Abstract
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has overwhelmed hospital systems globally, resulting in less experienced staff caring for critically ill patients within the intensive care unit (ICU). Many guidelines have been developed to guide nutrition care.
Aim
To identify key guidelines or practice recommendations for nutrition support practices in critically ill adults admitted with COVID-19, to describe similarities and differences between recommendations, and to discuss implications for clinical practice.
Methods
A literature review was conducted to identify guidelines affiliated with or endorsed by international nutrition societies or dietetic associations which included recommendations for the nutritional management of critically ill adult patients with COVID-19. Data were extracted on pre-defined key aspects of nutritional care including nutrition prescription, delivery, monitoring and workforce recommendations, and key similarities and discrepancies, as well as implications for clinical practice were summarized.
Results
Ten clinical practice guidelines were identified. Similar recommendations included: the use of high protein, volume restricted enteral formula delivered gastrically and commenced early in ICU and introduced gradually, while taking into consideration non-nutritional calories to avoid overfeeding. Specific advice for patients in the prone position was common, and non-intubated patients were highlighted as a population at high nutritional risk. Major discrepancies included the use of indirect calorimetry to guide energy targets and advice around using gastric residual volumes (GRVs) to monitor feeding tolerance.
Conclusion
Overall, common recommendations around formula type and route of feeding exist, with major discrepancies being around the use of indirect calorimetry and GRVs, which reflect international ICU nutrition guidelines.
Keywords: Guideline, Recommendations, COVID-19, Nutrition support, Enteral nutrition, Dietitian
1. Introduction
Globally the Coronavirus Disease 2019 (COVID-19) pandemic has impacted more than 100 million people, with hospitalization reported to occur at a rate of 4.6 per 100,000 population. Approximately 90% of hospitalized patients have more than one co-morbidity, with the most frequent being obesity, hypertension, chronic lung disease, diabetes and cardiovascular disease, complicating nutritional management [1]. Five to ten percent of hospitalized patients with COVID-19 develop severe acute respiratory distress coronavirus 2 (SARS-CoV-2) and require an intensive care unit (ICU) admission [1,2]. Commonly, patients present with progressive symptoms, several days after contracting the illness. In severe cases, this may cause respiratory distress syndrome, heart failure, and septic shock, which can lead to multi-organ failure and uncontrolled acute inflammation causing pulmonary tissue damage [3]. Critically ill patients with COVID-19 are at high nutritional risk due to critical illness itself, its medical management (e.g. organ support, sedation, ventilation) and significant metabolic changes such as persistent fever and hypermetabolism [4]. Patients may stay in the ICU for anywhere between a few days to months and the majority require mechanical ventilation with high doses of sedation [2,5]. Furthermore, patients often present with several unique physiological symptoms which are likely to impact nutritional intake including a loss of taste and smell, poor appetite and gastrointestinal (GI) symptoms in around 10% of cases, such as diarrhoea, nausea, and vomiting [6,7]. It has been reported that up to 65% of patients admitted to the ICU with COVID-19 are malnourished [[8], [9], [10]].
Due to the nature of this pandemic, the number of admissions has overwhelmed many ICUs and healthcare workers have been considerably impacted. As a result, less experienced staff have been required to work in the ICU. Due to both the complexity of the patients and the environment, guidelines detailing the nutritional management of these patients are vital to ensure safe patient care [11]. To date there is no international consensus on the optimal nutrition care of critically ill patients with COVID-19. The objective of this review was to identify key guidelines or practice recommendations which have been published for nutrition support practices in critically ill adults admitted with COVID-19. The secondary objectives were to describe similarities and differences between the recommendations, and to discuss implications for clinical practice.
2. Materials and methods
Guidelines, practice recommendations, or consensus recommendations affiliated with or produced by a professional nutrition society or dietetic association known to authors at commencement of the review were assessed against eligibility criteria. In addition, a literature search of Medical Literature Analysis and Retrieval System Online (MEDLINE) on Ovid was conducted from January 2020 to 22 January 2021 to identify further relevant guidelines that were published in scientific journals. Search strings included terms related to 1) intensive care; 2) nutrition support; and 3) guidelines/practice recommendations. The search strategy was based on two previously published search strategies from an affiliated institution [12,13] and is shown in the Supplemental Table S1. The websites of the International Confederation of Dietetic Associations (40 member countries) (https://www.internationaldietetics.org/NDAs.aspx) and reference lists of review articles identified via MEDLINE were also searched for relevant guidelines.
Guidelines and practice recommendations were included if they met all of the below inclusion criteria and none of the exclusion criteria:
2.1. Inclusion
-
1.
Included recommendations for the nutritional management of patients with COVID-19.
-
2.
Contained recommendations for critically ill adults.
-
3.
Related to care provided directly within the ICU setting.
-
4.
Were affiliated with an international nutrition or dietetic society.
2.2. Exclusion
-
1.
Were an opinion piece, narrative or systematic review.
-
2.
Were not published in English.
-
3.
Related solely for patients following an intensive care stay (e.g. standalone post-ICU recommendations were excluded).
Data were extracted on pre-defined key aspects of nutrition care identified as important by the authors during a pandemic and for patients with COVID-19, including: nutrition risk screening; nutrition prescription; timing, route, and mode of feeding; formula type; monitoring of nutrition intervention; recommendations for specific patient populations or conditions and; recommendations on service provision such as equipment considerations and workforce. Key similarities and discrepancies, as well as implications for clinical practice are discussed.
3. Results
3.1. Summary of published guidelines
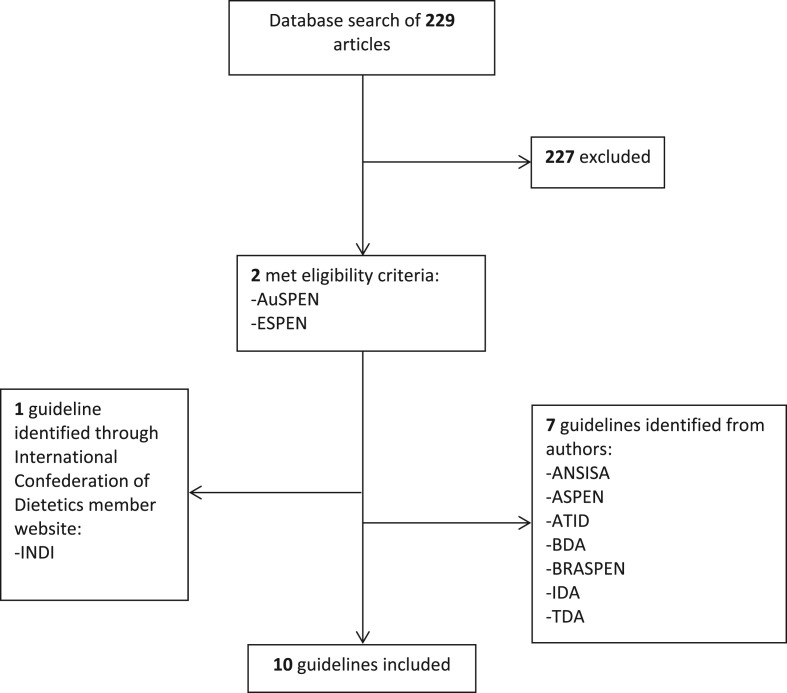
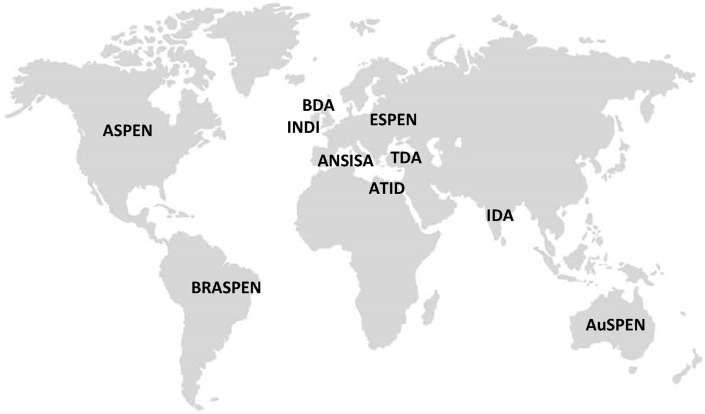
Seven guidelines were known to authors at commencement of the review and were included. To identify further guidelines unknown to authors, a database search was conducted which identified 229 non-duplicate articles of which two met all inclusion criteria and none of the exclusion criteria, and a final guideline was identified through searching the websites of the International Confederation of Dietetic Associations (CONSORT diagram in Fig. 1 ). Overall, 10 guidelines were included in this review from the following associations: American Society For Parenteral and Enteral Nutrition (ASPEN) [14]; Australian Society For Parenteral and Enteral Nutrition (AuSPEN) [15]; Brazilian Society of Parenteral and Enteral Nutrition (BRASPEN) [16]; British Dietetic Association (BDA) [17]; European Society for Clinical Nutrition and Metabolism (ESPEN) [18]; Indian Dietetic Association (IDA) [19]; Irish Nutrition and Dietetic Institute (INDI) [20]; Israeli Dietetic Association (ATID) [21]; ANSISA: National Association of Specialists in Food Science (ANSISA; Italy) [22]; Turkish Dietetic Association (TDA) [23] (Supplemental Table S2; Fig. 2 ).
Fig. 1.
CONSORT diagram of included guidelines. ANSISA: National Association of Specialists in Food Science (Italy); ASPEN: American Society for Parenteral and Enteral Nutrition; AuSPEN: Australasian Society for Parenteral and Enteral Nutrition; BDA: British Dietetic Association; BRASPEN: Brazilian Society of Parenteral and Enteral Nutrition; ESPEN: European Society for Clinical Nutrition and Metabolism; INDI: Irish Nutrition and Dietetic Institute; IDA: Indian Dietetic Association; ATID: Israeli Dietetic Association; TDA: Turkish Dietetic Association.
Fig. 2.
Geographical location of guideline development. ASPEN: American Society for Parenteral and Enteral Nutrition; AuSPEN: Australasian Society for Parenteral and Enteral Nutrition; BRASPEN: Brazilian Society of Parenteral and Enteral Nutrition; BDA: British Dietetic Association; ESPEN: European Society for Clinical Nutrition and Metabolism; IDA: Indian Dietetic Association; INDI: Irish Nutrition and Dietetic Institute; ATID: Israeli Dietetic Association; ANSISA: National Association of Specialists in Food Science (Italy); TDA: Turkish Dietetic Association.
The guideline development process was reported for five guidelines (ASPEN, AuSPEN, BDA, ESPEN and ATID) [14,15,17,18,21]. Recommendations were developed using clinician experiences with COVID-19 (BDA, ATID) [17,21], extrapolated from previous international guidelines for nutrition provision in critically ill patients without COVID-19 (ESPEN, ATID, ASPEN, AuSPEN) [14,15,18,21], expert consensus (ESPEN, AuSPEN) [15,18], infection control practices (ATID) [21], and based on physiological processes reported in patients with COVID-19 (AuSPEN) [15]. No guideline provided levels of evidence for their recommendations.
3.2. Common recommendations and major discrepancies
3.2.1. Nutrition risk screening
Seven guidelines state the importance of nutrition risk screening in critically ill patients with COVID-19 (Table 1 ) [[14], [15], [16],18,[20], [21], [22]]. Three guidelines recommend a specific validated tool: NRS-2002 (ESPEN) [18]; MST or MUST (INDI) [20] and; NUTRIC (ATID) [21]. A further two state specific patient populations that should be considered at higher nutritional risk: ASPEN recommend identifying pre-existing malnutrition or risk factors for re-feeding syndrome [14] and AuSPEN provide specific criteria to categorize patients as high or low nutritional risk to target early intervention [15]. In addition, two (AuSPEN and BRASPEN) acknowledge that the safety of staff must be considered while completing nutrition risk screening in a pandemic setting [15,16].
Key points.
-
-
Guidelines state nutritional risk screening is important to identify high risk patients requiring intervention
-
-
There is no agreement between guidelines as to which nutrition risk screening tool should be implemented
-
-
Guidelines recommend that the conduct of nutrition risk screening considers the safety of staff and reduction of bedside assessments, using coordinated care and remote working arrangements
Table 1.
Summary of content presented in each nutrition guideline for critically ill patients admitted with COVID-19.
| Guideline or practice recommendation society | Nutrition risk screening | Nutrition requirements/prescription | Timing of initiation | Route of feeding | Mode of feeding | Formula prescription | Monitoring | Specific patient populations/conditions | Equipment considerations | Workforce recommendations |
|---|---|---|---|---|---|---|---|---|---|---|
| ANSISA | √ | √ | Χ | √ | √ | √ | Χ | Χ | Χ | Χ |
| ASPEN | √ | √ | √ | √ | √ | √ | √ | √ | Χ | Χ |
| AuSPEN | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| BDA | Χ | Χ | Χ | √ | √ | √ | √ | √ | √ | √ |
| BRASPEN | √ | √ | √ | √ | Χ | √ | √ | Χ | Χ | Χ |
| ESPEN | √ | √ | √ | √ | Χ | Χ | √ | √ | Χ | √ |
| IDA | Χ | √ | √ | √ | √ | √ | Χ | √ | Χ | √ |
| INDI | √ | Χ | √ | √ | √ | √ | √ | √ | Χ | Χ |
| ATID | √ | √ | √ | √ | Χ | √ | √ | √ | Χ | √ |
| TDA | √ | √ | Χ | √ | Χ | √ | Χ | Χ | Χ | Χ |
ANSISA: National Association of Specialists in Food Science (Italy); ASPEN: American Society for Parenteral and Enteral Nutrition; AuSPEN: Australasian Society for Parenteral and Enteral Nutrition; BDA: British Dietetic Association; BRASPEN: Brazilian Society of Parenteral and Enteral Nutrition; ESPEN: European Society for Clinical Nutrition and Metabolism; INDI: Irish Nutrition and Dietetic Institute; IDA: Indian Dietetic Association; ATID: Israeli Dietetic Association; TDA: Turkish Dietetic Association.
3.2.2. Nutrition requirements and prescriptions
Five guidelines discuss the use of indirect calorimetry for measuring energy expenditure and one (ESPEN) recommends that indirect calorimetry is used where safely available [18]. Three (ASPEN, AuSPEN, BRASPEN) recommend against the use of indirect calorimetry due to risk of staff exposure to the virus, potential spread of disease, and/or workforce related demands [[14], [15], [16]]. The ATID guideline did not provide an explicit recommendation on the use of indirect calorimetry but mentioned the use of indirect calorimetry when considering energy prescription [21].
The majority of guidelines (eight of the 10) provide recommendations on the prescription of energy and protein in patients with COVID-19 using a predictive equation. All eight guidelines support the slow and progressive delivery of energy and protein during the first 5–7 days of critical illness, although approaches to this vary (Table 2 ) [[14], [15], [16],18,19,[21], [22], [23]]. Two guidelines (ANSISA and AuSPEN) provide algorithms for commencement and management of nutrition therapy; AuSPEN provides an algorithm with set rates for the first five days of nutrition therapy [15], while ANSISA provides guidance for continuous infusion rates [22]. Two guidelines (BDA and INDI) do not provide recommendations for energy and protein prescriptions; BDA encourages use of local practices and guideline recommendations [17] and INDI provides algorithms for out-of-hours EN and PN initiation [20].
Key points.
-
-
Two guidelines support the use of indirect calorimetry in patients with COVID-19, five do not make a recommendation and three state it should not be used
-
-
Slow and progressive delivery of energy and protein is recommended during the first 5–7 days of critical illness in patients with COVID-19
-
-
Energy and protein prescriptions vary, with 20–30 kcal/kg/day and 1.2–2.0 g/day protein most frequently recommended
-
-
Five guidelines recommend that non-nutritional calories from propofol and/or dextrose administration be considered when evaluating energy delivery
Table 2.
Guidelines and key recommendations.
| Guidelines | Energy requirements | Protein requirements | Route of feeding | Formula prescription | Initiation and considerations | Monitoring (GRVs) |
|---|---|---|---|---|---|---|
| ANSISA (Italian) | 20–25 kcal/kg/day | 1.2–2 g/kg/day | No recommendation for commencement of EN EN contraindication: PN is recommended within 3–7 days Supp PN: Consider if EN does not meet requirements |
High-protein energy EN, low carbohydrates, omega-3 enriched, no fiber as first preference | Start with <70% of requirements and increase progressively | N/A |
| ASPEN | First week: 15–20 kcal/kg/daya | 1.2–2.0 g/kg/daya | EN is preferred to PN EN contraindication: High nutrition risk: Commence PN as early as possible Low nutrition risk: may delay PN for 5–7 days |
Standard high protein (≥20% protein) polymeric isosmotic EN in acute phase | 24–36 h of ICU admission (or within 12 h of intubation) Low dose EN (hypocaloric or trophic), advancing to full dose EN over the first week |
Do not routinely monitor GRVs |
| AuSPEN | Day 1–5: Standard feed rate 50 ml/h 1.25 kcal/ml Day 6+: 25 kcal/kg/day (up to 30 kcal/kg/day for severely unwell patients + prolonged admission)b |
≥1.2 g/kg/dayb | No recommendation for commencement of EN Supp PN: Consider where post-pyloric EN is not possible and intake is consistently <50% of targets over 5–7 days |
Use energy-dense EN formula (1.25–1.5 kcal/ml) | Low nutrition risk: within 24 h of ICU admission High nutrition risk: Assess prior to EN commencement |
300 ml cut-off (8 hourly). Stop monitoring in non-prone patients if GRVs are <300 ml for >48 h |
| BDA | N/A | N/A | Consider an NGT on admission, post-pyloric tube if persistently high GRVs PN: consider if post-pyloric feeding is not available |
Avoid large volumes/high rates of EN. Consider 1.3/1.5 kcal/ml EN | N/A | Use local cut-off for non-prone patients and 300 ml cut-off (4 hourly) for prone patients |
| BRASPEN | Day 1–4: 15–20 kcal/kg/day Day 5+: 25 kcal/kg/day |
Day 1–2: <0.8 g/kg/day Day 3–5: 0.8–1.2 g/kg/day Day 6+: >1.5 g/kg/day |
EN preferred route in critical illness EN contraindication: PN should be initiated as early as possible. Supp PN: after 5–7 days where intake cannot reach >60% of requirements |
Use of EN with omega 3, borage oils, and antioxidants is not indicated | 24–48 h of admission | N/A |
| ESPEN | Use IC where safe, if so: Day 1–3: <70% of measured REE Day >3–7: progression to 80–100% measured REE If using predictive equation: <70% estimated target for first week |
1.3 g/kg/dayc delivered progressively | Oral + ONS preferred, followed by EN EN contraindication: PN to be considered Supp PN: case-by-case basis if not tolerating full dose EN during the first week in ICU |
N/A | 24-48 during hospitalization | 500 ml cut-off |
| IDA | First week: 15–20 kcal/kg/day | 1.3–1.5 g/kg/day (up to 2 g/kg/day with high metabolic demands) | EN preferred to PN EN contraindication: PN to be initiated as early as possible in high nutrition risk patient |
Standard high protein (>20% protein) polymeric and isosmotic EN | 24–36 h of ICU admission (or within 12 h of intubation) Low dose EN (hypocaloric or trophic), advancing to full dose EN over the first week |
Do not routinely monitor GRVs |
| INDI | N/A | N/A | EN preferred to PN EN contraindication: High nutrition risk: Commence PN as early as possible Low nutrition risk: Commence PN day 3–7 |
Consider double-strength EN Higher protein EN with lower energy content, if on high dose propofol (>15 ml/h) |
24–48 h once hemodynamically stable | Only check GRVs for surgical, prone positioned, intestinal failure, and multi-organ failure patients and those who had vomited in the previous 24 h |
| ATID | Day 1–2: up to 70% of 25 kcal/kg/day Day 3–7: 25 kcal/kg/dayd Day >7: 25–30 kcal/kg/dayd |
Day 1–2: up to 70% of ≥1.3 g/kg/day Day 3–7: ≥1.3 g/kg/dayd Elderly: 1.5–2.0 g/kg/day Day >7: 1–1.5 g/kg/dayd |
No recommendation for commencement of EN Supp PN: Consider days 3–7 in the case of malnutrition/severe EN intolerance |
High protein EN Concentrated formulas (1.5–2 kcal/ml) recommended for patients who require fluid restriction |
Within 48 h | 500 ml cut-off (6 hourly) |
| TDA | 25–30 kcal/kg/day | 1.2–2.0 g/kg/day | Oral feeding is preferred EN for intubated patients, recommend post-pyloric tube |
Whole-protein preparations with relatively high calories can be selected | Start with low dosage and gradually increase | N/A |
Abbreviations: EN, enteral nutrition; ONS, oral nutrition supplementation; PN, parenteral nutrition, ANSISA: National Association of Specialists in Food Science (Italy); ASPEN: American Society for Parenteral and Enteral Nutrition; AuSPEN: Australasian Society for Parenteral and Enteral Nutrition; BDA: British Dietetic Association; BRASPEN: Brazilian Society of Parenteral and Enteral Nutrition; ESPEN: European Society for Clinical Nutrition and Metabolism; INDI: Irish Nutrition and Dietetic Institute; IDA: Indian Dietetic Association; ATID: Israeli Dietetic Association; TDA: Turkish Dietetic Association.
Adjusted targets recommended in obesity.
Adjusted body weight should be used for overweight and obese patients as per usual site method.
1.3 g/kg “adjusted body weight” protein equivalents per day is recommended. Adjusted body weight is calculated as ideal body weight + (actual body weight - ideal body weight) ∗ 0.33.
Different targets recommended in obesity using an adjusted body weight.
3.2.3. Timing of initiation
Seven guidelines recommend the initiation of EN within 48 h of admission, in line with broader critical illness nutrition practice guidelines [24,25]. Two guidelines (ASPEN and IDA) discuss timing of initiation in patients with sepsis or circulatory shock, recommending early EN at a trophic rate be considered [14,19]. The ASPEN guideline states COVID-19 should not be considered a contraindication to early trophic EN, unless combined with escalating vasopressor use and EN intolerance [14]. The remaining three guidelines do not provide specific recommendations for the timing of initiation of EN [17,22,23], with the TDA referring to “early intestinal nutrition” but providing no further guidance [23].
Key points.
-
-
The early initiation of EN within 48 h of admission is recommended in seven of 10 guidelines
-
-
The use of early trophic EN in patients with sepsis or circulatory shock (if not combined with increasing vasopressor needs or EN intolerance) is recommended in two guidelines
3.2.4. Route of feeding
All of the included guidelines recommend the enteral route (oral or EN) in preference of PN for nutrition therapy [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]. Four (ASPEN, AuSPEN, BDA, ESPEN) guidelines recommend the commencement of EN via the nasogastric (NG) route [14,15,17,18]. One guideline (TDA) recommends post-pyloric EN as the first line feeding route, stating that critically ill patients often experience GI intolerance [23]. In the four guidelines where NG feeding is recommended, progression to post-pyloric EN is not recommended unless adequate management of GI intolerance is first attempted (such as the use of prokinetics) due to the risk to staff with tube insertions [14,15,17,18]. The remaining five guidelines recommended nutrition via the EN route but do specify whether delivery should be via a NG or post-pyloric tube [16,[19], [20], [21], [22]]. In the case of uncontrolled shock and hemodynamic instability, four (ASPEN, ESPEN, IDA and ATID) guidelines recommend that EN is withheld and gradually recommenced upon patient stabilization [14,18,19,21].
Six (ANSISA, ASPEN, BRASPEN, ESPEN, IDA, INDI) guidelines provide recommendations for the commencement of PN where EN is contraindicated (Table 2) [14,16,[18], [19], [20],22]; with three (ASPEN, IDA, INDI) recommending that PN is initiated as soon as possible in high nutrition risk and/or malnourished patients [14,19,20]. The commencement of supplemental PN is mentioned in five (ANSISA, AuSPEN, BRASPEN, ESPEN, ATID) guidelines (Table 2), but the recommended timing varies; four (AuSPEN, BRASPEN, ESPEN, ATID) guidelines recommending consideration of commencement within the first week of ICU where intake is suboptimal and one (ANSISA) not providing a specific recommendation regarding the timing of supplemental PN initiation [22]. Two (BDA and TDA) guidelines do not explicitly discuss the use of PN; one guideline (BDA) stating that it may be required where post-pyloric EN is not available and the remaining guideline (TDA) mentioning the use of PN in elderly patients at high aspiration risk or GI intolerance [17,23].
Key points.
-
-
Guidelines recommend the enteral route (oral or EN) is preferred over PN
-
-
Five guidelines discuss route of EN, with four recommending NG feeding followed by post-pyloric nutrition, where EN intolerance management strategies have not been successful
-
-
Four guidelines recommend that EN is withheld in the case of uncontrolled shock and gradually recommenced on stabilization
-
-
In the case of a contraindication to EN, commencing PN as soon as possible in high nutrition risk patients is recommended in three of the five guidelines that discuss this topic
-
-
Recommendations regarding the commencement of supplemental PN vary; with four guidelines recommending use within seven days of ICU admission in patients unable to meet nutrition requirements
3.2.5. Mode of feeding
Six guidelines (ANSISA, ASPEN, AuSPEN, BDA, IDA, INDI) include recommendations on the mode of feeding, with all guidelines recommending continuous EN [14,15,17,19,20,22]. ASPEN were the only society to provide evidence to justify this recommendation, stating a reduction in diarrhoea and less frequent patient interaction for staff with continuous EN, decreasing exposure of healthcare professionals to COVID-19 [14].
Key point.
-
-
Guidelines recommend continuous EN as the preferred mode of nutrition for patients with COVID-19
3.2.6. Enteral formula prescription
All but one guideline provided a recommendation on the type of EN formula to prescribe for critically ill patients admitted with COVID-19 [[14], [15], [16], [17],[19], [20], [21], [22], [23]]. Four guidelines provide a specific recommendation on caloric density of EN (ANSISA, AuSPEN, BDA, ATID) with disparate recommendations, ranging from 1.25 to 2 kcal/ml [15,17,21,22]. Six guidelines (ANSISA, ASPEN, AuSPEN, IDA, INDI, ATID) recommend using a high protein formula [14,15,[19], [20], [21], [22]] with three guidelines encouraging the use of protein supplementation delivered through a bolus [20], modular protein [21], or as a single bolus to cluster care [14].
ANSISA, ASPEN, and the IDA recommend using fiber-free formula early in the ICU admission [14,19,22]. Conflicting advice on the use of omega-3 enriched formula are present, with ANSISA and the IDA recommending omega-3 [19,22] while the BRASPEN guideline states the use of EN with omega-3 is not indicated [16]. Similarly, conflicting advice on carbohydrate load to manage dysglycemia is present: ANSISA recommend a low carbohydrate formula [22] and the TDA recommends the use of nutritional preparations which are beneficial to glycemic control in hyperglycaemic patients [23], while the ATID states there is no widespread recommendation for the use of EN low in carbohydrates compared to standard EN to achieve glycemic control [21].
Key points.
-
-
An energy-dense formula (>1.25 kcal/ml) is generally recommended within guidelines, with a more highly concentrated formula recommended for restrictive fluid management
-
-
The guidelines largely recommend a high protein (≥20% protein) enteral formula
-
-
Conflict exists within guidelines on the use of glucose-lowering formula (including lower carbohydrate preparations) and omega-3 enrichment
3.2.7. Monitoring
3.2.7.1. Gastric residual volumes
Seven guidelines make recommendations about using gastric residual volumes (GRVs) to monitor EN tolerance [15,[17], [18], [19], [20], [21]]. Five of these guidelines (AuSPEN, BDA, ESPEN, INDI, ATID) recommended monitoring GRVs at various timepoints using different cut-offs, as shown in Table 2 [15,17,18,20,21]. Both ASPEN and the IDA do not recommend routinely monitoring GRVs, citing that GRV monitoring is not reliable for detection of delayed gastric emptying, can impact nutrition delivery, and is a risk of viral transmission to the healthcare provider [14,19].
3.2.7.2. Electrolytes
Four guidelines mention that re-feeding syndrome risk should be considered in critically ill patients admitted with COVID-19 (ASPEN, BRASPEN, ESPEN, ATID), as poor appetite and intake and gastrointestinal symptoms are common before hospital admission. These guidelines recommend close monitoring and replacement of potassium, phosphate, and magnesium when commencing nutrition support [14,16,18,21].
3.2.7.3. Triglycerides
Four guidelines (ASPEN, ESPEN, INDI, ATID) recommend monitoring serum triglycerides when patients are receiving propofol and/or PN [14,18,20,21]. The ASPEN guideline also recommended that when interpreting elevated triglyceride levels, clinicians should be aware that a subset of COVID-19 patients develop a cytokine storm that resembles hemophagocytichistiocytosis (secondary HLH) [14], and this may be the cause of hypertriglyceridemia (rather than propofol-induced) [24].
3.2.7.4. Nutrition adequacy
To prevent under- or over-feeding, the majority of guidelines recommend close monitoring of nutrition adequacy (energy and protein delivery compared to estimated or measured requirements) [[14], [15], [16], [17], [18], [19], [20], [21], [22]]. Five guidelines (ASPEN, AuSPEN, BDA, INDI, ATID) highlight the importance of monitoring the delivery of non-nutritional calories (e.g. glucose, propofol) [14,15,17,20,21].
Key points.
-
-
Guidelines are conflicted regarding what GRV cut-off should be used and if GRVs are a useful indicator of enteral tolerance
-
-
The monitoring of electrolytes and triglycerides during the commencement and continuation of nutrition support is recommended in five and four guidelines respectively
-
-
The guidelines recommend that clinicians monitor nutrition adequacy (including non-nutritional calories) to prevent under- or over-feeding
3.2.8. Specific patient populations
3.2.8.1. Prone positioning
Seven guidelines recommend early EN, given continuously, while patients are in the prone position [[14], [15], [16], [17], [18],20,21]. Five of the seven guidelines (ASPEN, AuSPEN, BRASPEN, BDA, ATID) highlight that patients in the prone position may have increased GI intolerance, and prokinetics and insertion of post-pyloric tubes should be considered as necessary [[14], [15], [16], [17],21].
3.2.8.2. Patients receiving extracorporeal membrane oxygenation
Three guidelines (AuSPEN, ASPEN, ATID) make specific recommendations for patients receiving extracorporeal membrane oxygenation (ECMO) [15,17,23]. Two (ASPEN, ATID) specifically recommend early EN in this patient group [14,21]. The AuSPEN and ATID guidelines highlight that patients on ECMO are likely to have high metabolic needs (e.g. after ICU day 5 up to 30 kcal/kg and 1.5 – 2 g protein/kg day in normal-weight individuals) [15,21]. The ASPEN guideline highlights that in the past there was concern about lipid infiltration into the oxygenator when patients were receiving PN; however, with newer ECMO circuits it is stated that this is no longer a concern [14].
3.2.8.3. Non-intubated critically ill patient
Seven guidelines make recommendations for non-intubated patients [15,17,18,[20], [21], [22], [23]]. The overarching theme is that these patients are at high nutrition risk (e.g. due to poor appetite, fatigue, difficulty breathing, dysphagia) and that a high energy and high protein diet and oral nutrition supplements should be provided. Escalation to enteral nutrition should occur if energy and protein intakes are inadequate (e.g. meeting <50–65% targets after 5 days). Three guidelines (AuSPEN, BDA, ATID) specifically recommend avoiding early removal of nasogastric tubes post extubation [15,17,21].
Key points.
-
-
Seven guidelines recommend early EN in patients in the prone position, while monitoring GI tolerance closely
-
-
Two guidelines recommend early EN for patients receiving ECMO
-
-
Guidelines report that patients who are not intubated are considered at high nutrition risk and should receive a high energy high protein diet and oral nutritional supplements ± EN
3.2.9. Workforce and equipment recommendations
3.2.9.1. Equipment
Two guidelines (AuSPEN and BDA) recommend assessment of equipment needs early and development of plans in the event of equipment and nutrition formula shortage [15,17].
3.2.9.2. Workforce
Two guidelines (BDA and AuSPEN) make specific recommendations regarding dietetic workforce capacity during the COVID-19 pandemic [15,17]. This includes rapidly identifying additional staff who could be used in the case of significant admission numbers (such as the use of appropriately trained allied health assistance staff, or training of dietetic staff who have transferable skills in specific ICU nutrition processes). It is also recommended that training be commenced early with an appropriate education package that has been developed by an experienced critical care dietitian, and that the most experienced critical care dietitians see the sickest patients. Five guidelines (BDA, AuSPEN, ESPEN, INDI and ATID) mention use of remote working processes to protect staff from infection risk of COVID-19 [15,17,18,20,21]. Two guidelines (AuSPEN and ESPEN) specifically mention appropriate personal protective equipment (PPE) training for nutrition staff [15,18].
Key points.
-
-
Consideration to equipment, nutrition formula requirements and workforce capacity issues was infrequently discussed
-
-
Five guidelines discussed remote working and revision of processes to facilitate this
-
-
Specific mention of PPE training for nutrition staff was infrequent
3.2.10. Post-ICU
While not the primary focus of this article, four guidelines provided a section on nutritional management in the post-ICU or recovery phase within the ICU focussed guideline [15,17,18,21].
4. Discussion
4.1. Implementation into clinical practice
The application of recommendations within included guidelines is likely to be dependent on the clinical setting, the resources and available workforce, the individual patient presentations, and the stage of the pandemic. Due to the limited data available to inform optimal patient care, clinicians should utilize the available recommendations as a guide. However, where possible, focus should still be on the individual assessment and monitoring of nutrition support within the limitations of the available workforce [26]. When considering how to implement these recommendations, ICUs should consider the range of guidelines available and develop local procedures that take into consideration their patient cohort and workforce capabilities. ICUs may need to consider the standard operating procedures within their unit and what they are willing to change in the setting of a pandemic to minimize PPE use and reduce staff exposure [27].
Across most guidelines the maintenance of nutrition screening has been highlighted as important to continue to identify patients who are at the greatest nutritional risk. Malnourished patients and those with complex co-morbidities should be priorities for more specialized nutrition care [26]. However, all critically ill patients with COVID-19 are likely to be at nutritional risk and; therefore, early nutritional interventions should be integrated into the overall medical therapy [26]. The majority of the guidelines support the initiation of early EN in mechanically ventilated patients via the gastric route. Nutritional targets for protein tend to align with standard critical care nutrition guidelines, with a focus on high protein provision. Clinicians should therefore consider which nutrition formula they have available to try to reach these targets. Where the guidelines conflict, including in relation to the measurement of GRVs and the use of indirect calorimetry, clinicians should deliberate the risks and consider what is acceptable within their unit. All guideline recommendations need to be considered in the context of a lack of specific data and are predominately based on expert opinion rather than high level evidence, and it is hence reasonable to adapt them to the local context.
It should be recognized that the included guidelines were developed early in the pandemic (March–September 2020) and; therefore, may not reflect the most recently available evidence for managing patients with COVID-19 in ICU. New data is rapidly emerging to guide nutrition clinical care and should be considered [28,29]. In addition, as hospital systems become more coordinated, and patient numbers more manageable, the ability to safely implement higher-level practices to provide optimal nutrition care will improve, and these practices may be able to be reintroduced. For example, while the use of indirect calorimetry was not recommended early in the pandemic, primarily due to the increased risk of staff exposure to the virus. However, studies using indirect calorimetry have since been conducted safely as the pandemic and management strategies have progressed [28]. Reintroduction of these higher risk procedures should be considered based on site capacities, levels of expertise, and follow best practice recommendations [30].
4.2. Limitations
Limitations of the review include that only guidelines available in the English language were included, as it is likely that a number of country-specific associations would have guidelines in their native language only. Similarly, only websites of nutrition or dietetic societies that are members of the International Confederation of Dietetics were searched; however, it should be recognized that medical or intensive care specific societies may also have practice guidelines that incorporate a section on nutrition management.
5. Conclusions
Clinical recommendations for patients with COVID-19 were similar across guidelines and to those in the general ICU population including the use high protein, volume restricted enteral formula delivered gastrically and commenced early in ICU, while taking into consideration non-nutritional calories to avoid overfeeding. A number of discrepancies exist, including the use of IC to determine energy prescriptions, and monitoring of GI intolerance using GRVs, with these discrepancies also existing within international ICU nutrition guidelines. Further evidence generation is required to support many of the recommendations.
Author contributions
All authors (LC, OTB, KL, KF, ER) contributed to conceptualization; data curation; formal analysis; investigation; methodology; writing- original draft and writing-review & editing.
Grants and funding
Nil to declare.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgements
Authors would like to thank Matthew Summers, Rhea Louis, and Sarah McEwen for their assistance with figure preparation and International Confederation of Dietetic Associations website searches.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2021.05.003.
Appendix ASupplementary data
The following is the Supplementary data to this article:
Multimedia component 1
References
- 1.Minnelli N., Gibbs L., Larrivee J., Sahu K.K. Challenges of maintaining optimal nutrition status in COVID-19 patients in intensive care settings. J Parenter Enter Nutr. 2020;44(8):1439–1446. doi: 10.1002/jpen.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. J Am Med Assoc. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Quintela A., Milton-Laskibar I., Trepiana J., Gomez-Zorita S., Kajarabille N., Leniz A., et al. Key aspects in nutritional management of COVID-19 patients. J Clin Med. 2020;9(8) doi: 10.3390/jcm9082589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapple L., Fetterplace K., Ridley E. Nutrition for critically ill patients with COVID-19: ICU management and practice. ICU Management & Practice. 2020;20(1):52–57. [Google Scholar]
- 5.Richards-Belle A., Orzechowska I., Gould D.W., Thomas K., Doidge J.C., Mouncey P.R., et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46(11):2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. NEJM. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Liao B., Guo Y., Li F., Lei C., Zhang F., et al. Clinical characteristics of patients infected with the novel 2019 Coronavirus (SARS-Cov-2) in Guangzhou, China. Open Forum Infect Dis. 2020;7(6):ofaa187. doi: 10.1093/ofid/ofaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Filippo L., De Lorenzo R., D’Amico M., Sofia V., Roveri L., Mele R., et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr. 2021;40(4):2420–2426. doi: 10.1016/j.clnu.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mechanick J.I., Carbone S., Dickerson R.N., Hernandez B.J.D., Hurt R.T., Irving S.Y., et al. Clinical nutrition research and the COVID-19 pandemic: a scoping review of the ASPEN COVID-19 Task Force on nutrition research. J Parenter Enter Nutr. 2021;45(1):13–31. doi: 10.1002/jpen.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C., et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melnyk B.M. Important information about Clinical Practice Guidelines: key tools for improving quality of care and patient outcomes. Worldviews Evidence-Based Nurs. 2015;12(1):1–2. doi: 10.1111/wvn.12079. [DOI] [PubMed] [Google Scholar]
- 12.Seddon N., Chapple L.S., Tatucu-Babet O.A., Ridley E.J. Reporting of randomized controlled trials investigating an enteral or parenteral nutrition intervention in critical illness according to the CONSORT Statement: a systematic review and recommendation of minimum standard reporting criteria. J Parenter Enter Nutr. 2020 doi: 10.1002/jpen.2038. Available online ahead of print DOI. [DOI] [PubMed] [Google Scholar]
- 13.Tatucu O., Nguo K., Lambell K., Romero L., Earthman C., Ridley E. 2020. Doubly labelled water for determining total energy expenditure in adult critically ill and acute care hospitalised inpatients: a scoping review protocol. osf.io/4sp7e. [DOI] [PubMed] [Google Scholar]
- 14.Martindale R., Patel J.J., Taylor B., Warren M., McClave S. American Society for Parenteral and Enteral Nutrition; May 2020. Nutrition therapy in the patient with COVID-19 disease requiring ICU care. Version 26. [Google Scholar]
- 15.Chapple L.S., Fetterplace K., Asrani V., Burrell A., Cheng A.C., Collins P., et al. Nutrition management for critically and acutely unwell hospitalised patients with coronavirus disease 2019 (COVID-19) in Australia and New Zealand. Aust Crit Care. 2020;33(5):399–406. doi: 10.1016/j.aucc.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos L.F., Barreto P.A., Duprat G., Goncalves C.R.C., de Matos L.B.N., Zambelli C.M.S.F., et al. BRASPEN’s nutritional statement for coping with COVID-19 in hospitalized patients. Supported by Brazilian Intensive Care Medicine Association. Version 23. March 2020. Version 23. [Google Scholar]
- 17.Bear D., Terblanche E. 11 May 2020. Critical care specialist group (CCSG) of the BDA guidance on management of nutrition and dietetic services during the COVID-19 pandemic. Critical care specialist group for the British dietetic association. Version 2.1. [Google Scholar]
- 18.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Indian guidance for COVID-19 patients. Indian Dietetic Association; 2020. http://idaindia.com/mnt-guirdelines-for-covid-19/ Accessed online. [Google Scholar]
- 20.Irish Nutrition and Dietetic Institute . 2020. COVID-19 Dietetic care pathway Version 1 and guides to commencing enteral and parenteral nutrition in adult patients in intensive care with suspected or confirmed COVID-19 Version 2. Published March 2020. [Google Scholar]
- 21.Anbar R., Poulin D., Dolgich-Maza M., Refaeli R., Gilad D. Israeli Dietetic Association; April 2020. Feeding the critically Ill mechanically ventilated patient during the COVID-19 epidemic. [Google Scholar]
- 22.Cena H., Maffoni S., Braschi V., Brazzo S., Pallavicini C., Vietti I., et al. Position paper of the Italian association of medical specialists in dietetics and clinical nutrition (ANSISA) on nutritional management of patients with COVID-19 disease. Mediterr J Nutr Metabol. 2020;13(2):113–117. [Google Scholar]
- 23.Turkish dietetic association's recommendations on nutrition and COVID-19. Turkish dietetic association. 2020. http://www.efad.org/media/1956/turkish-dietetic-association-nutrition-recommendations-about-coronavirus-covid-1919.pdf Available online. [Google Scholar]
- 24.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) J Parenter Enteral Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 25.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Thibault R., Seguin P., Tamion F., Pichard C., Singer P. Nutrition of the COVID-19 patient in the intensive care unit (ICU): a practical guidance. Crit care. 2020;24(1):447. doi: 10.1186/s13054-020-03159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martindale R., Patel J.J., Taylor B., Arabi Y.M., Warren M., McClave S.A. Nutrition therapy in critically ill patients with coronavirus disease 2019. JPEN J Parenter Enteral Nutr. 2020;44(7):1174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittle J., Molinger J., MacLeod D., Haines K., Wischmeyer P.E. for the LEEP-COVID study group. Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care. 2020;24(1):581. doi: 10.1186/s13054-020-03286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cereda E., Guzzardella A., Klersy C., Belliato M., Pellegrini A., Sciutti F., et al. Early caloric deficit is associated with a higher risk of death in invasive ventilated COVID-19 patients. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.02.020. Available online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer P., Pichard C., De Waele E. Practical guidance for the use of indirect calorimetry during COVID 19 pandemic. Clin Nutr Exp. 2020;33:18–23. doi: 10.1016/j.yclnex.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1