Abstract
Simple Summary
Tight regulation of gene expression is critical for various biological processes such as proliferation, development, differentiation, and death; its dysregulation is linked to the pathogenesis of diseases. Gene expression is dynamically regulated by numerous factors at DNA, RNA, and protein levels, and RNA binding proteins (RBPs) and non–coding RNAs play important roles in the regulation of RNA metabolisms. RBPs govern a diverse spectrum of RNA metabolism by recognizing and binding to the secondary structure or the certain sequence of target mRNAs, and their malfunctions caused by aberrant expression or mutation are implicated in disease pathology. HuD, an RBP in the human antigen (Hu) family, has been studied as a pivotal regulator of gene expression in neuronal systems; however, accumulating evidence reveals the significance of HuD in non–neuronal systems including certain types of cancer cells or endocrine cells in the lung, pancreas, and adrenal gland. In addition, the abnormal function of HuD suggests its pathological association with neurological disorders, cancers, and diabetes. Thus, this review discusses HuD–mediated gene regulation in neuronal and non–neuronal systems to address how it works to orchestrate gene expression and how its expression is controlled in the stress response of pathogenesis of diseases.
Abstract
HuD (also known as ELAVL4) is an RNA–binding protein belonging to the human antigen (Hu) family that regulates stability, translation, splicing, and adenylation of target mRNAs. Unlike ubiquitously distributed HuR, HuD is only expressed in certain types of tissues, mainly in neuronal systems. Numerous studies have shown that HuD plays essential roles in neuronal development, differentiation, neurogenesis, dendritic maturation, neural plasticity, and synaptic transmission by regulating the metabolism of target mRNAs. However, growing evidence suggests that HuD also functions as a pivotal regulator of gene expression in non–neuronal systems and its malfunction is implicated in disease pathogenesis. Comprehensive knowledge of HuD expression, abundance, molecular targets, and regulatory mechanisms will broaden our understanding of its role as a versatile regulator of gene expression, thus enabling novel treatments for diseases with aberrant HuD expression. This review focuses on recent advances investigating the emerging role of HuD, its molecular mechanisms of target gene regulation, and its disease relevance in both neuronal and non–neuronal systems.
Keywords: HuD, RNA–binding protein, neuronal systems, non–neuronal systems, disease pathology
1. Introduction
RNA–binding proteins (RBPs) are responsible for the formation of ribonucleoprotein (RNP) complexes by binding to specific sequences or secondary structures of target RNAs. RBPs regulate the life cycle of RNAs, including alternative splicing, maturation, editing, transport, localization, turnover, and translation, thereby acting as an important regulators of gene expression [1,2,3,4]. Canonical RBPs usually include RNA–binding domains (RBDs), such as RNA recognition motif (RRM), K–homology (KH) domains, CCHC–type zinc–finger domains, helicase domains, and glycine–rich domains [5,6]. Non–canonical RBPs lack common RBDs and interact with RNA molecules via intrinsically disordered regions or mono–/di–nucleotide–binding domains [7,8,9]. Mutations or alterations in the expression of certain RBPs are linked to the development of human genetic diseases (reviewed in [10]). Further, impaired expression, mislocalization, and aggregation of RBPs are involved in the pathogenesis of various diseases, such as neurodegeneration, cancer, and metabolic diseases [11,12,13,14,15,16].
HuD, otherwise known as ELAVL4, is an RBP belonging to the Hu/ELAVL (Hu antigen/embryonic lethal, abnormal vision, Drosophila–like) family. While HuR is ubiquitously distributed across tissues, HuD, along with HuB and HuC, exhibits tissue–specific expression, particularly in neurons [17,18,19]. The HuD gene generates a variety of mRNA variants through alternative splicing and encodes ~40~42 kDa proteins in humans, mice, and rats [20,21,22]. HuD contains three RRMs (RRM1, RRM2, and RRM3) and a linker region between RRM2 and RRM3, through which it interacts with the AU–rich element (ARE) sequence of target mRNAs, thereby affecting their splicing, polyadenylation, transport, stability, and translation [23].
HuD plays diverse and important roles in neuronal processes, including neuronal development, plasticity, survival, function, and disease processes [23,24,25]. Many studies have emphasized the significance of HuD in the neuronal system; however, it also functions as a pivotal regulator of gene expression in non–neuronal tissues, including lung, testis, pituitary gland, and pancreatic endocrine cells [26,27,28,29]. Therefore, comprehensive knowledge of its expression, abundance, molecular targets, and regulatory mechanisms is needed to broaden our understanding of HuD as a versatile regulator of gene expression. This review focuses on recent studies elucidating the role of HuD, the molecular mechanisms underlying its target gene regulation, and its association with disease in both neuronal and non–neuronal systems.
2. General Characteristics of HuD
HuD was identified and characterized as a neuronal form of the Hu family, along with HuB and HuC [17]. HuD is well–conserved among vertebrates and located on chromosomes one, four, and five in humans, mice, and rats, respectively [30,31,32]. The complexity of the 5′ sequence of HuD transcripts and alternative exon splicing may generate several HuD mRNA variants [19,22,33,34] (reviewed in [24]). HuD proteins are ~40~42 kDa in size and have three highly conserved RRMs [23,25]. RRM1 and RRM2 associate with ARE–containing target mRNAs, while RRM3 is known to interact with poly(A) or ARE regions of target mRNAs [35,36,37]. Neuronal Hu proteins HuB, HuC, and HuD share 80% amino acid sequence homology compared to HuR, thus executing a similar role in RNA regulation [19]. The N–terminal and linker regions located between RRM2 and RRM3 seem to be responsible for the Hu family characteristics of each protein, including nuclear–cytoplasmic shuttling, protein–protein interactions, and binding to target mRNAs [24]. HuD variants reportedly display different amino acid sequences at their nuclear localization signal (NLS) or nuclear export signal (NES) in the linker region, which are responsible for temporal and spatial regulation of neuronal differentiation [38].
HuD is primarily found in the brain and regulates neural development, synaptic plasticity, and nerve generation [19,23,27,34]. However, increasing evidence has demonstrated its expression in non–neuronal cells, including small cell lung carcinoma (SCLC), oral squamous cell carcinoma (OSCC), β– and α–cells in the pancreatic islets, thymocytes, cells in the adrenal medulla, and spermatogonial cells in the testis [26,27,28,29,31,39,40,41,42]. This indicates that HuD expression is not ubiquitous, nor is it restricted to neurons and certain types of endocrine cells. To fully understand the cell–type specific roles of HuD, its molecular targets and gene regulatory mechanisms need to be determined.
HuD functions as an important regulator of gene expression by regulating a diverse spectrum of RNA metabolisms, including stability, translation, splicing, polyadenylation, nucleo–cytoplasmic shuttling, and intracellular localization of target mRNAs. HuD increases the stability of target mRNAs by competing with decay factors such as AU binding factor 1 (AUF1) and tristetraprolin (TTP); conversely, it also destabilizes target mRNAs in cooperation with microRNAs [20,24,43,44,45]. Although several studies have shown the role of HuD in mRNA stability, the detailed mechanisms of HuD–mediated regulation of mRNA turnover have not been fully elucidated. Further, HuD can affect translation of target mRNAs in a positive or negative manner. HuD promotes translation of target mRNAs by interacting with eIF4a and poly(A)–binding protein (PABP) [46]. Conversely, HuD functions as a translational repressor by associating with the internal ribosome entry site (IRES) of p27 mRNA or the stem–loop structure in the 5′UTR of proinsulin2 (Ins2) mRNA [28,47]. In addition to its regulatory role in mRNA turnover and translation, HuD is also involved in post–transcriptional control via exon inclusion or exclusion by splicing, alternative polyadenylation, and site–specific localization of various target mRNAs, thereby contributing to dynamic regulation of gene expression [28,48,49,50,51,52]. In addition, HuD regulates mRNA metabolisms through cooperative interactions with other RBPs including AUF1, insulin–like growth factor 2 mRNA binding protein 1 (IGF2BP1, also known as IMP1 and ZBP1), Ras–GAP SH3 domain binding protein (G3BP), survival of motor neurons (SMN), and PABP [53,54,55,56,57]. A list of target mRNAs and their HuD–mediated regulatory mechanisms is summarized in Table 1.
Table 1.
Target RNAs and their regulatory mechanisms.
| Target | Study Systems | Regulatory Mechanism |
Function | Ref. |
|---|---|---|---|---|
| I. Neuronal cells or brain | ||||
| Acetylcholinesterase (AChE) | Rat pheochromocytoma–derived cell PC12 Superior cervical ganglion (SCG) from rat Brain from HuD O/E mice |
mRNA stability ↑ | [73,74] | |
| Amyloid Precursor Protein (APP) | Human neuroblastoma SK–N–F1 Brain from HuD O/E mice Brain from AD patient |
mRNA stability ↑ | APP → Aβ processing ↑ | [76] |
| Human neuroblastoma SK–N–SH | Alternative splicing(Exon 7 and 8 exclusion ↑) | [57] | ||
| β–site APP–cleaving enzyme 1 (BACE1) and BACE–AS | Human neuroblastoma SK–N–F1 Brain from HuD O/E mice Brain from AD patient |
mRNA stability ↑ | APP → Aβ processing ↑ | [76] |
| Brain Derived Neurotrophic Factor (BDNF) long 3’UTR | Hippocampal neuron from E18 rat Hippocampal, cortical neuron from E17 mice Mouse catecholaminergic neural tumor cell CAD Brain from HuD O/E mice |
mRNA stability ↑ | Dendritic maturation ↑ | [68,69] |
| Calcitonin Gene–Related Peptide (CGPR) pre–mRNA | Human cervical tumor Hela Chinese hamster ovary (CHO) cell Mouse testicular teratoma F9 Rat pheochromocytoma–derived cell PC12 Human neuroblastoma SK–N–SH Mouse teratocarcinoma P19 Rat medullary thyroid carcinoma CA77 |
Alternative splicing (Exon4 exclusion ↑) |
[48] | |
| Calcium/Calmodulin Dependent Protein Kinase II Alpha (CaMKⅡα) | Hippocampal neuron from E18–19 rat | mRNA stability ↑ | [84] | |
| CDKN1A (p21) | Rat pheochromocytoma–derived cell PC12 | mRNA stability ↑ | Cell proliferation ↓ | [83] |
| circHomer protein homolog1a (cirHomer1a) | Brain (frontal cortex) from HuD K/O and O/E mice | Synaptic expression ↑ | [60,86] | |
| Growth Associated Protein 43 (GAP–43) | Rat pheochromocytoma–derived cell PC12 Mouse embryonic stem cell AB2.2 Cortical neuron from E19 rat Rat DRG/mouse neuroblastoma hybrid cell F11 Brain from rat Brain from HuD K/O and O/E mice |
mRNA stability ↑ Transportation into neurites ↑ |
Neurite outgrowth ↑ | [43,54,61,62,63,64,65,66,67] |
| Glutaminase (Gls) | Brain (cortex) from HuC, HuD double K/O mice | Alternative splicing (Gls–long isoform ↓) |
[88] | |
| MYCN | Human neuroblastoma NBL–W–N Mouse fibroblast NIH 3T3 |
mRNA stability ↑ | [81,82] | |
| Neprilysin (NEP) | Human neuroblastoma SK–N–SH | mRNA stability ↑ | Aβ levels ↓ by NEP | [77] |
| Nerve Growth Factor (NGF) | Hippocampal neuron from E18 rat | mRNA stability ↑ | Dendritic maturation ↑ | [68] |
| Neuritin 1 (Nrn1/Cpg15) | Rat pheochromocytoma–derived cell PC12 Human neuroblastoma SH–SY5Y DRG neuron from rat Cortical neuron from E18 rat Hippocampal neuron from E18 rat Rat DRG/mouse neuroblastoma hybrid cell F11 Brain from HuD KO mice |
Axonal localization ↑ mRNA stability ↑ |
[55,71,72] | |
| Neurofibromatosis type 1 (NF–1) pre–mRNA | Human cervical tumor cell Hela Rat medullary thyroid carcinoma CA77 Mouse embryonic stem cell R1 Cerebellar neurons from mice |
Alternative splicing (Exon23a skipping ↑) Local transcription rate ↑ (NF–1 gene exon 23a) |
[49,51] | |
| Neuroserpin | Brain from rat Rat pheochromocytoma–derived cell PC12 |
mRNA stability ↑ (?) | [45] | |
| Neurotrophin 3 (NT–3) | Hippocampal neuron from E18 rat | mRNA stability ↑ | Dendritic maturation ↑ | [68] |
| NOVA Alternative Splicing Regulator 1 (NOVA–1) | Mouse motor neuronal cell NSC34 | mRNA stability ↑ Translation ↑ |
Splicing activity | [70] |
| Musashi–1 (MSI1) | Neural stem/progenitor cell (NSC) in SVZfrom mice Human neuroblastoma SH–SY5Y |
mRNA stability ↑ | [85] | |
| Potassium voltage–gated channel subfamily A member 1 (Kv1.1) | Cortical neuron from E18–19 rat | Translation ↑ | [87] | |
| Special AT–rich DNA–binding protein 1 (SATB1) | Neural stem/progenitor cell (NSC) in SVZ from HuD KO mice |
mRNA stability ↑ | NSC differentiation ↑ | [75] |
| Superoxide Dismutase 1 (SOD1) long 3′UTR | Human neuroblastoma SH–SY5Y Brain from ALS patients |
mRNA stability ↑ | [80] | |
| Tau | Rat pheochromocytoma–derived cell PC12Mouse teratocarcinoma P19 | Transportation into neurites ↑ | Neurite outgrowth ↑ | [53,78,79] |
| Others: mTORC–responsive genes | Mouse motor neuronal cell NSC34 | Translation ↑ | [52] | |
| II. Non–neuronal cells or other tissues | ||||
| Autophagy Related Gene 5 (ATG5) | Mouse insulinoma βTC6 Pancreatic islet from HuD KO mice, db/db mice |
Translation ↑ | Autophagosome formation ↑ | [89] |
| CDKN1B (p27) | Human embryonic kidney cell 293T and human cervical tumor Hela Mouse insulinoma βTC6 and MIN6 Pancreatic NET from patients |
Translation ↑ or ↓ | Cell proliferation ↑ or ↓ | [47,90] |
| HuD mRNA | Human cervical tumor HelaRat medullary thyroid carcinoma CA77 | Alternative splicing(Exon 6 inclusion ↑) | [91] | |
| Hu Antigen R (HuR) | Mouse teratocarcinoma P19 | Alternative polyadenylation ↑ | [50] | |
| Insulin Induced Gene 1 (Insig1) | Mouse insulinoma βTC6 | Translation ↑ | TG accumulation ↓ | [92] |
| Insulinoma–Associated Protein 1 (INSM1) | Mouse insulinoma βTC6 | mRNA stability ↓ | [93] | |
| Ikaros (IK) | Thymocyte from N3–Ictg, N3–Ictg/pTα−/− and pTα–/– mice Human T–All cell line Molt–3 |
Alternative splicing (Ik–6, 8, 5/7, 9 ↑) | T cell lymphomagenesis | [42] |
| Matrix Metallopeptidase–2 and –9 (MMP–2 and –9) | Human oral squamous cell carcinoma HSC3 | mRNA stability ↑ (?) | [39] | |
| Mitofusin 2 (Mfn2) | Mouse insulinoma βTC6Pancreatic islet from HuD KO mice | Mitochondria fusion ↑ | [94] | |
| Potassium Voltage–Gated Channel Subfamily H Member 2 (KCNH2) | Human embryonic kidney 293 | Alternative polyadenylation ↓ | Kv11.1a isoform expression ↑ Kv11.1 channel current ↑ |
[95] |
| Preproglucagon (Gcg) | Mouse glucagonoma αTC1 Pancreatic islet from HuD KO mice |
Translation ↑ | Glucagon biosynthesis | [29] |
| Preproinsulin2 (Ins2) | Mouse insulinoma βTC6 Pancreatic islet from HuD KO mice |
Translation ↓ | Insulin biosynthesis | [28,56] |
| Vascular Endothelial Growth Factor–A and –D (VEGF–A and VEGF–D) | Human oral squamous cell carcinoma HSC3 | mRNA stability ↑ (?) | [39] |
↑ means its upregulation (increase). ↓ means its downregulation (decrease). → means from APP to Aβ.
3. Regulation of RNA Metabolism by HuD
Comprehensive understanding of HuD–mediated gene regulation requires identification of its target mRNAs and elucidation of the regulatory mechanisms of RNA metabolism. Several studies have extensively investigated interactions between HuD, its target mRNAs, and HuD–mediated post–transcriptional regulation in neuronal systems, thereby demonstrating the pivotal role of HuD as a neuronal regulator (summarized in Table 1). Additionally, systemic approaches have attempted to identify molecular targets of HuD on a large scale [52,58,59]. For example, HITS–CLIP (high–throughput sequencing of RNA isolated by crosslinking immunoprecipitation) was employed to determine the binding sites targeted by neuronal ELAVLs (nELAVLs), but not specifically HuD, on over 8000 transcripts from the human brain [59]. HuD–associated mRNAs have been analyzed by ribonucleoprotein immunoprecipitation (RIP) followed by microarray in the brains of transgenic mice [58], and also by CRAC (crosslinking and analysis of cDNA) in motor neuron cells expressing His–HA–HuD [52]. Recently, a series of HuD–associating circular RNAs (circRNAs) from HuD transgenic mice were identified by RIP analysis followed by circRNA arrays [60]. These analyses revealed that HuD interacts with a variety of mRNAs as well as non–coding RNAs, via their ARE regions and provided useful information concerning the roles of HuD in RNA regulation. Most studies demonstrated the neuronal function of HuD; however, growing evidence indicates that HuD plays essential roles in HuD–expressing non–neuronal cells, such as pancreatic β–cells and SCLC. In this section, we provide an update on the molecular targets of HuD and HuD–mediated regulatory mechanisms in both neuronal and non–neuronal systems.
3.1. Regulation of RNA Metabolism by HuD in Neuronal Systems
Since HuD was first discovered in the brain, its role as an essential regulator governing post–transcriptional control of neuronal gene expression has been extensively reported in drosophila and vertebrates (reviewed in [23,24]). HuD affects diverse neuronal gene expression by regulating mRNA turnover, translation, and splicing. Several studies have demonstrated the role of HuD as a stabilizer of neuronal mRNAs. For example, HuD increases the stability of growth associated protein 43 (GAP43) mRNA in neurons and promotes neurite outgrowth [43,61,62,63,64,65,66,67]. HuD also mediates post–transcriptional control of essential target mRNAs for the brain or neuronal functions, including brain–derived neurotrophic factor (BDNF) [68,69], nerve growth factor (NGF) [68], neurotropin–3 (NT–3) [68], neuro–oncological ventral antigen 1 (Nova1) [70], neuritin 1 [71,72], neuroserpin [45], acetylcholinesterase (AchE) [73,74], and special adenine–thymine (AT)–rich DNA–binding protein 1 (SATB1) [75], thereby regulating neuronal differentiation, neurogenesis, dendritic maturation, neuronal plasticity, synaptic transmission, and dynamic signaling pathways in neuronal systems. Further, HuD regulates the expression of mRNAs involved in the pathogenesis of neurodegenerative diseases or cancer, including amyloid precursor protein (APP), β–site APP–cleaving enzyme 1 (BACE1), lncRNA BACE1AS [76], neprilysin (NEP) [77], tau [78,79], superoxide dismutase 1 (SOD1) [80], and MYCN [81,82]. In addition to targets found in neuronal tissues, HuD promotes stabilization of target mRNAs also expressed in other tissues, such as p21 [83], Ca2+/Calmodulin–dependent protein kinase II α (CaMKIIα) [84], and musashi 1 (MSI1) [85]. Additionally, an interesting study recently demonstrated that circRNAs, such as cirHomer1a, could be molecular targets of HuD [60,86].
With a few exceptions, HuD generally promotes expression of target genes by enhancing translation of their mRNAs. HuD–mediated translational enhancement of Nova1 [70], potassium voltage–gated channel subfamily A member 1 (also known as Kv1.1) [87], and several mTORC–responsive genes [52] has been demonstrated in neuronal cells. In addition to turnover and translation of target mRNAs, HuD is also involved in regulating alternative splicing of calcitonin gene–related peptide (CGRP) pre–mRNA [48], neurofibromatosis type 1 (NF1) pre–mRNA [49], APP mRNA [57], and glutaminase mRNA [88].
3.2. Regulation of RNA Metabolism by HuD in Non–Neuronal Systems
HuD generally increases the stability of target mRNAs in neuronal cells; however, it decreases the amount of insulinoma–associated 1 (INSM1) mRNA in cooperation with miR–203a in pancreatic β–cells [93]. In OSCC cell line HSC3 cells, HuD knockdown downregulated vascular endothelial growth factor (VEGF)–A and –D, and matrix metallopeptidase (MMP)–2 and –9 mRNAs [39]. These studies determined that target mRNA abundance is altered by HuD knockdown and suggest that HuD regulates mRNA turnover by interacting with decay factors such as microRNAs, AUF1, and TTP in a co–operative or a competitive manner. However, the direct involvement of HuD in the regulation of mRNA stability warrants further investigation.
Several studies have reported that HuD mediates translational control of several target mRNAs in pancreatic β–cells. HuD suppresses translation of preproinsulin 2 (Ins2) mRNA, while enhancing the expression of preproglucagon (Gcg), insulin–induced gene 1 (INSIG1), autophagy–related gene 5 (ATG5), p27, and mitofusin 2 (Mfn2) mRNAs [28,29,89,90,92,94]. These results imply that HuD has a function in the maintenance of glucose homeostasis and β–cell function, and its dysregulation might be involved in the pathogenesis of metabolic diseases such as diabetes.
In addition to translational control of target mRNAs, HuD is also involved in the regulation of splicing and polyadenylation. HuD regulates its own expression by promoting exon 6 inclusion of HuD mRNA [91]. Additionally, HuD alters the Ikaros (IK) isoform profile by regulating alternative splicing of IK mRNAs in mouse thymocytes and human T–acute lymphoblastic leukemia (T–ALL) cell line Molt–3 cells in a Notch3–dependent manner [42]. Further, HuD increases the Kv11.1 channel current by affecting alternative polyadenylation of mRNA transcripts of potassium voltage–gated channel subfamily H member 2 (KCNH2), which encodes the Kv11.1 potassium channel [95].
HuD–mediated RNA regulation in non–neuronal cells is relatively unknown compared to that in neuronal systems, but further studies will enable us to explore the specific role of HuD in certain types of cells expressing HuD.
4. Disease Relevance of HuD and Its Regulatory Mechanisms
HuD plays important roles in the dynamic regulation of gene expression by affecting RNA metabolism, and its aberrant expression has been reported in several diseases, including neurodegenerative diseases, diabetes, and cancer. Despite the significance of HuD in gene regulation, little is known regarding control of HuD expression in response to stress or the implication of HuD in disease pathogenesis. Herein, we describe the current knowledge of HuD disease relevance as well as the regulatory mechanisms affecting HuD expression, which are summarized in Table 2.
Table 2.
Disease relevance of HuD.
| Disease | Disease Relevance of HuD | Ref. |
|---|---|---|
| Alzheimer’s diseases (AD) | HuD mRNA and HuD protein ↑ in superior temporal gyrus (STG) of AD patients | [76] |
| HuD protein ↑ in the brain of AD patients | [96] | |
| nELAVL protein ↓ in hippocampus of AD patients | [97] | |
| Parkinson’s diseases (PD) | Several SNPs (rs967582, 2494876, 3902720) were identified. | [98,99,100] |
| Epilepsy | HuD mRNA↑ in dentate gyrus of kainic acid–induced seizures model | [67] |
| Dendritic localization of HuD protein ↑ in hippocampal neurons of pilocarpine–induced seizure model | [101] | |
| Schizophrenia | HuD mRNA ↑ in the dorsolateral prefrontal cortex from patients with chronic schizophrenia | [102] |
| Amyotrophic lateral sclerosis (ALS) | HuD mRNA and HuD protein ↑ in motor cortex of sporadic ALS patients | [80] |
| HuD protein ↑ in human iPSCs carrying the FUSP525L mutation | [103] | |
| Neuroblastoma | HuD mRNA was detected in primary NB tumor samples. | [104] |
| Small cell lung carcinoma (SCLC) | HuD protein ↑ in serum from SCLC patients | [105,106,107] |
| HuD mRNA ↑ in primary tissue from SCLC patients | [108] | |
| HuD mRNA ↑ in blood from SCLC patients | [109] | |
| Oral squamous cell carcinoma (OSCC) | HuD (+) group is associated with poor prognosis of OSCC patients. | [39] |
| Pancreatic neuroendocrine tumor (PNET) | HuD (–) group is associated with poor prognosis of PNET patients. | [90] |
| Type 2 diabetes mellitus (T2DM) | HuD mRNA and HuD protein ↓ in islet from db/db mice | [94] |
↑ means its upregulation (increase). ↓ means its downregulation (decrease).
4.1. Disease Relevance of HuD
Several studies have implicated HuD in the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig’s disease). Augmented expression of HuD in the brain of patients with AD has been reported [76,96]. Increased expression of HuD may contribute to AD development by increasing expression of mRNAs involved in amyloid–β peptide (Aβ) production, including APP and BACE1 [76]. However, another study reported a reduction of nELAV in the hippocampus of patients with AD and downregulation of HuD after treatment with Aβ42 in human neuroblastoma SH–SY5Y cells [97]. Inconsistent aberrant levels of HuD in AD can be attributed to different brain tissues analyzed between study groups, and further investigation is warranted to clarify the relevance of HuD in AD. In PD, several single–nucleotide polymorphisms (SNPs) were identified in HuD [98,99,100]. Genetic variations in HuD (rs967582, rs2494876, rs3902720) have been associated with age–at–onset (AAO) in PD, while the biological roles of these variations in the regulation of HuD protein abundance or binding affinity to its target mRNAs have not yet been determined. A recent study reported increased levels of HuD proteins in human induced pluripotent stem cells (iPSCs) carrying the P525L mutation on the FUS gene, which causes ALS [103]. In addition, augmented HuD expression in the motor cortex of patients with sporadic ALS has been associated with superoxide dismutase (SOD) dysregulation [80].
Aberrant expression of HuD also has been determined in various neurological disorders, including epilepsy and schizophrenia. Upregulation of HuD mRNA in the dendritic gyrus after kainic acid–induced seizures and increased dendritic localization of HuD protein in hippocampal neurons, following pilocarpine–induced seizures, were reported in animal models [67,101]. nELAVL null mice displayed the spontaneous epileptic seizure activity resulted from the impaired splicing of genes regulating cellular glutamate level [88]. Additionally, abnormal overexpression of HuD mRNA was observed in the dorsolateral prefrontal cortex of patients with chronic schizophrenia [102]. Although the factors leading to HuD dysregulation or its impact on the regulation of alternative splicing and turnover of target mRNAs are unclear, these reports suggest that abnormal expression of HuD is linked to the pathogenesis of various neurological diseases.
Differential expression of HuD is associated with certain types of cancer, including SCLC, OSCC, neuroblastoma (NB), and pancreatic neuroendocrine tumor (PNET). In patients with SCLC, a more aggressive form of lung cancer, HuD protein was found in patient serum [105,106,107] and HuD mRNA was detected in primary tissues and blood [108,109]. In OSCC, HuD expression was associated with differentiation, metastasis, and invasion of cancer cells, and HuD–positive OSCC cases were associated with a poor survival rate [39]. High HuD mRNA levels were also reported in primary tumor tissues of patients with NB and in several NB cell lines [104,110]. Higher HuD expression was associated with a better clinical outcome in NB, which suggests a role of HuD in decreasing malignancy [104]. Concurring with these results, another study revealed a positive correlation between tumoral HuD loss and significantly reduced survival of patients with PNET. The HuD level was significantly corelated with tumor size and progression of PNET [90]. Although changes in HuD expression in the process of cancer and tumor development are unclear, aberrant HuD levels may provide useful markers for disease diagnosis or prognosis.
In addition to cancer, the disease relevance of HuD has been demonstrated in diabetes, one of the metabolic diseases resulting from impaired glucose homeostasis. Using an animal model of type 2 diabetes mellitus (T2DM), the levels of HuD mRNA and protein were reduced in the pancreas of db/db mice, suggesting HuD caused β–cell dysfunction [92].
As reported above, HuD is abnormally expressed in several diseases. Although differential regulation of HuD in pathological conditions has not been fully elucidated, understanding the molecular mechanisms fine–tuning HuD expression is critical for therapeutic intervention.
4.2. Regulation of HuD Expression
Elucidating the molecular mechanisms modulating HuD expression is required to fully understand how HuD–mediated gene regulation affects RNA metabolism. Several studies have described the regulation of HuD expression by a variety of factors at the transcriptional, post–transcriptional, and post–translational levels.
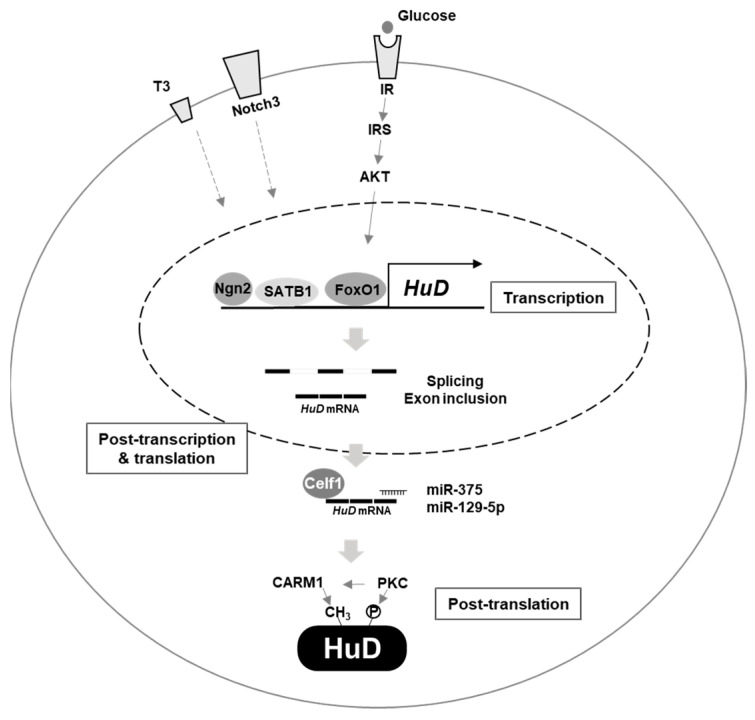
First, several regulatory mechanisms of HuD transcription have been identified. Neurogenin 2 (Ngn2), the basic helix–loop–helix transcription factor, promotes transcription of HuD by binding to E–boxes in its promoter region, which is essential during neuronal differentiation of P19 cells [22]. SATB1, one of the target mRNAs of HuD, also functions as a transcriptional activator of HuD [75]. Interestingly, HuD and SATB1 cooperatively regulate neural stem and progenitor cell neuronal differentiation via a positive feedback network; HuD stabilizes SATB1 mRNA, and SATB1 promotes transcription of HuD. Activation of Notch3 signaling contributes to upregulation of HuD expression in thymocytes, which in turn, promotes HuD–mediated splicing of IK mRNAs [42]. In mouse pancreatic β–cells, insulin signaling was shown to be responsible for upregulated HuD expression through the IR–IRS–Akt–FoxO1 axis after glucose stimulation [28]. Additionally, thyroid hormone T3 represses transcription of HuD in rat PC12 and mouse N2a cells, and T3 level was inversely correlated with HuD mRNA in the rat brain [111].
Second, HuD expression can be also regulated at the post–transcriptional level. Alternative splicing of HuD mRNAs generate different HuD isoforms exhibiting variable localization patterns, which have been suggested to play different roles in neuronal differentiation and development [22,38]. Neuronal Hu proteins are responsible for exon 6 inclusion of HuD mRNA [91]; however, detailed mechanisms regulating the alternative splicing of HuD mRNA by cis–elements or specific trans–factors have not been fully elucidated. microRNA–375 (miR–375) downregulates HuD expression by destabilizing HuD mRNA and suppressing its translation, thereby affecting neuronal differentiation [27]. microRNA–129–5p (miR–129–5p) decreases HuD expression and inhibits neurite outgrowth [112]. A recent study reported that RBP Celf1 functions as a translational repressor of HuD during neocortical neurogenesis [113]. Celf1 suppresses translation of HuD mRNA by binding to its 5′UTR region in glutamatergic neurons. Isoform–specific translational repression of HuD mRNAs by Celf1 has been shown to play an important role in neurodevelopment.
Third, HuD protein can be regulated by post–translational modification, including methylation and phosphorylation. Coactivator–associated arginine methyltransferase 1 (CARM1), also known as PRMT4, methylates Arg residues of HuD protein (Arg236 in PC12 cells and Arg248 residue in MN–1 cells), leading to decreased stability of HuD–mediated p21 mRNA [114,115]. Methylation of HuD by CARM1 seems to be essential for the transition of neuronal precursor cells from proliferation to differentiation by negatively regulating HuD–mediated gene expression. In addition to methylation, phosphorylation of neuronal Hu proteins by protein kinase C (PKC) has been reported [67,76,115]. PKCα induces phosphorylation of the Thr residue in neuronal Hu proteins, which in turn, promotes GAP–43 mRNA stabilization in SH–SY5Y cells [116]. PKC contributes to neuronal differentiation by affecting HuD–mediated RNA metabolism, which directly regulates binding between HuD and target mRNAs, or by regulating factors that affect HuD functions, such as CARM1, in an indirect manner [68,77].
As described above, several factors regulating HuD expression have been identified (Figure 1), but the detailed mechanisms of regulation warrant further investigation. Additional studies examining specific regulators directing HuD abundance or activity are expected to provide novel insights to facilitate the development of treatments for diseases caused by HuD malfunction.
Figure 1.
Regulation of HuD expression. Several factors affect HuD expression. Ngn2, SATB1, IR/IRS/AKT/FoxO1, notch3, and thyroid hormone T3 regulate transcription of HuD gene. Celf1, miR–375, miR–129–5p and alternative splicing are involved in post–transcriptional and translational control of HuD mRNA. CARM1 and PKC mediate post–translational regulation of HuD protein.
5. Concluding Remarks and Perspectives
RBPs function as critical effectors of gene expression and their malfunctions are implicated in disease pathology, including RBP gene mutations, altered RBP expression, and aggregation and sequestration of RBPs with RNAs or other proteins. Therefore, approaches that restore the abundance or function of RBPs have great potential for clinical applications [10,117]. HuD, an RBP in the Hu family, is a versatile protein that regulates various aspects of RNA metabolism, including splicing, stability, and translation of target mRNAs, and is therefore involved in various cellular processes, including cell growth, apoptosis, differentiation, and metabolism. The majority of studies have focused on the role of HuD in neuronal systems; however, accumulating evidence indicates that HuD is also expressed in non–neuronal cells, such as pancreatic β–cells, thymocytes, and SCLC, and its differential expression is implicated in the pathogenesis of several diseases. In this review, we summarized the current knowledge of molecular targets, disease relevance, and regulatory mechanisms of HuD.
Despite continued studies elucidating HuD–mediated gene regulatory mechanisms, several questions remain unanswered. Which characteristics of HuD are distinct from those of other Hu proteins? What mechanism contributes to cell–type specific expression and function of HuD? What signals or cellular conditions regulate HuD expression at the transcriptional, post–transcriptional, and post–translational level during disease development? Besides HuD abundance, which mechanisms determine subcellular localization and binding affinity to its interacting partners? What signals or stimuli affect the binding of HuD to target mRNA? What mechanisms determine competitive or cooperative association between HuD, miRNA, and other RBPs on target mRNAs? Addressing these questions based on systemic and/or integrated approaches using multi–omics analysis will enhance our knowledge of HuD–mediated gene regulation.
Although neuronal and non–neuronal cells express HuD, we still do not know how the detailed mechanisms regulating HuD expression or HuD–mediated RNA regulation are different among cell types. What are the common characteristics of HuD–expressing cells? Do common signaling pathways direct HuD expression in neuronal and non–neuronal systems or not? Do both systems have a common mechanism or cell type–specific mechanisms in mRNA regulation? Further studies enable us to fully explore the gene networks regulated by HuD, thus improving our understanding of diseases associated with aberrant HuD expression for therapeutic intervention.
Author Contributions
M.J. and E.K.L. prepared the original draft of the manuscript, reviewed, and edited the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Programs through the National Research Foundation of Korea (NRF) funded by the Minister of Education and the Minister of Science and ICT (2020R1F1A1048425 and 2021R1A2C1004128).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McKee A.E., Silver P.A. Systems perspectives on mRNA processing. Cell Res. 2007;17:581–590. doi: 10.1038/cr.2007.54. [DOI] [PubMed] [Google Scholar]
- 2.Lukong K.E., Chang K.W., Khandjian E.W., Richard S. RNA–binding proteins in human genetic disease. Trends Genet. 2008;24:416–425. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell S.F., Parker R. Principles and properties of eukaryotic mRNPs. Mol. Cell. 2014;54:547–558. doi: 10.1016/j.molcel.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Singh G., Pratt G., Yeo G.W., Moore M.J. The Clothes Make the mRNA: Past and Present Trends in mRNP Fashion. Annu. Rev. Biochem. 2015;84:325–354. doi: 10.1146/annurev-biochem-080111-092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstberger S., Hafner M., Tuschl T. A census of human RNA–binding proteins. Nat. Rev. Genet. 2014;15:829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunde B.M., Moore C., Varani G. RNA–binding proteins: Modular design for efficient function. Nat. Rev. Mol. Cell Biol. 2007;8:479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castello A., Fischer B., Eichelbaum K., Horos R., Beckmann B.M., Strein C., Davey N.E., Humphreys D.T., Preiss T., Steinmetz L.M., et al. Insights into RNA biology from an atlas of mammalian mRNA–binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Hentze M.W., Castello A., Schwarzl T., Preiss T. A brave new world of RNA–binding proteins. Nat. Rev. Mol. Cell Biol. 2018;19:327–341. doi: 10.1038/nrm.2017.130. [DOI] [PubMed] [Google Scholar]
- 9.Moore S., Jarvelin A.I., Davis I., Bond G.L., Castello A. Expanding horizons: New roles for non–canonical RNA–binding proteins in cancer. Curr. Opin. Genet. Dev. 2018;48:112–120. doi: 10.1016/j.gde.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebauer F., Schwarzl T., Valcarcel J., Hentze M.W. RNA–binding proteins in human genetic disease. Nat. Rev. Genet. 2020 doi: 10.1038/s41576-020-00302-y. [DOI] [PubMed] [Google Scholar]
- 11.Nussbacher J.K., Batra R., Lagier-Tourenne C., Yeo G.W. RNA–binding proteins in neurodegeneration: Seq and you shall receive. Trends Neurosci. 2015;38:226–236. doi: 10.1016/j.tins.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlon E.G., Manley J.L. RNA–binding proteins in neurodegeneration: Mechanisms in aggregate. Genes Dev. 2017;31:1509–1528. doi: 10.1101/gad.304055.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira B., Billaud M., Almeida R. RNA–Binding Proteins in Cancer: Old Players and New Actors. Trends Cancer. 2017;3:506–528. doi: 10.1016/j.trecan.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Qin H., Ni H., Liu Y., Yuan Y., Xi T., Li X., Zheng L. RNA–binding proteins in tumor progression. J. Hematol. Oncol. 2020;13:90. doi: 10.1186/s13045-020-00927-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magro M.G., Solimena M. Regulation of beta–cell function by RNA–binding proteins. Mol. Metab. 2013;2:348–355. doi: 10.1016/j.molmet.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nutter C.A., Kuyumcu-Martinez M.N. Emerging roles of RNA–binding proteins in diabetes and their therapeutic potential in diabetic complications. Wiley Interdiscip. Rev. RNA. 2018;9 doi: 10.1002/wrna.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo A., Dalmau J., Manley G., Rosenfeld M., Wong E., Henson J., Posner J.B., Furneaux H.M. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA–binding domains and is homologous to Elav and Sex–lethal. Cell. 1991;67:325–333. doi: 10.1016/0092-8674(91)90184-Z. [DOI] [PubMed] [Google Scholar]
- 18.Robinow S., Campos A.R., Yao K.M., White K. The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science. 1988;242:1570–1572. doi: 10.1126/science.3144044. [DOI] [PubMed] [Google Scholar]
- 19.Okano H.J., Darnell R.B. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J. Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinman M.N., Lou H. Diverse molecular functions of Hu proteins. Cell Mol. Life Sci. 2008;65:3168–3181. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombrita C., Silani V., Ratti A. ELAV proteins along evolution: Back to the nucleus? Mol. Cell Neurosci. 2013;56:447–455. doi: 10.1016/j.mcn.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Bronicki L.M., Belanger G., Jasmin B.J. Characterization of multiple exon 1 variants in mammalian HuD mRNA and neuron–specific transcriptional control via neurogenin 2. J. Neurosci. 2012;32:11164–11175. doi: 10.1523/JNEUROSCI.2247-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrone–Bizzozero N., Bird C.W. Role of HuD in nervous system function and pathology. Front. Biosci. (Schol Ed.) 2013;5:554–563. doi: 10.2741/S389. [DOI] [PubMed] [Google Scholar]
- 24.Bronicki L.M., Jasmin B.J. Emerging complexity of the HuD/ELAVl4 gene; implications for neuronal development, function, and dysfunction. RNA. 2013;19:1019–1037. doi: 10.1261/rna.039164.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akamatsu W., Fujihara H., Mitsuhashi T., Yano M., Shibata S., Hayakawa Y., Okano H.J., Sakakibara S., Takano H., Takano T., et al. The RNA–binding protein HuD regulates neuronal cell identity and maturation. Proc. Natl. Acad. Sci. USA. 2005;102:4625–4630. doi: 10.1073/pnas.0407523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Alessandro V., Muscarella L.A., la Torre A., Bisceglia M., Parrella P., Scaramuzzi G., Storlazzi C.T., Trombetta D., Kok K., De Cata A., et al. Molecular analysis of the HuD gene in neuroendocrine lung cancers. Lung. Cancer. 2010;67:69–75. doi: 10.1016/j.lungcan.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Abdelmohsen K., Hutchison E.R., Lee E.K., Kuwano Y., Kim M.M., Masuda K., Srikantan S., Subaran S.S., Marasa B.S., Mattson M.P., et al. miR–375 inhibits differentiation of neurites by lowering HuD levels. Mol. Cell Biol. 2010;30:4197–4210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee E.K., Kim W., Tominaga K., Martindale J.L., Yang X., Subaran S.S., Carlson O.D., Mercken E.M., Kulkarni R.N., Akamatsu W., et al. RNA–binding protein HuD controls insulin translation. Mol. Cell. 2012;45:826–835. doi: 10.1016/j.molcel.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn S., Tak H., Kang H., Ryu S., Jeong S.M., Kim W., Lee E.K. The RNA–binding protein, HuD regulates proglucagon biosynthesis in pancreatic alpha cells. Biochem. Biophys. Res. Commun. 2020;530:266–272. doi: 10.1016/j.bbrc.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Inman M.V., Levy S., Mock B.A., Owens G.C. Gene organization and chromosome location of the neural–specific RNA binding protein Elavl4. Gene. 1998;208:139–145. doi: 10.1016/S0378-1119(97)00615-X. [DOI] [PubMed] [Google Scholar]
- 31.Sekido Y., Bader S.A., Carbone D.P., Johnson B.E., Minna J.D. Molecular analysis of the HuD gene encoding a paraneoplastic encephalomyelitis antigen in human lung cancer cell lines. Cancer Res. 1994;54:4988–4992. [PubMed] [Google Scholar]
- 32.Steller U., Kohls S., Muller B., Soller R., Muller R., Schlender J., Blohm D.H. The RNA binding protein HuD: Rat cDNA and analysis of the alternative spliced mRNA in neuronal differentiating cell lines P19 and PC12. Brain Res. Mol. Brain Res. 1996;35:285–296. doi: 10.1016/0169-328X(95)00231-G. [DOI] [PubMed] [Google Scholar]
- 33.Clayton G.H., Perez G.M., Smith R.L., Owens G.C. Expression of mRNA for the elav–like neural–specific RNA binding protein, HuD, during nervous system development. Brain Res. Dev. Brain Res. 1998;109:271–280. doi: 10.1016/S0165-3806(98)00074-1. [DOI] [PubMed] [Google Scholar]
- 34.Abe R., Uyeno Y., Yamamoto K., Sakamoto H. Tissue–specific expression of the gene encoding a mouse RNA binding protein homologous to human HuD antigen. DNA Res. 1994;1:175–180. doi: 10.1093/dnares/1.4.175. [DOI] [PubMed] [Google Scholar]
- 35.Keene J.D. Why is Hu where? Shuttling of early–response–gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA. 1999;96:5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma W.J., Chung S., Furneaux H. The Elav–like proteins bind to AU–rich elements and to the poly(A) tail of mRNA. Nucleic Acids Res. 1997;25:3564–3569. doi: 10.1093/nar/25.18.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., Tanaka Hall T.M. Structural basis for recognition of AU–rich element RNA by the HuD protein. Nat. Struct. Biol. 2001;8:141–145. doi: 10.1038/84131. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi S., Yano M., Igarashi M., Okano H.J., Okano H. Alternative role of HuD splicing variants in neuronal differentiation. J. Neurosci. Res. 2015;93:399–409. doi: 10.1002/jnr.23496. [DOI] [PubMed] [Google Scholar]
- 39.Sasahira T., Kurihara M., Yamamoto K., Ueda N., Nakashima C., Matsushima S., Bhawal U.K., Kirita T., Kuniyasu H. HuD promotes progression of oral squamous cell carcinoma. Pathobiology. 2014;81:206–214. doi: 10.1159/000366022. [DOI] [PubMed] [Google Scholar]
- 40.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., et al. Proteomics. Tissue–based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 41.Juan-Mateu J., Rech T.H., Villate O., Lizarraga-Mollinedo E., Wendt A., Turatsinze J.V., Brondani L.A., Nardelli T.R., Nogueira T.C., Esguerra J.L., et al. Neuron–enriched RNA–binding Proteins Regulate Pancreatic Beta Cell Function and Survival. J. Biol. Chem. 2017;292:3466–3480. doi: 10.1074/jbc.M116.748335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellavia D., Mecarozzi M., Campese A.F., Grazioli P., Talora C., Frati L., Gulino A., Screpanti I. Notch3 and the Notch3–upregulated RNA–binding protein HuD regulate Ikaros alternative splicing. EMBO J. 2007;26:1670–1680. doi: 10.1038/sj.emboj.7601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beckel-Mitchener A.C., Miera A., Keller R., Perrone-Bizzozero N.I. Poly(A) tail length–dependent stabilization of GAP–43 mRNA by the RNA–binding protein HuD. J. Biol. Chem. 2002;277:27996–28002. doi: 10.1074/jbc.M201982200. [DOI] [PubMed] [Google Scholar]
- 44.Barreau C., Paillard L., Osborne H.B. AU–rich elements and associated factors: Are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuadrado A., Navarro-Yubero C., Furneaux H., Kinter J., Sonderegger P., Munoz A. HuD binds to three AU–rich sequences in the 3’–UTR of neuroserpin mRNA and promotes the accumulation of neuroserpin mRNA and protein. Nucleic Acids Res. 2002;30:2202–2211. doi: 10.1093/nar/30.10.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukao A., Sasano Y., Imataka H., Inoue K., Sakamoto H., Sonenberg N., Thoma C., Fujiwara T. The ELAV protein HuD stimulates cap–dependent translation in a Poly(A)– and eIF4A–dependent manner. Mol. Cell. 2009;36:1007–1017. doi: 10.1016/j.molcel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Kullmann M., Gopfert U., Siewe B., Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5’UTR. Genes Dev. 2002;16:3087–3099. doi: 10.1101/gad.248902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu H., Hasman R.A., Barron V.A., Luo G., Lou H. A nuclear function of Hu proteins as neuron–specific alternative RNA processing regulators. Mol. Biol. Cell. 2006;17:5105–5114. doi: 10.1091/mbc.e06-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu H., Hinman M.N., Hasman R.A., Mehta P., Lou H. Regulation of neuron–specific alternative splicing of neurofibromatosis type 1 pre–mRNA. Mol. Cell Biol. 2008;28:1240–1251. doi: 10.1128/MCB.01509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansfield K.D., Keene J.D. Neuron–specific ELAV/Hu proteins suppress HuR mRNA during neuronal differentiation by alternative polyadenylation. Nucleic Acids Res. 2012;40:2734–2746. doi: 10.1093/nar/gkr1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou H.L., Hinman M.N., Barron V.A., Geng C., Zhou G., Luo G., Siegel R.E., Lou H. Hu proteins regulate alternative splicing by inducing localized histone hyperacetylation in an RNA–dependent manner. Proc. Natl. Acad. Sci. USA. 2011;108:E627–E635. doi: 10.1073/pnas.1103344108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tebaldi T., Zuccotti P., Peroni D., Kohn M., Gasperini L., Potrich V., Bonazza V., Dudnakova T., Rossi A., Sanguinetti G., et al. HuD Is a Neural Translation Enhancer Acting on mTORC1–Responsive Genes and Counteracted by the Y3 Small Non–coding RNA. Mol. Cell. 2018;71:256–270 e210. doi: 10.1016/j.molcel.2018.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atlas R., Behar L., Elliott E., Ginzburg I. The insulin–like growth factor mRNA binding–protein IMP–1 and the Ras–regulatory protein G3BP associate with tau mRNA and HuD protein in differentiated P19 neuronal cells. J. Neurochem. 2004;89:613–626. doi: 10.1111/j.1471-4159.2004.02371.x. [DOI] [PubMed] [Google Scholar]
- 54.Yoo S., Kim H.H., Kim P., Donnelly C.J., Kalinski A.L., Vuppalanchi D., Park M., Lee S.J., Merianda T.T., Perrone–Bizzozero N.I., et al. A HuD–ZBP1 ribonucleoprotein complex localizes GAP–43 mRNA into axons through its 3’ untranslated region AU–rich regulatory element. J. Neurochem. 2013;126:792–804. doi: 10.1111/jnc.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akten B., Kye M.J., Hao Le T., Wertz M.H., Singh S., Nie D., Huang J., Merianda T.T., Twiss J.L., Beattie C.E., et al. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc. Natl. Acad. Sci. USA. 2011;108:10337–10342. doi: 10.1073/pnas.1104928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pandey P.R., Sarwade R.D., Khalique A., Seshadri V. Interaction of HuDA and PABP at 5’UTR of mouse insulin2 regulates insulin biosynthesis. PLoS ONE. 2018;13:e0194482. doi: 10.1371/journal.pone.0194482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fragkouli A., Koukouraki P., Vlachos I.S., Paraskevopoulou M.D., Hatzigeorgiou A.G., Doxakis E. Neuronal ELAVL proteins utilize AUF–1 as a co–partner to induce neuron–specific alternative splicing of APP. Sci. Rep. 2017;7:44507. doi: 10.1038/srep44507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bolognani F., Contente-Cuomo T., Perrone-Bizzozero N.I. Novel recognition motifs and biological functions of the RNA–binding protein HuD revealed by genome–wide identification of its targets. Nucleic Acids Res. 2010;38:117–130. doi: 10.1093/nar/gkp863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheckel C., Drapeau E., Frias M.A., Park C.Y., Fak J., Zucker–Scharff I., Kou Y., Haroutunian V., Ma’ayan A., Buxbaum J.D., et al. Regulatory consequences of neuronal ELAV–like protein binding to coding and non–coding RNAs in human brain. Elife. 2016;5 doi: 10.7554/eLife.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dell’Orco M., Oliver R.J., Perrone-Bizzozero N. HuD Binds to and Regulates Circular RNAs Derived from Neuronal Development– and Synaptic Plasticity–Associated Genes. Front. Genet. 2020;11 doi: 10.3389/fgene.2020.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung S., Eckrich M., Perrone-Bizzozero N., Kohn D.T., Furneaux H. The Elav–like proteins bind to a conserved regulatory element in the 3’–untranslated region of GAP–43 mRNA. J. Biol. Chem. 1997;272:6593–6598. doi: 10.1074/jbc.272.10.6593. [DOI] [PubMed] [Google Scholar]
- 62.Mobarak C.D., Anderson K.D., Morin M., Beckel-Mitchener A., Rogers S.L., Furneaux H., King P., Perrone-Bizzozero N.I. The RNA–binding protein HuD is required for GAP–43 mRNA stability, GAP–43 gene expression, and PKC–dependent neurite outgrowth in PC12 cells. Mol. Biol. Cell. 2000;11:3191–3203. doi: 10.1091/mbc.11.9.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson K.D., Morin M.A., Beckel-Mitchener A., Mobarak C.D., Neve R.L., Furneaux H.M., Burry R., Perrone-Bizzozero N.I. Overexpression of HuD, but not of its truncated form HuD I+II, promotes GAP–43 gene expression and neurite outgrowth in PC12 cells in the absence of nerve growth factor. J. Neurochem. 2000;75:1103–1114. doi: 10.1046/j.1471-4159.2000.0751103.x. [DOI] [PubMed] [Google Scholar]
- 64.Anderson K.D., Sengupta J., Morin M., Neve R.L., Valenzuela C.F., Perrone-Bizzozero N.I. Overexpression of HuD accelerates neurite outgrowth and increases GAP–43 mRNA expression in cortical neurons and retinoic acid–induced embryonic stem cells in vitro. Exp. Neurol. 2001;168:250–258. doi: 10.1006/exnr.2000.7599. [DOI] [PubMed] [Google Scholar]
- 65.Smith C.L., Afroz R., Bassell G.J., Furneaux H.M., Perrone-Bizzozero N.I., Burry R.W. GAP–43 mRNA in growth cones is associated with HuD and ribosomes. J. Neurobiol. 2004;61:222–235. doi: 10.1002/neu.20038. [DOI] [PubMed] [Google Scholar]
- 66.Bolognani F., Tanner D.C., Merhege M., Deschenes–Furry J., Jasmin B., Perrone-Bizzozero N.I. In vivo post–transcriptional regulation of GAP–43 mRNA by overexpression of the RNA–binding protein HuD. J Neurochem. 2006;96:790–801. doi: 10.1111/j.1471-4159.2005.03607.x. [DOI] [PubMed] [Google Scholar]
- 67.Bolognani F., Tanner D.C., Nixon S., Okano H.J., Okano H., Perrone-Bizzozero N.I. Coordinated expression of HuD and GAP–43 in hippocampal dentate granule cells during developmental and adult plasticity. Neurochem. Res. 2007;32:2142–2151. doi: 10.1007/s11064-007-9388-8. [DOI] [PubMed] [Google Scholar]
- 68.Lim C.S., Alkon D.L. Protein kinase C stimulates HuD–mediated mRNA stability and protein expression of neurotrophic factors and enhances dendritic maturation of hippocampal neurons in culture. Hippocampus. 2012;22:2303–2319. doi: 10.1002/hipo.22048. [DOI] [PubMed] [Google Scholar]
- 69.Allen M., Bird C., Feng W., Liu G., Li W., Perrone-Bizzozero N.I., Feng Y. HuD promotes BDNF expression in brain neurons via selective stabilization of the BDNF long 3’UTR mRNA. PLoS ONE. 2013;8:e55718. doi: 10.1371/journal.pone.0055718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ratti A., Fallini C., Colombrita C., Pascale A., Laforenza U., Quattrone A., Silani V. Post–transcriptional regulation of neuro–oncological ventral antigen 1 by the neuronal RNA–binding proteins ELAV. J. Biol. Chem. 2008;283:7531–7541. doi: 10.1074/jbc.M706082200. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z.H., Li S.J., Qi Y., Zhao J.J., Liu X.Y., Han Y., Xu P., Chen X.H. HuD regulates the cpg15 expression via the 3’–UTR and AU–rich element. Neurochem Res. 2011;36:1027–1036. doi: 10.1007/s11064-011-0443-0. [DOI] [PubMed] [Google Scholar]
- 72.Gomes C., Lee S.J., Gardiner A.S., Smith T., Sahoo P.K., Patel P., Thames E., Rodriguez R., Taylor R., Yoo S., et al. Axonal localization of neuritin/CPG15 mRNA is limited by competition for HuD binding. J. Cell Sci. 2017;130:3650–3662. doi: 10.1242/jcs.201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deschenes-Furry J., Belanger G., Perrone-Bizzozero N., Jasmin B.J. Post–transcriptional regulation of acetylcholinesterase mRNAs in nerve growth factor–treated PC12 cells by the RNA–binding protein HuD. J. Biol. Chem. 2003;278:5710–5717. doi: 10.1074/jbc.M209383200. [DOI] [PubMed] [Google Scholar]
- 74.Deschenes-Furry J., Mousavi K., Bolognani F., Neve R.L., Parks R.J., Perrone-Bizzozero N.I., Jasmin B.J. The RNA–binding protein HuD binds acetylcholinesterase mRNA in neurons and regulates its expression after axotomy. J. Neurosci. 2007;27:665–675. doi: 10.1523/JNEUROSCI.4626-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang F., Tidei J.J., Polich E.D., Gao Y., Zhao H., Perrone–Bizzozero N.I., Guo W., Zhao X. Positive feedback between RNA–binding protein HuD and transcription factor SATB1 promotes neurogenesis. Proc. Natl. Acad. Sci. USA. 2015;112:E4995–E5004. doi: 10.1073/pnas.1513780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang M.J., Abdelmohsen K., Hutchison E.R., Mitchell S.J., Grammatikakis I., Guo R., Noh J.H., Martindale J.L., Yang X., Lee E.K., et al. HuD regulates coding and noncoding RNA to induce APP––>Abeta processing. Cell Rep. 2014;7:1401–1409. doi: 10.1016/j.celrep.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim C.S., Alkon D.L. PKCepsilon promotes HuD–mediated neprilysin mRNA stability and enhances neprilysin–induced Abeta degradation in brain neurons. PLoS ONE. 2014;9:e97756. doi: 10.1371/journal.pone.0097756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Behar L., Marx R., Sadot E., Barg J., Ginzburg I. cis–acting signals and trans–acting proteins are involved in tau mRNA targeting into neurites of differentiating neuronal cells. Int. J. Dev. Neurosci. 1995;13:113–127. doi: 10.1016/0736-5748(95)00001-W. [DOI] [PubMed] [Google Scholar]
- 79.Aranda–Abreu G.E., Behar L., Chung S., Furneaux H., Ginzburg I. Embryonic lethal abnormal vision–like RNA–binding proteins regulate neurite outgrowth and tau expression in PC12 cells. J. Neurosci. 1999;19:6907–6917. doi: 10.1523/JNEUROSCI.19-16-06907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dell’Orco M., Sardone V., Gardiner A.S., Pansarasa O., Bordoni M., Perrone-Bizzozero N.I., Cereda C. HuD regulates SOD1 expression during oxidative stress in differentiated neuroblastoma cells and sporadic ALS motor cortex. Neurobiol. Dis. 2021;148:105211. doi: 10.1016/j.nbd.2020.105211. [DOI] [PubMed] [Google Scholar]
- 81.Ross R.A., Lazarova D.L., Manley G.T., Smitt P.S., Spengler B.A., Posner J.B., Biedler J.L. HuD, a neuronal–specific RNA–binding protein, is a potential regulator of MYCN expression in human neuroblastoma cells. Eur. J. Cancer. 1997;33:2071–2074. doi: 10.1016/S0959-8049(97)00331-6. [DOI] [PubMed] [Google Scholar]
- 82.Manohar C.F., Short M.L., Nguyen A., Nguyen N.N., Chagnovich D., Yang Q., Cohn S.L. HuD, a neuronal–specific RNA–binding protein, increases the in vivo stability of MYCN RNA. J. Biol. Chem. 2002;277:1967–1973. doi: 10.1074/jbc.M106966200. [DOI] [PubMed] [Google Scholar]
- 83.Joseph B., Orlian M., Furneaux H. p21(waf1) mRNA contains a conserved element in its 3’–untranslated region that is bound by the Elav–like mRNA–stabilizing proteins. J. Biol. Chem. 1998;273:20511–20516. doi: 10.1074/jbc.273.32.20511. [DOI] [PubMed] [Google Scholar]
- 84.Sosanya N.M., Cacheaux L.P., Workman E.R., Niere F., Perrone-Bizzozero N.I., Raab-Graham K.F. Mammalian Target of Rapamycin (mTOR) Tagging Promotes Dendritic Branch Variability through the Capture of Ca2+/Calmodulin–dependent Protein Kinase II alpha (CaMKIIalpha) mRNAs by the RNA–binding Protein HuD. J. Biol. Chem. 2015;290:16357–16371. doi: 10.1074/jbc.M114.599399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ratti A., Fallini C., Cova L., Fantozzi R., Calzarossa C., Zennaro E., Pascale A., Quattrone A., Silani V. A role for the ELAV RNA–binding proteins in neural stem cells: Stabilization of Msi1 mRNA. J. Cell Sci. 2006;119:1442–1452. doi: 10.1242/jcs.02852. [DOI] [PubMed] [Google Scholar]
- 86.Zimmerman A.J., Hafez A.K., Amoah S.K., Rodriguez B.A., Dell’Orco M., Lozano E., Hartley B.J., Alural B., Lalonde J., Chander P., et al. A psychiatric disease–related circular RNA controls synaptic gene expression and cognition. Mol. Psychiatry. 2020;25:2712–2727. doi: 10.1038/s41380-020-0653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sosanya N.M., Huang P.P.C., Cacheaux L.P., Chen C.J., Nguyen K., Perrone-Bizzozero N.I., Raab-Graham K.F. Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR–129 repression by mTORC1. J. Cell Biol. 2013;202:53–69. doi: 10.1083/jcb.201212089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ince-Dunn G., Okano H.J., Jensen K.B., Park W.Y., Zhong R., Ule J., Mele A., Fak J.J., Yang C., Zhang C., et al. Neuronal Elav–like (Hu) proteins regulate RNA splicing and abundance to control glutamate levels and neuronal excitability. Neuron. 2012;75:1067–1080. doi: 10.1016/j.neuron.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim C., Ahn S., Lee E.K. RNA binding protein HuD and microRNA–203a cooperatively regulate insulinoma–associated 1 mRNA. Biochem. Biophys. Res. Commun. 2020;521:971–976. doi: 10.1016/j.bbrc.2019.11.030. [DOI] [PubMed] [Google Scholar]
- 90.Kim C., Lee H., Kang H., Shin J.J., Tak H., Kim W., Gorospe M., Lee E.K. RNA–binding protein HuD reduces triglyceride production in pancreatic beta cells by enhancing the expression of insulin–induced gene 1. Biochim. Biophys. Acta. 2016;1859:675–685. doi: 10.1016/j.bbagrm.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim C., Kim W., Lee H., Ji E., Choe Y.J., Martindale J.L., Akamatsu W., Okano H., Kim H.S., Nam S.W., et al. The RNA–binding protein HuD regulates autophagosome formation in pancreatic beta cells by promoting autophagy–related gene 5 expression. J. Biol. Chem. 2014;289:112–121. doi: 10.1074/jbc.M113.474700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong Y., Tak H., Kim C., Kang H., Ji E., Ahn S., Jung M., Kim H.L., Lee J.H., Kim W., et al. RNA binding protein HuD contributes to beta–cell dysfunction by impairing mitochondria dynamics. Cell Death Differ. 2020;27:1633–1643. doi: 10.1038/s41418-019-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim C., Jeong D.E., Heo S., Ji E., Rho J.G., Jung M., Ahn S., Kim Y.J., Kim Y.S., Nam S.W., et al. Reduced expression of the RNA–binding protein HuD in pancreatic neuroendocrine tumors correlates with low p27(Kip1) levels and poor prognosis. J. Pathol. 2018;246:231–243. doi: 10.1002/path.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang H., Molfenter J., Zhu H., Lou H. Promotion of exon 6 inclusion in HuD pre–mRNA by Hu protein family members. Nucleic Acids Res. 2010;38:3760–3770. doi: 10.1093/nar/gkq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gong Q., Stump M.R., Zhou Z. Regulation of Kv11.1 potassium channel C–terminal isoform expression by the RNA–binding proteins HuR and HuD. J. Biol. Chem. 2018;293:19624–19632. doi: 10.1074/jbc.RA118.003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Subhadra B., Schaller K., Seeds N.W. Neuroserpin up–regulation in the Alzheimer’s disease brain is associated with elevated thyroid hormone receptor–beta1 and HuD expression. Neurochem. Int. 2013;63:476–481. doi: 10.1016/j.neuint.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amadio M., Pascale A., Wang J., Ho L., Quattrone A., Gandy S., Haroutunian V., Racchi M., Pasinetti G.M. nELAV proteins alteration in Alzheimer’s disease brain: A novel putative target for amyloid–beta reverberating on AbetaPP processing. J. Alzheimers Dis. 2009;16:409–419. doi: 10.3233/JAD-2009-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noureddine M.A., Qin X.J., Oliveira S.A., Skelly T.J., van der Walt J., Hauser M.A., Pericak-Vance M.A., Vance J.M., Li Y.J. Association between the neuron–specific RNA–binding protein ELAVL4 and Parkinson disease. Hum. Genet. 2005;117:27–33. doi: 10.1007/s00439-005-1259-2. [DOI] [PubMed] [Google Scholar]
- 99.Haugarvoll K., Toft M., Ross O.A., Stone J.T., Heckman M.G., White L.R., Lynch T., Gibson J.M., Wszolek Z.K., Uitti R.J., et al. ELAVL4, PARK10, and the Celts. Mov. Disord. 2007;22:585–587. doi: 10.1002/mds.21336. [DOI] [PubMed] [Google Scholar]
- 100.DeStefano A.L., Latourelle J., Lew M.F., Suchowersky O., Klein C., Golbe L.I., Mark M.H., Growdon J.H., Wooten G.F., Watts R., et al. Replication of association between ELAVL4 and Parkinson disease: The GenePD study. Hum. Genet. 2008;124:95–99. doi: 10.1007/s00439-008-0526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Santis R., Alfano V., de Turris V., Colantoni A., Santini L., Garone M.G., Antonacci G., Peruzzi G., Sudria–Lopez E., Wyler E., et al. Mutant FUS and ELAVL4 (HuD) Aberrant Crosstalk in Amyotrophic Lateral Sclerosis. Cell Rep. 2019;27:3818–3831 e3815. doi: 10.1016/j.celrep.2019.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tiruchinapalli D.M., Caron M.G., Keene J.D. Activity–dependent expression of ELAV/Hu RBPs and neuronal mRNAs in seizure and cocaine brain. J. Neurochem. 2008;107:1529–1543. doi: 10.1111/j.1471-4159.2008.05718.x. [DOI] [PubMed] [Google Scholar]
- 103.Hakak Y., Walker J.R., Li C., Wong W.H., Davis K.L., Buxbaum J.D., Haroutunian V., Fienberg A.A. Genome–wide expression analysis reveals dysregulation of myelination–related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dalmau J., Furneaux H.M., Gralla R.J., Kris M.G., Posner J.B. Detection of the anti–Hu antibody in the serum of patients with small cell lung cancer––a quantitative western blot analysis. Ann. Neurol. 1990;27:544–552. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- 105.Dalmau J., Furneaux H.M., Cordon–Cardo C., Posner J.B. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am. J. Pathol. 1992;141:881–886. [PMC free article] [PubMed] [Google Scholar]
- 106.Wang F., Lu J., Li S., Huo X., Liu X., Du X., Li C., Wang J., Chen Z. Application of Serum ELAVL4 (HuD) Antigen Assay for Small Cell Lung Cancer Diagnosis. Anticancer Res. 2017;37:4515–4522. doi: 10.21873/anticanres.11848. [DOI] [PubMed] [Google Scholar]
- 107.King P.H. Differential expression of the neuroendocrine genes Hel–N1 and HuD in small–cell lung carcinoma: Evidence for down–regulation of HuD in the variant phenotype. Int. J. Cancer. 1997;74:378–382. doi: 10.1002/(SICI)1097-0215(19970822)74:4<378::AID-IJC3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 108.D’Alessandro V., Muscarella L.A., Copetti M., Zelante L., Carella M., Vendemiale G. Molecular detection of neuron–specific ELAV–like–positive cells in the peripheral blood of patients with small–cell lung cancer. Cell Oncol. 2008;30:291–297. doi: 10.3233/clo-2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ball N.S., King P.H. Neuron–specific hel–N1 and HuD as novel molecular markers of neuroblastoma: A correlation of HuD messenger RNA levels with favorable prognostic features. Clin. Cancer Res. 1997;3:1859–1865. [PubMed] [Google Scholar]
- 110.Chagnovich D., Fayos B.E., Cohn S.L. Differential activity of ELAV–like RNA–binding proteins in human neuroblastoma. J. Biol. Chem. 1996;271:33587–33591. doi: 10.1074/jbc.271.52.33587. [DOI] [PubMed] [Google Scholar]
- 111.Cuadrado A., Navarro-Yubero C., Furneaux H., Munoz A. Neuronal HuD gene encoding a mRNA stability regulator is transcriptionally repressed by thyroid hormone. J. Neurochem. 2003;86:763–773. doi: 10.1046/j.1471-4159.2003.01877.x. [DOI] [PubMed] [Google Scholar]
- 112.Loffreda A., Nizzardo M., Arosio A., Ruepp M.D., Calogero R.A., Volinia S., Galasso M., Bendotti C., Ferrarese C., Lunetta C., et al. miR–129–5p: A key factor and therapeutic target in amyotrophic lateral sclerosis. Prog. Neurobiol. 2020;190:101803. doi: 10.1016/j.pneurobio.2020.101803. [DOI] [PubMed] [Google Scholar]
- 113.Popovitchenko T., Park Y., Page N.F., Luo X., Krsnik Z., Liu Y., Salamon I., Stephenson J.D., Kraushar M.L., Volk N.L., et al. Translational derepression of Elavl4 isoforms at their alternative 5’ UTRs determines neuronal development. Nat. Commun. 2020;11:1674. doi: 10.1038/s41467-020-15412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hubers L., Valderrama-Carvajal H., Laframboise J., Timbers J., Sanchez G., Cote J. HuD interacts with survival motor neuron protein and can rescue spinal muscular atrophy–like neuronal defects. Hum. Mol. Genet. 2011;20:553–579. doi: 10.1093/hmg/ddq500. [DOI] [PubMed] [Google Scholar]
- 115.Fujiwara T., Mori Y., Chu D.L., Koyama Y., Miyata S., Tanaka H., Yachi K., Kubo T., Yoshikawa H., Tohyama M. CARM1 regulates proliferation of PC12 cells by methylating HuD. Mol. Cell Biol. 2006;26:2273–2285. doi: 10.1128/MCB.26.6.2273-2285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pascale A., Amadio M., Scapagnini G., Lanni C., Racchi M., Provenzani A., Govoni S., Alkon D.L., Quattrone A. Neuronal ELAV proteins enhance mRNA stability by a PKCalpha–dependent pathway. Proc. Natl. Acad. Sci. USA. 2005;102:12065–12070. doi: 10.1073/pnas.0504702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nussbacher J.K., Tabet R., Yeo G.W., Lagier-Tourenne C. Disruption of RNA Metabolism in Neurological Diseases and Emerging Therapeutic Interventions. Neuron. 2019;102:294–320. doi: 10.1016/j.neuron.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.