Abstract
Catheter-associated urinary tract infections (CAUTIs) are among the leading nosocomial infections in the world and have led to the extensive study of various strategies to prevent infection. However, despite an abundance of anti-infection materials having been studied over the last forty-five years, only a few types have come into clinical use, providing an insignificant reduction in CAUTIs. In recent decades, marine resources have emerged as an unexplored area of opportunity offering huge potential in discovering novel bioactive materials to combat human diseases. Some of these materials, such as antimicrobial compounds and biosurfactants synthesized by marine microorganisms, exhibit potent antimicrobial, antiadhesive and antibiofilm activity against a broad spectrum of uropathogens (including multidrug-resistant pathogens) that could be potentially used in urinary catheters to eradicate CAUTIs. This paper summarizes information on the most relevant materials that have been obtained from marine-derived microorganisms over the last decade and discusses their potential as new agents against CAUTIs, providing a prospective proposal for researchers.
Keywords: marine microorganisms, urinary catheter, antibiofilm, antifouling, coating
1. Introduction
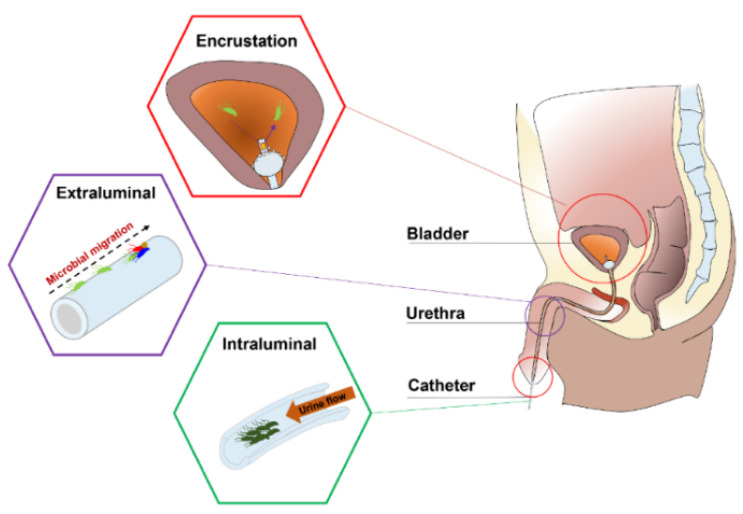
Urinary catheters are hollow, partially flexible tubes that are designed to drain urine from the bladder. The earliest use of urinary catheters can be traced back to the third century B.C., but the modern indwelling catheter, called the ‘Foley’ catheter, was designed by Frederick B. Foley in the mid-1930s [1]. To date, over 100 million urinary catheters are used worldwide per year since catheterization rates remain high at 20% in non-intensive care units and 61% in intensive care units (ICUs) [2]. Despite the care taken to avoid contamination, catheters are still susceptible to infections as they provide direct access for uropathogens from the outside environment into the urinary tract, impairing local host defence mechanisms of the bladder [3,4]. Opportunistic uropathogens (Table 1) are mainly faecal or skin microbiota from the patients that can enter the bladder through the catheter lumen (34%) or along the catheter–urethral interface (66%) causing infections and complications, such as encrustation, bladder stones, bacteriuria, pyelonephritis, septicaemia and endotoxic shock (Figure 1) [3,5,6,7]. According to the European Centre for Disease Prevention and Control (ECDC), catheter-associated urinary tract infections (CAUTIs) account for 27% of all hospital-acquired infections in developed countries, and over 1 million cases occur in the USA and Europe [1,8]. In the UK, CAUTIs cost the NHS GBP 1–2.5 billion and account for approximately 2100 deaths annually [9].
Table 1.
Common pathogens causing CAUTI.
| Short-Term Catheterization | Type | References |
| Escherichia coli | GN bacterium | [18] |
| Serratia spp. | GN bacterium | [19] |
| Staphylococcus epidermidis | GP bacterium | [20] |
| Enterococcus spp. | GP bacterium | [21] |
| Bacillus subtilis | GP bacterium | [3] |
| Long-Term Catheterization | Type | References |
| Providencia aeruginosa | GN bacterium | [1] |
| Proteus mirabilis | GN bacterium | [7] |
| Providencia stuartii | GN bacterium | [22] |
| Morganella morganii | GN bacterium | [6] |
| Klebsiella pneumoniae | GN bacterium | [23] |
| Staphylococcus aureus | GP bacterium | [24] |
| Candida spp. | Fungus | [25] |
GN: Gram-negative; GP: Gram-positive.
Figure 1.
Anatomical cross-section of the renal system in a male showing CAUTIs.
Depending on clinical indications, the duration of catheterization may be short- (<7 days) or long-term (>28 days). Early work showed that 10–50% of patients undergoing short-term catheterization developed bacteriuria, and all patients undergoing long-term catheterization became infected, regardless of whether the catheter system was open or closed [10]. As a basic survival strategy, bacteria that encounter a catheter surface submerged in urine become attached within minutes [11]. These attached bacteria begin to phenotypically change, producing extracellular polymeric substances (EPS) (mainly exopolysaccharides and proteins) that allow the emerging biofilm community to develop a complex, three-dimensional structure within hours. Once established, the biofilms are very difficult to eradicate and exhibit a high tolerance to antibiotics and other biocidal treatments, making them a continuous focus for infections that can only be eliminated by the constant removal of the catheters [3,7,12,13,14,15]. Furthermore, in the presence of urease-positive bacteria (e.g., Proteus mirabilis), urease catalyses the hydrolysis of urea into carbon dioxide and ammonia, which increases the urine pH, leading to the precipitation of calcium and magnesium phosphate crystals (encrustation) and the formation of crystalline biofilms on the catheter, which eventually results in the complete blockage of the catheter [7,16,17]. Previous attempts to prevent CAUTIs include improving sterile techniques to inhibit the access of microbes into the urinary tract and limiting microbial accumulation on the catheter surface by intermittent catheterization. However, clinical evidence shows that these efforts do not lead to a noticeable reduction in CAUTIs [15]. Therefore, developing novel urinary catheters with antibiofilm and antiencrustation properties remains the most direct and promising strategy for this significant clinical problem.
2. Current Anti-Infection Strategies against CAUTIs and Challenges
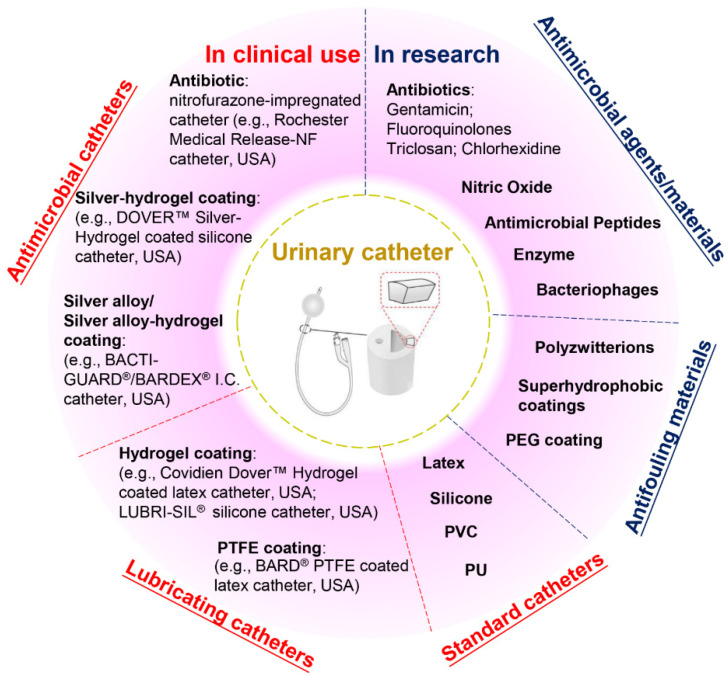
Current commercial urinary catheters can be generally classified as standard and antimicrobial catheters (Figure 2). Owing to their superior malleability, several materials used for making standard catheters include polyvinyl chloride (PVC), polyurethane (PU), silicone and latex [20]. Of these, silicone has emerged as the material of choice for urinary catheters due to distinct advantages, including excellent biocompatibility, no allergic reactions, superior chemical and thermal stability and good mechanical strength [2,26]. Morris et al. [27] compared the antiencrustation performance of 18 types of catheter and found that all-silicone urinary catheters took the longest time to encrust and block. The main reason for this lies in that the all-silicone catheter has a wider lumen, allowing a faster urine flow, which prevents the accumulation of crystalline deposits. However, recent studies demonstrated that there was no significant difference between the development of infections or bacterial adherence on silicone catheters as compared to other types of catheters [20,26,28,29].
Figure 2.
Classification of urinary catheters in clinical use and recently reported antimicrobial and antifouling materials for the prevention of CAUTIs.
Given that bacterial adhesion is the critical step in the progression of biofilm formation, numerous attempts have been made to endow the catheters with antiadhesive or antimicrobial properties, or both. Antiadhesive coatings are designed to prevent microbial adhesion through mechanisms of steric repulsion, electrostatic repulsion or low surface energy instead of killing the microbes [15]. To date, hydrogel- and polytetrafluoroethylene (PTFE)-coated catheters are commercially available, but clinical studies demonstrate that their efficacies against CAUTI are insignificant when compared with standard catheters [2,4,26,30]. Despite their lubricating features that may help improve patient comfort, hydrogel- or PTFE-coated catheters are only suitable for short- or medium-term catheterization (<28 days). Antimicrobial coatings are characterized by bactericidal or bacteriostatic activities that protect the catheters from microbial adhesion and migration. Recent reviews of commercial antimicrobial catheters identified two main strategies used: silver-based coatings and antibiotic impregnation [1,7,31]. However, all these catheters have only been reported to yield positive results for short-term application [7,20,32].
Silver is among the few FDA-approved antimicrobial materials for urinary catheter coatings, and its antimicrobial activity is associated with the release of silver ions. To date, silver has been applied in catheter coatings in the forms of bulk silver, silver alloy (silver/gold or palladium) and silver-hydrogels. Silver is very prone to oxidation in aqueous conditions, and the release of silver ions from these coatings often undergoes an initial burst-release phase followed by a slow-release phase. In clinical trials, the long-term antimicrobial efficacy of these silver-based coatings has proven limited [1,28,33]. In this scenario, attempts have been made to introduce silver nanoparticles into catheter coatings to attain enhanced antimicrobial efficacy. However, concerns have also been raised about the potential toxicity towards patients due to the fast and excessive release of silver ions [33,34]. In comparison, despite studies suggesting that the overuse of antibiotics may result in the development of antibiotic resistance, certain types of antibiotic-impregnated catheters have been proven to be more effective than silver-based antimicrobial catheters in preventing CAUTIs [3,20,35,36,37]. For example, nitrofurazone-impregnated catheters are commercially available, and studies comparing silver-alloy coated catheters, silver-hydrogel coated catheters, and nitrofurazone-impregnated catheters have found that nitrofurazone could effectively reduce the risk of symptomatic CAUTI and bacteriuria in short-term catheterization by impairing bacterial adherence and planktonic growth, while silver-based catheters have only demonstrated minimal effects [28,38]. Pickard et al. [33] compared the ability of silver-alloy-coated catheters and nitrofural-impregnated catheters for the reduction in incidence of symptomatic CAUTIs in adults requiring short-term catheterization via a clinical model, and the results demonstrated that nitrofural-impregnated catheters were more effective than the silver-alloy-coated catheters. In addition, impregnating antibiotics into catheters provides a cost-effective strategy to manufacture antimicrobial catheters, as this can be achieved by simply submerging swollen catheters in antibiotic-containing solutions for antibiotic encapsulation [31]. However, there are still certain concerns about using nitrofurazone in urinary catheters, such as patient discomfort [38] and the potential risks of developing tumours [39] and resistance in bacteria. This has hindered research in this field, and there is a growing demand for developing new antibiotics instead.
Apart from silver and antibiotics, recent research has also focused on a variety of antifouling materials (e.g., poly(ethylene glycol) (PEG) and polyzwitterions) [2,40] and biocidal materials (e.g., nitric oxide, antimicrobial peptides, enzymes and bacteriophages) [41,42,43,44] for urinary catheter coatings and has reported with varying levels of success, which offers potential for complete protection against CAUTIs. However, the promising performance of anti-infection coatings under laboratory conditions has not been translated into clinical success to date. Therefore, exploring novel anti-infection materials for urinary catheters will remain a current and broad interest in the upcoming decade.
3. Marine Microbiota as a Source of Novel Anti-Infection Materials
For short-term urinary catheters, the use of antibiotics has proven to be a cost-efficient strategy to prevent CAUTIs in clinical trials, but a major concern is the development of antibiotic resistance, particularly the increasing emergence of new forms of multidrug resistance among uropathogens that can render these antibiotics useless after repeated applications. Therefore, the identification of new resources for novel antibiotics with new modes of action has become the research focus over the past two decades. Currently, over 75% of the antibiotics currently available on the market are derived from terrestrial organisms, and the discovery of new antibiotics has declined considerably (only two new classes of antibiotics have been commercialized since 1962) due to difficulties in identifying novel and effective compounds [45,46,47].
Oceans cover 71% of the Earth’s surface, but less than 5% have been explored to date, presenting an unexplored area of opportunity [48,49]. In recent decades, significant progress in the clinical development of marine-derived drugs has been achieved, and the discovery of novel antimicrobial substances from marine organisms has indicated their potential to combat existing medical device-related infections [50,51,52,53,54]. However, there are concerns that the overexploration of marine organisms may affect the balance between ecological constraints and economic activity. In this scenario, marine microbiota are emerging as a viable source of bioactive materials [55]. Diverse marine habitats provide unique conditions for marine microorganisms to develop into complex and diverse assemblages, and the isolation and extraction of bioactive secondary metabolites from such organisms are relevant to the discovery of novel anti-infection agents [56,57,58,59]. Therefore, microbes isolated from previously unexplored marine habitats may lead to the discovery of novel structures with potent antibiotic activity, and marine microbial-derived antibiotics are believed to be a promising alternative to overcome existing problems [60,61,62].
For long-term catheters, microbes are more prone to colonize and build biofilms on the surfaces with the attenuation of antimicrobial efficacy. Catheters with only biocidal activity are considered insufficient to eradicate CATUIs, as recent studies demonstrated that a ’foundation layer’ composed of both dead and live bacteria can form on the catheter surface in long-term catheterization, which protects bacteria from contact with underlying biocidal substances [4,63]. To solve this problem, more research has focused on endowing catheter surfaces with both biocidal and antiadhesion properties [4,9,15,64]. Biosurfactants (BSs) are amphipathic secondary metabolites produced by microorganisms and have become a promising anti-infection material for medical applications [65,66,67]. Recent studies of the anti-infection functions of biosurfactants have identified three main routes, including killing microbes or inhibiting microbial growth, resisting microbial adhesion and disrupting biofilm formation [68,69,70]. For example, several BSs isolated from terrestrial-derived microbes, such as surfactin [71] and iturin [72], display potent antimicrobial activities, which make them relevant molecules in combating infections. Van Hoogmoed et al. [73] reported that biosurfactants released by Streptococcus thermophilus significantly inhibited Candida spp. adhesion to silicone rubber, which indicates their potential for use as a defence against colonizing strains on urinary catheters. These biosurfactants are mostly obtained from terrestrial-derived microorganisms, while biosurfactants produced by marine microorganisms have been less explored. Over the last decade, marine microbial-derived biosurfactants have attracted increasing interest, as marine microbes may exhibit unique metabolic and physiological capabilities producing novel metabolites with potent biological activities. To date, a considerable number of marine microbial-derived biosurfactants with antimicrobial, antiadhesive and antibiofilm activities have been obtained, and several have been proven to be effective against a broad spectrum of uropathogens, including Gram-positive and Gram-negative bacteria, as well as the yeast Candida albicans, which could potentially offer a new solution to the problems associated with long-term catheterization.
In this review, we summarize the diversity of marine microbial-derived antibiotics and biosurfactants discovered over the past 10 years and discuss their potential for use in combatting CAUTIs in short-term and long-term urinary catheters.
4. Marine Microbial-Derived Antibiotics with Broad-Spectrum Antimicrobial Activity
Antibiotics are low-molecular-weight compounds produced by microorganisms that kill or inhibit the growth of other microorganisms at low concentrations [74]. The current understanding of the biological activity of antibiotics is mainly cantered on their primary cellular targets. The mechanisms of antibiotic action can be classified into 5 categories: (1) inhibition of cell wall synthesis (the most common mechanism), (2) inhibition of protein synthesis, (3) alteration of cell membranes, (4) inhibition of nucleic acid synthesis and (5) antimetabolite activity [75,76]. However, not all antibiotics have broad-spectrum antimicrobial activity, and their efficiency decays along with catheterization due to a limited shelf-life. Some microbes may be inherently resistant to certain antibiotics, while they may also mutate and/or exchange genetic material with other microbes, leading to the development of multidrug resistance [77,78]. Furthermore, some antibiotics may exhibit toxicity at or close to their therapeutic dose [47]. Considering the diversity of uropathogen species, the ideal antibiotic for urinary catheters should exhibit broad-spectrum antimicrobial activity and avoid developing resistance without inducing cytotoxicity in patients.
Generally, antibiotic compounds based on their structures and/or biosynthetic origins can be classified as alkaloids [79], quinones [80], phenols [81], polyketides [82], terpenes [83], polyketides [84,85] and peptides [86]. Table 2 lists the recent discoveries of marine microbial-derived compounds with broad-spectrum antimicrobial activity. Although very few of them have been developed into clinical trial phases, several have demonstrated to be effective against a broad spectrum of uropathogens, including Gram-positive and Gram-negative bacteria, fungi, as well as methicillin-resistant Staphylococcus aureus (MRSA). Therefore, they could be an alternative to conventional antibiotics for short-term catheters against CAUTIs caused by those pathogens.
Table 2.
Marine microbial-derived compounds with broad-spectrum antimicrobial activity.
| Compound | Molecular Class | Source | Target | Reference |
|---|---|---|---|---|
| Gageotetrins A–C | Peptide | Bacillus subtillis 109GGC020 | B/F | [90] |
| Gageopeptides A–D | Peptide | Bacillus subtillis 109GGC020 | B/F | [91] |
| Ieodoglucomide 1, 2 | Peptide | Bacillus licheniformis 09IDYM23 | B/F | [88] |
| Bacteriocin | Peptide | Lactobacillus murinus AU06 | B | [96] |
| Actinomycins D, V, X0β | Peptide | Streptomyces sp. ZZ338 | F | [102] |
| Mohangamides A, B | Peptide | Streptomyces sp. SNM55 | F | [103] |
| Gageomacrolactins A–C | Macrolide | Bacillus subtillis 109GGC020 | B/F | [89] |
| Glycosylated macrolactins A1, B1 | Macrolide | Streptomyces sp. (KJ371985) | B | [94] |
| Bonactin | Acyclic ester | Streptomyces sp. BD21-2 | B/F | [86] |
| Butenolide | Lactone | Streptomyces sp. | B | [108] |
| Mollemycin A | Peptide-polyketide | Streptomyces sp. CMB-M0244 | B | [98] |
| Thiomarinols A-G | Polyketide | Alteromonas rava SANK 73390 | B | [99] |
| Branimycin B, C | Polyketide | Pseudonocardia carboxydivorans M-227 | B | [100] |
| UN | Polyketide | Streptomyces sp. JRG-04 | B | [101] |
| Janthinopolyenemycin A, B | Polyketide | Janthinobacterium spp. ZZ145 and ZZ148 | F | [105] |
| Kocumarin | Benzoic acid | Kocuria marina CMG S2 | B/F | [93] |
| Marinocine | Protein | Marinomonas mediterranea MMB-1 | B | [109] |
| 5HM2F | Furan | Bacillus subtilis KC433737 | F | [104] |
| Trichodin A | Pyridone | Trichoderma sp. MF106 | F | [106] |
B: Gram-positive and Gram-negative bacteria; F: fungi; UN: unnamed material.
Over the last two decades, the most studied group of microbes producing antimicrobial substances is the Firmicutes phylum (particularly the genus Bacillus) [87]. Tareq et al. [88,89,90,91] reported the discovery of four types of bioactive molecules with broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria and fungi. Gageostatins A–C (Figure 3a) and gageopeptides A–D (Figure 3b) are two types of non-cytotoxic linear lipopeptides isolated from a marine Bacillus subtilis 109GGC020. The authors proposed a biosynthetic pathway based on nonribosomal peptide synthetases (NRPS) for the production of gageotetrins. Comparative studies showed that gageostatins A–C were more active against fungi than bacteria with minimum inhibitory concentration (MIC) values of 0.02–0.04 μM. Gageopeptides A–D exhibited potent antifungal and moderately broad antibacterial activity, while not showing cytotoxicity to human myeloid leukaemia K-562 and mouse leukemic macrophage RAW 264.7 cell lines. Gageomacrolactins A–C (Figure 3c) are three macrolactin derivatives obtained from the secondary metabolites of marine Bacillus subtilis 109GGC020 and displayed strong broad-spectrum activity against Gram-negative and Gram-positive bacteria and fungi. By comparing the structure-function of the gageomacrolactins A–C with that of known macrolactins 4–7, the authors demonstrated that antibacterial activity was not affected by the position of the epoxide group but highly dependent on the hydroxyl group at C-15 of the macrolactone ring. Ieodoglucomides 1 and 2 (Figure 3d) are two unique glycolipopeptides produced by a marine Bacillus licheniformis 09IDYM23 which act as moderate antimicrobial molecules. Podilapu et al. [92] reported the successful synthesis of ieodoglucomides A and B through a high-yielding route using per-O-TMS glucosyl iodide, making them promising candidates for industrial application.
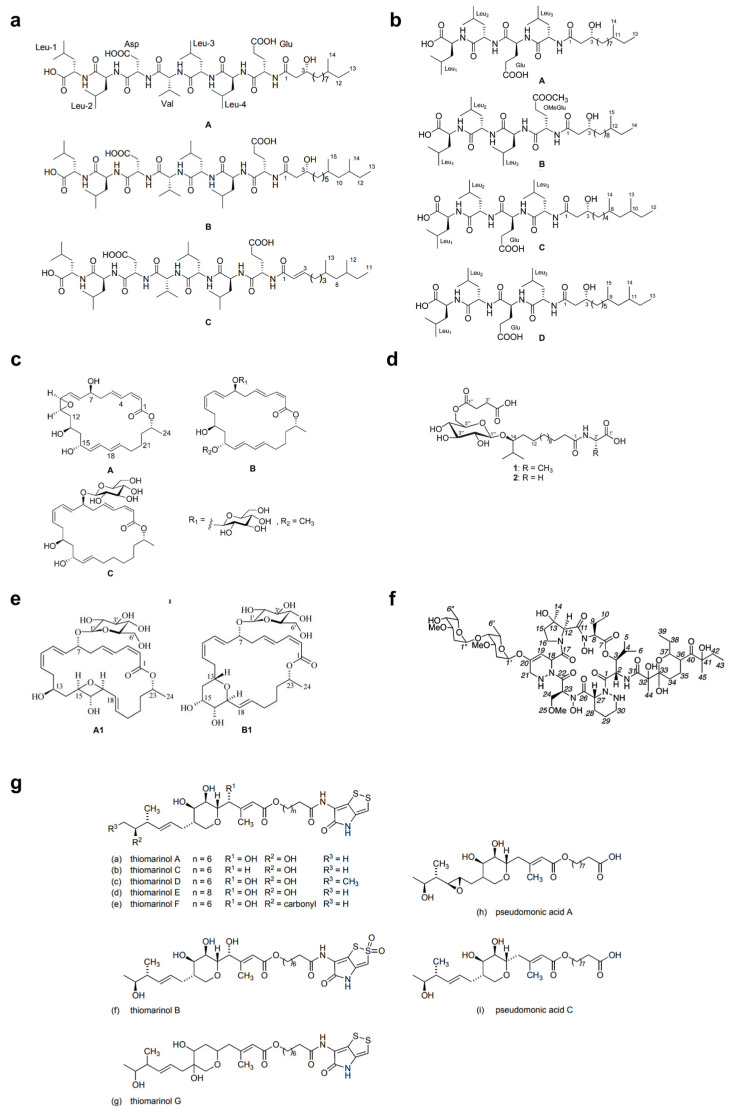
Figure 3.
Structures of (a) gageosatins A–C [90], (b) gageopeptides A–D [91], (c) gageomacrolactins A–C [89], (d) ieodoglucomides 1 and 2 [88], (e) glycosylated macrolactins A1 and B1 [94], (f) mollemycin A [98], and (g) thiomarinols A–G [99].
Apart from Bacillus spp., Uzair et al. [93] reported the isolation of 4-[(Z)-2 phenyl ethenyl] benzoic acid (kocumarin) from the marine Kocuria marina CMG S2, which exhibited pronounced and rapid growth inhibition against fungi and pathogenic bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). Although its antimicrobial mechanism has not been clearly elucidated, research on the functional groups involved in the molecular mechanism would reveal further insights into this atypical class of antibiotics. Schumacher et al. [84] described the first antimicrobial polyketide (bonactin) isolated from the liquid culture of a Streptomyces sp. BD21-2, a compound displaying antifungal and broad-spectrum antibacterial activity against microbes, including Bacillus megaterium, Micrococcus luteus, Klebsiella pneumoniae, Staphylococcus aureus, Alcaligenes faecalis, Escherichia coli and Saccharomyces cerevisiae. However, such marine microbial-derived compounds with activity against both bacteria and fungi are still very rare to date.
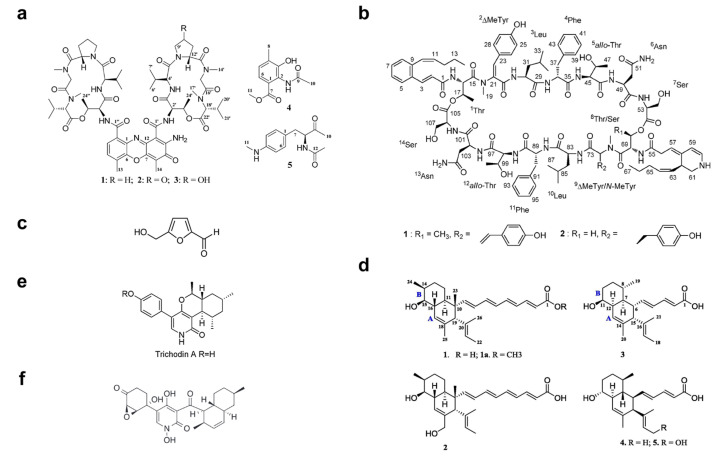
Over the past 10 years, numerous molecules possessing broad-spectrum antibacterial or antifungal activities have been isolated from marine microorganisms. Glycosylated macrolactins A1 and B1 (Figure 3e) were isolated from a marine Streptomyces sp. KJ371985, which inhibited Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus with MICs of 0.03–0.22 μM. These compounds inhibit the peptidyl transferase activity and binding of the acceptor substrate to bacterial ribosomes. The higher solubility in the polar solvents of sugar-containing macrolactins could be an advantage in clinical applications [94]. Bacteriocins are natural peptides synthesized by bacteria for the purpose of killing/inhibiting other bacterial strains whilst not harming the producing bacteria through specific immunity proteins [95]. Elayaraja et al. [96] purified a bacteriocin from marine Lactobacillus murinus AU06, which exhibited a broad inhibitory spectrum against both Gram-positive and -negative bacteria. Marinocine is a broad-spectrum antibacterial protein synthesized by the melanogenic marine bacterium Marinomonas mediterranea, which generates hydrogen peroxide that kills bacteria [97]. However, recent studies demonstrated that the molecular basis of the antibacterial activity was L-lysine dependent, and activity was inhibited under anaerobic conditions. Mollemycin A (Figure 3f) is an antibacterial glyco-hexadepsipeptide-polyketide isolated from an Australian marine-derived Streptomyces sp. (CMB-M0244), which exhibited exceptionally potent and selective growth inhibitory activity against Gram-positive and Gram-negative bacteria (IC50 10–50 nM) but did not show any antifungal activity against Candida albicans. The cytotoxicity test also showed that mollemycin A was proportionately less cytotoxic toward human neonatal foreskin fibroblast cells [98]. Thiomarinols A–G (Figure 3g) were discovered as a class of polyketide antibiotics from marine Alteromonas rava SANK 73390, which displayed broad-spectrum activity against Gram-positive and Gram-negative bacterial species [99]. These thiomarinols act through inhibiting bacterial isoleucyl-transfer RNA synthetase and have pronounced activity against MRSA, with MICs ≤ 0.01 μg/mL [60]. Similar antibacterial compounds were also obtained from actinobacteria, such as Pseudonocardia carboxydivorans M-227 [100] and Streptomyces sp. JRG-04 [101]. Fungi (primarily Candida species) account for 20–30% of CAUTI cases, and Candida albicans is the most prevalent pathogen found in CAUTI biofilms [1,37]. Antifungal (particularly anti-Candida) compounds derived from marine microbes have been reported for metabolites produced by Streptomyces sp. ZZ338 [102], Streptomyces sp. SNM55 [103], Bacillus subtilis KC433737 [104], Janthinobacterium spp. ZZ145 and ZZ148 [105], Trichoderma sp. MF106 [106] and Stagonosporopsis cucurbitacearumstrain G019 [107]. These microbial strains, respectively produced actinomycins D, V and X0β (Figure 4a) (MIC, 9.83–9.96 μM); mohangamides A and B (Figure 4b) (IC50, 4.4 and 20.5 μM); 5-hydroxymethyl-2-furaldehyde (5HM2F) (Figure 4c) (MBIC, 400 μg/mL); janthinopolyenemycin A and B (Figure 4d) (MIC, 15.6 μg/ML, MBC, 31.25 μg/mL); trichodin A (Figure 4e) (IC50, 25.38 ± 0.41 μM); and didymellamide A (Figure 4f) (MIC, 3.1 μg/mL).
Figure 4.
Structures of (a) actinomycins D, V and X0β [102], (b) mohangamides A and B [103], (c) 5-hydroxymethyl-2-furaldehyde (5HM2F) [104], (d) janthinopolyenemycin A and B [105], (e) trichodin A [106], and (f) didymellamide A [107].
5. Marine Microbial-Derived Biosurfactants: New Agents against CAUTIs
Biosurfactants (BSs) are amphipathic secondary metabolites produced by microorganisms that consist of both hydrophilic and hydrophobic moieties [45]. Compared to conventional chemical surfactants, these biomolecules have many advantages, such as low toxicity; high biodegradability; biocompatibility; low critical micelle concentrations (CMC); an ability to function over wide ranges of pH, temperature and salinity; as well as greater selectivity, and can be produced from renewable, cheaper substrates [110,111]. Such characteristics allow BSs to play a key role in multidisciplinary applications in industrial and environmental fields and make them a green alternative to their chemical counterparts. Furthermore, some BSs have been reported for their specific bioactivities, which play an essential role in the survival of microbial producers against other competing microbes [69,112]. In nature, BSs can be secreted extracellularly or remain attached to cell surfaces, reducing surface tension at the interface, thereby reducing surface contamination and aiding microbial motility in potentially hostile environments [113]. BSs may also have a range of therapeutic and biomedical benefits and could be used instead of conventional antibiotics to combat infections [114]. Current research regarding BSs has mostly focused on microbes from terrestrial sources (particularly soil-isolated microbes such as species of Bacillus, Pseudomonas and yeasts) [45,112], while practical applications of BSs in healthcare are still limited [115]. For this reason, marine habitats have again emerged as a potential source for isolating new BSs, and the discovery of new BS-producing microorganisms is attracting increasing interest.
BSs according to their molecular weight can be grouped into two categories: (a) high-molecular-mass (HMW) molecules (also called bioemulsifiers), such as polysaccharides, proteins, lipopolysaccharides, lipoproteins and lipoheteropolysaccharides, and (b) low-molecular-mass (LWM) molecules (generally 500 to 1500 Da), which can be further subdivided into glycolipids, lipopeptides, phospholipids, polymeric compounds and neutral lipids based on their chemical composition and microbial origin [45,65,116]. Recent research has mainly focused on the LWM BSs, and several have been reported to display antimicrobial, antiadhesive and antibiofilm activities against a broad spectrum of uropathogens (including multidrug-resistant pathogens), indicating their use as promising anti-infection materials for urinary catheters (Table 3).
Table 3.
Marine microbial-derived BSs with antimicrobial, antiadhesive or antibiofilm activities.
| Source | Type | Activity | Reference |
|---|---|---|---|
| Staphylococcus saprophyticus SBPS 15 | Glycolipid | Antibacterial activity against Klebsiella Pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Salmonella paratyphi and Staphylococcus aureus Antifungal activity against Aspergillus niger, Candida albicans and Cryptococcus neoformans |
[121] |
| Serratia marcescens | Glycolipid | Antibacterial activity against Pseudomonas aeruginosa and Bacillus pumilus Antifungal activity against Candida albicans Antiadhesive activity against Pseudomonas aeruginosa, Bacillus pumilus and Candida albicans |
[122] |
| Brevibacterium casei MSA19 | Glycolipid | Antibacterial activity against Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Vibrio parahaemolyticus and Vibrio vulnificus Antibiofilm activity against mixed and individual cultures of Escherichia coli, Pseudomonas aeruginosa and Vibrio spp. |
[123] |
| Streptomyces sp. MAB36 | Glycolipid | Antibacterial activity against Bacillus cereus, Enterococcus faecalis, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Shigella dysenteriae and Shigella boydii Antifungal activity against Candida albicans |
[124] |
| Brachybacterium paraconglomeratum MSA21 | Glycolipid | Antibacterial activity against Bacillus subtilis, Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae, Micrococcus luteus, Pseudomonas aeruginosa, Proteus mirabilis, Streptococcus sp., Staphylococcus aureus and Staphylococcus epidermidis Antifungal activity against Candida albicans |
[125] |
| Buttiauxella sp. M44 | Glycolipid | Antibacterial activity against Escherichia coli, Salmonella enterica, Bacillus cereus, Bacillus subtilis and Staphylococcus aureus Antifungal activity against Candida albicans and Aspergillus niger |
[126] |
| Staphylococcus lentus SZ2 | Glycolipid | Antiadhesive activity against Vibrio harveyi and Pseudomonas aeruginosa Antibiofilm activity against Vibrio harveyi and Pseudomonas aeruginosa |
[134] |
|
Bacillus velezensis H3 |
Lipopeptide | Antibacterial activity against Staphyloccocus aureus, Mycobacterium, Klebsiella peneumoniae and Pseudomonas aeruginosa Antifungal activity against Candida albicans |
[71] |
| Halobacterium salinarum | Lipopeptide | Antibacterial activity against Escherichia coli, Bacillus sps., Pseudomonas sp., Streptococcus sp. And Staphylococcus aureus Antifungal activity against Aspergillus niger and Candida albicans |
[127] |
| Nocardiopsis alba MSA10 | Lipopeptide | Antibacterial activity against Enterococcus faecalis, Klebsiella pneumoniae, Micrococcus luteus, Proteus mirabilis, Staphylococcus aureus and Staphylococcus epidermidis Antifungal activity against Candida albicans |
[128] |
| Aneurinibacillus aneurinilyticus SBP-11 | Lipopeptide | Antibacterial activity against Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis and Vibrio cholerae | [129] |
| Bacillus licheniformis NIOT-AMKV06 | Lipopeptide | Antibacterial activity against Enterococcus faecalis, Klebsiella pneumoniae, Micrococcus luteus, Proteus mirabilis, Salmonella typhi, Shigella flexneri, Staphylococcus aureus and Vibrio cholera | [131] |
| Pontibacter korlensis strain SBK-47 | Lipopeptide | Antibacterial activity against Streptococcus mutans, Micrococcus luteus, Salmonella typhi and Klebsiella oxytoca Antiadhesion potential against Bacillus subtilis, Staphylococcus aureus, Salmonella typhi and Vibrio cholerae |
[130] |
| Bacillus tequilensis CH | Lipopeptide | Antibiofilm activity against Escherichia coli and Streptococcus mutans | [149] |
|
Bacillus Amyloliquefaciens anti-CA |
Lipopeptide | Antibiofilm activity against Pseudomonas aeruginosa and Bacillus cereus | [148] |
| Bacillus circulans | Lipopeptide | Antiadhesive activity against Escherichia coli, Micrococcus flavus, Serratia marcescens, Salmonella typhimurium, Proteus vulgaris, Citrobacter freundii, Alcaligenes faecalis, and Klebsiella aerogenes | [147] |
| Aspergillus ustus MSF3 | Glycolipoprotein | Antibacterial activity against Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Micrococcus luteus, Pseudomonas aeruginosa, Proteus mirabilis, Staphylococcus aureus, Staphylococcus epidermidis and haemolytic Streptococcus Antifungal activity against Candida albicans |
[150] |
| Streptomyces sp. B3 | Mixture of proteins, carbohydrates and lipids | Antibacterial activity against Escherichia coli and Pseudomonas aeruginosa Antifungal activity against Candida albicans |
[151] |
| Oceanobacillus iheyensis BK6 | Extracellular polysacchrides | Antibiofilm activity against Staphylococcus aureus | [152] |
5.1. Antimicrobial Activity
Biosurfactants have been reported to exhibit antimicrobial activity via different mechanisms of action, which primarily destroy the cell wall or plasma membrane by disrupting their integrity and permeability [65,68,117,118]. More specifically, their amphiphilic characteristics and affinity for lipid bilayers allow BSs to interact with cell membranes, leading to cell lysis and metabolite leakage, which ultimately results in cell death [116]. To date, various marine microbes have been reported to produce antimicrobial BSs, of which glycolipids and lipopeptides are the two primary isolated families that display broad-spectrum antimicrobial activity.
Glycolipids are a class of carbohydrate molecules made of mono-, di-, tri- and tetra-saccharides in combination with long-chain aliphatic acids or hydroxyaliphatic acids [119], of which trehalolipids, rhamnolipids and sophorolipids are of the most interest [120]. Reported marine microbial-derived glycolipid BSs with broad-spectrum antimicrobial activity against both bacteria and fungi are listed in Table 3. A glycolipid BS isolated from a marine Staphylococcus saprophyticus SBPS 15 exhibited dual functions: (1) excellent antibacterial and antifungal activities against a broad spectrum of clinical human pathogens, including Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus), Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae and Salmonella paratyphi) and fungi (Aspergillus niger, Candida albicans and Cryptococcus neoformans), and (2) potent surface tension-reducing activity (32 mN/m). This BS also showed superior stability over a broad range of pH (3–9) and temperature (up to 80 °C) [121]. Another glycolipid BS purified from extracts of a tropical marine strain of Serratia marcescens demonstrated similar dual functions, inhibiting the growth of Candida albicans and Pseudomonas aeruginosa with MIC values of >25.0 μg/mL and preventing adhesion by up to 99% [122]. The glycolipid BS also displayed biofilm disrupting activities against the test strains, which were believed to result from synergistic antimicrobial and surfactant activity. Glycolipid BSs were also isolated from the marine actinobacteria Brevibacterium casei MSA19, Streptomyces sp. MAB36 and Brachybacterium paraconglomeratum MSA21. The MSA19 glycolipid was bacteriostatic and could inhibit microbial attachment and disrupt both fungal and bacterial biofilms in both individual strains and mixed cultures at 30 µg/mL [123]. The MAB36 glycolipid also possessed strong antimicrobial activity against pathogenic bacteria and fungi and demonstrated excellent stability over a wide range of pH, temperature and ion concentrations [124]. The BS produced by the sponge-associated Brachybacterium paraconglomeratum MSA21 displayed broad antibiotic activity and it was suggested that the discovery of polyketide synthase (pks II) genes might pave the way for making new BSs by exploiting the microbial genes and enzymes, making the green production of BSs possible in the future [125]. Another glycolipid BS was isolated from a marine halotolerant bacterium (Buttiauxella sp. M44) that exhibited significant stability over a broad range of pH (7–8), temperature (20–60 °C) and salinity (0–3%) and potent antimicrobial activity against both bacteria and fungi [126]. Moreover, the combination of a cheap energy and carbon source (e.g., molasses) as well as a response surface method (RSM) could favor production and expand possible uses in the future.
Lipopeptides (LPs) are composed of peptide chains (short linear or cyclic structures) with lipid moieties and are the most widely reported class of biosurfactants with antimicrobial activity. Bacillus species are the most studied LP-producing strains that have been reported to produce several antimicrobial lipopeptide biosurfactants (LPBs), such as surfactin, iturin and fengycin, although most of these strains are from terrestrial ecosystems [70,72]. Liu et al. [71] reported the first surfactin (mainly composed of the nC14- and anteiso-C15-surfactin) isolated from marine Bacillus velezensis H3, which exhibited antimicrobial activity against a broad range of pathogens, including Staphyloccocus aureus, Klebsiella peneumoniae, Pseudomonas aeruginosa and Candida albicans. The surfactin demonstrated a lower inhibitory effect than the antibiotic polymixin B but greater antifungal activity against Candida albicans than the antibiotic vancomycin. Similar antibacterial and antifungal activities were also found for the BSs produced by Halobacterium salinarum [127]. Another surfactin produced by the marine actinobacterium Nocardiopsis alba MSA10 was shown to effectively inhibit the growth of Enterococcus faecalis, Klebsiella pneumoniae, Micrococcus luteus, Proteus mirabilis, Staphylococcus aureus, Staphylococcus epidermidis and Candida albicans, however, without effects on Escherichia coli and Pseudomonas aeruginosa [128]. Apart from surfactin, Balan et al. [129] first reported the discovery of aneurinifactin from the marine Aneurinibacillus aneurinilyticus SBP-11, which showed broad-spectrum antibacterial activity against Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis and Vibrio cholerae. The antibacterial mechanism was proposed to be derived from the anchoring of the BS on the bacterial cell membrane that disrupted its integrity, resulting in the production of hydroxyl radicals, which in turn caused lipid peroxidation and pore formation in the membrane [129]. However, antifungal activity was not investigated. It was also reported that a LPB (pontifactin) produced by marine Pontibacter korlensis SBK-47 displayed high antibacterial activity against Streptococcus mutans, Micrococcus luteus, Salmonella typhi and Klebsiella oxytoca and moderate activity against Klebsiella pneumonia and Vibrio cholerae. The molecule also showed antiadhesion potential against Bacillus subtilis, Staphylococcus aureus, Salmonella typhi and Vibrio cholerae [130]. Lawrance et al. [131] discovered an antibacterial LPB from the marine sponge-associated bacterium Bacillus licheniformis NIOT-AMKV06, which displayed significant bacteriostatic activity against a range of human pathogens, including Enterococcus faecalis, Klebsiella pneumoniae, Micrococcus luteus, Proteus mirabilis, Salmonella typhi, Shigella flexneri, Staphylococcus aureus and Vibrio cholerae. The production of these biosurfactants in heterologous host strains was achieved by cloning three gene clusters (sfp, sfpO and srfA) in Escherichia coli, with production being increased three-fold over the original strain. In addition, a lipopeptide BS produced by the marine Bacillus circulans is the only one found to be effective against multidrug-resistant (MDR) clinical strains while not having any haemolytic activity, indicating its potential to combat infections caused by such pathogens [132].
5.2. Antiadhesive and Antibiofilm Activities
Biosurfactants have also been reported to inhibit microbial adhesion and biofilm formation without exerting antimicrobial activity [69,133,134]. Despite the precise mechanisms of such activity not being fully understood, biosurfactants may affect interactions between microbes and surfaces through several modes of action: (1) modification of the physico-chemical properties (e.g., surface charge, hydrophobicity and surface energy) of the surface, which reduces microbial adhesion [111]; (2) suppression of the expression of biofilm-related genes, which inhibits microbial adhesion [135]; (3) promotion of the solubilization of biofilms encouraging bacterial detachment [136]; and (4) the interference of quorum sensing leading to decreased biofilm formation [70]. Numerous studies have shown that the prior adhesion of BSs to catheter surfaces (surface conditioning) reduced microbial adhesion and colonization [137,138,139].
In general, bacterial adhesion to a surface is regulated by diverse factors (e.g., growth medium, substrate and cell surface). The most frequently cited theory is the two-step adhesion model in which the adhesion process is divided into two distinct phases: reversible adhesion and irreversible adhesion [11,12,140]. Reversible adhesion describes a dynamic process in which bacteria can easily attach to or detach from a surface due to a combination of surface–cell interactions including van der Waals, electrostatic double-layer, Lewis acid-base, Brownian motion and hydrophobic interactions [64,141]. Walencka et al. [142] reported that BSs can affect cell-to-surface interactions by altering surface tension and charge to overcome the initial electrostatic repulsion barrier. For instance, Meylheuc et al. [143] reported that a stainless-steel surface coated with the BS produced by Pseudomonas fluorescens significantly reduced microbial adhesion. The presence of BS in the conditioned surface remarkably reduced the surface energy and enhanced surface hydrophilicity, leading to a decrease in attraction due to a reduction in the van der Waals forces and an increase in electron–donor/electron–acceptor characteristics. Jemil et al. [144] also reported that electrostatic repulsion between negatively charged surfaces (coated with anionic lipopeptides) and the negatively charged microbial surface could aid in inhibiting microbial adhesion. However, following reversible adhesion, microbial attachment gradually becomes stronger with time through a range of interfacial rearrangements (e.g., removal of interfacial water, protein conformational changes and an increase in hydrophobic interactions) and the production of adhesins, eventually form biofilms [145]. Despite numerous studies highlighting the difficulties in eliminating biofilms, BSs have been shown to disrupt or remove biofilms by penetrating and absorbing at the interface between the solid substrate and the biofilm, thereby reducing interfacial tension [134,146].
Antiadhesive and antibiofilm activities have also been reported for marine microbial-derived BSs. Hamza et al. [134] reported a non-toxic glycolipid BS (SLSZ2) derived from a marine epizootic bacterium Staphylococcus lentus SZ2 that effectively prevented the adhesion of Vibrio harveyi and Pseudomonas aeruginosa without showing bactericidal activity. In addition, this BS efficiently inhibited biofilm formation and disrupted the pre-formed biofilms of both strains by ~80%. Another glycolipid BS produced from a marine Symphylia sp. also exhibited antiadhesive activity against a range of pathogens (Candida albicans, Pseudomonas aeruginosa and Bacillus pumilus) and could disrupt pre-formed biofilms of these cultures in a concentration-dependent manner [122]. Similarly, a glycolipid BS isolated from the marine actinobacterium Brevibacterium casei MSA19 disrupted biofilm formation in Escherichia coli, Pseudomonas aeruginosa and Vibrio spp. under dynamic conditions. Moreover, biofilm disruption activity was consistent against mixed and individual biofilm bacteria at ~30 µg/mL. The lipopeptide BS produced by marine Bacillus circulans exhibited promising antiadhesive activity against several potential pathogenic strains, and the BS-coated surface effectively reduced microbial adhesion by up to 89% at a concentration of 0.1 g/L. Moreover, the pre-formed biofilms were removed with efficiencies between 59 and 94% for all the strains tested, demonstrating its potential in biomedical applications [147]. Song et al. [148] reported a lipopeptide BS produced by marine Bacillus amyloliquefaciens anti-CA that inhibited biofilm formation and dispersed pre-formed biofilms of Pseudomonas aeruginosa PAO1 and Bacillus cereus. The obtained data indicated that the BS could suppress the expression of the PslC gene which is associated with exopolysaccharide production in Pseudomonas aeruginosa PAO1. Pradhan et al. [149] also reported a lipopeptide BS produced by a marine Bacillus tequilensis CH which effectively inhibited pathogenic biofilms (Escherichia coli and Streptococcus mutans) on both hydrophilic and hydrophobic surfaces at a concentration of 50 μg/mL.
6. Opportunities, Challenges and Future Perspectives
Currently, research on anti-infection materials for urinary catheters is still increasing, and there is a growing demand for catheters with stronger antibiofilm and antiencrustation capabilities. Compared to the conservative strategy of optimizing existing products (e.g., widening the internal lumen [1], replacing bulk silver with silver nanoparticles [4], and redesign of micro/nano-scale surface topography [153]), the exploration of novel anti-infection materials from marine resources has clearly become an exciting potential solution to address some current limitations. Owing to the vast diversity of marine microorganisms, a number of new bioactive molecules with antimicrobial, antiadhesive and antibiofilm activities have been discovered that could be potentially combined with urinary catheters to combat CAUTIs.
The antimicrobial strategy aims to endow urinary catheters with microbicidal or microbiostatic properties to prevent the contact of microbes with the catheter surface or inhibit microbial migration along the catheter, thus preventing biofilm formation and encrustation. For short-term catheters, the use of antibiotics has proven to be the most efficient and cost-effective strategy, whereas the application of conventional antibiotics (e.g., nitrofurazone) has been questioned due to the safety concerns and the presence of antibiotic resistance. Despite attempts having been made to develop new antibiotics to solve this problem, the difficulty in identifying novel and effective compounds has led to a slowdown of research in this field. In recent decades, the isolation of microbes from previously unexplored marine habitats has led to the discovery of a number of novel antimicrobial compounds with new modes of action to combat the current antibiotic resistance threat. Several of these have been proven to possess broad-spectrum antimicrobial activities against a wide range of uropathogens, which could be potentially applied to urinary catheters to eradicate CAUTIs. Technically, these antimicrobial molecules could be directly deposited on the catheter surfaces or applied in coatings. Considering that CAUTIs may be derived from both extraluminal and intraluminal routes, it would be ideal to endow both surfaces with antimicrobial properties, despite extraluminal infections being clinically more common. For example, commercial catheters have been conventionally impregnated with antimicrobials, such as antibiotics, and function via a release model. However, this is only a temporary solution for short-term catheterization, and microbial contamination can resume once the antibiotics are removed. Given that the efficacy of antimicrobial materials is often concentration dependent, a challenge in this type of catheter modification strategy is to achieve and preserve adequate local delivery of the antimicrobials during catheterization in vivo without inducing resistance and patient cytotoxicity. This could be achieved by developing a novel release system with controlled release kinetics instead of directly altering the drug loading. For example, hydrogel coatings, due to their lubricating properties, have been widely applied in urinary catheters. Milo et al. [154] reported a smart infection-responsive hydrogel coating for urinary catheters. The coating is a dual-layered polymeric system consisting of a lower ‘reservoir’ layer of poly(vinyl alcohol) (PVA) hydrogel (containing bacteriophage), capped by an upper layer of the pH-responsive polymer. The upper layer swells when urinary pH is elevated due to P. mirabilis infection, exposing the PVA reservoir layer to urine, resulting in the release of bacteriophage from the coating to prevent the encrustation and blockage of urinary catheters. In this way, the release of antimicrobials could be controlled in a smart way to prevent/retard CAUTIs and associated complications. Researchers will need to further conduct thorough in vitro and in vivo leaching and cytotoxicity studies to determine the optimum operational conditions and identify any problematic side effects.
In long-term catheterization, extensive biofilms containing >5 × 109 viable cells/cm2 can be found on catheters and can be responsible for the persistence of the infections [155]. Moreover, the biofilms are often composed of multispecies consortia, which have been reported to be more virulent and have a higher tolerance to antibiotics [7]. Therefore, in addition to killing the uropathogens, attempts have also been made to combine catheter surfaces with antiadhesive materials to retard the occurrence of CAUTIs. To date, only PTFE and hydrogel have entered clinical use, while their efficacy in resisting CAUTI and encrustation is still controversial [4,17,33]. Other antiadhesive materials, such as polyzwitterions, have recently emerged as promising candidates, although their instability in long-term catheterization hinders practical application [1]. In this scenario, research on marine microbes has led to the discovery of novel antiadhesive molecules with greater activity and stability. For instance, marine microbial-derived antiadhesive materials, such as biosurfactants and cyanobacterial extracellular polymers [59,156], can inhibit microbial adhesion via multimechanisms. Currently, several biosurfactants have been reported to influence both cell-to-cell and cell-to-surface interactions that can inhibit microbial adhesion as well as disrupt biofilm formation [67,70,112]. Technically, biosurfactants could be doped in coatings and act on microbial cells via a contact mode. These features may aid in preventing biofilm formation and encrustation as well as extending the lifetime of indwelling urinary catheters. Furthermore, they could be employed in combination with other strategies (e.g., use of antimicrobial materials and micro/nano patterning) to achieve synergistic effects.
In fact, although these marine microbial-derived molecules have shown potential in combating CAUTI-related pathogens and/or biofilms, it should be noted that their clinical effectiveness still needs to be verified in future. Currently, there has been a lack of accomplished research in this area, and this paper provides a prospective proposal for researchers. Currently, the most significant obstacles hindering the development of marine microbial-derived products are supply issues. This is mainly due to difficulties associated with the isolation and growth of producing microorganisms. In addition, for marine microbial-derived biosurfactants, most of these materials are unidentified mixtures of compounds, and the further pharmaceutical development of such mixtures is very challenging. To date, the development of new chemical and physicochemical approaches and tools has led to great advances in the isolation and structure elucidation of novel minor marine secondary metabolites, which could not be isolated/detected in the past [54]. On the other hand, urinary catheters are still considered as low-cost clinical care products, although commercial ‘anti-infective’ catheters are usually priced two to five times higher than standard catheters. Therefore, in addition to the cost of employing new materials, a cost-effective coating technology should also be addressed.
7. Conclusions
Marine microorganisms can produce metabolites of varied chemical structures displaying antimicrobial, antiadhesive and antibiofilm activities. These compounds present enormous potential for the discovery of new agents to overcome the current challenges associated with CAUTI. Owing to technical limitations in the past, bioactive compounds derived from marine microorganisms have not yet progressed into clinical trials. However, recent advances in oceanographic science and metabolome screening have led to a boost in the discovery of new species and metabolic profiles as well as new molecules with potent anti-infection activities against CAUTI-associated pathogens. Research efforts should be applied to the exploration of marine microbes in the search for new bioactive compounds for the development of novel anti-infection agents.
Acknowledgments
The authors acknowledge the financial supports from the UK Engineering and Physical Sciences Research Council (EP/P00301X/1).
Author Contributions
S.Z. and X.L. are first co-authors. Conceptualized and writing, S.Z. and X.L.; Revision and editing, G.M.G. and Q.Z.; Supervision and resources, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the UK Engineering and Physical Sciences Research Council (EP/P00301X/1).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singha P., Locklin J., Handa H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017;50:20–40. doi: 10.1016/j.actbio.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen M.J., Flores-Mireles A.L. Urinary catheter coating modifications: The race against catheter-associated infections. Coatings. 2020;10:23. doi: 10.3390/coatings10010023. [DOI] [Google Scholar]
- 3.Jacobsen S.M., Stickler D.J., Mobley H.L., Shirtliff M.E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008;21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S., Wang L., Liang X., Vorstius J., Keatch R., Corner G., Nabi G., Davidson F., Gadd G.M., Zhao Q. Enhanced antibacterial and antiadhesive activities of silver-PTFE nanocomposite coating for urinary catheters. ACS Biomater. Sci. Eng. 2019;5:2804–2814. doi: 10.1021/acsbiomaterials.9b00071. [DOI] [PubMed] [Google Scholar]
- 5.Stickler D.J. Clinical complications of urinary catheters caused by crystalline biofilms: Something needs to be done. J. Intern. Med. 2014;276:120–129. doi: 10.1111/joim.12220. [DOI] [PubMed] [Google Scholar]
- 6.Cortese Y.J., Wagner V.E., Tierney M., Devine D., Fogarty A. Review of catheter-associated urinary tract infections and in vitro urinary tract models. J. Heal. Eng. 2018;14:1–16. doi: 10.1155/2018/2986742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramstedt M., Ribeiro I.A.C., Bujdakova H., Mergulhao F.J.M., Jordao L., Thomsen P., Alm M., Burmolle M., Vladkova T., Can F., et al. Evaluating efficacy of antimicrobial and antifouling materials for urinary tract medical devices: Challenges and recommendations. Macromol. Biosci. 2014;19:e1800384. doi: 10.1002/mabi.201800384. [DOI] [PubMed] [Google Scholar]
- 8.Saint S., Gaies E., Fowler K.E., Harrod M., Krein S.L. Introducing a catheter-associated urinary tract infection (CAUTI) prevention guide to patient safety (GPS) Am. J. Infect. Control. 2014;42:548–550. doi: 10.1016/j.ajic.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milo S., Nzakizwanayo J., Hathaway H.J., Jones B.V., Jenkins A.T.A. Emerging medical and engineering strategies for the prevention of long-term indwelling catheter blockage. Proc. Inst. Mech. Eng. Part H. 2019;233:68–83. doi: 10.1177/0954411918776691. [DOI] [PubMed] [Google Scholar]
- 10.Stickler D.J. Bacterial biofilms and the encrustation of urethral catheters. Biofouling. 1996;9:13. doi: 10.1080/08927019609378311. [DOI] [Google Scholar]
- 11.Dunne W.M., Jr. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002;15:155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donlan R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Hooton T.M., Bradley S.F., Cardenas D.D., Colgan R., Geerlings S.E., Rice J.C., Saint S., Schaeffer A.J., Tambayh P.A., Tenke P., et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International clinical practice guidelines from the Infectious Diseases Society of America. CID. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 15.Yu K., Lo J.C., Yan M., Yang X., Brooks D.E., Hancock R.E., Lange D., Kizhakkedathu J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials. 2017;116:69–81. doi: 10.1016/j.biomaterials.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Stickler D., Ganderton L., King J., Nettleton J., Winters C. Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol. Res. 1993;21:407–411. doi: 10.1007/BF00300077. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S., Liang X., Gadd G.M., Zhao Q. Superhydrophobic coatings for urinary catheters to delay bacterial biofilm formation and catheter-associated urinary tract infection. ACS Appl. Bio Mater. 2020;3:282–291. doi: 10.1021/acsabm.9b00814. [DOI] [PubMed] [Google Scholar]
- 18.Pelling H., Nzakizwanayo J., Milo S., Denham E.L., MacFarlane W.M., Bock L.J., Sutton J.M., Jones B.V. Bacterial biofilm formation on indwelling urethral catheters. Lett. Appl. Microbiol. 2019;68:277–293. doi: 10.1111/lam.13144. [DOI] [PubMed] [Google Scholar]
- 19.Efthimiou I., Skrepetis K. Prevention of Catheter-Associated Urinary Tract Infections. Recent Advances in the Field of Urinary Tract Infections. IntechOpen; London, UK: 2013. [(accessed on 20 September 2020)]. Available online: https://www.intechopen.com/books/recent-advances-in-the-field-of-urinary-tract-infections/prevention-of-catheter-associated-urinary-tract-infections/ [Google Scholar]
- 20.Al-Qahtani M., Safan A., Jassim G., Abadla S. Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: A review on recent updates. J. Infect. Public Health. 2019;12:760–766. doi: 10.1016/j.jiph.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Guiton P.S., Hung C.S., Hancock L.E., Caparon M.G., Hultgren S.J. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 2010;78:4166–4175. doi: 10.1128/IAI.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J.R., Johnston B., Kuskowski M.A. In vitro comparison of nitrofurazone- and silver alloy-coated foley catheters for contact-dependent and diffusible inhibition of urinary tract infection-associated microorganisms. Antimicrob. Agents Chemother. 2012;56:4969–4972. doi: 10.1128/AAC.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng D., Li X., Liu P., Luo M., Chen S., Su K., Zhang Z., He Q., Qiu J., Li Y. Epidemiology of pathogens and antimicrobial resistance of catheter-associated urinary tract infections in intensivecare units: A systematic review and meta-analysis. Am. J. Infect. Control. 2018;46:e81–e90. doi: 10.1016/j.ajic.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Walker J.N., Flores-Mireles A.L., Pinkner C.L., Schreiber H.L.I., Joens M.S., Park A.M., Potretzke A.M., Bauman T.M., Pinkner J.S., Fitzpatrick J., et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl. Acad. Sci. USA. 2017;114:E8721–E8730. doi: 10.1073/pnas.1707572114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher L.E., Hook A.L., Ashraf W., Yousef A., Barrett D.A., Scurr D.J., Chen X., Smith E.F., Fay M., Parmenter C.D., et al. Biomaterial modification of urinary catheters with antimicrobials to give long-term broadspectrum antibiofilm activity. J. Control. Release. 2015;202:57–64. doi: 10.1016/j.jconrel.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence E.L., Turner I.G. Materials for urinary catheters: A review of their history and development in the UK. Med. Eng. Phys. 2005;27:443–453. doi: 10.1016/j.medengphy.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Morris N.S., Stickler D.J., Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br. J. Urol. 1997;80:58–63. doi: 10.1046/j.1464-410X.1997.00185.x. [DOI] [PubMed] [Google Scholar]
- 28.Desai D.G., Liao K.S., Cevallos M.E., Trautner B.W. Silver or nitrofurazone impregnation of urinary catheters has a minimal effect on uropathogen adherence. J. Urol. 2010;184:2565–2571. doi: 10.1016/j.juro.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen A.B., Dagli M., Stavropoulos S.W.J., Mondschein J.I., Soulen M.C., Shlansky-Goldberg R.D., Solomon J.A., Chittams J.L., Trerotola S.O. Silicone and polyurethane tunneled linfusion catheters: A comparison of durability and breakage rates. J. Vasc. Interv. Radiol. 2011;22:638–641. doi: 10.1016/j.jvir.2011.01.433. [DOI] [PubMed] [Google Scholar]
- 30.Kazmierska K.A., Thompson R., Morris N., Long A., Ciach T. In vitro multicompartmental bladder model for assessing blockage of urinary catheters: Effect of hydrogel coating on dynamics of Proteus mirabilis growth. Urology. 2010;76:e515–e520. doi: 10.1016/j.urology.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Liu L., Shi H., Yu H., Yan S., Luan S. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater. Sci. 2020;8:4095–4108. doi: 10.1039/D0BM00659A. [DOI] [PubMed] [Google Scholar]
- 32.Trautner B.W., Hull R.A., Darouiche R.O. Prevention of catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 2005;18:37–41. doi: 10.1097/00001432-200502000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickard R., Lam T., MacLennan G., Starr K., Kilonzo M., McPherson G., Gillies K., McDonald A., Walton K., Buckley B., et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: A multicentre randomised controlled trial. Lancet. 2012;380:1927–1935. doi: 10.1016/S0140-6736(12)61380-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S., Liang X., Gadd G.M., Zhao Q. A sol-gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. Appl. Surf. Sci. 2021;535:147675. doi: 10.1016/j.apsusc.2020.147675. [DOI] [Google Scholar]
- 35.Wu K., Yang Y., Zhang Y., Deng J., Lin C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int. J. Nanomed. 2015;10:7241–7252. doi: 10.2147/IJN.S92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feneley R.C.L., Hopley I.B., Wells P.N.T. Urinary catheters: History, current status, adverse events and research agenda. J. Med. Eng. Technol. 2015;39:459–470. doi: 10.3109/03091902.2015.1085600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam T.B., Omar M.I., Fisher E., Gillies K., MacLennan S. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database Syst. Rev. 2014;23:CD004013. doi: 10.1002/14651858.CD004013.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiraku Y., Sekine A., Nabeshi H., Midorikawa K., Murata M., Kumagai Y., Kawanishi S. Mechanism of carcinogenesis induced by a veterinary antimicrobial drug, nitrofurazone, via oxidative DNA damage and cell proliferation. Cancer Lett. 2004;215:141–150. doi: 10.1016/j.canlet.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Sankar S., Rajalakshmi T. Application of poly ethylene glycol hydrogel to overcome latex urinary catheter related problems. Biofactors. 2007;30:217–225. doi: 10.1002/biof.5520300403. [DOI] [PubMed] [Google Scholar]
- 41.Carlsson S., Weitzberg E., Wiklund P., Lundberg J.O. Intravesical nitric oxide delivery for prevention of catheter-associated urinary tract infections. Antimicrob. Agents Chemother. 2005;49:2352–2355. doi: 10.1128/AAC.49.6.2352-2355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X., Li P., Saravanan R., Basu A., Mishra B., Lim S.H., Su X., Tambyah P.A., Leong S.S. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014;10:258–266. doi: 10.1016/j.actbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Lehman S.M., Donlan R.M. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob. Agents Chemother. 2015;59:1127–1137. doi: 10.1128/AAC.03786-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thallinger B., Brandauer M., Burger P., Sygmund C., Ludwig R., Ivanova K., Kun J., Scaini D., Burnet M., Tzanov T., et al. Cellobiose dehydrogenase functionalized urinary catheter as novel antibiofilm system. J. Biomed. Mater. Res. B Appl. Biomater. 2016;104:1448–1456. doi: 10.1002/jbm.b.33491. [DOI] [PubMed] [Google Scholar]
- 45.Santos D.K., Rufino R.D., Luna J.M., Santos V.A., Sarubbo L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016;17:401. doi: 10.3390/ijms17030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lovell F.M. The structure of a bromine-rich marine antibiotic. J. Am. Chem. Soc. 1966;88:4510–4511. doi: 10.1021/ja00971a040. [DOI] [Google Scholar]
- 47.Nweze J.A., Mbaoji F.N., Huang G., Li Y., Yang L., Zhang Y., Huang S., Pan L., Yang D. Antibiotics development and the potentials of marine-derived compounds to stem the tide of multidrug-resistant pathogenic bacteria, fungi, and protozoa. Mar. Drugs. 2020;18:145. doi: 10.3390/md18030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernan V.S., Greenstein M., Carter G.T. Mining marine microorganisms as a source of new antimicrobials and antifungals. Curr. Med. Chem. Anti-Infect. Agents. 2004;3:181–195. doi: 10.2174/1568012043353883. [DOI] [Google Scholar]
- 49.Visbeck M. Ocean science research is key for a sustainable future. Nat. Commun. 2018;9:690. doi: 10.1038/s41467-018-03158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penesyan A., Kjelleberg S., Ega S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs. 2010;8:438–459. doi: 10.3390/md8030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stowe S.D., Richards J.J., Tucker A.T., Thompson R., Melander C., Cavanagh J. Anti-biofilm compounds derived from marine sponges. Mar. Drugs. 2011;9:2010–2035. doi: 10.3390/md9102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mi Y., Zhang J., He S., Yan X. New peptides isolated from marine cyanobacteria, an overview over the past decade. Mar. Drugs. 2017;15:132. doi: 10.3390/md15050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seal B.S., Drider D., Oakley B.B., Brussow H., Bikard D., Rich J.O., Miller S., Devillard E., Kwan J., Bertin G., et al. Microbial-derived products as potential new antimicrobials. Vet. Res. 2018;49:66. doi: 10.1186/s13567-018-0563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyshlovoy S.A., Honecker F. Marine compounds and cancer: The first two decades of XXI century. Mar. Drugs. 2020;18:20. doi: 10.3390/md18010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satheesh S., Ba-akdah M.A., Al-Sofyani A.A. Natural antifouling compound production by microbes associated with marine macroorganisms—A review. Electron. J. Biotechn. 2016;21:26–35. doi: 10.1016/j.ejbt.2016.02.002. [DOI] [Google Scholar]
- 56.Mandakhalikar K.D., Chua R.R., Tambyah P.A. New technologies for prevention of catheter associated urinary tract infection. Curr. Treat. Options. Infect. Dis. 2016;8:24–41. doi: 10.1007/s40506-016-0069-5. [DOI] [Google Scholar]
- 57.Younis K.M., Usup G., Ahmad A. Secondary metabolites produced by marine streptomyces as antibiofilm and quorum-sensing inhibitor of uropathogen Proteus mirabilis. Environ. Sci. Pollut. Res. Int. 2016;23:4756–4767. doi: 10.1007/s11356-015-5687-9. [DOI] [PubMed] [Google Scholar]
- 58.Wang J., Nong X.H., Zhang X.Y., Xu X.Y., Amin M., Qi S.H. Screening of antibiofilm compounds from marine-derived fungi and the effects of Secalonic Acid D on Staphylococcus aureus biofilm. J. Microbiol. Biotechnol. 2017;27:1078–1089. doi: 10.4014/jmb.1609.09053. [DOI] [PubMed] [Google Scholar]
- 59.Costa B., Mota R., Tamagnini P., Martins M.C.L., Costa F. Natural cyanobacterial polymer-based coating as a preventive strategy to avoid catheter-associated urinary tract infections. Mar. Drugs. 2020;18:279. doi: 10.3390/md18060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahman H., Austin B., Mitchell W.J., Morris P.C., Jamieson D.J., Adams D.R., Spragg A.M., Schweizer M. Novel anti-infective compounds from marine bacteria. Mar. Drugs. 2010;8:498–518. doi: 10.3390/md8030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tortorella E., Tedesco P., Esposito F.P., January G.G., Fani R., Jaspars M., De Pascale D. Antibiotics from deep-sea microorganisms: Current discoveries and perspectives. Mar. Drugs. 2018;16:355. doi: 10.3390/md16100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pereira F. Have marine natural product drug discovery efforts been productive and how can we improve their efficiency? Expert. Opin. Drug Discov. 2019;14:717–722. doi: 10.1080/17460441.2019.1604675. [DOI] [PubMed] [Google Scholar]
- 63.Stickler D.J., Morgan S.D. Observations on the development of the crystalline bacterial biofilms that encrust and block Foley catheters. J. Hosp. Infect. 2008;69:350–360. doi: 10.1016/j.jhin.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 64.Zhang S., Liang X., Gadd G.M., Zhao Q. Advanced titanium dioxide-polytetrafluorethylene (TiO2-PTFE) nanocomposite coatings on stainless steel surfaces with antibacterial and anti-corrosion properties. Appl. Surf. Sci. 2019;490:231–241. doi: 10.1016/j.apsusc.2019.06.070. [DOI] [Google Scholar]
- 65.Ndlovu T., Rautenbach M., Vosloo J.A., Khan S., Khan W. Characterization and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express. 2017;7:108. doi: 10.1186/s13568-017-0363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harshada K. Biosurfactant: A potent antimicrobial agent. J. Microbiol. Exp. 2014;1:173–177. doi: 10.15406/jmen.2014.01.00031. [DOI] [Google Scholar]
- 67.Gudiña E.J., Rocha V., Teixeira J.A., Rodrigues L.R. Antimicrobial and anti-adhesive properties of biosurfactant produced by lactobacilli isolates, biofilm formation and aggregation ability. J. Gen. Appl. Microbiol. 2013;59:425–436. doi: 10.2323/jgam.59.425. [DOI] [PubMed] [Google Scholar]
- 68.Rienzo M.A.D.D., Stevenson P., Marchant R., Banat I.M. Antibacterial properties of biosurfactants against selected Gram-positive and Gram-negative bacteria. FEMS. Microbiol. Lett. 2016;363:fnv224. doi: 10.1093/femsle/fnv224. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues L., Banat I.M., Teixeira J., Oliveira R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006;57:609–618. doi: 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- 70.Paraszkiewicz K., Moryl M., Płaza G., Bhagat D., Satpute S.K., Bernat P. Surfactants of microbial origin as antibiofilm agents. Int. J. Environ. Heal. Res. 2019;11:1–20. doi: 10.1080/09603123.2019.1664729. [DOI] [PubMed] [Google Scholar]
- 71.Liu X., Ren B., Chen M., Wang H., Kokare C.R., Zhou X., Wang J., Dai H., Song F., Liu M., et al. Production and characterization of a group of bioemulsifiers from the marine Bacillus velezensis strain H3. Appl. Microbiol. Biotechnol. 2010;87:1881–1893. doi: 10.1007/s00253-010-2653-9. [DOI] [PubMed] [Google Scholar]
- 72.Bonmatin J.M., Laprevote O., Peypoux F. Diversity among microbial cyclic lipopeptides: Iturins and surfactins. Activity-Structure relationships to design new bioactive agents. Comb. Chem. High. Throughput Screen. 2003;6:541–556. doi: 10.2174/138620703106298716. [DOI] [PubMed] [Google Scholar]
- 73.Busscher H.J., Van Hoogmoed C.G., Geertsema-Doornbusch G.I., Van Der Kuijl-Booij M., Van Der Mei H.C. Streptococcus thermophilus and its biosurfactants inhibit adhesion by Candida spp. on silicone rubber. Appl. Environ. Microbiol. 1997;63:3810–3817. doi: 10.1128/AEM.63.10.3810-3817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kasanah N., Hamann M.T. Development of antibiotics and the future of marine microorganisms to stem the tide of antibiotic resistance. Curr. Opin. Investig. Drugs. 2004;5:827–837. [PMC free article] [PubMed] [Google Scholar]
- 75.Kapoor G., Saigal S., Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017;33:300–305. doi: 10.4103/joacp.JOACP_349_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rourke A.O., Beyhan S., Choi Y., Morales P., Chan A.P., Espinoza J.L., Dupont C.L., Meyer K.J., Spoering A., Lewis K., et al. Mechanism-of-action classification of antibiotics by global transcriptome profiling. Antimicrob. Agents Chemother. 2020;64:e01207–e01219. doi: 10.1128/AAC.01207-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies J., Davies D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun D., Jeannot K., Xiao Y., Knapp C.W. Editorial: Horizontal gene transfer mediated bacterial antibiotic resistance. Front. Microbiol. 2019;10:1933. doi: 10.3389/fmicb.2019.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rabin N., Zheng Y., Opoku-Temeng C., Du Y., Bonsu E., Sintim H.O. Agents that inhibit bacterial bioflm formation. Future Med. Chem. 2015;7:647–671. doi: 10.4155/fmc.15.7. [DOI] [PubMed] [Google Scholar]
- 80.Liang Y., Xie X., Chen L., Yan S., Ye X., Anjum K., Huang H., Lian X., Zhang Z. Bioactive polycyclic quinones from marine Streptomyces sp. 182SMLY. Mar. Drugs. 2016;14:10. doi: 10.3390/md14010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramesh C., Vinithkumar N.V., Kirubagaran R. Marine pigmented bacteria: A prospective source of antibacterial compounds. J. Nat. Sci. Biol. Med. 2019;10:104–113. doi: 10.4103/jnsbm.JNSBM_201_18. [DOI] [Google Scholar]
- 82.Wiese J., Imhoff J.F. Marine bacteria and fungi as promising source for new antibiotics. Drug Dev. Res. 2019;80:24–27. doi: 10.1002/ddr.21482. [DOI] [PubMed] [Google Scholar]
- 83.Hughes C.C., Fenical W. Antibacterials from the sea. Chemistry. 2010;16:12512–12525. doi: 10.1002/chem.201001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schumacher R.W., Talmage S.C., Miller S.A., Sarris K.E., Davidson B.S., Goldberg A. Isolation and structure determination of an antimicrobial ester from a marine sediment-derived bacterium. J. Nat. Prod. 2003;66:1291–1293. doi: 10.1021/np020594e. [DOI] [PubMed] [Google Scholar]
- 85.Habbu P., Warad V., Shastri R., Madagundi S., Kulkarni V.H. Antimicrobial metabolites from marine microorganisms. Chin. J. Nat. Med. 2016;14:101–116. doi: 10.1016/S1875-5364(16)60003-1. [DOI] [PubMed] [Google Scholar]
- 86.Manivasagan P., Venkatesan J., Sivakumar K., Kim S.K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014;169:262–278. doi: 10.1016/j.micres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 87.Stincone P., Brandelli A. Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 2020;40:306–319. doi: 10.1080/07388551.2019.1710457. [DOI] [PubMed] [Google Scholar]
- 88.Tareq F.S., Kim J.H., Lee M.A., Lee H., Lee Y., Lee J.S., Shin H.J. Ieodoglucomides A and B from a marine derived bacterium Bacillus licheniformis. Org. Lett. 2012;14:1464–1467. doi: 10.1021/ol300202z. [DOI] [PubMed] [Google Scholar]
- 89.Tareq F.S., Kim J.H., Lee M.A., Lee H.S., Lee J.S., Lee Y.J., Shin H.J.V. Antimicrobial gageomacrolactins characterized from the fermentation of the marine-derived bacterium Bacillus subtilis under optimum growth conditions. J. Agric. Food Chem. 2013;61:3428–3434. doi: 10.1021/jf4009229. [DOI] [PubMed] [Google Scholar]
- 90.Tareq F.S., Lee M.A., Lee H.S., Lee Y.J., Lee J.S., Hasan C.M., Islam M.T., Shin H.J. Gageotetrins A–C, noncytotoxic antimicrobial linear lipopeptides from a marine bacterium Bacillus subtilis. Org. Lett. 2014;16:928–931. doi: 10.1021/ol403657r. [DOI] [PubMed] [Google Scholar]
- 91.Tareq F.S., Lee M.A., Lee H.S., Lee Y.J., Lee J.S., Hasan C.M., Islam M.T., Shin H.J. Non-cytotoxic antifungal agents: Isolation and structures of gageopeptides A–D from a Bacillus strain 109GGC020. J. Agric. Food. Chem. 2014;62:5565–5572. doi: 10.1021/jf502436r. [DOI] [PubMed] [Google Scholar]
- 92.Podilapu A.R., Emmadi M., Kulkarni S.S. Expeditious synthesis of ieodoglucomides A and B from the marine-derived bacterium Bacillus licheniformis. Eur. J. Org. Chem. 2018;2018:3230–3235. doi: 10.1002/ejoc.201800209. [DOI] [Google Scholar]
- 93.Uzair B., Menaa F., Khan B.A., Mohammad F.V., Ahmad V.U., Djeribi R., Menaa B. Isolation, purification, structural elucidation and antimicrobial activities of kocumarin, a novel antibiotic isolated from actinobacterium Kocuria marina CMG S2 associated with the brown seaweed Pelvetia canaliculata. Microbiol. Res. 2018;206:186–197. doi: 10.1016/j.micres.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 94.Mondol M.A.M., Shin H.J. Antibacterial and antiyeast compounds from marine-derived bacteria. Mar. Drugs. 2014;12:2913–2921. doi: 10.3390/md12052913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang S.C., Lin C.H., Sung C.T., Fang J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014;5:241. doi: 10.3389/fmicb.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elayaraja S., Annamalai N., Mayavu P., Balasubramanian T. Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian. Pac. J. Trop. Biomed. 2014;4:S305–S311. doi: 10.12980/APJTB.4.2014C537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lucas-Elío P., Gómez D., Solano F., Sanchez-Amat A. The antimicrobial activity of marinocine, synthesized by marinomonas mediterranea, is due to hydrogen peroxide generated by its lysine oxidase activity. J. Bacteriol. 2006;188:2493–2501. doi: 10.1128/JB.188.7.2493-2501.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raju R., Khalil Z.G., Piggott A.M., Blumenthal A., Gardiner D.L., Skinner-Adams T.S., Capon R.J. Mollemycin A: An antimalarial and antibacterial glyco-hexadepsipeptide-polyketide from an Australian marine-derived Streptomyces sp. (CMB-M0244) Org. Lett. 2014;16:1716–1719. doi: 10.1021/ol5003913. [DOI] [PubMed] [Google Scholar]
- 99.Murphy A.C., Gao S.S., Han L.C., Carobene S., Fukuda D., Song Z., Hothersall J., Cox R.J., Crosby J., Crump M.P., et al. Biosynthesis of thiomarinol A and related metabolites of Pseudoalteromonas sp. SANK 73390. Chem. Sci. 2014;5:397–402. doi: 10.1039/C3SC52281D. [DOI] [Google Scholar]
- 100.Braña A.F., Sarmiento-Vizcaíno A., Pérez-Victoria I., Otero L., Fernández J., Palacios J.J., Martín J., Cruz M.D.L., Díaz C., Vicente F., et al. Branimycins B and C, antibiotics produced by the abyssal Actinobacterium Pseudonocardia carboxydivorans M-227. J. Nat. Prod. 2017;80:569–573. doi: 10.1021/acs.jnatprod.6b01107. [DOI] [PubMed] [Google Scholar]
- 101.Govindarajan G., Satheeja S.V., Jebakumar S.R. Antimicrobial potential of phylogenetically unique actinomycete, Streptomyces sp. JRG-04 from marine origin. Biologicals. 2014;42:305–311. doi: 10.1016/j.biologicals.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 102.Mangamuri U., Muvva V., Poda S., Naragani K., Munaganti R.K., Chitturi B., Yenamandra V. Bioactive metabolites produced by Streptomyces Cheonanensis VUK-A from Coringa mangrove sediments: Isolation, structure elucidation and bioactivity. 3 Biotech. 2016;6:63. doi: 10.1007/s13205-016-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bae M., Kim H., Moon K., Nam S., Shin J., Oh K., Oh D. Mohangamides A and B, new dilactone-tethered pseudo-dimeric peptides inhibiting Candida albicans isocitrate lyase. Org. Lett. 2015;17:712–715. doi: 10.1021/ol5037248. [DOI] [PubMed] [Google Scholar]
- 104.Subramenium G.A., Swetha T.K., Iyer P.M., Balamurugan K., Pandian S.K. 5-hydroxymethyl-2-furaldehyde from marine bacterium Bacillus subtilis inhibits biofilm and virulence of Candida albicans. Microbiol. Res. 2018;207:19–32. doi: 10.1016/j.micres.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 105.Anjum K., Sadiq I., Chen L., Kaleem S., Li X., Zhang Z., Lian X. Novel antifungal janthinopolyenemycins A and B from a co-culture of marine-associated Janthinobacterium spp. ZZ145 and ZZ148. Tetrahedron Lett. 2018;59:3490–3494. doi: 10.1016/j.tetlet.2018.08.022. [DOI] [Google Scholar]
- 106.Wu B., Oesker V., Wiese J., Schmaljohann R., Imhoff J.F. Two new antibiotic pyridones produced by a marine fungus, Trichoderma sp. strain MF106. Mar. Drugs. 2014;12:1208–1219. doi: 10.3390/md12031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haga A., Tamoto H., Ishino M., Kimura E., Sugita T., Kinoshita K., Takahashi K., Shiro M., Koyama K. Pyridone alkaloids from a marine-derived fungus, Stagonosporopsis cucurbitacearum, and their activities against azole-resistant Candida albicans. J. Nat. Prod. 2013;76:750–754. doi: 10.1021/np300876t. [DOI] [PubMed] [Google Scholar]
- 108.Yin Q., Liang J., Zhang W., Zhang L., Hu Z.L., Zhang Y., Xu Y. Butenolide, a marine-derived broad-spectrum antibiofilm agent against both Gram-positive and Gram-negative pathogenic bacteria. Mar. Biotechnol. 2019;21:88–98. doi: 10.1007/s10126-018-9861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Böhringer N., Fisc K.M., Schillo D., Bara R., Hertzer C., Grein F., Eisenbarth J.H., Kaligis F., Schneider T., Wägele H., et al. Antimicrobial potential of bacteria associated with marine sea slugs from North Sulawesi, Indonesia. Front. Microbiol. 2017;8:1092. doi: 10.3389/fmicb.2017.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosa C.F.C., Freire D.M.G., Ferraz E.C. Biosurfactant microfoam: Application in the removal of pollutants from soil. J. Environ. Chem. Eng. 2015;3:89–94. doi: 10.1016/j.jece.2014.12.008. [DOI] [Google Scholar]
- 111.Giri S.S., Ryu E.C., Sukumaran V., Park S.C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microbial. Pathog. 2019;132:66–72. doi: 10.1016/j.micpath.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 112.Kubicki S., Bollinger A., Katzke N., Jaeger K.E., Loeschcke A., Thies S. Marine Biosurfactants: Biosynthesis, structural diversity and biotechnological applications. Mar. Drugs. 2019;17:408. doi: 10.3390/md17070408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun W., Wang Y., Zhang W., Ying H., Wang P. Novel surfactant peptide for removal of biofilms. Coll. Surf. B. Biointerfaces. 2018;172:180–186. doi: 10.1016/j.colsurfb.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 114.Fenibo E.O., Ijoma G.N., Selvarajan R., Chikere C.B. Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms. 2019;7:581. doi: 10.3390/microorganisms7110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gudiña E.J., Teixeira J.A., Rodrigues L.R. Biosurfactants produced by marine microorganisms with therapeutic applications. Mar. Drugs. 2016;14:38. doi: 10.3390/md14020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Naughton P.J., Marchant R., Naughton V., Banat I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019;127:12–28. doi: 10.1111/jam.14243. [DOI] [PubMed] [Google Scholar]
- 117.Rodrigues A.I., Gudiña E.J., Teixeira J.A., Rodrigues L.R. Sodium chloride effect on the aggregation behaviour of rhamnolipids and their antifungal activity. Sci. Rep. 2017;7:12907. doi: 10.1038/s41598-017-13424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sen S., Borah S.N., Bora A., Deka S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb. Cell Factories. 2017;16:1–14. doi: 10.1186/s12934-017-0711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Desai J.D., Banat I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997;61:47–64. doi: 10.1128/.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Banat I.M., Franzetti A., Gandolfi I., Bestetti G., Martinotti M.G., Fracchia L., Smyth T.J., Marchant R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010;87:427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- 121.Mani P., Dineshkumar G., Jayaseelan T., Deepalakshmi K., Kumar C.G., Balan S.S. Antimicrobial activities of a promising glycolipid biosurfactant from a novel marine Staphylococcus saprophyticus SBPS 15. 3 Biotech. 2016;6:163. doi: 10.1007/s13205-016-0478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dusane D.H., Pawar V.S., Nancharaiah Y.V., Venugopalan V.P., Kumar A.R., Zinjarde S.S. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling. 2011;27:645–654. doi: 10.1080/08927014.2011.594883. [DOI] [PubMed] [Google Scholar]
- 123.Kiran G.S., Sabarathnam B., Selvin J. Biofilm disruption potential of a glycolipid biosurfactant from marine Brevibacterium casei. FEMS. Immunol. Med. Microbiol. 2010;59:432–438. doi: 10.1111/j.1574-695X.2010.00698.x. [DOI] [PubMed] [Google Scholar]
- 124.Manivasagan P., Sivasankar P., Venkatesan J., Sivakumar K., Kim S. Optimization, production and characterization of glycolipid biosurfactant from the marine actinobacterium, Streptomyces sp. MAB36. Bioprocess Biosyst. Eng. 2014;37:783–797. doi: 10.1007/s00449-013-1048-6. [DOI] [PubMed] [Google Scholar]
- 125.Kiran G.S., Sabarathnam B., Thajuddin N., Selvin J. Production of glycolipid biosurfactant from spongeassociated marine actinobacterium Brachybacterium paraconglomeratum MSA21. J. Surfactants. Deterg. 2014;17:531–542. doi: 10.1007/s11743-014-1564-7. [DOI] [Google Scholar]
- 126.Marzban A., Ebrahimipour G., Danesh A. Bioactivity of a novel glycolipid produced by a Halophilic Buttiauxella sp. and improving submerged fermentation using a response surface method. Molecules. 2016;21:1256. doi: 10.3390/molecules21101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sumaiya M., Devi C.A., Leela K. A study on biosurfactant production from marine bacteria. Int. J. Sci. Res. 2017;7:139–145. [Google Scholar]
- 128.Gandhimathi R., Kiran G.S., Hema T.A., Selvin J., Raviji T.R., Shanmughapriya S. Production and characterization of lipopeptide biosurfactant by a sponge-associated marine actinomycetes Nocardiopsis alba MSA10. Bioprocess Biosyst. Eng. 2009;32:825–835. doi: 10.1007/s00449-009-0309-x. [DOI] [PubMed] [Google Scholar]
- 129.Balan S.S., Kumar C.G., Jayalakshmi S. Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: Purification, characterization and its biological evaluation. Microbiol. Res. 2017;194:1–9. doi: 10.1016/j.micres.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 130.Balan S.S., Kumar C.G., Jayalakshmi S. Pontifactin, a new lipopeptide biosurfactant produced by a marine Pontibacter korlensis strain SBK-47: Purification, characterization and its biological evaluation. Process. Biochem. 2016;51:2198–2207. doi: 10.1016/j.procbio.2016.09.009. [DOI] [Google Scholar]
- 131.Lawrance A., Balakrishnan M., Joseph T.C., Sukumaran D.P., Valsalan V.N., Gopal D., Ramalingam K. Functional and molecular characterization of a lipopeptide surfactant from the marine sponge-associated eubacteria Bacillus licheniformis NIOT-AMKV06 of Andaman and Nicobar Islands, India. Mar. Pollut. Bull. 2014;82:76–85. doi: 10.1016/j.marpolbul.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 132.Anestopoulos I., Kiousi D.E., Klavaris A., Maijo M., Serpico A., Suarez A., Sanchez G., Salek K., Chasapi S.A., Zompra A.A., et al. Marine-derived surface active agents: Health-promoting properties and blue biotechnology-based applications. Biomolecules. 2020;10:885. doi: 10.3390/biom10060885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tahmourespour A., Salehi R., Kermanshahi R.K. Lactobacillus Acidophilus-derived biosurfactant effect on GTFB and GTFC expression level in Streptococcus Mutans biofilm cells. Braz. J. Microbiol. 2011;42:330–339. doi: 10.1590/S1517-83822011000100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hamza F., Satpute S., Banpurkar A., Kumar A.R., Zinjarde S. Biosurfactant from a marine bacterium disrupts biofilms of pathogenic bacteria in a tropical aquaculture system. FEMS Microbiol. Ecol. 2017;93:140. doi: 10.1093/femsec/fix140. [DOI] [PubMed] [Google Scholar]
- 135.Ohadi M., Forootanfar H., Dehghannoudeh G., Eslaminejad T., Ameri A., Shakibaie M., Adeli-Sardou M. Antimicrobial, anti-bioflm, and anti-proliferative activities of lipopeptide biosurfactant produced by Acinetobacter junii B6. Microb. Pathog. 2020;138:103806. doi: 10.1016/j.micpath.2019.103806. [DOI] [PubMed] [Google Scholar]
- 136.Silva S.S.E., Carvalho J.W.P., Aires C.P., Nitschke M. Disruption of Staphylococcus aureus biofilms using rhamnolipid biosurfactants. J. Dairy. Sci. 2017;100:7864–7873. doi: 10.3168/jds.2017-13012. [DOI] [PubMed] [Google Scholar]
- 137.Mireles J.R., Toguchi A., Harshey R.M. Salmonella enterica serovar typhimurium swarming mutants with altered biofilm-forming abilities: Surfactin inhibits biofilm formation. J. Bacteriol. 2001;183:5848–5854. doi: 10.1128/JB.183.20.5848-5854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Janek T., Krasowska A., Czyżnikowska Ż., Łukaszewicz M. Trehalose lipid biosurfactant reduces adhesion of microbial pathogens to polystyrene and silicone surfaces: An experimental and computational approach. Front. Microbiol. 2018;9:2441. doi: 10.3389/fmicb.2018.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Falagas M.E., Makris G.C. Probiotic bacteria and biosurfactants for nosocomial infection control: A hypothesis. J. Hosp. Infect. 2009;71:301–306. doi: 10.1016/j.jhin.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 140.Hermansson M. The DLVO theory in microbial adhesion. Colloids Surf. B. 1999;14:105–119. doi: 10.1016/S0927-7765(99)00029-6. [DOI] [Google Scholar]
- 141.Katsikogianni M., Missirlis Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells. Mater. 2004;8:37–57. doi: 10.22203/eCM.v008a05. [DOI] [PubMed] [Google Scholar]
- 142.Walencka E., Rozalska S., Sadowska B., Rozalska B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008;53:61–66. doi: 10.1007/s12223-008-0009-y. [DOI] [PubMed] [Google Scholar]
- 143.Meylheuc T., Oss C.J.V., Bellon-Fontaine M.N. Adsorption of biosurfactant on solid surfaces and consequences regarding the bioadhesion of Listeria monocytogenes LO28. J. Appl. Microbiol. 2001;91:822–832. doi: 10.1046/j.1365-2672.2001.01455.x. [DOI] [PubMed] [Google Scholar]
- 144.Jemil N., Ayed H.B., Manresa A., Nasri M., Hmidet N. Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiol. 2017;17:144. doi: 10.1186/s12866-017-1050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kimkes T.E.P., Heinemann M. How bacteria recognise and respond to surface contact. FEMS Microbiol. Rev. 2020;44:106–122. doi: 10.1093/femsre/fuz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gomes M.Z.d.V., Nitschke M. Evaluation of rhamnolipid and surfactin to reduce the adhesion and remove biofilms of individual and mixed cultures of food pathogenic bacteria. Food Control. 2012;25:441–447. doi: 10.1016/j.foodcont.2011.11.025. [DOI] [Google Scholar]
- 147.Das P., Mukherjee S., Sen R. Antiadhesive action of a marine microbial surfactant. Colloids Surf. B Biointerfaces. 2009;71:183–186. doi: 10.1016/j.colsurfb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 148.Song B., Wang Y., Wang G., Liu G.L., Li W., Yan F. The lipopeptide 6-2 produced by Bacillus amyloliquefaciens anti-CA has potent activity against the biofilm-forming organisms. Mar. Pollut. Bull. 2016;108:62. doi: 10.1016/j.marpolbul.2016.04.062. [DOI] [PubMed] [Google Scholar]
- 149.Pradhan A.K., Pradhan N., Mall G., Panda H.T., Sukla L.B., Panda P.K., Mishra B.K. Application of lipopeptide biosurfactant isolated from a halophile: Bacillus tequilensis CH for inhibition of biofilm. Appl. Biochem. Biotechnol. 2013;171:1362–1375. doi: 10.1007/s12010-013-0428-3. [DOI] [PubMed] [Google Scholar]
- 150.Kiran G.S., Hema T.A., Gandhimathi R., Selvin J., Thomas T.A., Ravji T.R., Natarajaseenivasan K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf. B. Biointerfaces. 2009;73:250–256. doi: 10.1016/j.colsurfb.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 151.Khopade A., Ren B., Liu X.Y., Mahadik K., Zhang L., Kokare C. Production and characterization of biosurfactant from marine Streptomyces species B3. J. Colloid Interface Sci. 2012;367:311–318. doi: 10.1016/j.jcis.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 152.Kavita K., Singh V.K., Mishra A., Jha B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr. Polym. 2014;101:29–35. doi: 10.1016/j.carbpol.2013.08.099. [DOI] [PubMed] [Google Scholar]
- 153.Gu H., Lee S.W., Carnicelli J., Zhang T., Ren D. Magnetically driven active topography for long-term biofilm control. Nat. Commun. 2020;11:2211. doi: 10.1038/s41467-020-16055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Milo S., Hathaway H., Nzakizwanayo J., Alves D.R., Esteban P.P., Jonesbe B.V., Jenkins A.T.A. Prevention of encrustation and blockage of urinary catheters by Proteus mirabilis via pH-triggered release of bacteriophage. J. Mater. Chem. B. 2017;5:5403–5411. doi: 10.1039/C7TB01302G. [DOI] [PubMed] [Google Scholar]
- 155.Jordan R.P.C., Nicolle L.E. Biofilms in Infection Prevention and Control. Academic Press; Cambridge, MA, USA: 2014. pp. 287–309. [Google Scholar]
- 156.Costa B., Mota R., Parreira P., Tamagnini P., Martins M.C.L., Costa F. Broad-spectrum anti-adhesive coating based on an extracellular polymer from a marine cyanobacterium. Mar. Drugs. 2019;17:243. doi: 10.3390/md17040243. [DOI] [PMC free article] [PubMed] [Google Scholar]