Abstract
Introduction:
This study aimed to (a) review what theories have been applied to the development of digital self-management interventions for people with neurological disorders; (b) examine their effectiveness to improve depression, anxiety, fatigue and self-efficacy; and (c) identify the optimal mode of intervention delivery.
Methods:
Electronic databases (SCOPUS, MEDLINE, EMBASE, CINAHL, Cochrane Library and Clinicaltrials.gov) were searched. Two investigators independently screened studies and extracted data. Study quality and use of theory were also assessed
Results:
A total of 944 studies were screened, and 16 randomised controlled trials were included. Theory-based digital self-management interventions were effective in reducing depression (standardised mean difference (SMD) = −0.77, 95% confidence interval (CI) −1.04 to −0.49), anxiety (SMD = −0.88, 95% CI −1.54 to −0.21) and fatigue (SMD = −0.62, 95% CI −0.96 to −0.27) and in enhancing self-efficacy (SMD = 1.15, 95% CI 0.11–2.18). Cognitive–behavioural theory (CBT)-based interventions were effective in reducing depression (SMD = −0.81, 95% CI −1.22 to −0.39), anxiety (SMD = −1.15, 95% CI −1.85 to −0.44) and fatigue (SMD = −0.75, 95% CI −0.97 to −0.54) and in improving self-efficacy (SMD = 0.84, 95% CI 0.63–1.05), whereas social cognitive theory (SCT)-based interventions were effective in reducing depression (SMD = −0.73, 95% CI −1.17 to −0.28). Partially digital interventions were more effective than fully digital interventions.
Discussion:
Our findings support the use of theory to guide the development of digital self-management interventions to increase intervention effectiveness. In particular, CBT-based interventions have a positive impact on depression, anxiety, fatigue and self-efficacy, whereas SCT-based interventions have a positive impact on depression.
Keywords: Neuropsychiatry, telehealth, teleneurology, self-management, behavioural theory
Introduction
People with neurological disorders experience a wide variety of symptoms, including depression, anxiety, fatigue and impaired self-efficacy.1,2 Ineffective management of these symptoms results in adverse outcomes such as poor quality of life3 and caregiver distress.4 Self-management interventions are designed as supportive care that engages patients to take an active role in the management of their health5,6 and are effective in improving the symptoms of neurological disorders.7–9 However, physical and geographical barriers limit access to interventions for patients who live in remote areas and individuals who are restricted to their residences due to significant disabilities.10
The recent expansion of covered telehealth services by the Centers for Medicare and Medicaid Services and the passage of the Furthering Access to Stroke Telemedicine Act have vastly changed the landscape of medicine, including the delivery of traditional consultation and supportive neurological care via telemedicine.11,12 The potential of telemedicine for overcoming pragmatic barriers, reducing burden on the health-care system and continuing health services is greatly recognised. This potential is more salient now in the face of the coronavirus disease 2019 (COVID-19) pandemic.13–15
Digital self-management interventions allow for real-time remote monitoring and provision of immediate feedback, reduce administrative costs and are customisable to personalised patient needs.16,17 Thus, such interventions are particularly relevant to cope with mental health or other chronic health needs. Interventions designed to promote behavioural change, including self-management, can benefit from the use of theory.18 Greater use of theory in the development of digital self-management interventions increases their overall effectiveness.16 Reviews have supported the use of theory-based digital self-management interventions for improving clinical outcomes in diverse populations.5,16 The purpose of this review was to fill the knowledge gap about the promise of theory-based digital self-management interventions for people with neurological disorders. More specifically, the objectives of this review were threefold: (a) to review what theories have been applied in the development of these digital interventions; (b) to investigate the effectiveness of theory-based digital self-management interventions to improve depression, anxiety, fatigue and self-efficacy in people with neurological disorders; and (c) to identify the optimal mode of intervention delivery. This review primarily included randomised controlled trials (RCTs) because RCTs provide the best evidence for the effectiveness of health interventions.19
Methods
The protocol for the current study was registered at PROSPERO (registration number: CRD42019126508; www.crd.york.ac.uk/prospero/).
Definitions
We define ‘digital interventions’ as programmes that are accessed via a digital platform, such as a computer, website, SMS, email, videoconferencing, electronic wearable device or mobile application (app).20 According to McLean et al.,5 self-management is defined as care taken by individuals towards their own health and well-being consisting of the actions they take to: (a) lead a healthy lifestyle; (b) meet their social, emotional and psychological needs; (c) care for their long-term condition; and (d) prevent further illness. A theory refers to a set of concepts, definitions and propositions that predicts or explains phenomena by illustrating the relations among constructs.21 Neurological disorders are defined by the World Health Organization as disorders of the central and peripheral nervous system, such as multiple sclerosis, spinal cord injury, traumatic brain injury and Alzheimer’s disease.22
Search strategy
Search strategies were created by a medical librarian (A.H.) using a combination of controlled terms and keywords and were executed in Ovid-Medline 1946−, Embase 1947−, Scopus 1823−, CINAHL 1937−, Cochrane Library and Clinicaltrials.gov. Detailed information about the search terms used is available in Supplementary Appendix A. The search was completed in March 2020.
Study selection
Figure 1 summarises the study selection process. Two investigators (S.L. and S.B.) independently screened titles, abstracts and keywords from the electronic searches. Any discrepancies were resolved through discussion with a third investigator (A.W.). Full texts of potential studies were reviewed, and their reference lists were hand searched for additional trials. We included articles that: (a) were original articles published in peer-reviewed journals, (b) included participants with neurological disorders, (c) included adult participants, (d) were RCTs, (e) assessed the effectiveness of digital self-management interventions, (f) explicitly mentioned the use of theory in intervention development and (g) were written in English. We excluded: (a) abstracts, conference proceedings, data sets, reviews, protocols and non-English articles; (b) articles featuring participants without neurological disorders; (c) articles featuring children and adolescents; (d) non-RCT studies; (e) articles that did not involve digital self-management interventions; (f) articles that did not explicitly mention the use of theory in intervention development; and (g) articles that did not assess the effectiveness of a digital self-management intervention (e.g. economic evaluation). In the case of multiple publications derived from the same study, we selected the article with the latest or most sufficient statistical information to compute the effect sizes of interventions. The most sufficient statistical information included means and standard deviations of outcomes at each time point, as well as the sample size of each group.
Figure 1.
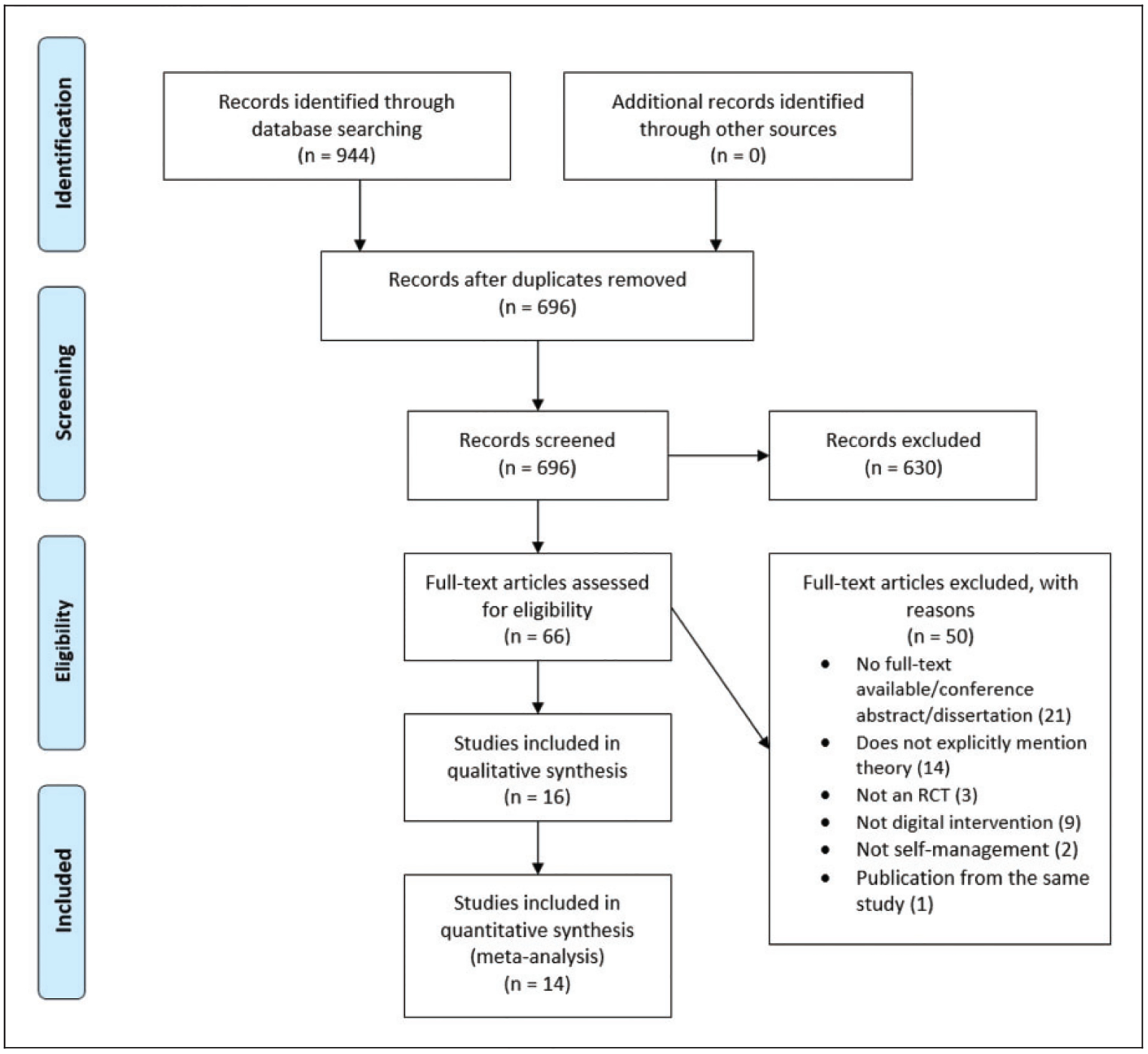
Flow diagram of study selection.
Data extraction and quality appraisal
Two investigators (S.L. and S.B.) used a structured form to extract data independently based on: study characteristics, patient characteristics, intervention characteristics and statistics. We contacted the first and corresponding authors via email to retrieve any missing information from the included articles. Based on the mode of delivery, each intervention was classified as ‘fully digital’ or ‘partially digital’ (i.e. a combination of digital and non-digital elements, such as the inclusion of resource books or in-person, face-to-face interaction). For the form of coaching, we classified each intervention as ‘digital’ (i.e. interactive feedback was delivered based on algorithms), ‘human’ (i.e. interactive feedback was delivered by therapist/peer coach) or ‘combined’ (i.e. a combination of digital and human coaching). To assess the degree of theory use, we used the Theory Coding Scheme (TCS)23 to code each intervention. The TCS consists of 19 items assessing six domains: reference to underpinning theory (items 1–3), targeting of related theoretical components (items 2, 5 and 7–11), selecting participants or tailoring interventions based on theory (items 4 and 6), measurement of constructs (items 12 and 13), theory testing (items 14–18) and theory refinement (item 19). We computed the percentage score based on the formula used by Lycett et al.16: (number of TCS items satisfied/19 TCS items)×100%; a higher percentage reflects a more extensive use of theory.
Two investigators (S.L. and S.B.) independently appraised the methodological quality of each study using the Physiotherapy Evidence Database (PEDro) scale,24 with any discrepancies resolved through discussion to reach a consensus. The PEDro scale is an 11-item scale assessing the risk of bias and statistical reporting of RCTs. Except for item 1, which assesses external validity, one point is given to each satisfied item. The total score ranges from 0 to 10 points, with higher scores indicating higher quality. A study is considered to be of sufficient quality when its total PEDro score is at least four points.25
Study outcome measures
Study outcomes were selected a priori, including the most common neuropsychiatric symptoms (i.e. depression, anxiety and fatigue)26 and a common conceptual element underpinning self-management interventions (i.e. self-efficacy).27
Statistical analyses
For each study, we estimated a standardised mean difference (SMD) using Cohen’s d test. The SMD is the mean difference in the score at post test between the intervention and control groups divided by the pooled standard deviation. If both intention-to-treat and per-protocol data were presented, the former estimate was used. A negative SMD indicates a greater reduction of the outcome measures at the end point compared to baseline in the intervention group. All meta-analyses were performed by pooling the SMD between trials using random-effects models, which account for the inherent heterogeneity of studies. Due to the small number of trials (i.e. <10) available for each outcome, stratified analyses were performed by the use of theory and mode of digital delivery to assess the effects of these factors on estimates.28 The I2 statistics were calculated a posteriori as an estimate of between-trial SMD heterogeneity. Caution is warranted in interpreting the I2 statistics, as their power to detect heterogeneity is relatively low, given the small number of trials.29 No tests of funnel plot asymmetry were performed due to the low power of this test with a small sample of studies.30
Results
Study selection
The online database search identified 944 citations. Duplicates were removed, and the titles, abstracts and keywords of the remaining 696 unique citations were screened. Sixty-six studies were considered for full-text review. Figure 1 summarises the review process and reasons for the exclusion of trials. Finally, we included 16 studies for systematic review and 14 studies for meta-analysis. Two studies31,32 were excluded from the meta-analysis because both arms employed the same digital platform.
Study characteristics
Table 1 summarises the characteristics of the 16 included studies. Seven neurological disorders, predominantly multiple sclerosis (n = 6), were presented. Studies were largely conducted in the USA (n = 9). Sample sizes ranged from 11 to 368 and included a total of 1674 participants. The mean age of the sample ranged from 37.6 to 55.8 years, and between 21.3% and 89.2% of participants were female. The length of interventions varied across studies and ranged from 4 to 24 weeks. One intervention33 was self-paced with a mean completion time of 14.4 weeks, and another intervention lasted 16 weeks or longer, depending on the participant’s long-term goal.34 Interventions were primarily implemented in online sessions (n = 13), were primarily fully digital (n = 11) and primarily used human coaching (n = 11).
Table 1.
Characteristics of included studies (N = 16).
| Author | Year | Country | Mean age (years) | N | Female (%) | Neurological disorder | Theory | Intervention | Length (weeks) | Control | Coaching |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boele35,e | 2018 | Netherlands | 44.9 | 82 | 54.9 | Glioma | CBT | Online sessions | 5 | WLC | Human |
| Bromberg40,e | 2011 | USA | 42.6 | 185 | 89.2 | Migraine | SCT | Online sessions | 4 | TAU | Digital |
| Conroy44,e | 2018 | USA | 51.0 | 51 | 58.8 | Multiple sclerosis | Wagnera | Online sessions | 24 | Exercise prescriptions | Human |
| Dilorio45,e | 2011 | USA | 40.9 | 148 | 73.6 | Epilepsy | Mixedb | Online sessions | 6 | WLC | Digital |
| Gahari10,e | 2010 | Australia | 50.3 | 95 | 81.1 | Multiple sclerosis | SCT | Online sessions | 7 | No intervention | Human |
| Kannan43,e | 2019 | USA | 55.8 | 30 | 70.0 | Multiple sclerosis | MCT | Online sessions | 8 | WLC | Digital |
| Knoop34,f | 2008 | The Netherlands | 37.6 | 171 | 79.3 | Chronic fatigue syndrome | CBT | Booklet+email/telephone | 16 or moreg | WLC | Human |
| Raina32,e | 2016 | USA | 46.2 | 38 | 44.7 | Traumatic brain injury | Mixedc | Online sessions via Web camera | 8 | Health education via Web camera | Human |
| Houlihan42,f | 2017 | USA | 45.7 | 84 | 26.2 | Spinal cord injury | HETd | Resource guide+telephone/text/email | 24 | Resource guide+TAU | Human |
| janse36,f | 2016 | Netherlands | Not reported | 100 | 68.0 | Chronic fatigue syndrome | CBT | Booklet+email | 24 | WLC | Human |
| Moss-Morris37,f | 2012 | UK | Not reported | 45 | 80.0 | Multiple sclerosis | CBT | Online sessions+email+telephone | 10 | TAU | Human+digital |
| Sajatovic41,f | 2018 | USA | 41.3 | 120 | 68.1 | Epilepsy | SCT | Face-to-face session+online sessions via web conferencing | 10 | WLC | Human |
| Sorbi33,e | 2015 | Netherlands | 43.6 | 368 | 86.0 | Migraine | CBT | Online sessions | 14.4h | WLC | Human |
| Thompson39,e | 2019 | USA | 41.2 | 107 | 66.4 | Epilepsy | CBT | Online sessions/telephone | 8 | TAU | Human |
| Tiejen38,e | 2018 | USA | 45.0 | 11 | 21.3 | Multiple sclerosis | CBT | Online sessions | 8 | No intervention | Digital |
| Van Kessel31,e | 2016 | New Zealand | 45.0 | 39 | 74.4 | Multiple sclerosis | CBT | Online sessions | 8 | Online sessions | Human |
Wagner’s Chronic Care Model.
SCT, motivational interviewing and trans-theoretical model of behaviour change.
Problem-solving therapy and behaviour activation therapy.
Health empowerment theory.
Fully digital intervention.
Partially digital intervention.
Intervention will last longer if patients formulated long-term goals.
Mean completion time of the self-paced intervention.
SCT: Social Cognitive Theory; CBT: Cognitive–Behavioural Theory; MCT: motor control theory; WLC: waitlist control; TAU: treatment as usual.
Methodological quality
All 16 included studies scored ≥4 points on the PEDro scale, indicating sufficient quality of all included studies. The mean PEDro score of all studies was 5.8 (range 4–7 points). Most studies (n = 14) did not have blinding of participants, therapists and assessors (Table 2).
Table 2.
Summary of quality appraisal using the PEDro scale.
| Author | Year | Eligibility criteria were specified | Subjects were randomly allocated to groups | Allocation was concealed | Groups were similar at baseline regarding the most important prognostic indicators | Blinding of all subjects | Blinding of all therapists who administered the therapy | Blinding of all assessors who measured at least one key outcome | Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups | All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analysed by ‘intention to treat’ | Results of between-group statistical comparisons are reported for at least one key outcome | Study provides both point measures and measures of variability for at least one key outcome | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boele | 2018 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Bromberg | 2011 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Conroy | 2018 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Dilorio | 2011 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 |
| Gahari | 2010 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Kannan | 2019 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| Knoop | 2008 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Raina | 2016 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Houlihan | 2017 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| anse | 2016 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Moss-Morris | 2012 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Sajatovic | 2018 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Sorbi | 2015 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Thompson | 2019 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Tiejen | 2018 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Van Kessel | 2016 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
1: yes; 0: no or unclear.
PEDro: Physiotherapy Evidence Database
Use of theory
Theories used in the development of interventions included cognitive–behavioural theory (CBT; n = 8),31,33–39 social cognitive theory (SCT; n = 3),10,40,41 health empowerment theory (n = 1),42 motor control theory (n = 1)43 and Wagner’s Chronic Care Model (n = 1).44 Mixed theories were used in Raina et al.32 (problem-solving therapy and behaviour activation theory) and DiIorio et al.45 (SCT, motivational interviewing and trans-theoretical model of behaviour change). The use of theory as assessed by the TCS ranged from 36.8% to 63.2%; 10 studies were coded >50% (Table 3). All studies mentioned theory (item 1), adopted quality measures (item 13) and performed randomisation (item 14). Table 3 provides the summary of TCS application.
Table 3.
Summary of Theory Coding Scheme application.
| Author | Year | Theory Coding Scheme Item | % Theory applied | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 17 | |||
| Boele | 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 52.6 | ||||||
| Bromberg | 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 47.4 | |||||||
| Conroy | 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 47.4 | |||||||
| Dilorio | 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 42.1 | ||||||||
| Gahari | 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 52.6 | ||||||
| Kannan | 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 36.8 | |||||||||
| Knoop | 2008 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 47.4 | |||||||
| Raina | 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 47.4 | |||||||
| Houlihan | 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 63.2 | ||||
| Janse | 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 63.2 | ||||
| Moss-Morris | 2012 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 57.9 | |||||
| Sajatovic | 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 57.9 | |||||
| Sorbi | 2015 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 52.6 | ||||||
| Thompson | 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 63.2 | ||||
| Tiejen | 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 63.2 | ||||
| Van Kessel | 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 52.6 | ||||||
Item 1: mentioned theory; item 2: mentioned a target construct as the predictor of behaviour; item 3: developed interventions based on a single theory; item 4: screened or selected recipients based on the score or level on a theory-relevant construct/predictor; item 5: used theory to choose or develop intervention techniques; item 6: used theory/predictors to tailor intervention techniques to different recipients; item 7: associated all intervention techniques with at least one theory-relevant construct/predictor; item 8: associated at least one intervention technique with at least one theory-relevant construct/predictor; item 9: linked a cluster of techniques to a cluster of theory-relevant constructs/predictors; item 10: associated all theory-relevant construct/predictors with at least one intervention technique; item 11: associated at least one theoretical construct/predictor with at least one intervention technique; item 12: assessed theory-relevant constructs/predictors; item 13: adopted quality measures; item 14: randomised participants; item 15: identified significant changes in theory-relevant constructs/predictors; item 17: discussed results based on the underpinning theory. Items 16, 18 and 19 were not applied in any study.
Impact of interventions on depression, anxiety, fatigue, and self-efficacy
Figure 2 shows the impact of interventions on end-point outcomes for depression, anxiety, fatigue and self-efficacy. Six studies showed a significant difference for end-point depression in favour of the intervention group (SMD = −0.77, 95% confidence interval (CI) −1.04 to −0.49, I2 = 39.8%). Three studies showed a significant difference for end-point anxiety in favour of the intervention group (SMD = −0.88, 95% CI −1.54 to −0.21, I2 = 81.6%). Five studies saw a significant difference for end-point fatigue in favour of the intervention group (SMD = −0.62, 95% CI −0.96 to −0.27, I2 = 62.4%). Finally, five studies showed a significant difference for end-point self-efficacy in favour of the intervention group (SMD = 1.15, 95% CI 0.11–2.18, I2 = 96.6%).
Figure 2.
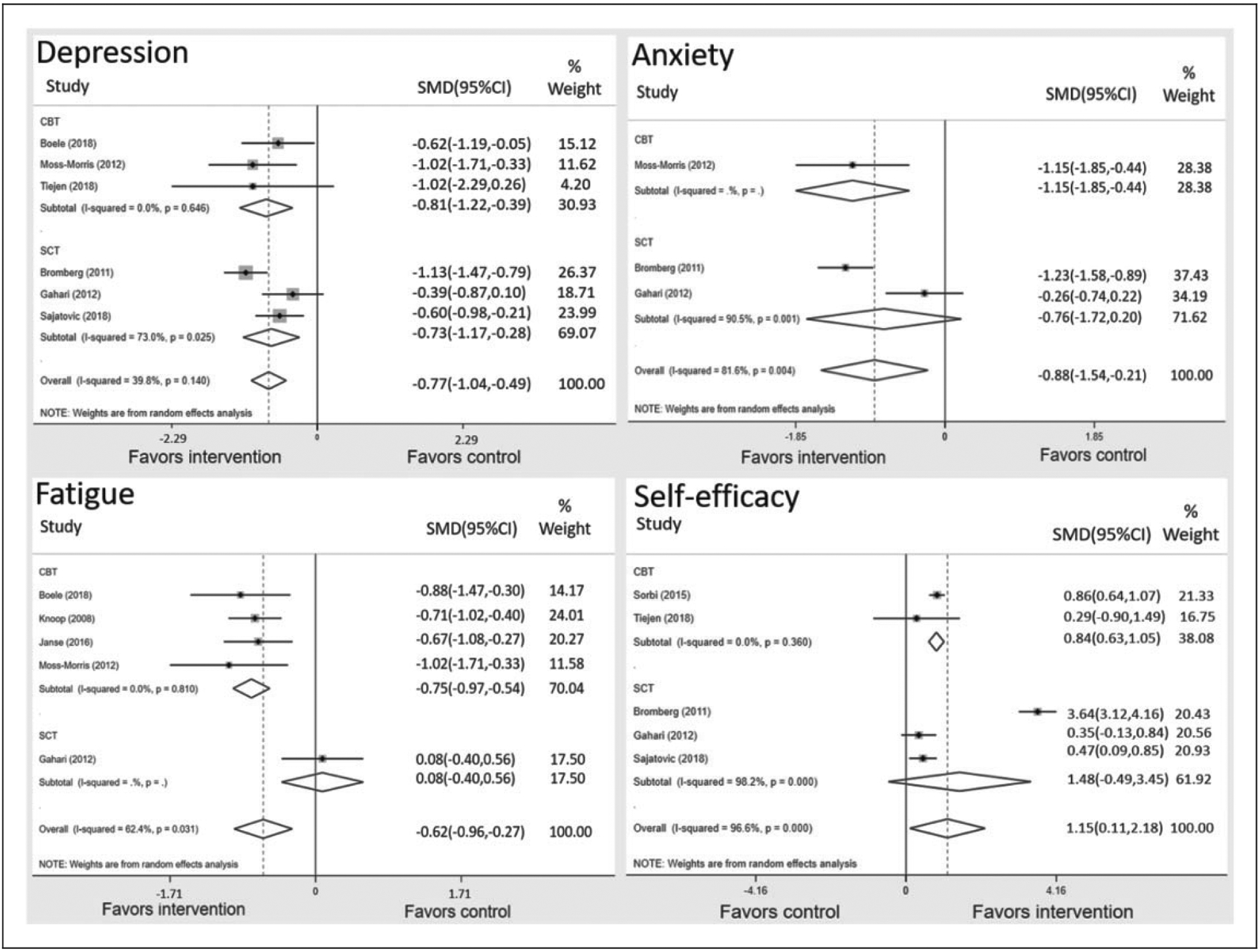
Forest plots of SMD between theory-based digital self-management interventions and control conditions. SMD: standardised mean difference; CBT: cognitive–behavioural theory; SCT: social cognitive theory.
Impact of theories on depression, anxiety, fatigue, and self-efficacy
Figure 2 shows the impact of different theories on end-point outcomes for depression, anxiety, fatigue and self-efficacy. To mitigate symptoms of depression, interventions based on CBT and SCT both yielded significant pooled estimates in favour of the intervention group; CBT-based interventions (SMD = −0.81, 95% CI −1.22 to −0.39) yielded a larger pooled estimate than those based on SCT (SMD = −0.73, 95% CI −1.17 to −0.28). To ameliorate symptoms of anxiety, CBT-based interventions yielded significant between group differences in favour of the intervention group (SMD = −1.15, 95% CI −1.85 to −0.44), and SCT-based interventions showed a lack of significance (SMD = −0.76, 95% CI −1.72 to 0.20). To alleviate symptoms of fatigue, interventions based on CBT yielded a significant pooled estimate (SMD = −0.75, 95% CI −0.97 to −0.54) that was not found in the interventions based on SCT (SMD = 0.08, 95% CI −0.40 to 0.56). To improve self-efficacy, interventions based on CBT yielded a significant pooled estimate (SMD = 0.84, 95% CI 0.63–1.05), which was not observed in interventions based on SCT (SMD = 1.48, 95% CI −0.49 to 3.45).
Impact of mode of digital delivery on depression, anxiety, fatigue, and self-efficacy
Figure 3 shows the impact of the mode of digital delivery on end-point outcomes for depression, anxiety, fatigue and self-efficacy. Partially digital interventions yielded significant estimates to improve all outcomes, whereas fully digital interventions yielded a significant estimate to alleviate only depression. To mitigate symptoms of depression, fully digital interventions (SMD = −0.77, 95% CI −1.19 to −0.35) yielded a slightly larger pooled estimate than partially digital interventions (SMD = −0.70, 95% CI −1.07 to −0.34). To ameliorate symptoms of anxiety, partially digital interventions yielded a significant pooled estimate (SMD = −1.15, 95% CI −1.85 to −0.44), which was not observed in fully digital interventions (SMD = −0.76, 95% CI −1.72 to 0.20). To alleviate symptoms of fatigue, partially digital interventions (SMD = −0.73, 95% CI −0.97 to −0.50) yielded a significant pooled estimate that was not observed in fully digital interventions (SMD = −0.39, 95% CI −1.33 to 0.56). To improve self-efficacy, partially digital interventions yielded a significant pooled estimate (SMD = 0.47, 95% CI 0.09–0.85), which was not observed in fully digital interventions (SMD = 1.31, 95% CI −0.11 to 2.73).
Figure 3.
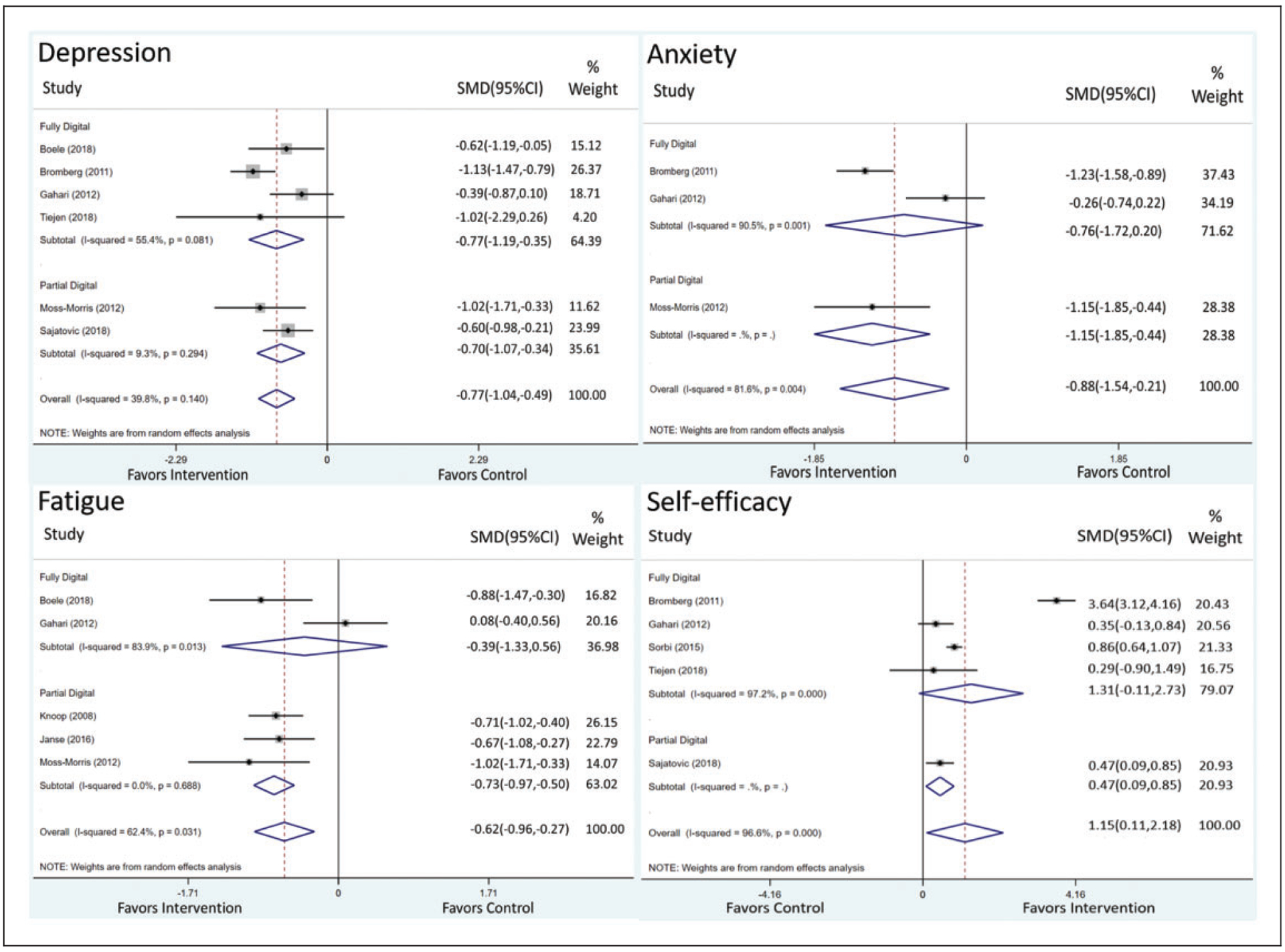
Impact of mode of delivery on theory-based digital self-management interventions on depression, anxiety, fatigue and self-efficacy.
Discussion
Supportive neurological care implemented via traditional in-person self-management interventions is resource intensive and necessitates a significant consideration for pragmatic issues, including geographical, physical and fiscal barriers.10,11 As an alternative, delivery of supportive care via telemedicine could be a cost-effective solution to improve access for patients with neurological disorders.11,12,46 Although telemedicine adoption has been hindered by a lack of reimbursement, Congressional Acts (e.g. The Furthering Access to Stroke Telemedicine Act) were recently passed, and the list of covered telehealth services by the U.S. Center for Medicare and Medicaid Services has been expanded to facilitate the incorporation of telemedicine into clinical practice. Furthermore, an emergent expansion of Medicare telehealth coverage under the 1135 waiver authority and the Coronavirus Preparedness and Response Supplemental Appropriations Act in response to the COVID-19 pandemic may further support the value of telemedicine. As a timely example, results obtained in this study concede that digital self-management interventions may be used to support people recovering from COVID-19 or other medical conditions.
Although digital delivery of interventions holds considerable promise and is believed to be transforming traditional health care,47 the effectiveness of digital interventions must be systematically reviewed before implementation in clinical practice. Our findings suggest that digital self-management interventions are effective in improving depression, anxiety, fatigue and self-efficacy among people with neurological disorders. According to the self-control model of depression,48 psychosocial distress is attributed to a disruption of the self-regulating process. Empowering people with self-management skills modulates this process and increases their ability to cope with distress and neuropsychiatric symptoms. Our findings are encouraging, but caution should be taken. First, as most of the control conditions in the included trials (9/16) were waitlist or no intervention, more active control trials are needed to assess whether digital self-management interventions are comparable or superior to in-person interventions. Second, most studies did not blind the participants and research personnel. Expectations regarding the effectiveness of interventions can engender the risk of bias. Future studies should adopt better blinding techniques (e.g. sham procedures) to improve evidence quality.
The current review extends previous research by evaluating the effects of different theories for digital self-management interventions on depression, anxiety, fatigue and self-efficacy. In general, we found that most studies (n = 10) had a decent use of theories (i.e. >50% as assessed by the TCS) in the development of interventions. Nevertheless, most studies lacked details regarding the linkages between theory-relevant constructs and intervention procedures. Additionally, most studies did not reference the components of the selected theory while describing the interventions. These missing pieces are essential for informing modifications of existing interventions and the development of more effective interventions by identifying the essential theoretical components related to specific outcomes. We recommend the use of the TCS to ensure that the theoretical basis of any future intervention is adequately described and to inform better the design of digital interventions that could adduce support of the benefits of future interventions.
Interventions used in the included studies differed in their use of theory, but CBT (n = 8) was used more frequently than SCT (n = 3). Comparatively, CBT-based digital interventions yielded larger effects in improving depression, anxiety, fatigue and self-efficacy than those based on SCT. The core premise of CBT is to reduce psychosocial distress and problematic behaviours by changing maladaptive thoughts.49 Our findings concur with previous literature supporting CBT for improving self-management50 and neuropsychiatric symptoms.51 To understand the mechanisms contributing to the superior outcomes of CBT-based interventions better, we conducted sensitivity analyses to identify the active ingredients underlying these interventions.52 Problem solving, goal setting, exercise and education are key elements of CBT-based interventions to change outcomes.52 Among the eight CBT-based interventions used in the reviewed studies, education (n = 7)31,33,34,36–39 and problem solving (n = 6)31,33,35,37–39 were the most frequently adopted active ingredients, followed by goal setting (n = 3)34,36,37 and physical exercise (n = 1).36 Education equips participants with knowledge about their condition and symptom management, and problem solving focuses on building the problem-solving skills necessary to overcome barriers in life.53 Hence, widespread use of these principles may support future digital self-management interventions aimed at improving depression, anxiety, fatigue and self-efficacy in people with neurological disorders.
Regarding mode of digital delivery, most interventions consisted of online sessions (n = 13) and were fully digital (n = 11). Features of these online sessions included online educational content on disease-specific self-management, self-assessment of symptoms, self-report of activities and/or interaction with therapists via web conferencing. Although we found that both fully digital and partially digital interventions ameliorate depression and that partially digital interventions yield significant estimates in improving anxiety, fatigue and self-efficacy, there were very limited studies included in the ‘partially digital’ group in each analysis. A stronger effect in partially digital interventions is consistent with a prior meta-analysis studying weight-loss interventions.54 These results indicate that digital interventions should incorporate other modes of delivery (e.g. providing a print booklet related to intervention content and including patient–clinician interaction sessions) for increased efficacy.
Several limitations should be considered. First, the overall number of studies for each of the outcome domains was small, which precludes the investigation of publication bias. We may have missed unpublished negative findings and overestimated the reported effect estimates. It also should be noted that some interventions included in this review were developed using a combination of theories and/or behavioural change approaches (e.g. motivational interviewing). Because the purposes and philosophies for behavioural changes underpinning these interventions are different across theories, conclusions for the overall effectiveness of digital self-management intervention should be interpreted with caution. Next, we only included studies of adults, and studies on certain leading neurological disorders were missing, which limits the generalisability of our findings. Moreover, we did not examine specific ‘management’ aspects of digital interventions. Future studies can be systematically evaluated to test which aspects can benefit most from the digital delivery of interventions. Due to insufficient follow-up data, we did not examine the long-term effects of digital interventions. However, this study has strengths, including the meta-analytic approach and the use of a coding scheme to assess the extent to which theory was used in the development of interventions.
This review provides promising evidence for the effectiveness of theory-based digital self-management interventions to improve depression, anxiety, fatigue and self-efficacy in people with neurological disorders. This study also informs the use of relevant theory to guide the future development of supportive care in digital medicine. Although more high-quality trials are warranted before solid conclusions can be drawn, digital self-management interventions have the potential to be effective, accessible and cost-effective alternatives to traditional interventions.
Supplementary Material
Acknowledgements
We acknowledge Megen Devine at Washington University for her editorial assistance with this manuscript.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with regard to the research, authorship and/or publication of this article: S.C.L.L., S.B., M.W.M.F. and A.H. declare no conflicting interests. G.E.N. declares the following conflicting interests from National Institutes of Health, Otsuka America, Inc., the Center for Brain Research in Mood Disorders, the Center for Diabetes Translational Research, the Institute for Public Health and the McDonnell Center for Neuroscience at Washington University and the Barnes Jewish Hospital Foundation for research support. G.E.N. also serves as a consultant for Sunovion, Alkermes and Supernus Pharmaceuticals, Inc. E. J.L. declares the following conflicting interests from National Institutes of Health, the Patient Centered Outcomes Research Institute, McKnight Brain Research Foundation, Taylor Family Institute for Innovative Psychiatric Research and Center for Brain Research in Mood Disorders (Department of Psychiatry, Washington University), Barnes Jewish Foundation, MagStim, Aptinyx, Takeda and Lundbeck for research support. E.J.L also serves as a consultant for Janssen and Jazz Pharmaceuticals. C.B. declares the following conflicting interests from National Institutes of Health, the Harvey Friedman Center for Aging at Washington University and Schultz Family Support Fund for research support. A.W.K.W. declares the following conflicting interests from National Institutes of Health, the American Occupational Therapy Foundation and Craig H. Neilsen Foundation for research support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Center for Medical Rehabilitation Research (K01HD095388) and the American Occupational Therapy Foundation (AOTFIRG20Wong). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Supplemental material
Supplemental material for this article is available online.
References
- 1.Lyketsos CG, Kozauer N and Rabins PV. Psychiatric manifestations of neurologic disease: where are we headed? Dialogues Clin Neurosci 2007; 9: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes AJ, Beier M, Hartoonian N, et al. Self-efficacy as a longitudinal predictor of perceived cognitive impairment in individuals with multiple sclerosis. Arch Phys Med Rehabil 2015; 96: 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong BO, Kang HJ, Bae KY, et al. Determinants of quality of life in the acute stage following stroke. Psychiatry Investig 2012; 9: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figved N, Myhr KM, Larsen JP, et al. Caregiver burden in multiple sclerosis: the impact of neuropsychiatric symptoms. J Neurol Neurosurg Psychiatry 2007; 78: 1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLean G, Band R, Saunderson K, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens 2016; 34: 600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster G, Taylor SJ, Eldridge S, et al. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2007; CD005108. [DOI] [PubMed] [Google Scholar]
- 7.Plow MA, Finlayson M and Rezac MJP. A scoping review of self-management interventions for adults with multiple sclerosis. PM R 2011; 3: 251–262. [DOI] [PubMed] [Google Scholar]
- 8.Huis In Het Veld J, Verkaik R, Van Meijel B, et al. Self-management by family caregivers to manage changes in the behavior and mood of their relative with dementia: an online focus group study. BMC Geriatr 2016; 16: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veenhuizen Y, Cup EHC, Jonker MA, et al. Self-management program improves participation in patients with neuromuscular disease: a randomized controlled trial. Neurology 2019; 93: e1720–e1731. [DOI] [PubMed] [Google Scholar]
- 10.Ghahari S, Leigh Packer T and Passmore AE. Effectiveness of an online fatigue self-management programme for people with chronic neurological conditions: a randomized controlled trial. Clin Rehabil 2010; 24: 727–744. [DOI] [PubMed] [Google Scholar]
- 11.Guzik AK and Switzer JA. Teleneurology is neurology. Neurology 2020; 94: 16–17. [DOI] [PubMed] [Google Scholar]
- 12.Hatcher-Martin JM, Adams JL, Anderson ER, et al. Telemedicine in neurology: Telemedicine Work Group of the American Academy of Neurology update. Neurology 2020; 94: 30–38. [DOI] [PubMed] [Google Scholar]
- 13.Choon-Huat Koh G and Hoenig H. How should the rehabilitation community prepare for 2019-nCoV? Arch Phys Med Rehabil 2020; 101: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare 2020; 26: 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Snoswell CL, Harding LE, et al. The role of telehealth in reducing the mental health burden from COVID-19. Telemed J E Health 2020; 26: 377–379. [DOI] [PubMed] [Google Scholar]
- 16.Lycett HJ, Raebel EM, Wildman EK, et al. Theory-based digital interventions to improve asthma self-management outcomes: systematic review. J Med Internet Res 2018; 20: e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bove R, Garcha P, Bevan CJ, et al. Clinic to in-home telemedicine reduces barriers to care for patients with MS or other neuroimmunologic conditions. Neurology 2018; 5: e505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337: a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barton S Which clinical studies provide the best evidence? The best RCT still trumps the best observational study. BMJ 2000; 321: 255–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollis C, Falconer CJ, Martin JL, et al. Annual research review: digital health interventions for children and young people with mental health problems – a systematic and meta-review. J Psychol Psychiatr 2017; 58: 474–503. [DOI] [PubMed] [Google Scholar]
- 21.Glanz K and Rimer BK. Theory at a glance: a guide for health promotion practice. 2nd ed. Washington, DC: U.S. Department of Health and Human Services, 2005. [Google Scholar]
- 22.World Health Organization. What are neurological disorders? https://www.who.int/news-room/q-a-detail/what-are-neurological-disorders (2016; accessed 20 June 2020).
- 23.Michie S and Prestwich A. Are interventions theory-based? Development of a Theory Coding Scheme. Health Psychol 2010; 29: 1. [DOI] [PubMed] [Google Scholar]
- 24.Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003; 83: 713–721. [PubMed] [Google Scholar]
- 25.Van Peppen RP, Kwakkel G, Wood-Dauphinee S, et al. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil 2004; 18: 833–862. [DOI] [PubMed] [Google Scholar]
- 26.Hackett ML, Köhler S, O’Brien JT, et al. Neuropsychiatric outcomes of stroke. Lancet Neurol 2014; 13: 525–534. [DOI] [PubMed] [Google Scholar]
- 27.Dishman RK, Motl RW, Sallis JF, et al. Self-management strategies mediate self-efficacy and physical activity. Am J Prev Med 2005; 29: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harbord RM and Higgins JP. Meta-regression in Stata. Stata J 2008; 8: 493–519. [Google Scholar]
- 29.Hedges LV and Pigott TD. The power of statistical tests in meta-analysis. Psychol Methods 2001; 6: 203. [PubMed] [Google Scholar]
- 30.Higgins JP and Green S. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley, 2006. [Google Scholar]
- 31.Van Kessel K, Wouldes T and Moss-Morris R. A New Zealand pilot randomized controlled trial of a web-based interactive self-management programme (MSInvigor8) with and without email support for the treatment of multiple sclerosis fatigue. Clin Rehabil 2016; 30: 454–462. [DOI] [PubMed] [Google Scholar]
- 32.Raina KD, Morse JQ, Chisholm D, et al. Feasibility of a cognitive behavioral intervention to manage fatigue in individuals with traumatic brain injury: a pilot study. J Head Trauma Rehabil 2016; 31: E41–E49. [DOI] [PubMed] [Google Scholar]
- 33.Sorbi MJ, Kleiboer AM, Van Silfhout HG, et al. Medium-term effectiveness of online behavioral training in migraine self-management: a randomized trial controlled over 10 months. Cephalalgia 2015; 35: 608–618. [DOI] [PubMed] [Google Scholar]
- 34.Knoop H, Van Der Meer JWM and Bleijenberg G. Guided self-instructions for people with chronic fatigue syndrome: randomised controlled trial. Br J Psychiatry 2008; 193: 340–341. [DOI] [PubMed] [Google Scholar]
- 35.Boele FW, Klein M, Verdonck-De Leeuw IM, et al. Internet-based guided self-help for glioma patients with depressive symptoms: a randomized controlled trial. J Neurooncol 2018; 137: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janse A, Wiborg JF, Bleijenberg G, et al. The efficacy of guided self-instruction for patients with idiopathic chronic fatigue: a randomized controlled trial. J Consult Clin Psychol 2016; 84: 377–388. [DOI] [PubMed] [Google Scholar]
- 37.Moss-Morris R, McCrone P, Yardley L, et al. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther 2012; 50: 415–421. [DOI] [PubMed] [Google Scholar]
- 38.Tietjen K, Wilson M, Amiri S, et al. Online depressive symptom self-management: comparing program outcomes for adults with multiple sclerosis versus those with other chronic diseases. J Neurosci Nurs 2018; 50: 13–19. [DOI] [PubMed] [Google Scholar]
- 39.Thompson NJ, McGee RE, Garcia-Williams A, et al. The impact of a depression self-management intervention on seizure activity. Epilepsy Behav 2020; 103:106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bromberg J, Wood ME, Black RA, et al. A randomized trial of a web-based intervention to improve migraine self-management and coping. Headache 2012; 52: 244–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sajatovic M, Colon-Zimmermann K, Kahriman M, et al. A 6-month prospective randomized controlled trial of remotely delivered group format epilepsy self-management versus waitlist control for high-risk people with epilepsy. Epilepsia 2018; 59: 1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houlihan BV, Brody M, Everhart-Skeels S, et al. Randomized trial of a peer-led, telephone-based empowerment intervention for persons with chronic spinal cord injury improves health self-management. Arch Phys Med Rehabil 2017; 98: 1067–1076.e1. [DOI] [PubMed] [Google Scholar]
- 43.Kannan M, Hildebrand A, Hugos CL, et al. Evaluation of a web-based fall prevention program among people with multiple sclerosis. Mult Scler Relat Disord 2019; 31: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conroy SS, Zhan M, Culpepper WJ, et al. Self-directed exercise in multiple sclerosis: evaluation of a home automated tele-management system. J Telemed Telecare 2018; 24: 410–419. [DOI] [PubMed] [Google Scholar]
- 45.DiIorio C, Bamps Y, Walker ER, et al. Results of a research study evaluating WebEase, an online epilepsy self-management program. Epilepsy Behav 2011; 22: 469–474. [DOI] [PubMed] [Google Scholar]
- 46.Schneider RB and Biglan KM. The promise of telemedicine for chronic neurological disorders: the example of Parkinson’s disease. Lancet Neurol 2017; 16: 541–551. [DOI] [PubMed] [Google Scholar]
- 47.Meskó B, Drobni Z, Bényei É, et al. Digital health is a cultural transformation of traditional healthcare. Mhealth 2017; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rehm LP. Self-management therapy for depression. Adv Behav Res Ther 1984; 6: 83–98. [Google Scholar]
- 49.Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther 1970; 1: 184–200. [Google Scholar]
- 50.Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med 2015; 175: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofmann SG, Asnaani A, Vonk IJ, et al. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res 2012; 36: 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu C-Y, Rodakowski JL, Terhorst L, et al. A scoping review of nonpharmacological interventions to reduce disability in older adults. Gerontologist 2020; 60: e52–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Zurilla TJ and Nezu AM. Problem-solving therapy. In: Dobson KS (ed). Handbook of cognitive–behavioral therapies. 3rd ed. New York: Guilford Press, 2010, pp.197–225. [Google Scholar]
- 54.Schippers M, Adam PC, Smolenski DJ, et al. A meta-analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration. Obes Rev 2017; 18: 450–459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


