Abstract
Purpose:
Clinical studies have shown that breast cancer risk is increased in hypertensive women. The underlying molecular mechanism remains undetermined. The current study tests our hypothesis that G protein coupled receptor kinase 4 (GRK4) is a molecule that links hypertension and breast cancer. GRK4 plays an important role in regulation of renal sodium excretion. Sustained activation of GRK4 as in the circumstances of single nucleotide polymorphism (SNPs) causes hypertension. Expression of GRK4 in the kidney is regulated by cMyc, an oncogene that is amplified in breast cancer.
Methods:
Western analysis was used to evaluate GRK4 protein expression in seven breast cancer cell lines. GRK4 gene single nucleotide polymorphism in breast cancer cell lines and in breast cancer cDNA arrays were determined using TaqMan Genotyping qPRC. The function of GRK4 was evaluated in MCF-7 cells with cMyc knock-down and GRK4 re-expression and in MDA-MB-468 cells expressing inducible GRK4 shRNA lentivirus constructs. Nuclei counting and 5-Bromo-2’-deoxy-uridine (BrdU) labeling were used to evaluate cell growth and proliferation.
Results:
Genotyping of GRK4 SNPs in breast cancer cDNA arrays (n = 94) revealed that the frequency of carrying two hypertension related SNPs A142 V or R65 L is threefold higher in breast cancer patients than in healthy people (P = 7.53E-11). GRK4 protein is expressed in seven breast cancer cell lines but not the benign mammary epithelial cell line, MCF-10A. Three hypertension related SNPs in the GRK4 gene were identified in the breast cancer cell lines. Except for BT20, all other breast cancer lines have 1-3 GRK4 SNPs of which A142 V occurs in all 6 lines. MDA-MB-468 cells contain homozygous A142 V and R65 L SNPs. Knocking down cMyc in MCF-7 cells significantly reduced the growth rate, which was rescued by re-expression of GRK4. We then generated three stable GRK4 knock-down MDA-MB-468 lines using inducible lentiviral shRNA vectors. Doxycycline (Dox) induced GRK4 silencing significantly reduced GRK4 mRNA and protein levels, growth rates, and proliferation. As a marker of cell proliferation, the percentage of BrdU-labeled cells decreased from 45 ± 3% in the cells without Dox to 32 ± 5% in the cells treated with 0.1 µg/mL Dox.
Conclusions:
GRK4 acts as an independent proliferation promotor in breast cancer. Our results suggest that targeted inhibition of GRK4 could be a new therapy for both hypertension and breast cancer.
Keywords: G protein coupled receptor kinase 4 (GRK4), breast cancer, cell proliferation, hypertension
Introduction
The issue of whether hypertension is a risk factor for cancer mortality was raised more than 40 years ago.1 While association between hypertension and cancer risk has been confirmed by a number of studies, the relationship varies in cancer types and genders.2 Breast cancer is the most commonly occurring cancer in American women and the second most common cause of cancer-related deaths.3 Association of breast cancer risk and hypertension has been controversial.4-7 A recent meta-analysis of 30 studies with 11,643 cases of breast cancer has shown a statistically significant association between hypertension and increased breast cancer risk (relative risk [RR]: 1.15; 95% confidence interval [CI]: 1.08-1.22).8 Increased breast cancer risk is specific for postmenopausal hypertensive women, which has been corroborated by other studies.9 These results lend support to the positive connection between breast cancer and hypertension, but the underlying molecular mechanism remains unclear.
The kidney is an important organ for water and electrolyte homeostasis in the body. Approximately 75% of sodium excretion from the kidney is regulated by dopaminergic receptors (D1R and D5R).10 Under normal circumstances, high salt intake activates D1R, which increases sodium excretion mediated by second messenger cyclic AMP. D1R signaling is terminated via phosphorylation of the receptor by G protein-coupled receptor kinase 4 (GRK4). It has been demonstrated that sustained activation of GRK4 leads to uncoupling between D1R and its downstream effector, adenylyl cyclase, and impairs the function of D1R.11,12 This uncoupling is observed in renal proximal tubules from humans with essential hypertension and from rodents with genetic hypertension.11-13 Three single nucleotide polymorphisms (SNP) in GRK4 gene, R65L (rs2960306), A142V (rs2014323), and A486V (rs1801058), have been identified in human essential hypertension.14 These variants alone or in combination have increased kinase activities and cause hypertension when expressed in transgenic mice.14 Therefore, GRK4 is a risk factor for hypertension because of its negative regulation of the renal dopaminergic system.15,16
GRK4 is one of the seven-member family of GRKs. There are four GRK4 isoforms due to alternative splicing of exons 2 or 14 or both.17 Expression of GRK4 is restricted to the testis, kidney, and brain.18 Interestingly, Matsubayashi et al reported that GRK4 isoforms are expressed in human breast cancer tissues but not in normal epithelia. Overexpression of GRK4 in breast cancer cells increases proliferation rates.19 A global differential allele-specific expression analysis in mammary epithelial transcriptome has identified two SNPs of GRK4, A142V and R65L, as breast cancer risk loci.20 These data suggest that GRK4 might be a risk factor for both hypertension and breast cancer.
The current studies employed breast cancer cell lines to determine the potential role of GRK4 in cell growth and proliferation of breast cancer. We found that GRK4 protein is expressed in all breast cancer cell lines examined but not in benign breast epithelial cells. GRK4 acts as an independent factor promoting cancer cell proliferation.
Materials and Methods
Cell culture
Breast cancer cell lines were originally obtained from ATCC. MCF-7 cells were cultured in IMEM with 5% fetal bovine serum (FBS). T47D and ZR75 cells were cultured in RPMI1640 containing 10% FBS. MDA-MB-48, MDA-MB-231, BT-20, and SK-Br3 cells were cultured in DMEM containing 10% FBS. Penicillin (100 units/ml) and streptomycin (100 µg/ml) were added to all cultures. Cell authentication was performed by Laragen in 2019. The STR alleles of all cell lines used in this study match ATCC database.
Human breast cancer cDNA array
Two sets of breast cancer cDNA array kits (BCRT III and IV) were purchased from Origene Technologies (Rockville, MD). BCRT III and IV contained 48 and 44 breast cancer cDNA samples, respectively. All samples are grades II or III breast cancer.
GRK4 gene SNP assay
Genomic DNA was extracted with the ZR Quick gDNA mini prep kit (Zymo Research, Irvine, CA). Total DNA was quantified using a NanoDrop Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The samples were normalized to 10 ng DNA/well on a plastic 96-well plate and mixed with TaqMan Genotyping Master Mix (Thermo Fisher Scientific) using GRK4 gene single nucleotide polymorphism sites rs2960306, rs1024323, and rs1801058 (Thermo Fisher Scientific).21 The samples were amplified on the CFX Connect Real-Time System (Bio-Rad, Hercules, CA) and analyzed using SnpMan software.22
cMyc knock down and GRK4 expression
MCF-7 cells were transfected with pLKO.1 vector containing cMyc shRNA (Sigma-Aldrich, St. Louis, MO) or pLKO.1 vector without cMyc shRNA using Lipofectamine 3000 (Thermo Fisher Scientific). Twenty-four hours later, the medium was replaced with full culture medium containing puromycin, 2 µg/ml. The resultant cMyc knockdown cells were transfected with pcDNA 4/TO vector (Thermo Fisher Scientific) constructed with cDNAs of three different GRK4 isoforms: GRK4α, GRK4γ, and GRK4γ with three SNPs. The transfected cells were selected by exposure to zeocine, 400 µg/ml.
GRK4 knock down
MDA-MB-468 cells contain two homozygous SNPs of the GRK4 gene that indicates higher GRK4 activity. MDA-MB-468 cells were plated in 35 mm dishes at the density of 200,000 cells per dish. Next day, the culture medium was replaced with serum- and antibiotics-free DMEM, containing SMARTvector inducible lentiviral GRK4 shRNA at the concentration of 0.3 multiplicity of infection (MOI). Twenty-two hours later, the cells were fed with full culture medium containing puromycin 0.5 µg/mL. Mature antisense sequences of three GRK4 shRNA are listed below. #167: TAGAAAACGGCTCTCTGCT; #323: TTCCAAGCCGCAACACAGC; #466: TAGAGCCATAGCTTCACCT.
Immunoblotting
Cells grown in 60 mm dishes were washed with cold PBS. To each dish, 0.5 mL lysis buffer was added (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, 1% Triton X-100, 1 mM β-glycerophosphate, 1 µg/mL leupeptin and aprotinin, and 1 mM phenylmethylsulfonyl fluoride (PMSF)). The dishes were incubated on ice for 5 minutes before collection. Cells were then pulse sonicated and centrifuged at 14,000 rpm for 10 min at 4°C. Cell lysates were stored at -80°C until analysis. The total protein content of the lysate was determined using a standard Bradford assay using the reagent from Bio-Rad (Hercules, CA). Fifty microgram total protein was loaded and separated on 10% SDS polyacrylamide gel and then transferred to a nitrocellulose membrane. The membrane was probed with primary antibodies dissolved in Tris-buffered saline, containing 5% BSA, followed by incubation with near-infrared fluorescence labeled secondary antibody (1:5,000) (LI-COR Biotechnology, Lincoln, NE). Bands of specific proteins were scanned and quantitated using the LI-COR Odyssey scanner and software (Lincoln, NE). Antibodies for GRK4 D-11 (all isoforms), K-20 (α isoform), and I-20 (γ isoform) were from Santa Cruz Biotechnology (Dallas, TX). Specificity of D-11 antibody was validated by a known polyclonal antibody H-70 (Santa Cruz Biotechnology).23
Determination of GRK4 mRNA levels by quantitative real-time PCR (q-PCR)
Total RNA was extracted and purified using the Qiagen RNeasy Mini Kit (Valencia, CA) and reverse transcribed. Transcription of GRK4 was determined by q-PCR using the SYBR Green method. GAPDH was used as a housekeeping gene for quantification. Relative mRNA copies were calculated by comparing to vehicle control using ΔΔCt method.24 Sequences of primers used were as follows. GRK4 primers: Forward 5’-TTGCTACCGGGTGTGTCTCC-3’ and Reverse 5’-GGTCTGGAAACCGGGGTATGT-3.’ GAPDH primers: Forward 5’-ACCCACTCCTCCACCTTTG-3’; Reverse 5’-CTCTTGTGCTCTTGCTGGG-3.’
Cell growth assay
For growth rate determination, cells were plated in 35 mm dishes at the density of 30,000 cells per dish in their culture media. Starting from day 2, two dishes of each line were taken out each day and nuclei were released according the protocol described previously and counted using a Coulter Counter.25 Treatment medium was refreshed every another day. The doubling time was calculated by the formula: (log2N2-log2N1)/(T2-T1), where N2 and N1 are cell numbers at time points T2 and T1, respectively.
Cell proliferation assay
Proliferation assays were carried out using 5-bromo-2’-deoxy-uridine Labeling and Detection Kit I (Roche Diagnostics, Indianapolis, IN), following the manufacturer’s instructions. Briefly, cells were plated into 6-well plates on sterile cover slips at the density of 2 ×105 cells per well. Two days after seeding, 5-bromo-2’-deoxy-uridine (BrdU) was added to the culture medium at the concentration of 10 µM and incubated for 30 min, followed by incubation with anti-BrdU antibody and secondary fluorescent antibody. The cover slips were mounted to glass slides using VECTASHIELD Antifade Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA). Images of the cells were acquired using Olympus IX81 microscope and Metamorph software. BrdU positive cells were quantified by manual counting using ImageJ software. Three to 5 fields (20 X objective) or 10 fields (60 X objective) of each treatment were counted.
Statistical analysis
Statistical comparisons were made using two-tailed Student’s t tests for cell counts and χ2 test for BrdU incorporation assays. Results were considered statistically significant if the p value was < .05.
Results
GRK4 gene SNPs in breast cancer samples
Three GRK4 SNPs (R65L, A142V, and A486V) were determined in 92 breast cancer cDNA samples using the TaqMan genotyping real time PCR method as described in the section of Materials and Methods. The results were compared with the data from 769 healthy people recruited for salt sensitivity study at The University of Virginia.
When the percentage of wild type, heterozygous and homozygous of each individual SNP was compared, we found no difference between breast cancer and healthy people in terms of SNPs of R65L and A486V and a slightly lower percentage of heterozygous SNPs of A142V in breast cancer patients (data not shown).
Another parameter that should be taken into account is the number of SNPs since our previous study has shown the relationship between number of SNPs on GRK4 gene and the ability of causing hypertension.14 We first counted SNP numbers including all three sites on the basis of allele and categorized into groups of 0, 1, 2-3, and ⩾ 4 SNPs. There was no difference between breast cancer and healthy people (data not shown). Next, SNP numbers of R65L and A142V were counted because percentage of A486V SNP was almost identical in breast cancer and healthy people. Interestingly, more breast cancer samples contain single SNP of either R65L or A142V which is threefold higher than the healthy people (P = 7.53E-11, Table 1). In addition, 15 out of 30 breast cancer cases with 2 to 3 SNPs contain both R65L and A142V. These data suggest that these two SNPs play a role in breast cancer development or progression.
Table 1.
Number of GRK4 SNPs in breast cancer samples.
| # of SNP | Healthy people (%) | Breast cancer (%) |
|---|---|---|
| 0 | 249 (32.38) | 36 (39.13) |
| 1 | 78 (10.14) | 27 (29.35)* |
| 2-3 | 343 (44.60) | 29 (31.52) |
| ⩾ 4 | 99 (12.87) | 0 (0) |
| Total | 769 (100) | 92 (100) |
Abbreviations: SNP, single nucleotide polymorphisms.
P = 7.526E-11 (χ2 analysis).
Expression of GRK4 and its gene SNPs in breast cancer cell lines
Under normal circumstances, GRK4 is mainly expressed in the testis, kidney, and brain. To evaluate the role of GRK4 in breast cancer cells, we further examined expression levels of GRK4 protein in seven breast cancer cell lines and one benign mammary epithelial cell line. GRK4 was expressed in all breast cancer cell lines with various levels but not in benign breast epithelial cells, MCF-10A (Figure 1). The genotyping result has shown that except for BT-20, the rest of the six breast cancer cell lines have the A142V SNP on one or two alleles (Table 2). Three cell lines have R65L SNP. A486V SNP is only found in SK-Br3. BT-20 is the only cell line we tested that does not have GRK4 SNP and SK-Br3 has all three SNPs on one allele. MDA-MB-468 cells have R65 L and A142 V SNPs on both alleles presumably having higher GRK4 activities. Therefore, this cell line was chosen for our functional studies.
Figure 1.
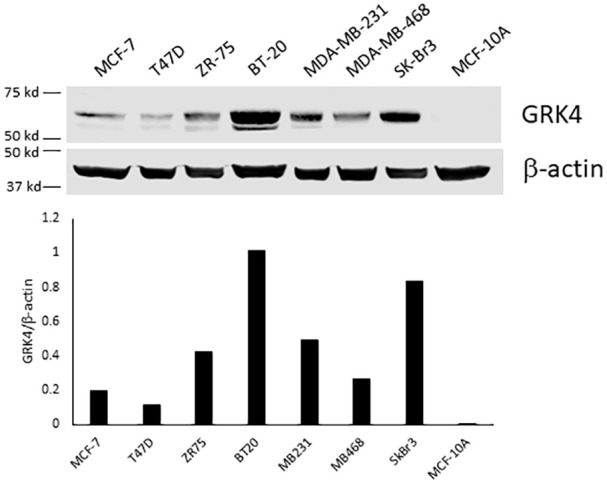
Western blot analysis of GRK4 in breast cancer and benign mammary epithelial cell lines. The cells were grown to confluence in serum containing medium without any treatment. Cell lysate containing 50 µg protein was separated on 10% SDS polyacrylamide gel and then transferred to a nitrocellulose membrane. The membrane was probed with monoclonal anti-GRK4 antibody (D-11). The experiment has been repeated 3 times with different GRK4 antibodies with similar results. Shown here is a representative blot.
Table 2.
GRK4 SNPs in breast cancer cell lines.
|
R65 L
Allele 1/Allele 2 |
A142 V
Allele 1/Allele 2 |
A486 V
Allele 1/Allele 2 |
|
|---|---|---|---|
| MCF-7 | +/- | +/- | -/- |
| T47D | -/- | +/+ | -/- |
| ZR-75 | -/- | +/- | -/- |
| BT-20 | -/- | -/- | -/- |
| MDA-MB-231 | -/- | +/- | -/- |
| MDA-MB-468 | +/+ | +/+ | -/- |
| SK-Br3 | +/- | +/- | +/- |
Expression of GRK4 increases growth rate of cMyc knock-down MCF-7 cells
To examine the role of GRK4 in cell growth, we first determined if GRK4 overexpression can accelerate growth of breast cancer cells. We chose MCF-7 as a model. This cell line expressed relatively low levels of GRK4 protein (Figure 1) and is a less aggressive line.
We have previously found that GRK4 expression in renal proximal tubule cells is regulated by cMyc, a transcription factor.26 In addition, cMyc also acts as an oncogene regulating cell cycle progression, apoptosis and cell transformation in different types of cancer including breast cancer.27,28 To downregulate endogenous GRK4 expression, MCF-7 cells were first transfected with different cMyc shRNA plasmids. Three stable cMyc knock-down lines had been generated in which cMyc mRNA levels had been reduced 50-70% (S1A Figure). GRK4 mRNA levels were slightly or moderately reduced in cMyc knockdown lines suggesting that additional factors might be involved in potential control of GRK4 expression in breast cancer cells. Among three lines, only line 1614 showed significant reduction in GRK4 mRNA (S1B Figure). Therefore, line 1614 was used for ectopic expression of GRK4 isoforms, GRK4α, γ, and γ with three SNPs (GRK4γ TM). Western blot analysis using isoform specific antibodies demonstrated stable expression of three GRK4 isoforms in cMyc knock down MCF-7 cells with the expression level of GRK4γ TM lower than the other two isoforms (S1C Figure).
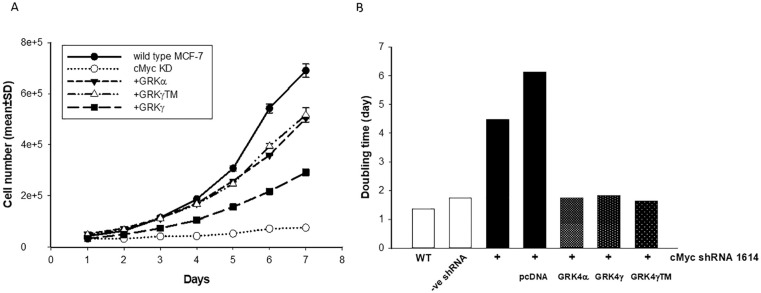
cMyc knock-down significantly decreased the growth rate of MCF-7 cells (Figure 2A). Doubling time raised from 1.74 days (-ve shRNA) to 4.48 days (Figure 2B). Expression of GRK4 in cMyc knock-down MCF-7 cells resumed the growth rate nearly to the level of wild type MCF-7 (Figure 2). The cells expressing GRK4α and GRK4γ TM showed similar increase in growth rate. Wild type GRK4γ was less potent than the other two isoforms (Figure 2). These data demonstrate that GRK4 acts as an independent factor to support growth of breast cancer cells.
Figure 2.
Growth of wild type and cMyc KD MCF-7 cells with or without GRK4 isoform expression. (A). Growth rate. The cells were plated in 35 mm dishes at the density of 30,000 cells/dish. Cell number was counted each day at same time for 7 days. Each point represents average cell number of two dishes. The experiment was repeated twice with similar results. Shown here is a representative result. Each data point is an average of duplicate counts ± SD. (B). Doubling time. Doubling time of each line was calculated using growth rate data in (A). WT, wild type MCF-7; -ve shRNA, MCF-7 cells transfected with negative control shRNA.
GRK4 knock-down reduces growth rate of MDA-MB-468 cells
To further confirm the role of GRK4 in breast cancer proliferation, we performed knock-down of GRK4 in MDA-MB-468 cells and examined cell growth. This cell line is the most aggressive line among seven breast cancer cell lines tested. More importantly, this cell line has two homozygous GRK4 SNPs that implicate higher GRK4 activity (Table 2).
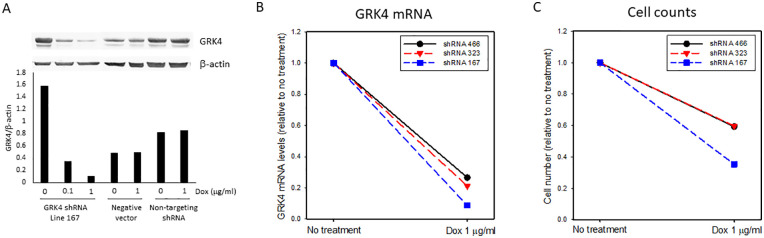
MDA-MB-468 cells were transduced with each of three GRK4 shRNA lentivirus constructs and three stable lines with inducible GRK4 shRNA were generated after puromycin selection. In all three lines, doxycycline induced dose-dependent expression of GRK4 shRNAs as indicated by expression of GFP (S2 Figure). Accordingly, GRK4 protein (Figure 3A and S3A Figure) and mRNA levels were reduced by 70% to 90% after 2 weeks of treatment with doxycycline (1 µg/mL) (Figure 3B). Growth rate of three cell lines were also reduced in the cells treated with doxycycline (1 µg/mL) compared with the cells without exposure to doxycycline (Figure 3C). In striking contrast, expression of GRK2, a member of GRK from different subfamily, was not altered in these cells (S3B Figure). There was no reduction in GRK4 mRNA levels in the cells expressing non-targeting shRNA (S4 Figure). These data confirmed the specificity of GRK4 shRNA constructs used in this model.
Figure 3.
Silencing GRK4 reduced growth rate of MDA-MB-468 cells. (A) GRK4 protein levels in the cells expressing GRK4 shRNA 167, empty vector, and non-targeting shRNA; (B) GRK4 mRNA levels in three GRK4 knock down cell lines after doxycycline treatment for 2 weeks; (C) Cell counts. The cells were treated with or without doxycycline (1 µg/ml) for 7 days and then were plated in 6-well plates at the density of 30,000 cells/well. Cell number was counted 7 days later (Total 14-day treatment with doxycycline).
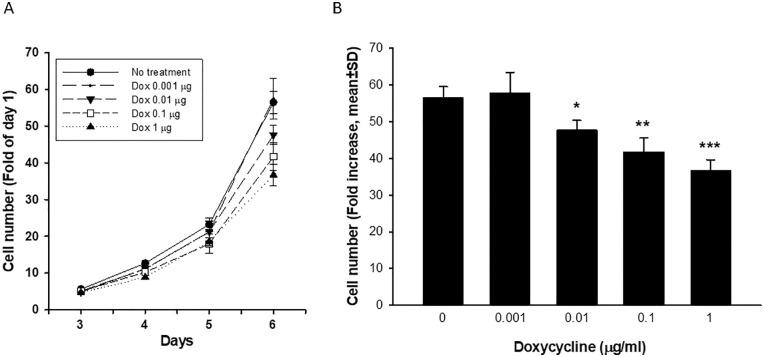
Among three GRK4 knock down lines, line 167 exhibited the greatest reduction in GRK4 mRNA (Figure 3B). We used this line to do more in depth exploration of the role of GRK4. Fifteen-day treatment with doxycycline dose-dependently reduced the growth rate (Figure 4A, S5 Figure). Compared to the cells without exposure to doxycycline, the final cell number of doxycycline treated cells reduced by 15.7% (0.01 µg/mL), 26.8% (0.1 µg/mL) and 35% (1 µg/mL), respectively (Figure 4B). In contrast, there was no change in growth rate in the cells transduced with negative control vector or wild type MDA-MB-468 treated with doxycycline (S6B, C Figure). A slight reduction in cell number of the cells expressing non-targeting shRNA treated with 1 µg/mL doxycycline was observed (S6A Figure). The extent of reduction was much smaller than that with GRK4 knock down (Figure 4A) and lower concentration doxycycline did not inhibit cell growth of the cells expressing non-targeting shRNA. These data indicated that reduction in growth rate in line 167 results from GRK4 knock down.
Figure 4.
Reduction in growth rate of MDA-MB-468 cells expressing GRK4 shRNA 167 treated with various concentrations of doxycycline for 15 days in total. (A) growth rate; (B) Cell counts at the end of experiment. Cell growth was expressed as fold increase compared with the number on day 1 of each treatment (Mean ± SD, n = 4). *P < .005, **P < .001, ***P < .0001 compared with vehicle control. The experiment was repeated four times with different length of doxycycline treatment. The results were consistent (S5 Figure). Shown here is a representative result.
GRK4 knock down decreases cell proliferation
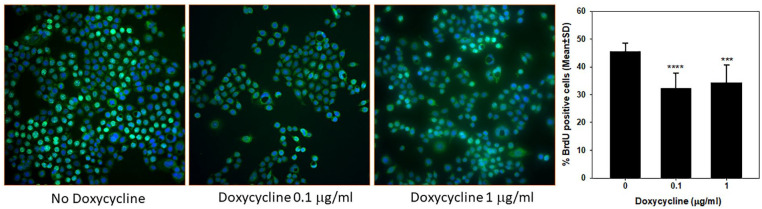
Reduced growth rate could be a result of decreased cell proliferation or increased cell death or both. To dissect out these possibilities, we first determined the effect of GRK4 knock down on cell proliferation by BrdU labeling. Line 167 of GRK4 knock down cells were pretreated with doxycycline for 6 to 9 days before being plated on coverslips. BrdU incorporated into DNA was detected by a specific anti-BrdU antibody. As shown in Figure 5, the nuclei of the cells at the S phase of cell cycle were labeled green. About 45% of non-treated cells were BrdU positive which was reduced to 32% and 34% in the cells treated doxycycline at 0.1 µg/mL and 1 µg/mL respectively (Figure 5). GRK4 knock-down did not affect apoptosis (S7 Figure). There was no difference in proliferation of the cells expressing non-targeting shRNA treated with or without doxycycline (S8 Figure). These results indicate that promoting cell proliferation is the predominant role of GRK4 in breast cancer cells.
Figure 5.
Reduction in proliferation of MDA-MB-468 cells expressing GRK4 shRNA 167 and treated with various concentrations of doxycycline for 7 days in total. Proliferating cells with BrdU incorporated into DNA were detected by FITC-conjugated antibody. Proliferation rate was expressed as percentage of cells with nuclear green fluorescence staining. ***P < .0005; ****P < 5×10−8 compared with no doxycycline treatment. The experiment was repeated twice with similar results. Shown here is a representative result.
Discussion
We found that GRK4 is expressed in breast cancer cells (all seven cell lines examined) but not in benign mammary epithelial cells. Six out of seven cancer lines have at least one SNP in GRK4 gene (Table 2) that has been demonstrated to be associated with an increased risk of hypertension.14 GRK4 SNPs were also detected in the cDNA array of 92 breast cancer patients. Compared with healthy people, breast cancer patients were three-fold more frequent to carry single SNP at R65 or A142 site. The data from the in vitro models further demonstrated that overexpression of GRK4 in cMyc knock-down MCF-7 cells significantly increased the growth rate. Knock-down of endogenous GRK4 in MDA-MB-468 cells slowed down cell growth which was predominantly a result of reduction in proliferation rather than induction of apoptosis. Thus, our data demonstrated that GRK4 plays a role in promoting cell proliferation of breast cancer.
It has been reported that GRK4 protein is over expressed in breast cancer tissues19 but GRK4 SNP types in breast cancer are largely unknown. Our data from breast cancer cDNA array and breast cancer cell lines have shown that GRK4 SNPs exist in breast cancer cells. Among three GRK4 SNPs, single SNP on R65 or A142 was much higher in breast cancer samples (Table 1). This result is consistent with that from breast cancer cell lines. In six SNP positive lines, all have either homozygous or heterozygous A142V and three cell lines contain R65L SNP (Table 2). A486V was found in one cell line only. It should be mentioned that heterozygous SNP may lead to over expression of the enzyme with higher activity because of differential allele-specific expression. Gao et al found 2.37 and 3.01-fold expression of A142V and R65L respectively in mammary epithelial cells derived from breast cancer patients.20 These data indicate that R65L and A142V are relevant SNPs in breast cancer.
Since the causal relationship between GRK4 and hypertension was confirmed in a transgenic mouse model14 and that GRK4 is transcriptionally regulated by cMyc in human kidney proximal tubule cells,26 and the fact that cMyc is a proto-oncogene that is amplified in various cancers including breast cancer causing cell proliferation, we hypothesized that this pathway may be implicated in the link between hypertension and breast cancer. Silencing cMyc in MCF-7 cells provides a useful model to evaluate the role of GRK4 in cancer cell growth. cMyc knock down almost completely impaired the ability of MCF-7 cells to grow. Expression of all three GRK4 isoforms resumed cell growth to different extents. These results indicated that GRK4 is a driving force of cell proliferation in MCF-7 cells. The effect of GRK4α on cell proliferation seems stronger than the γ isoform (Figure 2). The cells expressing GRK4γ with three SNPs grew as rapidly as those expressing GRK4α suggesting that the higher the kinase activity the stronger the growth promoting effect. These data are in line with those from the studies with renal proximal tubule cells where GRK4γ with three SNPs are associated with salt sensitivity of blood pressure.29 GRK4α could be a major player in cancer cells because it is the most abundant isoform expressed in all cancer cell lines examined (Figure 1).
The growth promotion function of GRK4 in breast cancer cells was further demonstrated in MDA-MB-468 cells. This cell line is a triple negative breast cancer line, e.g. without expression of estrogen, progesterone receptors and HER2, therefore is a very aggressive breast cancer cell line. A unique character of this cell line is that it contains two homozygous SNPs on GRK4 gene (R65L and A142V). This characteristic makes the cell line an ideal model for evaluating the function of GRK4. Indeed, knock down of GRK4 significantly reduced the growth rate of MDA-MB-468 cells. Growth retardation was predominantly a result of reduction in the population of proliferating cells. There was little effect for GRK4 on apoptosis. The results from MCF-7 and MDA-MB-468 models demonstrate the GRK4 plays an important role in cell proliferation in breast cancer cells. These data confirm the clinical findings that GRK4 is over expressed in invasive breast cancers but not the normal breast tissues19 and high expression levels as well as R65L and A142V SNPs of GRK4 is associated with higher breast cancer risk.20 Our results demonstrate that GRK4 is an independent factor that promotes proliferation of hormone-dependent and more aggressive triple negative breast cancer cells. This effect of GRK4 might be specific for cancer cells because a study using human embryonic kidney cells (HEK) has shown that overexpression of GRK4 inhibits cell growth via senescence.30 On the other hand, the difference between cancer cells and HEK cells could origin from different GRK4 constructs used. We used a non-tagged GRK4 in our studies since it improves upon previous studies that used a C-terminal GFP fusion tag that could disrupt pamitoylation and thus subcellular localization of GRK4 and possibly provide an unwanted dominant negative function.
In summary, this paper has provided experimental evidences demonstrating that (1) GRK4 is upregulated in breast cancer cells, (2) breast cancer tissues have higher frequency to carry single hypertension related SNPs which render higher or sustained GRK4 activity, and (3) GRK4 acts as an independent factor to promote proliferation of breast cancer cells. These findings warrant future studies to examine the presence of GRK4 SNPs in breast tumors excised from patients with or without hypertension. A positive correlation between GRK4 variants and hypertension would suggest that a specific GRK4 inhibitor could be pursued as a therapy for hypertensive breast cancer patients. Because of restricted expression of GRK4 in normal tissues, targeting GRK4 should be a safe and effective approach to wipe out the oncogenic effect of cMyc without disturbing other functions of this important transcription factor. A broader implication of GRK4 in chronic disease could be pursued by examining the comorbidity between GRK4 SNPs and obesity31 and the relationship between obesity and breast cancer risk.32
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Heart Lung and Blood Institute HL074940, and National Institute of Diabetes Digestive Diseases and Kidney DK039308.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Experimental design: John J. Gildea, Wei Yue; Experiment performance: Hanh T. Tran, Ji-ping Wang, Katherine Schiermeyer, Peng Xu, Wei Yue; Data analysis: Katherine Schiermeyer, Peng Xu, Wei Yue; Manuscript preparation: Wei Yue, John J. Gildea and Robin A. Felder.
ORCID iD: Wei Yue  https://orcid.org/0000-0002-7313-8516
https://orcid.org/0000-0002-7313-8516
References
- 1. Dyer A, Berkson D, Stamler J, Lindberg H, Stevens E. High blood-pressure: a risk factor for cancer mortality? Lancet. 1975;305:1051-1056. [DOI] [PubMed] [Google Scholar]
- 2. Stocks T, Van Hemelrijck M, Manjer J, et al. Blood pressure and risk of cancer incidence and mortality in the metabolic syndrome and cancer project. Hypertension. 2012;59:802-810. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [DOI] [PubMed] [Google Scholar]
- 4. Peeters P, van Noord P, Hoes A, Fracheboud J, Gimbrère C, Grobbee D. Hypertension and breast cancer risk in a 19-year follow-up study (the DOM cohort). J Hypertens. 2000;18:249-254. [DOI] [PubMed] [Google Scholar]
- 5. Soler M, Chatenoud L, Negri E, Parazzini F, Franceschi S, la Vecchia C. Hypertension and hormone-related neoplasms in women. Hypertension. 1999;34:320-325. [DOI] [PubMed] [Google Scholar]
- 6. Lindgren A, Pukkala E, Tuomilehto J, Nissinen A. Incidence of breast cancer among postmenopausal, hypertensive women. Int J Cancer. 2007;121:641-644. [DOI] [PubMed] [Google Scholar]
- 7. Pereira A, Garmendia ML, Alvarado ME, Albala C. Hypertension and the risk of breast cancer in Chilean women: a case-control study. Asian Pac J Cancer Prev. 2012;13:5829-5834. [DOI] [PubMed] [Google Scholar]
- 8. Han H, Guo W, Shi W, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep. 2017;7:44877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esposito K, Chiodini P, Capuano A, et al. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013;20:1301-1309. [DOI] [PubMed] [Google Scholar]
- 10. Goldberg LI. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev. 1972;24:1-29. [PubMed] [Google Scholar]
- 11. Nishi A, Eklof A-C, Bertorello A, Aperia A. Dopamine regulation of renal Na+,K(+)-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension. 1993;21:767-771. [DOI] [PubMed] [Google Scholar]
- 12. Hussain T, Lokhandwala MF. Renal dopamine DA1 receptor coupling with G(S) and G(q/11) proteins in spontaneously hypertensive rats. Am J Physiol. 1997;272:F339-F346. [DOI] [PubMed] [Google Scholar]
- 13. Sanada H, Jose PA, Hazen-Martin D, et al. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036-1042. [DOI] [PubMed] [Google Scholar]
- 14. Felder RA, Sanada H, Xu J, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Felder RA, Jose PA. Mechanisms of Disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2:637-650. [DOI] [PubMed] [Google Scholar]
- 16. Harris RC. Abnormalities in renal dopamine signaling and hypertension: the role of GRK4. Curr Opin Nephrol Hypertens. 2012;21:61-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Premont RT, Macrae AD, Stoffel RH, et al. Characterization of the G protein-coupled receptor kinase GRK4: identification of four splice variants. J Biol Chem. 1996;271:6403-6410. [DOI] [PubMed] [Google Scholar]
- 18. Premont RT, Macrae AD, Aparicio SAJR, Kendall HE, Welch JE, Lefkowitz RJ. The GRK4 subfamily of G protein-coupled receptor kinases: alternative splicing, gene organization, and sequence conservation. J Biol Chem. 1999;274:29381-29389. [DOI] [PubMed] [Google Scholar]
- 19. Matsubayashi J, Takanashi M, Oikawa K, et al. Expression of G protein-coupled receptor kinase 4 is associated with breast cancer tumourigenesis. J Pathol. 2008;216:317-327. [DOI] [PubMed] [Google Scholar]
- 20. Gao C, Devarajan K, Zhou Y, Slater CM, Daly MB, Chen X. Identifying breast cancer risk loci by global differential allele-specific expression (DASE) analysis in mammary epithelial transcriptome. BMC Genomics. 2012;13:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hui L, DelMonte T, Ranade K. Genotyping using the TaqMan assay. Current Protocols in Human Genetics. 2008;56:21011-121018. [DOI] [PubMed] [Google Scholar]
- 22. Konopac M, Dusatkova P, Cinek O. SNPman: a program for genotype calling using run data from TaqMan allelic discrimination. Bioinformatics (Oxford, England). 2011;27:2306-2308. [DOI] [PubMed] [Google Scholar]
- 23. Andresen BT. Chapter 31. Characterization of G protein-coupled receptor kinase 4 and measuring its constitutive activity in vivo. In: Conn PM, ed. Methods in Enzymology. Vol. 484. New York: Academic Press; 2010:631-651. [DOI] [PubMed] [Google Scholar]
- 24. Liu GJ, Wu YS, Brenin D, et al. Development of a high sensitivity, nested Q-PCR assay for mouse and human aromatase. Breast Cancer Res Treat. 2008;111:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yue W, Wang J-P, Conaway M, Masamura S, Li Y, Santen RJ. Activation of the MAPK pathway enhances sensitivity of MCF-7 breast cancer cells to the mitogenic effect of estradiol. Endocrinology. 2002;143:3221-3229. [DOI] [PubMed] [Google Scholar]
- 26. Gildea JJ, Tran HT, Van Sciver RE, Bigler Wang D, Carlson JM, Felder RA. A novel role for c-Myc in G protein coupled receptor kinase 4 (GRK4) transcriptional regulation in human kidney proximal tubule cells. Hypertension. 2013;61:1021-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253-264. [DOI] [PubMed] [Google Scholar]
- 28. Xu J, Chen Y, Olopade OI. MYC and breast cancer. Genes Cancer. 2010;1:629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens. 2013;22:65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiao P, Huang X, Huang L, et al. G protein-coupled receptor kinase 4-induced cellular senescence and its senescence-associated gene expression profiling. Exp Cell Res. 2017;360:273-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee M, Kim MK, Kim S-M, Park H, Park HK. Gender-based differences on the association between salt-sensitive genes and obesity in Korean children aged between 8 and 9 years. PLoS ONE. 2015;10:e0120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feliciano EMC, Chen WY, Bradshaw PT, et al. Adipose tissue distribution and cardiovascular disease risk among breast vancer survivors. J Clin Oncol. 2019;37:2528-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]