Abstract
Introduction: Trimethylamine N-oxide (TMAO) may play a key mediator role in the relationship between the diet, gut microbiota and cardiovascular diseases, particularly in people with kidney failure. The aim of this review is to evaluate which foods have a greater influence on blood or urinary trimethylamine N-oxide (TMAO) levels. Methods: 391 language articles were screened, and 27 were analysed and summarized for this review, using the keywords “TMAO” AND “egg” OR “meat” OR “fish” OR “dairy” OR “vegetables” OR “fruit” OR “food” in December 2020. Results: A strong correlation between TMAO and fish consumption, mainly saltwater fish and shellfish, but not freshwater fish, has been demonstrated. Associations of the consumption of eggs, dairy and meat with TMAO are less clear and may depend on other factors such as microbiota or cooking methods. Plant-based foods do not seem to influence TMAO but have been less investigated. Discussion: Consumption of saltwater fish, dark meat fish and shellfish seems to be associated with an increase in urine or plasma TMAO values. Further studies are needed to understand the relationship between increased risk of cardiovascular disease and plasma levels of TMAO due to fish consumption. Interventions coupled with long-term dietary patterns targeting the gut microbiota seem promising.
Keywords: TMAO, trimethylamine N-oxide, foods, fish, meat, eggs, dairy, microbiota
1. Introduction
Trimethylamine N-oxide (TMAO) has been identified as an osmolyte molecule of the fish world [1]. In many marine species, it acts as a protein stabiliser that counteracts the concentration of urea. The oxidised form decomposes into trimethylamine (TMA) which is responsible for the characteristic odour of putrefied fish [2]. The free TMAO from seafood is directly absorbed into the systemic circulation without metabolism by the gastrointestinal microbiome. In humans, the gut microbiota is responsible for the formation of TMA [3]. The metabolic pathway allows the conversion of choline, betaine and carnitine molecules into TMA, whilst a liver enzyme belonging to the flavin-containing monooxygenases (FMO) family is responsible for its conversion into TMAO [4].
In recent years, numerous studies have suggested that high blood TMAO levels might be associated with heart disease, atherosclerosis, diabetes and cancer [5,6]. Compelling evidence suggests that circulating TMAO may promote atherosclerosis by altering the clearance of cholesterol in the liver, promoting inflammation and oxidation of LDL cholesterol by up-regulation of the macrophage scavenger and foam cell formation [7]. The current literature shows that TMAO may be a major risk factor for cardiovascular disease (CVD), especially in individuals who have already had a cardiovascular event or have a kidney disease [8,9].
The factors that can influence the plasma concentration of TMAO are different and complex [10]. For instance, TMAO precursors are among the main bacterial products produced by the intestinal microbiota, and it is likely that the microbiota is essential for TMAO metabolism [6].
The correlation between fish intake and TMAO has been demonstrated since the discovery of this molecule. For other foods, there is an extensive conflicting literature. The aim of our review is to evaluate all the studies in the literature to establish which foods are associated with an increase in plasma or urinary TMAO.
Search Strategy and Selection Criteria
The present research was conducted and reported based on the PRISMA guidelines. We searched PubMed, Web of Science, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) electronic databases using the following keywords as title/abstract fields: (“Trimethylamine N-oxide” OR “TMAO”) AND (“egg” OR “meat” OR “fish” OR “dairy” OR “vegetables” OR “fruit” OR “food”). We researched papers from 1 January 1990 to 1 December 2020. Studies published in languages other than English were not considered.
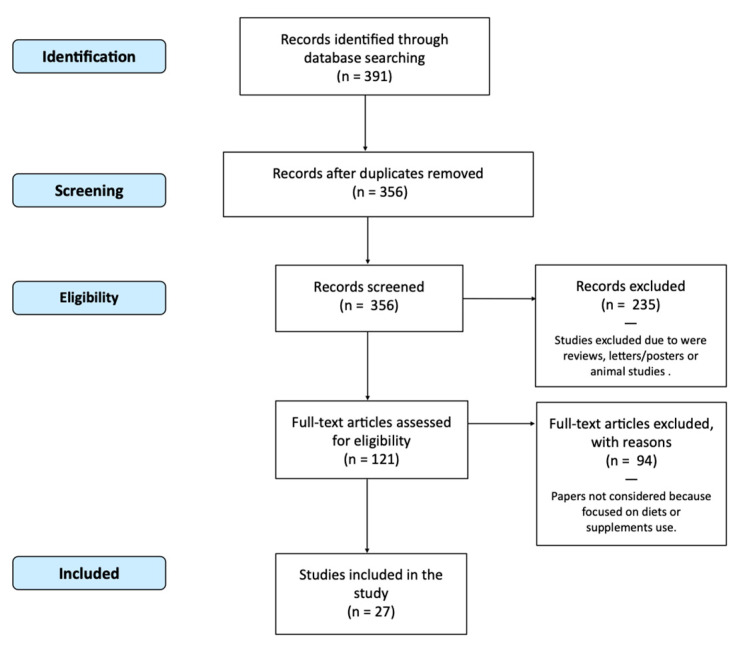
Figure 1 depicts the flow of the step-by-step process of applying the inclusion and exclusion criteria to generate a final number of studies for analysis in the review. Studies evaluating food intake in correlation with urinary or plasma TMAO were considered. Reviews, letters, comments, animal studies, and abstracts for posters were excluded. Thus, studies that focused on diets, supplements or nutraceutical use were not considered. Studies evaluating only TMAO measurement techniques or biochemical aspects without focusing on food were excluded.
Figure 1.
Flow Chart (PRISMA) of Studies Included.
391 studies with these characteristics were obtained; 35 papers were excluded because they were duplicate studies. Of the remaining 356, an additional 235 were excluded with use of the criteria above after a review of the title and abstract, leaving 121 articles for full text review. Ninety-four papers were not considered because they focused on diets, supplements or nutraceutical use. 27 papers were finally selected. The full texts of the available studies were analysed and the results are reported in this review.
2. Results
27 studies were considered for this review [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Their major features such as study design, methodology used for TMAO determination, and the effects of foods on TMAO are given in Table 1.
Table 1.
Differences of urinary or plasmatic trimethylamine (TMAO) production from foods following human ingestion.
| First Author |
Year | Study Design |
Sample | Methodology for TMAO Determination $ |
Red Meat (e.g., Beef, Lamb) |
Meat Products (e.g., Sausages, Bacon) | White Meat (e.g., Chicken) |
Fish | Eggs | Milk and Other Dairy Food | Plant-Based Foods | Reference | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Svensson BG | 1994 | Comparative study | Urine | LC/MS | ↑↑ | [11] | Swedish Work Environment Fund and others | ||||||
| Zhang AQ | 1999 | Clinical trial | Urine | TMA/DMA | = | = | = | ↑↑ | = | = | = | [12] | The Leverhulme Trust |
| Tang WHW | 2013 | Prospective | Plasma and Urine | UHPLC-MS/MS | ↑↑ | [13] | National Institutes of Health and its Office of Dietary Supplements | ||||||
| Miller CA | 2014 | RCT | Plasma | LC/MS | ↑↑ | [14] | Egg Nutrition Centre | ||||||
| West AA | 2014 | Clinical trial | Plasma | UHPLC-ESI-MS/SM | = | [15] | American Egg Board and the Agriculture Research Institute at California State Polytechnic University, Pomona | ||||||
| Zheng H | 2015 | Cross-sectional | Urine | NMR | = | [16] | The Danish Council for Strategic Research, Arla Foods, and the Danish Dairy Research Foundation in the project | ||||||
| Rohrmann S | 2016 | Cross-sectional | Plasma | LC/MS | = | = | = | = | ↑ | [17] | Advancement of Human Nutrition | ||
| Di Marco DM | 2017 | Crossover randomised | Plasma | LC/MS | = | [18] | Egg Nutrition Centre | ||||||
| Iannotti LL | 2017 | RCT | Plasma | LC/MS | ↑↑ | [19] | The Mathile Institute for the Advancement of Human Nutrition | ||||||
| Kruger R | 2017 | Comparative study | Plasma | LC/MS | ↑ | ↑ | ↑↑ | [20] | Federal Ministry of Food and Agriculture | ||||
| Cho CE | 2017 | RCT | Urine and Plasma | LC-MS/MS | ↑ | ↑ | ↑↑ | = | [21] | Egg Nutrition Centre and Beef Checkoff | |||
| Lemos BS | 2018 | Crossover randomised | Plasma | LC-MS/MS | = | [22] | Egg Nutrition Centre | ||||||
| Missimer A | 2018 | RCT | Plasma | LC/MS | = | [23] | Egg Nutrition Centre | ||||||
| Schmedes M | 2018 | RCT | Plasma | LC/MS | ↑↑ § | [24] | Aarhus University project “Seafood protein in the prevention of the metabolic syndrome” | ||||||
| Pignanelli M | 2019 | Prospective cohort | Plasma | UHPLC-MS/MS | ↑↑ | [25] | Canadian Institutes of Health Research | ||||||
| Wang Z | 2019 | RCT | Plasma Urine |
LC /MS | ↑↑ | [26] | National Institutes of Health and the Office of Dietary Supplements | ||||||
| Yu D | 2019 | Case-control multicentre | Urine | LC/MS | ↑↑ # | ↑↑ # | ↑↑ | = | = | = | [27] | National Institutes of Health (and others) | |
| Andraos S | 2020 | Cross-sectional | Plasma | UHPLC-MS/MS | ↑↑ | = (children) ↑↑ (adults) |
= (children) ↑↑ (adults) |
↑↑ | = | [28] | The New Zealand-Australia Life Course Collaboration on Genes, Environment, Nutrition and Obesity | ||
| Burton KJ | 2020 | Crossover randomised | Plasma and Urine | Plasma: UHPLC-MS/MS Urine: NMR (urine) |
↑↑ (fermented) ç ↑ (non-fermented) |
[29] | Joint Programming Initiative: A Healthy Diet for a Healthy Life | ||||||
| De Souza RJ | 2020 | Cross-sectional | Plasma | MSI-CE-MS | ↑↑ | ↑↑ | ↑↑ | ↑↑ | [30] | Canadian Institutes of Health Research (CIHR) | |||
| Gessner A | 2020 | Community-based | Plasma | LC/MS | = | = | ↑↑ ^ | = | = | [31] | None declared | ||
| Gibson R | 2020 | Cross-sectional | Urine | NMR | ↑↑ | [32] | None declared | ||||||
| Hagen IV | 2020 | RCT | Plasma and Urine | UHPLC-MS/MS | ↑↑ | [33] | Bergen Medical Research Foundation | ||||||
| Hamaya R | 2020 | Retrospective | Plasma | UPLC-ESI-MS/MS | = | ↑↑ | ↑ | [34] | US Highbush Blueberry Council | ||||
| Macpherson ME | 2020 | Prospective cohort | Plasma | UHPLC-MS/MS | = | = | = | = | = | = | [35] | South-Eastern Norway-Regional Health Authority | |
| Yin X | 2020 | RCT | Urine | NMR | ↑ | ↑↑ | [36] | NutriTech and the European Research Council | |||||
| Zhu C | 2020 | Crossover randomised | Plasma | LC/MS | = | [37] | Egg Nutrition Council |
* Effects on urinary and/or plasmatic TMAO based on study conclusions. $ Please refer to Table S1 in the Supplementary Materials for an explanation of the acronyms. (↑↑) food is strongly associated with TMAO concentrations;(↑) food is positively associated with TMAO concentrations; (=) food does not significantly impact TMAO concentrations; CVD; cardiovascular disease; RCT; randomised controlled trial; ç yogurt and cheese; # only fried; § (lean); ^ only men.
2.1. Fish
15 studies evaluated the possible relationship between fish and TMAO. 13 studies demonstrated that levels of urinary or plasma TMAO and/or TMA are significantly associated with the intake of fish [11,12,20,21,24,27,28,30,31,32,33,34,36]. One of the earliest studies evaluated 46 different foods and demonstrated that only fish and other sea-products significantly increase urinary TMAO [12]. Cho et al. [21] supposed that the rapid rise in circulating TMAO in response to fish consumption might demonstrate that the absorption of intact dietary TMAO is independent of the microbiota. In one paper, the association of fish and shellfish intake with TMAO plasma concentrations was limited to men only [31]. Only two papers demonstrated no association. Rohrmann et al. [17] revealed that meat, egg or fish consumption is not associated with TMAO, choline or betaine concentrations. Thus, a prospective cohort study showed that dietary intake of fish does not significantly impact the TMAO value in immunodeficient subjects [35].
Table 2 shows the four studies that specifically evaluated the influence on TMA and TMAO of eating different types of fish. TMAO was higher in subjects that eat saltwater fish and shellfish but not freshwater fish [27]. These data were confirmed in another study that evidenced higher TMAO values in the group that consumed shellfish and dark meat fish (tuna steak, mackerel, salmon, sardines, bluefish and swordfish) [36]. Another paper showed that cod intake has stronger effects on plasma and urine TMAO concentrations than salmon intake [33]. One study evaluated cooking methods and demonstrated that TMAO values are strongly associated with deep-fried fish consumption [27].
Table 2.
Associations of different types of fish intake with TMAO.
| First Author | Year | Study Design | Cod | Farmed Salmon | Halibut | Herring | Mackerel | Sardine | swordfish | Shellfish | Clam | Tuna | Trout | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang AQ # | 1999 | Clinical trial | ↑↑ 5135.3 |
↑↑ 8230.2 |
↑↑ 4345 |
↑ 1424.1 |
↑ 2769.4 |
↑ 1562 |
= 377.1 |
= 301.8 |
= 495.2 |
[12] | ||
| Yu D | 2019 | Case-control multicentre | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | = | [27] |
| Hagen IV | 2020 | RCT | ↑↑ | ↑ | [33] | |||||||||
| Hamaya R | 2020 | Retrospective | ↑↑ | ↑? | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | [34] |
TMA and TMAO: ↑↑ > 4000; ↑ 1000–3999; = less than 1000 mmol/8 h; # urinary TMAO production from foods following human ingestion (227 g); * only fried; RCT: randomised clinical trial.
2.2. Eggs
17 studies that assessed the correlation between egg consumption and increased TMAO were evaluated. Robust correlations between TMAO and eggs were demonstrated in six studies [13,14,19,25,30,34]. Pignanelli et al. [25] described that the intake of egg yolk contributes significantly to plasma levels of TMAO. The phosphatidylcholine in egg yolk, via action of the intestinal microbiome, may be the major contributor to production of TMAO. The increase in TMAO induced by egg consumption has also been related to cardiovascular risk. Tang et al. studied the effects on plasma TMAO after a test dose of two hard-boiled eggs and found that patients in the highest quartile for TMAO had a 2.5-fold increase in the three-year risk of myocardial infarction or stroke [13]. In contrast, studies mainly starting from 2014 have assessed little or no effect of egg consumption on TMAO levels [12,15,17,18,21,22,23,27,31,35,37]. Some of these studies have revealed that plasma choline and betaine increase dose-dependently with egg intake. Many of these studies have been funded by the egg industry [15,18,21,22,23,37].
2.3. Meat
12 studies [12,17,20,21,26,27,28,30,31,34,35,36] evaluated the possible correlation between meat consumption and an increase of TMAO. Seven studies showed that the levels of urinary or plasma TMAO and/or TMA are significantly associated with the intake of meat. In most of these studies, the differences between white meat, red meat and preserved meat were not evaluated. Yu et al. showed that TMAO is strongly associated with deep-fried meat but not with red meat or poultry [27]. In one study, the effect on TMAO of meat and fish was compared. Fish was shown to induce a two-fold increase in urinary TMAO compared to meat [36]. Five papers [14,19,33,36,37] demonstrated that ingestion of meats has no measurable effects on plasma or urinary TMAO.
2.4. Dairy
Eight studies that assessed the correlation between fermented and non-fermented dairy consumption and increased TMAO were evaluated. Two papers [17,29] showed a positive association between dairy food consumption and plasma TMAO concentrations. Lower circulating and urinary TMAO with fermented dairy consumption compared to non-fermented dairy consumption has been shown [29].
2.5. Plant-Based Foods
Three papers evaluated plant-based food consumption [14,37]. Ingestion of fruits, vegetables, cereals [12] and fibre [35] did not significantly impact TMAO concentrations. Different foods were evaluated by Yu et al. [27]; they showed that consumption of soy foods or legumes does not modify urinary or plasma TMAO values.
3. Discussion
It has been supposed that TMAO may be atherogenic, prothrombotic and inflammatory [38]. Compared to healthy subjects, patients suffering from CVD have higher blood TMAO levels [39]. Thus, TMAO predicted CVD and mortality in a prospective cohort study [40]. CVD risk was related to blood serum TMAO levels, even after accounting for covariates such as meat, fish, cholesterol and energy intake. TMAO may be a cardinal halfway marker that connects dietary foods and fat with gut microbiota metabolism [41]. Recent studies have identified plasma TMAO as a dose-dependent risk factor for CVD that may promote atherosclerosis through the increase of cholesterol storage in macrophages [13,42,43]. TMAO is also related to metabolic syndromes and cancers [44]. However, from the review of the literature, there is no agreement whether TMAO may be a proatherogenic compound or, conversely, a marker of CVD. As proposed by Papandreou et al. [45], TMAO levels in CVD could be the result of disease-related dysbiosis and this would account for the observed intra-individual variability in TMAO levels. Furthermore, the microbiome that is implicated in TMAO metabolism may play an autonomous role in disease progression, making TMAO a biomarker and not an inducer of CVD. The production of TMAO could be an expression of individual differences in the gut microbiome [21].
A number of studies have provided hypotheses as to which processes induce systemic TMAO levels. Wang et al. [26] have suggested enhanced dietary precursors, increased microbial TMA/TMAO production from carnitine, and reduced renal TMAO excretion. They also revealed that ending consumption of red meat cuts down plasma TMAO within four weeks. Koeth et al. hypothesised [43] that in omnivores dietary L-carnitine is converted via a first fast generation of the atherogenic intermediate γ-butyrobetaine, succeeded by transformation into TMA via microbiota.
It is important to understand which foods or which other nutritional strategies (e.g., cooking method or selective microbiome intervention) have a greater effect on plasma TMAO levels, as this would allow for better nutritional prescribing, particularly in patients with modestly impaired renal function [9] and type-2 diabetes mellitus (T2DM) [46] (in which TMAO has been demonstrated to have even more deleterious effects on CVD risk). Some authors have proposed that a simpler method of reducing TMAO might be to eat fewer foods containing TMA precursors and increase those that favour non-TMA-producing bacteria (e.g., vegetables/fruit) or suppression of FMO3 activity (e.g., vegetables containing indole) [47].
Our review found that the majority of studies demonstrated a strong correlation between TMAO content (per gram of protein) and fish consumption. For other foods, especially those of animal origin, results are conflicting. Fruits, vegetables, cereals and fibre have been less investigated but would not appear to have any effect on TMAO. These data could be further evidence confirming that, in subjects with renal insufficiency, substituting animal-based proteins with plant-based proteins has shown reductions in the severity of hypertension, hyperphosphatemia and metabolic acidosis [48]. The majority of studies do not report a correlation between dairy consumption and TMAO. Consumption of fermented dairy products such as yogurt and cheese appears to have less effect on TMAO, probably because of the beneficial effect on gut microflora [29]. With regard to meat (white, red or processed) and eggs, many studies suggest a possible correlation with increased TMAO, but further independently conducted randomised controlled trials (RCTs) are needed to establish the full correlation. Nevertheless, compared to meat, fish has been shown to cause a two-fold increase in urinary TMAO.
Fish consumption induces a rapid rise in circulating TMAO because the absorption of intact dietary TMAO occurs independently of the gut microbiota [3]. Similar to that of omega-3 polyunsaturated fatty acids, the TMAO content in fish is different for each species (Table 2) and is mainly present in deep-sea varieties such as cod, haddock and halibut. The TMAO content of other fish, including salmon, depends on several factors including whether bred or wild and the period of capture [33].
How is it possible that fish consumption, which according to most studies reduces the risk of CVD [49], induces such a clear increase in plasma and urinary TMAO? Several hypotheses have been proposed to explain this apparent contradiction. A recent review by Ufnal M, et al. [50] postulated that increased plasma TMAO could be a compensatory effect that prevents cells from hydrostatic and osmotic stresses. The increase in plasma TMAO in CVD may be similar to that observed for plasma natriuretic peptide B, which is considered a marker of CVD risk but also a compensatory response that results in beneficial effects for the overloaded heart. Thus, a recent study in animal models has shown that a TMAO supplementation could even be beneficial in counteracting hypertension-related heart failure [51]. Similarly, in another recent review it was suggested that TMAO elevation may be a compensatory mechanism in response to disease. TMAO may act as a molecular chaperone to antagonise the disease progression [45]. The TMAO-fish paradox could be explained by the presence of heart-healthy nutrients in fish that would offset the negative effects of TMAO [52]. In fact, it would be wrong to focus only on the presence of TMAO and leave out other important elements. It has been seen that the omega-3 polyunsaturated fatty acids EPA and DHA are the basis of cardioprotective effects. These have an important metabolic action, reducing triglyceride values and improving the lipid profile through various mechanisms that have positive effects on cardiovascular health [53,54]. Practical implications also suggest that the method of preparing may influence the TMAO plasma level. Association of TMAO has been found with deep-fried meat or fish, but not with stir-fried meat or fish or deep-fried wheat or rice [27]. Elevated TMAO has also been linked to substantially increased risk for type 2 diabetes and metabolic syndrome. Indeed, as it has been demonstrated in models of ischaemic injury that the elevation of TMAO may be the result of injury caused by disease that would induce the up-regulation of TMAO by the FMO gene regulation [45].
DiNicolantonio et al. suggested that the correlations between TMAO and T2DM risk may be stronger than those for CVD risk. They also supposed that the increased risk for vascular events in subjects with elevated TMAO may be mediated largely by hepatic insulin resistance [55]. These assumptions could open a new scenario on TMAO as a marker associated with the intake of fish and meats. Thus, as we have shown in a previous review, higher intake of animal protein might be related to an increased risk of T2DM and CVD [56].
The variability in plasma and urine TMAO between foods could also be related to the methodology used to assess TMAO. As shown in Table 1, most papers evaluated only plasma TMAO without considering TMA or urinary excretion. As proposed by Papandreou et al. it would probably be more useful to use plasma TMA/TMAO ratio, because it is a more accurate and standardized marker [45].
Finally, we acknowledge our narrative review suffers from some limitations, mainly resulting from the heterogeneity of studies analysed in terms of research design and characteristics of subjects included. A potential limitation of the present revision is the fact that the search was limited to a few databases and only the English language; nevertheless, a large number of studies were identified. In order to find a correlation between individual foods and TMAO, we decided not to consider studies that included more than one food or dietary protocol. The decision to focus research on individual foods clearly restricted the results and thus the possibility of giving clear answers.
4. Conclusions
This review shows that most studies reveal a correlation between the consumption of many fish types (saltwater fish, dark meat fish and shellfish) and urine or plasma TMAO values. A correlation of increased TMAO with other animal-origin foods such as meat and eggs has been highlighted by most of the studies considered.
The food choice, whilst certainly very important, cannot be considered as the only factor for the total control of blood TMAO values. Gut microbiomes act directly and indirectly on the metabolism of TMAO and its precursors. In this sense, interventions targeting the gut microbiota seem promising.
Further studies, independent of the food industry, are needed to establish more definitive correlations between foods, microbiota and TMAO levels.
Abbreviations
| TMAO | trimethylamine N-oxide |
| TMA | trimethylamine |
| RCT | randomised controlled trial |
| CVD | cardiovascular disease |
| T2DM | type-2 diabetes mellitus |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13051426/s1, Table S1. Methodology for TMAO determination.
Author Contributions
Conceptualization, M.L. and A.D.L.; methodology, M.L. and L.D.R.; software, G.A. and D.M.; validation, M.L. and M.G.T.; formal analysis, M.F. and M.C.; Writing—Original draft preparation, M.L. and G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this manuscript are publicly available from previous publications and fully disclosed in Table 1 and Table 2 of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Velasquez M.T., Ramezani A., Manal A., Raj D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins. 2016;8:326. doi: 10.3390/toxins8110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker R., Chaykin S. The Biosynthesis of Trimethylamine-N-Oxide. J. Biol. Chem. 1962;237:1309–1313. doi: 10.1016/S0021-9258(18)60325-4. [DOI] [PubMed] [Google Scholar]
- 3.Canyelles M., Tondo M., Cedó L., Farràs M., Escolà-Gil J.C., Blanco-Vaca F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018;19:3228. doi: 10.3390/ijms19103228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fennema D., Phillips I.R., Shephard E.A. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab. Dispos. 2016;44:1839–1850. doi: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farhangi M.A. Gut microbiota-dependent trimethylamine N-oxide and all-cause mortality: Findings from an updated systematic review and meta-analysis. Nutrients. 2020;78:110856. doi: 10.1016/j.nut.2020.110856. [DOI] [PubMed] [Google Scholar]
- 6.Haghikia A., Li X.S., Liman T.G., Bledau N., Schmidt D., Zimmermann F., Kränkel N., Widera C., Sonnenschein K., Haghikia A., et al. Gut Microbiota–Dependent Trimethylamine N -Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arter. Thromb. Vasc. Biol. 2018;38:2225–2235. doi: 10.1161/ATVBAHA.118.311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang W.W., Wang Z., Kennedy D.J., Wu Y., Buffa J.A., Agatisa-Boyle B., Li X.S., Levison B.S., Hazen S.L. Gut Microbiota-Dependent TrimethylamineN-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruppen E.G., Garcia E., Connelly M.A., Jeyarajah E.J., Otvos J.D., Bakker S.J.L., Dullaart R.P.F. TMAO is Associated with Mortality: Impact of Modestly Impaired Renal Function. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-13739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farhangi M.A., Vajdi M. Novel findings of the association between gut microbiota–derived metabolite trimethylamine N-oxide and inflammation: Results from a systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2020;60:2801–2823. doi: 10.1080/10408398.2020.1770199. [DOI] [PubMed] [Google Scholar]
- 10.Obeid R., Awwad H.M., Rabagny Y., Graeber S., Herrmann W., Geisel J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am. J. Clin. Nutr. 2016;103:703–711. doi: 10.3945/ajcn.115.121269. [DOI] [PubMed] [Google Scholar]
- 11.Svensson B., Åkesson B., Nilsson A., Paulsson K. Urinary excretion of methylamines in men with varying intake of fish from the baltic sea. J. Toxicol. Environ. Health Part A. 1994;41:411–420. doi: 10.1080/15287399409531853. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A., Mitchell S., Smith R. Dietary Precursors of Trimethylamine in Man: A Pilot Study. Food Chem. Toxicol. 1999;37:515–520. doi: 10.1016/S0278-6915(99)00028-9. [DOI] [PubMed] [Google Scholar]
- 13.Tang W.W., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller C., Corbin K.D., Da Costa K.-A., Zhang S., Zhao X., Galanko J., Blevins T., Bennett B.J., O’Connor A., Zeisel S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014;100:778–786. doi: 10.3945/ajcn.114.087692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West A.A., Shih Y., Wang W., Oda K., Jaceldo-Siegl K., Sabaté J., Haddad E., Rajaram S., Caudill M.A., Burns-Whitmore B. Egg n-3 Fatty Acid Composition Modulates Biomarkers of Choline Metabolism in Free-Living Lacto-Ovo-Vegetarian Women of Reproductive Age. J. Acad. Nutr. Diet. 2014;114:1594–1600. doi: 10.1016/j.jand.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Zheng H., Yde C.C., Clausen M.R., Kristensen M., Lorenzen J.K., Astrup A., Bertram H.C. Metabolomics Investigation To Shed Light on Cheese as a Possible Piece in the French Paradox Puzzle. J. Agric. Food Chem. 2015;63:2830–2839. doi: 10.1021/jf505878a. [DOI] [PubMed] [Google Scholar]
- 17.Rohrmann S., Linseisen J., Allenspach M., von Eckardstein A., Müller D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016;146:283–289. doi: 10.3945/jn.115.220103. [DOI] [PubMed] [Google Scholar]
- 18.DiMarco D.M., Missimer A., Murillo A.G., Lemos B.S., Malysheva O.V., Caudill M.A., Blesso C.N., Fernandez M.L. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-oxide is Unchanged in a Healthy Population. Lipids. 2017;52:255–263. doi: 10.1007/s11745-017-4230-9. [DOI] [PubMed] [Google Scholar]
- 19.Iannotti L.L., Lutter C.K., Waters W.F., Riofrío C.A.G., Malo C., Reinhart G., Palacios A., Karp C., Chapnick M., Cox K., et al. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: A randomized controlled trial in Ecuador. Am. J. Clin. Nutr. 2017;106:1482–1489. doi: 10.3945/ajcn.117.160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krüger R., Merz B., Rist M.J., Ferrario P.G., Bub A., Kulling S.E., Watzl B. Associations of current diet with plasma and urine TMAO in the KarMeN study: Direct and indirect contributions. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700363. [DOI] [PubMed] [Google Scholar]
- 21.Cho C.E., Taesuwan S., Malysheva O.V., Bender E., Tulchinsky N.F., Yan J., Sutter J.L., Caudill M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017;61:1600324. doi: 10.1002/mnfr.201600324. [DOI] [PubMed] [Google Scholar]
- 22.Lemos B.S., Medina-Vera I., Malysheva O.V., Caudill M.A., Fernandez M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J. Am. Coll. Nutr. 2018;37:716–723. doi: 10.1080/07315724.2018.1466213. [DOI] [PubMed] [Google Scholar]
- 23.Missimer A., Fernandez M.L., DiMarco D.M., Norris G.H., Blesso C.N., Murillo A.G., Vergara-Jimenez M., Lemos B.S., Medina-Vera I., Malysheva O.V., et al. Compared to an Oatmeal Breakfast, Two Eggs/Day Increased Plasma Carotenoids and Choline without Increasing Trimethyl AmineN-Oxide Concentrations. J. Am. Coll. Nutr. 2017;37:140–148. doi: 10.1080/07315724.2017.1365026. [DOI] [PubMed] [Google Scholar]
- 24.Schmedes M., Balderas C., Aadland E.K., Jacques H., Lavigne C., Graff I.E., Eng Ø., Holthe A., Mellgren G., Young J.F., et al. The Effect of Lean-Seafood and Non-Seafood Diets on Fasting and Postprandial Serum Metabolites and Lipid Species: Results from a Randomized Crossover Intervention Study in Healthy Adults. Nutrients. 2018;10:598. doi: 10.3390/nu10050598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pignanelli M., Bogiatzi C., Gloor G., Allen-Vercoe E., Reid G., Urquhart B.L., Ruetz K.N., Velenosi T.J., Spence J.D. Moderate Renal Impairment and Toxic Metabolites Produced by the Intestinal Microbiome: Dietary Implications. J. Ren. Nutr. 2019;29:55–64. doi: 10.1053/j.jrn.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z., Bergeron N., Levison B.S., Li X.S., Chiu S., Jia X., Koeth R., Li L., Wu Y., Tang W.H.W., et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Hear. J. 2019;40:583–594. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu D., Shu X., Rivera E.S., Zhang X., Cai Q., Calcutt M.W., Xiang Y., Li H., Gao Y., Wang T.J., et al. Urinary Levels of Trimethylamine-N-Oxide and Incident Coronary Heart Disease: A Prospective Investigation Among Urban Chinese Adults. J. Am. Hear. Assoc. 2019;8:e010606. doi: 10.1161/JAHA.118.010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andraos S., Lange K., Clifford S., Jones B., Thorstensen E.B., Kerr J., Wake M., Saffery R., Burgner D.P., O’Sullivan J.M. Plasma Trimethylamine N-Oxide and Its Precursors: Population Epidemiology, Parent–Child Concordance, and Associations with Reported Dietary Intake in 11- to 12-Year-Old Children and Their Parents. Curr. Dev. Nutr. 2020;4:nzaa103. doi: 10.1093/cdn/nzaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton K.J., Krüger R., Scherz V., Münger L.H., Picone G., Vionnet N., Bertelli C., Greub G., Capozzi F., Vergères G. Trimethylamine-N-Oxide Postprandial Response in Plasma and Urine Is Lower After Fermented Compared to Non-Fermented Dairy Consumption in Healthy Adults. Nutrients. 2020;12:234. doi: 10.3390/nu12010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Souza R.J., Shanmuganathan M., Lamri A., Atkinson S., Becker A., Desai D., Gupta M., Mandhane P.J., Moraes T.J., Morrison K.M., et al. Maternal Diet and the Serum Metabolome in Pregnancy: Robust Dietary Biomarkers Generalizable to a Multiethnic Birth Cohort. Curr. Dev. Nutr. 2020;4 doi: 10.1093/cdn/nzaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gessner A., Di Giuseppe R., Koch M., Fromm M.F., Lieb W., Maas R. Trimethylamine-N-oxide (TMAO) determined by LC-MS/MS: Distribution and correlates in the population-based PopGen cohort. Clin. Chem. Lab. Med. 2020;58:733–740. doi: 10.1515/cclm-2019-1146. [DOI] [PubMed] [Google Scholar]
- 32.Gibson R., Lau C.-H.E., Loo R.L., Ebbels T.M.D., Chekmeneva E., Dyer A.R., Miura K., Ueshima H., Zhao L., Daviglus M.L., et al. The association of fish consumption and its urinary metabolites with cardiovascular risk factors: The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP) Am. J. Clin. Nutr. 2019;111:280–290. doi: 10.1093/ajcn/nqz293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagen I.V., Helland A., Bratlie M., Midttun Ø., McCann A., Sveier H., Rosenlund G., Mellgren G., Ueland P.M., Gudbrandsen O.A. TMAO, creatine and 1-methylhistidine in serum and urine are potential biomarkers of cod and salmon intake: A randomised clinical trial in adults with overweight or obesity. Eur. J. Nutr. 2019;59:2249–2259. doi: 10.1007/s00394-019-02076-4. [DOI] [PubMed] [Google Scholar]
- 34.Hamaya R., Ivey K.L., Lee D.H., Wang M., Li J., Franke A., Sun Q., Rimm E.B. Association of diet with circulating trimethylamine-N-oxide concentration. Am. J. Clin. Nutr. 2020;112:1448–1455. doi: 10.1093/ajcn/nqaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacPherson M.E., Hov J.R., Ueland T., Dahl T.B., Kummen M., Otterdal K., Holm K., Berge R.K., Mollnes T.E., Trøseid M., et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Associates with Inflammation in Common Variable Immunodeficiency. Front. Immunol. 2020;11:2217. doi: 10.3389/fimmu.2020.574500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin X., Gibbons H., Rundle M., Frost G., McNulty B.A., Nugent A.P., Walton J., Flynn A., Brennan L. The Relationship between Fish Intake and Urinary Trimethylamine-N-Oxide. Mol. Nutr. Food Res. 2020;64:e1900799. doi: 10.1002/mnfr.201900799. [DOI] [PubMed] [Google Scholar]
- 37.Zhu C., Sawrey-Kubicek L., Bardagjy A.S., Houts H., Tang X., Sacchi R., Randolph J.M., Steinberg F.M., Zivkovic A.M. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr. Res. 2020;78:36–41. doi: 10.1016/j.nutres.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Abbasi J. TMAO and Heart Disease: The New Red Meat Risk? JAMA. 2019;321:2149–2151. doi: 10.1001/jama.2019.3910. [DOI] [PubMed] [Google Scholar]
- 39.Guasti L., Galliazzo S., Molaro M., Visconti E., Pennella B., Gaudio G.V., Lupi A., Grandi A.M., Squizzato A. TMAO as a biomarker of cardiovascular events: A systematic review and meta-analysis. Intern. Emerg. Med. 2021;16:201–207. doi: 10.1007/s11739-020-02470-5. [DOI] [PubMed] [Google Scholar]
- 40.Ivashkin V.T., Kashukh Y. Impact of L-carnitine and phosphatidylcholine containing products on the proatherogenic metabolite TMAO production and gut microbiome changes in patients with coronary artery disease. Vopr Pitan. 2019;88:25–33. doi: 10.24411/0042-8833-2019-10038. [DOI] [PubMed] [Google Scholar]
- 41.Mente A., Chalcraft K., Ak H., Davis A.D., Lonn E., Miller R., Potter M.A., Yusuf S., Anand S.S., McQueen M.J. The Relationship Between Trimethylamine-N-Oxide and Prevalent Cardiovascular Disease in a Multiethnic Population Living in Canada. Can. J. Cardiol. 2015;31:1189–1194. doi: 10.1016/j.cjca.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.-M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nat. Cell Biol. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu R., Wang Q., Li L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genom. 2015;16:S4. doi: 10.1186/1471-2164-16-S7-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papandreou C., Moré M., Bellamine A. Trimethylamine N-Oxide in Relation to Cardiometabolic Health—Cause or Effect? Nutrients. 2020;12:1330. doi: 10.3390/nu12051330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Croyal M., Saulnier P.-J., Aguesse A., Gand E., Ragot S., Roussel R., Halimi J.-M., Ducrocq G., Cariou B., Montaigne D., et al. Plasma Trimethylamine N-Oxide and Risk of Cardiovascular Events in Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020;105:2371–2380. doi: 10.1210/clinem/dgaa188. [DOI] [PubMed] [Google Scholar]
- 47.Naghipour S., Cox A.J., Peart J.N., Du Toit E.F., Headrick J.P. TrimethylamineN-oxide: Heart of the microbiota–CVD nexus? Nutr. Res. Rev. 2020:1–22. doi: 10.1017/S0954422420000177. [DOI] [PubMed] [Google Scholar]
- 48.Joshi S., Shah S., Kalantar-Zadeh K. Adequacy of Plant-Based Proteins in Chronic Kidney Disease. J. Ren. Nutr. 2019;29:112–117. doi: 10.1053/j.jrn.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Petermann-Rocha F., Parra-Soto S., Gray S., Anderson J., Welsh P., Gill J., Sattar N., Ho F.K., Celis-Morales C., Pell J.P. Vegetarians, fish, poultry, and meat-eaters: Who has higher risk of cardiovascular disease incidence and mortality? A prospective study from UK Biobank. Eur. Hear. J. 2021;42:1136–1143. doi: 10.1093/eurheartj/ehaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ufnal M., Nowiński A. Is increased plasma TMAO a compensatory response to hydrostatic and osmotic stress in cardiovascular diseases? Med. Hypotheses. 2019;130:109271. doi: 10.1016/j.mehy.2019.109271. [DOI] [PubMed] [Google Scholar]
- 51.Gawrys-Kopczynska M., Konop M., Maksymiuk K., Kraszewska K., Derzsi L., Sozanski K., Holyst R., Pilz M., Samborowska E., Dobrowolski L., et al. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. eLife. 2020;9:e57028. doi: 10.7554/eLife.57028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdelhamid A.S., Brown T.J., Brainard J.S., Biswas P., Thorpe G.C., Moore H.J., Deane K.H., Summerbell C.D., Worthington H.V., Song F., et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Datab. Syst. Rev. 2020;3:CD003177. doi: 10.1002/14651858.CD003177.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Visioli F., Poli A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients. 2020;12:3782. doi: 10.3390/nu12123782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tørris C., Småstuen M.C., Molin M. Nutrients in Fish and Possible Associations with Cardiovascular Disease Risk Factors in Metabolic Syndrome. Nutrients. 2018;10:952. doi: 10.3390/nu10070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiNicolantonio J.J., Mccarty M., Okeefe J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart. 2019;6:e000890. doi: 10.1136/openhrt-2018-000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lombardo M., Bellia C., Moletto C., Aulisa G., Padua E., Della-Morte D., Caprio M., Bellia A. Effects of Quality and Quantity of Protein Intake for Type 2 Diabetes Mellitus Prevention and Metabolic Control. Curr. Nutr. Rep. 2020;9:329–337. doi: 10.1007/s13668-020-00324-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this manuscript are publicly available from previous publications and fully disclosed in Table 1 and Table 2 of the manuscript.