Abstract
Background:
The COVID-19 pandemic reached Germany in spring 2020. No proven treatment for SARS-CoV-2 was available at that time, especially for severe COVID-19-induced ARDS. We determined whether the infusion of mesenchymal stromal cells (MSCs) would help to improve pulmonary function and overall outcome in patients with severe COVID-19 ARDS. We offered MSC infusion as an extended indication to all critically ill COVID-19 patients with a Horovitz index <100. We treated 5 out of 23 patients with severe COVID-19 ARDS with an infusion of MSCs. One million MSCs/kg body weight was infused over 30 minutes, and the process was repeated in 3 patients twice and in 2 patients 3 times.
Result:
Four out of 5 MSC-treated patients compared to 50% of control patients (9 out of 18) received ECMO support (80%). The MSC group showed a higher Murray score on admission than control patients, reflecting more severe pulmonary compromise (3.5 ± 0.2 versus 2.8 ± 0.3). MSC infusion was safe and well tolerated. The MSC group had a significantly higher Horovitz score on discharge than the control group. Compared to controls, patients with MSC treatment showed a significantly lower Murray score upon discharge than controls. In the MSC group, 4 out of 5 patients (80%) survived to discharge and exhibited good pulmonary function, whereas only 8 out of 18 patients (45%) in the control group survived to discharge.
Conclusion:
MSC infusion is a safe treatment for COVID-19 ARDS that improves pulmonary function and overall outcome in this patient population.
Keywords: COVID-19, acute respiratory distress syndrome, ARDS, mesenchymal stem cell therapy, inflammation, sepsis
Background
The COVID-19 pandemic reached Germany in spring 2020, with the public lockdown starting in early March. At that time, the understanding of the pathology of COVID-19 was sparse, and no pharmacological agent was available to influence the severity of ARDS in COVID-19. Therefore, we primarily treated these patients supportively with lung-protective ventilation, a conservative fluid strategy, and prone positioning.1–3 Among other potential treatment options, bone marrow-derived human mesenchymal stromal cell (MSC) therapy has been reported as a potential new treatment for ARDS that may be beneficial in COVID-19 ARDS.4 MSCs are multipotent cells that secrete growth factors for endothelial and epithelial cells as well as anti-inflammatory cytokines and antimicrobial peptides. In addition, they are characterized by low immunogenicity. These characteristics are directly relevant to the pathological abnormalities underlying SARS-COV-2-induced lung injury and COVID-19 ARDS.
Phase 1 and Phase 2 clinical trials in humans and preclinical studies in small animals demonstrated the potential efficacy and safety of MSC administration for the treatment of ARDS. In addition, MSCs have also been tested in human patients for other diseases that clinically resemble COVID-19 in parts of its pathology.4,5 We have also used MSCs in the past with good clinical results to treat other conditons associated with vascular pathological changes.6 Based on this, we decided that it would be worth using this therapy in critically ill COVID-19 ARDS patients. This article summarizes the results of this approach, comparing patients with severe COVID-19 ARDS who received MSCs with patients who received standard therapy without MSCs.
Methods
Patients
The treated patients were admitted to the intensive care unit after initial presentation in our or an external hospital emergency department with symptoms of severe dyspnea. The patients were tested for SARS-CoV-2 in the emergency room with positive results upon admission. Since these patients presented with severe dyspnea, the initial treatment consisted of oxygen supplementation followed by intubation due to the severity of the lung compromise. Protective ventilation, prone positioning, and fluid restriction were initiated. In 4 patients in the MSC group (80%) and 9 in the control group (50%), hypoxia was severe with increasing lactate levels. Therefore, the decision for placement of veno-venous extracorporeal membrane oxygenation was made, and veno-venous cannulation was performed. Corresponding laboratory and vital parameters were routinely measured in the intensive care unit. Approval was obtained from the local ethics committee for the evaluation and reporting of the patient data and results (see approval 432/2020BO). We only offered COVID-19 patients with a Horovitz index <100 on admission to intensive care MSC treatment since we assumed that detrimental conditions with a significant prognosis should warrant this novel treatment. Only these patients were included in the analysis. We offered this treatment to 10 further patients, but the legal representative did not agree on this treatment approach.
Source and Preparation of MSCs
Pooled cryopreserved MSCs from 8 donors generated via plastic adherence as previously described7 were purchased from the blood bank Frankfurt (Obnitix, Medac, Germany). The adherent MSCs were thawed and then further expanded using the Quantum Cell Expansion system (TerumoBCT, Inc., Lakewood, USA) as described.8 Briefly, the hollow fiber bioreactor was coated with fibronectin for 18 hours. After this incubation period, fibronectin was washed out, and the thawed MSCs were suspended in DMEM supplemented with 10% platelet lysate, 1% glutamine, and 2000 U/l heparin. The cell suspension was then loaded onto the hollow fiber bioreactor and incubated in the closed culture system in the presence of 20% O2, 5% CO2, and 75% N2. After 24 hours, the MSCs adhered, and the hollow fiber bioreactor was continuously supplied with fresh medium at a flow rate of 0.1 ml/min up to 1.6 ml/min. Glucose and lactate concentrations were measured on a daily basis. After the lactate level reached approx. 0.6 mM, the MSCs were washed out and resuspended in 0.9% NaCl + 0.5% human albumin. A 10-15-fold expansion of the MSCs in approx. 1 week could be achieved with this approach. The expanded MSCs were tested for endotoxin and mycoplasma contamination. The MSCs were then aliquoted and were given either freshly or cryopreserved. Purity (measured by the markers CD 105, 73, and 90) and vitality were >90%.
Statistical Evaluation
Patients in the MSC-treated group were compared to non-MSC-treated patients, with the nonparametric Mann-Whitney U-test being performed only on admission and discharge dates. Since the length of stay varied between the groups and MSC therapy was performed on different days after admission to the ICU, no further statistical evaluation was performed during the ICU course. A P-value < 0.05 was considered significant.
Results
Baseline Characteristics
Three males and 2 females were treated with MSCs. With a mean age of 39 years, they were significantly younger than the control group. The patients in the MSC group showed a nonsignificant tendency to have a higher BMI. Only one of the 5 MSC patients was diagnosed with arterial hypertension, and none of these patients had taken medication in the past. Given the reports of an increased incidence of patients with arterial hypertension infected with SARS-COV-2, we report here that 13 of the 18 control patients (72%) presented with arterial hypertension (see Table 1).
Table 1.
Baseline Characteristics and CoMorbidities.
| Control (n = 18) | MSC (n = 5) | P value | |
|---|---|---|---|
| Sex | 0.62 | ||
| Male | 13 | 3 | |
| Female | 5 | 2 | |
| Age, years | 59 (IQR 54 to 79) | 39 (IQR 32 to 50) | 0.01* |
| Weight, kg |
92 (IQR 74 to 120) | 95 (IQR 80 to 108) | 0.85 |
| BMI, kg/m2 | 29 (IQR 26 to 35) | 35 (IQR 26 to 39) | 0.48 |
| Chronic diseases | |||
| Arterial hypertension | 13/18 | 1/5 | |
| Congestive heart failure | 2/18 | 0/5 | |
| Coronary heart disease | 2/18 | 0/5 | |
| Chronic atrial fibrillation | 2/18 | 0/5 | |
| COPD | 1/18 | 0/5 | |
| Pulmonary diseases (e.g. Asthma) | 1/18 | 0/5 | |
| Smoker | 3/18 | 0/5 | |
| Diabetes mellitus | 2/18 | 0/5 | |
| Chronic medication | |||
| ACE inhibitor | 4/18 | 0/5 | |
| AT1 receptor blocker | 5/18 | 0/5 | |
| Betablockers | 9/18 | 0/5 | |
| Other anti-hypertensive drugs | 2/18 | 0/5 | |
| Calcium anatagonist | 4/18 | 1/5 | |
| Antiplatelet drugs | 4/18 | 0/5 | |
| Oral corticoids | 1/18 | 0/5 | |
| Immunosuppressive medication | 0/18 | 0/5 | |
Abbreviations: AKIN, classification based on the acute kidney injury network; ARF, acute renal failure; BPM, beats per minute; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; VV-ECMO, veno-venous extracorporeal membrane oxygenation.
* Wilcoxon rank sum test.
Laboratory, Hemodynamic, and Pulmonary Baseline Characteristics
The baseline laboratory values in the MSC group of survivors did not differ from the laboratory baseline values of the controls when the leukocytes and leukocyte subgroups were examined. The baseline inflammation markers did not differ between the groups at admission. No difference was found in the D-dimer and lymphocyte counts. When examining cardiovascular variables, we found no difference in MSC patients compared to controls. Patients treated with MSCs showed a significantly higher Murray score for lung injury at admission than the controls and thus overall more severe pulmonary compromise on admission to intensive care than the controls (Table 2).
Table 2.
Laboratory Values on Admission.
| Control (n = 18) | MSC (n = 5) | P value | |
|---|---|---|---|
| Leukocytes | 9,675 (IQR 7,155 to 14,060) | 7,630 (IQR 5,215 to 10,625) | 0.20 |
| Neutrophils | 8,621 (IQR 6,822 to 11,331) | 6,645 (IQR 3,722 to 8,446) | 0.16 |
| Lymphocytes | 657 (IQR 251 to 988) | 882 (IQR 610 to 1,196) | 0.43 |
| Hematocrit | 36.9 (IQR 29.8 to 39.9) | 31.8 IQR 27.6 to 38.5) | 0.36 |
| Thrombocytes | 256 (IQR 190 to 297) | 194 (IQR 172 to 301) | 0.51 |
| D-Dimer | 4.4 (IQR 2.5 to 18) | 2.7 (IQR 1.5 to 4.8) | 0.27 |
| PCT | 1.6 (IQR 0.8 to 5.1) | 1.5 (IQR 0.6 to 6.1) | 0.96 |
| CRP | 28.3 (IQR 21.6 to 34.5) | 18.5 (IQR 16.6 to 35.3) | 0.34 |
| IL-6 | 443.5 (IQR 77.4 to 845.3) | 144 (82.6 to 899.6) | 0.71 |
| Lactate | 1.3 (IQR 1.1 to 2.7) | 1 (IQR 0.9 to 1.3) | 0.14 |
| Vital signs and pulmonary function at ICU admission | |||
| Heart rate, bpm | 90 (IQR 82 to 109) | 103 (IQR 94 to 122) | 0.22 |
| Mean arterial pressures, mmHg | 82 (IQR 65 to 90) | 82 (IQR 79 to 96) | 0.58 |
| Temperature, °C | 37.3 (IQR 36.6 to 38.2) | 39 (IQR 38 to 39.4) | 0.02* |
| PaO2/FiO2 quotient | 68 (IQR 58 to 84) | 87 (IQR 68 to 92) | 0.29 |
| Murray Lung Injury Score (without chest X-Ray) | 2.8 (IQR 2.0 to 3.6) | 3.5 (IQR 3.3 to 4) | 0.20 |
Abbreviations: AKIN, classification based on the acute kidney injury network; ARF, acute renal failure; BPM, beats per minute; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; VV-ECMO, veno-venous extracorporeal membrane oxygenation.
* Wilcoxon rank sum test.
ICU Therapy, Morbidity, and Mortality
The patients in the control group showed a shorter stay in intensive care than the MSC group, but this was not significant (P = 0.07).
This is due to the higher incidence of deceased patients within this group and a higher percentage of ECMO use in the MSC group. Furthermore, the higher use of ECMO gives an indication of the severity of ARDS in the MSC group. The incidence of kidney injury and hepatic failure in the MSC group did not differ from the incidence in the control group of patients. Patients in the MSC group showed lower mortality than those in the control group (20% vs. 55.6%) (Table 3).
Table 3.
ICU Therapy and Morbidity.
| Control (n = 18) | MSC (n = 5) | P value | |
|---|---|---|---|
| LOS ICU, days | 15 (IQR 6 to 29) | 49 (IQR 18 to 54) | 0.07 |
| Acute kidney Injury | 4 | 1 | |
| No AKI | 1 | 0 | |
| AKIN I | 1 | 1 | |
| AKIN II | 12 | 3 | |
| AKIN III | |||
| Acute liver dysfunction | 59% | 60% | 1.0 |
| Veno-venous ECMO support | 9/18 | 4/5 | |
| ICU mortality | 10/18 (55.6%) | 1/5 (20%) | 0.32 |
| Causes of death acute liver failure | 4 | – | |
| Multiorgan failure | 3 | 1 | |
| Palliative care | 2 | – | |
| Requested cerebral bleeding | 1 | – |
Abbreviations: AKIN, classification based on the acute kidney injury network; ARF, acute renal failure; BPM, beats per minute; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; VV-ECMO, veno-venous extracorporeal membrane oxygenation.
* Wilcoxon rank sum test.
Laboratory Trends
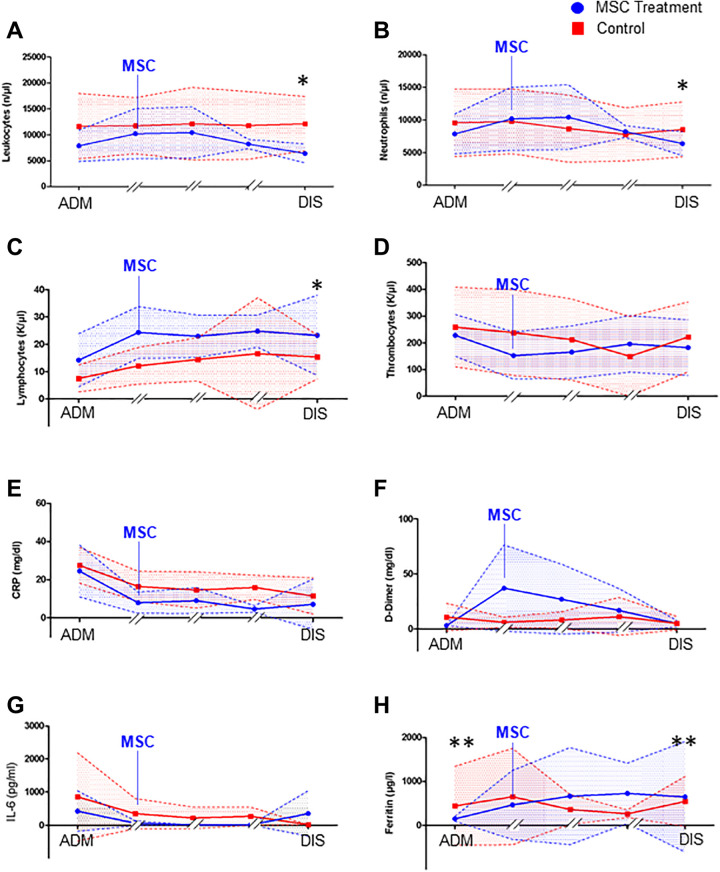
Next, we tested whether the values of the significant laboratory markers differed in trend during the course of treatment in the intensive care unit. This was not easily possible, as the average 15-day stay in the control group was different from the stay of the MSC-treated patient. Furthermore, MSC therapy was started at different times during treatment in the ICU. We decided to plot the admission data, the data on the treatment day and the data from day 1 and day 5 after MSC therapy and the discharge day for the MSC group. For the control group, we plotted the admission date, day 1, day 5, day 10 and the discharge day during their median stay of 15 days. A statistical comparison was made only for the admission and discharge days, the only 2 times we could confirm for each patient. We found that the values for CRP and IL-6 did not differ significantly between the groups during ICU treatment. Ferritin levels in the MSC group showed a significant increase at discharge. Leukocytes, leukocyte subgroups, and lymphocytes did not differ at admission. However, a significant reduction in leukocytes and neutrophils was found at discharge in the MSC group compared to the control group, indicating a reduction in inflammation. Furthermore, we observed a significant increase in lymphocytes at discharge in the MSC group, suggesting that the acquired immune system is activated. D-dimer values and platelet values did not differ between the 2 groups. The patients who received MSC treatment on the day of therapy showed an increase in D-dimer levels, which is not surprising since we used the increase in D-dimers as a marker for COVID-19 disease activity. The decision to administer an MSC infusion was based on the increase in D-dimer levels during the clinical course (Figure 1).
Figure 1.
Laboratory values with schematic timeline of the intensive care unit course of MSC-treated patients (blue) and control patients (red). The admission values (ADM) and discharge values (DIS) are compared statistically with nonparametric testing. (A) Total leukocyte counts, (B) neutrophil counts, (C) lymphocyte counts, (D) thrombocyte counts, (E) CRP values in mg/dl during the ICU course, (F) D-Dimer values in mg/dl during, (G) IL-6 serum values, and (H) ferritin values during the ICU course of MSC treated patients and controls. (With n = 5 in the MSC group and n = 18 in the control group, Mann-Whitney U test with *P < 0.05).
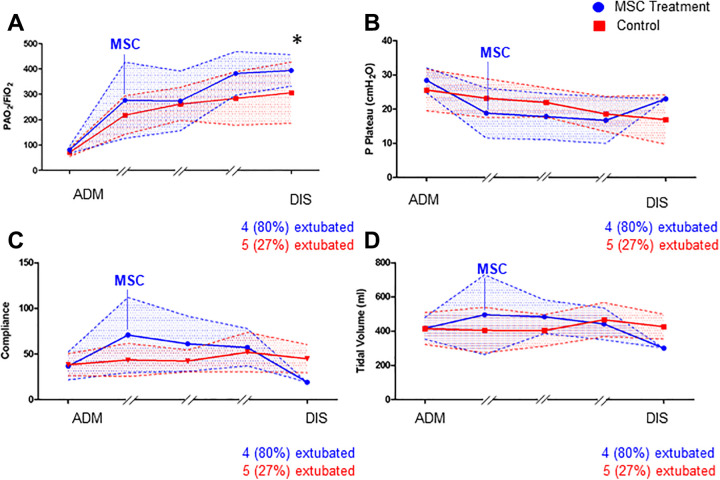
Ventilation Parameters and Murray Score
Patients in both the MSC group and the control group were enrolled with a Horovitz quotient <100, since by definition, all patients were in the severe ARDS group. Horovitz increased in both groups during ICU therapy, but in the MSC group, patients had more pronounced improvements and showed a significantly higher Horovitz than the control group at discharge (Figure 2A). This was also evident in the improvement in compliance, although both groups showed similar compliance on admission (Figure 2C). The discharge time in Figure 2A-D reflects only one patient in the MSC group, the deceased patient. The other 4 patients were already extubated at this time point. This general improvement in pulmonary function was reflected in the MSC group by the Murray score (Figure 3). At admission, the score was higher in the patients treated with MSCs, which reflected the overall worse pulmonary function at that time.
Figure 2.
Schematic graph of ventilation parameters during the intensive care unit course of MSC-treated patients (blue) and control patients (red). The admission values (ADM) and discharge values (DIS) were compared statistically with nonparametric testing. (A) Horovitz index during the ICU course, (B) plateau pressure in cmH2O, (C) compliance in ml/mmHg, and (D) tidal volume in ml (with n = 5 in the MSC group with 4 out of 5 patients extubated at discharge and n = 18 in the control group with n = 5 extubated at discharge, Mann-Whitney U Test with *P < 0.05).
Figure 3.
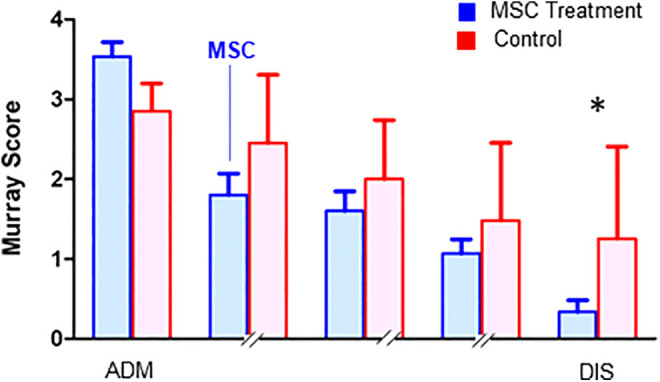
Murray score to determine overall pulmonary function during the intensive care unit course of MSC-treated patients (blue) and control patients (red). The admission values (ADM) and discharge values (DIS) were compared statistically with nonparametric testing (with n = 5 in the MSC group with 4 out of 5 patients extubated at discharge and n = 18 in the control group with n = 5 extubated at discharge, Mann-Whitney U test with *P < 0.05).
We observed a significant improvement in the Murray score in this group, which was particularly evident at the time of discharge. At discharge, the MSC-treated patients showed a significantly lower Murray score of 0.3 ± 0.1 than the control patients, who presented an average score of 1.3 ± 1.1.
Discussion
COVID-19-associated pneumonia and ARDS are feared complications of SARS-CoV-2 infection. Clinical and therapeutic options have been scarce to date and were not available, especially at the beginning of the pandemic. In view of the knowledge of the pathology of COVID-19, we offered critically ill patients and their guardians the opportunity to be treated with MSCs during their intensive care course. Five of the patients’ guardians agreed to the infusion of MSCs; therefore, we treated these patients with MSCs when the clinical condition of these patients showed deterioration. We found this approach to have good clinical outcomes and report here on this series of MSC treatments to share our experience in evaluating this treatment option in severe SARS-CoV-2 infection.
Animal studies have shown a beneficial effect of MSC treatments in models of ARDS.9,10 However, preclinical testing of MSCs on the effect of SARS-CoV-2-induced ARDS could not be pursued during the developing pandemic, as it is time-consuming and involves high laboratory safety requirements. The administration of MSCs in the treatment of ARDS in humans has been reported in non-SARS-CoV-2 ARDS in the past in Phase 1 and Phase 2a safety trials. In one trial, 60 patients were enrolled and randomized to either an MSC-based treatment strategy or placebo. The authors reported that MSC infusion was a safe treatment with a low number of adverse events. They showed a nonsignificant increase in mortality due to ARDS in the MSC treatment group with a hazard ratio just above 1.4 The interpretation of this study was complicated by the fact that the treatment group showed higher scores on the Acute Physiology and Chronic Health Evaluation III. Lung function tests also indicated that the MSC treatment group was sicker than the control group. We confirm parts of this study in our small population here. In contrast to this study, we did not find increased mortality in the MSC treatment group but rather an improved overall outcome that was consistent with earlier data. This was also reflected in the Murray score for the determination of general lung function. The authors also reported an extended stay in intensive care in the MSC group, which we also found in our treatment group. However, this mainly reflects the early deaths in the non-MSC cohort. In line with previous publications, we also found no significant side effects after MSC infusion, and we found that this approach is safe and not associated with serious adverse events. In an earlier Phase 1 trial with a single intravenous infusion of allogenic human bone marrow-derived MSCs, good tolerance was reported in 9 patients with moderate-to-severe ARDS. In this trial, the authors used a single infusion of MSCs as the treatment approach.5 They reported a reduction in the lung injury score 3 days after treatment with MSCs. We also found an improved Murray score, which changed significantly at the end of our observation period but with a tendency toward better values in the MSC group even earlier. Therefore, we conclude from these earlier studies and the data we report here that infusion of MSCs is a valuable treatment option for ARDS that should be pursued further. Ongoing clinical trials will show whether a possible effect of MSCs on COVId-19 pathology in the near future.11
The pathology of COVID-19-induced pneumonia and ARDS differs from the known pathological changes of other causes of ARDS.12,13 Thus, the treatment of MSCs cannot be translated from these other causes of ARDS without considering the underlying pathology. To date, 2 reports have used MSCs in the treatment of COVID-19 ARDS. In the first study, the authors described the administration of MSCs in 31 COVID-19 patients.14 A high clearance rate for SARS-CoV-2 in their patient population is described, but no data on the severity of pulmonary compromise are shown, nor is a comparison made with a potential control group at their treatment center. A second report included 7 patients treated with MSCs and showed that this improved the overall condition of the treated patients and especially the immune function.15 In contrast to these reports, we only included critically ill patients in our evaluation. A significant number of our patients were treated with extracorporeal membrane oxygenation, 50% in the control population and 80% in the MSC group. In the control group, we reported mortality in severe ARDS of 55%, while MSC-treated individuals had a mortality of 20%. Both groups were equally sick when admitted to intensive care. Other studies have reported mortality rates in their total COVID-19 ICU population of 39% to 60%.16–19 These reports of course do not only include patients with severe ARDS according to the Berlin Definition. In particular, we included patients with severe ARDS in our study, as this patient population has a high expected mortality rate due to severe pulmonary compromise but is likely to benefit most from this intervention.20
We understand that our report has limitations of course. First, we cannot report on a randomized control group, but we have a significant number of patients with severe COVID-19 ARDS treated in the same facility, and all patients showed severe ARDS. Given that we had to prepare for the SARS-CoV-2 pandemic on relatively short notice, a prospective treatment protocol was not possible. Second, the patients in the MSC group were younger and showed a longer course in intensive care than the reported controls. Age influences the outcome during ARDS with younger patients showing a overall better outcome. Yet our MSC treated group showed more significant pulmonary compromise as determined in the Murray Score. This is of course a limitation of this study, but it also shows that this patient population was more severely ill during the course of ICU therapy than the reported control patients. In addition, although they had a longer ICU course, the MSC group showed significantly better pulmonary function as determined by the Murray score at discharge. We therefore sought to show the progression of the patients within their ICU course. At least this approach shows the overall development of patients in intensive care units for their key laboratory and ventilation values. The control group could be judged to be different from the MSC group, which is true in some respects. Third, we have not yet used MSCs for the treatment of ARDS, and our experience with this treatment could be considered limited. However, members of our group have used MSCs to treat many other diseases for many years before this pandemic, and therefore, there was much prior experience in our institution with the preparation and use of MSCs.6,21–23
Conclusion
We show that treatment with MSC infusion is safe and improves pulmonary function in the severe form of COVID-19 ARDS. This approach is an option for improving pulmonary function and overall outcome in patients with severe COVID-19 ARDS.
Footnotes
Authors’ Note: Helene Häberle and Harry Magunia share authorship. HH—obtained consent, performed therapy, wrote parts of the manuscript; HM—evaluated and analyzed data, wrote parts of the manuscript; PL—prepared MSCs, wrote parts of the manuscript; HG—evaluated and analyzed data; AK—evaluated and analyzed data, wrote parts of the manuscript; MK—evaluated and analyzed data, wrote parts of the manuscript; TB—evaluated and analyzed data, wrote parts of the manuscript; NM—admitted patients, analyzed data; PR—treated patients, involved in concept of the study, wrote parts of the manuscript; RH—prepared MSCs, wrote parts of the manuscript; VM—overall design of the study, wrote parts of the manuscript. The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request. All authors gave consent for publication. Approval was obtained from the local ethics committee for the evaluation and reporting of the patient data and results (see approval 432/2020BO).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report was funded by the DFG CRC/TR 240, the Transregio-SFB 240 consortium “Platelets—Molecular, cellular and systemic functions in health and disease” (Projekt # 374031971), TP B08 to V.M., and TP B07 (to P.R.), and DFG RO 3671/8-2 to P.R. and DFG MI1506/5-1 to V.M.
ORCID iDs: Harry Magunia  https://orcid.org/0000-0001-9576-6399
https://orcid.org/0000-0001-9576-6399
Andreas Körner  https://orcid.org/0000-0002-9643-7498
https://orcid.org/0000-0002-9643-7498
References
- 1. Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. [DOI] [PubMed] [Google Scholar]
- 2. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342(18):1301–1308. [DOI] [PubMed] [Google Scholar]
- 3. National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. [DOI] [PubMed] [Google Scholar]
- 4. Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muller I, Kordowich S, Holzwarth C, et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40(1):25–32. [DOI] [PubMed] [Google Scholar]
- 7. Bader P, Kuci Z, Bakhtiar S, et al. Effective treatment of steroid and therapy-refractory acute graft-versus-host disease with a novel mesenchymal stromal cell product (MSC-FFM). Bone Marrow Transplant. 2018;53(7):852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rojewski MT, Fekete N, Baila S, et al. GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device quantum cell expansion system. Cell Transplant. 2013;22(11):1981–2000. [DOI] [PubMed] [Google Scholar]
- 9. Asmussen S, Ito H, Traber DL, et al. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax. 2014;69(9):819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2(12):1016–1026. [DOI] [PubMed] [Google Scholar]
- 11. ClinicalTrials.gov. 2021. Accessed January 11, 2021. https://www.clinicaltrials.gov/ct2/results?cond=COVID-19&term=Mesenchymal+Stromal+Cells&cntry=&state=&city=&dist=
- 12. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Z, Chen Y, Luo X, He X, Zhang Y, Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit Care. 2020;24(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with Covid-19 pneumonia. Aging Dis. 2020;11(2):216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-Cov-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. [DOI] [PubMed] [Google Scholar]
- 21. Bohringer J, Santer R, Schumacher N, et al. Enzymatic characterization of novel arylsulfatase a variants using human arylsulfatase A-deficient immortalized mesenchymal stromal cells. Hum Mutat. 2017;38(11):1511–1520. [DOI] [PubMed] [Google Scholar]
- 22. Doring M, Kluba T, Cabanillas Stanchi KM, et al. Longtime outcome after intraosseous application of autologous mesenchymal stromal cells in pediatric patients and young adults with avascular necrosis after steroid or chemotherapy. Stem Cells Dev. 2020;29(13):811–822. [DOI] [PubMed] [Google Scholar]
- 23. Muller I, Kustermann-Kuhn B, Holzwarth C, et al. In vitro analysis of multipotent mesenchymal stromal cells as potential cellular therapeutics in neurometabolic diseases in pediatric patients. Exp Hematol. 2006;34(10):1413–1419. [DOI] [PubMed] [Google Scholar]