Abstract
Background & Aims
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly expanded; however, clinical trials excluded patients taking immunosuppressive medications such as those with inflammatory bowel disease (IBD). Therefore, we explored real-world effectiveness of coronavirus disease 2019 (COVID-19) vaccination on subsequent infection in patients with IBD with diverse exposure to immunosuppressive medications.
Methods
This was a retrospective cohort study of patients in the Veterans Health Administration with IBD diagnosed before December 18, 2020, the start date of the Veterans Health Administration patient vaccination program. IBD medication exposures included mesalamine, thiopurines, anti-tumor necrosis factor biologic agents, vedolizumab, ustekinumab, tofacitinib, methotrexate, and corticosteroid use. We used inverse probability weighting and Cox’s regression with vaccination status as a time-updating exposure and computed vaccine effectiveness from incidence rates.
Results
The cohort comprised 14,697 patients, 7321 of whom received at least 1 vaccine dose (45.2% Pfizer, 54.8% Moderna). The cohort had median age 68 years, 92.2% were men, 80.4% were White, and 61.8% had ulcerative colitis. In follow-up data through April 20, 2021, unvaccinated individuals had the highest raw proportion of SARS-CoV-2 infection (197 [1.34%] vs 7 [0.11%] fully vaccinated). Full vaccination status, but not partial vaccination status, was associated with a 69% reduced hazard of infection relative to an unvaccinated status (hazard ratio, 0.31, 95% confidence interval, 0.17–0.56; P < .001), corresponding to an 80.4% effectiveness.
Conclusions
Full vaccination (> 7 days after the second dose) against SARS-CoV-2 infection has an ∼80.4% effectiveness in a broad IBD cohort with diverse exposure to immunosuppressive medications. These results may serve to increase patient and provider willingness to pursue vaccination in these settings.
Keywords: SARS-CoV-2 Vaccine, Inflammatory Bowel Disease, Effectiveness, Immunosuppressive Medications, Veterans Affairs Healthcare System
Abbreviations used in this paper: CD, Crohn’s disease; CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; IBD, inflammatory bowel disease; IPW, inverse probability weight(s) (ed) (ing); IQR, interquartile range; NLP, natural language processing; PS, propensity score; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SMD, standardized mean difference; TNF, tumor necrosis factor; UC, ulcerative colitis; VHA, Veterans Health Administration
What You Need to Know.
Background and Context
The real-world effectiveness of severe acute respiratory syndrome coronavirus 2 vaccination was evaluated in a large population with inflammatory bowel disease, many of whom were taking immunosuppressive medications.
New Findings
Full vaccination, but not partial vaccination, against severe acute respiratory syndrome coronavirus 2 was significantly associated with reduced infection, and no meaningful differences were noted in comparisons of key immunosuppressive medication classes.
Limitations
This study cohort comprised a predominantly older male population, and there remains a possibility of residual confounding.
Impact
Full vaccination effectively reduces severe acute respiratory syndrome coronavirus 2 infection in a large cohort with inflammatory bowel disease, many of whom were on immunosuppressive medications, which should promote patient and provider willingness to pursue immunization in this setting.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic is a grave threat to public health, with more than 28 million people reported to have been infected and more than half a million deaths in the United States alone as of April 2, 2021.1 Inflammatory bowel disease (IBD), consisting of ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic inflammatory disorder of the gastrointestinal tract of unknown etiology. The pathophysiology of IBD involves dysregulation of the mucosal immune system and is usually treated with immunomodulatory and/or immunosuppressive medications, which can lead to an increased risk of infection.2, 3, 4 To date, however, the incidence of SARS-CoV-2 among all patients with IBD appears to be comparable to that observed in the general population.5, 6, 7, 8
To curb the ongoing pandemic caused by SARS-CoV-2 infection, vaccine development has been undertaken at an unprecedented pace, and numerous candidates have been authorized or are under development.9 At present, 2 vaccines are in wide clinical use in the US, the BNT162b2 messenger RNA coronavirus disease 2019 (COVID-19) vaccine from Pfizer and the messenger RNA-1273 SARS-CoV-2 vaccine from Moderna.10 , 11 Both vaccines have been shown to have greater than 90% efficacy, and to date, more than 100 million vaccines have been administered in the US. However, the seminal clinical trials excluded patients taking immunosuppressive medications or those with immunosuppressive conditions, thus the effectiveness in the population of patients with IBD is unknown.
To evaluate the effectiveness of SARS-CoV-2 vaccination in the IBD population and the potential impact of immunosuppressive medications, we identified in the Veterans Health Administration (VHA) a national cohort of patients with IBD. Our secondary aims were to evaluate the impact of vaccination on severe SARS-CoV-2 infection and all-cause mortality. The VHA is the largest integrated health care system in the US, serving more than 9 million veterans every year.12 As of April 22, 2021, more than 2.1 million veterans have been fully vaccinated.13 The VHA has also established a database of all patients who have tested positive for SARS-CoV-2, and all medication records are maintained in a central pharmacy data set, making it an ideal health care system in which to conduct such a study.
Materials and Methods
Study Design and Data Source
This was a retrospective cohort study using VHA data from December 18, 2020 (index date), the date when the VHA began providing COVID-19 vaccinations to patients, and extending through April 20, 2021. The VHA contains granular demographic, laboratory, comorbidity, pharmacy, and other patient-level data that are centralized across 170 US centers.
We identified all patients with a diagnosis of UC or CD before the index date by using a previously validated algorithm based on administrative codes.14 We included patients age ≥ 18 years who had not previously been diagnosed with SARS-CoV-2 infection in the VHA system (as determined by polymerase chain reaction testing, antibody testing, or through natural language processing [NLP] of clinician notes15), who were taking an IBD medication of interest (defined below), and who had at least 6 months of VHA outpatient visit data before the index date, thereby identifying patients who generally obtain care through the VHA. We excluded patients who received the Janssen COVID-19 vaccine given limited sample size and follow-up data.
This study received Institutional Review Board approval from the Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, Pennsylvania.
Ascertainment of Exposures
Detailed demographic (age, sex, race), substance use (smoking status, alcohol abuse, drug abuse), and comorbidity data based on administrative codes (obesity, hypertension, diabetes, prior arrhythmia, heart failure, chronic obstructive pulmonary disease, renal failure, and metastatic malignancy)16 were obtained for each patient immediately before the index date. VHA center data were used to classify US geographic region.
To identify IBD medication exposures, we used VHA pharmacy records to identify prescription fills for medications of interest in a 3-month window before the index date, consistent with previously published methods.16 Mutually exclusive IBD medication groups were categorized as follows: mesalamine alone, thiopurines (azathioprine or mercaptopurine, with or without mesalamine), anti-tumor necrosis factor (anti-TNF) agents alone, anti-TNF agents plus immunomodulator therapy (thiopurines or methotrexate), vedolizumab, ustekinumab, tofacitinib, or methotrexate alone. We separately ascertained data on corticosteroid exposure in a 3-month window before the index date, which was treated as a binary variable. This included prescriptions for prednisone, prednisolone, methylprednisolone, or budesonide. Finally, we obtained COVID-19 vaccination data from the VHA Corporate Data Warehouse. This included the vaccine brand (Pfizer, Moderna, and Janssen), the date of the first vaccination dose, and the date of the second vaccination dose.
Ascertainment of Outcomes
The primary outcome was time to SARS-CoV-2 infection, which was determined from the results of polymerase chain reaction testing performed in the VHA as well as through an established NLP-based program that captures SARS-CoV-2 infections identified in non-VHA health systems.15 Secondary outcomes included all-cause mortality, which was ascertained using the vital status file in the VHA data set, and severe SARS-CoV-2 infection, which was defined as COVID-19–related hospitalization or death. This definition and methodology of severe SARS-CoV-2 infection is consistent with recently published literature using the VHA cohort.16
Statistical Analysis
Descriptive statistics are reported as medians and interquartile ranges (IQRs) for continuous variables and as percentages for categorical variables. Cohort comparisons were made among unvaccinated and vaccinated individuals (either 1 or 2 doses received) using Wilcoxon’s rank sum or χ2 test, as indicated. To demonstrate the timing of vaccine administration relative to the index date, we plotted overlaid histograms of the first and second vaccine doses separately for each vaccine brand. For subsequent analyses, we used a concept of “effective vaccination status,” where a patient was considered to be partially vaccinated starting 14 days after the first vaccine dose and fully vaccinated starting 7 days after the second dose. These time points are consistent with estimates of protection from recent literature17 and from the seminal vaccination clinical trials.10 , 11 We reported raw proportions of patients who developed each primary and secondary outcome, stratified by effective vaccination status and vaccine manufacturer.
To address covariate imbalance and potential confounding between groups while preserving sample size and power, we chose an inverse probability weighted (IPW) modeling approach. First, a propensity score (PS) was created for the outcome of receiving any vaccination by using all available patient characteristics, including US geographic region, in a logistic regression model. IPWs were then computed for each patient as 1/(PS) for individuals who received a vaccine dose and 1/(1 − PS) for individuals who did not.18 The standardized mean differences (SMDs) between groups in unadjusted and IPW-adjusted cohorts were then calculated and plotted for each variable, with an SMD ±0.1 considered to represent adequate covariate balance.19
To evaluate the association between effective vaccination status and the outcomes of interest, we used Cox’s proportional hazards regression. Effective vaccination status (unvaccinated, partially vaccinated, or fully vaccinated) was treated as a time-updating exposure to minimize the possibility of immortal time bias.20 , 21 Thus, the index time for a vaccinated individual would be the date of partial or full vaccination status, and risk adjustment would also account for unvaccinated time periods. For the primary outcome, we computed unadjusted and IPW-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the association between effective vaccination status (unvaccinated, partially vaccinated, fully vaccinated) and SARS-CoV-2 infection. The corresponding vaccine effectiveness was calculated as: ([1 − incidencevaccinated/incidenceunvaccinated] × 100). This was done for both partial vaccination and full vaccination. Observations were right censored at death during follow-up or at maximum follow-up.
Given the possibility of the differential impact of immunosuppressive IBD medications on vaccination effectiveness, we tested several a priori interaction terms, including between vaccination status and (1) steroid use, (2) mesalamine alone vs immunosuppressive medications, and (3) anti-TNF use with immunomodulators or steroids vs other medications. We also tested for an interaction between vaccination status and vaccine manufacturer. For the secondary outcomes, unadjusted and IPW-adjusted estimates were similarly provided for the association between vaccination status and (1) severe SARS-CoV-2 infection and (2) all-cause mortality. Cox-adjusted survival curves were presented for each IPW-adjusted model, and estimates of vaccine effectiveness in mitigating these outcomes were similarly provided using the approach detailed above. The proportional hazards assumption was tested using Schoenfeld’s residuals, and no obvious violations were observed.
Sensitivity Analysis
Because of the possibility of undiagnosed SARS-CoV-2 infection leading to death during follow-up, we performed additional analyses to evaluate the potential impact of this outcome misclassification on the primary analysis. A random number generator was used to reclassify increasing percentages of patients who died during follow-up without a COVID-19 diagnosis as having been infected with SARS-CoV-2. IPW-adjusted Cox’s regression models were performed for 5%, 10%, 15%, 20%, 25%, and 30% mortality event reclassifications. Adjusted HRs and 95% CIs were plotted for visual comparison. Data management and analyses were performed using SAS (SAS Institute, Carey, NC) and Stata 16.1/IC (StataCorp, College Station, TX) software. For all hypothesis tests, we used an α threshold of 5% for statistical significance.
Results
Baseline Cohort Characteristics and Vaccination Data
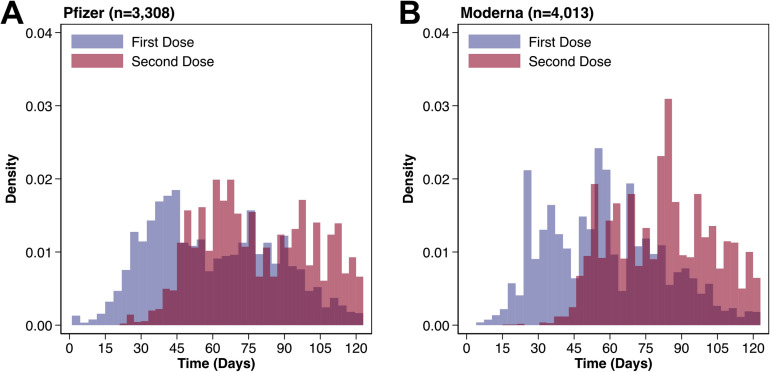
After application of screening criteria, we identified 14,929 patients with IBD taking medications of interest before the index date. We excluded 232 patients who received the Janssen vaccine, yielding an analytic cohort of 14,697 patients. Most of the patients had ulcerative colitis (61.8%), with 54.8% taking mesalamine alone, 19.9% anti-TNF agents alone, and 10.7% thiopurines. Unvaccinated individuals were younger (median age, 64 vs 71 years; P < .001) and had fewer comorbidities compared with vaccinated individuals (each medical comorbidity P < .001; Table 1 ). Vaccinated individuals were more likely to reside in the Northeast or Midwest compared with unvaccinated individuals (P < .001). Of the 7321 patients who received at least 1 vaccine dose, 3308 (45.2%) were given Pfizer and 4013 (54.8%) Moderna. Through maximum follow-up, 91.2% of Pfizer (n = 3017) and 88.7% of Moderna (n = 3561) patients received both vaccine doses, with the median duration between doses of 21 days (IQR 21, 21 days) and 28 days (IQR 28, 28 days), respectively (Figure 1 ).
Table 1.
Baseline Cohort Characteristics
| Factor | Unvaccinated | Received vaccination (any) | P value |
|---|---|---|---|
| (n = 7376) | (n = 7321) | ||
| Age, y | 64 (47, 73) | 71 (60, 75) | <.001 |
| Age category, y | <.001 | ||
| <65 | 3787 (51.3) | 2486 (34.0) | |
| 65–80 | 2776 (37.6) | 3819 (52.2) | |
| >80 | 813 (11.0) | 1016 (13.9) | |
| Male sex | 6766 (91.7) | 6777 (92.6) | .06 |
| Race | .004 | ||
| White | 5922 (80.3) | 5896 (80.5) | |
| Black | 832 (11.3) | 906 (12.4) | |
| Hispanic | 308 (4.2) | 271 (3.7) | |
| Other | 314 (4.3) | 248 (3.4) | |
| Current smoker | 529 (7.2) | 582 (7.9) | .07 |
| Alcohol abuse | 279 (3.8) | 310 (4.2) | .16 |
| Drug abuse | 183 (2.5) | 181 (2.5) | .97 |
| IBD type | .08 | ||
| CD | 2870 (38.9) | 2746 (37.5) | |
| UC | 4506 (61.1) | 4575 (62.5) | |
| IBD medication group | .005 | ||
| Mesalamine alone | 4026 (54.6) | 4022 (54.9) | |
| Thiopurine | 774 (10.5) | 793 (10.8) | |
| Anti-TNF alone | 1545 (20.9) | 1374 (18.8) | |
| Anti-TNF + IM | 296 (4.0) | 307 (4.2) | |
| Vedolizumab | 444 (6.0) | 529 (7.2) | |
| Ustekinumab | 79 (1.1) | 75 (1.0) | |
| Tofacitinib | 61 (0.8) | 49 (0.7) | |
| Methotrexate | 151 (2.0) | 172 (2.3) | |
| Steroid use | 414 (5.6) | 498 (6.8) | .003 |
| Obesity | 770 (10.4) | 986 (13.5) | <.001 |
| Hypertension | 3294 (44.7) | 4201 (57.4) | <.001 |
| Diabetes mellitus | 1504 (20.4) | 2122 (29.0) | <.001 |
| Arrhythmia | 713 (9.7) | 1001 (13.7) | <.001 |
| Heart failure | 249 (3.4) | 412 (5.6) | <.001 |
| COPD | 866 (11.7) | 1150 (15.7) | <.001 |
| Renal failure | 372 (5.0) | 609 (8.3) | <.001 |
| Metastatic malignancy | 21 (0.3) | 53 (0.7) | <.001 |
| US region | <.001 | ||
| West | 1434 (19.4) | 1446 (19.8) | |
| Midwest | 1689 (22.9) | 1899 (25.9) | |
| Northeast | 972 (13.2) | 1213 (16.6) | |
| South | 3281 (44.5) | 2763 (37.7) |
NOTE. Data are presented as median (IQR) or as n (%).
COPD, chronic obstructive pulmonary disease; IM, immunomodulator.
Figure 1.
Distribution of vaccination doses for each vaccine brand, relative to the index date of the VHA Vaccination Campaign (December 18, 2020).
Primary Analysis
After PS creation and IPW including all variables in Table 1, the SMD for each variable was reduced to less than ±0.05, representing excellent covariate balance (Figure 2 ). Raw proportions of SARS-CoV-2 infection, accounting for time-updating effective vaccination status, are reported in Table 2 . Over a median follow-up of 123 days (IQR 70, 123 days), unvaccinated individuals experienced the numerically highest rate of SARS-CoV-2 infection (197 [1.34%]). By contrast, over a median follow-up of 20 days (IQR 14, 21 days), there were 14 SARS-CoV-2 infections (0.28%) among individuals with partially vaccinated status, and over a median follow-up of 38 days (IQR 20, 55 days), there were 7 infections (0.11%) among individuals with fully vaccinated status (Table 2). Of the 7 infections occurring after full vaccination, 4 patients were taking mesalamine alone, 1 a thiopurine with mesalamine, 1 vedolizumab, and 1 tofacitinib.
Figure 2.
SMDs in unadjusted and IPW-adjusted cohorts. After IPW, the SMD for each variable incorporated into the propensity score was reduced to ±0.05 (red vertical lines), representing excellent balance of covariates between unvaccinated and vaccinated patients. COPD, chronic obstructive pulmonary disease; IM, immunomodulator.
Table 2.
Raw Proportion of Outcome Events, Stratified by Effective Vaccination Statusa,b and Vaccine Manufacturer
| Variable | No. | SARS-CoV-2 infection | Severe SARS-CoV-2 infection | All-cause mortality |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Unvaccinated state | 14,697 | 197 (1.34) | 47 (0.32) | 97 (0.66) |
| Pfizer | ||||
| Partially vaccinated | 3194 | 7 (0.22) | 2 (0.06) | 5 (0.16) |
| Fully vaccinated | 2873 | 3 (0.10) | 1 (0.03) | 0 (0.00) |
| Moderna | ||||
| Partially vaccinated | 3918 | 7 (0.18) | 1 (0.03) | 4 (0.10) |
| Fully vaccinated | 3380 | 4 (0.12) | 2 (0.06) | 2 (0.06) |
No., number.
Because vaccination exposure status is treated as a time-updating covariate in analyses, most patients contribute some follow-up time to multiple exposure categories. Vaccination exposure categories are therefore not mutually exclusive. Similarly, because a small proportion of patients were vaccinated near the end of the follow-up period, some patients who received a vaccine dose did not contribute follow-up time to partially vaccinated or fully vaccinated exposure categories.
Individuals were considered partially vaccinated 14 days after the first vaccine dose and fully vaccinated 7 days after the second vaccine dose.
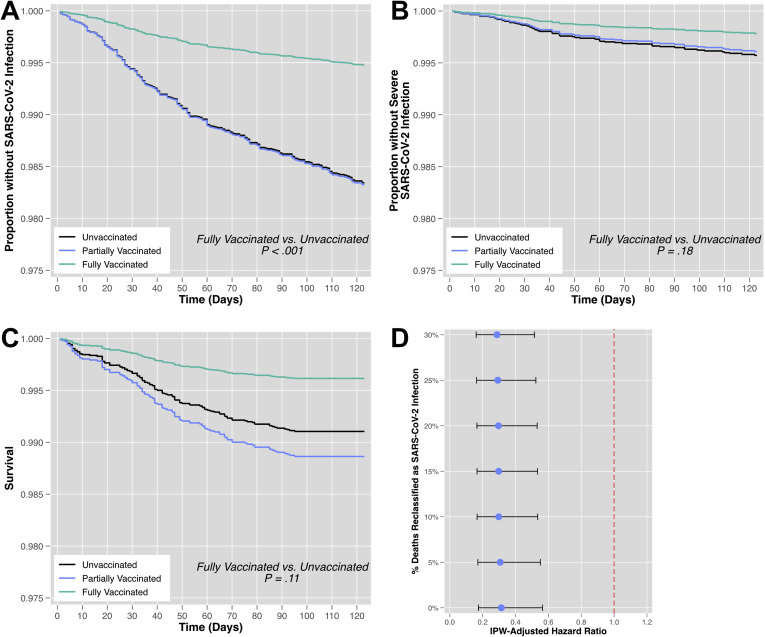
In unadjusted and IPW-adjusted Cox’s regression analysis, full vaccination status, but not partial vaccination status, was protective against SARS-CoV-2 infection. For example, in the IPW-adjusted model, full vaccination was associated with a 69% reduction in the hazard of infection (HR, 0.31; 95% CI, 0.17–0.56; P < .001) relative to unvaccinated individuals (Table 3 , Figure 3 A). IPW-adjusted incidence rates for each vaccination status in the cohort are reported in Table 4 . The corresponding vaccine effectiveness was 25.1% for partial vaccination status and 80.4% for full vaccination status. In a sensitivity analysis where increasing percentages of mortality events were randomly reclassified as SARS-CoV-2 infections, similar results were observed in the IPW-adjusted HRs for full vaccination vs unvaccinated status (Figure 3 D).
Table 3.
Cox’s Regression Models for SARS-CoV-2 Infection, Severe SARS-CoV-2 Infection, and All-Cause Mortality
| Variable | Vaccination status | Unadjusted model | IPW-adjusted model | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| SARS-CoV-2 infection | Unvaccinated (Ref) | (Ref) | (Ref) | ||
| Partially vaccinated | 1.01 (0.57–1.81) | .97 | 1.01 (0.68–1.50) | .96 | |
| Fully vaccinated | 0.31 (0.14–0.69) | .004a | 0.31 (0.17–0.56) | <.001a | |
| Severe SARS-CoV-2 infection | Unvaccinated (Ref) | (Ref) | (Ref) | ||
| Partially vaccinated | 0.99 (0.29–3.32) | .99 | 0.91 (0.39–2.14) | .84 | |
| Fully vaccinated | 0.65 (0.18–2.34) | .51 | 0.51 (0.19–1.36) | .18 | |
| All-cause mortality | Unvaccinated (Ref) | (Ref) | (Ref) | ||
| Partially vaccinated | 1.74 (0.84–3.60) | .13 | 1.27 (0.75–2.16) | .38 | |
| Fully vaccinated | 0.49 (0.11–2.09) | .33 | 0.43 (0.15–1.19) | .11 | |
Ref, reference.
Statistically significant at the α = 5% level.
Figure 3.
Cox’s adjusted survival curves for primary and secondary outcomes, stratified by effective vaccination status. (A) In IPW-adjusted analysis, full vaccination status was significantly associated with reduced SARS-CoV-2 infection relative to an unvaccinated status. In IPW-adjusted analysis, there was no significant association (B) between vaccination status and severe SARS-CoV-2 infection and (C) between vaccination status and all-cause mortality. (D) In a sensitivity analysis where increasing percentages of death events among uninfected patients were randomly reclassified as SARS-CoV-2 infection events, the IPW-adjusted significant association between full vaccination status and reduced SARS-CoV-2 infection was unchanged. The horizontal lines indicate the 95% CI.
Table 4.
Vaccine Effectiveness for Primary and Secondary Outcomes in Inverse Probability Weight-Adjusted Models
| Variable | Vaccination status | Person-time, d | Outcome events | Incidence rate (per 1000 person-days) | Vaccine effectiveness (vs unvaccinated state), %a |
|---|---|---|---|---|---|
| SARS-CoV-2 infection | Unvaccinated (Ref) | 2,861,990.10 | 416.84 | 0.146 | … |
| Partially vaccinated | 256,445.62 | 27.97 | 0.109 | 25.1 | |
| Fully vaccinated | 443,805.61 | 12.66 | 0.029 | 80.4b | |
| Severe SARS-CoV-2 Infection | Unvaccinated (Ref) | 2,882,437.00 | 108.23 | 0.038 | … |
| Partially vaccinated | 254,438.67 | 6.04 | 0.024 | 36.8 | |
| Fully vaccinated | 425,365.65 | 4.78 | 0.011 | 70.1 | |
| All-cause mortality | Unvaccinated (Ref) | 2,945,906.30 | 241.50 | 0.082 | … |
| Partially vaccinated | 266,056.47 | 15.76 | 0.059 | 27.8 | |
| Fully vaccinated | 381,159.52 | 3.98 | 0.010 | 87.3 |
Ref, reference.
Calculated as: ([1 − incidencevaccinated/incidenceunvaccinated] × 100).
The associated comparison in Cox’s regression analysis was statistically significant.
Finally, no significant interactions were found in models between vaccination status and (1) steroid use (P = .64), (2) mesalamine use vs immunosuppressive agents (P = .46), (3) or anti-TNF use with immunomodulators or steroids vs other agents (P = .34). Similarly, there were no significant differences in the relationship between vaccination status and SARS-CoV-2 infection for the Pfizer vs Moderna vaccine series (P = .09).
Secondary Analyses
During follow-up, individuals with an unvaccinated status had the numerically highest raw proportions of severe SARS-CoV-2 infection (47 [0.32%]) and all-cause mortality (97 [0.66%]), relative to individuals with partial or full vaccination status (Table 2). In both unadjusted and IPW-adjusted Cox’s regression analyses, no significant association was found between vaccination status and (1) severe SARS-CoV-2 infection or (2) all-cause mortality (Table 3, Figure 3 B and C). Although the associated Cox’s regression analyses were not statistically significant, the IPW-adjusted incidence rates and computed vaccine effectiveness in mitigating secondary outcomes are reported in Table 4.
Discussion
In this national cohort of patients with IBD with diverse exposure to immunosuppressive agents treated in the VHA, we found that full vaccination status, but not partial vaccination status, was associated with a 69% reduction in the hazard of SARS-CoV-2 infection. This corresponded to 80.4% vaccine effectiveness from 7 days after the second dose. Model estimates were not significantly affected by use of different key immunosuppressive medication classes, although we note that no patients taking anti-TNF agents, ustekinumab, or methotrexate experienced SARS-CoV-2 infection > 7 days after the second vaccine dose. Full vaccination status demonstrated the numerically lowest incidence rates of severe SARS-CoV-2 infection and all-cause mortality; however, these associations were not statistically significant.
Current data indicate that patients with IBD do not inherently have an increased risk of developing SARS-CoV-2 infection or associated complications of COVID-19.5, 6, 7, 8 , 22 However, ∼30% of patients with IBD are aged >65 years, and approximately one-third have comorbidities, including those associated with adverse outcomes for COVID-19 such as cardiovascular diseases and diabetes.22 It is imperative to control the spread of infection among this population.
COVID-19 vaccines approved for use in the United States have gone through development at an unprecedented pace and have been shown to be very efficacious in clinical trials.10 , 11 However, the Pfizer vaccine trial did not include patients who were undergoing “treatment with immunosuppressive therapy or diagnosed with an immunocompromising condition.”10 Similarly, the Moderna trial excluded those with “immunosuppressive or immunodeficient state, [or those] receiving systemic immunosuppressants or immune-modifying drugs for >14 days in total within 6 months prior to screening.”11 This has resulted in a lack of data on the impact of immune-modifying therapies used in the management of IBD on the effectiveness of vaccinations and potential reluctance to pursue vaccination in this setting.
Two recent studies have shown that immunosuppressive medications may affect the serologic response to SARS-CoV2 and to vaccinations. Kennedy et al23 found that infliximab is associated with attenuated serologic responses to SARS-CoV-2 that was further blunted by concomitant immunomodulators use. Wong et al24 reported 100% seropositivity after 2-dose Pfizer and Moderna COVID-19 vaccination among patients with IBD on biological therapies. However, despite achieving antibody levels consistent with those thought to confer protection, they also found an association of lower antibody levels in patients on vedolizumab for all antibodies tested and with anti-TNF agents for anti- receptor binding domain total Ig only. Therefore, it is imperative to obtain real-world data on vaccination outcomes in patients with IBD to help inform patients and their treating physicians.
In this study we found that completion of a vaccination series was effective in reducing SARS-CoV-2 infection among patients with IBD with diverse exposure to immunosuppressive medications. Approximately 45% of patients received the Pfizer vaccine with the remainder receiving the Moderna vaccine, and no differences in effectiveness were noted between the vaccines. The level of protection observed among patients with IBD in this study was slightly lower than that reported in the clinical trials consisting of a more general population,10 , 11 with 80.4% effectiveness vs > 90% using similar metrics in the trials.
As detailed in the limitations below, the estimate of vaccine effectiveness in this study may be conservative for several reasons; however, there are also biologically plausible explanations for reduced effectiveness in an IBD cohort. Recent studies as highlighted above have also shown that the serologic response to SARS-COV 2 and the vaccines may be affected by IBD medications. Additionally, partial vaccination status after receiving a single vaccine dose alone was not significantly associated with reductions in SARS-CoV-2 infection relative to an unvaccinated status, in contrast to prior data from a more general cohort,17 underscoring the importance of completion of the vaccination series in patients with IBD.
Importantly, we did not identify any significant interactions between vaccination and key immunosuppression medication classes, suggesting that SARS-CoV-2 vaccine effectiveness is similar in patients with IBD regardless of the particular immunosuppressive agents being used. However, of the patients taking immunosuppressive medications, the most common class was anti-TNF agents, and not a single case of SARS-CoV-2 infection was identified in fully vaccinated patients in this group.
Finally, while we did not identify a statistically significant difference in the secondary outcomes of severe SARS-CoV-2 infection and all-cause mortality, the incidence rates for these outcomes were numerically lowest in the fully vaccinated group. Future studies with larger sample size and/or longer follow-up are needed to evaluate this further.
Our findings have strong and immediate clinical importance. Patient willingness to receive vaccination is based on the perceived effectiveness of the vaccine as well as health care provider recommendations.21 This study supports the effectiveness of vaccination in patients with IBD taking diverse immunosuppressive agents and may allay fears in this regard. While this prospect requires further study, similar results may be expected in patients without IBD who are taking similar immunosuppression agents, where patient willingness to pursue vaccination may be increased through demonstration of vaccine effectiveness.
Major strengths of this study include the use of a national cohort of IBD patients monitored in the VHA system, serving ∼9 million veterans every year.12 Every patient in the VHA has a SARS-CoV-2 status designation in the electronic health record (positive, negative, or not tested), even if diagnosed to be positive outside the VHA. The VHA has also developed a central database that updates all SARS-CoV-2 diagnoses and vaccination status, with > 2.1 million veterans fully vaccinated to date. These features contribute to high confidence that vaccination events and SARS-CoV-2 diagnoses have been captured with minimal misclassification in this cohort.
An additional strength of this study is the use of nationwide VHA Pharmacy records for gathering IBD medication data. The VHA has a central pharmacy database, meaning that medications prescribed at different Veterans Affairs centers are linked for a given patient, thus decreasing the chance of missing or misclassified prescribed medications.
Notwithstanding, we acknowledge several important limitations in this study. First, although we demonstrated excellent IPW-adjusted covariate balancing across a range of important patient-level and geographic predictors, there remains a possibility of residual confounding.
Second, there are inherent external validity limitations to the VHA cohort, because it is largely composed of an older male population. Hence, there is also a higher proportion of patients with UC because older-onset UC is more common than CD, with rates higher in elderly men than in women.25 However, it is not clear that these features would be salient contributors to differences in vaccine effectiveness. There may also be external validity limitations as applied to patients without IBD who are taking immunosuppressive medications, because this study was intentionally focused on the IBD population where there may be independent influences of the condition itself on vaccination effectiveness. Future studies may seek to address vaccine effectiveness in immunosuppressed patients more generally.
Third, patients are not proactively screened for SARS-CoV-2 in the VHA but rather are tested when symptomatic or for preventative measures such as before an elective procedure. Hence, our patient population could be biased toward symptomatic patients with COVID-19 and could miss a substantial proportion of asymptomatic patients. This potential misclassification would likely lead to an underestimate in effect size, because vaccinated individuals may be more proactive with regard to their health care and therefore more likely to seek SARS-CoV-2 testing for any reason compared with unvaccinated individuals, although it is difficult to be certain without a systemic approach to testing, which may partly explain the lower efficacy rates seen in our study. Furthermore, the robust VHA approach to capture both VHA and non-VHA diagnosed SARS-CoV-2 infections (via NLP) should serve to minimize misclassification.
Fourth, patients vaccinated outside the VHA system could lead to exposure misclassification; however, this would be expected to bias estimates towards a null hypothesis, suggesting that the observed results may again be conservative.
Finally, there is the possibility of misclassification of medication exposures, and records of medications prescribed outside the VHA may be incomplete. This issue may be most salient for steroid use, which may be dynamic over even short periods of time. However, veterans have a strong adherence in using the VHA pharmacy, and depending on their benefit status, the medications are typically free or cheaper than non-VHA alternatives.22 , 23 Furthermore, we used a narrow ascertainment window for medications before the index date, and given the relatively short follow-up time, the impact of medication exposure misclassification in this study would be minimal.
Conclusion
In a large national cohort of patients with IBD with diverse exposure to immunosuppressive agents we found that full vaccination, but not partial vaccination, was significantly associated with reduced SARS-CoV-2 infection with an 80.4% effectiveness > 7 days after the second dose. Among the 6578 patients with full vaccination status, no SARS-CoV-2 infections were identified among those taking anti-TNF agents, ustekinumab, or methotrexate. To the best of our knowledge, this is the first study to report these findings, and they should provide positive reinforcement to IBD patients taking immunosuppressive agents who may otherwise be reluctant to receive vaccination.
Acknowledgements
The authors thank Rebecca A. Hubbard and James D. Lewis for useful conversations that informed the methodological approach in this study.
CRediT Authorship Contributions
Nabeel Khan, MD (Conceptualization: Lead; Formal analysis: Lead; Methodology: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead; Interpretation of data: Lead). Nadim Mahmud, MD, MS, MPH, MSCE (Conceptualization: Lead; Formal analysis: Lead; Methodology: Lead; Writing – original draft: Lead; Writing – review & editing: Lead; Data acquisition and interpretation: Lead).
Footnotes
Conflicts of interest This author discloses the following: Nabeel Khan has received an unrestricted research grant from Pfizer, Luitpold, Takeda Pharmaceuticals, and Samsung BioEpis. The remaining author discloses no conflicts.
Funding No funding was received for this study.
References
- 1.Centers for Disease Control and Prevention COVID-19 Mortality Overview: Provisional Death Counts for Coronavirus Disease 2019 (COVID-19), 2021. https://www.cdc.gov/nchs/covid19/mortality-overview.htm
- 2.Graham D.B., Xavier R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature. 2020;578:527–539. doi: 10.1038/s41586-020-2025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchgesner J., Lemaitre M., Carrat F. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–346.e10. doi: 10.1053/j.gastro.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Irving P.M., Gibson P.R. Infections and IBD. Nat Clin Pract Gastroenterol Hepatol. 2008;5:18–27. doi: 10.1038/ncpgasthep1004. [DOI] [PubMed] [Google Scholar]
- 5.Allocca M., Fiorino G., Zallot C. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol. 2020;18:2134–2135. doi: 10.1016/j.cgh.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taxonera C., Sagastagoitia I., Alba C. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52:276–283. doi: 10.1111/apt.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubatan J., Levitte S., Balabanis T. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology. 2020;159:1141–1144.e2. doi: 10.1053/j.gastro.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan N., Patel D., Xie D. Are patients with inflammatory bowel disease at an increased risk of developing SARS-CoV-2 than patients without inflammatory bowel disease? Results from a Nationwide Veterans’ Affairs Cohort Study. Am J Gastroenterol. 2021;116:808–810. doi: 10.14309/ajg.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 9.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 10.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Department of Veterans Affairs National Center for Veterans Analysis and Statistics. Volume. https://www.va.gov/health/ 2021, 2021. Available at:
- 13.U.S. Department of Veterans Affairs Department of Veterans Affairs COVID-19 National Summary. Volume. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary 2021, 2021. Available at:
- 14.Khan N., Patel D., Trivedi C. Overall and comparative risk of herpes zoster with pharmacotherapy for inflammatory bowel diseases: a nationwide cohort study. Clin Gastroenterol Hepatol. 2018;16:1919–1927.e3. doi: 10.1016/j.cgh.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Chapman A.B., Peterson K.S., Turano A. A Natural Language Processing System for National COVID-19 Surveillance in the US Department of Veterans Affairs 2020. https://www.aclweb.org/anthology/2020.nlpcovid19-acl.10.pdf
- 16.Khan N, Mahmud N, Trivedi C, et al. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut. Published online March 22, 2021. 10.1136/gutjnl-2021-324356. [DOI] [PMC free article] [PubMed]
- 17.Dagan N., Barda N., Kepten E. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J., Weinhandl E.D., Gilbertson D.T. Issues regarding ‘immortal time’ in the analysis of the treatment effects in observational studies. Kidney Int. 2012;81:341–350. doi: 10.1038/ki.2011.388. [DOI] [PubMed] [Google Scholar]
- 21.Jones M., Fowler R. Immortal time bias in observational studies of time-to-event outcomes. J Crit Care. 2016;36:195–199. doi: 10.1016/j.jcrc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Melmed G.Y., Agarwal N., Frenck R.W. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–154. doi: 10.1038/ajg.2009.523. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy N.A., Goodhand J.R., Bewshea C. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 24.Wong S-Y, Dixon R, Pazos VM, et al. Serological response to mRNA COVID-19 vaccines in IBD patients receiving biological therapies. Gastroenterology. Published online April 20, 2021. 10.1053/j.gastro.2021.04.025. [DOI]
- 25.Taleban S., Colombel J.-F., Mohler M.J. Inflammatory bowel disease and the elderly: a review. J Crohns Colitis. 2015;9:507–515. doi: 10.1093/ecco-jcc/jjv059. [DOI] [PubMed] [Google Scholar]