Abstract
When considering the development pathway for a genetically modified cell therapy product, it is critically important that the product is engineered consistent with its intended human use. For scientists looking to develop and commercialize a new technology, the decision to select a genetic modification method depends on several practical considerations. Whichever path is chosen, the developer must understand the key risks and potential mitigations of the cell engineering approach. The developer should also understand the clinical implications: permanent/memory establishment versus transient expression, and clinical manufacturing considerations when dealing with transplantation of genetically engineered cells. This review covers important topics for mapping out a strategy for developers of new cell-based therapeutics. Biological, technological, manufacturing, and clinical considerations are all presented to map out development lanes for the initiation and risk management of new gene-based cell therapeutic products for human use.
Keywords: genetic engineering, cell therapy, gene therapy, manufacturing, viral vector, product development
Introduction
The recent integration of cell and molecular medicine has led to breakthroughs in engineered cell therapeutics and the rapid development of new therapeutic modalities. Most notably in regard to the latter, this has evolved into the genetic engineering of human leukocytes to combine the specific and universal binding modality of an antibody with the cytolytic and anamnestic properties of T-cells. This led to the conceptualization and development of what are known as Chimeric Antigen Receptor (CAR)-T cells and represent the current state of adoptive immunotherapy with genetically modified cells. The first two FDA approved products, Kymriah for treatment of B-cell acute lymphoblastic leukemia and Yescarta, for large B-cell lymphoma represent the impressive and momentous shift into the application of gene editing technologies for production of successful cell-based therapeutics and the first engineered cells of their kind to be transplanted for treatment of diseases.
The field of biologics has experienced a transformative phase with advancements in the ability to reconstruct and manipulate DNA, which has allowed for direct targeting and modification of genomic sequences in most eukaryotic cells1. Gene editing technology has been implemented to enhance the capacity for in vitro gene modeling through generation of engineered animal models for pharmacological modeling and improved understanding of human diseases. Manipulation of human cells prior to transplantation through targeted insertion or deletion of genes in cell genomes has been used for improved targeting and/or efficiency in vivo. Important considerations such as off-target effects, desired genomic integration site, disruption of gene function or genomic instability have to be considered when choosing a genome-editing technology for a cell therapy product.
This review highlights the biological and technological considerations essential to achieve the manufacturing of a reproducible and robust engineered cell therapy product. The design of these novel engineered cell types in the decision-making process of defining the target product profile (TPP) include the desired genetic vehicle or technology, the manufacturing method, clinical considerations, sustainability, cost of goods, business considerations, and risk mitigation. This review further includes a discussion of the manufacturing operations that should be considered when designing gene-modified cell therapeutics. Finally, business considerations are highlighted with respect to the selection of vectors and manufacturing paradigms where intellectual property exists that may require licensing opportunities to further enable one’s therapeutic program(s) or provide needed freedom-to-operate for future commercialization. Ultimately, these considerations feed heavily into significant financial decisions for drug development, which are worthy of study for new developers and the profitability of the proposed therapeutic approach.
Genome Modification for Cell Therapy
History of Genetic Modification of Cells
The current state-of-the-art of genetically-modified cell therapeutics has had decades of history underpinning this new class of medicine first approved in the United States by the FDA in 20172. Adoptive cell therapy, which was developed as an application of immune surveillance theory, dates back to the concepts published by Paul Ehrlich and Lewis Thomas3. Historically, patient blood transfusions were the first cell transplantation therapy for the treatment of many kinds of blood disorders, including anemias, leukemias, lymphomas, and immunodeficiencies. Initial cell therapies consisted of allogeneic bone marrow transplants for leukemia patients post-myeloablation to both leverage blood reconstitution and induce a Graft-versus-Leukemia effect. Strong response rates were encouraging but the high incidence of Graft-versus-Host Disease (GvHD) limited applicability4. Refinement of this approach identified the synergistic roles of CD34+ cells and T cells in donor lymphocyte infusions in the treatment of cancer. These studies lead to the discovery of Tumor Infiltrating Lymphocytes (TILs), Lymphokine Activated Killer cells (LAKs), and Natural Killer (NK) cells which recognize specific antigens residing within tumors and mediate cancer regression. These findings collectively pointed to the potential of cellular immunotherapy for cancer. Early work involving genetically modified T cells were used to downregulate or upregulate certain binding domains or proteins to enhance cell function5. Efforts at the University of Pennsylvania, the Children’s Hospital of Philadelphia and their collaborators ultimately spurred the development of genetically modified cell therapies to recognize cancer specific antigens, which, in 2017, resulted in FDA approval and commercialization of the first CAR T-cell product for the treatment of leukemia. Further advances and elaborate complexities continue to be added to the CAR platform that take advantage of the molecular interplay of antigen receptors, lymphocytes, and tumor cells. Therapeutic responses to CAR T-cell therapy in patients have been dramatic, although at the cost of high rates and adverse events that can become severe6. For the first FDA approved CAR products, patients saw toxicities related to the systemic release of high levels of cytokines, which include cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), which can become fatal in some patients. Additional on-target, off-tumor effects can be seen in some cases where toxicities result from specific interactions between the CAR and its target antigen on non-malignant cells. In order to avoid general systemic toxicities, CAR T-cells must reach an efficacy threshold without exceeding cytokine secretion, and thus development of methods to meet this therapeutic window for different CARs will need to be established to overcome these limitations. These ongoing immunotherapy developments will continue to push the limits of specificity and efficacy onto additional and more difficult targets to become a routine treatment in cancer.
Lentiviral and Gamma-Retroviral Vector Permanent Expression Systems
Historically, gamma-retroviral and lentiviral vectors have a long history in gene editing for transplanted cells as the first vectors used in the clinical setting because they permanently express the genetic construct of interest. The earliest gamma-retroviruses were derived in the mid 1980s from avian and murine oncogenic retroviruses. The lentiviral vector constructs in use today were derived from non-human primates, feline, equine, and/or human immunodeficiency viruses type 1 and 2 (HIV-1, 2). Vectors were engineered to be non-replicating and self-inactivating, and thus less pathogenic to humans7. Since the early 2000s, lentiviral vectors have become the preferred system over gamma-retrovirus as early clinical trials using gamma-retrovirus resulted in cases of leukemia due to insertional mutagenesis8. Lentiviral vectors share similarities in efficacy to gamma-retroviral vectors, yet lentivirus has one significant point of differentiation that broadens their use in that they can transduce non-dividing cells.
Recent scientific advances in cancer immunotherapy in the clinic have shown success, particularly in the case of therapies involving the delivery of genes using viral vectors to directly kill or redirect autologous T cells to induce a potent anti-tumor immune response. While gamma-retroviral vectors were the first vehicles to be used for the delivery of CAR constructs to immune cells, safety concerns surrounding insertional mutagenesis potential moved the field to safer and potentially more effective lentiviral vectors. Lentiviral vectors are now the primary vectors used clinically for delivery of CAR-engineered cells and have been used to treat other types of inherited and acquired disorders, including hematologic disorders. Administration of lentiviral vectors is typically performed ex vivo to directly achieve high transduction in target cells which further adds to a beneficial safety margin as the virus is not directly transmitted to the patient. The modified cells are subsequently transplanted to the patient after expansion. Additionally, ex vivo administration allows the developer greater control in the dosing of a predetermined (range of) modified cells versus in vivo gene therapy in which the number of viral vectors is known but the number of resulting modified cells cannot be accurately measured.
Transient Expression with Adenovirus and Adeno-Associated Virus
Adenovirus and AAV had been initially developed for use in in vivo transient expression in patients, either directly delivered to the site of need (e.g., lungs for treatment of cystic fibrosis) or systemically for metabolic disorders where multiple target cells require a “hit” that direct organ targeting may not necessarily achieve or where the target organ may be easier to reach via systemic delivery, such as the liver which has a natural affinity for AAV uptake. Regardless, off-target effects associated with these vectors are heavily considered and influence not just the choice of vector, but also specific serotypes and construct design9. Unfortunately, many first-generation strategies did not demonstrate significant clinical meaningfulness and can be summarized as a combination of limitations in transgene selection to disease target, patient immunogenicity to the vectors, manufacturing challenges, and vector administration. Many of the technical challenges associated with these vectors, in particular scalable manufacturing, remain significant issues in the industry today yet with recent success in the field are receiving more attention than they had in past years10.
Over 1,000 different clinical trials between the two platforms have been conducted resulting in a robust safety profile for both AAV and adenovirus-based vector systems across a multitude of applications, including repeated dosing for infectious disease through systemic delivery of vectors into terminally ill patients. Both vector systems are also amenable to capsid engineering to modify tropism and further enhance specificity to target cells, as there are currently over 12 identified serotypes11. Novel non-human vector serotypes of both adeno- and AAV vectors have included chimpanzee and gorilla species in efforts to avoid host cell immunity, as most individuals have been exposed to native human viral serotypes at some point in their lives. Naturally occurring AAV vectors can be selected to more efficiently target particular cell types and directed evolution has further been employed to enhance specificity11.
Unlike retroviral and lentiviral vectors, an attractive benefit of adenovirus and AAV is their inability to integrate into the host genome, thus alleviating many of the safety concerns associated with the use of permanent integrating vectors. Human adenovirus does not contain any complementary DNA sequences that result in homologous recombination in human cells and thus expression is transient and not long lasting. Because of this, adenovirus has become a promising vector for vaccines and oncolytic viral therapy, where the replicating mechanism of action of adenovirus is combined with the expression of potent cytokines from the same vector12. Unlike adenovirus which is generally considered non-integrating, AAV does have the potential to integrate at a very low frequency with concerns for host cell mutagenesis resulting in malignant transformation being raised13. While AAV vectors are considered safe, a more thorough understanding of the biology of these vectors is needed to fully understand the risks. Table 1 outlines a number of viral vectors that have been safely applied in human trials of cell or gene therapy, each having their own specific applications in the field.
Table 1.
Vector Types and Characteristic Properties.
| Vector | Genome & size (kb) | Capacity (kb) | Diameter (nm) | Tropism | Expression |
|---|---|---|---|---|---|
| Adenovirus | dsDNA; 36 | 4–7 | 70–90 | D, ND | ST, Transient |
| AAV | ssDNA; 4.9 | 2.4–4.5 | 18–26 | D, ND | LT, Transient |
| HSV | dsDNA 120–200 | 30 | 150–200 | D, ND | LT, Transient |
| Lentivirus | RNA; 7–9 | 8 | 80–120 | D, ND | LT, Stable |
| Retrovirus | RNA; 7–10 | 8 | 80–130 | D | LT, Stable |
Abbreviations: D, dividing; ND, non-dividing; ST, short term; LT, long term.
Non-Viral Gene Editing Systems
The emergence over decades of highly unique and versatile genome-editing technologies has provided researchers with the ability to introduce sequence-specific nucleic acid modifications into the genomes of a broad spectrum of cell types and organisms14. The core technology most commonly used to facilitate genome editing is clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9). The system has evolved over time and now consists of only the Cas9 nuclease and a single target guide RNA (gRNA) containing essential elements. As of now, researchers have utilized several tools that allow for genome editing apart from the most notable CRISPR-Cas9 system, including zinc finger nucleases, (ZFNs), transcription activator-like effector nucleases (TALENs), and the next generation Arc nuclease platform, termed ARCUS. ZFNs have been around since as early as 1996, when the first zinc finger protein domain demonstrated site-specific nuclease cutting at strictly defined DNA sites in vitro for the first time15. However, the complexity and cost of this method when it comes to design and construction of specific protein domains for genome editing of a particular DNA locus as well as high probability for inaccurate cleavage of target DNA makes it less desirable for current and future applications. More recently, the development of the TALEN and ARCUS system have improved the simplicity and efficiency of gene editing methods in human, plant, and animal subtypes16. The TALEN system operates on the principle of bacterial secretion of effector proteins (TALEs) which are composed of a central domain for DNA binding, signaling, and activation domain responsible for target gene transcription. The activity of each TALE domain is restricted to one nucleotide, therefore does not affect binding specificity of neighboring TALEs unlike ZFNs17. The ARCUS system, most newly developed of all the gene editing technologies, is an ARC nuclease synthetic enzyme, similar to a homing endonuclease which gives it the unusual ability to precisely recognize long DNA sequences and trigger gene conversion events to modify the genome through insertion in a precise way18. Table 2 provides a more detailed overview of each of the different non-viral genome editing technologies used today and when each method is preferred for the desired application. Gene editing systems have been used to generate disease models for drug library screening, rapidly engineer cells for transplantation in immune treatment and surveillance, and targeted gene knockout, to name a few19–21.
Table 2.
| Genome editing technologies | Method of editing | Highest efficiency achieved | Benefit | Disadvantage | Off-targets | Clinical/preclinical usage | When is it preferred? |
|---|---|---|---|---|---|---|---|
| Zinc finger nucleases | Introducing a double stranded break at a specified location in the genome using DNA | Lower efficiency / high specificity suitable for Knocking out gene function23,25 | High level of specificity because pairs of ZFNs must be designed for dimerization and cleavage23,25 | Expensive and finicky to make23 | Off-target sites cleaved when ZFN pairs are expressed in cells (binding of nuclease to unintended sites that share sequence homology with on-target site).23,25 | C: Confer resistance to HIV virus in AIDS patients (Tebas et al. 2014)25,27,30 | Targeted gene replacement21,25 |
| Transcription activation-like effector nucleases (TALENs) | Introducing a double stranded break at a specified location in the genome using DNA binding domain and Fokl DNA-cleaving domain Protein-DNA Recognition.24,25,29 | Modify with high efficiency, however sensitive to cytosine methylation (DNA silencing)29 | Simpler and easier to engineer than Zinc fingers23,25 | Large molecules, therefore, can be difficult to deliver efficiently / Methylation Sensitive29,30 | Little evidence of mismatch tolerance or off-target activity demonstrated25,29 | PC: Engineer lymphocytes for treatment of acute lymphoblastic leukemia (Poirot et al. 2015)25,27,30 | Less mutagenesis unconstrained design23,25,29 |
| CRISPR-Cas9 | RNA-DNA recognition24,26,29 | Highest efficiency because it is capable of modifying targets at high frequency29 | Simple to design and use (flexible)26,29 | Been shown to associate with many off target sites29 | More potential for off-target effects than TALENs and ZFNs29 | C: T cells with PD-1 knockout and early CAR-T studies22,25,26,30 | Quick gene-knock out for pilot study, target design simplicity25,26 |
| Arc nuclease platform (ARCUS) | Enzyme-DNA recognition (Homing endonuclease)18 | Designed in silico for maximum gene editing efficiency18 | Non-destructive enzymes that modify in very precise manner18 | Relatively new technology not fully explored18 | Little evidence of random off-target events18 | None yet18 | Extremely specific target with no off-targets18 |
Overview of Transfection and Transduction Technologies
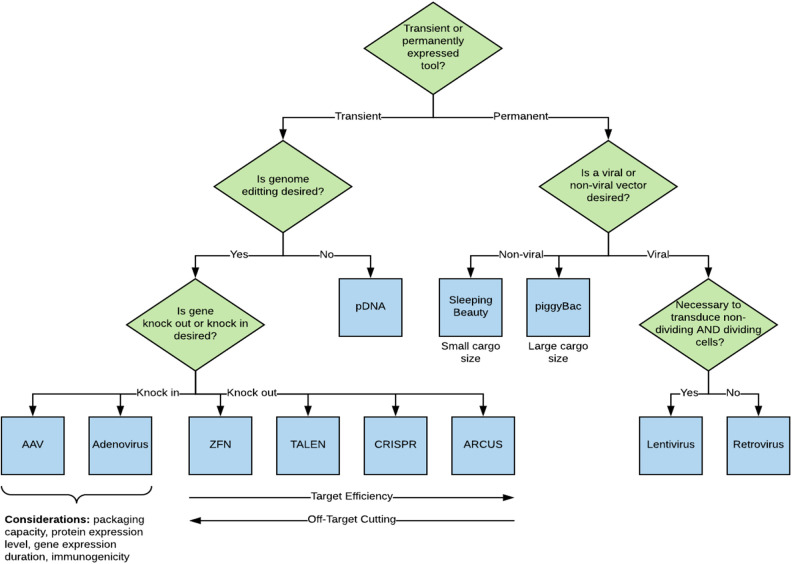
With advancements in genome modification technologies, there have been simultaneous advancements in the ability to deliver genetic payloads using specific gene delivery vehicles into cells31. There are numerous modalities, in both early development and commercial-use phases, that can be used for introducing genetic changes into a cell. Methods can generally be categorized as either viral (transduction) or non-viral (transfection). Transfection and transduction technologies form the foundation and technical scalability of the gene modification manufacturing process with gene insertion methodology being a key point when considering long or short-term applications (Fig. 1), namely transient versus permanent expression in cells.
Fig. 1.
Decision tree and rationale for the selection of a transfection methodology.
Each of these methods have advantages and disadvantages that are dependent on the disease application, number of patients, treatment modalities, and overall cost of goods. For gene modification, the first CAR-T candidates were developed using a gamma-retroviral vector, which delivers a permanent payload to the target cells as the gene of interest integrates into the genome. Other uses of viral vectors in immunotherapy aimed to enhance the immune response through a programmed cell death receptor (PD-1)22. The most used viral vectors for stable expression are the gamma-retroviral and lentiviral vectors. Other vectors such as adenovirus, adeno-associated virus and herpes simplex virus, have also been used for gene therapy applications but typically show lower transduction efficiencies into target cells or do not stably integrate compared to retroviral and lentiviral vectors.
Engineering of stem cells has been achieved with viral transduction methods that efficiently and stably introduce genetic material into cells through integration of the therapeutic gene into a cell’s genome. The creation of induced pluripotent stem cells (iPSCs) was an incredible feat accomplished through genetic modification of defined factors which provides a renewable source of patient autologous cells that can retain identical genetic information as well as give rise to many cell types of the body as desired. iPSCs are often used now as a replacement for embryonic stem cells (ESCs) because they overcome the ethical and technical limitations of ESCs32.
Despite advances in large-scale suspension cell line production using transfection-based manufacturing processes or through stable producer cell lines, the costs of generating large quantities of high titer viral vectors has remained relatively high. Current research on the use of integrating vectors is focused on improving vector design, characterizing insertion site preferences, and thus reducing patient risk associated with integrating vectors33. For additional safety, several groups have developed viral vectors or constructs that include a suicide switch within the transgene cassette based on an inducible caspase that allows selective elimination of engineered cells in vivo post-administration should treatment go awry. Here, a balance between efficacy and safety is yet to be determined, as is the ability of the insurance marketplace to shoulder the burden of costly and effective therapies.
Non-viral methods include a multitude of developing technologies that can be customized based on target cell type and genetic payload size. All aim to increase permeability of the cell membrane to promote uptake of the DNA or RNA and include electroporation, mechanoporation, and sonoporation. Non-viral alternatives are being developed for ex vivo transfection using liposomes, electroporation, or other techniques as described further in this document.
Non-Viral Transfection Methodologies
Transfection techniques designed to transfer nucleic acids into cells include both chemical-based products (such as liposomes), and non-chemical methods which typically involve the temporary formation of pores in the cellular membrane; allowing for the translocation of material from outside the cell to enter the cell (such as electroporation). The delivery of mRNA via transfection is a technique that can be used to transiently modify cells, with the full expectation that only short-lived expression of the new genetic material will occur. For stable expression of transgenes, transfection has been used to introduce plasmids that encode transposon elements and the transposase enzyme (such as Sleeping Beauty or PiggyBac) is relatively cost-effective in early clinical development. Additional methods include proprietary delivery solutions that permeabilize the cell membrane (e.g., chemoporation), allowing for diffusion of the delivery material into the cell, prior to reversing the permeabilization.
Non-viral vectors are typically cationic lipids, polymers and peptides that are able to complex or encapsulate genetic information for transfer within cells34. By complexing anionic nucleotides with cationic polymers or particles, DNA or RNA is protected from nucleases for in vivo and in vitro gene delivery. The cationic nature of these compact particles provides an additional advantage from a transfection efficiency perspective. First, cationic particles interact with the negatively charged cell surface due to the presence of glycosaminoglycans (GAGs)35. Next, these interactions either permeabilize the cell membrane or promote cell update via endocytic mechanism36. Therefore, at non-toxic doses, these non-viral vectors provide an efficient mechanism for gene delivery. The most common non-viral method is using polyethylenimine (PEI) because of its simplicity, size/shape tunability, strong DNA compaction capacity (polyplexes), and well known endocytic uptake mechanism37. However, with new and increasing developments of lipid-based nanoparticles, cationic liposomes that complex DNA (lipoplexes) are increasingly being utilized as non-viral transfection vectors38.
Some of these non-viral approaches have recently been implemented to advance two exciting developments in the field of gene delivery; delivery of siRNA (small interfering RNA),39 and delivery of whole CRISPR-Cas9 genome-editing systems40. In one recent and historic example, FDA approval was awarded to Alnylam Pharmaceuticals for two siRNA therapeutics. First, there was Onpattro, which consisted of a 21-mer double-stranded siRNA encapsulated within a cationic lipid nanoparticle and most recently, Givlaari was approved in 201941. This encouraging development came after years of lipid nanoparticle development which enabled high liver transfection efficiency for the treatment of transthyretin amyloidosis and acute hepatic porphyria. Similarly, recent attention has focused on developing delivery systems for CRISPR-Cas9 systems. To do this, one must either deliver whole Cas9 endonuclease plus single-guide RNA (sgRNA) or deliver Cas9 mRNA plus sgRNA42–44. While delivery of either plasmids or mRNA to synthesize Cas9 in situ provides several advantages, direct delivery of recombinant and cationic Cas9 complexed with sgRNA (ribonucleoprotein complexes) is most common and simple to implement. Moving forward, each of these delivery approaches for both siRNA and CRISPR-Cas9 will require significant optimization of nanoparticle formulations before these approaches are clinically viable.
Electroporation applies an electric field to cells to increase membrane permeability, allowing chemicals or genetic material to pass into the cell. It allows efficient transfection of hard-to-transfect cell lines and primary cells with different payloads including DNA vectors, short hairpin RNA (shRNA), microRNA (miRNA), small interfering RNA (siRNA), oligonucleotides, proteins or even small molecules. The versatility of such electroporation systems allows transfection of numerous cell lines in a variety of scales. This technique also offers the possibility of using gene-editing strategies such as CRISPR/Cas9 to further modify or improve engineered T cell products. Modern electroporation platforms are designed to significantly reduce cell damage while maintaining high efficiency. Most modern electroporation platforms have protocols specific to the cell type in which the electroporation parameters (waveform, electric field, and pulse length) are optimized for high efficiency and improved viability of transfected cells. In the realm of large scale GMP manufacturing of cell therapies, electroporation is the transfection method that is furthest ahead of other ‘-poration’ methods as commercial devices are designed to be closed and automated. Additionally, for electroporation cells need to be highly concentrated which, for applications with large numbers of cells, require the development of methods that do not rely on non-scalable centrifugation and open processing.
Mechanoporation is another technology that creates small pores in the membrane via compression of the cell. Microfluidic channels can be used to compress cells allowing for delivery of material while maintaining good cell viability and function. Mechanoporation may be the most diverse classification of transfection methods and various strategies are used in conjunction with sonoporation, electroporation, and chemoporation. Microinjection and ballistic gene delivery are suitable for desired cell numbers in the 100 s to 1,000 s. Microinjection requires an operator to insert a micro-sized pipette tip with DNA in carrier solution into a specific location within the cell and like many methods benefits from optimization of other relevant parameters such as DNA concentration, injection volume, and intracellular delivery location among others. Soluporation is a new type of chemoporation that has been developed by Avectas technology, a business that is focused on improving the cost, manufacturing and patient outcomes for next generation cellular therapies. Their SoluporeTM Technology is a non-viral cell engineering technology that permeabilizes the target cell membrane to allow efficient transfer of cargo into cells while retaining high levels of cell viability and functionality45. Technologies geared toward hard-to-transfect cells and uses a combination of shear induced deformation to porate the membrane and then ejects the cells with genetic material through a nozzle to initiate transfection. Systems such as this have shown high delivery efficiency in a variety of cell types and can handle a sample upwards to a few million cells46.
Sonoporation typically uses ultrasonic waves to generate an oscillatory velocity causing small bubbles to form in the fluid; the expansion and collapse of the bubbles creates shear at the membrane surface which induces membrane currents and forms pores in the membrane47,48. Microbubbles are also often used to increase acoustic cavitation in the cell solution. Historically, sonoporation was used for intradermal drug delivery, but in recent years more studies have adapted this technology for in vitro gene transfer. The instruments available for large scale sonoporation for in vitro manufacturing of cell therapies are limited, but this allows early cell therapy innovation teams to optimize parameters specific to the application. Some systems allow the operator to vary the pulse repetition interval, treatment time, waveform, and power intensity and reports have shown that it can be used to achieve higher transfection efficiencies when used with microbubbles and cause minimal loss of cell viability49. The total cells porated in one shot is much lower than that of electroporation and mechanoporation as the tip size of the units are in the millimeter size range and due to the fact that cells must be in a monolayer. In addition, there are not any automated large-scale systems available.
For any delivery method, effective delivery must be balanced with maintaining cell viability. Each method employs a unique approach very often dependent on the cell type and purpose, whether for research or human therapeutic use. Transfection utility of physiologically relevant cells remains a challenge, with the critical bottleneck of achieving reproducibly efficient transfection balanced against cytotoxicity combined with matching throughput capability from research to commercial scale-up. Innovative efforts combining efficient transfer of genetic material into cells and allowing high throughput processing will narrow the gap between cell therapy product (CTP) manufacturing capacities meeting high patient number CTP demands. Many articles have been written describing different transfection techniques. Kim T. et al. provides an overview on biological, chemical, and physical transfection methods and the advantages and limitations of each method and Kaestner, L. et al. provides some guidance to aid in transfection method choice based on factors such as in vivo versus ex vivo therapy, and large versus small populations of cells50,51.
Clinical Considerations
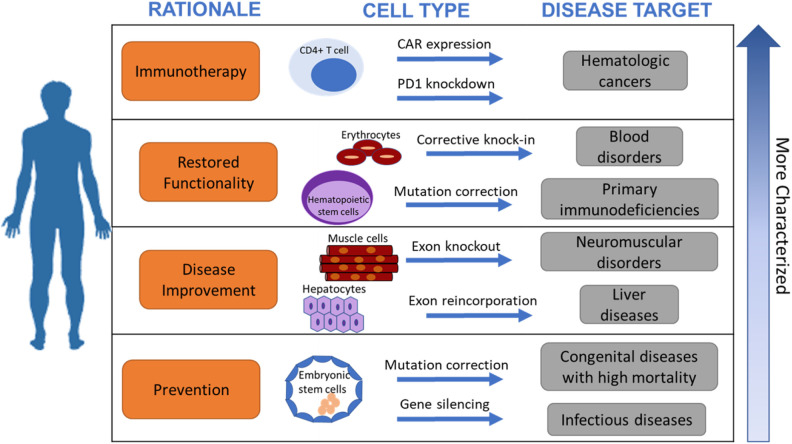
In the previous section we outlined the different tools that can be used for cellular engineering with some insights into their clinical success. In this section we will elaborate on the clinical considerations that should be factored in when choosing a method for cellular engineering when the intended use is for human transplantation to treat diseases. The different approaches impact the complexity of the manufacturing process and thus have a direct impact on cost of goods. Additionally, the choice of method will have direct implications for the clinical treatment regimen of the therapeutic product. Fig. 2 outlines the rationale of choosing a gene modification system for a desired cell type and target clinical disease.
Fig. 2.
Rationale for choosing a target clinical cell type and intended disease target for cell transplantation.
Achieving Transient or Permanent Expression
Transient expression
Transient gene expression in which the gene of interest is expressed for a short (transient) period of time, can be achieved via non-integrating viral vectors (AAV, Adeno-associated) and also via the direct introduction of mRNA (non-viral), or, in some instances, the introduction of non-integrating DNA plasmids52. None of these methods result in permanent knock-in of the gene into the host cell genomic DNA though long-term expression may be achieved in cells which are terminally differentiated and do not divide, such as nerves or retinal cells53. For mRNA-based gene therapy, the genetic material is expressed and translated in the cytosol. Because mRNA-based therapies are not being transcribed under the control of a promoter, this approach may be preferred over the use of viral vectors in cases where a lower level of protein expression is desired54. Mazhar Aldi provides a review of the molecular approaches to gene manipulation55. Transient gene expression may also be preferable if the engineered cell is intended to execute its targeted behavior and is no longer needed thereafter. An example of this is the controlled persistence of activated T-cells. Once the activated T-cells eliminate their targets they are directed to delete themselves via cues from the coordinated immune response56. Alternatively, in the liver, the engineered cell may persist, but due to subsequent rounds of cell division the introduced gene ultimately becomes exponentially diluted.
Another instance in which long-term expression may not be required is when the engineered cells are intended to induce changes in other cells. Here, while the engineered cell itself is not necessary or sufficient to execute the desired clinical effect, these cells initiate a chain of events in the endogenous cells of the patient. Mesenchymal stromal cells (MSCs) are considered a transient cell therapy that can influence the differentiation of immune cells upon administration that then amplify a systemic change in an immune response. Once the sequential actions have been set in motion the initiating cell is no longer needed. As before, the genetic payload could be eliminated via apoptosis or necrosis of host cells, or via serial dilution of the introduced genetic material through cell doubling.
Permanent expression
A permanent (stable) change to the gene expression in the patient is warranted where lifelong expression to correct a genetic defect is needed, with many metabolic disorders being prime targets for such approaches. Permanent expression can be achieved primarily using lentiviral and retroviral vectors. This can also be achieved using transposase systems, such as Sleeping Beauty or PiggyBac, and consist of naked DNA that encodes for the machinery necessary to enter the cellular nucleus and insert a payload into the genome. Unlike viral vector systems, transposon systems contain a targeting sequence which allows control over where the gene is inserted, although specificity is something that requires verification. This approach could be beneficial in correcting defects in large genes, where the use of viral vectors may be limited here due to packaging limitations of the virus. Jaitip, T. et al and Partow, K. et al provided a comprehensive review of transposase systems57,58.
Not to be overlooked are the methods of inserting genetic material into the host cell DNA that use combinatorial systems to achieve permanent expression in the cell. UCART19, an anti-CD19 CAR-T product being developed by Cellectis, utilizes the TALENs to knock-out the expression of the TCR in T-cells to create an allogeneic “off-the-shelf” cell therapy. TALENs can be introduced into T cells by electroporation of mRNAs encoding guide RNA (gRNA). Another allogeneic CAR-T therapy is based on engineered endonuclease to knock-out TCR expression. The nuclease is expressed after mRNA encoding the endonuclease protein enters the cell through electroporation. The nuclease creates a double stranded break in the T-cell receptor alpha constant (TRAC) locus and the CAR gene is inserted at the target site by means of homologous directed recombination (HDR) after delivery by recombinant adeno-associated virus serotype 6 (rAAV6) as this serotype has a high affinity for CD8+ and CD4+ T-cells59–61. In both cases, the nuclease is only transiently expressed but causes a permanent change in the host-DNA. While AAV is non-integrating, using this approach AAV can still be used to achieve permanent expression of a gene.
Clinical Implications of Transient or Permanent Expression
For permanent expression, the host cell genome must be modified as described above. Through preclinical and clinical development, the candidate therapeutic cells will have to be rigorously tested for safety. There are some safety concerns that exist when permanently changing the cellular genome. One concern is the risk of malignancy caused by the random insertion of the transgene. Insertional mutagenesis can occur due to or disrupting a viable gene, and/or by promoting the expression of oncogenic genes as a result of the insertion into the genome. Mout, R. et al offers a full review of delivery methods in gene delivery and the potential for random insertion and the associated risks62. There are tools that exist to increase the specificity of the insertion at a particular site. Though the risk of random insertion can be greatly reduced, it is rarely eliminated. For an ex vivo gene therapy developer, the risks associated with random insertion of the targeted gene will require a higher level of diligence in the clinical trial design. For instance, clinical trial investigators often include long-term follow up of the patient to evaluate if there is an unintended consequence of the gene insertion as patients may be more likely to develop ancillary cancers due to insertional mutagenesis or due to double-strand DNA breaks, as described earlier.
Another risk associated with gene modification is that of on-target/off-tumor responses. For immuno-oncology products (e.g. CAR-T or engineered TCRs), without increased levels of specificity engineered cells attack cells expressing the gene of interest, including non-cancerous cells63. For CAR-T cells targeting CD19, all CD19-expressing cells are targets, leaving patients B-cell aplastic for some time. This results in the patient having difficulty mounting appropriate immune responses to pathogens as they may have poor or no ability to make antibodies64. Additionally, for engineered TCRs targeting cancer testis antigens there also exists a risk of collateral damage to non-cancerous cells65. For a review of the various CAR targets being developed, and the potential risks associated with those targets, see the paper published by Salter, A66. For hematological malignancies and solid tumors, see the publication by Wang, Y, et al67.
An additional risk is that of unintended toxicity associated with sustained expression of the endogenous TCR in engineered immuno-oncology products. The engineered cells are effectively bi-specific. Therefore, the pronounced activation of the CAR-expressing cells also yields cells that are activated and targeted against unknown antigens/targets. In addition, there is also a chance that stimulation of both the targeted CAR and the endogenous TCR leads to T-cell exhaustion, muting the desired target-specific response68. A potential mitigation strategy to develop a focused targeted receptor product is to use gene editing tools to knock-out the endogenous TCR rendering the cells mono-specific via the introduced CAR or engineered TCR. The field has quickly caught onto the notion that, in addition to reducing the toxicity of autologous immuno-oncology products, knock-out of the endogenous TCR lends the potential to generate universal immuno-oncology products derived from healthy donor cells61,69. Additionally, a versatile approach to control the specificity and reduce the risks of off-target effects is the split, universal and programmable CAR system (SUPRA-CAR)70. This platform uses leucine zippers to generate zip-CAR T cells that can be redirected toward tumor antigens in conjunction with a zip-scFv. This allows the full activation of T cells to only be achieved in the presence of multiple antigens.
The permanency of the gene expression affects the clinical design of the study. For ex vivo gene therapies with permanent expression, most products achieve their desired effect with a single dose. However, for therapies involving transient gene expression, repeat dosing may be required as part of the treatment regimen71. The potential need for repeat dosing is an important consideration for patient-specific and autologous therapies, especially if there is a chance of a therapy-related immune response making repeat dosing problematic if not impossible. For autologous therapies, multiple doses of a single batch of gene modified cells are generated, cryopreserved and maintained for subsequent administrations. The alternative would be to produce a new batch of cells for each dose (or set of doses) if only one dose could be generated per batch. This would be the case if the product was required to be administered fresh and not after cryopreservation. In addition to a significant cost burden, this scenario could lead to regulatory hurdles and/or clinical complications. Furthermore, it may not be feasible to obtain multiple samples from a chronically ill patient.
For patient-specific batches of genetically modified cells derived from healthy volunteer donors (e.g. HLA-matched products), the patient may need to receive doses from multiple batches. For example, in a dose escalating clinical trial design, the first subjects may not receive enough allogeneic cells to fully combat the disease and may need to receive another dose. In this case, it would be desirable to treat a patient from a single batch of cells from the same donor, though it may negate the cost-savings associated with the use of universal batches of allogeneic product. The storage and chain of custody logistics associated with setting aside product uniquely for a patient would render the costs and challenges no different than that of autologous therapies. Clinically, the use of donor-derived cells would be beneficial in that patient by not having to repeatedly provide blood or tissue for the therapy.
Integrated Viral and Cell Therapy Manufacturing and Quality Considerations
Whether a cell therapy developer is manufacturing in-house or at a third-party Contract Development and Manufacturing Organization (CDMO), there are several key considerations when using viral vectors as part of the manufacturing process. As opposed to other genetic modification methods, viral vectors are most often needed at larger scales to achieve the desired results. Manufacturing limitations should be considered early on in the process to streamline development and regulatory approval. With each process there is risk of cross contamination which needs to be appropriately managed. This is particularly the case when utilizing multiple viral vectors, fulfilling both clinical and commercial demand, or when manufacturing multiple products in the same facility. There are three potential sources of cross contamination that must be managed, including:
Airborne particulates generated from open process manipulations
Direct transfer through product and non-product contact surfaces
Human error during product manufacturing (e.g. process deviations or labeling)
In addition to cross contamination, the introduction of viral vectors into a cell therapy manufacturing process also requires additional material testing procedures to facilitate the release of the viral vector into a GMP facility. To ensure that the patients receiving these cell therapies are not inadvertently exposed to replicating virus, testing for replication competent virus (RCV) specific to the platform used to produce the vector is required before the viral vector material can be released for GMP production of a cell therapy product72. During preclinical development of a cell therapy manufacturing process, the process may be developed using a viral vector preparation that has not been tested for RCV. Moving this process to GMP, this timing must be carefully managed as RCV testing must be done over a minimum of five passages on a permissive cell line and can therefore take anywhere from 30 to 60 days, which has the potential to disrupt product development timelines to achieve the first patient treatment. Typically, performing this test to release the viral vector for use in the GMP facility is sufficient, however, there have been concerns that a replication incompetent viral vector could be reactivated during the manufacturing process73. For this reason, product developers may elect to perform RCV tests at multiple stages throughout the product manufacturing process, to ensure reactivation has not occurred based on published FDA recommendations72.
A key limiting factor to utilizing gene transfer tools, particularly in the case of viral vectors, is the need for a consistent and large-scale viral manufacturing method that complies with current GMP standards. Transition to semi-automated, closed-system processing are of great interest due to their uniformity and ease of creating batches of virus that can then be purified and utilized in translational, preclinical and clinical settings, without the need for hands-on manipulation. Various methods have been under consideration in order to facilitate production of larger volumes of vector while maintaining high titer. Some of these methods include the use of fixed bed reactors, hollow fiber reactors, suspension culture processes, and stable producer cell lines that reduce the complexity of transfection associated with many viral vectors, such as AAV. Table 3 outlines the current approaches and challenges associated with large scale manufacturing, highlighting key areas of improvement for manufacturing systems of large-scale clinical grade viral vectors.
Table 3.
| Process challenge | Areas for improvement |
|---|---|
| In fixed bed bioreactors it is difficult to collect the virus from cells. | Fluidics modeling can optimize flow patterns and rates to collect virus. |
| Culture results using serum free media are not comparable to results using serum. | Early, upfront work with CROs to optimize and test serum free media formulations per cell type. |
| Difficulty scaling up culture volumes. | Invest in early process development work; this de-risks potential for large-scale failed batches. |
| Limited generation time of viral particles 72-96 post transfection. | Stable producer cell lines produce vector over extended amounts of time. |
| Cationic lipids (PEI and Lipofectamine) can be toxic to cells. | Optimize cell density, lipid concentration, pH, and temperature. Set tight specifications in early development work. |
| High yields are difficult to reach for certain gene constructs. | Use of infection-based systems such as baculovirus (1014 vg/L). |
| Unprocessed supernatants often contain vector titers from 1 to 5x107 TU / ml; relatively dilute for downstream use | Addition of a filtration or volume reduction step to collect vectors from supernatant and concentrate virus. |
Facility Design to Mitigate Risks
There are a number of ways in which challenges related to the use of viral vectors in the manufacture of cell therapy products can be mitigated through the use of an effective facility design and implementation of personnel, material and waste flows that limit the risk for cross-contamination (Table 4). The optimal facility design is dependent on the product manufacturing strategy, whether utilizing multiple viral vectors, manufacturing multiple products or manufacturing for multiple clinical phases. Given the rapid evolution of the cell therapy field, it is critical to design a facility that is flexible and can accommodate several levels of manufacturing capacity and can accommodate various manufacturing approaches. To ensure this flexibility, manufacturing operations at a multi-product GMP facility will need to allow the development and implementation of new production strategies and provide infrastructure for the manufacturing of novel and/or additional product supplements and viral vectors. Once a product receives approval, it will no longer be necessary to manufacture such product in a multi-product facility. Whether or not multi-product facilities will have the capacity to simultaneously support commercial and non-commercial manufacturing, and fulfill all the requirements, remains to be seen. Support of initial manufacturing operations for the commercial markets may allow more effective projections of future manufacturing demands and operational requirements for the full-scale commercial product.
Table 4.
| Manufacturing challenge | Potential mitigations |
|---|---|
| Cross contamination from airborne particles |
|
| Cross contamination from direct transfer through product and non-product contact surfaces |
|
| Cross contamination due to human error during product manufacturing |
|
| Additional RCR/RCL testing prior to GMP release of materials |
|
Minimizing the risk of viral vector cross contamination through the use of a dedicated and redundant HVAC systems for the viral manufacturing cleanrooms is necessary. Furthermore, the use of dedicated systems for each manufacturing cleanroom suite will enable a more robust containment strategy, by allowing for cleanroom suites to be operated independently of each other. In addition, the use of containment “sinks” within each clean room will ensure that containment is maintained in that suite at all times. The risk of cross contamination can also be controlled operationally through the implementation of unidirectional vector materials flow into the clean room and waste flow out of the manufacturing facility.
Manufacturing Controls to Mitigate Risks
Typically, manufacturing processes for cell therapy products contain open processing steps, which not only increase the risk of product contamination, but also introduce risk that the viral vector that is used in the product may contaminate other products. Risk of cross contamination is minimized by scheduling production so that only one viral vector can be manipulated in a cleanroom suite at a given time, and through the use of appropriately validated cleaning procedures. If the same manufacturing process is used for multiple indications with different viral vectors, and therefore require two different viral vectors to be utilized in the same clean room, it should be possible to stagger the production such that the different products are not openly manipulated at the same time. No two products employing different vectors shall be manipulated in the cleanroom at the same time during a GMP production campaign.
Alternatively, it may be possible to develop closed manufacturing processes that require only a minimal number of open processing steps, so that production scheduling is not unnecessarily constrained. An example would be closed system cell culture and sampling so that multiple products can be expanded and processed simultaneously. Early planning for potential constraints in production is critical so that capacity can be maximized, and overall product manufacturing costs can be reduced. As production volumes increase toward late-phase clinical and commercial manufacturing, it may be possible to physically separate the different processes to avoid these additional manufacturing controls and increase capacity utilization of the manufacturing facility. This is beneficial from both a risk mitigation as well as a cost savings perspective.
The second manufacturing control that mitigates the risk of utilizing viral vectors in cell therapy manufacturing is the use of validated cleaning procedures. To enable an effective line clearance between manufacturing processes utilizing different viral vectors or gene modification strategies, a fully validated decontamination procedure should be put in place. This procedure must show that the viral vector is completely neutralized after the cleaning procedure. Cleaning procedures still affect the manufacturing capacity as time is required between the manufacturing of different products for cleaning procedure81. Cleaning effectiveness procedures are also required for spill containment protocols along with appropriate secondary containment measures for products containing viral vector material.
The third manufacturing control is labelling and tracking of product and material throughout the manufacturing facility. For the manufacturing of patient-specific cell therapies, the introduction of 21 CFR Part 11 compliant electronic tracking software has the potential to reduce manufacturing costs through a reduction in labor. It also assures that the appropriate material is used in the correct manufacturing process and is considered to be part of a robust materials management system. This system can be used to ensure control of raw materials, including viral vectors, where the use of unique identifiers assure the right viral vector is used in the right manufacturing process. Within a cleanroom in which multiple products utilizing viral vectors are being manufactured, the manufacturing control strategy could include product color coding or use of assigned incubator shelves for each product codified into the manufacturing batch record, to prevent product mix up and cross contamination.
There are several quality and scalability challenges associated with the use of viral vectors in cell therapy manufacturing. Despite these challenges, incorporating the mitigation strategies described in Table 4 early into the product development plan, combined with effective collaboration and partnership with key suppliers, will ensure successful product manufacture during clinical and commercial production. Additionally, it is important to build flexibility into the quality and scalability strategy that allow for innovations toward safer more streamlined approaches to manufacturing. This could include end-to-end electronic tagging, robotics, and/or larger scale cellular bio-processing technologies to name a few examples of what may be on the horizon for the future of manufacturing in cell-based medicines.
Sustainability Considerations
Vector Manufacturing
Recent advances in cell and gene therapy along with accelerated pathways have compressed timelines for developing commercial manufacturing processes for viral vectors. As demand for cell therapy products continue to rise, there is a growing question around the availability of vectors at the right quality, cost, and scale. Viral vector manufacturing alone is its own science and in combination with cell therapy adds further complication to an already challenging process. Due to the large numbers of containers required to meet the requirements for a GMP clinical run, developers have traditionally used scale-out approaches to address demand using conventional adherent-cell systems (T-flasks, multi-layer flasks, and roller bottles). Besides space concerns, the use of increased numbers of culture vessels results in increased processing time and open manipulation steps which increases contamination risk during aseptic processing. A number of alternatives systems are available that are larger and allow for mostly closed processing including single-use bioreactor systems such as stirred tank reactors, commonly utilized in antibody production, and perfusion cultures. The Quantum Cell Expansion System (Terumo BCT, Lakewood CO) is a functionally closed and automated system using hollow fiber technology and has shown to produce lentiviral vector yields upwards to 4x109 vg per run74. The iCellis® single-use fixed-bed reactor (Pall Corporation; Port Washington, New York) offers another automated system that has both small- and large-scale options for vector manufacturing.
During production, downstream processing constraints such as labor intensity and scalability issues surround ultracentrifugation, which is still widely used for viral vector preparation for Phase I level work. There is a trend to move away from non-scalable strategies, although the lack of clarity around the quantity of vector required for dosing makes this challenging. However, with patient numbers increasing as they progress through their clinical program, and with more products moving toward commercial application, the need for more viral vector continues to increase. Small batch sizes challenge manufacturers to create robust processes that can produce an equivalent product every time and when organizations advance in their clinical development, scale-up of these processes is an inherent challenge.
As with cell therapy production, the raw materials used for vector production are equally vital and must be sourced with rigorous quality checks to minimize the risk of introducing adventitious agents into the production process. Critical raw materials for vector production include the cells, the cell culture medium, serum and plasmids. The need for these resources will increase proportionally with the increase in vector manufacturing. With the field moving toward commercialization, it is expected that the associated quality requirements may also increase for raw materials from research-grade to, in some cases, GMP-grade. Along with additional testing to reduce risk, sourcing of these supplies remains a considerable sustainability risk. If animal-derived materials, such as fetal bovine serum (FBS) or porcine trypsin, are required during manufacturing of clinical-grade cell therapeutics, there may be additional requirements that must be met by the FDA. The main regulatory concerns associated with the use of xenogeneic serum include the risk of contamination with non-human pathogens and inducing an unwanted immune response as xeno-toxic effects. For clinical-grade FBS, every batch or lot of FBS must be traceable back to its country, slaughterhouse and herd of origin in USDA approved import countries82. Additionally, all lots must be tested for adventitious agents (viral contamination), sterility (bacterial and fungal elements), endotoxin levels, mycoplasma content and other constituents in vitro83. In vivo animal toxicity and inhalation-based toxicity studies are also required, with many groups focused on developing advanced in vitro models for these tests that are more human-relevant84. While regulatory agencies address safety, it is up to the cell manufacturer to establish metrics around performance, as FBS has traditionally been both a major cost driver and a source of process variability. Efforts to implement xenogeneic-free methods in the production of the viral vector as well as the cell-based product should be undertaken as early in process development as feasible.
For all materials and processes associated with therapeutics intended for human use, including viral vectors, testing strategies are vital to ensure product identity, potency, purity and safety. Vector stocks must be certified to be free of RCV or any other viral contaminant that may arise as a result of the manufacturing process. After cell transduction, the cell therapy is also tested for sterility, absence of mycoplasma (especially where serum is employed in the manufacturing process), RCV, and endotoxin81. If the cell therapy product can be cryopreserved prior to administration, traditional assays for sterility and mycoplasma (which can take up to 28 days to perform) can be used. However, in some applications, a shorter time to administration may be needed as freshly prepared (non-cryopreserved) products need to be administered within days of product formulation. For these reasons, rapid microbial methods (RMM) are available that allow for the testing and release of product within the short time frame available. Efforts are underway to identify approaches for safely employing RMM into the production of cell-based medicines.
Cost of Goods and Business Considerations
Proprietary platform technologies that facilitate engineered cell product development are a common practice in the industry. Many companies and organizations have business models that license access to their platforms which may offer shorter paths to critical inflection points (e.g., clinical trials) or provide the freedom-to-operate in a field which they may have a dominant position. Typically, licensors of such technologies seek financial compensation for their investments made via upfront and/or annual payments for rights to use the platform. This may also include milestone payments linked to development activities, which tend to escalate as clinical development progresses and are often significant financial payments. All considered, these payments and obligations may be difficult to accept once presented with them, yet they may shorten development timelines which could be more sensical than attempting to “reinvent the wheel” by avoiding them. With respect to freedom-to-operate, licensing a technology which may be required for commercialization may also be needed to successfully raise funds or engage in other significant commercial events, such as going public, large partnerships, or acquisition. The company should be aware of these issues and incorporate them into their overall product development strategy. With aggressive competition, shortened timelines, and an overall high risk of failure to start as is any early stage therapeutic opportunity, the decision to license technologies that speed up and de-risk product development should be strongly considered rather than outright discounted.
Conclusion and Risk Mitigation
Cell engineering has become a critical feature of new and developing cell therapy products. Here we focus on immune cell therapies in detail and it is worth noting that multiple cell types are under development for transplantation in regenerative medicine to address a myriad of disease states. Currently approved CAR-T therapies use autologous T-cells, but efforts to use allogeneic or universal CAR-T cells are ongoing. If successful, the use of off-the-shelf universal CAR-T cell products could offer a dramatic increase in the affordability and widespread use of CARs. Additional cell types that are being developed with promising early results include Natural Killer Cells (NKCs), Dendritic Cells (DCs), mesenchymal stem cells (MSCs) and Natural Killer T-cells (NKTs). The design of these engineered cell types in the decision-making process of defining the target product profile (TPP) include the desired genetic vehicle or technology, the manufacturing method, clinical considerations, sustainability, cost of goods, business considerations and risk mitigation. Ultimately, these considerations feed heavily into significant financial decisions for drug development, which are worthy of study for new developers and the profitability of the proposed therapeutic approach.
The clinical impact of a chosen cell engineering methodology largely involves the permanence of treatment. Developers need to employ strict safety standards and diligence in clinical trial design to evaluate and balance these risks to ensure safe and efficacious clinical outcomes. For transient expression, meeting the quality demands of repeat dosing requires more strict quality control by reducing batch-to-batch variability or producing enough product for each patient to be treated multiple times from the same batch. Due to current clean room limitations and operational methods, and the high costs of manufacturing in such settings, developers need to consider implementing managed and controlled flexibility in their overall manufacturing activities. For scaling with multiple products, emphasis should be placed on the flexibility of the manufacturing facility itself to incorporate future products and product supplements. Scale and quality continue to be challenges in manufacturing yet continuous improvements in these areas are being made both by product innovators, contractors, and supporting technologies used in these processes. Patient numbers increase through each clinical phase, meaning developers need to be prepared for high demand much earlier than commercial-scale manufacturing. Similarly, the sooner a platform achieves commercial-grade quality in operations and manufacturing, the easier the regulatory process becomes by avoiding protocol revisions and amendments. This requires rigorous operational control and testing strategies employed as early as possible which while costly early on, lessens the burden of future challenges organizations will face as they continue to grow, which may include difficult financing environments, increased regulatory scrutiny, and up through potential late-stage product failure. Having robust systems in place alleviates these and other issues that many organizations may someday face. The Discovery Labs’ Center for Breakthrough Medicines is currently the world’s largest cell and gene therapy contract development and manufacturing organization (CDMO) who’s goal is to bring these therapies from benchtop to bedside in an accelerated and affordable manner. The goal is to provide researchers with a one source for process development, plasmid and viral vector production, analytical testing, cell bioprocessing, platform IP, and the space to operate. There is a long line of smaller cell and gene therapies hoping to utilize this space early on, and the competition will only continue to grow.
Overall, during cell therapy product development, the rationale for choosing a specific cell engineering methodology is largely dependent on both biological and manufacturing considerations. On one side, the choice is dependent on the disease state, the cell type being modified for transplantation, whether the expression should be permanent or transient and the ease and cost of the gene system being deployed. Secondly, for large scale cell therapy product production and use in a clinical setting, you must take into account manufacturing and risk controls, as well as potential for off target effects and long-term safety of patients. The growing world of gene engineering technology allows the developer a wide variety of choices for their own designer cell therapeutic, and once a methodology is selected the end product will positively contribute to the emerging field of cell-based therapies for treatment.
Footnotes
Authors Note: Lauren M. Timmins and Alexandra M. Burr authors contributed equally.
Authors Contribution: Conceptualization, T.R. J.H., D.S., B.P., Manuscript Preparation, All authors
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosure: KC, RK, LJC, TRJH, and DS are employees of private commercial entities with equity shareholding. AG, BP are founders of for-profit companies with equity shareholding and licensed patents to these organizations for commercialization. The remaining authors have no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was conducted with private funding and grant support under Grant No. R21AI134116, R01EB012521 and T32EB016652-01A1 awarded by the National Institutes of Health.
ORCID iD: Lauren M. Timmins  https://orcid.org/0000-0002-8776-4665
https://orcid.org/0000-0002-8776-4665
Alexandra M. Burr  https://orcid.org/0000-0002-7954-3619
https://orcid.org/0000-0002-7954-3619
David Smith  https://orcid.org/0000-0003-2726-9192
https://orcid.org/0000-0003-2726-9192
References
- 1. Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braendstrup P, Levine BL, Ruella M. The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19. Cytotherapy. 2020;22(2):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ribatti D. The concept of immune surveillance against tumors. The first theories. Oncotarget. 2017;8(4):7175–7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Negrin RS. Graft-versus-host disease versus graft-versus-leukemia. Hematology Am Soc Hematol Educ Program. 2015;2015:225–230. [DOI] [PubMed] [Google Scholar]
- 5. Qian X, Wang X, Jin H. Cell transfer therapy for cancer: past, present, and future. J Immunol Res. 2014;2014:525913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 2020;17(3):147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchschacher GL, Jr, Wong-Staal F. Development of lentiviral vectors for gene therapy for human diseases. Blood 2000;95(8):2499–2504. [PubMed] [Google Scholar]
- 8. Escors D, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp (Warsz) 2010;58(2):107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naso MF, Tomkowicz B, Perry WL, 3rd, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31(4):317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, et al. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4(2):43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buning H, Huber A, Zhang L, Meumann N, Hacker U. Engineering the AAV capsid to optimize vector-host-interactions. Curr Opin Pharmacol. 2015;24:94–104. [DOI] [PubMed] [Google Scholar]
- 12. Loskog A. Immunostimulatory gene therapy using oncolytic viruses as vehicles. Viruses. 2015;7(11):5780–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gil-Farina I, Schmidt M. Interaction of vectors and parental viruses with the host genome. Curr Opin Virol. 2016;21:35–40. [DOI] [PubMed] [Google Scholar]
- 14. Gaj T, Sirk SJ, Shui SL, Liu J. Genome-editing technologies: principles and applications. Cold Spring Harb Perspect Biol. 2016;8(12):a023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daniel-Moreno A, Lamsfus-Calle A, Raju J, Antony JS, Handgretinger R, Mezger M. CRISPR/Cas9-modified hematopoietic stem cells-present and future perspectives for stem cell transplantation. Bone Marrow Transplant. 2019;54(12):1940–1950. [DOI] [PubMed] [Google Scholar]
- 16. Nemudryi AA, Valetdinova KR, Medvedev SP, Zakian SM. TALEN and CRISPR/Cas genome editing systems: tools of discovery. Acta Naturae. 2014;6(3):19–40. [PMC free article] [PubMed] [Google Scholar]
- 17. Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186(2):757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. 2018 July 31. Gilead to Develop Hepatitis B Therapies Using Precision’s ARCUS Genome Editing Platform. MaryAnnLiebert, Inc. Accessed 2019 July 31. [Google Scholar]
- 19. Horii T, Tamura D, Morita S, Kimura M, Hatada I. Generation of an ICF syndrome model by efficient genome editing of human induced pluripotent stem cells using the CRISPR system. Int J Mol Sci. 2013;14(10):19774–19781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez-Lage M, Puig-Serra P, Menendez P, Torres-Ruiz R, Rodriguez-Perales S. CRISPR/Cas9 for cancer therapy: hopes and challenges. Biomedicines. 2018;6(4):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santiago Y, Chan E, Liu PQ, Orlando S, Zhang L, Urnov FD, Holmes MC, Guschin D, Waite A, Miller JC, Rebar EJ, et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc Natl Acad Sci USA. 2008;105(15):5809–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bailey SR, Maus MV. Gene editing for immune cell therapies. Nat Biotechnol. 2019;37(12):1425–1434. [DOI] [PubMed] [Google Scholar]
- 23. Yee JK. Off-target effects of engineered nucleases. FEBS J 2016;283(17):3239–3248. [DOI] [PubMed] [Google Scholar]
- 24. Human Genome Editing: Science, Ethics, and Governance. 2017. [PubMed] [Google Scholar]
- 25. Gaj T, Gersbach CA, Barbas CF, III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Foss DV, Hochstrasser ML, Wilson RC. Clinical applications of CRISPR-based genome editing and diagnostics. Transfusion. 2019;59(4):1389–1399. [DOI] [PubMed] [Google Scholar]
- 27. Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med. 2018;20(5):e3015. [DOI] [PubMed] [Google Scholar]
- 28. Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A. 1996;93(3):1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ed Davis PD.2014. July 21. Genome Editing: Which Should I Choose, TALEN or CRISPR? In Genome Editing. Accessed 2019 July 21. [Google Scholar]
- 30. Wang L, Li F, Dang L, Liang C, Wang C, He B, Liu J, Li D, Wu X, Xu X, Lu A, et al. In vivo delivery systems for therapeutic genome editing. Int J Mol Sci. 2016;17(5):626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DiCarlo JE, Deeconda A, Tsang SH. Viral vectors, engineered cells and the CRISPR revolution. Adv Exp Med Biol. 2017;1016:3–27. [DOI] [PubMed] [Google Scholar]
- 32. Halevy T, Urbach A. Comparing ESC and iPSC-based models for human genetic disorders. J Clin Med. 2014;3(4):1146–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deakin CT, Alexander IE, Kerridge I. Accepting risk in clinical research: is the gene therapy field becoming too risk-averse? Mol Ther. 2009;17(11):1842–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109(2):259–302. [DOI] [PubMed] [Google Scholar]
- 35. Mislick KA, Baldeschwieler JD. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc Nat Acad Sci. 1996;93(22):12349–12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Nati Acad Sci. 1999;96(9):5177–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lungwitz U, Breunig M, Blunk T, Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60(2):247–266. [DOI] [PubMed] [Google Scholar]
- 38. Buck J, Grossen P, Cullis PR, Huwyler J, Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13(4):3754–3782. [DOI] [PubMed] [Google Scholar]
- 39. Dong Y, Siegwart DJ, Anderson DG. Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliv Rev. 2019;144:133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu C, Zhang L, Liu H, Cheng K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J Control Release. 2017;266:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garber K. Alnylam launches era of RNAi drugs. Nat Biotechnol. 2018;36(9):777. [DOI] [PubMed] [Google Scholar]
- 42. Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J, Dirstine T, Ciullo C, Lescarbeau R, Seitzer J, Shah RR, et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22(9):2227–2235. [DOI] [PubMed] [Google Scholar]
- 43. Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA, Mackley VA, Chang K, Rao A, Skinner C, Shobha T, et al. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat Biomed Eng. 2017;1:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller JB, Zhang S, Kos P, Xiong H, Zhou K, Perelman SS, Zhu H, Siegwart DJ. Non-Viral CRISPR/CAS gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed Engl. 2017;56(4):1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Avectas. 2020 cell engineering technology. In Solupore Technology. 2020.
- 46. Meacham JM, Durvasula K, Degertekin FL, Fedorov AG. Enhanced intracellular delivery via coordinated acoustically driven shear mechanoporation and electrophoretic insertion. Sci Rep. 2018;8(1):3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwarz G, Callewaert G, Droogmans G, Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J Physiol. 1992;458:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Netz RR, Schick M. Pore formation and rupture in fluid bilayers. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1996;53(4):3875–3885. [DOI] [PubMed] [Google Scholar]
- 49. Zhang L, Sun Z, Ren P, Lee RJ, Xiang G, Lv Q, Han W, Wang J, Ge S, Xie M. Ultrasound-targeted microbubble destruction (UTMD) assisted delivery of shRNA against PHD2 into H9C2 cells. PLoS One. 2015;10(8):e0134629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Anal Bioanal Chem. 2010;397(8):3173–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaestner L, Scholz A, Lipp P. Conceptual and technical aspects of transfection and gene delivery. Bioorg Med Chem Lett. 2015;25(6):1171–1176. [DOI] [PubMed] [Google Scholar]
- 52. Nightingale SJ, Hollis RP, Pepper KA, Petersen D, Yu XJ, Yang C, Bahner I, Kohn DB. Transient gene expression by nonintegrating lentiviral vectors. Mol Ther. 2006;13(6):1121–1132. [DOI] [PubMed] [Google Scholar]
- 53. Stern J, Temple S. Retinal pigment epithelial cell proliferation. Exp Biol Med (Maywood). 2015;240(8):1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Richner JM, Jagger BW, Shan C, Fontes CR, Dowd KA, Cao B, Himansu S, Caine EA, Nunes BTD, Medeiros DBA, Muruato AE, et al. Vaccine mediated protection against zika virus-induced congenital disease. Cell. 2017;170(2):273–283. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li KP, Shanmuganad S, Carroll K, Katz JD, Jordan MB, Hildeman DA. Dying to protect: cell death and the control of T-cell homeostasis. Immunol Rev. 2017;277(1):21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tipanee J, Chai YC, VandenDriessche T, Chuah MK. Preclinical and clinical advances in transposon-based gene therapy. Biosci Rep. 2017;37(6):BSR20160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kebriaei P, Izsvak Z, Narayanavari SA, Singh H, Ivics Z. Gene therapy with the sleeping beauty transposon system. Trends Genet. 2017;33(11):852–870. [DOI] [PubMed] [Google Scholar]
- 59. Wang J, DeClercq JJ, Hayward SB, Li PW, Shivak DA, Gregory PD, Lee G, Holmes MC. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 2016;44(3):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. MacLeod DT, Antony J, Martin AJ, Moser RJ, Hekele A, Wetzel KJ, Brown AE, Triggiano MA, Hux JA, Pham CD, Bartsevich VV, et al. Integration of a CD19 CAR into the TCR alpha chain locus streamlines production of allogeneic gene-edited CAR T Cells. Mol Ther. 2017;25(4):949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McCreedy BJ, Senyukov VV, Nguyen KT. Off the shelf T cell therapies for hematologic malignancies. Best Pract Res Clin Haematol. 2018;31(2):166–175. [DOI] [PubMed] [Google Scholar]
- 62. Mout R, Ray M, Lee YW, Scaletti F, Rotello VM. In vivo delivery of CRISPR/Cas9 for therapeutic gene editing: progress and challenges. Bioconjug Chem. 2017;28(4):880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wei J, Han X, Bo J, Han W. Target selection for CAR-T therapy. J Hematol Oncol. 2019;12(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, Obstfeld AE, Lacey SF, Melenhorst JJ, Nazimuddin F, Hwang WT, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128(3):360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thomas R, Al-Khadairi G, Roelands J, Hendrickx W, Dermime S, Bedognetti D, Decock J. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol. 2018;9:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Salter AI, Pont MJ, Riddell SR. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood. 2018;131(24):2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Y, Luo F, Yang J, Zhao C, Chu Y. New chimeric antigen receptor design for solid tumors. Front Immunol. 2017;8:1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, Hu Y, Wanhainen K, Qin H, Fry TJ. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. 2017;9(417):eaag1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ruella M, Kenderian SS. Next-generation chimeric antigen receptor t-cell therapy: going off the shelf. BioDrugs. 2017;31(6):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stoiber S, Cadilha BL, Benmebarek MR, Lesch S, Endres S, Kobold S. Limitations in the design of chimeric antigen receptors for cancer therapy. Cells. 2019;8(5):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y. Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Hum Gene Ther. 2013;24(8):717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guidance for Industry USDoH, Human Services F, Drug Administration CfBE, Research. Supplemental guidance on testing for replication-competent retrovirus in retroviral vector-based gene therapy products and during follow-up of patients in clinical trials using retroviral vectors. Hum Gene Ther 2001;12(3):315–320. [DOI] [PubMed] [Google Scholar]
- 73. Anson DS. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet Vaccines Ther. 2004;2(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sheu J, Beltzer J, Fury B, Wilczek K, Tobin S, Falconer D, Nolta J, Bauer G. Large-scale production of lentiviral vector in a closed system hollow fiber bioreactor. Mol Ther Methods Clin Dev 2015;2:15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van der Loo JC, Wright JF. Progress and challenges in viral vector manufacturing. Hum Mol Genet 2016;25(R1):R42–R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol Ther Methods Clin Dev. 2016;3:16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ausubel LJ, Hall C, Sharma A, Shakeley R, Lopez P, Quezada V, Couture S, Laderman K, McMahon R, Huang P, Hsu D, et al. Production of CGMP-grade lentiviral vectors. Bioprocess Int. 2012;10(2):32–43. [PMC free article] [PubMed] [Google Scholar]
- 78. Clement N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol Ther Methods Clin Dev. 2016;3:16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Choi Y, Chang J. Viral vectors for vaccine applications. Clin Exp Vaccine Res 2013;2(2):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Agency EM. Guideline on the Development and Manufacture of Lentiviral Vectors. Committee for Medicinal Products for Human Use. 2005.
- 81. FDA. Content and Review of Chemistry, Manufacturing and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications. Guidance for FDA Reviewers and Sponsors; 2008. [Google Scholar]
- 82. Minonzio GM, Linetsky EA.The Use of fetal bovine serum in cellular products for clinical applications: commentary. CellR4. 2014;2(6):e1307. [Google Scholar]
- 83. EMAECBr. Guidelines for Bovine Serum in Manufacture of Human Biological Medicinal Products. 2013.
- 84. Movia D, Bruni-Favier S, Prina-Mello A. In vitro alternatives to acute inhalation toxicity studies in animal models-a perspective. Front Bioeng Biotechnol. 2020;8:549. [DOI] [PMC free article] [PubMed] [Google Scholar]