Comparison 1. Modafinil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Subjective sleepiness, using ESS, week 3 | 2 | 101 | Mean Difference (IV, Random, 95% CI) | ‐5.08 [‐7.16, ‐3.01] |
| 1.2 Other subjective measures of sleepiness or hypersomnia | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 Number of naps/week, week 3 | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐5.50, 1.48] |
| 1.2.2 Duration of daytime sleep per week, week 3 | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 7.00 [‐81.59, 95.59] |
| 1.2.3 Exhaustion severity, week 3 | 1 | 31 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.13, ‐0.21] |
| 1.2.4 Nocturnal sleep time (from log), change from baseline | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.98, 0.48] |
| 1.3 CGI‐S, change from baseline to week 3 | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.93, ‐0.11] |
| 1.4 CGI‐C, "much" or "very much" improved at week 3 | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 5.14 [1.76, 15.00] |
| 1.5 Objective sleepiness, using MWT, week 3 | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 4.74 [2.46, 7.01] |
| 1.6 Quality of life or ability to function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6.1 Effectiveness/performance, week 3 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.7 Adverse events | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Number of participants with any adverse events | 2 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.28, 9.94] |
| 1.7.2 Number of participants who dropped out due to adverse events | 2 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 3.36 [0.13, 85.26] |
Comparison 2. Clarithromycin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Subjective sleepiness, using the ESS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 ESS, change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Other subjective measures of sleepiness or hypersomnia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2.1 SSS, change from baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2.2 Average nocturnal sleep time from sleep diary, weeks 1 and 2 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2.3 Difficulty waking in the morning, average of weeks 1 and 2 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Objective sleepiness using the PVT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.1 PVT reciprocal of reaction time, change from baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Quality of life or ability to function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4.1 FOSQ score, change from baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Adverse events | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5.1 Number of participants with any adverse events | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Inoue 2021.
| Study characteristics | ||
| Methods | Parallel‐group, randomized, placebo‐controlled, double‐blind trial | |
| Participants | 71 participants with idiopathic hypersomnia, diagnosed per ICSD‐2, 69 without long sleep time and 2 with long sleep time | |
| Interventions | Oral modafinil, 200 mg every morning, or placebo, for 3 weeks | |
| Outcomes | ESS, MWT sleep latency, weekly nap frequency | |
| Dates | April 2014 to August 2015 | |
| Funding Source | Alfresa Pharmaceuticals | |
| Declarations of Interest | Y Inoue has received honoraria or funds from Alfresa. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomization method by a third party |
| Allocation concealment (selection bias) | Low risk | Randomization by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and modafinil tablets were made indistinguishable from each other. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated to be double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Single dropout |
| Selective reporting (reporting bias) | Low risk | Trial registered at JAPIC Clinical Trials; outcomes listed are MWT and safety, both of which were included in the published manuscript. |
| Other bias | Low risk | None identified. |
Mayer 2015.
| Study characteristics | ||
| Methods | Parallel‐group, randomized, placebo‐controlled, double‐blind trial | |
| Participants | 33 participants (31 included in analyses) with idiopathic hypersomnia, diagnosed per ICSD‐2, all without long sleep time | |
| Interventions | Oral modafinil, 100 mg in the morning and 100 mg at noon, or placebo, for 3 weeks | |
| Outcomes | ESS, MWT sleep latency, CGI‐S, diary measures of duration of daytime sleep per week, number of naps per week, and effectiveness/performance | |
| Dates | 2009 to 2011 | |
| Funding Source | Cephalon | |
| Declarations of Interest | None | |
| Notes | Unpublished data provided by Professor Mayer for inclusion in review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned randomly in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled randomization, rather than randomization by study team |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication blinded by pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Manuscript does not explicitly say that outcome assessors were blinded; however, randomization and medication blinding were both performed by the pharmacy, and the study is described as "double‐blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31 of 33 participants analyzed, but it is likely that only 31 participants received an intervention (1 dropped out at baseline and 1 was a screen‐fail). |
| Selective reporting (reporting bias) | Low risk | Record entered in EudraCT on 29 January 2009; study ran 2009 to 2011. Outcome listed on EudraCT is ESS, and this is reported. |
| Other bias | Low risk | None identified. |
Trotti 2015.
| Study characteristics | ||
| Methods | Cross‐over, randomized, placebo‐controlled, double‐blind trial. Analyzed as a parallel‐group design in review | |
| Participants | 20 participants with central disorders of hypersomnolence completed the study. Only data from the 10 participants with idiopathic hypersomnia were included in review. | |
| Interventions | Clarithromycin, 500 mg orally with breakfast and with lunch | |
| Outcomes | ESS, PVT, FOSQ, SF‐36, SSS, and PSQI | |
| Dates | 2011 to 2012 | |
| Funding Source | Primary funder: American Academy of Sleep Medicine Foundation; additional research support from the National Institutes of Health | |
| Declarations of Interest | 2 study authors had a patent pending for the use of GABA‐ergic agents in the treatment of hypersomnia disorders. | |
| Notes | Unpublished data provided by Professor Trotti for inclusion in review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization |
| Allocation concealment (selection bias) | Low risk | Randomization performed by off‐site pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masking of treatment and matched placebo performed by off‐site pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masking of treatment and matched placebo performed by off‐site pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants with idiopathic hypersomnia who started the study also completed the study. |
| Selective reporting (reporting bias) | Low risk | Protocol published on ClinicalTrials.gov prior to study start. |
| Other bias | Low risk | None identified. |
CGI‐S: Clinical Global Impression ‐ Severity
ESS: Epworth Sleepiness Scale
FOSQ: Functional Outcomes of Sleep Questionnaire
GABA: gamma‐Aminobutyric acid
ICSD: International Classification of Sleep Disorders
JAPIC: Japan Pharmaceutical Information Center
MWT: Maintenance of Wakefulness Test
PVT: psychomotor vigilance test
PSQI: Pittsburgh Sleep Quality Index
SF‐36: 36‐Item Short Form Health Survey
SSS: Stanford Sleepiness Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| JapicCTI‐142615 | Open‐label extension of an included study (Inoue 2021). |
| NCT01183312 | Only a single participant diagnosed with idiopathic hypersomnia. |
| Nodine 1960 | Did not use current terminology for hypersomnia syndromes, included a mixture of different diagnoses, and was not a controlled trial. |
| Philip 2014 | Data not available separately for participants with idiopathic hypersomnia, only as a combined group with participants with narcolepsy. |
| Sagaspe 2019 | Data not available separately for participants with idiopathic hypersomnia, only as a combined group with participants with narcolepsy. |
Characteristics of studies awaiting classification [ordered by study ID]
EUCTR2017‐002127‐16.
| Methods | Double‐blind, placebo‐controlled, cross‐over study |
| Participants | Idiopathic hypersomnia |
| Interventions | GR3027, a GABA‐A‐receptor modulating steroid antagonist, or placebo |
| Outcomes | ESS, MWT |
| Notes |
NCT02512588.
| Methods | Randomized, placebo‐controlled, double‐blind, multiple‐cohort, fixed‐dose, multiple‐cross‐over, dose‐finding study |
| Participants | Idiopathic hypersomnia or narcolepsy type 2 (ICSD‐3) |
| Interventions | Oral BTD‐001 (pentetrazol) or placebo |
| Outcomes | ESS, MWT, pharmacokinetics |
| Notes |
NCT03533114.
| Methods | Double‐blind, placebo‐controlled, randomized withdrawal, multicenter study |
| Participants | Idiopathic hypersomnia (ICSD‐2 or ICSD‐3) |
| Interventions | JZP‐258, an oxybate mixed‐salts oral solution being developed as a low‐sodium alternative product for Xyrem, or placebo |
| Outcomes | ESS, PGI‐C, CGI‐C, FOSQ‐10, HSS |
| Notes |
CGI‐C: Clinical Global Impression ‐ Change
ESS: Epworth Sleepiness Scale
FOSQ: Functional Outcomes of Sleep Questionnaire
GABA: gamma‐Aminobutyric acid
HSS: Hypersomnolence Severity Scale
ICSD: International Classification of Sleep Disorders
MWT: Maintenance of Wakefulness Test
PGI‐C: Patient Global Impression of Change scale
Characteristics of ongoing studies [ordered by study ID]
NCT03542851.
| Study name | A study of oral BTD‐001 in adults with idiopathic hypersomnia (ARISE2) |
| Methods | Randomized, placebo‐controlled, double‐blind, cross‐over study |
| Participants | Adults with idiopathic hypersomnia |
| Interventions | Oral BTD‐001 (pentetrazol) or placebo |
| Outcomes | Idiopathic hypersomnia symptom diary, ESS, MWT |
| Starting date | 29 May 2018 |
| Contact information | Nichole Baio, nichole.baio@balance‐therapeutics.com |
| Notes |
NCT03597555.
| Study name | Sodium oxybate in idiopathic hypersomnia (SODHI) |
| Methods | Bicentric, randomized, double‐blind, placebo‐controlled study |
| Participants | Idiopathic hypersomnia (ICSD‐3) |
| Interventions | Sodium oxybate or placebo |
| Outcomes | ESS |
| Starting date | Not yet recruiting |
| Contact information | Yves Dauvilliers, y‐dauvilliers@chu‐montpellier.fr |
| Notes |
NCT03772314.
| Study name | Modafinil versus amphetamines for the treatment of narcolepsy type 2 and idiopathic hypersomnia |
| Methods | Double‐blind, randomized, single‐site |
| Participants | Narcolepsy type 2 or idiopathic hypersomnia (ICSD‐3) |
| Interventions | Modafinil or amphetamine salts |
| Outcomes | ESS, PGI‐C (sleepiness, sleep inertia, cognitive dysfunction) |
| Starting date | 15 April 2019 |
| Contact information | natalie.fernandez@emory.edu |
| Notes |
NCT04026958.
| Study name | Antibiotic‐mediated improvements in vigilance: mechanisms of action of clarithromycin in hypersomnia syndromes |
| Methods | Double‐blind, placebo‐controlled, randomized study |
| Participants | Idiopathic hypersomnia or narcolepsy type 2 (ICSD‐3) |
| Interventions | Clarithromycin or placebo |
| Outcomes | ESS, MWT |
| Starting date | Not yet recruiting |
| Contact information | natalie.fernandez@emory.edu |
| Notes |
NCT04091438.
| Study name | A study of a single intravenous infusion dose of TAK‐925 in participants with idiopathic hypersomnia |
| Methods | Double‐blind, placebo‐controlled, randomized, cross‐over trial |
| Participants | Idiopathic hypersomnia |
| Interventions | TAK‐925 or placebo |
| Outcomes | Treatment‐emergent adverse events |
| Starting date | 2019 |
| Contact information | medinfoUS@takeda.com |
| Notes | Phase 1b |
CGI‐S: Clinical Global Impression ‐ Severity
ESS: Epworth Sleepiness Scale
FOSQ: Functional Outcomes of Sleep Questionnaire
HSS: Hypersomnolence Severity Scale
ICSD: International Classification of Sleep Disorders
MWT: Maintenance of Wakefulness Test
PGI‐C: Patient Global Impression of Change scale
Differences between protocol and review
For continuous data assessing the same outcome using different measures (e.g. two different quality of life scales), we planned to use standardized mean difference with 95% confidence interval. We did not identify any such data.
Several measures collected in the included studies and included in this review were not specifically listed in our protocol as outcome measures, but were nevertheless included in the review as they are alternate measures of our prespecified outcomes of interest. For our prespecified outcome 'other subjective measures of hypersomnia,' we included number of naps, exhaustion, and Clinical Global Impression of Severity. For the outcome 'quality of life or other measures of ability to function in work, school, or other important activities,' we included a rating of effectiveness/performance and the Functional Outcomes of Sleep Questionnaire.
We had planned to perform meta‐analysis of measures of treatment effect separately for each medication and medication class (in the case of trials of different medications within the same class, e.g. modafinil and armodafinil). However, an insufficient number of included studies precluded meta‐analyses by medication class.
We prespecified an analysis for publication biases by funnel plot if at least 10 trials were included for a given intervention; however, there were too few included studies to conduct this analysis.
We prespecified subgroup analyses for those participants with long sleep versus normal sleep duration and by method of diagnosis of idiopathic hypersomnia (e.g. comparing those meeting Multiple Sleep Latency Test (MSLT) criteria versus those with a clinical diagnosis lacking MSLT criteria; International Classification of Sleep Disorders (ICSD) versus Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria; and MSLT versus measured total sleep time of > 660 minutes). However, insufficient data precluded these analyses.
We prespecified sensitivity analyses, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), to evaluate the effect of including versus excluding studies at high risk of bias. We did not identify any studies at high risk of bias.
Contributions of authors
LT: extracting study data, evaluating risk of bias, requesting data from study authors, performing analyses, GRADE assessment, and drafting of the manuscript.
LB: extracting study data, evaluating risk of bias, GRADE assessment, and revision of the manuscript.
CM: GRADE assessment and revision of the manuscript.
RH: extracting study data, evaluating risk of bias, GRADE assessment, and revision of the manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institutes of Health, USA
K23 NS083748 (to LMT)
National Institute for Health Research (NIHR), UK
-
National Institutes of Health, USA
R01 NS111280 (to LMT)
Declarations of interest
LT: has performed clinical trials of medications used for daytime sleepiness in idiopathic hypersomnia, including one study included in this review (Trotti 2015). Her institution has received funding for clinical trials of medications for daytime sleepiness (Jazz Pharmaceuticals, Balance Therapeutics). She currently receives grant support from the National Institutes of Health and the American Academy of Sleep Medicine Foundation for clinical trials evaluating medications for the treatment of daytime sleepiness in idiopathic hypersomnia and related disorders (NCT03772314; NCT04026958).
LB: none known.
CM: none known.
RH: was the site Principal Investigator of a clinical trial of a medication for daytime sleepiness in idiopathic hypersomnia (NCT02512588).
New
References
References to studies included in this review
Inoue 2021 {published data only (unpublished sought but not used)}
- Inoue Y, Tabata T, Tsukimori N. Efficacy and safety of modafinil in patients with idiopathic hypersomnia without long sleep time: a multicenter, randomized, double-blind, placebo-controlled, parallel-group comparison study. Sleep Medicine (in press). [DOI: 10.1016/j.sleep.2021.01.018] [DOI] [PubMed]
Mayer 2015 {published and unpublished data}
- Mayer G, Benes H, Young P, Bitterlich M, Rodenbeck A. Modafinil in the treatment of idiopathic hypersomnia without long sleep time—a randomized, double-blind, placebo-controlled study. Journal of Sleep Research 2015;24(1):74-81. [DOI: 10.1111/jsr.12201] [PMID: ] [DOI] [PubMed] [Google Scholar]
Trotti 2015 {published and unpublished data}
- Trotti LM, Saini P, Bliwise DL, Freeman AA, Jenkins A, Rye DB. Clarithromycin in gamma-aminobutyric acid-related hypersomnia: a randomized, crossover trial. Annals of Neurology 2015;78(3):454-65. [DOI: 10.1002/ana.24459] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
JapicCTI‐142615 {published data only}
- JapicCTI-142615. Long-term study of CN-801 in patients with idiopathic hypersomnia. www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-142615 (first received 23 July 2014).
NCT01183312 {published and unpublished data}
- NCT01183312. Flumazenil for the treatment of primary hypersomnia. clinicaltrials.gov/show/NCT01183312 (first received 17 August 2010).
Nodine 1960 {published data only}
- Nodine JH, Bodi T, Slap J, Levy HA, Siegler PE. Preliminary trial of a new stimulant SCH 5472 in ambulatory patients with depression, exhaustion, or hypersomnia syndrome. Antibiotic Medicine & Clinical Therapy 1960;7:771-6. [PMID: ] [PubMed] [Google Scholar]
Philip 2014 {published data only (unpublished sought but not used)}
- Philip P, Chaufton C, Taillard J, Capelli A, Coste O, Leger D, et al. Modafinil improves real driving performance in patients with hypersomnia: a randomized double-blind placebo-controlled crossover clinical trial. Sleep 2014;37(3):483-7. [DOI: 10.5665/sleep.3480] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sagaspe 2019 {published data only}
- Sagaspe P, Micoulaud-Franchi JA, Coste O, Leger D, Espie S, Davenne D, et al. Maintenance of wakefulness test, real and simulated driving in patients with narcolepsy/hypersomnia. Sleep Medicine 2019;55:1-5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
EUCTR2017‐002127‐16 {published data only}
- EUCTR2017-002127-16. A phase IIa study of GR3027 in patients with idiopathic hypersomnia (IH) involving an open-label part to assess safety, tolerability and pharmacokinetics (PK) of a single oral GR3027 dose in female patients and a randomized, double-blind, placebo-controlled crossover study to assess safety, tolerability, exposure and exploratory efficacy of multiple oral doses of GR3027 in male and female IH patients. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2017-002127-16 (first received 5 July 2017).
NCT02512588 {published data only}
- NCT02512588. A study of safety and efficacy of BTD-001 in treatment of patients with idiopathic hypersomnia (IH) or narcolepsy type 2. clinicaltrials.gov/show/NCT02512588 (first received 31 July 2015).
NCT03533114 {published data only}
- NCT03533114. A multicenter study of the efficacy and safety of JZP-258 in the treatment of idiopathic hypersomnia (IH) with an open-label safety extension. clinicaltrials.gov/show/NCT03533114 (first received 22 May 2018).
References to ongoing studies
NCT03542851 {published data only}
- NCT03542851. A randomized, placebo-controlled, double-blind, crossover study of oral BTD-001 in adults with idiopathic hypersomnia. clinicaltrials.gov/show/NCT03542851 (first received 31 May 2018).
NCT03597555 {published data only}
- NCT03597555. Sodium oxybate for idiopathic hypersomnia. clinicaltrials.gov/show/NCT03597555 (first received 24 July 2018).
NCT03772314 {published data only}
- NCT03772314. Modafinil versus amphetamines for the treatment of narcolepsy type 2 and idiopathic hypersomnia. clinicaltrials.gov/ct2/show/NCT03772314 (first received 11 December 2018).
NCT04026958 {published data only}
- NCT04026958. Clarithromycin mechanisms in hypersomnia syndromes. clinicaltrials.gov/ct2/show/NCT04026958.
NCT04091438 {published data only}
- NCT04091438. A phase 1b randomized, double-blind, placebo-controlled, crossover study of a single intravenous infusion dose of TAK-925 in patients With idiopathic hypersomnia. clinicaltrials.gov/ct2/show/NCT04091438.
Additional references
Ali 2009
- Ali M, Auger RR, Slocumb NL, Morgenthaler TI. Idiopathic hypersomnia: clinical features and response to treatment. Journal of Clinical Sleep Medicine 2009;5(6):562-8. [PMC free article] [PubMed] [Google Scholar]
Anderson 2007
- Anderson KN, Pilsworth S, Sharples LD, Smith IE, Shneerson JM. Idiopathic hypersomnia: a study of 77 cases. Sleep 2007;30(10):1274-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2004
- Banerjee D, Vitiello MV, Grunstein RR. Pharmacotherapy for excessive daytime sleepiness. Sleep Medicine Review 2004;8(5):339–54. [DOI] [PubMed] [Google Scholar]
Bassetti 1997
- Bassetti C, Aldrich MS. Idiopathic hypersomnia: a series of 42 patients. Brain 1997;120:1423-35. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- www.covidence.org.
EMA Modafinil 2010
- European Medicines Agency. Modafinil. ww.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Modafinil/human_referral_000236.jsp (accessed 3 October 2016).
Gowda 2014
- Gowda CR, Lundt LP. Mechanism of action of narcolepsy medications. CNS Spectrums 2014;19:25-33. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEPro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), multiple access dates; most recent 3/8/21. Available at gradepro.org.
Heal 2014
- Heal DJ, Gosden J, Smith SL. Dopamine reuptake transporter (DAT) “inverse agonism”—a novel hypothesis to explain the enigmatic pharmacology of cocaine. Neuropharmacology 2014;87:19-40. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT , Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.handbook.cochrane.org edition. The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
ICSD‐3 2014
- Sateia J. International Classification of Sleep Disorders, 3rd edition. Darien (IL): American Academy of Sleep Medicine, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krahn 2015
- Krahn LE, Hershner S, Loeding LD, Maski KP, Rifkin DI, Selim B, et al. Quality measures for the care of patients with narcolepsy. Journal of Clinical Sleep Medicine 2015;11(3):335-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Leu‐Semenescu 2014
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Medicine 2014;15(6):681-7. [DOI] [PubMed] [Google Scholar]
Leu‐Semenescu 2016
- Leu-Semenescu S, Louis P, Arnulf I. Benefits and risk of sodium oxybate in idiopathic hypersomnia versus narcolepsy type 1: a chart review. Sleep Medicine 2016;17:38-44. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu J, Greco MA. Sleep circuitry and the hypnotic mechanism of GABAA drugs. Journal of Clinical Sleep Medicine 2006;2(2):S19-26. [PubMed] [Google Scholar]
Maski 2021
- Maski K, Trotti LM, Kotagal S, Auger RR, Rowley JA, Hashmi SD, Watson NF. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine in press. [DOI: 10.5664/jcsm.9328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montplaisir 2001
- Montplaisir J, Fantini L. Idiopathic hypersomnia: a diagnostic dilemma. A commentary of "Idiopathic hypersomnia" (M. Billiard and Y. Dauvilliers). Sleep Medicine Reviews 2001;5:361-2. [DOI] [PubMed] [Google Scholar]
Morgenthaler 2007
- Morgenthaler TI, Kapur VK, Brown T, Swick TJ, Alessi C, Aurora RN, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007;30(12):1705-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nittur 2013
- Nittur N, Konofal E, Dauvilliers Y, Franco P, Leu-Semenescu S, Cock VC, et al. Mazindol in narcolepsy and idiopathic and symptomatic hypersomnia refractory to stimulants: a long-term chart review. Sleep Medicine 2013;14(1):30-6. [DOI] [PubMed] [Google Scholar]
Ohayon 2013
- Ohayon MM, Reynolds CF 3rd, Dauvilliers Y. Excessive sleep duration and quality of life. Annals of Neurology 2013;73(6):785-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozaki 2012
- Ozaki A, Inoue Y, Hayashida K, Nakajima T, Honda M, Usui A, et al. Quality of life in patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time: comparison between patients on psychostimulants, drug-naïve patients and the general Japanese population. Sleep Medicine 2012;13(2):200-6. [DOI] [PubMed] [Google Scholar]
Pizza 2013
- Pizza F, Ferri R, Poli F, Vandi S, Cosentino FI, Plazzi G. Polysomnographic study of nocturnal sleep in idiopathic hypersomnia without long sleep time. Journal of Sleep Research 2013;22(2):185-96. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 2.5.1. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Rye 2012
- Rye DB, Bliwise DL, Parker K, Trotti LM, Saini P, Fairley J, et al. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Science Translational Medicine 2012;4(161):161ra151. [DOI: 10.1126/scitranslmed.3004685] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Shinno 2011
- Shinno H, Ishikawa I, Yamanaka M, Usui A, Danjo S, Inami Y, et al. Effect of levothyroxine on prolonged nocturnal sleep time and excessive daytime somnolence in patients with idiopathic hypersomnia. Sleep Medicine 2011;12(6):578-83. [DOI] [PubMed] [Google Scholar]
Šonka 2015
- Šonka K, Šusta M, Billiard M. Narcolepsy with and without cataplexy, idiopathic hypersomnia with and without long sleep time: a cluster analysis. Sleep Medicine 2015;16(2):225-31. [DOI] [PubMed] [Google Scholar]
Trotti 2013
- Trotti LM, Saini P, Freeman AA, Bliwise DL, García PS, Jenkins A, et al. Improvement in daytime sleepiness with clarithromycin in patients with GABA-related hypersomnia: clinical experience. Journal of Psychopharmacology 2013;28(7):697-702. [DOI] [PubMed] [Google Scholar]
Trotti 2016
- Trotti LM, Saini P, Koola C, LaBarbera V, Bliwise DL, Rye DB. Flumazenil for the treatment of refractory hypersomnolence: clinical experience with 153 patients. Journal of Clinical Sleep Medicine 2016;12(10):1389-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernet 2009
- Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep 2009;32(6):753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernet 2010
- Vernet C, Leu-Semenescu S, Buzare MA, Arnulf I. Subjective symptoms in idiopathic hypersomnia: beyond excessive sleepiness. Journal of Sleep Research 2010;19(4):525-34. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Trotti 2017
- Trotti LM, Becker LA, Friederich Murray C, Hoque R. Medications for daytime sleepiness in individuals with idiopathic hypersomnia. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012714. [DOI: 10.1002/14651858.CD012714] [DOI] [PMC free article] [PubMed] [Google Scholar]


