Abstract
Background: weight loss as a result of lifestyle intervention is effective when treating non-alcoholic fatty liver disease (NAFLD). We estimated the effects of PNPLA3 rs738409 and HSD17B13 rs6834314 variants in response to diet therapy in Japanese patients with NAFLD. Methods: we analyzed the correlation between the change in liver stiffness and change in body weight in 140 patients administered diet therapy for 1-year, according to PNPLA3 and HSD17B13 genotypes. Results: the bodyweight (BW) reduction rate was greater in patients with the PNPLA3 genotype CC than CG and GG (p = 0.035). Change in liver stiffness measurement (LSM) was significantly associated with a change in BW in PNPLA3 CG/GG (r = 0.279/0.381), but not in PNPLA3 CC (p = 0.187). Change in LSM was correlated with change in BW only in patients with HSD17B13 AG/GG (r = 0.425), but not the AA genotype (p = 0.069). A multivariate analysis identified that a change in LSM was correlated with a change in BW in carriers of HSD17B13 AG/GG (B = 3.043, p = 0.032), but not HSD17B13 AA. The change in LSM of patients with a BW reduction of more than 7% (0.50) was significantly greater than that of patients with a BW reduction of less than 7% (0.83) (p = 0.038). Conclusions: in Japanese patients with NAFLD, HSD17B13 rs6834314 polymorphism is associated with the change in LSM by lifestyle intervention. The approach, including genetic assessments, may contribute to the establishment of appropriate therapeutic strategies to treat NAFLD.
Keywords: NAFLD, PNPLA3, HSD17B13, SNP, diet therapy
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are hepatic phenotypes of metabolic syndrome, which are common causes of chronic liver disease and, ultimately, liver cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC), in the absence of significant alcohol consumption [1,2]. A large cohort study identified hepatic fibrosis as the only predictive factor among those evaluated for liver-related events and mortality in patients with NAFLD [3]. Bodyweight (BW) reduction by lifestyle intervention (e.g., calorie restriction or exercise) is the most effective management option for NAFLD. It ameliorates aminotransferases and improves histological severity, including hepatic fibrosis [4,5,6]. Previous studies reported that BW reduction of more than 10% from baseline could induce NASH resolution and fibrosis regression in most NAFLD patients. However, BW reduction of 5% can only improve histological severity in 35–45% of NAFLD patients [7].
Genetic factors are also involved in the histological progression in individuals with liver diseases. The single-nucleotide polymorphism (SNP) rs738409 in the patatin-like phospholipase domain containing 3 (PNPLA3) gene is associated with NAFLD and NASH [8,9,10]. We previously reported in a longitudinal study that advanced fibrosis and the PNPLA3 rs738409 GG genotype are predictive factors for HCC development in Japanese patients with NAFLD [11]. The mechanism underlying the effects of PNPLA3 on hepatic fibrosis and hepatocarcinogenesis is still unclear. Plausible mechanisms include the effects of PNPLA3 on lipid droplet remodeling, very-low-density lipoprotein secretion, retinol metabolism via retinyl-palmitate lipase activity, and the mRNA level of Fas ligand [12]. Recently, Abul-Husn et al. reported that a splice variant (rs72613567) of the 17-β-hydroxysteroid dehydrogenase 13 gene (HSD17B13) has a protective effect on the development of chronic liver disease [13]. Another study investigated the association between HSD17B13 and liver disease and showed that the HSD17B13 rs72613567 TA variant reduced the risks of alcohol-, NAFLD-, and hepatitis C-related cirrhosis and alcohol-related HCC [14]. In NAFLD subjects, the minor allele rs6834314 of HSD17B13 was associated with increased steatosis, but decreased inflammation and ballooning via its hepatic retinol dehydrogenase activity [15]. Furthermore, the carriage of this variant attenuated the risk of developing liver injury conferred by PNPLA3 polymorphisms. We recently reported that carriage of the HSD17B13 rs6834314 G allele attenuated the effect of the PNPLA3 rs738409 GG genotype on advanced hepatic fibrosis [16].
The genetic variants were associated not only with disease progression, but also with response to therapy. In a pilot study by Sevastianova et al., it was reported that patients with PNPLA3 genotype GG showed greater reduction of intrahepatic triglyceride content (IHTG) than patients with genotype CC by a short period hypocaloric diet intervention [17]. Shen et al. also reported that the presence of G allele was associated with greater reduction in IHTG, body weight, waist-to-hip ratio, total cholesterol, and low-density lipoprotein (LDL) cholesterol by a 1-year lifestyle modification [18]. As we mentioned, the PNPLA3 and HSD17B13 genotypes are in a mutual relationship in pathology of NAFLD, and improvement of liver fibrosis is more important than that of steatosis. Thus, an investigation of the effects of these polymorphisms in response to lifestyle intervention in Japanese patients is needed.
In this study, we adopted vibration-controlled transient elastography (VCTE) for evaluating liver stiffness measurement (LSM). Because of its non-invasive aspects and convenience, VCTE is frequently used in clinical settings for fibrosis staging in NAFLD [19]. VCTE is a simple and readily available examination to carry out, but obesity can be a reason for failed VCTE. Giuffrè M, et al. reported that skin-to-liver distance affected the LSM in obese patients. We should take into consideration skin-to-liver distance when interpreting LSM in NAFLD [20].
The aims of this study were to (1) identify the association between carriage of PNPLA3 rs738409 genotype and change in bodyweight (BW) and LSM, and (2) determine whether such HSD17B13 rs6834314 genotype influences the effect of the PNPLA3 rs738409 G variant, concerning the response to diet therapy in Japanese patients with biopsy-proven NAFLD.
2. Materials and Methods
2.1. Patients
A total of 283 Japanese patients who were diagnosed with NAFLD by liver biopsy at the Department of Gastroenterology and Hepatology, Kyoto Prefectural University of Medicine (Kyoto, Japan), from January 2002 to March 2019, were enrolled in this study. 0We diagnosed NAFLD based on the liver biopsy finding of steatosis in ≥5% of hepatocytes and the exclusion of other liver diseases, including viral hepatitis, autoimmune hepatitis, and drug-induced liver disease. Patients with a daily alcohol consumption >30 g for men and >20 g for women were excluded. This study was approved by the Ethical Review Board of Kyoto Prefectural University of Medicine (ERB-C-1416). All patients provided written informed consent at the time of liver biopsy, and the study was conducted in accordance with the Declaration of Helsinki (2013).
2.2. Physical Examination, Laboratory, and Clinical Parameters
Laboratory assays included blood cell counts and measurements of serum concentrations of albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ glutamyl transpeptidase, total cholesterol, triglycerides (TG), high-density lipoprotein (HDL) cholesterol, LDL cholesterol, fasting plasma glucose, and hemoglobin A1c. These parameters were measured using standard clinical chemical laboratory techniques. Body mass index (BMI) was calculated as weight in kilograms/(height in meters)2. The Fibrosis-4 (FIB-4) index was calculated as follows: ([age (years) × AST (IU/L)]/platelet count [109/L]) × (ALT [IU/L])1/2. FIB-4 index was reported associated with LSM [21]. Patients were examined in a fasting state at rest in the supine position. The FibroScan (EchoSens, Paris, France) standard M probe was placed on the skin in an intercostal space and LSM was carried out. The operators were experienced gastroenterologists who were conversant with transient elastography. Valid LSM was defined as follows: (i) at least 10 valid measurements with >60% success rate; and (ii) <30% of interquartile range. At the same time, the controlled attenuation parameter (CAP) was measured, only when LSM was valid.
Patients taking oral hypoglycemic agents and those with a fasting glucose concentration >126 mg/dL or a random glucose concentration >200 mg/dL were diagnosed with diabetes. Patients with serum levels of low-density lipoprotein cholesterol >140 mg/dL, TG >150 mg/dL, and/or high-density lipoprotein cholesterol <40 mg/dL were diagnosed with dyslipidemia.
2.3. DNA Preparation and SNP Genotyping
Genomic DNA was extracted from blood samples using the DNeasy Blood and Tissue kit (Qiagen, Tokyo, Japan). The SNPs rs738409 and rs6834314 were genotyped in each sample using TaqMan SNP genotyping assays (Applied Biosystems, Foster City, CA, USA) with commercially available predesigned SNP-specific primers for PCR amplification and extension reactions, according to the manufacturer’s protocol.
2.4. Liver Histology
The liver biopsy specimens were stained with hematoxylin and eosin, and Masson trichrome stain. The specimens were evaluated by two hepatic pathologists who were blinded to the clinical findings. An adequate liver biopsy sample was defined as that with a length >1.5 cm and/or more than 11 portal tracts. The steatosis severities of <5, 5–33, >33–66, and >66% were assigned steatosis scores of 0, 1, 2, and 3, respectively. Lobular inflammation grades 1, 2, and 3, and ballooning scores of 0, 1, and 2, were defined as mild, moderate, and severe, respectively. The NAFLD activity score was calculated as the sum of the steatosis, lobular inflammation, and hepatocellular ballooning scores. The severity of hepatic fibrosis was staged as follows: stage 1, zone 3 perisinusoidal fibrosis; stage 2, zone 3 perisinusoidal and portal fibrosis; stage 3, zone 3 perisinusoidal, portal, and bridging fibrosis; and stage 4, cirrhosis [22,23,24]. In this study, hepatic stages 3 and 4 were defined as advanced fibrosis.
2.5. Statistical Analysis
The categorical clinical data between groups were compared by the chi-squared test or the linear-by-linear trend test; quantitative variables were analyzed using paired t-test, and one-way ANOVA, as appropriate. All changes between baseline and 1 year after were analyzed using two-tailed paired t-tests. The correlation analysis was carried out with Spearman’s correlation analysis. The correlation coefficient was determined; weak, 0.00- 0.39; moderate, 0.40–0.59; strong, 0.60–1.0. Linear regression was used to determine the independent factors associated with quantitative change in LSM. The statistical analyses were performed using SPSS version 22 (SPSS Inc., Chicago, IL, USA). All p-values less than 0.05 by two-tailed tests were considered statistically significant.
3. Results
3.1. Patient Characteristics
Table 1 and Table 2 summarize the demographic profiles and laboratory and histologic data of the 140 patients in this study, according to the PNPLA3 or HSD17B13 genotype. The 140 patients, comprising 69 (49.3%) females, had a median age of 55 years and median BMI of 27.6 kg/m2. Of the 140 patients, 52 (37.1%), 45 (32.1%), 17 (12.1%), 17 (12.1%), and 9 (6.4%) were hepatic fibrosis stages 0, 1, 2, 3, and 4 (cirrhosis), respectively. In this cohort, the frequencies of the PNPLA3 rs738409 genotypes were CC in 30 (21.4%) patients, CG in 58 (41.4%), and GG in 52 (37.1%). The frequencies of the HSD17B13 rs6834314 genotypes were 54.1% AA in 72 (51.4%), AG in 57 (40.7%), and GG in 11(7.9%), respectively. Because the number of patients with GG were small, we combined AG and GG in this study. The serum levels of TG and HDL cholesterol were significantly different according to the PNPLA3 genotype (Table 1). The serum levels of AST, ALT, and FIB-4 index were significantly lower in patients with the HSD17B13 genotype AG/GG compared with genotype AA (Table 2).
Table 1.
Characteristics of the NAFLD patients according to the PNPLA3 genotype.
| Variable | PNPLA3 | |||
|---|---|---|---|---|
| CC n = 30 |
CG n = 58 |
GG n = 52 |
p-Value | |
| HSD17B13, AA/AG, GG | 15/15 | 30/28 | 27/25 | 0.984 |
| Sex, female | 12 (40.0%) | 31 (53.4%) | 26 (50.0%) | 0.485 |
| Age, years | 54 (23–81) | 55 (19–75) | 60 (17–76) | 0.291 |
| BMI, kg/m2 | 28.3 (23.3–45.2) | 27.2 (20.8–44.0) | 26.4 (17.6–41.0) | 0.083 |
| Hypertension | 14 (46.7%) | 25 (43.1%) | 20 (38.5%) | 0.429 |
| Diabetes | 15 (50.0%) | 25 (43.1%) | 23 (44.2%) | 0.819 |
| Hyperlipidemia | 19 (63.3%) | 36 (62.1%) | 25 (48.1%) | 0.248 |
| Albumin, g/dL | 4.5 (3.8–5.0) | 4.5 (3.5–5.2) | 4.4 (3.7–5.0) | 0.825 |
| AST, IU/L | 32.5 (12–93) | 42.5 (18–162) | 47.5 (22–155) | 0.186 |
| ALT, IU/L | 46 (18–146) | 53.5 (19–233) | 56 (14–166) | 0.507 |
| GGT, IU/L | 71.5 (18–279) | 59 (14–533) | 59.5 (14–202) | 0.833 |
| Platelet count, ×103/μL | 239.5 (90–420) | 226.5 (81–377) | 213 (75–444) | 0.210 |
| Total cholesterol, mg/dL | 195.5 (148–347) | 205 (137–281) | 201 (94–311) | 0.548 |
| TG, mg/dL | 190 (55–721) | 142.5 (49–739) | 129 (53–644) | 0.039 |
| LDL–C, mg/dL | 116 (33–252) | 127 (59–219) | 115 (36–177) | 0.478 |
| HDL–C, mg/dL | 47 (21–67) | 55 (30–93) | 50 (29–96) | 0.015 |
| FPG, mg/dL | 112 (77–232) | 106.5 (76–172) | 112 (71–236) | 0.484 |
| HbA1c, % | 6.1 (5.2–11.2) | 6.0 (5.0–8.2) | 6.0 (5.2–8.7) | 0.396 |
| FIB-4 index | 1.10 (0.28–7.83) | 1.38 (0.33–6.43) | 1.40 (0.39–7.73) | 0.100 |
| CAP, dB/m | 323.5 (230–400) | 308.5 (232–400) | 299 (159–394) | 0.052 |
| LSM, kPa | 8.9 (3.3–27.0) | 8.6 (2.9–39.7) | 9.6 (3.5–46.4) | 0.778 |
| Fibrosis stage (0/1/2/3/4) | 15/9/4/2/0 | 19/23/6/5/5 | 18/13/7/10/4 | 0.276 |
Results are presented as n (%) for qualitative data or as medians for quantitative data. Abbreviations: PNPLA3, patatin-like phospholipase domain containing 3; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ–glutamyl transferase; TG, triglyceride; LDL–C, low-density lipoprotein cholesterol; HDL–C, high-density lipoprotein cholesterol; FPG, fasting plasma glucose; FIB-4 index, Fibrosis-4 index.
Table 2.
Characteristics of the NAFLD patients according to the HSD17B13 genotype.
| Variable | HSD17B13 | ||
|---|---|---|---|
| AA n = 72 |
AG/GG n = 68 |
p-Value | |
| PNPLA3, CC/CG/GG | 15/30/27 | 15/28/25 | 0.984 |
| Sex, female | 38 (52.8%) | 31 (45.6%) | 0.404 |
| Age, years | 55 (17–81) | 54 (23–70) | 0.925 |
| BMI, kg/m2 | 27.0 (17.6–45.2) | 28.2 (17.9–34.0) | 0.809 |
| Hypertension | 31 (43.1%) | 24 (35.3%) | 0.865 |
| Diabetes | 33 (45.8%) | 23 (33.8%) | 0.866 |
| Hyperlipidemia | 45 (62.5%) | 29 (42.6%) | 0.232 |
| Albumin, g/dL | 4.4 (3.5–5.2) | 4.5 (3.8–4.8) | 0.306 |
| AST, IU/L | 50 (19–162) | 38.5 (12–64) | 0.002 |
| ALT, IU/L | 62 (21–233) | 47.5 (14–165) | 0.021 |
| GGT, IU/L | 66 (18–533) | 56.5 (14–279) | 0.316 |
| Platelet count, ×103/μL | 213 (81–420) | 237.5 (75–444) | 0.060 |
| Total cholesterol, mg/dL | 195 (95–347) | 204.5 (94–311) | 0.108 |
| TG, mg/dL | 154 (49–721) | 141 (53–739) | 0.400 |
| LDL–C, mg/dL | 113.5 (36–252) | 124 (33–219) | 0.276 |
| HDL–C, mg/dL | 49 (21–82) | 51 (30–96) | 0.191 |
| FPG, mg/dL | 112 (71–222) | 107 (77–236) | 0.854 |
| HbA1c, % | 6.0 (5.2–8.5) | 6.1 (5.0–11.2) | 0.851 |
| FIB-4 index | 1.51 (0.33–7.83) | 1.15 (0.28–7.73) | 0.033 |
| CAP, dB/m | 313 (174–400) | 304.5 (159–400) | 0.370 |
| LSM, kPa | 9.4 (3.7–29.6) | 8.1 (2.9–46.4) | 0.158 |
| Fibrosis stage (0/1/2/3/4) | 20/26/11/10/5 | 32/19/6/7/4 | 0.210 |
Abbreviations are listed in the footnote to Table 1. HSD17B13, 17–β hydroxysteroid dehydrogenase 13.
3.2. Changes in Parameters from Baseline to after 1 Year Diet Therapy and Associations with the PNPLA3 and HSD17B13 Genotypes
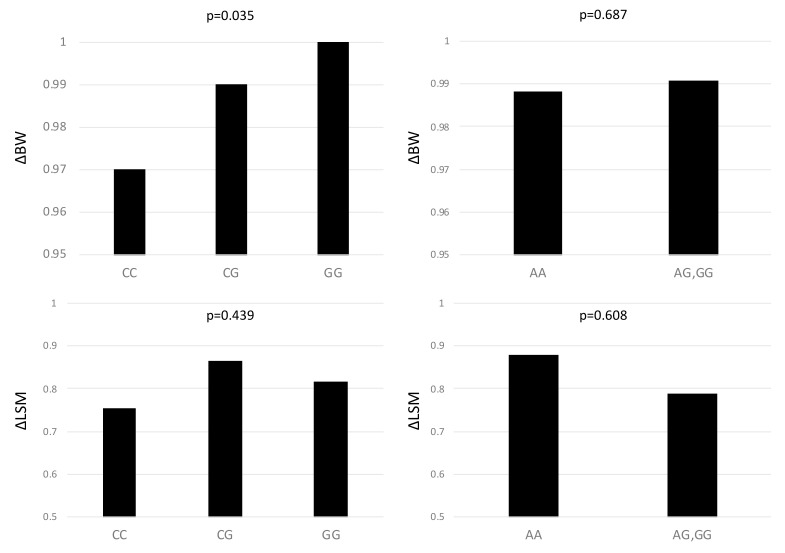
After 1 year of diet therapy, BMI, serum levels of AST, ALT, and GGT, CAP, and LSM were significantly reduced (Table 3). Figure 1 showed change in BW (ΔBW), in LSM (ΔLSM), according to PNPLA3 and HSD17B13 polymorphism. The PNPLA3 genotype was associated with a greater reduction rate of BW (CC: 2.9%, CG: 1.3%, GG: 0.2%) (p = 0.035). HSD17B13 polymorphism had no impact on ΔBW (p = 0.687). The ΔLSM was not associated with both PNPLA3 and HSD17B13 polymorphism (p = 0.439, p = 0.608, respectively). The ΔLSM had no difference in each fibrosis stage, in both PNPLA3 and HSD17B13 polymorphisms.
Table 3.
The characteristics of 140 patients with nonalcoholic fatty liver disease at baseline and after 1 year diet therapy.
| Variable | Baseline | After 1 Year | p-Value |
|---|---|---|---|
| PNPLA3, CC/CG/GG | 30/58/52 | ||
| HSD17B13, AA/AG/GG | 72/57/11 | ||
| BMI, kg/m2 | 27.6 (17.6–45.2) | 26.7 (17.6–46.2) | <0.001 |
| Albumin, g/dL | 4.5 (3.5–5.2) | 4.4 (3.6–5.3) | 0.358 |
| AST, IU/L | 42 (12–162) | 33 (14–121) | <0.001 |
| ALT, IU/L | 54 (14–233) | 40 (8–216) | <0.001 |
| GGT, IU/L | 59.5 (14–533) | 50.5 (9–456) | <0.001 |
| Platelet count, ×103/μL | 222.5 (76–444) | 227 (56–472) | 0.124 |
| Total cholesterol, mg/dL | 201.5 (94–347) | 193 (88–312) | 0.493 |
| TG, mg/dL | 145 (49–739) | 136 (49–714) | 0.117 |
| LDL–C, mg/dL | 119 (33–252) | 120 (33–231) | 0.131 |
| HDL–C, mg/dL | 50 (21–96) | 50.5 (22–116) | 0.330 |
| FPG, mg/dL | 109 (71–236) | 106.5 (61–387) | 0.830 |
| HbA1c, % | 6.0 (5.0–11.2) | 6.0 (4.9–11.8) | 0.534 |
| FIB-4 index | 1.29 (0.28–7.83) | 1.31 (0.27–11.58) | 0.001 |
| CAP, dB/m | 309.5 (159–400) | 298 (129–400) | 0.020 |
| LSM, kPa | 8.8 (2.9–46.4) | 6.5 (2.4–32.4) | <0.001 |
Figure 1.
The changes in body weight and liver stiffness measurement according to PNPLA3 rs738409 genotypes and HSD17B13 rs6834314 in 140 patients with non-alcoholic fatty liver disease.
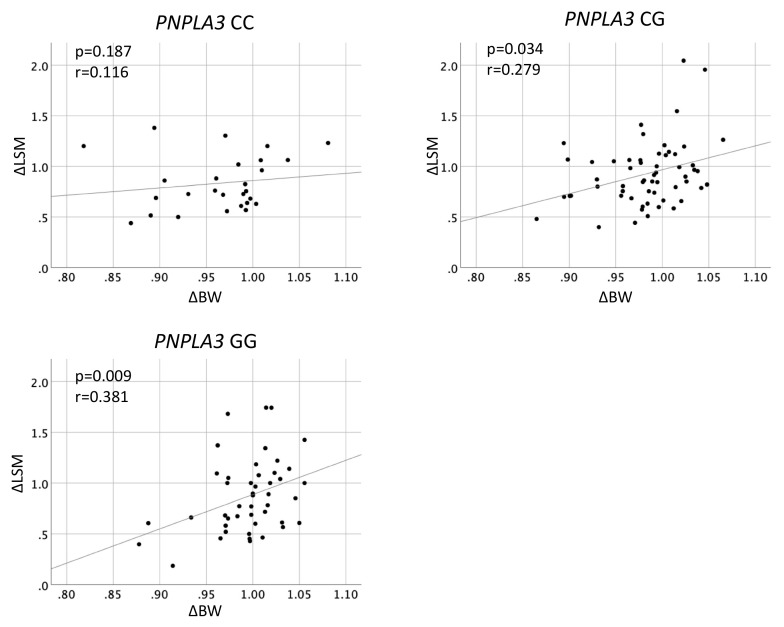
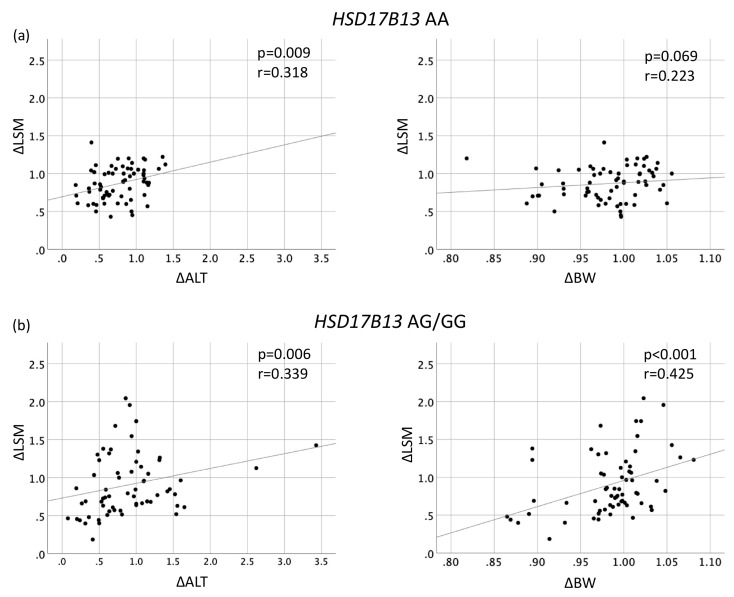
Figure 2 shows the associations between ΔBW and ΔLSM according to the PNPLA3 genotype. The ΔLSM had significant positive correlation with ΔBW in PNPLA3 genotype CG (r = 0.279, p = 0.034) and GG (r = 0.381, p = 0.009). In contrast, there was no correlation between ΔLSM and ΔBW in genotype CC (p = 0.187). The ΔLSM had significant positive correlation with ΔBW in HSD17B13 genotype AG/GG (r = 0.425, p < 0.01), but not in AA (p = 0.069) (Figure 3).
Figure 2.
Correlation between changes in body weight and in liver stiffness measurement according to PNPLA3 rs738409 genotypes in 140 patients with non-alcoholic fatty liver disease.
Figure 3.
Correlation between changes in liver stiffness measurement and changes in serum ALT level, and in body weight in patients with (a) HSD17B13 rs6834314 AA, (b) HSD17B13 rs6834314 AG/GG.
3.3. Factors Associated with Change in LSM According to HSD17B13 Polymorphism
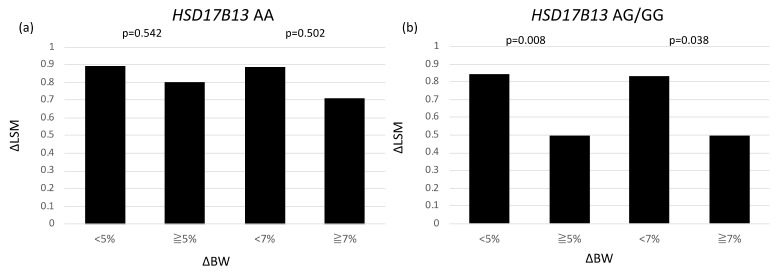
To estimate the factors, which effect ΔLSM due to HSD17B13 genotypes, we examined the correlations of ΔLSM with ΔALT and ΔBW in patients with HSD17B13 AA and AG/GG genotypes (Figure 3). In patients with HSD17B13 AA genotype, ΔLSM was associated with ΔALT (r = 0.318, p = 0.009), but not with ΔBW (p = 0.069) (Figure 3a). On the other hand, ΔLSM was significantly correlated with both ΔALT (r = 0.339, p = 0.006) and ΔBW (r = 0.425, p < 0.001) (Figure 3b). We performed multivariate linear regression analysis to evaluate the associations of change in BW, ALT level, AST level, GGT level, TC level, TG level, and PNPLA3 genotype with change in LSM to the HSD17B13 genotype (Table 4). Among patients with the HSD17B13 AA genotype, both changes in ALT and BW did not correlate with change in LSM. On the other hand, among patients with the HSD17B13 AG/GG genotype, change in BW (per 1 kg; β 3.043, 95% CI 0.276–5.811, p = 0.032) was independently associated with change in LSM. The change in LSM in patients with a reduction of BW more than 5% (0.80) and 7% (0.71) were not different from those in patients without achieving it (0.89, 0.89, respectively) (Figure 4a). Among patients with the HSD17B13 AG/GG genotype, the change in LSM of patients who achieved a BW reduction of more than 5% (0.50) was significantly greater than that of patients with a BW reduction of less than 5% (0.84) (p = 0.008). Furthermore, the change in LSM of patients with a BW reduction of more than 7% (0.50) was significantly greater than that of patients with a BW reduction of less than 7% (0.83) (p = 0.038) (Figure 4b).
Table 4.
Factors associated with change in live stiffness measurement according to the HSD17B13 genotype.
| Factors | β | 95% CI | p-Value | |
|---|---|---|---|---|
| HSD17B13 AA | PNPLA3 genotype | −0.058 | −0.136 to −0.021 | 0.145 |
| Change in BW | 0.454 | −0.770 to −1.678 | 0.460 | |
| Change in ALT | −0.111 | −0.416 to −0.193 | 0.468 | |
| Change in AST | 0.194 | −0.044 to −0.432 | 0.109 | |
| Change in GGT | 0.070 | −0.012 to −0.152 | 0.094 | |
| Change in TC | −0.087 | −0.419 to −0.246 | 0.604 | |
| Change in TG | 0.083 | −0.044 to −0.211 | 0.197 | |
| HSD17B13 AG/GG | PNPLA3 genotype | 0.032 | −0.107 to −0.172 | 0.645 |
| Change in BW | 3.043 | 0.276 to −5.811 | 0.032 | |
| Change in ALT | 0.001 | −0.509 to −0.510 | 0.998 | |
| Change in AST | −0.050 | −0.672 to −0.572 | 0.873 | |
| Change in GGT | 0.272 | −0.181 to −0.725 | 0.233 | |
| Change in TC | 0.179 | −0.434 to −0.793 | 0.560 | |
| Change in TG | −0.121 | −0.363 to −0.121 | 0.320 |
Figure 4.
Change in live stiffness measurement, according to body weight reduction in patients with (a) HSD17B13 rs6834314 AA, (b) HSD17B13 rs6834314 AG/GG.
4. Discussion
Lifestyle modifications, especially restrictions on calorie intake, are recommended as the most important types of therapy by the NAFLD practice guidelines [25]. Several trials examined the association between BW reduction and improvement of histological features, including fibrosis. In a meta-analysis, patients who lost at least 5% of BW had improved hepatic steatosis; those with more than 7% of BW reduction achieved NAFLD activity score improvement, and BW loss of more than 10% was associated with improvement in all features of NASH [26,27]. However, only 50% of patients were able to achieve at least 7% BW loss in that study. The PNPLA3 rs738409 G allele is widely known to be associated with more severe NAFLD. At the same time, it is also associated with a greater reduction in hepatic fat content by diet therapy [4,17,18], pharmacotherapy with the DPP-4 inhibitor [28], and bariatric surgery [29,30]. Shen et al. conducted a randomized control study to estimate the effects of PNPLA3 polymorphism in treating lifestyle modifications in NAFLD [18]. They reported that NAFLD patients carrying the G allele of PNPLA3 rs738409 demonstrated a greater reduction of BW and IHTG compared to those with the C allele. In the present study, the reduction of BW was greater in patients with PNPLA3 genotype CC than in patients with genotype CG/GG. Although the reduction of BW was greater according to the predominance of C allele, among patients with BW loss of more than 5%, the reduction of LSM was significantly greater according to the predominance of G allele, GG (0.46), CG (0.82), and CC (0.79) (p = 0.021). This discrepancy in BW change may be based on the difference in the restriction of caloric intake. In the previous study, authors provided an individual menu plan consisting of moderate carbohydrates, low fat, low-glycemic index, and low-calorific products with fruits and vegetables in appropriate portions. The patients attended dietary consultation sessions weekly during the first 4 months, and monthly in the following 8 months. We gave a dietary consultation session at baseline. This difference led to a greater BW reduction in a previous study than our study. The higher prevalence of patients with advanced fibrosis in this study may be another reason of the difference between two studies. Our study includes 18.6% of patients with advanced fibrosis, which is greater than the previous study. The reduction in CAP was significantly greater in patients without advanced fibrosis than with advanced fibrosis in our study.
In the present study, we focused in the association between change in LSM and BW reduction due to HSD17B13 genotype. After 1-year of diet therapy, the LSM significantly decreased from baseline and the change in LSM was positively associated with change in BW (data not shown). Change in LSM was not different between both PNPLA3 and HSD17B13 genotypes. Although the BW at baseline and change in BW were not different between HSD17B13 AA and AG/GG, the change in LSM was significantly associated with change in BW only in patients with the AG/GG genotype not the AA genotype. This result means the reduction of BW is a good therapeutic target for improvement of liver fibrosis in patients with the AG/GG genotype, but not the AA genotype. Indeed, when the patients achieved BW reduction of more than 5% or 7%, recommended in guidelines, the reduction of LSM was significantly greater in patients with the AG/GG genotype than the AA genotype. We previously reported that carriage of the HSD17B13 G allele attenuated the effect of the PNPLA3 GG genotype in advanced liver fibrosis [16]. To our knowledge, no report has investigated the effect of the HSD17B13 polymorphisms in response to BW reduction in Asian patients with NAFLD. The results of this study suggest that carriage of HSD17B13 rs6834314 AA showed resistance to the therapeutic effects of BW reduction in the improvement of liver fibrosis.
The HSD17B13 was identified as a locus associated with elevated levels of ALT in a large-scale genome-wide association study and presumed to reflect hepatic fat accumulation [31]. HSD17B13 encodes a hepatic lipid droplet protein; it was found upregulated in patients with NAFLD [32]. Ma et al. reported that HSD17B13 rs6834314 was associated with the histological features of NAFLD, such as hepatic steatosis, inflammation, and ballooning [15]. They demonstrated that HSD17B13 exhibits retinol dehydrogenase activity, and that the single amino acid mutation at rs6834314 is associated with loss of HSD17B13 enzymatic activity despite normal protein expression and localization [15]. Retinoid metabolism may play a role in hepatic steatosis and liver injury in patients with NAFLD. Thus, the patients with HSD17B13 G allele tend to accumulate IHTG, but have protective effects against liver injury. Peripheral lipolysis was identified as the main source of IHTG and hepatic steatosis [33,34]. The reduction in BW following lifestyle modification implies the reduction of lipolysis in peripheral tissues and of free fatty acid delivery to the liver. This beneficial effect might be shown more significantly in patients with the HSD17B13 AG/GG genotype than the AA genotype.
This study has some limitations. It was a retrospective cohort and hospital-based study, and such studies are potentially subject to selection bias. Second, the lifestyle intervention in this study was not sufficient, in that the total amount of BW reduction was smaller than previous studies. However, only a few patients with NAFLD can achieve and keep BW reduction of more than 7%. This study reflects the reality of lifestyle intervention. Because of the small sample size, we could not evaluate the difference in ΔLSM and correlation with ΔBW in each fibrosis stages. The LSM is known to be affected by, e.g., serum level of AST, ALT, splenoportal dynamics, and obesity [20,35,36,37]. We could not exclude the possibility of these effects on LSM. The strengths of this study include the diagnostic method used and the relatively large number of subjects. Confirmation of the NAFLD diagnosis by liver biopsy reveals histological findings and avoids the uncertainty associated with ultrasonography for NAFLD diagnosis.
5. Conclusions
In conclusion, the results of this cohort study suggest that the HSD17B13 rs6834314 polymorphism is associated with change in LSM, which might affect liver fibrosis by lifestyle intervention. The incomplete response to diet therapy was thought to be due to inadequate BW reduction. We suggest that the target rate of BW reduction, in order to improve liver histology, needs to be set by considering genetic assessments. The approach, including genetic assessments, may contribute to the establishment of appropriate therapeutic strategies to treat NAFLD.
Author Contributions
Conceptualization, Y.S. and Y.I.; methodology, Y.S., N.T.; formal analysis, Y.S.; investigation, Y.S.: data curation, Y.S., K.Y. (Kota Yano), A.T., S.O., S.K., K.O., A.U., K.Y. (Kanji Yamaguchi), M.M., and Y.I.; writing—original draft preparation, Y.S.; writing—review and editing, Y.S., N.T., K.Y. (Kota Yano), A.T., S.O., S.K., K.O., A.U., K.Y. (Kanji Yamaguchi), M.M. and Y.I.; supervision, Y.I.; project administration, Y.S. and Y.I.; funding acquisition, Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Kyoto Prefectural University of Medicine (ERB-C-1416).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to restriction by the institutional ethics committee.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe S., Hashimoto E., Ikejima K., Uto H., Ono M., Sumida Y., Seike M., Takei Y., Takehara T., Tokushige K., et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J. Gastroenterol. 2015;50:364–377. doi: 10.1007/s00535-015-1050-7. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., Mills P.R., Keach J.C., Lafferty H.D., Stahler A., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R., Fava J.L., Wing R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haufe S., Engeli S., Kast P., Böhnke J., Utz W., Haas V., Hermsdorf M., Mähler A., Wiesner S., Birkenfeld A.L., et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. 2011;53:1504–1514. doi: 10.1002/hep.24242. [DOI] [PubMed] [Google Scholar]
- 6.Wong V.W., Chan R.S., Wong G.L., Cheung B.H., Chu W.C., Yeung D.K., Chim A.M., Lai J.W., Li L.S., Sea M.M., et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: A randomized controlled trial. J. Hepatol. 2013;59:536–542. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Romero-Gómez M., Zelber-Sagi S., Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017;67:829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N., Guo X., Loomba R., Goodarzi M.O., Haritunians T., Kwon S., Cui J., Taylor K.D., Wilson L., Cummings O.W., et al. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology. 2010;139:1567–1576. doi: 10.1053/j.gastro.2010.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawaguchi T., Sumida Y., Umemura A., Matsuo K., Takahashi M., Takamura T., Yasui K., Saibara T., Hashimoto E., Kawanaka M., et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS ONE. 2012;7:e38322. doi: 10.1371/journal.pone.0038322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan X., Waterworth D., Perry J.R., Lim N., Song K., Chambers J.C., Zhang W., Vollenweider P., Stirnadel H., Johnson T., et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am. J. Hum. Genet. 2008;83:520–528. doi: 10.1016/j.ajhg.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seko Y., Sumida Y., Tanaka S., Mori K., Taketani H., Ishiba H., Hara T., Okajima A., Umemura A., Nishikawa T., et al. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: Association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol. Res. 2017;47:1083–1092. doi: 10.1111/hepr.12840. [DOI] [PubMed] [Google Scholar]
- 12.Singal A.G., Manjunath H., Yopp A.C., Beg M.S., Marrero J.A., Gopal P., Waljee A.K. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: A meta-analysis. Am. J. Gastroenterol. 2014;109:325–334. doi: 10.1038/ajg.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abul-Husn N.S., Cheng X., Li A.H., Xin Y., Schurmann C., Stevis P., Liu Y., Kozlitina J., Stender S., Wood G.C., et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N. Engl. J. Med. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., Trépo E., Nahon P., Cao Q., Moreno C., Letouzé E., Imbeaud S., Bayard Q., Gustot T., Deviere J., et al. A HSD17B13 variant protects from hepatocellular carcinoma development in alcoholic liver disease. Hepatology. 2019;70:231–240. doi: 10.1002/hep.30623. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y., Belyaeva O.V., Brown P.M., Fujita K., Valles K., Karki S., de Boer Y.S., Koh C., Chen Y., Du X., et al. 17-Beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology. 2019;69:1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seko Y., Yamaguchi K., Tochiki N., Yano K., Takahashi A., Okishio S., Kataoka S., Okuda K., Umemura A., Moriguchi M., et al. Attenuated effect of PNPLA3 on hepatic fibrosis by HSD17B13 in Japanese patients with non-alcoholic fatty liver disease. Liver Int. 2020;40:1686–1692. doi: 10.1111/liv.14495. [DOI] [PubMed] [Google Scholar]
- 17.Sevastianova K., Kotronen A., Gastaldelli A., Perttilä J., Hakkarainen A., Lundbom J., Suojanen L., Orho-Melander M., Lundbom N., Ferrannini E., et al. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am. J. Clin. Nutr. 2011;94:104–111. doi: 10.3945/ajcn.111.012369. [DOI] [PubMed] [Google Scholar]
- 18.Shen J., Wong G.L., Chan H.L., Chan R.S., Chan H.Y., Chu W.C., Cheung B.H., Yeung D.K., Li L.S., Sea M.M., et al. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2015;30:139–146. doi: 10.1111/jgh.12656. [DOI] [PubMed] [Google Scholar]
- 19.Giuffré M., Colecchia A., Crocé L.S. Elastography: Where are we now? Minerva Gastroenterol. Dietol. 2020 doi: 10.23736/S1121-421X.20.02773-7. [DOI] [PubMed] [Google Scholar]
- 20.Giuffrè M., Giuricin M., Bonazza D., Rosso N., Giraudi P.J., Masutti F., Palmucci S., Basile A., Zanconati F., de Manzini N., et al. Optimization of Point-Shear Wave Elastography by Skin-to-Liver Distance to Assess Liver Fibrosis in Patients Undergoing Bariatric Surgery. Diagnostics. 2020;7:795. doi: 10.3390/diagnostics10100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foschi F.G., Domenicali M., Giacomoni P., Dall’Aglio A.C., Conti F., Borghi A., Bevilacqua V., Napoli L., Mirici F., Cucchetti A., et al. Is there an association between commonly employed biomarkers of liver fibrosis and liver stiffness in the general population? Ann. Hepatol. 2020;19:380–387. doi: 10.1016/j.aohep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver diseases: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 25.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 26.Musso G., Cassader M., Rosina F., Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 27.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., Friedman S.L., Diago M., Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Kan H., Hyogo H., Ochi H., Hotta K., Fukuhara T., Kobayashi T., Naeshiro N., Honda Y., Kawaoka T., Tsuge M., et al. Influence of the rs738409 polymorphism in patatin-like phospholipase 3 on the treatment efficacy of non-alcoholic fatty liver disease with type 2 diabetes mellitus. Hepatol. Res. 2016;46:E146–E153. doi: 10.1111/hepr.12552. [DOI] [PubMed] [Google Scholar]
- 29.Krawczyk M., Jiménez-Agüero R., Alustiza J.M., Emparanza J.I., Perugorria M.J., Bujanda L., Lammert F., Banales J.M. PNPLA3 p.I148M variant is associated with greater reduction of liver fat content after bariatric surgery. Surg. Obes. Relat. Dis. 2016;12:1838–1846. doi: 10.1016/j.soard.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Palmer C.N., Maglio C., Pirazzi C., Burza M.A., Adiels M., Burch L., Donnelly L.A., Colhoun H., Doney A.S., Dillon J.F., et al. Paradoxical lower serum triglyceride levels and higher type 2 diabetes mellitus susceptibility in obese individuals with the PNPLA3 148M variant. PLoS ONE. 2012;7:e39362. doi: 10.1371/journal.pone.0039362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers J.C., Zhang W., Sehmi J., Li X., Wass M.N., Van der Harst P., Holm H., Sanna S., Kavousi M., Baumeister S.E., et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat. Genet. 2011;43:1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotronen A., Peltonen M., Hakkarainen A., Sevastianova K., Bergholm R., Johansson L.M., Lundbom N., Rissanen A., Ridderstråle M., Groop L., et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Korenblat K.M., Fabbrini E., Mohammed B.S., Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotronen A., Juurinen L., Tiikkainen M., Vehkavaara S., Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–130. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Giuffrè M., Fouraki S., Comar M., Masutti F., Crocè L.S. The Importance of Transaminases Flare in Liver Elastography: Characterization of the Probability of Liver Fibrosis Overestimation by Hepatitis C Virus-Induced Cytolysis. Microorganisms. 2020;29:348. doi: 10.3390/microorganisms8030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giuffrè M., Fouraki S., Campigotto M., Colombo A., Visintin A., Buonocore M.R., Aversano A., Budel M., Tinè F., Abazia C., et al. Alanine aminotransferase and spleno-portal dynamics affect spleen stiffness measured by point shear-wave elastography in patients with chronic hepatitis C in the absence of significant liver fibrosis. J. Ultrasound. 2021;24:67–73. doi: 10.1007/s40477-020-00456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giuffrè M., Bedogni G., Abazia C., Masutti F., Tiribelli C., Crocè L.S. Spleen stiffness can be employed to assess the efficacy of spontaneous portosystemic shunts in relieving portal hypertension. Ann. Hepatol. 2020;19:691–693. doi: 10.1016/j.aohep.2020.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to restriction by the institutional ethics committee.