Abstract
Gastric cancer is a common digestive tract malignancy that is mainly treated with surgery combined with perioperative adjuvant chemoradiotherapy and biological targeted therapy. However, the diagnosis rate of early gastric cancer is low and both postoperative recurrence and distant metastasis are thorny problems. Therefore, it is essential to study the pathogenesis of gastric cancer and search for more effective means of treatment. The nuclear factor-κB (NF-κB) signaling pathway has an important role in the occurrence and development of gastric cancer and recent studies have revealed that microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) are able to regulate this pathway through a variety of mechanisms. Understanding these interrelated molecular mechanisms is helpful in guiding improvements in gastric cancer treatment. In the present review, the functional associations between miRNAs, lncRNAs and the NF-κB signaling pathway in the occurrence, development and prognosis of gastric cancer were discussed. It was concluded that miRNAs and lncRNAs have complex relations with the NF-κB signaling pathway in gastric cancer. miRNAs/target genes/NF-κB/target proteins, signaling molecules/NF-κB/miRNAs/target genes, lncRNAs/miRNAs/NF-κB/genes or mRNAs, lncRNAs/target genes/NF-Κb/target proteins, and lncRNAs/NF-κB/target proteins cascades are all important factors in the occurrence and development of gastric cancer.
Keywords: gastric cancer, NF-κB signaling pathway, microRNAs, long non-coding RNAs
1. Introduction
According to the World Health Organization report, gastric cancer is the 6th most fatal cancer type worldwide, with 783,000 deaths in 2018(1). To date, the early diagnosis rate of gastric cancer has remained low and the golden standard for its diagnosis is endoscopic pathological biopsy. Gastric cancer is mainly treated through surgery, chemoradiotherapy, immunotherapy and molecular targeted therapies (2). As a novel treatment method, immunotherapy includes vaccination, immune checkpoint inhibitors and adoptive T-cell therapy, but numerous treatments are still in the research phase (3). With the development of medical theory and technology, the 5-year survival rate of patients with early gastric cancer has reached as high as 95% (4). Regrettably, treatment methods for advanced gastric cancer are still limited and the 5-year survival rate of such patients following neoadjuvant chemoradiotherapy combined with immunotherapy and targeted therapy remains low. At present, the overall treatment principle is to prepare more reasonable and comprehensive therapeutic regimens through a full assessment of the patients' condition to improve their quality of life (5).
The nuclear factor-κB (NF-κB) family is a class of transcription factors that possess multiple biological functions. There are 5 important genes in the NF-κB signaling pathway, including NF-κB1 (p50/p105), NF-κB2 (p52/p100), RelA (p65), RelB and c-Rel (6). The combination of IκBα (a prototypical member of the IκB family) and NF-κB complexes, such as NF-κB1 p50-RelA and NF-κB1 p50-c-Rel dimers, can inhibit the nuclear translocation of NF-κB complexes. The canonical pathway is activated by immune-related receptors (7). Subsequently, the IKK complex forms and mediates IκBα phosphorylation and ubiquitin (Ub)-dependent proteasomal degradation, resulting in nuclear translocation of the NF-κB dimers. The noncanonical NF-κB pathway is a NF-κB-inducing kinase, which activates downstream kinase IKKα to process p100 to p52, thereby forming the RelB/p52 complexes to activate the NF-κB signaling pathway (7). It has been indicated that the NF-κB signaling pathway regulates the cell cycle, apoptosis, migration, adhesion, inflammatory response and immune response (8). A large number of studies have demonstrated that the NF-κB signaling pathway is abnormally activated in different types of malignancies and that it has important roles in promoting tumor growth, invasion and migration, as well as in inhibiting apoptosis and enhancing chemoradiotherapy sensitivity (6). Furthermore, the NF-κB signaling pathway may serve as a key effector pathway that maintains the characteristics of cancer stem cells (6,9). Epithelial-mesenchymal transition (EMT) has been considered to be closely related to the migration and invasion of malignant cells. During the transdifferentiation of epithelial cells into mesenchymal cells, cell-cell adhesions are weakened, the cell adhesion molecule, E-cadherin, is downregulated and the mesenchymal molecule vimentin is up-regulated, leading to increased cell migration and invasion. It has been indicated that the high expression of cyclooxygenase-2 may lower the expression of E-cadherin through the NF-κB/Snail pathway, thereby enhancing the invasion and migration of tumor cells (10). The NF-κB pathway is continuously activated in the cytoplasm and nucleus of gastric cancer cells, and its activation is significantly higher than that in para-carcinoma normal tissues (11). The activation degree of the NF-κB pathway has significant correlations with the size, degree of malignancy, depth of infiltration, lymphatic and peritoneal metastasis and prognosis of gastric cancer patients. As confirmed in numerous clinicopathological specimens, the RelA (p65) protein has an increased expression in gastric adenocarcinoma tissues, where it is involved in tumor growth, invasion and metastasis (12,13).
Studies have proved that microRNAs (miRNAs/miRs) are able to act as oncogenes or tumor suppressor genes in tumors (14-16). It was also determined that non-coding (nc)RNAs serum profiles are different between gastric cancer patients and healthy individuals. miRNAs were indicated to have stable and simple detection indexes, which highlight their importance as biomarkers for early diagnosis of gastric cancer (17). miR-331, miR-21, miR-20b, miR-125a, miR-137, miR-141, miR-146a, miR-196a, miR-206, miR-218, miR-486-5p and miR-506, have been confirmed as tumor markers with potential value in gastric cancer diagnosis and prognostic prediction (18). Increasing evidence suggests that the imbalance of miRNAs has important links with tumor proliferation, migration, invasion, apoptosis, drug resistance and angiogenesis. miR-21, miR-23a, miR-27a, miR-106b-25, miR-130b, miR-199a, miR-215, miR-222-221 and miR-370 promote gastric cancer occurrence and development, while miR-29a, miR-101, miR-125a, miR-129, miR-148b, miR-181c, miR-212, miR-218, miR-335, miR-375, miR-449, miR-486 and miR-512 suppress gastric cancer progression (18).
In recent years, the potential value of long ncRNAs (lncRNAs) as tumor markers has been confirmed (18), and the important regulatory roles of lncRNAs in tumor occurrence and development have been recognized. lncRNAs are involved in various malignant biological behaviors; therefore, lncRNAs are expected to be used in tumor treatments (19-22). In addition, chemical modification of lncRNAs may be associated with cancer development (23).
Non-coding RNA serum profiles are different between patients with gastric cancer and healthy individuals, which have significant effects on prognosis. Additionally, the close association of the NF-κB signaling pathway with gastric cancer occurrence, development and chemoradiotherapeutic sensitivity may imply that miRNAs and lncRNAs share certain cascades with the NF-κB signaling pathway. In the present review, the cascades of miRNAs and lncRNAs with the NF-κB signaling pathway in gastric cancer were comprehensively explored.
2. miRNAs and the NF-κB signaling pathway
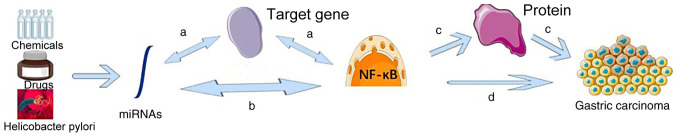
The general regulatory mechanisms of miRNAs and the NF-κB signaling pathway in gastric cancer are presented in Fig. 1 (24). miRNAs can regulate target genes to activate or inhibit the NF-κB signaling pathway. The NF-κB signaling pathway is able to influence miRNA expression by regulating target genes. miRNAs can also activate or inhibit the NF-κB signaling pathway directly, with the NF-κB signaling pathway influencing miRNA expression directly. NF-κB or miRNAs can therefore influence the development of gastric cancer by regulating downstream proteins or affecting the development of gastric cancer directly (24,25).
Figure 1.
Regulatory mechanisms of miRNAs and the NF-κB signaling pathway in gastric cancer. (a) miRNAs regulate target genes to activate or inhibit the NF-κB signaling pathway. The NF-κB signaling pathway is able to influence miRNA expression by regulating target genes. (b) miRNAs activate or inhibit the NF-κB signaling pathway directly, and the NF-κB signaling pathway is also able to influence miRNA expression directly. (c) The NF-κB signaling pathway influences the development of gastric cancer by regulating downstream proteins. (d) The NF-κB signaling pathway may influence the development of gastric cancer directly. miRNA, microRNA; NF-κB, nuclear factor-κB.
miR-20a activation of the NF-κB signaling pathway
The imbalance of miRNAs is frequently considered an important cause of tumor drug resistance and the continuous activation of the NF-κB signaling pathway also has an important role in the decline of tumor chemoradiotherapeutic sensitivity (26). It was previously indicated that miR-20a expression is significantly increased in a variety of gastric cancer cell lines and patient tissues, and that its ectopic expression facilitates the development of gastric cancer by promoting the proliferation, migration and invasion of gastric cancer cells (27). miR-20a has also been reported to be upregulated in the generated cisplatin (DDP)-resistant gastric cancer cell line SGC7901/DDP. Overexpression of miR-20a leads to inhibition of the expression of the cylindromatosis (CYLD) gene, activates the NF-κB signaling pathway, increases the expression of p65, livin and survivin, reduces apoptosis of gastric cancer cells and enhances their chemoresistance (28).
miR-300 activation of the NF-κB signaling pathway
Shikimic acid (SA), also known as Chinese anise, is a type of hydroaromatic compound that is present in bracken ferns and that increases the risk of gastric and esophageal cancers. Through in vitro experiments, Ma and Ning (29) confirmed that SA was able to upregulate miR-300 expression in the estrogen receptor-positive breast cancer cell line MCF-7, and inhibit IκBα protein, thereby upregulating the NF-κB signaling pathway and enhancing breast cancer cell proliferation. It was previously indicated that miR-300 is able to suppress EMT in malignant tumors, improve the prognosis of patients with laryngeal squamous cell carcinoma and inhibit the metastasis of oral squamous cell carcinoma (30-32). The ability of SA to increase the risk of gastric cancer may be related to such a mechanism, but relevant research is still limited.
miR-224 activation of the NF-κB signaling pathway
Hypoxia is an important factor in the occurrence and development of a variety of cancer types. Hypoxia-inducible factor-1 (HIF-1) is able to regulate various miRNAs, while certain miRNAs are also able to target HIF-1. Currently, miR-224 is considered as an oncogene that is highly expressed in various tumor types and that promotes the proliferation and invasion of tumor cells (33,34). The interactions between HIF-1 and miRNAs may have important roles in angiogenesis, apoptosis, cell cycle regulation, proliferation, migration and tumor drug resistance (35). He et al (36) confirmed that miR-224 is highly expressed in gastric cancer tissues and metastatic lymph nodes. Furthermore, it was confirmed that hypoxia and HIF-1α are able to upregulate the expression of miR-224 in gastric cancer cells, while miR-224 downregulated the expression of Ras association (RalGDS/AF-6) domain family 8 (RASSF8) through target genes. RASSF8 is closely related to the NF-κB signaling pathway, as both NF-κB transcriptional activity and p65 translocation were enhanced following RASSF8 knockout, which promoted the proliferation, migration and invasion of gastric cancer cells (36).
miR-21 activation of the NF-κB signaling pathway
miR-21 expression is upregulated in most tumor tissues, where it has an important function in tumor cell adhesion, migration, invasion and angiogenesis. The expression of miR-21 also correlates with patient prognosis, histological type, lymph node metastasis and TNM stage. miR-21 is also able to regulate the radiosensitivity of gastric cancer cells and directly downregulate the expression of phosphatase and tensin homolog deleted on chromosome ten (PTEN) (37). Smoking is a risk factor for a variety of cancers and nicotine is able to regulate cell proliferation, apoptosis, migration and invasion through related signaling pathways, which promote tumor occurrence and development. Shin et al (38) proved that nicotine is able to activate the NF-κB signaling pathway through the EP2/4 receptor and promote the expression of miR-21 and miR-16, therefore facilitating the proliferation of gastric cancer cells. Of note, a study indicated that miR-21 may be downregulated by celastrol, a natural component from a plant, resulting in the inhibition of the activation of the PI3K/Akt/NF-κB signaling pathway and the promotion of gastric cancer cells apoptosis (39). According to related research, chromobox protein homolog 7 (CBX7) upregulates the Akt/NF-κB/miR-21 signaling pathway via the inhibition of p16, which positively regulates the characteristics of gastric cancer stem cells. In addition, it has been proved that the Akt/NF-κB/miR-21 signaling pathway is a key effector pathway for CBX7 in inducing the characteristics of gastric cancer stem cells (40). Furthermore, the NF-κB signaling pathway and miR-21 appear to activate each other through different pathways, resulting in a joint promotion of gastric cancer progression.
miR-17-92 activation of the NF-κB signaling pathway
It is thought that miR-17-92, also known as oncomiR-1, is an important carcinogen (41). Overexpression of miR-17-92 promoted the proliferation and reduced apoptosis of gastric cancer cells in nude mice, and accelerated tumor growth in vivo (42). Tumor necrosis factor receptor-associated factor 3 (TRAF3) is thought to be related to patients' prognosis (43). TRAF3 expression levels in gastric cancer tissues are far lower than those in para-carcinoma normal tissues and its silencing may promote proliferation and markedly enhance migration and invasion of gastric cancer cells. As previously reported, TRAF3 is the target gene of miR-17-92 and its levels may be decreased by miR-17-92 overexpression, resulting in the activation of the NF-κB signaling pathway through non-classical and classical pathways, which promoted the proliferation, migration and invasion, and inhibited apoptosis of gastric cancer cells (42).
miR-577 activation of the NF-κB signaling pathway
miR-577 expression is closely associated with metastasis and poor prognosis of patients with gastric cancer. It has been confirmed that gastric cancer cells overexpressing miR-577 are spindle-shaped and display a strong invasive ability. miR-577 does not affect the proliferation of gastric cancer cells, but may promote the migration, invasion and chemoresistance of gastric cancer cells. Serum deprivation response protein (SDPR) is a tumor suppressor gene, and its expression in gastric cancer tissues is lower than that in normal tissues, which may be related to gastric cancer prognosis. miR-577 is able to inhibit the expression of its target gene SDPR and boost the activation of the ERK-NF-κB signaling pathway through forming an SDPR-ERK protein complex, which induces EMT. Furthermore, a positive feedback loop is also formed to upregulate miR-577 expression (44).
miR-216a-3p activation of the NF-κB signaling pathway
Runt-related transcription factor-1 (RUNX1) has been identified as a cancer suppressor gene and its expression is downregulated in gastric cancer tissues (45). miR-216a-3p is highly expressed in the gastric cancer cell lines AGS, MKN-45 and HGC-27. Analysis of 140 cases of gastric cancer tissue specimens suggested that miR-216a-3p expression levels are associated with the degree of malignancy and prognosis of gastric cancer. Furthermore, high expression of miR-216a-3p was indicated to activate the NF-κB signaling pathway and increase the expression of its downstream proteins, MMP2, MMP9, cyclin D1 and Bcl-2, through inhibition of RUNX1 expression, which enhanced the proliferation, migration and invasion of gastric cancer cells (46).
miR-362 activation of the NF-κB signaling pathway
According to the public research database about cancer-related miRNA microarrays, miR-362 expression is increased in gastric cancer cells. CYLD is a cancer suppressor gene that inhibits proliferation and induces apoptosis of tumor cells (47). A study suggested that miR-362, through inhibiting the expression of its target gene CYLD, facilitates the activation of the NF-κB signaling pathway, promotes the proliferation and inhibits apoptosis of gastric cancer cells, and induces cisplatin resistance (48).
miR-10b activation of the NF-κB signaling pathway
miR-10b expression correlates with the size, invasiveness, lymph node metastasis, distant metastasis and prognosis of gastric cancer (49). CUB and Sushi multiple domains-1 (CSMD1) expression is downregulated in gastric cancer tissues and associated with the prognosis of patients with gastric cancer. miR-10b is able to inhibit the expression of part of CSMD1, which further activates the NF-κB signaling pathway and promotes the EMT of gastric cancer cells, leading to enhanced migration and invasion of gastric cancer cells. In nude mouse experiments, the growth and metastasis of gastric cancer was promoted by overexpression of miR-10b (50).
miR-223-3p activation of miRNAs by the NF-κB signaling pathway
Through the secretion of cytotoxin-associated gene A (CagA), CagA-positive Helicobacter pylori is able to stimulate the activation of the NF-κB signaling pathway in gastric cancer cells and upregulate miR-223-3p expression, leading to partial inhibition of the expression of AT-rich interacting domain containing protein 1A (ARID1A). It has been previously confirmed that ARID1A is a tumor suppressor gene that restrains the invasion and migration of tumor cells via upregulating the levels of E-cadherin and p21. In patients infected with CagA-positive Helicobacter pylori, the occurrence and development of gastric cancer may be promoted through the NF-κB/miR-223-3p/ARID1A axis and miR-223-3p may offer a feasible method for preventing and treating gastric cancer (51).
miR-135b-5p activation of miRNAs by the NF-κB signaling pathway
It has been indicated that inflammation and infection account for ~25% of carcinogenic factors. Long-term chronic inflammation-induced cell DNA damage and epigenetic changes may be present in inflammation-related cancers, such as Helicobacter pylori-infected gastric cancer (52). In tissues of trefoil factor 1-knockout mice and the gastric cancer MKN45 and SNU1 cell lines, Helicobacter pylori was indicated to promote the expression of miR-135b-5p through the activation of the NF-κB signaling pathway and downregulation of Kruppel-like factor 4 (KLF4), which resulted in the inhibition of apoptosis and enhancement of drug resistance of gastric cancer cells. It was confirmed that KLF4 acts as a potential cancer suppressor gene in gastric cancer and that it has a role in cisplatin resistance (53).
miR-425 activation of miRNAs by the NF-κB signaling pathway
As a malignant tumor suppressor in vivo, PTEN is mutated or lost in a variety of cancer types. IL-1β is an inflammatory factor that activates the NF-κB signaling pathway in gastric cancer cells, which reduces the expression of target gene PTEN through upregulating miRNA-425 and promotes the growth of gastric cancer cells (54). According to related studies, both miR-21 and miR-32 downregulate the expression of PTEN in gastrointestinal tumors, which facilitates the migration and invasion of tumor cells (55,56).
miR-107 activation of miRNAs by the NF-κB signaling pathway
As suggested in previous related studies, miR-107 is a cancer suppressor gene, which inhibits tumor cell growth in colon and pancreatic cancers, as well as head-neck tumors (57). However, it was reported that miR-107 expression is upregulated in gastric cancer tissues (58). Overactivation of the NF-κB pathway may promote the expression of miR-107, while miR-107 is able to downregulate the downstream target gene forkhead box protein O1 (FOXO1), thereby enhancing the proliferation of gastric cancer cells. The expression of FOXO1 may be regulated by a variety of miRNAs as dysregulated miR-223, miR-139 and miR-370 expression may exert a series of influences on the progression of gastric cancer via regulating FOXO1(59).
miR-210 activation of miRNAs by the NF-κB signaling pathway
The uncontrolled expression of phosphatase of regenerating liver-3 (PRL-3) is closely related to the overall survival rate and tumor progression. It is believed that miR-210 is able to regulate the expression of target genes, E2F transcription factor 3, homeobox A1 (HOXA1) and HOXA3, which are crucial molecules that regulate cell proliferation and cell cycle progression. It has been indicated that the transcriptional level of PRL-3 in gastric cancer tissues positively correlates with the level of miR-210 expression. Through gene enrichment analysis and in vitro experiments, Zhang et al (60) confirmed that PRL-3 is able to increase HIF-1α levels through the activation of the NF-κB signaling pathway and the upregulation of miR-210 in a HIF-1α-dependent manner, which enhance the invasion and migration of gastric cancer.
To sum up, the above-mentioned studies appear to confirm that miRNAs act as oncogenes that may be involved in the activation of the NF-κB signaling pathway, which in turn, is able to upregulate the expression of related miRNAs. The interaction between the two jointly promotes the occurrence and development of gastric cancer. However, related mechanisms are yet to be revealed and whether inhibiting the NF-κB signaling pathway or the expression of related miRNAs is a novel target in gastric cancer treatment still requires to be experimentally validated.
miR-338 inhibition of the NF-κB signaling pathway
Li et al (61) screened differentially expressed genes from 90 samples in the Gene Expression Omnibus database and classified gastric cancer into 4 subtypes based on different target miRNAs. The most important feature of the second subtype was the miR-338/C-C motif chemokine ligand (CCL)21/NF-κB signaling pathway. miR-198/protein inhibitor of activated STAT4/NF-κB and miR-370/CCL21/NF-κB also belonged to this subtype. It was indicated that miR-338 is able to inhibit the proliferation and migration of gastric cancer cells (61).
miR-7 inhibition of the NF-κB signaling pathway
As reported in a previous study, miR-7 is a tumor suppressor gene that suppresses the proliferation, survival, migration and invasion of a variety of cancer cell types (62). Using quantitative isobaric Tags for Relative and Absolute Quantitation, gene expression microarray analysis and bioinformatics, it was revealed that FOS and RelA are important functional targets of miR-7 in gastric cancer cells. Analysis of 106 gastric cancer tissue samples indicated that gastric cancer tissues with low miR-7 expression had a more malignant phenotype and that miR-7 expression negatively correlates with RelA and FOS expression. Gastric cancer cell lines overexpressing miR-7 have a weakened proliferation ability, a significantly increased early apoptotic rate and an enhanced sensitivity to chemotherapeutic drugs (63). In nude mouse experiments, tumorigenesis is markedly reduced following miR-7 overexpression. It was also suggested that the expression of RelA, FOS, proliferating cell nuclear antigen and cyclin D1 markedly declined in tumor tissues. Furthermore, miR-7 overexpression was determined to inhibit IKKε expression, which inhibited the activation of RelA, leading to the suppression of the activation of the NF-κB signaling pathway (63). The results revealed that Helicobacter pylori infection is able to induce the expression of IKKε and increase the expression of RelA in normal gastric mucosa cells, thereby inhibiting the expression of miR-7. Through such a mechanism, the NF-κB signaling pathway may be overactivated in patients with Helicobacter pylori infection, suggesting that persistent chronic inflammation is caused and ultimately transforms cells into malignant tumor cells (63). Furthermore, dysregulated expression of miR-7 was observed in a variety of tumor tissues (64). Ye et al (65) used the Cancer Genome Atlas Stomach Adenocarcinoma and National Center for Bioinformatics Gene Expression Omnibus databases (accession no. 10.1186/s13046-019-1074-6), and determined that there was a negative correlation between miR-7 expression and NF-κB RelA (p65) protein expression in primary gastric cancer tissues. Downregulated miR-7 corresponded to poor prognosis (66), and mature miR-7 was significantly reduced in the gastric cancer HGC-27 and MKN-28 cell lines. In the nude mouse model, hepatic and pulmonary metastasis of gastric cancer was inhibited in the miR-7 overexpression group of mice. It was argued that overexpression of miR-7 may not only inhibit the expression of its downstream protein targets, intercellular adhesion molecule-1, MMP2, MMP9, VEGF and vascular cell adhesion molecule-1 through restraining the NF-κB signaling pathway, but also through reducing inflammatory cell infiltration. As mentioned above, inflammation has a close link with the occurrence and development of tumors; therefore, miR-7 may provide a feasible method for the prevention and treatment of gastric cancer (65).
miR-218 inhibition of the NF-κB signaling pathway
In patients with metastatic gastric cancer, the transcription factor POU class 2 homeobox 2 (POU2F2) was indicated to be abnormally upregulated, while the expression of miR-218 was downregulated. miR-218 was also demonstrated to inhibit the migration and invasion of tumor cells. Researchers confirmed that POU2F2 overexpression may activate the slit guidance ligand (SLIT)2/Roundabout guidance receptor 1 (ROBO1) signaling pathway and promote EMT of gastric cancer cells, thereby facilitating invasion and migration of gastric cancer cells, while its expression was able to be further enhanced by overactivation of NF-κB signaling pathway. According to a bioinformatics analysis, miR-218 is able to directly act on the target gene POU2F2, inhibit the NF-κB signaling pathway and reduce the expression of POU2F2 through IKK-β suppression. ROBO1 is a single-channel transmembrane receptor that is able to boost tumor metastasis. Of note, SLIT3 was able to partially reduce miR-218 expression in metastatic gastric cancer, thereby relieving the inhibitory effect of miR-218 on ROBO1. Overall, miR-218 may be an important target that may offer a novel direction in the diagnosis and treatment of gastric cancer (67).
miR-185 inhibition of the NF-κB signaling pathway
The expression of gastrokine 1 (GKN1) and GKN2 in gastric cancer tissues and the AGS gastric cancer cell line was lower than that in para-carcinoma tissues and normal gastric epithelial cell (68,69). GKN2 maintains the homeostasis of gastric mucosal epithelium and may delay the development of gastric cancer (70). It was previously indicated that GKN1 promotes apoptosis of gastric cancer cells and suppresses their proliferation by causing cell cycle arrest at the G2/M phase. GKN1 may promote miR-185 expression and decrease the expression of C-myc, a specific protein involved in maintaining gastric mucosal homeostasis, GKN2 is able to completely reverse the function of GKN1 in gastric cancer AGS cell line and that GKN1 inhibits the activity of the NF-κB signaling pathway via facilitating the expressions of IκB and GKN2(71).
miR-146a inhibition of the NF-κB signaling pathway
miR-146a, a dependent gene of the NF-κB signaling pathway, is able to alleviate the inflammatory response. Crone et al (72) indicated that there was no expression of miR-146a in normal gastric mucosa. Another study confirmed that as a cancer suppressor gene, miR-146a is able to inhibit the expression of transforming growth factor-activated kinase-1, resulting in the increase of IκBα expression and the suppression of the activation of the NF-κB signaling pathway. This event led to restricted expression of the downstream protein Bcl-2 and the promotion of apoptosis in the gastric cancer SGC7901 cell line (73). Another study indicated that the expression of miR-146a was upregulated in 73% of gastric cancer tissue samples and that miR-146a also downregulated the expressions of caspase recruitment domain-containing protein 10 (CARD10), COP9 constitutive photomorphogenic homolog subunit 8 (COPS8) and interleukin-1 receptor-associated kinase 1 (IRAK1) in vitro (72). It has previously been confirmed that IRAK1 is involved in the activation of the NF-κB signaling pathway (74,75). In this study, it was proved that miR-146a overexpression is able to decrease CARD10 and COPS8 expression levels, which prevented G protein-coupled receptor from activating the NF-κB signaling pathway. Furthermore, miR-146a was able to downregulate the expression of IL-8, IL-23A, CCL5, colony-stimulatory factor-1 and platelet-derived growth factor subunit B, which are involved in facilitating the occurrence and development of tumors (72). The morbidity rate of Helicobacter pylori infection is high and half of the world's population may be a carrier of this bacterium (76), which has been proved to be able to promote or inhibit the expression of certain miRNAs and enhance the proliferation and invasion of gastric cancer cells (77-79). In the serum of patients with gastric cancer with Helicobacter pylori infection, miR-375, miR-146 and let-7 were downregulated, while miR-19 and miR-21 were upregulated and the Wnt/β-catenin, IRAK and NF-κB signaling pathways were activated (80). In patients with Helicobacter pylori infection, IL-17A expression was increased, which is able to increase the levels of downstream products, including IL-8 and growth-regulated oncogene α (GRO-α), and enhance the local inflammatory response through upregulating the NF-κB signaling pathway. However, IL-17A also promoted the expression of miR-146a, which was able to reduce the expression levels of GRO-α and IL-8 in the gastric cancer SGC7901 cell line. In addition, miR-146a is a dependent gene of the NF-κB signaling pathway that may inhibit the activation of the NF-κB signaling pathway via reducing the expression of TRAF6 and IRAK1, thus reducing the release of inflammatory factors and inhibiting the inflammatory response (81).
miR-195 inhibition of the NF-κB signaling pathway
Surgery is currently the preferred treatment method for gastric cancer (5). Studies have indicated that certain anesthetics may affect the activation of the NF-κB signaling pathway through their influence on the expression of miRNAs (82-84). Propofol is a commonly used intravenous anesthetic in clinical anesthesia, characterized by rapid onset and relatively few side effects. Studies have demonstrated that propofol possesses an anti-tumor effect against a variety of tumor types through its inhibitory effect on the proliferation of gastric cancer cells via regulating MMP2 and inhibitor of growth protein 3 (85,86). As a tumor suppressor, miR-195 is downregulated in various types of cancer tissue and cells (87), but its expression in gastric cancer cells may be upregulated by propofol, which leads to the activation of the NF-κB and JAK/STAT signaling pathways, inhibition of cell proliferation, migration and invasion, and induction cell apoptosis (82). However, the molecular mechanisms underlying the inhibitory effects of propofol on gastric cancer growth remain to be fully elucidated.
miR-145 inhibition of the NF-κB signaling pathway
The amide lidocaine is also commonly used as a local anesthetic that exerts an important therapeutic effect on rapid ventricular arrhythmia. A study confirmed that lidocaine was able to restrain the growth of a variety of tumor types and that the tumor suppressor miR-145 inhibits the growth of gastric cancer cells (88). Lidocaine inhibits the activation of the NF-κB and MEK/ERK signaling pathways by upregulating the expression of miR-145 in the gastric cancer MKN45 cell line, indicating that miR-145 is a potential therapeutic target for gastric cancer (83). An intravenous defined dose of lidocaine exerts a certain treatment effect on post-operative chronic pain, post-operative cognitive dysfunction and malignant tumors (89). Perioperative anesthetics have important effects on gastric cancer, and therefore, understanding the mechanisms of action of drugs on tumors may serve as an important guidance for the peri-operative use of anesthetics in radical gastrectomy.
miR-128b inhibition of the NF-κB signaling pathway
miR-128b expression declines in gastric cancer tissues and SGC7901, GC-823 and HGC-27 cell lines, compared with that in normal tissues and gastric mucosal epithelial GES1 cell lines. It has been indicated that miR-128b is able to inhibit the target gene pyruvate dehydrogenase kinase 1, thereby suppressing the Akt/NF-κB signaling pathway, promoting apoptosis and restraining proliferation, migration and invasion of gastric cancer cells (90).
miR-3664-5P inhibition of the NF-κB signaling pathway
miR-3664-5P is downregulated in gastric cancer tissues and cell lines and its expression positively correlates with gastric cancer differentiation, degree of malignancy, tumor size and patient prognosis. Metadherin (MTDH), a transmembrane protein, is currently considered to be an oncogene in a variety of cancer types, which is able to enhance tumor cell proliferation, invasion, migration, angiogenesis and chemoresistance. Bioinformatics analysis and a luciferase reporter gene assay confirmed that MTDH is a target gene of miR-3664-5P and that its expression is decreased following upregulation of miR-3664-5P, resulting in the inhibition of the activation of the NF-κB signaling pathway. Furthermore, miR-3664-5P is able to restrain the growth and pulmonary metastasis of gastric cancer cells in nude mice (91).
The above-mentioned miRNAs act as tumor suppressors in gastric cancer cells and participate in the regulation on the NF-κB signaling pathway, which is involved in regulating the expression of a variety of genes. However, the imbalance of miRNAs and their regulatory mechanisms in gastric cancer remain to be fully clarified. The mutual regulatory pathways of miRNAs and the NF-κB signaling pathway in gastric cancer are summarized in Tables I and II. These schematic summaries may contribute to subsequent related research and provide a basis for the development of novel methods for preventing, detecting and treating gastric cancer.
Table I.
miRNAs that regulate the NF-κB signaling pathway in gastric cancer.
| miRNA | Target gene | NF-κB | Protein | Function | (Refs.) |
|---|---|---|---|---|---|
| miR-20a | CYLD (-) | (+) | Livin, survivin (+) | Proliferation, migration, invasion (+); apoptosis, chemosensitivity (-) | (27,28) |
| miR-21 | - | (+) | Caspase-3 (-) | Apoptosis (-) | (39) |
| miR-17-92 | TRAF3 (-) | (+) | - | Proliferation, migration, invasion (+) Apoptosis (-) | (42) |
| miR-577 | SDPR (-) | (+) | E-cadherin (-) | Migration, invasion (+); Chemosensitivity (-) | (44) |
| miR-216a-3p | RUNX1 (-) | (+) | MMP2, MMP9, CyclinD1, Bcl-2 (+) | Proliferation, migration, invasion (+) | (46) |
| miR-362 | CYLD (-) | (+) | - | Proliferation (+); Apoptosis, chemosensitivity (-) | (48) |
| miR-10b | CSMD1 (-) | (+) | c-Myc, cyclin D1 (+) | Migration, invasion (+) | (50) |
| miR-224 | RASSF8 (-) | (+) | - | Proliferation, migration, invasion (+) | (36) |
| miR-3664-5p | MTPH (-) | (-) | IL-8, MMP9, VEGF (-) | Proliferation, migration, invasion (-); Apoptosis (+) | (91) |
| miR-7 | RELA (-) | (-) | VCAM-1, VEGF, MMP-9 MMP-2, ICAM-1 (-) | Proliferation, migration, invasion (-) | (63,65) |
| miR-218 | - | (-) | POU2F2/SLIT2/ROBO1 (-) | Migration, invasion (-) | (67) |
| miR-195 | - | (-) | MMP-2, MMP-9, Bcl-2, cyclinD1, vimentin (-) Bax, P21, caspase-3, 9 (+) | Proliferation, migration, invasion (-); Apoptosis (+) | (82) |
| miR-145 | - | (-) | MMP-2, MMP-9, Bcl-2, cyclinD1, vimentin (-); P21, caspase-3, 7, 9 (+) | Proliferation, migration, invasion (-); Apoptosis (+) | (83) |
| miR-128b | PDK1 (-) | (-) | Bcl-2 (-) | Proliferation, invasion (-); Apoptosis (+) | (90) |
| miR-146a | CARD10, COPS8 (-) | (-) | - | Proliferation (-) | (72) |
| miR-146a | TRAF6/IRAK1 (-) | (-) | GRO-α, IL-8 (-) | Inflammation (-) | (81) |
| miR-146a | TAK1 (-) | (-) | Bcl-2 (-) | Apoptosis (+) | (73) |
(-), inhibition; (+), promotion; -, currently unknown; miRNA/miR, microRNA; NF-κB, nuclear factor-κB.
Table II.
Regulation of miRNAs through the NF-κB signaling pathway in gastric cancer.
| Signaling molecule | NF-κB | miRNAs | Target genes | Function | (Refs.) |
|---|---|---|---|---|---|
| CagA (+) | (+) | miR-223-3p | ARID1A (-)/E-cadherin, P21 (-) | Migration, invasion (+) | (51) |
| HP (+) | (+) | miR-135-5p | KLF4 (-) | Apoptosis, chemosensitivity (-) | (53) |
| IL-1β | (+) | miR-425 | PTEN (-) | Proliferation (+) | (54) |
| - | (+) | miR-107 | FOXO1 (-) | Proliferation (+) | (59) |
| PRL-3 | (+) | miR-210 | - | Migration, invasion (+) | (60) |
| EP2/4 | (+) | miR-21, miR-16 | - | Proliferation (+) | (38) |
| CBX7 | (+) | miR-21 | - | Stem cell-like properties (-) | (40) |
(-), inhibition; (+), promotion; -, currently unknown; miRNA/miR, microRNA; NF-κB, nuclear factor-κB.
3. lncRNAs and the NF-κB signaling pathway
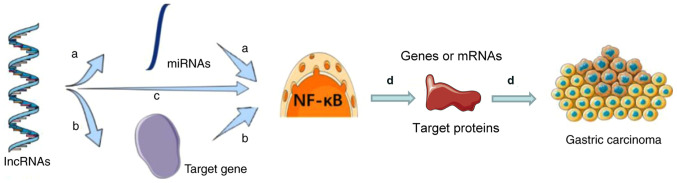
There is also an important link between lncRNAs and gastric cancer. For instance, in the blood and tissue samples of patients with cancer, long intergenic non-protein coding RNA CYTOR (LINC00152) and H19 expression levels positively correlate with the incidence rate of gastric cancer. NcRNA 1 (GClnc1), LINC02864 and TMEM132D-AS1 are associated with the survival rate of patients with gastric cancer and dysregulated lncRNAs may be closely related to Helicobacter pylori infection (92). Furthermore, lncRNAs are involved in the regulation of important signaling pathways, such as the NF-κB, PI3K/AKT, Wnt/β-catenin and ERK/MAPK signaling pathways (93). In the chapter below, the regulation of lncRNAs of the NF-κB signaling pathway is discussed. The general regulatory mechanisms of lncRNAs and the NF-κB signaling pathway in gastric cancer are illustrated in Fig. 2. lncRNAs regulate miRNAs to activate or inhibit the NF-κB signaling pathway. lncRNAs also regulate target genes to activate or the inhibit NF-κB signaling pathway. Furthermore, lncRNAs can activate or inhibit the NF-κB signaling pathway directly. The NF-κB signaling pathway therefore influences the development of gastric cancer by regulating downstream genes, mRNAs or proteins (94).
Figure 2.
Regulatory mechanisms of lncRNAs and the NF-κB signaling pathway on gastric cancer. (a) lncRNAs regulate miRNAs to activate or inhibit the NF-κB signaling pathway. (b) lncRNAs regulate target genes to activate or the inhibit NF-κB signaling pathway. (c) lncRNAs activate or inhibit the NF-κB signaling pathway directly. (d) The NF-κB signaling pathway influences the development of gastric cancer by regulating downstream genes, mRNAs or proteins. miRNA, microRNA; lncRNA, long non-coding RNA; NF-κB, nuclear factor-κB.
CDKN2B-AS1 (lncRNA ANRIL)
It was previously indicated that ten-eleven translocation-2 (TET2) is an important regulator of certain key physiological functions of the body. The TET2 protein has a low expression level in gastric cancer cells, where it is involved in apoptosis. Its expression also correlates with the prognosis of patients with gastric cancer. CDKN2B-AS1 (lncRNA ANRIL) is located at the human CDKN2A/B locus at 9p21.3(95). It is closely related to the occurrence and development of various tumor types and it is highly expressed in tumor tissues of certain patients with gastric cancer, where it promotes migration and inhibits apoptosis of gastric cancer cells. Using a nude mouse model of metastasis, researchers confirmed that TET2 protein inhibits pulmonary metastasis of gastric cancer through suppressing lncRNA ANRIL. The anti-tumor properties of TET2 depend on lncRNA ANRIL, while this later promotes the progression of gastric cancer through activating the NF-κB signaling pathway. Furthermore, the NF-κB signaling pathway is able to upregulate the expression of p21, baculoviral IAP repeat containing 2, X-linked inhibitor of apoptosis, cyclin D1, CDK2, proline rich acidic protein 1, MMP2 and anosmin 1. Therefore, lncRNA ANRIL may be a potential therapeutic target for gastric cancer (96).
BANCR
It has been widely accepted that lncRNAs act as miRNA sponges that affect the expression of genes/mRNAs. BANCR is an lncRNA containing 693 nucleotides that has four exons located on chromosome 9(97), whose level in human gastric cancer tissues is 5 times higher than that in normal gastric tissues. BGC-803 and MGC-823 cells have a higher level of BANCR than that of GES-1 cells. It has been confirmed that BANCR activates the NF-κB signaling pathway, facilitates proliferation and suppresses apoptosis of gastric cancer cells through inhibiting the expression of miRNA-9(98).
LINC01410
In gastric cancer cells, LINC01410 was indicated to inhibit the expression of miR-532-5p, which is involved in the inhibition of neutrophil cytosolic factor 2 (NCF2) expression, thus suppressing the activation of the NF-κB pathway. Additionally, Related genes downstream of the NF-κB pathway such as MMP1, MMP2, MMP9 and CASP8 and FADD like apoptosis regulator are downregulated. Through affecting the peripheral angiogenesis and metastasis of gastric cancer cells, the LINC01410/miR-532/NCF2/NF-κB axis inhibits the progression of gastric cancer (99).
KRT19P3
Certain lncRNAs may also inhibit the development of gastric cancer. KRT19P3 is downregulated in gastric cancer tissues. Through its binding to COP9 signalosome subunit 7A and upregulation of IκBα, KRT19P3 inhibits the activation of the NF-κB signaling pathway, which facilitates apoptosis and suppresses the proliferation of gastric cancer cells. As an important molecular marker for patients with gastric cancer, KRT19P3 is expected to become a novel therapeutic tumor target (100).
4. Conclusion
Gastric cancer, a disease with high morbidity and mortality rates worldwide, has imposed a severe clinical burden (101). A comprehensive exploration of the mechanisms of gastric cancer has been performed and is ongoing, and novel and early screening methods have been proposed. Surgery is still the major treatment method for gastric cancer, but numerous targeted drugs and immunotherapy drugs are being used for treatment of gastric cancer. Physicians and researchers around the world are making continuous efforts to put forward novel ideas regarding the etiology, pathogenesis, diagnosis and treatment of gastric cancer.
In the present review, recent and relevant research results were summarized. The NF-κB signaling pathway is present in almost all cells and is involved in numerous physiological functions. This pathway is overactivated in gastric cancer and in most cases, it participates in the occurrence and development of gastric cancer and is associated with unfavorable prognosis of patients with gastric cancer. The activation of the NF-κB signaling pathway may induce an immunosuppressive microenvironment that enhances tumor development, while its inhibition may be a promising approach for the treatment of gastric cancer (102). miRNAs and lncRNAs were originally considered meaningless molecules, but they have now been confirmed to have crucial roles in various functions of the human body. In gastric cancer, the NF-κB signaling pathway has an important link with miRNAs and lncRNAs, which exert important effects on the occurrence and development of gastric cancer. Furthermore, the interactions of miRNAs/target genes/NF-κB/target proteins, signaling molecules/NF-κB/miRNAs/target genes, lncRNAs/miRNAs/NF-κB/genes or mRNAs, lncRNAs/target genes/NF-κB/target proteins and lncRNAs/NF-κB/target proteins pathways also appear to offer opportunities for subsequent research and clinical treatments.
Acknowledgements
Not applicable.
Funding Statement
Funding: This project was supported by the Chinese National Science Foundation (grant no. 81172210), the China Postdoctoral Science Foundation (grant no. 2012M521528), the open fund of the Chinese Hunan Provincial Education Department Innovation Platform (grant no. 15K109) and the fund of Hunan Provincial Science and Technology Department (grant no. 2018SK51702).
Availability of data and materials
Not applicable.
Authors' contributions
WH and RC conceived the study, RC and MY performed the literature search and data analysis and drafted the manuscript and BW critically revised the manuscript. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Biagioni A, Skalamera I, Peri S, Schiavone N, Cianchi F, Giommoni E, Magnelli L, Papucci L. Update on gastric cancer treatments and gene therapies. Cancer Metast Rev. 2019;38:537–548. doi: 10.1007/s10555-019-09803-7. [DOI] [PubMed] [Google Scholar]
- 3.Abozeid M, Rosato A, Sommaggio R. Immunotherapeutic strategies for gastric carcinoma: A review of preclinical and clinical recent development. Biomed Res Int. 2017;2017(5791262) doi: 10.1155/2017/5791262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schernberg A, Rivin Del Campo E, Rousseau B, Matzinger O, Loi M, Maingon P, Huguet F. Adjuvant chemoradiation for gastric carcinoma: State of the art and perspectives. Clin Transl Radiat Oncol. 2018;10:13–22. doi: 10.1016/j.ctro.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(1010428317714626) doi: 10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 6.Zhang HD, Jiang LH, Sun DW, Li J, Ji ZL. The role of miR-130a in cancer. Breast Cancer. 2017;24:521–527. doi: 10.1007/s12282-017-0776-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Lu X, Zhang X. Noncanonical NF-κB signaling pathway in liver diseases. J Clin Transl Hepatol. 2021;9:81–89. doi: 10.14218/JCTH.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smale ST. Hierarchies of NF-κB target-gene regulation. Nat Immunol. 2011;12:689–694. doi: 10.1038/ni.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12(86) doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Liu M, Liu X, Huang S, Li L, Song B, Li H, Ren Q, Hu Z, Zhou Y, Qiao L. COX-2 regulates E-cadherin expression through the NF-kappaB/Snail signaling pathway in gastric cancer. Int J Mol Med. 2013;32:93–100. doi: 10.3892/ijmm.2013.1376. [DOI] [PubMed] [Google Scholar]
- 11.Koyama S. Differential expression of intracellular apoptotic signaling molecules in tumor and tumor-infiltrating lymphocytes during development of invasion and/or metastasis of gastric carcinoma. Dig Dis Sci. 2003;48:2290–2300. doi: 10.1023/b:ddas.0000007865.96569.9a. [DOI] [PubMed] [Google Scholar]
- 12.Yamanaka N, Sasaki N, Tasaki A, Nakashima H, Kubo M, Morisaki T, Noshiro H, Yao T, Tsuneyoshi M, Tanaka M, Katano M. Nuclear factor-kappaB p65 is a prognostic indicator in gastric carcinoma. Anticancer Res. 2004;24:1071–1075. [PubMed] [Google Scholar]
- 13.Lee BL, Lee HS, Jung J, Cho SJ, Chung HY, Kim WH, Jin YW, Kim CS, Nam SY. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin Cancer Res. 2005;11:2518–2525. doi: 10.1158/1078-0432.CCR-04-1282. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X. MicroRNA: Function, detection, and bioanalysis. Chem Rev. 2013;113:6207–6233. doi: 10.1021/cr300362f. [DOI] [PubMed] [Google Scholar]
- 15.Su W, Mo Y, Wu F, Guo K, Li J, Luo Y, Ye H, Guo H, Li D, Yang Z. MiR-135b reverses chemoresistance of non-small cell lung cancer cells by downregulation of FZD1. Biomed Pharmacother. 2016;84:123–129. doi: 10.1016/j.biopha.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Nezu Y, Hagiwara K, Yamamoto Y, Fujiwara T, Matsuo K, Yoshida A, Kawai A, Saito T, Ochiya T. MiR-135b, a key regulator of malignancy, is linked to poor prognosis in human myxoid liposarcoma. Oncogene. 2016;35:6177–6188. doi: 10.1038/onc.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majeed W, Iftikhar A, Khaliq T, Aslam B, Muzaffar H, Atta K, Mahmood A, Waris S. Gastric carcinoma: Recent trends in diagnostic biomarkers and molecular targeted therapies. Asian Pac J Cancer Prev. 2016;17:3053–3060. [PubMed] [Google Scholar]
- 18.Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol. 2018;24:2818–2832. doi: 10.3748/wjg.v24.i26.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018;109:2093–2100. doi: 10.1111/cas.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng L, Yuan X, Jiang B, Tang Z, Li G. lncRNAs: Key players and novel insights into cervical cancer. Tumour Biol. 2016;37:2779–2788. doi: 10.1007/s13277-015-4663-9. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Meng H, Bai Y, Wang K. Regulation of lncRNA and its role in cancer metastasis. Oncol Res. 2016;23:205–217. doi: 10.3727/096504016X14549667334007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinescu S, Ignat S, Lazar AD, Constantin C, Neagu M, Costache M. Epitranscriptomic Signatures in lncRNAs and their possible roles in cancer. Genes (Basel) 2019;10(52) doi: 10.3390/genes10010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghafouri-Fard S, Abak A, Fattahi F, Hussen BM, Bahroudi Z, Shoorei H, Taheri M. The interaction between miRNAs/lncRNAs and nuclear factor-κB (NF-κB) in human disorders. Biomed Pharmacother. 2021;138(111519) doi: 10.1016/j.biopha.2021.111519. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Guan H. Noncoding RNAs regulating NF-κB signaling. Adv Exp Med Biol. 2016;927:317–336. doi: 10.1007/978-981-10-1498-7_12. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Li Z, Bai L, Lin Y. NF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front Biosci (Landmark Ed) 2011;16:1172–1185. doi: 10.2741/3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Zhang Z, Yu M, Li L, Du G, Xiao W, Yang H. Involvement of miR-20a in promoting gastric cancer progression by targeting early growth response 2 (EGR2) Int J Mol Sci. 2013;14:16226–16239. doi: 10.3390/ijms140816226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu M, Zhou X, Du Y, Huang Z, Zhu J, Xu J, Cheng G, Shu Y, Liu P, Zhu W, Wang T. MiR-20a induces cisplatin resistance of a human gastric cancer cell line via targeting CYLD. Mol Med Rep. 2016;14:1742–1750. doi: 10.3892/mmr.2016.5413. [DOI] [PubMed] [Google Scholar]
- 29.Ma X, Ning S. Shikimic acid promotes estrogen receptor(ER)-positive breast cancer cells proliferation via activation of NF-κB signaling. Toxicol Lett. 2019;312:65–71. doi: 10.1016/j.toxlet.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Xie F, Bao X, Chen W, Xu Q. MiR-300 inhibits epithelial to mesenchymal transition and metastasis by targeting Twist in human epithelial cancer. Mol Cancer. 2014;13(121) doi: 10.1186/1476-4598-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang Y, Zhang Y, Sun Y, Wen Y, Sun F. MicroRNA-300 suppresses metastasis of oral squamous cell carcinoma by inhibiting epithelial-to-mesenchymal transition. Onco Targets Ther. 2018;11:5657–5666. doi: 10.2147/OTT.S173236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He FY, Liu HJ, Guo Q, Sheng JL. Reduced miR-300 expression predicts poor prognosis in patients with laryngeal squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2017;21:760–764. [PubMed] [Google Scholar]
- 33.Cui R, Kim T, Fassan M, Meng W, Sun HL, Jeon YJ, Vicentini C, Tili E, Peng Y, Scarpa A, et al. MicroRNA-224 is implicated in lung cancer pathogenesis through targeting caspase-3 and caspase-7. Oncotarget. 2015;6:21802–21815. doi: 10.18632/oncotarget.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He X, Zhang Z, Li M, Li S, Ren L, Zhu H, Xiao B, Shi R. Expression and role of oncogenic miRNA-224 in esophageal squamous cell carcinoma. BMC Cancer. 2015;15(575) doi: 10.1186/s12885-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen G, Li X, Jia YF, Piazza GA, Xi Y. Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol Sin. 2013;34:336–341. doi: 10.1038/aps.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He C, Wang L, Zhang J, Xu H. Hypoxia-inducible microRNA-224 promotes the cell growth, migration and invasion by directly targeting RASSF8 in gastric cancer. Mol Cancer. 2017;16(35) doi: 10.1186/s12943-017-0603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Yang YB, Zhang XH, Yu XL, Wang ZB, Cheng XC. MicroRNA-21 gene and cancer. Med Oncol. 2013;30(376) doi: 10.1007/s12032-012-0376-8. [DOI] [PubMed] [Google Scholar]
- 38.Shin VY, Jin H, Ng EK, Cheng AS, Chong WW, Wong CY, Leung WK, Sung JJ, Chu KM. NF-κB targets miR-16 and miR-21 in gastric cancer: Involvement of prostaglandin E receptors. Carcinogenesis. 2011;32:240–245. doi: 10.1093/carcin/bgq240. [DOI] [PubMed] [Google Scholar]
- 39.Sha M, Ye J, Zhang LX, Luan ZY, Chen YB, Huang JX. Celastrol induces apoptosis of gastric cancer cells by miR-21 Inhibiting PI3K/Akt-NF-κB signaling pathway. Pharmacology. 2014;93:39–46. doi: 10.1159/000357683. [DOI] [PubMed] [Google Scholar]
- 40.Ni S, Zhao L, Wang X, Wu ZH, Hua RX, Wan CH, Zhang JY, Zhang XW, Huang MZ, Gan L, et al. CBX7 regulates stem cell-like properties of gastric cancer cells via p16 and AKT-NF-κB-miR-21 pathways. J Hematol Oncol. 2018;11(17) doi: 10.1186/s13045-018-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Li Y, Qi P, Ma Z. Biology of MiR-17-92 cluster and its progress in lung cancer. Int J Med Sci. 2018;15:1443–1448. doi: 10.7150/ijms.27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, Cheng L, Xu J, Guo F, Chen W. MiR-17-92 functions as an oncogene and modulates NF-κB signaling by targeting TRAF3 in MGC-803 human gastric cancer cells. Int J Oncol. 2018;53:2241–2257. doi: 10.3892/ijo.2018.4543. [DOI] [PubMed] [Google Scholar]
- 43.Yang XD, Sun SC. Targeting signaling factors for degradation, an emerging mechanism for TRAF functions. Immunol Rev. 2015;266:56–71. doi: 10.1111/imr.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Y, Wu J, Wu Q, Li X, Wu J, Zhang J, Rong X, Rao J, Liao Y, Bin J, et al. MiR-577 regulates TGF-β induced cancer progression through a SDPR-Modulated positive-feedback loop with ERK-NF-κB in gastric cancer. Mol Ther. 2019;27:1166–1182. doi: 10.1016/j.ymthe.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li N, Zhang QY, Zou JL, Li ZW, Tian TT, Dong B, Liu XJ, Ge S, Zhu Y, Gao J, Shen L. MiR-215 promotes malignant progression of gastric cancer by targeting RUNX1. Oncotarget. 2016;7:4817–4828. doi: 10.18632/oncotarget.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Zhang J, Zheng Y, Ma C, Liu XE, Sun X. MiR-216a-3p inhibits the proliferation, migration, and invasion of human gastric cancer cells via targeting RUNX1 and activating the NF-κB signaling pathway. Oncol Res. 2018;26:157–171. doi: 10.3727/096504017X15031557924150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathis BJ, Lai Y, Qu C, Janicki JS, Cui T. CYLD-mediated signaling and diseases. Curr Drug Targets. 2015;16:284–294. doi: 10.2174/1389450115666141024152421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia JT, Chen LZ, Jian WH, Wang KB, Yang YZ, He WL, He YL, Chen D, Li W. MicroRNA-362 induces cell proliferation and apoptosis resistance in gastric cancer by activation of NF-κB signaling. J Transl Med. 2014;12(33) doi: 10.1186/1479-5876-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang YY, Ye ZY, Zhao ZS, Li L, Wang YX, Tao HQ, Wang HJ, He XJ. Clinicopathologic significance of miR-10b expression in gastric carcinoma. Hum Pathol. 2013;44:1278–1285. doi: 10.1016/j.humpath.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Chen XL, Hong LL, Wang KL, Liu X, Wang JL, Lei L, Xu ZY, Cheng XD, Ling ZQ. Deregulation of CSMD1 targeted by microRNA-10b drives gastric cancer progression through the NF-κB pathway. Int J Biol Sci. 2019;15:2075–2086. doi: 10.7150/ijbs.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang F, Xu Y, Liu C, Ma C, Zou S, Xu X, Jia J, Liu Z. NF-κB/miR-223-3p/ARID1A axis is involved in Helicobacter pylori CagA-induced gastric carcinogenesis and progression. Cell Death Dis. 2018;9(12) doi: 10.1038/s41419-017-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murata M. Inflammation and cancer. Environ Health Prev. 2018;23(50) doi: 10.1186/s12199-018-0740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shao L, Chen Z, Soutto M, Zhu S, Lu H, Romero-Gallo J, Peek R, Zhang S, El-Rifai W. Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. FASEB J. 2019;33:264–274. doi: 10.1096/fj.201701456RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma J, Liu J, Wang Z, Gu X, Fan Y, Zhang W, Xu L, Zhang J, Cai D. NF-kappaB-dependent microRNA-425 upregulation promotes gastric cancer cell growth by targeting PTEN upon IL-1β induction. Mol Cancer. 2014;13(40) doi: 10.1186/1476-4598-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang SM, Huang C, Li XF, Yu MZ, He Y, Li J. MiR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162–168. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Wu W, Yang J, Feng X, Wang H, Ye S, Yang P, Tan W, Wei G, Zhou Y. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol Cancer. 2013;12(30) doi: 10.1186/1476-4598-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo Z, Zheng Y, Zhang W. Pleiotropic functions of miR107 in cancer networks. Onco Targets Ther. 2018;11:4113–4124. doi: 10.2147/OTT.S151236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Q, Chen C, Guan H, Kang W, Yu C. Prognostic role of microRNAs in human gastrointestinal cancer: A systematic review and meta-analysis. Oncotarget. 2017;8:46611–46623. doi: 10.18632/oncotarget.16679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li F, Liu B, Gao Y, Liu Y, Xu Y, Tong W, Zhang A. Upregulation of MicroRNA-107 induces proliferation in human gastric cancer cells by targeting the transcription factor FOXO1. FEBS Lett. 2014;588:538–544. doi: 10.1016/j.febslet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Zhang C, Tian W, Meng L, Qu L, Shou C. PRL-3 promotes gastric cancer migration and invasion through a NF-κB-HIF-1α-miR-210 axis. J Mol Med (Berl) 2016;94:401–415. doi: 10.1007/s00109-015-1350-7. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Bai W, Zhang X. Identifying heterogeneous subtypes of gastric cancer and subtype-specific subpaths of microRNA-target pathways. Mol Med Rep. 2018;17:3583–3590. doi: 10.3892/mmr.2017.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalinowski FC, Brown RA, Ganda C, Giles KM, Epis MR, Horsham J, Leedman PJ. MicroRNA-7: A tumor suppressor miRNA with therapeutic potential. Int J Biochem Cell Biol. 2014;54:312–317. doi: 10.1016/j.biocel.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 63.Zhao XD, Lu YY, Guo H, Xie HH, He LJ, Shen GF, Zhou JF, Li T, Hu SJ, Zhou L, et al. MicroRNA-7/NF-κB signaling regulatory feedback circuit regulates gastric carcinogenesis. J Cell Biol. 2015;210:613–627. doi: 10.1083/jcb.201501073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li M, Pan M, You C, Dou J. The therapeutic potential of miR-7 in cancers. Mini Rev Med Chem. 2019;19:1707–1716. doi: 10.2174/1389557519666190904141922. [DOI] [PubMed] [Google Scholar]
- 65.Ye T, Yang M, Huang D, Wang X, Xue B, Tian N, Xu X, Bao L, Hu H, Lv T, Huang Y. MicroRNA-7 as a potential therapeutic target for aberrant NF-κB-driven distant metastasis of gastric cancer. J Exp Clin Canc Res. 2019;38(55) doi: 10.1186/s13046-019-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen WQ, Hu L, Chen GX, Deng HX. Role of microRNA-7 in digestive system malignancy. World J Gastrointest Oncol. 2016;8:121–127. doi: 10.4251/wjgo.v8.i1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang SM, Tie J, Wang WL, Hu SJ, Yin JP, Yi XF, Tian ZH, Zhang XY, Li MB, Li ZS, et al. POU2F2-oriented network promotes human gastric cancer metastasis. Gut. 2016;65:1427–1438. doi: 10.1136/gutjnl-2014-308932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo Y, Zhang T, Shi Y, Zhang J, Li M, Lu F, Zhang J, Chen X, Ding S. Helicobacter pylori inhibits GKN1 expression via the CagA/p-ERK/AUF1 pathway. Helicobacter. 2020;25(e12665) doi: 10.1111/hel.12665. [DOI] [PubMed] [Google Scholar]
- 69.Chung Nien Chin S, O'Connor L, Scurr M, Busada JT, Graham AN, Alipour Talesh G, Tran CP, Sarkar S, Minamoto T, Giraud AS, et al. Coordinate expression loss of GKN1 and GKN2 in gastric cancer via impairment of a glucocorticoid-responsive enhancer. Am J Physiol Gastrointest Liver Physiol. 2020;319:G175–G188. doi: 10.1152/ajpgi.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z, Xue H, Dong Y, Zhang J, Pan Y, Shi L, Xiong P, Zhu J, Li W, Zheng W, et al. GKN2 promotes oxidative stress-induced gastric cancer cell apoptosis via the Hsc70 pathway. J Exp Clin Cancer Res. 2019;38(338) doi: 10.1186/s13046-019-1336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim O, Yoon JH, Choi WS, Ashktorab H, Smoot DT, Nam SW, Lee JY, Park WS. GKN2 contributes to the homeostasis of gastric mucosa by inhibiting GKN1 activity. J Cell Physiol. 2014;229:762–771. doi: 10.1002/jcp.24496. [DOI] [PubMed] [Google Scholar]
- 72.Crone SG, Jacobsen A, Federspiel B, Bardram L, Krogh A, Lund AH, Friis-Hansen L. MicroRNA-146a inhibits G protein-coupled receptor-mediated activation of NF-κB by targeting CARD10 and COPS8 in gastric cancer. Mol Cancer. 2012;11(71) doi: 10.1186/1476-4598-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Y, Zhou B, Xu L, Fan H, Xie J, Wang D. MicroRNA-146a promotes gastric cancer cell apoptosis by targeting transforming growth factor β-activated kinase 1. Mol Med Rep. 2017;16:755–763. doi: 10.3892/mmr.2017.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng DF, Sun R, Liu GY, Peng LX, Zheng LS, Xie P, Lin ST, Mei Y, Qiang YY, Li CZ, et al. S100A14 suppresses metastasis of nasopharyngeal carcinoma by inhibition of NF-κB signaling through degradation of IRAK1. Oncogene. 2020;39:5307–5322. doi: 10.1038/s41388-020-1363-8. [DOI] [PubMed] [Google Scholar]
- 75.Wee ZN, Yatim SM, Kohlbauer VK, Feng M, Goh JY, Bao Y, Lee PL, Zhang S, Wang PP, Lim E, et al. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat Commun. 2015;6(8746) doi: 10.1038/ncomms9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diaconu S, Predescu A, Moldoveanu A, Pop CS, Fierbinteanu-Braticevici C. Helicobacter pylori infection: Old and new. J Med Life. 2017;10:112–117. [PMC free article] [PubMed] [Google Scholar]
- 77.Matsushima K, Isomoto H, Inoue N, Nakayama T, Hayashi T, Nakayama M, Nakao K, Hirayama T, Kohno S. MicroRNA signatures in Helicobacter pylori-infected gastric mucosa. Int J Cancer. 2011;128:361–370. doi: 10.1002/ijc.25348. [DOI] [PubMed] [Google Scholar]
- 78.Darnet S, Moreira FC, Hamoy IG, Burbano R, Khayat A, Cruz A, Magalhães L, Silva A, Santos S, Demachki S, et al. High-Throughput sequencing of miRNAs reveals a tissue signature in gastric cancer and suggests novel potential biomarkers. Bioinform Biol Insights. 2015;9 (Suppl 1):1–8. doi: 10.4137/BBI.S23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Assumpcao MB, Moreira FC, Hamoy IG, Magalhães L, Vidal A, Pereira A, Burbano R, Khayat A, Silva A, Santos S, et al. High-Throughput miRNA sequencing reveals a field effect in gastric cancer and suggests an epigenetic network mechanism. Bioinform Biol Insights. 2015;9:111–117. doi: 10.4137/BBI.S24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ranjbar R, Hesari A, Ghasemi F, Sahebkar A. Expression of microRNAs and IRAK1 pathway genes are altered in gastric cancer patients with Helicobacter pylori infection. J Cell Biochem. 2018;119:7570–7576. doi: 10.1002/jcb.27067. [DOI] [PubMed] [Google Scholar]
- 81.Li N, Wang J, Yu W, Dong K, You F, Si B, Tang B, Zhang Y, Wang T, Qiao B. MicroRNA-146a inhibits the inflammatory responses induced by interleukin-17A during the infection of Helicobacter pylori. Mol Med Rep. 2019;19:1388–1395. doi: 10.3892/mmr.2018.9725. [DOI] [PubMed] [Google Scholar]
- 82.Zhang W, Wang Y, Zhu Z, Zheng Y, Song B. Propofol inhibits proliferation, migration and invasion of gastric cancer cells by up-regulating microRNA-195. INT J Biol Macromol. 2018;120(Pt A):975–984. doi: 10.1016/j.ijbiomac.2018.08.173. [DOI] [PubMed] [Google Scholar]
- 83.Sui H, Lou A, Li Z, Yang J. Lidocaine inhibits growth, migration and invasion of gastric carcinoma cells by up-regulation of miR-145. BMC CANCER. 2019;19(233) doi: 10.1186/s12885-019-5431-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Xu L, Ge F, Hu Y, Yu Y, Guo K, Miao C. Sevoflurane postconditioning attenuates hepatic ischemia-reperfusion injury by limiting HMGB1/TLR4/NF-κB Pathway via modulating microRNA-142 in vivo and in vitro. Front Pharmacol. 2021;12(646307) doi: 10.3389/fphar.2021.646307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang C, Gao J, Yan N, Wu B, Ren Y, Li H, Liang J. Propofol inhibits the growth and survival of gastric cancer cells in vitro through the upregulation of ING3. Oncol Rep. 2017;37:587–593. doi: 10.3892/or.2016.5218. [DOI] [PubMed] [Google Scholar]
- 86.Peng Z, Zhang Y. doi: 10.4238/gmr.15027078. Propofol inhibits proliferation and accelerates apoptosis of human gastric cancer cells by regulation of microRNA-451 and MMP-2 expression. Genet Mol Res 15, 2016. [DOI] [PubMed] [Google Scholar]
- 87.Yu W, Liang X, Li X, Zhang Y, Sun Z, Liu Y, Wang J. MicroRNA-195: A review of its role in cancers. Onco Targets Ther. 2018;11:7109–7123. doi: 10.2147/OTT.S183600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang Z, Zhu W, Shu Y, Liu P. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett. 2014;588:1168–1177. doi: 10.1016/j.febslet.2014.02.054. [DOI] [PubMed] [Google Scholar]
- 89.Soto G, Naranjo González M, Calero F. Intravenous lidocaine infusion. Rev Esp Anestesiol Reanim. 2018;65:269–274. doi: 10.1016/j.redar.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L, Lei J, Fang ZL, Xiong JP. MiR-128b is down-regulated in gastric cancer and negatively regulates tumour cell viability by targeting PDK1/Akt/NF-κB axis. J Biosci. 2016;41:77–85. doi: 10.1007/s12038-016-9586-0. [DOI] [PubMed] [Google Scholar]
- 91.Jiao Y, Yang H, Qian J, Gong Y, Liu H, Wu S, Cao L, Tang L. MiR-3664-5P suppresses the proliferation and metastasis of gastric cancer by attenuating the NF-κB signaling pathway through targeting MTDH. Int J Oncol. 2019;54:845–858. doi: 10.3892/ijo.2019.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dastmalchi N, Khojasteh SMB, Nargesi MM, Safaralizadeh R. The correlation between lncRNAs and Helicobacter pylori in gastric cancer. Pathog Dis. 2019;77(ftaa004) doi: 10.1093/femspd/ftaa004. [DOI] [PubMed] [Google Scholar]
- 93.Peng WX, Koirala P, Mo YY. lncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta SC, Awasthee N, Rai V, Chava S, Gunda V, Challagundla KB. Long non-coding RNAs and nuclear factor-κB crosstalk in cancer and other human diseases. Biochim Biophys Acta Rev Cancer. 2020;1873(188316) doi: 10.1016/j.bbcan.2019.188316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kong Y, Hsieh CH, Alonso LC. ANRIL: A lncRNA at the CDKN2A/B Locus with roles in cancer and metabolic disease. Front Endocrinol (Lausanne) 2018;9(405) doi: 10.3389/fendo.2018.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng W, Zhang Y, Cai J, Zhang J, Liu X, Yin J, Bai Z, Yao H, Zhang Z. lncRNA-ANRIL promotes gastric cancer progression by enhancing NF-κB signaling. Exp Biol Med (Maywood) 2019;244:953–959. doi: 10.1177/1535370219860207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu X, Zheng H, Chan MT, Wu WKK. BANCR: A cancer-related long non-coding RNA. Am J Cancer Res. 2017;7:1779–1787. [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang ZX, Liu ZQ, Jiang B, Lu XY, Ning XF, Yuan CT, Wang AL. BRAF activated non-coding RNA (BANCR) promoting gastric cancer cells proliferation via regulation of NF-κB1. Biochem Bioph Res Commun. 2015;465:225–231. doi: 10.1016/j.bbrc.2015.07.158. [DOI] [PubMed] [Google Scholar]
- 99.Zhang JX, Chen ZH, Chen DL, Tian XP, Wang CY, Zhou ZW, Gao Y, Xu Y, Chen C, Zheng ZS, et al. LINC01410-miR-532-NCF2-NF-κB feedback loop promotes gastric cancer angiogenesis and metastasis. Oncogene. 2018;37:2660–2675. doi: 10.1038/s41388-018-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Zheng J, Zhang H, Ma R, Liu H, Gao P. Long non-coding RNA KRT19P3 suppresses proliferation and metastasis through COPS7A-mediated NF-κB pathway in gastric cancer. Oncogene. 2019;38:7073–7088. doi: 10.1038/s41388-019-0934-z. [DOI] [PubMed] [Google Scholar]
- 101.Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9:217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marta ZN, Agnieszka W, Jacek P, Jeleń A, Adrian K, Dagmara SK, Sałagacka-Kubiak A, Balcerczak E. NFKB2 gene expression in patients with peptic ulcer diseases and gastric cancer. Mol Biol Rep. 2020;47:2015–2021. doi: 10.1007/s11033-020-05299-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.