Abstract
Background
The world is in the midst of the COVID-19 pandemic. In this comprehensive review, we discuss the potential protective effects of (−)-epigallocatechin-3-gallate (EGCG), a major constituent of green tea, against COVID-19.
Scope and approach
Information from literature of clinical symptoms and molecular pathology of COVID-19 as well as relevant publications in which EGCG shows potential protective activities against COVID-19 is integrated and evaluated.
Key findings and conclusions
EGCG, via activating Nrf2, can suppress ACE2 (a cellular receptor for SARS-CoV-2) and TMPRSS2, which mediate cell entry of the virus. Through inhibition of SARS-CoV-2 main protease, EGCG may inhibit viral reproduction. EGCG via its broad antioxidant activity may protect against SARS-CoV-2 evoked mitochondrial ROS (which promote SARS-CoV-2 replication) and against ROS burst inflicted by neutrophil extracellular traps. By suppressing ER-resident GRP78 activity and expression, EGCG can potentially inhibit SARS-CoV-2 life cycle. EGCG also shows protective effects against 1) cytokine storm-associated acute lung injury/acute respiratory distress syndrome, 2) thrombosis via suppressing tissue factors and activating platelets, 3) sepsis by inactivating redox-sensitive HMGB1, and 4) lung fibrosis through augmenting Nrf2 and suppressing NF-κB. These activities remain to be further substantiated in animals and humans. The possible concerted actions of EGCG suggest the importance of further studies on the prevention and treatment of COVID-19 in humans. These results also call for epidemiological studies on potential preventive effects of green tea drinking on COVID-19.
Keywords: EGCG, COVID-19, SARS-CoV-2, Prevention, Treatment, Tea
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- DAMPs
damage-associated molecular patterns
- EGCG
epigallocatechin-3-gallate
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- GPX
glutathione peroxidase
- GST
glutathione S-transferase
- HMGB1
high mobility group box 1
- ICU
intensive care unit
- LPS
lipopolysaccharides
- Mpro
main protease
- NETs
neutrophil extracellular traps
- NOX
NADPH oxidases
- Nrf2
nuclear factor erythroid 2 p45-related factor 2
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- SOD
superoxide dismutase
1. Introduction
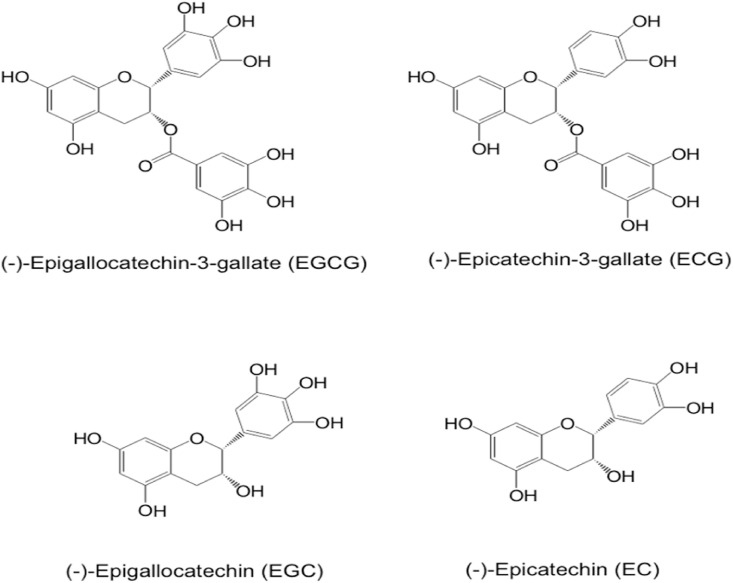
Green tea, made from the leaves of the plant Camelia sinensis, is a popular drink worldwide. Historically, tea was used as a medicinal herb to treat a variety of diseases. In scientific studies during the past decades, green tea and its characteristic polyphenols, catechins, have been shown to have activities in the prevention of obesity, diabetes, cardiovascular diseases, cancer, and other diseases (Yang & Hong, 2013; Yang & Zhang, 2019). Tea catechins have also been shown to have anti-viral activities (Kaihatsu, Yamabe, & Ebara, 2018; Steinmann, Buer, Pietschmann, & Steinmann, 2013; Xu, Xu, & Zheng, 2017), as well as protective activities against diseases caused by oxidative stress and inflammation. Many of these activities may help alleviate the devastating pandemic of COVID-19, caused by the virus SARS-CoV-2. Just in the United States alone, there have been more than 26 million cases of COVID-19 and 0.44 million deaths by February 2021, currently with an all-time high daily death rate of over 3000 per day. There have been three recent articles suggesting the possible prevention and treatment of COVID-19 by tea (Chowdhury & Barooah, 2020; Menegazzi et al., 2020; Mhatre, Srivastava, Naik, & Patravale, 2020). The major active constituent is (−)-epigallocatechin-3-gallate (EGCG), the most abundant catechin in green tea. Green tea also contains other catechins, such as (−)-epigallocatechin, (−)-epicatechin-3-gallate and (−)-epicatechin. The structures of these catechins are shown in Fig. 1 . This article is a comprehensive review of the mechanisms by which EGCG may inhibit SARS-CoV-2 infection and the different syndromes associated with COVID-19. The published results in the literature are critically evaluated – with the perspective of biological differences between rodents and humans, dose response relationships and possible side effects – to assess the possibility that EGCG or tea consumption can be useful for the prevention, alleviation and treatment of this pandemic disease.
Fig. 1.
Chemical structure of major green tea catechins.
2. EGCG, biological activities, and general anti-viral activities
EGCG, with a polyphenolic structure, is well recognized as a strong antioxidant through its activities in quenching reactive radicals and chelating metal ions to prevent the formation of reactive oxygen species (ROS). However, this redox-active molecule also undergoes auto-oxidation to produce superoxide radical and hydrogen peroxide. In cell lines, it can also be oxidized in the mitochondria to produce ROS (Tao, Forester, & Lambert, 2014; Tao, Park, & Lambert, 2015). At moderate levels of EGCG, the ROS produced can be beneficial via induction of nuclear factor erythroid 2 p45-related factor 2 (Nrf2) - mediated antioxidant and cytoprotective enzymes (Dong et al., 2016; Na et al., 2008; Sun et al., 2017; Yang et al., 2018), generally referred to as the indirect antioxidant activity of EGCG. These enzymes play far more important roles in cytoprotection than the free radical scavenging activity of EGCG. However, higher levels of ROS can cause oxidative stress, cellular damage and side effects, mainly hepatotoxicity (Yang & Zhang, 2019). In vivo, EGCG can also be oxidized to quinones, which can react with sulfhydryl groups of cellular proteins, leading to the loss of function.
EGCG, with eight phenolic groups, provides multiple electron acceptors and donors for hydrogen bonding to a variety of molecules, especially to proteins. This is one of the reasons why EGCG has been shown to bind to many different proteins with high affinity and inhibit their activities. In studies in cell free systems, this is especially true as the inhibitory activity is stronger when lower concentrations of proteins are used in the assay. Without detailed characterization of the specificity or reversibility of the binding, these types of studies may result in the false identification of proteins as being “targets” for EGCG. The different activities and low bioavailability of EGCG makes extrapolation of results from in vitro studies to in vivo situations difficult.
With these chemical reactivities, EGCG has been shown to have many different biological effects, including anti-viral activities and these have been reviewed (Kaihatsu et al., 2018; Steinmann et al., 2013; Xu, Xu, & Zheng, 2017). EGCG has been shown to possess a broad spectrum of antiviral activities against RNA viruses such as hepatitis C virus, human immunodeficiency virus, Ebola virus and influenza virus, Zika virus, Dengue virus, West Nile viruses, Chikungunya virus, human porcine reproductive and respiratory virus; as well as DNA viruses such as herpes simplex virus, human papillomavirus, and hepatitis B virus. The structure-activity relationship of different catechins has been studied; EGCG has the highest activity, and the 3-galloyl and 5′-OH groups of EGCG appear crucial for the anti-viral activity (Kaihatsu et al., 2018).
EGCG mainly inhibits the early stages of viral infection, such as attachment, entry, and membrane fusion, by interfering with either viral membrane proteins or host cellular proteins or both. EGCG-fatty acid monoesters, which bind more effectively to viral and cellular membranes, have been shown to improve the anti-viral activity of EGCG against influenza and other viruses (Kaihatsu et al., 2018; Mhatre et al., 2020; Steinmann et al., 2013; Xu, Xu, & Zheng, 2017). Most of these studies were conducted in vitro under conditions that may be very different from those situations in humans, and these results should be interpreted with caution.
Many attempts have been made in developing EGCG into a therapeutic drug. Of note is that Veregen, a green tea polyphenol ornament preparation with EGCG as the major constituent, has been approved by the Food and Drug Administration (FDA) and European Medicine Agent (EMA) as a drug for topical treatment of external genital and anal warts caused by papillomavirus (Hara, 2011). The key reason of the success is topical application. How to deliver an effective dose of EGCG to the site of anti-viral action is a challenging issue in therapeutic application.
3. EGCG may reduce SARS-CoV-2 infection via activation of Nrf2
Nrf2, the cytoprotective transcription factor, regulates expression of a wide array of genes involved in antioxidation, detoxification, inflammation, immunity and antiviral responses (Mendonca & Soliman, 2020). Nrf2 knockdown in differentiated human nasal epithelial cells increases virus entry and replication, while Nrf2 activators, such as EGCG and sulforaphane, decrease viral entry and replication (Kesic, Simmons, Bauer, & Jaspers, 2011). Many other studies also showed that genetic and pharmacological manipulations to activate the Nrf2 pathway can inhibit viral replication and prevent virus-induced oxidative damage and inflammation (Lee, 2018).
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as the receptor for cell entry and serine protease TMPRSS2 for spike protein priming (Hoffmann et al., 2020). These are the two crucial steps for cell entry of coronaviruses. Nrf2-activator PB125® downregulates the mRNA expression of both ACE2 and TMPRSS2 in human HepG2 cells (McCord, Hybertson, Cota-Gomez, Geraci, & Gao, 2020). Genetic deletion of Nrf2 or pharmacological inhibition of Nrf2 upregulates ACE2 expression in renal proximal tubule cells; whereas its activator, oltipraz, downregulates ACE2 expression (Zhao et al., 2018). Genes associated with Nrf2-dependent antioxidant response are highly suppressed in lung biopsies from COVID-19 patients, and Nrf2 inducers (4-octyl-itaconate and dimethyl fumarate) inhibit SARS-CoV-2 replication and inflammatory response (Cuadrado et al., 2020; Olagnier et al., 2020). These lines of evidence suggest that Nrf2 activation is a promising strategy to prevent the infection of SARS-CoV-2 and reduce the severity of COVID-19.
A large number of studies have shown that EGCG induces Nrf2-mediated antioxidant enzyme expression (Dong et al., 2016; Na et al., 2008; Na & Surh, 2008). In differentiated human nasal epithelial cells, pre-incubation with EGCG (1 μM) decreases influenza virus entry and replication, via activating Nrf2 (Kesic et al., 2011). The suppressive effects of EGCG cannot be observed in cells with knocked-down Nrf2 expression.
As discussed above, EGCG as an Nrf2 activator can inhibit the entry of SARS-CoV-2 into host cells (McCord et al., 2020), and prime host cells against SARS-CoV-2 infection (Kesic et al., 2011). In addition, through the activation of Nrf-2 regulated heme oxygenase 1, EGCG can mediate antiviral responses by increasing the expression of type 1 interferons (Cuadrado et al., 2020; Espinoza, González, & Kalergis, 2017) and alleviating SARS-CoV-2-initiated inflammatory responses through crosstalk of Nrf2 and NF-κB in inflamed tissues, where innate immune cells are recruited (Cuadrado et al., 2020). It remains to be demonstrated whether EGCG can activate Nrf2 to such an extent in vivo to exert these possible actions.
4. EGCG may suppress SARS-CoV-2 replication via inhibiting main protease (Mpro)
The replicase gene of SARS-CoV-2 encodes two overlapping polyproteins for viral replication and transcription. The pp1a and pp1ab polyproteins undergo extensive proteolytic processing, mainly mediated by a 33.8-kDa main protease (Mpro), to yield functional polypeptides. Mpro, also known as the 3C-like protease, plays a vital role in mediating the life cycle of SARS-CoV-2. There is no human homolog of Mpro. These features make it an attractive target for antiviral drug development. Mpro is a three-domain (domains I to III) cysteine protease and has a non-canonical Cys145-His41 dyad located in the cleft between domains I and II. Synthetic compounds with high activity in modifying Cys145 of Mpro exhibit strong inhibitory effect on the enzymatic activity of Mpro and anti-infection potency against SARS-CoV-2 (Dai et al., 2020). In a study evaluating potential medicinal herbs for Mpro inhibition, green tea extract is highly effective in inhibiting Mpro of SARS-COV-2 (Upadhyay et al., 2020). Green tea extract or EGCG shows a dose-dependent inhibitory activity against Mpro of SARS-CoV-2 in vitro, with an IC50 value of 2.8 μg/mL or 7.5 μM, respectively (Zhu & Xie, 2020). These concentrations will be compared with EGCG concentrations in humans in Section 12. Molecular docking shows that EGCG has higher binding affinity (−7.6 kcal/mol) than a well-recognized Mpro inhibitor N3 (−7.0 kcal/mol), and suggests that EGCG strongly interacts with His41 and Cys145, the catalytic moiety of Mpro of SARS-CoV-2 (Ghosh, Chakraborty, Biswas, & Chowdhuri, 2020). Another in-silico study also identified EGCG as a potential inhibitor of Mpro (Sharma & Deep, 2020). A recent study found that EGCG from 1 to 20 μg/mL inhibited Mpro activity and replication of HCoV-OC43 (a type of beta coronavirus, similar to SARS-CoV-2) in a dose-dependent manner, and even 1 μg/mL EGCG was able to significantly reduce levels of HCoV-OC43 proteins in the infected cells (Jang et al., 2021).
EGCG auto-oxidation leads to the formation of EGCG quinone, which can react with protein cysteinyl thiol to form quinone proteins (Ishii et al., 2008; Zhang et al., 2017). Via quinone protein formation, EGCG can irreversibly inhibit glyceraldehyde-3-phosphate dehydrogenase (Ishii et al., 2008). It is possible that EGCG can inhibit Mpro of SARS-CoV-2 by covalent bonding to Cys145 and this possibility remains to be investigated.
In addition to Mpro, EGCG interferes with SARS-CoV-2 spike-receptor interaction and blocks the entry of SARS-CoV-2 pseudotyped lentiviral vectors with an IC50 value of 2.5 μg/mL (Henss et al., 2021). Studies in silico suggest that EGCG may also inhibit papain-like protease protein via binding to its S1 ubiquitin-binding site and impede COVID-19 (Chourasia, Koppula, Battu, Ouseph, & Singh, 2021). Based on these studies, ChourasIia et al. suggested that EGCG may serve as a broad spectrum therapeutic in asymptomatic and symptomatic COVID-19 patients (Chourasia et al., 2021).
5. Oxidative stress prevention and mitochondria protection by EGCG
In general, respiratory viruses augment ROS production in host cells (Khomich, Kochetkov, Bartosch, & Ivanov, 2018). SARS-CoV-2 increases oxidative stress of host cells by at least the three following mechanisms.
-
a.
Suppressing antioxidant enzymes and activate pro-oxidant enzymes. Respiratory syncytial virus infection decreases antioxidant and detoxifying enzymes such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPX) and glutathione S-transferase (GST), and suppresses Nrf2 expression in murine lungs. The infection also significantly decreases these enzymes in the airways of children with severe bronchiolitis. Severity of clinical illness in infected infants correlates with the decrease of these enzymes (Hosakote et al., 2011). Similarly, decreased expression of SOD3 in the lungs of elderly COVID-19 patients correlates with disease severity (Laforge et al., 2020). Blood SELENOP which is secreted from the liver for selenium delivery is dramatically lowered in severe COVID-19 patients (Moghaddam et al., 2020), probably due to pronounced hypoxia and/or marked IL-6 elevation that suppress hepatic SELENOP (Becker et al., 2014; Martitz et al., 2015). Thus, severe COVID-19 patients have an impaired biosynthesis of selenoproteins, most of which have antioxidant functions via selenocysteins in their active sites (Zhang, Saad, Taylor, & Rayman, 2020). Respiratory viruses are known to induce ROS-generating enzymes such as NADPH oxidases (NOX) (Fink, Duval, Martel, Soucy-Faulkner, & Grandvaux, 2008; Kaul, Biagioli, Singh, & Turner, 2000; Khomich et al., 2018; To et al., 2017). Inhibition of NOX2 activity ameliorates influenza A virus-induced lung inflammation (Vlahos et al., 2011), while NOX2 activation is associated with severe disease and thrombotic events in COVID-19 patients (Violi et al., 2020).
-
b.
Inducing neutrophil extracellular traps (NETs) and ROS burst. Neutrophils are recruited early to sites of infection to destroy viruses intracellularly by “cellular atomic bomb” composed of potent oxidants and free radicals (superoxide anion, hydrogen peroxide, hypochlorous acid, peroxynitrite and hydroxyl radicals) (Kalyanaraman, 2020). Moreover, neutrophils can ensnare viruses to kill them extracellularly via formation of NETs, which are web-like structures of DNA and proteins expelled from neutrophils. NETs as an aggressive defense mechanism are a double-edged sword. Timely and moderate NETs are beneficial in host defense against viruses; however, excessive and sustained NETs lead to ROS burst causing collateral lung damages (Barnes et al., 2020; Laforge et al., 2020; Schönrich, Raftery, & Samstag, 2020; Wu, 2020). Neutrophil infiltration has been shown in autopsied COVID-19 patients. Enhanced release of NETs occurs in severe cases of COVID-19 (Wang et al., 2020; Zuo et al., 2020a; Zuo et al., 2020b). Even sera from COVID-19 patients can cause NET release from control neutrophils in vitro (Zuo et al., 2020b).
-
c.
Evoking hypoxia-associated ROS production. Pronounced hypoxia is a hallmark of severe SARS-CoV-2 infection (Potus et al., 2020). In COVID-19 patients, the severity of hypoxemia is independently associated with in-hospital mortality and an important predictor of intensive care unit (ICU) admission (Kashani, 2020; Xie et al., 2020). The lung is responsible for maintaining an adequate oxygenation in the organism. Hypoxia enhances the generation of superoxide anions and increases release of ROS from the inner mitochondrial membrane to the intermembrane space in the lung, resulting in disruption of redox balance (Araneda & Tuesta, 2012; Schumacker, 2011). Hypoxia also increases pulmonary ROS production via inducing NOX4, xanthine oxidase/reductase or endothelial/inducible nitric oxide synthases (Araneda & Tuesta, 2012). Hypoxia-induced pulmonary oxidative stress promotes vasoconstriction, edema, inflammation, vascular remodeling and pulmonary hypertension (Araneda & Tuesta, 2012; Fresquet et al., 2006; Hoshikawa et al., 2001; Liu, Zelko, Erbynn, Sham, & Folz, 2006).
Oxidative stress caused by SARS-CoV-2 not only promotes tissue damage but also accelerates virus replication. Monocytes from COVID-19 patients exhibited mitochondrial dysfunction (Gibellini et al., 2020). Age-related mitochondrial dysfunction is proposed as an enhancing factor in COVID-19 disease (Moreno Fernandez-Ayala, Navas, & Lopez-Lluch, 2020). SARS-CoV-2-infected monocytes have increased mitochondrial ROS production. Treatment of the monocytes with antioxidants such as mitoquinol or N-acetylcysteine almost fully inhibits SARS-CoV-2 replication (Codo et al., 2020). ROS are strong inducers of HIF-1α, which is a potent inducer of glycolysis. SARS-CoV-2-infected monocytes thus express high levels of HIF-1α and become highly glycolytic (Codo et al., 2020). Inhibition of rate-limiting enzyme of glycolysis (hexokinase) by 2-deoxy-d-glucose prevents SARS-CoV-2 replication (Bojkova et al., 2020; Codo et al., 2020). Likewise, HIF-1α inhibitor blocks SARS-CoV-2 replication, whereas HIF-1α stabilization increases SARS-CoV-2 replication (Codo et al., 2020). Concerning the ROS/HIF-1α/glycolysis axis pivotal for SARS-CoV-2 replication, modulation at each level has shown promising result.
Respiratory virus-induced oxidative damage and ROS-facilitated SARS-CoV-2 replication justify antioxidant therapeutic strategy for vulnerable COVID-19 patients (Laforge et al., 2020; Schönrich et al., 2020; Wu, 2020). N-acetylcysteine thus has been proposed to be used as preventive and adjuvant therapy of COVID-19 (De Flora, Balansky, & La Maestra, 2020). EGCG can directly scavenge multiple types of ROS and induce antioxidant and detoxifying enzymes, such as heme oxygenase 1, quinone reductase, glutamate cysteine ligase, GST, thioredoxin reductase, glutaredoxin, glutathione reductase, SOD, catalase and GPX (Dong et al., 2016; Na & Surh, 2008). In addition, EGCG can suppress NOX expression or activity in various disease models (Ahn, Kim, & Ha, 2010; Li et al., 2006; Nishikawa, Wakano, & Kitani, 2007; Sarkar, Chakraborti, Chowdhury, Bhuyan, & Chakraborti, 2019; Yao et al., 2009). Concerning viral infection, EGCG alleviates enterovirus 71-induced oxidative stress and impedes related enterovirus 71 reproduction in Vero cells (Ho, Cheng, Weng, Leu, & Chiu, 2009).
EGCG prevents mitochondrial dysfunction in diseases such as Down syndrome (Scala et al., 2021; Vacca & Valenti, 2015; Valenti et al., 2018). It has been reported that in primary cultures of rat neuronal cells, EGCG selectively accumulates in the mitochondria, where it acts locally as a free radical scavenger to protect cells from apoptosis induced by mitochondrial oxidative stress (Schroeder et al., 2009). Pre-treatment of rats with EGCG (30 mg/kg, i.g. for 21 days) prevents cardiac mitochondria from oxidative damage in isoproterenol-induced myocardial infarction (Devika & Stanely Mainzen Prince, 2008). Oxidative stress induced by hypoxia contributes to the development of pulmonary vascular remodeling and pulmonary hypertension (Araneda & Tuesta, 2012; Fresquet et al., 2006; Hoshikawa et al., 2001; Liu et al., 2006). EGCG (50, 100 or 200 mg/kg/d, i.g.) dose-dependently suppresses hypoxia-induced elevation of right ventricular systolic pressure, pulmonary vascular remodeling and right ventricular hypertrophy in rats (Zhu et al., 2017), suggesting that EGCG could ameliorate hypoxia-induced oxidative stress. Whether EGCG can impede SARS-CoV-2 triggered NETs and hypoxia associated oxidative stress in humans remains to be investigated.
6. Suppression of endoplasmic reticulum stress by EGCG
Coronavirus replication induces endoplasmic reticulum (ER) stress and the unfolded protein response in infected cells (Chan et al., 2006; Liao et al., 2013; Sureda et al., 2020; Versteeg, van de Nes, Bredenbeek, & Spaan, 2007). SARS-CoV-2 is known to simultaneously suppress the expression of SELENOF, SELENOM, SELENOK and SELENOS in Vero cells (Wang et al., 2021). Since hepatic SELENOP is essential for selenium delivery to other tissues for selenoprotein synthesis (Becker et al., 2014; Martitz et al., 2015), suppression of SELENOP in SARS-CoV-2 infection could cause synergistic suppression of ER-resident SELENOF, SELENOM, SELENOK and SELENOS via transcriptional and translational inhibition. SELENOF and SELENOM catalyze the reduction or rearrangement of disulfide bonds in ER-localized proteins and facilitate ER protein-folding. Impaired SELENOF and SELENOM increase misfolded proteins, causing ER stress. SELENOK and SELENOS promote ER-associated degradation (ERAD) of errant proteins. Impaired SELENOK and SELENOS attenuate ERAD of misfolded proteins (Labunskyy, Hatfield, & Gladyshev, 2014). Therefore, SARS-CoV-2 infection induces a strong ER stress. This idea is supported by the observations that serum glucose-regulated protein 78 (GRP78), an ER stress marker, increases by 7-fold in SARS-COV-2 Infected patients compared to healthy controls (Köseler, Sabirli, Gören, Türkçüer, & Kurt, 2020), and GRP78 mRNA levels are four times higher in the blood of SARS-CoV-2 positive versus SARS-CoV-2 negative pneumonia patients (Palmeira et al., 2020).
GRP78, as a sensor of ER stress, has been recognized as an essential chaperone required for the life cycle of DNA or RNA viruses (Booth et al., 2016; Dimcheff, Faasse, McAtee, & Portis, 2004; Earl, Moss, & Doms, 1991; Goodwin et al., 2011; Rayner et al., 2020; Xu, Bellamy, & Taylor, 1998). ER stress induced by viral infection promotes GRP78 membrane translocation. Cell surface GRP78 mediates the entry of viruses, Ebola, and molecular docking shows that SARS-CoV-2 spike protein can bind cell surface GRP78 (Ibrahim, Abdelmalek, Elshahat, & Elfiky, 2020; Palmeira et al., 2020). During viral replication within cells, ER-localized GRP78, which is increased in response to high load of unfolded viral proteins, maintains ER homeostasis for proper folding, processing and assembly of viral proteins, such as spike protein (Chan et al., 2006; Yeung et al., 2008). However, mature virions hijack GRP78 as an accessory host factor for enhanced infectivity (Ha, Van Krieken, Carlos, & Lee, 2020). For these reasons, GRP78 is a promising therapeutic target for coronavirus infection (Wan, Song, Li, & He, 2020). Indeed, AR-12 (OSU-03012), which inhibits ATPase activity of GRP78 and decreases GRP78 protein expression, dose-dependently inhibits SARS-CoV-2 spike protein expression in transfected or infected cells (Rayner et al., 2020). Moreover, AR-12 suppresses replication of various viruses, and reduces liver injury and prolongs survival in rabbits infected with hemorrhagic fever viruses (Booth et al., 2016).
Direct and specific interaction between EGCG and GRP78 has been demonstrated in cell-free system and in cultured cells. EGCG binding at the ATP-binding site of GRP78 inhibits ATPase activity and changes GRP78 from active monomer to inactive dimer and oligomer forms (Ermakova et al., 2006). It has been suggested that, via inhibiting GRP78, EGCG suppresses Ebola virus replication (Reid et al., 2014) and avian reovirus S1133-induced apoptosis (Lin et al., 2015). Moreover, EGCG has been shown to downregulate GRP78 protein expression induced by cisplatin in mice (Chen et al., 2015), by amyloid β-induced in neuronal cells and in vivo (Du et al., 2018), by high glucose in podocytes and by hydrogen peroxide or calcium disterbance in mouse retinal pigment epithelial cells (Karthikeyan et al., 2017; Xiang et al., 2017). These interesting activities of EGCG in suppressing GRP78 protein levels and the catalytic activity, if can be demonstrated in humans, EGCG may be useful in protecting against SARS-CoV-2 infection.
7. Impediment of cytokine storm by EGCG
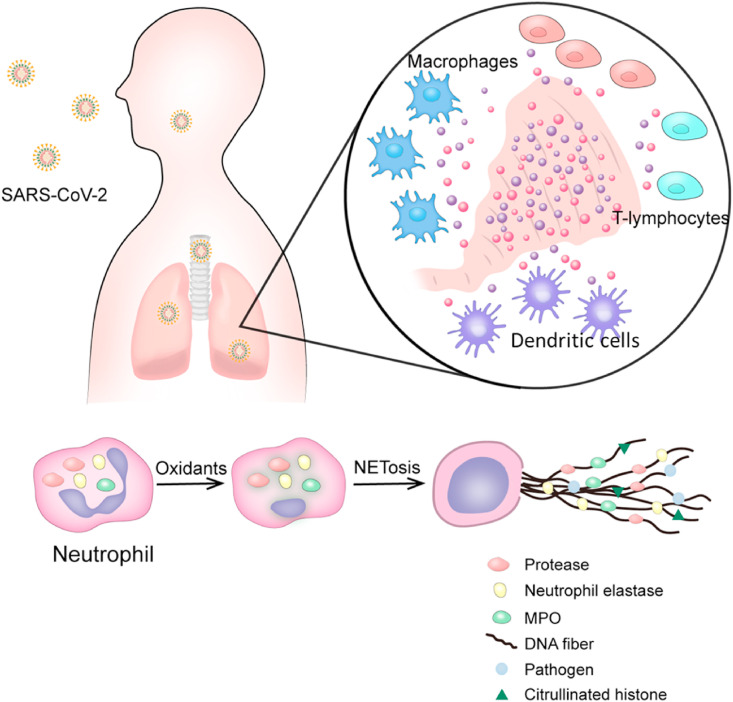
The cytokine storm is characterized as a sudden acute increase in circulating levels of different pro-inflammatory cytokines. SARS-CoV-2 infection in some individuals induces a hyperactive and uncontrolled immune response. Several types of immune cells including T-lymphocytes, macrophages, dendritic cells and neutrophils secret immense amounts of pro-inflammatory cytokines (IFNα, IFNγ, IL-6, IL-1β, IL-12, IL-18, IL-33, TNFα, TGFβ, etc.) and chemokines (CXCL8, CXCL9, CXCL10, CCL2, CCL3, CCL5, etc.), leading to acute respiratory distress syndrome (ARDS) and systemic inflammatory responses (see Fig. 2 ). Cytokine storm is a life-threatening condition requiring immediate ICU admission. Cytokine storm is directly correlated with multi-organ failure and high case fatality rate. In addition to anti-viral therapies, anti-inflammatory therapies that suppress cytokine storm are crucial for reducing mortality of severe and critical COVID-19 patients. IL-6 is frequently increased in COVID-19 patients, and its levels are positively associated to COVID-19 mortality. Tocilizumab (a recombinant humanized anti-human IL-6 receptor monoclonal antibody) has shown therapeutic results in severe and critical COVID-19 patients (Bohn et al., 2020; Coperchini, Chiovato, Croce, Magri, & Rotondi, 2020; Ragab, Salah Eldin, Taeimah, Khattab, & Salem, 2020; Tang, Liu, et al., 2020; Xu et al., 2020).
Fig. 2.
Cytokine storm development after SARS-CoV-2 infection. SARS-CoV-2 induces a maladaptive and uncontrolled generalized immune response. Immune cells like T-lymphocytes, macrophages and dendritic cells secrete immense amounts of cytokines and chemokines. Neutrophils and neutrophil extracellular traps (NETs) further fuel the hyper-inflammation that again stimulates NETs, causing a feedback snowballing effect and eventually leading to ARDS. Modified from (Kalyanaraman, 2020) and (Coperchini et al., 2020).
Many studies have shown that EGCG is highly effective in attenuating acute lung injury (ALI)/ARDS induced by virus, lipopolysaccharides (LPS) and other factors. Following the infection of H9N2 swine influenza virus in mice, administration of EGCG (10 mg/kg, i.g.) daily for 5 consecutive days significantly prolongs mouse survival and reduces death rate from 65% to 35%; attenuates lung histological lesions (inflammatory cell margination and infiltration, alveolar and interstitial edema, and bronchiolitis); decreases lung wet/dry ratio; suppresses total WBC count and leukocyte differential counts in bronchoalveolar lavage fluid; and lowers cytokines levels in the lung by downregulating TLR4 and NF-κB (Xu, Liu, et al., 2017). EGCG (40 mg/kg, i.g.) administered 3 days prior to influenza A virus infection in mice (followed with additional 2 daily EGCG treatments) significantly reduces death rate from 83.3% to 33.3%, decreases viral titers in the lung by 74-fold, and alleviates virus-evoked lung lesion (Ling et al., 2012).
In an LPS-induced mouse model of ALI, EGCG (15 mg/kg, i.p.) 1 h before and 3 h after LPS instillation mitigates ALI, neutrophils infiltration and the increase of M1/M2 macrophages subtype ratio (Almatroodi et al., 2020). In a similar model, EGCG (10 mg/kg, i.p.) administered 1 h before LPS injection in mice lowers the LPS-induced high PaCO2/RR ratio; histological lesions; and the elevation of IL-6, TNFα and IL-1β in the lung, serum and bronchoalveolar lavage fluid (Wang, Fan, & Zhang, 2019). Similarly, EGCG (10 mg/kg, i.p.) 1 h before intratracheal administration of LPS in mice, reduces histological severities and the elevation of TNFα, MIP-2 and neutrophils in the lung (Bae et al., 2010).
EGCG is also effective in other rodent models of ALI/ARDS: 1) EGCG (10 mg/kg, i.v.), immediately after the infliction of thermal injury in rats, significantly reduces plasma concentrations of inflammatory mediator and severity of ARDS (Liu et al., 2017). 2) EGCG (10 mg/kg, i.p.) 10 min before inflicting hip fracture in rats significantly reduces the elevated lung injury (Zhang et al., 2010; Zhao, Liu, Li, Gong, & Zhang, 2017) and infiltration of inflammatory cells in the bronchoalveolar lavage fluid (Zhao et al., 2017). 3) EGCG (20 mg/kg, i.p.), 1, 24 and 48 h after paraquat administration in mice, attenuates ALI, the activation of NF-κB and upregulation of toll-like receptor 2, 4 and 9, as well as their adaptors MyD88 and TRAF6 in the lung (Shen, Wu, Liu, Zhao, & Zhao, 2017). 4) EGCG (10 mg/kg, i.p.) 30 min before seawater instillation into the lung in rats significantly reduces hypoxemia, pulmonary edema, lung vascular leak, and levels of TNFα and IL-1 (Liu et al., 2014). 5) EGCG (25 mg/kg, administered as green tea extract i.p.) 1 h prior to pleural injection of carrageenan in mice, reduces acute inflammatory responses (interstitial haemorrhage, polymorphonuclear leukocyte infiltration, increased TNFα and activated STAT1) in lung tissues (Di Paola et al., 2005). Altogether, low to moderate doses of EGCG have consistently shown an inhibitory effect of ALI/ARDS in rodent models initiated by various challenges including RNA viruses. If such activities can be demonstrated in humans, EGCG may be useful to curb SARS-CoV-2 triggered cytokine storm and ARDS.
8. Sepsis prevention by EGCG
Sepsis, an infection-triggered systemic inflammatory response syndrome, is a common complication of severe and critical COVID-19 patients. High mobility group box 1 (HMGB1), released by activated monocytes, macrophages or neutrophils, acts as a late mediator of sepsis and is a crucial therapeutic target for preventing sepsis-triggered lethality (Yang et al., 2004). Administration of antibodies against HMGB1 attenuates endotoxin lethality in mice (Wang et al., 1999). Elevated serum levels of HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients (Chen et al., 2020). HMGB1 has been postulated as a therapeutic target of COVID-19 (Andersson, Ottestad, & Tracey, 2020; Menegazzi et al., 2020; Street, 2020; Tang, Comish, & Kang, 2020).
HMGB1 is a redox-sensitive protein containing three conserved cysteine residues at the amino acid sequence of 23, 45 and 106. Redox modification of these cysteines affects its extracellular chemokine- or cytokine-inducing properties (Yang et al., 2015). Specifically, fully reduced HMGB1 binds to CXCL12 and stimulates immune cell infiltration; disulfide HMGB1 (with a Cys23-Cys45 disulfide bond and a reduced Cys106) activates immune cells to produce cytokines/chemokines; and sulfonyl HMGB1 (all three cysteines are oxidized) loses its activity in modulating chemokines and cytokines (Venereau et al., 2012; Yang et al., 2015). EGCG can conjugate with purified HMGB1 protein to form quinone proteins, which can be visualized by nitrobluetetrazolium staining. In the presence of dithiothreitol, quinone protein formation is prevented, suggesting that free cysteines of HMGB1 participate in the covalent conjugation (Li et al., 2011). EGCG also reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages (Li et al., 2011). In addition to the redox modification of cysteines, the inhibitory effect of EGCG on STAT1 activation (Menegazzi et al., 2001, 2020; Townsend et al., 2004) may also play a role in reducing cytoplasmic HMGB1. JAK/STAT1 pathway regulates HMGB1 cytoplasmic accumulation (Lu et al., 2014). JAK/STAT1 inhibition decreases HMGB1 release and enhances survival in animal models of lethal sepsis and endotoxemia (Kim et al., 2009; Lu et al., 2014).
There are two studies demonstrating that treatment with as low as 4 mg/kg EGCG (i.p.), following the onset of sepsis (induced by cecal ligation and puncture) in mice, effectively reduces mortality, possibly involving HMGB1 inhibition (Li et al., 2007, 2011). Another study in rodent models found that treatment with EGCG, after cecal ligation and puncture, significantly improve survival and septic shock, as indicated by hypotension (Wheeler et al., 2007). In mouse model of LPS-induced lethal endotoxemia, EGCG (4 mg/kg, i.p) or green tea polyphenols (500 mg/kg, i.g.) significantly increase survival rate (Li et al., 2007; Yang, de Villiers, McClain, & Varilek, 1998). These results need to be further substantiated in rodent models and humans before considering studies in COVID-19 patients.
9. Thrombosis inhibition by EGCG
Coagulation activation and thrombotic events are common in COVID-19 patients (Katneni et al., 2020). Endotheliopathy has been identified in COVID-19 patients (Goshua et al., 2020; O'Sullivan, Gonagle, Ward, Preston, & O'Donnell, 2020). Coagulation factor III, an important mediator for coagulopathy, interacts with circulating coagulation factor VII to trigger extrinsic coagulation (DiNicolantonio & McCarty, 2020). Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression via activation of NOX (DiNicolantonio & McCarty, 2020). Indeed, NOX2 is activated in COVID-19 patients, especially those with thrombotic events (Violi et al., 2020). Increased plasma levels of tissue factors have been detected in COVID-19 patients (Skendros et al., 2020). Circulating platelet-neutrophil, -monocyte and -T-cell aggregates are significantly elevated in COVID-19 patients. Platelets from COVID-19 patients aggregate faster (Manne et al., 2020). SARS-CoV-2 and its spike protein directly activate platelets, which contribute to thrombus formation and inflammatory responses in COVID-19 patients (Zhang, Liu, et al., 2020).
EGCG inhibits TNFα - or histamine-induced tissue factor expression in cultured human aortic endothelial cells in a concentration-dependent manner (up to 30 μM). EGCG also decreases TNFα-induced tissue factor expression in cultured human aortic vascular smooth muscle cells and human umbilical venous endothelial cells. EGCG administration at the dose of 30 mg/kg (i.p.) daily for 7 days in mice inhibits tissue factor activity of carotid arteries (Holy et al., 2010). EGCG can inhibit ADP, collagen, epinephrine or calcium ionophore A23187 induced human platelet aggregation in a dose-dependent manner (Kang et al., 1999). EGCG also has antithrombotic activities in mice: 1) oral administration of a single dose of EGCG (10 or 50 mg/kg), 90 min prior to tail vein injection of epinephrine plus collagen, protects against death caused by pulmonary thrombosis in a dose-dependent manner; and 2) i.p. injection of EGCG (4 and 10 mg/kg) dose-dependently prolongs tail bleeding time of conscious mice by 2- and 3-fold, respectively (Kang et al., 1999). If the inhibition of tissue factor upregulation and anti-platelet activity of EGCG can be demonstrated in humans, EGCG may be useful in the prevention against thrombosis formation in COVID-19 patients.
10. Inhibition of lung fibrosis by EGCG
A systematic review of pathophysiological timeline in COVID-19 shows three main histological patterns in the lung, namely epithelial, vascular and fibrotic with interstitial fibrosis. The epithelial and vascular patterns can be found in all stages, while interstitial fibrous changes generally appear at 3 weeks after the onset of symptoms (Polak, Van Gool, Cohen, von der Thüsen, & van Paassen, 2020). Emerging evidence shows that many patients have persistent respiratory symptoms months after their initial illness (Fraser, 2020), and suggests that SARS-CoV-2 infection may have pulmonary fibrosis sequelae (George, Wells, & Jenkins, 2020).
The bleomycin-induced pulmonary fibrosis model is characterized by initial inflammation and secondary fibrosis, being similar to the pathological features of COVID-19. EGCG (20 mg/kg, i.p.) exhibits anti-fibrotic property in a rat model of pulmonary fibrosis induced by bleomycin (Sriram, Kalayarasan, Manikandan, Arumugam, & Sudhandiran, 2015; Sriram, Kalayarasan, & Sudhandiran, 2008, 2009a, 2009b). The mechanisms include augmenting Nrf2 defense and suppressing NF-κB activation. EGCG treatment (25 mg/kg, i.p.) also significantly inhibits irradiation-induced pulmonary fibrosis in rats (You et al., 2014). Oral administration of green tea extract in drinking water (equivalent to EGCG doses of 300–400 mg/kg) to mice significantly reduces pulmonary fibrosis induced by intratracheal challenge of fluorescein isothiocyanate; interstitial and peribronchial fibrosis is nearly completely prevented (Donà et al., 2003). In rats, green tea extract (150 mg/kg, i.g.) prevents cyclophosphamide-induced pulmonary fibrosis in rats (Hamdy, El-Maraghy, & Kortam, 2012) and green tea extract (1% in diet) ameliorates paraquat-induced pulmonary fibrosis (Kim et al., 2006). In cultured precision-cut lung slices from explants of patients with idiopathic pulmonary fibrosis undergoing transplantation, EGCG attenuates TGFβ1 signaling and new collagen accumulation, activates matrix metalloproteinase-dependent collagen I turnover (Wei et al., 2021). EGCG (600 mg given orally for 14 days) to patients with idiopathic pulmonary fibrosis, reverses profibrotic biomarkers in their diagnostic biopsies and serum samples (Chapman et al., 2020). Overall, with demonstrated activities in alleviating pulmonary fibrosis in rodent models and humans, EGCG and green tea extract may be useful for the prevention and treatment of pulmonary fibrosis in COVID-19 patients.
11. Reduction of COVID-19 diabetes comorbidity risk by EGCG
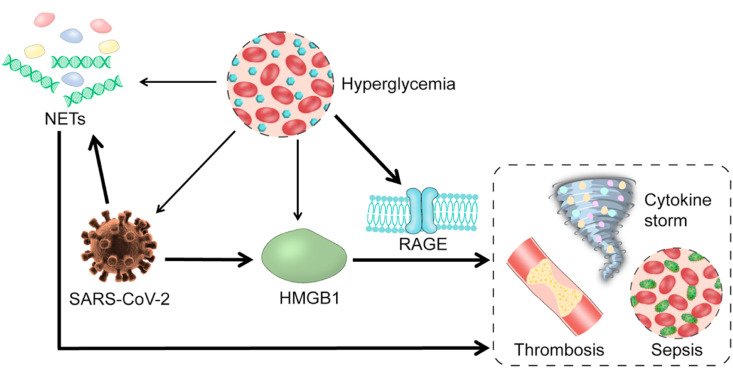
It is known that COVID-19 severity is magnified in people with underlying medical conditions. COVID-19 patients with diabetes comorbidity are at great risk of lengthy hospitalization and dire consequences, including ICU admission and mortality. Hyperglycemia upregulates receptors for advanced glycation end products (RAGE) and increases RAGE ligands such as HMGB1 (Le Bagge, Fotheringham, Leung, & Forbes, 2020). Most healthy tissues express low basal levels of RAGE, while pulmonary tissues have very high basal levels of RAGE (Rojas, Gonzalez, & Morales, 2020). RAGE is a major mediator of pulmonary inflammatory responses (Oczypok, Perkins, & Oury, 2017). RAGE regulates a positive feed-forward loop of RAGE and NF-κB, resulting in a sustained NF-κB activation (Bierhaus et al., 2001). RAGE may play a pivotal role in SARS-CoV-2-mediated inflammatory response in the lung (Rojas et al., 2020) and has been suggested to be a therapeutic target for inhibition in COVID-19 patients with diabetes (De Francesco, Vella, & Belfiore, 2020). As outlined in Fig. 3 , SARS-CoV-2 increases extracellular HMGB1 (Chen et al., 2020), which binds pulmonary RAGE to participate in triggering cytokine storm, sepsis and thrombosis (Yang et al., 2004). In diabetic conditions, hyperglycemia increases RAGE expression and HMGB1 levels (Le Bagge et al., 2020), amplifying SARS-CoV-2/HMGB1/RAGE axis. Moreover, hyperglycemia increases SARS-CoV-2 replication via reshaping cell metabolism in favor of glycolysis (Codo et al., 2020). Therefore, hyperglycemia and SARS-CoV-2 can synergistically enhance HMGB1-RAGE signaling transduction, leading to far worse outcomes. In addition, type 2 diabetic patients have an increased level of NETs (Carestia et al., 2016; Menegazzo et al., 2015; Park et al., 2016; Wang et al., 2018; Wong et al., 2015). SARS-CoV-2-evoked NETs together with high basal NETs in diabetic patients also increase risk of ICU admission and mortality of COVID-19 patients with diabetes comorbidity.
Fig. 3.
Proposed mechanism by which COVID-19 is aggravated in diabetic conditions. The main feature of diabetes, hyperglycemia, promotes the replication of SARS-CoV-2 and the development of neutrophil extracellular traps (NETs). Hyperglycemia also strongly upregulates receptor for advanced glycation end products (RAGE) and increases the RAGE ligands — high mobility group box 1 (HMGB1). SARS-CoV-2 also strongly stimulates the formation of NETs and synthesis of HMGB1. Upon activation by its receptor (HMGB1), the RAGE signaling and NETs both induce cytokine storm, sepsis and thrombosis.
Mounting evidence demonstrates that EGCG is helpful in controlling blood glucose in diabetic subjects (Chen et al., 2009; Kao, Chang, Lee, & Chen, 2006; Sae-tan, Grove, & Lambert, 2011; Wolfram et al., 2006; Yang, Zhang, Zhang, Huang, & Wang, 2016; Zhao, Wu, Wang, Yang, & Zhang, 2020). Moreover, several studies in animal models and humans have shown that EGCG suppresses RAGE signaling pathway; specifically, 1) treatment with EGCG (10 mg/kg and 50 mg/kg, i.p.) reduces pulmonary injury and airway remodeling in PM2.5-exposed asthmatic rats, partially via regulation of the HMGB1/RAGE signaling pathway (Li et al., 2019); 2) EGCG dose-dependently (75, 150 and 300 mg/kg, i.g.) downregulates pancreatic RAGE in diabetic mice (Feng, Hou, Zhu, Zhu, & Jiang, 2019); 3) EGCG (2 mg/kg and 4 mg/kg, i.p.) improves restenosis in a rat model of carotid artery balloon injury via inhibiting HMGB1/RAGE and NF-κB activation (Yang et al., 2017); 4) EGCG (75 mg/kg, i.p. 3 times per week) alleviates hyperglycemia, downregulates RAGE expression and reduces advanced glycation end products in mice with high fat diet-induced obesity (Sampath, Rashid, Sang, & Ahmedna, 2017); 5) in patients with type II diabetes, EGCG at doses from 300 to 900 mg/day dose-dependently increases plasma levels of soluble RAGE, a RAGE variant acting as a ligand decoy that competes with RAGE (Huang et al., 2013). These inhibitory activities of EGCG, in rodent models and humans, suggest that EGCG could reduce the risk of ICU admission and mortality of COVID-19 patients with diabetes comorbidity.
12. Dose and safety issues of EGCG for COVID-19 prevention and treatment
The biological activities and toxic effects of EGCG in mice have been extensively studied. Generally, the effect of an i.p. dose approximates that of a 10-fold oral dose. For example, 55–75 mg/kg (i.p.) or 600 mg/kg (i.g.) for five consecutive days caused hepatoxicity and elevated hepatic Nrf2 response, while one dose of 200 mg/kg (i.p.) or 2000 mg/kg (i.g.) caused lethal consequence associated with suppression of hepatic Nrf2, in the same mouse species (Wang, Wang, Wan, Yang, & Zhang, 2015). The maximum non-toxic dose of EGCG by i.p. injection was 45 mg/kg (Wang, Wang, et al., 2015; Wang, Wei, et al., 2015; Wang, Wang, et al., 2019; Wang, Yang, et al., 2019) or 400–450 mg/kg by i.g. administration in mice (Lambert et al., 2010). Accordingly, the non-toxic doses of EGCG at 10–40 mg/kg (i.p.) or 100–400 mg/kg (orally) are extensively used for elucidating functions of EGCG in mice (Bose et al., 2008; Gan et al., 2015; Kumazoe et al., 2013; Siddiqui et al., 2009; Yan, Zhao, Suo, Liu, & Zhao, 2012).
To prevent SARS-CoV-2 infection, the key step is to prevent the binding of viral spike proteins to cellular receptors (ACE2) and to inhibit cell host proteases (TMPRSS2). The activation of Nrf2 by EGCG at non-toxic doses, which has been demonstrated in animal models (Dong et al., 2016; Na et al., 2008; Sun et al., 2017; Yang et al., 2018), may reach a magnitude that can downregulate both ACE2 and TMPRSS2. However, these remain to be demonstrated. As described above, many studies in rodent models have demonstrated that EGCG at a non-toxic daily dose (under 30 mg/kg, i.p. or 300 mg/kg, i.g.) in mice can ameliorate hypoxia-induced oxidative stress, cytokine storm, and diabetes comorbidity as well as decrease GRP78 expression/activity, ER stress, thrombosis, sepsis, and lung fibrosis. These activities, if could be manifested in humans, would be beneficial in the prevention or alleviation of COVID-19 and associated syndromes.
The rodent dose can be converted to a human equivalent dose based on body weight divided by conversion factor of 12.9, because the mouse has a larger body surface area and higher metabolic rate per unit body weight (Reagan-Shaw, Nihal, & Ahmad, 2008). The above describes effective concentration of oral 300 mg/kg EGCG in mice is equal to 1392 mg EGCG daily for an adult with body weight of 60 kg. It was reported recently that daily 600 mg EGCG given orally to patients with idiopathic pulmonary fibrosis reversed profibrotic biomarkers in their diagnostic biopsies and serum samples, while FDA-approved drugs showed no beneficial effects (Chapman et al., 2020).
Two to 4 h following oral administrator of green tea polyphenols at a dose of 500 mg/kg in mice, the serum level of polyphenols reaches maximum value at 9–10 μg/mL (Yang et al., 1998). This level is higher than the IC50 (2.8 μg of green tea extract per mL) for the inhibition of Mpro activity of SARS-CoV-2 in vitro (Zhu & Xie, 2020). The inhibition of Mpro activity of SARS-CoV-2 by EGCG shows an IC50 of 7.5 μM (Zhu & Xie, 2020). In human studies, after ingesting 375 to 1200 mg of EGCG (as green tea extract or Polyphenon E) under fasting conditions, the maximum levels of EGCG were 4.3–5.6 μM (Chow et al., 2005; Nakagawa, Okuda, & Miyazawa, 1997). These human blood levels of EGCG approach, but are still lower than, the effective inhibitory concentration of EGCG against Mpro observed in vivo. There is also a possibility that some products formed from EGCG oxidation in vivo can inhibit Mpro activity of SARS-CoV-2 (Jang et al., 2020). The extrapolation of results from studies in vitro to situations in vivo, and the translation of animal studies to humans, are challenging issues. More studies on the dose response relationship of EGCG in humans are needed.
Following ingestion of tea catechins, the concentration of EGCG and other catechins can be rather high in the oral cavity. For example, in a study with volunteers, after each drinking 200 mL of warm tea (containing 1,200 mg of green tea extracts), followed by rinsing the mouth rigorously 10 times, the initial salivary concentrations of EGCG were 10–50 μM with elimination t1/2 values of 10–20 min. EGC and EC, were present in slightly higher concentrations (Yang, Lee, & Chen, 1999). In a second experiment, after subjects holding 96 mg EGCG in 60 mL in the mouth for 2 min, the saliva samples (collected similarly) initially contained 120–300 μM EGCG and decreased to 25–65 μM after 30 min. These results suggest that after drinking or gargling tea, the levels of EGCG and other catechins in the oral/nasal/pharyngeal cavity could be high enough to protect against viral infection. It has been shown in Japan that daily gargling a tea catechin solution significantly lowered the incidence of influenza infection in elderly (Yamada, Takuma, Daimon, & Hara, 2006). In a randomized double-blind trial of 200 healthcare workers, consumption of catechin capsules for 5 months had a protective effect against influenza virus compared to the placebo group (Matsumoto, Yamada, Takuma, Niino, & Sagesaka, 2011). These interesting studies need to be repeated in studies with more subjects and extended to other anti-viral studies in humans.
If higher doses of catechins are needed for alleviating the symptoms of COVID-19 disease, a concern is hepatotoxicity, which has been observed in animal models and individuals taking green tea extract-based dietary supplements for the purpose of weight reduction (Yang & Zhang, 2019). For long-term supplementation, a tolerable upper intake level was set at 300 mg of EGCG per day for humans in some European countries, such as France and Italy (Yates, Erdman, Shao, Dolan, & Griffiths, 2017). If tea is consumed as a beverage throughout the day, the tolerable upper intake level should be much higher (Yang & Zhang, 2019). Reversible hepatotoxicity caused by therapeutics for a period of a few months may be acceptable for a deadly disease. For example, in a study on the treatment of chronic lymphocytic leukemia (CLL), a high dose of Polyphenon E (containing 2000 mg EGCG, twice daily) was used for up to six months. Side effects, including transaminitis, abdominal pain and fatigue, were observed in some patients and some had to be switched to a lower dose. However, because of the clinical benefits (e.g. durable decline in absolute lymphocyte count), such side effects were considered to be tolerable (Shanafelt et al., 2013). Individuals that are susceptible to or already infected with SARS-CoV-2 are probably taking medications for prevention of chronic diseases or receiving anti-COVID-19 therapeutic drugs. One concern is that high dose of EGCG may interfere the transport, metabolism and efficacy of some of these drugs (Huang et al., 2020; Liu et al., 2020; Yang & Pan, 2012). However, such EGCG-drug interactions only occur with certain drugs, prior knowledge of such interactions could avoid complications.
13. Concluding remarks
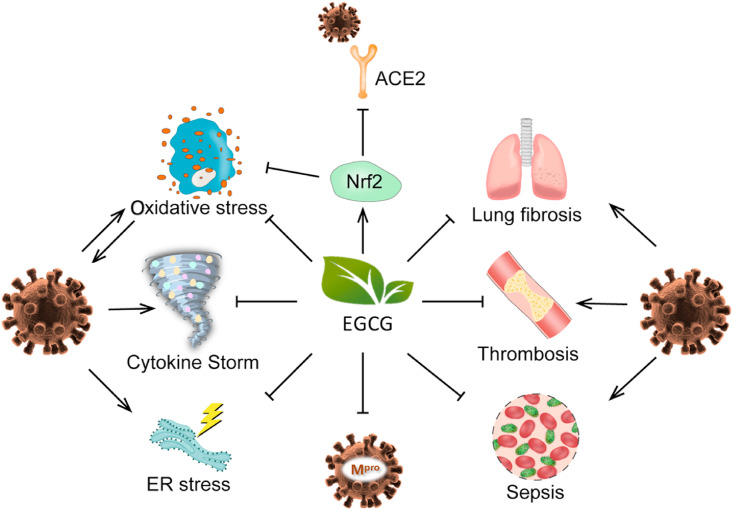
As discussed above, EGCG has shown many redox and specific inhibitory activities, in cell lines and rodent models, which may be applicable for the prevention and treatment of COVID-19. The possible anti-COVID-19 activities of EGCG are outlined in Fig. 4 . EGCG may suppress SARS-CoV-2 infection via suppressing the expression of cell surface ACE2 and TMPRSS2 via activating Nrf2. EGCG may also inhibit SARS-CoV-2 Mpro — a protease essential for viral reproduction. EGCG has direct and indirect antioxidant activities to possibly protect against SARS-CoV-2 evoked oxidative stress. EGCG could reduce ER stress and SARS-CoV-2 life cycle via inhibiting ER-resident GRP78 activity and expression. EGCG could also protect against cytokine storm-associated ALI/ARDS, thrombosis, sepsis and lung fibrosis. Overall, EGCG has shown activities with the potential to prevent against SARS-CoV-2 infection, suppress SARS-CoV-2 life cycle, and curb SARS-CoV-2 triggered cytokine storm, oxidative stress, ER stress, thrombosis, sepsis, and lung fibrosis. These possible concerted activities of EGCG suggest the importance of further studies in relevant animal models and humans. To our knowledge, such human studies have not been registered or conducted. Other catechins in green tea may have similar, but slightly lower, activities of EGCG. Tea consumption has been shown to decrease the risk for obesity, diabetes and cardiovascular diseases (Yang & Hong, 2013; Yang & Zhang, 2019), which enhance the risk of and aggravate COVID-19. It is important to conduct epidemiological studies to determine whether regular tea consumption (and the amount required) could decrease risk for SARS-CoV-2 infection and associated syndromes.
Fig. 4.
Proposed multiple-target actions by EGCG to prevent or alleviate COVID-19. EGCG via its direct antioxidant activity and induction of Nrf2 prevents or suppresses oxidative stress and inflammation. Through its downregulation of angiotensin-converting enzyme 2 (ACE2) via Nrf2 and direct inhibition of main protease (Mpro), EGCG inhibits the infection and replication of SARS-CoV-2. These activities in combination with direct inhibition of key enzymes or proteins, contribute to the prevention or alleviation of cytokine storm, ER stress, sepsis, thrombosis and lung fibrosis that are caused by SARS-CoV-2 infection.
Author contributions
Conception of idea: J.Z. Design of review outline: W.Y., C.S.Y. and J.Z. Sourcing literature of clinical symptoms and molecular pathology of COVID-19: Z.Z. and W.Y. Sourcing literature of EGCG and tea: X.Z., K.B., Y.H., C.S.Y. and J.Z. Drafting the article: Z.Z., X.Z., W.Y., C.S.Y. and J.Z. Preparing the figures: Z.Z. (Figs. 2–4) and C.S.Y. (Fig. 1). Reviewing and revising the manuscript: C.S.Y. and J.Z. Editing the manuscript: K.B. and Y.H.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant numbers: 31771971 and 32001013).
References
- Ahn H.Y., Kim C.H., Ha T.S. Epigallocatechin-3-gallate regulates NADPH oxidase expression in human umbilical vein endothelial cells. KOREAN JOURNAL OF PHYSIOLOGY and PHARMACOLOGY. 2010;14(5):325–329. doi: 10.4196/kjpp.2010.14.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almatroodi S.A., Almatroudi A., Alsahli M.A., Aljasir M.A., Syed M.A., Rahmani A.H. Epigallocatechin-3-gallate (EGCG), an active compound of green tea attenuates acute lung injury regulating macrophage polarization and krüpple-like-factor 4 (KLF4) Expression. Molecules. 2020;25(12):2853. doi: 10.3390/molecules25122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U., Ottestad W., Tracey K.J. Extracellular HMGB1: A therapeutic target in severe pulmonary inflammation including COVID-19? Molecular Medicine. 2020;26(1):42. doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda O.F., Tuesta M. Lung oxidative damage by hypoxia. Oxidative Medicine and Cellular Longevity. 2012;2012:856918. doi: 10.1155/2012/856918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H.B., Li M., Kim J.P., Kim S.J., Jeong C.W., Lee H.G.…Kwak S.H. The effect of epigallocatechin gallate on lipopolysaccharide-induced acute lung injury in a murine model. Inflammation. 2010;33(2):82–91. doi: 10.1007/s10753-009-9161-z. [DOI] [PubMed] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M.…Egeblad M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. Journal of Experimental Medicine. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N.P., Martitz J., Renko K., Stoedter M., Hybsier S., Cramer T., et al. Hypoxia reduces and redirects selenoprotein biosynthesis. Metall. 2014;6(5):1079–1086. doi: 10.1039/c4mt00004h. [DOI] [PubMed] [Google Scholar]
- Bierhaus A., Schiekofer S., Schwaninger M., Andrassy M., Humpert P.M., Chen J.…Nawroth P.P. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes. 2001;50(12):2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- Bohn M.K., Hall A., Sepiashvili L., Jung B., Steele S., Adeli K. Pathophysiology of COVID-19: Mechanisms underlying disease severity and progression. Physiology (Bethesda, Md. 2020;35(5):288–301. doi: 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S.…Münch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583(7816):469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth L., Roberts J.L., Ecroyd H., Tritsch S.R., Bavari S., Reid S.P.…Dent P. AR-12 inhibits multiple chaperones concomitant with stimulating autophagosome formation collectively preventing virus replication. Journal of Cellular Physiology. 2016;231(10):2286–2302. doi: 10.1002/jcp.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M., Lambert J.D., Ju J., Reuhl K.R., Shapses S.A., Yang C.S. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. Journal of Nutrition. 2008;138(9):1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carestia A., Frechtel G., Cerrone G., Linari M.A., Gonzalez C.D., Casais P., et al. NETosis before and after hyperglycemic control in type 2 diabetes mellitus patients. PloS One. 2016;11(12) doi: 10.1371/journal.pone.0168647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.P., Siu K.L., Chin K.T., Yuen K.Y., Zheng B., Jin D.Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. Journal of Virology. 2006;80(18):9279–9287. doi: 10.1128/jvi.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.A., Wei Y., Montas G., Leong D., Golden J.A., Trinh B.N.…Song J.W. Reversal of TGFbeta1-driven profibrotic state in patients with pulmonary fibrosis. New England Journal of Medicine. 2020;382(11):1068–1070. doi: 10.1056/NEJMc1915189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Bezzina R., Hinch E., Lewandowski P.A., Cameron-Smith D., Mathai M.L.…Weisinger R.S. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutrition Research. 2009;29(11):784–793. doi: 10.1016/j.nutres.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Chen B., Liu G., Zou P., Li X., Hao Q., Jiang B.…Hu Z. Epigallocatechin-3-gallate protects against cisplatin-induced nephrotoxicity by inhibiting endoplasmic reticulum stress-induced apoptosis. Experimental Biology and Medicine. 2015;240(11):1513–1519. doi: 10.1177/1535370215573394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Long X., Xu Q., Tan J., Wang G., Cao Y.…Zhou J. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cellular and Molecular Immunology. 2020;17(9):992–994. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourasia M., Koppula P.R., Battu A., Ouseph M.M., Singh A.K. EGCG, a green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules. 2021;26(5) doi: 10.3390/molecules26051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury P., Barooah A.K. Tea bioactive modulate innate immunity: In perception to COVID-19 pandemic. Frontiers in Immunology. 2020;11 doi: 10.3389/fimmu.2020.590716. 590716-590716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow H.H., Hakim I.A., Vining D.R., Crowell J.A., Ranger-Moore J., Chew W.M.…Alberts D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clinical Cancer Research. 2005;11(12):4627–4633. doi: 10.1158/1078-0432.Ccr-04-2549. [DOI] [PubMed] [Google Scholar]
- Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V.…Moraes-Vieira P.M. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-dependent axis. Cell Metabolism. 2020;32(3):437–446. doi: 10.1016/j.cmet.2020.07.007. e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & Growth Factor Reviews. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A., Pajares M., Benito C., Jimenez-Villegas J., Escoll M., Fernandez-Gines R.…Dinkova-Kostova A.T. Can activation of Nrf2 be a strategy COVID-19? Trends in Pharmacological Sciences. 2020;41(9):598–610. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y.…Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S., Balansky R., La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. The FASEB Journal. 2020;34(10):13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco E.M., Vella V., Belfiore A. COVID-19 and diabetes: The importance of controlling rage. Frontiers in Endocrinology. 2020;11:526. doi: 10.3389/fendo.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devika P.T., Stanely Mainzen Prince P. (-)Epigallocatechin-gallate (EGCG) prevents mitochondrial damage in isoproterenol-induced cardiac toxicity in albino wistar rats: A transmission electron microscopic and in vitro study. Pharmacological Research. 2008;57(5):351–357. doi: 10.1016/j.phrs.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Di Paola R., Mazzon E., Muià C., Genovese T., Menegazzi M., Zaffini R.…Cuzzocrea S. Green tea polyphenol extract attenuates lung injury in experimental model of carrageenan-induced pleurisy in mice. Respiratory Research. 2005;6(1):66. doi: 10.1186/1465-9921-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimcheff D.E., Faasse M.A., McAtee F.J., Portis J.L. Endoplasmic reticulum (ER) stress induced by a neurovirulent mouse retrovirus is associated with prolonged BiP binding and retention of a viral protein in the ER. Journal of Biological Chemistry. 2004;279(32):33782–33790. doi: 10.1074/jbc.M403304200. [DOI] [PubMed] [Google Scholar]
- DiNicolantonio J.J., McCarty M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart. 2020;7(1) doi: 10.1136/openhrt-2020-001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donà M., Dell'Aica I., Calabrese F., Benelli R., Morini M., Albini A., et al. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. The Journal of Immunology. 2003;170(8):4335–4341. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- Dong R., Wang D., Wang X., Zhang K., Chen P., Yang C.S., et al. Epigallocatechin-3-gallate enhances key enzymatic activities of hepatic thioredoxin and glutathione systems in selenium-optimal mice but activates hepatic Nrf2 responses in selenium-deficient mice. Redox Biology. 2016;10:221–232. doi: 10.1016/j.redox.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Liu M., Zhong X., Yao W., Xiao Q., Wen Q.…Wei M. Epigallocatechin gallate reduces amyloid β-Induced neurotoxicity via inhibiting endoplasmic reticulum stress-mediated apoptosis. Molecular Nutrition & Food Research. 2018;62(8) doi: 10.1002/mnfr.201700890. [DOI] [PubMed] [Google Scholar]
- Earl P.L., Moss B., Doms R.W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. Journal of Virology. 1991;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova S.P., Kang B.S., Choi B.Y., Choi H.S., Schuster T.F., Ma W.Y.…Dong Z. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Research. 2006;66(18):9260–9269. doi: 10.1158/0008-5472.Can-06-1586. [DOI] [PubMed] [Google Scholar]
- Espinoza J.A., González P.A., Kalergis A.M. Modulation of antiviral immunity by heme oxygenase-1. American Journal Of Pathology. 2017;187(3):487–493. doi: 10.1016/j.ajpath.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Feng Z., Hou X., Zhu C., Zhu J., Jiang C. Epigallocatechin gallate ameliorates morphological changes of pancreatic islets in diabetic mice and downregulates blood sugar level by inhibiting the accumulation of AGE-RAGE. Journal of Cellular Biochemistry. 2019;120(5):8510–8520. doi: 10.1002/jcb.28139. [DOI] [PubMed] [Google Scholar]
- Fink K., Duval A., Martel A., Soucy-Faulkner A., Grandvaux N. Dual role of Nox2 in respiratory syncytial virus- and Sendai virus-induced activation of NF-κB in airway epithelial cells. The Journal of Immunology. 2008;180(10):6911–6922. doi: 10.4049/jimmunol.180.10.6911. [DOI] [PubMed] [Google Scholar]
- Fraser E. Long term respiratory complications of COVID-19. British Medical Journal. 2020;370:m3001. doi: 10.1136/bmj.m3001. [DOI] [PubMed] [Google Scholar]
- Fresquet F., Pourageaud F., Leblais V., Brandes R.P., Savineau J.P., Marthan R., et al. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. British Journal of Pharmacology. 2006;148(5):714–723. doi: 10.1038/sj.bjp.0706779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Meng Z.J., Xiong R.B., Guo J.Q., Lu X.C., Zheng Z.W.…Li H. Green tea polyphenol epigallocatechin-3-gallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacologica Sinica. 2015;36(5):597–605. doi: 10.1038/aps.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. The lancet. Respiratory Medicine. 2020;8(8):807–815. doi: 10.1016/s2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Chakraborty A., Biswas A., Chowdhuri S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors - an in silico docking and molecular dynamics simulation study. Journal of Biomolecular Structure & Dynamics. 2020:1–13. doi: 10.1080/07391102.2020.1779818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini L., De Biasi S., Paolini A., Borella R., Boraldi F., Mattioli M.…Cossarizza A. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Molecular Medicine. 2020;12(12) doi: 10.15252/emmm.202013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin E.C., Lipovsky A., Inoue T., Magaldi T.G., Edwards A.P., Van Goor K.E.…DiMaio D. BiP and multiple DNAJ molecular chaperones in the endoplasmic reticulum are required for efficient simian virus 40 infection. mBio. 2011;2(3) doi: 10.1128/mBio.00101-11. e00101-00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P.…Lee A.I. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. The Lancet. Haematology. 2020;7(8):e575–e582. doi: 10.1016/s2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy M.A., El-Maraghy S.A., Kortam M.A. Modulatory effects of curcumin and green tea extract against experimentally induced pulmonary fibrosis: A comparison with N-acetyl cysteine. Journal of Biochemical and Molecular Toxicology. 2012;26(11):461–468. doi: 10.1002/jbt.21447. [DOI] [PubMed] [Google Scholar]
- Hara Y. Tea catechins and their applications as supplements and pharmaceutics. Pharmacological Research. 2011;64(2):100–104. doi: 10.1016/j.phrs.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Ha D.P., Van Krieken R., Carlos A.J., Lee A.S. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. Journal of Infection. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henss L., Auste A., Schurmann C., Schmidt C., von Rhein C., Muhlebach M.D., et al. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. Journal of General Virology. 2021;102(4) doi: 10.1099/jgv.0.001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H.Y., Cheng M.L., Weng S.F., Leu Y.L., Chiu D.T. Antiviral effect of epigallocatechin gallate on enterovirus 71. Journal of Agricultural and Food Chemistry. 2009;57(14):6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S.…Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy E.W., Stämpfli S.F., Akhmedov A., Holm N., Camici G.G., Lüscher T.F., et al. Laminin receptor activation inhibits endothelial tissue factor expression. Journal of Molecular and Cellular Cardiology. 2010;48(6):1138–1145. doi: 10.1016/j.yjmcc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Hosakote Y.M., Jantzi P.D., Esham D.L., Spratt H., Kurosky A., Casola A., et al. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. American Journal of Respiratory and Critical Care Medicine. 2011;183(11):1550–1560. doi: 10.1164/rccm.201010-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshikawa Y., Ono S., Suzuki S., Tanita T., Chida M., Song C., et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. Journal of Applied Physiology. 2001;90(4):1299–1306. doi: 10.1152/jappl.2001.90.4.1299. Bethesda, Md. : 1985. [DOI] [PubMed] [Google Scholar]
- Huang S.M., Chang Y.H., Chao Y.C., Lin J.A., Wu C.H., Lai C.Y.…Yen G.C. EGCG-rich green tea extract stimulates sRAGE secretion to inhibit S100A12-RAGE axis through ADAM10-mediated ectodomain shedding of extracellular RAGE in type 2 diabetes. Molecular Nutrition & Food Research. 2013;57(12):2264–2268. doi: 10.1002/mnfr.201300275. [DOI] [PubMed] [Google Scholar]
- Huang X., Zhang R., Yang T., Wei Y., Yang C., Zhou J.…Shi S. Inhibition effect of epigallocatechin-3-gallate on the pharmacokinetics of calcineurin inhibitors, tacrolimus, and cyclosporine A, in rats. Expert Opinion on Drug Metabolism and Toxicology. 2020:1–14. doi: 10.1080/17425255.2021.1837111. [DOI] [PubMed] [Google Scholar]
- Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. Journal of Infection. 2020;80(5):554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Mori T., Tanaka T., Mizuno D., Yamaji R., Kumazawa S.…Akagawa M. Covalent modification of proteins by green tea polyphenol (-)-epigallocatechin-3-gallate through autoxidation. Free Radical Biology & Medicine. 2008;45(10):1384–1394. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Jang M., Park Y.I., Cha Y.E., Park R., Namkoong S., Lee J.I., et al. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-Protease in vitro. Evidence-based Complementary and Alternative Medicine : eCAM. 2020;2020:5630838. doi: 10.1155/2020/5630838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Park R., Park Y.I., Cha Y.E., Yamamoto A., Lee J.I., et al. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochemical and Biophysical Research Communications. 2021;547:23–28. doi: 10.1016/j.bbrc.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaihatsu K., Yamabe M., Ebara Y. Antiviral mechanism of action of epigallocatechin-3-O-gallate and its fatty acid esters. Molecules. 2018;23(10):2475. doi: 10.3390/molecules23102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B. Do free radical NETwork and oxidative stress disparities in African Americans enhance their vulnerability to SARS-CoV-2 infection and COVID-19 severity? Redox Biology. 2020;37:101721. doi: 10.1016/j.redox.2020.101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W.S., Lim I.H., Yuk D.Y., Chung K.H., Park J.B., Yoo H.S., et al. Antithrombotic activities of green tea catechins and (-)-epigallocatechin gallate. Thrombosis Research. 1999;96(3):229–237. doi: 10.1016/s0049-3848(99)00104-8. [DOI] [PubMed] [Google Scholar]
- Kao Y.H., Chang H.H., Lee M.J., Chen C.L. Tea, obesity, and diabetes. Molecular Nutrition & Food Research. 2006;50(2):188–210. doi: 10.1002/mnfr.200500109. [DOI] [PubMed] [Google Scholar]
- Karthikeyan B., Harini L., Krishnakumar V., Kannan V.R., Sundar K., Kathiresan T. Insights on the involvement of (-)-epigallocatechin gallate in ER stress-mediated apoptosis in age-related macular degeneration. Apoptosis. 2017;22(1):72–85. doi: 10.1007/s10495-016-1318-2. [DOI] [PubMed] [Google Scholar]
- Kashani K.B. Hypoxia in COVID-19: Sign of severity or cause for poor outcomes. Mayo Clinic Proceedings. 2020;95(6):1094–1096. doi: 10.1016/j.mayocp.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katneni U.K., Alexaki A., Hunt R.C., Schiller T., DiCuccio M., Buehler P.W.…Kimchi-Sarfaty C. Coagulopathy and thrombosis as a result of severe COVID-19 infection: A microvascular focus. Thrombosis & Haemostasis. 2020 doi: 10.1055/s-0040-1715841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul P., Biagioli M.C., Singh I., Turner R.B. Rhinovirus-induced oxidative stress and interleukin-8 elaboration involves p47-phox but is independent of attachment to intercellular adhesion molecule-1 and viral replication. The Journal of Infectious Diseases. 2000;181(6):1885–1890. doi: 10.1086/315504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesic M.J., Simmons S.O., Bauer R., Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radical Biology and Medicine. 2011;51(2):444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomich O.A., Kochetkov S.N., Bartosch B., Ivanov A.V. Redox biology of respiratory viral infections. Viruses-Basel. 2018;10(8):392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kim S.J., Lee I.S., Lee M.S., Uematsu S., Akira S., et al. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. The Journal of Immunology. 2009;182(4):2458–2466. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- Kim H.R., Park B.K., Oh Y.M., Lee Y.S., Lee D.S., Kim H.K.…Lee S.D. Green tea extract inhibits paraquat-induced pulmonary fibrosis by suppression of oxidative stress and Endothelin-l expression. Lung. 2006;184(5):287–295. doi: 10.1007/s00408-005-2592-x. [DOI] [PubMed] [Google Scholar]
- Köseler A., Sabirli R., Gören T., Türkçüer I., Kurt Ö. Endoplasmic reticulum stress markers in SARS-CoV-2 infection and pneumonia: Case-control study. In Vivo. 2020;34(3 Suppl):1645–1650. doi: 10.21873/invivo.11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazoe M., Sugihara K., Tsukamoto S., Huang Y., Tsurudome Y., Suzuki T., et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. Journal of Clinical Investigation. 2013;123(2):787–799. doi: 10.1172/jci64768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: Molecular pathways and physiological roles. Physiological Reviews. 2014;94(3):739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P.…Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nature Reviews Immunology. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J.D., Kennett M.J., Sang S., Reuhl K.R., Ju J., Yang C.S. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food and Chemical Toxicology. 2010;48(1):409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bagge S., Fotheringham A.K., Leung S.S., Forbes J.M. Targeting the receptor for advanced glycation end products (RAGE) in type 1 diabetes. Medicinal Research Reviews. 2020;40(4):1200–1219. doi: 10.1002/med.21654. [DOI] [PubMed] [Google Scholar]
- Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxidative Medicine and Cellular Longevity. 2018;2018:6208067. doi: 10.1155/2018/6208067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Fung T.S., Huang M., Fang S.G., Zhong Y., Liu D.X. Upregulation of CHOP/GADD153 during coronavirus infectious bronchitis virus infection modulates apoptosis by restricting activation of the extracellular signal-regulated kinase pathway. Journal of Virology. 2013;87(14):8124–8134. doi: 10.1128/jvi.00626-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ashok M., Li J., Yang H., Sama A.E., Wang H. A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PloS One. 2007;2(11):e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen L., Guo F., Cao Y., Hu W., Shi Y.…Guo Y. Effects of epigallocatechin-3-gallate on the HMGB1/RAGE pathway in PM(2.5)-exposed asthmatic rats. Biochemical and Biophysical Research Communications. 2019;513(4):898–903. doi: 10.1016/j.bbrc.2019.03.165. [DOI] [PubMed] [Google Scholar]
- Li H.L., Huang Y., Zhang C.N., Liu G., Wei Y.S., Wang A.B.…Liang C.C. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radical Biology & Medicine. 2006;40(10):1756–1775. doi: 10.1016/j.freeradbiomed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Ling J.X., Wei F., Li N., Li J.L., Chen L.J., Liu Y.Y.…Yang Z.Q. Amelioration of influenza virus-induced reactive oxygen species formation by epigallocatechin gallate derived from green tea. Acta Pharmacologica Sinica. 2012;33(12):1533–1541. doi: 10.1038/aps.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.Y., Liu H.J., Chang C.D., Chen Y.C., Chang C.I., Shih W.L. Avian reovirus S1133-induced apoptosis is associated with Bip/GRP79-mediated Bim translocation to the endoplasmic reticulum. Apoptosis. 2015;20(4):481–490. doi: 10.1007/s10495-015-1085-5. [DOI] [PubMed] [Google Scholar]
- Liu W., Dong M., Bo L., Li C., Liu Q., Li Y.…Li Z. Epigallocatechin-3-gallate ameliorates seawater aspiration-induced acute lung injury via regulating inflammatory cytokines and inhibiting JAK/STAT1 pathway in rats. Mediators of Inflammation. 2014;2014:612593. doi: 10.1155/2014/612593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wang Z., Hou L., Tian X., Zhang X., Cai W. Predicting the effect of tea polyphenols on ticagrelor by incorporating transporter-enzyme interplay mechanism. Chemico-Biological Interactions. 2020;330:109228. doi: 10.1016/j.cbi.2020.109228. [DOI] [PubMed] [Google Scholar]
- Liu R., Xu F., Si S., Zhao X., Bi S., Cen Y. Mitochondrial DNA-induced inflammatory responses and lung injury in thermal injury rat model: Protective effect of epigallocatechin gallate. Journal of Burn Care and Research. 2017;38(5):304–311. doi: 10.1097/bcr.0000000000000501. [DOI] [PubMed] [Google Scholar]
- Liu J.Q., Zelko I.N., Erbynn E.M., Sham J.S., Folz R.J. Hypoxic pulmonary hypertension: Role of superoxide and NADPH oxidase (gp91phox) American Journal of Physiology - Lung Cellular and Molecular Physiology. 2006;290(1):L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- Li W., Zhu S., Li J., Assa A., Jundoria A., Xu J.…Wang H. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochemical Pharmacology. 2011;81(9):1152–1163. doi: 10.1016/j.bcp.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Antoine D.J., Kwan K., Lundbäck P., Wähämaa H., Schierbeck H.…Tracey K.J. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(8):3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C.…Campbell R.A. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martitz J., Becker N.P., Renko K., Stoedter M., Hybsier S., Schomburg L. Gene-specific regulation of hepatic selenoprotein expression by interleukin-6. Metall. 2015;7(11):1515–1521. doi: 10.1039/c5mt00211g. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yamada H., Takuma N., Niino H., Sagesaka Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial. BMC Complementary and Alternative Medicine. 2011;11:15. doi: 10.1186/1472-6882-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J.M., Hybertson B.M., Cota-Gomez A., Geraci K.P., Gao B. Nrf2 activator PB125® as a potential therapeutic agent against COVID-19. Antioxidants. 2020;9(6):518. doi: 10.3390/antiox9060518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca P., Soliman K.F.A. Flavonoids activation of the transcription factor Nrf2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants. 2020;9(8):659. doi: 10.3390/antiox9080659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegazzi M., Campagnari R., Bertoldi M., Crupi R., Di Paola R., Cuzzocrea S. Protective effect of epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled immune activation: Could such a scenario be helpful to counteract COVID-19? International Journal of Molecular Sciences. 2020;21(14):5171. doi: 10.3390/ijms21145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegazzi M., Tedeschi E., Dussin D., De Prati A.C., Cavalieri E., Mariotto S., et al. Anti-interferon gamma action of epigallocatechin-3-gallate mediated by specific inhibition of STAT1 activation. Federation of American Societies for Experimental Biology Journal. 2001;15(7):1309–1311. doi: 10.1096/fj.00-0519fje. [DOI] [PubMed] [Google Scholar]
- Menegazzo L., Ciciliot S., Poncina N., Mazzucato M., Persano M., Bonora B.…Fadini G.P. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetologica. 2015;52(3):497–503. doi: 10.1007/s00592-014-0676-x. [DOI] [PubMed] [Google Scholar]
- Mhatre S., Srivastava T., Naik S., Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153286. 153286-153286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L.…Schomburg L. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Fernandez-Ayala D.J., Navas P., Lopez-Lluch G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Experimental Gerontology. 2020;142:111147. doi: 10.1016/j.exger.2020.111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K., Okuda S., Miyazawa T. Dose-dependent incorporation of tea catechins, (-)-epigallocatechin-3-gallate and (-)-epigallocatechin, into human plasma. Bioscience Biotechnology & Biochemistry. 1997;61(12):1981–1985. doi: 10.1271/bbb.61.1981. [DOI] [PubMed] [Google Scholar]
- Na H.K., Kim E.H., Jung J.H., Lee H.H., Hyun J.W., Surh Y.J. (-)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Archives of Biochemistry and Biophysics. 2008;476(2):171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Na H.-K., Surh Y.-J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food and Chemical Toxicology. 2008;46(4):1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Nishikawa H., Wakano K., Kitani S. Inhibition of NADPH oxidase subunits translocation by tea catechin EGCG in mast cell. Biochemical and Biophysical Research Communications. 2007;362(2):504–509. doi: 10.1016/j.bbrc.2007.08.015. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J.M., Gonagle D.M., Ward S.E., Preston R.J.S., O'Donnell J.S. Endothelial cells orchestrate COVID-19 coagulopathy. The Lancet. Haematology. 2020;7(8):e553–e555. doi: 10.1016/s2352-3026(20)30215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oczypok E.A., Perkins T.N., Oury T.D. All the "RAGE" in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatric Respiratory Reviews. 2017;23:40–49. doi: 10.1016/j.prrv.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olagnier D., Farahani E., Thyrsted J., Blay-Cadanet J., Herengt A., Idorn M.…Holm C.K. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nature Communications. 2020;11(1):4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeira A., Sousa E., Köseler A., Sabirli R., Gören T., Türkçüer İ.…Vasconcelos M.H. Preliminary virtual screening studies to identify GRP78 inhibitors which may interfere with SARS-CoV-2 infection. Pharmaceuticals. 2020;13(6):132. doi: 10.3390/ph13060132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Kim J.E., Gu J.Y., Yoo H.J., Park S.H., Kim Y.I.…Kim H.K. Evaluation of circulating markers of Neutrophil Extracellular Trap (NET) formation as risk factors for diabetic retinopathy in a case-control association study. Experimental and Clinical Endocrinology & Diabetes. 2016;124(9):557–561. doi: 10.1055/s-0042-101792. [DOI] [PubMed] [Google Scholar]
- Polak S.B., Van Gool I.C., Cohen D., von der Thüsen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: A pathophysiological timeline and possible mechanisms of disease progression. Modern Pathology. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potus F., Mai V., Lebret M., Malenfant S., Breton-Gagnon E., Lajoie A.C.…Provencher S. Novel insights on the pulmonary vascular consequences of COVID-19. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2020;319(2):L277–l288. doi: 10.1152/ajplung.00195.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Frontiers in Immunology. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner J.O., Roberts R.A., Kim J., Poklepovic A., Roberts J.L., Booth L., et al. AR12 (OSU-03012) suppresses GRP78 expression and inhibits SARS-CoV-2 replication. Biochemical Pharmacology. 2020;182:114227. doi: 10.1016/j.bcp.2020.114227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. Federation of American Societies for Experimental Biology Journal. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Reid S.P., Shurtleff A.C., Costantino J.A., Tritsch S.R., Retterer C., Spurgers K.B., et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Research. 2014;109:171–174. doi: 10.1016/j.antiviral.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Rojas A., Gonzalez I., Morales M.A. SARS-CoV-2-mediated inflammatory response in lungs: Should we look at RAGE? Inflammation Research. 2020;69(7):641–643. doi: 10.1007/s00011-020-01353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-tan S., Grove K.A., Lambert J.D. Weight control and prevention of metabolic syndrome by green tea. Pharmacological Research. 2011;64(2):146–154. doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath C., Rashid M.R., Sang S., Ahmedna M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via Nrf2 pathway in mice with high fat diet-induced obesity. Biomedicine & Pharmacotherapy. 2017;87:73–81. doi: 10.1016/j.biopha.2016.12.082. [DOI] [PubMed] [Google Scholar]
- Sarkar J., Chakraborti T., Chowdhury A., Bhuyan R., Chakraborti S. Protective role of epigallocatechin-3-gallate in NADPH oxidase-MMP2-Spm-Cer-S1P signalling axis mediated ET-1 induced pulmonary artery smooth muscle cell proliferation. Journal of Cell Communication and Signaling. 2019;13(4):473–489. doi: 10.1007/s12079-018-00501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala I., Valenti D., Scotto D'Aniello V., Marino M., Riccio M.P., Bravaccio C.…Strisciuglio P. Epigallocatechin-3-Gallate plus omega-3 restores the mitochondrial complex I and FF-ATP synthase activities in PBMCs of young children with down syndrome: A pilot study of safety and efficacy. Antioxidants. 2021;10(3) doi: 10.3390/antiox10030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Advances in Biological Regulation. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder E.K., Kelsey N.A., Doyle J., Breed E., Bouchard R.J., Loucks F.A.…Linseman D.A. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxidants and Redox Signaling. 2009;11(3):469–480. doi: 10.1089/ars.2008.2215. [DOI] [PubMed] [Google Scholar]
- Schumacker P.T. Lung cell hypoxia: Role of mitochondrial reactive oxygen species signaling in triggering responses. Proceedings of the American Thoracic Society. 2011;8(6):477–484. doi: 10.1513/pats.201103-032MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt T.D., Call T.G., Zent C.S., Leis J.F., LaPlant B., Bowen D.A.…Kay N.E. Phase 2 trial of daily, oral polyphenon E in patients with asymptomatic, rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2013;119(2):363–370. doi: 10.1002/cncr.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Deep S. In-silico drug repurposing for targeting SARS-CoV-2 main protease (M(pro)) Journal of Biomolecular Structure & Dynamics. 2020:1–8. doi: 10.1080/07391102.2020.1844058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Wu N., Liu Z., Zhao H., Zhao M. Epigallocatechin-3-gallate alleviates paraquat-induced acute lung injury and inhibits upregulation of toll-like receptors. Life Sciences. 2017;170:25–32. doi: 10.1016/j.lfs.2016.11.021. [DOI] [PubMed] [Google Scholar]
- Siddiqui I.A., Adhami V.M., Bharali D.J., Hafeez B.B., Asim M., Khwaja S.I.…Mukhtar H. Introducing nanochemoprevention as a novel approach for cancer control: Proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Research. 2009;69(5):1712–1716. doi: 10.1158/0008-5472.Can-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P.…Ritis K. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. Journal of Clinical Investigation. 2020;130(11):6151–6157. doi: 10.1172/jci141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram N., Kalayarasan S., Manikandan R., Arumugam M., Sudhandiran G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-β1 signalling. Clinical and Experimental Pharmacology and Physiology. 2015;42(8):849–859. doi: 10.1111/1440-1681.12428. [DOI] [PubMed] [Google Scholar]
- Sriram N., Kalayarasan S., Sudhandiran G. Enhancement of antioxidant defense system by epigallocatechin-3-gallate during bleomycin induced experimental pulmonary fibrosis. Biological and Pharmaceutical Bulletin. 2008;31(7):1306–1311. doi: 10.1248/bpb.31.1306. [DOI] [PubMed] [Google Scholar]
- Sriram N., Kalayarasan S., Sudhandiran G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulmonary Pharmacology & Therapeutics. 2009;22(3):221–236. doi: 10.1016/j.pupt.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Sriram N., Kalayarasan S., Sudhandiran G. Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chemico-Biological Interactions. 2009;180(2):271–280. doi: 10.1016/j.cbi.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Steinmann J., Buer J., Pietschmann T., Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. British Journal of Pharmacology. 2013;168(5):1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street M.E. HMGB1: A possible crucial therapeutic target for COVID-19? Hormone Research in Paediatrics. 2020;93(2):73–75. doi: 10.1159/000508291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Liu X., Zhang H., Song Y., Li T., Liu X.…Wu H. Epigallocatechin gallate upregulates Nrf2 to prevent diabetic nephropathy via disabling Keap1. Free Radical Biology & Medicine. 2017;108:840–857. doi: 10.1016/j.freeradbiomed.2017.04.365. [DOI] [PubMed] [Google Scholar]
- Sureda A., Alizadeh J., Nabavi S.F., Berindan-Neagoe I., Cismaru C.A., Jeandet P.…Ghavami S. Endoplasmic reticulum as a potential therapeutic target for COVID-19 infection management? European Journal of Pharmacology. 2020;882:173288. doi: 10.1016/j.ejphar.2020.173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathogens. 2020;16(5) doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Frontiers in Immunology. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L., Forester S.C., Lambert J.D. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (-)-epigallocatechin-3-gallate, in oral cells. Molecular Nutrition & Food Research. 2014;58(4):665–676. doi: 10.1002/mnfr.201300427. [DOI] [PubMed] [Google Scholar]
- Tao L., Park J.Y., Lambert J.D. Differential prooxidative effects of the green tea polyphenol, (-)-epigallocatechin-3-gallate, in normal and oral cancer cells are related to differences in sirtuin 3 signaling. Molecular Nutrition & Food Research. 2015;59(2):203–211. doi: 10.1002/mnfr.201400485. [DOI] [PubMed] [Google Scholar]
- To E.E., Vlahos R., Luong R., Halls M.L., Reading P.C., King P.T.…Selemidis S. Endosomal Nox2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nature Communications. 2017;8(1):69. doi: 10.1038/s41467-017-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend P.A., Scarabelli T.M., Pasini E., Gitti G., Menegazzi M., Suzuki H.…Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. Federation of American Societies for Experimental Biology Journal. 2004;18(13):1621–1623. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- Upadhyay S., Tripathi P.K., Singh M., Raghavendhar S., Bhardwaj M., Patel A.K. Evaluation of medicinal herbs as a potential therapeutic option against SARS-CoV-2 targeting its main protease. Phytotherapy Research : PT. 2020;34(12):3411–3419. doi: 10.1002/ptr.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca R.A., Valenti D. Green tea EGCG plus fish oil omega-3 dietary supplements rescue mitochondrial dysfunctions and are safe in a down's syndrome child. Clinical Nutrition. 2015;34(4):783–784. doi: 10.1016/j.clnu.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Valenti D., Braidy N., De Rasmo D., Signorile A., Rossi L., Atanasov A.G., et al. Mitochondria as pharmacological targets in down syndrome. Free Radical Biology & Medicine. 2018;114:69–83. doi: 10.1016/j.freeradbiomed.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Venereau E., Casalgrandi M., Schiraldi M., Antoine D.J., Cattaneo A., De Marchis F.…Bianchi M.E. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. Journal of Experimental Medicine. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg G.A., van de Nes P.S., Bredenbeek P.J., Spaan W.J. The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. Journal of Virology. 2007;81(20):10981–10990. doi: 10.1128/jvi.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R., et al. Nox2 activation in Covid-19. Redox Biology. 2020;36:101655. doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos R., Stambas J., Bozinovski S., Broughton B.R.S., Drummond G.R., Selemidis S. Inhibition of Nox2 oxidase activity ameliorates influenza a virus-induced lung inflammation. PLoS Pathogens. 2011;7(2) doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Bloom O., Zhang M., Vishnubhakat J.M., Ombrellino M., Che J.…Tracey K.J. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang J., Fan S.M., Zhang J. Epigallocatechin-3-gallate ameliorates lipopolysaccharide-induced acute lung injury by suppression of TLR4/NF-κB signaling activation. Brazilian Journal of Medical and Biological Research. 2019;52(7) doi: 10.1590/1414-431x20198092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li Q., Yin Y., Zhang Y., Cao Y., Lin X.…Qiu Y. Excessive neutrophils and neutrophil extracellular traps in COVID-19. Frontiers in Immunology. 2020;11:2063. doi: 10.3389/fimmu.2020.02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang X., He Y., Jia L., Yang C.S., Reiter R.J., et al. Antioxidant and pro-oxidant activities of melatonin in the presence of copper and polyphenols in vitro and in vivo. Cells. 2019;8(8):903. doi: 10.3390/cells8080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Wang Y., Wan X., Yang C.S., Zhang J. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicology and Applied Pharmacology. 2015;283(1):65–74. doi: 10.1016/j.taap.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Wang D., Wei Y., Wang T., Wan X., Yang C.S., Reiter R.J., et al. Melatonin attenuates (-)-epigallocatehin-3-gallate-triggered hepatotoxicity without compromising its downregulation of hepatic gluconeogenic and lipogenic genes in mice. Journal of Pineal Research. 2015;59(4):497–507. doi: 10.1111/jpi.12281. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang L., Wang J., Zhang Y., Dong R., Wu X.…Zhang J. A mouse model of subacute liver failure with ascites induced by step-wise increased doses of (-)-epigallocatechin-3-gallate. Scientific Reports. 2019;9(1):18102. doi: 10.1038/s41598-019-54691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhou X., Yin Y., Mai Y., Wang D., Zhang X. Hyperglycemia induces neutrophil extracellular traps formation through an NADPH oxidase-dependent pathway in diabetic retinopathy. Frontiers in Immunology. 2018;9:3076. doi: 10.3389/fimmu.2018.03076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Huang J., Sun Y., Stubbs D., He J., Li W.…Zhang J. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food and Chemical Toxicology. 2021;153:112286. doi: 10.1016/j.fct.2021.112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q., Song D., Li H., He M.L. Stress proteins: The biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduction and Targeted Therapy. 2020;5(1):125. doi: 10.1038/s41392-020-00233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Dong W., Jackson J., Ho T.C., Le Saux C.J., Brumwell A.…Chapman H.A. Blocking LOXL2 and TGFbeta1 signalling induces collagen I turnover in precision-cut lung slices derived from patients with idiopathic pulmonary fibrosis. Thorax. 2021 doi: 10.1136/thoraxjnl-2020-215745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D.S., Lahni P.M., Hake P.W., Denenberg A.G., Wong H.R., Snead C.…Zingarelli B. The green tea polyphenol epigallocatechin-3-gallate improves systemic hemodynamics and survival in rodent models of polymicrobial sepsis. Shock. 2007;28(3):353–359. doi: 10.1097/shk.0b013e3180485823. [DOI] [PubMed] [Google Scholar]
- Wolfram S., Raederstorff D., Preller M., Wang Y., Teixeira S.R., Riegger C., et al. Epigallocatechin gallate supplementation alleviates diabetes in rodents. Journal of Nutrition. 2006;136(10):2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- Wong S.L., Demers M., Martinod K., Gallant M., Wang Y., Goldfine A.B.…Wagner D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nature Medicine. 2015;21(7):815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide-Biology and Chemistry. 2020;102:39–41. doi: 10.1016/j.niox.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C., Xiao X., Jiang B., Zhou M., Zhang Y., Li H., et al. Epigallocatechin-3-gallate protects from high glucose induced podocyte apoptosis via suppressing endoplasmic reticulum stress. Molecular Medicine Reports. 2017;16(5):6142–6147. doi: 10.3892/mmr.2017.7388. [DOI] [PubMed] [Google Scholar]
- Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G.…Somers V.K. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clinic Proceedings. 2020;95(6):1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A., Bellamy A.R., Taylor J.A. BiP (GRP78) and endoplasmin (GRP94) are induced following rotavirus infection and bind transiently to an endoplasmic reticulum-localized virion component. Journal of Virology. 1998;72(12):9865–9872. doi: 10.1128/jvi.72.12.9865-9872.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B.…Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M.J., Liu B.J., Wang C.L., Wang G.H., Tian Y., Wang S.H.…Xu T. Epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor and effectively alleviates acute lung injury induced by H9N2 swine influenza virus. International Immunopharmacology. 2017;52:24–33. doi: 10.1016/j.intimp.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Xu J., Xu Z., Zheng W.M. A review of the antiviral role of green tea catechins. Molecules. 2017;22(8):1337. doi: 10.3390/molecules22081337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Takuma N., Daimon T., Hara Y. Gargling with tea catechin extracts for the prevention of influenza infection in elderly nursing home residents: A prospective clinical study. Journal of Alternative & Complementary Medicine. 2006;12(7):669–672. doi: 10.1089/acm.2006.12.669. [DOI] [PubMed] [Google Scholar]
- Yang F., de Villiers W.J., McClain C.J., Varilek G.W. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. Journal of Nutrition. 1998;128(12):2334–2340. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- Yang B., Gao P., Wu X., Yu J., Li Y., Meng R.…Jin X. Epigallocatechin-3-gallate attenuates neointimal hyperplasia in a rat model of carotid artery injury by inhibition of high mobility group box 1 expression. Experimental and Therapeutic Medicine. 2017;14(3):1975–1982. doi: 10.3892/etm.2017.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.S., Hong J. Prevention of chronic diseases by tea: Possible mechanisms and human relevance. Annual Review of Nutrition. 2013;33(1):161–181. doi: 10.1146/annurev-nutr-071811-150717. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Ho C.T., Zhang J., Wan X., Zhang K., Lim J. Antioxidants: Differing meanings in food science and health science. Journal of Agricultural and Food Chemistry. 2018;66(12):3063–3068. doi: 10.1021/acs.jafc.7b05830. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Lee M.J., Chen L. Human salivary tea catechin levels and catechin esterase activities: Implication in human cancer prevention studies. Cancer Epidemiology, Biomarkers & Prevention. 1999;8(1):83–89. [PubMed] [Google Scholar]
- Yang H., Ochani M., Li J., Qiang X., Tanovic M., Harris H.E.…Tracey K.J. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.S., Pan E. The effects of green tea polyphenols on drug metabolism. Expert Opinion on Drug Metabolism and Toxicology. 2012;8(6):677–689. doi: 10.1517/17425255.2012.681375. [DOI] [PubMed] [Google Scholar]
- Yang H., Wang H., Ju Z., Ragab A.A., Lundbäck P., Long W., et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. Journal of Experimental Medicine. 2015;212(1):5–14. doi: 10.1084/jem.20141318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.S., Zhang J.S. Studies on the prevention of cancer and cardiometabolic diseases by tea: Issues on mechanisms, effective doses, and toxicities. Journal of Agricultural and Food Chemistry. 2019;67(19):5446–5456. doi: 10.1021/acs.jafc.8b05242. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Zhang J., Zhang L., Huang J., Wang Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Molecular Nutrition & Food Research. 2016;60(1):160–174. doi: 10.1002/mnfr.201500428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Zhao Y., Suo S., Liu Y., Zhao B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radical Biology & Medicine. 2012;52(9):1648–1657. doi: 10.1016/j.freeradbiomed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Yao J., Liu Y., Wang X., Shen Y., Yuan S., Wan Y., et al. UVB radiation induces human lens epithelial cell migration via NADPH oxidase-mediated generation of reactive oxygen species and up-regulation of matrix metalloproteinases. International Journal of Molecular Medicine. 2009;24(2):153–159. doi: 10.3892/ijmm_00000218. [DOI] [PubMed] [Google Scholar]
- Yates A.A., Erdman J.W., Jr., Shao A., Dolan L.C., Griffiths J.C. Bioactive nutrients - time for tolerable upper intake levels to address safety. Regulatory Toxicology and Pharmacology : Regulatory Toxicology and Pharmacology. 2017;84:94–101. doi: 10.1016/j.yrtph.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Yeung Y.S., Yip C.W., Hon C.C., Chow K.Y., Ma I.C., Zeng F., et al. Transcriptional profiling of Vero E6 cells over-expressing SARS-CoV S2 subunit: Insights on viral regulation of apoptosis and proliferation. Virology. 2008;371(1):32–43. doi: 10.1016/j.virol.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H., Wei L., Sun W.L., Wang L., Yang Z.L., Liu Y.…Zhang W.J. The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. International Journal of Molecular Medicine. 2014;34(1):92–102. doi: 10.3892/ijmm.2014.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Dong R., Sun K., Wang X., Wang J., Yang C.S., et al. Synergistic toxicity of epigallocatechin-3-gallate and diethyldithiocarbamate, a lethal encounter involving redox-active copper. Free Radical Biology & Medicine. 2017;113:143–156. doi: 10.1016/j.freeradbiomed.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y.…Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. Journal of Hematology & Oncology. 2020;13(1):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W.…Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biology. 2020;37:101715. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Ghosh A., Lo C.-S., Chenier I., Scholey J.W., Filep J.G.…Chan J.S.D. Nrf2 deficiency upregulates intrarenal angiotensin-converting enzyme-2 and angiotensin 1-7 receptor expression and attenuates hypertension and nephropathy in diabetic mice. Endocrinology. 2018;159(2):836–852. doi: 10.1210/en.2017-00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.D., Liu H., Li T., Gong Q., Zhang W.L. Epigallocatechin gallate attenuates hip fracture-induced acute lung injury by limiting mitochondrial DNA (mtDNA) release. Medical Science Monitor. 2017;23:3367–3372. doi: 10.12659/msm.902477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Wu X., Wang W., Yang C.S., Zhang J. Tea drinking alleviates diabetic symptoms via upregulating renal water reabsorption proteins and downregulating renal gluconeogenic enzymes in db/db mice. Molecular Nutrition & Food Research. 2020 doi: 10.1002/mnfr.202000505. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Xie D.Y. Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-CoV-2. Frontiers of Plant Science. 2020;11:601316. doi: 10.3389/fpls.2020.601316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T.T., Zhang W.F., Luo P., He F., Ge X.Y., Zhang Z., et al. Epigallocatechin-3-gallate ameliorates hypoxia-induced pulmonary vascular remodeling by promoting mitofusin-2-mediated mitochondrial fusion. European Journal of Pharmacology. 2017;809:42–51. doi: 10.1016/j.ejphar.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A.…Knight J.S. 2020. Neutrophil extracellular traps (NETs) as markers of disease severity in COVID-19. medRxiv, 2020.2004.2009.20059626. [DOI] [Google Scholar]
- Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A.…Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]