Abstract
Salmonella Gallinarum is one of the most important bacterial pathogens associated with diminished egg production in poultry. The aim of this study was to understand the occurrence, molecular traits and antimicrobial resistance patterns of Salmonella Gallinarum strains isolated from small-scale commercial layer flocks with low level biosecurity standards in Bangladesh. A total of 765 samples, including cloacal swabs (535), visceral organs (50), and droppings (180), were collected from chickens of 12 layer flocks in 11 districts. Salmonella Gallinarum was isolated and characterized through culture-based method, followed by biochemical tests, sero-grouping, PCR assays, sequencing, and antibiogram. The identity of biochemically detected isolates of Salmonella Gallinarum was confirmed via genus-specific 16S rRNA gene based PCR, followed by invA and spvC genes based PCR assays. Occurrence of Salmonella Gallinarum was detected in overall 25.75% (197/765) samples, with a significantly (p < 0.05) higher incidence in visceral organs (42%) in comparison to cloacal swab (24%) and droppings (26%). Sequencing and subsequent phylogenetic analysis of invA and spvC genes in representative strains of Salmonella Gallinarum revealed a close genetic lineage, with a sequence similarity of 98.05–99.21% and 97.51–99.45%, respectively, to previously published sequences of the corresponding genes from the same serogroup strains. Remarkably, 66.5% (131/197) of the isolated strains of Salmonella Gallinarum were found to be resistant to 3 to 6 antimicrobial agents, and interpreted as multidrug resistant (MDR). The findings of this study underscore an inherent need of appropriate control measures to curb the widespread incidence of MDR Salmonella Gallinarum in small-scale commercial layer flocks, thereby, facilitating enhanced egg production and further support to the food security and safety in low resource settings.
Keywords: Salmonella Gallinarum, invA and spvC genes, layer flocks, occurrence, multidrug resistance
1. Introduction
Eggs and meat from poultry are indispensable protein sources in peoples meals in Bangladesh [1]. However, the advancement in the poultry production is often interrupted by the overwhelming occurrence of infectious diseases in low resource settings of developing countries such as Bangladesh. Occurrence of these diseases, which incur a colossal loss due to less production of a quality product and also from the treatment cost, are attributable to noncompliance of good agriculture practices (GAP), low biosecurity, and inadequate hygienic measures in poultry farms [2,3]. Amongst the infectious pathogenic microbes, multidrug-resistant (MDR) Salmonella spp. has been regarded as a significant problem to the poultry sector in Bangladesh [4]. Salmonella species are rod shaped Gram-negative bacteria, which are predominantly classified as Salmonella enterica and Salmonella bongori. Salmonella enterica subsp. enterica comprises more than 1400 serovars, of which approximately 10% are reported from chicken [5,6]. Salmonella enterica subsp. enterica serovars Gallinarum and Pullorum, predominantly adapted in avian fauna, are closely related serovars, and indistinguishable by culture-based serotyping. However, stringent molecular assays such as enzyme based ribotyping and multiplex PCR method along with biochemical tests can distinguish these closely related serovars of S. enterica subsp. enterica [7,8]. Among the predominant infectious diseases in the poultry sector, Fowl Typhoid (FT) is a septicemic disease caused by Salmonella Gallinarum that mainly affects mature poultry flocks, particularly during the laying period. The disease is clinically manifested by reduced fertility, egg production, and hatchability, leading to increased mortality and considerable financial loss in poultry industries [9].
A variety of virulence factors have been reported for the pathogenic strains of Salmonella spp. Occurrence of inv genes, which aid the bacterial invasion of host cell, has been observed to be widespread, encompassing ca. 2000 serovars of Salmonella spp. [10]. This invasion gene, invA, is found to be genetically very similar among the closely related serovars of Salmonella, but remains highly conserved in the genome of Salmonella Gallinarum, and may be absent in Salmonella isolates under the serotypes: Enteritidis, Anatum, and Amsterdam [11,12]. Presence of virulence plasmid, containing pathogenic genes, including spv, is considered as an important trait for many pathogenic Salmonella serovars, viz., Typhimurium, Choleraesuis, Dublin, Enteritidis, and Gallinarum-Pullorum. The spv gene is claimed to facilitate the growth of Salmonella in the host environment by interacting with the host immune system [13,14,15]. Considering the above perspectives, PCR assays detecting the invasion gene and plasmid virulence gene might be beneficial for the rapid screening of Salmonella species, including Salmonella Gallinarum [11]. Therefore, multiplex PCR assays targeting invA and spvC genes have been developed and evaluated for the confirmation of Salmonella serovars [14,15,16,17]. At present, PCR-based confirmation of Salmonella Gallinarum targeting invA and spvC genes, using selective primers, S139-S141 [18], and SPV1-SPV2 [13], respectively, is considered to be highly efficient and suitable among the molecular detection tools [19].
In the recent decades, the escalating incidences of antimicrobial resistance (AMR) in pathogenic microbes, including Salmonella spp., has been linked to the indiscriminate use of antimicrobial drugs in poultry production [20]. Despite having an enormous potentiality of income generation activities to support the livelihood of millions of people, the recurrent infection of Salmonella spp., particularly Salmonella Gallinarum, in laying flocks has become a nascent threat for the poultry industry in Bangladesh [21]. A high incidence (53.5%) of Salmonella infections in adult layer chickens has been reported for the low resource settings in this country [22]. Moreover, a number of investigations have confirmed wide-scale occurrence of antimicrobial resistance in Salmonella spp., with variable incidence, between 20% and 100%, in poultry animals and associated environmental samples in Bangladesh [1,23,24,25,26]. Among different types of salmonellosis in poultry, Salmonella Gallinarum can cause more than 70% infection in layer chicken [27]. The economic benefit of poultry farmers is frequently disrupted due to a colossal loss in egg production, which can be attributable to MDR infection of Salmonella spp., especially Salmonella Gallinarum [10,28,29,30]. Notably, according to a previous report, majority (>80%) of the poultry-originated isolates of Salmonella spp. were resistant to commonly used antimicrobials, namely, amoxicillin, doxycycline, kanamycin, gentamicin, and tetracycline [31]. The rampant use of antimicrobials in poultry production, facilitating the emergence of multi-drug resistant (MDR) pathogens, is considered as a major concern to ‘One health’, a framework that holistically considers the health of animals, humans, and the environment while integrating the multifaceted drivers and consequences of infectious diseases [23].
Although a number of studies have revealed a widespread occurrence of Salmonella infection in poultry farms [24,26,32,33] little is known on the molecular traits and MDR patterns of Salmonella Gallinarum strains circulating among the layer flocks in Bangladesh. Without detailed information on the prevalence, and virulence traits, including AMR patterns of important pathogens, it is difficult to formulate intervention strategies to reduce disease burden in poultry production. Therefore, this surveillance study was conducted involving small-scale commercial poultry farms at different districts in five administrative divisions of Bangladesh to understand: (1) the occurrence, (2) molecular traits, and (3) diversity in antimicrobial resistance patterns of Salmonella Gallinarum in layer chicken. With respect to the development efforts aiming to enrich food security, and a more sustainable livelihood of small-scale poultry farmers, the findings of this study are considerably valuable to better understand the risks of infectious pathogens and formulating evidence-based control strategies to reduce disease burden in the poultry sector in Bangladesh and elsewhere.
2. Materials and Methods
2.1. Study Design and Location
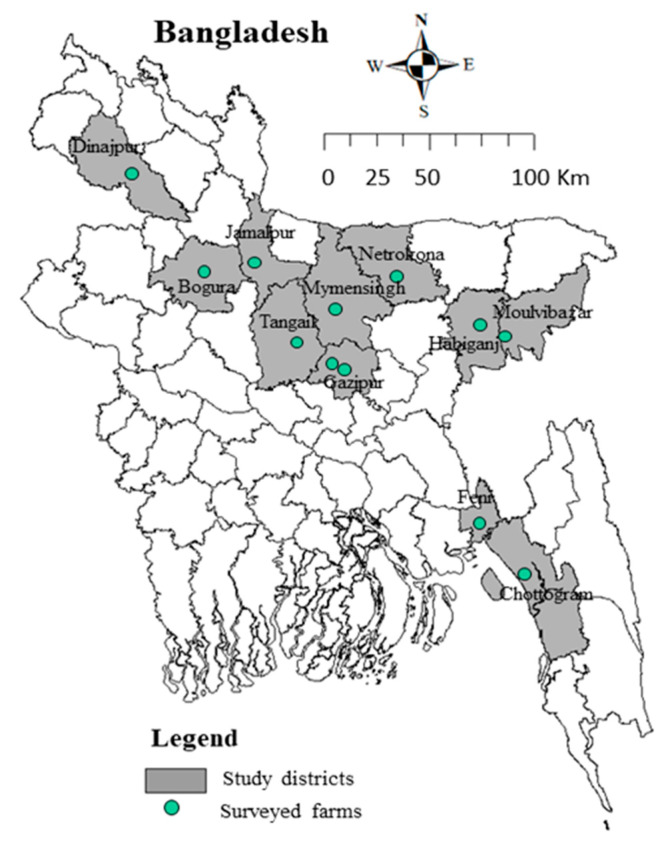
The study was conducted in 11 districts from five administrative divisions (Dhaka, Mymensingh, Rangpur, Sylhet, and Chattogram) of Bangladesh during March to September, 2020. A total of 12 small scale layer farms including three from the Dhaka division (two from Gazipur and one from Tangail districts), three from Mymensingh division (one each from Mymensingh, Jamalpur, and Netrokona districts), two from Rangpur division (one each from of Dinajpur and Bogura districts), two from Sylhet division (one each from Habiganj and Moulvibazar districts), and two from Chattogram division (one each from Chattogram and Feni districts) were enrolled (Figure 1). The farms represented the typical scenario of low-biosecurity standard, and were selected based on the following criteria: flock age of a minimum of 20 weeks, flock size >1000 birds, and lacking any vaccination measure against Salmonella Gallinarum. Details of the surveyed farms, including the type, location, and number of collected samples, are presented in Supplementary Table S1.
Figure 1.
Location of 12 layer flocks of 11 districts (five administrative divisions) of Bangladesh were taken under this survey that involved three from Dhaka, three from Mymensingh, two from Rangpur, two from Sylhet, and two from Chattogram division.
2.2. Sample Collection from the Layer Farms
The samples comprised of cloacal swabs (n = 535, 70.39%), poultry drooping (n = 180, 23.52%); and visceral organs (n = 50, 6.35%), which represented the composite samples of liver, spleen, ovary, and ovarian follicle from the dead layer birds. All samples were collected in sterile plastic containers, properly labeled and transferred in an insulated foam box maintaining cool chain at 4–6 °C to the Department of Microbiology and Hygiene laboratory of Bangladesh Agricultural University and processed within 24 h. During each sampling, information on different parameters as shown in the sample collection checklist (Supplementary Table S2) were obtained.
2.3. Isolation and Identification via Culture and Biochemical Tests
Isolation of Salmonella Gallinarum was done based on selective enrichment of the samples (cloacal swab, visceral organ, and droppings) in Rappaport Vassiliadis Soya Broth (RVS Broth) (HiMedia, Mumbai, India) following the protocol described earlier [34] with some modifications. A loopful of enriched broth was streaked on xylose lysine deoxycholate (XLD) agar media (HiMedia, Mumbai, India) and incubated at 43 ± 0.2 °C for 24 ± 2 h. Representative colonies of Salmonella cells grown on selective XLD agar media were separated individually by subculture on the same media. Thus, the obtained pure culture of each of the selected isolates were subjected to biochemical tests viz., sugar fermentation test, indole, and MR-VP tests [35]; and additionally, motility test was done using the hanging drop slide technique [36] to ascertain their identity as Salmonella spp.
2.4. Serogrouping of Salmonella Isolates
The isolates of Salmonella spp. obtained from the poultry samples were subjected to sero-grouping by rapid serum plate agglutination test (RSPAT) using commercially available Salmonella agglutinating antisera (S & A Reagents Lab Ltd., Bangkok, Thailand), and following the standard method described earlier [37].
2.5. Molecular Detection
Selected isolates of Salmonella spp. were subjected to detection by culture-based methods, and further confirmed by molecular tools, including PCR assays and sequencing. Culture lysate and DNA template of S. Gallinarum isolates were prepared using the standard procedure, as described previously [38]. A previously established 16S rRNA gene-based PCR, using primers and conditions shown in Table 1, was performed for confirmation of the genus Salmonella [39]. A reaction mixture of 25 μL volume, including 12.5 μL 2Χ master mixture, 2 μL genomic DNA, 1 μL each primer, and 8.5-μL nuclease-free water (Thermo Fisher Scientific, Waltham, MA, USA) was prepared and PCR amplification was carried out using a thermocycler (Astec, Fukuoka, Japan) as per the manufactures’ protocol. The PCR reaction comprised of an initial denaturation step at 94 °C for 5 min; followed by 30 cycles of DNA amplification, each including denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s; followed by a final extension step at 72 °C for 5 min. The PCR products were subjected to gel (2% agarose) electrophoresis (Invitrogen, Carlsbad, CA, USA), followed by staining of the gel with ethidium bromide (0.5 μg/mL) and de-staining in distilled water, each for 10 min.; and finally, the PCR amplicons in the gel were visualized under UV light, and images were captured using a gel documentation system (Biometra, Göttingen, Germany). Species-specific PCR assays, targeting invA and spvC genes, were employed to confirm the identity of Salmonella spp. isolates [13,18]. Details of the primers used in the PCR assays are listed in Table 1. Initially, a reaction mixture (25 μL) was prepared with template DNA, × 5 PremixTaq™ cradle, deoxynucleotide triphosphates (10 mM each), 25 mM MgCl2, 10 μM of each primer with Premix Taq™ DNA polymerase (Takara Bio Inc., Shiga, Japan), and nuclease-free water in volumes of 3 μL, 5 μL, 0.5 μL, 1.6 μL, 1 μL, 0.3 μL, and 12.6 μL, respectively. PCR amplification, agarose gel electrophoresis, and visualization of the PCR products were done following the standard protocol, as described previously.
Table 1.
List of primers used in this study.
| Gene | Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temperature | Reference |
|---|---|---|---|---|
| Sal 16S rRNA F | TGTTGTGGTTAATAACCGCA | 574 | 50 °C | [40] |
| Sal 16S rRNA R | CACAAATCCATCTCTGGA | |||
| invA-S139 | GTG AAATTATCGCCACGTTCGGGCAA | 284 | 64 °C | [17] |
| invA-S141 | TCATCGCACCGTCAAAGGAACC | |||
| spvC-SPV1 | ACTCCTTGCACAACCAAATGCGGA | 571 | 64 °C | [10] |
| spvC-SPV2 | TGTCTTCTGCATTTCGCCACCATCA |
2.6. Sequencing and Phylogenetic Tree Construction
PCR products of representative isolates, namely BAUSG3 and BAUSG6, were purified using the GeneJET Genomic DNA Purification Kit (Thermo Scientific™, Thermo Fisher Scientific, Waltham, MA, USA); and then sequenced using specific primers partially amplifying the invA and spvC genes (Table 1) in a Genetic Analyzer 3130 (Applied Biosystems™, Thermo Fisher Scientific, Waltham, MA, USA) as per manufacturer’s instructions. After initial quality check-up, trimming and editing, as required, the sequenced genes were uploaded in the GenBank. The ClustalW algorithm of the Molecular Evolutionary Genetics Analysis (MEGA) software (version 4.1.0) was applied to align the different sequences of the specific genes, including those obtained in this study and others selected through homology search using BLAST tool (www.ncbi.nlm.nih.gov/BLAST, accessed on 4 December 2020). Afterwards, a phylogenetic tree was constructed following the neighbour-joining (NJ) method [38]. The bootstrap test (100 replicates) was used to evaluate the percentage of replicate trees with related taxa assembled and that appeared as branches, identically [39]. The sequence identity was validated by comparison with the published sequences of the target genes available in the GenBank database (https://www.ncbi.nlm.nih.gov/, accessed on 10 January 2021).
2.7. Antimicrobial Susceptibility Screening
Antimicrobial susceptibility patterns of all isolates were determined according to the disc diffusion method [41]. A total of 13 antimicrobials, representing all the major classes, were used for this purpose. All antimicrobial discs were obtained from Oxoid, UK and applied at standard doses: Ciprofloxacin (5 μg), Neomycin (30 μg), Norfloxacin (10 μg), Levofloxacin (5 μg), Enrofloxacin (5 μg), Amoxycillin (10 μg), Amikacin (30 μg), Doxycycline (30 μg), Gentamicin (10 μg), Sulfamethoxazole (25 μg), Azithromycin (15 μg), Tetracycline (30 μg), and Fosfomycin (50 μg). The zones of growth inhibition were compared and interpreted as susceptible (S), intermediate resistant (I), or resistant (R) to the corresponding antimicrobials according to the Clinical and Laboratory Standards Institute [42]. Salmonella isolates that demonstrated resistant traits against three or more antimicrobial classes were considered as MDR [43]. Escherichia coli ATCC 25922 was used as a quality control organism. All interpretations were corroborated by conducting at least two replicates of the antimicrobial susceptibility testing by this assay.
2.8. Data Management and Statistical Analysis
All data obtained in this study were recorded in Excel spreadsheets and analyzed for descriptive statistics (frequency, proportion and 95% Confidence Interval [CI]). To estimate the variations in occurrence and antimicrobial resistance pattern of Salmonella Gallinarum, SPSS software (version 22.0, IBM Corp., Armonk, NY, USA) was used. Chi-squared test was performed where applicable to determine the level of significance of difference or association. A p value of <0.05 was considered statistically significant.
3. Results
3.1. Occurrence of Salmonella spp. and Salmonella Gallinarum
3.1.1. Sample Level Occurrence
Among the collected samples (N = 765), 28% (n = 214) were confirmed as positive for Salmonella spp. by conventional culture-based method. Among these isolates (n = 214) of Salmonella, 199 were confirmed to be Salmonella Gallinarum through selective biochemical tests, including carbohydrate fermentation, indole production, methyl red test, Voges-Proskauer reaction, and motility test. Isolates of Salmonella Gallinarum produced positive results in fermentation of glucose, maltose, and dulcitol, all without any gas production, which are typical biochmemical traits of this serovar, and useful to differentiate from closely related serovars (Table 2 and Table 3).
Table 2.
Occurrence of Salmonella isolates, determined by selective culture and biochemical tests, in different samples collected from 12 layer flocks from 5 divisions of Bangladesh.
| Method/Type of Sample | Number of Isolates | Occurrence (with 95% Confidence Interval) |
p-Value (Pearson’s Chi-Squared Test) |
|---|---|---|---|
| Selective culture (Salmonella spp.) | |||
| Cloacal swab (n = 535) | 138 | 25.8 (22.1–29.7) | 0.003 |
| Visceral organ (n = 50) | 24 | 48 (33.7–62.6) | |
| Droppings (n = 180) | 52 | 29 (22.4–36.1) | |
| Total sample (N = 765) | 214 | 28 (24.8–31.3) | |
| Biochemical identification (Salmonellla Gallinarum) | |||
| Cloacal swab (n = 138) | 130 | 24.3 (20.7–28.2) | 0.02 |
| Visceral organ (n = 24) | 21 | 42 (28.2–56.8) | |
| Droppings (n = 52) | 48 | 26.7 (20.4–33.8) | |
| Total sample (N = 214) | 199 | 26 (22.9–29.3) | |
Table 3.
Results of different biochemical test among culture positive isolates of Salmonella spp. (n = 214).
| Biochemical Tests | Salmonella Gallinarum |
Salmonella Typhimurium |
Salmonella Pullorum |
Others |
|---|---|---|---|---|
| Carbohydrate fermentation | ||||
| Glucose | + | + | + | - |
| (Acid) | (Acid and Gas) | (Acid and Gas) | - | |
| Dulcitol | + | + | - | - |
| (Acid) | (Acid) | |||
| Maltose | + | + | ± | - |
| (Acid) | (Acid and Gas) | (Acid and Gas) | ||
| Indole production | - | - | - | - |
| Methyl red test | + | + | + | - |
| Voges-Proskauer test | - | - | - | - |
| Motility | - | + | - | - |
| Total isolates | 199 | 6 | 0 | 9 |
+: Positive; -: Negative.
The 16S rRNA gene-based PCR confirmed the genus identity of 205 of 214 isolates, biochemically determined as Salmonella spp. Results of this genus-specific PCR showed that, overall, an estimated 26.80% (205 of 765) samples were contaminated with Salmonella spp., since the representative isolates from the positive samples generated the expected amplicon size of 574 bp (Table 4). However, invA and spvC-gene based PCR assays confirmed that approximately 25.80% (n = 197) samples/isolates were positive for Salmonella Gallinarum. The identity of these genes was further validated via sequencing, while phylogenetic analysis revealing their closest affinity to corresponding gene sequences reported previously from Salmonella Gallinarum strains. Observed variations in the occurrence of Salmonella spp. in different kinds of samples was found to be statistically significant (Table 2 and Table 4). Among the four kind of samples, a distinctive higher (p < 0.025) occurrence of Salmonella spp., including Salmonella Gallinarum (mean incidence rate 44% and 42%, respectively), was observed for the tested visceral organs in comparison to cloacal swab and dropping samples (mean incidence rate ca. 25 to 27% and 24 to 26%, respectively).
Table 4.
Occurrence of Salmonella isolates in different samples collected from 12 layer flocks from five divisions of Bangladesh as confirmed though molecular assays.
| Type of Sample | Number of Isolates | Occurrence (with 95% CI *) |
p-Value (Pearson’s Chi-Squared Test) |
|---|---|---|---|
| 16S rRNA gene based PCR (Salmonella spp.) | |||
| Cloacal swab (n = 535) | 134 | 25 (21.4–28.9) | 0.015 |
| Visceral organ (n = 50) | 22 | 44 (30–58.7) | |
| Droppings (n = 180) | 49 | 27.2 (20.9–34.3) | |
| Total sample (N = 65) | 205 | 26.8 (23.7–30.1) | |
| invA and spvC gene based PCR (Salmonella Gallinarum) | |||
| Cloacal swab (n = 535) | 129 | 24.1 (20.5–28) | 0.02 |
| Visceral organ (n = 50) | 21 | 42.0 (28.2–56.8) | |
| Droppings (n = 180) | 47 | 26.1 (20–33.2) | |
| Total sample (N = 765) | 197 | 25.8 (22.7–29) | |
* CI: Confidence Interval.
Of 205 isolates of Salmonella spp., when subjected to serogrouping using fur commercially available types of antisera in rapid serum plate agglutination test (RSPAT), the Salmonella spp. was classified into three serogroups, viz, with exclusive dominance of Group D (n = 199, 97%), and minor presence of Group B (n = 4, 2%) and Group C (n = 2, 1%) strains (Table 5).
Table 5.
Results of serogrouping in Salmonella positive isolates (n = 205).
| Isolate (n) | No. of Serogroup (%) | |||
|---|---|---|---|---|
| Poly A-I | Group B (O: 4, 5, 27) |
Group C (O: 6, 7, 8, 14, 20) |
Group D (O: 9, 46) |
|
| Salmonella spp. (205) | 205 (100) | 4 (2%) | 2 (1%) | 199 (97%) |
O: Somatic antigen of Salmonella.
3.1.2. Farm and Division Level Occurrence
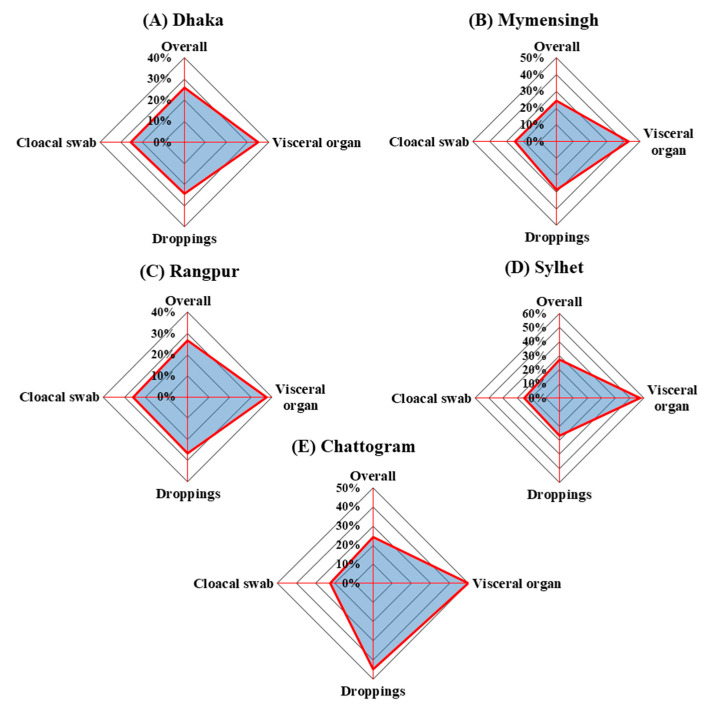
Occurrence of Salmonella Gallinarum was detected in 25.90 (57/220)%, 24.60 (41/61)%, 26.80(37/138)%, 27.40(32/117)%, and 24.40(30/123)% samples collected from Dhaka, Mymensingh, Rangpur, Sylhet, and Chattogram divisions, respectively, with an average occurrence of 25.8% (95% CI: 22.7–29). The observed variations in division-wise occurrence of the bacterium was found to be statistically non-significant (p = 0.97) (Figure 2). One the other hand, of the 12 flocks surveyed in this study, 75% (9/12) were found to be positive for Salmonella Gallinarum (Supplementary Table S1). However, the occurrence of Salmonella Gallinarum in different samples (cloacal swab, droppings, and visceral organ) at individual farms showed more wide-ranging variations, i.e., from 0% to 56.9% of total samples (Figure 2).
Figure 2.
Occurrence of Salmonella Gallinarum in different samples (cloacal swab (n = 535), visceral organ (n = 50) and drooping (n = 180)) collected from 12 layer flocks of five divisions: (A) Dhaka, (B) Mymensingh, (C) Rangpur, (D) Sylhet, and (E) Chattogram. Overall occurrence of Salmonella Gallinarum was estimated as 25.9%, 24.6%, 26.8%, 27.4%, and 24.4% in Dhaka, Mymensingh, Rangpur, Sylhet, and Chattogram divisions, respectively.
3.2. Sequencing and Phylogenetic Analysis
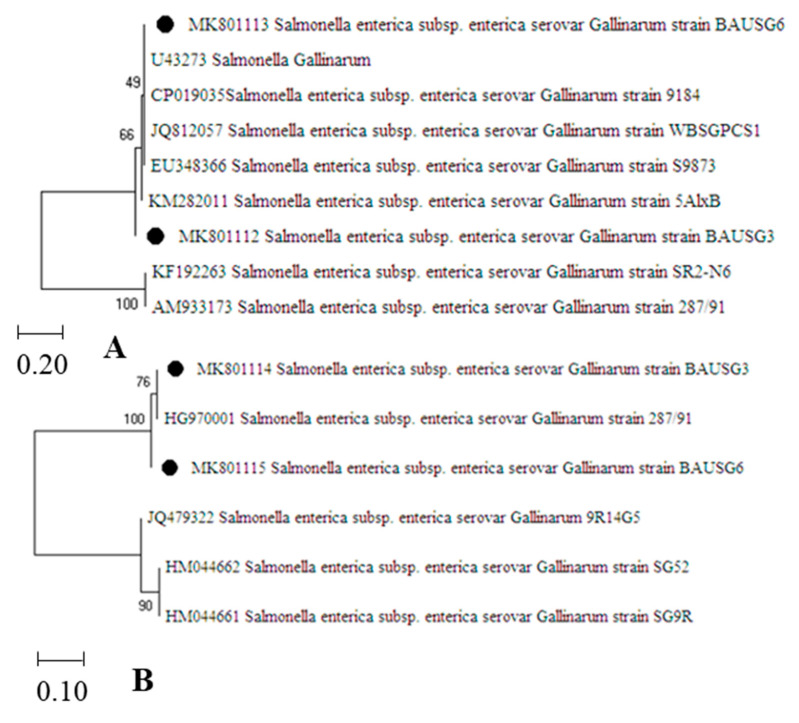
Gene (invA and spvC) based PCR assays, followed by sequencing and phylogenetic analysis of the representative target genes confirmed the identity of Salmonella Gallinarum strains. BLAST analysis confirmed a 100% similarity of invA gene between the isolated strains of Salmonella Gallinarum, namely BAUSG3 and BAUSG6. These nucleotide sequences of invA gene showed a close homology between 99.21% and 98.05% to earlier reported strains isolated from India (Accession number: JQ812057.1), China (EU348366.1), Korea (KF192263.1), Egypt (KM282011.1), UK(AM933173.1), and the USA (CP019035.1) (Figure 3A). Phylogenetic analysis of invA genes revealed the representative strains clustering to a lineage, which comprised of five previously published sequences of Salmonella Gallinarum strains from different countries. Similarly, nucleotide sequences of the spvC genes of Salmonella Gallinarum strains (BAUSG3 and BAUSG6) were found to be closely related with the previously published sequences of spvC of the same serogroup strains isolated from Korea and the UK (Figure 3B). According to BLAST analysis, a 97.8% similarity with five mismatches between the nucleotide sequences of the spvC genes was observed for the isolated strains, BAUSG3 and BAUSG6. However, these spvC genes showed between 99.82% and 97.51% homology to previously published sequences of this gene in strains isolated from different countries. The nucleotide sequences generated in this study were submitted in the GenBank and are available under accession numbers: MK801112 and MK801113 (invA1 and invA2, respectively) and MK801114 and MK801115 (spvC1 and spvC2, respectively).
Figure 3.
Phylogenetic analysis of (A) invasive gene (invA) and (B) plasmid virulence gene (spvC) of Salmonella Gallinarum strains (BAUSG3 and BAUSG6). The phylogenetic tree was constructed using the Neighbour-Joining (NJ) method with comparative alignment of 266 nucleotides of invA; and 572 nucleotides of spvC. BAUSG3 and BAUSG6 are marked as black circles in the phylogenetic tree, comparing their affinity to selected sequences of the target genes downloaded from the GenBank.
3.3. Antibiogram
3.3.1. Antimicrobial Susceptibility Status
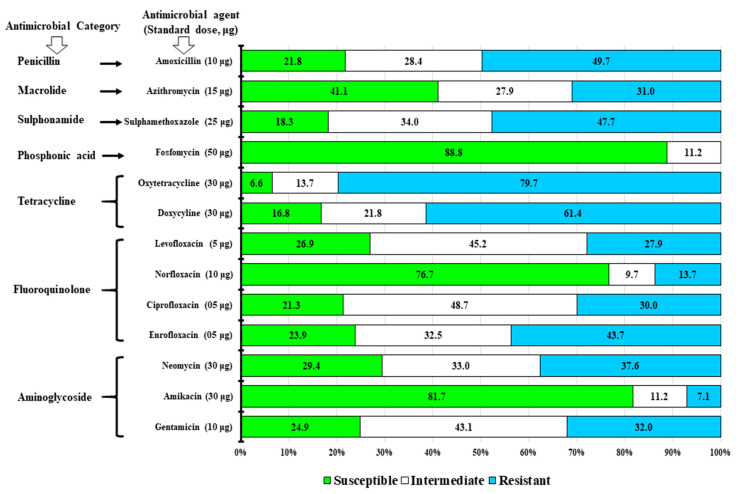
Among 197 isolated strains of Salmonella Gallinarum, confirmed by the PCR assays, 88.8%, 81.7%, and 76.7% were found to be susceptible to Fosfomycin, Amikacin, and Norfloxacin, respectively, while 48.7%, 45.2%, and 43.1% isolates could be considered as intermediately resistant to Ciprofloxacin, Levofloxacin, and Gentamicin, respectively. On the other hand, 79.7%, 61.4%, 49.7%, 47.7%, 43.7%, and 37.6% isolates of this serogroup were found to be fully resistant to Oxytetracycline, Doxycycline, Amoxycillin, Sulfamethoxazole, Enrofloxacin, and Neomycin, respectively (Figure 4).
Figure 4.
Antimicrobial susceptibility test results of Salmonella Gallinarum (n = 197) isolated from the non-vaccinated layer flocks. A total of 13 antimicrobial agents under seven groups of antimicrobials were used at standard doses (μg): Amoxycillin (10 µg), Azithromycin (15 µg), Sulfamethoxazole (25 µg), Fosfomycin (50 µg), Oxytetracycline (30 µg), Doxycycline (25 µg), Levofloxacin (5 µg), Norfloxacin (10 µg), Ciprofloxacin (5 µg), Enrofloxacin (5µg), Neomycin (30 µg), Amikacin (30 µg), and Gentamicin (10 μg). Three categories (susceptible, intermediate, and resistant) of the resistant pattern in accordance to Clinical and Laboratory Standards Institute (CLSI) [42] were estimated.
3.3.2. Antimicrobial Resistance Pattern
Among the isolated strains (n = 197) of Salmonella Gallinarum, 19.9% (n = 39) were observed to be resistant to one of the antimicrobial agents (OT, SXT, AMX, DO), whereas 13.7% (n = 27) showed resistance against two antimicrobials (OT-SXT and OT-AMX). Notably, a high prevalence of multidrug resistance (MDR), comprising 66.5% (131/197) of isolated strains, was observed. The MDR strains of Salmonella Gallinarum showed diverse patterns considering their resistance traits against three to six antimicrobial agents. Among these MDR strains, the observed resistance traits against three and four antimicrobials could be differentiated into two and one pattern(s), namely, OT-CN-AMX (11.7%, n = 23) and CIP-DO-AZM (9.6%, n = 19), and ENR-OT-SXT-AMX (8.1%, n = 16), respectively. Similarly, the MDR traits characterized by resistance to five antimicrobials were found to comprise of two patterns, N-DO-SXT-AZM-AMX (9.6%, n=19) and GEN-LEV-OT-SXT-AMX (8.1%, n = 16). The MDR strains showing resistance against six antimicrobials, could be also differentiated into two patterns: N-LEV-AZM-OT-SXT-AMX (10.7%, n = 21) and CN-N-ENR-DO-SXT-AMX (8.6%, n = 17) (Table 6).
Table 6.
Antimicrobial resistance patterns of Salmonella Gallinarum isolated from the layer flocks.
| Resistance Against Antimicrobials | Resistance Patterns | Salmonella Gallinarum Isolates (n = 197) | |
|---|---|---|---|
| No. (%) of Strains | Subtotal (No. (%)) |
||
| Against one to two antimicrobial agents | |||
| Against one | OT | 15 (7.6) | 39 (19.9) |
| SXT | 9 (4.6) | ||
| AMX | 7 (3.6) | ||
| DO | 8 (4.1) | ||
| Against two | OT, SXT | 9 (4.6) | 27 (13.7) |
| OT, AMX | 18 (9.1) | ||
| Against three or more antimicrobial agents (multidrug resistance) | |||
| Against three | CIP, DO, AZM | 19 (9.6) | 42 (21.3) |
| OT, CN, AMX | 23 (11.7) | ||
| Against four | ENR, OT, SXT, AMX | 16 (8.1) | 16 (8.1) |
| Against five | N, DO, SXT, AZM, AMX | 19 (9.6) | 35 (17.7) |
| GEN, LEV, OT, SXT, AMX | 16 (8.1) | ||
| Against six | CN, N, ENR, DO, SXT, AMX | 17 (8.6) | 38 (19.3) |
| N, LEV, AZM, OT, SXT, AMX | 21 (10.7) | ||
| Against three or more antimicrobials | 131 (66.5) | ||
N: Neomycin; GEN: Gentamicin; ENR: Enrofloxacin; CIP: Ciprofloxacin; LEV: Levofloxacin; DO: Doxycycline; OT: Oxytetracycline; SXT: Sulfamethoxazole; AZM: Azithromycin, and AMX: Amoxicillin.
4. Discussion
Salmonellosis is considered as one of the major causes of decreased meat and egg production in commercial poultry. The occurrence of disease has been reported to be connected with inadequate biosecurity measures in poultry farming practices in Bangladesh [20,44]. It has been difficult to evaluate the actual disease burden and adopt appropriate interventions in small-scale commercial poultry farms of Bangladesh due to limited systematic information on decreased egg production in layer flocks associated with salmonellosis, particularly, Salmonella Gallinarum infection. Of the surveyed farms scattered over different districts in five divisions in Bangladesh, a huge majority (75%, 9/12 farms) of the layer flocks were found infected with Salmonella Gallinarum, which could be connected to decreased egg production [18,29,45]. Considering its significance, Salmonella Gallinarum infection in the poultry sector has been included within the important notifiable diseases of the World Organization for Animal Health (OIE), whereas there has been an increased attention to implement a strict control measures regarding the import of birds and eggs [46].
Observations made in this study showed the occurrence of Salmonella spp., detected by biochemical tests and genus-specific 16S rRNA assay, in 26.8% (205/765, 95% CI: 23.7–30.1) samples of layer flocks. Interestingly, an overwhelming occurrence in these samples, 25.8% (197/765, 95% CI: 22.7–29), of Salmonella Gallinarum, confirmed by differential biochemical tests and molecular assays targeting virulence genes, invA and spvC was revealed. The observed large-scale contamination in visceral organs of Salmonella Gallinarum can be related to the typical invasive feature of Fowl Typhoid (FT), which causes lesions in multiple organs, including liver, heart, spleen, ovary, and intestine. In congruence, a higher prevalence of this virulent serogroup in visceral organs (42%, 21/50) in comparison to dropping (26.1%, 47/180) and cloacal swabs (24.1%, 129/535) was notable. A ubiquitous occurrence of invA and spvC genes observed for the isolated strains (n = 197) of Salmonella Gallinarum is in congruence to their prevalence reported previously among the virulent serotypes of S. enterica [10]. The observed similarity in the sequences of virulence invA and spvC genes of Salmonella Gallinarum strains obtained in this study indicates their genetic relatedness to the Salmonella Gallinarum strains isolated from poultry sources in different counties, including India, China, Korea, Egypt, UK, and the USA. Sequencing of invA and spvC in a higher number of Salmonella Gallinarum strains would enrich our knowledge to better understand the evolutionary trend linked to the geospatial prevalence of these virulent genes in Salmonella spp.
The overall occurrence of Salmonella Gallinarum in 25.8% (197/765) of the samples is lower than a previously reported occurrence in 53.5% of the samples of layer flocks in Bangladesh [21]. However, as observed in the present study, this pathogenic serovar can contaminate the majority samples (at least 57%) of the poultry flocks in an individual farm. On the other hand, a variable occurrence, between 11.5% and 24%, of Salmonella spp. reported for poultry samples in different geographical locations, e.g., France, Japan, Tanzania, and United Kingdom, respectively [47,48,49,50], could be attributable to geo-climatic variations. Nonetheless, differential anthropogenic risk factors, namely, age of the birds, flock size, feed, hygienic condition of the farm, and environmental determinants, including transmission from poultry litter, pest, and rodent may influence the preponderance of pathogenic microbes such as Salmonella Gallinarum in small-scale poultry farms [51,52].
Among the potential biosecurity interventions, systematic vaccination programs based on surveillance studies on the pathogenic microbes circulating in a particular region is a key approach to effectively control the infection of virulent strains, including Salmonella serotypes, in poultry flocks [53]. The poultry farms surveyed in this study were selected based on their non-vaccinated status, imposing a higher likelihood of diseases from Salmonella Gallinarum in reared layer flocks [54]. At present, a few imported commercial vaccines are available for immunization of layer poultry flocks in Bangladesh. Notably the SG 9R strain of Salmonella Gallinarum is being used as FT vaccine (viz. Nobilis® SG 9R, MSD Animal Health). However, this vaccine may not be antigenically well-matched with the circulating strains. Phylogenetic analysis of the nucleotide sequences of a virulence gene, spvC, obtained from a couple of Salmonella Gallinarum strains of this study, displayed its close relatedness to that of the SG 9R strain (accession number: HM044661). This kind of locally adapted strain could be considered as a more potential candidate strain to develop antigenically well-matched vaccine for Salmonella Gallinarum strains circulating in Bangladesh.
The rise of MDR among Salmonella spp. is a growing concern worldwide, particularly in developing countries, where multiple antibiotics are indiscriminately used at poultry farms for enhanced production [19,55,56,57]. The present study showed that 48.7%, 45.2%, and 43.1% isolates were intermediately resistant to Ciprofloxacin, Levofloxacin, and Gentamcin, respectively, however, 79.7%, 61.4%, 49.7%, 47.7%, 43.7%, and 37.6% isolates were fully resistant to Oxytetracycline, Doxycycline, Amoxycillin, Sulfamethoxazole, Enrofloxacin, and Neomycin. These findings are in congruence to the results of earlier studies [25,57,58,59]. Bangladesh is already included among the countries at high risk of AMR, according to the WHO [60]. Likewise, this study reporting 66.5% (n = 131) Salmonella Gallinarum isolates as resistant to three to six antimicrobials, indicates an alarming consequence to the extensive use of different antibiotic classes in layer flocks.
The low biosecurity standards in majority (>60%) of the small-scale commercial poultry farms, together with no vaccination status, and unscrupulous use of antibiotics might have substantiated the high occurrence of MDR strains of Salmonella Gallinarum in the layer flocks in Bangladesh [51,61]. Apart from further infections caused by this kind of MDR Salmonella Gallinarum strains, reportedly also resistant to prophylactic antibiotics [62], exposure of residual antimicrobials through the food chain is considered to impose a significant hazard to public health [63]. Therefore, training programs to enrich farmers’ knowledge on Good Agriculture Practices (GAP) including prudent use of antibiotics, immunization of layer birds and other biosecurity measures, including hygiene, water and waste management [24,64,65], would be vital to lessen the Salmonella Gallinarum infection in poultry farms.
The limited sampling scheme employed for each of the study farms did not capture any temporal variation in the occurrence of Salmonella Gallinaruam in layer flocks. Because of high phenotypic similarity between the closely related serovars, Salmonella Gallinarum and Salmonella Pulloram, a number of biochemical tests and serotyping were required to substantiate molecular detection targeting a couple of genes, including their sequencing and phylogenetic interpretation, of Salmonella Gallinarum isolates under this study. Culture-based biochemical identification methods have some inherent drawbacks, e.g., being labor-intensive and time consuming (requiring five to seven days). Another limitation of the methodological approach was the qualitative estimation of the bacterial occurrence, whereas a quantitative approach could capture more detail of the variations. On the other hand, sequencing and phylogenetic analysis of the target genes were done for only a few isolates. These could be considered as the primary limitations or constrains of this study.
5. Conclusions
The present study clearly shows a widespread occurrence of Salmonella Gallinarum strains with MDR traits in small-scale commercial layer flocks in all the major divisions of Bangladesh. The updated information on AMR patterns of Salmonella spp. circulating in the poultry flocks will contribute in efforts to lessen the multi-spectrum hazards, including treatment failure and production loss associated with the large-scale infection of MDR Salmonella Gallinarum in the poultry sector. Results obtained from the surveyed farms indicate an indispensable need of promoting biosecurity measures, and farmers’ training on GAP, including strict vaccination, and prudent use of antimicrobials. This study will eventually benefit the policy makers in divulging a strategic framework for the improvement of farmers’ livelihood, health and food security and safety in the context of the alarming MDR infections in poultry flocks at low resource settings.
Acknowledgments
We would like to thank Department of Microbiology and Hygiene, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh, for the support during conducting the research. We would like to acknowledge for assistance in this research from Department of Microbiology, Hajee Mohammad Danesh Science and Technology University, Dinajpur 5200, Bangladesh. We are also very grateful to the National Institute of Biotechnology, Savar, Dhaka, Bangladesh for the sequencing and phylogenetic tree analysis of Salmonella Gallinarum isolates. We are also thankful to the layer farmers and farm attendants for their kind co-operation during sample collection.
Supplementary Materials
The data that support the findings of this study are available online at https://www.mdpi.com/article/10.3390/vetsci8050071/s1. Table S1: Description of the surveyed farms with type of sample collection and farm level positivity status (N = 12), and Supplementary Table S2: Sample collection checklist.
Author Contributions
Conceptualization, M.R.A. and S.M.L.K.; methodology, A.K.M.Z.H. and J.A.; data analysis: A.K.M.Z.H. and S.S.I.; writing—original draft preparation, A.K.M.Z.H. and S.S.I.; writing—review and editing, S.B.N., S.Y. and S.M.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by a project (Project No. 2019/5/MoST) funded by the Ministry of Science and Technology, the Government of the People’s Republic of Bangladesh, Bangladesh Secretariat, Dhaka 1000, Bangladesh.
Institutional Review Board Statement
The Ethical Committee of the Bangladesh Agricultural University approved the study under reference No.: AWEEC/BAU/2020(24).
Informed Consent Statement
The farms were selected as per direction of the district and sub-district livestock officers of the Department of Livestock Services (DLS) under the Ministry of Fisheries and Livestock, Bangladesh, and the farmers’ willingness to participate in this study. Moreover, a written consent was taken prior to sample collection from each of the layer farmers of surveyed farms and to publish this paper using the outcomes of the sample test results.
Data Availability Statement
All data relevant to this study are included in this manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoque M.N., Mohiuddin R.B., Khan M.M.H., Hannan A., Alam M.J. Outbreak of Salmonella in poultry of Bangladesh and possible remedy. J. Adv. Biotechnol. Exp. Ther. 2019;2:87–97. doi: 10.5455/jabet.2019.d30. [DOI] [Google Scholar]
- 2.Hafez H.M. European perspectives on the control and eradication of some poultry diseases. In: Tserveni-Goussi A., Yanna-kopoulos A., Fortomaris P., Arsenos G., Sossidou E., editors. Advances and Challenges in Poultry Science. University Studio Press; Thessaloniki, Greece: 2008. [Google Scholar]
- 3.Dar M.A., Mumtaz P.T., Bhat S.A., Nabi M., Taban Q., Shah R.A., Khan H.M., Ahmed H.M. In: Genetics of Disease Resistance in Chicken, Application of Genetics and Genomics in Poultry Science. Liu X., editor. IntechOpen; London, UK: 2018. [(accessed on 4 March 2021)]. Available online: https://www.intechopen.com/books/application-of-genetics-and-genomics-in-poultry-science/genetics-of-disease-resistance-in-chicken. [Google Scholar]
- 4.Haider M., Chowdhury E., Khan M., Hossain M., Rahman M., Song H., Hossain M. Experimental Pathogenesis of Pullorum Disease with the Local Isolate of Salmonella enterica serovar. enterica subspecies Pullorum in Pullets in Bangladesh. Korean J. Poult. Sci. 2009;35:341–350. doi: 10.5536/KJPS.2009.35.4.341. [DOI] [Google Scholar]
- 5.Popoff M.Y., Bockemühl J., Brenner F.W. Supplement 1998 (no. 42) to the Kauffmann-White scheme. Res. Microbiol. 2000;151:63–65. doi: 10.1016/S0923-2508(00)00126-1. [DOI] [PubMed] [Google Scholar]
- 6.Popoff M.Y., LeMinor L. Antigenic Formulas of the Salmonella Serovars. 7th revision ed. World Health Organization Collaborating Centre for Reference and Research on Salmonella, Pasteur Institute; Paris, France: 1997. [Google Scholar]
- 7.Christensen J.P., Olsen J., Bisgaard M. Ribotypes of Salmonella enterica serovar Gallinarum biovars gallinarum and pullorum. Avian Pathol. 1993;22:725–738. doi: 10.1080/03079459308418960. [DOI] [PubMed] [Google Scholar]
- 8.Xiong D., Song L., Pan Z., Jiao X. Identification and Discrimination of Salmonella enterica Serovar Gallinarum Biovars Pullorum and Gallinarum Based on a One-Step Multiplex PCR Assay. Front. Microbiol. 2018;9:1718. doi: 10.3389/fmicb.2018.01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivaprasad H.L., Barrow P.A. In: Pullorum Disease and Fowl Typhoid. Diseases of Poultry. 12th ed. Saif Y.Y.M., Fadley A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Blackwell Publishing; Oxford, UK: 2008. pp. 620–634. [Google Scholar]
- 10.Chiu C.H., Ou J.T. Rapid identification of Salmonella serovars in feces by specific detection of virulence genes, invA and spvC, by an enrichment broth culture-multiplex PCR combination assay. Clin. Microbiol. 1996;34:2619–2622. doi: 10.1128/JCM.34.10.2619-2622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulig P.A., Danbara H., Guiney D.G., Lax A.J., Norel F., Rhen M. Molecular analysis of spv virulence genes of the Salmonella virulence plasmids. Mol. Microbiol. 1993;7:825–830. doi: 10.1111/j.1365-2958.1993.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 12.Malorny B., Hoorfar J., Bunge C., Helmuth C.B.R. Multicenter validation of the analytical accuracy of Salmonella PCR: Towards an international standard. Appl. Environ. Microbiol. 2003;69:290–296. doi: 10.1128/AEM.69.1.290-296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galán J.E., Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulig P.A., Caldwell A.L., Chiodo V.A. Identification, genetic analysis and DNA sequence of a 7.8-kb virulence region of the Salmonella typhimurium virulence plasmid. Mol. Microbiol. 1992;6:1395–1411. doi: 10.1111/j.1365-2958.1992.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 15.Ou J.T., Baron L.S., Dai X.Y., Life C.A. The virulence plasmids of Salmonella serovars typhimurium, choleraesuis, dublin, and enteritidis, and the cryptic plasmids of Salmonella serovars copenhagen and sendai belong to the same incompatibility group, but not those of Salmonella serovars durban, gallinarum, give, infantis and pullorum. Microb. Pathog. 1990;8:101–107. doi: 10.1016/0882-4010(90)90074-z. [DOI] [PubMed] [Google Scholar]
- 16.Turki Y., Mehr I., Ouzari H., Khessairi A., Hassen A. Molecular typing, antibiotic resistance, virulence gene and biofilm formation of different Salmonella enterica serotypes. J. Gen. Appl. Microbiol. 2014;60:123–130. doi: 10.2323/jgam.60.123. [DOI] [PubMed] [Google Scholar]
- 17.Rahn K., De Grandis S., Clarke R., McEwen S., Galan J., Ginocchio C., Curtiss Iii R., Gyles C. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol. Cell. Probes. 1992;6:271–279. doi: 10.1016/0890-8508(92)90002-F. [DOI] [PubMed] [Google Scholar]
- 18.Shivaprasad H. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 2000;19:405–416. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- 19.Kang M.S., Kim A., Jung B.Y., Her M., Jeong W., Cho Y.M., Oh J.Y., Lee Y.J., Kwon J.H., Kwon Y.K. Characterization of antimicrobial resistance of recent Salmonella enterica serovar Gallinarum isolates from chickens in South Korea. Avian Pathol. 2010;39:201–205. doi: 10.1080/03079451003767261. [DOI] [PubMed] [Google Scholar]
- 20.Kabir S.M.L. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman M.A., Samad M.A., Rahman M.B., Kabir S.M.L. Bacterio-pathological studies on salmonellosis, colibacillosis and pasteurellosis in natural and experimental infections in chickens. Bangladesh J. Vet. Med. 2004;2:1–8. doi: 10.3329/bjvm.v2i1.1926. [DOI] [Google Scholar]
- 22.Saha A., Sufian M., Hossain M., Hossain M. Salmonellosis in layer chickens: Pathological features and isolation of bacteria from ovaries and inner content of laid eggs. J. Bangladesh Agric. Univ. 2012;10:61–67. doi: 10.3329/jbau.v10i1.12095. [DOI] [Google Scholar]
- 23.Al Mamun M.A., Kabir S.M.L., Islam M.M., Lubna M., Islam S.S., Akhter A.H.M.T., Hossain M.M. Molecular identification and characterization of Salmonella species isolated from poultry value chains of Gazipur and Tangail districts of Bangladesh. Afr. J. Microbiol. Res. 2017;11:474–481. [Google Scholar]
- 24.Mridha D., Uddin M.N., Alam B., Akhter A.H.M.T., Islam S.S., Islam M.S., Khan M.S.R., Kabir S.M.L. Identification and characterization of Salmonella spp. from samples of broiler farms in selected districts of Bangladesh. Vet. World. 2020;13:275. doi: 10.14202/vetworld.2020.275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvej M.S., Nazir K.N.H., Rahman M.B., Jahan M., Khan M.F.R., Rahman M. Prevalence and characterization of multi-drug resistant Salmonella Enterica serovar Gallinarum biovar Pullorum and Gallinarum from chicken. Vet. World. 2016;9:65. doi: 10.14202/vetworld.2016.65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferdous T.A., Kabir S.M.L., Amin M.M., Hossain K.M.M. Identification and antimicrobial susceptibility of Salmonella species isolated from washing and rinsed water of broilers in pluck shops. Int. J. Anim. Veter. Adv. 2013;5:1–8. doi: 10.19026/ijava.5.5569. [DOI] [Google Scholar]
- 27.Berchieri Júnior A., Oliveira G.H.D., Pinheiro L.A.S., Barrow P.A. Experimental Salmonella Gallinarum infection in light laying hen lines. Braz. J. Microbiol. 2000;31:50–52. doi: 10.1590/S1517-83822000000100012. [DOI] [Google Scholar]
- 28.Khan M.F.R., Rahman M.B., Khan M.S.R., Nazir K.H.M.N.H., Rahman M. Antibiogram and plasmid profile analysis of isolated poultry Salmonella of Bangladesh. Pak. J. Biol. Sci. 2005;8:1614–1619. doi: 10.3923/pjbs.2005.1614.1619. [DOI] [Google Scholar]
- 29.Im M.C., Jeong S.J., Kwon Y.-K., Jeong O.-M., Kang M.-S., Lee Y.J. Prevalence and characteristics of Salmonella spp. isolated from commercial layer farms in Korea. Poult. Sci. 2015;94:1691–1698. doi: 10.3382/ps/pev137. [DOI] [PubMed] [Google Scholar]
- 30.Al-Salauddin A.S., Hossain M.F., Dutta A., Mahmud S., Islam M.S., Saha S., Kabir S.M.L. Isolation, identification, and antibiogram studies of Salmonella species and Escherichia coli from boiler meat in some selected areas of Bangladesh. Int. J. Basic Clin. Pharmacol. 2015;4:1000. doi: 10.18203/2319-2003.ijbcp20150881. [DOI] [Google Scholar]
- 31.Ansarey F.H. Prospects of poultry industry in Bangladesh; Proceedings of the Seminar and Reception on Animal Husbandry Education and Profession in Bangladesh—A Journey of 50 Years, (AHEPB’12); Dhaka, Bangladesh. 2012; pp. 62–65. [Google Scholar]
- 32.Van Schothorst M., Renaud A., Van Beek C. Salmonella isolation using RVS broth and MLCB agar. Food Microbiol. 1987;4:11–18. doi: 10.1016/0740-0020(87)90014-1. [DOI] [Google Scholar]
- 33.Islam M.K., Kabir S.M.L., Haque A.K.M.Z., Sarker Y.A., Sikder M.H. Molecular detection and characterization of Escherichia coli, Salmonella spp. and Campylobacter spp. isolated from broiler meat in Jamalpur, Tangail, Netrokona and Kishoreganj districts of Bangladesh. Afr. J. Microbiol. Res. 2018;12:761–770. [Google Scholar]
- 34.FDA . Bacteriological Analytical Manual. 8th ed. AOAC International; Gaithersburg, MD, USA: 1998. [Google Scholar]
- 35.Sannat C., Patyal A., Rawat N., Ghosh R., Jolhe D., Shende R., Hirpurkar S., Shakya S. Characterization of Salmonella Gallinarum from an outbreak in Raigarh, Chhattisgarh. Vet. World. 2017;10:144. doi: 10.14202/vetworld.2017.144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips I. Cowan and Steel’s Manual for the Identification of Medical Bacteria. J. Clin. Pathol. 1993;46:975. doi: 10.1136/jcp.46.10.975-a. [DOI] [Google Scholar]
- 37.Grimont P.A., Weill F.-X. Antigenic Formulae of the Salmonella Serovars. Volume 9. WHO Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur; Paris, France: 2007. [(accessed on 4 March 2021)]. pp. 1–166. Available online: https://www.pasteur.fr/sites/default/files/veng_0.pdf. [Google Scholar]
- 38.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 39.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 40.Lin C., Tsen H. Use of two 16S DNA targeted oligonucleotides as PCR primers for the specific detection of Salmonella in foods. J. Appl. Bacteriol. 1996;80:659–666. doi: 10.1111/j.1365-2672.1996.tb03271.x. [DOI] [PubMed] [Google Scholar]
- 41.Bayer A., Kirby W., Sherris J., Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 42.Clinical Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. CLSI Supplement M100S; Wayne, PA, USA: 2016. pp. 1–256. [Google Scholar]
- 43.Sweeney M.T., Lubbers B.V., Schwarz S., Watts J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018;73:1460–1463. doi: 10.1093/jac/dky043. [DOI] [PubMed] [Google Scholar]
- 44.Haider M.G., Rahman M.M., Hossain M.M., Rashid M., Sufian M.A., Islam M.M., Haque A.F.M.H. Production of formalin killed fowl typhoid vaccine using local isolates of Salmonella gallinarum in Bangladesh. J. Vet. Med. 2007:33–38. doi: 10.3329/bjvm.v5i1.1306. [DOI] [Google Scholar]
- 45.Bura Q.T., Magwisha H.B., Mdegela R.H. Seroprevalence and risk factors for Salmonella gallinarum infection in smallholder layers in Mwanza City, Tanzania. Livest. Res. Rural. Dev. 2014;26:10. [Google Scholar]
- 46.OIE . Fowl Typhoid and Pullorum Disease. World Organisation for Animal Health (OIE); Paris, France: 2019. Terrestrial Animal Health Code. Chapter 10.7. [Google Scholar]
- 47.Huneau-Salaün A., Marianne C., Françoise L., Isabelle P., Sandra R., Virginie M., Philippe F., Nicolas R. Risk factors for Salmonella enterica subsp. enterica contamination in 519 French laying hen flocks at the end of the laying period. Prev. Vet. Med. 2009;89:51–58. doi: 10.1016/j.prevetmed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Iwabuchi E., Maruyama N., Hara A., Nishimura M., Muramatsu M., Ochiai T., Hirai K. Nationwide survey of Salmonella prevalence in environmental dust from layer farms in Japan. J. Food Prot. 2010;73:1993–2000. doi: 10.4315/0362-028X-73.11.1993. [DOI] [PubMed] [Google Scholar]
- 49.Mdegela R.H., Yongolo M.G., Minga U.M., Olsen J.E. Molecular epidemiology of Salmonella gallinarum in chickens in Tanzania. Avian Pathol. 2000;29:457–463. doi: 10.1080/030794500750047216. [DOI] [PubMed] [Google Scholar]
- 50.Snow L., Davies R., Christiansen K., Carrique-Mas J., Wales A., O’connor J., Cook A., Evans S. Survey of the prevalence of Salmonella species on commercial laying farms in the United Kingdom. Vet. Rec. 2007;161:471–476. doi: 10.1136/vr.161.14.471. [DOI] [PubMed] [Google Scholar]
- 51.Neogi S.B., Islam M.M., Islam S.S., Akhter A.H.M.T., Sikder M.M.H., Yamasaki S., Kabir S.M.L. Risk of multi-drug resistant Campylobacter spp. and residual antimicrobials at poultry farms and live bird markets in Bangladesh. BMC Infect. Dis. 2020;20:1–14. doi: 10.1186/s12879-020-05006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sirdar M.M., Picard J., Bisschop S., Gummow B. A questionnaire survey of poultry layer farmers in Khartoum State, Sudan, to study their antimicrobial awareness and usage patterns. Onderstepoort J. Vet. Res. 2012;79:1–8. doi: 10.4102/ojvr.v79i1.361. [DOI] [PubMed] [Google Scholar]
- 53.Lee Y.J., Kang M.S. Protective efficacy of live Salmonella gallinarum 9R vaccine in commercial layer flocks. Avian Pathol. 2007;36:495–498. doi: 10.1080/03079450701691278. [DOI] [PubMed] [Google Scholar]
- 54.Yasmin S., Nawaz M., Anjum A.A., Ashraf K., Ullah N., Mustafa A., Ali M.A., Mehmood A. Antibiotic susceptibility pattern of Salmonellae isolated from poultry from different Districts of Punjab, Pakistan. Pak. Vet. J. 2019;40:98–102. [Google Scholar]
- 55.Lee S.K., Chon J.W., Song K.Y., Hyeon J.Y., Moon J.S., Seo K.-H. Prevalence, characterization, and antimicrobial susceptibility of Salmonella Gallinarum isolated from eggs produced in conventional or organic farms in South Korea. Poult. Sci. 2013;92:2789–2797. doi: 10.3382/ps.2013-03175. [DOI] [PubMed] [Google Scholar]
- 56.Sultana M., Bilkis R., Diba F., Hossain M.A. Predominance of multidrug resistant zoonotic Salmonella Enteritidis genotypes in poultry of Bangladesh. J. Poult. Sci. 2014;51:424–434. doi: 10.2141/jpsa.0130222. [DOI] [Google Scholar]
- 57.Seo K.W., Kim J.J., Mo I.P., Lee Y.J. Molecular characteristic of antimicrobial resistance of Salmonella Gallinarum isolates from chickens in Korea, 2014 to 2018. Poult. Sci. 2019;98:5416–5423. doi: 10.3382/ps/pez376. [DOI] [PubMed] [Google Scholar]
- 58.Nath S.K., Akter S., Dutta A., Sen A.B., Chakrabarty R., Gupta M.D. Prevalence and Antibiogram of Salmonella in Hisex Brown Strain at Commercial Poultry Farm in Chittagong. Int. J. Curr. Res. Biol. Med. 2015;2:14–19. [Google Scholar]
- 59.Olovo C.V., Reward E.E., Obi S.N., Ike A.C. Isolation, Identification and Antibiogram of Salmonella from Cloacal Swabs of Free Range Poultry in Nsukka, Nigeria. J. Adv. Microbiol. 2019;1:1–9. [Google Scholar]
- 60.Chereau F., Opatowski L., Tourdjman M., Vong S. Risk assessment for antibiotic resistance in South East Asia. BMJ. 2017;5:358. doi: 10.1136/bmj.j3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dolberg F. Poultry Sector Country Review. Bangladesh. [(accessed on 4 March 2021)];2008 Available online: http://www.fao.org/3/ai319e/ai319e.pdf.
- 62.Singer R.S., Finch R., Wegener H.C., Bywater R., Walters J., Lipsitch M. Antibiotic resistance—The interplay between antibiotic use in animals and human beings. Lancet Infect. Dis. 2003;3:47–51. doi: 10.1016/S1473-3099(03)00490-0. [DOI] [PubMed] [Google Scholar]
- 63.Babapour A., Azami L., Fartashmehr J. Overview of antibiotic residues in beef and mutton in Ardebil, North West of Iran. World Appl. Sci. J. 2012;19:1417–1422. [Google Scholar]
- 64.Akhter A.H.M.T., Islam S.S., Sufian M.A., Hossain M., Rahman S.M.M., Kabir S.M.L., Uddin M.G., Hossin S.M., Hossain M.M. Implementation of code of practices (CoP) in selected poultry farms of Bangladesh. Asian Australas. J. Food Saf. Secur. 2018;2:45–55. [Google Scholar]
- 65.Fraser R.W., Williams N., Powell L., Cook A. Reducing Campylobacter and salmonella infection: Two studies of the economic cost and attitude to adoption of on farm biosecurity measures. Zoonoses Public Health. 2010;57:e109–e115. doi: 10.1111/j.1863-2378.2009.01295.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to this study are included in this manuscript and Supplementary Materials.