To the Editor.
Gaucher’s disease (GD) is a rare lysosomal storage disorder (LSD) due to a defect in the β-glucocerebrosidase gene (GBA, MIM #606463) leading to a functional deficiency of β-glucocerebrosidase (GC-ase).(1, 2) GD affects approximately 1:40000–1:60000 people worldwide.(2) In GD, the deficiency of GC-ase leads to insufficient catabolism and accumulation of glucosylceramide (GC) in the lysosomes of macrophages.(1) These engorged macrophages accumulate in multiple organs, resulting in a multitude of morphological abnormalities including splenomegaly, hepatomegaly, cytopenias and bone disease.(1, 2) Visceral symptoms are common to all three Gaucher subtypes; types 2 and 3 are rarer, more severe forms, which also include neuronopathic disease.(1, 2) Macrophage targeted enzyme replacement therapy (ERT), available since the early 1990s, improves quality of life and reverses the visceral but not the neurological signs and symptoms of disease.(2)
Hypersensitivity reactions to enzyme replacement therapy have been reported, can be IgE-mediated, and desensitization protocols have been successful.(3) Though development of IgG to ERT happens in approximately 15%, allergic reactions are reported in 7% of patients with <1% reporting anaphylaxis to treatment (4) and overall sensitization as determined by IgE specific to imiglucerase in serum, appears to be uncommon. In one study 262 patients with Type 1 Gaucher disease were evaluated for development of humoral responses to imiglucerase (Cerezyme®, Sanofi Genzyme™) ERT. A total of 12.9% of treated patients developed IgG and 14 patients showed signs of immediate hypersensitivity reactions. IgE but not IgG associated hypersensitivity has been associated with mast cell degranulation and tryptase elevation. In this study neither IgE antibody to ERT nor tryptase elevations were detected in any of the 14 patients with reactions. Baseline tryptase is not reported and immediate post reaction serum samples were available for only 3 patients.(5) Tryptase, a hallmark of mast cell-mediated drug hypersensitivity reactions and mast cell-associated disorders, has not been studied in GD patients.
We present the case of an 18 month old boy with GD type 3 (2 GBA mutations, NM_000157.3, a novel c.849C>A, p.Y283*and previously published c.1603C>T, p.R535C) severe hepatosplenomegaly, anemia, thrombocytopenia and supra-nuclear gaze palsy. He was referred for drug allergy evaluation after he experienced anaphylaxis with systemic urticaria and hypotension during his second infusion of imiglucerase ERT, a recombinant human enzyme cultured in Chinese hamster ovary. His tryptase level at the time of his infusion reaction was 49 μg/L (normal:<11.5 μg/mL). Skin prick and serum IgE testing were performed in accordance with manufacturer recommendations. Intradermal injection of 1:1000 dilution of imiglucerase resulted in a 5 mm wheal (saline control: 1 mm wheal, histamine 3 mm). Anti-imiglucerase IgE antibody status was detected using enzyme linked immunosorbent assay was performed by the manufacturer and confirmed the suspected allergy. Desensitization was considered, but was not feasible given the time requirements for biweekly treatment.
The recombinant enzyme velaglucerase alpha (VPRIV®, Shire™ Inc.) produced in human fibroblasts, is almost identical in protein structure to imiglucerase. Though not FDA-approved for GD type 3 disease, a case report (6) of successful treatment of a GD type 3 patient with allergy to imiglucerase prompted investigation of whether velaglucerase alpha could be used for this patient. Both skin prick and intradermal testing at concentrations up to 1:100 showed no wheal response. Therefore, velaglucerase treatment was started. He has now received over 50 infusion of velaglucerase alfa every two weeks and has tolerated well thus far.
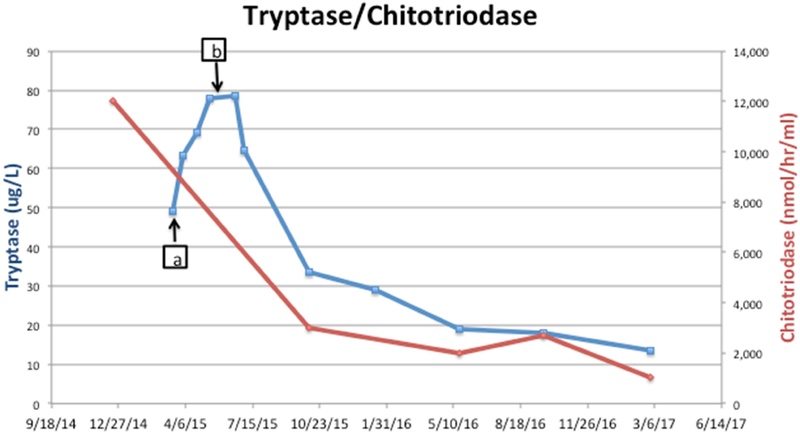
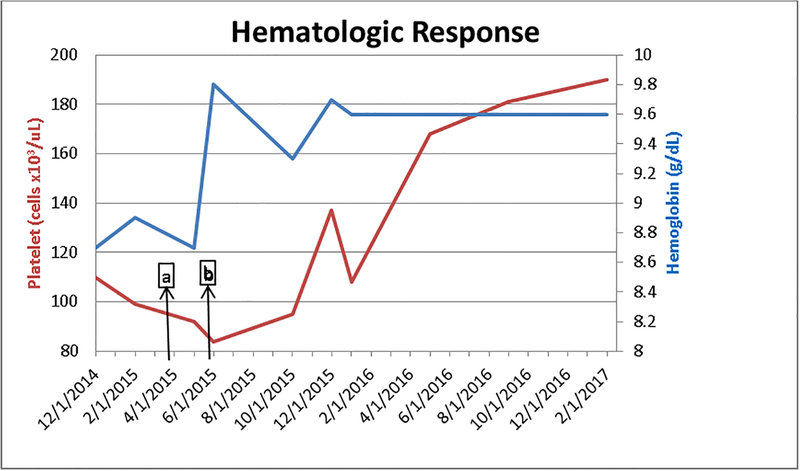
At the time of evaluation for drug allergy, 13 days after the initial reaction a tryptase level was obtained and was noted to be higher than at the time of initial reaction (63.2 μg/L). Tryptase elevations to that extent so far removed from the initial event suggested a second pathology beyond immediate hypersensitivity for elevated serum tryptase, such as a clonal mast cell disorder. However, bone marrow biopsy did not reveal evidence of mastocytosis or a clonal mast cell population. The marrow section had no evidence of myelodysplasia and displayed trilineage hematopoiesis. During the 2 months between discontinuation of imiglucerase and initiating velaglucerase alpha, the patient’s tryptase levels, drawn at 3 week intervals, continued to increase (figure 1). TPSAB1 copy number evaluation, performed as described (7), did not identify increased alpha-tryptase encoding copies which have been associated with hereditary alpha tryptasemia. Heterophile antibody interference can lead to false positive tryptase assay results.(8) Evaluation of common heterophile antibodies including human anti-mouse antibody, rheumatoid factor, and human chorionic gonadotropin levels in this patient were negative and thus did not support assay interference. With the initiation of velaglucerase alpha his tryptase levels (figure 1) decreased in tandem with improvement in his anemia and thrombocytopenia (figure 2), as well as plasma chitotriosidase activity (figure 1), a biomarker for Gaucher disease.(2) It is not known how alterations in GC may affect the mast cell compartment, but our findings indicate that in GD, tryptase production or metabolism may be altered. The pathology of GD is not just the burden of storage material, but also chronic macrophage activation.(2)
Figure 1.
Tryptase/chitotriosidase activity.
Figure 2:
Hemoglobin levels (g/dL) and Platelet Counts (cells × 103/uL) a. reaction to imiglucerase b. initiation of velaglucerase (8 weeks 5 days post reaction)
Markers of macrophage activation including chitotriodase and CCL-18, can be used in addition to hemoglobin levels and platelet count to evaluate GD severity and treatment response.(1, 2) Immune system dysfunction and activation, including hypergammaglobulinemia, increased autoantibodies, plasmacytosis, decreased NK cells, as well increased MHC Class II expression are well documented in GD.(9) It has been suggested that an excess accumulation of GC may activate or enhance macrophage function through selective calcium-channel dysregulation or the initiation of inflammatory pathways due to abnormal protein folding.(1) More recently Pandey et al have shown that increased GC storage induces complement activating IgG autoantibodies driving a pathway of C5a generation and C5aR1 activation fueling cellular GC accumulation with innate and adaptive immune cell recruitment and activation in GD.(10) Excess generation of C5a, other anaphylatoxins, or additional bioactive compounds points to a potential mechanism whereby secondary mast cell activation could be occurring in GD.
Alternatively, intrinsic mast cell or myeloid cell dysfunction may contribute to the elevated serum tryptase levels observed. There are no published studies of GBA activity in mast cells. However, human mast cells contain traditional lysosomes, as well as lysosomal secretory granules, and it would stand to reason that a defect in glucocerebrosidase in these organelles could lead to dysfunction. A study by Hammel et al. used electron transmission microscopy to examine the ultrastucture of human mast cells in lysosomal storage disorders. Membrane bound vacuoles with the ultrastructural features of secondary lysosomes, which either appeared empty or contained lamellated membrane were described for many LSDs.(11) In Hermansky-Pundlack Syndrome Type-1, the gene mutation, which affects lysosomal related organelles, has been shown to extend to the mast cell lineage leading to abnormal granule formation, activation, cytokine release, and matrix protein synthesis.(12) It has also been shown shown that leukemic myeloblasts can express tryptase leading to elevated serum tryptase through an unknown mechanism, further demonstrating that disruption in the myeloid lineage generally may lead to elevated serum tryptase levels. (13)
We can only speculate on which of these mechanisms may be involved in the pathogenesis of prolonged tryptase elevation in our patient. We are further limited by only having total tryptase levels in our patient; were mature tryptase levels elevated, the hypothesis of chronic mast cell degranulation would be strengthened. Guacher type 3 is a rare but more severe form of GD. It may be that serum tryptase elevations seen here are related to this more severe phenotype. In order to fully evaluate possible mechanisms of prolonged tryptase elevation and the extent to which mast cell activation, mast cell granule formation and/or tryptase metabolism might be affected by the disease, further studies including electron microscopy and functional studies will be needed.
Clinical Implications: This is the first report to our knowledge of tryptase elevation in Gaucher disease responsive to enzyme replacement therapy. It highlights the possibility that under certain circumstances, tryptase levels may be altered in Gaucher disease, and indicates the enzyme deficiency affects mast cells, an immune dysregulation not previously described.
Acknowledgments
Funding for this article: This study was supported in part by the Division of Intramural Research of the NIAID.
Abbreviations used:
- GD
Gaucher’s disease
- GBA
β-glucocerebrosidase gene
- GC-ase
β-glucocerebrosidase
- ERT
enzyme replacement therapy
- LSD
Lysosomal Storage Disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the authors have conflicts of interest related to this article.
References
- 1.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet (London, England). 2008;372(9645):1263–71. [DOI] [PubMed] [Google Scholar]
- 2.Stirnemann J, Belmatoug N, Camou F, Serratrice C, Froissart R, Caillaud C, et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. International journal of molecular sciences. 2017;18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsilochristou O, Gkavogiannakis NA, Ioannidou EN, Makris M. Successful rapid desensitization to imiglucerase in an adult patient with Gaucher disease and documented IgE-mediated hypersensitivity. The journal of allergy and clinical immunology In practice. 2015;3(4):624–6. [DOI] [PubMed] [Google Scholar]
- 4.Cerezyme.com. “About Cerezyme..Safety. Immunologic Adverse Events. Page available at. https://cerezyme.com/healthcare/aboutcerezyme/safety.aspx#isi. Accessed July 26, 2017.
- 5.Richards SM, Olson TA, McPherson JM. Antibody response in patients with Gaucher disease after repeated infusion with macrophage-targeted glucocerebrosidase. Blood. 1993. September 1;82(5):1402–9. [PubMed] [Google Scholar]
- 6.Vairo F, Netto C, Dorneles A, Mittelstadt S, Wilke M, Doneda D, et al. Enzyme Replacement Therapy in a Patient with Gaucher Disease Type III: A Paradigmatic Case Showing Severe Adverse Reactions Started a Long Time After the Beginning of Treatment. JIMD reports. 2013;11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nature genetics. 2016;48(12):1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargur R, Cowley D, Murng S, Wild G, Green K, Shrimpton A, et al. Raised tryptase without anaphylaxis or mastocytosis: heterophilic antibody interference in the serum tryptase assay. Clinical and experimental immunology. 2011;163(3):339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castaneda JA, Lim MJ, Cooper JD, Pearce DA. Immune system irregularities in lysosomal storage disorders. Acta neuropathologica. 2008;115(2):159–74. [DOI] [PubMed] [Google Scholar]
- 10.Pandey MK, Burrow TA, Rani R, Martin LJ, Witte D, Setchell KD, et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature. 2017;543(7643):108–12. [DOI] [PubMed] [Google Scholar]
- 11.Hammel I, Alroy J, Goyal V, Galli SJ. Ultrastructure of human dermal mast cells in 29 different lysosomal storage diseases. Virchows Archiv B, Cell pathology including molecular pathology. 1993;64(2):83–9. [DOI] [PubMed] [Google Scholar]
- 12.Kirshenbaum AS, Cruse G, Desai A, Bandara G, Leerkes M, Lee CC, Fischer ER, et al. Immunophenotypic and Ultrastructural Analysis of Mast Cells in Hermansky-Pudlak Syndrome Type-1: A Possible Connection to Pulmonary Fibrosis. PloS one. 2016. July 26;11(7):e0159177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperr WR, Jordan JH, Baghestanian M, Kiener HP, Samorapoompichit P, Semper H, et al. Expression of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia. Blood. 2001. October 1;98(7):2200–9. [DOI] [PubMed] [Google Scholar]