Abstract
Oxygen supplementation is rarely considered when anesthetizing laboratory mice, despite reports that mice become profoundly hypoxic under anesthesia. Little is known about the effects of hypoxia on anesthetic performance. This article focuses on the effects of oxygen supplementation on physiologic parameters and depth of anesthesia in male and female C57BL/6 mice. Anesthesia was performed via common injectable anesthetic protocols and with isoflurane. Mice anesthetized with injectable anesthesia received one of 3 drug protocols. Low-dose ketamine/xylazine (100/8 mg/kg) was chosen to provide immobilization of mice, suitable for imaging procedures. Medium-dose ketamine/xylazine/acepromazine (100/10/1 mg/kg) was chosen as a dose that has been recommended for surgical procedures. High-dose ketamine/xylazine/acepromazine (150/12/3 mg/kg) was chosen after pilot studies to provide a long duration of a deep plane of anesthesia. We also tested the effects of oxygen supplementation on the minimum alveolar concentration (MAC) of isoflurane in mice. Mice breathed supplemental 100% oxygen, room air, or medical air with 21% oxygen. Anesthetized mice that did not receive supplemental oxygen all became hypoxic, while hypoxia was prevented in mice that received oxygen. Oxygen supplementation did not affect the MAC of isoflurane. At the high injectable dose, all mice not receiving oxygen supplementation died while all mice receiving oxygen supplementation survived. At low and medium doses, supplemental oxygen reduced the duration of the surgical plane of anesthesia (low dose with oxygen: 22 ± 14 min; low dose without supplementation: 29 ± 18 min; medium dose with oxygen: 43 ± 18 min; medium dose without supplementation: 61 ± 27 min). These results suggest that mice anesthetized with injectable and inhalant anesthesia without supplemental oxygen are routinely hypoxic. This hypoxia prolongs the duration of anesthesia with injectable drug protocols and affects survival at high doses of injectable anesthetics. Because of variable responses to injectable anesthetics in mice, oxygen supplementation is recommended for all anesthetized mice.
Abbreviations: HR, heart rate; LORR, loss of righting reflex RR, respiratory rate; SpO2, peripheral oxygen saturation; MAC, minimum alveolar concentration
Anesthesia is frequently required for mice used in biomedical research, but anecdotal communications suggest that mice receive significantly less anesthetic monitoring and supportive care than do other research species. Monitoring of anesthetized mice is often minimal due to lack of specialized monitoring equipment, and the fact that many rodent surgeries are performed by a single person who acts as both surgeon and anesthetist. Supportive care during anesthesia is limited by a lack of supporting experimental evidence. The lack of monitoring and supportive care may increase the mortality rate in anesthetized mice.
Previous studies have shown that mice anesthetized with both inhalant and injectable anesthetics without supplemental oxygen become profoundly hypoxic.1,6,8,9,19,26,39,41 While mice in these studies appear to recover normally from anesthesia, little is known about the effects of hypoxia on physiologic parameters, anesthetic depth, and perioperative mortality. Respiratory complications, including hypoxia and hypoventilation, are second only to cardiovascular complications as a cause of perioperative mortality in veterinary species, and in humans, hypoxemia accounts for over 50% of deaths under anesthesia.4 To mitigate the risk of hypoxia under anesthesia, oxygen supplementation is commonly provided to anesthetized humans and animals, but is rarely provided to mice in research settings.6,19
All anesthetics affect respiratory function; ketamine and isoflurane are particularly known to cause respiratory depression in mice and rats by impairing the normal physiologic responses to hypoxemia and hypercapnia.9,12,20,23,28 The peripheral chemoreceptors, primarily in the carotid body, normally sense dropping arterial partial pressure of oxygen (PaO2) while central chemoreceptors located in the medulla sense changes in pH and rising partial pressure of carbon dioxide (PaCO2).22,23,29,40 Both sets of chemoreceptors compensate by initiating increases in respiratory rate and tidal volume.23,28,31,34,40 Injectable and inhalant anesthetic agents depress the function of these chemoreceptors, preventing the increases in respiration that compensate for hypoxia and hypoventilation.22,29
Pulse oximetry is commonly used to monitor peripheral oxygen saturation and detect the presence of hypoxia. Pulse oximeters use the difference in light absorption of oxygenated hemoglobin and deoxygenated hemoglobin in arterial blood to provide an estimate of arterial oxygen content, abbreviated as SpO2.17 An SpO2 of less than 90% to 95% generally corresponds to a PaO2 of less than 60 to 80 mm Hg, which is considered hypoxic in most species of mammals.7,17 Because of the small size of mice, species-specific pulse oximetry equipment is necessary to obtain this measurement. Therefore, measurement of SpO2 in anesthetized mice is not routinely performed, meaning that hypoxia under anesthesia generally goes unrecognized, and is likely more common than is appreciated by our field.
The purpose of this study was to confirm that mice become hypoxic after receiving a ketamine/xylazine based anesthetic admixture or isoflurane, which are commonly used anesthetics in mice and to investigate the effects of oxygen supplementation on anesthetic depth, physiologic values, and anesthetic requirements in these mice.9,35 We hypothesized that mice not receiving supplemental oxygen would be hypoxic, as indicated by lower SpO2 while anesthetized, and that supplemental oxygen would correct this hypoxia. We also hypothesized that oxygen supplementation would increase the doses of injectable and inhalant anesthesia necessary to maintain mice at a surgical plane of anesthesia.
Materials and Methods
Animals.
Experiments were performed using male (weight range 23 to 31 g) and female (weight range 17 to 23 g) C57BL/6 mice (Mus musculus, n = 52, Jackson Laboratories, Bar Harbor, ME). All experimental procedures were approved by the IACUC at the University of Pennsylvania. Mice were housed with up to 5 animals per cage in static polycarbonate microisolation cages with disposable bedding (1/8” Bed-o'Cobs, The Andersons, Maumee, OH) under a 12:12 light:dark cycle at 72°F in an AAALAC accredited facility. Mice were fed standard laboratory rodent chow (5001, LabDiet, St Louis, MO) and provided with untreated tap water by bottle. Dirty-bedding sentinel mice were used for routine health monitoring and were free of fur mites, pinworms, mouse hepatitis virus, mouse parvoviruses, rotavirus, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus, and polyomavirus.
Mice were weighed on a gram scale (Tanita Digital Scale, KD-160, Arlington Heights, IL) prior to anesthesia. Each mouse was anesthetized no more than twice, with at least a 14-d washout period between anesthetic events. Sixteen mice had previously been used in training and pilot experiments to determine the dosages used in the study.
Injectable anesthesia.
Combinations of ketamine HCl (Ketaset; Zoetis; Kalamazoo, MI), xylazine (AnaSed; Akorn; Lake Forest, Il), and acepromazine maleate (PromAce Injectable; Boehringer Ingelheim Vetmedica; St Joseph, MO) were diluted with sterile saline to provide the desired dose at 0.1 mL per 10 grams of body weight. Injections were administered intraperitoneally in the lower left or right quadrants of the abdomen using a 1 mL syringe and 25-gauge needle.
Mice were anesthetized with 1 of 3 drug combinations. The low-dose group received 100 mg/kg ketamine and 8 mg/kg xylazine, which provides immobilization with no spontaneous movement; this dose is suitable for an imaging procedure but does not reliably provide a surgical plane of anesthesia. The medium dose group received 100 mg/kg ketamine, 10 mg/kg xylazine, and 1 mg/kg acepromazine; this combination is commonly used for surgical procedures.1,5,15 The high-dose group received 150 mg/kg ketamine, 12 mg/kg xylazine, and 3 mg/kg acepromazine, which pilot studies demonstrated would result in a very deep plane of anesthesia for a long period of time. The low and medium dose groups each consisted of 5 males and 5 females, 6–12 wk-old. A crossover design was used in which each mouse was anesthetized twice at the same dose, once with and once without oxygen supplementation; the order of oxygen supplementation was randomized. The high- dose group consisted of 4 males and 2 females, 12–24 wk-old. Mice given the high dose were anesthetized only once, with or without oxygen supplementation (Table 1).
Table 1.
Animal number, sex, age at first experiment, and status of oxygen supplementation during the first and second experiments for animals receiving injectable anesthetics.
| Injectable anesthetic dose | Animal numbers | Sex | Age (weeks) | Oxygen (first experiment) | Oxygen (second experiment) |
| Low dose | 1–3 | Female | 9 | No | Yes |
| Low dose | 4–5 | Female | 9 | Yes | No |
| Low dose | 1 | Male | 9 | Yes | No |
| Low dose | 2–3 | Male | 10 | No | Yes |
| Low dose | 4 | Male | 11 | Yes | No |
| Low dose | 5 | Male | 11 | No | Yes |
| Medium dose | 1 | Female | 6 | No | Yes |
| Medium dose | 2 | Female | 6 | Yes | No |
| Medium dose | 3 | Female | 6 | No | Yes |
| Medium dose | 4 | Female | 7 | No | Yes |
| Medium dose | 5 | Female | 7 | Yes | No |
| Medium dose | 1 | Male | 8 | No | Yes |
| Medium dose | 2–3 | Male | 8 | Yes | No |
| Medium dose | 4–5 | Male | 9 | No | Yes |
| High dose | 1 | Female | 24 | No | N/A |
| High dose | 2 | Female | 24 | Yes | N/A |
| High dose | 3 | Male | 12 | No | N/A |
| High dose | 4 | Male | 12 | Yes | N/A |
| High dose | 5 | Male | 24 | No | N/A |
| High dose | 6 | Male | 24 | Yes | N/A |
Oxygen Delivery.
Mice were randomly assigned to receive either supplementation with 100% oxygen administered at 0.6 L/min delivered by face mask, or no oxygen supplementation (mice breathed room air). Because the mice either did or did not have a facemask (a facemask without oxygen delivery could impede the animals breathing), the researcher could not be blind to the oxygen delivery status.
Anesthetic Depth and Physiologic Monitoring.
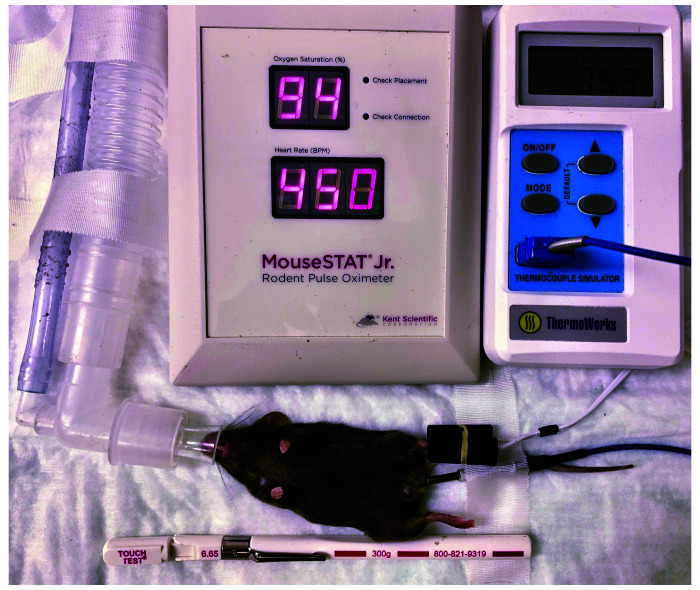
After induction of anesthesia, mice were continuously monitored for loss of righting reflex (LORR), which was defined as the mouse lying in dorsal recumbency without the ability to return to standing or sternal recumbency. Once this occurred, mice were moved to a circulating warm water blanket (Stryker T/Pump, Kalamazoo, MI), and an eye lubricant was applied (Akorn, Lake Forest, IL). Mice were instrumented with a rectal temperature probe (ThermaWorks, Alpine, UT), and rectal temperature was maintained at 35 to 37 °C with the use of the warm water blanket and a heat lamp. Heart rate (HR) and peripheral oxygen saturation (SpO2) were measured using a mouse-specific pulse oximeter (MouseSTAT Jr; Kent Scientific; Torrington, CT; Figure 1). Pulse oximetry measurements were taken independently from both hind feet at each time point. The pulse oximeter sensor was placed on the foot until a stable reading was obtained, after which the sensor was removed until the next time point. If the percent SpO2 values measured from the 2 feet at the same time point differed by more than 5%, the values at that time point were excluded from analysis. Consistent values were then averaged to provide a final value for that time point. Respiratory rate (RR) was measured by counting thoracic excursions, and respiratory effort was assessed subjectively. For all experiments, temperature, HR, RR, SpO2, and presence of paw withdrawal reflex were recorded every 5 min for the duration of anesthesia until the return of spontaneous movement. HR, SpO2, and RR were recorded prior to assessment of paw withdrawal reflex at each time point. For all experiments, a surgical plane of anesthesia was defined by the loss of paw withdrawal reflex after a brisk application of a 300 g noxious stimulus (Touch Test; North Coast Medical, Gilroy, CA) applied to the hind feet, alternating the left and right foot at each time point.18 A positive response was characterized as any movement by the mouse in response to the noxious stimulus. The return of the righting reflex was defined as the time when the mouse could rapidly right itself from its back 3 consecutive times. The time of LORR and return of righting reflex were recorded. After the return of the righting reflex, mice were monitored continuously until fully recovered from anesthesia and ambulatory.
Figure 1.
Mouse anesthetized and instrumented for physiologic monitoring. The face mask provides supplemental oxygen, and SpO2 and HR are measured using a mouse-specific pulse oximeter. Temperature is measured using a rectal temperature probe and thermal support was provided by a circulating water blanket and heat lamp. Paw withdrawal reflex was obtained by application of a 300-gram Touch Test device.
Statistical analysis of injectable anesthetic data.
A 3-way ANOVA was performed to determine the effects of oxygen supplementation, dose, and sex on the time of the LORR and time at a surgical plane of anesthesia. Tukey posthoc analysis was performed when significant differences were found. The SpO2, HR, and RR were analyzed by 3-way ANOVA with oxygen supplementation, sex, and time as the main effects separately for both of the 2 lower doses. A 2-way ANOVA was then performed to compare the low and medium dose groups for both HR and RR, with dose and oxygen supplementation as the main effects. When significant differences were detected, a Tukey posthoc analysis was performed. A P value of < 0.05 was considered statistically significant. The effect of oxygen supplementation on SpO2 was tested in the high-dose group by T-test. No other statistical analysis was performed for the high dose due to the small number of mice used based on the high death rate of mice not receiving oxygen supplementation.
MAC Determination using Isoflurane Anesthesia.
In this study, isoflurane was used for both anesthesia induction and maintenance. Isoflurane carrier gas of either 100% oxygen or medical grade air with 21% oxygen was used at a flow rate of 0.6 L/min. Because of the anesthetic machine design and differences in carrier gas containers, the researcher could not be blind to the carrier gas being used in each experiment.
The MAC for isoflurane (Piramal Critical Care, Bethlehem, PA) was determined using 5 female and 5 male young adult (6–12 wk old) C57BL/6 mice. Mice were weighed as described above. MAC was determined based on the concentration at which the mouse transitioned from a positive response to a noxious stimulus to a negative response. This concentration was calculated by averaging the highest isoflurane concentration with a positive response and the lowest isoflurane concentration with a negative response. If the mouse had both a positive and negative response at a single isoflurane concentration, this concentration was used as the estimate of the transitioning isoflurane concentration. As with the injectable anesthesia experiment, a cross over design was used, with each mouse being anesthetized twice, once with oxygen as the anesthetic carrier gas and once with compressed medical air, with the order of these carriers randomized for each mouse.
All mice were induced with 4.0% isoflurane in an anesthetic induction chamber until LORR occurred. After induction, mice were removed from the chamber, transitioned to a nose cone and placed on a circulating warm water blanket for the remainder of the study. The isoflurane concentration was set to the first experimental concentration, and mice were instrumented for anesthetic monitoring as described above. Rectal temperature was maintained between 35° and 37 °C using the circulating water blanket and heat lamp, as described above. Isoflurane concentration was measured throughout the experiment using an anesthetic gas monitor (Poet IQ2, Anesthetic Gas Monitor Criticare Systems, Waukesha, WI). Because this monitor was positioned at the fresh gas outlet, the isoflurane concentration provided an estimate of alveolar isoflurane concentration. Mice remained at the first of 3 experimental isoflurane concentrations for 15 min to allow for equilibration and at the second and third experimental concentrations for 10 min.9,36,38 Experimental isoflurane concentrations were randomly chosen between 1.5 and 2.1% (Table 2). Isoflurane concentrations were increased to prevent neural inertia from confounding the results.15,24,37 After an equilibration period, the depth of anesthesia was determined by applying the 300 g noxious stimulus twice to each hind foot, as described above, with a 1-min waiting period between applications on each foot. If the first test elicited a strongly positive response, no further tests were performed to avoid affecting the anesthetic event due to excessive stimulation. After mice were tested at the third experimental concentration, anesthesia was discontinued, and mice were monitored continuously until recovered.
Table 2.
Animal number, sex, age at first experiment, and status of oxygen supplementation during the first and second experiments for animals receiving inhalant anesthesia. Experimental isoflurane concentrations are numbered in the order they were tested during each experiment.
| Animal number | Sex | Age (weeks) | Oxygen (first experiment) | Oxygen (second experiment) | Isoflurane % 1 | Isoflurane % 2 | Isoflurane % 3 |
| 1–2 | Male | 8 | No | Yes | 1.7 | 1.8 | 2.0 |
| 3 | Male | 9 | Yes | No | 1.6 | 1.8 | 1.9 |
| 4 | Male | 9 | No | Yes | 1.6 | 1.8 | 1.9 |
| 5 | Male | 9 | Yes | No | 1.5 | 1.7 | 1.9 |
| 1 | Female | 9 | Yes | No | 1.7 | 1.8 | 2.0 |
| 2 | Female | 9 | Yes | No | 1.6 | 1.8 | 1.9 |
| 3 | Female | 9 | No | Yes | 1.6 | 1.8 | 2.0 |
| 4 | Female | 9 | No | Yes | 1.7 | 1.9 | 2.1 |
| 5 | Female | 9 | Yes | No | 1.7 | 1.8 | 1.9 |
Statistical Analysis and MAC Determination.
A 2-way repeated measures ANOVA was performed to determine the effects of oxygen supplementation and isoflurane concentration on the SpO2. A 2-way repeated measures ANOVA was performed to analyze the effects of sex and oxygen supplementation on MAC. For this experiment, the statistical analysis of the effects of oxygen supplementation on MAC was performed using bracketing analysis. We did not perform statistical analysis of HR or RR for this experiment because the repeated adjustment of the isoflurane concentration during each trial would make interpretation difficult. P < 0.05 was considered statistically significant.
Results
Injectable Anesthesia.
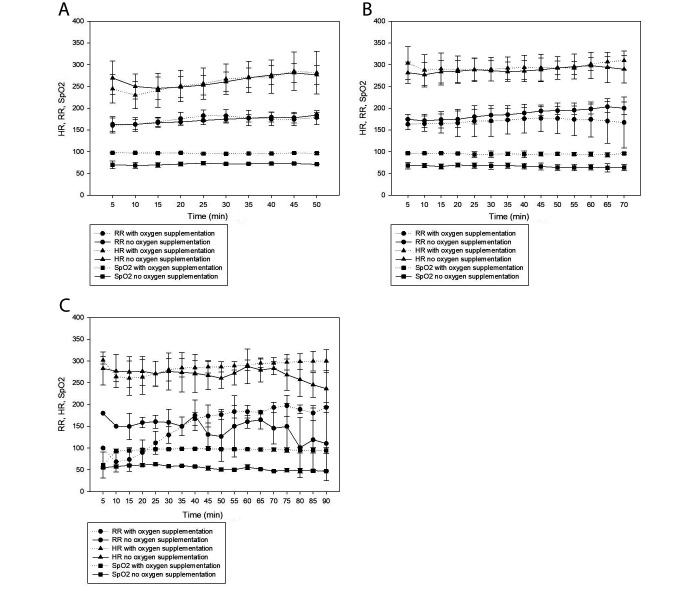
At the low and medium doses, male and female mice that did not receive supplemental oxygen were hypoxic (SpO2 = 69% ± 6%); hypoxia was prevented by oxygen supplementation (SpO2 = 96% ± 3%). The differences between SpO2 values of oxygen supplemented and non-supplemented mice were significantly different (P ≤ 0.001). Posthoc analysis indicated that mice at the medium dose and not receiving supplemental oxygen were significantly more hypoxic than were the mice at the low dose and not receiving oxygen supplementation (medium dose: 68% ± 6%, low dose: 71% ± 5%, P = 0.001). In the high-dose group, the control mice were again profoundly hypoxic (54% ± 6%) and the mice receiving oxygen supplementation were normoxic (94% ± 11%); these values were statistically different (P ≤ 0.001). Two of the 3 mice receiving supplemental oxygen in the high-dose group had an SpO2 of less than 90% for the first 10 min after induction, but by 10 min its value had increased to greater than 90%. One mouse had an additional period of mild hypoxia (SpO2 87% to 89%) from 75 to 110 min. The third mouse that received oxygen supplementation maintained an SpO2 of greater than 97% for the entire duration of anesthesia. Figure 2 shows the HR, RR, and SpO2 of male and female mice at each dose, with and without supplemental oxygen.
Figure 2.
HR, RR, and SpO2 of male and female C57BL/6J mice, with and without oxygen supplementation, under 3 doses of injectable anesthesia: (A) Low-dose ketamine/xylazine (100/8 mg/kg), (B) Medium-dose ketamine/xylazine/acepromazine (100/10/1 mg/kg), (C) High-dose ketamine/xylazine/acepromazine (150/12/3 mg/kg).
In the high-dose group, all three mice receiving supplemental oxygen survived and recovered normally, while the 3 mice which did not receive oxygen supplementation all died. One died within 6 min of anesthetic induction, so no data were collected prior to death. Two mice survived for over 90 min prior to death despite severe hypoxia (SpO2 of 43% to 65%). A fourth mouse died in the medium dose group when anesthetized for the second time and receiving supplemental oxygen. The mouse appeared to be responding normally to the anesthesia through 15 min (Figure 3). At this time point, the mouse had an abnormal drop in HR, RR and SpO2, and then appeared to stabilize with abnormally low physiologic parameters. At 43 min, the nose cone was briefly removed to confirm that the oxygen was flowing normally. Once this was established, the cone was returned to the mouse, and the values remained consistent with those measured before the cone was removed. The mouse underwent cardiac and respiratory arrest at 73 min. A necropsy did not reveal any gross abnormalities.
Figure 3.
Graph of the heart rate, respiratory rate and SpO2 of the mouse which died despite receiving oxygen supplementation after receiving the medium injectable dose.
At all 3 injectable doses, all mice showed LORR and absence of spontaneous movement after anesthesia induction. Table 3 reports the effects of oxygen supplementation on the durations of the LORR and the surgical plane for both low and medium doses. In the low and medium dose groups, control mice had a longer total time at a surgical plane of anesthesia than did mice receiving oxygen supplementation (P = 0.04). Oxygen supplementation did not affect the duration of the LORR (P = 0.186). Male mice had a longer LORR and a longer time at a surgical plane of anesthesia than did female mice (P ≤ 0.001 and P = 0.002, respectively). In the low-dose group, all male mice reached a surgical plane of anesthesia in both the control and oxygen supplemented condition, while 2 of 5 female control mice and 1 of 5 female mice with oxygen supplementation did not reach a surgical plane of anesthesia. In the medium-dose group, all mice reached a surgical plane of anesthesia. In the high-dose group, all mice reached a surgical plane of anesthesia; the mean duration of a surgical plane for the mice that received supplemental oxygen was 110 ± 14 min and the mean duration of LORR was 162 ± 28 min.
Table 3.
Response of C57BL/6 mice to injectable anesthesia with and without oxygen supplementation. Oxygen supplementation resulted in a significant decrease in the duration of LPWR (a surgical plane of anesthesia), but no significant difference in LORR. The males had a longer duration of LPWR and LORR than did females.
| Dose | Oxygen supplementation | Male LORR (min) | Male LPWR (min) | Female LORR (min) | Female LPWR (min) | Pooled LORR (min) | Pooled LPWR (min) |
| Low dose | No | 67 ± 7 | 39 ± 5 | 50 ± 10 | 20 ± 21 | 58 ± 12 | 29 ± 17 |
| Low dose | Yes | 62 ± 8 | 26 ± 10 | 52 ± 8 | 18 ± 17 | 57 ± 89 | 22 ± 14 |
| Medium dose | No | 111 ± 24 | 78 ± 20 | 74 ± 27 | 45 ± 23 | 92 ± 29 | 61 ± 26 |
| Medium dose | Yes | 87 ± 13 | 52 ± 22 | 73 ± 17 | 36 ± 13 | 80 ± 15 | 43 ± 17 |
LORR, loss of righting reflex; LPWR, loss of pedal withdrawal reflex.
Values are presented as minutes. Data are given as mean ± 1 SD.
Oxygen supplementation did not significantly affect HR at the low or medium doses (P = 0.346 and P = 0.140). Oxygen supplementation did not affect RR at the low dose (P = 0.116) but did significantly reduce RR at the medium dose (P = 0.003). In the medium-dose group, 4 control mice had subjectively increased respiratory effort as compared with mice receiving oxygen supplementation. Male mice had a significantly lower HR (P ≤ 0.001 and P = 0.041) and RR (P = 0.011 and P < 0.001) than did female mice. When the low and medium dose groups were compared, the HR of the medium dose was significantly higher than that of the low dose (P ≤ 0.001) and no significant difference was detected for RR (P = 0.520). Figure 2 shows the mean pooled SpO2, HR, and RR of mice at the low, medium, and high doses, with and without oxygen supplementation. In the high-dose group, statistical analysis of HR and RR was not performed due to the small sample size. All 3 mice receiving oxygen supplementation had a low RR of less than 100 breaths/min after induction, which gradually increased over the duration of anesthesia. All mice in the high-dose group, regardless of oxygen supplementation, had a HR between 200 to 350 beats/min for approximately the first 80 min of anesthesia at which point HR of the surviving mice began to increase as the mice recovered from anesthesia. Surviving mice at all 3 doses appeared to recover normally from anesthesia.
Inhalant Anesthesia.
All of the mice that received compressed air as the carrier gas were hypoxic (SpO2 = 79% ± 5%). Oxygen supplementation prevented this deficit (SpO2 = 97% ± 2%), and this difference was significant (P ≤ 0.001). The concentration of isoflurane did not significantly affect SpO2 (P = 0.35). Oxygen supplementation and sex did not significantly affect MAC in mice (P = 0.58 and P = 0.11, respectively). Average pooled MAC was 1.8% ± 0.1% in mice receiving oxygen supplementation and 1.8% ± 0.1% in control mice. One female mouse showed open-mouthed breathing with increased respiratory effort when anesthetized for the second time, without oxygen supplementation. This resolved within 30 s of discontinuing the isoflurane at the end of the experiment, and the mouse appeared to recover normally from anesthesia. Data from this trial was excluded from analysis.
Discussion
The current study showed that mice anesthetized with injectable and inhalant anesthesia without supplemental oxygen were consistently hypoxic as measured by SpO2 and that supplementation with oxygen corrected this deficit. In addition, supplemental oxygen conferred survival value to mice anesthetized with injectable anesthesia, as all of the mice anesthetized with the high dose without supplemental oxygen died, whereas all of the mice receiving oxygen supplementation at this dose survived. Oxygen supplementation shortened the time mice spent at a surgical plane of anesthesia under the low and medium-dose injectable protocols but did not affect the MAC of isoflurane for mice.
In all experiments, mice not receiving supplemental oxygen were consistently profoundly hypoxic. This finding is consistent with previous studies showing that without oxygen supplementation, mice are hypoxic under anesthesia.1,6,8,9,19,26,39,41 Long-term physiologic and cognitive effects resulting from this hypoxia may be minimal, as human studies have shown that transient hypoxia is well-tolerated if not accompanied by concurrent ischemia.2,42 However, if the anesthetized mice experience hypoxia with concurrent hypotension, which is difficult to confirm in anesthetized mice, this may predispose them to hypoxic brain injury.42 All of the mice in the current study appeared to recover normally, however further work examining potentially subtle long-term effects of this hypoxia will be necessary to clarify the effects of the hypoxia on the mice.
At the low and medium doses, control mice had a longer duration of surgical anesthesia, which is likely related to the effects of hypoxia on the brain. Hypoxia has previously been shown in humans to result in EEG changes, including slowing of EEG waveforms, which are similar to changes seen with a deepening plane of anesthesia.3 As oxygen supplementation is added to mouse anesthetic protocols, an important consideration will be to prepare for a decrease in the total duration of surgical anesthesia, potentially requiring supplemental dosing of anesthetics for longer procedures.20 Oxygen supplementation had no significant effect on the duration of the LORR, which is particularly relevant for imaging studies that do not require a surgical plane of anesthesia.
Even with oxygen supplementation, there is a significant risk associated with injectable anesthesia in mice. One mouse died in the medium-dose group while receiving supplemental oxygen. Death was preceded by a drop in HR, RR and SpO2. Previous work has shown that C57BL/6 mice with a respiratory rate below 120 breaths/min usually undergo arrest and die without intervention.20 This mouse was unique in that it maintained a respiratory rate below 120 breaths/min for over 50 min before arresting. Based on these observations, we hypothesize that this mouse would have died earlier had oxygen supplementation not been provided and that intervention during this period with atipamezole may have prevented the anesthetic arrest and saved the mouse (Table 1 and 2).
In the high-dose injectable anesthesia group, all mice not receiving supplemental oxygen died, while all mice receiving supplemental oxygen survived. Further trials were not performed due to the profound difference in survival for mice that received oxygen supplementation. This difference suggests that oxygen supplementation provides a survival benefit at higher doses of injectable anesthesia or in mice that have a profound response to the anesthetic related to the highly variable response of mice to injectable anesthetics. The mice that died in this group had a gradual reduction in RR for up to 10 min prior to death, as was noted in a previous study.20 If RR is monitored closely in mice, this drop may represent an opportunity to intervene and prevent death. To date, little information available on how to manage a mouse at dangerously deep planes of anesthesia due to injectable drugs. An obvious first choice would be the administration of oxygen if not already being provided and the administration of atipamezole to reverse the xylazine. However, if the surgery is still ongoing, these interventions could result in arousal during the surgery, which is unacceptable from a welfare point of view.20 Stopping surgical manipulations in an animal that is too deeply anesthetized may allow deepening of the plane of anesthesia, worsening the situation.25,27 Further work will be necessary to evaluate potential treatment options that address these complications in mice under injectable anesthesia.
The MAC for isoflurane of mice was not significantly different between mice receiving supplemental oxygen and those not receiving supplemental oxygen, despite the hypoxia seen in mice not receiving oxygen. These MAC values are consistent with values reported in previous studies that used hindlimb paw withdrawal reflex as a noxious stimulus in C57BL/6 mice.9,24 Our results contradict those of a previous study in dogs which found that hypoxia significantly decreased the MAC value when using halothane.11 However, in that study, the reduction in MAC did not occur until the PaO2 decreased below 38 mm Hg, corresponding to an SpO2 of approximately 65% in dogs,7,17 which is lower than what we saw in mice. Therefore, while the mice were hypoxic, they may not have been hypoxic enough to significantly affect MAC. In humans, loss of consciousness due to hypoxia typically occurs with a PaO2 of 20 to 35 mm Hg, depending on the cause of the hypoxic event,32 which is again lower than the hypoxia experienced by mice in the current study.
HR was higher in the medium dose group than in the low-dose group. Acepromazine is known to cause peripheral vasodilation, and in mice, the addition of acepromazine has previously been associated with more significant hypotension than ketamine/xylazine alone.5,31 Due to the baroreceptor reflex, hypotension resulting from acepromazine administration can cause a mild compensatory tachycardia.30 Despite the hypoxia noted in mice not receiving oxygen supplementation, lack of oxygen supplementation did not significantly affect RR in the low-dose group. In the medium-dose group, control mice had a higher RR than mice receiving oxygen supplementation. In the high-dose group, increased RR also occurred initially in the mice not receiving oxygen supplementation. Volatile anesthetics and some injectable anesthetics suppress the function of the carotid body chemoreceptors, which normally sense low arterial PaO2 and increase RR and tidal volume to compensate.22,23,29 Subjectively, in the medium-dose group, increased respiratory effort and abdominal breathing was noted in some, but not all, of the control mice. In the high-dose group, mice receiving supplemental oxygen initially had a very low RR, below 100 breaths/min, which was accompanied by a low SpO2 for the first 5 to 10 min. RR and SpO2 returned to normal over time, and all 3 mice recovered normally. This low RR may have been due to the high dose of ketamine, which has previously been shown to cause significant respiratory depression in mice.13,20,26
Sex differences were noted in HR and RR in the low and medium dose groups, with male mice having a lower HR and RR than female mice. This is possibly due to body weight differences, as females in these groups had an average weight of 18.2 g while males averaged 26.8 g. In many species, individuals with higher body weights tend to have lower heart rates.21 Each set of trials in our study was conducted over a 2-wk period, and male mice were anesthetized up to 1 wk after the female mice. This resulted in males being approximately 1 to 2 wk older than females during each trial. However, this small age difference is unlikely to be physiologically significant. Additional sex differences were also noted in anesthetic duration in the low and medium dose groups, with males having longer durations of LORR and time at a surgical plane of anesthesia than did females. This is consistent with some previous reports that male mice are more sensitive to ketamine-based anesthetic combinations in some strains of mice; however, other studies have not noted sex differences.10,14,16,33
In conclusion, this study demonstrates that mice anesthetized with injectable and inhalant anesthesia are profoundly hypoxic, as measured by pulse oximetry, when supplemental oxygen is not provided. Providing additional oxygen decreases the duration of surgical plane anesthesia in mice anesthetized with ketamine-xylazine drug protocols but does not alter the MAC for isoflurane in mice. At higher doses of injectable anesthetics or in mice that have an adverse response to the anesthetic cocktail, supplemental oxygen can help prevent mortality. Our findings also demonstrate the importance of monitoring anesthetized mice, as had intervention been provided when the drop in RR was noticed in the mouse that died (for example, reversal of xylazine with atipamezole), the mouse may have survived. The use of oxygen supplementation in mice is further supported by the wide variation of response to injectable anesthesia in mice, even within the same sex and dose, consistent with previous reports.1,5,20 Because predicting the individual response of mice to anesthesia is impossible, provision of oxygen supplementation is recommended for all anesthetized mice.
Acknowledgments
Funding for this project was provided by the Office of the Vice Provost for Research at the University of Pennsylvania.
References
- 1.Arras M, Autenried P, Rettich A, Spaeni D, Rülicke T. 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 51:443–456. [PubMed] [Google Scholar]
- 2.Bickler PE, Feiner JR, Lipnick MS, Batchelder P, MacLeod DB, Severinghaus JW. 2017. Effects of acute, profound hypoxia on healthy humans: implications for safety of tests evaluating pulse oximetry or tissue oximetry performance. Anesth Analg 124:146–153. 10.1213/ANE.0000000000001421. [DOI] [PubMed] [Google Scholar]
- 3.Bollen AR. 1962. The electroencephalogram in anaesthesia. Some aspects of hyperventilation. Br J Anaesth 34:890–896. 10.1093/bja/34.12.890. [DOI] [PubMed] [Google Scholar]
- 4.Brodbelt D. 2009. Perioperative mortality in small animal anaesthesia. Vet J 182:152–161. 10.1016/j.tvjl.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 6.Burnside WM, Flecknell PA, Cameron AI, Thomas AA. 2013. A comparison of medetomidine and its active enantiomer dexmedetomidine when administered with ketamine in mice. BMC Vet Res 9:1–9. 10.1186/1746-6148-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabro JM, Prittie JE, Palma DA. 2013. Preliminary evaluation of the utility of comparing SpO2 /FiO2 and PaO2 /FiO2 ratios in dogs. J Vet Emerg Crit Care (San Antonio) 23:280–285. 10.1111/vec.12050. [DOI] [PubMed] [Google Scholar]
- 8.Cesarovic N, Jirkof P, Rettich A, Nicholls F, Arras M. 2012. Combining sevoflurane anesthesia with fentanyl-midazolam or s-ketamine in laboratory mice. J Am Assoc Lab Anim Sci 51:209–218. [PMC free article] [PubMed] [Google Scholar]
- 9.Cesarovic N, Nicholls F, Rettich A, Kronen P, Hassig M, Jirkof P, Arras M. 2010. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim 44:329–336. 10.1258/la.2010.009085. [DOI] [PubMed] [Google Scholar]
- 10.Cruz JI, Loste JM, Burzaco OH. 1998. Observations on the use of medetomidine/ketamine and its reversal with atipamezole for chemical restraint in the mouse. Lab Anim 32:18–22. 10.1258/002367798780559383. [DOI] [PubMed] [Google Scholar]
- 11.Cullen DJ, Eger EI, 2nd. 1970. The effects of hypoxia and isovolemic anemia on the halothane requirement (MAC) of dogs. I. The effect of hypoxia. Anesthesiology 32:28–34. 10.1097/00000542-197001000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Dittmar MS, Fehm NP, Vatankhah B, Horn M. 2004. Ketamine/xylazine anesthesia for radiologic imaging of neurologically impaired rats: dose response, respiratory depression, and management of complications. Comp Med 54:652–655. [PubMed] [Google Scholar]
- 13.Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blümel G. 1984. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate). Res Exp Med (Berl) 184:159–169. 10.1007/BF01852390. [DOI] [PubMed] [Google Scholar]
- 14.Erickson RL, Terzi MC, Jaber SM, Hankenson FC, McKinstry-Wu A, Kelz MB, Marx JO. 2016. Intraperitoneal continuous-rate infusion for the maintenance of anesthesia in laboratory mice (Mus musculus). J Am Assoc Lab Anim Sci 55:548–557. [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB. 2010. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One 5:1–9. 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gergye CH, Zhao Y, Moore RH, Lee VK. 2020. A comparison of ketamine or etomidate combined with xylazine for intraperitoneal anesthesia in four mouse strains. J Am Assoc Lab Anim Sci 59:519–530. 10.30802/AALAS-JAALAS-19-000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haskins SC. 2015. Monitoring anesthetized patients. p 86–113. Chapter 4. In: Grimm KA, Robertson SA, Lamont LA, Tranquilli WJ, Greene SA, editors. Veterinary anesthesia and analgesia: The fifth edition of Lumb and Jones. John Wiley and Sons. 10.1002/9781119421375.ch4 [DOI] [Google Scholar]
- 18.Hill WA, Tubbs JT, Carter CL, Czarra JA, Newkirk KM, Sparer TE, Rohrbach B, Egger CM. 2013. Repeated administration of tribromoethanol in C57BL/6NHsd mice. J Am Assoc Lab Anim Sci 52:176–179. [PMC free article] [PubMed] [Google Scholar]
- 19.Hohlbaum K, Bert B, Dietze S, Palme R, Fink H, Thöne-Reineke C. 2018. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS One 13:e0203559. 10.1371/journal.pone.0203559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaber SM, Hankenson FC, Heng K, McKinstry-Wu A, Kelz MB, Marx JO. 2014. Dose regimens, variability, and complications associated with using repeat-bolus dosing to extend a surgical plane of anesthesia in laboratory mice. J Am Assoc Lab Anim Sci 53:684–691. [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs G, Knight DH. 1985. M-mode echocardiographic measurements in nonanesthetized healthy cats: effects of body weight, heart rate, and other variables. Am J Vet Res 46:1705–1711. [PubMed] [Google Scholar]
- 22.Jonsson MM, Lindahl SG, Eriksson LI. 2005. Effect of propofol on carotid body chemosensitivity and cholinergic chemotransduction. Anesthesiology 102:110–116. 10.1097/00000542-200501000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Kavanagh BP, Hedenstierna G. 2020. Respiratory physiology and pathophysiology. p 354–383. In: Gropper MA, Cohen NH, Eriksson LI, Fleisher LA, Leslie K, Wiener-Kronish JP, editors. Miller's anesthesia. Canada: Elsevier. [Google Scholar]
- 24.LaTourette PC, David EM, Pacharinsak C, Jampachaisri K, Smith JC, Marx JO. 2020. Effects of Standard and Sustained-release Buprenorphine on the Minimum Alveolar Concentration of Isoflurane in C57BL/6 Mice. J Am Assoc Lab Anim Sci 59:298–304. 10.30802/AALAS-JAALAS-19-000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latson TW, O'Flaherty D. 1993. Effects of surgical stimulation on autonomic reflex function: assessment by changes in heart rate variability. Br J Anaesth 70:301–305. 10.1093/bja/70.3.301. [DOI] [PubMed] [Google Scholar]
- 26.Massey CA, Richerson GB. 2017. Isoflurane, ketamine-xylazine, and urethane markedly alter breathing even at subtherapeutic doses. J Neurophysiol 118:2389–2401. 10.1152/jn.00350.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musizza B, Ribaric S. 2010. Monitoring the depth of anaesthesia. Sensors (Basel) 10:10896–10935. 10.3390/s101210896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njoku DB, Chitilian HV, Kronish K. 2020. Hepatic physiology, pathophysiology, and anesthetic considerations. p 420–430. Gropper MA, Cohen NH, Eriksson LI, Fleisher LA, Leslie K, Wiener-Kronish JP, editors. Miller's anesthesia. Canada: Elsevier. [Google Scholar]
- 29.Pandit JJ. 2014. Volatile anaesthetic depression of the carotid body chemoreflex-mediated ventilatory response to hypoxia: directions for future research. Scientifica (Cairo) 2014:1–15. 10.1155/2014/394270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parry BW, Anderson GA, Gay CC. 1982. Hypotension in the horse induced by acepromazine maleate. Aust Vet J 59:148–152. 10.1111/j.1751-0813.1982.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 31.Rankin DC. 2015. Sedatives and tranquilizers. Chapter 10.p 196–206. In: Grimm KA, Robertson SA, Lamont LA, Tranquilli WJ, Greene SA, editors. Veterinary anesthesia and analgesia: The fifth edition of Lumb and Jones. Ames (IA): John Wiley and Sons. 10.1002/9781119421375.ch10 [DOI] [Google Scholar]
- 32.Robson JG. 1964. The physiology and pathology of acute hypoxia. Br J Anaesth 36:536–541. 10.1093/bja/36.9.536. [DOI] [PubMed] [Google Scholar]
- 33.Schuetze S, Manig A, Ribes S, Nau R. 2019. Aged mice show an increased mortality after anesthesia with a standard dose of ketamine/xylazine. Lab Anim Res 35:1–7. 10.1186/s42826-019-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steffey EP, Mama KR, Brosnan RJ. Inhalation anesthetics. Chapter 16. p 297–331. In: Grimm KA, Robertson SA, Lamont LA, Tranquilli WJ, Greene SA, editors. Veterinary anesthesia and analgesia: The fifth edition of Lumb and Jones. Ames (IA): John Wiley and Sons. 10.1002/9781119421375.ch16 [DOI] [Google Scholar]
- 35.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154. 10.1258/la.2008.008020. [DOI] [PubMed] [Google Scholar]
- 36.Tao F, Skinner J, Yang Y, Johns RA. 2010. Effect of PSD-95/SAP90 and/or PSD-93/chapsyn-110 deficiency on the minimum alveolar anesthetic concentration of halothane in mice. Anesthesiology 112:1444–1451. 10.1097/ALN.0b013e3181dcd3dc. [DOI] [PubMed] [Google Scholar]
- 37.Tarnal V, Vlisides PE, Mashour GA. 2016. The neurobiology of anesthetic emergence. J Neurosurg Anesthesiol 28:250–255. 10.1097/ANA.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsukamoto A, Serizawa K, Sato R, Yamazaki J, Inomata T. 2015. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp Anim 64:57–64. 10.1538/expanim.14-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukamoto Y, Yamada N, Miyoshi K, Yamashita K, Ohsugi T. 2019. Anesthetic effect of a mixture of alfaxalone, medetomidine, and butorphanol for inducing surgical anesthesia in ICR, BALB/c, and C57BL/6 mouse strains. J Vet Med Sci 81:937–945. 10.1292/jvms.18-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittem T, Beths T, Bauquier SH. 2015. General pharmacology of anesthetic and analgesic drugs. Chapter 7. p 145–177. In: Grimm KA, Robertson SA, Lamont LA, Tranquilli WJ, Greene SA, editors. Veterinary anesthesia and analgesia: The fifth edition of Lumb and Jones. Ames (IA): John Wiley and Sons. 10.1002/9781119421375.ch7 [DOI] [Google Scholar]
- 41.Wilding LA, Hampel JA, Khoury BM, Kang S, Machado-Aranda D, Raghavendran K, Nemzek JA. 2017. Benefits of 21% oxygen compared with 100% oxygen for delivery of isoflurane to mice (Mus musculus) and rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 56:148–154. [PMC free article] [PubMed] [Google Scholar]
- 42.Williams M, Lee JK. 2014. Intraoperative blood pressure and cerebral perfusion: strategies to clarify hemodynamic goals. Paediatr Anaesth 24:657–667. 10.1111/pan.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]