Graphical abstract
Keywords: COVID-19, Nanomaterials, Prevention, Detection, Therapy
Abstract
In 2019, a novel type of coronavirus emerged in China called SARS-COV-2, known COVID-19, threatens global health and possesses negative impact on people's quality of life, leading to an urgent need for its diagnosis and remedy. On the other hand, the presence of hazardous infectious waste led to the increase of the risk of transmitting the virus by individuals and by hospitals during the COVID-19 pandemic. Hence, in this review, we survey previous researches on nanomaterials that can be effective for guiding strategies to deal with the current COVID-19 pandemic and also decrease the hazardous infectious waste in the environment. We highlight the contribution of nanomaterials that possess potential to therapy, prevention, detect targeted virus proteins and also can be useful for large population screening, for the development of environmental sensors and filters. Besides, we investigate the possibilities of employing the nanomaterials in antiviral research and treatment development, examining the role of nanomaterials in antiviral- drug design, including the importance of nanomaterials in drug delivery and vaccination, and for the production of medical equipment. Nanomaterials-based technologies not only contribute to the ongoing SARS- CoV-2 research efforts but can also provide platforms and tools for the understanding, protection, detection and treatment of future viral diseases.
1. Introduction
Worldwide health is confronting the high-risk circumstance due to the new extreme acute respiratory syndrome caused by coronavirus called COVID-19 pandemic. This virus is dangerous, because of the highly infectious nature of the disease and the risk of death from pneumonia in almost 6.89 % of COVID-19 patients (April 27, 2020) (Sivasankarapillai et al., 2020a). The infection has raised world concern due to its high transmission rate just as high movability and mortality (Megahed & Ghoneim, 2020a). The existence of coronavirus in environments even for a short time could cause a potential threat for all people involved in the environmental system (Gude & Muire, 2020). Indeed, during the COVID-19 pandemic, the use of disposable supplies such as facemasks and medical waste have increased the amount of pollution with Covid-19 in municipal waste. Therefore, presence of nanomaterial with antiviral property leads to kill the viruses and avoid spreading in the environment and urban life (Valizadeh, Hafezalkotob, Alizadeh, & Mozafari, 2021). In 2020, Rahmani and Mirmahaleh studied on prevention and treatment methods and effective parameters against COVID-19 by use of systematic literature review. Indeed, in this review paper, the author proposed a taxonomy tree to investigate the COVID-19 confronting methods and effects. Besides, they providing a systematic literature review based on the proposed taxonomy tree and finally they indicating the impact of medical and social methods for facing the COVID-19 outbreak (Rahmani & Hosseini Mirmahaleh, 2020). Another paper focused on how the antivirus –built environment prevents the virus transmission (Megahed & Ghoneim, 2020b). Besides, in this study, the importance of designing a healthy and sustainable built environment was investigated. Many unanswered questions need more multidisciplinary researches. So it is important to be familiar with protect nanomaterials that can be used in built environment (Megahed & Ghoneim, 2020b).
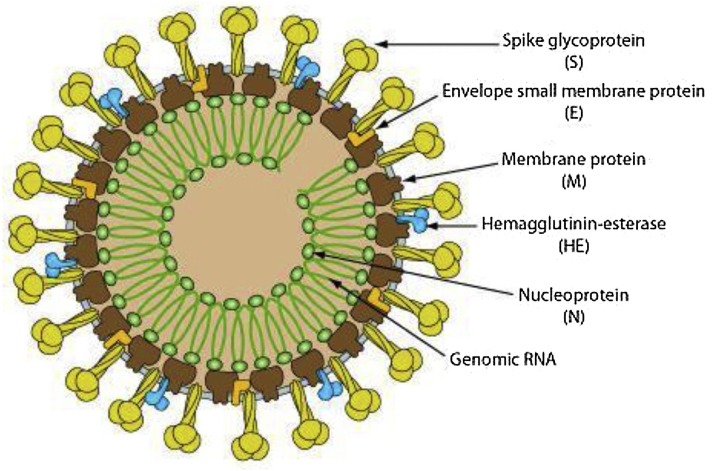
Besides, transmission from person to person COVID-19 occurs mostly through respiratory droplets, similar to the outbreak of the flu, produced during coughing, sneezing, and talking (Huang et al., 2020). Indeed, sometimes transmission takes place when a healthy individual is close to an infected person, with or without symptoms, or when be in contact with infected surfaces and then touches his or her eyes, nose, or mouth. (de Wit, van Doremalen, Falzarano, & Munster, 2016) This novel virus is 7th coronavirus belonging to the coronavirus family of Genus-β possesses 30–40 species (Schoeman & Fielding, 2019). The 5th and 6th coronaviruses were appeared in 2002 and 2012 called SARS-CoV and MERS, respectively. These three types of viruses have the same symptoms such as fever and cough, followed by respiratory tract disease (Schoeman & Fielding, 2019). In order to know how to face with Covid-19, we should know about the structure of coronaviruses. The genome of coronaviruses possesses four main structural proteins with different functions including spike (S) protein (connect the virus into the host cell), nucleocapsid (N) protein (gives a response to the host cell and engages in the replication cycle), membrane (M) protein (determines the shape of the cell), and envelope (E) protein (is hydrophobic viroporins) (Fig. 1 ) (McBride, van Zyl, & Fielding, 2014; Neuman et al., 2011; Venkatagopalan, Daskalova, Lopez, Dolezal, & Hogue, 2015). The outer layer of viroporins is lipid composed of hydrophobic phospholipids,5 which leads to interact with the host cell (Baglivo et al., 2020).
Fig. 1.
Schematic representation of the coronavirus structure with structural proteins. Reprinted with permission (Venkatagopalan et al., 2015).
The nanotechnology community can contribute significantly to the fight against coronavirus disease 2019 (COVID-19). Nanomaterials with antiviral properties known as nanoantimicrobials have an important role in the prevention, diagnosis, and treatment of virus disease. While serious supplies are dedicated to prevention, diagnosis, and treatment, more endeavors could be devoted to limit the infection spread. Therefore, applying nanomaterials in coating surfaces as antiviral covers is an important issue in the prevention of the spread of infection in the environment. This isn't the first and last pandemic of the disease: the nanomaterials society may proffer a few innovative solutions for challenging the ongoing and future worldwide wellbeing crises (Sportelli, Izzi et al., 2020). The methodologies that have been investigated looking to inactivate coronavirus predominantly follow techniques including the interaction of the outer layer of the virus with nanomaterials followed by inhibition of virus infection and or totally killing the virus. In these cases, the nanosystem with hydrophobic and antiviral properties can interact with the virus surface (Palestino, García-Silva, González-Ortega, & Rosales-Mendoza, 2020).
Besides, nanomaterials with significant properties and many privileges have been recently incorporated into medicine sections such as nanoparticle (NP)-based formulation, NP-based delivery system, and NP-based vaccine (Ferrari et al., 2015; Shao et al., 2016). Since, nanoparticles possess equal size with viruses, they can enter cells to enable expression of antigens from delivered nucleic acids (mRNA and DNA vaccines) and/or directly target immune cells for delivery of antigens (subunit vaccines). During vaccine development, the virus antigen can be either encapsulated in the nanosystem or conjugated to the surfaces of nanocarriers for administration along with adjuvant (Chintagunta, Kumar, Kumar, & Kumar, 2020; Kumar, Chintagunta, Kumar, Roy, & Kumar, 2020). Various delivery nanocarriers such as lipid nanoparticles and polymeric nanoparticles are being employed as antigen carriers. The effectiveness of the vaccine can be enhanced through optimization the size, morphology, and charge of the nanoparticles (Chintagunta, Nalluru, & Sampath Kumar, 2021). Recently, the incredible race in development of the COVID-19 vaccines within the global scientific community, caused to produce many types of vaccine (Milane & Amiji, 2021). SARS-CoV-2-based mRNA vaccines as nanotechnology-based formulations vaccines from Pfizer/BioNTech and Moderna employ lipid nanoparticles (McGill COVID19 Vaccine Tracker Team, 2021), while the University of Oxford/ Astrazeneca (from here on out referred to as Oxford/Astrazeneca) and CanSino incorporate antigen-encoding sequences within the DNA carried by Adenoviruses (Folegatti et al., 2020; Jackson et al., 2020; Novavax Announces Positive Phase 1 Data for Its COVID-19 Vaccine Candidate, 2021; Zhu et al., 2020). Novavax decorates recombinant S proteins of SARS-CoV-2 onto their proprietary virus like particle nanoparticles (Clinical Stage Pipeline, 2021).
Consequently, here we enforce the emphasis of nanomaterial-based technological solutions in various possible applications of the fight against the virus. Indeed, in this review paper we investigated the different types of nanomaterials and their effects on the environment to attach COVID-19. Therefore, safe, nontoxic and biocompatible nanomaterials might be an ideal choice in prevention, detection and remedy of virus. Besides, this review paper has been divided into some sections involving types of nanomaterials and their applications in biosensors, antiviral coating, airborne virus filtration, facemasks, drug delivery, and vaccines to fight COVID-19.
2. Carbon-based nanomaterials
While there aren’t many studies accessible up to date that gives a few carbon-based nanomaterial applications for fighting COVID-19 infection, their extraordinary physicochemical and antiviral characteristics recommend that these nanomaterials have an active and significant role against COVID-19. These nanomaterials including graphene and graphene oxide, carbon quantum dot, carbon nanotube, and fullerene with great properties especially sensing, antiviral and antimicrobial properties, are ideal choices with potential applications against COVID-19 in biosensor for diagnosis, antiviral coating, airborne virus filtration, facemask, and drug delivery (Ferrari et al., 2015).
2.1. Graphene and graphene oxide
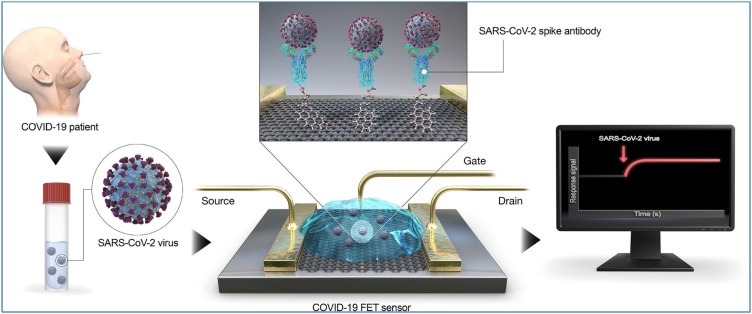
The nanomaterial graphene and graphene oxide with two dimensions have caught a lot of consideration because of their antimicrobial and antiviral properties. Antibody-conjugated graphene sheets not only can quickly recognize targeted proteins of the virus but also be remedial for huge population screening, and improvement of environmental sensors and filters (Palmieri & Papi, 2020). Besides, the functionalized graphene could be utilized as a disinfectant due to acceptable viral capture capacity that, combined with heat or light-mediated inactivation. Besides, graphene sensor arrays can be implemented on standard utility textiles and drug efficacy screening. Indeed, biosensing procedures utilizing antibodies to specifically catch the entire infection exist. Moreover, graphene-based field-effect transistors (FET) as potable sensors have been designed to analyze COVID-19 viral load in clinical nasopharyngeal samples, utilizing special antibody against its spike protein (Seo et al., 2020). Indeed, the spike antibody of SARS-CoV-2 was immobilized on the FET device by conjugating onto the graphene sheet through an interfacing molecule as probe linker (Fig. 2]). The FET-based biosensing device accomplishes the detection of SARS-CoV-2 by evaluation of its performance using a cultured virus, antigen protein, and nasopharyngeal swab samples from infected individuals (Ferrari et al., 2015).
Fig. 2.
Field-effect transistor (FET) sensor and its related operational process for the diagnosis of COVID-19. Reprinted with permission.
To make the graphene-based FET biosensors, the graphene sheets were covered with the COVID-19 antibody by probe linker (1-pyrenebutyric acid N-hydroxysuccinimide ester).
Based on the results, the fabricated FET sensors can detect the SARS-CoV-2 spike protein in phosphate-buffered saline and 100 fg mL−1 medical transfer system, at the level of 1 fg mL−1 concentration and limit of detection ∼1.6 × 101 pfu mL-1 and ∼2.42 × 102 pfu mL-1 for the cultured sample and medical test, respectively. This sensor reveals is highly sensitive to screening and diagnosis of novel coronavirus disease 2019 without any sample pretreatment and the presence of graphene leads to improve the signal to noise ratio (Seo et al., 2020). Besides, Zhang and co-authors (Zhang, Qi et al., 2020), reported an accurate, rapid, and easy diagnosis of COVID-19 by use of the graphene-based FET immunosensors. The speed of detection was about 2 min and the limit of detection was achieved down to 0.2 pM, in a real-time and label-free manner. Seo et al. (2020) also used the graphene-based FET to detect the COVID-19. They reported the LODs of this sensor were evaluated in nasopharyngeal swab samples in phosphate buffer saline, 1 fg/mL, in universal transport medium, 100 fg/mL, and in the culture medium, 1.6 × 101 pfu/mL.
To evaluate the graphene oxide (GO) as an antiviral agent, Ye et al. (Ye et al., 2015) applied some types of GO derivatives including graphite (Gt), graphite oxide (GtO), graphene oxide (GO), reduced graphene oxide (rGO), graphene oxide/poly(diallyldimethylammonium chloride) composite (GO-PDDA), and graphene oxide/polyvinylpyrrolidone composite (GO-PVP) to detect PEDV (porcine epidemic diarrhea virus, strain CH/YNKM-8/2013, a DNA virus) and PRV (pseudorabies virus, strain HNX, an RNA virus). Regarding the obtained results, Go and rGO with maximum antiviral activity were selected as the best antiviral candidates among them and GtO. Gt with minimum activity and GO-PDDA without any antiviral activity was ignored. Indeed, this activity is related directly to the presence of nanostructure of the materials. Besides, all GO materials revealed negative zeta potential except for the composite having PDDA that had positive zeta potential. Subsequently, the particle charges influence on the desired antiviral properties.
The demand for masks as prevention of virus transmission has increased everywhere throughout the world. On the other hand, the presence of used an enormous number of single-use masks is a threat to the environment. Therefore, taking advantage of graphene as breathable barrier layers on the facemasks leads to not only minimize the risk of transmission but also makes the reusable facemasks (Chauhan, Maekawa, & Kumar, 2017). Besides, GO nanoparticles as hydrophobic materials are used in filters, textiles, and facemasks to inactivate viruses (Spitz Steinberg, Cruz, Mahfouz, Qiu, & Hurt, 2017). Indeed, the maintenance of facemask is affected by water permeation of its surface, so when its surface is hydrophobic and dry the microorganisms couldn't penetrate the protective layers. So, this facemask is recyclable by photocatalysis or heat (Akhavan, Choobtashani, & Ghaderi, 2012; Song et al., 2015; Ullah et al., 2020). Also, the high surface temperature of the mask under sun illumination can effectively sterilize the surface infections. Indeed, viruses can be denatured after contact with graphene under mild temperature at 56 °C for 30 min. It is found that different advanced nanoparticles can be employed to fabricate multifunctional antiviral facemask (Zhong et al., 2020)
On the other hand, graphene as a regular inorganic particle with 2D structure possesses great drug loading potency to efficiently adsorb small molecular and macromolecular drugs because of its π-π conjugation at every single layer. This ability makes the graphene an excellent delivery platform for nucleic acid and protein delivery (McBride et al., 2014). Besides, these nanoparticles have potential to be used in vaccine technology. For example, in 2020, Gao et al., developed a new vaccine against COVID-19 by using the combination of self-developed nano adjuvant loaded with carnosine graphene oxide adjuvant loaded with CpG molecule and RBD protein antigen (Gao et al., 2020). Their achievements illustrated that this vaccine can fabricate high titer anti-SARS-CoV-2 RBD antibody neutralizing SARS-CoV-2 in mice within 2 weeks.
Since in vivo toxicity of graphene is still a matter of debate because of some difficulties due to the infinite combinations of dose, surface chemistry, exposure route utilized for the evaluation (Fadeel et al., 2018). In one of the researches, it was found that the contact of GO with the lungs of C57BL/6 mice leads to inordinate pulmonary inflammation (Sivasankarapillai et al., 2020a). Besides, the instability and aggregation of graphene in solution is a further challenge while the solution of drugs and vaccine needs stability (Ren et al., 2018).
2.2. Carbon nanotube
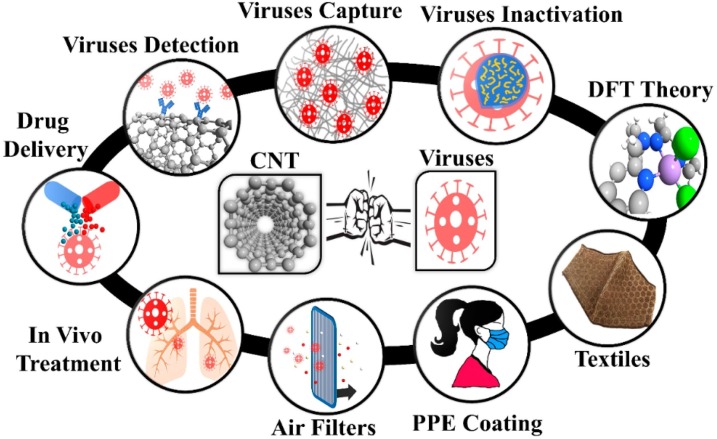
In recent years, carbon nanotubes (CNTs) with 10−100 nm dimensions, antiviral and antimicrobial activity, and good light-heat conversion efficiency was widely applied for biology and biomedical sciences thanks to their large surface volume ratio, slight density, small pore size, flexibility, resistance to acids and bases, great mechanical strength, ability to create reactive oxygen species, resistance to respiratory droplets, and biological compatibility with several drugs (Aasi, Aghaei, Moore, & Panchapakesan, 2020). Excellent properties of CNTs that provide novel proposals facing COVID-19, are included high storage space, high surface area, high biocompatibility, great permeability of biological barriers, good bioabsorption rate, multi-energy surface/tube chemical functional group capability, and targeted biomolecule modification potency, etc. Besides, CNTs are applied as diagnosis system, filtering, virus inactivation agent. Moreover, CNTs have been applied as drug delivery systems (Mohajeri, Behnam, & Sahebkar, 2019; Zhu et al., 2019), anti-HIV agents (Iannazzo et al., 2015; Liu, Winters, Holodniy, & Dai, 2007), detect and capture viruses and viral proteins (Ahmed, Kim, Suzuki, Lee, & Park, 2016; Brady-Estévez, Kang, & Elimelech, 2008; Iannazzo et al., 2015; Liu et al., 2007; Lee, Chander, Goyal, & Cui, 2011; Ting et al., 2018a; Yeh et al., 2016; Zhang et al., 2007). Fig. 3 gives a representation of the potential application of CNTs against various infections including influenza and respiratory viruses such as SARS-CoV-1 and SARS-CoV-2.
Fig. 3.
CNT in different sections of viral capture/inactivation. PPE: Personal Protective Equipment; CNT: Carbon nanotubes; DFT: Density Functional Theory. Reprinted with permission.
Microfluidic is a progressive innovation that controls limited quantities of fluidics (10−9-10−18 L), by that means, becoming sensitive in the detection of viruses. Yeh et al. proposed a movable and high-throughput microfluidic VIRRION platform including CNT arrays witch (Yeh et al., 2020). VIRRION sensor adequately caught diverse infections by size, yet also performed continuous nondestructive distinguishing proof of infection and virus, utilizing surface improved Raman spectroscopy (SERS) coupled to a machine learning and database. Besides, Zheng et al. developed portable devices with CNTs to capture infections based on their sizes, sensitively (Yeh et al., 2016). Dependence of the virus size by sensors coupled with CNT can selectively capture and detect the infections in diluted samples by a factor of 100. The most advantages of this method are not only high sensitivity but also simple operation and isolation process because of no requirement of any antibody for viral identification.
Another potential application of CNT against COVID-19 is using these nanoparticles in the fabrication of N95 facemasks, which prevent person-to-person transmission viruses (Vo & Zhuang, 2013; Vo et al., 2014; Vo, Zhuang, Birch, & Birch, 2016; Zou & Yao, 2015).
However, CNTs possess many benefits, they have some disadvantages because of its genotoxicity properties that lead to interact directly with DNA in vivo (animal models) and cellular levels (Ahmed et al., 2016; Brady-Estévez et al., 2008; Iannazzo et al., 2015; Kim et al., 2012; Lee et al., 2011; Ting et al., 2018a; Vo & Zhuang, 2013; Vo et al., 2014, 2016; Yeh et al., 2016; Yeh et al., 2020; Zhang et al., 2007; Zou & Yao, 2015). Based on the fulfilled researches, because of the genotoxicity of multi-walled CNTs, pulmonary administration of these nanomaterials leads to insistent oxidative stress by prompting chronic inflammation (Cho et al., 2012).
2.3. Fullerene
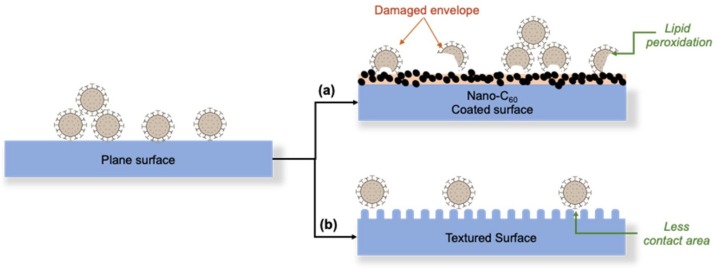
The fullerene (C60) as a carbon-based nanoparticle with many characteristics including antiviral activity, antiradical and antioxidant properties, and also hydrophobic character (Gacem, Gacem, & Ould-El-Hadj-Khelil, 2020). With hydrophobic characteristics, fullerene possesses colloidal form in aqueous samples. When this colloidal solution is illuminated under UVA light, the singlet oxygen is produced. The oxidative reaction starts the lipid degradation with the peroxidation step. The concentration of fullerene in solution, effects on the deterioration of the lipid layers. A similar observation of lipid peroxidation by C60 was made on bacterial phospholipid membrane (Alvarez, 2007). Fig. 4 depicts the illustration of damage envelope of the virus via lipid peroxidation with fullerene coating.
Fig. 4.
Two possible methods to fight COVID-19. (a) By inactivating the lipid layer by coating the surface with nano-C60. (b) By reducing the contact area with the virus by surface texturing. Reprinted with permission.
Based on Fig. 4 (Siddiquie, Agrawal, & Joshi, 2020), the surface contact of the textured surface is coated by C60 that leads to lipid peroxidation on the phospholipid layer that exists in the outer layer of COVID-19. Besides, the hydrophobic properties of C60 lead to minimizing contact between texture surface and virus outer layer because of the existence of entrapped air bubbles. Consequently, because of the decrement of adhesion between virus and texture surfaces by C60, these nanomaterials as coating materials are introduced as a possible solution against COVID-19, as they will be hydrophobic as well as toxic to the envelope for the virus. On the other hand, lipid peroxidation by a water-soluble C60 leads to break the lipid of the outer layer of the virus because of the hydrophobic property of the lipid (Sayes et al., 2005).
In the authors’ view point, by investigation of carbon nanoparticles (G, GO, CNT and F), it can be concluded that these types of nanomaterials better to be used as biosensor, filtration and other applications, which have not contact with living cells directly (like drug delivery system or vaccine). Indeed, the toxicity and biocompatibility of these types of nanoparticles in the living cells need to be more surveyed. Therefore, by employing carbon nanomaterials as antiviral agents in facemask, the generation of disposal facemask becomes extinct.
3. Quantum dots (QDs)
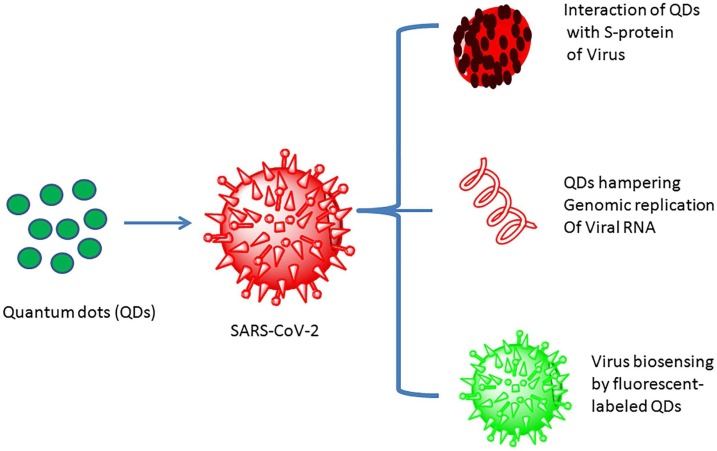
Quantum dots (QDs) as semiconductor nanoparticles with 1−10 nm diameters and tunable optical wavelength, were attached with high fluorescent probes that is an important factor to detect the long-term fluorescence imaging of different cellular processes (Boles, Ling, Hyeon, & Talapin, 2016; Mudshinge, Deore, Patil, & Bhalgat, 2011). Indeed, QDs as a new nanoparticles not only used as fluorescent probe for molecular and cellular imaging but also applied as interception systems to prevent the entry and interaction of COVID-19 with the host cell membrane (Peer et al., 2007). Therefore, QDs materials have potential to inactivate the viruses through interaction with S-protein of virus, prevention genomic replication of viral RNA and also usage as fluorescent probes (Fig. 5 ).
Fig. 5.
Schematic representation of the actions exerted by QDs on SARS-CoV-2. QD, quantum dot; S protein, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2. Reprinted with permission (Vo et al., 2016).
Besides, the employment of green methods to synthesis QDs makes them a suitable choice for antiviral applications especially in vivo infection (Lin, Bao, & Wu, 2019). Taking into consideration, that QDs optimization and functionalization with novel functional molecules against SARS-CoV-2 would extrapolate nanostructures for COVID-19 remedial to inactive the life-threatening infections in the near future.
3.1. Carbon quantum dots
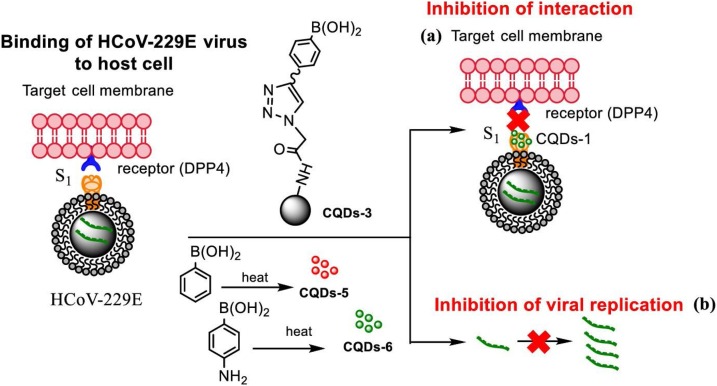
Carbon quantum dots (CQDs) as predominant imaging probes (chemosensors and biosensors) with antiviral activity can be used not only as sensing microbes, biomolecules, and infections, but also as biocompatible inactivation system for pathogenic human coronavirus infections. The CQDs are about 10 nm with high solubility in water, were fabricated via hydrothermal carbonization of carbon precursors. For the detection of coronaviruses, some innovative approaches have focused on the application of CQDs. In one of the studies (Łoczechin et al., 2019), the antiviral activities of seven types of CQDs were used to remedy human coronavirus contagions. Łoczechin et al. prepared different types of CQDs by the use of hydrothermal carbonization and conjugation of boronic acid. It was revealed that the virus inhibition is perhaps owing to the interaction between CQDs functional groups with entry receptors of the virus (Fig. 6 ) (Łoczechin et al., 2019). Indeed, the positive charge of CQDs surface disable and disjoint the spike protein of the virus and then react with negative RNA of COVID-19 (Dong, Moyer, Yang, Sun, & Yang, 2017; Ting et al., 2018b).
Fig. 6.
Carbon quantum dots (CQDs) fabricated by use of hydrothermal carbonization and their impression on binding of HCoV-229E virus to cells: (a) prevention of protein S receptor junction, (b) prohibition of viral RNA genome replication. Redrawn with permission from. Reprinted with permission.
3.2. Zirconium quantum dots (Zr QDs)
Zirconium as a nontoxic transition metal element has been used in many biomedical fields because of its properties such as UV light capture, thermal stability, and mechanical strength. Besides, nanosize of Zr has special physical and chemical characteristics due to its high surface area and the confinement of electronic states in comparison with its bulk regime (Liu et al., 2016; Puigdollers, Illasand, & Pacchioni, 2016; Vennemann, Alessandrini, & Wiemann, 2017; Wang et al., 2016).
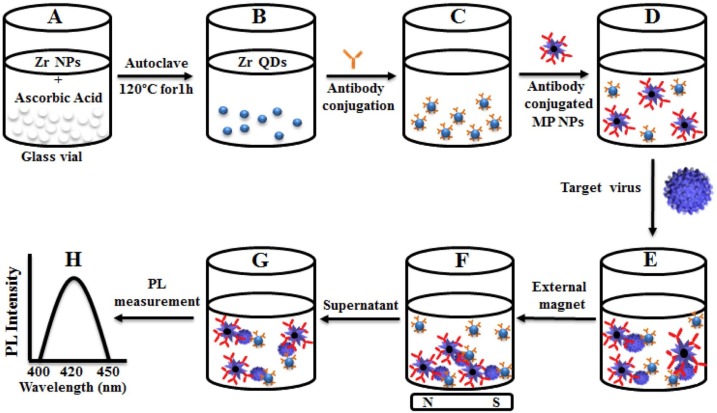
However, some studies worked on the fabrication and characterization of zirconium nanoparticles, to date there has been no report on the synthesis of zirconium quantum dots (Zr QDs). In one of the studies, Ahmed et al. researched the detection of infectious bronchitis virus (IBV) by the use of Zr QDs magnetoplasmonic (MP) NPs. They reported a one-step fabrication of Zr QDs from Zr nanoparticle by the assistance of an autoclave (Ahmed, Kang, Oh, Lee, & Neethirajan, 2018). Regarding the obtained results, Zr QDs possess blue fluorescence emission applied in the biosensing of the infectious bronchitis virus (IBV). As stated in Fig. 7 , conjugated antibody-Zr QDs and antibody -MP NPs were observed separately till the time of adding infections. At this time, the antibody-Zr QDs and antibody -MP NPs were attached together and make a nanocomposite (Zr QDs-MP NPs) to carry the viruses or infections and subsequently by use of the external magnet, the composite was separated (Nikaeen, Abbaszadeh, & Yousefinejad, 2020).
Fig. 7.
Schematic representation of virus sensor design based on Zr nanomaterials. (A) Zr NPs and reducing agent kept in vial; (B) Zr QDs formation; (C) antibody conjugated QDs; (D) the addition of antibody-conjugated MP NPs; (E) formation of nanostructured magnetoplasmonic-fluorescence with the addition of target virus, then separated (F); (G) the nanohybrid-conjugated part was dispersed and the optical properties measured (H). MP: Magnetoplasmonic. Reprinted with permission.
In alternative research, Weng and Neethirajan developed a rapid detection biosensor based on antibody-functionalized MoS2 for analyzing IBV (Weng & Neethirajan, 2018). In contrast to traditional tests, their immunosensor supplies significant privileges including higher sensitivity and lower analysis time, as well as acceptable linearity and validation using the ELISA technique. Consequently, the synthesis of novel QDs and their application as an optical-based bioassay may open new doors for research and further optical and biomedical applications.
Overall, the employment of QDs against coronavirus is one of the best choices due to its great remedial efficiency. Moreover, QDs can be used as a powerful imaging probe and sensor in diagnosis and prognostic. In addition, the drugs can be coated on the surface of QDs to target COVID-19. However, caution should be exercised to avoid renal filtration and other side effects.
4. Metal-based nanoparticles
One of the most important nanomaterials with effective biomedical applications is metal-based nanoparticles because of their capacity to fill in as productive drug delivery systems, but also to permit stimuli-responsive qualities and characteristic ability of certain kinds (for example Magnetic or Gold Nanoparticles) to be observed following in vivo organization to human body using noninvasive clinical imaging (Yoon et al., 2017).
While metal-based NP is widely examined in preclinical and clinical researches for the recognition, diagnosis, and remedy of numerous infections, a few concerns are yet emerging about their safe medical applications (Bayda et al., 2018). To solving this problem, the functionalized metal-based nanoparticles with different kinds of biocompatible materials are studied due to their nontoxic properties.
4.1. Gold nanoparticles
Gold nanoparticles (Au NP) have demonstrated specific attention in vaccine advancement as they can without any difficulties activate the immune system utilizing disguise by antigen introducing cells (Salazar-Gonzalez, Gonzalez-Ortega, & Rosales-Mendoza, 2015). Besides, Au NPs can be applied for intranasal delivery and then diffused into lymph nodes thus activating CD8+ (T-killer) cell-mediated immune response (Marques Neto, Kipnis, & Junqueira-Kipnis, 2017). Moreover, because of the high atomic number of Au NP, this nanoparticle with high stability and biocompatibility can be utilized as a contrast agent for X-ray based medical imaging, especially in Computed Tomography (CT) (Iranpour et al., 2018).
In 2010, Papp and co-authors understood that composite of Au NPs-sialic acids (SA) prevent the virus attachment to the host cells. Indeed, the infections are identified via the surface protein hemagglutinin of SA as a prerequisite for cellular entry on the target cell membrane (Papp et al., 2010). Besides, in 2011, Staroverov et al. assessed the defensive resistant reaction animated by the organization of gold nanoparticles (Au NPs) conjugated with a sort of coronavirus known as swine transmissible gastroenteritis virus (TGEV)) in vaccinated mice and bunnies (Staroverov et al., 2011). TGEV-Au NP was discovered to evoke higher gathering of interferon γ (a protein released by host cells, usually in response to the entry of a virus, which has the property of inhibiting virus replication) and prevalent titers of neutralizing antibody in immunized creatures. The vaccination with the collide of antigen- Au NPs expanded the spread of T cells ten times in comparison with the reaction to the free antigen. In this way, AuNPs conjugated to infection could be considered as a possible antiviral candidate for immune systems.
The diagnosis of disease cells by the use of electrocatalytic characteristics of Au NPs hydrogen evolution has been studied (Maltez-da Costa et al., 2012). This biosensor works through responses of surface proteins of host cells with the conjugation of particular antibodies with gold nanoparticles. The same method can also be used for virus diagnosis, utilizing the recognized antigens and obtainable antibodies.
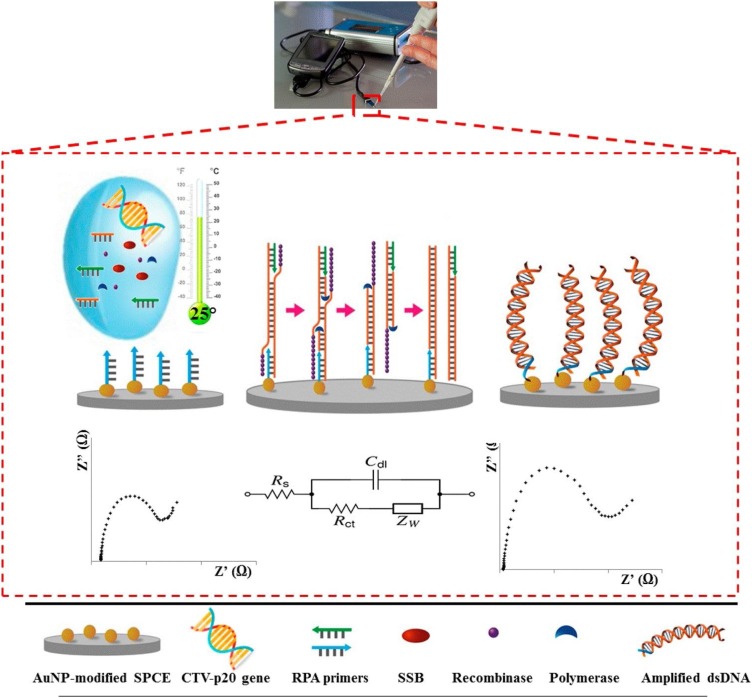
SPCEs modification with AuNPs and thiolated nucleic acid immobilization were performed and reported by Khater et al. 2017 (Khater, Escosura-Muñiz, Altet, & Merkoçi, 2019). Firstly, the electrodes were pretreated by applying oxidative potentials in acetate buffer, and then rinsed and dried. After that, carbon working electrodes were immersed into a Au solution and a constant negative potential (−0.4 V, 200 s) was applied for obtaining a homogenous shapes of well distributed spherical AuNPs. AuNP-modified SPCEs were then incubated with SH-(AT7)-F1 prepared with MCH solution for 2 h at room temperature and then rinsed and dried. subsequently, Recombinase polymerase amplification (RPA) solutions were prepared for the surface amplification and detection of the target sequence of (P20 gene) on the AuNP-modified SPCEs. Besides, the AuNP-modified SPCEs with RPA solutions containing water or other unrelated DNAs as negative controls were evaluated. A scheme of the developed nucleic acid amplification/detection system is shown at Fig. 8 .
Fig. 8.
Schematic of recombinase polymerase reinforcement-based Citrus tristeza virus diagnosis by taking advantage of Au NPs-modified DNA strands and electrochemical impedance spectroscopic tracing. Reprinted with permission.
In 2013, Ulianas et al. introduced a regenerable electrochemical DNA biosensor based on a new sort of acrylic microspheres and gold nanoparticles (AuNPs) composite coated onto a screen printed electrode (SPE) has been improved to detect the 35S promoter from cauliflower mosaic virus (CaMV 35S) gene in soybean. The Au NPs played a role to assist the electron conductivity from the intercalated anthraquinone-2- sulfonic acid monohydrate sodium salt (AQMS) to the electrode surface. Without the inclusion of Au NPs in the composite, only very little current response was observed (Ulianas et al., 2014).
Point-of-care tests are applied to detect the positive cases without laboratory organization or transmittance of samples to centralized facilities (Drain et al., 2014). Antigen Lateral flow detection for SARS-CoV-2 is one point-of-care approach under development for diagnosing COVID-19 (Xiang et al., 2020). In this system, a membrane strip with two lines including antibody-Au NPs in one line, and capture antibodies in the other line. The blood or urine samples are deposited on the membrane, and the proteins are drawn across the strip by capillary action. As it passes the first line, the antigens bind to the antibody-Au NPs, and the complex flows together through the membrane. As they reach the second line, the complex is immobilized by the capture antibodies, and a red or blue line becomes visible. Individual gold nanoparticles are red in color, but a solution containing clustered gold nanoparticles is blue due to the coupling of the plasmon band.
Lately, the dual-functional plasmonic biosensor consists of the effect of the plasmonic photothermal (PPT) and the sensing transduction of localized surface plasmon resonance (LSPR), is used for the diagnosis of nucleic acid from COVID-19 (Qiu et al., 2020a).
This system is unified on a chip by Au-S bonding between two-dimensional gold nanoislands (AuNIs) and thiol-cDNA receptor of RNA-dependent RNA polymerase (RdRp), polyprotein ORF1ab, or the E gene sequence. This plasmonic chip can generate local PPT heat that develops rapid and delicate recognition of nucleic acids by enhancing the hybridization kinetics of fully matching strands. In another study, sensitive detection of COVID-19 was fulfilled via nucleic acid hybridization by the use of 2D AuNIs decorated complementary DNA receptors (Nasrollahzadeh, Sajjadi, Soufi, Iravani, & Varma, 2020). Similar to the previous study, here, the thermos-plasmonic heat was fabricated on the chip to enhance detecting execution, whereby illumination at their plasmonic resonance frequency and the ensuing local PPT heat enhanced the in situ hybridization temperature and activate the exact diagnosis of two equivalent gene ordering. This biosensor demonstrated significant precision to analyzed COVID-19 arrangements with lower limit of detection of about 0.22 pM concentration witch leads to specific target recognition of multigene (Qiu et al., 2020b). In another study, the Au NP coated with mercaptoethanesulfonate was used as an antiviral agent for tBVC`he inactivation of the HSV type 1 virus. This nanocomposite seduces cell surface receptor heparan sulfate and binds to the virus competitively, which prevents attachment of infections to the target cells.
The equal or larger sizes of nanoparticles than virus diameter efficiently prevent virus infection. Probably, bigger nanoparticles proficiently cross-link virions, whilst ultra fine nanoparticles just decorate the surface of viruses (Vonnemann et al., 2014). Computational models demonstrate that normal or disease cells grab the NPs to better predict the pharmacokinetic and pharmacodynamic characteristics of these nanomaterials (Drain et al., 2014; Iranpour et al., 2018; Khater et al., 2019; Maltez-da Costa et al., 2012; Mullard, 2020; Nasrollahzadeh et al., 2020; Papp et al., 2010; Qiu et al., 2020a, 2020b; Staroverov et al., 2011; Ulianas et al., 2014; Vonnemann et al., 2014; Xiang et al., 2020). For instance, Lunnoo and co-workers utilized a coarse-grained molecular dynamics (MD) simulation to perceive the internalization routes of different morphologies of gold nanoparticles (Lunnoo, Assawakhajornsak, & Puangmali, 2019).
Regarding the vaccine technology, AuNPs have become the choice for immunotherapy applications because their physicochemical properties prevent antibody production against the platform material (Ahmad et al., 2017; Niikura et al., 2013). Besides, some studies in vitro and in vivo have stated that diverse immune cells are stimulated by AuNPs (Dykman & Khlebtsov, 2017). In 2020, Sekimukai et al. assessed the efficiency of AuNPs as vaccine adjuvants (Iwata‐Yoshikawa et al., 2014). Besides, an alternative research illustrated a technique to attach viral antigens to gold nanoparticles in order to enhance the vaccine technology against COVID-19 (Chen, Huang et al., 2016). Indeed, it is hypothesized that to produce coronavirus nanoparticles vaccine by use of AuNPs, S or N protein from coronavirus can be attached on the AuNPs capped with polysaccharides (Fig. 9 ) (Chen, Han, Wang, & Zhao, 2020).
Fig. 9.
The proposed schematics illustrating the S or N protein from coronavirus loaded onto polysaccharides capped AuNPs (Chen et al., 2020).
4.2. Iron oxide and Ferrite-based Nanoparticles
The US Food and Drug Administration (FDA) have ratified iron oxide nanoparticles (IONPs) as biocompatible material to cure anemia formerly, and studies have shown the antiviral characteristic of these nanomaterials in vitro. The antiviral property of IONPs has been repeatedly presented (Abo‐zeid & Williams, 2020; Arias et al., 2018). The antiviral properties of IONPs have been studied in eradicating of some viruses, including influenza virus (H1N1) (Kumar et al., 2019), Dengue virus (Murugan et al., 2017), and rotavirus (Gutierrez et al., 2009). According to the findings, the assumption is that the antiviral activity of IONPs is due to interaction with virus surface proteins and interference with virus binding or entry into the host cell, resulting in neutralization. Consequently, this nanoparticle can be used as a suitable and secure option for the rapid diagnosis and remedy of the SARS-COV-2 virus in patients.
In 2020, Abo-zied et al. (Abo-Zeid, Ismail, McLean, & Hamdy, 2020) fulfilled a docking study to probe the Fe2O3 and Fe3O4 as IONPs with the spike protein receptor-binding domain (S1- RBD) of SARS-CoV-2 that is needed for virus adhesion to the host cell receptors as stated previously. Besides, a similar study was carried out for antiviral activity of IONPs hepatitis C virus (HCV) glycoproteins E1 and E2. Consequently, both Fe2O3 and Fe3O4 interacted effectively with the S1-RBD of the SARS-CoV-2 virus and to glycoproteins E1 and E2 of HCV. Fe3O4 formed a more stable complex with S1-RBD whereas Fe2O3 favored HCV E1 and E2. The revealed interactions of IONPs are associated with conformational changes in viral structural proteins and subsequent inactivation of the virus.
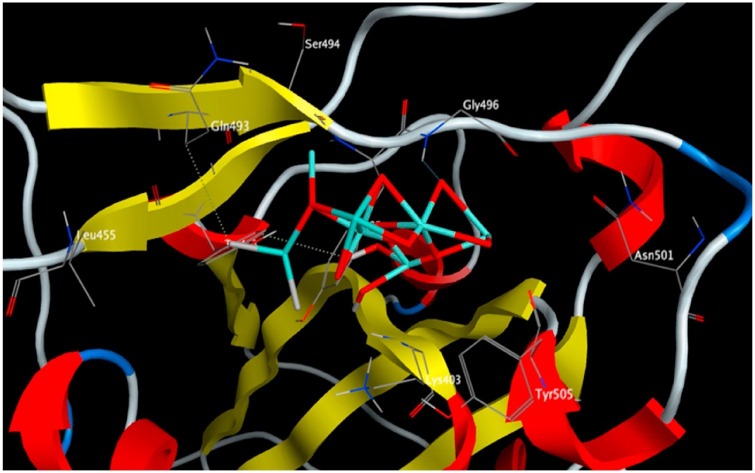
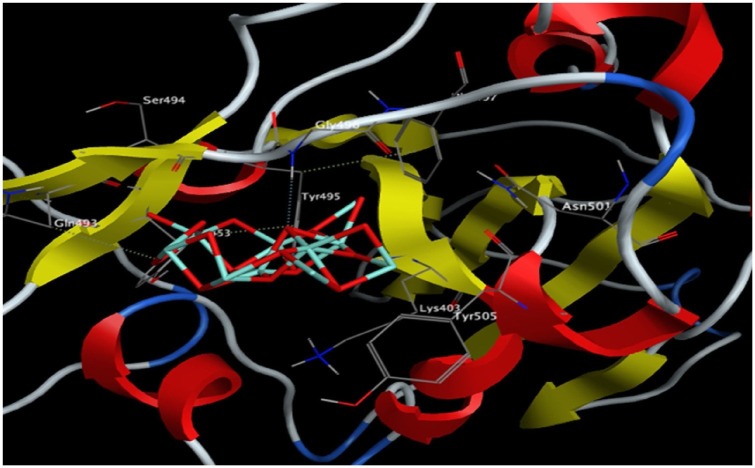
The interaction of Fe2O3 and Fe3O4 with the key amino acids in the S1-RBD of SARS-CoV-2 are reported in Table 1 and illustrated in Fig. 9, Fig. 10 . The binding free energy of Fe3O4 is lower than Fe2O3 indicating the higher stability of the Fe3O4 S1-RBD complex. Thus, S1-RBD favors interaction with Fe3O4 over Fe2O3. The interaction of Fe3O4 with S1-RBD involved the formation of four hydrogen bonds, with a total intermolecular energy of -11.40 Kcal/mol (Table 1). In addition, hydrophobic interactions of Fe3O4 were detected with Leu455, Ser494 and Phe497 (Table 1). In contrast, Fe2O3 interactions involved the formation of three hydrogen bonds with a total intermolecular energy of -7.55 Kcal/mol and hydrophobic interactions were identified with Tyr495, Phe497, Tyr505 (Table 1).
Table 1.
The Docking Interaction Parameters of Both Fe2O3 and Fe3O4 with S1-RDB of SARS-CoV-2 (Abo-Zeid et al., 2020).
| Ligands | Binding free energy (Kcal/mol) | Total Intermolecular energy (Kcal/mol) | Interacting amino acids | Hydrogen bonds | Hydrophobic interactions |
|---|---|---|---|---|---|
| Fe2O3 | −8.97 | −7.55 | Gly496, Gln493, Tyr 453 | 3 | Tyr495, Phe497, Tyr505 |
| Fe3O4 | −10.66 | −11.40 | Gly496, Gln493, Tyr 453 | 4 | Leu455, Ser494, Phe 497 |
Fig. 10.
3D interaction diagram showing Fe2O3 docking interactions with the key amino acids in the S-RBD of SARS-COV-2. Reprinted with permission.
Besides, Cagno and their colleagues (Cagno et al., 2018), reported that AuNPs and IONPs, coated with organic ligands, disrupt the ultrastructure of multiple viruses and break down viral particles, leading to the inhibition of the encapsulated and naked viruses.
Cave sediments commonly contain iron-oxides that have proven invaluable in paleomagnetic studies (Blatnik et al., 2020; Luiszer, 2009), The surface charge of limestone is related to the cation Ca2+ (although HCO3−, CO3 2-, H+, and OH- ions contribute to surface chemistry), and has been experimentally demonstrated to have a pI (isoelectric point) between 8.0 and 9.5, with commonly measured pH values of 8.3 (Banks et al., 2010; Somasundaran & Agar, 1967). Indeed, strong poliovirus attraction has been demonstrated on positively charged iron oxide and dolomite surfaces, indicating that limestone surfaces and iron-rich cave sediments are capable of binding high levels of SARS-CoV-2 particles. Also, the presence of bicarbonate and pH > 8 in calcareous and limestone surfaces will unavoidably inactivate the coronavirus activation (Lamarre & Talbot, 1989; Moore, Taylor, Sturman, Reddy, & Fuhs, 1981). Furthermore, Singh and co-workers have revealed an electrochemical DNA sensor based on chemically Chitosan–iron oxide for the detection of infection bacterium (Singh et al., 2011).
Ferrite-based nanomaterials (consist of iron oxide) as an important type of functional particles have been studied due to their excellent poetries including large surface area, high surface to volume ratio, strong adsorption capacity, facile recovery, etc. (Casbeer, Sharma, & Li, 2012; Polshettiwar et al., 2011; Velinov et al., 2016).
Owning to these special properties, these types of nanoparticles have been used in some applications such as catalysis, drug delivery, water treatment, sensors, memory devices, electrical components, etc. (Harris et al., 2009; Jumeri et al., 2014; Sharma & Singhal, 2013).
Recently, taking advantage of Ferrite-based nanoparticles for biological and biomedical applications has been growing rapidly. Ferrite-based materials have attracted attention because they are ferromagnetic and yet insulating. Having these two types of characteristics makes these materials suitable in magnetic fields without unwanted effects of eddy currents.
The advances in magnetic materials coupled with nanotechnology lead to diagnostic technologies in nanoscale and decrease the limit of detection to the early stage of infection diagnosis. Besides, the detection of the COVID-19 virus is fulfilled via real-time PCR based on the extraction of RNA from nasopharyngeal cells using functionalized magnetic nanoparticles. This method can detect about 10,000 tests per day to cover a wide range of the population. However, the main drawback of this technique is the requirement for dedicated magnetic nanoparticles with a strong negative charge for viral RNA purification to diagnosis the existence of the COVID-19 virus. Edeas, Saleh, and Peyssonnaux (2020) introduced a simplified low-cost technique to fabricate 100 g of magnetic nanoparticles in 1 L of the solution to the analysis of about 50,000 COVID-19 tests that assist in decreasing the expense of obtained nanoparticles for diverse biomolecular applications supporting developing country budgets constraints and chemical availability, especially during the COVID-19 International Health Emergency.
Magnetic biosensor areas have attracted special attention rather than other types of biosensors. Volume-based and surface-based magnetic biosensors have been applied for the diagnosis of viruses, pathogens, cancer biomarkers, metallic ions, etc. (Aytur et al., 2006; Gao et al., 2019; Hash et al., 2019; Klein et al., 2019; Lin, Lu, Ge, Cai, & Grimes, 2010; Rettcher et al., 2015; Su et al., 2019; Wang, Yang, Lei, Lei, & Zhou, 2014, 2014b; Wu et al., 2020; Zou et al., 2019). Indeed, magnetic biosensors are coupled with antibodies or DNA/RNA probes that can attach to the target cells specifically, and then, the amount of the target cells affects the magnetic signals. Because, most of the biological environmental materials don't have magnetic properties, therefore, this type of biosensor reveals low background noise in comparison with other biosensors such as optical, plasmonic, and electrochemical biosensors. Moreover, because signals are not influenced by the sorts of the analyte matrix, the detection process is so accurate and reliable (Schotter et al., 2004).
4.3. Copper nanoparticles
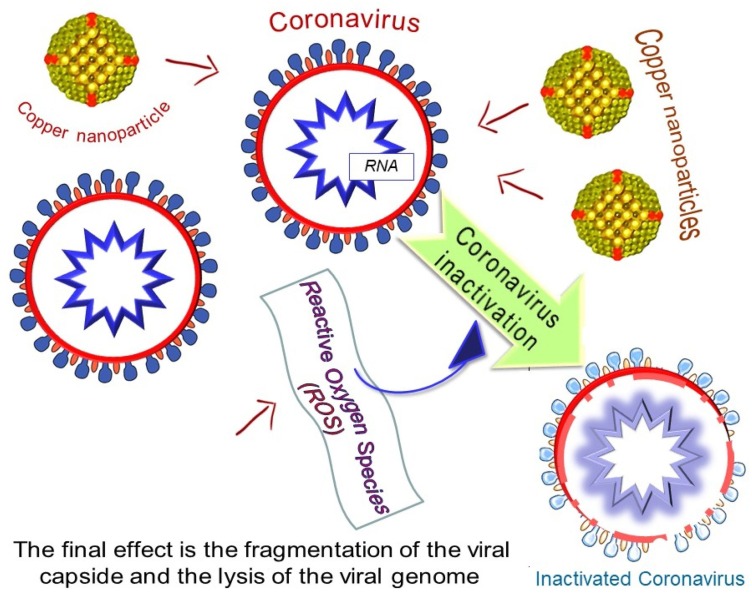
It was observed that copper could reduce the activities of the virus in a very short time, as reported on CoV-229E in 2015 (Warnes, Little, & Keevil, 2015). The combination of brasses and at least 70 % of copper were found effectively to inactivate the virus. The inactivation rate was influenced by the percentage of copper. Fig. 11 , shows the Cu NPs effects on the virus inactivation based on collected knowledge by Poggio et al. (2020).
Fig. 11.
3D interaction diagram showing Fe3O4 docking interactions with the key amino acids in the S-RBD of SARS-COV-2. Reprinted with permission.
The formulation could be varied for tailoring antimicrobial properties using nanostructures metal species to combat COVID-19 (Cioffi & Rai, 2012; Sportelli, Picca, & Cio, 2016). The usage of nanoparticles metals salt such as copper salt and/ or solutions can contribute to antiviral results (Broglie et al., 2015; Fujimori et al., 2012; Khodashenas & Ghorbani, 2014; Krzyzowska et al., 2017; Sucipto et al., 2017). This could be useful in designing the PPE material. For example, the treatment of PPEs using copper ions could assist in inhibiting virus scattering on the PPEs. In general, the metal ions could lessen the activity of CoV on these substrates. Meanwhile, it is helpful to perform the surface treatment with Cu, or copper brasses. The release of ionic metal is influencing the virus deactivation (Cioffi, Ditaranto, Sabbatini, Torsi, & Zambonin, 2009, 2015). The inactivation is due to the release of Cu ion and the production of reactive oxygen species (ROS) (Van Doremalen et al., 2020). The inserting of Cu nanoparticles in polymer matrices could alter the metal release process and can minimize the risk of contaminating the environment (Cioffi & Rai, 2012). Additionally, a strong antiviral property can be achieved via simple routes to combine nanoparticles' copper core and a quaternary ammonium shell (Sportelli, Longano et al., 2020). The PPE treatment using copper nanoparticles and also, copper oxides and salts were reported in a few publications. It is obvious that insufficient and suitable PPE can be accountable for the death of front liners. Bhattacharjee et al. studied the topic in 2019, by considering other pandemic diseases (Bhattacharjee, Joshi, Chughtai, & Macintyre, 2019). They specified that the modification of non-woven tissues using metal-grafted graphene oxide (GO), exhibited to have very effective antimicrobial properties. Graphene was used as composites and photocatalyst when combining with metals such as Cu, Fe, Zn, and Ag (Chen, Hsueh, Hsieh, Tzou, & Chang, 2016). Also, there were studies explored the usage of GO with nanoparticle to treat PPE (Perreault, de Faria, Nejati, & Elimelech, 2015). It was found that nanometal such as silver and copper, when loaded on GO can work effectively as antiviral material (Chen, Hsueh et al., 2016; Hang et al., 2015). In 2010, new anti-influenza respirators were invented using a similar approach. Meanwhile, Borkow et al. developed copper oxide-impregnated masks, which can reduce the risk of being infected by the influenza virus, while maintaining the filtration effectiveness (Borkow, Zhou, Page, & Gabbay, 2010). However, similar methods were not yet applied in masks and other PPE (Sunada, Minoshima, & Hashimoto, 2012). It was found that Cu(II) ions on eukaryotic cells, will contribute to low toxicity (Shaligram & Campbell, 2013). Newly, it was found that polyurethane/ CuO nanocomposites can effectively work as antimicrobial filters for air purification. Also, CuO microparticles were known as a good additive for PU filters, compared to the nano-sized particles (Ungur & Hruza, 2017). Hence decreasing risks of toxicity due to the nano element. The usage of the active material of SiO2 –Ag towards MS2 bacteriophage was an example of an antiviral air filtering (Krähling, Stein, Spiegel, Weber, & Mühlberger, 2009).
Copper is known for its antimicrobial activity since ancient times (Grass, Rensing, & Solioz, 2011), and high Cu amount on surfaces has proven their antiviral efficiency. This proves the Cu effectiveness of poliovirus (Murray & Laband, 1979). More lately, the Cu effectiveness to deactivate coronaviruses recommends possible alike effectiveness towards SARS-CoV-2 (Warnes, Little, & Keevil, 2015). However, it was found that deactivation of the virus is much faster on brasses with Cu presence, compared to other smooth surfaces. The results showed that the viral genome becomes damaged. This could ensure the inactivation irreversibility (Warnes et al., 2015b). This is due to of toxicity of copper ions released and ROS generated from Cu attacks viral proteins and lipids (Grass et al., 2011). Also, both SARS-CoV-1 and SARS-CoV-2 are deactivated on Cu surfaces faster, compared to other studied surfaces (van Doremalen et al., 2020). The deactivation mechanism is due to damage of viral proteins and lipids. Using Cu alloys in addition to stainless steel could contribute better efficient antiviral surfaces. For air disinfection, the supported catalysts Al2O3 impregnated Cu and Ag were found capable to deactivate virus (Han et al., 2005).
Cu and CuO nanoparticles have the potential to release metal ions (Al-Gaashani, Radiman, Tabet, & Daud, 2011; Shi et al., 2017). The kinetic of Cu ion release was fasten due to the high aspect ratio with higher reactivity. These nanoparticles could be used in textile fabrics. Hence the product with antiviral properties could be designed. It was observed that CuO-impregnated masks can act as anti-influenza virus PPE (H1N1 and H9N2) (Borkow et al., 2010). Hence, the usage of these materials toward virus SARS-CoV-2 should be explored further.
4.4. Silver nanoparticles
The Ag nanoparticles' antiviral properties are well known. This is due to the capability to hinder the viral entry in host cells. Also, the metal interaction with a viral genome was found to inhibit viral replication (Kerry et al., 2019). Gold nanoparticles covered with silver nanoparticles were found be able to bind the HIV envelope glycoprotein gp120, hence inhibit infection (Di Gianvincenzo et al., 2010; Elechiguerra et al., 2005a). A study on size-dependent interaction with the virus was also studied (Elechiguerra et al., 2005a). The functionalized AgNPs may have the capability too to stop virus infection, via several mechanisms (Baram-Pinto, Shukla, Perkas, Gedanken, & Sarid, 2009; Orlowski, Tomaszewska, Gniadek, Baska, Nowakowska, Sokolowska, Nowak, Donten, Celichowski, Grobelny, 2014). It is noted that the smaller size particle would reach the cell membrane easier. Hence, this could inhibit virus replication (Rai et al., 2016). For example, usage of nano-titanium dioxide and silver ions was reported to study the effective minimal inhibitory concentration (MIC) (Zachar, 2020). This includes the study on its effectiveness in various respiratory system target locations. These include early-stage home treatment, and to lower the risk of ventilator-associated pneumonia (VAP) in hospital ICU. The dosage is influenced by the size of Ag nanoparticle. It was concluded that MIC can be achieved, after accounting for deposition losses (Van Doremalen et al., 2020). The antiviral and antibacterial characteristics of silver nanoparticles have been well accepted in medical applications (Sim, Barnard, Blaskovich, & Ziora, 2018), for example, paint containing silver (Kaiser, Zuin, & Wick, 2013) and in food plates (Sohal, O’Fallon, Gaines, Demokritou, & Bello, 2018) as a biocide. These properties lead to inactivate many infections such as HIV-1 (Elechiguerra et al., 2005b), monkeypox virus (Rogers, Parkinson, Choi, Speshock, & Hussain, 2008), bacteriophages UZ1 and MS2 (De Gusseme et al., 2010; Park et al., 2018), murine norovirus MNV1 (De Gusseme et al., 2010; Park et al., 2018), HSV (Orlowski et al., 2014), HBV (Lu et al., 2008) and, recently, in porcine epidemic diarrhea virus (PEDV) (Du et al., 2018). Three mechanisms were expected to act as antiviral action. First, the release of some toxic agent of Ag(I) forms (including Ag + ions). Also, it illustrates a powerful affinity toward sulfur. Hence, it has good interactions with thiol in the active sites of enzymes. Besides, Ag NPs may interact with virus protein spikes, and it is possible to aggregate in host cells. Further interaction with enzymes, which involve virus replication will resist the process. This was suggested by Zodrow et al. and by De Gusseme et al. (2010); Zodrow et al. (2009). Furthermore, Ag2S nanoclusters (NCs) also showed effective inhibition of RNA copy of the Porcine Epidemic Diarrhea Virus. It was also seen that subjecting living cells to silver ions, at a similar concentration did not hinder virus copy. It can be concluded that the antiviral property was independent of the Ag(I) release (Du et al., 2018). Nevertheless, it is worth taking note that the mechanisms entering the cells by both Ag + ions and Ag particles are different. Thus, the distribution within cells would be different, which leads to different toxicity action toward the viruses. Ag NCs may accumulate in intracellular areas, where else Ag(I), could aggregate in other areas of the cells, or be quickly eliminated. Second, the antiviral action of Ag NPs would come from physical interaction with the surface of viruses, hence this could hinder their docking on host cells and limit their infectivity. This was studied by Elechiguerra et al. for the HIV-1 case and by Orlowski et al. for HSV-2 (Orlowski et al., 2014). From Elechiguerra et al. work, they observed that the size of nanoparticle sizes did not affect that physical interaction with the virus. However, the larger size would be more preferred to act as an antiviral agent, based on Orlowski et al. They concluded that this can be achieved by blocking the virus attachment to the host cell. The same mechanism was proposed by De Gusseme et al. when studied combined the release of Ag(I) (De Gusseme et al., 2010). It was also said that the local release of ROS could damage the envelope and/or membrane of the virus, in which this is due to Ag nanoparticles docking on the virus surface. The usage of Ag nanoparticles in many medical appliances and equipment. Also, the Ag application can be seen as fillers in paints, materials for air filters, and face masks. It was also found that filters containing Ag NPs possess good antiviral capability towards bacteriophage MS2 (Joe, Park, & Hwang, 2016).
4.5. Zinc nanoparticles
Zinc is used in many biological processes due to its multifunction capability as an indicating molecule, cofactor, and also, a physical element. The element involved the metabolism of lipids and carbohydrates. And also involved in the functioning of the nervous system, reproductive, and cardiovascular (Prasad, 2017). Zinc plays an important role in our immune system, such as to regulate proliferation, differentiate, maturation, and functioning leukocytes and lymphocytes (Wessels, Maywald, & Rink, 2017). Zinc also shows a signaling role in the modulation of the inflammatory response (Maywald, Wessels, & Rink, 2017). It is also a component of nutritional immunity (Haase & Rink, 2014). The immune system can be affected significantly by changing the zinc status. This will result in increased exposure to diseases such as malaria, pneumonia, and measles (Gammoh & Rink, 2017). It is interesting to discuss the zinc potential usage during the pandemic. Zinc has good antiviral properties, and also immune-modulatory (Zhang & Liu, 2020). However, to the best of our knowledge, the results or analysis of the effect of zinc usage to combat COVID-19 are not available. It was found that Zn2+ cations when combined with Zn ionophore pyrithione, could inhibit virus reproduction (Te Velthuis et al., 2010). This indicated that Zn2+ could act as an antiviral agent. For COVID-19 treatment, it has been noted that chloroquine could act as an antiviral agent (Wang et al., 2020). However, further study is required to perceive the antiviral mechanism. (Liu et al., 2020) Previous studies showed that chloroquine could act as a zinc ionophore (Xue et al., 2014). The authors also proposed that chloroquine-mediate zinc influx could also act anticancer agents (Xue et al., 2014). Also, it was predicted that that increasing intracellular Zn2+ concentration by chloroquine can combat SARS-CoV-2. Similar positive effects may be expected when zinc supplementation without chloroquine is used (Guastalegname & Vallone, 2020). Supposedly, a similar effect can be predicted when applying other zinc ionophores (Dabbagh-Bazarbachi et al., 2014) with lower toxicity. This required further studies to support the prediction.
Another approach is to use targeting Zn ions to combat COVID-19. Mainly, it was observed that disulfiram-induced Zn2+ release from papain-like protease can result in protein destabilization in both MERS-CoV and SARS-CoV (Lin et al., 2018). Zn-ejector drugs also could potentially function as antiviral agents (Sargsyan, Chen, Grauffel, & Lim, 2020) and as targeted oxidation strategy in antiviral treatment (Xu, Tong, Wu, Zhao, & Lin, 2020). For reaching the target cells, both SARS-CoV-2 and SARS-CoV are having a similar requirement of angiotensin-converting enzyme 2 (ACE2) (Hoffmann et al., 2020). Hence, ACE2 receptor modulation could be considered to possess a good therapeutic strategy in COVID-19 treatment (Zhang, Penninger, Li, Zhong, & Slutsky, 2020). It was concluded that by Speth, Carrera, Jean‐Baptiste, Joachim, and Linares (2014), for rat lungs, 100 μM zinc exposure could lessen recombinant human ACE-2 activity. However, in their studies, the modulating effect of zinc appears to be hypothetical (Chilvers et al., 2001). Though neither coronavirus HCoV 229E (Maret, 2015) nor HCoV-OC43 (Essaidi-Laziosi et al., 2018) infection resulted in a clear decrease in the frequency of ciliary beat, it was found that impaired mucociliary clearance would result from HCoV 229E induced ciliary dyskinesia. This may not change the removal of the viral particle only but also predispose to bacterial co-infection (Pittet, Hall-Stoodley, Rutkowski, & Harmsen, 2010). A study on Zn-deficient rats, supplementation of Zn was found to improve ciliary length in bronchial epithelium (Darma et al., 2020). Also, it can increase the ciliary beat frequency in vitro (Woodworth et al., 2011). Thus, zinc could potentially improve nCo-2019-induced mucociliary clearance dysfunction. For respiratory epithelium, zinc was proven to be vital due to its anti-inflammatory and antioxidant activities (Truong‐Tran, Carter, Ruffin, & Zalewski, 2001), along with the regulation of tight junction proteins ZO-1 and Claudin-1 (Roscioli et al., 2017). Hence, this could increase the barrier functions. Sequentially, reduction in barrier function and downregulation of tight junction protein complexes intensifies the viral inflammatory processes (Wittekindt, 2017). Besides, the formation of ARDS and alveolar edema could be caused by TJ perm loss selectivity in the airways. This resulted in an uninhibited leakage of high molecular weight proteins and water into the airways (Günzel & Yu, 2013).
Even with limited studies on the result of Zn on COVID-19, its antiviral properties were confirmed for other viral sicknesses. This can be achieved via viral particle entry modulation, replication, translation of viral protein, fusion, and additional release for some viruses (Ishida, 2019; Read, Obeid, Ahlenstiel, & Ahlenstiel, 2019). It was concluded that the increment of intracellular Zn levels via the usage of Zn ionophores considerably changes picornavirus replication (Krenn et al., 2009). These conclusions were from earlier studies from around the 1970s (Korant, Kauer, & Butterworth, 1974). The increment of interferon α (IFNα) creation by leukocytes resulted from Zn treatment (Cakman, Kirchner, & Rink, 1997). Also in rhinovirus-infected cells, it potentiates its antiviral action (Berg, Bolt, Andersen, & Owen, 2001). It was observed that the antiviral activity mechanism could be stimulated by Zn2+ (Lin & Young, 2014). These findings increased attention towards Zn potential usage in common cold prevention and treatment. Singh and Das have done a review work (Singh & Das, 2013) in which it was shown that Zn supplementation can be used as an effective cold treatment (Hemilä, 2017). Zn acetate was the best form (Hemilä, 2011). Some studies relate Zn status and infection of respiratory syncytial virus (RSV). It was observed that children with RSV pneumonia have lower whole blood zinc (Che & Sun, 2016). Also, lessened zinc metabolism would result in increased exposure to RSV infection (Johnson, Harris, Ping, Gauthier, & Brown, 2019). Consecutively, Zn compounds were observed to prevent respiratory syncytial virus replication (Suara & Crowe, 2004). In an influenza study, it was proven that Zn insufficiency could result in higher mortality (Kaynar et al., 2019). Also, it is important to consider bacterial coinfection risk. Further clinical data to be studied to use Zn supplementation to treat respiratory virus infections.
Meanwhile, few studies have reported the nanoparticles of zinc oxide antibacterial properties (Pasquet et al., 2014). ZnO was proven to hinder both biofilm formation and growth by S. pneumoniae (Bhattacharyya et al., 2018). A similar effect was detected for other bacterial agents such as K. pneumoniae (Reddy, Nisha, Joice, & Shilpa, 2014), methicillin-resistant S. aureus (Kadiyala, Turali-Emre, Bahng, Kotov, & VanEpps, 2018), and P. aeruginosa (Ann et al., 2014). Nevertheless, nanoparticles ZnO are toxic to lung, hence limit its usage as an antibacterial agent (Sahu, Kannan, Vijayaraghavan, Anand, & Khanum, 2013), also weakening macrophages phagocytic activity in the respiratory system (Lin et al., 2014). It is important to note that Zn is needed for bacteria to grow and colonize when the study involves S. pneumonia and Zn (Bayle et al., 2011). Biofilm formation also required Zn bioavailability (Brown et al., 2017).
4.6. TiO2 nanoparticle
It was studied that photocatalytic nanoparticle could deactivate SARS-CoV-2. Titanium dioxide (TiO2) is the most common one. The material is inert, low toxicity, and is not prone to photo corrosion when irradiated with UV light (Maeda & Domen, 2007), We can find TiO2 in paints, (Kaiser et al., 2013) water purification, and for self-cleaning windows (Dunnill & Parkin, 2011). Paints with nanoparticle could clean air due to the ability of photocatalytic TiO2 to remove volatile organic compounds (VOCs) under UV light. But, the usage in the paint could be a problem due to the release of toxins in the air (Kaiser et al., 2013). If possible, in combatting SARS-CoV-2, TiO2 photocatalysis may assist in deactivating the virus via surface decontamination using aerosol, paint, water, and air treatment system containing these particles. The mechanism of this photocatalytic process is well known and explained by the excitation of an electron from the valence band (VB) to the conduction band (CB, which lead to initiate reactions to produce ROS such as superoxide anion and hydroxyl radical (Byrne et al., 2015). The presence of hydroxyl radicals due to the water molecules oxidation contributed to the disinfection activity of TiO2. This happens due to protein modifications, damages of DNA, cell wall, and membrane of the virus (Byrne et al., 2015). TiO2 also can be used for an antibacterial agent. Bogdan et al. found that many studies proved that the inactivation of viruses would be more susceptible, compared to bacteria (Bogdan, Zarzyńska, & Pławińska-Czarnak, 2015). There were studies concluded that enveloped viruses were more threatened than non-enveloped viruses. However, there were also opposite findings (Bogdan et al., 2015). To the best knowledge of the author, there is only one journal article shared the usage photocatalytic titanium apatite filter (PTAF) to combat SARS-CoV-2 (Han et al., 2004). It showed that there is the possibility of using the treatment method.
There were also efforts to develop second-generation photocatalysts, which combine other components with TiO2, such as S-doped and N-doped. This resulted in the effective deactivation of bacteria and some viruses. The review work done by other researchers can be found in these articles (Byrne et al., 2015; Dunnill & Parkin, 2011). However, to the best of our knowledge, there is no testing on viruses yet using the second generation photocatalyst treatment. It was found that nanoparticle Ag on the TiO2 particle could increase the antiviral productivity towards MS2 via hydroxyl production (Liga, Bryant, Colvin, & Li, 2011). Also, being reported that the usage of Ag- and Cu-doping on TiO2 nanowire could eliminate bacteria in drinking water, than using Ag-TiO2, TiO2, or Cu-TiO2 both in dark and being exposed to UV light.
This is due to combination both enhanced photoactivity, and the lower bandgap of (Ag, Cu)-TiO2 (Behnajady & Eskandarloo, 2013). The free Ag and Cu in water treatment also could impose antiviral (Rao et al., 2016). Another approach can be done by mixing TiO2 with SiO2 NPs. This resulted in the effective antiviral ability of TiO2 due to the large specific surface area of SiO2, compared to TiO2 alone (Liga, Maguire-Boyle, Jafry, Barron, & Li, 2013). Also, it was observed that TiO2 doped with Pt, coated on glass slides had better efficiency in combating influenza A presence in aerosols. It is great to note that Byrnes et al. (Byrne et al., 2015) has already put many efforts in developing photocatalytic materials for the deactivation of SARS-CoV-2. Most importantly, the materials used are non-toxic to us (Weiss et al., 2020). It was observed that nanofibers coated with TiO2 and being deposited on a surface of the filter, using the electrospinning process was capable to deactivate virus under natural sunlight and UV radiation. In this study, the electrospraying of NPs onto the nanofibers was used (Lee et al., 2010). The experimental data showed that the materials possess exceptional photoinduced hydrophilicity, photocatalytic, and antibacterial activity.
4.7. Two-dimensional carbides and nitrides (MXenes)
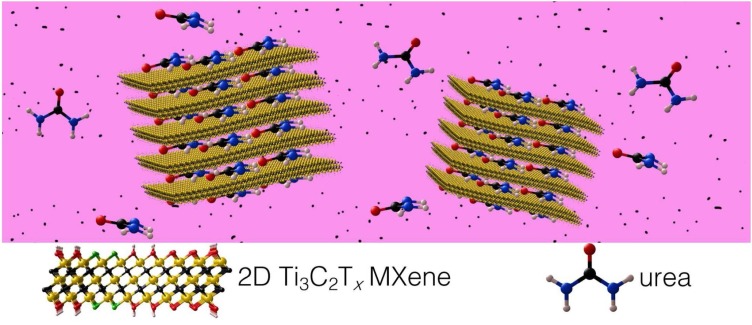
Among the emerging new substances, Two-Dimensional Carbides and Nitrides (MXENES) with hydrophilic and high negative charge used as a coating on facemask to capture and inactivate viruses. The formula of MXenes is Mn+1XnTx, where M is an early transition metal (Ti, Zr, V, Mo, etc.), X is C and/or N, Tx represents the surface functional groups ( O, OH, F, C— — —l), and n ranges between 1 and 4 (Anasori, Lukatskaya, & Gogotsi, 2017; Deysher et al., 2020). A wide range of MXenes involving Ti3C2Tx, Ta4C3Tx, Nb2CTx, are biocompatible (Huang, Li, Lin, Han, & Huang, 2018; Lin, Gao, Dai, Chen, & Shi, 2017). Besides, high surface area and porosity of MXenes leads to not only adsorb amino acids strongly (e.g. Ti3C2Tx MXene (Chen et al., 2018), but also, binding to viral spike peplomers and immobilizing the virus. Indeed, MXenes are applied as a strong magnet for proteins. Moreover, these materials have been illustrated to be photocatalytically active, meaning that when viruses are absorbed on their surface, they use light to destroy the adsorbed virus at the same time (Low, Zhang, Tong, Shen, & Yu, 2018; Nguyen et al., 2020; Wong, Tan, Yang, & Xu, 2018). MXenes with plasmon resonance in visible or IR range can convert the light to heat, efficiently. Depending on the type of MXene can be excited by specific wavelengths of light and also can be sterilized with that wavelength and kill viruses left on the surface. For instance, Ti3C2Tx is excited by red light (780 nm plasmon resonance) and sterilized by the use of a red/ infrared lamp as well as solar light. Besides, MXenes have antibacterial characteristics because of the combination of their charge transferability and hydrophilicity (Pandey et al., 2018; Rasool et al., 2016, 2017). Besides, the most common and most inexpensive type of titanium carbide MXenes has no destructive or negative environmental or toxicological effects on the ecosystem (Nasrallah, Al-Asmakh, Rasool, & Mahmoud, 2018).
In addition to the known pulmonary findings, it was estimated that the 30–40 percentage of COVID-19 patients with kidney disease who need hemodialysis therapy that prevents uremic toxins in the blood which may lead to death (Naicker et al., 2020; US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States, 2019). MXenes can regenerate dialysate by removing toxins that build up in the setting of kidney failure. MXene as biocompatible has slit pores between the negatively charged MXene sheets can easily absorb urea which was eliminated by from dialysate by dialysis difficultly (Fig. 12 ) (Meng et al., 2018). Therefore, MXenes probably be qualified to address key limitations of current ambulatory dialysis systems by offering efficient urea adsorption, small size, and lightweight.
Fig. 12.
Coronavirus inactivation by copper nanoparticles. Reprinted with permission.
4.8. Metal-Organic Framework (MOF)
To prevent the viral disease spread, using air filtration could be considered as a noneffective control process. Nonetheless, many purifiers on the market are fabricated from only dense fibrous filters that can effectively remove particles but have not antibacterial or antimicrobial properties (Yu et al., 2020). Li and co-workers fabricated a series of metal-organic frameworks (MOFs) as a super adsorbent with photocatalytic antimicrobial and bacterial characteristics to make a nanofiber membrane (Li et al., 2019). This material can effectively produce biocidal reactive oxygen species (ROS) underexposure of sunlight (see Fig. 13 A). Especially, Zn-based MOF (ZIF-8) inactivates of about 99.999 % of Escherichia coli (E.Coli) for 2 h in saline solution under simulated solar irradiation. This super adsorbent substance can be used as an antimicrobial filter in facemask, clothes, ventilator, and air purifier to prevent COVID-19 transmission.
Fig. 13.
Adsorption of urea by MXenes from aqueous solution. (a) Schematic of MXene nanosheets used as the adsorbent. Reprinted with permission.
Indeed, MOFs under ultraviolet light could inactivate the SARS-COV-2 by eliminating of crown-like spike proteins and then puncturing the lipid membrane, and removing the RNA contents for about 3 h. In contrast when photocatalysis properties of MOF under solar light target the RNA of the virus then a huge amount of hydroxyl radicals have released that lead to damage to the spike proteins (Yu et al., 2020).
In summary, in the aspect of metal nanoparticles (NPs) applications against COVID-19, most metal NPs based-virus diagnosis methods were designed based on the unique properties of metal nanoparticles. Because of their antiviral characteristic, most of them have been used in antiviral coating to prevent transmission of virus in any community. In particular, noble metal NPss like Au, Ag and Cu have special optical and electrical properties, which lead to employ metal NPs in biosensing application. Additionally, AuNPs has potential to be used in vaccine technology because of its biocompatibility.
5. Polymer-based nanoparticles
Polymer-based nanoparticles involving synthetics and natural polymers have excellent characteristics including tunable properties, feasible synthetic protocols, and good biocompatibility that is a suitable candidate for biomedical applications (Amjad, Kesharwani, Amin, & Iyer, 2017; Sivasankarapillai et al., 2020b). These types of nanomaterials with biosafety properties are widely used in biological applications such as viral delivery system, in vivo delivery and controlled release of viral vaccines (Amjad et al., 2017; Lee et al., 2015). Viral vaccines can be delivered in the form of DNA, mRNA or protein (Schmitt et al., 2020) all of which can be easily enzymatically degraded when entering the blood circulation (Carter, Wright, Gray, Rose, & Wilson, 2020; Pillai et al., 2020).
5.1. Synthetics polymer nanoparticles
Synthetics polymer-based nanoparticles with the possibility of tailoring their properties and functions are suitable for the delivery system. They have different structures such as linear, branched, and 3D networks, which were obtained by the addition of several monomers (Amjad et al., 2017; Carter et al., 2020; Chen et al., 2018; Lee et al., 2015; Li et al., 2019; Low et al., 2018; Meng et al., 2018; Naicker et al., 2020; Nasrallah et al., 2018; Nguyen et al., 2020; Pandey et al., 2018; Pillai et al., 2020; Rasool et al., 2016, 2017; Schmitt et al., 2020; Sivasankarapillai et al., 2020b; Susanna, Al Halifa, & Jennifer, 2017; US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States, 2019; Wong et al., 2018; Yu et al., 2020). By optimizing their dimension, morphology, and surface charge, releasing their cargo under external conditions would be controlled (Kamaly, Xiao, Valencia, Radovic-Moreno, & Farokhzad, 2012). Poly (lactic-co-glycolic acid) (PLGA) is one of the famous polymers of this category, which is approved by the FDA for application in the human body because of its excellent biocompatibility and biodegradability characteristics (Liga et al., 2013). To detect the COVID-19, Zhao and co-workers studied on the fabrication of poly (amino ester) with carboxyl groups (PC)-coated magnetic nanoparticles (pcMNPs) and developed the pcMNPs-based viral RNA extraction technology (Zhao et al., 2020). Indeed, this one-step and simplified method is a combination of the lysis and binding steps that produce pcMNPs-RNA complex, which was introduced into subsequent RT-PCR reactions. In this process, the viral RNA was purified among multiple samples within 20 min by the use of a simple manual method or an automated high-throughput approach. By recognizing two parts of viral RNA (ORFlab and N gene), a 10-COpy sensitivity and a strong linear correlation between 10 and 105 copies of SARS-COV-2 pseudovirus particles are achieved. This novel, simple, and highly efficient technique for viral RNA extraction can greatly decrease the turn-around time and operational necessities in current molecular detection of SARS-COV-2, especially for the quickly clinical diagnosis.
Besides, polymer nanoparticles can be used to fabricate facemask protection (Fig. 13B). Liu et al. designed a new self-powered electrostatic adsorption facemask based on the poly (vinylidene fluoride) electrospun nanofiber film and a triboelectric nanogenerator that have potential to perform of about 99.2 % particulates removal efficiency that is more efficient than the commercial mask (Liu et al., 2018).
5.2. Nanocellulose
Cellulose-based materials as natural polymer could be used against COVID-19 virus via many applications such as personal hygiene paper products and paper-based medical materials such as filtration, absorption, paper electrode, paper-based microfluidic chips, biosensors, and biological test (Abd Manan, Hong, Abdullah, Yusof, & Ahmad, 2019). Different forms of cellulose are included crystallites, nanocrystals, whiskers, and nanofibers that are applied widely in biomedical applications (Siro & Plackett, 2010; Virkutyte & Varma, 2011). Among them, cellulose nanofibers with nano dimensions extracted from cellulose fibers have been a focus of research due to its special properties including large surface area, high mechanical strength (Nishino, Matsuda, & Hirao, 2004) that leads to gain many attentions as a device for filtration and diagnosis infections.
Another development in nanocellulose-based kits involves mounting electronic components such as different kinds of sensors and displays to increase the sensitivity and accuracy of the kits (Mahesh, 2020).
Johnson and co-authors presented a model for thermoplastic 3D printing of facemasks coated with NaCl, or clay/biocellulose impregnated with NaCl to make a sterile deactivating area which may further help control the spread of COVID-19 (Johnson, Johnson, Witiw, & Mardon, 2020).
Metreveli et al. in 2014 (Metreveli et al., 2014), introduced a non-woven, μm-thick filter paper from cellulose nanofiber to fabricate unmodified nanofibrous polymer-based membrane. This 100 % natural membrane can remove viral particles regarding their size-exclusion principle, with log 10 reduction value (LRV) ≥ 6.3, thereby matching the performance of industrial synthetic polymer virus removal filters. To minimize the damage and dangers of influenza and coronaviruses now and in the future due to the frequent mutations of these viruses, and on the other hand, due to the lack of an efficient vaccine against these types of virus, the use of these active filters to remove viruses in public places is very effective.
5.3. Chitosan nanoparticles
Chitosan as the second most abundant natural polymer-based nanoparticles with the potential to modify into desired size and shape, are highly demanded to apply in biomedical applications (Halim et al., 2018; Sonaje et al., 2012). Chitosan with the linear and partially acetylated (1–4)-2-amino-2-deoxy-d-glucan structure widely employed as drug carriers and also ideal nucleic acid delivery vehicles due to their biocompatibility, biodegradability and non-toxicity properties (Wang et al., 2011). Slow/controlled release of chitosan nanoparticles improves drug solubility and stability, enhanced efficacy, and reduced toxicity of nanoparticles (Kang, Cho, & Yoo, 2009; Shi & Fan, 2002). Moreover, chitosan directly affects tumor cells and interfere with cell metabolism, inhibits cell growth, or induces cell apoptosis (Dolati et al., 2016). Upon conjugation with therapeutic compounds, Chitosan can improve the persistence of polymeric NP in the mucosal environment and penetration to mucosal tissue.
The presence of a cationic charge on chitosan tends to bind strongly to nucleic acids, which can be used for gene delivery, effectively (Mao et al., 2001). Gene delivery property of Chitosan can be used in preparation of gene-based antiviral vaccines. Raghuwanshi and co-workers fabricated a vaccine delivery system via the nasal path by the use of developed dendritic cell-targeted chitosan nanoparticles (Wikstrom & Stumbles, 2007). Their strategy combines the pDNA carrying capacity of chitosan and selective targeting specificity of bfFp to respiratory Dendritic cells (DCs). Ultrapure water-soluble biotinylated chitosan hydrochloride was utilized to formulate pVAXN loaded nanoparticles. These pVAXN loaded biotinylated chitosan nanoparticles were surface-functionalized with bifunctional fusion protein to achieve nasal DC targeting (Fig. 14 ) (Raghuwanshi, Mishra, Das, Kaur, & Suresh, 2012).
Fig. 14.
Biosafety materials for PPE with antimicrobial capacity. (A) Schematic illustration of the metal-organic framework (MOF)-based filter; (B) Schematic illustration of the filtration mechanism of the polymeric material mask. Reprinted with permission.
One of the most important developments in DNA-based vaccine technology is the improvement in the delivery with chitosan nanoparticles, which have provided an effective means for prevention of DNA vaccine degradation while the cationic nature enables binding to the negative charge of DNA (Xu, Yuen, & Lam, 2014). Chitosan possesses many desirable advantages for DNA vaccine delivery to the mucosal surface such as mucoadhesion, highly soluble, inert and non-immunogenic (Xu et al., 2014). DNA vaccination at the mucosal surfaces with the usage of chitosan nanoparticles is highly recommended since mucosal surfaces are a main place for the most pathogens that infect humans. Indeed, powerful immune responses happen at the site of vaccination with the induction of mucosal and systemic immunity. Besides, based on the fulfilled research, it was found that intranasal administration of plasmid DNA encoding nucleocapsid COVID-19 loaded into chitosan nanoparticles has positive and effective results (Fig. 15 ) (Raghuwanshi, Mishra, Das, Kaur, & Suresh, 2012).
Fig. 15.
Schematic representation of dendritic cell targeting strategy using bifunctional fusion protein (bfFp). The bfFp is a recombinant fusion protein consisting of truncated core-streptavidin fused with anti-DEC-205 single chain antibody (scFv). The core-streptavidin arm of fusion protein binds with biotinylated nanoparticles while anti-DEC-205 scFv imparts targeting specificity to DC DEC-205 receptor. Reprinted with permission.
Presence of some types of materials has negative effects on the environment and ecological system. Hence, using biocompatible and nontoxic nanoparticles against COVID-19 for a long time is very essential to reduce the negative impact of Covid-19 on the environment. Since, chitosan and cellulose nanoparticles have a natural source, are very good choices to use against COVID-19.
6. Lipid nanoparticles
However, for efficient nucleic acid delivery, some nanoparticles have been employed, lipid nanoparticles (LNPs) are a clinically advanced one which is approved by the U.S. Food and Drug Administration (FDA) (Adams et al., 2018). Besides, LNPs have potential to be efficient mRNA delivery platforms. These nanoparticles are composed of multiple lipid components such as ionizable amine lipid, phospholipid, cholesterol, and PEG lipid. Among them, ionizable amine lipids have main role in endosomal escape of nucleic acid. Several studies have been done regarding vaccine technology based mRNA encapsulated in lipid nanoparticles for many infectious diseases including HIV, CMV, rabies, influenza, zika, and most recently COVID-19 (Alberer et al., 2017; John et al., 2018; Sahin et al., 2020). For instance, Elia et al., in 2021, fabricated an mRNA-based vaccine by use of LNPs-encapsulated SARS-CoV-2 human Fc-conjugated receptor binding domain (RBD-hFc). Various ionizable lipids have been assessed in vivo in a luciferase (luc) mRNA reporter assay, and two leading LNPs formulations have been selected for the subsequent RBD-hFc mRNA vaccine technology. The data in the current research show that these nanoparticles possess the potential to be employed in LNP-based mRNA vaccines technology against COVID-19.
7. Discussion
The worldwide requirement for smart and impressive methods to fight COVID-19 leads the researchers towards employing nanotechnology instead of common methods. Nano-based approaches are effective on diagnosis, protection, detection and remedy of coronavirus. In the aspect of antiviral properties of nanomaterials, they have an effective role in coating layers such as facemask or medical clothes. The presence of nanomaterials in biosensors has many privileges like high detection capability, stability, simple design, reliability, and affordability. Employing nanomaterials including carbon nanomaterials, Cu, Ag, TiO2, MXene, MOFs and polymer nanoparticles in textile, facemask and filters possesses many advantages that not only minimize the transmission risk but also makes the reusable facemask, textile and filters. Besides, the nanoparticles including graphene and graphene oxide, carbon nanotube, quantum dots, gold nanoparticles, iron oxide, and polymer nanoparticles significantly improve biosensors’ performance for detecting the SARSCoV-2 virus, as they have shown their potential for detecting other viruses. Nanomedicine can dramatically improve (or enable) the safety and efficacy of a drug and vaccine technology. Lipid nanoparticles are highly biocompatible and biodegradable, and to date, the reports of adverse immune reactions have been low. Although, generally, the biodegradation of polymeric and metallic nanoparticles is slower, they may also be beneficial depending on the disease being treated and the drug being delivered. Besides, the most remarkable property of nanomaterials used in nanomedicine is the nontoxicity, high surface area to volume ratio; this enables highly efficient drug packaging. Indeed, the toxicity and biocompatibility of nanoparticles in the living cells need to be surveyed more. In brief, without the nanoparticles, the mRNA- and DNA-based vaccines would have little efficacy. Among different nanoparticles, old nanoparticles, chitosan and lipid nanoparticles have a main role in vaccine technology. Consequently, by employing nanomaterials, the COVID-19 can be better and sooner controlled in the environment and the society.
8. Conclusions and outlook
Global health is in critical condition with the serious risk of the new respiratory virus called COVID-19. In this review, different types of nanomaterials with the potential of antivirus applications against COVID-19 were reviewed and discussed. However, some issues need further research and attention. First, we introduced different types of nanomaterials and their potential applications such as detection biosensor, antiviral coating, airborne virus filtration, facemask, drug delivery, and vaccine technology against COVID-19. These nanomaterials are used widely for infiltration, diagnosis, and remedy of many infections. Recently, the biosensors with nanotechnology has been commercialized for COVID-19 detection. However, using the nanomaterials are compatible with a sustainable built environment, the toxicity of these types of nanomaterials in vivo is an important challenge. However, examining the toxicity of these substances in vivo is still a big problem in this direction, and using standard amounts of nanomaterials with the least toxicity and the most antiviral properties is a big challenge that needs more research.
The last but not least is that the nanoparticle ingredients have an important role in the mRNA- and DNA-based vaccines. The current huge phase 4 clinical test has commenced a theory how to overcome the clinical problem with nanomedicine. Then, the phase 4 clinical test solved the unknown risk of lipid nanoparticles in vaccine technology against COVID-19. Since nanomedicine has a significant role in COVID-19 vaccine solutions, translational nanomedicine has finally improved compared with earliest stages, and the future turns of events and applications are guaranteed to be surprising. Therefore, collecting informative data and analyzing side effects of each COVID-19 vaccine, will appear a brilliant path in future for progress of more complex and non-lipid nanomedicines. Although complete eradication of this virus requires much more extensive research, the use of antiviral nanomaterials is an effective and important step in reducing the prevalence of Covid-19. Therefore, based on scientific findings, antiviral nanomaterials should be used in medical instruments, fabrics reinforced with nanotechnology, facemasks and etc. Also, nanomaterial-based disinfectants have faster and higher inactivation properties, which are very effective in increasing safety in health centers and public places. Due to the extremely useful properties of nanomaterials in the elimination of viruses, this substance can be used in the preparation of diagnostic kits, vaccines, and also in treatment. Consequently, the use of nanomaterials in the biomedical application is an effective path to control this viral pandemic outbreak at local, national, and international levels.
Author contributions
Ferial Ghaemi designed the paradigm, collected the data and wrote the first draft and revised draft of the manuscript. Nor Yuliana Yuhana contributed to the specific sections. Mohd Yazid Bajuri contributed to prepare the sections related to the vaccines parts and medical progresses against COVID-19. Amirhassan Amiri and Massimiliano Ferrara supervised and directed the overall project.
Declaration of Competing Interest
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
References
- Aasi A., Aghaei S.M., Moore M.D., Panchapakesan B. Pt-, Rh-, Ru-z and Cu-single-wall carbon nanotubes are exceptional candidates for design of anti-viral surfaces: A theoretical study. International Journal of Molecular Sciences. 2020;21(15):5211. doi: 10.3390/ijms21155211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd Manan F.A., Hong W.W., Abdullah J., Yusof N.A., Ahmad I. Nanocrystalline cellulose decorated quantum dots based tyrosinase biosensor for phenol determination. Materials Science and Engineering: C. 2019;99:37–46. doi: 10.1016/j.msec.2019.01.082. [DOI] [PubMed] [Google Scholar]
- Abo‐zeid Y., Williams G.R. The potential anti‐infective applications of metal oxide nanoparticles: A systematic review. Wiley Interdisciplinary Reviews Nanomedicine and Nanobiotechnology. 2020;12(2):e1592. doi: 10.1002/wnan.1592. [DOI] [PubMed] [Google Scholar]
- Abo-Zeid Y., Ismail N.S., McLean G.R., Hamdy N.M. A molecular docking study repurposes FDA approved Iron oxide nanoparticles to treat and control COVID-19 infection. European Journal of Pharmaceutical Sciences. 2020;153 doi: 10.1016/j.ejps.2020.105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D., Gonzalez-Duarte A., O’Riordan W.D., Yang C.C., Ueda M., Kristen A.V., et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. The New England Journal of Medicine. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- Ahmad S., Zamry A.A., Tan H.T., Wong K.K., Lim J., Mohamud R. Targeting dendritic cells through gold nanoparticles: A review on the cellular uptake and subsequent immunological properties. Molecular Immunology. 2017;91:123–133. doi: 10.1016/j.molimm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Ahmed S.R., Kim J., Suzuki T., Lee J., Park E.Y. Enhanced catalytic activity of gold nanoparticle-carbon nanotube hybrids for influenza virus detection. Biosensors & Bioelectronics. 2016;85:503–508. doi: 10.1016/j.bios.2016.05.050. [DOI] [PubMed] [Google Scholar]
- Ahmed S.R., Kang S.W., Oh S., Lee J., Neethirajan S. Chiral zirconium quantum dots: A new class of nanocrystals for optical detection of coronavirus. Heliyon. 2018;4(8) doi: 10.1016/j.heliyon.2018.e00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhavan O., Choobtashani M., Ghaderi E. Protein degradation and RNA efflux of viruses photocatalyzed by graphene–tungsten oxide composite under visible light irradiation. The Journal of Physical Chemistry C. 2012;116(17):9653–9659. [Google Scholar]
- Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- Al-Gaashani R., Radiman S., Tabet N., Daud A.R. Synthesis and optical properties of CuO nanostructures obtained via a novel thermal decomposition method. Journal of Alloys and Compounds. 2011;509(35):8761–8769. [Google Scholar]
- Alvarez P.J.J. Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behavior. Environmental Science & Technology. 2007;41(7):2636–2642. doi: 10.1021/es062181w. [DOI] [PubMed] [Google Scholar]
- Amjad M.W., Kesharwani P., Amin M.C.I.M., Iyer A.K. Recent advances in the design, development, and targeting mechanisms of polymeric micelles for delivery of siRNA in cancer therapy. Progress in Polymer Science. 2017;64:154–181. [Google Scholar]
- Anasori B., Lukatskaya M.R., Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nature Reviews Materials. 2017;2:16098. [Google Scholar]
- Ann L.C., Mahmud S., Bakhori S.K.M., Sirelkhatim A., Mohamad D., Hasan H., et al. Antibacterial responses of zinc oxide structures against Staphylococcus aureus, Pseudomonas aeruginosa and Streptococcus pyogenes. Ceramics International. 2014;40(2):2993–3001. [Google Scholar]
- Arias L.S., Pessan J.P., Vieira A.P.M., Lima T.M.T.D., Delbem A.C.B., ;Monteiro D.R. Iron oxide nanoparticles for biomedical applications: a perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7:46. doi: 10.3390/antibiotics7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytur T., Foley J., Anwar M., Boser B., Harris E., Beatty P.R. A novel magnetic bead bioassay platform using a microchip-based sensor for infectious disease diagnosis. Journal of Immunological Methods. 2006;314:21–29. doi: 10.1016/j.jim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Baglivo M., Baronio M., Natalini G., Beccari T., Chiurazzi P., Fulcheri E., et al. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: a possible strategy for reducing SARSCOV-2 infectivity? Acta Bio-Medica. 2020;91:161–164. doi: 10.23750/abm.v91i1.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks E.D., Taylor N.M., Gulley J., Lubbers B.R., Giarrizo J.G., Bullen H.A., et al. Bacterial calcium carbonate precipitation in cave environments: A function of calcium homeostasis. Geomicrobiology Journal. 2010;27:444–454. [Google Scholar]
- Baram-Pinto D., Shukla S., Perkas N., Gedanken A., Sarid R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjugate Chemistry. 2009;20:1497–1502. doi: 10.1021/bc900215b. [DOI] [PubMed] [Google Scholar]
- Bayda S., Hadla M., Palazzolo S., Riello P., Corona G., Toffoli G., et al. Inorganic nanoparticles for cancer therapy: A transition from lab to clinic. Current Medicinal Chemistry. 2018;25:4269–4303. doi: 10.2174/0929867325666171229141156. [DOI] [PubMed] [Google Scholar]
- Bayle L., Chimalapati S., Schoehn G., Brown J., Vernet T., Durmort C. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Molecular Microbiology. 2011;82(4):904–916. doi: 10.1111/j.1365-2958.2011.07862.x. [DOI] [PubMed] [Google Scholar]
- Behnajady M.A., Eskandarloo H. Silver and copper Co-impregnated onto TiO2-P25 nanoparticles and its photocatalytic activity. Chemical Engineering Journal. 2013;228:1207–1213. [Google Scholar]
- Berg K., Bolt G., Andersen H., Owen T.C. Zinc potentiates the antiviral action of human IFN-α tenfold. Journal of Interferon & Cytokine Research. 2001;21(7):471–474. doi: 10.1089/10799900152434330. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S., Joshi R., Chughtai A.A., Macintyre C.R. Graphene modified multifunctional personal protective clothing. Advanced Materials Interfaces. 2019;6 doi: 10.1002/admi.201900622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Agarwal B., Goswami M., Maiti D., Baruah S., Tribedi P. Zinc oxide nanoparticle inhibits the biofilm formation of Streptococcus pneumoniae. Antonie Van Leeuwenhoek. 2018;111(1):89–99. doi: 10.1007/s10482-017-0930-7. [DOI] [PubMed] [Google Scholar]
- Blatnik M., Culver D.C., Gabrovšek F., Knez M., Kogovšek B., Kogovšek J., Liu H., Mayaud C., Mihevc A., Mulec J., Năpăruş-Aljančič M. In: Karstology in the classical karst. Knez M., Otoničar B., Petrič M., Pipan T., Slabe T., editors. Springer; Switzerland: 2020. Significant findings from karst sediments research; pp. 93–113. [Google Scholar]
- Bogdan J., Zarzyńska J., Pławińska-Czarnak J. Comparison of infectious agents susceptibility to photocatalytic effects of nanosized titanium and zinc oxides: A practical approach. Nanoscale Research Letters. 2015;10:1023. doi: 10.1186/s11671-015-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles M.A., Ling D., Hyeon T., Talapin D.V. The surface science of nanocrystals. Nature Materials. 2016;15(2):141–153. doi: 10.1038/nmat4526. [DOI] [PubMed] [Google Scholar]
- Borkow G., Zhou S.S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PloS One. 2010;5 doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Estévez A.S., Kang S., Elimelech M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small. 2008;4:481–484. doi: 10.1002/smll.200700863. [DOI] [PubMed] [Google Scholar]
- Broglie J.J., Alston B., Yang C., Ma L., Adcock A.F., Chen W., et al. Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PloS One. 2015;10 doi: 10.1371/journal.pone.0141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L.R., Caulkins R.C., Schartel T.E., Rosch J.W., Honsa E.S., Schultz-Cherry S., et al. Increased zinc availability enhances initial aggregation and biofilm formation of Streptococcus pneumoniae. Frontiers in Cellular and Infection Microbiology. 2017;7:233. doi: 10.3389/fcimb.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J.A., Dunlop P.S., Hamilton J.W., Fernandez-Ibanez P., Polo-Lopez I., Sharma P.K., et al. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules. 2015;20:5574–5615. doi: 10.3390/molecules20045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagno V., Andreozzi P., D’Alicarnasso M., Silva P.J., Mueller M., Galloux M., et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nature Materials. 2018;17:195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- Cakman I., Kirchner H., Rink L. Zinc supplementation reconstitutes the production of interferon-α by leukocytes from elderly persons. Journal of Interferon & Cytokine Research. 1997;17(8):469–472. doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- Carter D.C., Wright B., Gray W., Rose J.P., Wilson E. A unique protein self-assembling nanoparticle with significant advantages in vaccine development and production. Journal of Nanomaterials. 2020;2020 [Google Scholar]
- Casbeer E., Sharma V.K., Li X.Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Separation and Purification Technology. 2012;87:1–14. [Google Scholar]
- Chauhan N., Maekawa T., Kumar D.N.S. Graphene based biosensors—Accelerating medical diagnostics to new-dimensions. Journal of Materials Research. 2017;32(15):2860–2882. [Google Scholar]
- Che Z., Sun J. Investigation on relationship between whole blood zinc and Fe elements with children pneumonia caused by respiratory syncytial virus. International Journal of Laboratory Medicine. 2016;37(17):2401–2402. [Google Scholar]
- Chen C., Boota M., Urbankowski P., Anasori B., Miao L., Jiang J., et al. Effect of Glycine functionalization of 2D titanium carbide (MXene) on charge storage. Journal of Materials Chemistry A. 2018;6:4617–4622. [Google Scholar]
- Chen Y.-N., Hsueh Y.-H., Hsieh C.-T., Tzou D.-Y., Chang P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. International Journal of Environmental Research and Public Health. 2016;13:430. doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.W., Huang C.Y., Lin S.Y., Fang Z.S., Hsu C.H., Lin J.C., et al. Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials. 2016;106:111–118. doi: 10.1016/j.biomaterials.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Han W., Wang G., Zhao X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. International Journal of Biological Macromolecules. 2020;164 doi: 10.1016/j.ijbiomac.2020.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilvers M.A., McKean M., Rutman A., Myint B.S., Silverman M., O’Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. The European Respiratory Journal. 2001;18(6):965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- Chintagunta A.D., Kumar M., Kumar N.S., Kumar S.J. Springer; Singapore: 2020. Diagnostic strategies for COVID-19 and other coronaviruses. Differential diagnosis and possible therapeutics for coronavirus disease 2019; pp. 51–71. [Google Scholar]
- Chintagunta A.D., Nalluru S., Sampath Kumar N.S. Nanotechnology: an emerging approach to combat COVID-19. Emergent Materials. 2021:1–12. doi: 10.1007/s42247-021-00178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W.-S., Duffin R., Poland C.A., Duschl A., Oostingh G.J., MacNee W., et al. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology. 2012;6:22–35. doi: 10.3109/17435390.2011.552810. [DOI] [PubMed] [Google Scholar]
- Cioffi N., Rai M. 1st ed. Springer; Berlin/Heidelberg, Germany: 2012. Nano-antimicrobials: Progress and prospects. [Google Scholar]
- Cioffi, N., Ditaranto, N., Sabbatini, L., Torsi, L., Zambonin, P.G., Nanomaterials for Controlled Metal Release and Process for Their Production. European Patent Application EP 2123797 B1, 25 November 2009.
- Cioffi, N., Ditaranto, N., Sabbatini, L., Tantillo, G., Torsi, L., Zambonin, P.G., Bioactive Metal Nanomaterial Stabilized by Bioactive Agents and Preparation Process. European Patent Application EP 2157211 B1, 2 September 2015.
- Cntivirus-built environment: Lessons learned flinical Stage Pipeline – Novavax – Creating Tomorrow’s Vaccines Today. Novavax.com. https://novavax.com/ourpipeline#nvx-cov2373 (Accessed 14 August 2020).
- Dabbagh-Bazarbachi H., Clergeaud G., Quesada I.M., Ortiz M., O’Sullivan C.K., Fernández-Larrea J.B. Zinc ionophore activity of quercetin and epigallocatechin-gallate: From Hepa 1-6 cells to a liposome model. Journal of Agricultural and Food Chemistry. 2014;62(32):8085–8093. doi: 10.1021/jf5014633. [DOI] [PubMed] [Google Scholar]
- Darma A., Ranuh R.G., Merbawani W., Setyoningrum R.A., Hidajat B., Hidayati S.N., et al. Zinc supplementation effect on the bronchial cilia length, the number of cilia, and the number of intact bronchial cell in zinc deficiency rats. The Indonesian Biomedical Journal. 2020;12(1):78–84. [Google Scholar]
- De Gusseme B., Sintubin L., Baert L., Thibo E., Hennebel T., Vermeulen G., et al. Biogenic silver for disinfection of water contaminated with viruses. Applied and Environmental Microbiology. 2010;76:1082–1087. doi: 10.1128/AEM.02433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nature Reviews Microbiology. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deysher G., Shuck C.E., Hantanasirisakul K., Frey N.C., Foucher A.C., Maleski K., et al. Synthesis of Mo4VAlC4MAX phase and two-dimensional Mo4VC4MXene with 5 atomic layers of transition metals. ACS Nano. 2020;14:204–217. doi: 10.1021/acsnano.9b07708. [DOI] [PubMed] [Google Scholar]
- Di Gianvincenzo P., Marradi M., Martínez-Ávila O.M., Bedoya L.M., Alcamí J., Penadés S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorganic & Medicinal Chemistry Letters. 2010;20:2718–2721. doi: 10.1016/j.bmcl.2010.03.079. [DOI] [PubMed] [Google Scholar]
- Dolati S., Sadreddini S., Rostamzadeh D., Ahmadi M., Jadidi-Niaragh F., Yousefi M. Utilization of nanoparticletechnology in rheumatoid arthritis treatment. Biomedicine & Pharmacotherapy. 2016;80:30–41. doi: 10.1016/j.biopha.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Dong X., Moyer M.M., Yang F., Sun Y.P., Yang L. Carbon dots’ antiviral functions against noroviruses. Scientific Reports. 2017;7(1):519. doi: 10.1038/s41598-017-00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P.K., Hyle E.P., Noubary F., Freedberg K.A., Wilson D., Bishai W.R., et al. Diagnostic point-of-care tests in resource-limited settings. The Lancet Infectious Diseases. 2014;14(3):239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T., Liang J., Dong N., Lu J., Fu Y., Fang L., et al. Glutathione-capped Ag(2)S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Applied Materials & Interfaces. 2018;10:4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- Dunnill C.W., Parkin I.P. Nitrogen-doped TiO2 thin films: Photocatalytic applications for healthcare environments. Dalton Transactions. 2011;40:1635–1640. doi: 10.1039/c0dt00494d. [DOI] [PubMed] [Google Scholar]
- Dykman L.A., Khlebtsov N.G. Immunological properties of gold nanoparticles. Chemical Science. 2017;8:1719–1735. doi: 10.1039/c6sc03631g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeas M., Saleh J., Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? International Journal of Infectious Diseases. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., et al. Interaction of silver nanoparticles with HIV-1. Journal of Nanobiotechnology. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., et al. Interaction of silver nanoparticles with HIV-1. Journal of Nanobiotechnology. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essaidi-Laziosi M., Brito F., Benaoudia S., Royston L., Cagno V., Fernandes-Rocha M., et al. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. The Journal of Allergy and Clinical Immunology. 2018;141(6):2074–2084. doi: 10.1016/j.jaci.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel B., Bussy C., Merino S., Vázquez E., Flahaut E., Mouchet F., et al. Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano. 2018;12(11):10582–10620. doi: 10.1021/acsnano.8b04758. [DOI] [PubMed] [Google Scholar]
- Ferrari A.C., Bonaccorso F., Fal’Ko V., Novoselov K.S., Roche S., Bøggild P., et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale. 2015;7(11):4598–4810. doi: 10.1039/c4nr01600a. [DOI] [PubMed] [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 NCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori Y., Sato T., Hayata T., Nagao T., Nakayama M., Nakayama T., et al. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Applied and Environmental Microbiology. 2012;78:951. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacem M.A., Gacem H., Ould-El-Hadj-Khelil A. Elsevier Inc; Amsterdam: 2020. Nanocarbons: Antibacterial, antifungal, and antiviral activity and the underlying mechanism. Carbon nanomaterials for agri-food and environmental applications. [Google Scholar]
- Gammoh N.Z., Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Huo W., Zhang L., Lian J., Tao W., Song C., et al. Multiplex measurement of twelve tumor markers using a GMR multi-biomarker immunoassay biosensor. Biosensors & Bioelectronics. 2019;123:204–210. doi: 10.1016/j.bios.2018.08.060. [DOI] [PubMed] [Google Scholar]
- Gao A., Liang H., Shen Q., Zhou C., Chen X.M., Tian J.…Cui D. Designing a novel nano-vaccine against SARS-CoV-2. Nano Biomedicine and Engineering. 2020;12(4):321–324. [Google Scholar]
- Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Applied and Environmental Microbiology. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastalegname M., Vallone A. Could chloroquine/hydroxychloroquine be harmful in Coronavirus disease 2019 (COVID‑19) treatment? Clinical Infectious Diseases. 2020;71:888–889. doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gude V.G., Muire P.J. Sustainable Cities and Society. 2020;64 doi: 10.1016/j.scs.2020.102558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzel D., Yu A.S. Claudins and the modulation of tight junction permeability. Physiological Reviews. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez L., Li X., Wang J., Nangmenyi G., Economy J., Kuhlenschmidt T.B., et al. Adsorption of rotavirus and bacteriophage MS2 using glass fiber coated with hematite nanoparticles. Water Research. 2009;43(20):5198–5208. doi: 10.1016/j.watres.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Haase H., Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6:1175–1180. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- Halim A.S., Nor F.M., Saad A.Z.M., Nasir N.A.M., Norsa’adah B., Ujang Z. Efficacy of chitosan derivative films versus hydrocolloid dressing on superficial wounds. Journal of Taibah University Medical Sciences. 2018;13(6):512–520. doi: 10.1016/j.jtumed.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W., Zhang P.-H., Cao W.-C., Yang D.L., Taira S., Kamoto Y., et al. The inactivation effect of photocatalytic titanium apatie filter on SARS virus. Progress in Biochemistry and Biophysics. 2004;31:982–985. [Google Scholar]
- Han, Chen L., Duan S.M., Yang Q.X., Yang M., Gao C., et al. Efficient and quick inactivation of SARS coronavirus and other microbes exposed to the surfaces of some metal catalysts. Biomedical and Environmental Sciences. 2005;18:176–180. [PubMed] [Google Scholar]
- Hang X., Peng H., Song H., Qi Z., Miao X., Xu W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. Journal of Virological Methods. 2015;222:150–157. doi: 10.1016/j.jviromet.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Harris V.G., Geiler A., Chen Y., Yoon S.D., Wu M., Yang A., et al. Recent advances in processing and applications of microwave ferrites. Journal of Magnetism and Magnetic Materials. 2009;321:2035–2047. [Google Scholar]
- Hash S., Martinez-Viedma M.P., Fung F., Han J.E., Yang P., Wong C., et al. Nuclear magnetic resonance biosensor for rapid detection of Vibrio parahaemolyticus. Biomedical Journal. 2019;42:187–192. doi: 10.1016/j.bj.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H. Zinc lozenges may shorten the duration of colds: A systematic review. The Open Respiratory Medicine Journal. 2011;5:51–58. doi: 10.2174/1874306401105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H. Zinc lozenges and the common cold: A meta‑analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 2017;8 doi: 10.1177/2054270417694291. 2054270417694291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Krüger N., Mueller M.A., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019‑nCoV) uses the SARS‑coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020 [Google Scholar]
- Huang K., Li Z., Lin J., Han G., Huang P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chemical Society Reviews. 2018;47:5109–5124. doi: 10.1039/c7cs00838d. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannazzo D., Pistone A., Galvagno S., Ferro S., De Luca L., Monforte A.M., et al. Synthesis and anti-HIV activity of carboxylated and drug-conjugated multi-walled carbon nanotubes. Carbon. 2015;82:548–561. [Google Scholar]
- Iranpour P., Ajamian M., Safavi A., Iranpoor N., Abbaspour A., Javanmardi S. Synthesis of highly stable and biocompatible gold nanoparticles for use as a new X-ray contrast agent. Journal of Materials Science Materials in Medicine. 2018;29:48. doi: 10.1007/s10856-018-6053-5. [DOI] [PubMed] [Google Scholar]
- Ishida T. Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell‑virus growth inhibition. American Journal of Biomedical Science and Research. 2019:2. [Google Scholar]
- Iwata-Yoshikawa N., Uda A., Suzuki T., Tsunetsugu-Yokota Y., Sato Y., Morikawa S., et al. Effects of Toll‐like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV‐inactivated severe acute respiratory syndrome‐related coronavirus vaccine. Journal of Virology. 2014;88:8597–8614. doi: 10.1128/JVI.00983-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An MRNA Vaccine against SARS-CoV-2 - Preliminary Report. The New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2022483. 0, null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe Y.H., Park D.H., Hwang J. Evaluation of Ag nanoparticle coated air filter against aerosolized virus: Anti-viral efficiency with dust loading. Journal of Hazardous Materials. 2016;301:547–553. doi: 10.1016/j.jhazmat.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S., Yuzhakov O., Woods A., Deterling J., Hassett K., Shaw C.A., et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine. 2018;36:1689–1699. doi: 10.1016/j.vaccine.2018.01.029. [DOI] [PubMed] [Google Scholar]
- Johnson J.K., Harris F.L., Ping X.D., Gauthier T.W., Brown L.A.S. Role of zinc insufficiency in fetal alveolar macrophage dysfunction and RSV exacerbation associated with fetal ethanol exposure. Alcohol. 2019;80:5–16. doi: 10.1016/j.alcohol.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.C., Johnson P.A., Witiw R., Mardon A.A. Design and development of reusable facial deactivation masks for COVID-19. Pacific Journal of Science and Technology. 2020;21:13–15. [Google Scholar]
- Jumeri F.A., Lim H.N., Ariffin S.N., Huang N.M., Teo P.S., Fatin S.O., et al. Microwave synthesis of magnetically separable ZnFe2O4-reduced graphene oxide for wastewater treatment. Ceramics International. 2014;40(5):7057–7065. [Google Scholar]
- Kadiyala U., Turali-Emre E.S., Bahng J.H., Kotov N.A., VanEpps J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA) Nanoscale. 2018;10(10):4927–4939. doi: 10.1039/c7nr08499d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P., Zuin S., Wick P. Is nanotechnology revolutionizing the paint and lacquer industry? A critical opinion. The Science of the Total Environment. 2013;442:282–289. doi: 10.1016/j.scitotenv.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Kamaly N., Xiao Z., Valencia P.M., Radovic-Moreno A.F., Farokhzad O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chemical Society Reviews. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.L., Cho C.S., Yoo H.S. Application of chitosan microspheres for nasal delivery of vaccines. Biotechnology Advances. 2009;27(6):857–865. doi: 10.1016/j.biotechadv.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Kaynar A.M., Andreas A., Maloy A., Austin W., Pitt B.R., Gopal R., et al. Zinc deficiency worsens the long-term outcome and exacerbates inflammation in a murine model of influenza-MRSA superinfection. American Journal of Respiratory and Critical Care Medicine. 2019;199:A4130. [Google Scholar]
- Kerry R.G., Malik S., Redda Y.T., Sahoo S., Patra J.K., Majhi S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomedicine: Nanotechnology, Biology and Medicine. 2019;18:196–220. doi: 10.1016/j.nano.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater M., Escosura-Muñiz A.D., Altet L., Merkoçi A. In situ plant virus nucleic acid isothermal amplification detection on gold nanoparticle-modified electrodes. Analytical Chemistry. 2019;91:4790–4796. doi: 10.1021/acs.analchem.9b00340. [DOI] [PubMed] [Google Scholar]
- Khodashenas B., Ghorbani H.R. Synthesis of copper nanoparticles: An overview of the various methods. The Korean Journal of Chemical Engineering. 2014;31:1105–1109. [Google Scholar]
- Kim J.S., Sung J.H., Song K.S., Lee J.H., Kim S.M., Lee G.H., et al. Persistent DNA damage measured by comet assay of Sprague Dawley rat lung cells after five days of inhalation exposure and 1 month post-exposure to dispersed multi-wall carbon nanotubes (MWCNTs) generated by new MWCNT aerosol generation system. Toxicological Sciences. 2012;128:439–448. doi: 10.1093/toxsci/kfs161. [DOI] [PubMed] [Google Scholar]
- Klein T., Wang W., Yu L., Wu K., Boylan K.L., Vogel R.I., et al. Development of a multiplexed giant magnetoresistive biosensor array prototype to quantify ovarian cancer biomarkers. Biosensors & Bioelectronics. 2019;126:301–307. doi: 10.1016/j.bios.2018.10.046. [DOI] [PubMed] [Google Scholar]
- Korant B.D., Kauer J.C., Butterworth B.E. Zinc ions inhibit replication of rhinoviruses. Nature. 1974;248(5449):588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- Krähling V., Stein D.A., Spiegel M., Weber F., Mühlberger E. Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase r but is resistant to its antiviral activity. Journal of Virology. 2009;83:2298–2309. doi: 10.1128/JVI.01245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn B.M., Gaudernak E., Holzer B., Lanke K., Van Kuppeveld F.J.M., Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. Journal of Virology. 2009;83(1):58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzowska M., Tomaszewska E., Ranoszek-Soliwoda K., Bien K., Orlowski P., Celichowski G., Grobelny J. In: Nanostructures for oral medicine. Andronescu E., Grumezescu A.M., editors. Elsevier; Amsterdam, The Netherlands: 2017. Chapter 12—Tannic acid modification of metal nanoparticles: Possibility for new antiviral applications; pp. 335–363. ISBN 978-0-323-47720-47728. [Google Scholar]
- Kumar R., Nayak M., Sahoo G.C., Pandey K., Sarkar M.C., Ansari Y., et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. Journal of Infection and Chemotherapy. 2019;25(5):325–329. doi: 10.1016/j.jiac.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Kumar N.S.S., Chintagunta A.D., Kumar S.J., Roy S., Kumar M. Immunotherapeutics for Covid-19 and post vaccination surveillance. 3 Biotech. 2020;10(12):1–11. doi: 10.1007/s13205-020-02522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre A., Talbot P.J. Effect of pH and temperature on the infectivity of human coronavirus 229E. Canadian Journal of Microbiology. 1989;35:972–974. doi: 10.1139/m89-160. [DOI] [PubMed] [Google Scholar]
- Lee B.-Y., Behler K., Kurtoglu M.E., Wynosky-Dolfi M.A., Rest R.F., Gogotsi Y. Titanium dioxide-coated nanofibers for advanced filters. Journal of Nanoparticle Research. 2010;12:2511–2519. [Google Scholar]
- Lee D., Chander Y., Goyal S.M., Cui T. Carbon nanotube electric immunoassay for the detection of swine influenza virus H1N1. Biosensors & Bioelectronics. 2011;26:3482–3487. doi: 10.1016/j.bios.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-T., Ko E.-J., Hwang H.S., Lee J.S., Kim K.-H., Kwon Y.-M., et al. Respiratory syncytial virus-like nanoparticle vaccination induces long-term protection without pulmonary disease by modulating cytokines and T-cells partially through alveolar macrophages. International Journal of Nanomedicine. 2015;10:4491–4505. doi: 10.2147/IJN.S83493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Li J., Feng X., Li J., Hao Y., Zhang J., et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nature Communications. 2019;10(1):1–10. doi: 10.1038/s41467-019-10218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liga M.V., Bryant E.L., Colvin V.L., Li Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Research. 2011;45:535–544. doi: 10.1016/j.watres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Liga M.V., Maguire-Boyle S.J., Jafry H.R., Barron A.R., Li Q. Silica decorated TiO2 for virus inactivation in drinking water simple synthesis method and mechanisms of enhanced inactivation kinetics. Environmental Science & Technology. 2013;47:6463–6470. doi: 10.1021/es400196p. [DOI] [PubMed] [Google Scholar]
- Lin F.C., Young H.A. Interferons: success in anti-viral immunotherapy. Cytokine & Growth Factor Reviews. 2014;25(4):369–376. doi: 10.1016/j.cytogfr.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Lu Q., Ge S., Cai Q., Grimes C.A. Detection of pathogen Escherichia coli O157: H7 with a wireless magnetoelastic-sensing device amplified by using chitosan-modified magnetic Fe3O4 nanoparticles. Sensors and Actuators B, Chemical. 2010;147:343–349. [Google Scholar]
- Lin C.D., Kou Y.Y., Liao C.Y., Li C.H., Huang S.P., Cheng Y.W., et al. Zinc oxide nanoparticles impair bacterial clearance by macrophages. Nanomedicine. 2014;9(9):1327–1339. doi: 10.2217/nnm.14.48. [DOI] [PubMed] [Google Scholar]
- Lin H., Gao S., Dai C., Chen Y., Shi J. Two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II bio-windows. Journal of the American Chemical Society. 2017;139:16235–16247. doi: 10.1021/jacs.7b07818. [DOI] [PubMed] [Google Scholar]
- Lin M.H., Moses D.C., Hsieh C.H., Cheng S.C., Chen Y.H., Sun C.Y., et al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antiviral Research. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Bao Y.W., Wu F.G. Carbon dots for sensing and killing microorganisms. C—Journal of Carbon Research. 2019;5(2):33. [Google Scholar]
- Liu Z., Winters M., Holodniy M., Dai H. siRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angewandte Chemie International Edition. 2007;46:2023–2027. doi: 10.1002/anie.200604295. [DOI] [PubMed] [Google Scholar]
- Liu C., Hajagos T.J., Chen D., Chen Y., Kishpaugh D., Pei Q. Efficient one-pot synthesis of colloidal zirconium oxide nanoparticles for high-refractive-index nanocomposites. ACS Applied Materials & Interfaces. 2016;8(7):4795–4802. doi: 10.1021/acsami.6b00743. [DOI] [PubMed] [Google Scholar]
- Liu G., Nie J., Han C., Jiang T., Yang Z., Pang Y., et al. Self-powered electrostatic adsorption face mask based on a triboelectric nanogenerator. ACS Applied Materials & Interfaces. 2018;10:7126–7133. doi: 10.1021/acsami.7b18732. [DOI] [PubMed] [Google Scholar]
- Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‑CoV, MERS‑CoV, and 2019‑nCoV. Journal of Medical Virology. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łoczechin A., Séron K., Barras A., Giovanelli E., Belouzard S., Chen Y.T., et al. Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Applied Materials & Interfaces. 2019;11:42964–42974. doi: 10.1021/acsami.9b15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J., Zhang L., Tong T., Shen B., Yu J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. Journal of Catalysis. 2018;361:255–266. [Google Scholar]
- Lu L., Sun R.W., Chen R., Hui C.K., Ho C.M., Luk J.M., et al. Silver nanoparticles inhibit hepatitis B virus replication. Antiviral Therapy. 2008;13:253–262. [PubMed] [Google Scholar]
- Luiszer F.G. Vol. 15. 2009. pp. 119–132. (Speleogenesis of cave of the winds, manitou springs, Colorado. Select field guides to cave and karst lands of the United States). [Google Scholar]
- Lunnoo T., Assawakhajornsak J., Puangmali T. In silico study of gold nanoparticle uptake into a mammalian cell: Interplay of size, shape, surface charge, and aggregation. The Journal of Physical Chemistry C. 2019;123:3801–3810. [Google Scholar]
- Maeda K., Domen K. New non-oxide photocatalysts designed for overall water splitting under visible light. The Journal of Physical Chemistry C. 2007;111:7851–7861. [Google Scholar]
- Mahesh G. Vol. 57. 2020. pp. 28–29. (Paper-based diagnostics for COVID-19 developed by CSIR-IGIB). [Google Scholar]
- Maltez-da Costa M., de la Escosura-Muñiz A., Nogués C., Barrios L., Ibáñez E., Merkoçi A. Simple monitoring of cancer cells using nanoparticles. Nano Letters. 2012;12:4164–4171. doi: 10.1021/nl301726g. [DOI] [PubMed] [Google Scholar]
- Mao H.Q., Roy K., Troung-Le V.L., Janes K.A., Lin K.Y., Wang Y., et al. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. Journal of Controlled Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- Maret W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics. 2015;7:202–211. doi: 10.1039/c4mt00230j. [DOI] [PubMed] [Google Scholar]
- Marques Neto L.M., Kipnis A., Junqueira-Kipnis A.P. Role of metallic nanoparticles in vaccinology: Implications for infectious disease vaccine development. Frontiers in Immunology. 2017;8:239. doi: 10.3389/fimmu.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywald M., Wessels I., Rink L. Zinc signals and immunity. International Journal of Molecular Sciences. 2017;18:2222. doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride R., van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6(8):2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill COVID19 Vaccine Tracker Team. https://covid19.trackvaccines.org/vaccines/.
- Megahed N.A., Ghoneim E.M. Sustainable Cities and Society. 2020;61 doi: 10.1016/j.scs.2020.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megahed N.A., Ghoneim E.M. Antivirus-built environment: Lessons learned from Covid-19 pandemic. Sustainable Cities and Society. 2020;61 doi: 10.1016/j.scs.2020.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Seredych M., Chen C., Gura V., Mikhalovsky S., Sandeman S., et al. MXene sorbents for removal of urea from dialysate: A step toward the wearable artificial kidney. ACS Nano. 2018;12:10518–10528. doi: 10.1021/acsnano.8b06494. [DOI] [PubMed] [Google Scholar]
- Metreveli G., Wågberg L., Emmoth E., Belák S., Strømme M., Mihranyan A. A size‐exclusion nanocellulose filter paper for virus removal. Adv. Healthcare Mater. 2014;3(10):1546–1550. doi: 10.1002/adhm.201300641. [DOI] [PubMed] [Google Scholar]
- Milane L., Amiji M. Clinical approval of nanotechnology-based SARS-CoV-2 mRNA vaccines: impact on translational nanomedicine. Drug Delivery and Translational Research. 2021:1–7. doi: 10.1007/s13346-021-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri M., Behnam B., Sahebkar A. Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. Journal of Cellular Physiology. 2019;234:298–319. doi: 10.1002/jcp.26899. [DOI] [PubMed] [Google Scholar]
- Moore R.S., Taylor D.H., Sturman L.S., Reddy M.M., Fuhs G.W. Poliovirus adsorption by 34 minerals and soils. Applied and Environmental Microbiology. 1981;42:963–975. doi: 10.1128/aem.42.6.963-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudshinge S.R., Deore A.B., Patil S., Bhalgat C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharmaceutical Journal. 2011;19(3):29–141. doi: 10.1016/j.jsps.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A. Flooded by the torrent: The COVID-19 drug pipeline. Lancet. 2020;395:1245–1246. doi: 10.1016/S0140-6736(20)30894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.P., Laband S.J. Degradation of poliovirus by adsorption on inorganic surfaces. Applied and Environmental Microbiology. 1979;37:480–486. doi: 10.1128/aem.37.3.480-486.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan K., Wei J., Alsalhi M.S., Nicoletti M., Paulpandi M., Samidoss C.M., et al. Magnetic nanoparticles are highly toxic to chloroquine-resistant Plasmodium falciparum, dengue virus (DEN-2), and their mosquito vectors. Parasitology Research. 2017;116(2):495–502. doi: 10.1007/s00436-016-5310-0. [DOI] [PubMed] [Google Scholar]
- Naicker S., Yang C.-W., Hwang S.-J., Liu B.-C., Chen J.-H., Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney International. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah G., Al-Asmakh M., Rasool K., Mahmoud K.A. Ecotoxicological assessment of Ti3C2Tx (MXene) using zebrafish embryo model. Environmental Science Nano. 2018;5:1002–1011. [Google Scholar]
- Nasrollahzadeh M., Sajjadi M., Soufi G.J., Iravani S., Varma R.S. Nanomaterials and nanotechnology-associated innovations against viral infections with a focus on coronaviruses. Nanomaterials. 2020;10(6):1072. doi: 10.3390/nano10061072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman B.W., Kiss G., Kunding A.H., David B., Fazil Baksh M., Connelly S., et al. A structural analysis of M protein in coronavirus assembly and morphology. Journal of Structural Biology. 2011;174(1):11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.-H., Nguyen B.-S., Hu C., Nguyen C.C., Nguyen D.L.T., Nguyen Dinh M.T., et al. Novel architecture titanium carbide (Ti3C2Tx) MXene cocatalysts toward photocatalytic hydrogen production: A mini-review. Nanomaterials. 2020;10:602. doi: 10.3390/nano10040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K., Matsunaga T., Suzuki T., Kobayashi S., Yamaguchi H., Orba Y., et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- Nikaeen G., Abbaszadeh S., Yousefinejad S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine. 2020;15:15. doi: 10.2217/nnm-2020-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T., Matsuda I., Hirao K. All-cellulose composite. Macromolecules. 2004;37:7683–7687. [Google Scholar]
- Novavax Announces Positive Phase 1 Data for Its COVID-19 Vaccine Candidate, Novavax Inc. IR Site. https://ir.novavax.com/news-releases/news-release-details/novavax-announces-positivephase-1-data-its-covid-19-vaccine (Accessed 06 August 2020).
- Orlowski P., Tomaszewska E., Gniadek M., Baska P., Nowakowska J., Sokolowska J., et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PloS One. 2014;9 doi: 10.1371/journal.pone.0104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palestino G., García-Silva I., González-Ortega O., Rosales-Mendoza S. Can nanotechnology help in the fight against COVID-19? Expert Review of Anti-Infective Therapy. 2020;24:1–16. doi: 10.1080/14787210.2020.1776115. [DOI] [PubMed] [Google Scholar]
- Palmieri V., Papi M. Can graphene take part in the fight against COVID 19? Nano Today. 2020;7 doi: 10.1016/j.nantod.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R.P., Rasool K., Madhavan V.E., Aissa B., Gogotsi Y., Mahmoud K.A. Ultrahigh-flux and fouling-resistant membrane based on layered Silver/MXene(Ti3C2Tx) nanosheets. Journal of Materials Chemistry A. 2018;6:3522–3533. [Google Scholar]
- Papp I., Sieben C., Ludwig K., Roskamp M., Böttcher C., Schlecht S., et al. Inhibition of influenza virus infection by multivalent sialic-acid-Functionalized gold nanoparticles. Small. 2010;6:2900–2906. doi: 10.1002/smll.201001349. [DOI] [PubMed] [Google Scholar]
- Park S., Ko Y.S., Jung H., Lee C., Woo K., Ko G. Disinfection of waterborne viruses using silver nanoparticle-decorated silica hybrid composites in water environments. The Science of the Total Environment. 2018;625:477–485. doi: 10.1016/j.scitotenv.2017.12.318. [DOI] [PubMed] [Google Scholar]
- Pasquet J., Chevalier Y., Pelletier J., Couval E., Bouvier D., Bolzinger M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids and Surfaces A, Physicochemical and Engineering Aspects. 2014;457:263–274. [Google Scholar]
- Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- Perreault F., de Faria A.F., Nejati S., Elimelech M. Antimicrobial properties of graphene oxide nanosheets: Why size matters. ACS Nano. 2015;9:7226–7236. doi: 10.1021/acsnano.5b02067. [DOI] [PubMed] [Google Scholar]
- Pillai A.M., Sivasankarapillai V.S., Rahdar A., Joseph J., Sadeghfar F., Anuf A.R., et al. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. Journal of Molecular Structure. 2020;1211 [Google Scholar]
- Pittet L.A., Hall-Stoodley L., Rutkowski M.R., Harmsen A.G. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. American Journal of Respiratory Cell and Molecular Biology. 2010;42(4):450–460. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio C., Colombo M., Arciola C.R., Greggi T., Scribante A., Dagna A. Copper-alloy surfaces and cleaning regimens against the spread of SARS-CoV-2 in dentistry and orthopedics. From fomites to anti-infective nanocoatings. Materials. 2020;13(15):3244. doi: 10.3390/ma13153244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polshettiwar V., Luque R., Fihri A., Zhu H., Bouhrara M., Basset J.M. Magnetically recoverable nanocatalysts. Chemical Reviews. 2011;111:3036–3075. doi: 10.1021/cr100230z. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. In: Molecular, genetic, and nutritional aspects of Major and trace minerals. Collins J.F., editor. Academic Press; Cambridge: 2017. Discovery of zinc for human health and biomarkers of zinc deficiency; pp. 241–260. [Google Scholar]
- Puigdollers A.R., Illasand F., Pacchioni G. Structure and properties of zirconia nanoparticles from density functional theory calculations. The Journal of Physical Chemistry C. 2016;120:4392–4402. [Google Scholar]
- Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- Raghuwanshi D., Mishra V., Das D., Kaur K., Suresh M.R. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Molecular Pharmaceutics. 2012;9(4):946–956. doi: 10.1021/mp200553x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuwanshi D., Mishra V., Das D., Kaur K., Suresh M.R. Dendritic cells targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Molecular Pharmaceutics. 2012;9:946–956. doi: 10.1021/mp200553x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani A.M., Hosseini Mirmahaleh S.Y. Coronavirus disease (COVID-19) prevention and treatment methods and effective parameters: A systematic literature review. Sustainable Cities and Society. 2020 doi: 10.1016/j.scs.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M., Deshmukh S.D., Ingle A.P., Gupta I.R., Galdiero M., Galdiero S. Metal nanoparticles: The protective nanoshield against virus infection. Critical Reviews in Microbiology. 2016;42:46–56. doi: 10.3109/1040841X.2013.879849. [DOI] [PubMed] [Google Scholar]
- Rao G., Brastad K.S., Zhang Q., Robinson R., He Z., Li Y. Enhanced disinfection of Escherichia coli and bacteriophage MS2 in water using a copper and silver loaded titanium dioxide nanowire membrane. Frontiers of Environmental Science & Engineering. 2016;10:11. [Google Scholar]
- Rasool K., Helal M., Ali A., Ren C.E., Gogotsi Y., Mahmoud K.A. Antibacterial activity of Ti3C2Tx MXene. ACS Nano. 2016;10:3674–3684. doi: 10.1021/acsnano.6b00181. [DOI] [PubMed] [Google Scholar]
- Rasool K., Mahmoud K.A., Johnson D.J., Helal M., Berdiyorov G.R., Gogotsi Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2Tx (MXene) Nanosheets. Scientific Reports. 2017;7:1598. doi: 10.1038/s41598-017-01714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Advances in Nutrition. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy L.S., Nisha M.M., Joice M., Shilpa P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharmaceutical Biology. 2014;52(11):1388–1397. doi: 10.3109/13880209.2014.893001. [DOI] [PubMed] [Google Scholar]
- Ren X., Li J., Chen C., Gao Y., Chen D., Su M., et al. Graphene analogues in aquatic environments and porous media: Dispersion, aggregation, deposition and transformation. Environmental Science Nano. 2018;5(6):1298–1340. [Google Scholar]
- Rettcher S., Jungk F., Kühn C., Krause H.J., Nölke G., Commandeur U., et al. Simple and portable magnetic immunoassay for rapid detection and sensitive quantification of plant viruses. Applied and Environmental Microbiology. 2015;81:3039–3048. doi: 10.1128/AEM.03667-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.V., Parkinson C.V., Choi Y.W., Speshock J.L., Hussain S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Research Letters. 2008;3:129–133. [Google Scholar]
- Roscioli E., Jersmann H.P., Lester S., Badiei A., Fon A., Zalewski P., et al. Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2017;12:3503–3510. doi: 10.2147/COPD.S149589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 TCell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Sahu D., Kannan G.M., Vijayaraghavan R., Anand T., Khanum F. Nanosized zinc oxide induces toxicity in human lung cells. International Scholarly Research Notices. 2013 doi: 10.1155/2013/316075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez J.A., Gonzalez-Ortega O., Rosales-Mendoza S. Gold nanoparticles and vaccine development. Expert Review of Vaccines. 2015;14:1197–1211. doi: 10.1586/14760584.2015.1064772. [DOI] [PubMed] [Google Scholar]
- Sargsyan K., Chen T., Grauffel C., Lim C. 2020. Identifying COVID-19 drug-sites susceptible to clinically safe Zn-ejector drugs using evolutionary/physical principles. OSF Preprints. [Google Scholar]
- Sayes C.M., Gobin A.M., Ausman K.D., Mendez J., West J.L., Colvin V.L. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26(36):7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Schmitt V., Kesch C., Jackson J.K., Bidnur S., Beraldi E., Yago V., et al. Design and characterization of injectable poly(lactic-co-glycolic acid) pastes for sustained and local drug release. Pharmaceutical Research. 2020;37:36. doi: 10.1007/s11095-019-2730-4. [DOI] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virology Journal. 2019;16(1):1–22. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotter J., Kamp P.B., Becker A., Puhler A., Reiss G., Bruckl H. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosensors & Bioelectronics. 2004;19:1149–1156. doi: 10.1016/j.bios.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- Shaligram S., Campbell A. Toxicity of copper salts is dependent on solubility profile and cell type tested. Toxicology in Vitro. 2013;27:844–851. doi: 10.1016/j.tiv.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Shao M., Hussain Z., Thu H.E., Khan S., Katas H., Ahmed T.A., et al. Drug nanocarrier, the future of atopic diseases: Advanced drug delivery systems and smart management of disease. Colloids and Surfaces B. 2016;147:475–491. doi: 10.1016/j.colsurfb.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Sharma R., Singhal S. Structural, magnetic and electrical properties of zinc doped nickel ferrite and their application in photo catalytic degradation of methylene blue. Physica B, Condensed Matter. 2013;414:83–90. [Google Scholar]
- Shi X.Y., Fan X.G. Advances in nanoparticle system for deliverying drugsacross the biological barriers. Journal of China Pharmaceutical University. 2002;33:169–172. [Google Scholar]
- Shi M., de Mesy Bentley K.L., Palui G., Mattoussi H., Elder A., Yang H. The roles of surface chemistry, dissolution rate, and delivered dose in the cytotoxicity of copper nanoparticles. Nanoscale. 2017;9:4739–4750. doi: 10.1039/c6nr09102d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiquie R.Y., Agrawal A., Joshi S.S. Surface alterations to impart antiviral properties to combat COVID-19 transmission. The Transactions of Indian National Academy of Engineering. 2020;5:343–347. doi: 10.1007/s41403-020-00096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim W., Barnard R.T., Blaskovich M.A.T., Ziora Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017) Antibiotics (Basel, Switz.) 2018;7:93. doi: 10.3390/antibiotics7040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Das R.R. Zinc for the common cold. The Cochrane Database of Systematic Reviews. 2013:6. doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- Singh R., Verma R., Kaushik A., Sumana G., Sood S., Gupta R.K., et al. Chitosan–iron oxide nanocomposite platform for mismatch-discriminating DNA hybridization for Neisseria gonorrhoeae detection causing sexually transmitted disease. Biosensors & Bioelectronics. 2011;26:2967–2974. doi: 10.1016/j.bios.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Siro I., Plackett D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose. 2010;17:459–494. [Google Scholar]
- Sivasankarapillai V.S., Pillai A.M., Rahdar A., Sobha A.P., Das S.S., Mitropoulos A.C., Mokarrar M.H., Kyzas G.Z. Nanomaterials. 2020;10:852. doi: 10.3390/nano10050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankarapillai V.S., Pillai A.M., Rahdar A., Sobha A.P., Das S.S., Mitropoulos A.C., et al. On facing the SARS-CoV-2 (COVID-19) with combination of nanomaterials and medicine: Possible strategies and first challenges. Nanomaterials. 2020;10(5):852. doi: 10.3390/nano10050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal I.S., O’Fallon K.S., Gaines P., Demokritou P., Bello D. Ingested engineered nanomaterials: State of science in nanotoxicity testing and future research needs. Particle and Fibre Toxicology. 2018;15:29. doi: 10.1186/s12989-018-0265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaran P., Agar G.E. The zero point of charge of calcite. Journal of Colloid and Interface Science. 1967;24:433–440. [Google Scholar]
- Sonaje K., Chuang E.Y., Lin K.J., Yen T.C., Su F.Y., Tseng M.T., et al. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: Microscopic, ultrastructural, and computed-tomographic observations. Molecular Pharmaceutics. 2012;9:1271–1279. doi: 10.1021/mp200572t. [DOI] [PubMed] [Google Scholar]
- Song Z., Wang X., Zhu G., Nian Q., Zhou H., Yang D., et al. Virus capture and destruction by label‐free graphene oxide for detection and disinfection applications. Small. 2015;11(9–10):1171–1176. doi: 10.1002/smll.201401706. [DOI] [PubMed] [Google Scholar]
- Speth R., Carrera E., Jean‐Baptiste M., Joachim A., Linares A. Concentration‐dependent effects of zinc on angiotensin‐converting enzyme‐2 activity (1067.4) FASEB Journal. 2014:28. 1067.4. [Google Scholar]
- Spitz Steinberg R., Cruz M., Mahfouz N.G., Qiu Y., Hurt R.H. Breathable vapor toxicant barriers based on multilayer graphene oxide. ACS Nano. 2017;11(6):5670–5679. doi: 10.1021/acsnano.7b01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportelli M.C., Picca R.A., Cio N. Recent advances in the synthesis and characterization of nano-antimicrobials. Trends in Analytical Chemistry. 2016;84:131–138. [Google Scholar]
- Sportelli M.C., Izzi M., Kukushkina E.A., Hossain S.I., Picca R.A., Ditaranto N., et al. Can nanotechnology and materials science help the fight against SARS-CoV 2? Nanomater. 2020;10(4):802. doi: 10.3390/nano10040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportelli M.C., Longano D., Bonerba E., Tantillo G., Torsi L., Sabbatini L., et al. Electrochemical preparation of synergistic nanoantimicrobials. Molecules. 2020;25:49. doi: 10.3390/molecules25010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staroverov S.A., Vidyasheva I.V., Gabalov K.P., Vasilenko O.A., Laskavyi V.N., Dykman L.A. Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bulletin of Experimental Biology and Medicine. 2011;151(4):436. doi: 10.1007/s10517-011-1350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D., Wu K., Krishna V.D., Klein T., Liu J., Feng Y., et al. Detection of influenza a virus in swine nasal swab samples with a wash-free magnetic bioassay and a handheld giant magnetoresistance sensing system. Frontiers in Microbiology. 2019;10:1077. doi: 10.3389/fmicb.2019.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suara R.O., Crowe J.E. Effect of zinc salts on respiratory syncytial virus replication. Antimicrobial Agents and Chemotherapy. 2004;48(3):783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucipto T.H., Churrotin S., Setyawati H., Kotaki T., Martak F., Soegijanto S. Antiviral activity of copper(II)chloride dihydrate against dengue virus type-2 in vero cell. Indonesian Journal of Tropical and Infectious Disease. 2017;6:84–87. [Google Scholar]
- Sunada K., Minoshima M., Hashimoto K. Highly ecient antiviral and antibacterial activities of solid-state cuprous compounds. Journal of Hazardous Materials. 2012;235–236:265–270. doi: 10.1016/j.jhazmat.2012.07.052. [DOI] [PubMed] [Google Scholar]
- Susanna P., Al Halifa S., Jennifer P. Molecularly engineered polymer-based systems in drug delivery and regenerative medicine. Current Pharmaceutical Design. 2017;23:281–294. doi: 10.2174/1381612822666161021104239. [DOI] [PubMed] [Google Scholar]
- Te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathogens. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting D., Dong N., Fang L., Lu J., Bi J., Xiao S., et al. Multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Applied Nano Materials. 2018;1:5451–5459. doi: 10.1021/acsanm.0c00970. [DOI] [PubMed] [Google Scholar]
- Ting D., Dong N., Fang L., Lu J., Bi J., Xiao S., et al. Multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Applied Nano Materials. 2018;1:5451–5459. doi: 10.1021/acsanm.0c00970. [DOI] [PubMed] [Google Scholar]
- Truong‐Tran A.Q., Carter J., Ruffin R., Zalewski P.D. New insights into the role of zinc in the respiratory epithelium. Immunology and Cell Biology. 2001;79(2):170–177. doi: 10.1046/j.1440-1711.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- Ulianas A., Heng L.Y., Ahmad M., Lau H.Y., Ishak Z., Ling T.L. A regenerable screen-printed DNA biosensor based on acrylic microsphere–gold nanoparticle composite for genetically modified soybean determination. Sensors and Actuators B. 2014;190:694–701. [Google Scholar]
- Ullah S., Ullah A., Lee J., Jeong Y., Hashmi M., Zhu C., et al. Reusability comparison of melt-blown vs nanofiber face mask filters for use in the coronavirus pandemic. ACS Applied Nano Materials. 2020;3(7):7231–7241. doi: 10.1021/acsanm.0c01562. [DOI] [PubMed] [Google Scholar]
- Ungur G., Hruza J. Modified polyurethane nanofibers as antibacterial filters for air and water purification. RSC Advances. 2017;7:49177–49187. [Google Scholar]
- US renal data system 2018 annual data report: Epidemiology of kidney disease in the United States. American Journal of Kidney Diseases. 2019;73:A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valizadeh J., Hafezalkotob A., Alizadeh S.M., Mozafari P. Hazardous infectious waste collection and government aid distribution during COVID-19: A robust mathematical leader-follower model approach. Sustainable Cities and Society. 2021:102814. doi: 10.1016/j.scs.2021.102814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. The New England Journal of Medicine. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. The New England Journal of Medicine. 2020 doi: 10.1056/NEJMc2004973. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velinov N., Petrova T., Tsoncheva T., Genova I., Koleva K., Kovacheva D., et al. Auto-combustion synthesis, Mössbauer study and catalytic properties of copper-manganese ferrites. Hyperfine Interactions. 2016;237:1–11. [Google Scholar]
- Venkatagopalan P., Daskalova S.M., Lopez L.A., Dolezal K.A., Hogue B.G. Coronavirus envelope (E) protein remains at the site of assembly. Virology. 2015;478:75–85. doi: 10.1016/j.virol.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennemann A., Alessandrini F., Wiemann M. Differential effects of surface-functionalized zirconium oxide nanoparticles on alveolar macrophages, rat lung, and a mouse allergy model. Nanomaterials. 2017;7:280. doi: 10.3390/nano7090280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkutyte J., Varma R.S. Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilization and surface functionalization. Chemical Science. 2011;2:837–846. [Google Scholar]
- Vo E., Zhuang Z., Birch E., Zhao Q., Horvatin M., Liu Y. Measurement of mass-based carbon nanotube penetration through filtering facepiece respirator filtering media. The Annals of Occupational Hygiene. 2014;58:646–656. doi: 10.1093/annhyg/meu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo E., Zhuang Z., Birch E., Birch Q. Application of direct-reading and elemental carbon 25 analysis methods to measure mass-based penetration of carbon nanotubes through elastomeric half-face and filtering facepiece respirators. Aerosol Science and Technology. 2016;50:1044–1054. doi: 10.1080/02786826.2016.1216519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo E., Zhuang Z. Development of a new test system to determine penetration of multi-walled carbon nanotubes through filtering facepiece respirators. Journal of Aerosol Science. 2013;61:50–59. doi: 10.1016/j.jaerosci.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonnemann J., Sieben C., Wolff C., Ludwig K., Böttcher C., Herrmann A., et al. Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale. 2014;6:2353–2360. doi: 10.1039/c3nr04449a. [DOI] [PubMed] [Google Scholar]
- Wang J.J., Zeng Z.W., Xiao R.Z., Xie T., Zhou G.L., Zhan X.R., et al. Recent advances of chitosannanoparticles as drug carriers. International Journal of Nanomedicine. 2011;6:765–774. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yin W., He X., Wang Q., Guo M., Chen S. Good biocompatibility and sintering properties of zirconia nanoparticles synthesized via vapor-phase hydrolysis. Scientific Reports. 2016;6:35020. doi: 10.1038/srep35020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‑nCoV) in vitro. Cell Research. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang Y., Tu L., Klein T., Feng Y., Li Q., et al. Magnetic detection of mercuric ion using giant magnetoresistance-based biosensing system. Analytical Chemistry. 2014;86:3712–3716. doi: 10.1021/ac404015j. [DOI] [PubMed] [Google Scholar]
- Wang T., Yang Z., Lei C., Lei J., Zhou Y. An integrated giant magnetoimpedance biosensor for detection of biomarker. Biosensors & Bioelectronics. 2014;58:338–344. doi: 10.1016/j.bios.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6:e01697–15. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6:01697. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- Weng X., Neethirajan S. Immunosensor based on antibody-functionalized MoS 2 for rapid detection of avian coronavirus on cotton thread. IEEE Sensors Journal. 2018;18(11):4358–4363. doi: 10.1109/JSEN.2018.2829084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom M.E., Stumbles P.A. Mouse respiratory tract dendritic cell subsets and the immunological fate of inhaled antigens. Immunology and Cell Biology. 2007;85(3):182–188. doi: 10.1038/sj.icb.7100039. [DOI] [PubMed] [Google Scholar]
- Wittekindt O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflügers Archiv - European Journal of Physiology. 2017;469:135–147. doi: 10.1007/s00424-016-1917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Z.M., Tan T.L., Yang S.-W., Xu G.Q. Enhancing the photocatalytic performance of MXenes via stoichiometry engineering of their electronic and optical properties. ACS Applied Materials & Interfaces. 2018;10:39879–39889. doi: 10.1021/acsami.8b14325. [DOI] [PubMed] [Google Scholar]
- Woodworth B.A., Zhang S., Tamashiro E., Bhargave G., Palmer J.N., Cohen N.A. Zinc increases ciliary beat frequency in a calcium-dependent manner. American Journal of Rhinology & Allergy. 2011;24(1):6–10. doi: 10.2500/ajra.2010.24.3379. [DOI] [PubMed] [Google Scholar]
- Wu K., Saha R., Su D., Krishna V.D., Liu J., Cheeran M.C., et al. Magnetic immunoassays: A review of virus and pathogen detection before and amidst the coronavirus Disease-19 (COVID-19) arXiv preprint arXiv. 2020 2007.04809. [Google Scholar]
- Xiang J., Yan M., Li H., Liu T., Lin C., Huang S., et al. Evaluation of enzyme-linked immunoassay and colloidal gold-immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19) MedRxiv. 2020 [Google Scholar]
- Xu Y., Yuen P.W., Lam J.K. Intranasal DNA vaccine for protection against respiratory infectious diseases: The delivery perspectives. Pharmaceutics. 2014;6:378–415. doi: 10.3390/pharmaceutics6030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Tong J., Wu Y., Zhao S., Lin B.L. Targeted oxidation strategy (TOS) for potential inhibition of coronaviruses by disulfiram—A 70-year old anti-alcoholism drug. ChemRxiv. 2020 [Google Scholar]
- Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PloS One. 2014;9(10) doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q., et al. Antiviral activity of graphene oxide: How sharp edged structure and charge matter. ACS Applied Materials. 2015;7(38):21571–21579. doi: 10.1021/acsami.5b06876. [DOI] [PubMed] [Google Scholar]
- Yeh Y.-T., Tang Y., Sebastian A., Dasgupta A., Perea-Lopez N., Albert I., et al. Tunable and label-free virus enrichment for ultrasensitive virus detection using carbon nanotube arrays. Science Advances. 2016;2 doi: 10.1126/sciadv.1601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y.T., Gulino K., Zhang Y., Sabestien A., Chou T.W., Zhou B., et al. A rapid and label-free platform for virus capture and identification from clinical samples. Proceedings of the National Academy of Sciences. 2020;117(2):895–901. doi: 10.1073/pnas.1910113117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H.Y., Jeon S., You D.G., Park J.H., Kwon I.C., Koo H., et al. Inorganic nanoparticles for image-guided therapy. Bioconjugate Chemistry. 2017;28(1):124–134. doi: 10.1021/acs.bioconjchem.6b00512. [DOI] [PubMed] [Google Scholar]
- Yu Y., Bu F., Zhou H., Wang Y., Cui J., Wang X., et al. Biosafety materials: An emerging new research direction of materials science from COVID-19 outbreak. Materials Chemistry Frontiers. 2020;4:1930–1953. [Google Scholar]
- Zachar O. ScienceOpen Preprints; 2020. Formulations for COVID-19 early stage treatment via silver nanoparticles inhalation delivery at home and hospital. [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: A systematic review. Journal of Medical Virology. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-B., Kanungo M., Ho A.J., Freimuth P., Van Der Lelie D., Chen M., et al. Functionalized carbon nanotubes for detecting viral proteins. Nano Letters. 2007;7:3086–3091. doi: 10.1021/nl071572l. [DOI] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Qi Q., Jing Q., Ao S., Zhang Z., Ding M., et al. Electrical probing of COVID-19 spike protein receptor binding domain via a graphene field-effect transistor. arXiv preprint arXiv. 2020:12529. 2003. [Google Scholar]
- Zhao Z., Cui H., Song W., Ru X., Zhou W., Yu X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv. 2020 doi: 10.1016/j.talanta.2023.124479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H., Zhu Z., Lin J., Cheung C.F., Lu V.L., Yan F., et al. Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances. ACS Nano. 2020;14(5):6213–6221. doi: 10.1021/acsnano.0c02250. [DOI] [PubMed] [Google Scholar]
- Zhu S., Li J., Huang A.G., Huang J.Q., Huang Y.Q., Wang G.X. Anti-betanodavirus activity of isoprinosine and improved efficacy using carbon nanotubes based drug delivery system. Aquaculture. 2019;512:734377. [Google Scholar]
- Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., et al. Immunogenicity and safety of a recombinant adenovirus Type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo- controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zodrow K., Brunet L., Mahendra S., Li D., Zhang A., Li Q., et al. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Research. 2009;43:715–723. doi: 10.1016/j.watres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Zou Z., Yao M. Airflow resistance and bio-filtering performance of carbon nanotube filters and current facepiece respirators. Journal of Aerosol Science. 2015;79:61–71. [Google Scholar]
- Zou D., Jin L., Wu B., Hu L., Chen X., Huang G., et al. Rapid detection of Salmonella in milk by biofunctionalised magnetic nanoparticle cluster sensor based on nuclear magnetic resonance. International Dairy Journal. 2019;91:82–88. [Google Scholar]