Abstract
Background:
Emerging evidence suggests that post concussive symptoms, including mood changes, may be improved through morning blue-wavelength light therapy (BLT). However, the neurobiological mechanisms underlying these effects remain unknown. We hypothesize that BLT may influence the effective brain connectivity (EC) patterns within the default-mode network (DMN), particularly involving the medial prefrontal cortex (MPFC), which may contribute to improvements in mood.
Methods:
Resting-state functional MRI data were collected from 41 healthy-controls (HCs) and 28 individuals with mild traumatic brain injury (mTBI). Individuals with mTBI also underwent a diffusion-weighted imaging scan and were randomly assigned to complete either 6 weeks of daily morning BLT (N = 14) or amber light therapy (ALT; N = 14). Advanced spectral dynamic causal modeling (sDCM) and diffusion MRI connectometry were used to estimate EC patterns and structural connectivity strength within the DMN, respectively.
Results:
The sDCM analysis showed dominant connectivity pattern following mTBI (pre-treatment) within the hemisphere contralateral to the one observed for HCs. BLT, but not ALT, resulted in improved directional information flow (ie, EC) from the left lateral parietal cortex (LLPC) to MPFC within the DMN. The improvement in EC from LLPC to MPFC was accompanied by stronger structural connectivity between the 2 areas. For the BLT group, the observed improvements in function and structure were correlated (at a trend level) with changes in self-reported happiness.
Conclusions:
The current preliminary findings provide empirical evidence that morning short-wavelength light therapy could be used as a novel alternative rehabilitation technique for mTBI.
Trial registry:
The research protocols were registered in the ClinicalTrials.gov database (CT Identifiers NCT01747811 and NCT01721356).
Keywords: Diffusion tensor imaging, resting-state fMRI, effective brain connectivity, light therapy, mood
Introduction
A mild traumatic brain injury (mTBI) occurs when an individual experiences a physical impact, rotational force, or other traumatic damage to head1 that leads to a temporary alteration in consciousness or cognition.2,3 Within the brain, the physical force often leads to sheared axons and microscopic changes in brain tissue.4 These kinds of injuries are common in sports such as soccer, hockey, and boxing, and are also among the most prevalent injuries sustained by military service members. Within the military alone, recent statistics suggest that more than 417 500 military personnel have sustained a traumatic brain injury since the year 2000, with 82.4% of these being in the mild range (DVBIC; 2019 Q4: https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi). Individuals with an mTBI may exhibit independent or simultaneous alterations in brain function and structure.5,6 While most symptoms of concussion will resolve within a matter of days to weeks, for many individuals the injury may lead to persistent post-concussive symptoms, including mood disturbances7,8 and impairment of some cognitive abilities,3,9 including concentration/attention10 and memory problems.11 Moreover, mTBIs are also commonly associated with mood dysregulation, fatigue, and sleep problems, which can impede the recovery process.12-17 One novel and promising approach to overcome these problems is the use of morning blue-wavelength light therapy (ie, at ~480 nm) (BLT).17,18 Blue light stimulates intrinsically photosensitive retinal ganglion cells (ipRGCs) that project to the suprachiasmatic nucleus (SCN) of the hypothalamus, which serves as the primary regulator of the circadian rhythm.19,20 Such exposure each day is crucial to the maintaining a regular circadian rhythm, including the normal cycling of sleep and wake. Consequently, precisely timed BLT has been proposed as one possible, safe, and non-pharmacological way of improving sleep and post-concussive symptoms among individuals with a TBI.18,21 Prior work has shown that BLT appears to be effective at improving mood and reducing fatigue and daytime sleepiness,18,22 and at changing white-matter compactness within specific areas.23 Our recent work suggests that morning BLT may also have wide ranging effects including altered circadian timing of sleep, as well as changes in thalamic volume, functional connectivity, and white matter structural connectivity, which are in turn, correlated with improvements in cognitive abilities among those with mTBI.17 These findings suggest that BLT may provide a potential non-pharmacological key to effective recovery from injury for people with mTBI.
Mood dysregulation, including lower self-reported happiness and associated positive emotions, appears to be closely intertwined with the brain’s default-model network (DMN).24-28 The DMN is a distributed brain network that includes the posterior cingulate/precuneus region, medial prefrontal cortex (MPFC), and bilateral posterior parietal cortex. The DMN usually activates when an individual is at rest, cognitively disengaged, or during mind wandering, but is suppressed during effortful cognitive activities.29 The impact of mTBI on functional brain connectivity (representing temporal correlations) within the DMN has also been extensively studied.30-33 The frontal lobe, particularly the MPFC, an integral part of the DMN, is especially vulnerable to disruption by mTBI,6 and this particular region plays an important role in the generation and regulation of emotions.34 Notably, symptoms of mood disruption are also common following a brain injury.7,8,35,36 BLT has also been shown to be helpful at improving mood in various other populations,37-39 and it is likely that it would have similar mood enhancing effects in patients with mTBI.40 It is well known that mood and DMN activation are closely related.24,26,41,42 Despite this strong association between mood states and activation of the DMN, the effect of BLT on brain connectivity, particularly effective connectivity and structural connectivity measures within the DMN along with their association with mood states following an mTBI has not been explored.
Various neuroimaging methodologies allow the assessment of brain function and structure in vivo. Numerous studies have repeatedly demonstrated the inextricable association between brain function and structure as well as highlighted the importance of unique information provided by each methodology.43-45 Recently, more advanced neuroimaging approaches and analysis techniques, such as effective connectivity (EC)46 and diffusion-weighted structural imaging,47 have been developed to better understand small and large-scale neural networks. For instance, dynamic causal modeling (DCM)—an EC approach46,48-51 is usually implemented to estimate directed connectivity within the brain during resting-state52 and cognitive tasks.53,54 DCM relies on theoretical assumptions specifying a set of hypotheses in terms of different models. Using the Bayesian model reduction approach,49 an optimal model is found in DCM by calculating a degree of belief (called model exceedance probability) about a model having higher posterior probability than other models in the model space. EC represents an explicit functional influence that one neural system exerts over the other. Endogenous fluctuations (also called neural state noise or neural fluctuations) are the sources driving the neural activity and hence EC in the absence of the external stimuli. Next, the diffusion weighted approaches can identify white-matter pathways using metrics such as fractional anisotropy (FA), which refers to diffusivity or speed of water diffusion along white matter fiber bundles. Similarly, quantitative anisotropy (QA), is another diffusion weighted metric that refers to the density or quantity of water diffusion along white matter fiber bundles. QA is sensitive to density characteristics of these bundles (eg, fiber compactness).47,55 Compared to diffusivity measures, density measures are less vulnerable to partial volume effects and are better able to filter out image noise.47
Here, we proposed that a multimodal approach that is, measures of EC and structural connectivity (in terms of FA and QA) could be used to better understand the dynamics of neural networks following an mTBI and quantify the impact of BLT on the functional/structural architecture of the DMN and its association with mood. The present study focused on identifying differences in EC within the DMN for healthy controls (HCs) and post-mTBI before and after a course of light therapy. The first aim was to determine whether the connectivity pattern within the DMN is typically damaged following mTBI. Secondly, the goal was to quantify the effect of 6 weeks of daily morning exposure to BLT versus amber-light therapy (ALT) on the EC of the DMN and calculate the extent to which the post-treatment connectivity pattern resembled that of the HCs. From diffusion-weighted data, we investigated if there was any associated treatment effect (BLT vs ALT) on FA and QA measures of the DMN. Lastly, due to the close association between the DMN and mood,24,26 we investigated whether changes in EC or characteristics of white-matter (FA or QA) following either treatment were associated with changes in happiness. Due to the known impact of light therapy on mood and the close association between mood and DMN functioning, we hypothesized that BLT would strengthen the EC as well as structural characteristics of the DMN. Secondly, based on the prior evidence of mood improvement with light treatment, we hypothesized that changes in the EC and both FA and QA following BLT would be associated with changes in self-reported happiness. In our exploratory analyses, we predicted that the changes in EC (for both ALT and BLT groups) would be associated with changes in the characteristics of white matter in terms of FA and QA.
Methods
Participants
Forty-one healthy controls (HCs; mean age = 26.07 ± 5.01 years, 25 F) with no history of any head injury and 28 individuals with a history of mTBI (mean age = 21.50 ± 3.76 years, 15 F) (Table 1) underwent neuroimaging scans using a 3T Siemens Tim Trio scanner (Erlangen, Germany) located at the McLean Hospital Imaging Center. The final sample of 28 individuals with mTBI excludes a set of participants because of (i) participant missing identity (n = 1); (ii) disqualifying psychopathology (n = 1); (iii) unavailable MRI data due to claustrophobia upon entering the scanner (n = 1); (iv) unusable MRI data (n = 1); (v) unavailability of the participant for MRI scan (n = 2); and (vi) failure to complete required study procedure related to light-device use (ie, lack of treatment compliance) (n = 2). Included individuals with mTBI met the definition of an mTBI56 that is, participants with an mTBI had to have experienced at least 1 traumatically induced physiological disruption and/or structural injury caused by an external force (eg, blast wave, head impact) leading to an alteration in mental status (eg, disorientation, confusion, retrograde/anterograde amnesia), brief loss of consciousness (ie, less than 30 minutes; alteration of consciousness up to 24 hours), or post-traumatic amnesia up to 24 hours, and/or a Glasgow Coma Scale greater than or equal to 13 within 24 hours of injury (when measured). All eligible individuals with an mTBI were injured between 2 and 18 months (mean = 6.84 ± 4.0 months) prior to their screening and provided documentation from a relevant professional (eg, coach, trainer, or security guard) who either witnessed or was involved in the immediate response to the injury (eg, physician, nurse, medical technician, or police officer). The majority of the individuals with an mTBI (68%) were engaged in a physical activity (eg, rugby, soccer, karate, and hiking); whereas 32% individuals were engaged in a household or vehicular accident at the time of injury. Individuals with any history of neurological or psychiatric disorder prior to the mTBI were excluded. All the HCs were recruited from a separate study57-61 but completed an identical functional and structural neuroimaging scan in the same scanner. All the HCs were screened via a telephone interview and were included if they had no history of significant medical problems, current use of psychotropic medications, or illicit substances. A written informed consent was obtained from each participant prior to enrollment. Participants were financially compensated for their participation. The Institutional Review Board (IRB) at McLean Hospital, Partners Health Care (IRB protocol numbers: 2009-P-002230 for HCs study and 2010-P-001570 for mTBI study) and the U.S. Army Human Research Protections Office (HRPO protocol numbers: A-15731 for HCs study and A-16161 for mTBI study) approved the experimental protocols.
Table 1.
Demographics.
| Demographics | Healthy controls (N = 41) | MTBI (overall) (N = 28) | MTBI (BLT group) (N = 14) | MTBI (ALT group) (N = 14) | Significance (independent sample t-test/Chi-square) |
|---|---|---|---|---|---|
| Mean age (SD) (in years) | 26.07 (5.01) | 21.50 (3.76) | – | – | t (67) = 4.10, P < .001 |
| Sex (females/males) (N) | 25/16 | 15/13 | – | – | χ2(1) = 0.37, P = .54 |
| Mean age (SD) (in years) | – | – | 21.78 (4.42) | 21.21 (3.09) | t (26) = −0.40, P = .69 |
| Sex (females/males) (N) | – | – | 8/6 | 7/7 | χ2(1) = 0.14, P = .70 |
| Mean TSI (SD) (in months) | – | – | 6.57 (4.57) | 7.11 (3.53) | t (26) = 0.35, P = .73 |
| Days light used (SD) | – | – | 38.71 (4.30) | 37.21 (6.09) | t (26) = −0.75, P = .46 |
| Scores on MEQ (chronotype) | – | – | 51.93 (4.50) | 51.50 (6.28) | t (26) = −0.21, P = .84 |
| Levels of happiness at baseline (SD) | – | – | 71.43 (17.21) | 63.89 (17.33) | t (26) = −1.16, P = .26 |
Abbreviations: MEQ, morningness-eveningness questionnaire; MTBI, mild traumatic brain injury; SD, standard deviation; TSI, time since injury.
Procedure
Individuals in the HC group underwent 1 neuroimaging session that included a resting-state functional MRI (fMRI) and structural MRI scan. The mTBI group underwent a resting-state fMRI scan, structural MRI scan, and a diffusion-weighted MRI scan on 2 different occasions, separated by 6 weeks. After the first scanning session (baseline data collection), individuals in the mTBI group were randomly assigned to 1 of 2 light treatment conditions that involved using either: (1) a commercially available blue-light device (BLT: N = 14, mean age = 21.78 ± 4.42 years, 8 F; Philips Electronics GoLite Blu®) or (2) a placebo amber-light device (ALT: N = 14, mean age = 21.21 ± 3.09 years, 7 F; Philips Electronics custom light box); see Table 1. At the time of initial grant submission, we conducted a power analysis based on the assumption of a treatment (light group) × testing session (pre-treatment vs post-treatment) with a moderate effect size (f = 0.25) and α = .05, assuming at least a correlation of r = .60 between repeated measures. A sample size of N = 15 per group (N = 30 total) was found to provide power = 0.84. Randomization was based on a pre-established computer-generated randomization scheme that is, light devices were provided to the participants in a double-blind manner. In other words, both participants as well as all study staff were blind to the assignment of light device (ie, either amber or blue) to each participant. Light devices provided controlled exposure to a narrow band of either blue wavelength light (λ = 469 nm, illuminance = 214 lux, and single panel irradiance = 0.11 mW/cm2 at 80 cm) or amber wavelength light (λ = 578 nm, illuminance = 188 lux, and single panel irradiance = 0.04 mW/cm2 at 80 cm). All participants were instructed to use the device daily (30 minutes each morning, within 2 hours of awakening but starting before 11:00 A.M.) for 6 weeks. Participants were required to log in to a secure online website and indicate the timing of their light use each day.
Data acquisition
Happiness scores
Due to the close association between brain injuries and mood disturbances7,8 and between DMN and its core phenomenon of mind-wandering with happiness/sadness,24,26,62 the happiness scores (HAP), collected using ANAM4 (Automated Neuropsychological Assessment Metrics) Concussion Battery63 at baseline and post-treatment, were analyzed for the purpose of this study. The ANAM4 was developed and normed for use with U.S. military personnel. Construct validity and test-retest reliability of ANAM mood measures have been shown in previous studies.64 Participants were asked to select their predominant mood state, representing “how” they felt at that time using a 7-point (0 for not at all, midpoint 3 for somewhat, and 6 for very much) Likert scale.
Questionnaire for morningness and eveningness (MEQ)
Chronotype refers to the trait-like preference for certain activities earlier or later in the day. The chronotype of participants might affect their natural tendency to awaken at certain times and their preferred timing for using the light device. Therefore, trained research assistants administered the Horne-Ostberg Morningness-Eveningness Questionnaire65 to each participant. In the present sample, scores on the questionnaire ranged from 42 to 65, with a mean of 51.88 ± 5.52. Here, lower scores represent greater preference for evening activities, and higher scores represent the tendency to prefer morning activities (ie, fewer preferences for evening activities).
Resting-state fMRI (rsfMRI) and structural MRI data
RsfMRI and structural data were collected from all participants. During both scans, participants were instructed to relax and remain still. The resting-state scan for both groups (ie, HCs and mTBI group) lasted for 6 minutes, and data were acquired using a T2*-weighted echo-planner imaging (EPI) sequence, which consisted of 180 frames (voxel resolution = 3.5 × 3.5 × 3.5 mm3, field of view (FOV) = 384 mm, FA/TE/TR of 90°/30 ms/2000 ms). During the scan, participants were asked to keep their eyes open and to let their mind wander. Structural data from HCs were acquired using a 3D MPRAGE echo sequence (128 sagittal slices, voxel resolution = 1.33 × 1 × 1 mm3, field of view (FOV) = 256 mm), flip angle/TE/TR/inversion time of 12°/2.25 ms/2100 ms/1100 ms). Structural data from mTBI individuals were acquired using a 3D MPRAGE echo sequence (176 sagittal slices, voxel resolution = 1 × 1 × 1 mm3, field of view (FOV) = 256 mm, flip angle/TE/TR/inversion time of 12°/2.30 ms/2100 ms/1100 ms).
Diffusion-weighted imaging (DWI) data
The DWI data from mTBI individuals were acquired with maximum b-value = 1000 s/mm2, 72 directions, 8 b0 images that is, images with no diffusion weighting, voxel size = 1.75 × 1.75 × 3.5 mm3, flip angle/TR/TE/slice thickness of 90°/6340 ms/99 ms/3.5 mm, and 40 slices encompassing the whole brain.
Data analysis
Outlier detection
Two participants from the mTBI sample, one from the ALT group and the second from the BLT group, showed excessive head-motion during functional scans. Head-motion was considered excessive if images with movement from a preceding image exceeded 0.5 mm or if images had a global mean signal intensity greater than 3 standard deviations.17 Both of these participants were excluded from further analysis. None of the data points on the ANAM Happy mood scale were identified as outliers. Here, a data point was considered as an outlier only if the value exceeded more than 1.5 inter-quartile (IQ) range below or above the top or bottom quartiles.
Demographics
Potential group differences in age, sex, time-since injury (TSI), number of days light used within 2 hours of awakening, chronotype, or baseline levels of happiness were examined via 2 samples t tests (and chi-squared tests for sex differences). Group differences obtained meant that the variable would be treated as a covariate in subsequent analyses.
Adherence to light treatment
Adherence to light treatment was estimated by the compliance of each of the 28 participants in completing their daily online sleep and light diary. Based on the number of days the participant was enrolled in the study, we calculated an estimate of adherence as the percentage of potential treatment days during which the light was reportedly used. Group differences in adherence to light treatment were examined via 2 samples t test.
Image preprocessing
Raw DICOM rsfMRI, structural MRI, and DWI data were converted into NIFTI format using the dcm2nii program available in the MRIcron package. For DWI data, this conversion also generated b-value and b-vector files for each subject. RsfMRI data were preprocessed using the default analysis pipeline implemented in CONN Toolbox (V.18.a) (https://www.nitrc.org/projects/conn) based on SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) in MATLAB R2018a (MathWorks, Inc., MA, USA). The preprocessing steps included functional realignment (all scans were coregistered and resampled to the first scan), slice-timing correction, motion-correction (new reference image for functional data was created by averaging over all scans), direct segmentation and normalization (separate normalization of the functional and structural volumes to standard MNI-space and segmentation into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) tissue classes), Gaussian spatial smoothing at 6 mm full width at half maximum (FWHM), and band-pass filtering (0.008-0.09 Hz, default). During the direct segmentation and normalization step, data were also resampled with 2 mm isotropic voxels for functional data and 1 mm for structural data, using fourth order spline interpolation. For EC analysis, time-series from each region of interest from smoothed images was corrected for head motion and physiological noise by including the nuisance regressors including the 6 head-motion parameters, CSF extracted from the left ventricle, and WM extracted from the pons. Low-frequency neural signal drifts were further filtered using a 128-s high-pass filter.
Abbreviations of conditions: Our data consists of following 4 experimental conditions:
- APre and APost: for pre- and post-treatment condition of individuals with mTBI in ALT group
- BPre and BPost: for pre- and post-treatment condition of individuals with mTBI in BLT group
Regions of Interest (ROIs) and first level EC analysis
Four spherical ROIs within the DMN that is, posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), and left and right (bilateral) lateral parietal cortex (LLPC/RLPC) with a radius of 8 mm and peak MNI co-ordinates at (0, −52, 27), (−1, 54, 27), (−46, −66, 30), and (49, −63, 33) respectively,66 were used as ROIs. For estimating the EC within the DMN, the spectral dynamical causal modeling approach (spDCM)48,51 (spm_dcm_fmri_csd.m implemented in DCM12/SPM12) with default shrinkage priors48 was used. In the past, the spDCM has been shown to have reliable and robust subjectwise DMN EC estimates.67
Goodness of model fit
Explained variance was used as diagnostic to determine the goodness of model fit (spm_dcm_fmri_check.m in SPM12). In spDCM we fit observed BOLD cross-spectra which is calculated by using parametric multivariate autoregressive (MAR) modeling. The cross spectra are smooth (due to parametric model) with a 1/f distribution hence usually result in a very high explained variance. We did visual inspection and found that the variance in our current sample was ranged between 50% and 95% with most of the subjects having explained variance >85%.
Group level EC analysis
Individual connectivity parameters for the DMN across all of the participants were modeled at the second (group) level using a Bayesian general linear model (GLM), that is, parametric empirical Bayes (PEB), with a regressor for group’s mean value per connection.68,69 The PEB is a hierarchical Bayesian approach that models how individual (ie, within-subject) connections are associated with group or condition means. This technique treats intrinsic connectivity as a random between-subjects effect and is modeled by adding a random (Gaussian) factor to subject-specific parameters. A GLM of between subject effects generates the parameters of a within-subject DCM. The random effect modeling uses the full posterior density over the parameters from subject-wise DCM (ie, expected strength of each connection and the associated uncertainty [ie, posterior covariance]) to inform the second-level result (ie, group means).68 To evaluate how regions in the network interact, we implemented Bayesian model comparison approach to explore the model space (ie, a space of possible models/hypotheses, where each model assumed that a unique combination of the connectivity parameters could characterize all the participants).70 Models of interest were obtained by removing connections to produce reduced (or nested) instances of the full (or parent) model. With 16 (4 × 4 regions) intrinsic connections of the fully connected model, there is large number of possible reduced models in the model space. To address this, we used Bayesian Model Reduction (BMR) approach that enables the evidence and parameters of reduced models to be derived from a fully connected model very quickly (within seconds to minutes).49 The BMR approach enables an efficient and greedy search within the model space by scoring each reduced model based on the log model-evidence or free energy. In addition, the search algorithm further used BMR approach to prune connection parameters from the fully connected model. The process ran until there was no further improvement in model-evidence. The parameters of the best 256 models from this procedure were then averaged and weighted by their model evidence using Bayesian Model Averaging (BMA) approach.71 Only the parameters that exceeded 95% posterior probability (Pp) were interpreted. Also, because the intrinsic self-connections cannot be pruned, therefore, the self-connections were not interpreted.
Comparison between conditions: Mean centered “age” and “sex” were included as covariates for comparison between HCs and individuals with mTBI (ie, both pre- and post-treatment) group.
Hierarchical design analysis (2 × 2 design): Our data consists of a balanced 2 × 2 design with 4 experimental conditions: APre, APost, BPre, and BPost. We modeled the data in a 3-level hierarchy, using the PEB-of-PEBs approach72 with 3 levels: (1) session level (APre, APost, BPre, and BPost), (2) treatment level (APre vs APost and BPre vs BPost), and (3) group level (ALT vs BLT). Specifically, at the first level, we inverted DCMs for each of the 4 sessions for each subject. These connectivity parameters were modeled at the second (within condition ie, pre vs post) level with (Bayesian) GLM, with regressors modeling mean connectivity and the differences where a PEB model is created for each condition (ALT and BLT) separately. Finally, the second-level parameters from each condition were modeled at the third level in a GLM with regressors representing commonalities and differences across the 2 conditions (ALT vs BLT).
In brief, following 4 parameters are calculated for every connection within the network:
(i) The overall mean connectivity (ie, mean of the 2 group’s within subject means)
(ii) The main effect of treatment (ie, mean of the 2 group’s treatment effects)
(iii) The main effect of group (ie, difference in the group’s means)
(iv) The interaction of group and treatment (ie, difference in the 2 group’s treatment effects)
Correction for multiple comparisons: All the statistical testing is performed using PEB framework (which is a multivariate approach), hence there is only 1 test per PEB model. In order to focus on a smaller subset of connections, we used a very strict threshold of Pp > 0.95. However, it is not a test in itself but rather only a threshold. Although we are showing several group comparisons, they all represent various contrasts in a single design matrix. Hence only 1 multivariate statistical test (based on PEB) is used so there is no need for correction for multiple comparisons.
Diffusion-weighted image (DWI) processing: Fractional and quantitative anisotropy (FA/QA) analyses
For head-motion correction, standard eddy current correction (ECC) was performed on diffusion-weighted data using the FMRIB Software Library v6.0 software package (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). The motion-corrected data were exported to DSI Studio (http://dsi-studio.labsolver.org). DSI Studio converts the data into SRC format, which stores the DWI volumes, image dimensions, voxel size, and b-table. Each SRC file underwent thorough examination using quality control (QC) procedures to ensure the quality and integrity of data in terms of consistency across image dimensions, image resolution, DWI count, and neighboring DWI correlation (NDC). NDC represents the correlation coefficient of low-b diffusion volumes, which have similar gradient directions. Succinctly, DSI Studio computes a voxel-wise correlation coefficient between every 2 DWIs of closest b-vector (multiplied by b-value), and NDC represents the average of those coefficients. Here, higher NDC values represent good data quality, and vice-versa. NDC values for the current data set were greater than 0.95. None of the NDC values was identified as outlier (ie, with a value greater than 3 times the mean).
For estimating the structural connectivity in terms of FA and QA for the white-matter fiber tracts connecting the regions that showed an improvement in EC from pre-treatment to post-treatment, the diffusion MRI connectometry technique was implemented.73 This technique, implemented in DSI Studio, was used to extract FA and QA values from each subject for each group. Here an FA and QA component database was created in normalized standard space (HCP842 template for young adults) using the Q-space diffeomorphic reconstruction (QSDR)74 approach. QSDR is a model free approach that calculates the distribution of water diffusion using a high-resolution standard brain atlas in the ICBM-152 space. Automated registration to standard space was used for each subject. All the ROIs were dilated by 5 mm to extend to white matter. A seed count of 50 000 sub voxels (randomized) for each region was used for connectometry analysis. The default direction interpolation (trilinear) method and default-tracking algorithm (streamline) were used to perform tractography. To limit the tracts between specific regions, a length threshold between 50 and 200 mm, an angle threshold of 70°, differential tracking threshold of 0.30, and a default Otsu threshold of 0.60 were applied. Tract pruning was conducted using a single iteration.
Group differences in DTI parameters and HAP, and associations between estimated EC, FA, QA, and HAP
FA, QA, and HAP parameters were compared using paired sample t-tests. Subject-wise connectivity values for the identified connections, which showed an improvement in EC strength across a treatment, were extracted for further analysis. Pearson’s correlation analyses were conducted between characteristics of interest (ie, EC, FA, and QA for identified connections, and HAP) following treatment (ie, both APost and BPost) and age, sex, TSI, number of days the light device was used, and chronotype of participants that is, scores from MEQ) to identify potential covariates. Partial correlation analyses were conducted between differences (post-treatment vs pre-treatment) in EC and HAP, FA and HAP, QA and HAP, EC and FA, and EC and QA for the identified connections. Variables that showed significant associations with characteristics of interest (ie, either EC, FA, QA, or HAP) were included as covariates in above partial correlation analyses.
Results
Demographics
HC and mTBI groups differed significantly in age [independent sample t-test: t (67) = 4.10, P < .001], but not sex [Chi-square: χ2(1) = 0.37, P = .54]. Independent sample t-test/chi-square tests showed that the BLT and ALT groups did not differ significantly in age [t (26) = −0.40, P = .69], sex [χ2(1) = 0.14, P = .70], TSI [t (26) = 0.35, P = .73], number of days light used [t (26) = −0.75, P = .46] within 2 hours of awakening, chronotype [t (26) = −0.21, P = .84], or baseline levels of happiness [t (26) = −1.16, P = .26]; see Table 1.
Adherence to light treatment
The participants in the BLT group reported using their light during 90.78% (SD = 7.24%) of the possible treatment days, while the participants in the ALT group reported 85.34% (SD = 13.07%) use of the light, which was not significantly different between groups, t (20.29) = 1.36, P = .19.
EC within the DMN
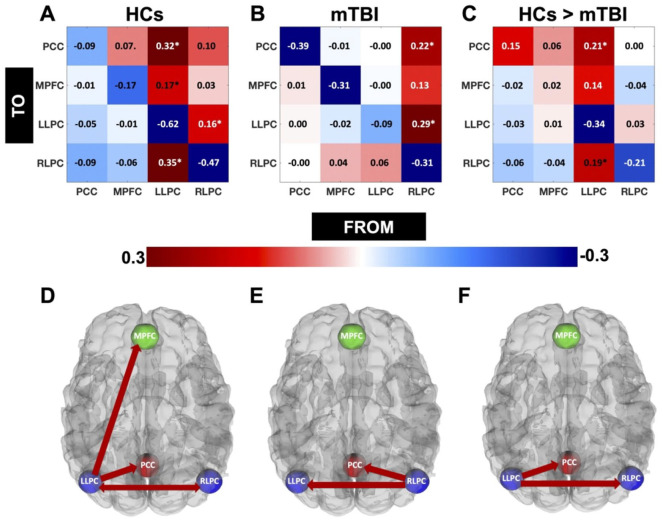
HCs and mTBI (pre-treatment): In Figure 1(A and B), the data show the subject average EC strength (in Hz) of all the connections within the DMN for HCs (A) and mTBI (B). In Figure 1C, the data show the strength of the connections which are stronger in HCs as compared to mTBI. Positive and negative values in Figure 1A and B represent the excitatory and inhibitory connections respectively. The positive values for Figure 1C represent the connections that are stronger in HCs than the mTBI group, whereas the negative values here represent the connections that are weaker in HCs than the mTBI group. Connections exceeding the Pp of 95% are indicated by “*” in Figure 1(A–C), and are shown in Figure 1D (HCs), 1E (mTBI), and 1F (HCs > mTBI). Here, we noticed that the network pattern for HCs was dominant within the left hemisphere, particularly involving the LLPC (Figure 1A and D), and for mTBI sample, it was dominant within the right hemisphere, particularly involving the RLPC (Figure 1B and E). Both of these regions (LLPC for HCs and RLPC for mTBI) acted as “sources” indicating out-going information flow. A direct comparison showed that the connections from LLPC to PCC and RLPC (ie, LLPC acted as a source indicating out-going information flow to PCC and RLPC) were stronger in HCs compared to mTBI sample (Figure 1C and F).
Figure 1.
EC for HCs and individuals with mTBI. Here we showed the subject average EC strength (in Hz) of all the connections within the DMN for HCs (A) and post mTBI (B). Positive and negative values here represent the exhibitory and inhibitory connections respectively. In Figure 1C, we showed the comparisons of EC strengths between HCs and post mTBI. The positive values here represent the connections that are stronger in HCs than post mTBI, whereas the negative values here represent the connections that are weaker in HCs than post mTBI. Connections exceeding the Pp of 95% are indicated by “*” in Figure 1(A to C), and are shown in Figure 1D (HCs), 1E (mTBI), and 1F (HCs > mTBI).
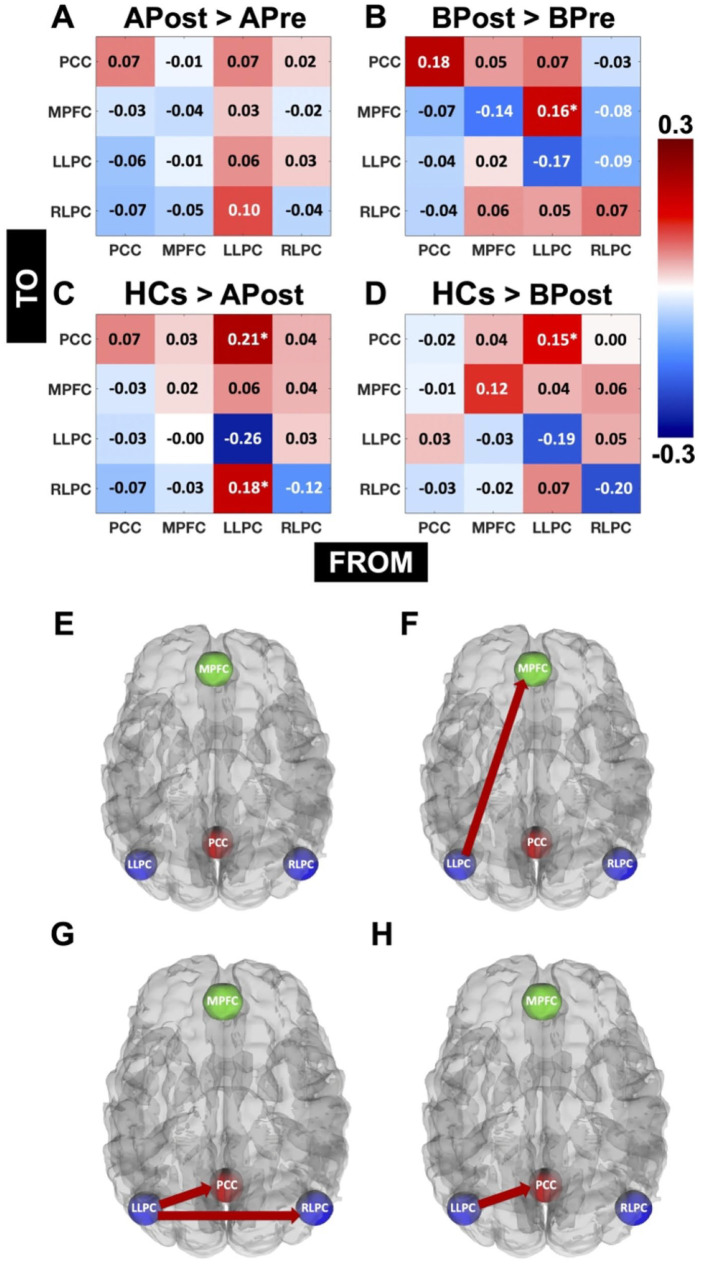
APost versus APre, BPost versus BPre, and HCs versus APost and BPost: In Figure 2(A–D), the data show the comparisons of EC strengths between APost and APre (A), BPost and BPre (B), HCs and APost (C), and HCs and BPost (D). The positive values in Figure 2A and B represent the connections that are stronger in APost and BPost than APre and BPre respectively, whereas the negative values here represent the connections that are weaker in APost and BPost than APre and BPre respectively. Similarly, the positive values for Figure 2C and D represent the connections that are stronger in HCs than APost and BPost respectively, whereas the negative values here represent the connections that are weaker in HCs than APost and BPost respectively. Connections exceeding the Pp of 95% are indicated by “*” in Figure 2 (A–D), and are shown in Figure 2E (APost > APre), F (BPost > BPre), G (HCs > APost), and H (HCs > BPost). Here, as expected, for individuals who underwent amber-light placebo therapy, the network pattern did not show any difference between post-treatment condition compared to pre-treatment at a Pp of 95%. Interestingly, for individuals who underwent blue-light therapy, the connectivity strength from LLPC to MPFC was greater for the post-treatment condition compared to pre-treatment at a Pp greater than 95%, that is, LLPC acted as a “source” to deliver information to MPFC. In addition, the information flow from LLPC to other regions, including PCC and RLPC was still greater for HCs than mTBI individuals who underwent amber-light placebo therapy. However, it was only 1 connection that is, from LLPC to PCC, which showed greater information flow in HCs than mTBI individuals who underwent blue-light therapy.
Figure 2.
Comparisons of EC for APost versus APre, BPost versus BPre, and HCs versus APost and BPost: Here we showed the comparisons of EC strengths between APost and APre (A), BPost and BPre (B), HCs and APost (C), and HCs and BPost (D). The positive values (A and B) represent the connections that are stronger in APost and BPost than APre and BPre respectively, whereas the negative values here represent the connections that are weaker in APost and BPost than APre and BPre respectively. Similarly, the positive values for Figure 2C and D represent the connections that are stronger in HCs than APost and BPost respectively, whereas the negative values here represent the connections that are weaker in HCs than APost and BPost respectively. Connections exceeding the Pp of 95% are indicated by “*” (A–D) and are shown in Figures 2E (APost > APre), F (BPost > BPre), G (HCs > APost), and H (HCs > BPost).
Hierarchical PEB analysis (first level: DCM, second and third levels: PEB): Next, we performed a hierarchical design analysis (2 × 2 design) using the PEB-of-PEBs approach. At a Pp greater than 95%, the BLT group did not show a difference in EC strength for any of the connections at pre-treatment (BPre) condition (ie, baseline) as compared to pre-treatment condition (ie, baseline) for ALT group (APre). Interestingly, at a Pp greater than 95%, there was group-treatment interaction, which showed greater EC strength from LLPC to PCC and RLPC to MPFC. These findings indicate that the impact of blue-light therapy in normalizing the DMN connectivity patterns are irrespective of any differences at the baseline.
FA, QA, and happiness
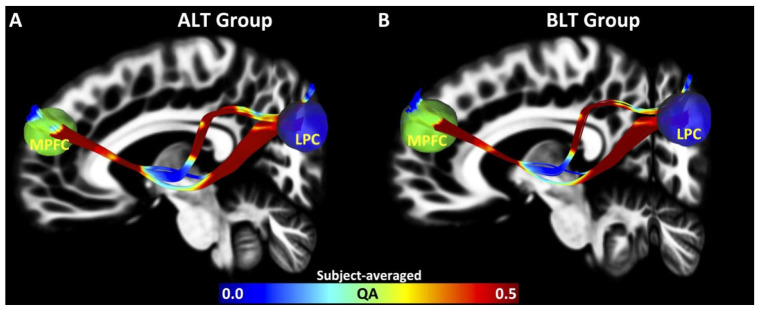
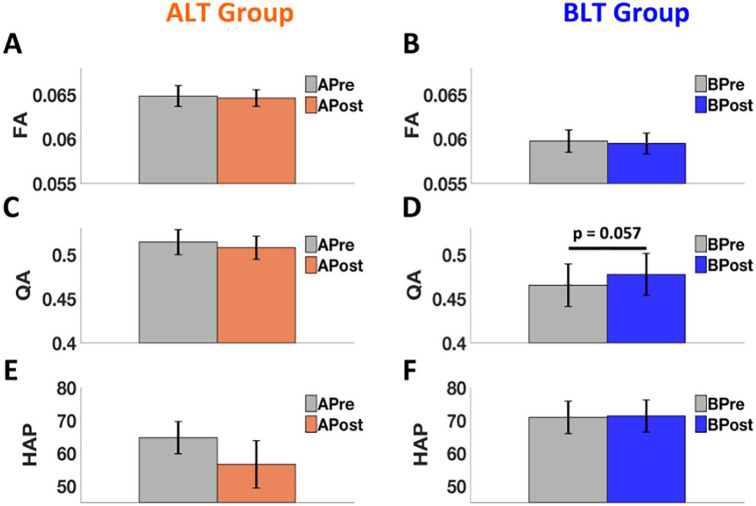
FA and QA: From EC analysis at Pp of 95%, it was observed that following amber-light placebo therapy, none of the connections showed an improvement in EC strength, whereas following blue-light therapy, 1 connection from LLPC to MPFC was improved and the overall connectivity pattern was almost normalized back to that observed in the HCs for BLT group. Therefore, the structural connectivity analysis was limited to the tracts connecting LLPC (seed region) and MPFC (region of interest). Pre-treatment subject averaged maps of structural connectivity (in terms of QA) between LLPC and MPFC are shown in Figure 3(A: ALT Group and B: BLT Group). The data showed that there was no significant difference in FA for tracts connecting LLPC and MPFC for either ALT group (APre vs APost) (paired t-test, t (12) = −0.31, P = .760) (Figure 4A) or BLT group (BPre vs BPost) (paired t-test, t (12) = −0.45, P = .662) (Figure 4B). There was no significant difference in QA for tracts connecting LLPC and MPFC for ALT group (APre vs APost) (paired t-test, t (12) = −0.75, P = .468) (Figure 4C), but there was a clear trend in expected direction of greater QA for BPost compared to BPre (paired t-test, t (12) = 2.1, P = .057) (Figure 4D).
Figure 3.
White matter tractography for ALT and BLT groups. Here we showed subject averaged maps of structural connectivity (in terms of QA) between LLPC and MPFC (A: ALT Group and B: BLT Group).
Figure 4.
Comparisons of FA, QA, and levels of happiness (HAP). We found that there was no significant difference in FA for tracts connecting LLPC and MPFC for either ALT group (APre vs APost) (paired t-test, P = .760) (A) or BLT group (BPre vs BPost) (paired t-test, P = .662) (B). Also, there was no significant difference in QA for tracts connecting LLPC and MPFC for ALT group (APre vs APost) (paired t-test, P = .468) (C), but there was a clear trend of greater QA for BPost compared to BPre (paired t-test, P = .057) (D). Lastly, we found non-significant reduction in levels of happiness for individuals who used amber-light therapy compared to their pre-treatment condition (paired t-test, P = .170) (E). However, levels of happiness sustained for individuals who used blue-light therapy compared to their pre-treatment condition (paired t-test: P = .924) (F). Error bars here represent “standard error of the mean.”
Happiness: No significant change in levels of happiness (from 3.89 ± 1.06 [pre] to 3.40 ± 1.56 [post] on raw scale, or 64.74 ± 17.73 [pre] to 56.62 ± 26.07 [post] on relative percent scale) for individuals who used amber-light placebo therapy compared to their pre-treatment condition (paired t-test, t (12) = −1.46, P = .170) (Figure 4E) was found. Similarly, levels of happiness did not change significantly (from 4.26 ± 1.07 [pre] to 4.28 ± 1.06 [post] on raw scale, or 70.94 ± 17.81 [pre] to 71.37 ± 17.73 [post] on relative percent scale) for individuals who used blue-light therapy compared to their pre-treatment condition (paired t-test: t (12) = 0.1, P = .924) (Figure 4F). Error bars in Figure 4 represent “standard error of the mean.” Here, relative percent of happiness scale as shown in Figure 4(E and F) represents the average of the responses across the adjectives of each category relative to the maximum possible rating.75
Associations among changes in EC, FA, QA, and HAP
Identification of covariates: Pearson’s correlation analyses between variables of interest (ie, EC, FA, and QA for 1 identified connection: LLPC to MPFC, and HAP) and age, sex, TSI, number of days the light device was used, and chronotype of participants (ie, scores from MEQ) showed significant associations between (a) EC and age (r = .42, P ⩽ .05), (b) EC and MEQ scores (r = .38, P ⩽ .05), (c) FA and sex (r = −.40, P ⩽ .05), (d) QA and sex (r = −.61, P ⩽ .05); see Table 2. Therefore, age, MEQ, and sex were included as covariates for further analyses.
Table 2.
Identification of covariates.
| Variables | Age | Sex | TSI | Days light used | MEQ scores |
|---|---|---|---|---|---|
| EC | 0.42* | −0.18 | 0.27 | −0.18 | 0.38* |
| FA | −0.04 | −0.40* | 0.08 | −0.04 | 0.25 |
| QA | −0.18 | −0.61* | −0.02 | −0.08 | 0.02 |
| HAP | −0.04 | −0.20 | 0.10 | 0.14 | 0.04 |
Abbreviations: EC, effective connectivity; FA, fractional anisotropy; HAP, happiness scores; MEQ, morningness-eveningness questionnaire; QA, quantitative anisotropy; TSI, time since injury.
P ⩽ .05.
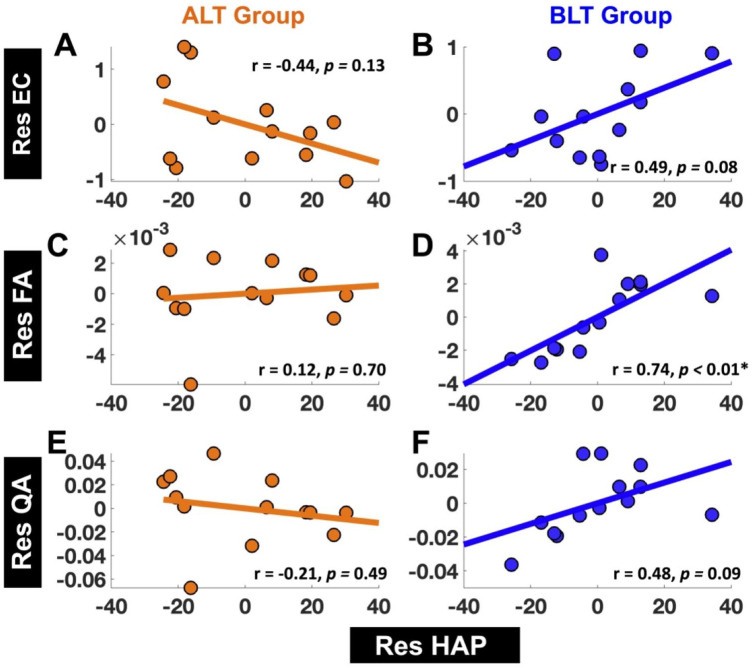
EC versus happiness: For ALT group, there was no significant association between residualized changes in EC (Res EC) from LLPC to MPFC and residualized changes in happiness (Res HAP) (r = −.44, 95% CI [−0.80 0.14]; P = .13) (Figure 5A). For the BLT group, there was a clear trend showing positive association between residualized changes in EC (Res EC) from LLPC to MPFC and residualized changes in happiness (Res HAP) (r = .49, 95% CI [−0.08 0.82]; P = .08) (Figure 5B). Correlation coefficients between “Res EC” and “Res HAP” were also significantly different between ALT and BLT groups (z = −2.25, P = .02).
Figure 5.
Associations between residualized changes (pre- to post-treatment) in happiness (Res HAP) versus residualized changes (pre-to post-treatment) in EC (Res EC), FA (Res FA), and QA (Res QA). We did not find significant association between residualized changes in happiness scores (Res HAP) and residualized changes in EC (Res EC) from LLPC to MPFC for ALT group (A), but there was a clear tend showing a positive association between Res HAP and Res EC from LLPC to MPFC for BLT group (B). There was no significant association between Res HAP and Res FA for tracts connecting LLPC and MPFC for ALT group (C), but there was significant positive association between Res HAP and Res FA for tracts connecting LLPC and MPFC for BLT group (D). Lastly, there was no significant association between Res HAP and Res QA for tracts connecting LLPC and MPFC for ALT group (E), but there was a clear trend showing a positive association between Res HAP and Res QA for tracts connecting LLPC and MPFC for BLT group (F).
FA versus happiness: For ALT group, there was no significant association between residualized changes in FA (Res FA) for tracts connecting LLPC and MPFC and residualized changes in happiness (Res HAP) (r = .12, 95% CI [−0.46 0.63], P = .70) (Figure 5C). For BLT group, there was significant positive association between residualized changes in FA (Res FA) for tracts connecting LLPC and MPFC and residualized changes in happiness (Res HAP) (r = .74, 95% CI [0.32 0.92], P < .01) (Figure 5D). Correlation coefficients between “Res FA” and “Res HAP” were different (at a trend level) between ALT and BLT groups (z = −1.86, P = .06).
QA versus happiness: For ALT group there was no significant association between residualized changes in QA (Res QA) for tracts connecting LLPC and MPFC and residualized changes in happiness (Res HAP) (r = −.21, 95% CI [−0.68 0.39], P = .49) (Figure 5E). For BLT group, there was a clear trend showing a positive association between residualized changes in QA (Res QA) for tracts connecting LLPC and MPFC and residualized changes in happiness (Res HAP) (r = .48, 95% CI [−0.09 0.82], P = .09) (Figure 5F).
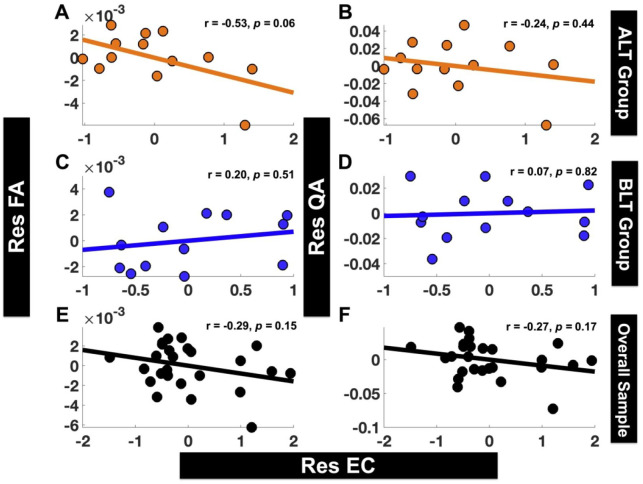
EC versus FA and QA: Correlation analyses were conducted between residualized changes in EC (Res EC) from LLPC to MPFC and residualized changes in structural brain connectivity (Res FA for FA and Res QA for QA) for the tracts connecting LLPC and MPFC. For ALT group, there was an association (at a trend level) between “Res EC” and “Res FA” (r = −.53, 95% CI [−0.83 0.03], P = .06) (Figure 6A), but not between “Res EC” and “Res QA” (r = −.24, 95% CI [−0.70 0.36], P = .44) (Figure 6B). For BLT group, there was no association between either “Res EC” and “Res FA” (r = .20, 95% CI [−0.39 0.68], P = .51) (Figure 6C), or between “Res EC” and “Res QA” (r = .07, 95% CI [−0.50 0.60], P = .82) (Figure 6D). The overall sample (combined ALT and BLT groups) also did not show significant association between “Res EC” and either “Res FA” or “Res QA” (Res EC vs Res FA: r = −.29, 95% CI [−0.61 0.11], P = .15 (Figure 6E); Res EC vs Res QA: r = −.27, 95% CI [−0.60 0.12], P = .17 (Figure 6F)).
Figure 6.
Associations between residualized changes (post- to pre-treatment) in EC (Res EC) versus residualized changes (post- to pre-treatment) in FA (Res FA) and QA (Res QA). Neither of the groups ALT (A and B) or BLT (C and D) showed significant association between Res EC from LLPC to MPFC and Res FA (A, C) or Res QA (B, D) for tracts connecting LLPC and MPFC. Overall sample also did not show significant association between Res EC from LLPC to MPFC and either Res FA (E) or Res QA (F) for tracts connecting LLPC and MPFC.
Discussion
The current findings suggest that relative to healthy-controls, there are identifiable group-level differences in EC within the DMN among individuals recovering from a recent mTBI. We found an improvement over the course of treatment for EC within the DMN, particularly from LLPC to MPFC, for those receiving blue-light therapy, and normalizing the overall connectivity pattern back to that seen in healthy controls. This pattern was not observed for the placebo group. Our next observation was that within the BLT group, individuals with mTBI also had greater (at a trend level) white-matter compactness following blue-light therapy compared to baseline. Finally, changes in the strength of EC from LLPC to MPFC (at trend level) and both FA (highly significant) and QA (at trend level) from pre to post treatment were associated with greater levels of happiness for the BLT group, but not ALT group. These findings are discussed in greater detail below.
Amber light as a control condition
Amber light may have some positive or negative photo-biomodulation effects. The issue of the most appropriate control condition was one to which we have given considerable thought. We concluded that there is no single ideal control, since light comes in many wavelengths, each with potential biological and psychological effects, and the absence of light is no different. There are arguments that could be made for using a no-intervention control, but our pilot work and work with other conditions (eg, PTSD) suggested that participants are significantly affected by the regimen of treatment and the psychological effects of using a light device (ie, simply using a device that emits light at the same time each day seems to be psychologically beneficial for some people, regardless of the wavelength). Thus, while we would likely have found stronger differences between groups with a no-intervention condition, our aim here was to demonstrate the specific effect of what we believe to be the critical wavelength (ie, blue) relative to other less effective wavelengths. At this point, this seemed to be the most appropriate control to demonstrate the efficacy of blue light relative to other similar conditions.76 Further, since this wavelength has been used in a number of our prior investigations,17,23,77–81 it appeared to be the most appropriate starting point. Nonetheless, additional control conditions, including a no-exposure condition, will be critical to fully understanding the effects of light on EC.
EC within the DMN for HCs and post-mTBI
In comparison to HCs, post-mTBI participants had weaker EC between several regions within the DMN. These findings extend previous reports published on functional brain connectivity following mTBI including weaker functional connectivity within the DMN involving the posterior cingulate cortex and parietal areas following mTBI.30 Decreased cortical volume as well as gray matter atrophy within the posterior regions of the brain have often been reported among individuals with mTBI.82,83 We observed that EC strength within the left hemisphere involving the PCC and the parietal cortex was weaker following mTBI, whereas EC strength within the right hemisphere among these same regions was stronger following mTBI. These findings suggest that following a concussion, the DMN may reorganize the affected connectivity patterns. The independent components involving distinct connectivity patterns within each hemisphere may play important roles in compensating for potential deficits following an mTBI. Previous brain connectivity studies, although not specific to mTBI, support this notion and suggest that the damaged brain connectivity within the affected hemisphere could be compensated for by the expression of stronger brain connectivity within the less affected hemisphere.84,85 In the current study, stronger EC for the mTBI group (prior to treatment), particularly in the hemisphere contralateral to the one where stronger connectivity was observed for HCs, may indicate compensatory reorganization of the DMN following injury. However, it should be noted that there might be some inconsistencies in overall DMN EC patterns in the current study compared to previously published data due to differences in sample size and sample populations.86,87
Effect of light-therapy on happiness, EC and white-matter characteristics
Happiness
First, it should be noted in this study that the happiness data were not available within the HCs sample so a direct comparison between HCs and individuals with mTBI regarding scores on happiness scale was not possible. However, established normative data for the ANAM4, collected from more than 100 000 active duty service members ranging from 17 to 65 years of age, suggests that the average relative percent score on the happiness scale for the mTBI group at baseline reported in this study (67.66 ± 17.37) was within the normal range of average scores between 64.4 ± 21.6 and 68.2 ± 21.2 collected from de-identified service members aged between 17 and 35.75 Therefore, non-significant changes in happiness levels following either therapy could be due to the normal levels of happiness in mTBI individuals at the baseline.
EC and QA
BLT showed an improvement in both effective and structural connectivity patterns within the DMN. Emerging evidence suggests that blue light exposure can have a wide range of effects on cognition88 as well as on functional and structural neural characteristics.23,89 It remains a question of interest as to why blue-light exposure would have a positive effect on effective and associated structural connectivity within the DMN. Significant additional work will be necessary to fully determine the underlying mechanisms of observed improvements following morning exposure to blue light. However, the following proposed theories indirectly explain the underlying mechanisms responsible for the observed changes in functional and structural neural characteristics following BLT for post-mTBI. The strongest evidence for the role of blue light involves its effects on sleep and circadian systems. Notably, dysfunctions in sleep patterns are among the most common problems observed in individuals with traumatic brain injury,90–94 and it has been reported that morning exposure to blue-light can lead to improved circadian timing and greater daytime alertness resulting in overall improvements in sleep quality in various groups.88,95,96 Among those with brain injury, sleep may modulate the mechanisms underlying the proliferation of oligodendrocyte precursor cells (which are critical for the repair of the myelin sheath surrounding axons),97 as well as the removal of neurotoxins that accumulate during waking hours via the glymphatic system.98 Blue-light may also cause a phase shift in the circadian timing of sleep onset by suppressing melatonin production from the pineal gland.99 The phase shift in the circadian timing of sleep onset may further lead to an earlier and more regular bedtime and wake time, allowing the individual to capitalize on sleep during the dark period of the night, when sleep is likely to be most restorative. This phenomenon may also play an important role in modulating neural repair processes following an mTBI. Our prior published work on the same sample that is used in the current study provides clear evidence of improvements in sleep quality and circadian phase shifting following morning BLT.12,17,23,78,100 Therefore, it is highly likely that the observed improvements from BLT within the DMN in the current study may be one of the consequences of improved sleep.
This is the first study demonstrating the effects of BLT on EC in conjunction with white-matter characteristics within the DMN, and its associated effects on mood. The focus here was to go beyond reestablishing the underlying mechanisms of how blue light induces benefits via sleep. Rather, we focused on implementing cutting-edge brain connectivity methods to quantify the impact of BLT on effective and structural connectivity parameters of the DMN. It would be interesting for future studies to simultaneously analyze the improvements in sleep and improvements in both effective and structural connectivity parameters following BLT.
Association between happiness and DMN EC
No direct difference between the light groups in mood scores was detected. Positive associations between residualized change in scores on the happiness scale and both residualized change in EC strength (ie, from the left lateral parietal cortex to the medial prefrontal cortex) as well as measures of white-matter integrity (FA) and compactness (QA) (ie, for white-matter fiber tracts between the same 2 regions) following BLT, but not following ALT, were found. In contrast, prior studies reported a negative association between DMN functional connectivity and happiness in healthy controls. In particular, Luo and colleagues reported that compared to happy individuals, individuals with lower levels of happiness had stronger functional connectivity within the DMN.24 Taruffi and colleagues found higher scores on a mind-wandering scale during sad music as compared to happy music, as well as to happy but slow music compared to happy fast music.26 In a web-app based study of 2250 participants, it was found that the mind-wandering phenomenon (a core phenomenon underlined by the DMN) tends to be most associated with feelings of unhappiness.62 It should be noted that all of these studies established the association between DMN (or mind wandering) and levels of happiness only in healthy-controls or in general, whereas the findings of the current study show the association between the 2 following a treatment for individuals recovering from an mTBI. Therefore, these findings do not contradict the previous literature; rather our results suggest that for individuals recovering from an mTBI, the improvements in EC within the DMN following blue-light therapy are associated with improvement in self-rated feelings of happiness.
Association between changes in EC, and changes in FA and QA
Contrary to our hypothesis, there was no association between residualized changes in EC and residualized changes in FA or QA. However, each of these parameters (ie, changes in EC, FA, and QA) hold an association (mostly at trend level) with changes in scores on the happiness scale. Previously, Greicius et al reported that resting state functional connectivity within the DMN reflects the underlying anatomical connectivity to a large degree.43 Greicius et al described that functional connectivity could exist in spite of the absence of direct structural connections, although the existence of anatomical connectivity in absence of functional connectivity is relatively less plausible, suggesting that overall both functional connectivity and anatomical connectivity can exist independently. These current findings are further supported by the fact that anatomical connectivity constrains, rather than determines, EC, because changes in EC depend on recent functional changes or transmission of neuronal signals within a synapse,101 even in the absence of associated structural change.102 Stephan et al suggested that due to the dependence of neuronal signal transmission on temporal components resulting from several mechanisms including membrane potential and opening/closing of ion gated channels, anatomical connectivity may not be engaged during a specific directed connection or signal flow.102 In sum, absence of association between functional (ie, EC) and diffusion-weighted (ie, FA and QA) substrates may indicate distinct independent neural mechanisms underlying significant changes in EC and white-matter characteristics (FA or QA) following blue-light therapy.
Limitations
Findings from this study should be viewed in light of several limitations. First, the changes in EC following an mTBI reported in this study might not be generalizable across heterogeneous mTBI profiles, because every mTBI may represent a unique injury and it is highly unlikely that specific connectivity values would reflect changes common to most mTBIs. Replication will be required to determine the stability of the patterns observed in the current study. Second, despite the non-significant sex differences, the HCs sample was fairly heterogeneous with regard to sex ratio (25 females and 16 males) relative to mTBI sample (15 females and 13 males), and it is possible that EC patterns may differ between groups due to the heterogeneity within HCs sample. However, to rule out this possibility, we statistically controlled heterogeneity related to sex ratio by using “sex” as a covariate. Third, the sample sizes of both treatment groups were relatively small, which may have yielded some findings which only reached a trend level of significance. Therefore, this study should be considered preliminary, and a replication with a larger sample would be beneficial in future studies. However, despite the small sample sizes for the treatment conditions, we found that BLT, not ALT, appears to strengthen EC within the DMN. Lastly, this study focused on only the DMN and happiness as the only measure of mood. We focused on happiness as it is one of the most important mood scales that is known to be associated with DMN. Future studies should make use of large-scale DCM technique51 to explore the impact of light therapy on other functional brain networks and additional mood scales.
Conclusions
The current results demonstrate that the DMN is susceptible to mild head injury. Moreover, empirical findings suggest that 6 weeks of morning blue-light therapy produce stronger EC as well as greater white-matter compactness within the DMN, especially between the lateral parietal and medial prefrontal regions, and sustained levels of happiness. However, the neural mechanisms causing the underlying associations between changes in functional/structural connectivity patterns and the changes in mood could be independent, as evidenced by non-significant associations between changes in EC and structural parameters. In sum, the present preliminary findings suggest that short-wavelength light therapy could be used as a novel alternative rehabilitation technique that can potentially strengthen the functional and structural pathways within the DMN.
Footnotes
Funding:The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from the U.S. Army Medical Research and Development Command to WDSK (W81XWH-11-1-0056 and W81XWH-09-1-0730). Opinions, interpretations, conclusions, and recommendations in this study are those of the authors and are not necessarily endorsed by the Department of Defense (DoD). AR is funded by the Australian Research Council Discovery Early Career Research Award Fellowship (DE170100128) and the Wellcome Trust.
Declaration of conflicting interests:The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SB analyzed the data and wrote the initial draft. ACR contributed to the statistical analysis and writing of the initial draft. AR contributed to spectral DCM analysis and contributed to the writing of the manuscript. MAM contributed to overall data analysis and writing of the manuscript. WDSK designed the study, obtained the funding, supervised all aspects of the study, and contributed to writing of the manuscript.
ORCID iD: Sahil Bajaj  https://orcid.org/0000-0003-0629-6036
https://orcid.org/0000-0003-0629-6036
References
- 1. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258. [DOI] [PubMed] [Google Scholar]
- 2. Blyth BJ, Bazarian JJ. Traumatic alterations in consciousness: traumatic brain injury. Emerg Med Clin North Am. 2010;28:571-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS One. 2017;12:e0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su E, Bell M. Diffuse axonal injury. In: Laskowitz D, Grant G, eds. Translational Research in Traumatic Brain Injury. Boca Raton, FL: CRC Press/Taylor and Francis Group; 2016. [Google Scholar]
- 5. Narayana PA. White matter changes in patients with mild traumatic brain injury: MRI perspective. Concussion. 2017;2:CNC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eierud C, Craddock RC, Fletcher S, et al. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 2014;4:283-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37:13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emery CA, Barlow KM, Brooks BL, et al. A systematic review of psychiatric, psychological, and behavioural outcomes following mild traumatic brain injury in children and adolescents. Can J Psychiatry Revue Can Psychiatr. 2016;61:259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malojcic B, Mubrin Z, Coric B, Susnic M, Spilich GJ. Consequences of mild traumatic brain injury on information processing assessed with attention and short-term memory tasks. J Neurotrauma. 2008;25:30-37. [DOI] [PubMed] [Google Scholar]
- 10. Barman A, Chatterjee A, Bhide R. Cognitive impairment and rehabilitation strategies after traumatic brain injury. Indian J Psychol Med. 2016;38:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exp Neuropsychol. 2005;27:977-1021. [DOI] [PubMed] [Google Scholar]
- 12. Raikes AC, Bajaj S, Dailey NS, et al. Diffusion Tensor Imaging (DTI) correlates of self-reported sleep quality and depression following mild traumatic brain injury. Front Neurol. 2018;9:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parcell DL, Ponsford JL, Rajaratnam SM, Redman JR. Self-reported changes to nighttime sleep after traumatic brain injury. Arch Phys Med Rehabil. 2006;87:278-285. [DOI] [PubMed] [Google Scholar]
- 14. Wickwire EM, Williams SG, Roth T, et al. Sleep, sleep disorders, and mild traumatic brain injury. what we know and what we need to know: findings from a National Working Group. Neurotherapeutics. 2016;13:403-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grima N, Ponsford J, Rajaratnam SM, Mansfield D, Pase MP. Sleep disturbances in traumatic brain injury: a meta-analysis. J Clin Sleep Med. 2016;12:419-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ponsford JL, Ziino C, Parcell DL, et al. Fatigue and sleep disturbance following traumatic brain injury—their nature, causes, and potential treatments. J Head Trauma Rehabil. 2012;27:224-233. [DOI] [PubMed] [Google Scholar]
- 17. Killgore WDS, Vanuk JR, Shane BR, Weber M, Bajaj S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol Dis. 2020;134:104679. [DOI] [PubMed] [Google Scholar]
- 18. Sinclair KL, Ponsford JL, Taffe J, Lockley SW, Rajaratnam SM. Randomized controlled trial of light therapy for fatigue following traumatic brain injury. Neurorehabil Neural Repair. 2014;28:303-313. [DOI] [PubMed] [Google Scholar]
- 19. Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: Increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070-1073. [DOI] [PubMed] [Google Scholar]
- 21. Raikes AC, Killgore WD. Potential for the development of light therapies in mild traumatic brain injury. Concussion. 2018;3:CNC57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol Psychiatry. 2006;59:502-507. [DOI] [PubMed] [Google Scholar]
- 23. Bajaj S, Vanuk JR, Smith R, Dailey NS, Killgore WDS. Blue-light therapy following mild traumatic brain injury: effects on white matter water diffusion in the brain. Front Neurol. 2017;8:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo Y, Kong F, Qi S, et al. Resting-state functional connectivity of the default mode network associated with happiness. Soc Cogn Affect Neurosci. 2016;11:516-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo Y, Huang X, Yang Z, Li B, Liu J, Wei D. Regional homogeneity of intrinsic brain activity in happy and unhappy individuals. PLoS One. 2014;9:e85181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taruffi L, Pehrs C, Skouras S, Koelsch S. Effects of sad and happy music on mind-wandering and the default mode network. Sci Rep. 2017;7:14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. sueroa CA, Mocking RJT, van Wingen G, Martens S, Ruhé HG, Schene AH. Aberrant default-mode network-hippocampus connectivity after sad memory-recall in remitted-depression. Soc Cogn Affect Neurosci. 2017;12:1803-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Satpute AB, Lindquist KA. The default mode network’s role in discrete emotion. Trends Cogn Sci. 2019;23:851-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou Y, Milham MP, Lui YW, et al. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265:882-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iraji A, Benson RR, Welch RD, et al. Resting state functional connectivity in mild traumatic brain injury at the acute stage: independent component and seed-based analyses. J Neurotrauma. 2015;32:1031-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alhourani A, Wozny TA, Krishnaswamy D, et al. Magnetoencephalography-based identification of functional connectivity network disruption following mild traumatic brain injury. J Neurophysiol. 2016;116:1840-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Santhanam P, Wilson SH, Oakes TR, Weaver LK. Effects of mild traumatic brain injury and post-traumatic stress disorder on resting-state default mode network connectivity. Brain Res. 2019;1711:77-82. [DOI] [PubMed] [Google Scholar]
- 34. Dixon ML, Thiruchselvam R, Todd R, Christoff K. Emotion and the prefrontal cortex: an integrative review. Psychol Bull. 2017;143:1033-1081. [DOI] [PubMed] [Google Scholar]
- 35. Dailey NS, Smith R, Bajaj S, et al. Elevated aggression and reduced white matter integrity in mild traumatic brain injury: a DTI study. Front Behav Neurosci. 2018;12:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil. 1998;13:24-39. [DOI] [PubMed] [Google Scholar]
- 37. Strong RE, Marchant BK, Reimherr FW, et al. Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress Anxiety. 2009;26:273-278. [DOI] [PubMed] [Google Scholar]
- 38. Holzman DC. What’s in a color? The unique human health effect of blue light. Env Heal Perspect. 2010;118:A22–A27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68:61–70. [DOI] [PubMed] [Google Scholar]
- 40. Killgore WDS. Lightening the mood: evidence for blue light exposure in the treatment of post-concussion depression. Expert Rev Neurother. 2020;20:1081-1083. [DOI] [PubMed] [Google Scholar]
- 41. Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of brain resting-state networks by sad mood induction. PLoS One. 2008;3:e1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soares JM, Marques P, Magalhaes R, Santos NC, Sousa N. The association between stress and mood across the adult lifespan on default mode network. Brain Struct Funct. 2017;222:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang HQ, Ding MZ. Linking functional connectivity and structural connectivity quantitatively: a comparison of methods. Brain Connect. 2016;6:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zimmermann J, Griffiths JD, McIntosh AR. Unique mapping of structural and functional connectivity on cognition. J Neurosci. 2018;38:9658-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13-36. [DOI] [PubMed] [Google Scholar]
- 47. Yeh FC, Verstynen TD, Wang Y, et al. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8:e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Friston KJ, Kahan J, Biswal B, Razi A. A DCM for resting state fMRI. Neuroimage. 2014;94:396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Friston KJ, Litvak V, Oswal A, et al. Bayesian model reduction and empirical Bayes for group (DCM) studies. Neuroimage. 2016;128:413-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273-1302. [DOI] [PubMed] [Google Scholar]
- 51. Razi A, Seghier ML, Zhou Y, et al. Large-scale DCMs for resting-state fMRI. Netw Neurosci. 2017;1:222-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bajaj S, Adhikari BM, Friston KJ, Dhamala M. Bridging the gap: dynamic causal modeling and granger causality analysis of resting state functional magnetic resonance imaging. Brain Connect. 2016;6:652-661. [DOI] [PubMed] [Google Scholar]
- 53. Bajaj S, Lamichhane B, Adhikari BM, Dhamala M. Amygdala mediated connectivity in perceptual decision-making of emotional facial expressions. Brain Connect. 2013;3:386-397. [DOI] [PubMed] [Google Scholar]
- 54. Bajaj S, Butler AJ, Drake D, Dhamala M. Brain effective connectivity during motor-imagery and execution following stroke and rehabilitation. NeuroImage Clin. 2015;8:572-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yeh FC, Vettel JM, Singh A, et al. quantifying differences and similarities in whole-brain white matter architecture using local connectome fingerprints. PLoS Comput Biol. 2016;12:e1005203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev. 2009;46:1-68.19533516 [Google Scholar]
- 57. Bajaj S, Alkozei A, Dailey NS, Killgore WDS. Brain aging: uncovering cortical characteristics of healthy aging in young adults. Front Aging Neurosci. 2017;9:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smith R, Bajaj S, Dailey NS, et al. Greater cortical thickness within the limbic visceromotor network predicts higher levels of trait emotional awareness. Conscious Cogn. 2018;57:54-61. [DOI] [PubMed] [Google Scholar]
- 59. Bajaj S, Raikes AC, Smith R, Vanuk JR, Killgore WDS. The role of prefrontal cortical surface area and volume in preclinical suicidal ideation in a non-clinical sample. Front Psychiatry. 2019;10:54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bajaj S, Killgore WDS. Sex differences in limbic network and risk-taking propensity in healthy individuals. J Neurosci Res. 2020;98:371-383. [DOI] [PubMed] [Google Scholar]
- 61. Bajaj S, Raikes A, Smith R, et al. The relationship between general intelligence and cortical structure in healthy individuals. Neuroscience. 2018;388:36-44. [DOI] [PubMed] [Google Scholar]
- 62. Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. [DOI] [PubMed] [Google Scholar]
- 63. Ibarra S. Automated neuropsychological assessment metrics. In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of clinical neuropsychology. New York, NY: Springer; 2011. [Google Scholar]
- 64. Johnson DR, Vincent AS, Johnson AE, Gilliland K, Schlegel RE. Reliability and construct validity of the Automated Neuropsychological Assessment Metrics (ANAM) mood scale. Arch Clin Neuropsychol. 2008;23:73-85. [DOI] [PubMed] [Google Scholar]
- 65. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97-110. [PubMed] [Google Scholar]
- 66. Raichle ME. The restless brain. Brain Connect. 2011;1:3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Almgren H, Van de Steen F, Kühn S, Razi A, Friston K, Marinazzo D. Variability and reliability of effective connectivity within the core default mode network: a multi-site longitudinal spectral DCM study. Neuroimage. 2018;183:757-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zeidman P, Jafarian A, Seghier ML, et al. A guide to group effective connectivity analysis, part 2: second level analysis with PEB. Neuroimage. 2019;200:12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zeidman P, Jafarian A, Corbin N, et al. A guide to group effective connectivity analysis, part 1: first level analysis with DCM for fMRI. Neuroimage. 2019;200:174-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. Neuroimage. 2009;46:1004-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Penny WD, Stephan KE, Daunizeau J, et al. Comparing families of dynamic causal models. PLoS Comput Biol. 2010;6:e1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Park HJ, Pae C, Friston K, et al. Hierarchical dynamic causal modeling of resting-state fMRI reveals longitudinal changes in effective connectivity in the motor system after thalamotomy for essential tremor. Front Neurol. 2017;8:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yeh FC, Badre D, Verstynen T. Connectometry: a statistical approach harnessing the analytical potential of the local connectome. Neuroimage. 2016;125:162-171. [DOI] [PubMed] [Google Scholar]
- 74. Yeh FC, Tseng WYI. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage. 2011;58:91-99. [DOI] [PubMed] [Google Scholar]
- 75. Vincent AS, Roebuck-Spencer T, Gilliland K, Schlegel R. Automated neuropsychological assessment metrics (v4) traumatic brain injury battery: military normative data. Mil Med. 2012;177:256-269. [DOI] [PubMed] [Google Scholar]
- 76. Gupta U, Verma M. Placebo in clinical trials. Perspect Clin Res. 2013;4:49-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Geerdink M, Walbeek TJ, Beersma DGM, Hommes V, Gordijn MCM. Short blue light pulses (30 Min) in the morning support a sleep-advancing protocol in a home setting. J Biol Rhythms. 2016;31:483-497. [DOI] [PubMed] [Google Scholar]
- 78. Raikes AC, Dailey NS, Shane BR, Forbeck B, Alkozei A, Killgore WDS. Daily morning blue light therapy improves daytime sleepiness, sleep quality, and quality of life following a mild traumatic brain injury. J Head Trauma Rehabil. 2020;35:E405-E421. [DOI] [PubMed] [Google Scholar]
- 79. Beaven CM, Ekstrom J. A comparison of blue light and caffeine effects on cognitive function and alertness in humans. PLoS One. 2013;8:e76707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Alkozei A, Smith R, Dailey NS, et al. Acute exposure to blue wavelength light during memory consolidation improves verbal memory performance. PLoS One. 2017;12:e0184884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Minguillon J, Lopez-Gordo MA, Renedo-Criado DA, Sanchez-Carrion MJ, Pelayo F. Blue lighting accelerates post-stress relaxation: results of a preliminary study. PLoS One. 2017;12:e0186399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Levine B, Kovacevic N, Nica EI, et al. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70:771-778. [DOI] [PubMed] [Google Scholar]
- 83. Yount R, Raschke KA, Biru M, et al. Traumatic brain injury and atrophy of the cingulate gyrus. J Neuropsychiatry Clin Neurosci. 2002;14:416-423. [DOI] [PubMed] [Google Scholar]
- 84. Bajaj S, Housley SN, Wu D, Dhamala M, James GA, Butler AJ. Dominance of the unaffected hemisphere motor network and its role in the behavior of chronic stroke survivors. Front Hum Neurosci. 2016;10:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Celeghin A, Diano M, de Gelder B, Weiskrantz L, Marzi CA, Tamietto M. Intact hemisphere and corpus callosum compensate for visuomotor functions after early visual cortex damage. Proc Natl Acad Sci U S A. 2017;114:E10475-E10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhou Y, Friston KJ, Zeidman P, Chen J, Li S, Razi A. The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb Cortex. 2018;28:726-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Razi A, Kahan J, Rees G, Friston KJ. Construct validation of a DCM for resting state fMRI. Neuroimage. 2015;106:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54:352-353. [DOI] [PubMed] [Google Scholar]
- 89. Alkozei A, Smith R, Pisner DA, et al. Exposure to blue light increases subsequent functional activation of the prefrontal cortex during performance of a working memory task. Sleep. 2016;39:1671-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Castriotta RJ, Murthy JN. Sleep disorders in patients with traumatic brain injury: a review. CNS Drugs. 2011;25:175-185. [DOI] [PubMed] [Google Scholar]
- 91. Orff HJ, Ayalon L, Drummond SP. Traumatic brain injury and sleep disturbance: a review of current research. J Head Trauma Rehabil. 2009;24:155-165. [DOI] [PubMed] [Google Scholar]
- 92. Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007;3:349-356. [PMC free article] [PubMed] [Google Scholar]
- 93. Viola-Saltzman M, Watson NF. Traumatic brain injury and sleep disorders. Neurol Clin. 2012;30:1299-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sullivan KA, Berndt SL, Edmed SL, Smith SS, Allan AC. Poor sleep predicts subacute postconcussion symptoms following mild traumatic brain injury. Appl Neuropsychol Adult. 2016;23:426-435. [DOI] [PubMed] [Google Scholar]
- 95. Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429-438. [DOI] [PubMed] [Google Scholar]
- 96. Wang H-B, Whittaker DS, Truong D, et al. Blue light therapy improves circadian dysfunction as well as motor symptoms in two mouse models of Huntington’s disease. Neurobiol Sleep Circadian Rhythm. 2017;2:39-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci. 2013;33:14288-14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brainard GC, Hanifin JP. Photons, clocks, and consciousness. J Biol Rhythm. 2005;20:314-325. [DOI] [PubMed] [Google Scholar]
- 100. Raikes AC, Satterfield BC, Killgore WDS. Evidence of actigraphic and subjective sleep disruption following mild traumatic brain injury. Sleep Med. 2019;54:62-69. [DOI] [PubMed] [Google Scholar]
- 101. Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355-405. [DOI] [PubMed] [Google Scholar]
- 102. Stephan KE, Tittgemeyer M, Knosche TR, Moran RJ, Friston KJ. Tractography-based priors for dynamic causal models. Neuroimage. 2009;47:1628-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]