Abstract
Extra virgin olive oil (EVOO) is defined as a functional food as it contains numerous phenolic components with well-recognized health-beneficial properties, such as high antioxidant and anti-inflammatory capacity. These characteristics depend on their structural/conformational behavior, which is largely determined by intra- and intermolecular H-bond interactions. While the vibrational dynamics of isolated compounds have been studied in a number of recent investigations, their signal in a real-life sample of EVOO is overwhelmed by the major constituent acids. Here, we provide a full characterization of the vibrational spectroscopic signal from commercially available EVOO samples using Inelastic Neutron Scattering (INS) and Raman spectroscopies. The spectra are dominated by CH2 vibrations, especially at about 750 cm−1 and 1300 cm−1. By comparison with the spectra from hydroxytyrosol and other minor phenolic compounds, we show that the best regions in which to look for the structure–activity information related to the minor polar compounds is at 675 and 1200 cm−1 for hydroxytyrosol, and around 450 cm−1 for all minor polar compounds used as reference, especially if a selectively deuterated sample is available. The regional origin of the EVOO samples investigated appears to be related to the different amount of phenolic esters versus acids as reflected by the relative intensities of the peaks at 1655 and 1747 cm−1.
Keywords: extra virgin olive oil, minor polar compounds, inelastic neutron scattering, Raman spectroscopy, UV-Vis spectroscopy
1. Introduction
Dietary products such as vegetable oils are known to contain biologically relevant components with recognized health-beneficial properties. Their phenolic constituents (namely phenolic acids and esters, e.g., cinnamic acid derivatives) display a well-known antioxidant activity, which makes them very effective chemo-preventive agents against oxidative-related diseases such as inflammation, cancer and neurodegenerative disorders [1,2,3,4,5].
Extra virgin olive oil (EVOO) is the main source of lipids in a Mediterranean diet (MD) [6,7,8,9]. Apart from the major triacylglycerols and unsaturated fatty acids, it comprises tyrosol (p-hydroxyphenylethanol), hydroxytyrosol (3,4-dihydroxyphenylethanol), lignans (e.g., (1)-pinoresinol and (1)-1-acetoxy-pinoresinol), secoiridoids (e.g., oleuropein and oleuropein aglycones) and relatively high amounts of at least 30 other minor polar phenolic acids/esters. The amount of these “non-nutrients” or minor polar compounds (MPCs) in EVOO may vary, depending on several factors, such as olive cultivar, location, climate, degree of maturation, agronomic and technological aspects of production [10]. Thanks to the protective role of these phenolic components against carcinogenesis, a direct relationship has been found between olive oil consumption and the incidence of different types of cancers [11] such as leukaemia [12], breast [13,14], prostate [15], lung [16], colon [17,18], bladder [19], carcinomas, cardiovascular diseases, and chronic kidney disease [20]. Recently, several studies have pointed out the nutraceutical role of EVOO in chronic non-communicable diseases (CNCDs) such as metabolic syndrome [21,22]. It is generally accepted that the cardioprotective properties of the MD are partly related to EVOO consumption [23,24]. Moreover, beneficial biological activities of polyphenols recovered from olive oil by-products and leaves have been described [21,22]. MPCs seem to exert a preventive and adjuvant action against CNCDs [23,25,26,27,28,29]. The study and analysis of the clinical and preclinical evidence of the cardiovascular beneficial effects of each constituent has been presented in Ref. [30].
Because of the beneficial properties of EVOO phenolic constituents, intense research has been directed towards the characterization of their structure–activity relationships (SAR). Vibrational spectroscopy is a powerful technique with which to characterize phenolic structures as, for example, it can provide information on the formation and chain length of alkyl esters and the intermolecular hydrogen-bond interactions inducing the dimerization of esters [31]. Complementary information can be obtained by the combined use of Raman spectroscopy and Inelastic Neutron Scattering (INS). In particular, INS exhibits a unique sensitivity to hydrogen, thus being an excellent technique for tackling H-bonding properties, such as the out-of-plane (O-H⋯O) mode, involving almost exclusively the motion of a hydrogen atom relative to the undeformed molecule [32]. Furthermore, INS yields intense features in the low-wavenumber region of the spectrum, where vibrations associated with hydrogen-type interactions mostly occur, thus providing complementary information to optical methods, such as infrared or Raman [33]. However, while a number of isolated components have been characterized using these techniques, as discussed later, an investigation of SAR of the MPC in a bulk sample of EVOO has not yet been presented. This is mainly due to the dominant vibrational signal arising from the major components of saturated and unsaturated fats, such as oleic, linoleic, and palmitic acids. While the spectroscopic fingerprint of isolated MPCs is a valuable piece of information, their vibrational frequencies and molecular structure are expected to change when a given MPC is considered within a real EVOO sample where intermolecular interactions are expected to be different from those present in the isolated extract of a given MPC [32].
The aim of this work is to assess possible strategies for the application of vibrational techniques, such as INS and Raman spectroscopies, to investigate the spectroscopic fingerprints of MPCs in EVOO samples. However, as the intensity in both INS and Raman spectra is proportional to the amount of a given molecule in the sample under investigation, the signal from MPCs is expected to be too weak to allow a detailed analysis of the peak position and, consequently, of the SAR. Therefore, the characterization of the predominant signal from major components in EVOO is a fundamental step towards the investigation of the spectroscopic fingerprints from MPCs. In particular, especially in the case of INS, once a promising energy region is recognized, where the signal from MPCs overlaps with a weakly structured background from major components, selective deuteration of the sample can be attempted to suppress the signal from the major components. In fact, the ratio of the neutron scattering cross sections of hydrogen and deuterium is about a factor of 11 [33]. As a result, one can eventually obtain the SAR of MPCs within EVOO samples, rather than from a pure extract following chemical separation.
In the present work, several EVOOs from the regions of Toscana, Umbria [34], Puglia, Lazio, and Abruzzo (Italy) were analyzed using INS and Raman spectroscopies. To the best of the authors’ knowledge, this was the first study of this type on dietary products by neutron scattering spectroscopy. The olive oil samples were obtained from olives grown organically or biodynamically with an organic oil label within the Italian and international market.
2. Materials and Methods
2.1. Materials
A series of EVOO samples were obtained from products commercially available in Italy: Toscana-Abruzzo blend (EVOO1); Toscana monocultivar 2019 (EVOO2); Umbria 2019 (EVOO3); Toscana monocultivar 2018 (EVOO4); Puglia monocultivar 2019 (EVOO5); Abruzzo monocultivar 2019 (EVOO6); Lazio blend 2018 (EVOO7); and Toscana monocultivar from biodynamic cultivation 2018 (EVOO8). Moreover, two standard samples were used: hydroxytyrosol (STD1) was obtained from olive pomace after EVOO production through a separation process by membrane technology followed by a concentration step under reduced pressure at low temperature, and STD 2 that was a fraction obtained from extraction and concentration from EVOO1 [21,24,35,36,37,38]. The EVOO samples were fully characterized prior to the spectroscopic measurements, namely regarding their acidity (% oleic acid), peroxide (mEqO2/kgoil) and total polyphenol content (mgtyrosol/kgoil), performed by using CDR-Oxitester system (CDR srl., Florence, Italy) and specific reagent kits [21]. A quasi-quantitative analysis of MPCs was performed by HPLC-DAD-MS using five-point regression curves built with the specific available standard references as reported in [21,35,36]. Solvents and reagents were purchased from Sigma Aldrich (Milan, Italy), hydroxytyrosol, tyrosol and oleuropein were furnished by Extrasynthèse, (Genay, France), and water was purified by a Milli-Q Plus system from Millipore (Milford, MA, USA).
2.2. INS Spectroscopy
INS measurements were carried out at the ISIS Pulsed Neutron and Muon Source of the STFC Rutherford Appleton Laboratory (Didcot, UK), using the time-of-flight high-resolution broad-range spectrometer TOSCA [39,40,41,42,43]. The samples were placed into indium-sealed thin-wall flat aluminum cans, with a 4 × 4 cm2 surface perpendicular to the neutron beam. To reduce the impact of the Debye–Waller factor on the observed spectral intensity, the samples were cooled to temperatures below 20 K. Data were recorded in the energy range from 0 to 4000 cm−1, and converted into the conventional scattering law, S(Q,ω) vs. energy transfer (in cm−1), using the MANTID program (version 5.1) [44]. All INS data were corrected by subtraction of an empty-can background and were normalized relative to the band at 720 cm−1 (assigned to the ρ(CH2) vibrational mode).
2.3. Raman Spectroscopy
Raman experiments were performed at the CSGI laboratories of the Chemistry Department of the University of Florence, using a Renishaw inVia Qontor confocal microRaman system equipped with 785 nm (solid state type, IPS R-type NIR785, 100 mW, 1200 L/mm grating) and 532 nm (Nd:YAG solid state type, 50 mW, 1800 L/mm grating) lasers, a research-grade Leica DM2700 microscope with a LWD50x objective (NA 0.55, WD 8.0 mm), LWD 100× (NA 0.75, WD 4.7 mm) and 100× (NA 0.85, WD 0.27 mm) objectives, and using a front-illuminated charge-coupled device (CCD) camera as a detector (256 × 1024 pixels, working temperature −70 °C). The 785 nm near-infrared laser was used as the excitation radiation, with a laser power at the sample varying between 0.6 and 3 mW, since these samples are very sensitive to the laser power, which can easily induce phenol dimerization and degradation processes. Spectra were collected in the range from 150 to 3500 cm−1 using the extended-range mode, an acquisition time of 10 s, and a single scan per sample. Raw data were pre-processed using the Renishaw software WiRE (version 5.2), and the baseline was corrected and normalized using the band at 720 cm−1 (assigned to the ρ(CH2) vibrational mode) as reference. The spectra were compared using Origin Pro 9.0 (OriginLab, Northampton, MA, USA).
2.4. UV-Vis Absorbance
Hexane solutions of EVOO8 and sunflower oil were investigated in the range 200–400 nm using a UV-Vis Varian Cary 50 spectrophotometer, with a resolution of 2 nm. Mixtures of a maximum of 20 μL of oil in 50 mL of hexane were used to avoid signal saturation. Hexane was chosen as the most suitable solvent to avoid absorbance bands from the solvent for wavelengths below 300 nm.
3. Results and Discussion
Table 1 shows the results of the EVOO HPLC-DAD-MS analysis and the result of acidity, peroxide content and total polyphenols analyzed with the CDR-Oxitester instrument. Furthermore, two standard samples were characterized. STD1 comprising 994.08 mg/g of hydroxytyrosol, and STD2 fraction obtained from extraction and concentration of EVOO1 containing hydroxytyrosol (12.38 mg/g), tyrosol (8.29 mg/g), elenolic acid (76.32 mg/g), 10-hydroxy-oleocanthal (287.62 mg/g), oleocanthal (189.16 mg/g), oleuropein aglycone (95.44 mg/g), ligstroside aglycone (23.29 mg/g) and ligustaloside A + B (171.99 mg/g), for a total of 864.49 mg/g MPCs.
Table 1.
Minor polar compounds (MPCs) present in the extra virgin olive oils (EVOOs).
| Constituents (mg/L) | EVOO1 | EVOO2 | EVOO3 | EVOO4 | EVOO7 | EVOO8 |
|---|---|---|---|---|---|---|
| hydroxytyrosol | 3.12 ± 0.95 | 0.51 ± 0.02 | 0.98 ± 0.03 | 6.88 ± 0.28 | 1.47 ± 0.04 | 1.88 ± 0.06 |
| Tyrosol | 1.02 ± 0.04 | 1.23 ± 0.05 | 0.21 ± 0.01 | 6.22 ± 0.19 | 1.86 ± 0.06 | 1.87 ± 0.07 |
| Elenolic acid derivatives | 9.31 ± 0.28 | 60.8 ± 1.8 | 12.5 ± 0.4 | 36.6 ± 1.5 | 21.2 ± 0.64 | 29.3 ± 0.9 |
| Elenolic acid | 150 ± 4 | 31.5 ± 1.3 | 111 ± 3 | 106 ± 4 | 117 ± 3 | 197 ± 6 |
| 10-hydroxy-oleocanthal | 315 ± 12 | 362 ± 11 | 61.0 ± 1.8 | 168 ± 7 | 67.5 ± 2.0 | 124 ± 4 |
| Oleocanthal | 198 ± 6 | 192 ± 6 | 46.2 ± 1.4 | 79.4 ± 3.2 | 94.1 ± 2.8 | 44.0 ± 1.3 |
| Secoiridoid derivatives | 96.4 ± 2.9 | 17.1 ± 0.7 | 30.9 ± 0.9 | 47.3 ± 1.9 | 48.4 ± 1.4 | 36.4 ± 1.1 |
| Lignans | 208 ± 8 | 160 ± 5 | 107 ± 3 | 90.2 ± 3.6 | 62.1 ± 1.9 | 129 ± 4 |
| Oleuropein aglicone | 164 ± 5 | 67.7 ± 2 | 105 ± 3 | 108 ± 4 | 83.4 ± 2.5 | 143 ± 4 |
| Total MPCs | 1146 ± 34 | 893 ± 27 | 475 ± 14 | 649 ± 19 | 497 ± 14 | 707 ± 21 |
| Acidity (% oleic acid) | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.24 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| Peroxides (mEq O2/kgoil) | 4.98 ± 0.20 | 5.21 ± 0.21 | 5.01 ± 0.15 | 8.82 ± 0.26 | 8.81 ± 0.26 | 7.81 ± 0.23 |
| Polyphenols (mgtyrosol/kgoil) | 890 ± 36 | 791 ± 31 | 354 ± 10 | 423 ± 13 | 342 ± 10 | 443 ± 13 |
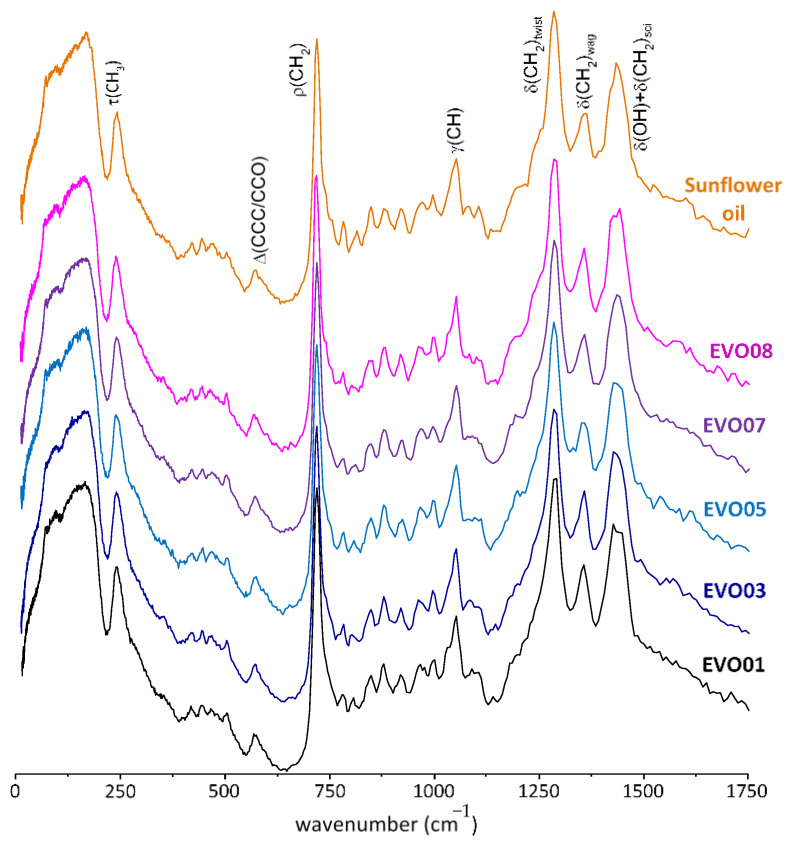
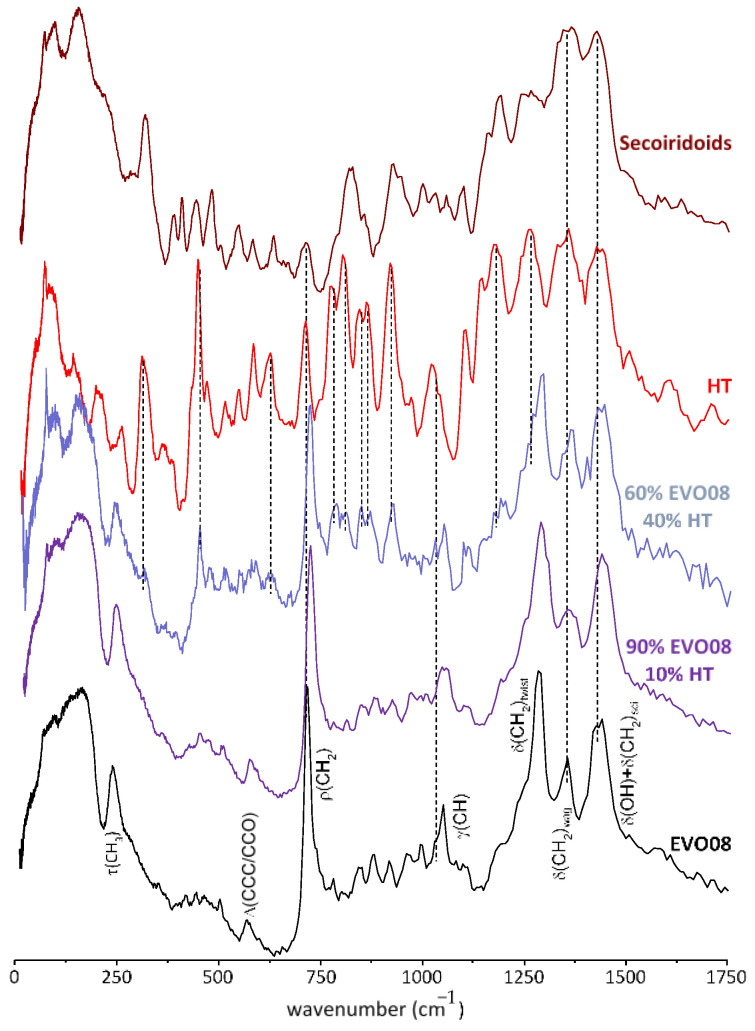
Figure 1 reports INS spectra obtained for some EVOO samples as well as sunflower oil. It is clearly seen that all the oil samples under analysis have very similar INS spectral signatures, which are dominated by the vibrational modes from unsaturated fatty acids, i.e., the major oil components: oleic (ca. 80%), linoleic and palmitic acids (ca. 20%) [45,46]. In addition, the profiles reveal the presence of hydroxytyrosol and secoiridoids (mainly oleuropein and oleuropein aglycone) (see Figure 2) that are important constituents of this vegetal oil (both from olives and olive leaves), [47] taken as reference compounds in this work. In particular, Figure 2 reports the spectra of 40% and 10% w/w STD1/EVOO8 mixtures. These spectra can be used as guides for the eyes to monitor the change in intensity of the characteristic peaks in pure hydroxytyrosol, present only as ca. 2 mg/L in EVOO8. It is interesting to note how in the region of the wagging δ(CH2) mode at ca. 1370 cm−1 and of the ρ(CH2) peak at ca. 720 cm−1, all spectra present well defined signals, which are the common signature of both MPCs and oil’s major components. On the other hand, hydroxytyrosol peaks between 1100 and 1200 cm−1 fall in a region where no intense structural features can be observed in EVOO8, and this is also the case for the peak at 675 cm−1, quite evident in hydroxytyrosol and in the 60%/40% EVOO8/STD1 mixture, corresponding to a smooth background signal in EVOO8. Moreover, a sharp peak at ca. 450 cm−1 in hydroxytyrosol corresponds to a region of EVOO samples with a weakly structured background in EVOO. To measure the SAR and the hydroxytyrosol’s dynamical effects in EVOO samples, the INS signals around 675 cm−1 and 1150 cm−1 are the most promising, as the spectral features from MPCs are isolated from the other bands ascribed to the major oil components (saturated and unsaturated fatty acids and esters).
Figure 1.
INS spectra (0–1750 cm−1) of some of the extra virgin olive oils (EVOOs) studied in the present work, and of sunflower oil.
Figure 2.
INS spectra (0–1750 cm−1) of extra virgin olive oil (EVOO8), hydroxytyrosol (HT) and secoiridoids, and two different mixtures of EVOO8 and HT (9:1 and 6:4)).
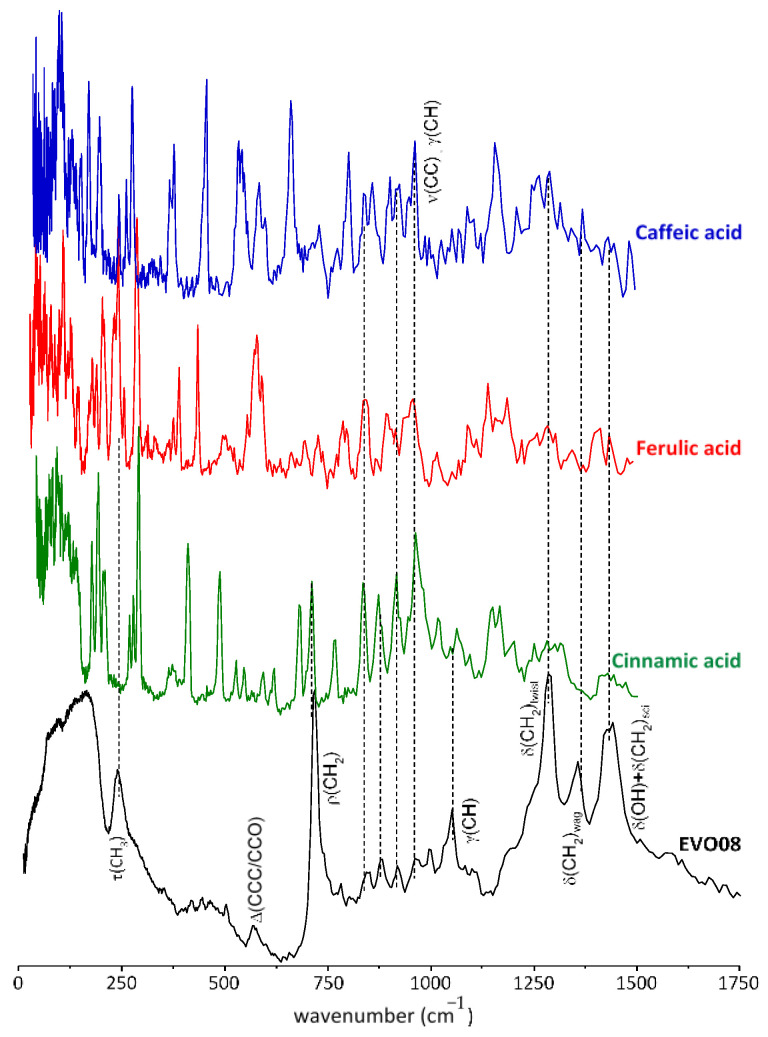
EVOO phenolic constituents with recognized health-beneficial properties (e.g., coumaric, caffeic, ferulic and sinapic acids/esters), that were previously found to yield very definite vibrational signatures including INS characteristic bands [48] (Figure 3), are also MPCs in the present EVOO samples. However, their vibrational modes are largely obscured by those from the predominant non-phenolic elements. Figure 3 shows a comparison of the INS spectra from caffeic, ferulic, and cinnamic acids with that of EVOO8. In the case of caffeic acid, the most promising peak is the one at ca. 670 cm−1, where the signal from EVOO samples is relatively weak and unstructured, at energies slightly lower than the ρ(CH2) band. Moreover, for all MPCs in Figure 3, the region centered around 450 cm−1 is particularly interesting for the three reference molecules that present sharp peaks, possibly the γ(CH) band, although they fall in a weakly structured background in EVOO. Therefore, one can speculate that, by selectively deuterating the major components of EVOO, all four reference MPCs might be detected in this region.
Figure 3.
INS spectra (0–1750 cm−1) of extra virgin olive oil (EVOO8) and some representative dietary phenols (cinnamic, caffeic and ferulic acids).
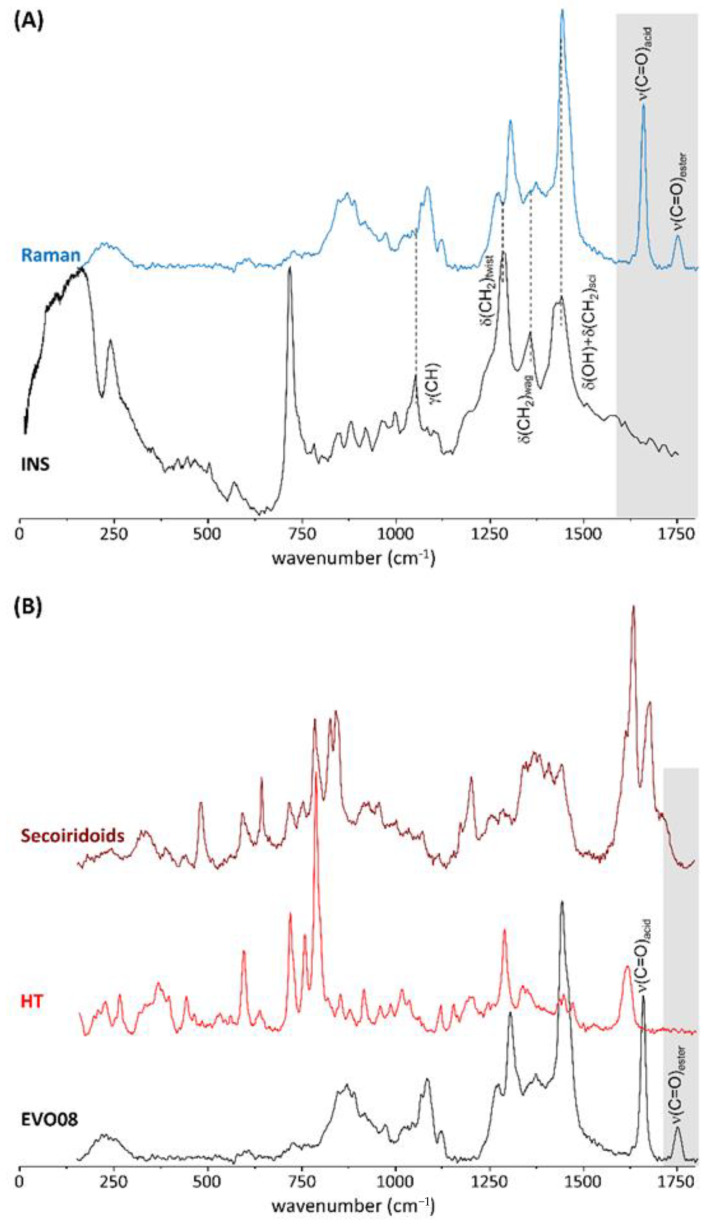
Figure 4 shows the Raman spectra recorded for the same samples. The spectra show some typical features of phenolic acids and esters [31,49,50,51]. In particular, the ν (C = O) mode detected at ca. 1747 cm−1, assigned to the esterified species, is not observed in the INS spectra as it does not involve hydrogen motion, as shown in Figure 4A. Also, the same peak is not present in either the secoiridoids or hydroxytyrosol constituents (Figure 4B). A detailed analysis of the Raman features of the carbonyl stretching modes characteristic of phenolic acids and esters, respectively at 1655 and 1747 cm−1, in the series of EVOOs and sunflower oil (Table 2), provides insight into the relative amount of phenolic esters versus acids in these samples. The I1655/I1747 ratio lies within the range from 4.39 to 4.59 for the olive oils, while a higher value of about 5 was obtained for sunflower oil, evidencing a higher content in esters for the EVOOs. The two monocultivar species from the Toscana region (Italy) (EVOO2 and EVOO4) display the lowest I1655/I1747 ratio (respectively 4.39 and 4.43), revealing a slightly larger amount of phenolic esters as compared to the other EVOOs.
Figure 4.
(A)—INS and Raman spectra (0–1750 cm−1) of extra virgin olive oil (EVOO8); (B)—Raman spectra (0–1750 cm−1) of EVOO8 and the reference compounds hydroxytyrosol and secoiridoids.
Table 2.
Raman intensity ratios for the bands at 1655 and 1747 cm−1 (νC = O from phenolic acids and esters, respectively), for the extra virgin olive oils and sunflower oil in the present study.
| Sample | Source | I1655/I1747 |
|---|---|---|
| EVOO1 | Toscana–Abruzzo blend | 4.52 |
| EVOO2 | Toscana monocultivar 2019 | 4.39 |
| EVOO3 | Umbria 2019 | 4.58 |
| EVOO4 | Toscana monocultivar 2018 | 4.43 |
| EVOO5 | Puglia monocultivar 2019 | 4.59 |
| EVOO6 | Abruzzo monocultivar 2019 | 4.50 |
| EVOO7 | Lazio blend 2018 | 4.55 |
| EVOO8 | Toscana monocultivar from biodynamic cultivation 2018 | 4.53 |
| Sunflower oil | 5.02 |
The larger value of the I1655/I1747 ratio for sunflower oil, relative to other EVOO samples, is compatible with results reported in the literature [52], which revealed an intensity decrease in the peak at 1655 cm−1 for higher quality EVOOs with a lower unsaturation degree, while the peak at 1747 cm−1 was found to be relatively constant amongst samples. In our analysis, the significance of the changes in the ratio of the intensities at 1655 and 1747 cm−1 was checked against the ratios of peak intensities in the region 800–1200 cm−1. In the latter, that includes the CC stretching (869 cm−1) and bending (1079 cm−1) modes from the –(CH2)n– chains, the relative peak intensities were not found to vary significantly amongst the several samples, as expected [52].
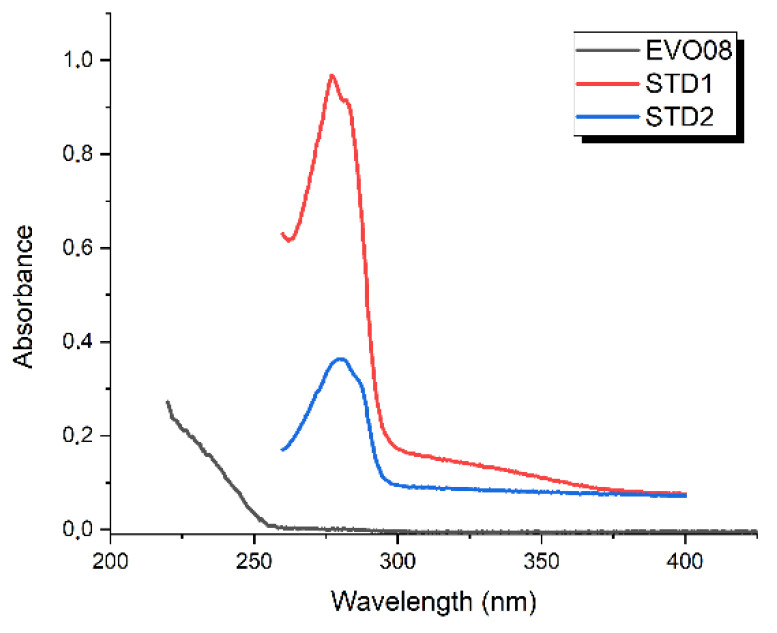
Finally, Figure 5 shows the UV-Vis absorbance spectra of the EVOO8, STD1, and STD2 samples. The spectra of both STD1 and STD2 show a pronounced peak at about 280 nm, very similar to that found for oleuropein [10], making it difficult to discriminate among the several MPCs present in the EVOOs using this technique. Moreover, the 280 nm absorption becomes completely negligible in the case of the olive oil samples, as shown in Figure 5 for the particular case of EVOO8, which shows a sharp increase in the absorbance curve for wavenumbers lower than 255 nm.
Figure 5.
UV-Vis absorbance spectra of EVOO8 (black), STD1 (red) and STD2 (blue), in hexane solution (20 μL sample to 50 mL solvent).
4. Outlook and Conclusions
We have presented a spectroscopic investigation of a set of commercially available extra virgin olive oils (EVOOs) using Inelastic Neutron Scattering and Raman vibrational techniques. As the properties of different EVOO samples are related mainly to their minor phenolic components (MPCs), we find that different EVOO samples display similar INS and Raman signals. However, by comparison to the spectra from hydroxytyrosol and other MPCs, we show that the best regions to gain structure–activity information related to the MPCs are in the 675 and 1200 cm−1 range for hydroxytyrosol and around 450 cm−1 for caffeic, ferulic, and cinnamic acids. Our results provide a fundamental preliminary step in the investigation of the structure–activity relationship of minor polar compounds in real-life EVOO samples. From our results, future investigations based on INS, combined with selective partial deuteration to silence the vibrational contribution from the CH2 wagging modes of the major components in EVOO samples, or the structured signal around 450 cm−1, would open the way to a direct investigation of modes near these energies from the minor polar compounds. Finally, our results represent a step forward for accurate future investigations of the vibrational dynamics of olive oil samples subject to external stimuli, such as temperature or environmental parameters (e.g., soil composition and climate), as well as for the identification of minor polar compounds therein. For example, the detailed knowledge of the vibrational density of states, i.e., the signal measured by inelastic neutron scattering, could be used to analyze energy-resolved and/or time-resolved neutron imaging experiments [53] providing, e.g., molecular-specific information of the oil extraction process.
Acknowledgments
The authors gratefully acknowledge the financial support from Regione Lazio (IR approved by Giunta Regionale n., Grant No. G10795, 7 August 2019, published by BURL n. 69 27 August 2019), ISIS@MACH (I), and the ISIS Neutron and Muon Source (UK) of Science and Technology Facilities Council (STFC); the financial support from the Consiglio Nazionale delle Ricerche within CNR-STFC [Grant Agreement No. 2014–2020 (N 3420)], concerning collaboration in scientific research at the ISIS Neutron and Muon Source (UK) of Science and Technology Facilities Council (STFC), is gratefully acknowledged. The STFC Rutherford Appleton Laboratory are thanked for access to neutron beam facilities. MPMM and LAEBC are grateful for financial support from the Portuguese Foundation for Science and Technology (UIDB/00070/2020). We are indebted to the Centro di Ricerca Alimenti e Nutrizione (CREA) for scientific support, to Moreno Landrini, Mayor of Spello (Perugia, Italy) and to Frantoio Di Spello Uccd (Perugia, Italy). The invaluable technical assistance of C. D’Ottavi is gratefully acknowledged. Lazio Region PSR-Lazio 2014–2020 Misura 16.1 “SonninoNutraOil”-“Valorization of quality and functional nutraceutical properties of Sonnino’s extra virgin olive oil”.
Author Contributions
R.S.—Conceptualization; C.A.—Conceptualization, investigation, Writing—original draft preparation; P.B.—investigation; L.A.E.B.d.C.—data analysis; S.L.—Conceptualization, investigation, contribution to manuscript writing; M.P.M.M.—data analysis and contribution to manuscript writing; G.M.—investigation; A.N.—Resources; R.P.—investigation; S.F.P.—investigation; E.P.—investigation; G.R.—investigation, Writing—original draft preparation; A.R.—Resources; N.D.D.—Resources; All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the NAST Centre, Università degli Studi di Roma “Tor Vergata”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study (TOSCA, RB2000118) are openly available at https://doi.org/10.5286/ISIS.E.RB2000118-1 (accessed on 21 April 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gomes C.A., da Cruz T.G., Andrade J.L., Milhazes N., Borges F., Marques M.P.M. Anticancer Activity of Phenolic Acids of Natural or Synthetic Origin: A Structure−Activity Study. J. Med. Chem. 2003;46:5395–5401. doi: 10.1021/jm030956v. [DOI] [PubMed] [Google Scholar]
- 2.Fresco P., Borges F.I.G.M., Diniz C.G., Marques M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006;26:747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 3.Fresco P., Borges F., Marques M.P.M., Diniz C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010;16:114–134. doi: 10.2174/138161210789941856. [DOI] [PubMed] [Google Scholar]
- 4.Servili M., Sordini B., Esposto S., Urbani S., Veneziani G., di Maio I., Selvaggini R., Taticchi A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants. 2014;3:1–23. doi: 10.3390/antiox3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batista de Carvalho A.L.M., Caselli F., Rodrigues V., Paiva-Martins F., Marques M.P.M. Antiproliferative Activity of Olive Oil Phenolics against Human Melanoma Cells. Lett. Drug Des. Discov. 2017;14:1053–1059. [Google Scholar]
- 6.Di Daniele N., di Renzo L., Noce A., Iacopino L., Ferraro P.M., Rizzo M., Sarlo F., Domino E., de Lorenzo A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014;27:529–536. doi: 10.1007/s40620-014-0067-y. [DOI] [PubMed] [Google Scholar]
- 7.Andreoli A., Lauro S., di Daniele N., Sorge R., Celi M., Volpe S.L. Effect of a moderately hypoenergetic Mediterranean diet and exercise program on body cell mass and cardiovascular risk factors in obese women. S.L. Eur. J. Clin. Nutr. 2008;62:892–897. doi: 10.1038/sj.ejcn.1602800. [DOI] [PubMed] [Google Scholar]
- 8.Di Daniele N., Noce A., Vidiri M.F., Moriconi E., Marrone G., Annicchiarico-Petruzzelli M., D’Urso G., Tesauro M., Rovella V., de Lorenzo A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget. 2017;8:8947–8979. doi: 10.18632/oncotarget.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Daniele N., Petramala L., di Renzo L., Sarlo F., della Rocca D.G., Rizzo M., Fondacaro V., Iacopino L., Pepine C.J., de Lorenzo A. Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol. 2013;50:409–416. doi: 10.1007/s00592-012-0445-7. [DOI] [PubMed] [Google Scholar]
- 10.Oliveras-López M.J., Innocenti M., Giaccherini C., Ieri F., Romani A., Mulinacci N. Study of the phenolic composition of spanish and italian monocultivar extra virgin olive oils: Distribution of lignans, secoiridoidic, simple phenols and flavonoids. Talanta. 2007;73:726–732. doi: 10.1016/j.talanta.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Owen R.W., Haubner R., Würtele G., Hull W.E., Spiegelhalder B., Bartsch H. Olives and olive oil in cancer prevention. Eur. J. Cancer Prev. 2004;13:319. doi: 10.1097/01.cej.0000130221.19480.7e. [DOI] [PubMed] [Google Scholar]
- 12.Fabiani R., de Bartolomeo A., Rosignoli P., Servili M., Selvaggini R., Montedoro G.F., di Saverio C., Morozzi G. Virgin Olive Oil Phenols Inhibit Proliferation of Human Promyelocytic Leukemia Cells (HL60) by Inducing Apoptosis and Differentiation. J. Nutr. 2006;136:614–619. doi: 10.1093/jn/136.3.614. [DOI] [PubMed] [Google Scholar]
- 13.Han J., Talorete T.P.N., Yamada P., Isoda H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology. 2009;59:45–53. doi: 10.1007/s10616-009-9191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sepporta M.V., Fuccelli R., Rosignoli P., Ricci G., Servili M., Morozzi G., Fabian R. Oleuropein inhibits tumour growth and metastases dissemination in ovariectomised nude mice with MCF-7 human breast tumour xenografts. J. Funct. Foods. 2014;8:269–273. doi: 10.1016/j.jff.2014.03.027. [DOI] [Google Scholar]
- 15.Hodge A.M., English D.R., McCredie M.R.E., Severi G., Boyle P., Hopper J.L., Giles G.G. Foods, nutrients and prostate cancer. Cancer Causes Control. 2004;15:11–20. doi: 10.1023/B:CACO.0000016568.25127.10. [DOI] [PubMed] [Google Scholar]
- 16.Fortes C., Forastiere F., Farchi S., Mallone S., Trequattrinni T., Anatra F., Schmid G., Perucci C. The Protective Effect of the Mediterranean Diet on Lung Cancer. Nutr. Cancer. 2003;46:30–37. doi: 10.1207/S15327914NC4601_04. [DOI] [PubMed] [Google Scholar]
- 17.Stoneham M., Goldacre M., Seagroatt V., Gill L. Olive oil, diet and colorectal cancer: An ecological study and a hypothesis. J. Epidemiol. Community Health. 2000;54:756–760. doi: 10.1136/jech.54.10.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashim Y.Z.H.-Y., Rowland I.R., McGlynn H., Servili M., Selvaggini R., Taticchi A., Esposto S., Montedoro G., Kaisalo L., Wahala K., et al. Inhibitory effects of olive oil phenolics on invasion in human colon adenocarcinoma cells in vitro. Int. J. Cancer. 2008;122:495–500. doi: 10.1002/ijc.23148. [DOI] [PubMed] [Google Scholar]
- 19.Coccia A., Mosca L., Puca R., Mangino G., Rossi A., Leandro E. Extra-virgin olive oil phenols block cell cycle progression and modulate chemotherapeutic toxicity in bladder cancer cells. Oncol. Rep. 2016;36:3095–3104. doi: 10.3892/or.2016.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabiani R., Rosignoli P., de Bartolomeo A., Fuccelli R., Servili M., Montedoro G.F., Morozzi G. Oxidative DNA Damage Is Prevented by Extracts of Olive Oil, Hydroxytyrosol, and Other Olive Phenolic Compounds in Human Blood Mononuclear Cells and HL60 Cells. J. Nutr. 2008;138:1411. doi: 10.1093/jn/138.8.1411. [DOI] [PubMed] [Google Scholar]
- 21.Romani A., Bernini R., Noce A., Urciuoli S., di Lauro M., Zaitseva A.P., Marrone G., di Daniele N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules. 2020;25:4757. doi: 10.3390/molecules25204757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noce A., Marrone G., Urciuoli S., di Daniele F., di Lauro M., Zaitseva A.P., di Daniele N., Romani A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients. 2021;13:581. doi: 10.3390/nu13020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romani A., Ieri F., Urciuoli S., Noce A., Marrone G., Nediani C., Bernini R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients. 2019;11:1776. doi: 10.3390/nu11081776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romani A., Campo M., Urciuoli S., Marrone G., Noce A., Bernini R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols From Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020;7:120. doi: 10.3389/fnut.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noce A., di Lauro M., di Daniele F., Zaitseva A.P., Marrone G., Borboni P., di Daniele N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients. 2021;13:630. doi: 10.3390/nu13020630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noce A., Marrone G., di Lauro M., Urciuoli S., Zaitseva A.P., Jones G.W., di Daniele N., Romani A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020:1807941. doi: 10.1155/2020/1807941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noce A., Bocedi A., Campo M., Marrone G., di Lauro M., Cattani G., di Daniele N., Romani A. A Pilot Study of a Natural Food Supplement as New Possible Therapeutic Approach in Chronic Kidney Disease Patients. Pharmaceuticals. 2020;13:148. doi: 10.3390/ph13070148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noce A., Marrone G., di Daniele F., di Lauro M., Zaitseva A.P., Jones G.W., de Lorenzo A., di Daniele N. Potential Cardiovascular and Metabolic Beneficial Effects of ω-3 PUFA in Male Obesity Secondary Hypogonadism Syndrome. Nutrients. 2020;12:2519. doi: 10.3390/nu12092519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dessì M., Noce A., Bertucci P., Noce G., Rizza S., de Stefano A., di Villahermosa S.M., Bernardini S., de Lorenzo A., di Daniele N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014;13:54. doi: 10.1186/1476-511X-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flori L., Donnini S., Calderone V., Zinnai A., Taglieri I., Venturi F., Testai L. The Nutraceutical Value of Olive Oil and Its Bioactive Constituents on the Cardiovascular System. Focusing on Main Strategies to Slow Down Its Quality Decay during Production and Storage. Nutrients. 2019;11:1962. doi: 10.3390/nu11091962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calheiros R., Machado N.F.L., Fiuza S.M., Gaspar A., Garrido J., Milhazes N., Borges F., Marques M.P.M. Antioxidant phenolic esters with potential anticancer activity: A Raman spectroscopy study. J. Raman Spec. 2008;39:95–107. doi: 10.1002/jrs.1822. [DOI] [Google Scholar]
- 32.Mitchell P.C.H., Parker S.F., Ramirez-Cuesta A.J., Tomkinson J. Vibrational Spectroscopy with Neutrons: With Applications in Chemistry, Biology, Materials Science and Catalysis. World Scientific; Singapore: 2005. [DOI] [Google Scholar]
- 33.Sears V.F. Neutron scattering lengths and cross sections. Neutron News. 1992;3:26–37. doi: 10.1080/10448639208218770. [DOI] [Google Scholar]
- 34.Frantoio Di Spello Uccd (Perugia, Italy) [(accessed on 21 April 2021)]; Available online: https://www.frantoiodispello.it/en/contacts/
- 35.Romani A., Lapucci C., Cantini C., Ieri F., Mulinacci N., Visioli F. Evolution of Minor Polar Compounds and Antioxidant Capacity during Storage of Bottled Extra Virgin Olive Oil. J. Agric. Food Chem. 2007;55:1315–1320. doi: 10.1021/jf062335r. [DOI] [PubMed] [Google Scholar]
- 36.Romani A., Pinelli P., Ieri F., Bernini R. Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L. Sustainability. 2016;8:1002. doi: 10.3390/su8101002. [DOI] [Google Scholar]
- 37.Romani A., Scardigli A., Pinelli P. An environmentally friendly process for the production of extracts rich in phenolic antioxidants from Olea europaea L. and Cynara scolymus L. matrices. Eur. Food Res. Technol. 2017;243:1229–1238. doi: 10.1007/s00217-016-2835-5. [DOI] [Google Scholar]
- 38.Garcia-Castello E., Cassano A., Criscuoli A., Conidi C., Drioli E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010;44:3883. doi: 10.1016/j.watres.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 39.ISIS Facility INS/TOSCA. [(accessed on 18 March 2021)]; Available online: https://www.isis.stfc.ac.uk/Pages/tosca.aspx.
- 40.Parker S.F., Carlile C.J., Pike T., Tomkinson J., Newport R.J., Andreani C., Ricci F.P., Sacchetti F., Zoppi M. TOSCA: A world class inelastic neutron spectrometer. Phys. B. 1997;241–243:154–156. doi: 10.1016/S0921-4526(97)00536-X. [DOI] [Google Scholar]
- 41.Parker S.F., Lennon D., Albers P.W. Vibrational Spectroscopy with Neutrons: A Review of New Directions. Appl. Spec. 2011;65:1325–1341. doi: 10.1366/11-06456. [DOI] [Google Scholar]
- 42.Parker S.F., Fernandez-Alonso F., Ramirez-Cuesta A.J., Tomkinson J., Rudic S., Pinna R.S., Gorini G., Castanon J.F. Recent and future developments on TOSCA at ISIS. J. Phys. Conf. Ser. 2014;554:012003. doi: 10.1088/1742-6596/554/1/012003. [DOI] [Google Scholar]
- 43.Pinna R.S., Rudić S., Parker S.F., Armstrong J., Zanetti M., Škoro G., Waller S.P., Zacek D., Smith C.A., Capstick M.J., et al. The neutron guide upgrade of the TOSCA spectrometer. Nucl. Instrum. Methods Phys. Res. A. 2018;896:68–74. doi: 10.1016/j.nima.2018.04.009. [DOI] [Google Scholar]
- 44.Arnold O., Bilheux J.C., Borreguero J.M., Buts A., Campbell S.I., Chapon L., Doucet M., Draper N., Leal R.F., Gigg M.A., et al. Mantid—Data analysis and visualization package for neutron scattering and μSR experiments. Nuclear Instrum. Methods Phys. Res. Sect. A. 2014;764:156–166. doi: 10.1016/j.nima.2014.07.029. [DOI] [Google Scholar]
- 45.Mishra S., Chaturvedi D., Kumar N., Tandon P., Siesler H.W. An ab initio and DFT study of structure and vibrational spectra of γ form of Oleic acid: Comparison to experimental data. Chem. Phys. Lipids. 2010;163:207–217. doi: 10.1016/j.chemphyslip.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Machado N.F.L., de Carvalho L.A.E.B., Otero J.C., Marques M.P.M. The autooxidation process in linoleic acid screened by Raman spectroscopy. J. Raman Spec. 2012;43:1991–2000. doi: 10.1002/jrs.4121. [DOI] [Google Scholar]
- 47.Paiva-Martins F., Rodrigues V., Calheiros R., Marques M.P.M. Characterization of antioxidant olive oil biophenols by spectroscopic methods. J. Sci. Food Agric. 2010;91:309–314. doi: 10.1002/jsfa.4186. [DOI] [PubMed] [Google Scholar]
- 48.Marques M.P.M., de Carvalho L.A.E.B., Valero R., Machado N.F.L., Parker S.F. An inelastic neutron scattering study of dietary phenolic acids. Phys. Chem. Chem. Phys. 2014;16:7491–7500. doi: 10.1039/C4CP00338A. [DOI] [PubMed] [Google Scholar]
- 49.Van Besien E., Marques M.P.M. Ab initio conformational study of caffeic acid. J. Molec. Struct. 2003;625:265–275. doi: 10.1016/S0166-1280(03)00026-5. [DOI] [Google Scholar]
- 50.Machado N.F.L., Calheiros R., Gaspar A., Garrido J., Borges F., Marques M.P.M. Antioxidant phenolic esters with potential anticancer activity: Solution equilibria studied by Raman spectroscopy. J. Raman Spec. 2009;40:80–85. doi: 10.1002/jrs.2083. [DOI] [Google Scholar]
- 51.Zou M., Zhang X.-F., Qi X.-H., Ma H.-L., Dong Y., Liu C.-W., Guo X., Wang H. Rapid Authentication of Olive Oil Adulteration by Raman Spectrometry. J. Agric. Food Chem. 2009;57:6001–6006. doi: 10.1021/jf900217s. [DOI] [PubMed] [Google Scholar]
- 52.Duraipandian S., Petersen J.C., Lassen M. Authenticity and Concentration Analysis of Extra Virgin Olive Oil Using Spontaneous Raman Spectroscopy and Multivariate Data Analysis. Appl. Sci. 2019;9:2433. doi: 10.3390/app9122433. [DOI] [Google Scholar]
- 53.Romanelli G., Minniti T., Škoro G., Krzystyniak M., Taylor J., Fornalski D., Fernandez-Alonso F. Visualization of the Catalyzed Nuclear-Spin Conversion of Molecular Hydrogen Using Energy-Selective Neutron Imaging. J. Phys. Chem. C. 2019;123:11745–11751. doi: 10.1021/acs.jpcc.9b01858. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study (TOSCA, RB2000118) are openly available at https://doi.org/10.5286/ISIS.E.RB2000118-1 (accessed on 21 April 2021).