Abstract
SARS-CoV-2 is a type of beta-CoV that develops acute pneumonia, which is an inflammatory condition. A cytokine storm has been recognized as one of the leading causes of death in patients with COVID-19. ALI and ARDS along with multiple organ failure have also been presented as the consequences of acute inflammation and cytokine storm. It has been previously confirmed that SARS-CoV, as another member of the beta-CoV family, activates NLRP3 inflammasome and consequently develops acute inflammation in a variety of ways through having complex interactions with the host immune system using structural and nonstructural proteins. Numerous studies conducted on Tranilast have further demonstrated that the given drug can act as an effective anti-chemotactic factor on controlling inflammation, and thus, it can possibly help the improvement of the acute form of COVID-19 by inhibiting some key inflammation-associated transcription factors such as NF-κB and impeding NLRP3 inflammasome. Several studies have comparably revealed the direct effect of this drug on the prevention of inappropriate tissue’s remodeling; inhibition of neutrophils, IL-5, and eosinophils; repression of inflammatory cell infiltration into inflammation site; restriction of factors involved in acute airway inflammation like IL-33; and suppression of cytokine IL-13, which increase mucosal secretions. Therefore, Tranilast may be considered as a potential treatment for patients with the acute form of COVID-19 along with other drugs.
Keywords: Coronavirus, COVID-19, Tranilast, NLRP3 Inflammasome, Inflammation, SARS-CoV-2
Introduction
Coronaviruses (CoVs) are known as potential pathogens for humans and vertebrates, which affect respiratory system, gastrointestinal (GI) tract, liver, and central nervous system (CNS) of humans, livestock, birds, bats, mice, and many other wild animals [1]. Alpha- and beta-CoVs typically infect mammals, while gamma- and delta-CoVs mostly infect birds and fish, and rarely mammals [2]. Before 2019, only six CoVs had been introduced as causing infection in humans and developing respiratory diseases. In this respect, human CoV 229E (HCoV-229E), HCoV-NL63, HCoV-HKU1, and HCoV-OC43 (HCoV-OC43 only develops the upper respiratory tract infection (URTI)) can cause severe infections in infants, children, and adults, but in rare cases. The severe acute respiratory syndrome CoV (SARS-CoV) and the Middle East respiratory syndrome (MERS) can also affect the lower respiratory tract, which have previously caused human SARS in China (2002) and in the Middle East (2012), respectively [3,4]. In December 2019, patients with acute respiratory infections were reported in Wuhan city, Hubei Province, China. Many patients had direct or indirect links with the Huanan Seafood Wholesale Market, which is based in Wuhan city and believed to be the hotspot of the outbreak of the novel CoV (SARS-CoV-2) [5]. Studies performed in this field have further shown that SARS-CoV-2 has many genetic similarities to the SARS-CoV. Analysis of the gene sequence of SARS-CoV-2, as well as the resemblance of spike (S) proteins on the virus surface have also led to this prediction that SARS-CoV-2, similar to SARS-CoV, would likely use angiotensin-converting enzyme 2 (ACE2) receptors, in order to penetrate into the cell. In a study, sequencing of five patients had correspondingly revealed that the sequence of SARS-CoV-2 genome is 96% identical to that of the bat CoV [5,6]. CoVs are also the biggest ribonucleic acid (RNA) viruses, whose genome contains a positive-sense single-stranded RNA (+ssRNA) with about 30 kb in length, a 5′ cap structure, and a poly (A) tail. It was shown that SARS-CoV-2, which belongs to beta-CoVs, can similarly cause severe respiratory syndromes by involving the lower respiratory tract. Even though Tranilast (INN, brand name Rizaben) is known as an anti-allergy drug, its efficacy in other diseases is being investigated in various clinical trials, as illustrated in Table 1. In some diseases such as gout, hyperuricemia, active rheumatoid arthritis, and sarcoidosis, both immune responses and inflammation play destructive roles [10]. Moreover, in some cases, including gout and hyperuricemia, the destructive role of NLRP3 inflammasomes in immunopathogenesis is now well-established. Given the presence of destructive inflammation, cytokine storm, and NLRP3 inflammasome hyperactivity in COVID-19, conducting a clinical trial to evaluate the efficacy and safety of Tranilast in the treatment of this disease is needed. In the present review we discuss the anti-inflammatory and immunomodulatory role of Tranilast in COVID-19 and also highlights the underlying mechanisms of action.
Table 1.
Studies indicating the role of Tranilast in COVID-19 patients.
| Targets | Mechanisms | Main conclusion of the study | Ref |
|---|---|---|---|
| NLRP3 inflammasome | Tranilast inhibit NLRP3 inflammasome | One of main therapeutic target in the severe COVID-19 treatment in the patients with a weakened immune system is the NLRP3 inflammasome and related downstream pathways. | [7] |
| NLRP3 inflammasome | Tranilast inhibit NLRP3 inflammasome | The inflammatory cytokine signaling caused by SARS-CoV-2 can be mitigated following the administration of Tranilast and other therapeutic agents by targeting NLRP3 inflammasome, resulting in and immediate improvement of patient outcomes. | [8] |
| NLRP3 inflammasome | Tranilast targets NLRP3 or NLRP3–ASC interaction to block the assembly of NLRP3 inflammasome | There is a possibility of an initial defect in the immune systems of obese people with metabolic disorders that indicate an increased susceptibility to SARS-CoV-2 infection, which is attributed to the occurrence of metaflammation, i.e. an increase in systemic metabolic inflammation. | [9] |
A body of literature
Immunopathogenesis of COVID-19
Undoubtedly, obtaining a better understanding on the immunopathogenesis as well as the interaction of the immune system and the virus can be enormously helpful. Identifying the immune responses in patients with severe COVID-19 may thus result in significant advances in developing effective treatment methods [11]. SARS-CoV2 leads to dysregulation of adaptive and innate immune responses through various mechanisms. On the one hand, the virus impairs effective antiviral immune responses, and on the other, it evokes severe inflammatory responses in patients with severe and critical COVID-19. Various studies associated with the pathogenesis of COVID-19 lymphopenia reported the hyperactivity and exhaustion of T lymphocytes and NK cells, the destructive role of inflammatory neutrophils and macrophages, aberrant differentiation of T cell helper subsets, the elevated Th17 cell frequencies, the exacerbation of neutrophil inflammation, increased neutrophil-lymphocyte ratio (NLR) as an important marker of severe inflammation, change in antibody production trend with overproduction of IgG and total immunoglobulins, and overexpression of chemokines, adhesion molecules and proinflammatory cytokines [12–18]. Notably, one of the leading causes of death in patients with COVID-19 is cytokine storm. Although a large number of patients with this disease only show mild symptoms of the disease, some of them manifest very severe symptoms under this condition. In general, patients over the age of 65 years old suffering from underlying diseases are at much higher risk of mortality. Besides, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [2] are the two conditions characterized by acute pulmonary insufficiency, acute onset of respiratory failure, and low arterial oxygen levels, which are major pathological complications from cytokine storm in patients with COVID-19 [11,19–21]. Therefore, SARS-CoV-2 has a higher rate of infection, so it can even affect a wider range of cells independent from ACE2 expression, which is a strong evidence for those studies reporting liver failure, CNS involvement, and GI distress [22–25].
NLRP3 inflammasome as a source of cytokine storm and inflammation in COVID-19
NLRP3 inflammasome is among the most important components of the innate immune system that significantly enhance inflammation by increasing the production of interleukin 1 beta (IL-1β), IL-18, and gasdermin D (GSDMD). It also plays a key role in the pathogenesis of many diseases associated with destructive inflammation. Many studies conducted on viral infections have further confirmed the over-activation of NLRP3 inflammasome, which consequently results in destructive and systemic inflammations in patients. SARS-CoV-2 proliferation can be comparably considered in a wide range of cells along with numerous observations of direct and indirect activations of the NLRP3 Inflammasome by other CoVs. This inflammasome is likely to be triggered by acute cytokine storm, which develops following ARDS and multiple organ failure (MOF), and eventually leads to the death of patients [8]. A large number of clinical trials have been conducted on controlling acute inflammation in COVID-19 patients, but most of these treatments have merely focused on the related branches and ignored the root and the motor trigger of inflammation. ALI and ARDS are often characterized by neutrophil accumulation in lungs as well as the increased production of inflammatory cytokines, chemokines, proteases, and oxidants [26]. Studies have further demonstrated that a large number of infectious and noninfectious macrophages are present in the lungs of patients with COVID-19, indicating the important role of macrophages in the immunopathogenesis of this viral disease. Additionally, high levels of pre-inflammatory and suppressive cytokines such as IL-2, IL-7, granulocyte colony-stimulating factor (G-CSF), interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1a (MIP-1a), and tumor necrosis factor alpha (TNF-α) have been reported in acute cases [27]. A recent study has correspondingly suggested that the NLRP3 inflammasome activation is a central mechanism for the induction and the progression of cytokine storms and MOF in a streptococcal toxic-shock syndrome (TSS) model. In this regard, NLRP3 inflammasome plays a key role in the inductions of ALI and ADRS [28–30]. Correspondingly, other studies have revealed that IL-1β, as a potent pre-inflammatory cytokine resulted from the activity of NLRP3 Inflammasome, is also involved in the pathogenesis of ARDS (Figure 1) [31].
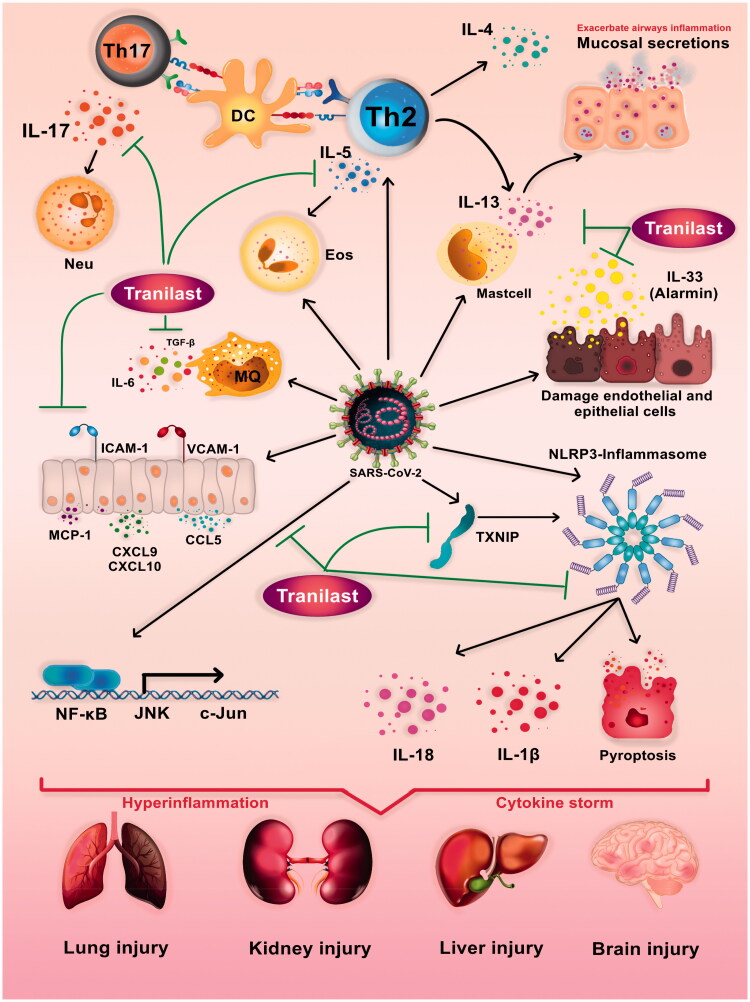
Figure 1.
Putative roles of Tranilast in modulating immune response and Potential mechanisms of SARS-CoV-2-induced immunopathology. SARS-CoV2 leads to dysregulation of adaptive and innate immune responses resulting in severe inflammation, cytokine storm and multiple organ dysfunction syndromes. Hyperactivity of inflammasome and dependent pathways, overexpression of chemokines and adhesion molecules, overproduction of proinflammatory cytokines, aberrant differentiation of CD4+ T-lymphocytes, and destructive role of Th17 and Th2 are all key factors in COVID-19 immunopathogenesis. Tranilast has the potential to prevent the exacerbation of COVID-19 by affecting the different pathways such as NLRP3 inflammasome (NLRP3, Caspase1, TXNIP), signaling pathways (NF-κB), cytokines (IL-33, IL-5, IL-17, IL-13, TGF-β), and chemokines (CCL5, CXCL9, CXCL9) and cell adhesion molecules (ICAM1).
Beta-CoVs activate NLRP3 inflammasome in different ways
SARS-CoV, which has more than 80% structural and phylogenetical similarities to SARS-CoV-2, activates NLRP3 inflammasome in different ways. Proper ionic concentration is also of great importance to maintain cellular homeostasis in host cells. Thus, NLRP3 inflammasome could recognize the alarmin once homeostasis is disrupted and then it will be activated. The flow of potassium ions also activates NLRP3 inflammasome [32,33]. Besides, viroporins are small and highly hydrophobic viral proteins disrupting the homeostasis of intracellular ionic concentrations and consequently activating NLRP3 inflammasome in various types of viruses [34–37]. SARS-CoV viroporin 3a can thus form a cell membrane ion channel to release potassium and sodium ions, replace calcium, and activate more NLRP3 inflammasome [38]. Moreover, SARS-CoV viroporin E penetrates into the Golgi apparatus and the membranes of other cellular organelles, thereby activating NLRP3 inflammasome. Additionally, SARS-CoV protein N activates NLRP3 inflammasome by transferring intracellular calcium storage into the cytosol. It is noteworthy that SARS-CoV ORF8b protein also causes intracellular accumulation through its valine at residue 77 [39]. The latest structural models of mature peptides in SARS-CoV-2 produced by Contact-guided Iterative Threading ASSEmbly Refinement (C-I-TASSER) clearly show that SARS-CoV-2, which is responsible for COVID-19, can encode both ORF3a and E viroporins. Therefore, SARS-CoV-2 not only enhances the first step, but also activates NLRP3 inflammasome. In this regard, various studies have further revealed the positive inhibitory role of NLRP3 inflammasome in preventing the destructive effects of cytokine storm [40]. Moreover, different natural, chemical, and hormonal compounds able to inhibit NLRP3 inflammasome directly and indirectly, thereby reducing inflammation, have also been identified [41].
Importance of the NLRP3 inflammasome inhibition in the treatment of inflammatory diseases
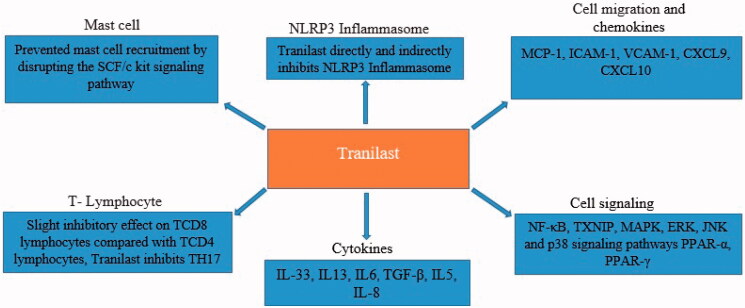
NLRP3 inflammasome is one of the most important areas in immunology. So, future medical research should focus on the treatment of inflammatory diseases by examining the molecular mechanisms of the NLRP3 inflammasome activation to identify the effective inhibitors of NLRP3 inflammasome or its inhibitory pathways. Considerably, a number of NLRP3 inflammasome inhibitors have been reported to date, including those that directly impede NLRP3 or indirectly hinder the components of the inflammasome or the related signaling pathways [42]. IL-1β inhibition also is one of the successful experiments performed for the treatment of NLRP3 inflammasome-associated inflammatory disorders. Canakinumab (as a IL-1β neutralizing antibody), anakinra (viz. a recombinant human IL-1 receptor antagonist that prevents the binding of IL-1β and IL-1α), and rilonacept (as an IL-1 decoy receptor binding to IL-1β and IL-1α) are the approved medications by Food and Drug Administration (FDA) in the treatment of various inflammatory diseases [43]. Two similar biologic drugs, named as IL-18 inhibitor (that is, GSK1070806) and IL-1α neutralizing antibody viz. MABp1), are at the early stages of their developments [44]. It is important to note that IL-1β inhibition or a defect in IL-1 receptor type 1 (IL-1R1) would not lead to the cessation of mortality in a mouse model of calcyphosine (CAPS), suggesting that other inflammatory mediators released during the NLRP3 inflammasome activation process might be vital for disease progression [45]. Therefore, directly targeting NLRP3 inflammasome with small molecules is a far less invasive, a more cost-effective, and a more specific way of blocking cytokines. In this respect, a diarylsulfonylurea-containing compound (viz. MCC950), originally reported as CP-456773 sodium salt (CRID3)/CP-456773, is known as the strongest and the most specific NLRP3 inflammasome inhibitor [46]. The therapeutic efficacy of MCC950 on a variety of preclinical models of immunopathology, including CAPS, in vitro experimental autoimmune encephalomyelitis (EAE) [47], Alzheimer’s disease (AD) [48], brain injury [49], atherosclerosis [50], cardiac arrhythmia [51], myocardial infarction (MI) [52], diabetes mellitus (DM) [53], hepatitis, steatorrhea [54], and colitis, has been confirmed [55]. Although a phase-II clinical trial of MCC950 has been suspended for the treatment of rheumatoid arthritis (RA) due to hepatotoxicity, these performed studies can be considered as a compelling reason to target NLRP3 inflammasome in the treatment of inflammatory diseases [56]. Undoubtedly, if the drugs used for human purposes could directly and indirectly inhibit NLRP3 inflammasome, they would be considered as the ultimate treatment candidates for inflammatory disorders. Accordingly, Tranilast is one of these drugs, which was approved as an anti-allergic agent and tryptophan metabolite analogue in Korea and Japan in 1982 for the treatment of allergies, asthma, and hypertrophic scars (approximately 40 years ago) [10]. Recently, using this drug, the mechanism involved in the direct inhibition of NLRP3 has been identified. Some studies have further revealed that Tranilast binds to NACHT domain and stops the interaction between NLRP3-NLRP3 and subsequent oligomerization without affecting its ATPase activity [57]. Tranilast also acts to inhibit NLRP3 inflammasome specifically without disrupting the activation of the other known inflammasomes such as NLRC2 and AIM2. This drug not only inhibits NLRP3 inflammasome in a direct manner, but also effectively suppresses other inflammatory responses in various ways. In fact, Tranilast restrains inflammation in an effective manner through multiple mechanisms and also prevents inappropriate tissue remodeling, which is an adverse effect. Accordingly, understanding immune responses in patients with severe COVID-19 may lead to significant advances in developing effective treatment methods [11]. In this regard, a large number of clinical trials are attempting to manage acute inflammation in these patients. At the beginning of the pandemic COVID-19, numerous studies investigating other pathogenic beta-coronaviruses have highlighted the idea that the increased activity and adjustment disorder in NLRP3 inflammasome can play key roles in the occurrence of cytokine storms in patients with COVID-19 [7,58]. For example, in a study, the role of pyroptosis and inflammasome activations in lymphopenic liver had been investigated in patients with COVID-19, confirming that the inhibitions of pyroptosis and NLRP3 inflammasome could subsequently lead to liver disorders and could also be a potential therapeutic target for patients with COVID-19 [59]. A single-cell mathematical model of SARS-CoV-2 induced pyroptosis and the anti-inflammatory response to the drug tranilast demonstrated that tranilast delays the formation of the NLRP3 inflammasome, and thus may reduce pyroptosis [60]. The results of these studies are presented in the form of review articles in which the efficacy of NLRP3 inflammasome inhibitors like Tranilast, has been suggested (Table 1).
Effect of tranilast on cytokines and chemokines
The regulation of immune system responses, its proper functioning, and ideal interaction between immune and non-immune cells resulted from the expression of a range of different cytokines and chemokines by different cells, play key roles in the physiological conditions of the body and the immunopathogenesis of many diseases such as cancer, autoimmunity, allergies, and inflammatory diseases [61,62]. It was shown that cytokines and chemokines also contribute to the virus and the host immune system involvement, so they can be considered as potential therapeutic targets [63]. Likewise, Tranilast is able to affect the expression patterns of cytokines and chemokines (Figures 1 and 2). Accordingly, IL-33 belonging to IL-1 family of cytokines is secreted as an alarmin, especially from the damaged endothelial and epithelial cells, which increases the expression of chemokines. Genetically, this cytokine is most strongly associated with asthma exacerbation, which plays a prominent role in the incidence and worsening of pulmonary inflammation [64,65]. It also increases reactive oxygen species (ROS), as one of the activators of NLRP3 inflammasome. Several recent studies have reported that a higher expression of chemokines in the lung tissue and the pulmonary alveolar macrophages play a destructive role in COVID-19 [66]. The protein kinase B (AKt) signaling pathway has been documented to increase NF-κB expression, which on the other hand, strengthen type 2 inflammation. Tranilast is reportedly able to inhibit the secretion of IL-33 from lipopolysaccharide-stimulated macrophages. Therefore, it can protect patients from the occurrence of acute inflammation [64]. In this regard, a study had demonstrated that Tranilast is able to inhibit mast cell degranulation and prevent IL-13 production from mast cells derived from bone marrow stimulated by IL-33 and 2,4-dinitrophenyl group (DNP)/bovine serum albumin (BSA) stimulants. In patients with ARDS, IL-13 can further exacerbate their condition by escalating mucosal secretions, indicating the effectiveness of Tranilast in the inhibition of airway inflammation [65]. Moreover, IL-6 is one of the inflammatory cytokines leading to acute inflammation in patients with COVID-19. IL-6 secretion from fibroblasts similarly plays a destructive role in exacerbating inflammation during carpal tunnel syndrome. One study has found that Tranilast effectively and dose-dependently inhibits the proliferation of fibroblasts and their secretion from IL-6 [67]. Another study had evaluated the therapeutic effect of Tranilast with beclomethasone in an asthmatic mice model with acute airway inflammation. It was indicated that Tranilast is able to prevent sub mucosal tissue thickening and remodeling by reducing transforming growth factor beta (TGF-β) as a growth factor [68]. In this regard, a study has found that Tranilast can inhibit IL-5 secretion in mice infected with Toxocara canis (Tc). Additionally, this drug can dose-dependently decrease the production of IL-5 in lung and spleen’s cells obtained from Tc-infected mice cultured under stimulation with antigens. Daily administration of Tranilast (100 mg/kg) for one week had significantly mitigated the expression of IL-5 messenger ribonucleic acid (mRNA) expression in the lung. Furthermore, this treatment had decreased the IL-5 serum level [69]. Considering the destructive role of eosinophils in exacerbating airway inflammation in patients with asthma and the effectiveness of IL-5 on the production and recruitment of these cells [70], one clinical trial that investigated the efficacy of anti-IL-5 monoclonal antibody (Mepolizumab) for the treatment of patients with COVID-19 has been registered in the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (NCT04275245) [71]. Regular administration of beclomethasone can thus reduce rhinitis and nasal mucosal responses to allergens. One study with autopsies of COVID-19 patients have found that excessive wall thickness of the airways was one of the exacerbating factors, especially in ALI and ARDS [72]. Viral infection of the cornea can also lead to the inflammation and ulceration, which eventually cause blindness. In this regard, a study had evaluated the effects of Tranilast on the expression of a number of pro-inflammatory molecules in human corneal fibroblasts [73]. As a result, this drug had time-dependently reduced the expressions of IL-6, IL-8, MCP-1, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), MMP-1, and MMP-3 in poly (I: C)-stimulated corneal fibroblasts [73]. Since the increased levels of inflammatory cytokines and chemokines, adhesion molecules in epithelial and endothelial cells, and alveolar macrophages and other lung tissue-resident immune cells are known as the main exacerbating causes of destructive inflammation and pulmonary insufficiency, the findings of the present study may raise hopes for the effectiveness of Tranilast in the treatment of patients with COVID-19. Hepatic encephalopathy was also shown to affect a wide range of the CNS disorders occurring after acute liver damage, liver failure, or hepatic portal venous shunt. this disorder causes the liver inability to produce and excrete urea from ammonia, which have debilitating effects on the CNS [74]. During this disorder, inflammation plays a destructive role [75]. Subsequently, Thioacetamide injection can significantly increase IL-6 and IL-13 serum levels, and negatively change oxidative/antioxidant balance, and impair liver function, synthesis, metabolism, and hepatic excretion. In another study, Tranilast administration for a 15-day period had restored normal oxidant/antioxidant balance, reduced IL-6 and IL-13 serum levels, and significantly improved liver function. Accordingly, the anti-inflammatory, antioxidant, and immunomodulatory effects of Tranilast could justify its protective effects on neurohepatic damage [76]. These findings could raise hopes for the administration of Tranilast in the treatment of patients with COVID-19, according to the reports of neurohepatic damage of SARS-CoV-2 in patients with COVID-19. Some studies have further shown that Tranilast reduces the expression of chemokine (C-X-C motif) ligand 9 (CXCL9) and CXCL10, in a way that they appear to act as the targets for Tranilast [77]. In this regard, one study had found that Tranilast could reduce the expression of T cell receptor alpha constant (TRAC), eotaxin-1 chemokines, and VCAM-1, expressed by the TNF induction on the surface of corneal fibroblasts. Correspondingly, these effects were similar to the ones for dexamethasone, which are currently being examined in clinical trials for their effectiveness in the treatment of COVID-19 [78]. In severe cases of COVID-19, the TGF-β appeared to lead to further exacerbation of the disease by increasing the differentiation of T-helper cell 17 (Th17) lineage along with IL-6 and other inflammatory cytokines by affecting the airway wall thickening [79]. Tranilast also neutralizes the effects of inflammatory cytokines, which can even prevent the exacerbation of acute inflammation caused by COVID-19 through the inhibition of chemokines and cell infiltration. Syntheses of chemokines and adhesion molecules by corneal fibroblasts result in the development of corneal lesions in severe light sensitivity. Oxidative stress, cytokines, and chemokines such as MCP-1, TGF-β, and IL-2, comparably play important roles in the pathogenesis of insulin resistance. Interestingly, it has been shown that this drug helps in reducing insulin resistance through its antioxidant properties and decreasing expressions of MCP-1, TGF-β, and IL-2. Therefore, Tranilast can be effective on reducing insulin resistance [80]. A group of researchers had further investigated the effect of Tranilast on the production of MCP-1 by mesangial cells, and as a result, suggested that this drug could inhibit MCP-1 production due to IL-1 stimulation. It should be noted that the expression of MCP-1 is significantly higher in patients with COVID-19. Therefore, the anti-chemokine role of Tranilast can help other promising results in the treatment of those patients under such conditions regarding the key role of the increased expression of chemokines like MCP-1 in the acute form of the disease [81]. Besides, one study had additionally used the model of deoxycorticosterone acetate (DOCA)-salt hypertension to investigate the therapeutic effects of Tranilast on the inhibition of myocardial fibrosis as well as infiltrating monocytes and macrophages into the heart under hypertensive conditions. By passing only two weeks from the administration of DOCA-salt, the expression levels of some cytokines and factors such as TGF-β1, plasminogen activator inhibitor-1 (PAI-1), MCP-1, and IL-6 had significantly elevated. Thereafter, the administration of Tranilast was able to reduce these factors, and suppress myocardial fibrosis and collagen accumulations caused by hypertension [82]. The results of this study illustrated how Tranilast could be effective on pathological conditions caused by inflammation and aberrant remodeling process, while reducing inflammation and inhibiting tissue’s disorders. Recent studies have further confirmed the relationship among growth levels in some types of proinflammatory cytokines (as TNF-α, IL-1α, and 1β), TH2-realted cytokines (as IL-4, 5, and 13), and inflammatory chemokines such as CXCL1/GRO-α, CCL11/Eotaxin, CXCL9/MIG, CXCL10/IP-10, CCL2, CXCL1, CXCL5, and CCL27/CTACK in different forms of COVID-19. Increase in cytokines including IL-2, 6, 9, 18 and IFN-γ as well as chemokines (viz. CCL2/MCP-1, CCL3/MIP-1α, CCL7/MCP-3, CXCL8/IL-8, CCL5/RANTES, CCL4/MIP-1β, and CXCL12/SDF-1α) was also shown to be associated with mortality in patients with COVID-19 [66,83–85]. Accordingly, it is not impossible to recognize a variety of cytokines and chemokines along with their downstream and upstream signaling pathways as potential therapeutic targets in the treatment of COVID-19. As mentioned earlier, Tranilast can help patients being recovered, so it can consequently prevent cytokine storms through exerting its effect on the expression of key transcription factors and signaling complexes such as NLRP3 inflammasome, various cytokines and chemokines (Figure 2), and other drugs and therapies.
Figure 2.
Inhibitory effects of tranilast on inflammation, to manage COVID‐19. Studies have shown that SARS-CoV-2 elevates inflammation and activates NLRP3 inflammasome, which subsequently lead to a cytokine storm and destructive inflammation as well as causing ALI/ARDS in patients with COVID‐19. Tranilast can potentially play an effective role on the regulation of immune system, NLRP3 inflammasome, and inflammation, and provide support for Patients with both severe and moderate COVID‐19.
Effect of tranilast on signaling pathways
In recent years, some attempts were made to understand signaling pathways involved in the immune system responses, which made it possible to answer many unanswered questions. Accordingly, accurate knowledge of signaling pathways has helped developing a variety of new drugs by increasing awareness on disease pathogens [86]. Tranilast can significantly affect those signaling pathways involved in the processes of inflammation and fibrosis (Figure 1). In this respect, a study had evaluated the therapeutic effects of Tranilast on graft-versus-host disease (GvHD). The drug had also helped the treatment of the disease by suppressing NF-κB and thioredoxin interacting protein (TXNIP), which is a multifunctional protein involved in tumor suppression, oxidative stress, inflammatory responses, inflammation inhibiting, and fibrosis formation [87]. Studies have correspondingly confirmed that TXNIP is the bridge between ROS and NLRP3 inflammasome activations. The mice with TXNIP gene encoding mutation had also depicted a significant disruption in NLRP3 inflammasome activation as well as IL-1β production [88]. In the case of acute kidney injury (AKI) caused by inflammation in rats, NLRP3 inflammasome activation had been mediated by TXNIP signaling pathway [89]. As confirmed in various studies, TXNIP enhances the effects of NF-κB in different ways [90]. Therefore, reducing NF-κB and TXNIP can be effective on the alleviation of acute inflammation in COVID-19, especially by inhibiting NLRP3. Another study performed in a cyclosporine-induced nephrotoxicity model of rats had further found that Tranilast can improve this disease and prevent its progression through the regulation of TGF/Smad signaling pathways [91]. Ischemia-reperfusion injury (IRI) also occurs when blood returns to the tissue after discontinuation, which is associated with a life-threatening inflammatory response. In a study conducted on rats with cerebral IRI, the therapeutic effects of Tranilast had been evaluated. As a result, this drug had effectively reduced the rate of neuronal apoptosis [92]. Moreover, it had moderated the secretion of pre-inflammatory cytokines as well as the expression of NF-κB, which is one of the main inflammatory transcription factors [92]. Interestingly, although it had lowered innate immune responses, increased the expression of peroxisome proliferator-activated receptor alpha (PPAR-α) and PPAR-γ as the two nuclear transcription regulators. Such nuclear receptors, since they create a wide range of effects to stabilize health status in an individual, are expressed in various tissues. PPAR-α activation has been also reported to cause neuroprotection in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (MS) [93,94]. In another study, Tranilast had inhibited the production of MMP-1, 2, 3 induced by IL-1 in human corneal fibroblasts by the inhibition of some signaling pathways such as mitogen-activated protein kinase (MAPK) and NF-κB, as the main pathways for inflammatory responses [95]. This drug had appeared to inhibit MCP-1 production by impairing NF-κB and c-Jun N-terminal kinase (JNK) signaling pathways [81]. Notably, in response to stimuli like interferon-gamma (IFN-γ), microglial cells increase their expression levels of inducible nitric oxide synthase (iNOS), so the consequently produce higher levels of NO [96]. A study had further examined the effect of Tranilast on the above-mentioned pathway in mouse N9 microglial cell line, suggesting that exposure of cells to nontoxic doses of Tranilast could effectively reduce the expression level of IFNγ-induced iNOS, in parallel with a reduction in NO production level by cells. Besides all these mentioned effects, Tranilast had also inhibited NF-κB by activating its inhibitory kinase (IkB) [97]. Normally, NF-κB is always under the control of its IkB inhibitor. Along with developing pattern recognition receptor (PRR) alarmin and signaling, the IkB has finally given up the NF-κB control. Tranilast also prevents it occurrence, and does not allow the release of NF-κB from IkB captivity. In one study, Tranilast had inhibited the expressions of NF-κB-dependent adhesion molecules in vascular endothelial cells. On the other hand, interestingly, this inhibitory effect had not been observed in the expression of major histocompatibility complex (MHC) I molecules [98]. Similar to the observations in human studies, a recent study on SARS-CoV-2-infected monkeys demonstrated a severe cell infiltration and lymphocyte accumulation in pulmonary capillaries [4]. Therefore, inhibiting the expression of adhesion molecules, which is necessary for leukocyte infiltration, is helpful in severe and destructive inflammation of COVID-19 patients. However, no inhibitory effect on MHC-I can be taken into account as a positive point. The MHC-I also plays a vital role in antigen presentation in viral infections and also in tumors [99]. Besides, viruses use the reduced MHC-I expression as a way to escape immune responses. Therefore, the results of this study had shown the anti-inflammatory effect of Tranilast along with no impact on viral antigen presentation. It was also shown that Tranilast inhibits extracellular signal-regulated kinase (ERK), JNK, and p38 signaling pathways [78]. In addition, this drug can suppress inflammation by weakening JNK-activator protein-1 (AP-1) signaling through the inhibition of phosphorylation [73].
Effect of tranilast on function of the immune system cells
Inflammation is a process during which the main actors are different Immune cells. Therefore, many drugs are designed and manufactured to regulate or alter the function of these cells [100]. There are some studies that examined the effect of Tranilast on the function of the cells involved in the inflammation [101]. Mast cells are also important effective cells on the innate immunity that initiate inflammation due to their placement on mucosal surfaces, and they are also known as cells that produce inflammatory cytokines [102]. Studies have further shown that TNF is stored in mast cell secretory granules as one of the main pro-inflammatory cytokines. Ligation of TNF receptor type 1 results in the recruitment of an adaptor protein called TRADD gene, which can in turn activate TNF receptor associated factor (TRAF) molecules (E3 ubiquitin ligases) and receptor-interacting serine/threonine-protein kinase 1 (RIPK1). Moreover, the downstream consequences include the activation of NF-κB and the JNK MAPK pathway and the induction of apoptotic death. Tranilast can further act as an effective anti-chemotactic factor, and also help in controlling inflammation and thus possibly improve the acute form of COVID-19 by inhibiting key inflammation-associated transcription factors like NF-κB. This drug can also inhibit ERK, JNK, and p38 signaling pathways [78]. In a study that investigated the role of Tranilast in improving kidney interstitial fibrosis in rats, this drug had prevented mast cell recruitment by disrupting the stem cell factor (SCF)/c-kit signaling pathway [103]. It should be noted that mast cells are equipped with various PRRs, in order to detect viral pathogen-associated molecular patterns (PAMPs) and they also play key roles in airway inflammation resulted from beta-CoVs [104]. Inflammatory bowel diseases (IBD) such as colitis can be further considered as one type of destructive damages caused by immune responses and acute inflammation. In this regard, a study had used Tranilast to treat colitis in rats. Thereafter, by oral administration of Tranilast, cell infiltration rate had been reduced in tissue lesions up to 92%, which had consequently decreased sub-mucosal thickness [105]. The results of this study become more important when reviewing previous studies performed on ALI and ARDS in COVID-19 patients, which reported severe cell infiltration and pathological thickening of the airways. It can be imagined that to what extent Tranilast can help treating severe COVID-19. This drug has been shown to be effective on animal models of MS and RA, as two autoimmune diseases. It has also been demonstrated that it has a slight inhibitory effect on CD8+ T cell (TCD8) lymphocytes compared with TCD4 ones [77]. An increase in the number of neutrophils and their presence in the lung tissue can thus lead to a more severe lung damage. Therefore, a study had examined the effects of Tranilast on the activation process of the stimulated neutrophils. The results had further shown the decreased levels of expression and production of matrix metalloproteinases such as MMP-7, MMP-8, MMP-9, and TIMP factors needed for tissue’s remodeling [106]. It is noteworthy that a prominent feature of COVID-19 is a drop in the numbers of TCD8 and TCD4 lymphocytes [107]. In many inflammatory diseases like COVID-19, neutrophils also play a destructive role in the immunopathogenesis of the disease. Moreover, high-density neutrophils have been found in the lungs of patients with COVID-19 [108]. The productions of IL-17 and TH17 correspondingly play key roles in enhancing the neutrophils’ function. Several studies have further shown the abnormal differentiation of TH17 lymphocytes in COVID-19. Due to this reason, IL-17 has been introduced as a potential therapeutic target in the treatment of COVID-19 [109]. In this respect, the production of TH2 cytokines such as IL-4, 5, and 13 in patients with severe forms of COVID-19 has also been redoubled [66], which exacerbated the disease. Consequently, Tranilast can be mediated through its effect on TCD4 lymphocyte differentiation as well as the reduced chemokines like CXCL8, which moderate the intensity of the inflammatory neutrophils and macrophages’ function. Alternatively, it helps in boosting the disease process by mitigating the expression and production of cytokines, including IL-4, 5, and 13 (Figure 1).
The effect of tranilast in pulmonary diseases
Interstitial pulmonary fibrosis resulted from lung damage, is a fatal phenomenon that occurs due to the uncontrolled remodeling. Accordingly, alveolar macrophages play a key role in the development of pulmonary fibrosis. The uncontrolled remodeling also is a serious complication of COVID-19, especially in its severe forms associated with lung involvement and damage. It was indicated that the tranilast can effectively control the pulmonary fibrosis by inhibiting lung alveolar macrophages in rats [76]. A study examined the effect of tranilast on the TGFβ2-stimulated A549 cells as well as murine pulmonary fibrosis model, and finally reported that the tranilast controls the pulmonary fibrosis by inhibiting the TGF-β signaling pathway. The elevated TGF-β levels have been reported in patients with COVID-19 and the TGF-β inhibition has also been suggested as a potential treatment for this disease [110]. The tranilast inhalation can effectively inhibit acute pulmonary inflammation in rats. This inhaled form can be considered as a candidate to control the pulmonary inflammation in COVID-19 patients due to its higher solubility and the need for lower drug concentrations [111,112]. The injection of oleic acid (OA) into pigs has developed lung injury similar to ARDS/ALI along with the increased vascular permeability and hypoxia. The oral tranilast administration could also alleviate the severity of lung damage in the OA-induced acute lung injury. Thus, tranilast might prove to be an effective candidate for the treatment of ARDS/ALI, which is a fatal complication of SARS-CoV-2 [113].
Combination therapy suppresses COVID-19 cytokine storm
Different signaling pathways, types of cytokines and chemokines, and types of transcription factors are involved in the immunopathogenesis of COVID-19. Therefore, the combination therapy is proposed to be appropriate for this disease. The majority of the registered clinical trials have also considered drug regimens consisting of a variety of drugs. Corticosteroids and other immunosuppressants are currently being used to control cytokine storm, each counteracting with some of the causative agents of cytokine storm [114]. The corticosteroids effectively operate by inhibiting the release of proinflammatory cytokines and the outflow of inflammatory leukocytes from the arteries [115]. On the other hand, the tranilast can be prescribed along with corticosteroids. The tranilast inhibits the NLRP3-inflammasome activation, whose hyperactivity is one of the main causes of the formation of cytokine storm. Thus, tranilast and corticosteroids and any other type of immunosuppressant in combination can inhibit the cytokine storm. Corticosteroids, tranilast, and other similar drugs are more cost-effective than monoclonal antibodies, recombinant drugs under investigation, and cell therapy, which are ultimately are important findings due to the pandemic availability of the drug to all countries, including those low-income and less developed countries.
Safety and side effects of tranilast in other clinical trials
In a study, two patients with muscular dystrophy and cardiomyopathy had been treated with Tranilast (300 mg/day), as a TRPV2 inhibitor, for a 3-month period, and the given drug had effectively inhibited TRPV2 in patients with heart failure. Some side effects such as deterioration of renal failure, the increased heart rate, and premature ventricular contractions had been also observed [116]. In another study, two patients with type-2 diabetes had been treated with Tranilast (300 mg/day). In these patients, pretreatment skin samples had shown extended reticular dermis and wastes containing thick collagen. Following the treatment’s period, the skin quality had begun to improve and ultrasound imaging had revealed a reduction in skin thickness. The positive effects of Tranilast in these patients could be attributed to the role of this drug in regulating collagen synthesis. However, no severe side effects had been observed in each one of these patients [117]. Correspondingly, some studies had reported hepatotoxicity by the drug in high doses as well as the exacerbation of renal failure. However, in the clinical trials cited in this study, patients were treated with 300–600 mg of the drug for the periods of several months or even one year (Table 2). Nevertheless, the goal of treating patients with COVID-19 by the use of Tranilast is a short-term treatment to prevent cytokine storm and lethal systemic inflammation, similar to other anti-inflammatory and immunosuppressive drugs. Therefore, treatment duration in these patients is much shorter than that of the above-mentioned studies. In fact, the main objective of the treatment with Tranilast is to prevent cytokine storms and exacerbate inflammation caused by NLRP3 inflammasome. It should be noted that hospitalized patients are routinely evaluated for their kidney and liver functions, since they are being treated with various drugs. Considering the immunopathogenesis of COVID-19 and the occurrence of severe cytokine storms within the first days of hospitalization, patients are treated with Tranilast only for several days to prevent inflammasome activity. Besides, clinical trials that evaluated the efficacy and safety of the treatment of patients with COVID-19 via Tranilast in Iran (IRCT20200419047128N1) and China (ChiCTR2000030002) are being performed, whose results can help in better understanding this issue.
Table 2.
Clinical trials registered in Clinical Trials.gov evaluating efficacy and safety of Tranilast in a variety of diseases.
| Condition or disease | Intervention/treatment | Phase | Participants | Clinical trial identifier |
|---|---|---|---|---|
| Mucinoses | 0.1g each time, three times a day,12 months | Early Phase 1 | 56 | NCT03490708 |
| Scleredema Adultorum | 0.1g each time, three times a day,6 months | Early Phase 1 | 56 | NCT03512873 |
| Sarcoidosis | 0.1g each time, three times a day | Early Phase 1 | 56 | NCT03528070 |
| Gout Hyperuricemia | Combination Tranilast and Febuxostat | Phase 2 | 24 | NCT00995618 |
| Active Rheumatoid Arthritis | Tranilast, 300 mg/day Tranilast, 150 mg/day |
Phase 2 | 250 | NCT00882024 |
| Gout Hyperuricemia | Tranilast and Allopurinol | Phase 2 | 24 | NCT01052987 |
| Gout Hyperuricemia | 1-Tranilast 300 mg QD Allopurinol 400 mg QD. 2-Tranilast, 300 mg QD Allopurinol 600 mg QD | Phase 2 | 112 | NCT01109121 |
| Allergic Conjunctivitis | Olopatadine 0.1% one drop in one eye Tranilast 0.5% one drop in one eye |
Phase 4 | 50 | NCT00818805 |
Conclusion
According to many findings associated with Tranilast, this drug that has been prescribed for many years can help in controlling inflammation, which even act as an effective antichemotactic factor, thereby resulting in an improvement in severe COVID-19 by preventing key inflammatory transcription factors like NF-κB and inhibiting NLRP-3 inflammasome. Some studies have further demonstrated the direct effect of this drug on the prevention of inappropriate tissue remodeling, which is one of the pathological events of this disease. The inhibitions of neutrophils, IL-5, and subsequently eosinophils can accordingly make Tranilast an effective drug in the regimen of patients with severe diseases. Numerous studies have comparably documented the effective role of Tranilast on the inhibition of the infiltration of inflammatory cells into the inflammation site. On the other hand, this drug along with other medications can be considered as a potential treatment for patients affected with a severe kind of COVID-19, because it inhibits factors involved in acute airway inflammation such as IL-33 and cytokine, which greatly multiply mucosal secretions like IL-13.
Acknowledgement
The authors wish to acknowledge the support prepared by Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Funding Statement
This work was supported by the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Author contributions
All authors passed four criteria for authorship contribution based on recommendations of the International Committee of Medical Journal Editors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Cui J, Li F, Shi Z-L.. Origin and evolution of pathogenic Coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gralinski LE, Menachery VD.. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skariyachan S, Challapilli SB, Packirisamy S, et al. Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for Middle East respiratory syndrome coronavirus infections. Front Microbiol. 2019;10:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis Of COVID-19, MERS And SARS In A Non-Human Primate Model. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new Coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Berg DF, Te Velde AA.. Severe COVID-19: NLRP3 inflammasome dysregulated. Front Immunol. 2020;11:1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman TL, Swartz TH.. Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol. 2020;11:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertocchi I, Foglietta F, Collotta D, et al. The hidden role of NLRP3 inflammasome in obesity-related COVID-19 exacerbations: lessons for drug repurposing. Br J Pharmacol. 2020;177(21):4921–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darakhshan S, Pour AB.. Tranilast: a review of its therapeutic applications. Pharmacol Res. 2015;91:15–28. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Liu S, Liu J, et al. COVID-19: immunopathogenesis and immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang R, Wang X, Ni L, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020; 250:117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Geng M, Peng Y, et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YC, Bai WZ, Hashikawa T.. The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. [DOI] [PubMed] [Google Scholar]
- 24.Ong J, Young BE, Ong S.. COVID-19 in gastroenterology: a clinical perspective. Gut. 2020;69(6):1144–1145. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Shi L, Wang F-S.. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grommes J, Soehnlein O.. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17(3–4):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prompetchara E, Ketloy C, Palaga T.. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Z, Chen Z, Hu L, et al. Calreticulin blockade attenuates murine acute lung injury by inducing polarization of M2 subtype macrophages. Front Immunol. 2020;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Li R, Wang Z, et al. Dehydrocostus lactone inhibits NLRP3 inflammasome activation by blocking ASC oligomerization and prevents LPS-mediated inflammation in vivo. Cellular Immunology. 2020;349:104046. [DOI] [PubMed] [Google Scholar]
- 30.Hou X, Xu G, Wang Z, et al. Glaucocalyxin A alleviates LPS-mediated septic shock and inflammation via inhibiting NLRP3 inflammasome activation. Int Immunopharmacol. 2020;81:106271. [DOI] [PubMed] [Google Scholar]
- 31.Nosaka N, Martinon D, Moreira D, et al. Autophagy protects against developing increased lung permeability and hypoxemia by down regulating inflammasome activity and IL-1β in LPS plus mechanical ventilation-induced acute lung injury. Front Immunol. 2020;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrilli V, Papin S, Dostert C, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differentiation. 2007;14(9):1583–1589. [DOI] [PubMed] [Google Scholar]
- 33.Ichinohe T, Pang IK, Iwasaki A.. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11(5):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez ME, Carrasco L.. Viroporins. FEBS Lett. 2003;552(1):28–34. [DOI] [PubMed] [Google Scholar]
- 35.Ito M, Yanagi Y, Ichinohe T.. Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome. PLoS Pathog. 2012;8(8):e1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Triantafilou K, Kar S, Vakakis E, et al. Human respiratory syncytial virus viroporin SH: a viral recognition pathway used by the host to signal inflammasome activation. Thorax. 2013;68(1):66–75. [DOI] [PubMed] [Google Scholar]
- 37.Triantafilou K, Kar S, van Kuppeveld FJ, et al. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am J Respir Cell Mol Biol. 2013;49(6):923–934. [DOI] [PubMed] [Google Scholar]
- 38.Chen I-Y, Moriyama M, Chang M-F, et al. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 2019;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi C-S, Nabar NR, Huang N-N, et al. SARS-Coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahim I, Djerdjouri B, Sayed RK, et al. Melatonin administration to wild‐type mice and nontreated NLRP 3 mutant mice share similar inhibition of the inflammatory response during sepsis. J Pineal Res. 2017;63(1):e12410. [DOI] [PubMed] [Google Scholar]
- 41.Zahid A, Li B, Kombe JK, et al. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol. 2019;10:2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanson KV, Deng M, Ting JP-Y.. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinarello CA, Simon A, Van Der Meer JW.. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discovery. 2012;11(8):633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozaki E, Campbell M, Doyle SL.. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflam Res. 2015;8:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brydges SD, Mueller JL, McGeough MD, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30(6):875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laliberte RE, Perregaux DG, Hoth LR, et al. Glutathione S-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1β posttranslational processing. J Biol Chem. 2003;278(19):16567–16578. [DOI] [PubMed] [Google Scholar]
- 47.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dempsey C, Araiz AR, Bryson K, et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain, Behav Immun. 2017;61:306–316. [DOI] [PubMed] [Google Scholar]
- 49.Ismael S, Nasoohi S, Ishrat T.. MCC950, the selective NLRP3 inflammasome inhibitor protects mice against traumatic brain injury. J Neurotrauma. 2018;35:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Heijden T, Kritikou E, Venema W, et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E–deficient mice—brief report. Arterioscler Thromb Vasc Biol. 2017;37(8):1457–1461. [DOI] [PubMed] [Google Scholar]
- 51.Monnerat G, Alarcón ML, Vasconcellos LR, et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun. 2016;7(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Hout GP, Bosch L, Ellenbroek GH, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Euro Heart J. 2017;38(11):828–836. [DOI] [PubMed] [Google Scholar]
- 53.Zhai Y, Meng X, Ye T, et al. Inhibiting the NLRP3 inflammasome activation with MCC950 ameliorates diabetic encephalopathy in db/db mice. Molecules. 2018;23(3):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mridha AR, Wree A, Robertson AA, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66(5):1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perera AP, Fernando R, Shinde T, et al. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangan MS, Olhava EJ, Roush WR, et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(8):588–606. [DOI] [PubMed] [Google Scholar]
- 57.Huang Y, Jiang H, Chen Y, et al. Tranilast directly targets NLRP3 to treat inflammasome‐driven diseases. EMBO Mol Med. 2018;10(4):e8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bertocchi I, Foglietta F, Collotta D, et al. The hidden role of NLRP3 inflammasome in obesity‐related COVID‐19 exacerbations: lessons for drug repurposing. Br J Pharmacol. 2020;177(21):4921–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kroemer A, Khan K, Plassmeyer M, et al. Inflammasome activation and pyroptosis in lymphopenic liver patients with COVID-19. J Hepatol. 2020;73(5):1258–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamis SJ, Macfarlane Fr A. single-cell mathematical model of SARS-CoV-2 induced pyroptosis and the anti-inflammatory response to the drug tranilast. arXiv preprint arXiv:200804172. 2020.
- 61.Kaplansky G, Bongrand P.. Cytokines and chemokines. Cell Mol Biol. 2001;47(4):569–574. [PubMed] [Google Scholar]
- 62.Amedei A, Prisco D.. The use of cytokines and chemokines in the cancer immunotherapy. Recent Pat Anticancer Drug Discov. 2013;8(2):126–142. [PubMed] [Google Scholar]
- 63.Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA)-Mol Cell Res. 2014;1843(11):2563–2582. [DOI] [PubMed] [Google Scholar]
- 64.Hiraide S, Yanagawa Y, Iizuka K.. Tranilast inhibits interleukin-33 production by macrophages. Eur J Pharmacol. 2018;818:235–240. [DOI] [PubMed] [Google Scholar]
- 65.Tsuji K, Fukuda K, Fukushima A.. Inhibition by Tranilast of the synergistic induction of degranulation and IL-13 expression by IL-33 and FcvarepsilonRI cross-linking in mast cells. Ocul Immunol Inflamm. 2017;25(6):841–843. [DOI] [PubMed] [Google Scholar]
- 66.Xu Z-S, Shu T, Kang L, et al. Temporal profiling of plasma cytokines, chemokines and growth factors from mild, severe and fatal COVID-19 patients. Signal Transduct Targ Ther. 2020;5(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawamoto H, Iwatsuki K, Kurimoto S, et al. Interleukin-6 secretion by fibroblasts in carpal tunnel syndrome patients is associated with trigger finger and inhibited by tranilast. Muscle Nerve. 2020;61(3):408–415. [DOI] [PubMed] [Google Scholar]
- 68.Nader MA, Gameil N, Abdelaziz RR, et al. Effect of tranilast in comparison with beclomethasone in chronic murine model of asthma. Exp Lung Res. 2016;42(6):296–306. Aug [DOI] [PubMed] [Google Scholar]
- 69.Hiratochi M, Takamoto M, Tatemichi S, et al. Inhibition of interleukin 5 production with no influence on interleukin 4 production by an anti-allergic drug, tranilast, in Toxocara canis-infected mice. Int J Immunopharmacol. 2000;22(6):463–471. [DOI] [PubMed] [Google Scholar]
- 70.Tashkin DP, Wechsler ME.. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bian H, Zheng Z-H, Wei D, et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. MedRxiv. 2020.
- 72.Menter T, Haslbauer JD, Nienhold R, et al. Post‐mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, Kan M, Li A, et al. Inhibitory effects of Tranilast on cytokine, chemokine, adhesion molecule, and matrix metalloproteinase expression in human corneal fibroblasts exposed to Poly(I:C). Curr Eye Res. 2016;41(11):1400–1407. [DOI] [PubMed] [Google Scholar]
- 74.Görg B, Karababa A, Häussinger D.. Hepatic encephalopathy and astrocyte senescence. J Clin Exp Hepatol. 2018;8(3):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Azhari H, Swain MG.. Role of peripheral inflammation in hepatic encephalopathy. J Clin Exp Hepatol. 2018;8(3):281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdelaziz RR, Elkashef WF, Said E.. Tranilast reduces serum IL-6 and IL-13 and protects against thioacetamide-induced acute liver injury and hepatic encephalopathy. Environ Toxicol Pharmacol. 2015;40(1):259–267. [DOI] [PubMed] [Google Scholar]
- 77.Hertenstein A, Schumacher T, Litzenburger U, et al. Suppression of human CD4+ T cell activation by 3,4-dimethoxycinnamonyl-anthranilic acid (tranilast) is mediated by CXCL9 and CXCL10. Biochem Pharmacol. 2011;82(6):632–641. [DOI] [PubMed] [Google Scholar]
- 78.Adachi T, Fukuda K, Kondo Y, et al. Inhibition by tranilast of the cytokine-induced expression of chemokines and the adhesion molecule VCAM-1 in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2010;51(8):3954–3960. [DOI] [PubMed] [Google Scholar]
- 79.Chen W. A potential treatment of COVID-19 with TGF-β blockade. Int J Biol Sci. 2020;16(11):1954–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Namazi MR, Soma J.. Tranilast: a novel weapon against insulin resistance. Med Hypotheses. 2005;64(6):1135–1137. [DOI] [PubMed] [Google Scholar]
- 81.Chikaraishi A, Hirahashi J, Takase O, et al. Tranilast inhibits interleukin-1β-induced monocyte chemoattractant protein-1 expression in rat mesangial cells. Eur J Pharmacol. 2001;427(2):151–158. [DOI] [PubMed] [Google Scholar]
- 82.Kagitani S, Ueno H, Hirade S, et al. Tranilast attenuates myocardial fibrosis in association with suppression of monocyte/macrophage infiltration in DOCA/salt hypertensive rats. J Hypertens. 2004;22(5):1007–1015. [DOI] [PubMed] [Google Scholar]
- 83.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu H, Chan JF-W, Wang Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020;71(6):1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Catanzaro M, Fagiani F, Racchi M, et al. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Sig Transduct Target Ther. 2020;5(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mukai S, Ogawa Y, Saya H, et al. Therapeutic potential of tranilast for the treatment of chronic graft-versus-host disease in mice. PLoS One. 2018;13(10):e0203742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou R, Tardivel A, Thorens B, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136. [DOI] [PubMed] [Google Scholar]
- 89.Deng H, Chen F, Wang Y, et al. The role of activated NLRP3 inflammatory body in acute kidney injury in rats caused by sepsis and NLRP3-TXNIP signaling pathway. Saudi J Biol Sci. 2020;27(5):1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muri J, Thut H, Feng Q, et al. Thioredoxin-1 distinctly promotes NF-κB target DNA binding and NLRP3 inflammasome activation independently of Txnip. eLife. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tao Y, Hu L, Li S, et al. Tranilast prevents the progression of chronic cyclosporine nephrotoxicity through regulation of transforming growth factor β/Smad pathways. Transplant Proc. 2011;43(5):1985–1988. [DOI] [PubMed] [Google Scholar]
- 92.Zhuo Y, Zhuo J.. Tranilast treatment attenuates cerebral ischemia-reperfusion injury in rats through the inhibition of inflammatory responses mediated by NF-κB and PPARs. Clin Transl Sci. 2019;12(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bluher M, Engeli S, Kloting N, et al. Hormones, autacoids, neurotransmitters and growth factors. J Pharmacol Exp Ther. 2006;318:563–570.16702440 [Google Scholar]
- 94.Wójtowicz S, Strosznajder AK, Jeżyna M, et al. The Novel role of PPAR Alpha in the Brain: promising target in therapy of Alzheimer’s disease and other neurodegenerative disorders. Neurochem Res. 2020;45(5):972–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y, Xu D, Li J, et al. Inhibition of interleukin-1β-induced matrix metalloproteinase expression in human corneal fibroblasts by tranilast. Curr Eye Res. 2014;39(9):885–893. [DOI] [PubMed] [Google Scholar]
- 96.Saha RN, Pahan K.. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal. 2006;8(5–6):929–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Platten M, Wick W, Wischhusen J, et al. N-[3, 4-dimethoxycinnamoyl]‐anthranilic acid (tranilast) suppresses microglial inducible nitric oxide synthase (iNOS) expression and activity induced by interferon‐γ (IFN‐γ). Br J Pharmacol. 2001;134(6):1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spiecker M, Lorenz I, Marx N, et al. Tranilast inhibits cytokine-induced nuclear factor κB activation in vascular endothelial cells. Mol Pharmacol. 2002;62(4):856–863. [DOI] [PubMed] [Google Scholar]
- 99.D’Alicandro V, Romania P, Melaiu O, et al. Role of genetic variations on MHC class I antigen-processing genes in human cancer and viral-mediated diseases. Mol Immunol. 2019;113:11–15. [DOI] [PubMed] [Google Scholar]
- 100.Vigano S, Perreau M, Pantaleo G, et al. Positive and negative regulation of cellular immune responses in physiologic conditions and diseases. Clin Dev Immunol. 2012;2012:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wen Q, Zhou L, Chen H, et al. N-(3′, 4′ dimethoxycinnamonyl) anthranilic acid alleviated experimental colitis by inhibiting autoimmune response and inducing CD4+ CD25+ regulatory T cells production. J Gastroenterol Hepatol. 2013;28(8):1330–1338. Aug [DOI] [PubMed] [Google Scholar]
- 102.Krystel-Whittemore M, Dileepan KN, Wood JG.. Mast cell: a multi-functional master cell. Front Immunol. 2016;6:620–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yin DD, Luo JH, Zhao ZY, et al. Tranilast prevents renal interstitial fibrosis by blocking mast cell infiltration in a rat model of diabetic kidney disease. Mol Med Rep. 2018;17(5):7356–7364. [DOI] [PubMed] [Google Scholar]
- 104.Agier J, Pastwińska J, Brzezińska-Błaszczyk E.. An overview of mast cell pattern recognition receptors. Inflamm Res. 2018;67(9):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seto Y, Kato K, Tsukada R, et al. Protective effects of tranilast on experimental colitis in rats. Biomed Pharmacother. 2017;90:842–849. [DOI] [PubMed] [Google Scholar]
- 106.Shimizu T, Kanai KI, Kyo Y, et al. Effect of tranilast on matrix metalloproteinase production from neutrophils in‐vitro. J Pharm Pharmacol. 2006;58(1):91–99. [DOI] [PubMed] [Google Scholar]
- 107.Wang F, Hou H, Luo Y, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5(10):e137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps (NETs) as markers of disease severity in COVID-19. medRxiv. 2020.
- 109.De Biasi S, Meschiari M, Gibellini L, et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kato M, Takahashi F, Sato T, et al. Tranilast inhibits pulmonary fibrosis by suppressing TGFβ/SMAD2 pathway. Drug Des Devel Ther. 2020;14:4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Onoue S, Aoki Y, Kawabata Y, et al. Development of inhalable nanocrystalline solid dispersion of tranilast for airway inflammatory diseases. J Pharm Sci. 2011;100(2):622–633. [DOI] [PubMed] [Google Scholar]
- 112.Kawabata Y, Yamamoto K, Debari K, et al. Novel crystalline solid dispersion of tranilast with high photostability and improved oral bioavailability. Eur J Pharm Sci. 2010;39(4):256–262. [DOI] [PubMed] [Google Scholar]
- 113.Ishitsuka Y, Moriuchi H, Yang C, et al. Preventive effect of tranilast on oleic acid-induced lung injury in guinea pigs. Biol Pharm Bull. 2004;27(9):1451–1454. [DOI] [PubMed] [Google Scholar]
- 114.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miao Y, Fan L, Li J-Y.. Potential treatments for COVID-19 related cytokine storm – beyond corticosteroids. Front Immunol. 2020;11:1445–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matsumura T, Matsui M, Iwata Y, et al. A pilot study of tranilast for cardiomyopathy of muscular dystrophy. Intern Med. 2018;57(3):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun M, Yang F, Hou M.. Successful treatment of scleredema diabeticorum with tranilast: three case reports. Diabetes Care. 2018;41(4):e40–e41. [DOI] [PubMed] [Google Scholar]