Abstract
COVID-19 pandemic has challenged public health systems worldwide, particularly affecting developing countries in Latin America like Ecuador. In this report, we exposed the fundamental role of the Ecuadorian universities to improve COVID-19 surveillance in the country, with an overall contribution over 15% of the total SARS-CoV-2 RT-PCR tests done. We highlight the role of our university during the first semester of the COVID-19 pandemic, contributing to a massive free SARS-CoV-2 testing up to almost 10% of the total diagnosis completed in the country, mainly focus on underserved urban, rural and indigenous communities. Finally, we described our contribution to a high quality and low-cost SARS-CoV-2 RT-PCR diagnostic in Ecuador.
Keywords: Ecuador, SARS-CoV-2, RT-PCR, Surveillance, Neglected communities
1. COVID-19 pandemic in Latin America and Ecuador
Humanity is facing the largest pandemic and the worst public health crisis since the “Spanish flu” in 1918. The Coronaviruses Disease 2019 (COVID-19) pandemic, caused by infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has challenged public health systems worldwide since the initial outbreak in the Chinese city of Wuhan in December 2019 [1,2]. By January 31st 2021, SARS-CoV-2 has caused more than 100 million infections and 2.2 million deaths worldwide (https://coronavirus.jhu.edu/map.html).
Latin America has been deeply affected by COVID-19 pandemic, as many of the public health systems in these countries have been traditionally neglected in government funds. So, when the COVID-19 pandemic landed in Latin America, testing laboratories and hospitals were quickly overflowed [3]. Among the Latin American countries, Ecuador has suffered a severe countrywide COVID-19 outbreak since the first case was reported on February 29th 2020 in the city of Guayaquil. By January 31st 2021, the Ministry of Health (MoH) of Ecuador reported 250,828 cases and 10,177 deaths caused by SARS-CoV-2. However, with a population over 17 million people and a total number of 885,074 PCR tests realized during the whole COVID-19 pandemic period, Ecuador is among the leading countries for positivity rate (28.3%) and poor testing capacities (an average 2626 PCR test/day) despite the unvaluable efforts from the "Instituto Nacional de Investigación y Salud Pública" to keep up SARS-CoV-2 testing with a limited allocation of personnel and funds. So, the true number of SARS-CoV-2 cases and deaths are probably much higher than the officially recognized ones [2].
2. The role of Ecuadorian universities supporting SARS-CoV-2 surveillance: One Health research groups facing a One Health problem
Under this scenario, several universities have played an important role to improve SARS-CoV-2 testing capacities in Ecuador by adapting their research facilities and staff toward clinical diagnosis that were successfully certified by the Ecuadorian public health authorities. Up to January 31st 2021, the “Pontificia Universidad Católica del Ecuador” with more than 50.000 PCR tests done, “Escuela Superior Politécnica del Litoral” and “Universidad de Las Américas”, with more than 30,000 PCR tests each, are leading the universities contribution to SARS-CoV-2 PCR testing. Additionally, “Universidad Central del Ecuador” with more than 10,000 PCR tests, “Universidad Tecnológica Equinocial”, “Ikiam Universidad Regional Amazónica”, “Universidad Yachay Tech” and “Universidad Nacional de Loja” with more than 5000 tests each, have contributed to SARS-CoV-2 surveillance in Ecuador (personal communication to the authors). Also, other universities with certified clinical microbiology laboratories prior to COVID-19 pandemic has been actively performing SARS-CoV-2 testing, like “Universidad San Francisco de Quito” and “Universidad de Especialidades Espiritu Santo”.
Among all the universities, our laboratory at the “Universidad de Las Américas” (UDLA) leads the number of free SARS-CoV-2 tests during the first semester of COVID-19 pandemic. Since April 2020 until September 2020, 22,545 PCR tests were done at our facilities or in collaboration with "Agencia de Regulación y Control de la Bioseguridad y Cuarentena para Galápagos" at Galapagos Islands, 91.8% of them performed at no cost for the patients to help some of the most neglected communities in Ecuador. This effort was possible thanks to UDLA internal funds and the donations received by “Fondo Sumar Juntos” (“Banco de Pichincha”) and “Fondo Salvar Vidas” (“Banco de Guayaquil”).
Up to January 31st 2021, we estimate that at least 140,000 SARS-CoV-2 RT-PCR tests were developed at research laboratories at Ecuadorian universities that were successfully converted to clinical laboratories, representing more than 15% of the SARS-CoV-2 diagnosis completed in Ecuador at that time.
The COVID-19 pandemic is caused by the zoonotic virus SARS-CoV-2 and its dramatic impact worldwide supports the need of the One Health approach to address current and upcoming human and animal public health issues. Actually, the leading research teams from the universities involved in SARS-CoV-2 diagnosis in Ecuador come from a previous experience on tropical medicine and One Health topics like arboviruses, chagas disease, tuberculosis, brucellosis, leptospirosis among other zoonotic diseases. So, those research groups already had the technical and scientific capacities to support the extraordinary surge in demand for diagnostic testing of human samples not ever seen before the COVID-19 pandemic; but not that uncommon on animal infectious diseases surveillance. Moreover, those skills include good biosafety and biosecurity training, and high throughput and quality testing, all of them absolutely mandatory to improve successful COVID-19 surveillance programs.
3. The role of the universities improving SARS-CoV-2 surveillance in neglected provinces and communities
UDLA was the first university in the country to undertake SARS-CoV-2 diagnostic out of the public and private network of clinical microbiology laboratories, and that was done in collaboration with “Agencia de Regulación y Control de la Bioseguridad y Cuarentena para Galápagos” (ABG), by deploying personnel, equipment and reagents to the Galapagos Islands since April 2020.
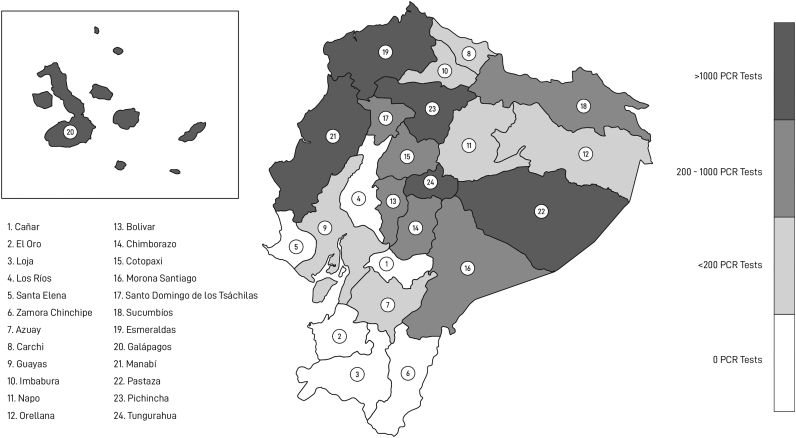
By the end of May 2020, our research laboratories in the capital city of Quito were converted to a SARS-CoV-2 diagnostic facility, where we have been running SARS-CoV-2 RT-PCR tests since then. Moreover, our medical brigades have mostly tested asymptomatic or mild symptomatic community dwelling population from neglected urban, rural, and indigenous communities in 18 out of the 24 provinces of Ecuador (Fig. 1 and Table 1).
Fig. 1.
Distribution of SARS-CoV-2 RT-PCR tests done by “Universidad de Las Américas” among 18 provinces of Ecuador.
Table 1.
SARS-CoV-2 RT-PCR tests done by UDLA and total number of tests done in Ecuador since the beginning of the COVID-19 pandemic till September 12th 2020. Distribution among the provinces of Ecuador, positivity rates and ratio UDLA/ECUADOR is detailed.
| PROVINCE | UDLA positive cases | UDLA total PCR test | UDLA positive rate (%) | ECUADOR total PCR test | ECUADOR positive rate (%) | UDLA/ECUADOR total PCR test (%) |
|---|---|---|---|---|---|---|
| Pichincha | 2138 | 10,401 | 20.6 | 55,480 | 49.9 | 18.8 |
| Manabí | 492 | 2469 | 19.9 | 20,268 | 44.9 | 12.2 |
| Pastaza | 999 | 2194 | 45.5 | 3547 | 56.8 | 61.9 |
| Galápagos | 123 | 2008 | 6.1 | 2325 | 11.8 | 86.4 |
| Esmeraldas | 97 | 1265 | 7.7 | 10,324 | 39.6 | 12.3 |
| Tungurahua | 623 | 1084 | 57.5 | 11,899 | 32.5 | 9.1 |
| Chimborazo | 82 | 603 | 13.6 | 3948 | 51.6 | 15.3 |
| Morona S. | 171 | 525 | 32.6 | 9561 | 26.4 | 5.5 |
| Sucumbíos | 236 | 520 | 45.5 | 5700 | 41.7 | 9.1 |
| Bolívar | 34 | 455 | 7.5 | 4633 | 37.0 | 9.8 |
| Cotopaxi | 83 | 361 | 23.0 | 6692 | 49.5 | 5.4 |
| S. Domingo | 40 | 268 | 14.9 | 9864 | 49.4 | 2.7 |
| Napo | 58 | 191 | 30.4 | 2389 | 50.1 | 8.0 |
| Imbabura | 8 | 40 | 20.0 | 8241 | 39.9 | 0.5 |
| Azuay | 3 | 71 | 4.2 | 21,846 | 34.5 | 0.3 |
| Carchi | 1 | 21 | 4.8 | 5498 | 39.7 | 0.4 |
| Guayas | 3 | 38 | 7.9 | 58,933 | 33.9 | 0.06 |
| Orellana | 7 | 31 | 22.6 | 3177 | 51.9 | 0.1 |
| Total | 5198 | 22,545 | 23.06% | 244,325 | 40.0% | 9.2% |
The strategy of the MoH for SARS-CoV-2 testing has mainly focused on symptomatic population attending hospitals, with a limited average testing capacity below 3000 samples per day. According to the last COVID-19 pandemic epidemiological bulletin from the MoH [4], during the first semester of the COVID-19 pandemic (up to September 12th 2020) there have been 116,451 reported cases of SARS-CoV-2 infection among 244,325 people tested. That means a high positivity rate of 40% (Table 1), clearly above the 5% recommended by the World Health Organization.
As we have detailed above, a total number of 22,545 samples were analyzed for SARS-CoV-2 RT-PCR diagnostic at the laboratory of UDLA or in collaboration with ABG during the first semester of the COVID-19 pandemic. That number of tests represents 9.23% of the total SARS-CoV-2 RT-PCR diagnosis performed in Ecuador during that period of time. Samples were collected countrywide, with more than 1000 tests done at 6 of the 18 provinces attended (Fig. 1). Our contribution to SARS-CoV-2 surveillance was particularly relevant for the provinces of Pichincha, Manabí, Esmeraldas and Chimborazo, where more than 10% of the total SARS-CoV-2 RT-PCR tests were done at our laboratory (Table 1) [5,6]. Moreover, 61.9% of the total SARS-CoV-2 RT-PCR tests done at the Amazonian province of Pastaza were done at our laboratory. Notably, our medical brigades carried out massive COVID-19 surveillance at Waorani People communities, one of the most endangered Amazonian ethnic groups [7]. Our coordinated actions with Waorani leaders to report SARS-CoV-2 outbreaks on their communities supported the lawsuit, previously filed on May 21st 2020, and directed to Ecuador's President Lenin Moreno and Vice President Otto Sonnenholzner, for the lack of protective actions against COVID-19 spread. In a public statement at the time of filing the lawsuit, the Waorani emphasized that their actions were aimed first and foremost at protecting their elders or “Pikenani”, as well as their uncontacted relatives. The Provincial Court of Pichincha finally ruled in favor of the Waorani people's rights to health and granted partial precautionary measures requiring the Ecuadorian government to take urgent actions to contain the COVID-19 pandemic in Waorani territory.
In close collaboration with our colleagues from ABG, we both were responsible for 86.4% of the SARS-CoV-2 diagnostics done at the Galapagos Islands (Fig. 1, Table 1). Up to date, Galapagos Islands is the province of Ecuador with the highest number of SARS-CoV-2 tests per capita. Moreover, this intervention made Galapagos Islands the only province of Ecuador achieving the WHO recommendation of a positivity rate below 5% during the first months of COVID-19 pandemic; actually, by the end of May 2020, Galapagos was declared free of SARS-CoV-2 for several weeks [8]. It is important to notice that ABG is a public institution within the Ecuadorian Ministry of Environment, and not the MoH, who controls companion and livestock animal health in Galapagos Islands with a One Health scope. Moreover, ABG and UDLA previous experience and collaboration on zoonotic diseases surveillance and One Health research on Galapagos Islands prior to COVID-19 pandemic was fundamental to improve the most successful SARS-CoV-2 diagnosis program in Ecuador.
Overall, we have identified 5198 positive cases of SARS-CoV-2 infection. Our positivity rate of 23.06% was significantly lower than the 40% for the whole country. Moreover, that was the case for all the provinces where we carried out SARS-CoV-2 testing, except for Tungurahua and Sucumbíos provinces where the positivity rate was above the one reported for MoH (Table 1).
Largely, UDLA has contributed with almost 10% of the total SARS-CoV-2 testing during the first semester of the COVID-19 pandemic in Ecuador. Moreover, as our surveillance was mainly focused on asymptomatic or mild symptomatic community dwelling individulas not attending medical facilities at neglected urban, rural and indigenous communities, the high positivity rate of 23,06% obtained at our lab supports the hypothesis that massive community transmission of SARS-CoV-2 has been happening in Ecuador [5].
4. The role of the universities improving high quality and low cost SARS-CoV-2 RT-PCR testing in Ecuador
Our SARS-CoV-2 diagnosis laboratory at UDLA has also improved the quality and accessibility to SARS-CoV-2 diagnostic for the Ecuadorian population. We have reported cheaper sample collection and processing protocols prior to SARS-CoV-2 RT-PCR diagnosis, by using cotton swabs for sampling, heat shock for RNA extraction or sample pooling prior to PCR [[9], [10], [11]]. Moreover, since the Ecuadorian government regulatory agency certifying SARS-CoV-2 diagnostic kits lacks of validation laboratories, we have carried an intensive research activity to evaluate up to 12 commercial kits for SARS-CoV-2 RT-PCR and RT-LAMP diagnosis, and also serological and antigen tests [[12], [13], [14], [15], [16], [17], [18], [19]]. We have identified 5 commercial kits with a poor clinical performance and a limit of detection much higher than what it was detailed at manufacturer manual [12,[14], [15], [16],18]. Overall, we found a worrying trend among the 12 commercial RT-PCR and RT-LAMP kits analyzed: only the 7 kits that have Emergency Use Authorization at their country of production accomplished the sensitivity and limit of detection promised by manufacturer. Considering that those 5 low quality kits are produced in developed countries, we expressed our deep ethical concern toward those companies that sell SARS-CoV-2 diagnosis kits to Ecuador that are not authorized for clinical use with their fellow citizens [14,18].
Additionally, UDLA has developed “ECUGEN SARS-CoV-2 RT-PCR kit”, the first SARS-CoV-2 RT-PCR kit made in Ecuador and to our knowledge the second one in Latin America. This has allowed to several laboratories to reduce SARS-CoV-2 RT-PCR diagnostic costs. Actually, UDLA laboratory for SARS-CoV-2 diagnosis offered one of the most affordable price for SARS-CoV-2 RT-PCR diagnosis in Ecuador (40 USD; minimum wage in Ecuador is below 400 USD/month). Also, to our knowledge, we and our colleagues from ABG are the only laboratories that report viral loads in the country.
5. Conclusions
The COVID-19 pandemic continues to challenge Latin American countries including Ecuador. While the MoH of Ecuador has a limited testing capacity for SARS-CoV-2 surveillance, public and private universities have played a crucial role in supporting more than 15% of SARS-CoV-2 diagnostic in Ecuador. The contribution of UDLA has been particularly relevant during the first semester of the COVID-19 pandemic with almost 10% of the SARS-CoV-2 tests done at our laboratories and more 20,000 tests done at no cost for the Ecuadorian population. We have also contributed to make SARS-CoV-2 diagnosis affordable and sensitive in Ecuador, through new products, protocols and clinical evaluation studies of commercial tests.
We are very proud of the labor of the university system to improve SARS-CoV-2 surveillance in Ecuador. All this work was done without any financial support from the Government of Ecuador. We hope that the experience described in this article will inspire the Government of Ecuador and other Latin American countries to count on the universities to face future public health threats like this COVID-19 pandemic. Moreover, the story of success described on this article was possible due to the leadership of research groups related to One Health topics within the universities involved, proving themselves as highly qualified to face a One Health problem such as the COVID-19 pandemic .
Ethical approval and consent to participate
Written consent was obtained for all the individuals enrolling SARS-CoV-2 testing at UDLA. As non personal information was used and data was anonymized before analysis, this study falls under the category of “exempted” by the Bioethics Commission from UDLA.
Funding
This study was supported by Fondo Sumar Juntos” (Banco del Pichincha), Fondo Salvar Vidas” (Banco de Guayaquil) and by “Universidad de Las Américas”.
Authors' contributions
All authors contributed to data collection, analysis and conceptualization of the manuscript. MAGB wrote the first draft of the manuscript. All the authors revised and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We thank UDLA authorities for their support to conduct SARS-CoV-2 surveillance at our research laboratories, specially to our Canciller Dr. Carlos Larreategui Nardi. We thank to our colleagues from "Agencia de Regulación y Control de la Bioseguridad y Cuarentena para Galápagos" to count on us for the improvement of SARS-CoV-2 diagnosis at Galapagos Islands at early stages of the COVID-19 pandemic; particularly to Dra Marilyn Cruz for her leadership to protect Galapagos Island population againts COVID-19 pandemic, and the rest of the ABG lab team: Alberto Velez, Paulina Castillo, Patricio Vega Mariño y Carlos Masaquiza. Our experience in Galapagos was fundamental to success later on doing SARS-CoV-2 testing in our lab in Quito. We thank to all the regional and municipal governments, non profit organizations, indigenous organizations and regional authorities of MoH for their support during our community surveillance. United we made it.
References
- 1.Ortiz-Prado E., Simbaña-Rivera K., Gómez-Barreno L., Rubio-Neira M., Guaman L.P., Kyriakidis N.C., Muslin C., Jaramillo A.M.G., Barba-Ostria C., Cevallos-Robalino D., Sanches-SanMiguel H., Unigarro L., Zalakeviciute R., Gadian N., López-Cortés A. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020 Sep;98(1):115094. doi: 10.1016/j.diagmicrobio.2020.115094. (Epub 2020 May 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz-Prado E., Simbaña-Rivera K., Barreno L.G., Diaz A.M., Barreto A., Moyano C., Arcos V., Vásconez-González E., Paz C., Simbaña-Guaycha F., Molestina-Luzuriaga M., Fernández-Naranjo R., Feijoo J., Henriquez-Trujillo A.R., Adana L., López-Cortés A., Fletcher I., Lowe R. Epidemiological, socio-demographic and clinical features of the early phase of the COVID-19 epidemic in Ecuador. PLoS Negl. Trop. Dis. 2021 Jan 4;15(1) doi: 10.1371/journal.pntd.0008958. (eCollection 2021 Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez P.J., Huete-Perez J.A., Bottazzi M.E. COVID-19 in the Americas and the erosion of human rights for the poor. PLoS Negl. Trop. Dis. 2020;14(12) doi: 10.1371/journal.pntd.0008954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolletin Epidemiologico COVID-19 Ministerio de Salud Pública. September 2020. https://www.salud.gob.ec/wp-content/uploads/2020/09/Boletin-196_Nacional_MSP.pdf Avalaible at.
- 5.Ortiz-Prado Esteban, Henriquez-Trujillo Aquiles R., Rivera-Olivero Ismar A., Lozada Tannya, Miguel Angel Garcia-Bereguiain, on behalf of the UDLA-COVID-19 Team High prevalence of SARS-CoV-2 infection among food delivery riders. A case study from Quito, Ecuador. Sci. Total Environ. 2021;770:145225. doi: 10.1016/j.scitotenv.2021.145225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz-Prado Esteban, Henriquez-Trujillo Aquiles R., Rivera-Olivero Ismar A., Freire-Paspuel Byron, Vallejo-Zaneta Paolo Alexander, Lozada Tannya, Miguel Angel Garcia-Bereguiain; on behalf of “UDLA COVID-19 Team” Massive SARS-CoV-2 RT-PCR testing on rural communities in Manabi province (Ecuador) reveals severe COVID19 outbreaks. Am. J. Trop. Med. Hyg. 2021 Feb 8;104(4):1493–1494. doi: 10.4269/ajtmh.20-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz-Prado Esteban, Rivera-Olivero Ismar A., Freire-Paspuel Byron, Lowe Rachel, Lozada Tannya, Henriquez-Trujillo Aquiles R., Garcia-Bereguiain Miguel Angel, on behalf of “UDLA COVID-19 Team” Int. J. Infect. Dis. 2021 Apr;105:234–235. doi: 10.1016/j.ijid.2021.02.039. [DOI] [PubMed] [Google Scholar]
- 8.Freire-Paspuel B., Vega-Mariño P., Velez A., Castillo P., Masaquiza C., Cedeño-Vega R., Lozada T., Cruz M., Garcia-Bereguiain M.A. “One Health” inspired SARS-CoV-2 surveillance: the Galapagos Islands experience. One Health. 2020 Oct;20:100185. doi: 10.1016/j.onehlt.2020.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freire-Paspuel B., Vega-Mariño P., Velez A., Castillo P., Gomez-Santos E.E., Cruz M., Garcia-Bereguiain M.A. Cotton-tipped plastic swabs for SARS-CoV-2 RT-qPCR diagnosis to prevent supply shortages. Front. Cell. Infect. Microbiol. 2020 Jun 23;10:356. doi: 10.3389/fcimb.2020.00356. (eCollection 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno Alfredo, de Mora Domenica, Freire-Paspuel Byron, Orlando Alberto, Bereguiain Miguel Angel Garcia. Analytical and clinical evaluation of a heat shock SARS-CoV-2 diagnosis method without RNA extraction for N and E genes RT-qPCR. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.06.038. (Accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freire-Paspuel Byron, Vega-Mariño Patricio, Velez Alberto, Castillo Paulina, Cruz Marilyn, Garcia-Bereguiain Miguel Angel. Sample pooling of RNA extracts to speed up SARS-CoV-2 diagnosis using CDC FDA EUA RT-qPCR kit. Virus Res. 2020;290 doi: 10.1016/j.virusres.2020.198173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freire-Paspuel Byron, Vega-Mariño Patricio, Velez Alberto, Castillo Paulina, Cruz Marilyn, Garcia-Bereguiain Miguel Angel. Evaluation of nCoV-QS (MiCo BioMed) for RT-qPCR detection of SARS-CoV-2 from nasopharyngeal samples using CDC FDA EUA qPCR Kit as a gold standard: an example of the need of validation studies. J. Clin. Virol. 2020 May 22;128:104454. doi: 10.1016/j.jcv.2020.104454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freire-Paspuel B., Vega-Mariño P., Velez A., Cruz M., Perez F., Garcia-Bereguiain M.A. Analytical and clinical comparison of Viasure (CerTest Biotec) and 2019-nCoV CDC (IDT) RT-qPCR kits for SARS-CoV2 diagnosis. Virology. 2020 Nov 18;553:154–156. doi: 10.1016/j.virol.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freire-Paspuel Byron, Garcia-Bereguiain Miguel Angel. Low Clinical Performance of "Isopollo COVID19 detection kit" (Monitor, South Korea) for RT-LAMP SARS-CoV-2 diagnosis: a call for action against low quality products for developing countries. Int. J. Infect. Dis. 2021 Jan 9 doi: 10.1016/j.ijid.2020.12.088. S1201-9712(21)00017-5. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freire-Paspuel Byron, Garcia-Bereguiain Miguel Angel. Poor sensitivity of "AccuPower SARS-CoV-2 real time RT-PCR kit (Bioneer, South Korea)". Virol. J. 2020 Nov 14;17(1):178. doi: 10.1186/s12985-020-01445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freire-Paspuel Byron, Garcia-Bereguiain Miguel Angel. Analytical and clinical evaluation of "AccuPower SARS-CoV-2 Multiplex RT-PCR kit (Bioneer, South Korea)" and "Allplex 2019-nCoV Assay (Seegene, South Korea)" for SARS-CoV2 RT-PCR diagnosis: Korean CDC EUA as a quality control proxy for developing countries. Front. Cell Infect. Microbiol. 2021 doi: 10.3389/fcimb.2021.630552. (Accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freire-Paspuel Byron, Bruno Alfredo, Orlando Alberto, Garcia-Bereguiain Miguel Angel. Clinical performance and analytical sensitivity of three SARS-CoV-2 nucleic acid diagnostic tests. Am. J. Trop. Med. Hyg. 2021 Feb 26;104(4):1516–1518. doi: 10.4269/ajtmh.20-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freire-Paspuel Byron, Garcia-Bereguiain Miguel Angel. Clinical performance and analytical sensitivity of two SARS-CoV-2 nucleic acid diagnostic tests used in Ecuador. Am. J. Trop. Med. Hyg. 2021 Mar 5;104(5):1672–1675. doi: 10.4269/ajtmh.20-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freire-Paspuel Byron, Garcia-Bereguiain Miguel Angel. Analytical sensitivity and clinical performance of a triplex RT-qPCR assay using CDC N1, N2 and RP targets for SARS-CoV-2 diagnosis. Int. J. Infect. Dis. 2020 Oct 25 doi: 10.1016/j.ijid.2020.10.047. S1201-9712(20)32250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]