Abstract
Intestinal commensal bacteria are considered good indicators for monitoring antimicrobial resistance. We investigated the antimicrobial resistance profiles and resistance trends of Enterococcus faecium and Enterococcus faecalis isolated from food animals in Korea between 2010 and 2019. E. faecium and E. faecalis, isolated from chickens and pigs, respectively, presented a relatively high resistance rate to most of the tested antimicrobials. We observed high ciprofloxacin (67.9%), tetracycline (61.7%), erythromycin (59.5%), and tylosin (53.0%) resistance in E. faecium isolated from chickens. Similarly, more than half of the E. faecalis isolates from pigs and chickens were resistant to erythromycin, tetracycline and tylosin. Notably, we observed ampicillin, daptomycin, tigecycline and linezolid resistance in a relatively small proportion of enterococcal isolates. Additionally, the enterococcal strains exhibited an increasing but fluctuating resistance trend (p < 0.05) to some of the tested antimicrobials including daptomycin and/or linezolid. E. faecalis showed higher Multidrug resistance (MDR) rates than E. faecium in cattle (19.7% vs. 8.6%, respectively) and pigs (63.6% vs. 15.6%, respectively), whereas a comparable MDR rate (≈60.0%) was noted in E. faecium and E. faecalis isolated from chickens. Collectively, the presence of antimicrobial-resistant Enterococcus in food animals poses a potential risk to public health.
Keywords: antimicrobial resistance, E. faecium, E. faecalis, food animals, public health
1. Introduction
Enterococci are commensal bacteria of the gastrointestinal tract of animals and humans. They can also be detected in the environment and in foods of animal origin. They are considered emerging pathogens of humans and are often associated with invasive nosocomial infections [1]. Enterococci have emerged as good indicators of antibiotic resistance. They can acquire resistance genes from other bacteria, which can also spread to other commensal and pathogenic bacteria through horizontal transfer of mobile genetic elements [2]. Besides, the frequent use of antimicrobials in humans and animals select for resistant enterococci [3].
There are 55 enterococci species reported so far based on 16S rDNA sequences. Enterococcus faecium and Enterococcus faecalis are the most commonly isolated species, accounting for more than 80% of isolates [4]. E. faecium and E. faecalis have become increasingly important pathogens worldwide because they are associated with life-threatening hospital-acquired infections, whereas the remaining Enterococcus spp. are infrequent causes of human clinical infections [3]. E. faecalis is the most pathogenic species, while E. faecium is commonly involved in the acquisition and transfer of antimicrobial resistance [5].
Food animals have been suggested as a possible intermediate vector in the transmission mode of antimicrobial-resistant enterococci. Cross-contamination of edible carcass tissues during the slaughter process represents a significant food safety hazard [6]. Tyson et al. [7] and Boehm et al. [8] demonstrated that more than 90% of food samples from animals are contaminated with Enterococcus at the slaughterhouse, predominantly with E. faecium and E. faecalis. Therefore, it is important to continuously evaluate the antimicrobial resistance profiles of E. faecium and E. faecalis strains found in food animals.
Monitoring the resistance profiles and temporal trends of antimicrobial resistance in Enterococcus species isolated from food animals provides useful information for understanding the level of resistance of gut microbial flora as well as for the empirical selection of antimicrobial agents to treat infected patients. Several researchers have reported the antimicrobial resistance profiles of Enterococcus isolated from food animals worldwide [9,10,11,12,13,14]. In South Korea (Korea), only a few studies have been conducted to determine the resistance profiles of E. faecium and E. faecalis isolates from food animals [15,16,17,18,19,20]. These studies were conducted in isolates collected from some parts of the country for a very short duration, and hence the resistance trends of the Enterococcus isolates remained unexplored. Thus, we performed this study to determine the antimicrobial resistance profiles and resistance trends of E. faecium and E. faecalis isolated from healthy cattle, pigs and chickens throughout Korea between 2010 and 2019.
2. Materials and Methods
2.1. Bacterial Isolates
A total of 3360 E. faecium isolates (572 isolates from cattle, 1385 from pigs, and 1403 from chickens) and 4218 E. faecalis isolates (910 isolates from cattle, 1556 from pigs, and 1752 from chickens) were obtained from 16 laboratories/centers participating in the Korean Veterinary Antimicrobial Resistance Monitoring System between 2010 and 2019. The isolates were recovered from the feces of animals in various slaughterhouses (Supplementary Table S1). No more than five fecal samples were collected from each farm. Sample processing and enterococcal isolation were performed according to Lim et al. [20] using buffered peptone water and Enterococcus agar media (Becton, Dickinson, Sparks, MD). Enterococcus species were identified by Polymerase Chain Reaction (PCR) [21] or matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF, Biomerieux, Marcy L’Etoile, France). One isolate per sample was selected for the antimicrobial susceptibility test.
2.2. Antimicrobial Susceptibility Study
The antimicrobial susceptibility profiles of the enterococcal isolates were determined by the broth microdilution method [22], using commercially available antibiotic-containing plates (Sensititre, Trek Diagnostics, Cleveland, OH, USA). The following antimicrobials were tested: ampicillin (1–16 µg/mL), chloramphenicol (2–32 µg/mL), ciprofloxacin (0.25–16 µg/mL), daptomycin (0.5–32 µg/mL), erythromycin (1–64 µg/mL), florfenicol (2–32 µg/mL), gentamicin (128–2048 µg/mL), kanamycin (128–2048 µg/mL), linezolid (0.5–16 µg/mL), quinupristin/dalfopristin (1–32 µg/mL), salinomycin (2–32 µg/mL), streptomycin (128–2048 µg/mL), tetracycline (2–128 µg/mL), tigecycline (0.12–2 µg/mL), tylosin (1–64 µg/mL) and vancomycin (2–32 µg/mL). Enterococcus faecalis ATCC 29212 was used as a quality reference strain. The MIC values were interpreted according to the guidelines of the Clinical and Laboratory Standards Institute [22], the National Antimicrobial Resistance Monitoring System [23], and the Danish Integrated Antimicrobial Resistance Monitoring and Research Programme [24]. MIC50 and MIC90 values were defined as the lowest concentration of antimicrobials at which 50% and 90% of the isolates were inhibited, respectively. Isolates that were resistant to at least three subclasses of antimicrobials, excluding resistance to quinupristin/dalfopristin in E. faecalis, were considered multidrug-resistant.
2.3. Data Analysis
Analysis of the antimicrobial resistance rates and Pearson correlation was conducted using Excel (Microsoft-Excel, 2016, Microsoft Corporation, Redmond, WA, USA) and Rex software (Version 3.0.3, RexSoft Inc., Seoul, Korea). p values less than 0.05 were considered significant.
3. Results
3.1. Antimicrobial Resistance
In general, a more frequent occurrence of resistance to most of the tested antimicrobials was observed among the E. faecalis isolates compared with E. faecium (Table 1). More than 50% of the E. faecalis isolates were resistant to macrolides and tetracycline, whereas we noted a moderate resistance rate to these antimicrobials in E. faecium. Both enterococcal species demonstrated a very low resistance rate (≤10.0%) to ampicillin, daptomycin, tigecycline, linezolid and salinomycin. However, all isolates were susceptible to vancomycin.
Table 1.
Antimicrobial resistance rate in E. faecium (n = 3360) and E. faecalis (n = 4218) recovered from cattle, pigs, and chickens between 2010 and 2019 in Korea.
| Resistance Rate % (Number of Isolates) | ||||||||
|---|---|---|---|---|---|---|---|---|
| E. faecium | E. faecalis | |||||||
| Antimicrobials | Cattle | Pigs | Chickens | Subtotal | Cattle | Pigs | Chickens | Subtotal |
| (n = 572) | (n = 1385) | (n = 1403) | (n = 3360) | (n = 910) | (n = 1556) | (n = 1752) | (n = 4218) | |
| Ampicillin | 0.3 (2) | 0.1 (1) † | 11.4 (160) # | 4.8 (163) | 0 (0) | 0.1 (1) | 0.1 (2) | 0.1 (3) |
| Chloramphenicol | 2.3 (13) * | 6.9 (95) † | 18.8 (264) # | 11.1 (372) | 12.1 (110) * | 50.0(778) † | 20.9 (367) # | 29.8 (1255) |
| Ciprofloxacin | 23.1 (132) * | 13.6 (189) † | 67.9 (953) # | 37.9 (1274) | 7.9 (72) * | 13.7 (213) † | 48.6 (852) # | 27.0 (1137) |
| Daptomycin | 5.6 (32) | 6.9 (95) † | 11.1 (156) # | 8.4 (283) | 1.3 (12) | 0.6 (10) | 0.6 (10) # | 0.8 (32) |
| Erythromycin | 19.9 (114) * | 31.5 (436) † | 59.5 (835) # | 41.2 (1385) | 20.4 (186) * | 67.1 (1044) † | 63.0 (1104) # | 55.3 (2334) |
| Florfenicol | 1.6 (9) * | 8.4 (116) † | 18.7 (262) # | 11.5 (387) | 4.5 (41) * | 45.8 (712) † | 14.3 (250) # | 23.8 (1003) |
| Gentamicin | 0.2 (1) | 0.7 (10) † | 2.4 (33) # | 1.3 (44) | 7.5 (68) * | 18.9 (294) † | 10.3 (181) # | 12.9 (543) |
| Kanamycin | 6.3 (36) * | 9.5 (132) | 10.1 (142) # | 9.2 (310) | 13.4 (122) * | 44.3 (690) † | 21.3 (374) # | 28.1 (1186) |
| Linezolid | 0.3 (2) | 0 (0) † | 5.2 (73) # | 2.2 (75) | 0.2 (2) * | 1.4 (22) | 1.1 (20) # | 1.0 (44) |
| Quinupristin/dalfopristin | 8.7 (50) | 11.9 (165) † | 28.0 (393) # | 18.1 (608) | ND | ND | ND | ND |
| Salinomycin | 0.2 (1) | 0.4 (5) † | 11.0(154) # | 4.8 (160) | 0 (0) | 0 (0) † | 2.8 (49) # | 1.2 (49) |
| Streptomycin | 6.8 (39) * | 12.4 (172) † | 30.8 (432) # | 19.1 (643) | 18.8 (171) * | 50.9 (792) † | 31.8 (558) # | 36.1 (1521) |
| Tetracycline | 25.9 (148) * | 20.1 (279) † | 61.7 (866) # | 38.5 (1293) | 41.8 (380) * | 78.3 (1219) | 76.1 (1333) # | 69.5 (2932) |
| Tigecycline | 6.8 (39) | 5.8 (81) | 5.5 (77) | 5.9 (197) | 7.5 (68) * | 11.5 (179) | 10.6 (185) # | 10.2 (432) |
| Tylosin | 4.9 (28) * | 13.8 (191) † | 53.0 (743) # | 28.6 (962) | 20.1 (183) * | 66.6 (1036) | 63.6 (1115) # | 55.3 (2334) |
| Vancomycin | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MDR | 8.6 (49) * | 15.6 (216) † | 60.9 (854) # | 33.3 (1119) | 19.7 (179) * | 63.6 (990) † | 59.7 (1046) # | 52.5 (2215) |
* p < 0.05 compared with the resistance rates in pigs, # p < 0.05 compared with the resistance rates in chickens, and † p < 0.05 compared with the resistance rates in pigs.
E. faecium isolates recovered from chickens demonstrated a very high resistance rate to most of the tested antimicrobials compared with those isolated from cattle and pigs (Table 1). More than half of the E. faecium isolates from chickens were resistant to ciprofloxacin, erythromycin, tetracycline, and tylosin. E. faecium isolates from cattle presented relatively low resistance rates (<10.0%) to the tested antimicrobials except to tetracycline (25.9%), ciprofloxacin (23.1%), and erythromycin (19.9%). Similarly, pig isolates exhibited low resistance rates (<15.0%) to most of the tested antimicrobials, except to erythromycin (31.5%) and tetracycline (20.1%). We observed linezolid (0.0–5.2%), daptomycin (5.6–11.1%), and tigecycline (5.5–6.8%) resistance in a relatively small percentage of E. faecium isolates. Indeed, the linezolid resistance rate was high in chicken isolates (5.2%) compared with those of cattle (0.3%) and pigs (0.0%). Additionally, we identified high-level gentamicin (1.3%, MIC ≥ 500 µg/mL), kanamycin (15.0%, MIC ≥ 500 µg/mL), and streptomycin (17.1%, MIC ≥ 2000 µg/mL) resistance in E. faecium isolated mainly from chickens and pigs (Supplementary Table S2). The MIC50 and MIC90 of the tested antimicrobials against E. faecium isolated from cattle, chickens, and pigs are summarized in Supplementary Table S3A–C.
E. faecalis isolated from chickens and pigs exhibited high resistance rate to most of the tested antimicrobials, especially to tetracycline (76.1–78.3%), erythromycin (63.0–67.1%), and tylosin (63.6–66.6%) (Table 1). In contrast, cattle isolates presented a moderate or low resistance rate to these antimicrobials. We identified linezolid (1%), daptomycin (0.8%), and tigecycline (10.2%) resistance in a very small proportion of E. faecalis isolates. High-level gentamicin (14%), kanamycin (28.5%), and streptomycin (34.2%) resistances were noted in E. faecalis isolated predominantly from chickens and pigs (Supplementary Table S4). The MIC50 and MIC90 of the tested antimicrobials against E. faecalis isolated from cattle, chickens, and pigs are summarized in Supplementary Table S5A–C.
3.2. Antimicrobial Resistance Trends
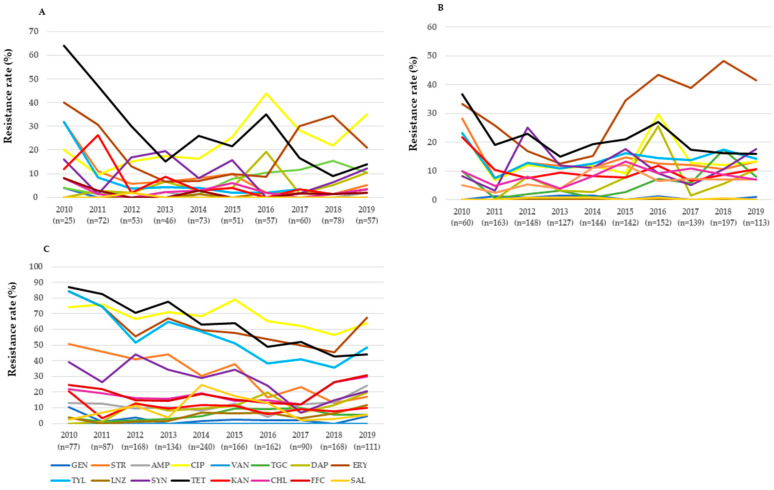
We observed variations in the antimicrobial resistance trend between the two strains as well as in the same strain from different sources. E. faecium isolates exhibited an increasing but fluctuating trend of resistance to ciprofloxacin and tigecycline (isolates from cattle), erythromycin (from pigs), and daptomycin and linezolid (from chickens) (Figure 1 and Supplementary Tables S6–S8). In contrast, we noted a decreasing but fluctuating resistance trend to some of the tested antimicrobials in E. faecium isolated from cattle (tetracycline, streptomycin, and tylosin) and chickens (streptomycin, ciprofloxacin, tetracycline, erythromycin, tylosin, and quinupristin/dalfopristin). Although we did not find a significant change in the daptomycin resistance trend in E. faecium isolated from cattle and pigs, resistance rates peaked in 2016.
Figure 1.
Antimicrobial resistance trend of E. faecium isolates recovered from cattle (A), pigs (B), and chickens (C) in Korea from 2010 to 2019. Abbreviations: AMP, ampicillin; CIP, ciprofloxacin; CHL, chloramphenicol; DAP, daptomycin; ERY, erythromycin; FFC, florfenicol; GEN, gentamicin; KAN, kanamycin; LIN, linezolid; SAL, salinomycin; STR, streptomycin; SYN, quinupristin/dalfopristin; TET, tetracycline; TGC tigecycline, TYL, tylosin; and VAN, vancomycin.
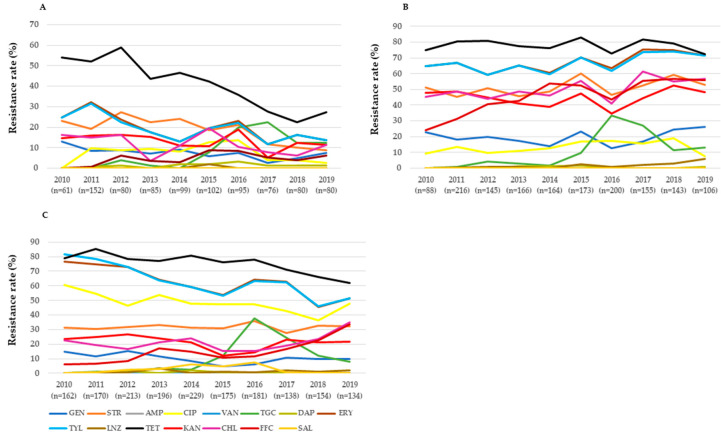
E. faecalis isolates presented an increasing but fluctuating resistance trend to tigecycline (isolates from cattle), erythromycin, tigecycline, linezolid, and florfenicol (from pigs), and florfenicol (from chickens) (Figure 2 and Supplementary Tables S9–S11). Nevertheless, a decreasing but fluctuating resistance trend was noted in E. faecalis isolated from cattle (gentamicin, streptomycin, erythromycin, tylosin and tetracycline) and chickens (ciprofloxacin, erythromycin, tylosin and tetracycline). In addition, we noted a stable daptomycin resistance rate in E. faecalis isolated from cattle, chickens and pigs.
Figure 2.
Antimicrobial resistance trend of E. faecalis isolates recovered from cattle (A), pigs (B), and chickens (C) in Korea from 2010 to 2019. Abbreviations: AMP, ampicillin; CIP, ciprofloxacin; CHL, chloramphenicol; DAP, daptomycin; ERY, erythromycin; FFC, florfenicol; GEN, gentamicin; KAN, kanamycin; LIN, linezolid; SAL, salinomycin; STR, streptomycin; TET, tetracycline; TGC, tigecycline, TYL, tylosin; and VAN, vancomycin.
3.3. Multidrug Resistance (MDR) and Antimicrobial Resistance Patterns
The majority of E. faecium (72.0%) and E. faecalis (80.7%) isolates were resistant to one or more of the tested antimicrobials (Table 2 and Table 3). Notably, 33.3% of the E. faecium and 52.5% of the E. faecalis isolates were resistant to multiple antimicrobials (Table 1). MDR was high in E. faecium isolated from chickens (60.9%), and in E. faecalis recovered from pigs (63.6%) and chickens (59.7%). Besides, resistance to five or more antimicrobials was noted in E. faecium isolated from chickens (44.7%), and in E. faecalis from pigs (56.6%) and chickens (34.7%) (Table 2 and Table 3). A decreasing (p < 0.05) but fluctuating MDR trend was found in E. faecium and E. faecalis isolated from cattle and chickens, whereas the converse was noted in E. faecalis from pigs (Supplementary Tables S6, S8–S10 and S11).
Table 2.
Frequent resistance patterns in E. faecium isolates (n = 3360) recovered from cattle, pigs, and chickens between 2010 and 2019 in Korea.
| Animal Species | No. of Antimicrobials | No. of Isolates (%) | Most Frequent Resistance Pattern |
|---|---|---|---|
| Cattle (n = 572) | 0 | 216 (37.8) | |
| 1 | 199 (34.8) | ERY (n = 63) | |
| 2 | 107 (18.7) | CIP TET (n = 43) | |
| 3 | 22 (3.8) | CHL STR TET (n = 3) | |
| 4 | 7 (1.2) | ERY STR TET TYL (n = 4) | |
| 5 | 3 (0.5) | CIP ERY KAN TET TYL (n = 1), ERY KAN STR TET TYL (n = 1), ERY SYN STR TET TYL (n = 1) | |
| 6 | 9 (1.6) | CIP ERY KAN STR TET TYL (n = 3) | |
| 7 | 4 (0.7) | AMP CHL CIP ERY STR TET TYL (n = 1), CHL CIP FFC ERY STR TET TYL (n = 1), CHL FFC ERY KAN STR TET TYL (n = 1), CIP ERY KAN SYN STR TET TYL (n = 1) | |
| 8 | 4 (0.7) | CHL CIP FFC ERY KAN STR TET TYL (n = 1), CHL CIP FFC ERY LZD STR TET TYL (n = 1), CHL CIP FFC ERY SYN STR TET TYL (n = 1), CHL FFC ERY KAN SYN STR TET TYL (n = 1) | |
| 9 | 0 (0) | ||
| 10 | 1 (0.2) | AMP CHL CIP FFC ERY KAN SYN STR TET TYL (n = 1) | |
| Pigs (n = 1385) | 0 | 605 (43.7) | |
| 1 | 406 (29.3) | ERY (n = 179) | |
| 2 | 137 (9.9) | CIP TET (n = 25) | |
| 3 | 49 (3.5) | ERY TET TYL (n = 8) | |
| 4 | 26 (1.9) | CHL FFC ERY TYL (n = 3), CIP ERY TET TYL (n = 3), ERY STR TET TYL (n = 3) | |
| 5 | 44 (3.2) | ERY KAN STR TET TYL (n = 9) | |
| 6 | 56 (4.0) | CHL FFC ERY STR TET TYL (n = 17) | |
| 7 | 28 (2.0) | CHL FFC ERY KAN STR TET TYL (n = 8) | |
| 8 | 25 (1.8) | CHL FFC ERY KAN SYN STR TET TYL (n = 13) | |
| 9 | 6 (0.4) | CHL CIP FFC ERY KAN SYN STR TET TYL (n = 4) | |
| 10 | 3 (0.2) | AMP CHL CIP FFC DAP ERY SYN STR TET TYL (n = 1), CHL CIP FFC ERY GEN KAN SYN STR TET TYL (n = 1), CHL CIP FFC ERY KAN SYN STR TET TGC TYL (n = 1) | |
| Chicken (n = 1403) | 0 | 134 (9.6) | |
| 1 | 205 (14.6) | CIP (n = 80) | |
| 2 | 158 (11.3) | CIP TET (n = 73) | |
| 3 | 132 (9.4) | CIP STR TET (n = 24) | |
| 4 | 146 (10.4) | CIP ERY TET TYL (n = 53) | |
| 5 | 195 (13.9) | CIP ERY STR TET TYL (n = 48) | |
| 6 | 191 (13.6) | CIP ERY SYN STR TET TYL (n = 38) | |
| 7 | 101 (7.2) | AMP CIP ERY SYN STR TET TYL (n = 7) | |
| 8 | 73 (5.2) | CHL CIP FFC ERY SYN STR TET TYL (n = 12) | |
| 9 | 51 (3.6) | AMP CHL CIP FFC ERY SYN STR TET TYL (n = 8) | |
| 10 | 16 (1.1) | AMP CHL CIP FFC ERY LZD SAL STR TET TYL (n = 3) | |
| 11 | 1 (0.1) | CHL CIP FFC ERY KAN LZD SYN SAL STR TET TYL (n = 1) |
Abbreviations: AMP, Ampicillin; CIP, Ciprofloxacin; CHL, Chloramphenicol; DAP, Daptomycin; ERY, Erythromycin; FFC, Florfenicol; GEN, Gentamicin; KAN, Kanamycin; LNZ, Linezolid; SAL, Salinomycin; STR, Streptomycin; SYN, Quinupristin/Dalfopristin; TET, Tetracycline; TYL, Tylosin; TGC, Tigecycline; VA, Vancomycin.
Table 3.
Frequent resistance patterns in E. faecalis isolates (n = 4218) recovered from cattle, pigs, and chickens between 2010 and 2019 in Korea.
| Animal Species | No. of Antimicrobials | No. of Isolates (%) | Most Frequent Resistance Pattern |
|---|---|---|---|
| Cattle (n = 910) | 0 | 454 (49.9) | |
| 1 | 184 (20.2) | TET (n = 132) | |
| 2 | 66 (7.3) | STR TET (n = 32) | |
| 3 | 46 (5.1) | ERY TET TYL (n = 19) | |
| 4 | 28 (3.1) | CHL ERY TET TYL (n = 10) | |
| 5 | 32 (3.5) | ERY KAN STR TET TYL (n = 17) | |
| 6 | 33 (3.6) | ERY GEN KAN STR TET TYL (n = 7) | |
| 7 | 47 (5.2) | CHL ERY GEN KAN STR TET TYL (n = 24) | |
| 8 | 18 (2.0) | CHL CIP ERY GEN KAN STR TET TYL (n = 6) | |
| 9 | 2 (0.2) | CHL CIP FFC ERY KAN LZD STR TET TYL (n = 1), | |
| CHL FFC DAP ERY GEN KAN STR TET TYL (n = 1) | |||
| Pigs (n = 1556) | 0 | 216 (13.9) | |
| 1 | 186 (12.0) | TET (n = 139) | |
| 2 | 79 (5.1) | STR TET (n = 24) | |
| 3 | 88 (5.7) | ERY TET TYL (n = 45) | |
| 4 | 104 (6.7) | CHL ERY TET TYL (n = 20) | |
| 5 | 133 (8.5) | CHL FFC ERY TET TYL (n = 53) | |
| 6 | 269 (17.3) | CHL FFC ERY STR TET TYL (n = 67) | |
| 7 | 248 (15.9) | CHL FFC ERY KAN STR TET TYL (n = 147) | |
| 8 | 156 (10.0) | CHL FFC ERY GEN KAN STR TET TYL (n = 73) | |
| 9 | 66 (4.2) | CHL CIP FFC ERY GEN KAN STR TET TYL (n = 37) | |
| 10 | 11 (0.7) | CHL CIP FFC ERY GEN KAN STR TET TGC TYL (n = 6) | |
| Chickens (n = 1752) | 0 | 144 (8.2) | |
| 1 | 264 (15.1) | TET (n = 202) | |
| 2 | 143 (8.2) | CIP TET (n = 39) | |
| 3 | 222 (12.7) | ERY TET TYL (n = 83) | |
| 4 | 369 (21.1) | CIP ERY TET TYL (n = 193) | |
| 5 | 277 (15.8) | CIP ERY STR TET TYL (n = 52) | |
| 6 | 141 (8.0) | CIP ERY GEN KAN TET TYL (n = 17) | |
| 7 | 97 (5.5) | CHL CIP ERY KAN STR TET TYL (n = 16) | |
| 8 | 64 (3.7) | CHL CIP ERY GEN KAN STR TET TYL (n = 23) | |
| 9 | 24 (1.4) | CHL CIP FFC ERY GEN KAN STR TET TYL (n = 14) | |
| 10 | 7 (0.4) | CHL CIP FFC ERY GEN KAN LZD STR TET TYL (n = 4) |
Abbreviations: AMP, Ampicillin; CIP, Ciprofloxacin; CHL, Chloramphenicol; DAP, Daptomycin; ERY, Erythromycin; FFC, Florfenicol; GEN, Gentamicin; KAN, Kanamycin; LNZ, Linezolid; SAL, Salinomycin; STR, Streptomycin; TET, Tetracycline; TYL, Tylosin; TGC, Tigecycline; VA, Vancomycin.
A total of 434 and 262 MDR combination patterns were observed in E. faecium and E. faecalis isolates, respectively. Ciprofloxacin resistance with (5.2%) or without (5.7%) tetracycline was frequently noted in E. faecium isolated from chickens, whereas resistance to erythromycin was predominant in cattle (11%) and pig (12.9%) isolates (Table 2). Tetracycline resistance was frequently noted in E. faecalis isolated from cattle (14.5%) (Table 3). Tetracycline resistance with (11.0%) or without (11.5%) ciprofloxacin and macrolides were most frequent in E. faecalis isolated from chickens. The most frequent MDR pattern in E. faecalis isolated from pigs was resistance to seven antimicrobials (9.4%), including macrolides (Table 3).
4. Discussion
Knowledge of the distribution of antimicrobial-resistant bacteria in food animals and the food chain is vital for determining the potential risk to human health. Korea relies on testing isolates from food animals and foods of animal origin to determine the development and trends of antimicrobial resistance in the food chain. Consequently, this study provides a better understanding of the antimicrobial resistance profiles of the two most common enterococcal isolates recovered from healthy food animals slaughtered in Korea.
In this study, a considerable proportion of enterococcal isolates exhibited resistance to tetracycline and ciprofloxacin. Consistent with this study, high tetracycline resistance was reported in E. faecium and E. faecalis isolated from chickens and pigs in Korea [15,16,18,19,25], other Asian countries [13,26], Europe [27,28,29], and North America [30,31]. The ciprofloxacin resistance rate in E. faecium (67.9%) and E. faecalis (48.6%) isolated from chickens were higher than previous reports in Korea [15,16,18,25] but contradict reports from other countries [27,28,30,31,32,33]. About 85–100 tons of tetracyclines and 35–40 tons of quinolones (mainly enrofloxacin for poultry) were sold annually for the livestock industry in Korea during the study period [34]. Thus, the widespread use of these antimicrobials in livestock production provides selective pressure and accelerates the emergence of resistant Enterococcus strains.
The chloramphenicol and florfenicol resistance in E. faecium isolated from chickens (18.7–18.8%) and E. faecalis isolated from pigs (45.8–50.0%) were higher than those described in previous studies in Korea [15,18,25]. We also observed an increasing florfenicol resistance trend in E. faecalis isolated from pigs and chickens. Several studies in Asia and Europe reported variable chloramphenicol resistance rates in E. faecium (8–53%) and E. faecalis (16–53%) isolated from cattle, pigs and chickens [26,32,35,36]. The average annual consumption of florfenicol in Korean livestock especially in chickens and pigs was increased by about 50% in the last five years compared to the amount during 2010–2014 [34]. Thus, the frequent use of florfenicol in food animals might select for chloramphenicol and florfenicol resistance. Our recent studies also identified the oxazolidinone and phenicol resistance genes (fexA, optrA, and poxtA) in Enterococcus strains recovered from food animals and their carcasses in Korea. The optrA and poxtA genes were transferred to recipient strains from about 48% and 28% of optrA and poxtA-carrying enterococcal strains, respectively. Further, the fexA gene was co-transferred with the optrA gene in all optrA-positive transconjugants [17,37,38]. Therefore, horizontal dissemination of phenicol resistance genes among enterococcal isolates might also contribute to the increase in chloramphenicol and florfenicol resistance.
Enterococcus strains identified in this study demonstrated a relatively low resistance rate to the tested aminoglycosides; except to kanamycin and streptomycin in E. faecalis isolated from pigs. Additionally, high-level gentamicin, kanamycin and streptomycin resistance were noted among the Enterococcus strains. Previous studies in Asian countries [13,18,25,26] and Europe [27,29,36] have reported highly variable gentamicin (8–95%), kanamycin (38–62%), and streptomycin (62–100%) resistance rates in E. faecium and E. faecalis isolated from various food animals. Although enterococci are intrinsically resistant to clinically achievable concentrations of aminoglycosides, they are considered the antimicrobials of choice to treat human enterococcal infections when combined with cell wall inhibitors [4]. The widespread application of aminoglycosides in food animals (50, 71, and 296 tons in cattle, chickens and pigs, respectively, between 2010 and 2019) in Korea could be associated with the emergence of resistance [34]. Of note, the emergence of high-level aminoglycoside-resistant strains cannot be ignored because it could threaten the existing efficacy of the broad-spectrum activity of aminoglycosides.
Resistance to penicillin may narrow the therapeutic options for enterococcal infections [39]. Consistent with previous reports in Korea [18,25] and other countries [10,13,31,35,40], 4.8% of E. faecium isolates, especially those isolated from chickens, and 0.1% of E. faecalis were resistant to ampicillin. In contrast, previous studies have reported a relatively high ampicillin resistance in E. faecium from poultry in Germany (28%) [27], and E. faecalis from pigs in Thailand (44%) and Laos (12%) [26]. Ampicillin and penicillin are the most active β-lactams against Enterococcus inhibiting the synthesis of peptidoglycan [41]. Although we do not have information about the resistance determinants, intrinsic tolerance to the action of β-lactamase in E. faecium is associated with the presence of a species-specific chromosomal gene, pbp5, which encodes a class B penicillin-binding protein (PBP) with low affinity for ampicillin. Maintenance of peptidoglycan in the stationary phase mediated by an l,d-transpeptidase (Ldtfm) and overproduction of β-lactamase have also been implicated in ampicillin resistance in E. faecium [41]. Similarly, the overproduction of β-lactamase and point mutations of penicillin-binding protein (PBP4) have been shown to confer ampicillin resistance in E. faecalis [41,42].
Macrolide-lincosamide-streptogramin antibiotics constitute an alternative therapy for the treatment of insidious enterococcal infections [43]. We noted high erythromycin and tylosin resistance in E. faecium isolated from chickens and E. faecalis from pigs and chickens. Worryingly, despite fluctuations, we found an increasing erythromycin resistance trend in E. faecium and E. faecalis obtained from pigs. Consistent with this study, a considerable proportion of E. faecium and E. faecalis isolates obtained from food animals, especially chickens and pigs, in Korea demonstrated resistance to erythromycin (31–90%) and tylosin (50–94%) [16,18,19]. Our findings were also consistent with previous studies in Asia [26,40,44], Europe, and the United States [28,29,31,32,33]. Recently, the Korea Animal Health Products Association reported an increase in the annual sales of macrolides, especially tylosin, for livestock uses. The extensive use of macrolides (209 tons in pigs between 2010 and 2019), especially tylosin in Korean livestock husbandry can cause selective pressure and lead to resistance [34]. Cross-resistance between erythromycin and tylosin could also contribute to the increase in the proportion of macrolide-resistant isolates. Further, Noh et al. [15] and Yoon et al. [45] have identified the erm(A) and erm(B) genes in virulent and multi-resistant strains of E. faecalis from chickens in Korea, indicating the dissemination of macrolide resistance genes among enterococcal isolates.
The quinupristin/dalfopristin resistance rate in E. faecium isolated from chickens was consistent with Kim et al. [16] in Korea and Unal et al. [32] in Canada, but much lower than other studies reported in Canada (89.7%) [30] and the United States (63%) [31]. The quinupristin/dalfopristin resistance rate in E. faecium isolated from pigs and cattle was comparable to the findings of Ramos et al. [28] in Portugal. Quinupristin/dalfopristin is not approved for animal use in Korea. The use of virginiamycin, a streptogramin showing cross-resistance with quinupristin/dalfopristin, could be linked to the occurrence of quinupristin/dalfopristin resistance in E. faecium [46]. For E. faecalis, the situation appears different, because this bacterium is intrinsically resistant to quinupristin/dalfopristin through activity conferred by the expression of the lsa gene [39]. Quinupristin/dalfopristin-resistant Enterococcus in food animals may play a role in the emergence of human infections through the food chain, indicating the need for continuous and careful monitoring.
We also investigated antibiotic susceptibility to four antibiotics, namely daptomycin, tigecycline, linezolid and vancomycin, representing four different classes and not registered for use in veterinary medicine in Korea [34]. Consistent with previous reports in Korea [16,17,37,38] and other countries [47,48,49,50], the occurrence of linezolid resistance is still rare among enterococcal isolates from food animals. The rarity of linezolid resistance among enterococci might be due to the fact that resistance develops as a spontaneous mutation in the multiple copies 23S rRNA gene [48]. A small proportion (≤10%) of E. faecium and E. faecalis isolates exhibited resistance to daptomycin and tigecycline, which are considered critical to humans. Daptomycin resistance in E. faecium and E. faecalis is typically coordinated by the three-component cell envelope stress response system, LiaFSR [51]. The daptomycin resistance rate found in this study agreed with previous reports in enterococcal isolates from ducks and food animal carcasses in Korea [17,38] and cattle in Australia [10]. However, the tigecycline resistance rate contradicts the above reports. Vancomycin resistance in E. faecium and E. faecalis isolated from food animals has been reported in many countries [52]. In this study; however, all of the enterococcal isolates were susceptible to vancomycin. Overall, enterococcal isolates resistant to newer and critically important antimicrobials might transfer to humans through the food chain and makes the treatment of MDR infections a daunting clinical challenge.
The majority of E. faecium and E. faecalis isolates were resistant to at least one antimicrobial agent, and numerous resistance patterns were found in both species. The MDR rates in E. faecium and E. faecalis isolated from pigs and chickens were higher than those found by Kwon et al. [18] in Korea. In contrast, the MDR rate in Enterococcus isolates from chickens, pigs, and cattle from this study contradicts various reports in Europe [29,32] and other Asian countries [13,26]. Consistent with Novais et al. [6], Kim et al. [16] and Kwon et al. [18], MDR patterns usually include tetracycline, erythromycin and/or ciprofloxacin. The occurrence of MDR in Enterococcus species might be related to the propensity of the bacteria to be involved in various forms of conjugation. This can lead to the widespread dissemination of resistance determinants through plasmids [53]. Additionally, the hardiness of enterococci species may likely contribute to resistance development by enhancing the survivability of MDR strains in the environment. This has the potential of enhancing transmission from animals to humans [53]. Multidrug-resistant enterococcal isolates pose a serious threat to public health as the same class of antibiotics is being used in the treatment of most bacterial diseases in humans.
In conclusion, we found enterococcal isolates that exhibited resistance to several antimicrobials, including those considered critical for humans. The occurrence of a high percentage of multidrug-resistant E. faecium and E. faecalis in food animals is alarming, especially given the fact that very few antimicrobial agents can be used to control enterococcal infection. Such resistance is likely to be passed from food animals to humans through the food chain. Therefore, the prudent use of antimicrobials in food animals will be crucial in limiting the public health hazards of Enterococcus in Korea.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9050925/s1. Table S1. E. faecium and E. faecalis isolates recovered from cattle, pigs, and chickens between 2010 and 2019 in Korea. Table S2. MIC distribution of the tested antimicrobials against E. faecium isolated from cattle, chickens, and pigs between 2010 and 2019 in Korea. Table S3. The MIC50 and MIC90 of the tested antimicrobials against E. faecium isolated from cattle (A), pigs (B), chickens (C) between 2010 and 2019 in Korea. Table S4. MIC distribution of the tested antimicrobials against E. faecalis isolated from cattle, chickens, and pigs between 2010 and 2019 in Korea. Table S5 A. The MIC50 and MIC90 of the tested antimicrobials against E. faecalis isolated from cattle (A), pigs (B), chickens (C) between 2010 and 2019 in Korea. Table S6. Antimicrobial resistance rate in E. faecium isolated from cattle between 2010 and 2019 in Korea. Table S7. Antimicrobial resistance rate in E. faecium isolated from pigs between 2010 and 2019 in Korea. Table S8. Antimicrobial resistance rate in E. faecium isolated from chickens between 2010 and 2019 in Korea. Table S9. Antimicrobial resistance rate in E. faecalis isolated from cattle between 2010 and 2019 in Korea. Table S10. Antimicrobial resistance rate in E. faecalis isolated from pigs between 2010 and 2019 in Korea. Table S11. Antimicrobial resistance rate in E. faecalis isolated from chickens between 2010 and 2019 in Korea.
Author Contributions
Conceptualization, S.-K.L., and M.H.K.; methodology, M.H.K., J.-H.C., H.Y.K., and D.C.M.; software, A.F.M., J.-H.C., and S.-J.K.; validation, A.F.M., S.-J.K.; and J.-H.C.; formal analysis M.H.K., and S.-J.K.; investigation, M.H.K., H.Y.K., H.-J.S., J.-H.C., A.F.M., and S.-J.K.; data curation, H.-J.S., D.C.M., S.-S.Y., and S.-J.K.; writing—original draft preparation, A.F.M., and M.H.K.; writing—review and editing, A.F.M., D.C.M., and S.-K.L.; supervision, S.-S.Y., and S.-K.L.; project administration, D.C.M., and H.Y.K.; funding acquisition; S.-K.L., and D.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food, and Rural Affairs, South Korea, grant B-1543081-2017-24-01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hammerum A.M. Enterococci of animal origin and their significance for public health. Clin. Microbiol. Infect. 2012;18:619–625. doi: 10.1111/j.1469-0691.2012.03829.x. [DOI] [PubMed] [Google Scholar]
- 2.Sparo M., Urbizu L., Solana M.V., Pourcel G., Delpech G., Confalonieri A., Ceci M., Sánchez Bruni S.F. High-level resistance to gentamicin: Genetic transfer between Enterococcus faecalis isolated from food of animal origin and human microbiota. Lett. Appl. Microbiol. 2012;54:119–125. doi: 10.1111/j.1472-765X.2011.03182.x. [DOI] [PubMed] [Google Scholar]
- 3.Hao H., Sander P., Iqbal Z., Wang Y., Cheng G., Yuan Z. The risk of some veterinary antimicrobial agents on public health associated with antimicrobial resistance and their molecular basis. Front. Microbiol. 2016;7:1626. doi: 10.3389/fmicb.2016.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres C., Alonso C.A., Ruiz-Ripa L., León-Sampedro R., del Campo R., Coque T.M. Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol. Spectrum. 2018;6:ARBA 0032. doi: 10.1128/microbiolspec.arba-0032-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson O. Vancomycin-resistant enterococci in farm animals–occurrence and importance. Infect. Ecol. Epidemiol. 2012;2:16959. doi: 10.3402/iee.v2i0.16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novais C., Freitas A.R., Silveira E., Antunes P., Silva R., Coque T.M., Peixe L. Spread of multidrug-resistant Enterococcus to animals and humans: An underestimated role for the pig farm environment. J. Antimicrob. Chemother. 2013;68:2746–2754. doi: 10.1093/jac/dkt289. [DOI] [PubMed] [Google Scholar]
- 7.Tyson G.H., Nyirabahizi E., Crarey E., Kabera C., Lam C., Rice-Trujillo C., McDermott P.F., Tate H. Prevalence and antimicrobial resistance of enterococci isolated from retail meats in the United States, 2002 to 2014. Appl. Environ. Microbiol. 2018;84:e01902-17. doi: 10.1128/AEM.01902-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehm A.B., Sassoubre L.M. Enterococci as indicators of environmental fecal contamination. In: Gilmore M.S., Clewell D.B., Ike Y., Shankar N., editors. Enterococci: From Commensals to Leading Causes of Drug-Resistant Infection. Eye and Ear Infirmary; Boston, MA, USA: 2014. [PubMed] [Google Scholar]
- 9.Foka F.E., Ateba C.N., Lourenco A. Detection of virulence genes in multidrug-resistant enterococci isolated from feedlots dairy and beef cattle: Implications for human health and food safety. Biomed. Res. Int. 2019;2019:5921840. doi: 10.1155/2019/5921840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow R.S., McMillan K.E., Duffy L.L., Fegan N., Jordan D., Mellor G.E. Antimicrobial resistance status of Enterococcus from Australian cattle populations at slaughter. PLoS ONE. 2017;12:e0177728. doi: 10.1371/journal.pone.0177728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertelloni F., Salvadori C., Moni A., Cerri D., Mani P., Ebani V.V. Antimicrobial resistance in Enterococcus spp. isolated from laying hens of backyard poultry flocks. Ann. Agric. Environ. Med. 2015;22:665–669. doi: 10.5604/12321966.1185771. [DOI] [PubMed] [Google Scholar]
- 12.Delpech G., Pourcel G., Schell C., De Luca M., Basualdo J., Bernstein J., Grenovero S., Sparo M. Antimicrobial resistance profiles of Enterococcus faecalis and Enterococcus faecium isolated from artisanal food of animal origin in Argentina. Foodborne Pathog. Dis. 2012;9:939–944. doi: 10.1089/fpd.2012.1192. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Liu K., Lai J., Wu C., Shen J., Wang Y. Prevalence and antimicrobial resistance of Enterococcus species of food animal origin from Beijing and Shandong Province, China. J. Appl. Microbiol. 2013;114:555–563. doi: 10.1111/jam.12054. [DOI] [PubMed] [Google Scholar]
- 14.Layton B.A., Walters S.P., Lam L.H., Boehm A.B. Enterococcus species distribution among human and animal hosts using multiplex PCR. J. Appl. Microbiol. 2010;109:539–547. doi: 10.1111/j.1365-2672.2010.04675.x. [DOI] [PubMed] [Google Scholar]
- 15.Noh E.B., Kim Y.B., Seo K.W., Son S.H., Ha J.S., Lee Y.J. Antimicrobial resistance monitoring of commensal Enterococcus faecalis in broiler breeders. Poult. Sci. 2020;99:2675–2683. doi: 10.1016/j.psj.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y.J., Park J.H., Seo K.H. Comparison of the loads and antibiotic-resistance profiles of Enterococcus species from conventional and organic chicken carcasses in South Korea. Poult. Sci. 2018;97:271–278. doi: 10.3382/ps/pex275. [DOI] [PubMed] [Google Scholar]
- 17.Tamang M.D., Moon D.C., Kim S.R., Kang H.Y., Lee K., Nam H.M., Jang G.C., Lee H.S., Jung S.C., Lim S.K. Detection of novel oxazolidinone and phenicol resistance gene optrA in enterococcal isolates from food animals and animal carcasses. Vet. Microbiol. 2017;201:252–256. doi: 10.1016/j.vetmic.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Kwon K.H., Hwang S.Y., Moon B.Y., Park Y.K., Shin S., Hwang C.Y., Park Y.H. Occurrence of antimicrobial resistance and virulence genes, and distribution of enterococcal clonal complex 17 from animals and human beings in Korea. J. Vet. Diagn. Investig. 2012;24:924–931. doi: 10.1177/1040638712455634. [DOI] [PubMed] [Google Scholar]
- 19.Hwang I.Y., Ku H.O., Lim S.K., Park C.K., Jung G.S., Jung S.C., Nam H.M. Species distribution and resistance patterns to growth-promoting antimicrobials of enterococci isolated from pigs and chickens in Korea. J. Vet. Diagn. Investig. 2009;21:858–862. doi: 10.1177/104063870902100616. [DOI] [PubMed] [Google Scholar]
- 20.Lim S.K., Kim T.S., Lee H.S., Nam H.M., Joo Y.S., Koh H.B. Persistence of vanA-type Enterococcus faecium in Korean livestock after ban on avoparcin. Microb. Drug Resist. 2006;12:136–139. doi: 10.1089/mdr.2006.12.136. [DOI] [PubMed] [Google Scholar]
- 21.Dutka-Malen S., Evers S., Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995;33:24–27. doi: 10.1128/JCM.33.1.24-27.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement, CLSI Document M100. CLSI; Wayne, PA, USA: 2018. [Google Scholar]
- 23.National Antimicrobial Resistance Monitoring System . NARMS Integrated Report: The National Antimicrobial Resistance Monitoring System: Enteric Bacteria. U.S. Food and Drug Administration; Rockville, MD, USA: 2014. [Google Scholar]
- 24.Danish Integrated Antimicrobial Resistance Monitoring and Research Program (DANMAP) Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food, and Humans in Denmark; DANMAP Annual Report; Denmark. [(accessed on 12 January 2021)];2016 :1600–2032. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/140535625/DANMAP_2016_LOW_241017.pdf.
- 25.Sung C.H., Chon J.W., Kwak H.S., Kim H., Seo K.H. Prevalence and antimicrobial resistance of Enterococcus faecalis and Enterococcus faecium isolated from beef, pork, chicken, and sashimi. Korean J. Food Sci. Anim. Resour. 2013;33:133–138. doi: 10.5851/kosfa.2013.33.1.133. [DOI] [Google Scholar]
- 26.Thu W.P., Sinwat N., Bitrus A.A., Angkittitrakul S., Prathan R., Chuanchuen R. Prevalence, antimicrobial resistance, virulence gene, and class 1 integrons of Enterococcus faecium and Enterococcus faecalis from pigs, pork, and humans in Thai-Laos border provinces. J. Glob. Antimicrob. Resist. 2019;18:130–138. doi: 10.1016/j.jgar.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Maasjost J., Mühldorfer K., De Jäckel S.C., Hafez H.M. Antimicrobial susceptibility patterns of Enterococcus faecalis and Enterococcus faecium isolated from poultry flocks in Germany. Avian Dis. 2015;59:143–148. doi: 10.1637/10928-090314-RegR. [DOI] [PubMed] [Google Scholar]
- 28.Ramos S., Igrejas G., Capelo-Martinez J.L., Poeta P. Antibiotic resistance and mechanisms implicated in fecal enterococci recovered from pigs, cattle, and sheep in a Portuguese slaughterhouse. Ann. Microbiol. 2012;62:1485–1494. doi: 10.1007/s13213-011-0402-7. [DOI] [Google Scholar]
- 29.Šeputiene V., Bogdaite A., Ružauskas M., Sužiedeliene E. Antibiotic resistance genes and virulence factors in Enterococcus faecium and Enterococcus faecalis from diseased farm animals: Pigs, cattle, and poultry. Pol. J. Vet. Sci. 2012;15:431–438. [PubMed] [Google Scholar]
- 30.Tremblay C.L., Letellier A., Quessy S., Boulianne M., Daignault D., Archambault M. Multiple-antibiotic resistance of Enterococcus faecalis and Enterococcus faecium from cecal contents in broiler chicken and turkey flocks slaughtered in Canada and plasmid colocalization of tetO and ermB genes. J. Food Prot. 2011;74:1639–1648. doi: 10.4315/0362-028X.JFP-10-451. [DOI] [PubMed] [Google Scholar]
- 31.Hayes J.R., English L.L., Carr L.E., Wagner D.D., Joseph S.W. Multiple-antibiotic resistance of Enterococcus spp. isolated from commercial poultry production environments. Appl. Environ. Microbiol. 2004;70:6005–6011. doi: 10.1128/AEM.70.10.6005-6011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ünal N., Aşkar Ş., Yildirim M. Antibiotic resistance profile of Enterococcus faecium and Enterococcus faecalis isolated from broiler cloacal samples. Turk. J. Vet. Anim. Sci. 2017;41:199–203. doi: 10.3906/vet-1607-26. [DOI] [Google Scholar]
- 33.Jackson C.R., Lombard J.E., Dargatz D.A., Fedorka-Cray P.J. Prevalence, species distribution, and antimicrobial resistance of enterococci isolated from US dairy cattle. Lett. Appl. Microbiol. 2011;52:41–48. doi: 10.1111/j.1472-765X.2010.02964.x. [DOI] [PubMed] [Google Scholar]
- 34.Animal and Plant Quarantine Agency . Korean Veterinary Antimicrobial Resistance Monitoring System. APQA; Gimcheon, Korea: 2019. [Google Scholar]
- 35.Pesavento G., Calonico C., Ducci B., Magnanini A., Lo Nostro A. Prevalence and antibiotic resistance of Enterococcus spp. isolated from retail cheese, ready-to-eat salads, ham, and raw meat. Food Microbiol. 2014;41:1–7. doi: 10.1016/j.fm.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Kasimoglu-Dogru A., Gencay Y.E., Ayaz N.D. Prevalence and antibiotic resistance profiles of Enterococcus species in chicken at slaughter level; absence of vanA and vanB genes in E. faecalis and E. faecium. Res. Vet. Sci. 2010;89:153–158. doi: 10.1016/j.rvsc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Na S.H., Moon D.C., Kim M.H., Kang H.Y., Kim S.J., Choi J.H., Mechesso A.F., Yoon S.S., Lim S.K. Detection of the phenicol–oxazolidinone resistance gene poxta in Enterococcus faecium and Enterococcus faecalis from food-producing animals during 2008–2018 in Korea. Microorganisms. 2020;8:1839. doi: 10.3390/microorganisms8111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Na S.H., Moon D.C., Choi M.J., Oh S.J., Jung D.Y., Kang H.Y., Hyun B.H., Lim S.K. Detection of oxazolidinone and phenicol-resistant enterococcal isolates from duck feces and carcasses. Int. J. Food Microbiol. 2019;293:53–59. doi: 10.1016/j.ijfoodmicro.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Hollenbeck B.L., Rice L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 2012;3:421–569. doi: 10.4161/viru.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usui M., Ozawa S., Onozato H., Kuge R., Obata Y., Uemae T., Ngoc P.T., Heriyanto A., Chalemchaikit T., Makita K., et al. Antimicrobial susceptibility of indicator bacteria isolated from chickens in Southeast Asian countries (Vietnam, Indonesia, and Thailand) J. Vet. Med. Sci. 2014;76:685–692. doi: 10.1292/jvms.13-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller W.R., Munita J.M., Arias C.A. Mechanisms of antibiotic resistance in enterococci. Expert. Rev. Anti. Infect. Ther. 2015;12:1221–1236. doi: 10.1586/14787210.2014.956092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono S., Muratani T., Matsumuoto T. Mechanism of resistance to imipenem and ampicillin in Enterococcus faecalis. Antimicrob. Agents Chemother. 2005;49:2954–2958. doi: 10.1128/AAC.49.7.2954-2958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazar V., Gheorghe I., Curutiu C., Savin I., Marinescu F., Cristea V.C., Dobre D., Popa G.L., Chifiriuc M., Popa M.L. Antibiotic resistance profiles in cultivable microbiota isolated from some Romanian natural fishery lakes included in Nature 2000 network. BMC Vet. Res. 2021;17:1–11. doi: 10.1186/s12917-021-02770-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimura H., Ishimaru M., Endoh Y.S., Kojima A. Antimicrobial susceptibilities of enterococci isolated from feces of broiler and layer chickens. Lett. Appl. Microbiol. 2000;31:427–432. doi: 10.1046/j.1365-2672.2000.00842.x. [DOI] [PubMed] [Google Scholar]
- 45.Yoon S., Son S.H., Kim Y.B., Seo K.W., Lee Y.J. Molecular characteristics of optrA-carrying Enterococcus faecalis from chicken meat in South Korea. Poult. Sci. 2020;99:6990–6996. doi: 10.1016/j.psj.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simjee S., Jensen L.B., Donabedian S.M., Zervos M.J. Enterococcus. In: Aarestrup F.M., editor. Antimicrobial Resistance in Bacteria of Animal Origin. ASM Press; Washington, DC, USA: 2006. pp. 315–328. [Google Scholar]
- 47.Mendes R.E., Hogan P.A., Jones R.N., Sader H.S., Flamm R.K. Surveillance for linezolid resistance via the Zyvox(R) Annual Appraisal of Potency and Spectrum (ZAAPS) Programme (2014): Evolving resistance mechanisms with stable susceptibility rates. J. Antimicrob. Chemother. 2016;71:1860–1865. doi: 10.1093/jac/dkw052. [DOI] [PubMed] [Google Scholar]
- 48.Wang L., He Y., Xia Y., Wang H., Liangm S. Investigation of mechanism and molecular epidemiology of linezolid-resistant Enterococcus faecalis in China. Infect. Genet. Evol. 2014;26:14–19. doi: 10.1016/j.meegid.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Flamm R.K., Mendes R.E., Ross J.E., Sader H.S., Jones R.N. An international activity and spectrum analysis of linezolid: ZAAPS Program results for 2011. Diagn. Microbiol. Infect. Dis. 2013;76:206–213. doi: 10.1016/j.diagmicrobio.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 50.Patel S.N., Memari N., Shahinas D., Toye B., Jamieson F.B., Farrell D.J. Linezolid resistance in Enterococcus faecium isolated in Ontario, Canada. Diagn. Microbiol. Infect. Dis. 2013;77:350–353. doi: 10.1016/j.diagmicrobio.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Prater A.G., Mehta H.H., Kosgel A.J., Miller W.R., Tran T.T., Arias C.A., Shamoo Y. Environment shapes the accessible daptomycin resistance mechanisms in Enterococcus faecium. Antimicrob. Agents Chemother. 2019;63:e00790-19. doi: 10.1128/AAC.00790-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arias C.A., Murray B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gousia P., Economou V., Bozidis P., Papadopoulou C. Vancomycin-resistance phenotypes, vancomycin-resistance genes, and resistance to antibiotics of enterococci isolated from food of animal origin. Foodborne Pathog. Dis. 2015;12:214–220. doi: 10.1089/fpd.2014.1832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.