Abstract
Valerianaceae, the sub-family of Caprifoliaceae, contains more than 300 species of annual and perennial herbs, worldwide distributed. Several species are used for their biological properties while some are used as food. Species from the genus Valeriana have been used for their antispasmodic, relaxing, and sedative properties, which have been mainly attributed to the presence of valepotriates, borneol derivatives, and isovalerenic acid. Among this genus, the most common and employed species is Valeriana officinalis. Although valerian has been traditionally used as a mild sedative, research results are still controversial regarding the role of the different active compounds, the herbal preparations, and the dosage used. The present review is designed to summarize and critically describe the current knowledge on the different plant species belonging to Valerianaceae, their phytochemicals, their uses in the treatment of different diseases with particular emphasis on the effects on the central nervous system. The available information on this sub-family was collected from scientific databases up until year 2020. The following electronic databases were used: PubMed, Scopus, Sci Finder, Web of Science, Science Direct, NCBI, and Google Scholar. The search terms used for this review included Valerianaceae, Valeriana, Centranthus, Fedia, Patrinia, Nardostachys, Plectritis, and Valerianella, phytochemical composition, in vivo studies, Central Nervous System, neuroprotective, antidepressant, antinociceptive, anxiolytic, anxiety, preclinical and clinical studies.
Keywords: Valerianaceae, anxiolytic, sedative, myorelaxant, antidepressant, biological activities, clinical studies, phytochemicals, valerian
1. Introduction
Valerianaceae, the sub-family of the family Caprifoliaceae (order Dipsacales), contains about 315 species of annual and perennial herbs, distributed, throughout the world, except Australia and New Zealand, usually at high elevations. Achene fruits, absence of endosperm, bilaterally symmetric or sporadically asymmetric and sympetalous flowers, three-carpellate and inferior ovaries, one fertile carpel at maturity, and an anatropous ovule are the main characteristics of Valerianaceae sub-family species [1]. Valerianaceae sub-family showed significant diversity in flowers and fruits morphology. Probably, the most remarkable variation in the morphology of flowers is related to the stamens number ranging from 4 to 1. Donoghue et al. [2] described a reduction from the ancestral condition of 4 to 3 stamens in the Valerianaceae core, followed by an additional reduction to two stamens in Fedia genus and one stamen in Centranthus genus.
The calyx can be completely lacking as in some Valeriana species, reduced to small teeth as in Fedia and Valerianella, leafy and persistent as in Nardostachys, or pappus-like and featherly as in some Valeriana species and in Centranthus. The genera of this sub-family include Valeriana L., Centranthus Lam. and DC., Fedia Gaertn., Patrinia Juss., Nardostachys DC., Plectritis (Lindl.) DC., and Valerianella Mill. Phytochemical studies revealed the presence of sesquiterpenes, valepotriates, alkaloids, flavonoids, organic acids, and their derivatives, as characteristic classes of constituents in Valerianaceae plants [3,4,5]. It is generally accepted that valepotriates are common in Valerianaceae species and are responsible for their sedative properties. The present review is designed to report the current knowledge on the plant species that belong to the sub-family Valerianaceae, their phytochemicals, and their uses in the treatment of several diseases with particular emphasis to the action on the central nervous system (CNS). All collected data have been obtained from different databases such as PubMed, Scopus, Sci Finder, Web of Science, Science Direct, NCBI, and Google Scholar.
2. Habitat, Distribution, and Traditional Uses of Valerianaceae Sub-Family
Valerianaceae species are mainly found around the Northern Hemisphere. Overall, their distribution matches that of other Dipsacales clades. Even though the Asiatic origin, actually the center of diversity Valerianaceae sub-family is located in South America, where several morphological forms, from rosette plants to annual vine-like species to microphyllous shrubs, are found in different habitats [2,6,7,8]. Several species of the genus Valeriana are abundant in the Andes. Peru is the richest country, but there is a high number of species also in Ecuador, Chile, Colombia, and Argentina [9]. Species present in northwestern Argentina, and northern Chile are generally inhabitants of the arid zone and are taxonomically related to species found in the northern Andes [9]. Patrinia species are principally distributed in China, Korea, Japan, and Siberia. Nardostachys grandiflora DC. (syn. Nardostachys chinensis and Nardostachys jatamansi) and Nardostachys scrophulariiflora (syn. Picrorhiza scrophulariiflora) are alpine perennial herbs found only in the Himalayas [10].
Numerous Valerianaceae species have a traditional use. Several species are used for their biological properties while some species are used as food. Species from the genus Valeriana have been used for their antispasmodic, relaxing, and sedative properties that have been mainly attributed to the presence of valepotriates, borneol derivatives, and isovalerenic acid [11]. Among this genus, the most common and employed species is Valeriana officinalis L. Its roots have long been traditionally used for their sleep-promoting, anxiolytic, sedative, and antispasmodic activities [12,13,14]. In Brazil, it has been used for its hypnotic, anticonvulsant, and anxiolytic properties [15]. In Europe, V. officinalis is used for treating anxiety and restlessness; in the United States, it is mainly employed for its sleep-promoting activity [12].
Patrinia species are commonly used in Asian medicine to treat peri-appendicular abscesses, dysentery, erysipelas, conjunctival congestion, lung carbuncle, post-partum disease, and leucorrhea [16]. The young leaves and shoots of Centranthus ruber (L.) DC. (Valeriana rubra L., commonly known as red valerian, red spur valerian, and spur valerian) are used in Italy as depurative [17]. N. chinensis has been used in some traditional medicines, including Chinese, Korean, and Ayurvedic medicine to treat epilepsy, hysteria, hyperglycemia, dyslipidemia, headache, stress indigestion, heart palpitations, mental weakness, cholera, and leprosy [18]. The leaves of Fedia cornucopieae (L.) Gaertn. are eaten in southern Italy (Sicily) raw in salads or cooked (browned in oil, fried, in omelets, and meatballs) [19].
3. Effect on the Central Nervous System
Among Valerianaceae plants, species from the genus Valeriana (e.g., V. officinalis L., V. jatamansi Jones, V. fauriei Briq., V. amurensis Smir. ex Kom, V. glechomifolia Meyer, V. polystachya Smith, etc.) are the most used to treat CNS-related disorders (Table 1). In the last decades, many studies have validated the traditional uses of these species. V. officinalis is the most used particularly for its sedative, anticonvulsant, tranquilizing, and anxiolytic properties. According to the European Medicine Agency, extracts from this species roots can be used to alleviate mild nervous anxiety and sleep disorders [20]. V. officinalis is available as an herbal supplement and is extensively utilized to cure anxiety disorders. Its anxiolytic effects have been studied by different authors using different models. Hattesohl et al. [21] investigated in vivo the myorelaxant, anxiolytic, sedative, and antidepressant properties of different extracts from this species. None of the studied extracts showed myorelaxant or sedative activity up to limit doses of 500 or 1000 mg/kg b.w. Nevertheless, some extracts showed pronounced anxiolytic activity in the elevated plus-maze assay and one extract, after subacute treatment, showed anti-depressant activity in the forced swimming assay, allowing to conclude that the anti-depressant and anxiolytic effects may contribute to the positive effects of valerian to improve sleep quality.
Table 1.
Selected studies reporting the effects of Valerianaceae species on the central nervous system.
| Plant Species | Extract/Compound | Effect/Main Findings | Model/Assays | Dose | Administration | Reference |
|---|---|---|---|---|---|---|
| Nardostachys chinensis Batalin (synonym of Nardostachys jatamansi (D.Don) DC.) | Valerena-4,7(11)-diene from roots | Anti-stress, inhibited stress-induced excitatory behaviors and reduced stress-induced blood corticosterone, cerebral serotonin, and dopamine levels | Mice, a model of acute stress (restraint stress for 15 min) | 300 µg/cage | Inhalation | [28] |
| Nardostachys chinensis Batalin (synonym of Nardostachys jatamansi (D.Don) DC.) | Sesquiterpenoids isolated from underground parts | Alleviate the Alzheimer’s disease-like symptom of paralysis in worms | Caenorhabditis elegans Alzheimer’s disease pathological model | 50 μM | Added to the culture medium | [29] |
| Nardostachys jatamansi (D. Don) DC. | Rhizomes 70% ethanol extract | Anti-stress effect, inhibited cold restraint stress-induced oxidative stress | Rats, the cold restraint stress model | 200 and 500 mg/kg | Orally | [30] |
| Nardostachys jatamansi (D. Don) DC. | Root fractions | Anti-inflammatory effects reduced lipopolysaccharide-induced inflammatory response | Lipopolysaccharide-induced inflammation in murine peritoneal macrophages and mice model of lipopolysaccharide-induced endotoxin shock | 10–100 μg/mL | Intraperitoneally | [31] |
| Nardostachys jatamansi (D. Don) DC. | Root 70% ethanol extract | Anxiolytic effects | Mice, models of anxiety (elevated plus maze, open field test, light-dark box test, and Vogel’s conflict test) | 250 mg/kg | Orally | [32] |
| Nardostachys jatamansi (D. Don) DC. | Sesquiterpeniods | Anti-neuroinflammatory effects | BV2 microglial cells | Several (10–80 μM) | In vitro | [33,34] |
| Valeriana amurensis P. Smir. ex Kim. | Isolated compounds from roots and rhizomes | Ameliorate amyloid-beta-induced cognitive dysfunction | Amyloid-beta1-42 induced Alzheimer’s disease mice model | 0.2–0.8 g/kg | Intrahippocampal injection | [35] |
| Valeriana amurensis P. Smir. ex Kim. | Petroleum ether, ethyl acetate, n-butanol, and aqueous extract, and kissoone B from roots and rhizomes | Anti-inflammatory and neuroprotective effects | Cell (THP-1 cells as surrogates for microglia, SH-SY5Ycells as surrogates for neurons, and U373 cells as surrogates for astrocytes) and mice models | 400 μM kissoone B and 100 μg/mL extracts | Intragastric | [36] |
| Valeriana fauriei Briq. | Sesquiterpenes from the roots | Antidepressant | Mice, Forced swim test | 20 mg/kg, during seven consecutive days | Orally | [37] |
| Valeriana fauriei Briq. | Commercial root extract | Reduction the incidence of prenatal stress related-psychiatric disorders | Rats, prenatal stress model, evaluation of behavioral patterns and changes in protein levels in the prefrontal cortex | 100 mg/kg/day, administered on postnatal days 35–56 | Orally | [38] |
| Valeriana fauriei Briq. | Aqueous extract | Antinociceptive effect | Mice, a model of fibromyalgia (induced by intermittent cold stress) | 100 mg/kg/day for 24 days | Orally | [39] |
| Valeriana glechomifolia Meyer | Diene valepotriates fraction from underground parts | Antidepressant, interaction with dopaminergic and noradrenergic neurotransmission | Mice, tail suspension test (TST), and forced swimming test (FST) | 0.25–20 mg/kg | Orally | [40] |
| Valeriana glechomifolia Meyer | Valepotriate-enriched extract from aerial and underground parts | Antidepressant potential, prevent lipopolysaccharide-induced sickness and depressive behavior | Mice submitted to a forced swimming session as a stressful stimulus (experimental model of depression associated with inflammation) | 10 mg/kg | Orally | [41] |
| Valeriana glechomifolia Meyer | Valepotriate-enriched fraction from aerial and underground parts | Anti-inflammatory activity, inhibition of leukocytes migration | Formalin test in CF1 mice and Wistar rat’s leukocytes migration assay | 1, 10 and 30 mg/kg; 0.1–1 g/mL | Orally | [42] |
| Valeriana jatamansi Jones | Bakkenolides from rosts | Neuroprotective effects | 1-Methyl-4-phenylpyridinium-induced neuronal cell death in human dopaminergic neuroblastoma SH-SY5Y cells | 1.5, 5 and 15 μM | In vitro | [43] |
| Valeriana jatamansi Jones | Iridoids from roots | Neuroprotective effects | 1-Methyl-4-phenylpyridinium-induced neuronal cell death in human dopaminergic neuroblastoma SH-SY5Y cells | 3, 10 and 30 μM | In vitro | [44] |
| Valeriana jatamansi Jones | Root ethanol extract | Anxiolytic action | Mice, elevated plus maze, light/dark box test, and spontaneous activity | 1.2, 2.4 and 4.8 g/kg, for 10 days | Orally | [45] |
| Valeriana jatamansi Jones | Root and rhizome (Zhi zhu xiang) 35% ethanol extract | Anti-anxiety activity | Empty bottle stimulated rats, open field test, and the elevated plus-maze test | 1.2 g/kg, for 7 days | Orally | [46] |
| Valeriana jatamansi Jones | Iridoid-rich fraction from roots and rhizomes | Antidepressive | Unpredictable mild stress mouse model | 5.73, 11.47 and 22.94 mg/kg | Orally | [47] |
| Valeriana officinalis L. | Extracts from roots | Anxiolytic and antidepressant effect | Mice elevated plus maze test and forced swimming test | 100–1000 mg/kg | Orally and intraperitoneally | [21] |
| Valeriana officinalis L. | Root ethanol extract and valerenic acid | Anxiolytic effects, reduction in anxious behavior | Rats, elevated plus maze | 3 mL/kg extract and 3 mg/kg valerenic acid | Intraperitoneally | [23] |
| Valeriana officinalis L. | Roots aqueous extract | Anticonvulsant effects | Mice, pentylenetetrazole-induced clonic seizure | 0.25, 0.5 and 1 g/kg | Intraperitoneally | [48] |
| Valeriana officinalis L. | Root extract and valerenic acid | Memory function, cell proliferation, neuroblast differentiation, serum corticosterone, and lipid peroxidation | Mice, D-galactose-induced aging model | 100 mg/kg extracts and 340 μg/kg valerenic acid | Orally | [49] |
| Valeriana officinalis L. | Root ethanol extract | Antinociceptive effects, pain modulation | Mice, Tail-Flick Test, Acetic Acid Writhing Test | 50, 200 and 800 mg/kg | Intraperitoneally | [50] |
| Valeriana officinalis L. | Root extract | Anti-stress effects | Mice, exposure to physical stress psychological in a communication box | 100 mg/kg/0.5 mL | Orally | [22] |
| Valeriana officinalis L. | Root aqueous and ethanol extracts | Anticonvulsant effects | Zebrafish (Danio rerio), an animal model used to study clonic-like behaviors | 1 mg/mL; 5 mg/mL | Dissolved in aquarium water | [51] |
| Valeriana officinalis L. | Root ethanol extract | Protective effects against ischemic injury in the hippocampal pyramidal neurons | Gerbils subjected to ischemia/reperfusion injury | 100 mg/kg | Orally | [52] |
| Valeriana officinalis var. latiofolia | Sesquiterpenes and a monoterpenoid from roots | Inhibition of acetylcholinesterase | In vitro and in vivo in mice | 0.65, 1.30 and 2.6 mg/kg/day, for 90 days | Intragastric | [53] |
| Valeriana officinalis L. | Root aqueous extract | Elucidation of mechanisms of neuroprotective action against rotenone-induced cellular damage | Theoretical analysis (microarray data) | - | - | [54] |
| Valeriana officinalis L. | Eighteen root compounds | Inhibition of GABA aminotransferase | Molecular docking and molecular dynamics simulations | - | - | [55] |
| Valeriana officinalis L. | Root aqueous extract | Protective action against rotenone effects (counteract Cortical spreading depression propagation velocity and C6 glioma cytotoxicity) | Cortical spreading depression (in vivo) and C6 glioma cell culture (in vitro) models | 250 mg/kg/day, for 15 days | Orally | [56] |
| Valeriana polystachya Smith | Extract from roots and rhizome, and isolated compounds from roots and rhizomes | Inhibition of acetylcholinesterase and prolyl oligopeptidase activities | In vitro | 200 μg/mL extract and 150 μM of isolated compounds | In vitro | [57] |
| Valeriana prionophylla Standl. | Roots and rhizomes 50% ethanol extract | Anxiolytic, antidepressant, and hypno-sedative effects | Swiss mice and male Wistar rats, open field, rota rod, elevated plus-maze, forced swimming, strychnine- and pentobarbital-induced sleeping time, pentylenetetrazole-induced seizures, and the inhibitory avoidance tests | 50, 100 and 150 mg/kg | Orally and intraperitoneally | [58] |
| Valeriana wallichii DC (synonym of Valeriana jatamansi Jones) | Roots and rhizomes dichloromethane extract | Antidepressant effect | Mice, acute toxicity, studies forced swim test, locomotor activity and measurement of biogenic amines | 10, 20 and 40 mg/kg | Orally | [59] |
| Valeriana wallichii DC (synonym of Valeriana jatamansi Jones) | Roots and rhizomes essential oil | Antidepressant effect | Mice, acute toxicity, studies forced swim test, locomotor activity, measurement of biogenic amines and effect of nitric oxide modulators | 10, 20 and 40 mg/kg | Orally | [60] |
| Valeriana wallichii DC (synonym of Valeriana jatamansi Jones) | Root aqueous extract | Sleep quality improvement | Rats, estimation of the sleep-wake profile, electroencephalogram delta activity, and estimation of regional brain monoamines. | 200 and 300 mg/kg | Orally | [61] |
| Valeriana wallichii DC (synonym of Valeriana jatamansi Jones) | Rhizome methanol extract | Neuroprotective effect | Mice, 1-methyl-4-phenyl-1,2,3,6-tet-rahydropyridine-induced Parkinson’s disease model | 50, 100 and 200 mg/kg | Orally | [62] |
The effect of oral administration of V. officinalis root extract on physical and psychological stress response was investigated in mice by using a communication box [22]. Obtained data suggested that this extract could suppress stress responses through the modulation of the changes in the turnover of norepinephrine and serotonin in the amygdala and hippocampus, and through the control of corticosterone plasma levels.
In a study by Murphy et al. [23] V. officinalis root extract was intraperitoneally administered to rats and the number of entries and time spent on the open arms of an elevated plus-maze was evaluated. A reduced anxious behavior was observed in extract-treated rats in comparison to the control group and valerenic acid was suggested as the main anxiolytic component in the extract. However, other studies indicate that other constituents, namely borneol, lignans, and flavonoids, also exhibited anxiolytic and sedative activity [24,25,26]. In addition, studies with other Valeriana species containing low amounts of valerenic acid (e.g., V. edulis) showed similar properties [27].
The anticonvulsant effects of various extracts from V. officinalis were investigated in mice using the temporal lobe epilepsy model showing that administration of aqueous extract significantly decreased seizure activity in amygdala-kindled rats [63]. The anticonvulsant effect of an aqueous extract against clonic seizure threshold induced by pentylenetetrazole in mice [48]. Using a different mode, adult zebrafish [51], observed that ethanol extract noticeably improved the anticonvulsant effect of the anti-epileptic drugs clonazepam and phenytoin thus benefiting epilepsy treatment.
Derived Neurotrophic Factor (BDNF) has a crucial role in the CNS. The levels of BDNF must be maintained at an adequate concentration to allow neurotransmission to occur at an ideal level and prevent several mental diseases. A significant increase in the BDNF expression compared to control in SH-SY5Y cell lines treated with V. officinalis extract was observed allowing us to conclude that the antidepressant effects are mostly due to valerenic acid [64,65].
Yoo et al. [52] investigated the neuroprotective action from V. officinalis root extracts in gerbils after transient cerebral ischemia. The results revealed that the oral pretreatment with 100 mg/kg of extract decreased microglial activation and lipid peroxidation protection against ischemic damage in the hippocampal pyramidal neurons. In another study, the same root extract (100 mg/kg) and valerenic acid (340 μg/kg) was orally administered to control mice and aged mice (previously treated with D-galactose) to evaluate their effects on cell proliferation, memory function, and neuroblast differentiation in the mouse dentate gyrus [49]. Both enhanced the preferential exploration of new objects (novel object recognition test) and the escape latency, platform crossings, swimming speeds, and spatial preference for the target quadrant (Morris water maze test). They also improved cognitive function, promoted cell proliferation, neuroblast differentiation, and decreased the plasma corticosterone levels in aged mice.
The neuroprotective properties of V. officinalis extract and its therapeutic potential for neurological disorders have been reported. The extract showed protective effects against toxicity induced by rotenone in Drosophila melanogaster [66]. Rotenone is a chemical substance widely used as a pesticide that acts within the respiratory chain causing oxidative damage. Animals’ treatment with this substance has been used as a model to study brain disorders linked with redox imbalance. Amaral de Brito et al. [56] recently observed that V. officinalis extract has protective effects against in vitro cytotoxicity induced by rotenone in rat glioma C6 cells, by a novel action on the cortical spreading depression.
The antinociceptive effects of a V. officinalis hydroalcoholic extract were demonstrated in adult male Balb/c mice [50]. When mice were injected intraperitoneally at the maximum dose tested (800 mg/kg), the somatic pain was successfully reduced, the antinociceptive activity was clearly expressed, and the number of abdominal writhings significantly decreased.
Acetylcholinesterase (AchE) inhibitors have been widely recognized as an effective treatment for Alzheimer’s disease (AD) and AChE inhibitory activity of compounds from V. officinalis (sesquiterpenes and a monoterpenoid) have been also validated in mice both in vitro and in vivo [53].
V. jatamansi (synonymous V. wallichii DC.), popularly named as Indian valerian, is considered as an Asian counterpart for the European V. officinalis, is another important medicinal plant from the genus Valeriana, broadly recognized for its uses in anxiety, insomnia, epilepsy, and hysteria treatment (Nadkarni, 2001). Many studies are reporting the pharmacological properties of this species including the sedative, tranquilizing and neuroprotective effect, and its capacity to attenuate stress, anxiety, and depression (Table 1) [67]. The anti-anxiety effect of its ethanol extract was studied on empty bottle-stimulated rats using the open field and the elevated plus-maze tests. Four bioactive compounds (hesperidin, isochlorogenic acid A, isochlorogenic acid B, and isochlorogenic acid C) were identified as the chemical markers for that effect [46]. There are some reports about the antidepressant effect of extracts and essential oils from this species [60,68,69,70]. The nitric oxide pathway is involved in the antidepressant properties of essential oil from a chemotype of this species in mice [60]. The essential oil components inhibit nitric oxide to a critical concentration changing the vesicular release of norepinephrine and serotonin, two neurotransmitters implicated in depression.
An aqueous roots extract of V. jatamansi was also investigated for its effects on the sleep pattern of Sprague-Dawley rats by analyzing sleep-wake profile, electroencephalogram delta action during sleep, and estimating regional brain monoamines [61]. The extract ameliorated sleep disturbances and improved sleep quality, which can be related to the modulation of regional brain monoamines.
Several reports are describing the neuroprotective effects of extracts and compounds from V. jatamansi [43,62]. The neuroprotective effects of bakkenolides and iridoids against 1-methyl-4-phenylpyridinium (MPP+)-induced neuronal cell death in human dopaminergic neuroblastoma SH-SY5Y cells were demonstrated [43,44]. 1-Metil-4-fenil-1,2,3,6-tetraidropiridine (MPTP), a precursor of the neurotoxin MPP+, is widely used to study PD insult. The neuroprotective effects against MPTP in mice by mitigating oxidative stress and inflammatory damage were also reported [62].
V. fauriei is also described as an antidepressant [37], vasorelaxant [71], and anxiolytic [72]. Yoon et al. [34] demonstrated its antinociceptive effects in a fibromyalgia animal model provoked by intermittent cold stress. Its effects in reducing the occurrence of prenatal depression and schizophrenia in rats were also reported [38].
V. amurensis (roots and rhizomes) has been extensively employed in Chinese medicine for the treatment of neurological disorders. The petroleum ether extract improves sleep by reducing the sleep latency and delaying the sleep duration of mice [73]. Using human cell lines and mice models Wu et al. [36] recently reported the anti-inflammatory and neuroprotective properties of kissoone B and extracts of this species, suggesting its capacity for neurological diseases treatment.
Nardostachys jatamansi (D. Don) DC. (synonymous Nardostachys chinensis Batalin) belongs to a different Valerianaceae genus and has also been extensively reported as affecting CNS disorders (Table 1). The anti-stress properties of this species have been investigated by several authors and using different animal models. Lyle et al. [74] using a rat model of chronic fatigue syndrome, observed a significant reversion of the locomotor activity and the anxiety state in stressed animals, after the oral administration of the extract (200 and 500 mg/kg b.w.). The extract showed the capacity to manage lipid peroxidation, nitrite, superoxide dismutase, and catalase activities indicating that the antioxidant properties of this extract can be responsible for its anti-stress effects. Later, Lyle et al. [30] used a cold restraint stress model to investigate the biochemical and neurochemical alterations induced by oral administration of a hydro-ethanolic extract in the same doses and observed alterations on rats enzymatic and non-enzymatic antioxidant system, and alleviated oxidative stress and neurochemical alterations induced by cold restraint stress.
This species has been traditionally used as a tranquilizer and sedative agent and many investigations demonstrating its anxiolytic and sedative properties. The sedative effect of extracts and isolated compounds applied by inhalation were previously reported [28,75,76]. Takemoto et al. [76] investigated the sedative effects of oxygenated compounds applied by inhalation in an excitatory mouse model treated with caffeine and showed that aristolen-1(10)-en-9-ol exerted its effects via the GABAergic system. The anxiolytic effects of N. jatamansi extract were investigated after oral administration to mice (250 mg/kg) by using different behavioral tests, such as elevated plus maze, light-dark box test, open field test, and Vogel’s conflict test [32]. A 7 days treatment with the extract showed significant anxiolytic effects and increased levels of brain monoamine and GABA neurotransmitter, suggesting that anxiolytic effects are mediated by activating the GABAergic receptor complex.
Recently, the potential of some Nardostachys species to alleviate AD-like symptoms in Caenorhabditis elegans AD pathological model was investigated and some compounds can delay AD worm paralysis [29,77].
Some reports are describing the serotonin transporter regulating the activity of compounds from Nardostachys species, like sesquiterpenoids and nardonaphthalenones [78,79]. Serotonin transporter is responsible for the reuptake of 5-hydroxytryptamine into presynaptic neurons and plays an important role in the pathophysiology of neuropsychiatric conditions, like depression, anxiety, or obsessive-compulsive disorder.
Many investigations have been performed in animals to assess the anticonvulsant properties of N. chinensis. An ethanol extract significantly increased the seizure threshold in a dose and time-dependent manner in a rats’ model of electric shock-induced seizures [80]. The anticonvulsant effect of N. jatamansi extract (400 mg/kg b.w.) in a maximal electric shock model was less than that of the standard sodium valproate (300 mg/kg b.w.) [81]. The neuroprotective effect of an ethanol extract from the roots of this species against Aβ toxicity was studied in vitro using a cell culture system and in vivo using a Drosophila AD model [82] and the results suggest that the neuroprotective effect observed can be linked with the extract antioxidant and anti-inflammatory properties and its inhibitory effect against extracellular-signal-regulated kinase signaling. Indeed, several extracts, fractions, and compounds from this species exhibited anti-neuro inflammatory by different mechanisms [31,32,33,34,83,84].
4. Molecular Mechanism of Action of Pharmacological Potential
V. officinalis extracts are one of the most popular herbal medications used to alleviate insomnia, anxiety, epilepsy, and other conditions of the CNS. Several studies have been conducted to clarify the mechanisms implicated in the pharmacological effects of these extracts. γ-Aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the CNS. GABA is crucial for normal brain function and a reduction in its concentration has been associated with several neurological conditions as is the case of epilepsy, AD, Parkinson’s disease, Huntington’s disease, etc. If GABA balance is perturbed, conditions like depression, sedation, anxiety, restlessness, and insomnia may also arise [85]. Due to the blood–brain barrier, GABA cannot be easily introduced into the CNS, thus the inhibition of GABA aminotransferase, the enzyme responsible for its degradation, has been the target for the adjustment of GABA amounts in the CNS. The mode of action of valerian compounds is associated with the modulation of GABAergic transmission via inhibition of GABA transaminase, interaction with GABA receptor/benzodiazepine, and intervention in uptake and intake of GABA in synaptosomes.
Studies with animal showed that V. officinalis potentiates the GABAergic neuronal transmission in the brain via the improvement of GABA release and inhibition of the degradation of GABA via the inhibition of GABA-transaminase [86,87,88]. A recent study of molecular docking and molecular dynamics simulation suggests that valerian compounds could be valuable resources for the development of GABA-transaminase inhibitors [55]. Valle-Mojica et al. [89] suggested an interaction with glutamatergic receptors as a possible mechanism by which valerian exhibit its action in the central nervous system. Valerian compounds like valerenic acid and valerenol also displayed allosteric modulation of GABA receptors [90,91]. In vitro studies indicated that both the activation of adenosine A1 receptor and GABAA receptors separately contribute to the valerian capacity to induce sleep [92]. It was also observed that factors like solvent extraction and the stability of the extract affect the selectivity for the interactions for glutamate receptors [93].
Recently Li et al. [47] demonstrated that the antidepressant activity of the iridoid-rich fraction from V. jatamansi in a chronic unpredictable mild stress mouse model is exerted by regulating several metabolic pathways such as the synthesis of neurotransmitters, the tricarboxylic acid cycle, and amino acid metabolism. Recent studies also using the same model indicated that that gut flora structures and regulation of serotonin, norepinephrine, substance P, and corticotropin-releasing factor in the brain and intestine, can be implicated in the antidepressant properties of this plant [94]. The enhancement of noradrenergic and/or dopaminergic transmission induced by valepotriates from this species in the mouse brain can be related to the facilitation of GABA [59].
Studies from Shahidi et al. [50] indicated for the first time that the antinociceptive effects of V. officinalis are mediated by the serotonergic and dopaminergic systems. Monoamine neurotransmitters in the central nervous system, mostly serotonin (5-hydroxytryptamine, 5-HT) and norepinephrine, are crucial in regulating cognition, emotion, and mood. These neurotransmitters also play an essential role in the stress response and the mechanism of antidepressant action. It was observed that V. officinalis extract can suppress physical and psychological stress responses by controlling the variations in these neurotransmitters turnover in the hippocampus and amygdala [22], and reduces the plasma corticosterone levels in D-galactose-induced aging mice [49]. Dichloromethane extracts from V. wallichii significantly improve norepinephrine and dopamine amounts with no changes in serotonin amounts [59]. The effects of V. adscendens extracts on the central nervous system have been associated with the interaction with serotoninergic, dopaminergic, and noradrenergic receptors [95]. On the other hand, studies from Lee et al. [39] suggest that the antinociceptive effects of V. fauriei can be related to modulatory effects on the BDNF signaling pathway in the hippocampus and medial prefrontal cortex. Recently, Choi et al. [96] demonstrated that this species exerts antidepressant effects through its anti-inflammatory and antioxidant effects by inhibiting BDNF expression.
Valepotriates from V. jatamansi inhibit Cav2.2 and Cav3.1 calcium channels in a selective way which is coherent with the analgesic action of this species in relieving gastrointestinal and rheumatic pain [97]. Moreover, studies by Dong et al. [98] indicated that its use for alleviating abdominal distention and pain may be mediated through Cav2.2 channel. Although the information about the anticonvulsant effects of valerian is scarce, studies from Rezvani et al. [63] suggested that it is mediated through the activation of the adenosine system.
Santos et al. [54] demonstrated that different compounds from V. officinalis extract may trigger distinct mechanisms involved in neuronal cell protection in PD. Hesperidin, and probably linarin, alleviate oxidative stress effects during ATP depletion due to its capability to binding SUR1. On the other hand, valerenic acid and apigenin avoid cortical hyperexcitation by stimulating neuronal cells from the substantia nigra to release GABA on the brain stem. V. amurensis showed protective effects on amyloid-beta (Aβ)-induced toxicity in PC12 cells [99] and capacity to improve Aβ-induced cognitive dysfunction in mice by two mechanisms, by enhancing acetylcholine and choline acetyltransferase activity and thus improving cerebral cholinergic function, and by protecting neurons from Aβ-induced apoptosis [35].
The anti-neuroinflammatory effects of N. jatamansi extracts and isolated compounds have been described by different authors. Ko et al. [83] reported that four nardosinone-type sesquiterpenes showed anti-neuroinflammatory action on lipopolysaccharide (LPS)-induced immortalized murine brain microglia BV2 cell lines by inhibiting NF-κB- and MAPK-mediated inflammatory pathways. The anti-neuroinflammatory effects of two sesquiterpenoids from this species, desoxo-narchinol A and narchinol B, in BV2 and the primary microglial cell, was also reported, which is related to the inhibition of LPS-induced expression of iNOS and COX-2 enzymes the suppression of pro-inflammatory cytokines (IL-1b, IL-6, and TNF-a). Yoon et al. [33] showed that the inhibition of the NF-κB signaling pathway was also implicated in the anti-neuroinflammatory effect of three sesquiterpenoids from this species. Recently, Kim et al. [84] reported the anti-neuroinflammatory effect of desoxo-narchinol A and narchinol B in microglial cells by up-regulating of nuclear transcription factor erythroid-2-related factor 2/heme oxygenase-1 signaling. The mechanisms of action of Valerianaceae plants have not been fully investigated in humans. The effects of V. officinalis extract on cortical excitability were evaluated in humans with transcranial magnetic stimulation [100] and it was observed that a single oral dose adjusts intracortical facilitatory circuits.
5. Other Pharmacological Potential of Valerianaceae
As aforementioned some Valerianaceae species, particularly from the Valeriana genus, are well investigated for their pharmacological potential on the central nervous, namely anxiolytic, antidepressive, antinociceptive, and anticonvulsant, etc. However, various Valeriana species are still understudied for other biological properties. Among all the species, V. officinalis, V. jatamansi (syn. Valeriana wallichii), V. hardwickii Wall, and V. stenoptera Diels. are the ones that show better potential for further investigation in drug discovery. The bioactivity of root extracts from V. officinalis is mainly associated with its anxiolytic compounds, as the valerenic acid and its biosynthetic precursors, valerenal and valerenadiene. β-Caryophyllene is another sesquiterpenoid present in the extracts but is more associated with the anti-inflammatory effect, and in this case, V. officinalis and V. wallichii still need to be more deeply studied. Regarding anti-viral, hepatoprotective, or immune stimulant activities, no relevant data was found in the last ten years. The most recent research with Valeriana spp. is published in anti-anxiety, anti-bacterial, anti-cancer, anti-depressive, and cardiovascular effects, and they are summarized below for a better understanding of the data available.
5.1. Antibacterial Effect
Regarding the antibacterial activity of Valeriana spp. extracts few data are available [101]. The aerial parts of V. wallichii DC (Valerianaceae) were evaluated by Khuda et al. [101], for their antifungal and antibacterial activities. The authors prepared two extracts with the aerial parts of the plant, one with chloroform and the other with hexane, and both showed significant bioactivity [101]. It was also reported antibacterial activity for Staphylococcus aureus and Pseudomonas aeruginosa by four sesquiterpenoids isolated from the roots of V. jatamansi Jones [102].
5.2. Anti-Cancer Effect
Current pharmacotherapy has critical tools to speed the development of new target therapies which will accelerate the final goal in the fight against cancer. Various compounds separated from V. jatamansi and V. officinalis roots were active for a variety of cellular cancer lines, both in vitro and in vivo assays. For instance, among the isolated constituents from V. jatamansi, the derivative IVHD-valtrate, is one of the most promising molecules that was tested against human ovarian cancer cells (A2780 and OVCAR-3), in vitro and in vivo. This compound showed inhibition of the growth and proliferation in a concentration-dependent manner. This compound also revealed low cytotoxicity to immortalized non-tumorigenic human ovarian surface epithelial cells (IOSE-144), which is very important for further research. The authors even refer that Preclinical results pointed out IVHD-valtrate as a potential therapeutic drug for this type of cancer [103].
Two years late, the same group evaluated jatamanvaltrates P-Y, nardostachin, and ten new valepotriates, but this time, the compounds were isolated from the whole plants and found cytotoxic activity against PC-3M cells [104]. They also examined the structure-activity relationship of these valepotriates and the results reveal a crucial 10-chlorine in the oxirane ring and the bond C3–C4. The compound valtrate was able to avoid migration of human breast cancer cells and induce apoptosis, both in vitro and in vivo. In another experiment, they discover that valtrals (A, B, and C), which are by-products of valepotriates resulting from a degradation reaction during the separation methodology from the ethanol extract prepared with the entire plant, showed selective cytotoxicity against colon cancer (HCT-8) cell lines and metastatic prostate cancer (PC-3M) [104]. Other tests performed with three new minor valepotriate isomers, jatamanvaltrates, all of them isolated from the entire plants of V. jatamansi, evidenced moderate cytotoxicity in the two cell lines cited above and also hepatoma (Bel7402) [105]. Jatamanvaltrate P, an iridoid ester, can inhibit the proliferation and growth, in a concentration-dependent manner, of MDA-MB-231, MDA-MB-453, and MDA-MB-468 (this last three lines corresponding to the triple-negative breast cancer) and MCF-7 cell lines. As the author’s highlight, this molecule exhibited an antitumor effect in MDA-MB-231 xenografts without noticeable toxicity, suggesting that it could be used in research for a future potential therapeutic drug against breast cancer [105]. A specific fraction obtained from V. jatamansi (no data provided about the parts of the plant) significantly inhibited the growth of breast cancer cells in a concentration-dependent manner by inducing apoptosis [106]. Among its therapeutic effects on insomnia and seizures the valeric acid, which is another active compound in valerian, has been reported to improve immunity against cancer [107]. Promising data also involve other compounds, in this case, fatty acids naturally occurring in seed oils such as conjugated linolenic acids (CLNs) which are likewise present in C. ruber and V. officinalis) [108].
5.3. Anti-Inflammatory Effect
Cravotto, et al. [109] summarized the available scientific information on the intense, in-progress investigation for novel plants, extracts, and compounds with intense anti-inflammatory activity and found out that V. officinalis has, among many others understudy, this potential too. Other species like V. jatamansi Jones also presented this bioactivity [70]. There is also a report on the anti-inflammatory activity, of a methanolic crude extract prepared from V. wallichii leaves (topical formulation cream) using an in vitro and in vivo screening. The authors presented data of a considerable in vitro anti-inflammatory activity obtained with the ethyl acetate fraction [110].
5.4. Antioxidant Effect
In natural products, different compounds are under screening for their antioxidant activity and the possible use for therapeutic strategies, for instance in degenerative diseases. Among the various therapeutic properties attributed to Valeriana spp., most of them correlated to valepotriates, the antioxidant properties were also investigated. According to Sudati et al. [111], V. officinalis extracts show a protective effect on lipid peroxidation (LPO) caused by different pro-oxidant reactive with neuro damage relevance. Dugaheh et al., [112] also studied the antioxidant effect of root extracts from V. officinalis, N. jatamansi, and V. sisymbriifolia. The best DPPH inhibition effect was obtained with V. officinalis extracts, but all of the tested plants inhibited beta-carotene oxidation.
Another antioxidant activity investigation carried out with the aerial parts and roots of V. jatamansi, collected in pre-flowering, flowering, and post-flowering phenological stages, pointed to higher results for the first one, which could be correlated to the maximum concentration of phenolics and flavonoids. These methanolic extracts include catechin, and various phenolic acids as gallic, chlorogenic, hydroxybenzoic, caffeic, and p-coumaric, which largely varied among the different phenological stages, and along the altitudes. It was also highlighted that the pre-flowering stage is the most suitable for harvesting the roots containing the maximum phytochemicals amounts and antioxidant activity even for the samples which have few phenolics as the ones collected at high altitudes [113].
5.5. Cardiovascular Effect
Cardiovascular diseases are an important cause of death worldwide. Much research has been conducted to treat and delay these pathologies; however, much more needs to be done.
According to a report, lowering of blood pressure and heart rate, antiarrhythmic, regulation of blood lipid levels, and anti-myocardial ischemia-reperfusion injury are bioeffects that can be attributed to Valeriana spp. them. The vasorelaxant effect in endothelium-denuded rings was obtained with a hexane extract from V. edulis. The authors speculate that this effect could be related to the presence of valepotriates obtained from the hexane extract rhizomes [11]. It was suggested by Gan et al. [114] that some plant extracts, compared with the “model group”, could decrease the percentage of infarct volume, improve neurological activity, accentuate the expression of VEGFR2 and number of new blood vessels in the cortex infarction around, given a possible further use to relive the acute cerebral ischemia-reperfusion injury [114].
6. Phytochemical Configuration of Valerianaceae
Different species under the sub-family Valerianaceae exhibits pharmacologic activities, especially in the central nervous system. These plants are known sources of different phytochemicals such as flavonoids, lignans, neolignans, and terpenoids [24,53,99,115]. Table 2 shows the various extraction methods and biological activities of compounds isolated from selected species under the sub-family Valerianaceae.
Table 2.
Extraction methods and biological activities of compounds isolated from selected species under the sub-family Valerianaceae.
| Major Compound | Known Biological Activity | Isolation Techniques Used * | Detection Methods ** | First Author and Year |
|---|---|---|---|---|
| Nardostachys jatamansi (D.Don) DC. | ||||
| (–)-(8R)-neonardochinoneA and (+)-(8S)neonardochinoneA | Anti-Alzheimer’s disease (AD) activity | Silica gel CC, MCI gel CC, and Sephadex LH-20 CC | HRESIMS NMR XRC |
[115] |
| Nardochinins A-D | Silica gel CC, MCI gel CC, and Sephadex LH-20 CC | HRESIMS NMR XRC |
[29] | |
| Kanshone C—inhibits SERT and Desoxo-nachinol A—enhances SERT | Natural serotonin regulator using SERT activity assay | Silica gel CC and preparative HPLC | HRESIMS NMR XRC |
[78] |
| Aristolen-1(10)-en-9-ol | Sedative effect via GABAergic system | Silica gel CC and preparative HPLC | GC-FID GC-MS |
[76] |
| Valerena-4,7(11)-diene | The stress-reducing effect in mice | Silica gel CC and preparative HPLC | GC-MS | [28] |
| Valerena-4,7(11)-diene and b-maaliene | Sedative effects in mice | Silica gel CC, gel permeation chromatography, and HPLC | GC NMR |
[116] |
| Aristolene, calarene, and valerena-4,7(11)-diene | Sedative effects in mice | Silica gel CC, gel permeation chromatography, and HPLC | GC/GC-MS NMR |
[75] |
| Kanshone L, Kanshone M | Anti-inflammatory effects in BV2 and primary microglial cells | Silica gel CC and preparative HPLC | NMR HRESIMS |
[34] |
| Sesquiterpenoids: Kanshone J and Kanshone K | Anti-inflammatory effects in BV2 and primary microglial cells | Solvent partition, CC, and HPLC | NMR HRESIMS |
[34] |
| Nardosinone, Isonardosinone, Kanshone E, Kanshone B | Anti-inflammatory effects in BV2 and microglial cells | Silica gel CC | NMR MS |
[83] |
| Compounds 5 and 6 | Cytotoxic activity against a neuroblastoma cell line | Silica gel CC | FT-IR MS NMR |
[117] |
| Patrinia scabiosifolia Link | ||||
| Caryophyllene oxide | Anti-inflammatory activity in BV-2 cells | Distillation | GC-MS | [118] |
| Valeriana amurensis P. Smirn. ex Kom. | ||||
| Kissoone B | Anti-inflammatory and neuroprotective effects | Percolation, Sephadex LH-20 CC, and paper chromatography | EIMS NMR |
[36] |
| Xiecaoside E and Lignin 11-17 | Neuroprotective effects in PC12 cells | Silica gel CC and preparative HPLC | FT-IR NMR |
[119] |
| Lignans (e.g., (þ) pinoresinol-4, 4′-di-O-β-D-glucopyranoside, (þ) 8-hydroxypinoresinol-4′-Oβ-D-glucopyranoside); Iridoids (e.g., patrinoside and kanokoside A) | Activity on cerebral cholinergic function and neuroprotective effect from an αβ-induced cognitive deficit in mice | Silica gel CC, octadecyl silica gel CC, and preparative HPLC | NMR EIMS |
[35] |
| Heishuixiecaoline A, B, and C, volvalerenal C, (+) pinoresinol-4,4′-di-O-β-D-glucopyranoside, (+) pinoresinol-8-O-β-D-glucopyranoside, and 8-hydroxypinoresinol-4,4′-di-O-β-D-glucopyranoside | Neuroprotective effects in PC12 cells | AB-8 macroporous resin CC and silica gel CC | HRESIMS NMR FT-IR |
[99] |
| Valeriana fauriei Briq. | ||||
| 8α-acetoxyl-3α,4α,10-trihydroxyl-guaia-1(2)-ene-12,6α-olide and 2-Ethylhexyl-4-hydroxybenzoate | An antidepressant activity using forced swim test in a mouse model | Silica gel CC and Sephadex LH-20 CC | FT-IR MS NMR |
[37] |
| Valeriana glechomifolia F.G. Mey. | ||||
| Valtrate, Acevaltrate, 1-β-acevaltrate, 1-β-aceacevaltrate and isovaltrate | Activity on depressive-like behavior in mice | Supercritical CO2 (SCCO2) extraction and HPLC | HPLC | [41] |
| Valtrate, acevaltrate, and 1-β-acevaltrate | Inhibition of Na+/K+-ATPase activity in the brain hemispheres of rat | Ultrasonic bath and preparative TLC | NMR | [120] |
| Valeriana jatamansi Jones | ||||
| Rupesin E | Anticancer and pro-apoptotic activity against glioma stem cells. | Silica gel CC, Sephadex LH-20 CC, and semi-preparative HPLC | NMR | [121] |
| (4β,8β)-8-methoxy-3-methoxy-10-methylene-2,9-dioxatricyclo[4.3.1.0]decan-4-ol and (1S,3R,5R,7S,8R,9S)-3,8-epoxy-1- O-ethyl-5-hydroxyvalechlorine | Neuroprotective effects in PC12 cells | Silica gel CC, semipreparative HPLC, Sephadex LH-20 CC, preparative TLC | NMR ECD FT-IR UV Vis HRESIMS |
[105] |
| Valepotriate | The anti-epileptic effect in mice | Silica gel CC | NMR HPLC |
[122] |
| Isopatrinioside and Valeriananoid F | Neuroprotective effects in PC12 cells | Silica gel CC, Sephadex LH-20 CC, preparative TLC, and preparative HPLC | NMR HRESIMS FT-IR MCP UV-Vis |
[123] |
| Valtrate | The anxiolytic effect in rats | Chromatography in AB-8 macroporous adsorption resin, silica gel CC, and TLC | NMR EIMS FT-IR UV Vis |
[124] |
| Iridoids: Jatadoids A and B | Neuroprotective effects in SH-SY5Y cells | Silica gel CC, TLC, ODS CC, and preparative HPLC | NMR HRESIMS |
[44] |
| Jatairidoids A–C | Neuroprotective effects in SH-SY5Y cells | Silica gel CC, ODS CC, and preparative HPLC | NMR HRESIMS EIMS FT-IR |
[125] |
| Valeriandoids A–C and chlorovaltrate | Neuroprotective effects in SH-SY5Y cells | Silica gel CC, ODS CC, and preparative HPLC | NMR HRESIMS EIMS FT-IR |
[44] |
| 2S(−)-hesperidin | Sedative and sleep-enhancing properties in mice | Silica gel CC | UV-Vis NMR EIMS |
[24] |
| 6-methylapigenin | Anxiolytic and sleep-enhancing properties in mice | Silica gel CC and C18 column chromatography | UV-Vis NMR EIMS |
[126] |
| Valerilactones A and B, and bakkenolide-H | Neuroprotective effects in human dopaminergic neuroblastoma SH-SY5Y cells | Silica gel CC, ODS CC, and preparative HPLC | NMR HRESIMS ESIMS FT-IR |
[127] |
| Valeriana officinalis L. | ||||
| Acetoxyvalerenic acid and valerinic acid | Sleep promoting properties in mice model | Soxhlet extraction, rotary vacuum evaporation, and C18 CC | HPLC UV-Vis |
[128] |
| Valerenic acid | GABAA receptor modulator using a larval zebrafish seizure model | ASE® 200 solvent extraction system | HPLC | [129] |
| Volvalerenal H, Volvalerenal I, Volvalerenal J, Volvalerenal acid K, and Densispicnins C | Acetylcholinesterase inhibitory activity | Silica gel CC, Sephadex LH-20 CC, preparative TLC, and preparative HPLC | NMR HRESIMS FT-IR |
[53] |
| Valeneomerin A, Valeneomerin B, Valeneomerin D | Neuroprotective effects against H2O2 induced oxidative stress in SH-SY5Y cells | Silica gel CC, RP-MPLC, and Sephadex LH-20 (MeOH) CC, and preparative TLC | NMR HRESIMS FT-IR XRC |
[130] |
| Linarin | Sedative and sleep-enhancing property | Silica gel CC | NMR EIMS UV-Vis |
[25] |
| Volvalerenals A-E and volvalerenic acids A-C | Weak acetylcholinesterase inhibitory activities | Silica gel CC, and Sephadex LH-20 CC | NMR HRESIMS EIMS XRC |
[131] |
| Valeriana laurifolia Kunth | ||||
| Valtrate acetoxyhydrine, valtrate, isovaleroyloxyhydrine, and valtrate chlorohydrine | Anticonvulsant property in mice | Silica gel CC, and preparative TLC | NMR HRESIMS EIMS FT-IR |
[132] |
* CC—column chromatography, HPLC—high-performance liquid chromatography, ODS—octadecyl silica; TLC—thin layer chromatography. ** ECD—Electronic circular dichroism; EIMS—Electrospray ionization mass spectrometry; FT-IR—Fourier-transform infrared spectroscopy; GC-FID—Gas chromatography with flame-ionization detection; HRESIMS—High-resolution electrospray ionization mass spectrometry; MCP—Modular circular polarimeters; MS—Mass spectroscopy; NMR—nuclear magnetic resonance; UV Vis—Ultraviolet-visible spectroscopy; XRC—Xray crystallography.
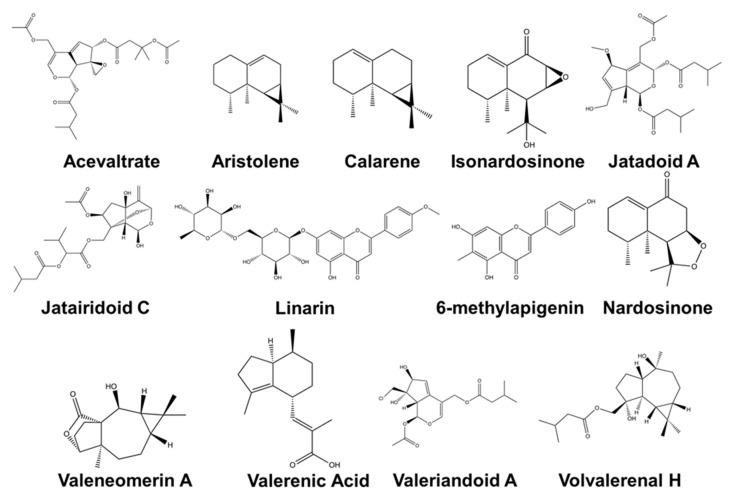
Phytochemical compounds from Valerianaceae have great potential as drugs for neurodegenerative diseases (Figure 1). The compounds (–)-(8R)-neonardochinone A, (+)-(8S) neonardochinone A, and nardochinins A–D isolated from N. jatamansi exhibit anti-AD activity using the humanized Caenorhabditis Elegans AD pathological model [115]. Lignans and iridoids isolated from V. amurensis showed neuroprotective activity against Aβ-induced toxicity in PC12 cells [35,99,119]. AD is associated with the production and deposition of the β-amyloid peptide (Aβ) in the brain [23]. Several compounds isolated from V. jatamansi such as jatadoids A and B, jatairidoids A and B, valeriandoids A–C, chlorovaltrate, valerilactones A and B, and bakkenolide-H have been studied to have neuroprotective properties in MPP+-induced Parkinson’s disease model in vitro [43,44,105,127]. Sesquiterpenoids from V. officinalis showed AChE inhibitory activity in vitro [53,131]. AChE inhibitors are clinically used to treat neuropsychiatric symptoms of AD, PD, dementia, and schizophrenia. These compounds are promising lead compounds for discovering drugs for AD and PD.
Figure 1.
Chemical structure of selected compounds isolated from plants under the sub-family Valerianaceae with potent biological activity in the central nervous system. The chemical structures were generated using the PerkinElmer ChemDraw Prime Software Version 20.0.0.38.
The other compounds from Valerianaceae are also known for their relaxant effects. Aristolen-1(10)-en-9-ol, calarene, β-maaliene, and valerena-4,7(11)-diene isolated from N. jatamansi showed significant sedative effects in mice studies [28,75,76,116]. Treatment with valerena-4,7(11)-diene prolonged the continuous sleep time of pentobarbital-treated mice by about 2.7 times [116]. Aristolen-1(10)-en-9-ol also exhibited a sedative effect comparable to that of diazepam and this sedative property is mediated through the GABAergic system [76]. Acetoxyvalerenic acid, valerenic acid, and linarin from V. officinalis also showed sedative and sleep-enhancing properties in animal studies [25,128]. 2S(−)-hesperidin, 6-methylapigenin isolated from V. jatamansi also showed sedative properties while valtrate exhibited anxiolytic effects and significantly reduced the corticosterone level in the rat serum [24,124,126].
The compounds isolated from plants under sub-family Valerianaceae are also known for their neuroprotective properties. Valeneomerin from V. officinalis showed neuroprotective properties against oxidative stress [130]. Different sesquiterpenes isolated from N. jatamansi (isonardosinone, kanshone B, E, J, K, L and M, and nardosinone), P. scabiosifolia (caryophyllene oxide and V. amurensis (kissoone B) prevented neuroinflammation in LPS-stimulated BV2 and primary microglial cells [34,36,83,118]. Neuroinflammation is associated with multiple neurodegenerative diseases such as AD, multiple sclerosis, and PD [133]. Isopatrinioside, valeriananoid F, and structural analogs of chlorovaltrate isolated from V. jatamansi exhibited neuroprotective properties against CoCl2-induced neuronal cell death in PC12 cell [105,123]. These compounds may be further developed to prevent chemical hypoxia-induced neurotoxicity.
Some compounds are also studied for their anticonvulsant, antidepressant, and stress-reducing properties. Monoterpenoids from V. glechomifolia and sesquiterpenoids from V. fauriei showed antidepressant activity in mice [37,41]. Valerena-4,7(11)-diene from N. jatamansi reduced stress in animal studies [28]. Valepotriates from V. jatamansi and valtrates from V. laurifolia showed anticonvulsant properties in mice [122,132]. These studies show the variety of phytochemical compounds isolated from plant species under the sub-family Valerianaceae. Interestingly, several compounds from subfamily Valerianaceae were more associated with a certain biological effect in the central nervous system. Sesquiterpenoids such as kanshone, kissoone, nardosinone, valerinic acid, etc., were shown to have anti-neuroinflammatory properties and sedative effects. Flavonoids such as linarin and methylapigenin were known to have sedative effects. On the other hand, monoterpenoids and glycosides were shown to have neuroprotective properties against oxidative stress and toxicants in neural cell lines. These compounds have huge potential to be developed as preventive and therapeutic interventions for different diseases of the CNS. Most of the studies for these compounds are still currently in the preclinical phase and warrant more clinical studies in the future.
7. Extraction and Isolation Procedure of Major Compounds from Valerianaceae
To ensure the reliability and replicability of preclinical studies using compounds from plants under the sub-family Valerianaceae, standardization of plant collection and identification; phytochemical compounds extraction, isolation and validation are warranted. Generally, plants undergo several processes before a pure compound can be isolated. This includes collection and authentication of the plant, extraction, purification, and structure determination. All the studies that were reviewed in this manuscript have utilized the roots and the rhizomes of the plant as the main source for the crude extract and the isolation of its potent pure compound. Extraction, fractionation, and purification of the roots and rhizomes of Valerianaceae have no difference as compared to other plant extraction. Each roots and rhizomes undergo air-drying, soaking, and chromatographic fractionation and purification. This is a general method in all natural products research to extract all the possible compounds within the plant. The roots and rhizomes are typically used for medicinal purposes because it is believed that the essential oils from its roots produce the biologic activity [134,135]. However, the use of roots and rhizomes is more destructive for the plants than collecting their leaves and flowers or buds [136]. It was reported that in the North-West Himalayan region, the population diversity of V. jatamansi is getting low. In the North-East Himalayan region, this herb is also classified as an endangered medicinal plant [137]. This requires a rigorous conservation measure to preserve these plants for future research and medicinal use.
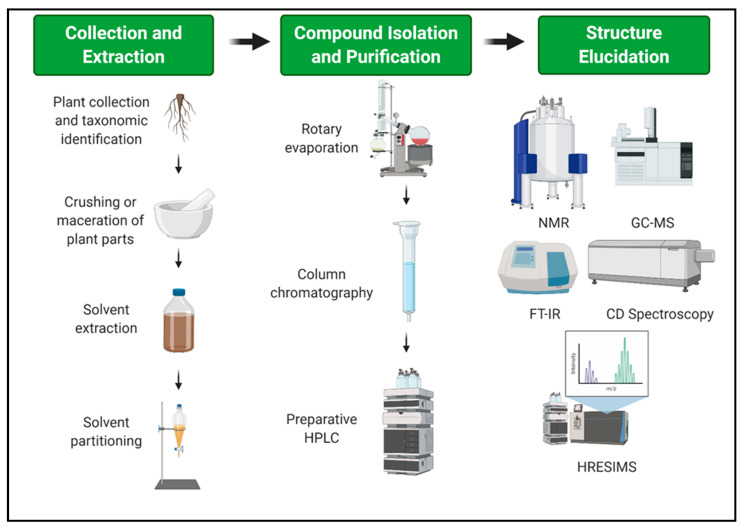
For the extraction process of biologically active compounds from plants under the sub-family Valerianaceae, Figure 2 shows the schematic diagram. The roots and the rhizomes were usually air-dried at room temperature. Some of the air-dried samples were further macerated and pulverized for greater absorption of the solvent. Air-dried plant samples were extracted with ethanol, methanol, hexane, and water solvent and underwent solvent partitioning with a range of nonpolar to polar solvents such as ether, ethyl acetate, and butanol. Most authors initially extracted the pulverized sample using an alcohol solvent such as ethanol or methanol because these are the most polar among the non-polar solvents and can extract several compounds [138]. Moreover, solvents such as water, methanol, butanol, ethyl acetate, and ether were used for solvent partitioning. This is because the compounds extracted have different polarity and solubility and through this range of solvents can a researcher identify the most active compound. The most common compound isolated belongs to the group of terpenoids. Most terpenoids are non-polar and volatile, thus, solvent partitioning involving this range of non-polar to polar solvents is important to isolate and purify the potent compound [134].
Figure 2.
Schematic representation of the general extraction, isolation, purification, and structure elucidation of biologically active compounds from plant species under the sub-family Valerianaceae. This figure was created with BioRender.com.
Each crude extract underwent a purification process through different chromatographic techniques such as the use of silica gel column chromatography, thin-layer chromatography (TLC), and preparative high-performance liquid chromatography (HPLC). TLC is commonly done among natural products research. It is the simplest and cheapest technique to separate several components in an extract and verify the identity and purity of the compound. Furthermore, TLC serves as a guide in setting the parameters for column chromatography as a means for preparative separation [139]. Meanwhile, column chromatography is widely used as an initial separation step for the phytochemical components because of its simplicity, high capacity, and low cost. In column chromatography, the mobile phase carries the bioactive components as they pass through the stationary phase that separates the components depending on their affinity [140]. HPLC, on the other hand, is an analytic technique used to separate, quantify, and identify inorganic and organic solutes. This technique is robust and versatile that requires high pressure to elute the analyte to the detector [141]. In isolating iridoids, it was noted that these three common techniques of purification: preparative TLC, silica gel CC, and preparative HPLC were used. It is a traditional way and more feasible way to isolate compounds before undergoing more advance chromatographic techniques.
As the solute is purified, the compound is identified through its structure and molecular weight. Some of the techniques used to determine the structure of the pure compound responsible for its biological activity are nuclear magnetic resonance (NMR), gas chromatography-mass spectrophotometry (GC-MS), high-resolution electrospray ionization mass spectrophotometry (HRESIMS), Fourier transform infrared spectroscopy (FT-IR), and circular dichroism (CD). Each one has its advantage over the other but they help each other in understanding more about the discovered compound. NMR is used to give an idea about the physical, chemical, and biological property of the compound through the identification of carbon present in the compound while MS is used to identify, quantify, elucidate the structure, and determine the molecular weight of the unknown compound [142]. Mass spectrometry is an analytical tool that can give qualitative and quantitative data about the analyte and it has several types. One of these is HRESIMS which is a robust technique that can analyze the minute volume of samples that are non-volatile and thermally stable compounds. It is used when conventional techniques cannot analyze the given sample while GC-MS is a combination of two techniques making it a powerful tool in the analysis of a certain compound [143]. Gas chromatography separates the individual components while mass spectrometry characterizes the components. On the other hand, FTIR is a tool to identify the functional groups and the structure of the molecule in a given extract [142]. Finally, the CD is absorption spectroscopy that uses circularly polarized light to determine the chirality of a given compound [144]. Therefore, there is no single process in doing natural product research. The process always depends on the characteristic of the compound to be discovered.
8. Preclinical and Clinical Effectiveness in Humans and Patents
Although valerian has traditionally been used as a mild sedative, research results are still controversial today regarding the role of the different active compounds, the herbal preparations, and the dosage used [24,145]. In fact, in vivo studies, showed that valerian can be used as an anti-depressant [92,146] (Table 3). In particular, as abovementioned the neurobiological mechanisms of the different bioactive compounds in Valerianaceae species can be due to the effects on GABA, serotoninergic, dopaminergic, noradrenergic, and adenosine A1 receptors [86,95,147,148]. V. glechomifolia extract containing 96% of valepotriates (10 mg/kg) showed anxiolytic and sedative properties reducing exploratory and behavior locomotion during open field exploration without affecting memory test. Otherwise the dose of 3 mg/kg V. glechomifolia selectively influenced the recognition memory without effects on other behavioral parameters [149]. The anti-depressant activity of V. glechomifolia in mice also appears to be due to the interaction with noradrenergic and dopaminergic neurotransmission. The extract of V. glechomifolia can enhance its antidepressant effects such as imipramine, desipramine, and bupropion without involving the neurotransmission of serotonin and activating the noradrenergic and dopaminergic systems [149]. Holzamann et al. [58] reported the general depressive activity in rats Wistar treated with 50–150 mg/kg p.o. of the extract of V. prionophylla Standl., used in Mesoamerican traditional medicine for treating sleep disorders. This effect seems to be related to the capacity of V. prionophylla to increase pentobarbital-induced sleep time and to decrease sleep latency [150].
Table 3.
In vivo studies on the effects of some species belonging to the Valerianaceae sub-family on the Central Nervous System.
| Compound/Species | Animal Model | Dosage | Outcomes | Ref. |
|---|---|---|---|---|
| Valerenic acid derivatives | Male mice (c57Bl/6N) | 3 mg/kg | Anxiolytic effect | [151] |
| Valerenic acid derivatives | Mutant mice (GABAA receptor b3 subunit mutation) | From 1 to 30 mg/kg | Anxiolitic effects | [152] |
| V. glechomifolia | Swiss male CF1 mice | 1, 3, and 10 mg/kg | Sedative effects | [149] |
| V. prionophylla | Swiss female mice; Wistar male rats | 50, 100, and 150 mg/kg | Anxiolytic; antidepressant; hypno-sedative effects | [58] |
| N. chinensis | Different animal model | Different dosages | Antidepressant; anticonvulsant; neuroprotective and antiparkinson activities; cognition and memory improvement | [153] |
| V. jatamansi | Kunming mice | Ethanolic extract | Anxiolytic effects; No drug dependence | [146] |
| V. officinalis | Zebrafish larvae | 0.3 g/kg, 0.9 g/kg | Regulation of neural-activity genes | [154] |
| V. officinalis | BALB/c mice | 1, 2.5, 5, and 7 mg/mL | Modulate GABAA subunit β3 receptors; sedative effects | [155] |
| V. edulis | Wistar male rats | 2.5 mg/10 g; 5 mg/10 g | Anticonvulsant properties | [156] |
The anxiolytic, sedative, and memory effects of extracts from Valerianaceae species may be due to the ability to interact with the GABAA receptor, possibly at the level of the subtypes (sub-unit β3; GABRB3) of receptors that mediate the effects of benzodiazepines, so producing the hypnotic and sedative activities. Administration of V. officinalis extracts at different dosages in BALB/c mice is related to changes in the levels of the GABRB3 protein. In particular, the extract induced an increase in the protein expression in comparison to the group of animals, which were given diazepam [155]. The anxiolytic effects have also been attributed to V. jatamansi; these effects may be due to the modulation of the levels of 5-HT, norepinephrine, dopamine, γ-aminobutyric acid, which by adjusting the axis hypothalamus-pituitary-adrenal axis employing β-endorphins and corticosterone [146].
The sedative and anticonvulsant effects of V. edulis and several valerian extracts are often related to high dosages and the different phytochemical compounds such as valerenic acids and flavonoids, present in the different Valerian species. In vivo experimental models confirmed that valerenic acid and valerian extracts have shown sedative effects [151,156,157,158]. In particular, Benke et al. [152] recently demonstrated a precise binding site on GABAA receptors that showed a high affinity for valerenol and valerenic acid. Several previous studies demonstrated that the properties of valerian are related not only by the interaction with GABAA receptors but also by the involvement of adenosine receptors [148,159,160].
Several authors reported that the administration of valepotriates or V. officinalis root extracts in zebrafish larvae induced the modulation of c-fos and of Npas4a, involved in regulating of the neural circuits [154,161,162,163]. The modulation of these genes, induced by valepotriates, together with their ability to bind GABAA receptors and histone deacetylase (HDAC) inhibitors confirm the neuroprotective effects of valerian extracts [164]. In addition to their anxiolytic and depressive effects, the extracts from V. officinalis can protect the neurons of the hippocampus from ischemic damage and restore behavioral deterioration. These protective effects are due to the ability of V. officinalis and its main constituents such as valerenic acid and acetylvalerenolic acid, to inhibit the activity of the nuclear factor (NF)-κB in vitro [165]. Other studies showed that the CNS effects of V. officinalis, V. jatamansi, and N. jatamansi can certainly also attributed to the inhibitory activity exerted on AChE [166].
Rahman and collaborators showed that the administration of an N. jatamansi ethanol extract to young and aged mice for 8 days enhanced memory and learning and determined a reversion of the amnesia induced by diazepam and scopolamine [153]. Among the Valerianaceae sub-family, few species have been clinically evaluated for their biological activities on CNS, despite the large traditional use, to treat insomnia, anxiety, epilepsy, and neurodegenerative diseases. The majority of controlled clinical trials available in the scientific literature are on the efficacy of improving sleep disorders of different valerian extracts. A fact sheet from the NIH Office of Dietary Supplements updated on March 2013 [167] is reported five rated randomized, controlled trials (Table 4).
Table 4.
Systematic reviews reporting the effectiveness of some species of Valerianaceae sub-family on CNS.
| Species | Systematic Review | Indication | Conclusions | Source |
|---|---|---|---|---|
| V. officinalis | 5 clinical trials | Sleep disorders | Not sufficient for determining the effectiveness | [167] |
| V. officinalis | 60 studies and meta-analysis | Sleep disorders | Sufficient for determining the effectiveness but standardization and quality control is needed | [173] |
| N. grandiflora | Preliminary clinical studies | Aggressiveness, restlessness, stubbornness, sleep disorders |
Further studies are needed | [153] |
The studies showed that V. officinalis root improved sleep quality [168], reduced sleep latency and improved the subjective sleep rating [169], decreased insomnia symptoms [170] and also improved sleep quality with results comparable to those obtained by the administration of 10 mg of oxazepam but with fewer side effects [171]. Conversely, subsequent randomized double-blind studies based on sleep parameters evaluated objectively with polysomnographic techniques detected no substantial differences on any of the measurements in comparison to the placebo group except for only one parameter [172]. The NHI document addressed to health professionals, concluded that qualitative results suggest that V. officinalis would be a promising strategy for insomnia and sleep-associated disorders subjective improvement. However, not all studies have produced positive outcomes and the real effectiveness of V. officinalis could not be proved by objective or quantitative measurements due to methodological limitations observed in most clinical studies, such as the small sample sizes, the unstandardized sources of valerian, the low rate of reproducibility of the results, etc.
Recently, Shinjyo et al. [173], based on a systematic review of 60 studies and meta-analyses, updated and re-evaluated the most reliable literature data to assess the effectiveness of V. officinalis in ameliorating sleep and sleep-associated disorders, yet controversial and not fully conclusive. The authors reported that the inconsistent and conflicting results of the clinical trials are maybe due to the quality and to the differences in herbal preparation in addition to the aforementioned methodological limits verified.
Among the 40 articles analyzed to assess the effectiveness of V. officinalis to treat sleep disorders, 30 addressed its efficacy in ameliorating sleep quality and sleep-associated problems. The additional analysis of seven articles also revealed the efficacy of V. officinalis in inducing positive effects on anxiety states in different stress conditions, also confirmed by another recent study on its anxiolytic activity in patients during dental surgery [174].
Other positive effects are reported in reducing the symptoms of obsessive-compulsive disorder and in preventing cognitive dysfunction rather than in reducing menopausal and postmenopausal hot flashes in women. Concluding, the results of this study suggested that the whole root rather than different extracts of V. officinalis is a valid and safe alternative for the treatment of sleep problems and anxiety. V. wallichii and V. edulis rich only in valepotriates and lacking in valerenic acids demonstrated sleep-promoting properties but the numbers of the studies are insufficient. The therapeutic effects on sleep were found with the use of doses of 450–1410 mg of the whole root per day for up to 8 weeks. These positive biological activities could be ensured by the standardization and characterization of the phytochemical profile of the diverse active compounds present in extracts and could be improved by the combination with different sleep-promoting herbs [173]. N. grandiflora is another species tested in a preliminary clinical study; the principle compound jatamansone obtained from the rhizomes significantly reduced aggressiveness and restlessness in hyperkinetic children [153].
Most common commercial Valerian preparations are generally quite safe for short-term use (for 4–6 weeks), 600 mg of valerian did not cause clinically significant effects on reaction time, alertness, and concentration the morning after ingestion [167]. No literature data is available that demonstrated the safety of long term use. In addition, little is known about their use in the woman during pregnancy and nursing and in children younger than 3 years old. However, the few side effects reported in the literature include agitation, restlessness, insomnia, headache, dizziness, itching and gastrointestinal upset. Particular attention must be paid to the potential interactions with sedative substances such as barbiturates, benzodiazepine, melatonin, alcohol, some herbs and dietary supplements with sedative properties (Hypericum perforatum L., Piper methysticum G. Forst) due to the potential additive effects. Cytotoxicity was detected only in vitro for valepotriates with no carcinogenic effects in animal models. These compounds are not always present in commercial valerian preparations. There is report that the roots and rhizomes of Valeriana officinalis have been used in official medicine in the form of various dosage for more than 240 years [175]. Moreover, Valeriana drugs in Russian medicine are used in mono form, which increases the importance of this plant for the treatment of a wide range of diseases [175]. Despite the wide use of these herbs in traditional medicine as well in the official medicine, more advanced clinical studies are still necessary on other plant species of this subfamily to establish the numerous biological activities associated with their pharmacological applications.
9. Conclusions
The studies reported and discussed in the present review article have indicated the multifaceted biological activities of many species from the Valerianaceae sub-family. Reviewed studies indicated that species from the genus Valeriana, particularly species like V. officinalis, V. jatamansi, V. fauriei, V. amurensis, V. glechomifolia, and V. polystachya have positive effects to treat disorders related to the Central Nervous System dysfunctions, such as insomnia, anxiety, and epilepsy. The mode of action of valerian compounds is mainly associated with the modulation of GABAergic transmission. Nevertheless, validated clinical studies are still necessary to establish the numerous and interesting biological activities associated with the pharmacological applications of the species.
Acknowledgments
Authors are grateful to respective institutions for support. G.D., J.-K.P. and H.-S.S. acknowledges Dongguk University, Republic of Korea for support. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667). S.G. and A.R. acknowledge the project INTERREG—MD.NET: When Brand Meets People and by National Funds through FCT—Foundation for Science and Technology under the Project UIDB/05183/2020. S.G. is funded by national funds through FCT, under the Norma Transitória—DL 57/2016/CP1361/CT0022; M.G.C. wish to thank to “Projeto Estratégico—(UI0204): UIDB/00313/2020 (Portugal)”.
Author Contributions
G.D., H.-S.S., R.T., S.G., O.A.G.T., M.G.C., R.A., G.A.M., A.R., J.A.H.R., M.Q.C. and J.-K.P., writing—original draft preparation, investigation, resources, data curation; G.D., R.T., S.G., O.A.G.T., M.G.C. and J.-K.P., writing—review and editing, methodology, formal analysis; J.-K.P., conceptualization, supervision, project administration, funding acquisition, visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667).
Data Availability Statement
All data related to the review manuscript are presented in the manuscript in the form of tables and figures.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Backlund A., Moritz T. Phylogenetic implications of an expanded valepotriate distribution in the Valerianaceae. Biochem. Syst. Ecol. 1998;26:309–335. doi: 10.1016/S0305-1978(97)00121-X. [DOI] [Google Scholar]
- 2.Donoghue M.J., Bell C.D., Winkworth R.C. The evolution of reproductive characters in Dipsacales. Int. J. Plant Sci. 2003;164:S453–S464. doi: 10.1086/376874. [DOI] [Google Scholar]
- 3.Lin S., Chen T., Liu X.-H., Shen Y.-H., Li H.-L., Shan L., Liu R.-H., Xu X.-K., Zhang W.-D., Wang H. Iridoids and lignans from Valeriana jatamansi. J. Nat. Prod. 2010;73:632–638. doi: 10.1021/np900795c. [DOI] [PubMed] [Google Scholar]
- 4.Tang Y., Liu X., Yu B. Iridoids from the Rhizomes and Roots of Valeriana jatamansi. J. Nat. Prod. 2002;65:1949–1952. doi: 10.1021/np0203335. [DOI] [PubMed] [Google Scholar]
- 5.Wang R., Xiao D., Bian Y.-H., Zhang X.-Y., Li B.-J., Ding L.-S., Peng S.-L. Minor iridoids from the roots of Valeriana wallichii. J. Nat. Prod. 2008;71:1254–1257. doi: 10.1021/np070598p. [DOI] [PubMed] [Google Scholar]
- 6.Bell C.D., Edwards E.J., Kim S.-T., Donoghue M.J. Dipsacales phylogeny based on chloroplast DNA sequences. Harv. Pap. Bot. 2001;6:481–499. [Google Scholar]
- 7.Bell C.D. Preliminary phylogeny of Valerianaceae (Dipsacales) inferred from nuclear and chloroplast DNA sequence data. Mol. Phylogenetics Evol. 2004;31:340–350. doi: 10.1016/j.ympev.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Bell C.D., Donoghue M.J. Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. Org. Divers. Evol. 2005;5:147–159. doi: 10.1016/j.ode.2004.10.014. [DOI] [Google Scholar]
- 9.Kutschker A., Morrone J.J. Distributional patterns of the species of Valeriana (Valerianaceae) in southern South America. Plant Syst. Evol. 2012;298:535–547. doi: 10.1007/s00606-011-0564-6. [DOI] [Google Scholar]
- 10.Olsen C. Trade and conservation of Himalayan medicinal plants: Nardostachys grandiflora DC. and Neopicrorhiza scrophulariiflora (Pennell) Hong. Biol. Conserv. 2005;125:505–514. doi: 10.1016/j.biocon.2005.04.013. [DOI] [Google Scholar]
- 11.Estrada-Soto S., Rivera-Leyva J., Ramírez-Espinosa J.J., Castillo-España P., Aguirre-Crespo F., Hernández-Abreu O. Vasorelaxant effect of Valeriana edulis ssp. procera (Valerianaceae) and its mode of action as calcium channel blocker. J. Pharm. Pharmacol. 2010;62:1167–1174. doi: 10.1111/j.2042-7158.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- 12.Houghton P.J. The scientific basis for the reputed activity of Valerian. J. Pharm. Pharmacol. 1999;51:505–512. doi: 10.1211/0022357991772772. [DOI] [PubMed] [Google Scholar]
- 13.Oshima Y., Matsuoka S., Ohizumi Y. Antidepressant principles of Valeriana fauriei roots. Chem. Pharm. Bull. 1995;43:169–170. doi: 10.1248/cpb.43.169. [DOI] [PubMed] [Google Scholar]
- 14.Morazzoni P., Bombardelli E. Valeriana officinalis: Traditional used and recent evaluation of activity. Fitoterapia. 1995;66:99–112. [Google Scholar]
- 15.Hendriks H., Bos R., Allersma D., Malingré T.M., Koster A.S. Pharmacological screening of valerenal and some other components of essential oil of Valeriana officinalis. Planta Med. 1981;42:62–68. doi: 10.1055/s-2007-971547. [DOI] [PubMed] [Google Scholar]
- 16.He X., Luan F., Zhao Z., Ning N., Li M., Jin L., Chang Y., Zhang Q., Wu N., Huang L. The genus Patrinia: A review of traditional uses, phytochemical and pharmacological studies. Am. J. Chin. Med. 2017;45:637–666. doi: 10.1142/S0192415X17500379. [DOI] [PubMed] [Google Scholar]
- 17.Cornara L., La Rocca A., Marsili S., Mariotti M. Traditional uses of plants in the Eastern Riviera (Liguria, Italy) J. Ethnopharmacol. 2009;125:16–30. doi: 10.1016/j.jep.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Rehman T., Ahmad S. Nardostachys chinensis Batalin: A review of traditional uses, phytochemistry, and pharmacology. Phytother. Res. 2019;33:2622–2648. doi: 10.1002/ptr.6447. [DOI] [PubMed] [Google Scholar]
- 19.Lentini F., Venza F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007;3:1–12. doi: 10.1186/1746-4269-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(HMPC/EMEA) Committee on Herbal Medicinal Product from European Medicines Agency Community Monograph on European Medicine Agency on Valerian Root, Valeriana officinalis L. Radix. European Medicines Agency. [(accessed on 28 November 2020)];2016 Available online: https://www.ema.europa.eu/en/documents/herbal-summary/valerian-root-summary-public_en.pdf.
- 21.Hattesohl M., Feistel B., Sievers H., Lehnfeld R., Hegger M., Winterhoff H. Extracts of Valeriana officinalis L. sl show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine. 2008;15:2–15. doi: 10.1016/j.phymed.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Jung H.Y., Yoo D.Y., Kim W., Nam S.M., Kim J.W., Choi J.H., Kwak Y.-G., Yoon Y.S., Hwang I.K. Valeriana officinalis root extract suppresses physical stress by electric shock and psychological stress by nociceptive stimulation-evoked responses by decreasing the ratio of monoamine neurotransmitters to their metabolites. BMC Complement. Altern. Med. 2014;14:1–8. doi: 10.1186/1472-6882-14-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy K., Kubin Z., Shepherd J., Ettinger R. Valeriana officinalis root extracts have potent anxiolytic effects in laboratory rats. Phytomedicine. 2010;17:674–678. doi: 10.1016/j.phymed.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Marder M., Viola H., Wasowski C., Fernández S., Medina J.H., Paladini A.C. 6-Methylapigenin and hesperidin: New valeriana flavonoids with activity on the CNS. Pharmacol. Biochem. Behav. 2003;75:537–545. doi: 10.1016/S0091-3057(03)00121-7. [DOI] [PubMed] [Google Scholar]
- 25.Fernández S., Wasowski C., Paladini A.C., Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 2004;77:399–404. doi: 10.1016/j.pbb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Granger R.E., Campbell E.L., Johnston G.A. (+)-And (−)-borneol: Efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors. Biochem. Pharmacol. 2005;69:1101–1111. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Oliva I., González-Trujano M.E., Arrieta J., Enciso-Rodríguez R., Navarrete A. Neuropharmacological profile of hydroalcohol extract of Valeriana edulis ssp. procera roots in mice. Phytother. Res. 2004;18:290–296. doi: 10.1002/ptr.1389. [DOI] [PubMed] [Google Scholar]
- 28.Takemoto H., Omameuda Y., Ito M., Fukuda T., Kaneko S., Akaike A., Kobayashi Y. Inhalation administration of valerena-4, 7 (11)-diene from Nardostachys chinensis roots ameliorates restraint stress-induced changes in murine behavior and stress-related factors. Biol. Pharm. Bull. 2014;37:1050–1055. doi: 10.1248/bpb.b14-00136. [DOI] [PubMed] [Google Scholar]
- 29.Wu P.-Q., Yu Y.-F., Zhao Y., Yu C.-X., Zhi D.-J., Qi F.-M., Fei D.-Q., Zhang Z.-X. Four novel sesquiterpenoids with their anti-Alzheimer’s disease activity from Nardostachys chinensis. Org. Biomol. Chem. 2018;16:9038–9045. doi: 10.1039/C8OB02319K. [DOI] [PubMed] [Google Scholar]
- 30.Lyle N., Chakrabarti S., Sur T., Gomes A., Bhattacharyya D. Nardostachys jatamansi protects against cold restraint stress induced central monoaminergic and oxidative changes in rats. Neurochem. Res. 2012;37:2748–2757. doi: 10.1007/s11064-012-0867-1. [DOI] [PubMed] [Google Scholar]
- 31.Bae G.-S., Heo K.-H., Choi S.B., Jo I.-J., Kim D.-G., Shin J.-Y., Seo S.-H., Park K.-C., Lee D.-S., Oh H. Beneficial effects of fractions of Nardostachys jatamansi on lipopolysaccharide-induced inflammatory response. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/837835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razack S., Kandikattu H.K., Venuprasad M., Amruta N., Khanum F., Chuttani K., Mishra A.K. Anxiolytic actions of Nardostachys jatamansi via GABA benzodiazepine channel complex mechanism and its biodistribution studies. Metab. Brain Dis. 2018;33:1533–1549. doi: 10.1007/s11011-018-0261-z. [DOI] [PubMed] [Google Scholar]
- 33.Yoon C.-S., Kim D.-C., Park J.-S., Kim K.-W., Kim Y.-C., Oh H. Isolation of Novel Sesquiterpeniods and Anti-neuroinflammatory Metabolites from Nardostachys jatamansi. Molecules. 2018;23:2367. doi: 10.3390/molecules23092367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon C.-S., Kim K.-W., Lee S.-C., Kim Y.-C., Oh H. Anti-neuroinflammatory effects of sesquiterpenoids isolated from Nardostachys jatamansi. Bioorg. Med. Chem. Lett. 2018;28:140–144. doi: 10.1016/j.bmcl.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q., Wang C., Shu Z., Chan K., Huang S., Li Y., Xiao Y., Wu L., Kuang H., Sun X. Valeriana amurensis improves Amyloid-beta 1-42 induced cognitive deficit by enhancing cerebral cholinergic function and protecting the brain neurons from apoptosis in mice. J. Ethnopharmacol. 2014;153:318–325. doi: 10.1016/j.jep.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Wu J., Guo J., Du X., Mcgeer P.L. Anti-inflammatory and neuroprotective effects of kissoone B and extracts of Valeriana amurensis. Rev. Bras. Farmacogn. 2020;30:474–481. doi: 10.1007/s43450-020-00037-1. [DOI] [Google Scholar]
- 37.Liu X.G., Gao P.Y., Wang G.S., Song S.J., Li L.Z., Li X., Yao X.S., Zhang Z.X. In vivo antidepressant activity of sesquiterpenes from the roots of Valeriana fauriei Briq. Fitoterapia. 2012;83:599–603. doi: 10.1016/j.fitote.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Lee H., Won H., Im J., Kim Y.O., Lee S., Cho I.-H., Kim H.-K., Kwon J.-T., Kim H.-J. Effect of Valeriana fauriei extract on the offspring of adult rats exposed to prenatal stress. Int. J. Mol. Med. 2016;38:251–258. doi: 10.3892/ijmm.2016.2589. [DOI] [PubMed] [Google Scholar]
- 39.Lee H., Im J., Won H., Kim J.Y., Kim H.K., Kwon J.T., Kim Y.O., Lee S., Cho I.H., Lee S.W. Antinociceptive effect of Valeriana fauriei regulates BDNF signaling in an animal model of fibromyalgia. Int. J. Mol. Med. 2018;41:485–492. doi: 10.3892/ijmm.2017.3203. [DOI] [PubMed] [Google Scholar]
- 40.Müller L.G., Salles L.A., Stein A.C., Betti A.H., Sakamoto S., Cassel E., Vargas R.F., von Poser G.L., Rates S.M. Antidepressant-like effect of Valeriana glechomifolia Meyer (Valerianaceae) in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;36:101–109. doi: 10.1016/j.pnpbp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Müller L.G., Borsoi M., Stolz E.D., Herzfeldt V., Viana A.F., Ravazzolo A.P., Rates S.M.K. Diene valepotriates from Valeriana glechomifolia prevent lipopolysaccharide-induced sickness and depressive-like behavior in mice. Evid. Based Complement. Altern. Med. 2015;2015 doi: 10.1155/2015/145914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Almeida T.M., Danielli L.J., Apel M.A., Cassel E., Vargas R.M., Von Poser G.L., Müller L.G., Rates S.M. A valepotriate-enriched fraction from Valeriana glechomifolia Meyer inhibits leukocytes migration and nociception in formalin test in rodents. Rev. Bras. Farmacogn. 2019;29:477–482. doi: 10.1016/j.bjp.2019.02.004. [DOI] [Google Scholar]
- 43.Xu J., Zhao P., Guo Y., Xie C., Jin D.-Q., Ma Y., Hou W., Zhang T. Iridoids from the roots of Valeriana jatamansi and their neuroprotective effects. Fitoterapia. 2011;82:1133–1136. doi: 10.1016/j.fitote.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Xu J., Li Y., Guo Y., Guo P., Yamakuni T., Ohizumi Y. Isolation, structural elucidation, and neuroprotective effects of iridoids from Valeriana jatamansi. Biosci. Biotechnol. Biochem. 2012;76:1401–1403. doi: 10.1271/bbb.120097. [DOI] [PubMed] [Google Scholar]
- 45.You J.-S., Peng M., Shi J.-L., Zheng H.-Z., Liu Y., Zhao B.-S., Guo J.-Y. Evaluation of anxiolytic activity of compound Valeriana jatamansi Jones in mice. BMC Complement. Altern. Med. 2012;12:1–9. doi: 10.1186/1472-6882-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S.-N., Ding Y.-S., Ma X.-J., Zhao C.-B., Lin M.-X., Luo J., Jiang Y.-N., He S., Guo J.-Y., Shi J.-L. Identification of bioactive chemical markers in Zhi zhu xiang improving anxiety in rat by fingerprint-efficacy study. Molecules. 2018;23:2329. doi: 10.3390/molecules23092329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y., Wu L., Chen C., Wang L., Guo C., Zhao X., Zhao T., Wang X., Liu A., Yan Z. Serum Metabolic Profiling Reveals the Antidepressive Effects of the Total Iridoids of Valeriana jatamansi Jones on Chronic Unpredictable Mild Stress Mice. Front. Pharmacol. 2020;11:338. doi: 10.3389/fphar.2020.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nouri M.H.K., Abad A.N.A. Gabaergic system role in aqueous extract of Valeriana officinalis L. root on PTZ-induced clonic seizure threshold in mice. Afr. J. Pharm. Pharmacol. 2011;5:1212–1217. doi: 10.5897/AJPP11.241. [DOI] [Google Scholar]
- 49.Nam S.M., Choi J.H., Yoo D.Y., Kim W., Jung H.Y., Kim J.W., Kang S.-Y., Park J., Kim D.-W., Kim W.J. Valeriana officinalis extract and its main component, valerenic acid, ameliorate D-galactose-induced reductions in memory, cell proliferation, and neuroblast differentiation by reducing corticosterone levels and lipid peroxidation. Exp. Gerontol. 2013;48:1369–1377. doi: 10.1016/j.exger.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Shahidi S., Bathaei A., Pahlevani P. Antinociceptive effects of Valeriana extract in mice: Involvement of the dopaminergic and serotonergic systems. Neurophysiology. 2013;45:448–452. doi: 10.1007/s11062-013-9392-3. [DOI] [Google Scholar]
- 51.Torres-Hernández B.A., Del Valle-Mojica L.M., Ortíz J.G. Valerenic acid and Valeriana officinalis extracts delay onset of Pentylenetetrazole (PTZ)-Induced seizures in adult Danio rerio (Zebrafish) BMC Complement. Altern. Med. 2015;15:1–10. doi: 10.1186/s12906-015-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo D.Y., Jung H.Y., Nam S.M., Kim J.W., Choi J.H., Kwak Y.-G., Yoo M., Lee S., Yoon Y.S., Hwang I.K. Valeriana officinalis extracts ameliorate neuronal damage by suppressing lipid peroxidation in the gerbil hippocampus following transient cerebral ischemia. J. Med. Food. 2015;18:642–647. doi: 10.1089/jmf.2014.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H.-W., He X.-H., Yuan R., Wei B.-J., Chen Z., Dong J.-X., Wang J. Sesquiterpenes and a monoterpenoid with acetylcholinesterase (AchE) inhibitory activity from Valeriana officinalis var. latiofolia in vitro and in vivo. Fitoterapia. 2016;110:142–149. doi: 10.1016/j.fitote.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Santos G., Giraldez-Alvarez L.D., Ávila-Rodriguez M., Capani F., Galembeck E., Neto A.G., Barreto G.E., Andrade B. SUR1 receptor interaction with hesperidin and linarin predicts possible mechanisms of action of Valeriana officinalis in Parkinson. Front. Aging Neurosci. 2016;8:97. doi: 10.3389/fnagi.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park J.-Y., Lee Y., Lee H.J., Kwon Y.-S., Chun W. In silico screening of GABA aminotransferase inhibitors from the constituents of Valeriana officinalis by molecular docking and molecular dynamics simulation study. J. Mol. Model. 2020;26:1–13. doi: 10.1007/s00894-020-04495-1. [DOI] [PubMed] [Google Scholar]
- 56.Amaral de Brito A.P., Galvão de Melo I.M.d.S., El-Bachá R.S., Guedes R.C.A. Valeriana officinalis Counteracts Rotenone Effects on Spreading Depression in the Rat Brain in vivo and Protects Against Rotenone Cytotoxicity Toward Rat Glioma C6 Cells in vitro. Front. Neurosci. 2020;14:759. doi: 10.3389/fnins.2020.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Ávila J.M., Pereira A.O., Zachow L.L., Gehm A.Z., Santos M.Z., Mostardeiro M.A., Back D., Morel A.F., Dalcol I.I. Chemical constituents from Valeriana polystachya Smith and evaluation of their effects on the acetylcholinesterase and prolyl oligopeptidase activities. Fitoterapia. 2018;131:80–85. doi: 10.1016/j.fitote.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Holzmann I., Cechinel Filho V., Mora T.C., Cáceres A., Martínez J.V., Cruz S.M., de Souza M.M. Evaluation of behavioral and pharmacological effects of hydroalcoholic extract of Valeriana prionophylla Standl. from Guatemala. Evid. Based Complement. Altern. Med. 2011;2011 doi: 10.1155/2011/312320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sah S.P., Mathela C.S., Chopra K. Antidepressant effect of Valeriana wallichii patchouli alcohol chemotype in mice: Behavioural and biochemical evidence. J. Ethnopharmacol. 2011;135:197–200. doi: 10.1016/j.jep.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 60.Sah S.P., Mathela C.S., Chopra K. Involvement of nitric oxide (NO) signalling pathway in the antidepressant activity of essential oil of Valeriana wallichii Patchouli alcohol chemotype. Phytomedicine. 2011;18:1269–1275. doi: 10.1016/j.phymed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Sahu S., Ray K., Kumar M.Y., Gupta S., Kauser H., Kumar S., Mishra K., Panjwani U. Valeriana wallichii root extract improves sleep quality and modulates brain monoamine level in rats. Phytomedicine. 2012;19:924–929. doi: 10.1016/j.phymed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Sridharan S., Mohankumar K., Jeepipalli S.P.K., Sankaramourthy D., Ronsard L., Subramanian K., Thamilarasan M., Raja K., Chandra V.K., Sadras S.R. Neuroprotective effect of Valeriana wallichii rhizome extract against the neurotoxin MPTP in C57BL/6 mice. Neurotoxicology. 2015;51:172–183. doi: 10.1016/j.neuro.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 63.Rezvani M.E., Roohbakhsh A., Allahtavakoli M., Shamsizadeh A. Anticonvulsant effect of aqueous extract of Valeriana officinalis in amygdala-kindled rats: Possible involvement of adenosine. J. Ethnopharmacol. 2010;127:313–318. doi: 10.1016/j.jep.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Björkholm C., Monteggia L.M. BDNF–a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonulalan E.-M., Bayazeid O., Yalcin F.-N., Demirezer L.-O. The roles of valerenic acid on BDNF expression in the SH-SY5Y cell. Saudi Pharm. J. 2018;26:960–964. doi: 10.1016/j.jsps.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sudati J.H., Vieira F.A., Pavin S.S., Dias G.R.M., Seeger R.L., Golombieski R., Athayde M.L., Soares F.A., Rocha J.B.T., Barbosa N.V. Valeriana officinalis attenuates the rotenone-induced toxicity in Drosophila melanogaster. Neurotoxicology. 2013;37:118–126. doi: 10.1016/j.neuro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Jugran A.K., Rawat S., Bhatt I.D., Rawal R.S. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother. Res. 2019;33:482–503. doi: 10.1002/ptr.6245. [DOI] [PubMed] [Google Scholar]
- 68.Shalam M., Shantakumar S., Narasu M.L. Pharmacological and biochemical evidence for the antidepressant effect of the herbal preparation Trans-01. Indian J. Pharmacol. 2007;39:231. doi: 10.4103/0253-7613.37273. [DOI] [Google Scholar]
- 69.Subhan F., Karim N., Gilani A.H., Sewell R.D. Terpenoid content of Valeriana wallichii extracts and antidepressant-like response profiles. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010;24:686–691. doi: 10.1002/ptr.2980. [DOI] [PubMed] [Google Scholar]
- 70.Sah S.P., Mathela C.S., Chopra K. Elucidation of possible mechanism of analgesic action of Valeriana wallichii DC chemotype (patchouli alcohol) in experimental animal models. Indian J. Exp. Biol. 2010;48:289–293. [PubMed] [Google Scholar]
- 71.Kim B., Ma S.-S., Jo C., Lee S., Choi H., Lee K., Ham I., Choi H.-Y. Vasorelaxant effect of the ethanol extract from Valeriana fauriei briquet root and rhizome on rat thoracic aorta. Pharmacogn. Mag. 2019;15:59. [Google Scholar]
- 72.Kim J.S., Ahn J.D., Cho S.-I. Effects of Valerianae Radix et Rhizoma extract on psychological stress in mice. Pharmacogn. Mag. 2015;11:381. doi: 10.4103/0973-1296.153093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J., Wu J., Liu L., Zhang Y., Wang F., Du X. Studies on improving sleep function and relative mechanism of mice by petroleum extract of Valeriana amurensis. Chin. J. Exp. Tradit. Med Formulae. 2013;19:245–249. [Google Scholar]
- 74.Lyle N., Gomes A., Sur T., Munshi S., Paul S., Chatterjee S., Bhattacharyya D. The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome. Behav. Brain Res. 2009;202:285–290. doi: 10.1016/j.bbr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Takemoto H., Ito M., Shiraki T., Yagura T., Honda G. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J. Nat. Med. 2008;62:41–46. doi: 10.1007/s11418-007-0177-0. [DOI] [PubMed] [Google Scholar]
- 76.Takemoto H., Ito M., Asada Y., Kobayashi Y. Inhalation administration of the sesquiterpenoid aristolen-1 (10)-en-9-ol from Nardostachys chinensis has a sedative effect via the GABAergic system. Planta Med. 2015;81:343–347. doi: 10.1055/s-0035-1545725. [DOI] [PubMed] [Google Scholar]
- 77.Wang W., Song X., Gao Z., Zhao H., Wang X., Liu M., Jia L. Anti-hyperlipidemic, antioxidant and organic protection effects of acidic-extractable polysaccharides from Dictyophora indusiata. Int. J. Biol. Macromol. 2019;129:281–292. doi: 10.1016/j.ijbiomac.2019.01.182. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y.-P., Ying S.-S., Zheng H.-H., Liu Y.-T., Wang Z.-P., Zhang H., Deng X., Wu Y.-J., Gao X.-M., Li T.-X. Novel serotonin transporter regulators: Natural aristolane-and nardosinane-types of sesquiterpenoids from Nardostachys chinensis Batal. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-15483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng X., Wu Y.-J., Chen Y.-P., Zheng H.-H., Wang Z.-P., Zhu Y., Gao X.-M., Xu Y.-T., Wu H.-H. Nardonaphthalenones A and B from the roots and rhizomes of Nardostachys chinensis Batal. Bioorg. Med. Chem. Lett. 2017;27:875–879. doi: 10.1016/j.bmcl.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Rao V.S., Rao A., Karanth K.S. Anticonvulsant and neurotoxicity profile of Nardostachys jatamansi in rats. J. Ethnopharmacol. 2005;102:351–356. doi: 10.1016/j.jep.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 81.Purushotham K., Pl B. Anticonvulsant profile of Nardostachys jatamansi roots in albino rats. Int. J. Basic Clin. Pharmacol. 2016:758–762. [Google Scholar]
- 82.Liu Q.F., Jeon Y., Sung Y.-W., Lee J.H., Jeong H., Kim Y.-M., Yun H.S., Chin Y.-W., Jeon S., Cho K.S. Nardostachys jatamansi ethanol extract ameliorates Aβ42 cytotoxicity. Biol. Pharm. Bull. 2018;41:470–477. doi: 10.1248/bpb.b17-00750. [DOI] [PubMed] [Google Scholar]
- 83.Ko W., Park J.-S., Kim K.-W., Kim J., Kim Y.-C., Oh H. Nardosinone-type sesquiterpenes from the hexane fraction of Nardostachys jatamansi attenuate NF-κB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 microglial cells. Inflammation. 2018;41:1215–1228. doi: 10.1007/s10753-018-0768-9. [DOI] [PubMed] [Google Scholar]
- 84.Kim K.-W., Yoon C.-S., Kim Y.-C., Oh H. Desoxo-narchinol a and narchinol b isolated from Nardostachys jatamansi exert anti-neuroinflammatory effects by up-regulating of nuclear transcription factor erythroid-2-related factor 2/heme oxygenase-1 signaling. Neurotox. Res. 2019;35:230–243. doi: 10.1007/s12640-018-9951-x. [DOI] [PubMed] [Google Scholar]
- 85.Sieghart W. Structure and pharmacology of γ-amino butyric acid_A receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 86.Dietz B.M., Mahady G.B., Pauli G.F., Farnsworth N.R. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Mol. Brain Res. 2005;138:191–197. doi: 10.1016/j.molbrainres.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Awad R., Levac D., Cybulska P., Merali Z., Trudeau V., Arnason J. Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol. 2007;85:933–942. doi: 10.1139/Y07-083. [DOI] [PubMed] [Google Scholar]
- 88.Felgentreff F., Becker A., Meier B., Brattström A. Valerian extract characterized by high valerenic acid and low acetoxy valerenic acid contents demonstrates anxiolytic activity. Phytomedicine. 2012;19:1216–1222. doi: 10.1016/j.phymed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 89.Valle-Mojica D., Lisa M., Ayala-Marín Y.M., Ortiz-Sanchez C.M., Torres-Hernández B.A., Abdalla-Mukhaimer S., Ortiz J.G. Selective interactions of Valeriana officinalis extracts and valerenic acid with [3H] glutamate binding to rat synaptic membranes. Evid. Based Complement. Altern. Med. 2011;2011 doi: 10.1155/2011/403591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Becker A., Felgentreff F., Schröder H., Meier B., Brattström A. The anxiolytic effects of a Valerian extract is based on valerenic acid. BMC Complement. Altern. Med. 2014;14:1–5. doi: 10.1186/1472-6882-14-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khom S., Hintersteiner J., Luger D., Haider M., Pototschnig G., Mihovilovic M.D., Schwarzer C., Hering S. Analysis of β-subunit-dependent GABAA receptor modulation and behavioral effects of valerenic acid derivatives. J. Pharmacol. Exp. Ther. 2016;357:580–590. doi: 10.1124/jpet.116.232983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sichardt K., Vissiennon Z., Koetter U., Brattström A., Nieber K. Modulation of postsynaptic potentials in rat cortical neurons by valerian extracts macerated with different alcohols: Involvement of adenosine A1-and GABAA-receptors. Phytother. Res. 2007;21:932–937. doi: 10.1002/ptr.2197. [DOI] [PubMed] [Google Scholar]
- 93.Valle-Mojica D., Lisa M., Cordero-Hernández J.M., González-Medina G., Ramos-Vélez I., Berríos-Cartagena N., Torres-Hernández B.A., Ortíz J.G. Aqueous and ethanolic Valeriana officinalis extracts change the binding of ligands to glutamate receptors. Evid. Based Complement. Altern. Med. 2011;2011 doi: 10.1155/2011/891819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L., Sun Y., Zhao T., Li Y., Zhao X., Zhang L., Wu L., Zhang L., Zhang T., Wei G. Antidepressant effects and mechanisms of the total iridoids of Valeriana jatamansi on the brain-gut Axis. Planta Med. 2020;86:172–179. doi: 10.1055/a-1068-9686. [DOI] [PubMed] [Google Scholar]
- 95.Nencini C., Cavallo F., Capasso A., De Feo V., De Martino L., Bruni G., Giorgi G., Micheli L. Binding studies for serotoninergic, dopaminergic and noradrenergic receptors of Valeriana adscendens Trel. extracts. J. Ethnopharmacol. 2006;108:185–187. doi: 10.1016/j.jep.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 96.Choi J.H., Lee M.J., Chang Y., Lee S., Kim H.-J., Lee S.W., Kim Y.O., Cho I.-H. Valeriana fauriei exerts antidepressant-like effects through anti-inflammatory and antioxidant activities by inhibiting brain-derived neurotrophic factor associated with chronic restraint stress. Rejuvenation Res. 2020;23:245–255. doi: 10.1089/rej.2018.2157. [DOI] [PubMed] [Google Scholar]
- 97.Jiang H.-H., Dong F.-W., Zhou J., Hu J.-M., Yang J., Nian Y. Ca v 2.2 and Ca v 3.1 calcium channel inhibitors from Valeriana jatamansi Jones. RSC Adv. 2017;7:45878–45884. doi: 10.1039/C7RA07327E. [DOI] [Google Scholar]
- 98.Dong F.-W., Jiang H.-H., Yang L., Gong Y., Zi C.-T., Yang D., Ye C.-J., Li H., Yang J., Nian Y. Valepotriates from the roots and rhizomes of Valeriana jatamansi Jones as novel N-type calcium channel antagonists. Front. Pharmacol. 2018;9:885. doi: 10.3389/fphar.2018.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Q., Wang C., Zuo Y., Wang Z., Yang B., Kuang H. Compounds from the roots and rhizomes of Valeriana amurensis protect against neurotoxicity in PC12 cells. Molecules. 2012;17:15013–15021. doi: 10.3390/molecules171215013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mineo L., Concerto C., Patel D., Mayorga T., Paula M., Chusid E., Aguglia E., Battaglia F. Valeriana officinalis root extract modulates cortical excitatory circuits in humans. Neuropsychobiology. 2017;75:46–51. doi: 10.1159/000480053. [DOI] [PubMed] [Google Scholar]
- 101.Khuda F., Iqbal Z., Khan A., Nasir F. Antimicrobial and anti-inflammatory activities of leaf extract of Valeriana wallichii DC. Pak J. Pharm. Sci. 2012;25:715–719. [PubMed] [Google Scholar]
- 102.Liu Y.-H., Wu P.-Q., Hu Q.-L., Pei Y.-J., Qi F.-M., Zhang Z.-X., Fei D.-Q. Cytotoxic and antibacterial activities of iridoids and sesquiterpenoids from Valeriana jatamansi. Fitoterapia. 2017;123:73–78. doi: 10.1016/j.fitote.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Li X., Chen T., Lin S., Zhao J., Chen P., Ba Q., Guo H., Liu Y., Li J., Chu R. Valeriana jatamansi constituent IVHD-valtrate as a novel therapeutic agent to human ovarian cancer: In vitro and in vivo activities and mechanisms. Curr. Cancer Drug Targets. 2013;13:472–483. doi: 10.2174/1568009611313040009. [DOI] [PubMed] [Google Scholar]
- 104.Lin S., Fu P., Chen T., Ye J., Su Y.-Q., Yang X.-W., Zhang Z.-X., Zhang W.-D. Minor valepotriates from Valeriana jatamansi and their cytotoxicity against metastatic prostate cancer cells. Planta Med. 2015;81:56–61. doi: 10.1055/s-0034-1383369. [DOI] [PubMed] [Google Scholar]
- 105.Wang R.-J., Chen H.-M., Yang F., Deng Y., Hui A., Xie X.-F., Li H.-X., Zhang H., Cao Z.-X., Zhu L.-X. Iridoids from the roots of Valeriana jatamansi Jones. Phytochemistry. 2017;141:156–161. doi: 10.1016/j.phytochem.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 106.Zhu Z., Shen W., Tian S., Yang B., Zhao H. F3, a novel active fraction of Valeriana jatamansi Jones induces cell death via DNA damage in human breast cancer cells. Phytomedicine. 2019;57:245–254. doi: 10.1016/j.phymed.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 107.Han R., Nusbaum O., Chen X., Zhu Y. Valeric acid suppresses liver cancer development by acting as a novel HDAC inhibitor. Mol. Ther. Oncolytics. 2020;19:8–18. doi: 10.1016/j.omto.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Honma T., Shiratani N., Banno Y., Kataoka T., Kimura R., Sato I., Endo Y., Kita K., Suzuki T., Takayanagi T. Seeds of Centranthus ruber and Valeriana officinalis Contain Conjugated Linolenic Acids with Reported Antitumor Effects. J. Oleo Sci. 2019;68:481–491. doi: 10.5650/jos.ess19007. [DOI] [PubMed] [Google Scholar]
- 109.Cravotto G., Boffa L., Genzini L., Garella D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010;35:11–48. doi: 10.1111/j.1365-2710.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- 110.Khuda F., Iqbal Z., Khan A., Nasir F., Shah Y. Anti-inflammatory activity of the topical preparation of Valeriana wallichii and Achyranthes aspera leaves. Pak. J. Pharm. Sci. 2013;26:451–454. [PubMed] [Google Scholar]
- 111.Sudati J.H., Fachinetto R., Pereira R.P., Boligon A.A., Athayde M.L., Soares F.A., de Vargas Barbosa N.B., Rocha J.B.T. In vitro antioxidant activity of Valeriana officinalis against different neurotoxic agents. Neurochem. Res. 2009;34:1372–1379. doi: 10.1007/s11064-009-9917-8. [DOI] [PubMed] [Google Scholar]
- 112.Dugaheh M.A., Meisami F., Torabian Z., Sharififar F. Antioxidant effect and study of bioactive components of Valeriana sisymbriifolia and Nardostachys jatamansii in comparison to Valeriana officinalis. Pak. J. Pharm. Sci. 2013;26:53–58. [PubMed] [Google Scholar]
- 113.Jugran A.K., Rawat S., Bhatt I.D., Rawal R.S. Essential oil composition, phenolics and antioxidant activities of Valeriana jatamansi at different phenological stages. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020:1–8. doi: 10.1080/11263504.2020.1810803. [DOI] [Google Scholar]
- 114.Gan L., Wei S., Xiang B., Wang W., Xue C. Effect of Valerian Ligusticum Pill on angiogenesis after injury of cerebral ischemia reperfusion in rats. Yao Xue Xue Bao Acta Pharm. Sin. 2016;51:1423–1428. [PubMed] [Google Scholar]
- 115.Wu P.-Q., Li B., Yu Y.-F., Zhao Y., Yu C.-X., Zhi D.-J., Qi F.-M., Su P.-J., Wang R.-Y., Fei D.-Q. (±)-Neonardochinone A, a pair of enantiomeric neoligans from Nardostachys chinensis with their anti-Alzheimer’s disease activities. Phytochem. Lett. 2020;39:39–42. doi: 10.1016/j.phytol.2020.07.009. [DOI] [Google Scholar]
- 116.Takemoto H., Yagura T., Ito M. Evaluation of volatile components from spikenard: Valerena-4, 7 (11)-diene is a highly active sedative compound. J. Nat. Med. 2009;63:380–385. doi: 10.1007/s11418-009-0340-x. [DOI] [PubMed] [Google Scholar]
- 117.Rekha K., Rao R.R., Pandey R., Prasad K.R., Babu K.S., Vangala J.R., Kalivendi S.V., Rao J.M. Two new sesquiterpenoids from the rhizomes of Nardostachys jatamansi. J. Asian Nat. Prod. Res. 2013;15:111–116. doi: 10.1080/10286020.2012.738673. [DOI] [PubMed] [Google Scholar]
- 118.Lin J., Cai Q.-Y., Xu W., Lin J.-M., Peng J. Chemical composition, anticancer, anti-neuroinflammatory, and antioxidant activities of the essential oil of Patrinia scabiosaefolia. Chin. J. Integr. Med. 2018;24:207–212. doi: 10.1007/s11655-016-2459-4. [DOI] [PubMed] [Google Scholar]
- 119.Wang C., Xiao Y., Yang B., Wang Z., Wu L., Su X., Brantner A., Kuang H., Wang Q. Isolation and screened neuroprotective active constituents from the roots and rhizomes of Valeriana amurensis. Fitoterapia. 2014;96:48–55. doi: 10.1016/j.fitote.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 120.Bettero G.M., Salles L., Rosário Figueira R.M., Poser G., Rates S.M., Noël F., Quintas L.E. In vitro effect of valepotriates isolated from Valeriana glechomifolia on rat P-type ATPases. Planta Med. 2011;77:1702–1706. doi: 10.1055/s-0030-1271084. [DOI] [PubMed] [Google Scholar]
- 121.Qi S.G., Quan L.Q., Cui X.Y., Li H.M., Zhao X.D., Li R.T. A natural compound obtained from Valeriana jatamansi selectively inhibits glioma stem cells. Oncol. Lett. 2020;19:1384–1392. doi: 10.3892/ol.2019.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu A., Ye X., Huang Q., Dai W.-M., Zhang J.-M. Anti-epileptic effects of valepotriate isolated from Valeriana jatamansi jones and its possible mechanisms. Pharmacogn. Mag. 2017;13:512. doi: 10.4103/0973-1296.211027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tan Y.-Z., Yong Y., Dong Y.-H., Wang R.-J., Li H.-X., Zhang H., Guo D.-L., Zhang S.-J., Dong X.-P., Xie X.-F. A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity. Phytochem. Lett. 2016;17:177–180. doi: 10.1016/j.phytol.2016.07.020. [DOI] [Google Scholar]
- 124.Shi S.-N., Shi J.-L., Liu Y., Wang Y.-L., Wang C.-G., Hou W.-H., Guo J.-Y. The anxiolytic effects of valtrate in rats involves changes of corticosterone levels. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/325948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu J., Guo Y., Xie C., Jin D.Q., Gao J., Gui L. Isolation and neuroprotective activities of acylated iridoids from Valeriana jatamansi. Chem. Biodivers. 2012;9:1382–1388. doi: 10.1002/cbdv.201100238. [DOI] [PubMed] [Google Scholar]
- 126.Wasowski C., Marder M., Viola H., Medina J.H., Paladini A.C. Isolation and identification of 6-methylapigenin, a competitive ligand for the brain GABAA receptors, from Valeriana wallichii. Planta Med. 2002;68:934–936. doi: 10.1055/s-2002-34936. [DOI] [PubMed] [Google Scholar]
- 127.Xu J., Yang B., Guo Y., Jin D.-Q., Guo P., Liu C., Hou W., Zhang T., Gui L., Sun Z. Neuroprotective bakkenolides from the roots of Valeriana jatamansi. Fitoterapia. 2011;82:849–853. doi: 10.1016/j.fitote.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 128.Choi H.-S., Hong K.-B., Han S.H., Suh H.J. Valerian/Cascade mixture promotes sleep by increasing non-rapid eye movement (NREM) in rodent model. Biomed. Pharmacother. 2018;99:913–920. doi: 10.1016/j.biopha.2018.01.159. [DOI] [PubMed] [Google Scholar]
- 129.Moradi-Afrapoli F., Ebrahimi S.N., Smiesko M., Hamburger M. HPLC-based activity profiling for GABAA receptor modulators in extracts: Validation of an approach utilizing a larval zebrafish locomotor assay. J. Nat. Prod. 2017;80:1548–1557. doi: 10.1021/acs.jnatprod.7b00081. [DOI] [PubMed] [Google Scholar]
- 130.Han Z.-Z., Zu X.-P., Wang J.-X., Li H.-L., Chen B.-Y., Liu Q.-X., Hu X.-Q., Yan Z.-H., Zhang W.-D. Neomerane-type sesquiterpenoids from Valeriana officinalis var. latifolia. Tetrahedron. 2014;70:962–966. doi: 10.1016/j.tet.2013.12.005. [DOI] [Google Scholar]
- 131.Wang P.-C., Ran X.-H., Chen R., Luo H.-R., Liu Y.-Q., Zhou J., Zhao Y.-X. Germacrane-type sesquiterpenoids from the roots of Valeriana officinalis var. latifolia. J. Nat. Prod. 2010;73:1563–1567. doi: 10.1021/np100452a. [DOI] [PubMed] [Google Scholar]
- 132.Giraldo S.E., Rincon J., Guerrero M.F., Lopez I., Jimenez I.A., Marder M., Wasowski C., Vergel N.E. Valepotriate Hydrines Isolated from an Anticonvulsant Fraction of Valeriana pavonii Poepp. & Endl. Lat. Am. J. Pharm. 2013;32:1224–1230. [Google Scholar]
- 133.Chen W.W., Zhang X., Huang W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016;13:3391–3396. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang Z., Kempinski C., Chappell J. Extraction and analysis of terpenes/terpenoids. Curr. Protoc. Plant Biol. 2016;1:345–358. doi: 10.1002/cppb.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perveen S., Al-Taweel A. Introductory chapter: Terpenes and terpenoids. Terpenes Terpenoids. 2018:1–12. doi: 10.5772/intechopen.79683. [DOI] [Google Scholar]
- 136.Tantengco O.A.G., Condes M.L.C., Estadilla H.H.T., Ragragio E.M. Ethnobotanical survey of medicinal plants used by ayta communities in Dinalupihan, Bataan, Philippines. Pharmacogn. J. 2018;10:859–870. doi: 10.5530/pj.2018.5.145. [DOI] [Google Scholar]
- 137.Dhiman B., Sharma P., Pal P.K. Biology, chemical diversity, agronomy, conservation and industrial importance of Valeriana jatamansi: A natural sedative. J. Appl. Res. Med. Aromat. Plants. 2020;16:100243. doi: 10.1016/j.jarmap.2020.100243. [DOI] [Google Scholar]
- 138.Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011;1:98–106. [Google Scholar]
- 139.Cai L. Thin layer chromatography. Curr. Protoc. Essent. Lab. Tech. 2014;8:6.3.1–6.3.18. doi: 10.1002/9780470089941.et0603s08. [DOI] [Google Scholar]
- 140.Zhang Q.-W., Lin L.-G., Ye W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mtewa A.G., Deyno S., Kasali F.M., Annu A., Sesaazi D.C. General extraction, isolation and characterization techniques in drug discovery: A review. Int. J. Sci. Basic Appl. Res. 2018;38:10–24. [Google Scholar]
- 142.Rasul M.G. Extraction, isolation and characterization of natural products from medicinal plants. Int. J. Basic Sci. Appl. Comput. 2018;2:1–6. [Google Scholar]
- 143.Ho C.S., Lam C., Chan M., Cheung R., Law L., Lit L., Ng K., Suen M., Tai H. Electrospray ionisation mass spectrometry: Principles and clinical applications. Clin. Biochem. Rev. 2003;24:3. [PMC free article] [PubMed] [Google Scholar]
- 144.Ranjbar B., Gill P. Circular dichroism techniques: Biomolecular and nanostructural analyses—A review. Chem. Biol. Drug Des. 2009;74:101–120. doi: 10.1111/j.1747-0285.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- 145.Gutierrez S., Ang-Lee M.K., Walker D.J., Zacny J.P. Assessing subjective and psychomotor effects of the herbal medication valerian in healthy volunteers. Pharmacol. Biochem. Behav. 2004;78:57–64. doi: 10.1016/j.pbb.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 146.Yu L., Ke-Ke X., Chao-Yong C., Rui-Tong Z., Ming L., Shao-Hua L., Ling-Zhen P., Tian-E Z., Zhi-Yong Y. A study of the substance dependence effect of the ethanolic extract and iridoid-rich fraction from Valeriana jatamansi Jones in mice. Pharmacogn. Mag. 2015;11:745. doi: 10.4103/0973-1296.165575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gooneratne N.S. Complementary and alternative medicine for sleep disturbances in older adults. Clin. Geriatr. Med. 2008;24:121–138. doi: 10.1016/j.cger.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schumacher B., Scholle S., Hölzl J., Khudeir N., Hess S., Müller C.E. Lignans isolated from valerian: Identification and characterization of a new olivil derivative with partial agonistic activity at A1 adenosine receptors. J. Nat. Prod. 2002;65:1479–1485. doi: 10.1021/np010464q. [DOI] [PubMed] [Google Scholar]
- 149.Maurmann N., Reolon G.K., Rech S.B., Fett-Neto A.G., Roesler R. A valepotriate fraction of Valeriana glechomifolia shows sedative and anxiolytic properties and impairs recognition but not aversive memory in mice. Evid. Based Complement. Altern. Med. 2011;2011 doi: 10.1093/ecam/nep232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morais L., Barbosa-Filho J., Almeida R. Central depressant effects of reticuline extracted from Ocotea duckei in rats and mice. J. Ethnopharmacol. 1998;62:57–61. doi: 10.1016/S0378-8741(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 151.Khom S., Baburin I., Timin E., Hohaus A., Trauner G., Kopp B., Hering S. Valerenic acid potentiates and inhibits GABAA receptors: Molecular mechanism and subunit specificity. Neuropharmacology. 2007;53:178–187. doi: 10.1016/j.neuropharm.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 152.Benke D., Barberis A., Kopp S., Altmann K.-H., Schubiger M., Vogt K.E., Rudolph U., Möhler H. GABAA receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology. 2009;56:174–181. doi: 10.1016/j.neuropharm.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 153.Rahman H., Shaik H.A., Madhavi P., Eswaraiah M.C. A review: Pharmacognostics and pharmacological profiles of Nardastachys jatamansi DC. Elixir Pharm. 2011;39:5017–5020. [Google Scholar]
- 154.Torres-Hernández B.A., Colón L.R., Rosa-Falero C., Torrado A., Miscalichi N., Ortíz J.G., González-Sepúlveda L., Pérez-Ríos N., Suárez-Pérez E., Bradsher J.N., et al. Reversal of pentylenetetrazole-altered swimming and neural activity-regulated gene expression in zebrafish larvae by valproic acid and valerian extract. Psychopharmacology. 2016;233:2533–2547. doi: 10.1007/s00213-016-4304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mulyawan E., Ahmad M.R., Islam A.A., Massi M.N., Hatta M., Arif S.K. Analysis of GABRB3 Protein Level After Administration of Valerian Extract (Valeriana officinalis) in BALB/c mice. Pharmacogn. J. 2020;12:821–827. doi: 10.5530/pj.2020.12.118. [DOI] [Google Scholar]
- 156.González-Trujano M.E., Contreras-Murillo G., López-Najera C.A., Hidalgo-Flores F.J., Navarrete-Castro A., Sánchez C.G., Magdaleno-Madrigal V.M. Anticonvulsant activity of Valeriana edulis roots and valepotriates on the pentylenetetrazole-induced seizures in rats. J. Ethnopharmacol. 2021;265:113299. doi: 10.1016/j.jep.2020.113299. [DOI] [PubMed] [Google Scholar]
- 157.Bent S., Padula A., Moore D., Patterson M., Mehling W. Valerian for sleep: A systematic review and meta-analysis. Am. J. Med. 2006;119:1005–1012. doi: 10.1016/j.amjmed.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Feo V., Faro C. Pharmacological effects of extracts from Valeriana adscendens Trel. II. Effects on GABA uptake and amino acids. Phytother. Res. 2003;17:661–664. doi: 10.1002/ptr.1225. [DOI] [PubMed] [Google Scholar]
- 159.Vissiennon Z., Sichardt K., Koetter U., Brattström A., Nieber K. Valerian extract Ze 911 inhibits postsynaptic potentials by activation of adenosine A1 receptors in rat cortical neurons. Planta Med. 2006;72:579–583. doi: 10.1055/s-2006-931561. [DOI] [PubMed] [Google Scholar]
- 160.Lacher S.K., Mayer R., Sichardt K., Nieber K., Müller C.E. Interaction of valerian extracts of different polarity with adenosine receptors: Identification of isovaltrate as an inverse agonist at A1 receptors. Biochem. Pharmacol. 2007;73:248–258. doi: 10.1016/j.bcp.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 161.Bloodgood B.L., Sharma N., Browne H.A., Trepman A.Z., Greenberg M.E. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature. 2013;503:121–125. doi: 10.1038/nature12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lin Y., Bloodgood B.L., Hauser J.L., Lapan A.D., Koon A.C., Kim T.-K., Hu L.S., Malik A.N., Greenberg M.E. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spiegel I., Mardinly A.R., Gabel H.W., Bazinet J.E., Couch C.H., Tzeng C.P., Harmin D.A., Greenberg M.E. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell. 2014;157:1216–1229. doi: 10.1016/j.cell.2014.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Phiel C.J., Zhang F., Huang E.Y., Guenther M.G., Lazar M.A., Klein P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 165.Jacobo-Herrera N.J., Vartiainen N., Bremner P., Gibbons S., Koistinaho J., Heinrich M. F-κB modulators from Valeriana officinalis. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006;20:917–919. doi: 10.1002/ptr.1972. [DOI] [PubMed] [Google Scholar]
- 166.Cornara L., Ambu G., Trombetta D., Denaro M., Alloisio S., Frigerio J., Labra M., Ghimire G., Valussi M., Smeriglio A. Comparative and functional screening of three species traditionally used as antidepressants: Valeriana officinalis L., Valeriana jatamansi Jones ex Roxb. and Nardostachys jatamansi (D. Don) DC. Plants. 2020;9:994. doi: 10.3390/plants9080994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.NIH Office of Dietary Supplements. [(accessed on 30 November 2020)]; Available online: https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional/#en13.
- 168.Leathwood P.D., Chauffard F., Heck E., Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol. Biochem. Behav. 1982;17:65–71. doi: 10.1016/0091-3057(82)90264-7. [DOI] [PubMed] [Google Scholar]
- 169.Leathwood P., Chauffard F. Aqueous extract of valerian reduces latency to fall asleep in man. Planta Med. 1985;51:144–148. doi: 10.1055/s-2007-969430. [DOI] [PubMed] [Google Scholar]
- 170.Vorbach E., Gortelmeyer R., Bruning J. Treatment of insomnia: Effectiveness and tolerance of a valerian extract. Psychopharmakotherapie. 1996;3:109–115. [Google Scholar]
- 171.Dorn M. Baldrian versus Oxazepam: Efficacy and Tolerability in Non-Organic and Non-Psychiatric Insomniacs. A Randomised, Double-Blind, Clinical, Comparative Study. Forsch. Komplement. Und Klass. Nat. 2000;7:79–84. doi: 10.1159/000021314. [DOI] [PubMed] [Google Scholar]
- 172.Donath F., Quispe S., Diefenbach K., Maurer A., Fietze I., Roots I. Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsychiatry. 2000;33:47–53. doi: 10.1055/s-2000-7972. [DOI] [PubMed] [Google Scholar]
- 173.Shinjyo N., Waddell G., Green J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid. Based Integr. Med. 2020;25:2515690X20967323. doi: 10.1177/2515690X20967323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pinheiro M.L.P., Alcântara C.E.P., de Moraes M., de Andrade E.D. Valeriana officinalis L. for conscious sedation of patients submitted to impacted lower third molar surgery: A randomized, double-blind, placebo-controlled split-mouth study. J. Pharm. Bioallied Sci. 2014;6:109. doi: 10.4103/0975-7406.129176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shikov A.N., Narkevich I.A., Flisyuk E.V., Luzhanin V.G., Pozharitskaya O.N. Medicinal plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021;268:113685. doi: 10.1016/j.jep.2020.1136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data related to the review manuscript are presented in the manuscript in the form of tables and figures.