In this issue, Rossaint and colleagues demonstrate that platelets contribute to the resolution of pulmonary inflammation by directly recruiting T regulatory (T reg) cells to the lungs and by transcriptionally reprogramming alveolar macrophages toward an anti-inflammatory phenotype.
Abstract
Platelets convey important nonhemostatic immune functions; however, their potential role in resolving pulmonary inflammation remains to be determined. In this issue of JEM, Rossaint et al. (2021. J. Exp. Med. https://doi.org/10.1084/jem.20201353) reveal that platelets contribute to the resolution of pulmonary inflammation by directly recruiting T regulatory (T reg) cells to the lungs and by transcriptionally reprogramming alveolar macrophages toward an anti-inflammatory phenotype.
Acute respiratory distress syndrome (ARDS) with pulmonary inflammation is a frequently occurring and life-threatening disorder (Matthay et al., 2012), with ∼40% of ARDS cases being fatal (Bellani et al., 2016). Pulmonary inflammation is characterized by increased pulmonary neutrophil recruitment, which can result in increased endothelial cell permeability, which leads to pulmonary edema and acute lung injury (Semple et al., 2019; Rebetz et al., 2018; Matthay et al., 2012). Macrophages also play an important pathogenic role (Kapur et al., 2019; Huang et al., 2018; Zeeuw van der Laan et al., 2020) as recently demonstrated by the fact that macrophages secrete the protein Osteopontin, which stimulates pulmonary neutrophil accumulation in the onset of antibody-mediated acute lung injury (Kapur et al., 2019). Interestingly, this pathogenic response may additionally be dependent on platelets, as demonstrated in various models of acute lung injury (Looney et al., 2009; Sreeramkumar et al., 2014). In the lungs, platelets may not only mediate injurious pathogenic responses but also protective responses depending on the specific setting of ARDS or the type of acute lung injury (Middleton et al., 2018). It was previously shown that platelets elicit an important role in the host defense to pneumonia-derived sepsis in mice caused by the gram-negative bacteria Klebsiella pneumoniae (de Stoppelaar et al., 2014). In that study, antibody-induced thrombocytopenia was found to be associated with impaired survival and increased bacterial growth in the lungs, distant organs, and circulation. This versatility of platelets illustrates their ability to regulate nonhemostatic immune functions, and indeed it has become clear in the last decade that platelets can convey a variety of immune-sensing functions, including targeting pathogens and communicating with a large variety of target cells (Kapur et al., 2015; Kapur and Semple, 2016).

Insights from Kapur and Semple.
Timely resolution of pulmonary inflammation is critical to limit uncontrolled host tissue destruction; however, it remains poorly understood how this is regulated. Rossaint and colleagues have now shed light on this by using a murine model of pneumonia induced by K. pneumoniae (Rossaint et al., 2021). They hypothesized that platelets may have an important role in the resolution of pulmonary inflammation. They depleted platelets in vivo 3–5 d after induction of pneumonia during the resolution phase, which resulted in persistent lung inflammation, increased collagen levels, and reduced lung compliance 2 wk after induction of pneumonia. This indeed suggested an important role for platelets in resolving pulmonary inflammation. Moreover, it was observed that platelet–neutrophil aggregates were present in peripheral blood during initiation of inflammation, but their frequency decreased from day 2, while from that moment, platelet–T reg cell aggregates appeared and increased until day 5, corresponding to the resolution phase. Analysis of cell surface marker expression demonstrated that the sheddase ADAM8 cleaved P-selectin glycoprotein ligand (PSGL)–1 on neutrophils during early inflammation, while PSGL-1 expression on circulating T reg cells increased, together allowing for the preferential binding of platelets to T reg cells during the onset of the resolution phase. In vivo T reg cell depletion inhibited the resolution of pulmonary inflammation, as observed by increased lung collagen content, reduced lung compliance, and persistently high numbers of neutrophils in bronchoalveolar lavage fluid (BALF). Furthermore, it was observed that T reg cell recruitment was associated with the emergence of the anti-inflammatory cytokines IL-10 and TGF-β in the BALF starting from day 3. Cellular in vitro co-culture experiments of T reg cells with platelets suggested that direct platelet–T reg cell interaction is required for IL-10 and TGF-β secretion, which could be blocked by PSGL-1 or P-selectin. Importantly, platelet depletion in vivo decreased the IL-10 and TGF-β levels in the BALF. In addition, the authors observed that increased numbers of macrophages were present in the BALF until at least 4–5 d after induction of pneumonia. They hypothesized that platelets may transcriptionally reprogram macrophages, and for that purpose they induced murine pneumonia, depleted platelets 3 d later, and subsequently isolated pro-inflammatory (CD68+, CD80+) and anti-inflammatory (CD68+, CD163+) macrophages from the BALF. They identified 61 genes in pro-inflammatory macrophages and 804 genes in anti-inflammatory macrophages that were differentially regulated upon platelet depletion. To demonstrate that platelets are crucially needed for the polarization and education of anti-inflammatory macrophages during resolution, platelets were depleted and macrophages were isolated from BALF 5 d after induction of pneumonia, and gene-expression profiles were analyzed. Macrophages showed a shift toward a pro-inflammatory state, which could subsequently be reversed by intratracheal instillation of platelets into the platelet-depleted mice. Interestingly, macrophages isolated from platelet-depleted mice also demonstrated a lower efferocytosis index (phagocytosis of apoptotic neutrophils by macrophages) compared with macrophages from nondepleted mice. Importantly, the efferocytosis could be restored by intratracheal administration of platelets into platelet-depleted mice. This suggests that platelet-reprogrammed alveolar macrophages functionally contribute to efferocytosis and thus clear dying neutrophils in the resolution of pulmonary inflammation.
Rossaint and colleagues thus shed light on the role of platelets in not only the initiation but also the resolution of bacterial lung inflammation (see figure). They identify an important resolving role for T reg cells, as platelet interaction with T reg cells was found to be required for their recruitment into the lung at the onset of the resolution phase, which was accompanied by elevated levels of IL-10 and TGF-β in the BALF. In this setting of K. pneumoniae–induced pulmonary inflammation, the critical requirement for IL-10 and TGF-β for resolution of lung inflammation was not directly investigated. In addition, the in vivo cellular source of IL-10 and TGF-β remains to be elucidated, despite having observed a higher mRNA expression of IL-10 and Tgfb1 in T reg cells compared with macrophages and despite in vitro co-culture experiments of T reg cells with platelets demonstrating that direct interaction of platelets with T reg cells can induce IL-10 and TGF-β production. Previously, it was found that T reg cells and dendritic cells are the key protecting cells in antibody-mediated acute lung injury, a response which was also found to be associated with increased levels of IL-10 (Kapur et al., 2017). In that study, in vivo depletion of T reg cells or dendritic cells resulted in low IL-10 levels, which was associated with induction of antibody-mediated acute lung injury. This observation was confirmed by using IL-10 knockout mice, which were found to be hyper-susceptible to induction of acute lung injury by antibodies (Kapur et al., 2017). Interestingly, prophylactic or therapeutic (after onset of symptoms) administration of IL-10 prevented and rescued, respectively, the development of antibody-induced acute lung injury (Kapur et al., 2017). Regarding the in vivo cellular source of IL-10 in protection against pulmonary inflammation and acute lung injury, it may be possible that dendritic cells are crucial cells (Kapur et al., 2017), which may also regulate the interplay between T reg cells and platelets as described by Rossaint and co-workers. This will, however, need to be further elucidated. Furthermore, it was shown that platelets transcriptionally reprogram macrophages (and T reg cells); however, it will be interesting to investigate how these cellular interactions may also alter the transcriptional landscape of platelets, and consequently how this may affect their immune functions. The current study also elegantly illustrates the dual immune nature of platelets, which through interaction with neutrophils facilitate the development of lung inflammation, while on the other hand through interaction with T reg cells and alveolar macrophages lead the way toward resolution of lung inflammation. Additional studies are now warranted to validate and further expand on these promising results. This may potentially have important implications for the use of anti-platelet drugs and may provide a basis for the development of novel treatment approaches for ARDS and pulmonary inflammation.
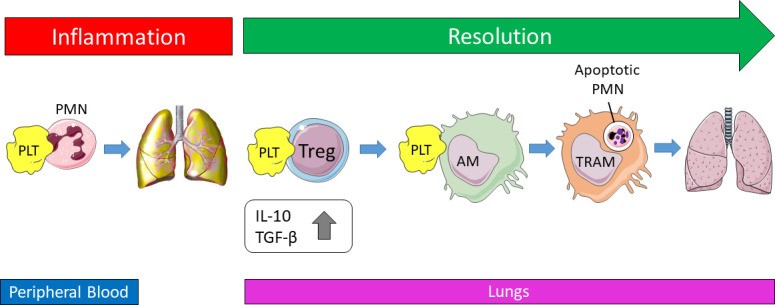
Platelets facilitate the onset of bacterial lung inflammation through interaction with neutrophils in peripheral blood, but switch to interacting with T reg cells during the start of the resolution phase, enabling platelet–T reg cell recruitment into the lungs. This is accompanied by the emergence of elevated levels of the anti-inflammatory cytokines IL-10 and TGF-β. Furthermore, platelets transcriptionally reprogram alveolar macrophages toward an anti-inflammatory phenotype, which allows significantly increased efferocytosis, which results in increased clearance of apoptotic neutrophils. Altogether, this leads to resolution of pulmonary inflammation. PLT, platelet; PMN, neutrophil; AM, alveolar macrophage; TRAM, transcriptionally reprogrammed alveolar macrophage.
References
- Bellani, G., et al. 2016. JAMA. 10.1001/jama.2016.0291 [DOI] [Google Scholar]
- de Stoppelaar, S.F., et al. 2014. Blood. 10.1182/blood-2014-05-573915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., et al. 2018. Mediators Inflamm. 10.1155/2018/1264913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur, R., and Semple J.W.. 2016. Blood Adv. 10.1182/bloodadvances.2016000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur, R., et al. 2015. J. Immunol. 10.4049/jimmunol.1500259 [DOI] [Google Scholar]
- Kapur, R., et al. 2017. Blood. 10.1182/blood-2016-12-758185 [DOI] [Google Scholar]
- Kapur, R., et al. 2019. Blood. 10.1182/blood.2019000972 [DOI] [Google Scholar]
- Looney, M.R., et al. 2009. J. Clin. Invest. 10.1172/JCI38432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay, M.A., et al. 2012. J. Clin. Invest. 10.1172/JCI60331 [DOI] [Google Scholar]
- Middleton, E.A., et al. 2018. Am. J. Respir. Cell Mol. Biol. 10.1165/rcmb.2017-0420TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebetz, J., et al. 2018. Transfus. Med. Hemother. 10.1159/000492950 [DOI] [Google Scholar]
- Rossaint, J., et al. 2021. J. Exp. Med. 10.1084/jem.20201353 [DOI] [Google Scholar]
- Semple, J.W., et al. 2019. Blood. 10.1182/blood-2018-10-860809 [DOI] [Google Scholar]
- Sreeramkumar, V., et al. 2014. Science. 10.1126/science.1256478 [DOI] [Google Scholar]
- Zeeuw van der Laan, E.A.N., et al. 2020. Curr. Opin. Hematol. 10.1097/MOH.0000000000000607 [DOI] [PubMed] [Google Scholar]


