Summary
The diagnosis of bland-looking spindle cell lesions of the breast is often challenging because there is a close morphological and immunohistochemical overlap among the different entities. The present review will discuss reactive spindle cell nodule/exuberant scar, nodular fasciitis, inflammatory pseudotumor, myofibroblastoma (classic type), lipomatous myofibroblastoma, palisaded myofibroblastoma, benign fibroblastic spindle cell tumor, spindle cell lipoma, fibroma, leiomyoma, solitary fibrous tumor, myxoma, schwannoma/neurofibroma, desmoid-type fibromatosis, dermatofibrosarcoma protuberans, low-grade fibromatosis-like spindle cell carcinoma, inflammatory myofibroblastic tumor and low-grade myofibroblastic sarcoma arising in the breast parenchyma. The pathologist should be aware of each single lesion to achieve a correct diagnosis to ensure patient a correct prognostic information and therapy. Accordingly representative illustrations and morphological/immunohistochemical diagnostic clues will be provided.
Key words: Spindle cell tumors, Breast parenchyma, Differential diagnosis, Diagnostic approach
Introduction
Bland-looking spindle cell lesions of the breast comprise a heterogeneous group of tumor-like and tumor entities, ranging from reactive to low-grade malignant neoplasms with metastatic potential 1. (Tab. I). Accordingly, differential diagnosis between the benign spindle cell lesions and the potentially aggressive tumors is mandatory to avoid overdiagnosis and overtreatment. However, this distinction is often challenging in daily practice, especially in needle core biopsies, due to the morphological and immunohistochemical overlap exhibited by the different lesions that often share bland-looking spindle cells with the morphological features of fibroblasts/myofibroblasts, arranged haphazardly or in short fascicles or with focal storiform growth pattern, and set in a variable fibro-myxoid stroma. Pathologists should be aware of the morphological and immunohistochemical differences between fibroblasts and myofibroblasts when dealing with a spindle cell lesion of the breast. Recognizing a lesion as predominantly fibroblastic or myofibroblastic in nature may help in the diagnostic approach. Fibroblastic lesions are mainly or entirely composed of elongated spindle cells with scant, pale to slightly eosinophilic cytoplasm, elongated nuclei with absent or only inconspicuous nucleoli. Fibroblasts are usually stained with vimentin and CD34, generic mesenchymal markers lacking any specificity of differentiation cell lineage. Focal and weak staining with α-smooth muscle actin can be seen. Conversely myofibroblasts -modified fibroblasts with the capability to contract- are plumper than fibroblasts, showing more abundant slightly to deeply eosinophilic cytoplasm and ovoid nuclei with evident small nucleoli. Unlike fibroblasts, myofibroblasts exhibit a more diffuse and strong staining for α-smooth muscle actin; some lesions, like myofibroblastoma, are typically stained with desmin more than with α-smooth muscle actin. Finally, the pathologist should be aware that the neoplastic cells of the breast carcinoma, as like in other carcinomas, may adopt a spindled morphology raising confusion with benign/low-grade mesenchymal lesions. This phenomenon is related to an epithelial-mesenchymal transition, i.e. a biologic process due to plasticity of the cells, that consists in the progressive loss of epithelial morphological and immunohistochemical features and gain of a mesenchymal cell profile, including the expression of vimentin and α-smooth muscle actin. Low-grade, fibromatosis-like spindle cell carcinoma is a prototypical example of an epithelial-mesenchymal transition 10.
Tab. I.
Bland-looking spindle cell lesions of the breast.
| Reactive lesions | |
| |
| Benign tumors | |
| Specific to mammary stroma | Not specific to mammary stroma |
|
|
| Low-grade tumors, locally aggressive | |
| |
| Low-grade tumors with metastatic potential | |
| |
In our opinion, the tumor-like and tumor spindle cell lesions of the breast are often underrecognized, with confusion in the distinction between reactive versus neoplastic (benign or low-grade malignant) lesions. Although some difficulties are due the fact that different names are often used to indicate the same entity, it is also true that a single name is applied to biologically different lesions. In addition, we think that potential diagnostic errors are likely to occur because the pathologist: i) is faced with an unfamiliar lesion (myofibroblastoma; low-grade myofibroblastic sarcoma; low-grade fibromatosis-like spindle cell carcinoma); ii) pathologist is not familiar with soft tissue pathology (nodular fasciitis; desmoid-type fibromatosis; solitary fibrous tumor; low-grade myofibroblastic sarcoma); iii) may encounter diagnostic difficulties when dealing with a typical soft tissue lesion/tumor occurring in an unexpected site, such as in the breast (nodular fasciitis, desmoid-type fibromatosis, solitary fibrous tumor, leiomyoma, schwannoma, spindle cell lipoma). The present overview focuses on the morphological and immunohistochemical features helpful to recognize each single entity in the wide spectrum of the bland-looking spindle cell lesions of breast parenchyma (Tab. II). Representative illustrations along with the main diagnostic clues are provided (Tab. III).
Tab. II.
Key diagnostic features.
| Reactive Spindle Cell Nodule/Exuberant Scar: circumscribed and, at least focally, infiltrative margins; previous biopsy/FNA; α-smooth muscle actin-positive spindle cells; foamy and hemosiderin-laden macrophages, lymphocytes and foreign body giant cells; fat necrosis |
| Nodular Fasciitis: circumscribed and, at least focally, infiltrative margins; α-smooth muscle actin-positive spindle cells; fibro-myxoid stroma, at least focally, with tissue culture-like appearance |
| Inflammatory Pseudotumor: circumscribed and, at least focally, infiltrative margins; α-smooth muscle actin-positive spindle cells closely intermingling with lymphocytes and plasma cells; previous history of local trauma/stimuli; ALK-1 is negative |
| Myofibroblastoma, Classic-type: circumscribed margins; desmin/CD34/α-smooth muscle actin-positive spindle cells; short intersecting fascicles interrupted by keloid-like collagen fibers |
| Lipomatous Myofibroblastoma: circumscribed margins; desmin/CD34/α-smooth muscle actin-positive spindle cells with finger-like pseudo-infiltration into an intratumoral lipomatous component |
| Palisaded/Schwannian Myofibroblastoma: circumscribed margins; desmin/CD34/α-smooth muscle actin-positive spindle cells with formation of Verocay-like bodies; S100 protein is negative |
| Benign Fibroblastic Spindle Cell Tumor: circumscribed margins; CD34-positive fibroblastic-like spindle cells; short intersecting fascicles; thick keloid-like collagen fibers; variable additional lipomatous component |
| Spindle Cell Lipoma: circumscribed margins; CD34-positive short spindle cells with bipolar cytoplasmic processes; variably admixed mature lipomatous component; at least focally, myxoid stroma with ropey collagen fibers |
| Fibroma: circumscribed margins; hypocellular, fibrosclerotic nodule with interspersed CD34-positive fibroblast-like spindle cells |
| Solitary Fibrous Tumor: circumscribed margins; CD34/STAT6-positive fibroblast-like spindle cells, haphazardly arranged (pattern-less growth pattern); branching vessels, often with perivascular hyalinization |
| Leiomyoma: circumscribed margins; interlacing fascicles of desmin/α-smooth muscle actin/h-caldesmon-positive spindle cells with the features of mature smooth muscle cells |
| Myxoma: circumscribed margins; vimentin positive spindle to stellate cells embedded in an abundant myxoid stroma; atypical bizarre cells, along with thick keloid-like collagen fibers, can be seen |
| Schwannoma/Neurofibroma: circumscribed margins; S100-positive spindle cells with formation of Verocay-bodies and alternating Antoni A and Antoni B areas (schwannoma); cells with wavy nuclei set in myxoid stroma with keloid-like collagen fibers (neurofibroma) |
| Desmoid-type Fibromatosis: finger-like infiltrative margins; α-smooth muscle actin and β-catenin-positive fibroblast/myofibroblast-like spindle cells arranged into long intersecting fascicles; the cells are often aligned parallel and are separated by collagenized stroma |
| Dermatofibrosarcoma Protuberans: circumscribed and, at least focally, infiltrative margins; CD34-positive fibroblast-like spindle cells; diffuse storiform growth pattern; low mitotic activity; finger-like or honeycomb infiltration of the adjacent fibro-fatty tissue |
| Low-grade, Fibromatosis-like Spindle Cell Carcinoma: finger-like infiltrative margins; p63/cytokeratin-positive spindle cells with the features of fibroblasts/myofibroblasts; at least focally, small cohesive clusters of cytokeratin/p63-positive epithelioid-polygonal cells |
| Inflammatory Myofibroblastic Tumor: circumscribed and, at least focally, infiltrative margins; α-smooth muscle actin-positive spindle cells admixed with lymphocytes and plasma cells; no association with previous history of local trauma/stimuli; ALK-1 expression in about 40-50% of cases |
| Low-grade Myofibroblastic Sarcoma: circumscribed and, at least focally, infiltrative margins; α-smooth muscle actin-positive myofibroblastic-like cells with mild/moderate nuclear pleomorphism and high mitotic activity (7 to 35 mitoses x 10 HPF); fascicular arrangement |
Tab. III.
Differential diagnoses between benign versus low-grade lesions.
| Nodular fasciits versus Desmoid-type Fibromatosis |
| Shared features: at least focally, infiltrative margins with entrapment of mammary ducts/lobules; α-smooth muscle actin-positive spindle cells in a variable fibro-myxoid stroma |
| Distinguishing features: desmoid-type fibromatosis shows long intersecting fascicles with cells aligned parallel, whereas nodular fasciitis exhibits cells haphazardly arranged or forming short fascicles with focal storiform growth pattern; unlike nodular fasciitis, desmoid-type fibromatosis is usually stained with β-catenin (nuclear staining) |
| Lipomatous Myofibroblastoma versus Desmoid-type Fibromatosis |
| Shared features: α-smooth muscle actin-positive spindle cells in a fibrous stroma, with finger-like extension into mature adipose tissue |
| Distinguishing features: desmoid-type fibromatosis exhibits infiltrative margins, whereas lipomatous myofibroblastoma shows pushing borders; adipose tissue is an integral part of the lipomatous myofibroblastoma, whereas adipose tissue in desmoid-type fibromatosis is mammary fat infiltrated by neoplastic cells; lipomatous myofibroblastoma is stained with desmin, CD34 and estrogen/progesterone receptors, whereas desmoid-type fibromatosis is negative to these markers, but positive for β-catenin |
| Classic-type Myofibroblastoma versus Low-grade Myofibroblastic Sarcoma |
| Shared features: circumscribed borders, α-smooth muscle actin-spindle cells arranged in short fascicles with variable fibro-myxoid stroma |
| Distinguishing features: low-grade myofibroblastic sarcoma is more cellular and the spindle cells show, at least focally, moderate nuclear pleomorpshism, nuclear overlapping, as well as high mitotic activity (7 to 35 mitoses x 10 HPF); myofibroblastoma is a tumor with absent to low mitotic activity (up to 2 mitoses x 10HPF), that variably co-expresses desmin, CD34 and estrogen/progesterone receptors |
| Desmoid-type Fibromatosis versus Low-grade Fibromatosis-like Spindle Cell Carcinoma |
| Shared features: infiltrative margins with entrapment of mammary ducts/lobules and fat; α-smooth muscle actin-positive spindle cells set in a fibrous stroma |
| Distinguishing features: desmoid-type fibromatosis shows long intersecting fascicles with cells often aligned parallel, but lacks pancytokeratins/p63-positive spindle to focally epithelioid-polygonal cells arranged in small cohesive clusters |
Reactive lesions
REACTIVE SPINDLE CELL NODULE/EXUBERANT SCAR 11-15
It should be suspected in presence of a fairly circumscribed nodule, arising after biopsy/FNAC or surgical procedures, composed of α-smooth muscle actin-positive spindle cells with the features of myofibroblasts, set in a variably fibro-myxoid stroma containing both foamy and hemosiderin-laden macrophages, lymphocytes and foreign body giant cells (Fig. 1A). Fat necrosis and entrapment or displacement of normal mammary glands/ducts are frequently encountered (Fig. 1A,B). The reactive spindle cells may be, at least focally, arranged into short fascicles (Fig. 1C), focally exhibiting a storiform growth pattern. Mitotic activity is low, ranging from 1 to 4 mitoses x 10 high power fields.
Fig. 1.
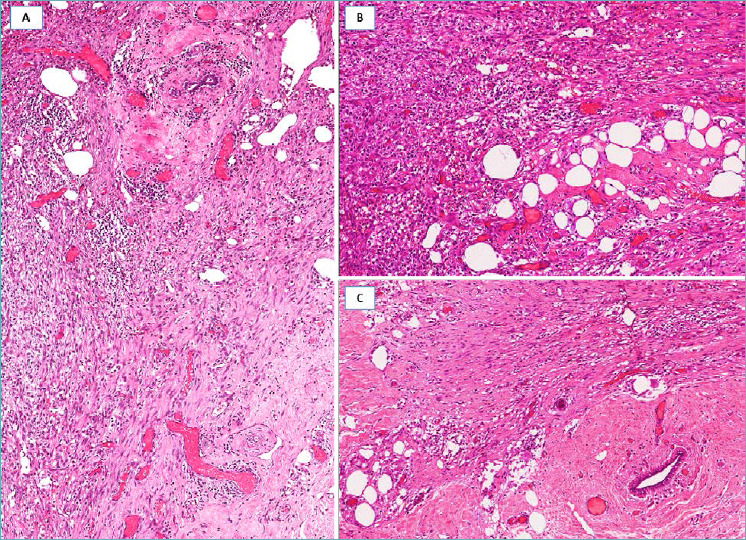
Reactive spindle cell nodule/exuberant scar. (A) Fibro-inflammatory tissue with spindle cells; (B) fat necrosis is a diagnostic clue; (C) fibro-sclerotic stroma with interspersed spindle cells, entrapping a mammary duct.
NODULAR FASCIITIS 16-20
It should be suspected in presence of a nodule with partially circumscribed margins, composed of a proliferation of α-smooth muscle actin-positive spindle cells with the features of myofibroblasts and brisk mitotic activity; the cells are arranged into short, not well-formed fascicles and focally in whorls or storiform growth pattern (Fig. 2A). The stroma, variably myxoid with microcystic degeneration to fibrous in nature (Fig. 2B) and containing red blood cells and lymphocytes, shows, at least focally, a tissue culture-like morphology (Fig. 2C). Mammary ducts/lobules can be entrapped, especially at the periphery of the lesion (Fig. 2A). Surgical excision is curative with rare local recurrence (< 2% of cases, including lesions incompletely excised).
Fig. 2.
Nodular fasciitis. (A) Spindle cell proliferation with fibrous stroma and entrapped mammary ducts at the periphery of the lesion; (B) area with myxo-edematous stroma containing inflammatory cells (tissue culture-like appearance); (C) extravasated erythrocytes can be seen.
INFLAMMATORY PSEUDOTUMOR 21-27
It should be suspected in the presence of a fairly circumscribed nodule arising in association with local trauma/stimuli; it is composed of α-smooth muscle actin-positive spindle cells with the features of myofibroblasts, closely intermingling with lymphocytes and plasma cells (Fig. 3A, B); cells are usually arranged in interlacing short bundles (Fig. 3C) or may exhibit swirling/storiform growth pattern; atypical/bizarre mono- or multi-nucleated cells can be, at least focally, encountered (Fig. 3D); defining the boundaries between a reactive (inflammatory pseudotumor) versus a true neoplastic process (inflammatory myofibroblastic tumor) still remains to be established. Surgical excision is curative.
Fig. 3.
Inflammatory pseudotumor (male patient with local breast trauma). (A) Spindle cells intermingling with inflammatory cells; (B) spindle cells are stained with α-smooth muscle actin; (C) spindle cells are arranged in a swirling growth pattern; (D) atypical/bizarre cells can be seen.
Benign tumors
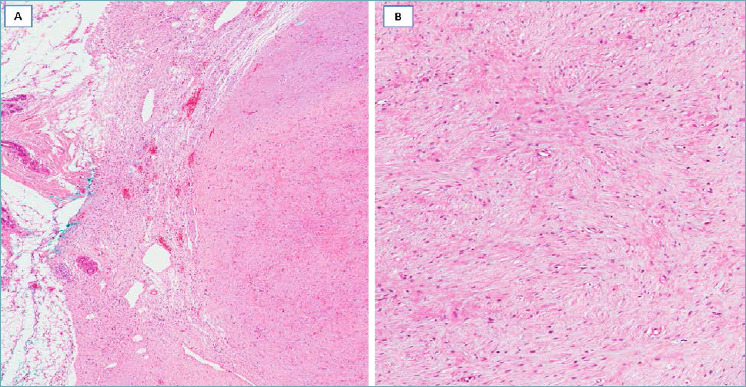
MYOFIBROBLASTOMA, CLASSIC-TYPE 28,29
It should be suspected in presence of a well-circumscribed nodule (Fig. 4A) composed of a proliferation of desmin/CD34/α-smooth muscle actin-positive spindle cells with the features of myofibroblasts, arranged into short, haphazardly intersecting fascicles interrupted by thick keloid-like collagen bands (Fig. 4B). Focal storiform or neural-like growth patterns can be seen, including a minor component of neoplastic cells with epithelioid morphology. Mitotic activity is low (0-2 mitoses x 10 high power field). The stroma is usually fibrous to focally myxoid and may contain islands of mature adipose tissue. Mast cells are variably interspersed among neoplastic cells. Usually no entrapment of mammary ducts/lobules is seen. Surgical excision is curative.
Fig. 4.
Myofibroblastoma, classic-type. (A) A spindle cell tumor with pushing margins and numerous keloid-like collagen fibers; (B) cells, with eosinophilic cytoplasm and oval nuclei, are arranged in short fascicles with interspersed keloid-like collagen fibers.
LIPOMATOUS MYOFIBROBLASTOMA 30-33
It should be suspected in presence of a well-circumscribed fibro-fatty nodule (Fig. 5A), composed of a proliferation of desmin/CD34/α-smooth muscle actin-positive spindle cells with the features of myofibroblasts that exhibit a finger-like pseudo-infiltration into an intratumoral lipomatous component (Fig. 5B). Although this pattern is reminiscent of desmoid-type fibromatosis or low-grade (fibromatosis-like) spindle cell carcinoma, tumor margins are pushing and not infiltrative (Fig. 5B); areas with the typical features of myofibroblastoma are identified, at least focally (Fig. 5C). Surgical excision is curative.
Fig. 5.
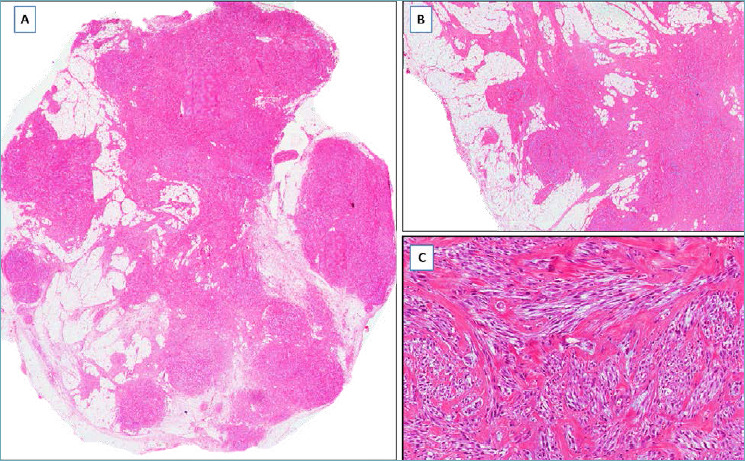
Lipomatous myofibroblastoma. (A) Fibrolipomatous tumor with pushing borders; (B) the fibrous component exhibits a finger-like infiltration into the lipomatous component, but the margins are circumscribed; (C) tumor area with the characteristics of classic-type myofibroblastoma: fascicles of spindle cells separated by keloid-like collagen bands.
PALISADED MYOFIBROBLASTOMA 34-36
It should be suspected in presence of a well-circumscribed nodule, histologically reminiscent of schwannoma (Fig. 6A). It is composed of desmin/CD34/α-smooth muscle actin-positive spindle cells exhibiting a prominent nuclear palisading, with formation of numerous Verocay-like bodies: two compact rows of well aligned nuclei separated by myxoid matrix (Fig. 6A-C). Mitotic activity is low (0-2 mitoses x 10 high power field). As in other myofibroblastoma variants, keloid-like eosinophilic collagen fibers are dispersed throughout the myxoid stroma and between neoplastic cells; areas with the typical features of myofibroblastoma can be seen, at least focally. Surgical excision is curative.
Fig. 6.
Palisaded/Schwannoma-like Myofibroblastoma. (A) Tumor with pushing borders, closely reminiscent of Schwannoma; (B) higher magnification showing nuclear palisading with formation of Verocay-like bodies; (C) cells, negative to S100 protein, are stained with α-smooth muscle actin, revealing their myofibroblastic nature.
BENIGN FIBROBLASTIC SPINDLE CELL TUMOR 37-44
It should be suspected in presence of a well-circumscribed nodule (Fig. 7A) composed of a proliferation of CD34-positive spindle cells with the features of fibroblasts arranged haphazardly, or in intersecting short fascicles with interspersed keloid-like collagen fibers (Fig. 7B); mitoses are absent to low (0-2 mitoses x 10 high power field). The stroma is collagenized and may contain a prominent lipomatous component (spindle cell lipoma-like morphology). Surgical excision is curative.
Fig. 7.
Benign fibroblastic spindle cell tumor. (A) A fibrous tumor with circumscribed borders; (B) spindle cells look like fibroblasts and are arranged in short fascicles set in a collagenized stroma.
SPINDLE CELL LIPOMA 45-48
It should be suspected in presence of a circumscribed nodule composed of a proliferation of CD34-positive, short spindle cells, variably admixed with mature adipocytes (Fig. 8A) and set in, at least focally, myxoid stroma containing ropey collagen fibers (Fig. 8B). In the myxoid areas the spindle cells often show long and thin bipolar cytoplasmic processes (Fig. 8B). Mitoses are absent or rare; mast cells are variably scattered throughout the tumor. Surgical excision is curative.
Fig. 8.
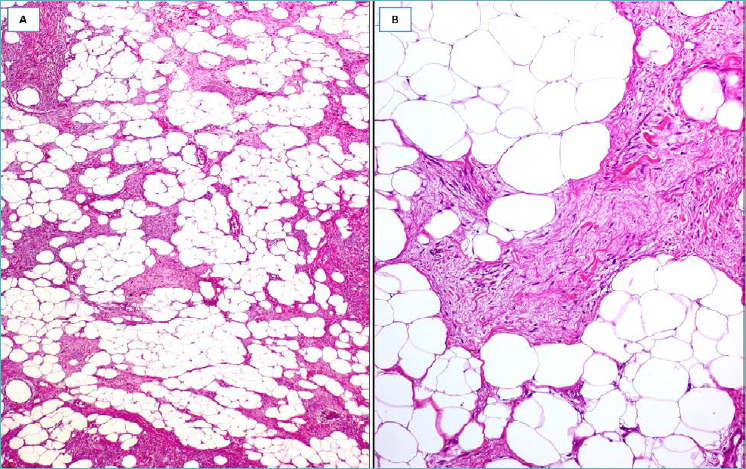
Spindle cell lipoma. (A) A fatty-tumor with interspersed fibro-myxoid areas; (B) higher magnification: myxoid area showing spindle cells with long cytoplasmic bipolar processes and ropey collagen fibers.
FIBROMA 43,49
It should be suspected in presence of a well-circumscribed, hypocellular nodule (Fig. 9A) composed of CD34-positive spindle cells with the features of fibroblasts, haphazardly dispersed in a heavily collagenized stroma (Fig. 9B) in which mammary ducts/lobules can be entrapped; mitotic activity is absent. Surgical excision is curative.
Fig. 9.
Fibroma. (A) A fibrous hypocellular tumor with circumscribed margins; (B) higher magnification showing fibroblast-like spindle cells set in a collagenized stroma.
LEIOMYOMA 50-53
It should be suspected in presence of a well-circumscribed nodule (Fig. 10A) composed of interlacing fascicles of desmin/α-smooth muscle actin/h-caldesmon-positive spindle cells with the features of mature smooth muscle cells (deeply eosinophilic cytoplasm with elongated nuclei with blunt ends) (Fig. 10B); absent to low mitotic activity. Surgical excision is curative.
Fig. 10.
Leiomyoma. (A) Spindle cell tumor with circumscribed borders and fascicular growth pattern; (B) higher magnification showing smooth muscle cells with deep eosinophilic cytoplasm.
SOLITARY FIBROUS TUMOR 43,44,54-61
It should be suspected in presence of a well-circumscribed nodule (Fig. 11A) composed of CD34/STAT6-positive spindle cells with the features of fibroblasts, low mitotic activity (<4 mitoses x 10 HPF), and haphazardly arranged in a fibrous to focally myxoid stroma containing branching vessels, often with perivascular hyalinization (Fig. 11B, C). Surgical excision is curative. Pathologists should always search for morphological features that can be associated with an aggressive clinical course, including >4 mitoses x 10 HPF, nuclear pleomorphism, hypercellularity, necrosis, sarcomatous dedifferentiation.
Fig. 11.
Solitary fibrous tumor. (A) Spindle cell tumor with pushing margins; (B) neoplastic cells are set in a fibrous stroma containing branching blood vessels with perivascular fibrosis; (C) neoplastic cells show diffuse nuclear staining with STAT-6.
MYXOMA 62-66
It should be suspected in presence of a well-circumscribed nodule (Fig. 12A) composed of vimentin-positive spindle- to stellate-shaped cells dispersed in abundant/exclusive myxoid stroma (Fig. 12B); stromal microcystic spaces simulating lipoblasts are seen. Atypical/bizarre cells, as well as keloid-like collagen fibers, can be occasionally observed (Fig. 12C). Mitotic activity is absent. Surgical excision is curative.
Fig. 12.
Myxoma. (A) A myxoid tumor with circumscribed margins; (B) higher magnification showing spindle and stellate cells embedded in abundant myxoid stroma; stromal microcystic spaces look like univacuolated lipoblasts; (C) neoplastic cells may exhibit nuclear atypia (bizarre cells) and the myxoid stroma may contain keloid-like collagen fibers.
SCHWANNOMA/NEUROFIBROMA 51,67-76
It should be suspected in presence of a nodular mass with circumscribed margins, composed of S100-positive spindle cells with wavy nuclei and absent to low mitotic activity; schwannoma shows interlacing fascicles and whorls, as well as palisading nuclei (Verocay bodies) and alternating hypercellular (Antoni A areas) and hypocellular (Antoni B) areas; in neurofibroma the spindle cells are usually haphazardly arranged in a slightly myxoid stroma containing thick collagen fibers. Surgical excision is curative.
Low-grade tumors, locally aggressive
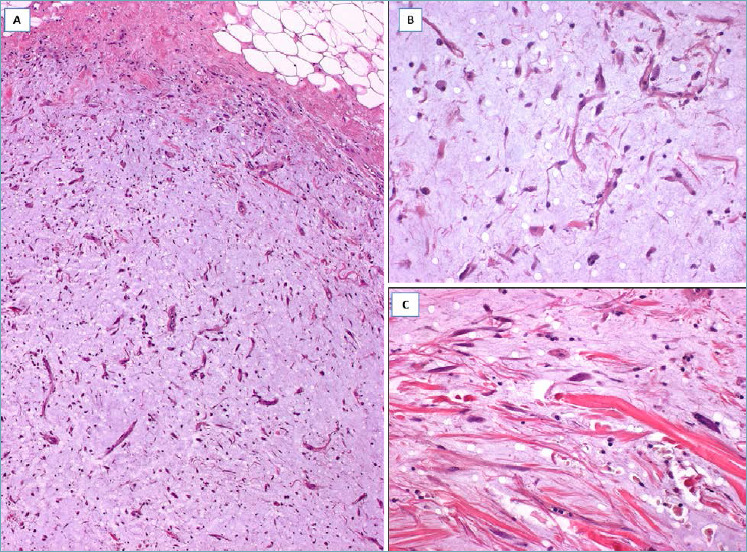
DESMOID-TYPE FIBROMATOSIS77-82
It should be suspected in presence of a nodular mass with finger-like infiltrative margins (Fig. 13A), composed of spindle cells with the features of both fibroblasts and myofibroblasts Characteristically the neoplastic cells, often aligned parallel, are arranged in long and sweeping fascicles set in a prominent fibrous to focally myxoid stroma (Fig. 13B,C). Mitoses are rare. These cells are variably stained with α-smooth muscle actin and β-catenin (nuclear staining in about 80% of cases) (Fig. 13C). Desmoid-type fibromatosis is a locally aggressive tumor that can recur locally but with no metastatic potential.
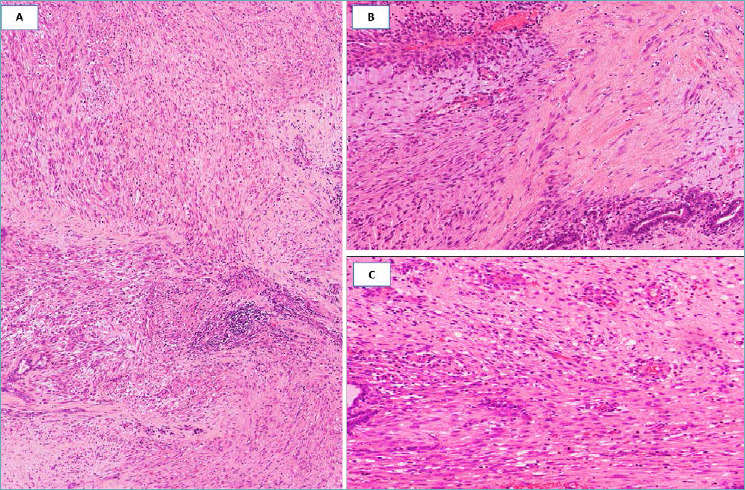
Fig. 13.
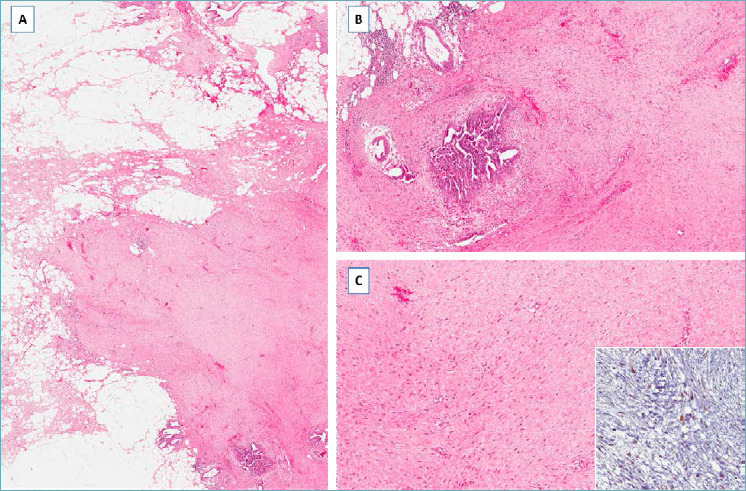
Desmoid-type Fibromatosis. (A) Fibrous proliferation with infiltrative margins; (B) bland-looking spindle cells entrap pre-existing mammary ducts; (C) higher magnification showing spindle cells aligned parallel and separated by a fibrous stroma. Neoplastic cells show nuclear expression of β-catenin (insert).
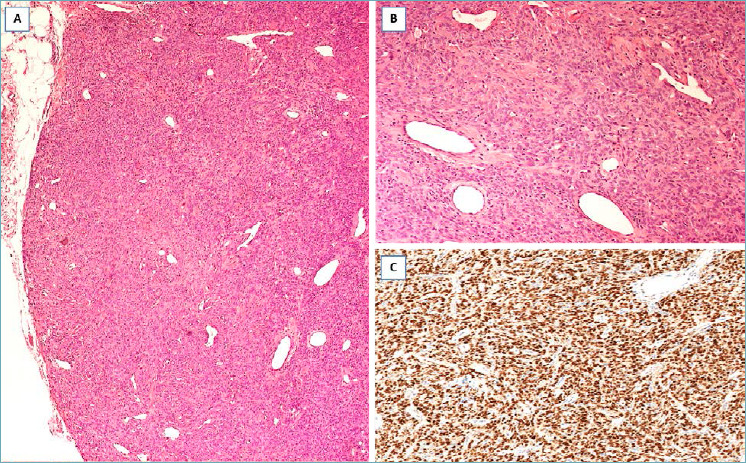
DERMATOFIBROSARCOMA PROTUBERANS 83-87
It should be suspected in presence of a nodular mass with relatively circumscribed margins, composed of CD34-positive spindle cells with the features of fibroblasts, low mitotic activity, and diffusely arranged in a storiform growth pattern with finger-like or honeycomb infiltration of the adjacent fibro-fatty tissue (Fig. 14 A-C). Radical excision is curative. Local recurrence is usually due to incomplete surgical excision.
Fig. 14.
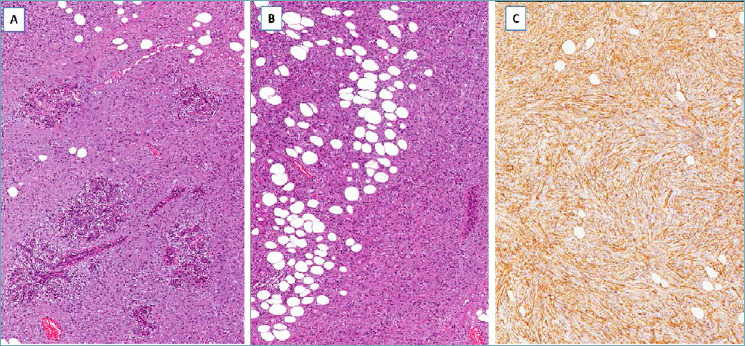
Dermatofibrosarcoma protuberans. (A) Spindle cell tumor surrounding pre-existing duct/lobular units; (B) the neoplastic cells diffusely infiltrate adipose tissue; (C) neoplastic cells are diffusely stained with CD34.
Low-grade tumors with metastatic potential
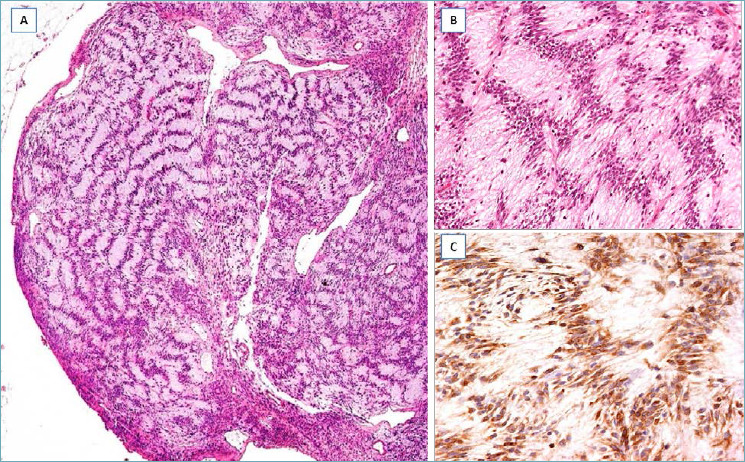
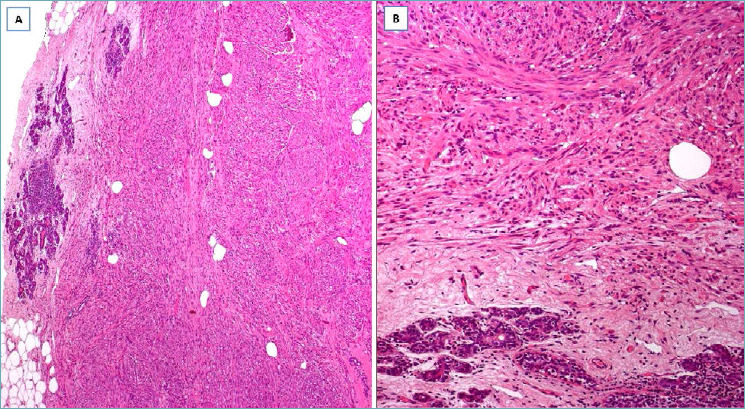
LOW-GRADE FIBROMATOSIS-LIKE SPINDLE CELL CARCINOMA 88-89
It should be suspected in presence of a nodular mass with finger-like infiltrative margins (Fig. 15A), composed of p63/cytokeratin-positive spindle cells (Fig. 15B) and low mitotic activity; variable co-expression of α-smooth muscle actin can be seen. The identification, at least focally, of epithelioid-polygonal cells arranged in small cohesive clusters (Fig. 15C), better highlighted by immunohistochemistry (Fig. 15D) is the main diagnostic clue for this type of carcinoma. A minority of squamous nests and/or small-sized neoplastic glands can be encountered. Estrogen/progesterone receptors and HER2 are negative (triple-negative carcinoma). This carcinoma can recur locally, with low metastatic potential (lymph node or distant metastases). Radical excision is curative in most cases.
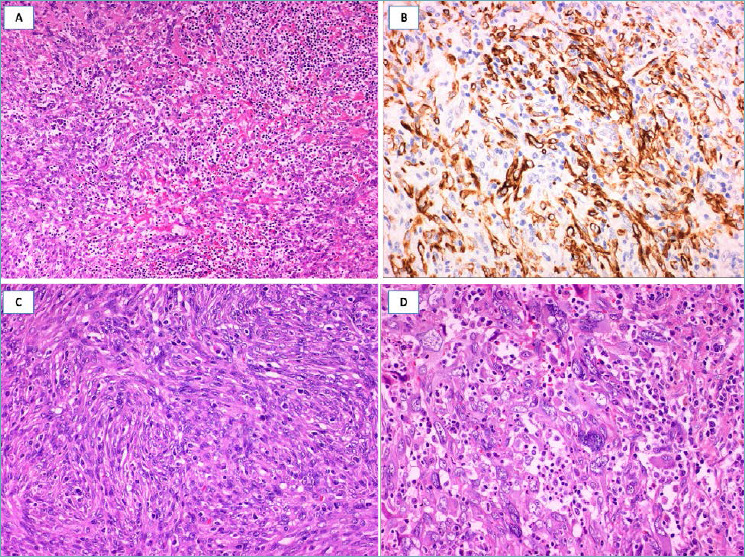
Fig. 15.
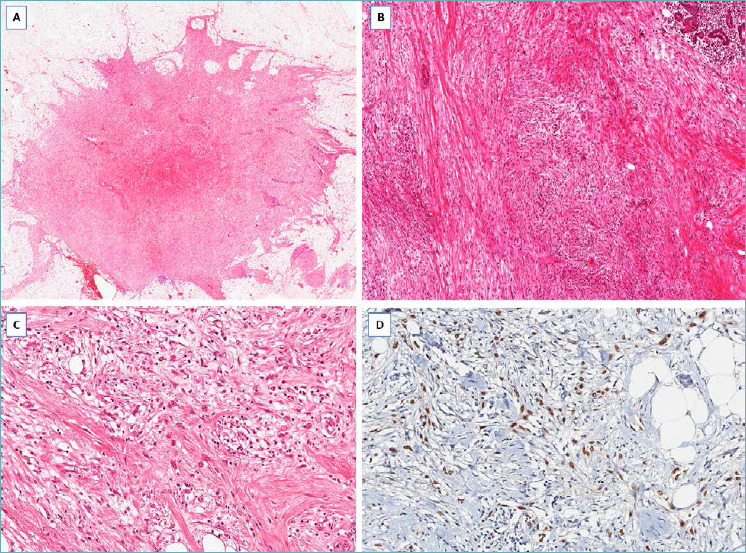
Low-grade fibromatosis-like spindle cell carcinoma. (A) Low-magnification showing a fibrous tumor with finger-like infiltrative margins; (B) bland-looking spindle cells are set in a fibrous stroma and exhibit a fascicular arrangement; (C) some tumor areas show single or small groups of round to epithelioid cells scattered throughout the fibrous stroma; (D) these neoplastic cells show nuclear expression of p63.
INFLAMMATORY MYOFIBROBLASTIC TUMOR 90-94
It should be suspected in presence of a nodular mass with relatively circumscribed margins, with morphological features similar to inflammatory pseudotumor, arising without apparent correlation with local trauma/stimuli; ALK-1 expression favors this diagnosis. Surgical excision is curative with local recurrence documented in about 15-20% of cases. Rarely distant metastases have been reported.
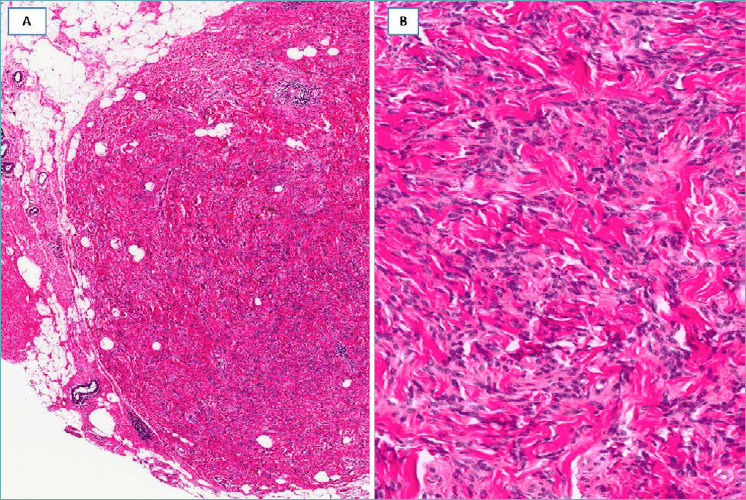
LOW-GRADE MYOFIBROBLASTIC SARCOMA 95-98
It should be suspected in presence of a nodular mass with relatively circumscribed margins, composed of a proliferation of mitotically active (from 7 to 35 mitoses x 10 HPF) spindle cells with the features of myofibroblasts, showing, at least focally, moderate nuclear pleomorphism, fascicular arrangement and variable staining for α-smooth muscle actin. This tumor, which can recur locally, has metastatic potential.
Figures and tables
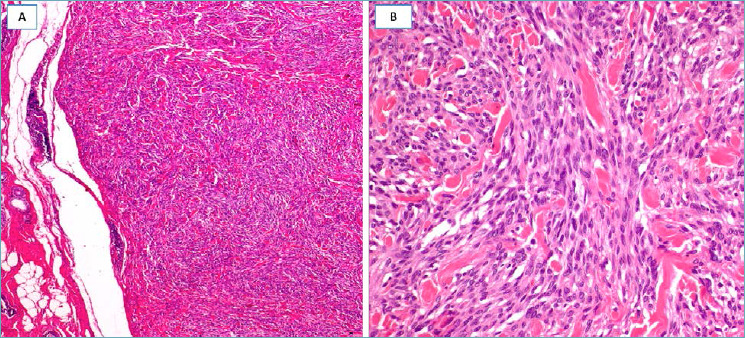
Fig. 16.
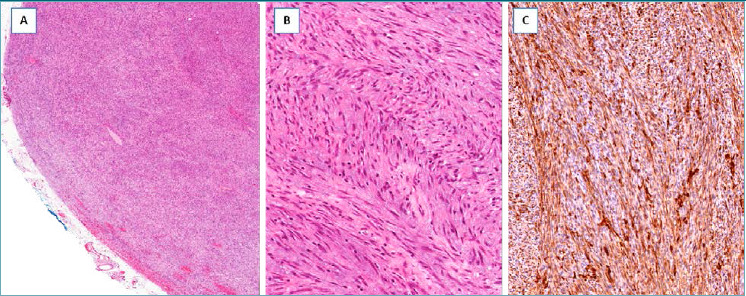
Low-grade myofibroblastic sarcoma. (A) Low-magnification showing a hypercellular tumor with pushing borders; (B) the neoplastic cells, with the morphological features of myofibroblasts, are arranged in short intersecting fascicles; (C) neoplastic cells are diffusely stained with α-smooth muscle actin.
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1.Al-Nafussi A. Spindle cell tumours of the breast: practical approach to diagnosis. Histopathology 1999;35:1-13. https://doi.org/10.1046/j.1365-2559.1999.00766.x 10.1046/j.1365-2559.1999.00766.x [DOI] [PubMed] [Google Scholar]
- 2.McMenamin ME, DeSchryver K, Fletcher CD. Fibrous Lesions of the Breast: A Review. Int J Surg Pathol 2000;8: 99-108. https://doi.org/10.1177/106689690000800204 10.1177/106689690000800204 [DOI] [PubMed] [Google Scholar]
- 3.Magro G, Michal M, Bisceglia M. Benign spindle cell tumors of the mammary stroma: diagnostic criteria, classification, and histogenesis. Pathol Res Pract 2001;197:453-66. https://doi.org/10.1078/0344-0338-00112 10.1078/0344-0338-00112 [DOI] [PubMed] [Google Scholar]
- 4.Magro G, Bisceglia M, Michal M, et al. Spindle cell lipoma-like tumor, solitary fibrous tumor and myofibroblastoma of the breast: a clinico-pathological analysis of 13 cases in favor of a unifying histogenetic concept. Virchows Arch 2002;440:249-60. https://doi.org/10.1007/s00428-001-0572-y 10.1007/s00428-001-0572-y [DOI] [PubMed] [Google Scholar]
- 5.Brogi E. Benign and malignant spindle cell lesions of the breast. Semin Diagn Pathol 2004;21:57-64. https://doi.org/10.1053/j.semdp.2003.10.007 10.1053/j.semdp.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Rakha EA, Aleskandarany MA, Lee AH, et al. An approach to the diagnosis of spindle cell lesions of the breast. Histopathology 2016;68:33-44. https://doi.org/10.1111/his.12865 10.1111/his.12865 [DOI] [PubMed] [Google Scholar]
- 7.Cheah AL, Billings SD, Rowe JJ. Mesenchymal tumours of the breast and their mimics: a review with approach to diagnosis. Pathology 2016;48:406-24. https://doi.org/10.1016/j.pathol.2016.05.006 10.1016/j.pathol.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Tay TKY, Tan PH. Spindle cell lesions of the breast - An approach to diagnosis. Semin Diagn Pathol 2017;34:400-9. https://doi.org/10.1053/j.semdp.2017.05.012 10.1053/j.semdp.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 9.Magro G. Differential diagnosis of benign spindle cell lesions. Surg Pathol Clin 2018;11:91-121. https://doi.org/10.1016/j.path.2017.09.005 10.1016/j.path.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Li H, Ren G. Epithelial-mesenchymal transition and drug resistance in breast cancer (Review). Int J Oncol. 2015;47:840-8. https://doi.org/10.3892/ijo.2015.3084 10.3892/ijo.2015.3084 [DOI] [PubMed] [Google Scholar]
- 11.Tabbara SO, Frierson HF, Jr, Fechner RE. Diagnostic problems in tissues previously sampled by fine-needle aspiration. Am J Clin Pathol 1991;96:76-80. https://doi.org/10.1093/ajcp/96.1.76 10.1093/ajcp/96.1.76 [DOI] [PubMed] [Google Scholar]
- 12.Lee KC, Chan JK, Ho LC. Histologic changes in the breast after fine-needle aspiration. Am J Surg Pathol 1994;18:1039-47. https://doi.org/10.1097/00000478-199410000-00007 10.1097/00000478-199410000-00007 [DOI] [PubMed] [Google Scholar]
- 13.Gobbi H, Tse G, Page DL, et al. Reactive spindle cell nodules of the breast after core biopsy or fine-needle aspiration. Am J Clin Pathol 2000;113,288-94. https://doi.org/10.1309/RPW4-CXCC-1JHM-0TL7 10.1309/RPW4-CXCC-1JHM-0TL7 [DOI] [PubMed] [Google Scholar]
- 14.Garijo MF, Val-Bernal JF, Vega A, et al. Postoperative spindle cell nodule of the breast: pseudosarcomatous myofibroblastic proliferation following endosurgery. Pathol Int 2008;58,787-91. https://doi.org/10.1111/j.1440-1827.2008.02312.x 10.1111/j.1440-1827.2008.02312.x [DOI] [PubMed] [Google Scholar]
- 15.Sciallis AP, Chen B, Folpe AL. Cellular Spindled histiocytic pseudotumor complicating mammary fat necrosis. Am J Surg Pathol 2012;36:1571-8. https://doi.org/10.1097/PAS.0b013e31825faa2b 10.1097/PAS.0b013e31825faa2b [DOI] [PubMed] [Google Scholar]
- 16.Kang A, Kumar JB, Thomas A, et al. A spontaneously resolving breast lesion: imaging and cytological findings of nodular fasciitis of the breast with FISH showing USP6 gene rearrangement. BMJ Case Rep 23: 2015. https://doi.org/10.1136/bcr-2015-213076 10.1136/bcr-2015-213076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paliogiannis P, Cossu A, Palmieri G, et al. Breast nodular fasciitis: a comprehensive review. Breast Care (Basel) 2016;11:270-74. https://doi.org/10.1159/000448185 10.1159/000448185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi S, Yasuda S, Takahashi N, et al. Nodular fasciitis of the breast clinically resembling breast cancer in an elderly woman: a case report. J Med Case Rep, 11,57. https://doi.org/10.1186/s13256-017-1219-1 10.1186/s13256-017-1219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naso JR, Chiu CG, Goecke ME, et al. Benign spindle cell lesions of the breast: a diagnostic approach to solitary fibrous tumour, nodular pseudoangiomatous stromal hyperplasia and nodular fasciitis. J Clin Pathol 2019;72:438-42. https://doi.org/10.1136/jclinpath-2018-205561 10.1136/jclinpath-2018-205561 [DOI] [PubMed] [Google Scholar]
- 20.Erinanc H, Türk E. The Rare Benign Lesion That Mimics a Malignant Tumor in Breast Parenchyma: Nodular Fasciitis of the Breast. Case Rep Pathol 2018;30:1612587. https://doi.org/10.1155/2018/1612587 10.1155/2018/1612587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yip CH, Wong KT, Samuel D. Bilateral plasma cell granuloma (inflammatory pseudotumour) of the breast. Aust N Z J Surg 1997;67:300-2. https://doi.org/10.1111/j.1445-2197.1997.tb01972.x 10.1111/j.1445-2197.1997.tb01972.x [DOI] [PubMed] [Google Scholar]
- 22.Ilvan S, Celik V, Paksoy M, et al. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the breast. APMIS 2005;113:66-9. https://doi.org/10.1111/j.1600-0463.2005.apm1130110.x 10.1111/j.1600-0463.2005.apm1130110.x [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Moon WK, Kim JH, et al. Inflammatory pseudotumor of the breast: a case report with imaging findings. Korean J Radiol 2009;10:515-8. https://doi.org/10.3348/kjr.2009.10.5.515 10.3348/kjr.2009.10.5.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SB, Kim HH, Shin HJ, et al. Inflammatory pseudotumor (myoblastic tumor) of the breast: a case report and review of the literature. J Clin Ultrasound 2010;38:52-5. https://doi.org/10.1002/jcu.20637 10.1002/jcu.20637 [DOI] [PubMed] [Google Scholar]
- 25.Hill PA. Inflammatory pseudotumor of the breast: a mimic of breast carcinoma. Breast J 2010;16: 549-50. https://doi.org/10.1111/j.1524-4741.2010.00967.x 10.1111/j.1524-4741.2010.00967.x [DOI] [PubMed] [Google Scholar]
- 26.Vecchio GM, Amico P, Grasso G, et al. Post-traumatic inflammatory pseudotumor of the breast with atypical morphological features: a potential diagnostic pitfall. Report of a case and a critical review of the literature. Pathol Res Pract 2011;207:322-6. https://doi.org/10.1016/j.prp.2011.01.009 10.1016/j.prp.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 27.Greenleaf EK, Williams NC, Leung AM. Inflammatory Pseudotumor of the Breast. Am Surg 2016;82:e106-7. PMID: 27215709. [PubMed] [Google Scholar]
- 28.Magro G. Mammary myofibroblastoma: a tumor with a wide morphologic spectrum. Arch Pathol Lab Med 2008;132:1813-20. https://doi.org/10.1043/1543-2165-132.11.1813 10.1043/1543-2165-132.11.1813 [DOI] [PubMed] [Google Scholar]
- 29.Magro G. Mammary myofibroblastoma: an update with emphasis on the most diagnostically challenging variants. Histology and Histopathology 2016;31:1-23. 10:14670 [PubMed] [Google Scholar]
- 30.Magro G, Michal M, Vasquez E, et al. Lipomatous myofibroblastoma: a potential diagnostic pitfall in the spectrum of the spindle cell lesions of the breast. Virch Arch. 2000;437:540-4. https://doi.org/10.1007/s004280000297 10.1007/s004280000297 [DOI] [PubMed] [Google Scholar]
- 31.Baxendine-Jones J, Theaker JM, Baldwin LJ. Predominant fatty variant of myofibrolastoma of breast. J Clin Pathol 2001;54:568-569. https://doi.org/10.1136/jcp.54.7.568 10.1136/jcp.54.7.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magro G, Longo FR, Salvatorelli L, et al. Lipomatous myofibroblastoma of the breast: case report with diagnostic and histogenetic considerations. Pathologica 2014;106:36-40. PMID: 25291864. [PubMed] [Google Scholar]
- 33.Rowe JJ, Cheah AL, Calhoun BC. Lipomatous tumors of the breast: A contemporary review. Semin Diagn Pathol. 2017;34:453-61. https://doi.org/10.1053/j.semdp.2017.05.008 10.1053/j.semdp.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 34.Magro G, Foschini MP, Eusebi V. Palisaded myofibroblastoma of the breast: a tumor closely mimicking schwannoma: report of 2 cases. Hum Pathol 2013;44:1941-6. https://doi.org/10.1016/j.humpath.2013.01.018 10.1016/j.humpath.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 35.Laforga JB, Escandón J. Schwannoma-Like (Palisaded) Myofibroblastoma: A Challenging Diagnosis on Core Biopsy. Breast J 2017;23:354-6. https://doi.org/10.1111/tbj.12744 10.1111/tbj.12744 [DOI] [PubMed] [Google Scholar]
- 36.Fabbri VP, Damiani S, Baccarini P, et al. Cytological Features of Palisaded Mammary-Type Myofibroblastoma. Int J Surg Pathol 2017;25:173-6. https://doi.org/10.1177/1066896916665699 10.1177/1066896916665699 [DOI] [PubMed] [Google Scholar]
- 37.Toker C, Tang CK, Whitely JF, et al. Benign spindle cell breast tumor. Cancer 1981;48:1615-22. https://doi.org/10.1002/1097-0142(19811001)48:7<1615::aid-cncr2820480724>3.0.co;2-i [DOI] [PubMed] [Google Scholar]
- 38.Böger A. Benign spindle cell tumor of the male breast. Pathol Res Pract 1984;178:395-9. https://doi.org/10.1016/S0344-0338(84)80035-7 10.1016/S0344-0338(84)80035-7 [DOI] [PubMed] [Google Scholar]
- 39.Chan KW, Ghadially FN, Alagaratnam TT. Benign spindle cell tumour of breast- a variant of spindled cell lipoma or fibroma of breast? Pathology 1984;16:331-6. https://doi.org/10.3109/00313028409068546 10.3109/00313028409068546 [DOI] [PubMed] [Google Scholar]
- 40.Magro G, Bisceglia M, Pasquinelli G. Benign spindle cell tumor of the breast with prominent adipocytic component. Ann Diagn Pathol 1998;2:306-11. https://doi.org/10.1016/s1092-9134(98)80023-3 10.1016/s1092-9134(98)80023-3 [DOI] [PubMed] [Google Scholar]
- 41.Bombonati A, Parra JS, Schwartz GF, et al. Solitary fibrous tumor of the breast. Breast J 2003;9:251. https://doi.org/10.1046/j.1524-4741.2003.09315.x 10.1046/j.1524-4741.2003.09315.x [DOI] [PubMed] [Google Scholar]
- 42.Dragoumis D, Atmatzidis S, Chatzimavroudis G, et al. Benign spindle cell tumor not otherwise specified (NOS) in a male breast. Int J Surg Pathol 2010;18:575-9. https://doi.org/10.1177/1066896908328576 10.1177/1066896908328576 [DOI] [PubMed] [Google Scholar]
- 43.Magro G, Angelico G, Righi A, et al. Utility of STAT6 and 13q14 deletion in the classification of the benign spindle cell stromal tumors of the breast. Hum Pathol 2018;81:55-64. https://doi.org/10.1016/j.humpath.2018.06.015 10.1016/j.humpath.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 44.Magro G, Spadola S, Motta F, et al. STAT6 expression in spindle cell lesions of the breast: An immunohistochemical study of 48 cases. Pathol Res Pract. 2018;214:1544-1549. https://doi.org/10.1016/j.prp.2018.07.011 10.1016/j.prp.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 45.Lew WY. Spindle cell lipoma of the breast: a case report and literature review. Diagn Cytopathol 1993;9:434-7. https://doi.org/10.1002/dc.2840090412 10.1002/dc.2840090412 [DOI] [PubMed] [Google Scholar]
- 46.Mulvany NJ, Silvester AC, Collins JP. Spindle cell lipoma of the breast. Pathology 1999;31:288-91. https://doi.org/10.1080/003130299105188 10.1080/003130299105188 [DOI] [PubMed] [Google Scholar]
- 47.Abdel-All HS. Breast spindle cell tumours: about eight cases. Diagn Pathol 2006;22:1:13. https://doi.org/10.1186/1746-1596-1-13 10.1186/1746-1596-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaffar R, Zaheer S, Vasenwala SM, et al. Spindle cell lipoma breast. Indian J Pathol Microbiol 2008;51:234-6. https://doi.org/10.4103/0377-4929.41666 10.4103/0377-4929.41666 [DOI] [PubMed] [Google Scholar]
- 49.Magro G, Angelico G, Spadola S, et al. Fibroma of the breast: a rare tumour in the spectrum of the benign spindle cell tumours of the mammary stroma. Pol J Pathol 2018;69:189-94. https://doi.org/10.5114/pjp.2018.76703 10.5114/pjp.2018.76703 [DOI] [PubMed] [Google Scholar]
- 50.Granic M, Stefanovic-Radovic M, Zdravkovic D, et al. Intraparenchimal Leiomyoma of the Breast. Arch Iran Med. 2015;18:608-12. https://doi.org/0151809/AIM.0012 10.0151809/AIM.0012 [DOI] [PubMed] [Google Scholar]
- 51.Jones MW, Norris HJ, Wargotz ES. Smooth muscle and nerve sheath tumors of the breast. A clinicopathologic study of 45 cases. Int J Surg Pathol 1994;2,85-92. https://doi.org/10.1177/106689699400200202 10.1177/106689699400200202 [DOI] [Google Scholar]
- 52.Vecchio GM, Cavaliere A, Cartaginese F, et al. Intraparenchymal leiomyoma of the breast: report of a case with emphasis on needle core biopsy-based diagnosis. Pathologica 2013;105,122-7. PMID: 24466762 [PubMed] [Google Scholar]
- 53.Krings G, McIntire P, Shin SJ. Myofibroblastic, fibroblastic and myoid lesions of the breast. Semin Diagn Pathol 201;34:427-37. https://doi.org/10.1053/j.semdp.2017.05.010 10.1053/j.semdp.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 54.Khalifa MA, Montgomery EA, Azumi N, et al. Solitary fibrous tumors: a series of lesions, some in unusual sites. South Med J 1997;90:793-9. https://doi.org/10.1097/00007611-199708000-00005 10.1097/00007611-199708000-00005 [DOI] [PubMed] [Google Scholar]
- 55.Falconieri G, Lamovec J, Mirra M, et al. Solitary fibrous tumor of the mammary gland: a potential pitfall in breast pathology. Ann Diagn Pathol 2004;8:121-5. https://doi.org/10.1016/j.anndiagpath.2004.03.002 10.1016/j.anndiagpath.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 56.Meguerditchian AN, Malik DA, Hicks DG, et al. Solitary fibrous tumor of the breast and mammary myofibroblastoma: the same lesion? Breast J 2008;14:287-92. https://doi.org/10.1111/j.1524-4741.2008.00588.x 10.1111/j.1524-4741.2008.00588.x [DOI] [PubMed] [Google Scholar]
- 57.Yang LH, Dai SD, Li QC, et al. Malignant solitary fibrous tumor of breast: a rare case report. Int J Clin Exp Pathol 2014;7:4461-6. PMID: 25120834 [PMC free article] [PubMed] [Google Scholar]
- 58.Salemis NS. Solitary fibrous tumor of the breast: A case report and the review of the literature. Breast J 2018;24:78-81. https://doi.org/10.1111/tbj.12841 10.1111/tbj.12841 [DOI] [PubMed] [Google Scholar]
- 59.Tsai SY, Hsu CY, Chou YH, et al. Solitary fibrous tumor of the breast: A case report and review of the literature. J Clin Ultrasound 2017;45:350-4. https://doi.org/10.1002/jcu.22415 10.1002/jcu.22415 [DOI] [PubMed] [Google Scholar]
- 60.Park BN, Woo OH, Kim C, et al. Recurrent solitary fibrous tumor of the breast: Magnetic resonance imaging and pathologic findings. Breast J 2018;24:1064-5. https://doi.org/10.1111/tbj.13131 10.1111/tbj.13131 [DOI] [PubMed] [Google Scholar]
- 61.Brenes J, Moreno A, Merchán MJ, et al. Solitary fibrous tumor of the breast: A rare neoplasm. Breast J 2018;24:417-9. https://doi.org/10.1111/tbj.12925 10.1111/tbj.12925 [DOI] [PubMed] [Google Scholar]
- 62.Tyler GT. Report of a case of pure myxoma of the breast. Ann Surg 1915;61:121-7. https://doi.org/10.1097/00000658-191501000-00022 10.1097/00000658-191501000-00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan YF, Yeung HY, Ma L. Myxoma of the breast: report of a case and ultrastrucutual study. Pathology 1986;18:153-7. https://doi.org/10.3109/00313028609090845 10.3109/00313028609090845 [DOI] [PubMed] [Google Scholar]
- 64.Balci P, Kabakci N, Isil T, et al. Breast myxoma: radiologic and histopathologic features. Breast J 2007;13:88-90. https://doi.org/10.1111/j.1524-4741.2006.00370.x 10.1111/j.1524-4741.2006.00370.x [DOI] [PubMed] [Google Scholar]
- 65.Magro G, Cavanaugh B, Palazzo J. Clinico-pathological features of breast myxoma: report of a case with histogenetic considerations. Virch Arch 2010;456:581-6. https://doi.org/10.1007/s00428-010-0902-z 10.1007/s00428-010-0902-z [DOI] [PubMed] [Google Scholar]
- 66.Nel J, O’Donnell M, Bradley R. Myxoma of the Breast in a Male Patient. Breast J 2016;22:468-9. https://doi.org/10.1111/tbj.12612 10.1111/tbj.12612 [DOI] [PubMed] [Google Scholar]
- 67.Charu V, Cimino-Mathews A. Peripheral nerve sheath tumors of the breast. Semin Diagn Pathol 2017;34:420-6. https://doi.org/10.1053/j.semdp.2017.05.011 10.1053/j.semdp.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 68.Bellezza G, Lombardi T, Panzarola P, et al. Schwannoma of the breast: a case report and review of the literature. Tumori 2007;93:308-11. PMID: 17679472 [PubMed] [Google Scholar]
- 69.Gultekin SH, Cody HS, Hoda SA. Schwannoma of the breast. South Med J 1996;89: 238-9. https://doi.org/10.1097/00007611-199602000-00018 10.1097/00007611-199602000-00018 [DOI] [PubMed] [Google Scholar]
- 70.Murat A, Kansiz F, Kabakus N, et al. Neurofibroma of the breast in a boy with neurofibromatosis type 1. Clin Imaging 2004;28:415-7. https://doi.org/10.1016/S0899-7071(04)00004-X 10.1016/S0899-7071(04)00004-X [DOI] [PubMed] [Google Scholar]
- 71.Lee EK, Kook SH, Kwag HJ, et al. Schwannoma of the breast showing massive exophytic growth: a case report. Breast 2006;15: 562-6. https://doi.org/10.1016/j.breast.2005.11.009 10.1016/j.breast.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 72.Dialani V, Hines N, Wang Y, et al. Breast schwannoma. Case Rep Med 2011;2011:930841. https://doi.org/10.1155/2011/930841 10.1155/2011/930841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thejaswini M, Padmaja K, Srinivasamurthy V, et al. Solitary intramammary schwannoma mimicking phylloides tumor: cytological clues in the diagnosis. J Cytol 2012;29:258-60. https://doi.org/10.4103/0970-9371.103947 10.4103/0970-9371.103947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parikh Y, Sharma KJ, Parikh SJ, et al. Intramammary schwannoma: a palpable breast mass. Radiol Case Rep 2016;11:129-33. https://doi.org/10.1016/j.radcr.2016.05.011 10.1016/j.radcr.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeyaretna DS, Oriolowo A, Smith MEF, et al. Solitary neurofibroma in the male breast. World J Surg Oncol 2007;27;5:23. https://doi.org/10.1186/1477-7819-5-23 10.1186/1477-7819-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson S, Kaplan SS, Poppiti RJ, et al. Solitary neurofibroma of the breast. Radiol Case Rep 2012;7:462. https://doi.org/10.2484/rcr.v7i4.462 10.2484/rcr.v7i4.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wargotz ES, Norris HJ, Austin RM, et al. Fibromatosis of the breast. A clinical and pathological study of 28 cases. Am J Surg Pathol 1987;11,38-44. https://doi.org/10.1097/00000478-198701000-00005 10.1097/00000478-198701000-00005 [DOI] [PubMed] [Google Scholar]
- 78.Rosen PP, Ernsberger D. Mammary fibromatosis. A benign spindle-cell tumor with significant risk for local recurrence. Cancer 1989;63:1363-9. https://doi.org/10.1002/1097-0142(19890401)63:7<1363::aid-cncr2820630722>3.0.co;2-b [DOI] [PubMed] [Google Scholar]
- 79.Magro G, Mesiti M. Breast and pectoralis musculo-aponeurotic fibromatosis: two independent lesions occurring in the same patient. A case report. Pathol Res Pract 1998;194:867-71. https://doi.org/10.1016/S0344-0338(98)80092-7 10.1016/S0344-0338(98)80092-7 [DOI] [PubMed] [Google Scholar]
- 80.Devouassoux-Shisheboran M, Schammel MDP, Man YG, et al. Fibromatosis of the breast. Arch Pathol Lab Med 2000;124:276-80. https://doi.org/10.1043/0003-9985(2000)124<0276:FOTB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 81.Magro G, Gurrera A, Scavo N, et al. Fibromatosis of the breast: a clinical, radiological and pathological study of 6 cases. Pathologica 2002;94:238-46. [DOI] [PubMed] [Google Scholar]
- 82.Wongmaneerung P, Somwangprasert A, Watcharachan K, et al. Bilateral desmoid tumor of the breast: case series and literature review. Int Med Case Rep J 2016;9:247-51. https://doi.org/10.2147/IMCRJ.S106325 10.2147/IMCRJ.S106325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valli R, Rossi G, Natalini G, et al. Dermatofibrosarcoma protuberans of the breast: a case report. Pathologica 2002;94:310-3. [DOI] [PubMed] [Google Scholar]
- 84.Karcnik TJ, Miller JA, Fromowitz F, et al. Dermatofibrosarcoma protuberans of the breast: a rare malignant tumor simulating benign disease. Breast J 1999;5:262-3. https://doi.org/10.1046/j.1524-4741.1999.99003.x 10.1046/j.1524-4741.1999.99003.x [DOI] [PubMed] [Google Scholar]
- 85.Callery CD, Rosen PP, Kinne DW. Sarcoma of the breast. A study of 32 patients with reappraisal of classification and therapy. Ann Surg 1985;201:527-32. https://doi.org/10.1097/00000658-198504000-00020 10.1097/00000658-198504000-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norris HJ, Taylor HB. Sarcomas and related mesenchymal tumors of the breast. Cancer 1968;22:22-8. https://doi.org/10.1002/1097-0142(196807)22:1<22::aid-cncr2820220105>3.0.co;2-r [DOI] [PubMed] [Google Scholar]
- 87.Vecchio GM, Broggi G, Mulè A, et al. Dermatofibrosarcoma protuberans: a tumor in the wide spectrum of the bland-looking spindle cell lesions of the breast. Pathologica 2019;111:87-91. https://doi.org/10.32074/1591-951X-22-19 10.32074/1591-951X-22-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sneige N, Yaziji H, Mandavilli SR, et al. Low-grade (fibromatosis-like) spindle cell carcinoma of the breast. Am J Surg Pathol 2001;25,1009-1016. https://doi.org/10.1097/00000478-200108000-00004 10.1097/00000478-200108000-00004 [DOI] [PubMed] [Google Scholar]
- 89.Dwyer JB, Clark BZ. Low-grade fibromatosis-like spindle cell carcinoma of the breast. Arch Pathol Lab Med 2015;139:552-7. https://doi.org/10.5858/arpa.2013-0555-RS 10.5858/arpa.2013-0555-RS [DOI] [PubMed] [Google Scholar]
- 90.Zhou Y, Zhu J, Zhang Y, et al. An inflammatory myofibroblastic tumour of the breast with ALK overexpression. BMJ Case Rep 2013;4: 2013. https://doi.org/10.1136/bcr-07-2011-4474 10.1136/bcr-07-2011-4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao HD, Wu T, Wang JQ, et al. Primary inflammatory myofibroblastic tumor of the breast with rapid recurrence and metastasis: a case report. Oncol Lett 2013;5:97-100. https://doi.org/10.3892/ol.2012.948 10.3892/ol.2012.948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bosse K, Ott C, Biegner T, et al. 23-Year-Old Female with an inflammatory myofibroblastic tumour of the breast: a case report and a review of the literature. GeburtshilfeFrauenheilkd 2014;74:167-70. https://doi.org/10.1055/s-0033-1360185 10.1055/s-0033-1360185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Markopoulos C, Charalampoudis P, Karagiannis E, et al. Inflammatory myofibroblastic tumor of the breast. Case Rep Surg 2015;2015:705127. https://doi.org/10.1155/2015/705127 10.1155/2015/705127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi EJ, Jin GY, Chung MJ, et al. Primary Inflammatory Myofibroblastic Tumors of the Breast with Metastasis: Radiographic and Histopathologic Predictive Factors. J Breast Cancer 2015;18:200-5. https://doi.org/10.4048/jbc.2015.18.2.200 10.4048/jbc.2015.18.2.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taccagni G, Rovere E, Masullo M, et al. Myofibrosarcoma of the breast: review of the literature on myofibroblastic tumors and criteria for defining myofibroblastic differentiation. Am J Surg Pathol 1997;21,489-96. https://doi.org/10.1097/00000478-199704000-00017 10.1097/00000478-199704000-00017 [DOI] [PubMed] [Google Scholar]
- 96.Gocht A, Bösmüller HC, Bässler R, et al. Breast tumors with myofibroblastic differentiation: clinico-pathological observations in myofibroblastoma and myofibrosarcoma. Pathol Res Pract 1999;195,1-10. https://doi.org/10.1016/S0344-0338(99)80087-9 10.1016/S0344-0338(99)80087-9 [DOI] [PubMed] [Google Scholar]
- 97.Lucin K, Mustać E, Jonjić N. Breast sarcoma showing myofibroblastic differentiation. Virchows Arch 2003;443:222-4. https://doi.org/10.1007/s00428-003-0853-8 10.1007/s00428-003-0853-8 [DOI] [PubMed] [Google Scholar]
- 98.Morgan PB, Chundru S, Hatch SS, et al. Uncommon malignancies: case 1. Low-grade myofibroblastic sarcoma of the breast. J Clin Oncol 2005;23,6249-51. https://doi.org/10.1200/JCO.2005.06.213 10.1200/JCO.2005.06.213 [DOI] [PubMed] [Google Scholar]


