Abstract
Introduction
The number of individuals worldwide with Alzheimer's disease (AD) is growing at a rapid rate. New treatments are urgently needed. We review the current pipeline of drugs in clinical trials for the treatment of AD.
Methods
We interrogated ClinicalTrials.gov, the federal registry of clinical trials to identify drugs in trials.
Results
There are 126 agents in 152 trials assessing new therapies for AD: 28 treatments in Phase 3 trials, 74 in Phase 2, and 24 in Phase 1. The majority of drugs in trials (82.5%) target the underlying biology of AD with the intent of disease modification; 10.3% are putative cognitive enhancing agents; and 7.1% are drugs being developed to reduce neuropsychiatric symptoms.
Discussion
This pipeline analysis shows that target biological processes are more diversified, biomarkers are more regularly used, and repurposed agents are being explored to determine their utility for the treatment of AD.
Keywords: Alzheimer's disease, amyloid, biomarkers, clinical trials, Common Alzheimer's Disease Research Ontology (CADRO), drug development, inflammation, National Institutes of Health, pharmaceutical companies, repurposed drugs, tau
1. INTRODUCTION
Alzheimer's disease (AD) is the sixth leading cause of death in the United States and the fifth leading cause among those over age 65. The current number of those with AD dementia is 5.8 million and this is anticipated to grow to 13.8 million in 2050 if effective interventions are not found. Based on 2018 death certificate data, 122,019 individuals succumbed from AD dementia that year, indicating an average daily death toll of 334. 1 AD dementia is preceded by a preclinical phase that may last for 15 to 20 years and a prodromal period that persists for 3 to 6 years prior to onset of dementia. 2 Preclinical, prodromal, and AD dementia are all populations in which clinical trials are ongoing; the US Food and Drug Administration (FDA) has provided guidance on defining AD populations from preclinical to late‐stage dementia to facilitate clinical trials and drug development across the continuum of AD. 3
The biology of AD is increasingly well understood and comprises a plethora of complex, progressive, interactive, destructive processes leading to cell dysfunction and death. 4 The Common Alzheimer's Disease Research Ontology (CADRO) provides a means of classifying targets for drug development relevant to AD. 5 There is an urgent need to develop new therapies for disease modification of AD and to address cognitive impairment and neuropsychiatric symptoms with symptom‐reducing agents. Progress is being made. Suvorexant had a successful Phase 3 trial for insomnia in AD and safety and efficacy data have been added to the package insert allowing clinicians to use this agent for sleep disturbances in AD using evidence‐based guidance. 6 Pimavanserin is under review by the FDA for treatment of dementia‐related psychosis, 7 and aducanumab is under review for treatment of progression of AD. 8 Regardless of the outcomes of these regulatory reviews, the trials and development data packages have advanced adequately to warrant regulatory review and indicate increasing confidence in trials to demonstrate efficacy and safety of AD therapeutics.
We conduct an annual review of the AD drug development pipeline with the intent of understanding the progress of the field in developing new therapeutics including new agents, targets, biomarkers, and trial design strategies. 9 , 10 , 11 , 12 , 13 Here we present the results of our analysis of the 2021 pipeline as represented on ClinicalTrials.gov.
2. METHODS
We used the FDA/US National Library of Medicine of the National Institutes of Health (NIH) clinical research registry, ClinicalTrials.gov, as the source of information for this review. The “common rule” governing ClinicalTrials.gov specifies that registration is required for studies that meet the definition of an “applicable clinical trial” (ACT) and were initiated after September 27, 2007 or initiated on or before that date and were still ongoing as of December 26, 2007. ACTs, as defined in section 402(j) of the Public Health Service Act, include controlled clinical investigations of any FDA‐regulated drugs, biological therapies, or devices for any disease or condition. ACTs generally include interventional studies (with one or more arms) of FDA‐regulated products that meet one of the following conditions: the trial has one or more sites in the United States; the trial is conducted under an FDA investigational new drug application exemption; or the trial involves a small molecule drug, biological therapy, or device that is manufactured in the United States or its territories and are studied for research purposes. 14 Studies of ClinicalTrials.gov suggest that compliance with the common rule is high. 15 , 16 The reporting of results of clinical trials is required, but trial sponsors are less adherent to this expectation. 17 The United States has more clinical trials than any other country, and ClinicalTrials.gov includes the majority of agents currently in clinical trials for AD; this review is therefore comprehensive but not exhaustive. There are other clinical trial registries and comparisons show that only a few agents registered in the European Union Clinical Trial Register, for example, are not found on ClinicalTrials.gov. 18 Phase 1 trials are often conducted outside of the United States, may not be registered on ClinicalTrials.gov, and may be under‐represented in our analysis.
RESEARCH IN CONTEXT
Systematic review: We reviewed drugs currently in clinical trials and registered in the mandated federal database, ClinicalTrials.gov.
Interpretation: There are 126 agents in clinical trials in 2021; the total number of treatments in trials is similar in 2021 and 2020. Most drugs in trials aim to achieve disease modification by targeting the underlying biological processes of Alzheimer's disease (AD). Understanding of the continuum of AD from preclinical stages to severe dementia is reflected in broadening of the trial populations to include preclinical AD, prodromal AD, and AD dementia. Repurposed agents play an important role in the pipeline, especially in Phase 2 trials. Trial participants make an enormous contribution to clinical trials (calculated to be ≈2.5 million participant‐weeks for currently ongoing trials) and research partners invest a similar amount of time in trials.
Future directions: To develop urgently needed drugs with timely delivery to the market, trials must be shorter, smaller, and less expensive. Use of biomarkers and more targeted clinical outcomes as well as improvements in trial site performance will contribute to achieving this goal. If new therapies are approved by regulatory authorities, more sponsors and more funding may be attracted to AD research with accelerated innovation.
HIGHLIGHTS
There are 126 agents in clinical trials for Alzheimer's disease.
Most of the agents in the trial target disease modification.
More than 38,000 participants are required for currently registered trials; cumulatively they will contribute more than 2.5 million participant‐weeks in trials.
Across all trials, biopharmaceutical companies sponsor 49% of trials and collaborate in another 14% of trials sponsored by public–private partnerships.
This review is based on trials present on ClinicalTrials.gov as of January 5, 2021; the tables and text of the review apply to the information available at that time. We comment on terminated trials if the information has become publicly available but is not yet reflected on ClinicalTrials.gov. We include all trials of agents in Phase 1, 2, and 3; if trials are presented as Phase 1/2 or Phase 2/3 in the ClinicalTrials.gov database we use that terminology in the review. We collect information on the trial agent; trial title; trial number in ClinicalTrials.gov; start date; projected end date; actual end date, if completed or terminated; primary completion date; calculated trial duration; duration of treatment exposure; number of subjects planned for enrollment; number of arms of the study (usually a placebo arm and one or more treatment arms with different doses); whether a biomarker was described; whether the agent was repurposed; subject characteristics (age range, acceptable range of cognitive impairment, etc.); and sponsorship (a biopharmaceutical company, NIH with academic medical centers, public–private partnership, or “other”). We included trials labeled as recruiting, active but not recruiting (e.g., trials that have completed recruitment and are continuing with the exposure portion of the trial), enrolling by invitation (e.g., open‐label extensions of trials), and not yet recruiting. We did not include trials listed as completed, terminated, suspended, unknown, or withdrawn as information on these trials and reasons for their current status are often not publicly revealed. We do not include trials of non‐pharmacologic therapeutic approaches such as cognitive therapies, caregiver interventions, supplements, and medical foods. We do not include trials of biomarkers; we note whether biomarkers were used in the trials discussed. We include stem cell therapies among the interventions reviewed (they are not integrated into Figure 1).
FIGURE 1.
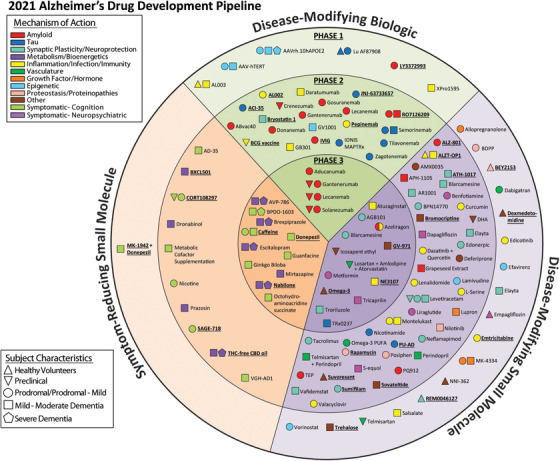
Agents in clinical trials for treatment of Alzheimer's disease in 2021 (from ClinicalTrials.gov as of the index date of January 5, 2021). The inner ring shows Phase 3 agents; the middle ring comprises Phase 2 agents; the outer ring presents Phase 1 therapies; agents in green areas are biologics; agents in purple are disease‐modifying small molecules; agents in orange are symptomatic agents addressing cognitive enhancement or behavioral and neuropsychiatric symptoms; the shape of the icon shows the population of the trial; the icon color shows the Common Alzheimer's Disease Research Ontology (CADRO)‐based class of the agent (“Other” category includes CADRO classes that have three or fewer agents in trials). Agents underlined are new to the pipeline since 2020. Figure: J Cummings; M de la Flor, PhD, Illustrator
For mechanism of action (MOA), we classified agents using the CADRO 5 approach. Some agents have more than one mechanism of action and, in these cases, we noted both mechanisms and depended on the available literature to identify a dominant mechanism. We use the terminology of “symptomatic” treatments for agents whose purpose was cognitive enhancement or control of neuropsychiatric symptoms without claiming to impact the biological causes of cell death in AD, and we used the terminology of “disease‐modifying” for treatments that intended to change the biology of AD and produce neuroprotection (often through a variety of intermediate mechanisms such as effects on amyloid or tau). 19 We used the features of the trials (e.g., clinical outcomes, trial duration, use of biomarkers, number of participants) to determine whether a trial was attempting to demonstrate disease medication or symptomatic benefit. We recognize that these definitions are arbitrary, and many therapies may have symptomatic and disease‐modifying effects. We divided disease‐modifying therapies (DMTs) into biologics and small molecules. Biologics are generally derived from living organisms and include antibodies, vaccines, antisense oligonucleotides (ASOs), and therapeutic proteins. “Small molecules” refers to drugs typically taken orally that are <500 daltons in size and can regulate a biological process. AD has preclinical, prodromal, and dementia phases, 20 and we note if the studies are prevention trials including participants with preclinical AD; prodromal trials with participants with mild cognitive impairment (MCI) who have biomarker evidence indicative of AD pathology; or have mild, moderate, or severe AD dementia.
3. RESULTS
3.1. Overview
There were 126 agents in 152 trials of treatments for AD (as of the index date of January 5, 2021). Twenty‐eight agents are in 41 Phase 3 trials, 74 agents are in 87 Phase 2 trials, and 24 agents are in 24 Phase 1 trials. Figure 1 shows all pharmacologic compounds currently in clinical trials for AD. DMTs are the most common agents being studied (104; 82.5% of the total number of agents in trial); 13 (10.3%) agents in trials target cognitive enhancement; and 9 (7.1%) are intended to treat neuropsychiatric and behavioral symptoms. Of the DMTs 16 (15.4%) have amyloid and 11 (10.6%) have tau as the primary target or as one of several potential effects. Considering DMTs only, there are 17 in Phase 3, 64 in Phase 2, 23 in Phase 1. Across all phases, DMTs comprise 31 (29.8%) biological therapies and 73 (70.2%) small‐molecule drugs. There are 50 repurposed agents in the pipeline—39.6% of the candidate agents.
3.2. Phase 3
In Phase 3 there are 28 agents in 41 trials (Figure 1, Figure 2, Table 1). There are 11 (39.3%) symptomatic agents in Phase 3: six (21.4%) cognitive enhancers and five (17.9%) targeting behavioral symptoms. There are 10 repurposed agents in Phase 3 trials. Among the 17 DMTs there are four biological therapies and 13 oral agents/small molecule therapies. All four of the biological therapies and one of the small molecules—four monoclonal antibodies and one receptor for advanced glycation end products (RAGE) antagonist—have amyloid as the primary or one of several targets (29.4% of DMTs). Other CADRO mechanisms represented among Phase 3 DMT therapies include tau (one agent; 5.9% of Phase 3 DMTs), inflammation/infection (two agents; 11.8%), oxidative stress (two agents; 11.8%), metabolism and bioenergetics (two agents; 11.8%), vascular factors (one agent; 5.9%), synaptic plasticity/neuroprotection (three agents; 17.6%), and gut–brain axis (one agent; 5.9%). Figure 2 shows the MOAs of agents in Phase 3. Four (23.5%) of the DMT agents in Phase 3 are repurposed agents approved for use in another indication. Since the 2020 review, seven Phase 3 trials have been completed or terminated and there are five new agents in this phase.
FIGURE 2.
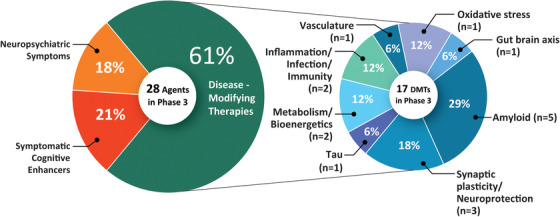
Mechanisms of action of agents in Phase 3 (as classified using the Common Alzheimer's Disease Research Ontology approach). Figure: J Cummings; M de la Flor, PhD, Illustrator
TABLE 1.
Agents in Phase 3 of Alzheimer's disease drug development (ClinicalTrials.gov accessed January 5, 2021)
| Agent | CAD ROMechanism class | Mechanism of action | Therapeutic purpose | Status (CT.gov ID) | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| Aducanumab | Amyloid | Monoclonal antibody directed at Aβ plaques and oligomers | DMT |
Enrolling by invitation |
Biogen | Mar 2020 | Oct 2023 |
|
AGB101 (low‐dose levetiracetam) |
Synaptic Plasticity/Neuroprotection | SV2A modulator; to reduce Aβ‐induced neuronal hyperactivity | DMT |
Recruiting |
AgeneBio, NIA | Jan 2019 | Dec 2022 |
|
Atuzaginstat (COR388) |
Inflammation/Infection | Bacterial protease inhibitor targeting gingipain produced by P. gingivalis to reduce neuroinflammation and hippocampal degeneration | DMT |
Active, not recruiting *(NCT03823404) |
Cortexyme | Mar 2019 | Dec 2022 |
| AVP‐786 | Neurotransmitter receptors | Sigma 1 receptor agonist; NMDA receptor antagonist | Neuropsychiatric symptoms agent (agitation) |
Recruiting |
Avanir | Oct 2017 | Jun 2021 |
|
Recruiting, extension study |
Avanir | Dec 2015 | Jun 2022 | ||||
|
Recruiting |
Avanir | Sep 2020 | Dec 2024 | ||||
|
Recruiting, extension study |
Avanir | Jul 2020 | Dec 2024 | ||||
| Azeliragon | Amyloid, inflammation | RAGE antagonist; to reduce Aβ transport into the brain; mitigate toxic effects of oligomers and reduce inflammation | DMT |
Active, not recruiting *(NCT03980730) |
vTv Therapeutics | Jun 2019 | Jul 2023 |
|
Blarcamesine (ANAVEX2‐73) |
Synaptic plasticity/neuroprotection | Sigma‐1 receptor agonist, M2 autoreceptor antagonist; to ameliorate oxidative stress, protein misfolding, mitochondrial dysfunction, and inflammation | DMT |
Recruiting *(NCT03790709) |
Anavex Life Sciences | Jul 2018 | Dec 2021 |
|
Recruiting *(NCT04314934) |
Anavex Life Sciences | Oct 2019 | Dec 2023 | ||||
| BPDO‐1603 | Undisclosed | Undisclosed | Cognitive enhancer |
Recruiting |
Hyundai Pharmaceutical | Feb 2020 | Mar 2023 |
| Brexpiprazole | Neurotransmitter Receptors | Atypical antipsychotic; D2 receptor partial agonist; serotonin‐dopamine modulator | Neuropsychiatric symptoms agent (agitation) |
Recruiting *(NCT03620981) |
Otsuka | Aug 2018 | Nov 2021 |
|
Recruiting, extension study |
Otsuka | Oct 2018 | Jul 2022 | ||||
|
Recruiting |
Otsuka | May 2018 | Apr 2022 | ||||
|
Recruiting, extension study |
Otsuka | Nov 2018 | May 2021 | ||||
| Caffeine | Metabolism and bioenergetics | Pleiotropic effect on CNS function | Cognitive enhancer |
Not yet recruiting |
University Hospital, Lille | Nov 2020 | Nov 2023 |
| Donepezil | Neurotransmitter receptors | Acetylcholinesterase inhibitor | Cognitive enhancer |
Not yet recruiting |
Assistance Publique ‐ Hôpitaux de Paris | Feb 2021 | Aug 2023 |
| Escitalopram | Neurotransmitter receptors | Selective serotonin reuptake inhibitor | Neuropsychiatric symptoms agent (agitation) |
Recruiting |
Johns Hopkins University, NIA | Jan 2018 | Aug 2022 |
| Gantenerumab | Amyloid | Monoclonal antibody directed at Aβ plaques and oligomers | DMT |
Active, not recruiting |
Roche | Mar 2014 | Apr 2021 |
|
Recruiting |
Roche | Jun 2018 | Nov 2023 | ||||
|
Active, not recruiting |
Roche | Aug 2018 | Nov 2023 | ||||
|
Recruiting, extension study |
Roche | May 2020 | Feb 2023 | ||||
|
Not yet recruiting, extension study |
Roche | Feb 2021 | Dec 2024 | ||||
| Gantenerumab & solanezumab | Amyloid | Monoclonal antibody directed at Aβ plaques and oligomers (gantenerumab); monoclonal antibody directed at Aβ monomers (solanezumab); given in separate arms of the trial | DMT |
Recruiting *(NCT01760005) |
Washington University, Eli Lilly, Roche, NIA, Alzheimer's Association | Dec 2012 | Jul 2022 |
| Ginkgo biloba | Metabolism and bioenergetics | Plant extract with antioxidant properties to improve mitochondrial function | Cognitive enhancer |
Recruiting *(NCT03090516) |
Nanjing Medical University | Aug 2016 | Mar 2020 |
| Guanfacine | Neurotransmitter receptors | Alpha‐2 adrenergic agonist | Cognitive enhancer |
Recruiting |
Imperial College London, UK National Institute of Health Research | Jan 2019 | Mar 2021 |
| GV‐971 | Gut–brain axis | Algae‐derived acidic oligosaccharides; changes microbiome to reduce peripheral and central inflammation | DMT |
Recruiting |
Shanghai Greenvalley | Oct 2020 | Oct 2026 |
| Icosapent ethyl (IPE) | Oxidative stress | Purified form of the omega‐3 fatty acid EPA; to improve synaptic function and reduce inflammation | DMT |
Recruiting *(NCT02719327) |
VA Office of Research and Development, University of Wisconsin, Madison | Jun 2017 | Nov 2021 |
|
Lecanemab (BAN2401) |
Amyloid | Monoclonal antibody directed at Aβ protofibrils | DMT |
Recruiting |
Eisai, Biogen | Mar 2019 | Aug 2024 |
|
Recruiting |
Eisai, Biogen, ACTC, NIA | Jul 2020 | Oct 2027 | ||||
| Losartan & amlodipine & atorvastatin + exercise | Vasculature | Angiotensin II receptor blocker (losartan), calcium channel blocker (amlodipine), cholesterol agent (atorvastatin) | DMT |
Active, not recruiting *(NCT02913664) |
University of Texas Southwestern | Feb 2017 | Mar 2022 |
| Metformin | Metabolism and bioenergetics | Insulin sensitizer to improve CNS glucose metabolism | DMT |
Not yet recruiting *(NCT04098666) |
Columbia University, NIA | Jan 2021 | Apr 2024 |
| Mirtazapine | Neurotransmitter receptors | Alpha‐1 antagonist | Neuropsychiatric symptoms agent (agitation) |
Active, not recruiting |
University of Sussex | Jan 2017 | Mar 2021 |
| Nabilone | Neurotransmitter receptors | Synthetic cannabinoid; antiemetic | Neuropsychiatric symptoms agent (agitation) | Not yet recruiting (NCT04516057) | Sunnybrook Health Sciences Center, ADDF | Oct 2020 | Oct 2025 |
| NE3107 | Inflammation |
MAPK‐1/3 inhibitor; reduces proinflammatory NFκB activation |
DMT |
Not yet recruiting |
Neurmedix | Apr 2021 | Jan 2023 |
| Octohydro‐aminoacridine succinate | Neurotransmitter receptors | Acetylcholinesterase inhibitor | Cognitive enhancer |
Recruiting |
Shanghai Mental Health Center | Aug 2017 | Feb 2021 |
|
Omega‐3 (DHA+EPA) |
Oxidative stress | Antioxidant |
DMT |
Recruiting |
University Hospital, Toulouse | Apr 2018 | Dec 2023 |
| Solanezumab | Amyloid | Monoclonal antibody directed at Aβ monomers |
DMT |
Active, not recruiting |
Eli Lilly, ATRI | Feb 2014 | Jan 2023 |
| Tricaprilin | Metabolism and bioenergetics | Caprylic triglyceride; to induce ketosis and improve mitochondrial and neuronal function | DMT |
Not yet recruiting |
Cerecin | Jan 2021 | Feb 2023 |
|
Troriluzole (BHV4157) |
Synaptic plasticity/neuroprotection | Glutamate modulator; prodrug of riluzole; to improve synaptic function | DMT |
Active, not recruiting *(NCT03605667) |
Biohaven Pharma, ADCS | Jul 2018 | Dec 2020 |
| TRx0237 |
Tau |
Tau protein aggregation inhibitor | DMT |
Recruiting |
TauRx Therapeutics | Jan 2018 | Jun 2023 |
Abbreviations: ACTC, Alzheimer's Clinical Trial Consortium; ADCS, Alzheimer's Disease Cooperative Study; ADDF, Alzheimer's Drug Discovery Foundation; ATRI, Alzheimer's Therapeutic Research Institute; Aβ, amyloid beta; CADRO, Common Alzheimer's Disease and Related Disorders Research Ontology; DMT, disease‐modifying therapy; EPA, eicosapentaenoic acid; GABA, gamma‐aminobutyric acid; MAPK, mitogen activated protein kinase; NFκB, nuclear factor kappa B; NIA, National Institute on Aging; SSRI, selective serotonin reuptake inhibitor; SV2A, synaptic vesicle protein 2A.
Notes: Twenty‐eight agents in 41 Phase 3 clinical trials currently ongoing as of January 5, 2021 according to ClinicalTrials.gov. Bolded terms represent new agents into the 2021 Phase 3 pipeline since 2020.
*Phase 2/3 trials.
There were five Phase 3 prevention trials enrolling cognitively normal participants known to be at risk for AD (preclinical AD). Two of these trials are assessing monoclonal antibodies (solanezumab, gantenerumab), there is one vaccine trial (CAD106), and two trials of small molecules (icosapent ethyl and a combination of losartan, amlodipine, and atorvastatin). There is one Phase 3 trial enrolling both preclinical patients and patients with MCI to mild AD dementia (DIAN‐TU trial); 13 trials in patients with prodromal AD/MCI or prodromal/mild AD dementia; 11 trials of patients with mild to moderate AD; and 11 trials of patients with mild‐to‐severe AD (most of the neuropsychiatric agents).
Phase 3 trials included a mean of 619 participants per trial and a total of 25,373 participants were needed for enrollment. Prevention trials included a mean of 684 participants and had a mean duration of 335 weeks (including the recruitment and the treatment period). DMT trials focusing on prodromal AD or prodromal AD/mild AD dementia had a mean of 772 participants and a mean duration of 240 weeks (including the recruitment and the treatment period). Trials of DMTs enrolling mild‐to‐moderate AD dementia participants included an average of 504 participants and had a mean duration of 177 weeks (including the recruitment and the treatment period). The mean treatment exposure period was 154 weeks for prevention trials, 87 weeks for prodromal AD or prodromal AD/mild AD dementia trials, and 31 weeks for mild‐to‐moderate AD dementia trials. The mean duration of cognitive enhancer trials was 161 weeks (22 treatment weeks), and they included an average of 367 participants. Trials of agents for behavioral symptoms had a mean duration of 210 weeks (15 treatment weeks) and included a mean of 447 subjects. Calculated recruitment periods for trials were: prevention (172 weeks), prodromal AD and prodromal AD/mild AD dementia (120 weeks), and AD dementia trial (123 weeks). Two thirds of Phase 3 trials took longer to complete than originally planned as recorded on ClinicalTrials.gov. The time required for recruitment of the patient population typically exceeded the treatment period by up to two‐ to five‐fold.
3.3. Phase 2
In Phase 2 there are 74 agents in 87 trials (Figure 1, Figure 3, Table 2). Thirty (40.5%) of the Phase 2 agents are repurposed from other indications. Of Phase 2 candidate treatments, there are 64 potential DMTs, six cognitive enhancing agents, and four drugs targeting behavioral symptoms. Among DMTs in Phase 2 there are 21 biologics and 43 small molecules. Using the CADRO approach, four of the small molecules and seven of the biologics in Phase 2 have amyloid reduction as one of the mechanisms (17.2% of DMTs). Other CADRO mechanisms represented among Phase 2 DMT therapies include tau (nine agents; 14.1% of Phase 2 DMTs), inflammation/infection/immunity (12 agents; 18.8%), transmitter systems and receptors (two agents; 3.1%), oxidative stress (one agent; 1.6%), cell death (2 agents; 3.1%), proteostasis (four agents; 6.3%), metabolism and bioenergetics (four agents; 6.3%), vascular factors (three agents; 4.7%), growth factors and hormones (1 agent; 1.6%), synaptic plasticity/neuroprotection (11 agents; 17.2%), epigenetic regulators (one agent; 1.6%), and neurogenesis (one agent; 1.6%). Figure 3 shows the MOAs of agents in Phase 2. There are six trials in Phase 2 involving cell therapies (see Table 4). Twenty‐six of the Phase 2 DMT agents are repurposed after approval for use in another indication. Since the 2020 review, 16 trials have been completed or terminated and there are 22 new agents in the Phase 2 pipeline.
FIGURE 3.
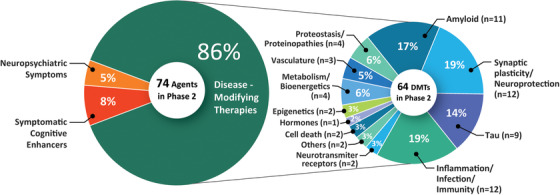
Mechanisms of action of agents in Phase 2. Figure: J Cummings; M de la Flor, PhD, Illustrator
TABLE 2.
Agents in Phase 2 of Alzheimer's disease drug development (ClinicalTrials.gov accessed January 5, 2021)
| Agent | CADRO mechanism class | Mechanism of action | Therapeutic purpose | Status (CT.gov ID) | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| ABvac40 | Amyloid | Active immunotherapy to remove Aβ | DMT |
Recruiting |
Araclon Biotech | Feb 2018 | Feb 2022 |
| ACI‐35 | Tau | Active immunotherapy targeting tau | DMT |
Recruiting *(NCT04445831) |
AC Immune, Janssen | Jul 2019 | Oct 2023 |
| AD‐35 | Neurotransmitter receptors | Acetylcholinesterase inhibitor | Cognitive enhancer |
Active, not recruiting |
Zhejiang Hisun Pharmaceutical | Oct 2018 | Dec 2020 |
|
Active, not recruiting |
Zhejiang Hisun Pharmaceutical | Dec 2018 | Jul 2021 | ||||
| AL002 | Inflammation | Monoclonal antibody targeting TREM2 receptors to promote microglial clearance of Aβ | DMT |
Recruiting |
Alector, AbbVie | Nov 2020 | Aug 2023 |
| ALZ‐801 | Amyloid | Prodrug of tramiprostate; inhibits Aβ aggregation into toxic oligomers | DMT |
Recruiting |
Alzheon | Sep 2020 | May 2023 |
|
ALZT‐OP1 (cromolyn + ibuprofen) |
Inflammation | Combination therapy addressing microglial modulation; promoting microglial clearance of Aβ | DMT |
Recruiting *(NCT04570644) |
AZTherapies | Aug 2020 | Dec 2020 |
| AMX0035 | Cell death | Reduce cell death associated with mitochondrial dysfunction; modulate neuroinflammation | DMT |
Active, not recruiting |
Amylyx Pharmaceuticals, ADDF, Alzheimer's Association | Aug 2018 | Dec 2020 |
| APH‐1105 | Amyloid | Alpha‐secretase modulator to reduce Aβ production | DMT |
Not yet recruiting |
Aphios | Jun 2021 | Dec 2022 |
| AR1001 |
Synaptic plasticity/ neuroprotection |
PDE‐5 inhibitor; improve synaptic plasticity | DMT |
Active, not recruiting |
AriBio Co. | Apr 2019 | Jan 2021 |
|
ATH‐1017 (NDX‐107) |
Synaptic plasticity/ neuroprotection |
Activates signaling via the hepatocyte growth factor system to regenerate neurons and enhance synaptic plasticity | DMT |
Recruiting |
Athira Pharma | Sep 2020 | Oct 2022 |
|
Recruiting |
Athira Pharma | Nov 2020 | Mar 2022 | ||||
| BCG vaccine | Inflammation/immunity |
Vaccination against tuberculosis infection; immunomodulator |
DMT |
Not yet recruiting |
Mindful Diagnostics and Therapeutics | Nov 2020 | Dec 2021 |
| Benfotiamine | Metabolism and bioenergetics | Synthetic thiamine to improve neuronal function | DMT |
Active, not recruiting |
Burke Medical Research Institute, Columbia University, NIA, ADDF | Nov 2014 | May 2021 |
|
Blarcamesine (ANAVEX 2‐73) |
Synaptic plasticity/ neuroprotection |
Sigma‐1 receptor agonist; M2 antagonist; ameliorate oxidative stress, protein misfolding, mitochondrial dysfunction and inflammation | DMT |
Active, not recruiting, extension study |
Anavex Life Sciences | Mar 2016 | Nov 2020 |
| BPN14770 | Synaptic plasticity/ neuroprotection |
PDE‐4 inhibitor; prolongs cAMP activity and improves neuronal plasticity |
DMT |
Active, not recruiting |
Tetra Discovery Partners | Apr 2019 | Feb 2020 |
| Bromocriptine | Neurotransmitter receptors | Dopamine agonist with anti‐Aβ effects | DMT |
Recruiting *(NCT04413344) |
Kyoto University | Jun 2020 | Mar 2022 |
| Bryostatin 1 |
Synaptic plasticity/ neuroprotection |
Protein Kinase C inhibitor; facilitates synaptogenesis | DMT |
Recruiting |
Neurotrope Bioscience, NIH, NIA | Aug 2020 | Nov 2022 |
|
BXCL501 |
Neurotransmitter receptors | Sublingual dexmedetomidine; selective α2‐adrenergic receptor agonist | Neuropsychiatric symptoms agent (agitation) |
Recruiting |
BioXcel Therapeutics | Dec 2019 | Apr 2020 |
|
Crenezumab |
Amyloid | Monoclonal antibody targeting soluble Aβ oligomers | DMT |
Active, not recruiting |
Genentech, NIA Banner Alzheimer's Institute |
Dec 2013 | Feb 2022 |
| CORT108297 | Hormones | Selective glucocorticoid receptor antagonist; reduce neuroendocrine stress responses | Cognitive enhancer |
Recruiting |
Johns Hopkins University | Feb 2021 | Dec 2023 |
| Curcumin + aerobic yoga | Inflammation | Herb with antioxidant and anti‐inflammatory properties | DMT |
Active, not recruiting |
VA Office of Research and Development | Jan 2014 | Dec 2020 |
| Dapagliflozin | Metabolism and bioenergetics | SGLT2 inhibitor; to improve insulin sensitivity and CNS glucose metabolism |
DMT |
Recruiting *(NCT03801642) |
University of Kansas | Jan 2019 | Oct 2022 |
| Daratumumab | Inflammation/immunity | Monoclonal antibody targeting CD38; regulates microglial activity | DMT |
Recruiting |
Northwell Health, Janssen | Nov 2019 | Jun 2022 |
| Dasatinib + quercetin | Inflammation/immunity | Tyrosine kinase inhibitor (dasatinib) and flavonoid (quercetin); senolytic therapy approach to reduce senescent cells and tau aggregation | DMT |
Recruiting* |
The University of Texas Health Science Center at San Antonio, Mayo Clinic | Feb 2020 | Aug 2023 |
|
Not yet recruiting |
Wake Forest University, The University of Texas Health Science Center at San Antonio, Mayo Clinic | Mar 2021 | Mar 2031 | ||||
| Deferiprone | Cell death | Iron chelating agent; reduce damaging reactive oxygen species | DMT |
Recruiting |
Neuroscience Trials Australia | Jan 2018 | Dec 2021 |
| DHA | Oxidative stress | Omega 3 fatty acid; improve synaptic function; antioxidant | DMT |
Recruiting |
University of Southern California, NIA, ADDF | Jul 2018 | Sep 2024 |
|
Donanemab (LY3002813) |
Amyloid | Monoclonal antibody specific for pyroglutamate form of Aβ | DMT |
Active, not recruiting |
Eli Lilly | Dec 2017 | Nov 2021 |
|
Recruiting |
Eli Lilly | Jun 2020 | Apr 2024 | ||||
|
Recruiting |
Eli Lilly | Nov 2020 | Mar 2023 | ||||
| Dronabinol | Neurotransmitter receptors | CB1 and CB2 endocannabinoid receptor partial agonist | Neuropsychiatric symptoms agent (agitation) |
Recruiting |
Mclean Hospital, Johns Hopkins University | Mar 2017 | May 2022 |
|
Edonerpic (T‐817MA) |
Synaptic plasticity/ neuroprotection |
Neurotrophic agent; activates sigma receptors to preserve synaptic plasticity; protect against Aβ toxicity | DMT |
Recruiting |
Toyama Chemical | Dec 2019 | Oct 2022 |
|
Elayta (CT1812) |
Synaptic plasticity/ neuroprotection |
Sigma‐2 receptor antagonist; competes with oligomeric Aβ binding; protect against Aβ‐induced synaptic toxicity | DMT |
Active, not recruiting |
Cognition Therapeutics | Oct 2018 | Jul 2020 |
|
Active, not recruiting *(NCT03493282) |
Cognition Therapeutics | Apr 2018 | Mar 2021 | ||||
| Gantenerumab | Amyloid | Monoclonal antibody directed at Aβ plaques and oligomers | DMT |
Recruiting |
Roche | Dec 2020 | Feb 2024 |
| GB301 | Inflammation/immunity | Regulatory T cells; reduce neuroinflammation | DMT |
Not yet recruiting *(NCT03865017) |
GMP BIO, BHT Lifescience Australia | Dec 2019 | Dec 2021 |
|
Gosuranemab (BIIB092) |
Tau | Monoclonal antibody targeting truncated form of tau | DMT |
Active, not recruiting |
Biogen | May 2018 | Mar 2024 |
| Grapeseed extract |
Proteostasis/ proteinopathies |
Polyphenolic compound; antioxidant; prevent aggregation of Aβ and tau | DMT |
Active, not recruiting |
Mount Sinai School of Medicine, NCCIH | Nov 2014 | Dec 2021 |
| GV1001 | Epigenetic | hTERT peptide vaccine; mimics extra‐telomeric functions to inhibit neurotoxicity, apoptosis, and reactive oxygen species | DMT |
Not yet recruiting |
GemVax & Kael | Sep 2019 | Feb 2022 |
| IONIS MAPTRx (BIIB080) | Tau | Antisense oligonucleotide targeting tau expression; MAPT RNA inhibitor | DMT |
Active, not recruiting *(NCT03186989) |
Ionis Pharmaceuticals |
Jun 2017 | May 2022 |
|
IVIG (NewGam 10%) |
Amyloid | Polyclonal antibody; remove amyloid | DMT |
Active, not recruiting |
Sutter Health | Jan 2011 | Dec 2019 |
| JNJ‐63733657 | Tau | Monoclonal antibody targeting soluble tau | DMT |
Not yet recruiting |
Janssen | Jan 2021 | Mar 2025 |
| Lamivudine (3TC) | Epigenetic | Nucleoside reverse transcriptase inhibitor; reduces genetic rearrangements | DMT |
Not yet recruiting *(NCT04552795) |
University of Texas Health Science Center at San Antonio | Feb 2021 | Jun 2022 |
|
Lecanemab (BAN2401) |
Amyloid | Monoclonal antibody directed at protofibrils | DMT |
Active, not recruiting |
Eisai | Dec 2012 | Feb 2025 |
| Lenalidomide | Inflammation/immunity | Reduce inflammatory cytokines; modulate innate and adaptive immune responses | DMT |
Recruiting |
Cleveland Clinic, NIA | Jul 2020 | Sep 2024 |
| Levetiracetam |
Synaptic plasticity/ neuroprotection |
SV2A modulator; improve synaptic function; reduce Aβ‐induced neuronal hyperactivity | DMT |
Active, not recruiting |
University of California, San Francisco | Jun 2014 | Dec 2021 |
|
Active, not recruiting |
UCB Pharma, University of Oxford, NHS Foundation Trust | Oct 2018 | Sep 2021 | ||||
|
Recruiting |
Medical College of Wisconsin, NIA | Apr 2019 | Mar 2021 | ||||
|
Recruiting |
Beth Israel Deaconess Medical Center | Aug 2019 | Nov 2023 | ||||
| Liraglutide | Metabolism and bioenergetics | Glucagon‐like peptide 1 receptor agonist; improve CNS glucose metabolism | DMT |
Active, not recruiting |
Imperial College London | Jan 2014 | Dec 2019 |
|
L‐Serine |
Inflammation | Dietary amino acid; reduce brain inflammation and preserve nerve cells | DMT |
Recruiting |
Dartmouth‐Hitchcock Medical Center | Mar 2017 | Dec 2021 |
| Lupron (leuprolide acetate depot) | Growth factors and hormones | GnRH receptor agonist; reduce effects of elevated GnRH and gonadotropins on the brain | DMT |
Recruiting |
New York University | Dec 2020 | Feb 2026 |
| Metabolic cofactor supplementation | Metabolism and bioenergetics | Mixture of N‐acetylcysteine, L‐carnitine tartrate, nicotinamide roboside, and serine to increase mitochondrial activity | Cognitive enhancer |
Recruiting |
Istanbul Medipol University Hospital, ScandiBio Therapeutics | Dec 2019 | Sep 2020 |
| Montelukast | Inflammation | Cysteinyl leukotriene type 1 (cysLT‐1) receptor antagonist; effects on inflammatory processes, neuronal injury, blood‐brain‐barrier integrity, and Aβ protein accumulation | DMT |
Recruiting (NCT03402503) –buccal film |
IntelGenx Corp. | Nov 2018 | Jul 2021 |
|
Recruiting (NCT03991988)—tablet |
Emory University | Sep 2019 | Jun 2022 | ||||
|
Neflamapimod (VX‐745) |
Synaptic plasticity/ neuroprotection |
p38 MAPK‐α inhibitor; enhance endolysosomal function to reduce synaptic dysfunction | DMT |
Recruiting |
EIP Pharma | Oct 2018 | Jan 2021 |
| Nicotinamide |
Tau |
HDAC inhibitor; to reduce tau‐induced microtubule depolymerization and tau phosphorylation | DMT |
Recruiting |
University of California, Irvine | Jul 2017 | Jun 2020 |
|
Nicotine transdermal patch |
Neurotransmitter receptors | Nicotinic acetylcholine receptor agonist | Cognitive enhancer |
Recruiting |
University of Southern California, NIA, ATRI, Vanderbilt University | Jan 2017 | Dec 2020 |
| Nilotinib | Proteostasis/ proteinopathies | Tyrosine kinase inhibitor; autophagy enhancer; promotes clearance of Aβ and tau | DMT |
Active, not recruiting |
Georgetown University | Jan 2017 | Feb 2020 |
| Omega‐3 PUFA | Vasculature | Polyunsaturated fatty acid; reduce damage to small blood vessels | DMT |
Active, not recruiting |
Oregon Health and Science University, NIA | May 2014 | Jun 2021 |
|
Pepinemab (VX15) |
Inflammation | Monoclonal antibody directed at semaphorin 4D to reduce inflammation | DMT |
Recruiting *(NCT04381468) |
Vaccinex, ADDF, Alzheimer's Association | Sep 2020 | Dec 2021 |
| Posiphen |
Proteostasis/ proteinopathies |
Inhibitor of APP and α‐synuclein | DMT |
Recruiting *(NCT02925650) |
QR Pharma, ADCS | Mar 2017 | Dec 2020 |
|
Recruiting *(NCT04524351) |
Annovis Bio, Parexel | Aug 2020 | Sep 2021 | ||||
| Prazosin | Neurotransmitter receptors | Alpha‐1 adrenoreceptor antagonist | Neuropsychiatric symptoms agent (agitation) |
Recruiting |
ADCS, NIA | Oct 2018 | Dec 2022 |
| PQ912 | Amyloid | Glutaminyl cyclase (QC) enzyme inhibitor to reduce pyroglutamate Aβ (pGlu‐Aβ) production | DMT |
Not yet recruiting |
Vivoryon Therapeutics AG, ADCS, NIA | Jun 2021 | Jan 2023 |
|
Recruiting |
Vivoryon Therapeutics AG, ADCS, NIA | Jul 2020 | Jul 2023 | ||||
| PU‐AD | Tau | Heat shock protein 90 inhibitor; to prevent aggregation and hyperphosphorylation of tau | DMT |
Active, not recruiting |
Samus Therapeutics | Jun 2020 | Dec 2022 |
| Rapamycin (sirolimus) |
Proteostasis/ proteinopathies |
mTOR inhibitor; ameliorate metabolic and vascular effects of aging | DMT |
Not yet recruiting |
The University of Texas Health Science Center at San Antonio | Jan 2021 | Aug 2023 |
|
RO7126209 (brain shuttle gantenerumab) |
Amyloid | Anti‐Aβ monoclonal antibody with enhanced BBB penetration | DMT |
Recruiting *(NCT04639050) |
Roche | Mar 2021 | Oct 2024 |
|
SAGE‐718 |
Neurotransmitter receptors | NMDA receptor positive allosteric modulator | Cognitive enhancer |
Not yet recruiting |
Sage Therapeutics | Dec 2020 | Jun 2021 |
|
Semorinemab (RO7105705) |
Tau | Monoclonal antibody to remove extracellular tau | DMT |
Active, not recruiting |
Genentech | Oct 2017 | Jun 2022 |
|
Active, not recruiting |
Genentech | Jan 2019 | Jun 2023 | ||||
|
S‐equol (AUS‐131) |
Metabolism and bioenergetics | Agonist of non‐hormonal estrogen receptor B located on mitochondria to potentiate mitochondrial function | DMT |
Recruiting |
Ausio Pharmaceuticals | May 2017 | Nov 2020 |
|
Sovateltide (PMZ‐1620) |
Neurogenesis | Endothelin B receptor agonist; augments activity of neuronal progenitor cells | DMT |
Recruiting |
Pharmazz | Mar 2018 | Aug 2021 |
|
Sumifilam (PTI‐125) |
Synaptic plasticity/ neuroprotection |
Filamin A protein inhibitor; stabilize the interaction of soluble Aβ and the alpha7 nicotinic acetylcholine receptor, reducing Aβ and synaptic dysfunction | DMT |
Recruiting |
Cassava Sciences, NIA | Mar 2020 | Apr 2022 |
| Suvorexant | Neurotransmitter receptors | Dual orexin receptor antagonist; improved sleep with effects on CSF Aβ | DMT |
Not yet recruiting |
Washington University School of Medicine | Jan 2021 | Jan 2025 |
| Tacrolimus |
Synaptic plasticity/ neuroprotection |
Calcineurin inhibitor; to prevent Aβ‐induced dendritic spine loss and synaptic dysfunction | DMT |
Not yet recruiting |
Massachusetts General Hospital | Dec 2021 | Jan 2023 |
| Telmisartan & Perindopril | Vasculature | Angiotensin II receptor blocker (telmisartan); angiotensin converting enzyme inhibitor (perindopril) | DMT |
Recruiting |
Sunnybrook Health Sciences Centre, ADDF |
Mar 2014 | Mar 2022 |
|
THC‐free CBD oil |
Neurotransmitter receptors | Cannabinoid with effects on cannabinoid receptors | Neuropsychiatric symptoms agent (agitation) |
Not yet recruiting |
Eastern Virginia Medical School, Ananda Hemp | Jul 2020 | Dec 2021 |
| Thiethylperazine (TEP) | Amyloid | Activates transport protein ABCC1 to remove Aβ | DMT |
Active, not recruiting |
Immungenetics AG | Nov 2017 | Jul 2021 |
|
Tilavonemab (ABBV‐8E12) |
Tau | Monoclonal antibody to remove tau and prevent propagation | DMT |
Active, not recruiting |
AbbVie | Oct 2016 | Jul 2021 |
|
Recruiting, extension study |
AbbVie | Mar 2019 | Jul 2026 | ||||
|
Vafidemstat (ORY‐2001) |
Synaptic plasticity/ neuroprotection | HDAC demethylase inhibitor and MAO‐B inhibitor; neuroprotective | DMT |
Active, not recruiting |
Oryzon Genomics, ADDF | May 2019 | Nov 2020 |
| Valacyclovir | Infection/immunity | Antiviral against HSV‐1 and ‐2 infection; to prevent Aβ aggregation and plaque deposition | DMT |
Recruiting |
New York State Psychiatric Institute, NIH, NIA | Feb 2018 | Aug 2022 |
| VGH‐AD1 | Undisclosed | Traditional Chinese herbal medicine | Cognitive enhancer |
Not yet recruiting * |
Taipei Veterans General Hospital, Taiwan | Feb 2020 | Dec 2020 |
|
Zagotenemab (LY3303560) |
Tau | Monoclonal antibody to remove tau and reduce tau propagation | DMT |
Active, not recruiting |
Eli Lilly | Apr 2018 | Oct 2021 |
Abbreviations: ADCS, Alzheimer's Disease Cooperative Study; ADDF, Alzheimer's Drug Discovery Foundation; APP, amyloid precursor protein; Aβ, amyloid beta; BBB, blood‐brain barrier; CADRO, Common Alzheimer's Disease and Related Disorders Research Ontology; cAMP, cycling adenosine monophosphate; CB, cannabinoid; DMT, disease‐modifying therapy; GnRH, gonadotropin‐releasing hormone; HSV, herpes simplex virus; hTERT, human telomerase reverse transcriptase; MAPK, mitogen‐activated protein kinase; mTOR, mammalian target of rapamycin; NCCIH, National Center for Complementary and Integrative Health; NIA, National Institute on Aging; NMDA, N‐methyl‐D‐aspartate; PDE, phosphodiesterase; PUFA, polyunsaturated fatty acids; SGLT2, sodium glucose transporter 2; SV2A, synaptic vesicle protein 2A; TREM2, Triggering Receptor Expressed On Myeloid Cells 2.
Notes: Seventy‐four agents in 87 Phase 2 clinical trials currently ongoing as of January 5, 2021 according to ClinicalTrials.gov. Bolded terms represent new agents into the 2021 Phase 2 pipeline since 2020.
*Phase 1/2 trials.
TABLE 4.
Stem cell therapy in clinical trials for Alzheimer's disease (ClinicalTrials.gov accessed January 5, 2021)
| Agent | Phase | Status (CT.gov ID) | Sponsor | Subject characteristics | Amyloid evidence at entry |
|---|---|---|---|---|---|
| Allogeneic human MSCs | 1 |
Recruiting |
University of Miami | Mild‐to‐moderate AD with MMSE of 20–26 | Amyloid PET |
| Allogeneic human MSCs | 1 |
Active, not recruiting |
Longeveron | Mild‐to‐moderate AD with MMSE of 18–24 | Amyloid PET |
| SNK01 (autologous natural killer cell) | 1 |
Not yet recruiting |
NKMax America | MCI or AD | Not required |
| Allogenic adipose MSC‐Exosomes | 1/2 |
Recruiting |
Ruijin Hospital, Cellular Biomedicine Group | Mild‐to‐moderate AD with MMSE of 10–24 | Not required |
| Autologous adipose‐derived MSCs | 1/2 |
Active, not recruiting |
Hope Biosciences | Preclinical/MCI | Amyloid PET |
| CB‐AC‐02 (placenta derived MSCs) | 1/2 |
Recruiting |
CHABiotech Co. | Mild‐to‐moderate AD with MMSE of 10–26 | Amyloid PET |
| Human umbilical cord blood‐derived MSCs (NEUROSTEM) | 1/2 |
Recruiting, extension study |
Medipost | Probable AD with KMMSE of 18–26 | Amyloid PET |
| Allogeneic human MSCs | 2 |
Recruiting |
Stemedica | Mild‐to‐moderate AD with MMSE of 12–24 | Amyloid PET |
| AstroStem (autologous adipose‐derived MSCs) | 2 |
Not yet recruiting |
Nature Cell Co. | Mild AD with MMSE of 20–24 | CSF amyloid |
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; KMMSE, Korea Mini‐Mental State Examination; MMSE, Mini‐Mental State Examination; MSC, mesenchymal stem cell; PET, positron emission tomography.
Two Phase 2 trials are prevention trials involving participants with preclinical AD (assessing crenezumab and levetiracetam); 49 trials involved patients with prodromal or prodromal/mild AD dementia; 30 were trials for mild‐to‐moderate AD; one trial included patients with mild, moderate, or severe AD; one trial included preclinical patients and patients with prodromal/mild AD; and one trial was for mild‐to‐moderate AD or healthy participants.
Phase 2 trials for DMTs included an average of 147 participants in each trial and were on average 197 weeks in duration including an average of 52 weeks of treatment and 121 weeks for recruitment. Phase 2 trials for cognitive‐enhancing agents included an average of 117 participants and were on average 103 weeks in duration including an average of 31 weeks of treatment. Phase 2 trials for agents targeting behavioral symptoms included 104 participants and were on average 145 weeks in duration including an average of 8 weeks of treatments. Of Phase 2 trials 78.6% took longer to complete than originally planned as recorded on ClinicalTrials.gov. All types of trials took between two and three times longer to recruit patients to the trial than to assess the effects of the treatment during the exposure period.
3.4. Phase 1
Phase 1 has 24 agents in 24 trials (Figure 1, Table 3). Repurposed agents comprise 41.6% (N = 10) of the Phase 1 pipeline. Of Phase 1 candidates, there are 23 potential DMTs; one cognitive enhancing agent; and no agents targeting behavioral symptoms. There are 17 DMT small molecules and six DMT biologics being assessed in Phase 1. Review of the CADRO categories reveals that none of the small molecules and only one of the biologics in Phase I has amyloid reduction as the major mechanism attributed to the agent (4.3% of DMTs). Other CADRO mechanisms represented among Phase 1 DMT therapies include tau (one agent; 4.3% of Phase 1 DMTs), inflammation (five agents; 21.7%), cell death (one agent; 4.3%), proteostasis (two agents; 8.7%), metabolism and bioenergetics (one agent; 4.3%), vascular factors (two agents; 8.7%), growth factors and hormones (two agents; 8.7%), synaptic plasticity/neuroprotection (two agents; 8.7%), epigenetic regulators (two agents; 8.7%), circadian rhythm (one agent; 4.3%), and neurogenesis (one agent; 4.3%). There are three trials in Phase 1 involving stem cell therapies (Table 4).
TABLE 3.
Agents in Phase 1 of Alzheimer's disease drug development (ClinicalTrials.gov accessed January 5, 2021)
| Agent | CADRO mechanism class | Mechanism of action | Therapeutic purpose | Status (CT.gov ID) | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| AAV‐hTERT | Epigenetic | Extending telomeres may benefit AD; reduce Aβ‐induced neurotoxicity; effects on multiple cellular pathways | DMT |
Recruiting |
Libella Gene Therapeutics | Oct 2019 | Jan 2021 |
| AAVrh.10hAPOE2 | Epigenetic | Conversion of the apoE protein isoforms in the CSF of APOE ε4 homozygotes from APOE ε4 to APOE ε2‐APOE ε4 | DMT |
Recruiting |
Cornell University | Oct 2019 | Dec 2021 |
| AL003 | Inflammation | Monoclonal antibody targeting SIGLEC‐3 (CD33); reactivates microglia and immune cells in the brain; improve microglial clearance of toxic proteins | DMT |
Recruiting |
Alector | Mar 2019 | Aug 2021 |
| Allopregnanolone (Allo) | Growth factors/hormones | GABA‐A receptor modulator; promote neurogenesis and reduce inflammation | DMT |
Recruiting |
University of Southern California, University of Arizona, Alzheimer's Association | Oct 2019 | Oct 2020 |
| BEY2153 | Proteostasis/proteinopathies | Aβ and tau aggregation inhibitor; inhibits neuronal death | DMT |
Recruiting |
BeyondBio | Aug 2020 | Oct 2021 |
| BDPP (bioactive dietary polyphenol preparation) |
Proteostasis/ proteinopathies |
Prevents Aβ and tau aggregation | DMT |
Recruiting |
Johns Hopkins University, Mount Sinai School of Medicine | Jun 2015 | Jun 2021 |
| Dabigatran | Vasculature | Direct thrombin inhibitor; reduce neurovascular damage | DMT |
Not yet recruiting |
University of Rhode Island, ADDF, Boehringer Ingelheim | Nov 2018 | Dec 2021 |
| Dexmedetomidine | Circadian rhythm | Selective α2‐adrenergic receptor agonist; neuroprotection | DMT |
Recruiting |
Neurological Associates of West Los Angeles | Apr 2019 | Dec 2021 |
|
Edicotinib (JNJ‐40346527) |
Inflammation | CSF‐1R antagonist; attenuates microglial proliferation and neurodegeneration | DMT |
Not yet recruiting |
Janssen, University of Oxford | Nov 2020 | Dec 2021 |
| Efavirenz | Epigenetics | NNRTI; promote cholesterol removal; enhance amyloid reduction | DMT |
Recruiting |
Case Western Reserve University, Cleveland Medical Center, Massachusetts General Hospital | May 2018 | Dec 2021 |
|
Elayta (CT1812) |
Synaptic plasticity/ neuroprotection |
Sigma‐2 receptor antagonist; competes with oligomeric Aβ binding at synapse | DMT |
Recruiting |
Cognition Therapeutics | May 2018 | Mar 2021 |
| Empagliflozin | Metabolism and bioenergetics | SGLT2 inhibitor; improve glycemic control; enhance neuronal function | DMT |
Recruiting |
NIA | Mar 2019 | Dec 2022 |
| Emtricitabine | Inflammation | NRTI; reduce neuroinflammation | DMT |
Not yet recruiting |
Butler Hospital, Alzheimer's Association, Brown University | Jan 2021 | Aug 2023 |
| Lu AF87908 | Tau | Monoclonal antibody to reduce tau | DMT |
Recruiting |
Lundbeck | Sep 2019 | May 2021 |
| LY3372993 | Amyloid | Monoclonal antibody to reduce Aβ | DMT |
Recruiting |
Eli Lilly | Jul 2020 | Feb 2022 |
| MK‐1942 + donepezil | Neurotransmitter receptors | Undisclosed (MK‐1942) | Cognitive enhancer |
Not yet recruiting |
Merck | Feb 2021 | Sep 2021 |
| MK‐4334 | Growth factors and hormones | Corticosteroid to reduce inflammation | DMT |
Not yet recruiting |
Merck | Sep 2019 | Feb 2020 |
| NNI‐362 | Neurogenesis | Enhance neurogenesis; activates progenitor cells | DMT |
Recruiting |
Neuronascent, NIA | Aug 2019 | Dec 2020 |
| REM0046127 |
Synaptic plasticity/ neuroprotection |
Regulates calcium dyshomeostasis; tau and Aβ reduction | DMT |
Recruiting |
reMYND, NeuroScios GmbH | Nov 2020 | Oct 2021 |
| Salsalate | Inflammation | Non‐steroidal anti‐inflammatory to reduce inflammation | DMT |
Active, not recruiting |
University of California, San Francisco | Jul 2017 | Jul 2021 |
| Telmisartan | Vasculature | Angiotensin II receptor blocker | DMT |
Recruiting |
Emory University | Apr 2015 | Oct 2021 |
| Trehalose | Cell death | Induces autophagy and promotes clearance of aggregated proteins | DMT |
Recruiting |
Mashhad University of Medical Sciences | Aug 2020 | Aug 2022 |
| Vorinostat | Epigenetics | Histone deacetylase (HDAC) inhibitor; enhanced synaptic plasticity | DMT |
Recruiting |
German Center for Neurodegenerative Diseases, University Hospital, Bonn, University of Gottingen | Sep 2017 | Mar 2022 |
| XPro1595 | Inflammation | TNF inhibitor; reduce neuroinflammation | DMT |
Recruiting |
Immune Bio, Alzheimer's Association | Nov 2019 | Dec 2020 |
Abbreviations: AAV, adeno‐associated virus; ADDF, Alzheimer's Drug Discovery Foundation; APOE, apolipoprotein E; Aβ, amyloid beta; CADRO, Common Alzheimer's Disease and Related Disorders Research Ontology; CSF, cerebrospinal fluid; CSF‐1R, colony‐stimulating factor 1 receptor; DMT, disease‐modifying therapy; GABA, gamma‐aminobutyric acid; hTERT, human telomerase reverse transcriptase; NIA, National Institute on Aging; NNRTI, non‐nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; SGLT2, sodium glucose co‐transporter 2; SIGLEC‐3, sialic acid‐binding Ig‐like lectin 3; TNF, tumor necrosis factor.
Notes: Twenty‐four agents in 24 Phase 1 clinical trials currently ongoing as of January 5, 2021 according to ClinicalTrials.gov. Bolded terms represent new agents into the 2021 Phase 1 pipeline since 2020.
Phase 1 trials have an average duration of 127 weeks (recruitment and treatment period) and include a mean of 43 participants in each trial.
3.5. Trial sponsors
Across all trials, 49% are sponsored by the biopharma industry, 29% by academic medical centers (usually with funding from NIH), 14% are public–private partnerships, and 7% by others. In Phase 3, 61% of trials are sponsored by the biopharma industry, 20% by academic medical centers (with funding from NIH), 12% are public–private partnerships, and 7% by others. In Phase 2, 47% of trials are sponsored by the biopharma industry, 31% by academic medical centers (with NIH funding), 15% are public–private partnerships, and 7% by others. Table 5 shows the sponsor of agents in each phase of development. Repurposed agents are more likely to have academic medical centers/NIH sponsors (59%) and less likely to have industry sponsors (16%; Table 5).
TABLE 5.
Trial sponsor for each phase of AD drug development and the number of trials of repurposed agents supported by each entity (ClinicalTrials.gov accessed January 5, 2021)
| N of trials (%) | |||||
|---|---|---|---|---|---|
| Sponsor | Phase 3 | Phase 2 | Phase 1 | All phases | Repurposed agents |
| Biopharma industry | 25 (61%) | 41 (47%) | 9 (38%) | 75 (49%) | 9 (16%) |
| Academic medical centers/NIH | 8 (20%) | 27 (31%) | 9 (38%) | 49 (29%) | 33 (59%) |
| Public–private partnerships (PPP) | 5 (12%) | 13 (15%) | 4 (17%) | 22 (14%) | 5 (9%) |
| Others | 3 (7%) | 6 (7%) | 2 (8%) | 11 (7%) | 9 (16%) |
Abbreviation: NIH, National Institutes of Health.
3.6. Biomarkers
Table 6 shows the biomarkers used as entry criteria or as outcome measures in current Phase 2 and Phase 3 AD clinical trials of DMTs as described on ClinicalTrials.gov; not all trial descriptions on the website note if biomarkers are included in the trial.
TABLE 6.
Biomarkers as outcome measures or as entry criteria in Phase 2 and Phase 3 DMT trials (ClinicalTrials.gov accessed January 5, 2021)
| N of trials (%) | ||
|---|---|---|
| Biomarker role in trial a | Phase 3 DMTs | Phase 2 DMTs |
| Biomarker as an outcome measure a | ||
| CSF amyloid | 15 (25%) | 10 (48%) |
| CSF tau | 17 (28%) | 9 (43%) |
| FDG‐PET | 7 (11%) | 1 (5%) |
| vMRI | 8 (13%) | 8 (38%) |
| Plasma amyloid | 7 (11%) | 2 (10%) |
| Plasma tau | 2 (3%) | 1 (5%) |
| Amyloid PET | 5 (8%) | 7 (33%) |
| Tau PET | 4 (7%) | 3 (14%) |
| Biomarker as an entry criterion a | ||
| Amyloid PET | 4 (17%) | 11 (14%) |
| CSF amyloid | 1 (4%) | 9 (12%) |
| Amyloid PET or CSF amyloid | 6 (25%) | 11 (14%) |
| Tau PET | 0 | 2 (3%) |
| CSF amyloid or CSF tau | 0 | 2 (3%) |
| Amyloid PET or CSF tau | 0 | 1 (1%) |
Abbreviations: CSF, cerebrospinal fluid; DMT, disease‐modifying therapy; FDG, fluorodeoxyglucose; PET, positron emission tomography; vMRI, volumetric magnetic resonance imaging.
Percentages refer to the percent of trials that used any biomarker as an outcome or the percent that used biomarkers as an entry criterion.
Of the 24 Phase 3 DMT trials, four trials (17%) used amyloid positron emission tomography (PET) as an entry criterion, one (4%) used cerebrospinal fluid (CSF) amyloid, and six (25%) used either amyloid PET or CSF‐amyloid. Thirteen (54%) of the Phase 3 trials did not use biomarkers for study entry. In Phase 2, 11 (14%) DMT trials used amyloid PET as an entry criterion, nine (12%) used CSF amyloid, and 11 (14%) used either amyloid PET or CSF amyloid. Two (3%) of the Phase 2 DMT trials used tau PET as an entry criterion, two (3%) used either CSF amyloid or CSF tau, and one (1%) used either amyloid PET or CSF tau. Forty (53%) of the Phase 2 trials did not require biomarker confirmation for study entry. There is one trial in Phase 3 of a cognitive enhancer trial that requires CSF amyloid or CSF tau for entry.
Of Phase 3 DMT trials, 15 (63%) use biomarkers as supportive outcomes. In Phase 2, nine DMT trials (12%) have biomarkers as primary outcomes and 29 (38%) have biomarkers as supportive outcomes. Three (13%) of the Phase 3 DMT trials include tau PET imaging as an outcome and nine (12%) of Phase 2 DMT trials include tau PET imaging as an outcome.
3.7. Trial participants
Across all currently active trials, the total number of participants needed is 38,826. Of these, 25,373 are in Phase 3 trials; 12,414 in Phase 2 trials; and 1039 in Phase 1 trials. Table 7 shows the major types of trials, the average duration of exposure for each type of trial, and the number of patients currently participating in each type of trial. This allows calculation of the total number of participant‐weeks across all active trials and shows that there are 2,540,014 participant weeks of time devoted to clinical trials. This sum does not include time devoted to screening prior to randomization or the number of participant‐weeks consumed in screen fails of individuals who do not progress to randomization.
TABLE 7.
Total person weeks contributed by participants for each type of trial (ClinicalTrials.gov accessed January 5, 2021)
| Phase | Type of trial | Average duration of treatment (Weeks) | Total number of participants | Total participant weeks devoted to clinical trials |
|---|---|---|---|---|
| Phase 3 | Prevention (preclinical AD) | 154 | 4103 | 631,862 |
| DMT (not prevention) | 82 | 14,150 | 1,160,300 | |
| Cognitive enhancing | 22 | 2200 | 48,400 | |
| Psychotropic | 15 | 4,920 | 73,800 | |
| Phase 2 | DMT | 52 | 11,181 | 581,412 |
| Cognitive enhancing | 31 | 817 | 25,327 | |
| Psychotropic | 8 | 416 | 3328 | |
| Phase 1 | All | 15 | 1039 | 15,585 |
| Total | 2,540,014 weeks | |||
Abbreviations: AD, Alzheimer's disease; DMT, disease‐modifying therapy.
3.8. Global distribution of trials
Table 8 shows the distribution of trials divided into North American (United States and Canada) only, non–North American only (excluding United States and Canada), and North American and non–North American combined. Phase 2 trials are more often conducted in North America (60%) only and involve North America in 80%. Thirty‐seven percent of Phase 3 trials involve North American only and 71% included North America and non–North American countries. Some Phase 1 trials conducted outside the United States may not be registered on ClinicalTrials.gov and may have gone undetected in this review. Across all phases, 54% of trials are conducted in North America only, 25% are conducted only outside North America, and 21% are conducted with both North American and non–North American sites. North America participates in 75% of all trials registered on ClinicalTrials.gov.
TABLE 8.
Global distribution of trials (ClinicalTrials.gov accessed January 5, 2021)
| No. trials (%) | |||
|---|---|---|---|
| Phase 3 | Phase 2 | Phase 1 | |
| North America (US & Canada) | 15 (37%) | 52 (60%) | 15 (63%) |
| Non–North America | 12 (29%) | 18 (21%) | 8 (33%) |
| Both | 14 (34%) | 17 (20%) | 1 (4%) |
4. DISCUSSION
In 2020, the FDA approved 53 new drugs across all therapeutic categories. Four of these were agents administered as part of imaging procedures including flortaucipir (Tauvid) to be used with PET imaging of the brain to estimate the density and distribution of aggregated tau neurofibrillary tangles in adult patients with cognitive impairment who are being evaluated for AD. Eleven of the approved treatments addressed central nervous system (CNS) diseases. 21 Five of the approved agents are DMTs involving neurodegeneration—2 for neuromyelitis optica, 1 for multiple sclerosis, 1 for Duchenne muscular dystrophy, and 1 for spinal muscular atrophy. Treatments for neuroblastoma, neurofibromatosis, migraine, on/off in Parkinson's disease, and an agent for sedation comprised the other CNS approvals. There were no new therapies for AD. Thirty‐six percent of the CNS approvals were for DMTs, suggesting that DMT‐related targets, biomarkers, and trial designs are progressing.
The AD drug development pipeline comprises 126 agents in 152 trials (Figure 1). There are 28 agents in Phase 3, 74 in Phase 2, and 24 in Phase 1. One hundred four putative DMTs are being assessed (17 in Phase 3, 64 in Phase 2, 23 in Phase 1). DMTs represent 83% of the pipeline of agents. There are 13 cognitive enhancers and nine drugs targeting neuropsychiatric symptoms in the pipeline. The 126 agents in the pipeline compares to 121 in the pipeline in 2020; 13 with one less agent in Phase 3, nine additional agents in Phase 2, and three less agents in Phase 1. Since the 2020 pipeline, 7 trials in Phase 3, 18 trials in Phase 2, and 9 trials in Phase 1 have been either completed, terminated, suspended, or the status is unknown. There are five prevention trials in Phase 3 and two prevention trials in Phase 2; the number of prevention trials in Phase 3 has remained constant over the past 6 years, while the number of Phase 2 prevention trials has varied (0 to 4).
Using the CADRO classification, review of the pipeline reveals a proliferation of mechanistic approaches to the treatment of AD (Figure 4). Phase 2 has both more agents in trials and a greater diversity of targets than other phases; this may reflect a more diversified approach to treatment targets in Phase 2 or a reduction of targets in Phase 3 because of the lack of success in Phase 2 outcomes. Amyloid and tau protein are important targets; inflammation, synaptic plasticity, neuroprotection, and bioenergetics/metabolism account for most of the rest of the current pipeline of agents.
FIGURE 4.
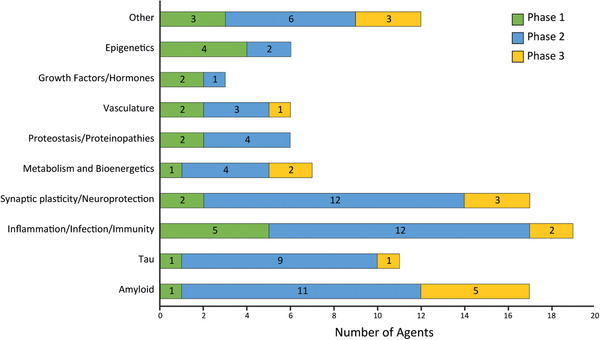
Mechanisms of action of disease‐modifying agents in all phases of clinical trials grouped according to the Common Alzheimer's Disease Research Ontology (CADRO). Figure: J Cummings; M de la Flor, PhD, Illustrator
Biomarkers play an increasing role in drug development and use the amyloid (A), tau (T), neurodegeneration (N)—A/T/N—biomarker framework. 22 , 23 Biomarkers are integrated into development programs for diagnostic confirmation (e.g., amyloid PET, CSF amyloid), analytic stratification (e.g., apolipoprotein E genotype), prognostic anticipation (e.g., tau PET, CSF phosphorylated tau [p‐tau]), assessment of neuroprotection and disease modification (magnetic resonance imaging, fluorodeoxyglucose [FDG] PET, CSF total tau, neurofilament light [NfL], neurogranin), or for safety monitoring in monoclonal antibodies and possibly other therapies. 22 Biomarkers are rarely used for diagnostic confirmation in trials for cognitive enhancing agents or drugs targeting neuropsychiatric symptoms. Lack of diagnostic precision in these trials may contribute to commonly observed challenges including robust placebo responses and failure of placebo groups to decline. 24 , 25 More use of biologic confirmation in AD trials or more trials in populations of “dementia” including several major types of dementia and less emphasis on precise definition are anticipated. The pimavanserin trial of dementia‐related psychosis is an example of the latter with five types of clinically defined dementia included in the trial population. 26
The rapid evolution of blood‐based biomarkers may have a significant impact on patient screening and diagnosis and is expected to eventually act as entry criteria or outcomes in some circumstances. Plasma amyloid beta protein (Aβ) 42/40 correlates highly with amyloid PET 27 and is commercially available for clinical application (PrecivityAD). Plasma p‐tau‐181 and plasma p‐tau 217 are elevated as AD advances and may be useful in trials to assess impact on tau pathology of trial participants. 28 , 29 Plasma NfL, a measure of neuronal degeneration, is increasingly elevated as AD progresses. 30 With these measures it is possible to characterize all of the ATN framework elements using peripheral markers. Data will soon be available regarding whether these measures can qualify participants for trials or will serve as screens indicating which participants should be further characterized by amyloid or tau PET or CSF measures.
Examination of sponsorship shows that most Phase 3 trials are supported by biopharmaceutical companies, whereas at Phase 2 47% of trials have biopharmaceutical sponsors: 31% are NIH/academic medical center trials, 15% are public–private partnerships, and 7% have other sponsors. This reflects the increased focus of biopharmaceutical companies on late‐stage development and outsourcing larger portions of the early‐stage drug development enterprise. 31 Repurposed agents are more common in trials in Phase 2 and more likely to be in trials sponsored by NIH/academic medical centers (Table 5). Repurposed agents rarely progress to Phase 3; the Phase 2 trials of these drugs produce invaluable data on molecules that can be reengineered to have better intellectual property protection, pathways, and processes that may be promising therapeutic targets, and effects on biomarkers or novel clinical outcomes. These trials provide important educational opportunities for a variety of trainees who will comprise the clinical trial workforce of the future. 32
The total number of participants in trials and the total number of trial weeks they contribute is dramatic. Participants required for currently active trials total 38,826; when calculated in terms of participant‐weeks in trials (Table 7), the total number in all ongoing trials is 2,540,014. There are at least an equivalent number of weeks contributed by research partners, doubling the total participant‐partner weeks to 5,080,028 weeks. This large number is a marked underestimate of total weeks because it does not include weeks spent in screening prior to randomization or weeks contributed by participants who are excluded during the screening period.
A continuing challenge to trial conduct is the slow rate of recruitment. The average length of time required for recruitment of participants exceeds the duration of the treatment period in nearly all trials. There has been no trend toward shortening recruitment times over the past 5 years despite expanded efforts to improve recruitment. Although sponsors plan for slow recruitment, 66.7% of Phase 3 trials and 78.6% of Phase 2 trials took longer to complete than originally anticipated as recorded on ClinicalTrials.gov. The COVID‐19 pandemic further exacerbated recruitment struggles with many clinics suspending research at least temporarily, delaying visits during this time, and experiencing attrition due to SARS‐CoV‐2 infections in patients and family members or an unwillingness to attend medical facilities. 33 These observations identify a gap in the trial system that requires remedy to accelerate trials and drug development.
Many trials are conducted globally to increase the number of sites and accelerate recruitment. This is especially important for large Phase 3 trials. Global trials allow diversified participant exposures and improved understanding of effects of ethnicity, standard of care, body size, nutrition, and educational level on trial outcomes. Table 8 shows that most trials conducted have US representation either as the sole country in which the trials are conducted or as part of a world‐wide global trial program. This is, in part, a reflection of the fact that ClinicalTrials.gov is a US trial registry (although note that most trials conducted by sponsors anywhere in the world are registered on ClinicalTrials.gov). Combined with the participant weeks in trials noted above, it is evident that participants and research partners throughout the world are making a remarkable contribution to AD drug development efforts through trial participation.
Next generation biotherapeutics (NGBs) include cell, gene, and nucleotide therapies. 34 There are nine trials of cell therapies in AD (Table 4), one oligonucleotide targeting tau expression, and several epigenetic modulators. Technologies have advanced to facilitate trials of gene therapy in AD and trials are anticipated in the pipeline. 35 Stem cell interventions may promote nerve cell regeneration, adding a dimension to therapeutic response beyond the slowing of cognitive decline targeted by DMTs in current trials. 36 , 37
Therapeutic concepts related to the larger universe of diseases of aging are beginning to influence the AD pipeline. Cell senescence occurs throughout the lifespan but plays a larger role with aging and is postulated to contribute to many diseases of aging including vascular disease, arthritis, and neurodegeneration. 38 Senolytic therapies directed at removing senescent cells are included in the AD pipeline. Three senolytics in current AD trials are metformin, rapamycin, and dasatinib plus quercetin; these therapies have shown benefit in non‐clinical models of aging and AD. 39 , 40 , 41 A toolkit of biomarkers relevant to trials of senolytics suggests measures relevant to peripheral effects of treatment that may be useful in some AD trials. 42 Many questions remain unresolved concerning the relationship of cellular senescence and neurodegeneration/AD; 43 trials of senolytics will provide key insights into the value of these agents as treatments for AD and late‐life cognitive decline.
5. SUMMARY
Clinical trials are the sole means by which new treatments for AD can be approved. Increasing the number of trials, enhancing trial efficiency, and improving trial success are critical to advancing new therapeutics for AD. Trials are being conducted in preclinical, prodromal, and AD dementia populations in an effort to prevent, delay the onset, slow the progression, or improve the cognitive and behavioral symptoms of AD. There are slightly more agents in the AD pipeline in 2021 compared to 2020. There is an increasing diversity of targets and corresponding therapeutic mechanisms of drugs in the AD pipeline. Participants are making a remarkable contribution of 2,540,014 participant‐weeks to support AD drug development, making a major contribution to treatment development efforts.
CONFLICTS OF INTEREST
JC has provided consultation to Acadia, Alkahest, AriBio, Avanir, Axsome, Behren Therapeutics, Biogen, Cassava, Cerecin, Cerevel, Cortexyme, EIP Pharma, Eisai, Foresight, GemVax, Genentech, Green Valley, Grifols, Janssen, Jazz, Merck, Novo Nordisk, Otsuka, ReMYND, Resverlogix, Roche, Signant Health, Sunovion, Suven, United Neuroscience, and Unlearn AI pharmaceutical and assessment companies. Dr. Cummings has stock options in ADAMAS, AnnovisBio, MedAvante, BiOasis, and United Neuroscience. Dr. Cummings owns the copyright of the Neuropsychiatric Inventory. Dr Cummings is supported by NIGMS grant P20GM109025; NINDS grant U01NS093334; NIA grant R01AG053798; and NIA grant P20AG068053. GL is a full‐time employee of Biogen. KZ provides consultation to Green Valley Pharmaceuticals. JF has no disclosures. KT has no disclosures.
Cummings J, Lee G, Zhong K, et al. Alzheimer's disease drug development pipeline: 2021. Alzheimer's Dement. 2021;7:e12179. 10.1002/trc2.12179
REFERENCES
- 1. Alzheimer's Association . 2020 Alzheimer's disease facts and figures. Alzheimer Dement. 2020;16:391‐460. [Google Scholar]
- 2. Vermunt L, Sikkes SAM, van den Hout A, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15:888‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Drug Administration . Early Alzheimer's disease: developing drugs for treatment guidance for industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER; ). 2018. [Google Scholar]
- 4. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer's disease. Lancet. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Alzheimer's and Related Dementia Research Portfolio; Accessed January 4, 2021; https://iadrp.nia.nih.gov/about/cadro [Google Scholar]
- 6. Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer's disease dementia and insomnia: a randomized trial. Alzheimers Dement. 2020;16:541‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer's disease psychosis: a phase 2, randomised, placebo‐controlled, double‐blind study. Lancet Neurol. 2018;17:213‐222. [DOI] [PubMed] [Google Scholar]
- 8. Sabbagh MN, Cummings J. Open peer commentary to “Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen December 2019”. Alzheimer's Dement. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cummings J, Morstorf T, Lee G. Alzheimer's drug‐development pipeline: 2016. Alzheimer's Dement (N Y). 2016;2:222‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer's disease drug development pipeline: 2017. Alzheimer's Dement (N Y). 2017;3:367‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cummings J, Lee G, Ritter A, Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimer's Dement (N Y). 2018;4:195‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimer's Dement (N Y). 2019;5:272‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2020. Alzheimer's Dement (N Y). 2020;6:e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FDA/US National Library of Medicine of the National Institutes of Health (NIH) . Clinicaltrials.gov.
- 15. Lassman SM, Shopshear OM, Jazic I, Ulrich J, Francer J. Clinical trial transparency: a reassessment of industry compliance with clinical trial registration and reporting requirements in the United States. BMJ Open. 2017;7:e015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phillips AT, Desai NR, Krumholz HM, Zou CX, Miller JE, Ross JS. Association of the FDA Amendment Act with trial registration, publication, and outcome reporting. Trials. 2017;18:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeVito NJ, Bacon S, Goldacre B. Compliance with legal requirement to report clinical trial results on clinicaltrials.gov: a cohort study. Lancet. 2020;395:361‐369. [DOI] [PubMed] [Google Scholar]
- 18. Bauzon J, Lee G, Cummings J. Repurposed agents in the Alzheimer's disease drug development pipeline. Alzheimer's Res Ther. 2020;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummings J, Fox N. Defining disease modifying therapy for Alzheimer's disease. J Prev Alzheimer's Dis. 2017;4:109‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG‐2 criteria. Lancet Neurol. 2014;13:614‐629. [DOI] [PubMed] [Google Scholar]
- 21. Food and Drug Administration . Advancing Health Through Innovation: New Drug Therapy Approvals 2020. US Food and Drug Administration. Silver Spring, MD. 2020. [Google Scholar]
- 22. Cummings J. The role of biomarkers in Alzheimer's disease drug development. Adv Exp Med Biol. 2019;1118:29‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lang F, Mo Y, Sabbagh M, et al. Intepirdine as adjunctive therapy to donepezil for mild‐to‐moderate Alzheimer's disease: a randomized, placebo‐controlled, phase 3 clinical trial (MINDSET). Alzheimer's Dement TRCI. 2021. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenberg PB, Drye LT, Porsteinsson AP, et al. Change in agitation in Alzheimer's disease in the placebo arm of a nine‐week controlled trial. Int Psychogeriatr. 2015;27:2059‐2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cummings J, Ballard C, Tariot P, et al. Pimavanserin: potential treatment For dementia‐related psychosis. J Prev Alzheimers Dis. 2018;5:253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schindler SE, Bollinger JG, Ovod V, et al. High‐precision plasma beta‐amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93:e1647‐e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moscoso A, Grothe MJ, Ashton NJ, et al. Time course of phosphorylated‐tau181 in blood across the Alzheimer's disease spectrum. Brain. 2020;144:325‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho‐tau217 for Alzheimer's disease vs other neurodegenerative disorders. JAMA. 2020;324:772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer's disease. JAMA Neurol. 2019;76:791‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dierks RML, Bruyere O, Reginster JY. Critical analysis of valuation and strategical orientation of merger and acquisition deals in the pharmaceutical industry. Expert Rev Pharmacoecon Outcomes Res. 2018;18:147‐160. [DOI] [PubMed] [Google Scholar]
- 32. Supino PG, Borer JS. Teaching clinical research methodology to the academic medical community: a fifteen‐year retrospective of a comprehensive curriculum. Med Teach. 2007;29:346‐352. [DOI] [PubMed] [Google Scholar]
- 33. Ousset PJ, Vellas B. Viewpoint: impact of the Covid‐19 outbreak on the clinical and research activities of memory clinics: an Alzheimer's disease center facing the Covid‐19 crisis. J Prev Alzheimer's Dis. 2020;7:197‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. IQVIA . The Changing Landscape of Research and Development. IVQIA Institute for Human Data Science. Parsippany, New Jersey. 2019. [Google Scholar]
- 35. Sudhakar V, Richardson RM. Gene therapy for neurodegenerative diseases. Neurotherapeutics. 2019;16:166‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vasic V, Barth K, Schmidt MHH. Neurodegeneration and neuro‐regeneration‐Alzheimer's disease and stem cell therapy. Int J Mol Sci. 2019:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han F, Bi J, Qiao L, Arancio O. Stem cell therapy for Alzheimer's disease. Adv Exp Med Biol. 2020;1266:39‐55. [DOI] [PubMed] [Google Scholar]
- 38. Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16:718‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Farr SA, Roesler E, Niehoff ML, Roby DA, McKee A, Morley JE. Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer's disease. J Alzheimers Dis. 2019;68:1699‐1710. [DOI] [PubMed] [Google Scholar]
- 41. Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020;19:513‐532. [DOI] [PubMed] [Google Scholar]
- 42. Justice JN, Ferrucci L, Newman AB, et al. A framework for selection of blood‐based biomarkers for geroscience‐guided clinical trials: report from the TAME. Biomarkers Workgroup Geroscience. 2018;40:419‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saez‐Atienzar S, Masliah E. Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nat Rev Neurosci. 2020;21:433‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]


