Abstract
Simple Summary
Carbapenems and colistin are reserved as the last-resort treatments of multidrug-resistant (MDR) infections in humans. Consequently, the emergence of carbapenem and colistin-resistant Klebsiella pneumoniae (K. pneumoniae) in poultry, contact workers and hospitalized patients is of grave concern for therapeutic options, and no data are available supporting this assumption on a regional or countrywide scale. We investigated the frequency and typing of extended-spectrum β-lactamase (ESBL) and carbapenemase-producing K. pneumoniae (ESBLK and CPK) in hospitalized patients, chickens from 10 poultry farms and their environment (water, food and litter) and farm workers in Egypt. All isolates from patients (13/90, 14.4%), workers (5/22, 22.7%), chickens (9/100, 9%) and the environment (10/60, 16.7%) harbored a single or multiple β-lactamase genes, blaSHV, blaTEM, blaCTX-M1 and blaOXA-1, often in combination with carbapenemase genes (blaVIM, blaNDM-1 or blaIMP; 45.9%), the mcr-1 gene (18.9%) or both (13.5%). Enterobacterial repetitive intergenic consensus (ERIC)-PCR genotyping highlighted potential inter and intraspecies clonal dissemination in the study area. The increased frequency and genetic relatedness of ESBLK and CPK from chickens and humans pose a public health threat that urges more prudent use of antimicrobials in chicken farms to avoid the propagation and expansion of both ESBLK and CPK from the chicken sources to humans.
Abstract
This study investigated the frequency of carbapenem and colistin resistance in ESBL-producing K. pneumoniae (ESBLK) isolates recovered from chickens and their environment, contact farm workers and hospitalized patients in Egypt. Further, the phenotypic and genotypic relationships between the community and hospital-acquired K. pneumoniae isolates in the same geographical area were investigated. From 272 total samples, 37 (13.6%) K. pneumoniae isolates were identified, of which 20 (54.1%) were hypervirulent. All isolates (100%) were multidrug-resistant (MDR) with multiple antibiotic resistance (MAR) indices ranging from 0.19 to 0.94. Colistin-resistant isolates (18.9%) displayed colistin MIC values >2 μg/mL, all harbored the mcr-1 gene. All isolates from patients (13/90, 14.4%), workers (5/22, 22.7%), chickens (9/100, 9%) and the environment (10/60, 16.7%) harbored a single or multiple β-lactamase genes, blaSHV, blaTEM, blaCTX-M1 and blaOXA-1, often in combination with carbapenemase genes (blaVIM, blaNDM-1 or blaIMP; 45.9%), the mcr-1 gene (18.9%) or both (13.5%). Enterobacterial repetitive intergenic consensus (ERIC)–PCR genotyping revealed 24 distinct ERIC types (ETs) with a discrimination index of 0.961. Six ETs showed clusters of identical isolates from chicken and human sources. The increased frequency and genetic relatedness of ESBLK and carbapenemase-producing K. pneumoniae (CPK) from chickens and humans pose a public health threat that urge more prudent use of antimicrobials in chicken farms to avoid the propagation and expansion of both ESBLK and CPK from the chicken sources to humans.
Keywords: K. pneumoniae, colistin, ESBL, carbapenems, chickens, humans, environment
1. Introduction
Klebsiella pneumoniae (K. pneumoniae) is a pervasive nosocomial and community associated-pathogen causing a wide range of infections in humans and animals [1,2]. Bacterial invasion in livestock animals poses a potential hazard to public health, as infected animals act as a reservoir of multidrug-resistant (MDR) isolates [1,3]. In Egypt, no regulations are controlling the use of antimicrobials in animals [4], which may be used as growth promoters or for the prevention and treatment of zoonotic diseases. This supports the evidence linking consumption of antimicrobials in livestock animals to their resistance in humans [5].
The emergence of extended-spectrum β-lactamase-producing K. pneumoniae (ESBLK) in Egypt is of serious concern. Previous studies suggested that food-producing animals, particularly chickens, have been considered a possible source for transmission of ESBLK to humans [6,7]. However, integrated studies on chickens and their environment, contact workers and hospitalized patients in the same area are still missing.
Carbapenems and polymyxin E (colistin) are considered the last-resort for treating infections caused by MDR K. pneumoniae isolates, particularly those producing extended-spectrum β-lactamase (ESBL) enzymes [8,9]. Carbapenem-resistant K. pneumoniae exhibited coresistance to a range of critically relevant antimicrobial classes resulting in few therapeutic possibilities [10]. The emergence of carbapenemases together with the mcr-1 colistin resistance gene constitutes a global risk to public health [11,12]. There are three mechanisms by which K. pneumoniae employs carbapenem resistance: (i) enzymatic hydrolysis via carbapenemases enzymes, (ii) overexpression of the efflux pump system and (iii) loss of porin expression [13]. Carbapenemases (i.e., Ambler molecular classes A, B and D β-lactamases) represent the most prevalent mechanism of carbapenem resistance. They hydrolyze a wide variety of β-lactams including penicillins, cephalosporins, monobactams, carbapenems and β-lactamases inhibitors through carbapenemase encoding genes, mainly of class B metallo-β-lactamases (MBL), including imipenem metallo-β-lactamases (blaIMP), New Delhi metallo-β-lactamases (blaNDM) and Verona integron-encoded metallo-β-lactamases (blaVIM) [14]. Carbapenemases are mostly detected in K. pneumoniae, with a lower extent in other enterobacterial species [15]. Although carbapenem and colistin-resistant K. pneumoniae isolates have been documented in numerous human studies in Egypt [8,16,17,18], no data are available on this issue from a veterinary overview. Moreover, the role of poultry and their environment in the maintenance and transmission of carbapenem-and colistin coresistant isolates is poorly explored.
Enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR) has been documented to have a virtuous differentiation power for molecular characterization and genotyping of bacterial isolates from human and animal origins [19,20]. Therefore, this study aimed to determine the frequency of carbapenem and colistin resistance in ESBLK isolates recovered from chickens and their environment, contact farm workers and hospitalized patients in Kafrelsheikh Governorate, Egypt, and to investigate the phenotypic and genotypic relationships between the community and hospital-acquired K. pneumoniae isolates in the same geographical area.
2. Materials and Methods
2.1. Study Design and Sampling
For determination of the potential risk for chicken-human cross-transmission, we detected the occurrence of carbapenemase and colistin resistance in ESBLK in hospitalized patients, workers who were in close contact with broiler chickens, broilers and their environment. The protocol of this study was approved by the ethics committee of Kafrelsheikh University, Kafrelsheikh, Egypt (KFS-2019/3). Written informed consent was attained from the patients, poultry workers and owners of farms for the participation in this study and the publication of any potentially identifiable data.
The sampling was carried out during the period from April 2019 to November 2019. Sputum and urine samples were collected from 90 hospitalized patients showing manifestations of respiratory and urinary tract infections. Moreover, 22 stool samples were collected from contact workers from 10 different broiler farms in Kafrelsheikh Governorate, Egypt. Additionally, 100 lung and trachea samples were collected from broilers showing respiratory manifestations. Furthermore, 60 environmental samples were included: two pooled samples for water, food and litter (n = 2 each) per farm were separately screened for carbapenem and colistin resistance in ESBLK.
2.2. Isolation and Identification of K. pneumoniae
Samples were directly streaked onto HiCrome™ Klebsiella selective agar (Himedia, India) at 37 °C for 24 h. The presumptive purple colonies were confirmed by growth on MacConkey’s agar and eosin methylene blue (Oxoid, Cambridge, UK) agar media and identified by biochemical tests [21]. The hypermucoviscous phenotype of colonies on an agar plate has been defined by a positive string test [22]. The 16S-23S rDNA internal transcribed spacer (ITS) of K. pneumoniae isolates were amplified with the species-specific primers reported previously [23]. K. Pneumoniae isolates grown on Columbia sheep blood agar (Oxoid, Cambridge, UK) plates for 24 h were subjected to matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) (BioMeri’eux, Marcy I’Etoile, France) identification using αcyano-4-hydroxycinnamic acid matrix solution as previously described [24]. MALDI-TOF MS running MYLA 3.1.0-15 software (BioM’erieux, Inc., Marcy I’Etoile, France) analyzed, compared the generated spectrum for each tested isolate with a library of standard reference spectra and calculated the confidence values.
2.3. Antimicrobial Susceptibility Testing
The disc diffusion test [25] was used for testing the susceptibility of K. pneumoniae isolates to a range of antimicrobials approved for both human and livestock use [26], including ampicillin (10 µg), amoxicillin-clavulanic acid (30 µg), ceftazidime (30 µg), cefepime (30 µg), amikacin (30 µg), nalidixic acid (30 µg), ciprofloxacin (5 µg), imipenem (10 µg), azithromycin (15 µg), aztreonam (30 µg), gentamicin (10 µg), tetracycline (30 µg), chloramphenicol (30 µg), nitrofurantoin (300 µg), trimethoprim-sulfamethoxazole (25 µg) and colistin (25 µg).
The broth microdilution method [27] was used for the determination of the minimum inhibitory concentrations (MICs) of colistin (Sigma-Aldrich, Seelze, Germany). A K. pneumoniae ATCC 700603 reference strain was included as quality control in the disc diffusion test and the fully colistin-susceptible E. coli ATCC 25922 and the mcr-1-positive E. coli NCTC 13846 with a colistin MIC of 4 mg/L were used in broth microdilution method.The results of antimicrobial susceptibility testing were interpreted according to the Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing (EUCAST) joint subcommittee [28]. The multiple antibiotic resistance (MAR) index for each isolate was calculated according to Tambekaret al. [29].
2.4. Phenotypic Screening for ESBLs and Carbapenemases Production
ESBL production was screened using the CLSI ESBL confirmatory test (double disc synergy test) with cefotaxime (CTX, 30 µg) and ceftazidime (CAZ, 30 µg) antimicrobial discs alone, and in combination with clavulanic acid (CA, 10 µg) (Becton, Dickinson, Sparks, MD, USA). A positive ESBL production was considered when an increase in the inhibition zone diameter for CTX or the CAZ disc + CA was ≥5 mm of the diameter around the CTX or CAZ [30]. The modified Hodge test (MHT) was performed to screen for carbapenemase production according to CLSI guidelines [31]. The EDTA-imipenem synergy test was used for MBL carbapenemase identification [32]. K. pneumoniae ATCCBAA-1705 (carbapenemase-positive) and ATCC700603 (SHV-18 producer) reference strains were used as positive controls. A β-lactamase-negative E. coli ATCC25922 strain was used as a negative control. The ESBL- and carbapenemases-producing isolates were subjected to further analysis including detection of the resistance determinants, mcr-1gene in colistin-resistant isolates and ERIC-genotyping.
2.5. Detection of ESBL, Carbapenemase-Encoding Genes, and Colistin Resistance Determinant mcr-1
Genomic DNA was extracted from overnight cultures of all presumptive ESBLK and carbapenemase-producing K. pneumoniae (CPK) isolates using QIAamp DNA Mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. The purity and concentrations of extracted DNA were assessed by using a nanodrop™ 2000 and 2000c (Thermo Fisher Scientific, Waltham, MA, USA). The ESBL genes (blaSHV, blaCTX-M, blaTEM and blaOXA-1) were detected using the previously described multiplex PCR [33]. PCR amplification of carbapenemase genes (blaIMP, blaVIM and blaNDM1) was performed using specific primers [34,35,36] listed in Table S1. The uniplex PCR was carried out in a 25-µL reaction mixture containing 12.5 µL of EmeraldAmp Max PCR Master Mix (Takara, Shigino-higashi, Joto-ku, Osaka, Japan), 1 µL of each primer (20 pmol; Metabion GmbH, Germany), 5.5 µL of nuclease-free water and 5 µL of DNA template using the Applied Biosystems® 2720 thermal cycler (Thermo Scientific, Waltham, MA, USA). The PCR products were electrophoresed on 1.5% agarose gel (Applichem GmbH, Darmstadt, Germany) containing 0.5 µg/mL ethidium bromide. The gel was photographed by Alpha Innotech gel documentation system (Biometra GmbH, Göttingen, Germany) and the data were analyzed through computer software.
Furthermore, colistin-resistant K. pneumoniae isolates were tested for the presence of the mcr-1 gene using the specific primers (Table S1) as described previously [37]. Genomic DNA from mcr-1-positive E. coli NCTC 13846 and colistin-susceptible E. coli ATCC 25922 were included in each run.
2.6. ERIC Genotyping
For analysis of fingerprinting profiles of different ESBL and carbapenemase-positive isolates, ERIC-PCR was applied using the ERIC-1R and ERIC-2 primers (Table S1), as previously described [38]. The ERIC-PCR band patterns were analyzed by GelJ software v.2.0 [39]. The comparison between fingerprinting profiles was conducted using the Dice coefficient, and a dendrogram was constructed using the unweighted pair group method with arithmetic mean. Simpson’s discrimination index for ERIC genotyping was estimated as previously described [40].
2.7. Data Analysis
Differences between frequencies of K. pneumonia in chicken and human samples were assessed by the chi-squared test. A univariate logistic regression model was used to estimate the odds ratios and significant associations between phenotypic and genetic antimicrobial resistance profiles and source (humans versus chickens) of the K. pneumoniae isolates. The analysis was done using SPSS v19 (IBM, Armonk, NY, USA), and associations at a p-value ≤ 0.05 were considered significant. To determine the distribution of the isolates from various hosts based on their antimicrobial resistance gene profiles, the occurrence of a particular gene in the isolates was entered as binary data (0 = absent, 1 = present), and this was used as inputs into non-metric multidimensional scaling (nMDS) analyses using the Sorensen distance. The nMDS biplot was produced to determine the association of the genes and isolates.
3. Results
3.1. Prevalence of K. pneumoniae in Chickens, Their Environment, Contact Workers and Hospitalized Patients in the Study Area
In total, 37 K. pneumoniae isolates were identified using the standard bacteriological tests, PCR amplification of 16S-23S rDNA ITS region (fragment size 130 bp) and MALDI-TOF MS. The latter provided correct species-level identification for K. pneumoniae isolates with a confidence value of 99.9%. As presented in Table 1, K. pneumoniae isolates were detected in 19 samples from diseased chickens (9/100; 9%) and their compartments (10/60; 16.7%), and 18 human samples including contact workers (5/22; 22.7%) and hospitalized patients (13/90; 14.4%). Six of ten poultry farms (60%) were positive for K. pneumoniae (Table S2). Of note, 20 isolates (54.1%) were hypervirulent, demonstrating a hypermucoviscous phenotype as confirmed by the string test (Table 2).
Table 1.
Frequency distribution of 37 multidrug-resistant K. pneumoniae isolates recovered from broiler farms and human cases in the study area.
| Isolates (%) * | No. of Isolates (%) | Chicken vs. Human | ||||||
|---|---|---|---|---|---|---|---|---|
| Chickens | Humans | |||||||
| C (n = 100) |
Ce (n = 60) |
TC (n = 160) |
Cw (n = 22) |
Hc (n = 90) |
TH (n = 112) |
X2 | p-Value | |
| MDR-K. pneumoniae (100) | 9 (9) | 10 (16.7) | 19 (11.9) | 5 (22.7) | 13 (14.4) | 18 (16.1) | 0.9 | 0.3 |
| ESBL-K. pneumoniae (100) | 9 (9) | 10 (16.7) | 19 (11.9) | 5 (22.7) | 13 (14.4) | 18 (16.1) | 0.9 | 0.3 |
| CR-K. pneumoniae (45.9) | 1 (1) | 2 (3.3) | 3 (1.9) | 4 (18.2) | 10 (11.1) | 14 (12.5) | 12.7 | >0.001 |
| CTR-K. pneumoniae (18.9) | 1 (1) | 1 (1.7) | 2 (1.25) | 1 (4.5) | 4 (4.4) | 5 (4.5) | 2.7 | 0.1 |
| ESBL and CR-K. pneumoniae (45.9) | 1 (1) | 2 (3.3) | 3 (1.9) | 4 (18.2) | 10 (11.1) | 14 (12.5) | 12.7 | >0.001 |
| ESBL and CTR-K. pneumoniae (18.9) | 1(1) | 1 (1.7) | 2 (1.25) | 1 (4.5) | 4 (4.4) | 5 (4.5) | 2.7 | 0.1 |
| CR and CTR-K. pneumoniae (13.5) | 0 | 0 | 0 | 1 (4.5) | 4 (4.4) | 5 (4.5) | - | - |
| ESBL, CR and CTR-K. pneumoniae (13.5) | 0 | 0 | 0 | 1 (4.5) | 4 (4.4) | 5 (4.5) | - | - |
* Percentage was calculated from total isolates; C: diseased chicken; Ce: chicken environment samples; TC: total chicken samples; Cw: chicken worker; Hc: human case; TH: total human samples; MDR: multidrug-resistant; ESBL: extended-spectrum β-lactamases; CR: carbapenemases-producing; CTR: colistin resistant; X2: chi-square.
Table 2.
Antimicrobial resistance phenotypes, their associated genes and ERIC fingerprinting patterns of hypervirulent ESBL and carbapenemase-producing K. pneumoniae isolates from chickens and humans in this study.
| Pattern No. | Source | Code No. |
Antimicrobial Resistance Pattern |
MAR Index | Colistin MIC (mg/L) | Genotype | ERIC Fingerprint |
|---|---|---|---|---|---|---|---|
| (Band Size) | |||||||
| 1 | Hc | 34 | Amp, AMC, CAZ, FEP, IPM, NA, CIP, AK, TE, C, ATM, AZM, SXT, F, CT | 0.94 | 16 | blaSHV, blaTEM, blaVIM, blaNDM-1, blaIMP, mcr-1 | E12 (109, 312, 423, 878, 1000, 1225) |
| 2 | Cw | 22 | Amp, AMC, CAZ, FEP, IPM, NA, CIP, AK, TE, C, AZM, SXT, F, CT | 0.88 | 8 | blaSHV, blaVIM, mcr-1 | E7 (872) |
| 3 | Cw | 23 | Amp, AMC, CAZ, FEP, NA, IPM, CIP, TE, C, AZM, SXT, F | 0.75 | 1 | blaSHV, blaNDM-1 | E7 (872) |
| 4 | C | 6 | Amp, AMC, CAZ, FEP, NA, TE, SXT, F | 0.5 | 0.25 | bla SHV | E7 (878) |
| 5 | Hc | 28 | Amp, AMC, CAZ, FEP, CN, IPM, NA, CIP, AK, TE, ATM, AZM, SXT, F, CT | 0.94 | 32 | blaSHV, blaCTX-M1, blaVIM, mcr-1 | E6 (298, 428, 860, 1148) |
| 6 | Hc | 29 | Amp, AMC, CAZ, FEP, CN, NA, CIP, AK, TE, C, ATM, AZM, SXT, F | 0.88 | 0.5 | bla TEM | E22 (157, 305, 401) |
| 7 | C | 4 | Amp, AMC, CAZ, FEP, CN, IPM, NA, AK, TE, C, ATM, SXT, F | 0.81 | 0.25 | blaTEM, blaNDM-1 | E24 (159, 412) |
| 8 | Hc | 25 | Amp, AMC, CAZ, FEP, CN, IPM, NA, CIP, AK, ATM, AZM, SXT, F | 0.81 | 1 | blaTEM, blaNDM-1, blaIMP | E24 (150, 380) |
| 9 | Ce | 12 | Amp, AMC, CAZ, FEP, IPM, NA, CIP, AK, TE, C, AZM, SXT, F | 0.81 | 0.5 | blaSHV, blaCTX-M1, blaNDM-1, blaIMP | E9 (127, 199, 294, 352, 436, 489, 748) |
| 10 | Ce | 15 | Amp, AMC, CAZ, FEP, IPM, NA, CIP, AK, TE, C, ATM, SXT, F | 0.81 | 0.25 | blaSHV, blaVIM | E20 (116, 168, 312) |
| 11 | Hc | 30 | Amp, AMC, CAZ, FEP, CN, IPM, NA, CIP, AK, TE, C, AZM, SXT | 0.81 | 2 | blaSHV, blaVIM, blaNDM-1 | E3 (144, 169, 320, 340, 432, 1205, 1346) E14 (107) |
| Cw | 20 | 1 | |||||
| 12 | Hc | 32 | Amp, AMC, CAZ, FEP, CN, IPM, NA, CIP, AK, ATM, AZM, SXT, F | 0.81 | 2 | blaSHV, blaCTX-M1, blaVIM, blaNDM-1 | E14 (117) |
| 13 | Hc | 26 | Amp, AMC, CAZ, FEP, CN, IPM, NA, CIP, AK, ATM, AZM, SXT, F, CT | 0.88 | 16 | blaSHV, blaOXA-1, blaVIM, mcr-1 | E19 (159, 244, 307, 429, 680) |
| 14 | Hc | 31 | Amp, AMC, CAZ, FEP, CN, NA, CIP, AK, ATM, TE, AZM, SXT, F | 0.81 | 1 | bla SHV | E5 (1187, 1307) |
| 15 | Ce | 19 | Amp, AMC, CAZ, FEP, NA, TE, CIP, C, AZM, SXT, F | 0.69 | 2 | bla SHV | E5 (1205, 1353) |
| 16 | C | 7 | Amp, AMC, CAZ, FEP, CN, NA, CIP, TE, C, AZM, SXT, F | 0.75 | 2 | bla SHV | E5 (1196, 1320) E5 (1214, 1353) E5 (1200,1333) E5 (1214, 1353) |
| C | 3 | 2 | |||||
| Ce | 14 | 1 | |||||
| Cw | 24 | 2 | |||||
| 17 | C | 5 | Amp, AMC, CAZ, FEP, NA, CIP, AK, TE, C, AZM, SXT, F | 0.75 | 0.5 | blaSHV, blaTEM | E23 (157) |
| 18 | Ce | 10 | Amp, AMC, NA, AK, TE, SXT, F | 0.44 | 0.5 | bla TEM | E23 (159) |
| Ce | 11 | 1 | |||||
| 19 | Hc | 37 | Amp, AMC, CAZ, FEP, CN, IPM, NA, CIP, ATM, TE, C, AZM, SXT | 0.81 | 1 | blaSHV, blaTEM, blaCTX-M1, blaVIM, blaNDM-1, blaIMP | E11 (188, 306) |
| 20 | Ce | 17 | Amp, AMC, CAZ, FEP, NA, CIP, AK, TE, C, SXT, F, CT | 0.75 | 4 | blaSHV, mcr-1 | E4 (1360) |
| 21 | Hc | 27 | Amp, AMC, CAZ, FEP, NA, AK, CN, CIP, ATM, AZM, SXT, F | 0.75 | 2 | bla TEM | E18 (160, 254, 301, 369, 424, 703, 884, 1197) |
| 22 | C | 2 | Amp, AMC, CAZ, FEP, TE, AK, C, ATM, AZM, SXT, F | 0.69 | 1 | bla SHV | E1 (679) |
| 23 | C | 9 | Amp, AMC, CAZ, FEP, NA, TE, C, CN, SXT, F, CT | 0.69 | 8 | blaTEM, blaCTX-M1, blaOXA-1, mcr-1 | E8 (891) |
| 24 | Hc | 35 | Amp, AMC, CAZ, IPM, FEP, NA, TE, CIP, C, AZM, SXT | 0.69 | 2 | blaCTX-M1, blaVIM, blaIMP | E10 (107, 188, 377, 492) |
| 25 | Ce | 18 | Amp, AMC, CAZ, FEP, NA, TE, AK, C, SXT, F | 0.63 | 1 | bla CTX-M1 | E17 (189, 266, 331, 373, 488, 565, 623, 964) |
| 26 | Hc | 33 | Amp, AMC, CAZ, FEP, NA, IPM, CIP, TE, C, ATM, AZM, SXT, F, CT | 0.88 | 16 |
blaTEM, blaOXA-1, blaVIM, blaNDM-1, mcr-1 |
E15 (167, 211, 316) |
| 27 | Cw | 21 | Amp, AMC, FEP, NA, IPM, CIP, TE, AZM, C, SXT | 0.63 | 0.25 | blaOXA-1, blaVIM, blaNDM-1 | E16 (396, 767, 1357) |
| 28 | Hc | 36 | Amp, AMC, CAZ, FEP, NA, IPM, CIP, TE, C, SXT | 0.63 | 2 | blaTEM, blaCTX-M1, blaVIM, blaNDM-1 | E13 (107, 878) |
| 29 | Ce | 13 | Amp, AMC, CAZ, NA, AK, C, SXT, F | 0.5 | 0.5 | bla TEM | E21(170, 320, 459) |
| 30 | Ce | 16 | Amp, AMC, CIP, NA, AK, ATM, SXT, F | 0.5 | 1 | blaTEM, blaOXA | E21(169, 320, 462) |
| 31 | C | 8 | AMP, NA, TE | 0.19 | 0.25 | bla OXA-1 | E21(170, 320, 466) |
| 32 | C | 1 | Amp, AMC, CAZ, FEB, TE, C, F | 0.44 | 1 | blaSHV, blaCTX-M1 | E2 (297, 409, 700, 1158) |
PT: Phenotypic resistance pattern; AMP: ampicillin; AMC: amoxicillin-clavulanic acid; CAZ: ceftazidime; FEB: cefepime; IPM: imipenem; NA: nalidixic acid; CIP: ciprofloxacin; AK: amikacin; CN: gentamicin; TE: tetracycline; C: chloramphenicol; ATM: aztreonam; AZM: azithromycin; SXT: trimethoprim-sulfamethoxazole; F: nitrofurantoin; CT: colistin; MAR: multiple antibiotic resistance index; Hc: human case; Cw: chicken worker; C: chicken; Ce: chicken environment. Patterns with bold numbers were found in more than one isolate. Isolates with bold code numbers are hypervirulent.
3.2. Antimicrobial Resistance of K. pneumoniae Isolates
All K. pneumoniae isolates were MDR, being resistant to 3–15 of the 16 tested antimicrobials with MAR indices ranged from 0.19 to 0. 94. The MDR isolates were assigned to a total of 32 distinct resistance patterns (Table 2). All hospitalized patients´ isolates showed high levels of MDR being resistant to 10–15 antimicrobials (MAR indices ranged from 0.63–0.94) and the farm workers’ isolates were resistant to 10–14 antimicrobials (MAR indices ranged from 0.63–0.88). The majority of chicken isolates (66.7%) were resistant to 11–13 antimicrobials with MAR indices 0.68–0.81. Moreover, 60% of the isolates from environment samples were resistant to 10–13 antimicrobials (MAR indices = 0.63–0.81). Overall, the isolates exhibited full resistance to ampicillin (100%) and 97.3% resistance to amoxicillin-clavulanic acid. Moreover, 94.6% of the isolates exhibited resistance to each of nalidixic acid and trimethoprim-sulfamethoxazole, whilst 86.5% were resistant to ceftazidime and cefepime. The resistance to tetracycline was found among 83.8% of the isolates, followed by nitrofurantoin (81.1%), ciprofloxacin (72.9%), chloramphenicol (70.3%), azithromycin (64.9%), amikacin (59.5%) and imipenem (45.9%). The lowest resistance rate was found to colistin (18.9%) followed by aztreonam (37.8%) and gentamicin (43.2%).
A univariate logistic regression model analysis (Table S3) showed that the acquisition of carbapenemase encoding genes (blaVIM and blaNDM) were more likely to be associated with human isolates than chicken ones (OR = 10.6–36, p = 0.002–0.01). A similar association was recorded for imipenem phenotypic resistance (77.8% vs. 15.8%, OR = 18.7, p = 0.001). Additionally, human isolates were more likely resistant to azithromycin, aztreonam and gentamicin antimicrobials than chicken isolates (OR = 4.4–29.1, p = 0.003–0.04) (Table S3). There was no significant association with regard to other antimicrobial resistance genes or tested antimicrobials among detected isolates.
3.3. Distributions of ESBLs, Carbapenemase Genes and Colistin Resistance Determinant mcr-1 among K. pneumoniae Isolates
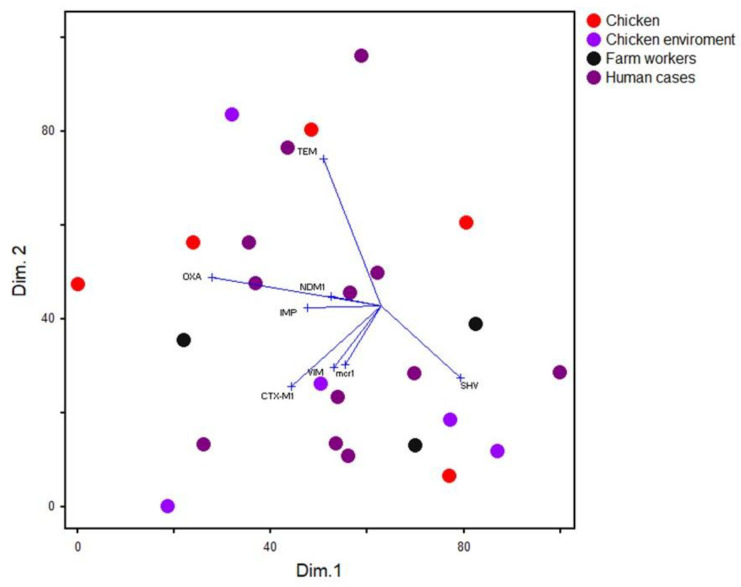
Molecular testing revealed that among the 37 phenotypically positive isolates for ESBLs, 17 blaSHV, 14 blaTEM, 9 blaCTX-M1 and 6 blaOXA-1 producers were identified (Table 2). Seventeen isolates with decreased susceptibility to imipenem, and showed positive MHT, produced MBL carbapenemases and possessed blaVIM (n = 13), blaNDM-1 (n = 12) and blaIMP (n = 5). As depicted in Table 1, Table 2 and Table 3 and Figure 1, all CPK isolates harbored carbapenemase genes in combination with other ESBL genes. As presented in Table 3, 25 profiles were found for the distributions of ESBL and carbapenemase genes among ESBLK and CPK isolates. Importantly, five human isolates (13.5%) harbored ESBLs, carbapenemases genes and colistin resistance determinant mcr-1. Moreover, two isolates with blaTEM, blaCTX, blaOXA-1 and blaSHV genotype profiles from chicken and their environmental samples also harbored the mcr-1 gene (Table 1, Table 2 and Table 3). Figure 1 presents the ESBL and carbapenemases genes found in humans, broilers and environment samples. In the broiler farms, the predominant ESBL and carbapenemases of ESBLK and CPK isolates from chickens and their environment were blaSHV (11/19; 57.9%) and blaNDM (2/19, 10.5%); blaSHV (4/5, 80%), blaVIM and blaNDM (3/5; 60% each) were predominant in poultry workers. blaSHV, blaTEM (7/13; 53.8% each) and blaVIM (9/13; 69.2%) were the most frequent genes in hospitalized patients in the study area. As presented in Figure 1, the nMDS analysis of K. pneumoniae isolates (n = 37) displayed a non-specific clustering pattern, as the chicken and human isolates largely overlapped. However, 12 isolates among all analyzed origins were omitted from the analysis as they were identical and demonstrated unique patterns.
Table 3.
Distribution of extended-spectrum β-lactamase, carbapenemase genes and colistin resistance determinant mcr-1 among 37 K. pneumoniae isolates from chickens and humans.
| No. | Isolates Group and Genotypes | Total | Chickens | Humans | ||||
|---|---|---|---|---|---|---|---|---|
| C | Ce | TC | Cw | Hc | TH | |||
| ESBL-producing isolates | ||||||||
| 1 | bla SHV | 8 (21.6) | 4 | 2 | 6 (75) | 1 | 1 | 2 (25) |
| 2 | blaSHV, blaCTX-M1 | 1 (2.7) | 1 | 1 (100) | ||||
| 3 | blaSHV, blaTEM | 1 (2.7) | 1 | 1 (100) | ||||
| 4 | bla TEM | 5 (13.5) | 3 | 3 (60) | 2 | 2 (40) | ||
| 5 | blaTEM, blaOXA-1 | 1 (2.7) | 1 | 1 (100) | ||||
| 6 | bla CTX-M1 | 1 (2.7) | 1 | 1 (100) | ||||
| 7 | bla OXA-1 | 1 (2.7) | 1 | 1 (100) | ||||
| ESBL- and CR-producing isolates | ||||||||
| 8 | blaSHV, blaVIM | 1 (2.7) | 1 | 1 (100) | ||||
| 9 | blaSHV, blaNDM-1 | 1 (2.7) | 1 | 1 (100) | ||||
| 10 | blaSHV, blaVIM, blaNDM-1 | 2 (5.4) | 1 | 1 | 2 (100) | |||
| 11 | blaSHV, blaCTX-M1, blaVIM, blaNDM-1 | 1 (2.7) | 1 | 1 (100) | ||||
| 12 | blaSHV, blaCTX-M1, blaNDM-1, blaIMP | 1 (2.7) | 1 | 1 (100) | ||||
| 13 | blaSHV, blaTEM, blaCTX-M1, blaVIM, blaNDM-1, blaIMP | 1 (2.7) | 1 | |||||
| 14 | blaTEM, blaNDM-1 | 1 (2.7) | 1 | 1 (100) | ||||
| 15 | blaTEM, blaNDM-1, blaIMP | 1 (2.7) | 1 | 1 (100) | ||||
| 16 | blaTEM, blaCTX-M1, blaVIM, blaNDM-1 | 1 (2.7) | 1 | 1 (100) | ||||
| 17 | blaCTX-M1, blaVIM, blaIMP | 1 (2.7) | 1 | 1 (100) | ||||
| 18 | blaOXA, blaVIM, blaNDM-1 | 1 (2.7) | 1 | 1 (100) | ||||
| ESBL-producing and CTR isolates | ||||||||
| 19 | blaSHV, mcr-1 | 1 (2.7) | 1 | 1 (100) | ||||
| 20 | blaTEM, blaCTX-M1, blaOXA-1, mcr-1 | 1 (2.7) | 1 | 1 (100) | ||||
| ESBL-, CR- producing and CTR isolates | ||||||||
| 21 | blaSHV, blaVIM, mcr-1 | 1 (2.7) | 1 | 1 (100) | ||||
| 22 | blaSHV, blaCTX-M1, blaVIM, mcr-1 | 1 (2.7) | 1 | 1 (100) | ||||
| 23 | blaSHV, blaOXA-1, blaVIM, mcr-1 | 1 (2.7) | 1 | |||||
| 24 | blaSHV, blaTEM, blaVIM, blaNDM-1, blaIMP, mcr-1 | 1 (2.7) | 1 | |||||
| 25 | blaTEM, blaOXA-1, blaVIM, blaNDM-1, mcr-1 | 1 (2.7) | 1 | 1 (100) | ||||
ESBL: extended-spectrum β-lactamase; CR: carbapenemases-producing; CTR: colistin resistant; C: chicken; Ce: chicken environment; TC: total chicken samples; Cw: chicken worker; Hc: human case; TH: total human samples.
Figure 1.
Non-metric multidimensional scaling biplot showing the overall distribution of isolates from various hosts based on the frequency distribution of antimicrobial resistance genes. Each dot refers to one isolate and the arrows refer to the association of each gene with either dimension 1 or 2.
In the univariate analysis, blaVIM and blaNDM were significantly associated with human isolates with increased odds (p = 0.002, 0.01; OR = 36, 10.6; and 95% CI = 3.8–337.9, 1.9–60.2, respectively).
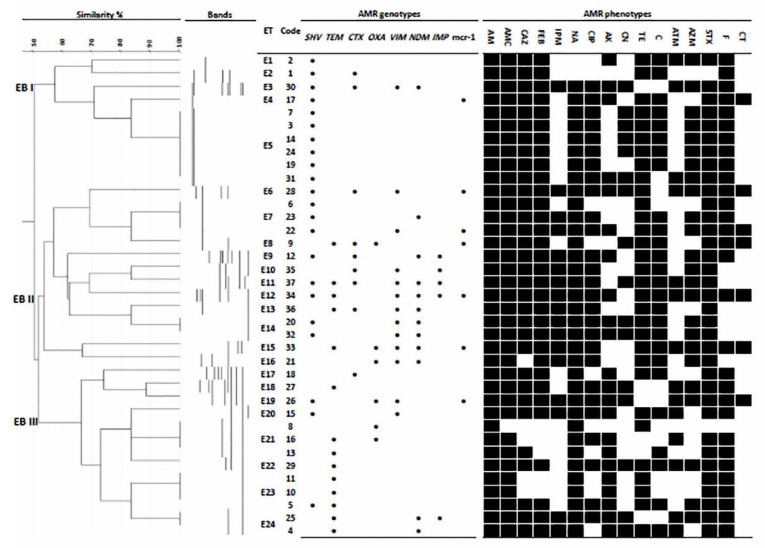
3.4. Genotyping and Epidemiological Association of Chicken and Human Isolates
The genetic relatedness and homology of ESBLK and CPK isolates from chickens, their environment, contact workers and hospitalized patients in the study area were investigated using ERIC-PCR. As revealed in Figure 2, ERIC genotyping classified 37 K. Pneumoniae isolates into three ERIC branches (EB): EBI, EBII and EBIII containing 13, 14 and 10 isolates of chickens and humans, respectively. These EBs contained 24 distinct ERIC types (ET) with band sizes ranged from 100 to 1400 bp with a discrimination index of 0.961. Six ETs (E5, E7, E14, E21, E23 and E24) showed clusters of two to six identical isolates per each ET (Figure 2). Of these, E5 contained four isolates (two from chickens, one from the chicken environment and one from farm workers) that shared identical antimicrobial resistance pattern (P16) (Figure 2).
Figure 2.
ERIC typing dendrogram of K. pneumoniae isolates from humans and chickens in the study area and their associated genetic and phenotypic antimicrobial resistance patterns. EB: ERIC branch; ET: ERIC type; black dot: positive for antibiotic resistance gene; black square: positive for antibiotic resistance phenotype; AMR: antimicrobial resistance; AMP: ampicillin; AMC: amoxicillin-clavulanic acid; CAZ: ceftazidime; FEB: cefepime; IPM: imipenem; NA: nalidixic acid; CIP: ciprofloxacin; AK: amikacin; CN: gentamicin; TE: tetracycline; C: chloramphenicol; ATM: aztreonam; AZM: azithromycin; SXT: trimethoprim-sulfamethoxazole; F: nitrofurantoin; CT: colistin.
4. Discussion
The emergence of colistin resistance in K. pneumoniae is a global public health concern, since this antibiotic is the last defense line against carbapenem-resistant isolates [41]. So far, there are no published data on colistin and carbapenem resistance in ESBLK isolated from chickens and their environment, community settings and hospitals in the same geographical region. In this study, K. pneumoniae was isolated from 9% of diseased chickens and 16.7% of environment samples, which is inconsistent with the findings reported in a previous study in Egypt, where K. pneumoniae was recovered from 35% of broiler chickens and 25% of water samples [42]. However, the prevalence of K. pneumoniae among contact poultry workers (22.7%) and hospitalized patients (14.4%) in the same region was lower than lately published reports in Egypt [7,42,43] or abroad, both in China [44] and Taiwan [45]. Hence, the direct transmission may be facilitated via close contact between chickens and humans as evidenced previously by Davis et al. [46]. The prevalence differences may be attributed to the geographical location, climatic circumstances, environmental contamination, sample types, chicken breed, management systems and growth conditions.
Antimicrobial resistance is an emergent affair of concern in human and veterinary medicine. In this study, K. pneumoniae resistance was detected most commonly to ampicillin (100%), followed by amoxicillin-clavulanic acid (94.6%), nalidixic acid and trimethoprim-sulfamethoxazole (94.6% each) and ceftazidime and cefepime (86.5% each). Almost half of the isolates (45.9%) showed imipenem resistance, whereas the lowest resistance rates were observed against colistin (18.9%), aztreonam (37.8%) and gentamicin (43.2%). In China, a previous study reported higher resistance rates of K. pneumoniae human isolates to β-lactams, quinolones and carbapenems, while all isolates exhibited 100% susceptibility to colistin [47]. Even worse, an earlier study in Shandong province, China documented pan drug-resistant K. pneumoniae isolates from both patients and chickens [48]. This disparity in resistance may be related to the antimicrobial agent frequently used for treatment in diverse geographical areas.
K. pneumoniae is one of the foremost imperative causes of MDR infections all over the world, resulting in inflated healthcare expenses and high mortalities [49]. Here, all K. pneumoniae isolates were MDR, being resistant to 3–15 commonly used antimicrobial agents either in Egypt or worldwide of diverse antimicrobial classes. Similarly in Indonesia, all K. pneumoniae isolates from chicken farms showed an MDR pattern [50], whereas the proportion of MDR K. Pneumoniae in Indonesian hospitalized patients was 54.49% [51]. However, 96% of K. pneumoniae isolates from humans and livestock including poultry demonstrated a nonwild-type phenotype to ≥ three antimicrobial classes in rural Cambodia [52]. The MDR pattern may be accredited to the unregulated use of antimicrobials in human and animal medicine [53], the facility in purchasing antimicrobials freely without prescription and the vertical or horizontal transmission of antimicrobial resistance genes via plasmids from humans to animals and vice versa [54]. Thus, the high level of MDR K. pneumoniae isolates warrants a commitment to establish purposeful antimicrobial stewardship.
ESBL-producing Enterobacteriaceae are virtually resistant to all β-lactams and may compel the use of last-resort antimicrobials as carbapenems and colistin [55]. Initially, hospital-associated K. pneumoniae was reported as an ESBL producer and, thereafter, community-acquired infections have emerged worldwide [56,57]. Herein, irrespective of the source, all K. pneumoniae isolates (n = 37) were phenotypically confirmed as ESBLs. According to PCR results, blaSHV was the slightly predominant β-lactamase gene (45.95%), followed by blaTEM (37.84%), blaCTX-M1 (24.32%) and blaOXA-1 (16.22%). Generally, it was documented that most K. pneumoniae isolates harbored SH-based non-ESBL β-lactamases as blaSHV [58]. In Germany, all K. pneumoniae isolates from broilers and their environment carried the same ESBL-gene, blaSHV−2 [59]. In contrast, in Indonesia, blaTEM (100%) was detected in all K. pneumoniae isolates of chicken origin, followed by blaCTX-M (90.9%). However, only 9.1% of the isolates harbored blaSHV [50].
In the current study, 17 out of 37 K. pneumoniae isolates were imipenem-resistant as well as MHT-positive (45.95%). Our CPK isolates possessed MBL carbapenemases comprising blaVIM (76.47%), blaNDM-1(70.59%) and blaIMP (29.41%). Concerning the source, the dominant enzymatic mechanism of carbapenemase resistance was blaNDM (10.5%) in broiler farms, whereas blaNDM and blaVIM (60% each) were the most frequent in poultry workers. Moreover, blaVIM (69.2%) was the predominant gene in the hospitalized patients in the study area. Thus, blaVIM was frequently involved in causing carbapenem resistance in humans. Likewise, CPK occurred at a relatively high rate amongst broilers (42.86%), drinking water (60%) and contact workers (56%) at poultry farms in Egypt, all of them possessed blaNDM [42]. However, an earlier report in Iran [60] was discordant with our results, in which blaNDM-1 was mostly detected in K. pneumoniae isolates from humans (75%), while blaVIM was not found in any examined CPK isolates.
Globally, in hospitalized patients, an elevated rate of carbapenem resistance was reported in Greece (68%; NDM, OXA-48 and KPC), followed by India (54%; NDM, OXA-48 and KPC) and eastern Mediterranean regions (54%; NDM and OXA-48) [61], the USA and China (11% each; NDM, OXA−48 and KPC) and Africa (4%; NDM and OXA-48) [62].
In this study, cocarriage of ESBLs and carbapenem resistance genes in K. pneumoniae isolates was 45.9%, ESBLs and colistin resistance genes was 18.9% and carbapenem and colistin resistance genes was 13.5%. As reported previously, 90% of CPK isolates had the carbapenemase phenotype, of which 50% were ESBL-producers [63]. In France, the coxistence of ESBL genes and the mcr-1 colistin resistance gene was reported in a hospitalized patient [64]. Also, a recent study in Iran encountered chromosomal-mediated colistin resistance among CPK isolates in hospitalized patients with bloodstream infections [65]. Consistent with our results, a recent study in the United States documented the coexistence of carbapenem and colistin resistance in 13% of K. pneumoniae isolates from hospitalized patients [66]. The most striking finding in this study was the cocarriage of ESBLs, carbapenem and colistin resistance genes in 13.5% of K. pneumoniae isolates from poultry workers (n = 1/22; 4.5%) and hospitalized patients (n = 4/90; 4.4%), as the first report in Egypt. The extensive use of carbapenems results in selection pressure and simplifies the spread of carbapenem-resistant isolates, for which colistin is one of the last treatment choices. Further colistin resistance in CPK, such as the reported outbreak in hospitalized patients in Germany [67], leaves limited treatment options, among them fosfomycin and tigecycline. Thus, regimens of colistin combined with carbapenem display a high level of synergism even in the existence of colistin resistance [68].
In this study, we present the first report in the genetic relatedness of community and hospital-acquired K. pneumoniae isolates using ERIC-PCR. In this context, data fingerprints revealed pronounced similarities between K. pneumoniae isolates recovered from chicken farms and hospitalized patients. These may originate from a common ancestral strain, demonstrating cross-transmission between chickens and humans. A recent study in Iran showed immense genetic diversity among K. pneumoniae isolates from hospitalized patients [69]. Moreover, another Indian study demonstrated heterogeneous K. pneumoniae human isolates while using ERIC-PCR, suggesting the involvement of multiple subtypes in infection [70]. In light of our results, additional studies are needed to confirm the transmission between poultry and humans and to explain the direction and machinery of transmission.
The limitation of this study is due to restricted funding resources. Therefore, we investigated the genetic relatedness among the community and hospital-acquired ESBL producing K. pneumoniae isolates based on ERIC-PCR typing, which is a less expensive and less laborious approach in routine diagnosis that allows rapid genotyping with a high discriminatory potential, and thus is more suitable on a local scale in developing countries. However, using advanced techniques such as multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE) would add more sensitivity and accuracy to the results and would allow comparison among different typing methods simultaneously. Future studies are warranted in this direction.
5. Conclusions
This is the first, at least in Egypt, of the coexistence of ESBLs, carbapenem and colistin resistance in K. pneumoniae isolates recovered from poultry workers and hospitalized patients. Moreover, our findings demonstrate great similarities between community and hospital-acquired K. pneumoniae isolates, which warrants the need to improve farm management strategies and implementation of proper hygiene practices in the study area.
Acknowledgments
We thank Taif University researchers supporting project TURSP-2020/07, Taif University, Taif, Saudi Arabia. We thank the staff members of Faculty of Veterinary Medicine, Kafrelsheikh University and Zagazig University, Egypt for their technical support during this study.
Supplementary Materials
The following are available online at www.mdpi.com/xxx/s1.Table S1: Oligonucleotide primer sequences used for PCR assays. Table S2: Frequency distribution of K. pneumoniae isolates recovered from broiler farms and human workers in the study area. Table S3: Source associated variations in phenotypic and genetic antibiotic resistance traits of K. pneumoniae isolates from chickens and humans in this study.
Author Contributions
Conceptualization, W.E., N.K.A.E.-A. and Y.H.T.; methodology, W.E., N.K.A.E.-A. and Y.H.T.; validation, S.M.M., E.M.A.R., R.E., H.A.S. and A.A.T.; formal analysis, W.E.; investigation, W.E., N.K.A.E.-A., Y.H.T., S.M.M., E.M.A.R., R.E., H.A.S. and A.A.T.; data curation, W.E., N.K.A.E.-A. and Y.H.T.; writing—original draft preparation, N.K.A.E.-A. and Y.H.T.; writing—review and editing, N.K.A.E.-A., Y.H.T. and W.E.; project administration and funding acquisition, H.A.S. and A.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Kafrelsheikh University, Kafrelsheikh, Egypt (KFS-2019/3).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors have no conflict of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cheng F., Li Z., Lan S., Liu W., Li X., Zhou Z., Song Z., Wu J., Zhang M., Shan W. Characterization of Klebsiella pneumoniae associated with cattle infections in southwest China using multi-locus sequence typing (MLST), antibiotic resistance and virulence-associated gene profile analysis. Braz. J. Microbiol. 2018;49:93–100. doi: 10.1016/j.bjm.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ripabelli G., Tamburro M., Guerrizio G., Fanelli I., Flocco R., Scutellà M., Sammarco M.L. Tracking multidrug-resistant Klebsiella pneumoniae from an Italian hospital: Molecular epidemiology and surveillance by PFGE, RAPD and PCR-Based resistance genes prevalence. Curr. Microbiol. 2018;75:977–987. doi: 10.1007/s00284-018-1475-3. [DOI] [PubMed] [Google Scholar]
- 3.Abd El-Aziz N.K., Gharib A.A. Coexistence of plasmid-mediated quinolone resistance determinants and AmpC-beta-lactamases in Escherichia coli strains in Egypt. Cell. Mol. Biol. 2015;61:29–35. doi: 10.14715/cmb/2015.61.5.5. [DOI] [PubMed] [Google Scholar]
- 4.Dahshan H., Abd-Elall A.M.M., Megahed A.M., Abd-El-Kader M.A., Nabawy E.E. Veterinary antibiotic resistance, residues, and ecological risks in environmental samples obtained from poultry farms, Egypt. Environ. Monit. Assess. 2015;187:2. doi: 10.1007/s10661-014-4218-3. [DOI] [PubMed] [Google Scholar]
- 5.WHO Report on the Consultative Meeting on Antimicrobial Resistance for Countries in the Eastern Mediterranean Region: From Policies to Action Sharm el Sheikh, Egypt, 12–14 November 2013. [(accessed on 28 April 2020)]; Available online: http://apps.who.int/iris/handle/10665/116211?show=full.
- 6.Overdevest I.T.M.A., Heck M., van der Zwaluw K., Huijsdens X., van Santen M., Rijnsburger M., Eustace A., Xu L., Hawkey P., Savelkou P., et al. Extended-spectrum β-lactamase producing Klebsiella spp. in chicken meat and humans: A comparison of typing methods. Clin. Microbiol. Infect. 2014;20:251–255. doi: 10.1111/1469-0691.12277. [DOI] [PubMed] [Google Scholar]
- 7.Elmowalid G.A., Ahmed A.M., Hassan M.N., Abd El-Aziz N.K., Abdelwahab A.M., Elwan S.I. Molecular Detection of New SHV β-lactamase variants in clinical Escherichia coli and Klebsiella pneumoniae isolates from Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2018;60:35–41. doi: 10.1016/j.cimid.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Alizadeh N., Rezaee M.A., Kafil H.S., Barhaghi M.H.S., Memar M.Y., Milani M., Hasani A., Ghotaslou R. Detection of carbapenem-resistant Enterobacteriaceae by chromogenic screening media. J. Microbiol. Methods. 2018;153:40–44. doi: 10.1016/j.mimet.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Petrosillo N., Taglietti F., Granata G. Treatment options for colistin resistant Klebsiella pneumoniae: Present and Future. J. Clin. Med. 2019;8:934. doi: 10.3390/jcm8070934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magiorakos A., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., OlssonLiljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel G., Bonomo R.A. “Stormy waters ahead”: Global emergence of carbapenemases. Front. Microbiol. 2013;4:48. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skov R.L., Monnet D.L. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Eurosurveillance. 2016;21:30155. doi: 10.2807/1560-7917.ES.2016.21.9.30155. [DOI] [PubMed] [Google Scholar]
- 13.Durante-Mangoni E., Andini R., Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin. Microbiol. Infect. 2019;25:943–950. doi: 10.1016/j.cmi.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Queenan A.M., Bush K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007;20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva N., Carvalho I., Currie C., Sousa M., Igrejas G., Poeta P. Extended-Spectrum-β-Lactamase and carbapenemase-producing Enterobacteriaceae in food-producing animals in Europe. In: Igrejas J.C.G., editor. Antibiotic Drug Resistance. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2019. pp. 261–273. [Google Scholar]
- 16.Zafer M.M., El-Mahallawy H.A., Abdulhak A., Amin M.A., Al-Agamy M.H., Radwan H.H. Emergence of colistin resistance in multidrug-resistant Klebsiella pneumoniae and Escherichia coli strains isolated from cancer patients. Ann. Clin. Microbiol. Antimicrob. 2019;18:40. doi: 10.1186/s12941-019-0339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Badawy M.F., El-Far S.W., Althobaiti S.S., Abou-Elazm F.I., Shohayeb M.M. The first Egyptian report showing the co-existence of blaNDM-25, blaOXA-23, blaOXA-181, and blaGES-1 among carbapenem-resistant K. Pneumoniae clinical isolates genotyped by BOX-PCR. Infect. Drug Resist. 2020;13:1237–1250. doi: 10.2147/IDR.S244064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel Salam S.A., Hager R. Colistin susceptibility among multidrug resistant Gram-negative bacilli isolated from Tertiary hospital in Egypt. Nov. Res. Microbiol. J. 2020;4:968–978. doi: 10.21608/nrmj.2020.118447. [DOI] [Google Scholar]
- 19.Wilson L.A., Sharp P.M. Enterobacterial repetitive intergenic consensus (ERIC) sequences in Escherichia coli: Evolution and implications for ERIC-PCR. Mol. Biol. Evol. 2006;23:1156–1168. doi: 10.1093/molbev/msj125. [DOI] [PubMed] [Google Scholar]
- 20.Tartor Y.H., EL-Naenaeey E.-S.Y., Abdallah H.M., Samir M., Yassen M.M., Abdelwahab A.M. Virulotyping and genetic diversity of Aeromonas hydrophila isolated from Nile tilapia (Oreochromis niloticus) in aquaculture farms in Egypt. Aquaculture. 2021;541:736781. doi: 10.1016/j.aquaculture.2021.736781. [DOI] [Google Scholar]
- 21.Markey B., Leonard F., Archambault M., Cullinane A., Maguire D. Clinical Veterinary Microbiology. 2nd ed. Mosby; London, UK: 2013. [Google Scholar]
- 22.Pomakova D.K., Hsiao C.B., Beanan J.M., Olson R., MacDonald U., Keynan Y., Russo T.A. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: An emerging and under-recognized pathogenic variant. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:981–989. doi: 10.1007/s10096-011-1396-6. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y., Liu C., Zheng W., Zhang X., Yu J., Gao Q., Hou Y., Huang X. PCR detection of Klebsiella pneumoniae in infant formula based on 16S-23S internal transcribed spacer. Int. J. Food Microbiol. 2008;125:230–235. doi: 10.1016/j.ijfoodmicro.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y., Siu G.K.H., Yeung A.S.F., Chen J.H.K., Ho P.L., Leung K.W., Tsang J.L.Y., Cheng V.C.C., Guo L., Yang J., et al. Performance of the VITEK MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for rapid bacterial identification in two diagnostic centres in China. J. Med. Microbiol. 2015;64:18–24. doi: 10.1099/jmm.0.080317-0. [DOI] [PubMed] [Google Scholar]
- 25.Bauer A.W., Kirby W.M., Sherris J.C., Truck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 26.Plumb D. Plumb’s Veterinary Drug Handbook. 8th ed. Wiley-Blackwell; Ames, IA, USA: 2015. (Pocket) [Google Scholar]
- 27.Rankin D. Test Methods: MIC Testing. In: Coyle B.M., editor. Manual of Antimicrobial Susceptibility Testing. American Society for Microbiology; Washington, DC, USA: 2005. pp. 53–62. [Google Scholar]
- 28.EUCAST . Recommendations for MIC Determination of Colistin (Polymyxin E) as Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. European Committee on Antimicrobial Susceptibility Testing; Växjö, Sweden: 2016. [(accessed on 20 March 2020)]. Available online: http://www.Eucast.Org. [Google Scholar]
- 29.Tambekar D., Dhanorkar D., Gulhane S., Khandelwal V., Dudhane M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006;5:1562–1565. doi: 10.4314/AJB.V5I17.43162. [DOI] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. Twenty Second Informational Supplement Update. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. CLSI Document M100-S22 U. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2014. CLSI Document M100-S24. [Google Scholar]
- 32.Pournaras S., Poulou A., Tsakris A. Inhibitor-based methods for the detection of KPC carbapenemase-producing Enterobacteriaceae in clinical practice by using boronic acid compounds. J. Antimicrob. Chemother. 2010;65:1319–1321. doi: 10.1093/jac/dkq124. [DOI] [PubMed] [Google Scholar]
- 33.Ogutu J.O., Zhang Q., Huang Y., Yan H., Su L., Gao B., Zhang W., Zhao J., Cai W., Li W., et al. Development of a multiplex PCR system and its application in detection of blaSHV, blaTEM, blaCTX-M-1, blaCTX-M-9 and blaOXA-1 group genes in clinical Klebsiella pneumoniae and Escherichia coli strains. J. Antibiot. 2015;68:725–733. doi: 10.1038/ja.2015.68. [DOI] [PubMed] [Google Scholar]
- 34.Qi C., Malczynski M., Parker M., Scheetz M.H. Characterization of genetic diversity of carbapenem-resistant Acinetobacter baumannii clinical strains collected from 2004 to 2007. J. Clin. Microbiol. 2008;46:1106–1109. doi: 10.1128/JCM.01877-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Y., Liang Z., Su X., Xiong Y. Characterization of carbapenemase genes in Enterobacteriaceae species exhibiting decreased susceptibility to carbapenems in a university hospital in Chongqing, China. Ann. Lab. Med. 2012;32:270–275. doi: 10.3343/alm.2012.32.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yong D., Toleman M.A., Giske C.G., Cho H.S., Sundman K., Lee K., Walsh T.R. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 38.Versalovic J., Koeuth T., Lupski R. Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial enomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heras J., Domínguez C., Mata E., Pascual V., Lozano C., Torres C., Zarazaga M. GelJ—A tool for analyzing DNA fingerprint gel images. BMC Bioinformatics. 2015;16:1–8. doi: 10.1186/s12859-015-0703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter P.R., Gaston M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988;26:2465–2466. doi: 10.1128/JCM.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kempf I., Fleury M.A., Drider D., Bruneau M., Sanders P., Chauvin C., Madec J.Y., Jouy E. What do we know about resistance to colistin in Enterobacteriaceae in avian and pig production in Europe? Int. J. Antimicrob. Agents. 2013;42:379–383. doi: 10.1016/j.ijantimicag.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Hamza E., Dorgham S.M., Hamza D.A. Carbapenemase-producing Klebsiella pneumoniae in broiler poultry farming in Egypt. J. Glob. Antimicrob. Resist. 2016;7:8–10. doi: 10.1016/j.jgar.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Elwakil B.H., Ali S.M., Hafez S.F., Bekhit A.A., El-Naggar M.Y., Olama Z.A. Resistance prevalence profile of Klebsiella pneumoniae in the Intensive Care Units of Al-Shatby pediatric hospital, Alexandria, Egypt. Nov. Res. Microbiol. J. 2019;3:535–545. doi: 10.21608/nrmj.2019.66746. [DOI] [Google Scholar]
- 44.Yang F., Deng B., Liao W., Wang P., Chen P., Wei J. High rate of multiresistant Klebsiella pneumoniae from human and animal origin. Infect. Drug Resist. 2019;12:2729. doi: 10.2147/IDR.S219155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juan C.H., Fang S.Y., Chou C.H., Tsai T.Y., Lin Y.T. Clinical characteristics of patients with pneumonia caused by Klebsiella pneumoniae in Taiwan and prevalence of antimicrobial-resistant and hypervirulent strains: A retrospective study. Antimicrob. Resist. Infect. Control. 2020;9:4. doi: 10.1186/s13756-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis G.S., Waits K., Nordstrom L., Weaver B., Aziz M., Gauld L., Grande H., Bigler R., Horwinski J., Porter S., et al. Intermingled Klebsiella pneumoniae populations between retail meats and human urinary tract infections. Clin. Infect. Dis. 2015;61:892–899. doi: 10.1093/cid/civ428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou X.H., Song X.Y., Ma X.B., Zhang S.Y., Zhang J.Q. Molecular characterization of multidrug-resistant Klebsiella pneumoniae isolates. Braz. J. Microbiol. 2015;46:759–768. doi: 10.1590/S1517-838246320140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun S., Gao H., Liu Y., Jin L., Wang R., Wang X., Wang Q., Yin Y., Zhang Y., Wang H. Co-existence of a novel plasmid-mediated efflux pump with colistin resistance gene mcr in one plasmid confers transferable multidrug resistance in Klebsiella pneumoniae. Emerg. Microbes Infect. 2020;9:1102–1113. doi: 10.1080/22221751.2020.1768805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kidd T.J., Mills G., Sá-Pessoa J., Dumigan A., Frank C.G., Insua J.L., Ingram R., Hobley L., Bengoechea J.A. A Klebsiella pneumoniae antibiotic resistance mechanism that subdues host defences and promotes virulence. EMBO Mol. Med. 2017;9:430–447. doi: 10.15252/emmm.201607336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayati M., Indrawati A., Mayasari N.L.P.I., Istiyaningsih I., Atikah N. Molecular detection of extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates of chicken origin from East Java, Indonesia. Vet. World. 2019;12:578–583. doi: 10.14202/vetworld.2019.578-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nirwati H., Sinanjung K., Fahrunissa F., Wijaya F., Napitupulu S., Hati V.P., Hakim M.S., Meliala A., Aman A.T., Nuryastuti T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019;13:1–8. doi: 10.1186/s12919-019-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atterby C., Osbjer K., Tepper V., Rajala E., Hernandez J., Seng S., Holl D., Bonnedahl J., Börjesson S., Magnusson U., et al. Carriage of carbapenemase- and extended-spectrum cephalosporinase-producing Escherichia coli and Klebsiella pneumoniae in humans and livestock in rural Cambodia; gender and age differences and detection of blaOXA-48in humans. Zoonoses Public Health. 2019;66:603–617. doi: 10.1111/zph.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elalamy R.A., Tartor Y.H., Ammar A.M., Eldesouky I.E., Esawy A.E.I. Molecular characterization of extensively drug-resistant Pasteurella multocida isolated from apparently healthy and diseased chickens in Egypt. Pak. Vet. J. 2020;40:319–324. doi: 10.29261/pakvetj/2020.020. [DOI] [Google Scholar]
- 54.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantón R., Akóva M., Carmeli Y., Giske C.G., Glupczynski Y., Gniadkowski M., Livermore D.M., Miriagou V., Naas T., Rossolini G.M., et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2012;18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 56.Paterson D.L., Bonomo R.A. Extended-spectrum β-lactamases: A clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Livermore D.M., Canton R., Gniadkowski M., Nordmann P., Rossolini G.M., Arlet G., Ayala J., Coque T.M., Kern-Zdanowicz I., Luzzaro F., et al. CTX-M: Changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 2007;59:165–174. doi: 10.1093/jac/dkl483. [DOI] [PubMed] [Google Scholar]
- 58.Karanika S., Karantanos T., Arvanitis M., Grigoras C., Mylonakis E. Fecal colonization with extended-spectrum Beta-lactamase-producing Enterobacteriaceae and risk factors among healthy individuals: A systematic review and metaanalysis. Clin. Infect. Dis. 2016;63:310–318. doi: 10.1093/cid/ciw283. [DOI] [PubMed] [Google Scholar]
- 59.Daehre K., Projahn M., Friese A., Semmler T., Guenther S., Roesler U.H. ESBL-producing Klebsiella pneumoniae in the broiler production chain and the first description of ST3128. Front. Microbiol. 2018;9:302. doi: 10.3389/fmicb.2018.02302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delarampour A., Ghalehnoo Z.R., Khademi F., Delarampour M., Vaez H. Molecular detection of carbapenem-resistant genes in clinical isolates of Klebsiella pneumoniae. Ann. Ig. 2019;31:349–355. doi: 10.7416/ai.2019.2296. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization . Antimicrobial Resistance Global Report on Surveillance. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 62.Lee C.R., Lee J.H., Park K.S., Kim Y.B., Jeong B.C., Lee S.H. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: Epidemiology, genetic context, treatment options, and detection methods. Front. Microbiol. 2016;7:895. doi: 10.3389/fmicb.2016.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eftekhar F., Naseh Z. Extended-spectrum beta-lactamase and carbapenemase production among burn and non-burn clinical isolates of Klebsiella pneumoniae. Iran. J. Microbiol. 2015;7:144–149. [PMC free article] [PubMed] [Google Scholar]
- 64.Caspar Y., Maillet M., Pavese P., Francony G., Brion J., Mallaret M., Bonnet R. Colistin resistance in ESBL-producing Klebsiella pneumoniae, France. Emerg. Infect. Dis. 2017;23:874–876. doi: 10.3201/eid2305.161942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haeili M., Feizabadi M.M. The threat of colistin resistance among carbapenem-resistant Klebsiella pneumoniae isolates in Iran. Iran. J. Microbiol. 2018;10:72–73. [PMC free article] [PubMed] [Google Scholar]
- 66.Rojas L.J., Salim M., Cober E., Richter S.S., Perez F., Salata R.A., Kalayjian R.C., Watkins R.R., Marshall S., Rudin S.D., et al. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: Laboratory detection and impact on mortality. Clin. Infect. Dis. 2017;64:711–718. doi: 10.1093/cid/ciw805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.European Centre for Disease Prevention and Control . Outbreak of Carbapenemase-Producing (NDM-1 and OXA-48) and Colistin-Resistant Klebsiella pneumoniae ST307, North-East Germany, 2019. ECDC; Stockholm, Sweden: 2019. [Google Scholar]
- 68.Erdem F., Abulaila A., Aktas Z., Oncul O. In vitro evaluation of double carbapenem and colistin combinations against OXA-48, NDM carbapenemase-producing colistin-resistant Klebsiella pneumoniae strains. Antimicrob. Resist. Infect. Control. 2020;9:70. doi: 10.1186/s13756-020-00727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sedighi P., Zarei O., Karimi K., Taheri M., Karami P., Shokoohizadeh L. Molecular typing of Klebsiella pneumoniae Clinical Isolates by Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction. Int. J. Microbiol. 2020:1–5. doi: 10.1155/2020/8894727. [DOI] [Google Scholar]
- 70.Shaikh S., Rizvi S.M.D., Anis R., Shakil S. Prevalence of CTX-M resistance marker and integrons among Escherichia coli and Klebsiella pneumoniae isolates of clinical origin. Lett. Appl. Microbiol. 2016;62:419–427. doi: 10.1111/lam.12567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.