Abstract
Kallikrein-related peptidase 6 (KLK6), a member of the kallikrein-related peptidase family, is involved in the regulation of epithelial-mesenchymal transition (EMT) in cancer cells and is highly expressed in gastric cancer tissues. The aim of the present study was to investigate the effect of KLK6 on the proliferation, migration and invasion of gastric cancer cells and to determine the underlying mechanism of its actions. The expression of KLK6 was measured in metastatic gastric cancer cells using western blotting and reverse transcription-quantitative PCR, and KLK6 was overexpressed or inhibited in HGC-27 cells using plasmid transfection. Cell proliferation, migration, invasion and EMT were also evaluated using Cell Counting Kit 8, Transwell and western blot analysis, respectively. In addition, a mouse xenograft model was constructed by injection of HGC-27 cells. The xenograft was treated with KLK6 interference or overexpression plasmids to study the in vivo effects of KLK6 on tumor development. The results demonstrated that KLK6 was highly expressed in HGC-27 cells and that KLK6 inhibition attenuated cell proliferation, migration and invasion and prevented gastric cancer tumor development. In addition, KLK6 inhibition reduced the expression of epithelial cell adhesion molecule and vimentin, reduced the phosphorylation of SMAD2 and SMAD3 and upregulated epithelial-cadherin expression. In conclusion, KLK6 inhibition suppressed the proliferation, migration and invasion of gastric cancer cells both in vitro and in vivo through the inhibition of EMT. These findings indicate that KLK6 a potential therapeutic target for gastric cancer therapy.
Keywords: gastric cancer, kallikrein-related peptidase 6, migration, invasion, epithelial-mesenchymal transition
Introduction
Gastric cancer is one of the most prevalent malignant tumors worldwide (1). The number of newly diagnosed cases of gastric cancer increased to 1.033 million worldwide in 2018, with 782,000 related deaths (2). With aging and socioeconomic development, cancer morbidity and mortality are rapidly increasing globally (3). A variety of advanced methods, including chemotherapy, surgical removal and radiotherapy, have been used for gastric cancer treatment, however, mortality remains high (4). An estimated 456,000 new cases of gastric cancer were diagnosed in China in 2018, with approximately 390,000 related deaths (5). Therefore, it is imperative to develop effective therapeutic strategies for gastric cancer treatment and gene therapy is a potentially beneficial option (6).
Kallikrein-related peptidase 6 (KLK6) is a member of the KLK family that was initially identified based on its abnormal expression in human ovarian and breast cancers (7). Accumulating evidence has demonstrated the role of KLK6 in carcinogenesis and its potential as a cancer biomarker (8). As a proteolytic protein, KLK6 regulates the invasive phenotype of tumor cells by degrading fibronectin, fibrinogen, collagen and laminin (9,10). Previous research indicated that KLK6 promoted cancer cell migration and invasion by modulating epithelial-mesenchymal transition (EMT) (11). However, the effect of KLK6 on EMT varies based on tumor classification. KLK6 expression in non-KL6 expressing breast cancer cells downregulated the expression of the EMT marker vimentin and upregulated the expression of the epithelial markers cytokeratin 8 and 19, suggesting an inhibitory effect of KLK6 against EMT in breast cancer cells (12). Conversely, KLK6 overexpression or expression in KLK6-knockdown colorectal cancer cells facilitated EMT (11).
Previous studies have demonstrated that KLK6 is upregulated in gastric cancer (13-15). However, the effect of KLK6 on the invasive phenotype and EMT status of gastric cancer cells requires further study. In the present study KLK6 was expressed at significantly higher levels in metastatic gastric cancer cells (HGC-27) than in primary gastric cancer cells (AGS and SNU-1). Subsequently, the effect of KLK6 on the invasive phenotype and EMT status of gastric cancer cells lines HGC-27 in vitro and in vivo in a mouse xenograft model were investigated.
Materials and methods
Cell culture
Human gastric cancer cell line HGC-27 (derived from a lymphatic metastasis) and primary gastric cancer cell lines AGS and SNU-1 were purchased from The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences. The cells were incubated in RPMI-1640 medium (Cytiva; cat. no. SH30809.01B) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.; cat. no. 10270-106) at 37˚C in an environment containing 5% CO2. The protein and mRNA expression levels of KLK6 in HGC-27, AGS and SNU-1 cells were measured using western blotting and reverse transcription-quantitative polymerase chain reaction (RT-qPCR), respectively.
Cell transfection
pSICOR interference vectors and pCDH-CMV- MCS-EF1-CopGFP-T2A-Puro overexpression vectors were supplied by Addgene, Inc. KLK6 mRNA was amplified via PCR (forward, 5'-GCTCTAGATGAAGAAGCTGATGGTG-3'; reverse, 5'-CGGGATCCTCACTTGGCCTGAATGGT-3') and inserted into the pCDH vector to produce pCDH-KLK6 overexpression (OV) plasmids. The short hairpin (sh) interference fragments (shKLK6-1, 5'-AGAATAAGTTGGTGCATGG-3'; shKLK6-2, 5'-CAGATGGTGATTTCCCTGAC-3'; shKLK6-3, 5'-GATCAAAGGAGAAGCCAGGA-3'; and shKLK6-4, 5'-CAGATACACGAACTGGATCC-3') were inserted into pSICOR vectors to produce pSICOR-shKLK6 plasmids. HGC-27 cells were transfected for 48 h with 0.8 µg pCDH-KLK6 (OV-KLK6), pSICOR-shKLK6 (shRNA) or their corresponding negative controls (OV-NC and sh-NC, respectively) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.; cat. no. 11668-027). Non-transfected cells served as the control (CON).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell viability was measured using the MTT assay. HGC-27 cells were seeded in a 96-well plate at a density of 5x103 cells/well and maintained overnight at 37˚C with 5% CO2. A 20 µl volume of MTT reagent (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd.; cat. no. C1736) was added to each well after 24, 48 and 72 h of transfection. The cells were incubated at 37˚C for 4 h in an atmosphere containing 5% CO2 and subsequently incubated with 150 µl of dimethyl sulfoxide for 10 min. The absorbance of the wells was measured using a microplate reader at 570 nm.
Flow cytometry
Flow cytometry was performed to evaluate apoptosis and cell cycle progression in HGC-27 cells. To assess cell apoptosis, 1x106 HGC-27 cells were resuspended in 1 ml of phosphate-buffered saline (PBS) and centrifuged at 400 x g for 5 min at 4˚C. The cells were then resuspended in 200 µl of PBS, stained with 10 µl of Annexin V-fluorescein isothiocyanate (BD Biosciences), and 10 µl of propidium iodide (PI; BD Biosciences) in the dark at 4˚C for 30 min. Thereafter, the cells were subjected to flow cytometry. To assess cell cycle progression, 1x107 HGC-27 cells were fixed in a mixture of 300 µl of PBS and 700 µl of absolute ethyl alcohol at -20˚C for 24 h. After two washes with PBS, the cells were resuspended in 100 µl of RNase A (BD Biosciences) and maintained at 37˚C for 30 min. The cells were then stained with 400 µl of PI (50 µg/ml) in the dark at 4˚C for 5 min and subjected to flow cytometry (NovoCyte, ACEA Biosciences, Inc.; Agilent Technologies, Inc.). The data were analyzed using NovoExpress software version 1.3.0 (ACEA Biosciences, Inc.; Agilent Technologies, Inc.). For apoptosis analysis, both early- and late-stage apoptosis (quadrants Q2-2 and Q2-4) were evaluated.
Wound healing
HGC-27 cells were cultured overnight in 6-well plates at a density of 1x106 cells/well. After 4 h of transfection, the cells were wounded by scratching the cell monolayer with a sterile 200-µl plastic pipette tip. The scratches were observed and imaged under an inverted fluorescence microscope (magnification, x100; Leica Microsystems, GmbH) after incubation in serum-free medium at 37˚C in an environment containing 5% CO2 for 0 and 24 h.
Cell migration and invasion assays
Cell migration and invasion were evaluated using two-chamber Transwell inserts (Corning, Inc.). The cells were starved in serum-free medium for 24 h and resuspended in RPMI-1640 medium (Cytiva; cat. no. SH30809.01B) containing 1% FBS. In the upper chambers, 0.5 ml of treated cells were seeded at a density of 1x105 cells/ml and the lower chambers were filled with 0.75 ml of RPMI-1640 medium supplemented with 10% FBS. The inserts for the cell invasion assay were pre-coated for 30 min at 37˚C with Matrigel (BD Biosciences; cat. no. 354230) between the lower and upper chambers. After 24 h of incubation at 37˚C, the cells were fixed at 25˚C with 4% paraformaldehyde for 10 min and stained with 1 ml of 0.5% crystal violet (Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd.; cat. no. C1701) for 30 min at room temperature. Non-invading or non-migrating cells were wiped away using cotton swabs, and the migrated or invaded cells were counted under an inverted fluorescence microscope (magnification, x100; Leica Microsystems GmbH).
RT-qPCR
RT-qPCR was performed to examine the mRNA expression levels of KLK6 in HGC-27, AGS and SNU-1 cells, as well as EMT-related genes in HGC-27 cells. Total RNA was extracted from HGC-27, AGS and SNU-1 cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.) and reverse-transcribed into cDNA using the M-MuLV kit (42˚C for 60 min; 70˚C for 15 min and held at 16˚C), according to the manufacturer's instructions (Takara Bio, Inc). The collected cDNA was amplified using a SYBR Green PCR kit (KAPA Biosystems, Inc.; Roche Diagnostics; cat. no. KM4101) following the manufacturer's instructions in a CFX-Connect 96 apparatus (Bio-Rad Laboratories, Inc.). The thermocycling conditions were as follows: 95˚C for 3 min; 39 cycles of denaturation at 95˚C for 5 sec, annealing at 56˚C for 10 sec, and extension at 72˚C for 25 sec; and final extension at 65˚C for 5 sec and 95˚C for 50 sec. The primer sequences were as follows: KLK6 forward, 5'-GAACTCATCCAGCCCCTT-3' and reverse, 5'-CATCCCC AGCACACAACA-3'; epithelial (E)-cadherin forward, 5'-GGC AAGGTTTTCTACAGC-3' and reverse, 5'-ATGTGGCA ATGCGTTCT-3'; vimentin forward, 5'-TTGAACGCAAAGT GGAATC-3'; and reverse, 5'-AGGTCAGGCTTGGAAACA-3'; GAPDH forward, 5'-CCACTCCTCCACCTTTG-3' and reverse, 5'-CACCACCCTGTTGCTGT-3'. GAPDH served as an endogenous control. Relative mRNA expression levels were calculated using the 2-∆∆Cq method (16).
Western blotting
Western blotting was performed to determine the protein expression levels of KLK6 in HGC-27, AGS and SNU-1 cells and EMT-related markers in HGC-27 cells. Total proteins were collected using radioimmunoprecipitation assay lysis buffer (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd.; cat. no. W1689) and quantified using a bicinchoninic acid assay kit (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd.; cat. no. W1712). Proteins (20 µg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (EMD Millipore). The membranes were blocked and incubated overnight at 4˚C with primary antibodies against epithelial cell adhesion molecules (EP-CAM, Bioswamp, cat. no. PAB30814, 1:1000), E-cadherin (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd.; cat. no. PAB43792, 1:1000), vimentin (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd; cat. no. PAB40646; 1:1,000), phosphorylated (p)-SMAD2 (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd; cat. no. PAB43294-P; 1:1,000), SMAD2 (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd; cat. no. PAB30712; 1:1,000), p-SMAD3 (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd; cat. no. PAB43521-P; 1:1,000), SMAD3 (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd; cat. no. PAB30705; 1:1,000), and GAPDH (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd; cat. no. PAB36269; 1:1,000) and were subsequently incubated for 1 h at room temperature with HRP-conjugated goat anti-rabbit IgG secondary antibodies (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd; cat. no. SAB43714; 1:20,000). The membranes were developed using ECL (EMD Millipore) and then observed using a Tanon-5200 apparatus (Tanon Science & Technology Co., Ltd.) and data were analyzed using Tanon GIS software version 4.2 (Tanon Science & Technology Co., Ltd.). GAPDH served as an internal reference.
Tumor xenografts in nude mice
Female BALB/c nude mice (age, 6 weeks; weight 18-20 g; n=15) were supplied by Changzhou Cavens Experimental Animal Co. Ltd. (ref. no. 1107301911000025) and divided into five groups (n=3 per group): CON, sh-NC, sh-KLK6, OV-NC and OV-KLK6. All mice were injected with 200 µl of HGC-27 cells at a density of 1x106 cells/m in the right axilla. When the tumor size reached 80-100 mm3 (12 days after injection), the mice were treated with 10 µg of PBS, sh-NC, sh-KLK6, OV-NC and OV-KLK6, respectively, by intratumoral injections once every three days for a total of four times. Animal health and behavior were monitored every day. When the tumor could be clearly identified (4 days after injection), tumor volume was measured every two days using the following formula: volume (mm3) = length x width2/2(17). After 10 days of treatment, the mice were euthanized with an intraperitoneal injection of sodium pentobarbital at 100 mg/kg of body weight (death was confirmed by the absence of heartbeat and breath), and the tumors were removed for further analysis. The humane endpoint was when the tumor maximum diameter reached >15 mm. No animals died during the course of the experiment. All animal procedures were approved by the ethics committee of Wuhan Myhalic Biotechnology Co., Ltd. (approval no. HLK-20181102-01), who also conducted the animal experiments.
Hematoxylin and eosin (H&E) staining
Pathological changes in the tumors were evaluated by H&E staining. The tissues were fixed in 10% formalin buffer at 25˚C for 48 h, embedded in paraffin and sectioned at a thickness of 4 µm. After dewaxing, the sections were stained with hematoxylin (Bioswamp, cat. no. I1709) at 25˚C for 3 min and then stained with eosin solution (Bioswamp Life Science Lab; Wuhan Beinlai Biotechnology Co., Ltd; cat. no. I1703) for 3 min. Pathological changes were assessed using a light microscope (magnification, x100), Leica Microsystems GmbH).
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Differences between two groups were analyzed using unpaired Student's t-test and those between more than two groups were analyzed using one-way analysis of variance, followed by Tukey's test. P<0.05 was considered statistically significant.
Results
KLK6 is highly expressed in HGC-27 cells and increases HGC-27 cell viability
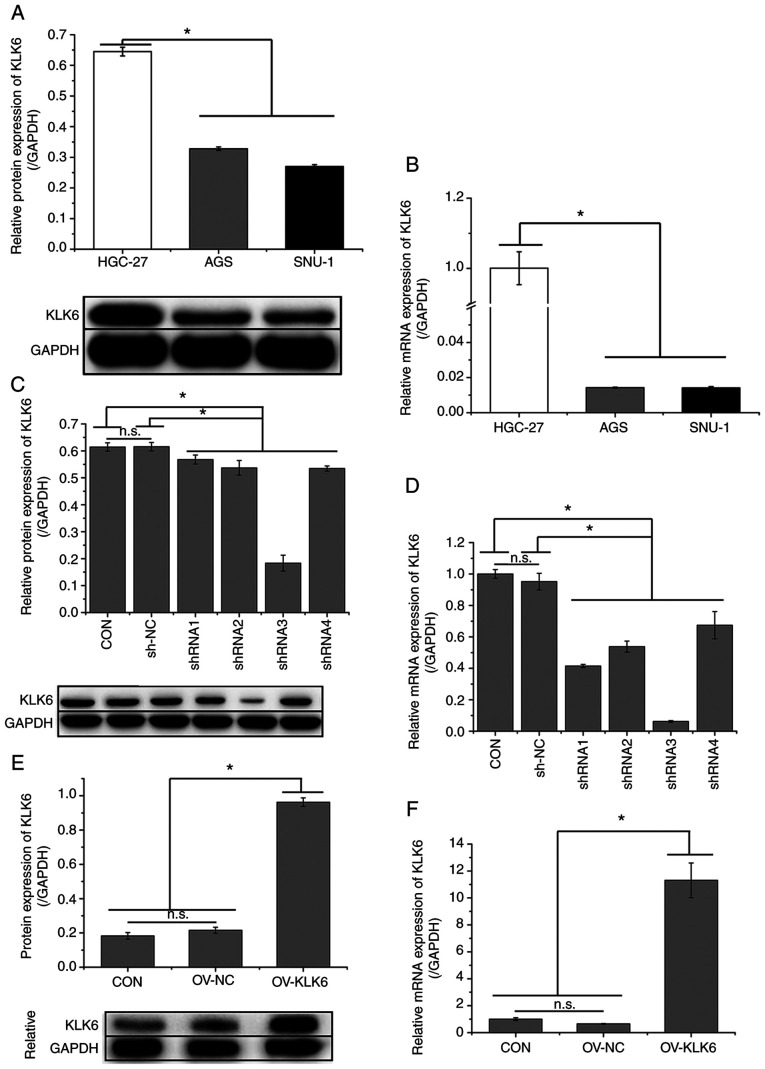
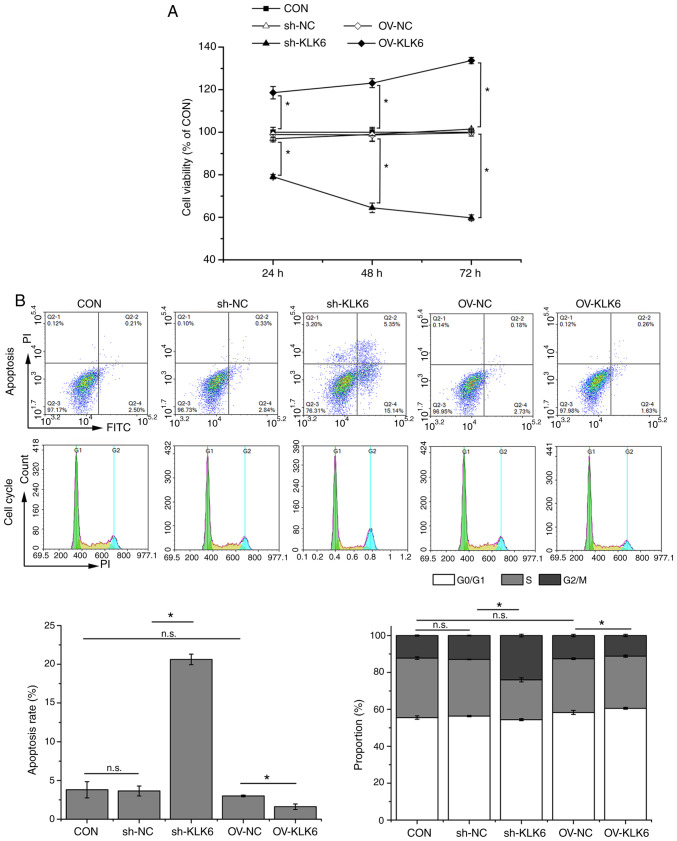
As indicated in Fig. 1A and B, the expression of KLK6 was significantly higher in metastatic gastric cancer cells (HGC-27) than in primary gastric cancer cells (AGS and SNU-1). Thus, the effect of KLK6 on gastric cancer was investigated in vitro and in vivo using HGC-27 cells. In HGC-27 cells, KLK6 was inhibited by pSICOR-shKLK6 plasmids and overexpressed by pCDH-KLK6 plasmids (Fig. 1C-F). As shRNA3 interference resulted in the greatest reduction in the expression of KLK6, it was selected for subsequent experiments. MTT assay demonstrated that KLK6 overexpression enhanced the viability of HGC-27 cells, whereas KLK6 inhibition attenuated cell viability in a time-dependent manner in comparison with controls (Fig. 2A). After 24 h of transfection, cell viability was significantly different between the CON group and the sh-KLK6 or OV-KLK6 groups. Therefore, subsequent experiments were performed 24 h after transfection. Flow cytometry indicated that KLK6 inhibition promoted apoptosis and G2/M phase arrest in HGC-27 cells, while KLK6 overexpression showed the opposite effect (Fig. 2B).
Figure 1.
KLK6 is highly expressed in HGC-27 cells. Relative (A) protein and (B) mRNA expression levels of KLK6 in HGC-27, AGS and SNU-1 cells. Relative (C) protein and (D) mRNA expression levels of KLK6 in HGC-27 cells after plasmid transfection for 24 h. Relative (E) protein and (F) mRNA expression of KLK6 in HGC-27 cells after overexpression plasmid transfection for 24 h. Data are presented as the mean ± SD (n=3). KLK6, kallikrein-related peptidase 6; Con, untreated control; shRNA, short hairpin RNA; NC, non-coding control; OV, overexpression plasmid; n.s., not significant. *P<0.05.
Figure 2.
KLK6 inhibition suppresses viability and increases apoptosis of HGC-27 cells. (A) HGC-27 cell viability after 24, 48, and 72 h of transfection. (B) Apoptosis rate and cell cycle stage of HGC-27 cells. Data are presented as the mean ± SD (n=3). KLK6, kallikrein-related peptidase 6; Con, untreated control; sh, short hairpin; NC, non-coding control; OV, overexpression plasmid; n.s., not significant. *P<0.05.
KLK6 inhibition attenuates HGC-27 cell migration and invasion
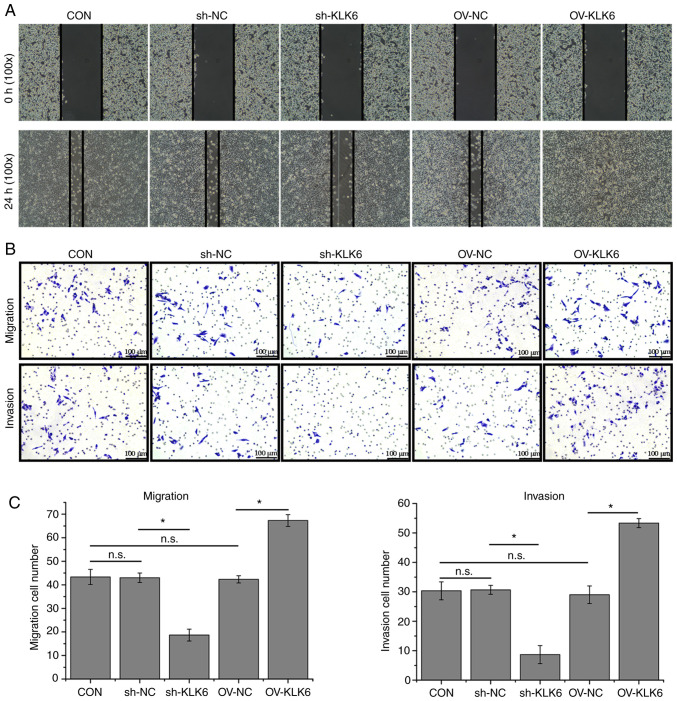
The wound healing ability of sh-KLK6-transfected HGC-27 cells was poorer than that of control cells, whereas it was increased by KLK6 overexpression (Fig. 3A). In addition, the Transwell assay showed that KLK6 inhibition reduced the number of migratory and invading cells in comparison with the control, whereas numbers were increased by KLK6 overexpression (Fig. 3B and C).
Figure 3.
KLK6 inhibition attenuates HGC-27 cell migration and invasion. (A) Wound healing of HGC-27 cells. (B) Migration and invasion of HGC-27 cells. (C) Quantification of cell migration and invasion. Data are presented as the mean ± SD (n=3). KLK6, kallikrein-related peptidase 6; Con, untreated control; sh, short hairpin; NC, non-coding control; OV, overexpression plasmid; n.s., not significant. *P<0.05.
KLK6 is involved in EMT regulation in HGC-27 cells
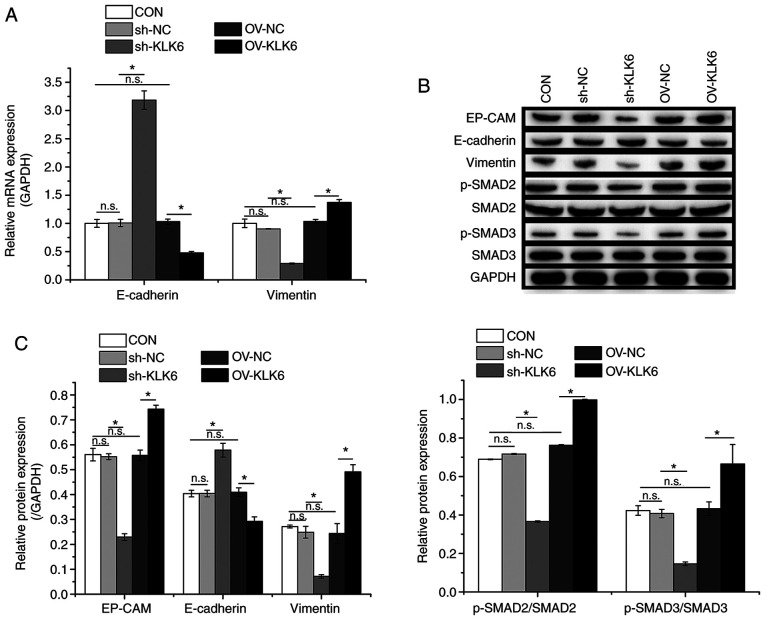
Western blotting and RT-qPCR were performed to evaluate the expression of EMT-related factors. As shown in Fig. 4, compared to the CON group, the protein and mRNA expression levels of E-cadherin were increased by sh-KLK6 but decreased by OV-KLK6 in comparison with controls, whereas vimentin showed the opposite trend as that of E-cadherin. Additionally, KLK6 inhibition suppressed the protein expression of EP-CAM and the phosphorylation of SMAD2 and SMAD3. These results demonstrated the regulatory effect of KLK6 on EMT in HGC-27 cells through modulation of EMT-related proteins.
Figure 4.
KLK6 inhibition suppresses EMT in HGC-27 cells. (A) Relative mRNA expression levels of E-cadherin and vimentin in HGC-27 cells. (B) Relative protein expression levels of EP-CAM, E-cadherin and vimentin and phosphorylation levels of SMAD2 and SMAD3 in HGC-27 cells. (C) Western blotting densitometry values. Data are presented as the mean ± SD (n=3). KLK6, kallikrein-related peptidase 6; EMT, epithelial-to-mesenchymal transition; E-cadherin, epithelial cadherin; EP-CAM, epithelial cell adhesion molecule; p-, phosphorylated; Con, untreated control; sh, short hairpin; NC, non-coding control; OV, overexpression plasmid; n.s., not significant. *P<0.05.
KLK6 inhibition suppresses gastric cancer development in vivo
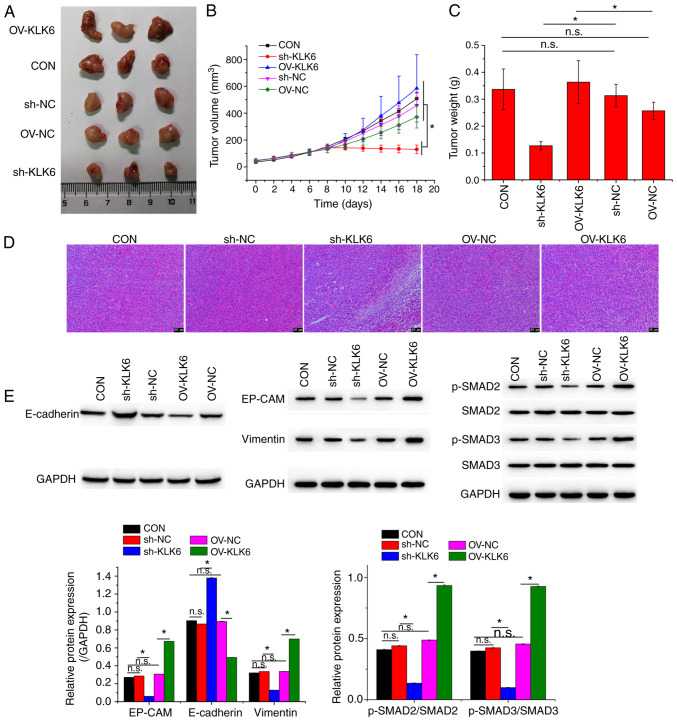
To investigate the effect of KLK6 on gastric cancer in vivo, a xenograft mouse model was constructed by injecting HGC-27 cells. Mice were treated with sh-KLK6, OV-KLK6 or the corresponding negative controls. The results demonstrated that both the mRNA and protein expression levels of KLK6 in tumor tissues were increased compared to those in normal tissues (Fig. 5). KLK6 overexpression promoted the growth of gastric cancer, while KLK6 inhibition blocked tumor growth (Fig. 6A-C). H&E staining revealed that KLK6 increased tumor progression (Fig. 6D). In addition, the expression of EMT-related proteins in tumors was measured. Compared to the CON and negative controls, the protein expression of EP-CAM and vimentin and the phosphorylation of SMAD2, and SMAD3 was downregulated by sh-KLK6, whereas that of E-cadherin was upregulated, with OV-KLK6 showing the opposite trend (Fig. 6E).
Figure 5.
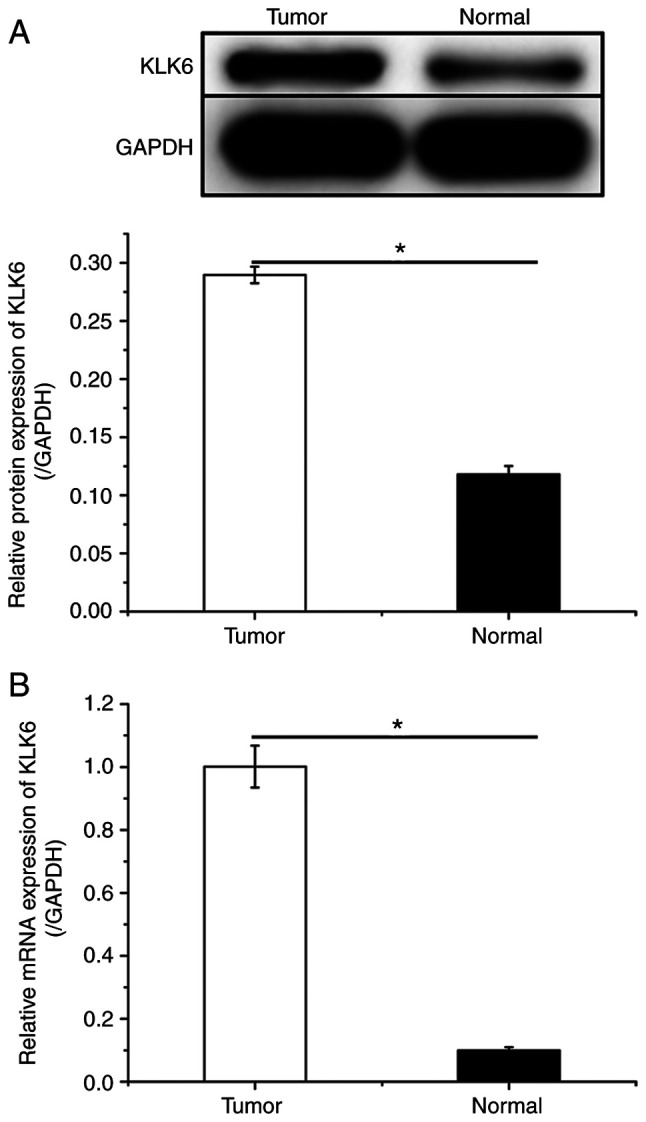
KLK6 is highly expressed in HGC-27-induced xenograft tumor tissue. Relative (A) protein and (B) mRNA expression levels of KLK6 in HGC-27-induced tumor tissue and normal tissue. Data are presented as the mean ± SD (n=3). KLK6, kallikrein-related peptidase 6. *P<0.05.
Figure 6.
KLK6 inhibition suppresses tumor growth. (A) Representative photographs, (B) volumes and (C) weights of corresponding tumors. (D) Pathological morphology of xenografted tumors. (E) Relative protein expression levels of EP-CAM, E-cadherin and vimentin and levels of phosphorylation of SMAD2 and SMAD3 in xenografted tumors. Data are presented as the mean ± SD (n=3). KLK6, kallikrein-related peptidase 6; EMT, epithelial-to-mesenchymal transition; E-cadherin, epithelial cadherin; EP-CAM, epithelial cell adhesion molecule; p-, phosphorylated; Con, untreated control; sh, short hairpin; NC, non-coding control; OV, overexpression plasmid; n.s., not significant. *P<0.05.
Discussion
In the present study it was demonstrated that KLK6 was expressed at significantly higher levels in metastatic gastric cancer cells (HGC-27) than in primary gastric cancer cells (AGS and SNU-1). KLK6 interference inhibited the proliferation, migration and invasion of gastric cancer cells in vitro and suppressed the growth of gastric cancer cell line tumors in vivo. In addition, KLK6 interference attenuated EMT in gastric cancer cells by regulating the expression and phosphorylation of EMT-related proteins (E-cadherin, vimentin, EP-CAM, SMAD2 and SMAD3). KLKs are a family of serine proteases that contains 15 members, which are associated with various physiological functions (18). Several KLKs, including KLK6, are involved in neoplastic malignant progression and transformation, through regulation of tumor chemoresistance, migration, invasion and growth (18,19). Aberrant expression of KLK6 is common in a number of malignancies, including head and neck squamous cell carcinoma (20) and colorectal (21), ovarian (22) and melanoma skin cancer (23). KLK6 is highly expressed in patients with head and neck squamous cell carcinoma and modulates cancer cell migration, invasion and chemoresistance by regulating EMT (20). KLK6 is also associated with drug resistance in gastric cancer. Kim et al (24) reported that KLK6 expression resulted in chemoresistance by inhibiting auranofin-induced apoptosis via autophagy activation in gastric cancer. In addition, KLK6 has been reported to be highly expressed in gastric cancer (15) and acts as a prognostic indicator for gastric cancer (25,26). Zhu et al (27) found that KLK6 facilitated gastric cancer cell migration, invasion and growth, consistent with the findings of the present study. Additionally, the present study demonstrated that KLK6 enhanced EMT in gastric cancer cells, both in vivo and in vitro, and promoted the progression of gastric cancer cell tumors in vivo.
EMT is a biological process in which epithelial cells transform to adopt a mesenchymal phenotype, resulting in the loss of cell-cell adhesion and cell polarization and in the acquisition of migration and invasion abilities (28). This process occurs during tissue regeneration, organ fibrosis and wound healing (29). EMT has been demonstrated to be a vital mechanism that causes epithelial cancer cells to acquire the migratory and invasive properties associated with metastatic characteristics (30). During EMT, the expression of epithelial markers, including E-cadherin, a type I classical cadherin that plays important roles in intercellular interactions (31), is suppressed. Meanwhile, mesenchymal markers, including vimentin, a cytoskeletal protein involved in regulating cell motility (32), are upregulated (33). The present study revealed that KLK6 interference increased E-cadherin expression and suppressed that of vimentin, suggesting an inhibitory effect of KLK6 on EMT in gastric cancer.
In addition, the present study demonstrated that KLK6 inhibition attenuated the phosphorylation of SMAD2 and SMAD3, both of which are transcription factors that are activated by transforming growth factor-β (29). Several studies have reported that SMAD2/3 signaling is associated with EMT. Tang et al (34) revealed that SMAD2/3 activation promoted EMT in lung adenocarcinoma. Kim et al (35) suggested that epidermal growth factor enhanced the phosphorylation of SMAD2/3, thereby inducing EMT in breast cancer cells. Wang et al (36) indicated that microfibril-associated protein 2 promotes EMT in gastric cancer by activating the SMAD2/3 signaling pathway. In addition, it was previously demonstrated that KLK6 activation altered the expression of EMT markers by promoting SMAD2/3 phosphorylation (11). These findings suggest that the effect of KLK6 on EMT in gastric cancer may be associated with the regulation of SMAD2/3 signaling.
In conclusion, the present study demonstrated that KLK6 is involved in the modulation of gastric cancer cell proliferation, migration and invasion via EMT regulation. The effect of KLK6 on EMT in gastric cancer is possibly mediated by SMAD2/3 signaling. Whether there are other factors involved in the mechanism of KLK6 in EMT regulation remains to be further elucidated. A limitation of the present study is that only three nude mice were used in each group for the in vivo studies, which may have affected the statistical analysis. However, all animal experiments were performed based on the findings of a preliminary experiment. More animals (at least 6 in each group) will be included in follow-up study designs, which will focus on investigating the effect of KLK6 on EMT-associated metabolic reprogramming in gastric cancer. The lack of co-immunoprecipitation experiments for validating the signaling pathway and the use of only one cell line are other limitations of this study, which will be iresolved in the follow-up study. Overall, the present findings suggest that KLK6 may be a future target for gastric cancer therapy.
Acknowledgements
Not applicable
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
DZ was responsible for the study design, as well as drafting and revision of the manuscript. YH, HL and WH performed the experiments. YH analyzed the data. DZ and YH were responsible for confirming the authenticity of raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures were approved by the Ethics Committee of Wuhan Myhalic Biotechnology Co., Ltd. (approval no. HLK-20181102-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Seo GH, Kang HY, Choe EK. Osteoporosis and fracture after gastrectomy for stomach cancer: A nationwide claims study. Medicine (Baltimore) 2018;97(e0532) doi: 10.1097/MD.0000000000010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Omran AR. The epidemiologic transition: A theory of the epidemiology of population change 1971. Milbank Q. 2005;83:731–757. doi: 10.1111/j.1468-0009.2005.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian Y, Li X, Li H, Lu Q, Sun G, Chen H. Astragalus mongholicus regulate the Toll-like-receptor 4 meditated signal transduction of dendritic cells to restrain stomach cancer cells. Afr J Tradit Complement Altern Med. 2014;11:92–96. doi: 10.4314/ajtcam.v11i3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39(22) doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gou WF, Yang XF, Shen DF, Zhao S, Liu YP, Sun HZ, Takano Y, Su RJ, Luo JS, Zheng HC. The roles of BTG3 expression in gastric cancer: A potential marker for carcinogenesis and a target molecule for gene therapy. Oncotarget. 2015;6:19841–19867. doi: 10.18632/oncotarget.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anisowicz A, Sotiropoulou G, Stenman G, Mok SC, Sager R. A novel protease homolog differentially expressed in breast and ovarian cancer. Mol Med. 1996;2:624–636. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang F, Hu ZD, Chen Y, Hu CJ. Diagnostic value of KLK6 as an ovarian cancer biomarker: A meta-analysis. Biomed Rep. 2016;4:681–686. doi: 10.3892/br.2016.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh MC, Grass L, Soosaipillai A, Sotiropoulou G, Diamandis EP. Human kallikrein 6 degrades extracellular matrix proteins and may enhance the metastatic potential of tumour cells. Tumour Biol. 2004;25:193–199. doi: 10.1159/000081102. [DOI] [PubMed] [Google Scholar]
- 10.Klucky B, Mueller R, Vogt I, Teurich S, Hartenstein B, Breuhahn K, Flechtenmacher C, Angel P, Hess J. Kallikrein 6 induces E-cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res. 2007;67:8198–8206. doi: 10.1158/0008-5472.CAN-07-0607. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Sells E, Pandey R, Abril ER, Hsu CH, Krouse RS, Nagle RB, Pampalakis G, Sotiropoulou G, Ignatenko NA. Kallikrein 6 protease advances colon tumorigenesis viainduction of the high mobility group A2 protein. Oncotarget. 2019;10:6062–6078. doi: 10.18632/oncotarget.27153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pampalakis G, Prosnikli E, Agalioti T, Vlahou A, Zoumpourlis V, Sotiropoulou G. A tumor-protective role for human kallikrein-related peptidase 6 in breast cancer mediated by inhibition of epithelial-to-mesenchymal transition. Cancer Res. 2009;69:3779–3787. doi: 10.1158/0008-5472.CAN-08-1976. [DOI] [PubMed] [Google Scholar]
- 13.Henkhaus RS, Gerner EW, Ignatenko NA. Kallikrein 6 is a mediator of K-RAS-dependent migration of colon carcinoma cells. Biol Chem. 2008;389:757–764. doi: 10.1515/BC.2008.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Xiong H, Li J, He Y, Yuan X. Correlation of hK6 expression with tumor recurrence and prognosis in advanced gastric cancer. Diagn Pathol. 2013;8(62) doi: 10.1186/1746-1596-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JJ, Kim JT, Yoon HR, Kang MA, Kim JH, Lee YH, Kim JW, Lee SJ, Song EY, Myung PK, et al. Upregulation and secretion of kallikrein-related peptidase 6 (KLK6) in gastric cancer. Tumour Biol. 2012;33:731–738. doi: 10.1007/s13277-011-0267-1. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Tang H, Xu L, Cen X, Yang L, Feng J, Li G, Zhu H, Gao S, Yu Y, Zhao Y, et al. CDK5 inhibition in vitro and in vivo induces cell death in myeloma and overcomes the obstacle of bortezomib resistance. Int J Mol Med. 2020;45:1661–1672. doi: 10.3892/ijmm.2020.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed N, Dorn J, Napieralski R, Drecoll E, Kotzsch M, Goettig P, Zein E, Avril S, Kiechle M, Diamandis EP, et al. Clinical relevance of kallikrein-related peptidase 6 (KLK6) and 8 (KLK8) mRNA expression in advanced serous ovarian cancer. Biol Chem. 2016;397:1265–1276. doi: 10.1515/hsz-2016-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talieri M, Zoma M, Devetzi M, Scorilas A, Ardavanis A. Kallikrein-related peptidase 6 (KLK6)gene expression in intracranial tumors. Tumour Biol. 2012;33:1375–1383. doi: 10.1007/s13277-012-0385-4. [DOI] [PubMed] [Google Scholar]
- 20.Schrader CH, Kolb M, Zaoui K, Flechtenmacher C, Grabe N, Weber KJ, Hielscher T, Plinkert PK, Hess J. Kallikrein-related peptidase 6 regulates epithelial-to-mesenchymal transition and serves as prognostic biomarker for head and neck squamous cell carcinoma patients. Mol Cancer. 2015;14(107) doi: 10.1186/s12943-015-0381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohlsson L, Lindmark G, Israelsson A, Palmqvist R, Öberg Å, Hammarström ML, Hammarström S. Lymph node tissue kallikrein-related peptidase 6 mRNA: A progression marker for colorectal cancer. Br J Cancer. 2012;107:150–157. doi: 10.1038/bjc.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P, Magdolen V, Seidl C, Dorn J, Drecoll E, Kotzsch M, Yang F, Schmitt M, Schilling O, Rockstroh A, et al. Kallikrein-related peptidases 4, 5, 6 and 7 regulate tumour-associated factors in serous ovarian cancer. Br J Cancer. 2018;119:1–9. doi: 10.1038/s41416-018-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krenzer S, Peterziel H, Mauch C, Blaber SI, Blaber M, Angel P, Hess J. Expression and function of the kallikrein-related peptidase 6 in the human melanoma microenvironment. J Invest Dermatol. 2011;131:2281–2288. doi: 10.1038/jid.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TW, Lee SJ, Kim JT, Kim SJ, Min JK, Bae KH, Jung H, Kim BY, Lim JS, Yang Y, et al. Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer. Oncotarget. 2016;7:85332–85348. doi: 10.18632/oncotarget.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagahara H, Mimori K, Utsunomiya T, Barnard GF, Ohira M, Hirakawa K, Mori M. Clinicopathologic and biological significance of kallikrein 6 overexpression in human gastric cancer. Clin Cancer Res. 2005;11:6800–6806. doi: 10.1158/1078-0432.CCR-05-0943. [DOI] [PubMed] [Google Scholar]
- 26.Kolin DL, Sy K, Rotondo F, Bassily MN, Kovacs K, Brezden-Masley C, Streutker CJ, Yousef GM. Prognostic significance of human tissue kallikrein-related peptidases 6 and 10 in gastric cancer. Biol Chem. 2014;395:1087–1093. doi: 10.1515/hsz-2014-0143. [DOI] [PubMed] [Google Scholar]
- 27.Zhu S, Shi J, Zhang S, Li Z. KLK6 Promotes Growth, Migration, and Invasion of Gastric Cancer Cells. J Gastric Cancer. 2018;18:356–367. doi: 10.5230/jgc.2018.18.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gloushankova NA, Zhitnyak IY, Rubtsova SN. Role of Epithelial-Mesenchymal Transition in Tumor Progression. Biochemistry (Mosc) 2018;83:1469–1476. doi: 10.1134/S0006297918120052. [DOI] [PubMed] [Google Scholar]
- 29.Liang X, He X, Li Y, Wang J, Wu D, Yuan X, Wang X, Li G. Lyn regulates epithelial-mesenchymal transition in CS-exposed model through Smad2/3 signaling. Respir Res. 2019;20(201) doi: 10.1186/s12931-019-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Fang J. Epigenetic regulation of epithelial-mesenchymal transition. Cell Mol Life Sci. 2016;73:4493–4515. doi: 10.1007/s00018-016-2303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bure IV, Nemtsova MV, Zaletaev DV. Roles of E-cadherin and Noncoding RNAs in the Epithelial-mesenchymal Transition and Progression in Gastric Cancer. Int J Mol Sci. 2019;20(20) doi: 10.3390/ijms20122870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang N, Liu D, Guo J, Sun Y, Guo T, Zhu X. Molecular mechanism of Poria cocos combined with oxaliplatin on the inhibition of epithelial-mesenchymal transition in gastric cancer cells. Biomed Pharmacother. 2018;102:865–873. doi: 10.1016/j.biopha.2018.03.134. [DOI] [PubMed] [Google Scholar]
- 33.Cevenini A, Orrù S, Mancini A, Alfieri A, Buono P, Imperlini E. Molecular Signatures of the Insulin-like Growth Factor 1-mediated Epithelial-Mesenchymal Transition in Breast, Lung and Gastric Cancers. Int J Mol Sci. 2018;19(19) doi: 10.3390/ijms19082411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y, Xuan Y, Qiao G, Ou Z, He Z, Zhu Q, Liao M, Yin G. MDM2 promotes epithelial-mesenchymal transition through activation of Smad2/3 signaling pathway in lung adenocarcinoma. OncoTargets Ther. 2019;12:2247–2258. doi: 10.2147/OTT.S185076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Kong J, Chang H, Kim H, Kim A. EGF induces epithelial-mesenchymal transition through phospho-Smad2/3-Snail signaling pathway in breast cancer cells. Oncotarget. 2016;7:85021–85032. doi: 10.18632/oncotarget.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JK, Wang WJ, Cai HY, Du BB, Mai P, Zhang LJ, Ma W, Hu YG, Feng SF, Miao GY. MFAP2 promotes epithelial-mesenchymal transition in gastric cancer cells by activating TGF-β/SMAD2/3 signaling pathway. OncoTargets Ther. 2018;11:4001–4017. doi: 10.2147/OTT.S160831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.