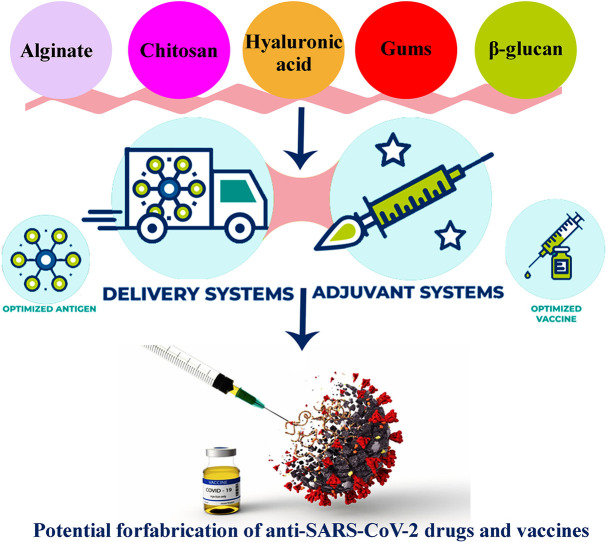
Abstract
Pathogen transmission is a widespread threat to global human health. Vaccines are very important during the outbreak of a pandemic. Destructive fractures caused by a sudden outbreak of COVID-19 have spurred vaccine production at an unprecedented rate. The strategy of an effective vaccine delivery system is opening up novel probabilities to make more immunization. Indeed, vaccination is the most successful way to prevent deaths from infectious diseases. In order to optimal immune response production or improvement in the effectiveness of vaccines, delivery systems or adjuvants are required. Natural polymers such as chitosan, alginate, hyaluronic acid, gums, and β-glucan with antiviral activity have good potential as adjuvant or delivery systems for vaccine formulation development and design vaccine delivery devices. According to the antiviral performance and immunomodulation of these biopolymers, they will play significant characters in the anti-COVID-19 field. In this mini-review, the recent progress in vaccine development by using biopolymers is presented which, provides a reference for their research on anti-COVID-19 drugs and vaccines.
Keywords: Vaccines, Alginate, Chitosan, Gums, Hyaluronic acid, β-Glucan
Graphical abstract
1. Introduction
Vaccination is one of the most successful, affordable, and sustainable methods for prevention and elimination of infectious diseases and so, inhibit the death of infectious diseases. Although new vaccines have been developed nowadays with better stability and longer duration, and less toxic side effects, they have drawbacks such as weak immunogenicity and low expression levels. In order to optimal immune response production carrier molecules or adjuvants are required. Indeed, to induce a better immune response, reduce preparation cost and vaccine dose, for improving the efficiency and efficacy of vaccine, adjuvants, and delivery systems are applied [[1], [2], [3], [4], [5]]. Adjuvants through augmenting the immunogenicity of weaker immunogens, increase the effect of the vaccine, and reduce antigen amount and required immunization frequency for protective immunity [6].
Biomacromolecular compounds such as sodium alginate, chitosan, and gums, which are mainly derived from plants, animals, fungi, and bacteria, have attracted much attention in medical science for the formulation of vaccines and delivery systems. Many advantages of these compounds are their nontoxic nature, bio-compatibility, bio-degradability, and antiviral properties. Till now, numerous applications of these biopolymers have been reported. By using these materials, safer and more effective vaccines are prepared. These biopolymers can prevent the degradation of antigens, improve the stability of antigen, cause slow release; and therefore, enhance the immunogenicity. These natural macromolecules display respectable inhibitory performance toward different viruses. Many of these biopolymers can directly interact with the virus's surface via negative charge, and inhibit the infectious capability of them or kill them. Sulfated polysaccharides with polyanionic features block the positive charge on the surface of the cell for inhibiting the adsorption of virus or invasion. Also, polysaccharides can inhibit viral transcription and replication. Furthermore, they are responsible for activating the host antiviral immunomodulatory system [[7], [8], [9], [10], [11], [12], [13], [14]].
As mentioned above, natural polymers as adjuvants or immune potentiators are useful for immunogenicity improvement, sustained release of drugs, and antigen stability. These polysaccharides, directly and indirectly, exhibit antiviral effects and activities by interfering with the viral life cycle or enhancing body immunity. Therefore, these compounds are expected to have excellent application in the preparation of a vaccine for emerging and pandemic threats [15,16].
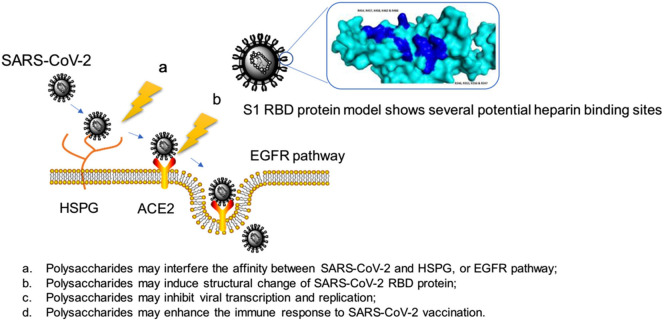
With the onset of acute respiratory syndrome, the SARS-CoV-2 has created a unique global disorder in the health, social stability, and economies of countries. This virus has severely affected human life. So far, various compounds with antimicrobial properties such as polysaccharides and nanoparticles have been used to control the disease and provide personal protective equipment such as gowns, gloves, and masks [17]. The possible mechanism of action of polysaccharides on the virus and their anti-SARS-CoV-2 effects is seen in Fig. 1 , which shows their influence by interfering with the life cycle of the virus [13]. However, the global scientific community is studying and preparing vaccines as the most effective solution to prevent SARS-CoV-2 infection, and control and spread the COVID-19 [18]. In this mini-review, the recent progress in vaccine development by using biopolymers including chitosan, alginate, hyaluronic acid, gums, and β-glucan, is presented which, provides a reference for their research on anti-SARS-CoV-2 drugs and vaccines.
Fig. 1.
The proposed mechanisms of polysaccharides anti-SARS-CoV-2 (Reproduced with permission from ref [13] Copyright 2020 Elsevier).
2. Chitosan as promising adjuvant and vaccine delivery system for viral threats
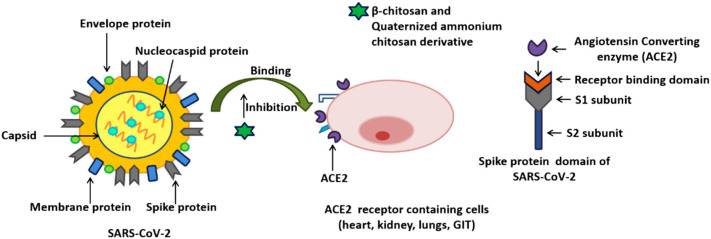
Chitosan and derivatives, as natural polymers with great and valuable features such as antiviral, anti-fungal, anti-bacterial, and antioxidant characteristics, affordability, bio-degradability, have been applied in diverse uses like biomedical. Recently, this biopolymer was used as a potential biomacromolecule against the SARS-CoV-2 virus, preparation antiviral vaccines, and adjuvants [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]]. Indeed, the virulence of this virus is mainly due to the capability of spike protein for binding to ACE2 receptors existing in the human respiratory tract and start an infection. These polymers and derivatives prevent the spike protein from binding to the ACE2 receptors and inhibit infection (Fig. 2 ). Chitosan enhances cell-mediated as well as humoral immune responses. Owing to positive charge and ionic interactions with the negatively charged cell membranes, chitosan causes superior uptake via antigen-presenting cells. Likewise, by electrostatic attractions with DNA as well as antigens with negative charges, it can be applied in vaccine delivery. Till now, it has been tested as a nano-carrier to deliver antigens and raise immune responses. Also, the performance of its adjuvants can be explained in three or more mechanisms: providing a depot for the antigen to slow release, facilitating the antigen targeting to immune cell and improve phagocytosis, modulation and enhancement immune response induced by the antigen alone [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]].
Fig. 2.
Proposed β-chitosan and quaternized ammonium chitosan derivatives preventing the SARS-CoV-2 infection by inhibiting the binding of spike protein to ACE2 receptors (recognized as primary receptor for SARS-CoV-2 binding). Alongside, interaction of ACE2 to receptor binding domain of SARS-CoV-2 has been displayed (Reproduced with permission from ref [20] Copyright 2021 Elsevier).
The hepatitis E virus is a causative agent of hepatitis, causes serious infections and risks and deaths worldwide. A safe and effective vaccine is the best way to deal with this global threat. In one study reported by Wei et al., in order to control and prevent hepatitis E infection, chitosan nanoparticles were used to prepare an effective vaccine [23]. In this study, stable chitosan/truncated capsid protein p146 nanoparticles were successfully prepared by the covalent cross-linking of chitosan and tripolyphosphate. After antigen loading, the size of the chitosan nanoparticles increased slightly to reach a 200–300 nm diameter to efficient uptake through the antigen-presenting cells. Nanoparticles with positive charge can be efficiently internalized through dendritic cells, and meaningfully stimulate the maturation of cells, signifying that delivery systems based on chitosan can act as adjuvants. Furthermore, for molecular interaction with the target cells, the surface charge has a critical role. It has been shown that the incorporation of antigen-presenting cells with cationic nanoparticles is more efficient than neutrally charged ones. So, this natural polymer was investigated as a mucosal delivery system for encapsulation of truncated capsid protein p146. Physical, chemical, cytotoxicity, and immunogenicity in mice were evaluated for this system and compared to the purified p146. Immunogenicity was analyzed by assessing cellular and humeral immune responses. The chitosan/p146 system with 200–300 nm and spherical morphology showed 65–73.9% encapsulation efficiency and 27.7–67.5% loading capacity. Additionally, the chitosan/p146 system exhibited low-cytotoxicity and a sustained-release influence. Moreover, expression levels, as well as mRNA transcription for the chitosan/p146 system, were better than purified p146, so, the fabricated nanoparticles were a vaccine candidate for potential hepatitis E.
In a study by Namasivayam et al. by in-situ ionic gelation technique, hepatitis B-surface antigen vaccine was manufactured by chitosan/polyethylene glycol nanocomposite (100–120 nm) as a harmless and efficient vaccine for improvement the immunogenicity [24]. For determination of the sustained release of antigen, an in-vitro dissolution study has been performed. The controlled release develops the temporal/spatial presentation of antigen, protects from physiological elimination, and improves patient compliance as well as quality control. By several mechanisms such as surface erosion, disintegration, diffusion, and desorption, the antigen release from the polymeric nanoparticles can take placed. In this work, the prepared nanocomposite showed advantages such as great antigen loading, remarkable stability, and release pattern, bio-compatibility, non-target effect, enhanced immunogenicity effect. By experimenting on animals, it showed no sign of toxicity and mortality. Overall, the findings showed successful preparation and the great potential of this immunizing agent for hepatitis B.
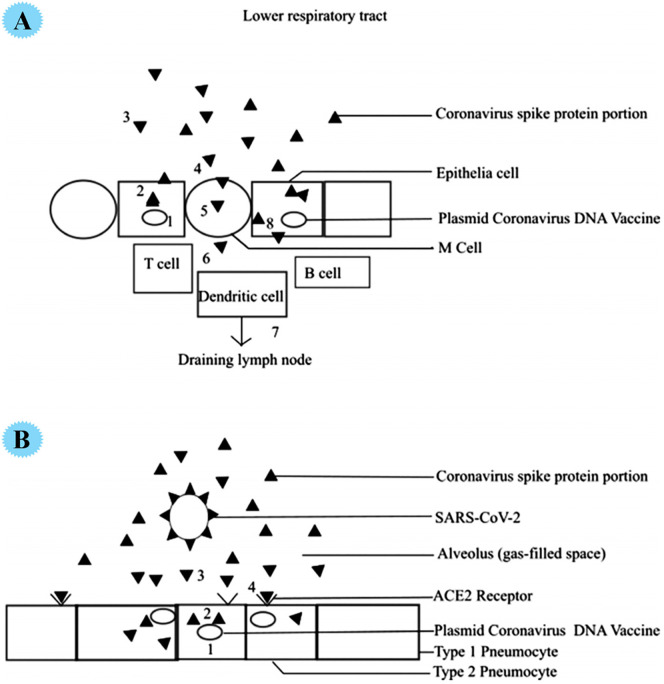
Surface spike protein in the SARS-CoV-2 virus is the main feature of it and shows a critical character in infection. It allows viral entry via mediating high specificity viral attachment to the host cell surface of the ACE2 receptor. Recently, for treatment and vaccination toward COVID-19, a new idea by using chitosan-coated DNA vaccine was reported (Fig. 3 ). The plasmid DNA vaccine is used as an effective vaccine to fight bacterial and viral diseases. Optimal plasmid DNA delivering to the target cell is a challenge for proper protein expression and immune response. In fact, the immunogenicity of these vaccines is low. Due to the repulsion of negatively charged phospholipids, the effectiveness of plasmid DNA vaccines is small, and they cannot cross the cell membrane. These vaccines are developed with the help of chitosan as a delivery system. This cationic polymer can be attached to a negative charge of DNA and provide an efficient means to prevent DNA vaccine from degradation. Chitosan contains several satisfactory properties for delivery DNA vaccine, for example, mucoadhesion, highly soluble, being inert as well as non-immunogenic. Also, a major site for pathogens and human contamination is the mucosal surface, so, chitosan-based DNA vaccination is highly desirable on the mucosal surface and it can effectively deliver the vaccine to the mucosal surfaces where the virus reproduces and site of viral infection. In a recent report, the utilization of inhaled plasmid DNA vaccine comprising spike protein DNA sequence of SARS-CoV-2 for treatment was stated. A 138-nm inhalation vaccine (similar to the SARS-CoV-2 virus) with the ability to reach deep into the lungs was reported in this work. Plasmid DNA-created spike proteins functioned for immunity creation and worked as an inexpensive antagonist toward attachment of coronavirus host cells [28].
Fig. 3.
A, DNA vaccine induction of humoral and cellular mucosal immune response. 1 transfection of epithelial cell 2 expression of S1 protein, 3 secretion of S1 protein, 4 S1 protein taken up by M cells, 5 transported to immune cells with 6 uptake by dendritic cells and MHC II antigen presentation to T-cells with subsequent draining into lymph node stimulating humoral response. 8. MHC 1 presentation stimulated cytotoxic lymphocyte response. B, DNA vaccine treatment with secreted S1 spike protein acting as a competitive antagonist by interfering with the coronavirus binding to ACE2 receptors. 1 transfected cell 2.expression of S1 protein, 3 secretion of the S1 protein, and 4 ACE2 receptor blockade by S1 protein preventing SARS-CoV-2 from binding to ACE2 receptor (Reproduced with permission from ref [28] Copyright 2020 John Wiley and Sons).
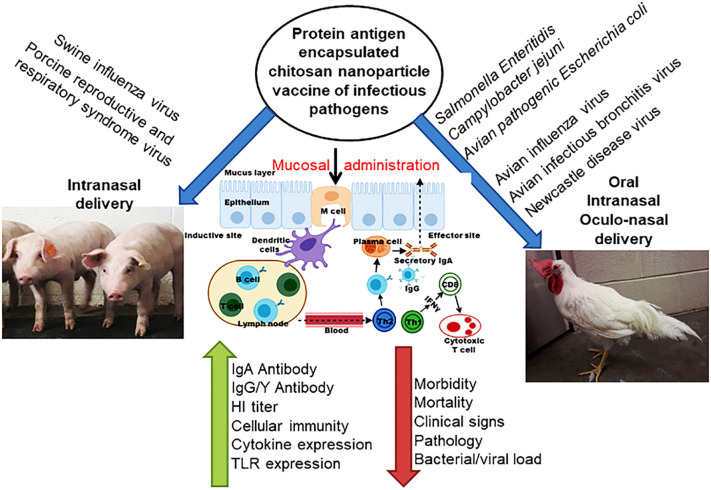
A major threat to human health is the infectious diseases of pigs and poultry that can be shared between humans and animals. On the other hand, chitosan, with its positive charges, is a natural mucosal adhesive whose nanoparticles are used to transmit vaccine antigens. Because the pathway of pathogenic pathogens is through the mouth and nose, so chitosan-based oral and nasal vaccines increase the immunogenicity of loaded antigens and induce strong antibodies and cellular immune responses, so, it protects against infection. To date, this natural polymer has been used as a delivery system for mucosal vaccines in poultry and pigs (Fig. 4 ) [31].
Fig. 4.
Schematic representation of chitosan based nanoparticle used for delivering bacterial and viral vaccine to mucosal sites of poultry and pigs, and the vaccine induced mucosal immune responses [31].
3. The role of alginate-based adjuvant and vaccine delivery system for pandemic strains
Another natural polysaccharide with great features and applications [32] is alginate. Recently this polymer was used for combination viral such as SARS-CoV-2 [21,33]. Hepatitis A is a liver disease caused by a virus that passes through the fecal-oral route. This disease is preventable and vaccination is the best way to prevent it. The disease is prevalent in areas that are poor sanitary conditions and economy. This infection is common in people under 10 years of age. This infection rarely causes death and its severity increases with age. To overcome the high cost and improve the immune system, adjuvants are used. In most countries, formaldehyde-inactivated vaccines as efficient vaccines for this disease are accessible. But, it has disadvantages such as the safety and efficacy of vaccines based on this substance. So, there is an urgent need to find a safe and effective alternative for improving vaccine release and developing immune responses. An ideal adjuvant creates a balanced Th1 and Th2 immune response. Both of these are necessary to protect against viral infections. Th1 and Th2 are related to cell-mediated immunity and humoral immune response, respectively.
In a recent study, an effective adjuvant for Hepatitis A vaccine was used and compared with traditional alum adjuvant [34]. The immune response of vaccines was evaluated. Indeed, in this work, immune responses to alum-, chitosan-, and alginate/chitosan-based vaccine were assessed in mice. Humoral and cellular immune responses were improved by using alginate-coated chitosan nanoparticles. After 28 days from vaccinations, splenocyte stimulation was evaluated. Vaccines prepared with the help of alginate/chitosan improved immunity via the development of seroconversion rate (100%), antibodies level of hepatitis A, as well as the splenocytes proliferation. The IgG level for the alginate/chitosan-based vaccine significantly was enhanced in comparison with other groups. Besides, antibody levels were increased in the presence of chitosan compared to alum.
Hepatitis B viral infection is a serious risk for healthcare workers. As mentioned, the alum-based vaccine has limitations, including poor response and cold-sensitive suspension. Alum also causes inflammation and local reactions at the injection site. The hepatitis vaccine is made from pure recombinant proteins, which unfortunately show little safety. Therefore, adjuvants are used to improving safety. So, AbdelAllah et al. used alginate adjuvant systems for better immunogenic and permitting to reduce in cost as well as dose [35]. Immunogenic response, as well as safety of the prepared adjuvants-based vaccine, were examined, compared with commercially available adjuvants, and good results were obtained.
Also, vaccine surface antigen adjuvanted with chitosan and alginate and a combination of them were tested in a mouse model. Mice were immunized with the subcutaneous vaccine and compared with control models without adjuvant. Seroconversion rate, serum antibody, IL-4, as well as IFN-γ levels were evaluated, and the outcomes exhibited higher immunogenic response for alginate/chitosan-based vaccine compared to alum-adjuvanted [36]. Chitosan and alginate are non-toxic and non-irritating natural polymers used in clinical trials and have advantages including outstanding immune stimulation, respectable tolerability, being safe and cheap, and positive clinical results. Considering the outcomes, the alginate/chitosan provided a meaningfully higher immunogenic response in comparison with controls.
Because influenza epidemics kill thousands around the world, so, improving the immunogenicity and performance of the vaccines is of great importance. In one study by Dehghan and co-workers, alginate nanoparticles were fabricated via ionic gelation technique and then, the influenza vaccine and adjuvants were incorporated into alginate nanoparticles [37]. Physico-chemical, as well as biological investigations, exhibited no influence of the encapsulation process on the construction and chemistry of protein. After injection, immune responses (humoral and cellular immune responses) were assessed in rabbits‘nostrils. Based on the obtained data, after a combination of CpG ODN (efficient adjuvant) with influenza virus, a strong humoral and cellular immune response was observed. Also, in another study, the hepatitis B vaccine and CpG ODN were inserted into alginate-coated chitosan to improve the immune response after the oral vaccination. High immunogenicity was observed after the injection. Higher values of the CD69 expression in CD4+ and CD8+ T-lymphocytes and lower values of this marker in B lymphocytes were observed [38].
For mucosal vaccine delivery, microfold and dendritic cells in lymphoid tissue can better take nanoparticulate antigens. Chitosan nanoparticles and derivatives (trimethylchitosan) are a proper carrier/adjuvant for this aim. By using anionic polymer such as alginate, immunostimulatory features and stability can enhance. So, in a study, inactivated PR8 influenza virus was inserted into chitosan and trimethylchitosan nanoparticles via direct virus coating. After nasal immunizations, the elicited IgG2a and IgG1 antibody titers in PR8/chitosan formulation were higher than PR8/trimethylchitosan. With coating, these formulations with alginate, Th1-type immune response (IgG2a/IgG1 ratio) for PR8/trimethylchitosan/alginate was higher than PR8/chitosan/alginate. Based on the outcomes, PR8/trimethylchitosan/alginate formulation was considered as an efficient carrier for nasal vaccines [15].
Katsikis et al. proposed a new strategy for delivering live H1N1 influenza vaccines that had the advantages of being safe, consuming small doses of virus, and faster than current vaccines [39]. It is created immunize by encapsulating vaccine in a polymer gel and releasing the virus in the body, once. In this study, through encapsulating of the vaccine in alginate, antigenicity of the vaccine was preserved, and strong robust CD8+ T cell responses were observed. The safety of subcutaneous vaccination was tested. This study verified that live virus encapsulated into alginate is a good choice for the development of universal CD8+ T cell vaccines during epidemic strains.
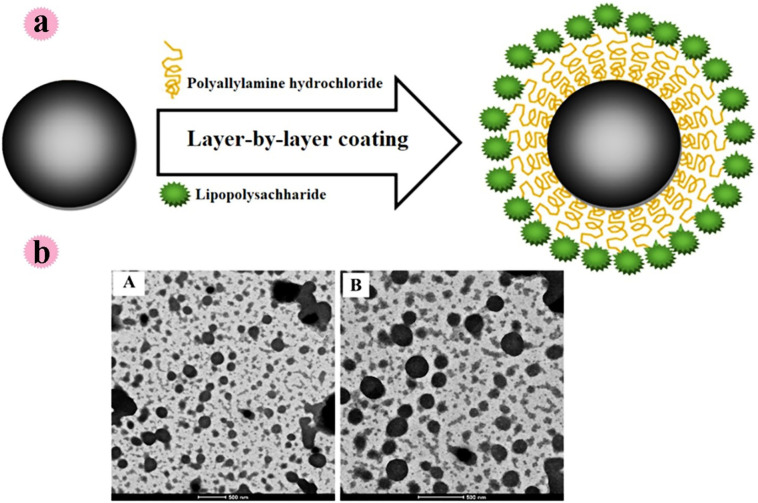
A strong immune response is produced by administering the vaccine to the mucosa. In a study by Saraf et al., for oral mucosal immunization, lipopolysaccharide adjuvant resulted in alginate-coated chitosan containing Hepatitis B surface antigen was fabricated. Alginate has been used to protect the stomach environment. The prepared spherical particles with a size of 605 nm (Zeta potential −26.2 mV) effectively protected the antigen in an acidic environment. The methodology for lipopolysaccharide anchoring with hepatitis B-alginate coated chitosan nanoparticles can be observed in Fig. 5a. Also, microscopic examinations for alginate coated chitosan and hepatitis B-alginate coated chitosan nanoparticles can see in this Fig. 5b. based on the outcomes, the prepared system showed good potential for delivery of vaccine in oral mucosal immunization [27].
Fig. 5.
a: Anchoring of LPS with chitosan nanoparticles using the layer-by-layer (LbL) technique, b: TEM images of chitosan nanoparticles: (A) LPS-HB-ACNPs; (B) ACNPs (Reproduced with permission from ref [27] Copyright 2020 Elsevier).
4. Application of hyaluronic acid during viral threats
Influenza viruses belong to the RNA virus family and are classified into three types (A, B, C) according to their antigenicity. Injectable influenza vaccines, which are used in the clinic, produce immunoglobulin G (IgG) in the blood and protect against viral infections. Indeed, one of the most effective ways to combat influenza epidemics is mucosal vaccination, which releases immunoglobulin A (IgA) on the mucosa along with the induction of immunoglobulin G (IgG) in the blood. In a study by Tanishita et al., hyaluronic acid was modified with tetraglycine-L-octaarginine, and then, mucosal adjuvant by using modified hyaluronic acid was prepared [40]. This research team performed the heterologous influenza A virus challenge to confirm the performance of this biopolymer as an adjuvant with cross-protective capacities. Immunoglobin is expected to prevent host infection. After nasal inoculation of inactivated viruses into mice, and then, exposure to infectious viruses, severe weight loss was observed. In fact, in the presence and absence of this biopolymer, mice were inoculated with inactivated viral particles. In mice inoculated with viral antigens and modified hyaluronic acid, viral infectious symptoms were difficult to observe due to the high induction of immunoglobulins that can interact with viruses. Based on the results, this polysaccharide derivative enables the host to increase immunity and the ability to protect against viral infection. So, this study showed that this biopolymer is a good candidate mucosal adjuvant to protect against viral infection.
Hyaluronic acid, a biodegradable biopolymer in the glycosaminoglycan category, is an extracellular matrix compound that is distributed throughout the body and degrades by enzymes (hyaluronidases). It is widely used in medicine as well as cosmetics. On the other hand, the conversion of the non-degradable platform into a degradable form is essential for clinical use. In another study, degradable cell-penetrating peptides were prepared to employ hyaluronic acid derivatives. To circumvent the latent risk of toxicity caused by the accumulation of doctaarginine-linked poly(N-vinylacetamide-co-acrylic acid) in the human body, the hyaluronic acid-based platform was prepared. In-vitro examines showed low toxicity of the hyaluronic acid derivative compared to poly(N-vinylacetamide-co-acrylic acid) derivatives. Overall, this study confirmed the safety of hyaluronic acid-based adjuvant for mucosal influenza vaccination [41].
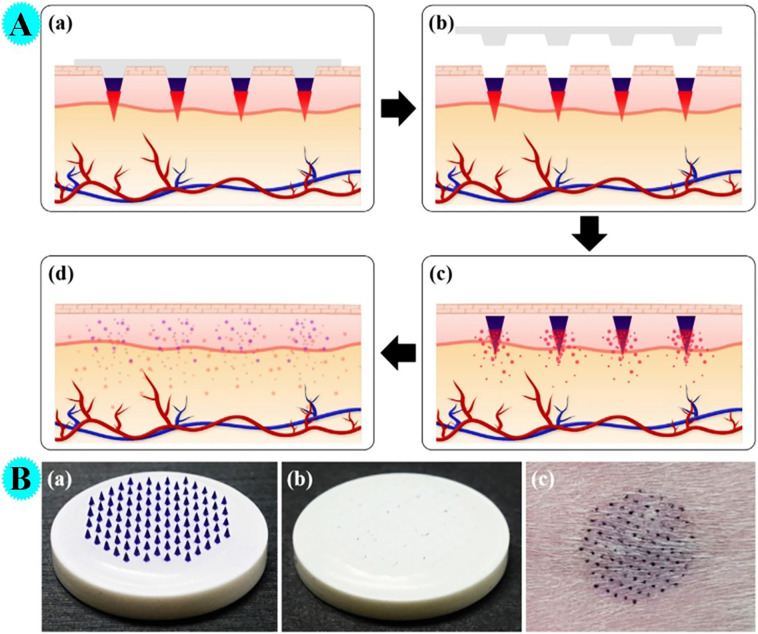
In a study by Choi et al. by using hyaluronic acid and polycaprolactone, microneedles (insertion-responsive microneedles) for transcutaneous influenza vaccination were prepared and the performance of them was evaluated (Fig. 6A) [42]. Vaccine antigens were covered on hyaluronic acid tips through fast freezing the tips before the dip-coating process. Ex-vivo experiments have shown that microneedles can penetrate the skin without fracture and release the drug properly. As can be observed in figure, vaccine-covered tips are easily removed from the base as soon as they are injected (Fig. 6B).
Fig. 6.
A: The working principle of drug-coated IRMNs. (a) Application of drug-coated IRMNs into skin. (b) Embedment of tips in the skin upon removal of bases. (c) Rapid dissolution of the drug-containing coating layer. (d) Complete dissolution of the tips. B: Optical images of vaccine-coated IRMNs. (a) IRMNs before insertion. (b) IRMNs after insertion showing successful separation of tips. (c) The site of IRMNs insertion on the guinea pig skin (Reproduced with permission from ref [42] Copyright 2018 Elsevier).
5. Gums as promising adjuvant and vaccine delivery systems
Compounds used as vaccine adjuvants are used to improve antigen immunogenicity. The most commonly used adjuvants approved by the Food and Drug Administration are aluminum-based salts. The formulation of new adjuvants is in progress. Nucleic acids, emulsions, or bacterial products have revealed the ability as an adjuvant, but preclinical trials exhibited not safe for human use. In fact, natural polymers play significant characters in the immune response and the creation of biocompatible formulations for vaccines. Natural polymers derived from plants and microbes are used as an adjuvant in vaccination. Xanthan gum, a natural polymer made from plants that has adhesive properties, is used in the pharmaceutical industry as a viscous. So far, it has been proven that this natural polymer can increase the immunity of recombinant antigens and make them resistant to pathogens. It has been used in bio-adhesive formulations for intranasal influenza virus immunizations. In a study, Schuch and coworkers studied the generated immune response by Xanthan adjuvant by using ovalbumin antigen. The induced immune response by this polymer associated with antigen in terms of antibody production and levels of IFN-γ was investigated. Also, the cytotoxicity of this natural polymer in rat fetal fibroblast cells was also investigated in-vitro and according to the results, no cytotoxicity was observed. Mice immunized with xanthan-antigen showed higher antibody IgG1 production compared to controls [43].
Adjuvants can be synthetic or natural components. These adjuvants associated with antigen can boost the immune system. Therefore, these adjuvants can reduce vaccine doses as well as antigen amounts. Eventually, the cost of producing the vaccine will be reduced. In a study, Silveira et al. evaluated the adjuvant performance of xanthan gum on the humoral and cellular immune response to DNA vaccines in mice. After intramuscular administration of the prepared vaccine, the level of antibodies showed an acceptable increase compared to the control sample. Also, IL-17 expression noteworthy was increased in vaccinated animals using xanthan gum. Based on the outcomes, in addition to enhancement in the immune response (humoral and cellular) by xanthan, due to the natural nature of this adjuvant, side effects are reduced [44].
Also, gums are effective in preventing influenza infection. Prevention of seasonal influenza infections is very important. During hospitalization, the elderly and those with an underlying disease may become infected with influenza and their health may be compromised. Short-chain fatty acids formed through intestinal bacteria via dietary fiber fermentation have been reported to affect the onset and severity of influenza. In one study, the relationship between probiotics (partially hydrolyzed guar gum PHGG) and the onset of influenza in hospitalized patients was studied. In this study, patients were divided into two groups: One group used PHGG consistently and the other group did not. According to the results of the analyses, people who got the influenza infection were in the group that did not use PHGG. These results showed that this PHGG has an effect on the intestinal environment and reduces the incidence of influenza [45].
In another work, Adeniran et al. examined natural gums (Cedrela and Khaya) as delivery agents for vaccines in veterinary diseases. In this examination, the numbers of chicks (200) were divided into 4 groups: A: IBDV (gums-infectious bursal disease virus) vaccine, B: just vaccine, C: just gums, D: nothing (no gum and vaccine). Hematological as well as histopathological responses were evaluated in this study. Based on the outcomes, chickens in the first group (A) showed better immunity and fewer clinical symptoms and there was no lesion in the limb after infection compared to the other groups. Severe clinical disease and lesions were observed in the latter group (D). Based on these results, natural gums have a good potential for the delivery of vaccines [46]. In another investigation, Mumin and coworkers, ex-vivo and in-vivo evaluated the mucoadhesive feature as well as the immunomodulatory influence of many gums on PPR (Peste des petits ruminants) vaccination in sheep and goats. The animals were divided into several categories and intranasally immunized by gum/vaccine combination. After vaccination, antibodies against the PPR virus were measured on different days. Antibodies were observed in all vaccinated species except the control sample. Based on the results, the 1:1 ratio of gum/vaccine combination showed a good effect [47].
The immune-potentiating feature of Cedrela odorata and Khaya senegalensis gums was investigated ex-vivo and in-vivo. Oyebanji et al. used these natural gums as delivery agents for chicken vaccination (oral and ocular routes) toward Newcastle disease [48]. Blood samples were collected and analyzed before and after the first vaccination at selected intervals. The lack of imbalance in hematological indicated the safety of delivery agents for vaccination of Newcastle disease vaccine.
6. The performance of β-glucan as adjuvant and vaccine delivery systems
As mentioned, vaccination is one of the utmost efficient strategies for preventing infectious diseases via the production of immunogenic antibodies. Vaccines and additives are effective in inducing cytotoxic lymphocytes as well as T cells, which are needed for fighting infection. A DNA vaccine is a known treatment applicant for the chronic hepatitis B vaccine, which, elicits a strong immune response in the host cell. For some pathogens, a vaccine that provides long-term immunity and the protective effect is challenging. β-glucan is a natural polymer found in the cell wall of bacteria and fungi and has been used as an adjuvant and vaccine development [49]. In immune modulation, as a pathogen-associated molecular pattern, β-glucan is recognized via transmembrane pattern recognition receptors. With the formation binding of Glucan-receptors phagocytosis of the glucan shell, the release of proinflammatory cytokines, chemokines, anti-microbial proteins have happened. In a study, Borges et al. showed the potential performance of glucan for preparation of delivery nanosystem as DNA vaccination platform [3].
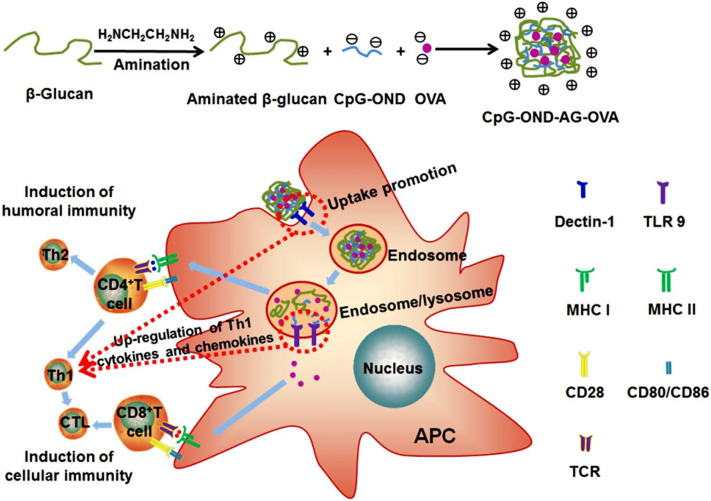
Currently, approved additives are not able to induce a cellular immune response. A proper vaccine adjuvant has many characteristics; as immunopotentiator for simulation of antigen-presenting cells for T cells activation, carrier for enhancement the uptake, sustained processing and antigens cytosolic delivery, being safe, as well as available. Aminated β-glucan (AβG) presents these roles, so, in a recent study, AβG nanoparticles were fabricated via ionic complexation technique. Antigens were encapsulated in it (AβG -Ovalbumin). Indeed, AβG acted as antigen-presenting cells targeted carrier and immunopotentiator. They were cross-linked with CpG-oligodeoxynucleotides as cross-linker and immunopotentiator (Fig. 7 ). After that, the adjuvant performance of these particles was estimated by in-vivo and in-vitro examinations. Based on the outcomes, the prepared particles showed strong immune responses (humoral and cellular) without toxicity. The strong adjuvant performance of the prepared particles toward infectious diseases was due to the synergistic impacts of aminated β-glucan and CpG-oligodeoxynucleotides [50].
Fig. 7.
Schematic illustration of the proposed dual targeting aminated b-glucan nanoparticles for induction of antigen specific immunity. Firstly, b-glucan was aminated, and then the nanoparticles were made from aminated b-glucan, CpG-OND and OVA by ionic complexation method. Having been vaccinated, the nanoparticles were internalized by APCs, and the uptake of the nanoparticles was mediated by dectin-1. Then, the nanoparticles were unfolded in acidic environment of endosome/lysosome, resulting in CpG-OND and OVA released from the nanoparticles. CpG-OND was recognized by TLR9 on endosome/lysosome. OVA in endosome/lysosome was loaded into MHC II where it can be presented to CD4+ through specific interaction with TCR on CD4+, inducing Th1 and Th2 responses. Th1 responses further promoted CTL responses. The OVA delivered to cytosol was loaded into MHC I where it can be presented to CD8+ through specific interaction with TCR on CD8+, inducing CTL responses. Synergistic stimulation of APCs by dectin-1 and TLR9 resulted in upregulation of Th1 cytokines and chemokines. Th1 cells and CTLs induced cellular immunity. Meanwhile, Th2 cells induced humoral immunity. The specific interaction of costimulatory molecules (CD80/CD86) on APCs with CD28 on CD4+/CD8+ was essential for induction of antigen specific immunity, and the expression levels of costimulatory molecules were also enhanced by synergistic stimulation of APCs by dectin-1 and TLR9 (Reproduced with permission from ref [50] Copyright 2018 Elsevier).
Natural polymers such as glucan as a template can be detected by receptors and provide a rapid and good immune response. Interestingly, the highest immune response is generated against cell wall polysaccharides such as glucan. Borges et al. examined the adjuvant potential of β-glucan -based particles as pathogen like adjuvant. In this study, the performance of chitosan particles, chitosan/glucan particles, and glucan particles were tested. After subcutaneous injection into mice with hepatitis B antigen, an increase in antibody was observed for all particles. However, just glucan particles could induce strong cytokine-mediated immunity. Indeed, glucan particles showed great performance as adjuvant and antiviral immunity components for the hepatitis B vaccine [49]. Also, in another study, a research team by combining natural polymers chitosan and glucan, prepared an adjuvant and studied their properties. For this aim, via the precipitation process, (precipitation of chitosan into an alkaline solution of glucan and genipin cross-link), chitosan:β-glucan particles were prepared. The prepared particles had benefits such as low cytotoxicity and high antigen loading. According to Mice vaccination studies, chitosan and chitosan: glucan particles showed a good adjuvant effect on hepatitis B antigen, however, the antibodies produced by chitosan: glucan was higher than chitosan particles [1].
7. Conclusions
The global scientific community is studying and preparing vaccines as the most effective solution to prevent SARS-CoV-2 infection, and control and spread the COVID-19. As stated in this study and summarized in Table 1 , natural polymers such as chitosan, alginate, gums, and so on have good potential for developing safe and new vaccines to prevent the spread of infectious diseases, including COVID-19 disease, due to their antiviral performance, non-toxic, and biocompatibility features. This mini-review presented the recent development in vaccine progress by using natural polymers which, provides a reference for their research on anti-COVID-19 drugs and vaccines.
Table 1.
Application of chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems.
| Ref | Results | vaccine adjuvants for: | Materials |
|---|---|---|---|
| [23] | 65–73.9% encapsulation efficiency, 27.7–67.5% loading capacity, sustained-release | Hepatitis E virus | Chitosan/truncated capsid protein p146 nanoparticles |
| [24] | Great antigen loading, remarkable stability, and enhanced immunogenicity effect | Hepatitis B virus | Chitosan/polyethylene glycol nanocomposite |
| [28] | Inexpensive antagonist toward attachment of coronavirus host cells | SARS-CoV-2 virus | Chitosan-coated DNA vaccine |
| [34] | Vaccines prepared with the help of alginate/chitosan improved the immunity (antibody levels were increased) | Hepatitis A virus | Alum-, chitosan-, and alginate/chitosan-based vaccine |
| [35] | The less expensive vaccine was prepared with a strong impact on the immune response | Hepatitis B virus | Chitosan/alginate |
| [36] | Higher immunogenic response for alginate/chitosan-based vaccine compared to Alum-adjuvanted | L. monocytogenes | Alginate/chitosan |
| [37] | Strong humoral and cellular immune response was observed | Influenza virus | Alginate nanoparticles |
| [38] | High immunogenicity was observed after injection | Hepatitis B virus | Alginate/chitosan |
| [15] | Improvement in immune response | Influenza virus | Trimethylchitosan/alginate |
| [39] | Improvement in immune response | H1N1 influenza virus | Alginate |
| [27] | Good potential for delivery of vaccine in oral mucosal immunization | Hepatitis B virus | Alginate |
| [40] | Increase immunity and the ability to protect against viral infection. | Influenza virus | Hyaluronic acid |
| [41] | Safety of adjuvant for mucosal influenza vaccination | Influenza virus | Hyaluronic acid |
| [42] | Release the drug properly without fracture | Influenza virus | Hyaluronic acid and polycaprolactone |
| [43] | Higher antibody IgG1 production compared to controls | Influenza virus | Xanthan gum/ovalbumin antigen |
| [44] | Acceptable increase in level of antibodies | DNA vaccine | Xanthan gum |
| [45] | and reduces in the incidence of influenza | Influenza virus | Partially hydrolyzed guar gum |
| [46] | Better immunity and fewer clinical symptoms | Infectious bursal disease virus | Natural gums (Cedrela and Khaya) |
| [50] | Strong immune responses (humoral and cellular) without toxicity | – | Aminated β-glucan |
| [49] | Antibodies produced by chitosan: glucan was higher than others | Hepatitis B virus | Chitosan and glucan |
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
We are immensely grateful for financial support from the Research Affairs Division of Isfahan University of Technology (IUT), Isfahan. I. R. Iran, and Iran Nanotechnology Initiative Council (INIC) Tehran, I. R. Iran. We would also like to show our gratitude to the National Elite Foundation (NEF), Tehran, I. R. Iran, and Center of Excellence in Sensors and Green Chemistry IUT.
References
- 1.Soares E., Jesus S., Borges O. Chitosan:β-glucan particles as a new adjuvant for the hepatitis B antigen. Eur. J. Pharm. Biopharm. 2018;131:33–43. doi: 10.1016/j.ejpb.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Abraham A., Ostroff G., Levitz S.M., Oyston P.C.F. A novel vaccine platform using glucan particles for induction of protective responses against Francisella tularensis and other pathogens. Clin. Exp. Immunol. 2019;198:143–152. doi: 10.1111/cei.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares E., Cordeiro R., Faneca H., Borges O. Polymeric nanoengineered HBsAg DNA vaccine designed in combination with β-glucan. Int. J. Biol. Macromol. 2019;122:930–939. doi: 10.1016/j.ijbiomac.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Miao J., Han X., Lu Y., Deng B., Lv F., Zhao Y., Ding C., Hou J. Development of a novel oil-in-water emulsion and evaluation of its potential adjuvant function in a swine influenza vaccine in mice. BMC Vet. Res. 2018;14:1–11. doi: 10.1186/s12917-018-1719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim J.W., Na W., Kim H.O., Yeom M., Park G., Kang A., Chun H., Park C., Oh S., Le V.P., Jeong H.H., Song D., Haam S. Cationic poly(amino acid) vaccine adjuvant for promoting both cell-mediated and humoral immunity against influenza virus. Adv. Healthc. Mater. 2019;8:1–8. doi: 10.1002/adhm.201800953. [DOI] [PubMed] [Google Scholar]
- 6.Genç R., Yakuboğullari N., Nalbantsoy A., Çöven F., Bedir E. Adjuvant potency of astragaloside vii embedded cholesterol nanoparticles for H3N2 influenza vaccine. Turk. J. Biol. 2020;44:304–314. doi: 10.3906/biy-2003-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee A.L.Z., Yang C., Gao S., Wang Y., Hedrick J.L., Yang Y.Y. Biodegradable cationic polycarbonates as vaccine adjuvants. ACS Appl. Mater. Interfaces. 2020;12:52285–52297. doi: 10.1021/acsami.0c09649. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X., Huang Y., Zheng C., Dong S., Liang W. Alginate-chitosan-PLGA composite microspheres enabling single-shot hepatitis B vaccination. AAPS J. 2010;12:519–524. doi: 10.1208/s12248-010-9213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan X., Zhou M., Yu S., Jin Z., Zhao K. An overview of biodegradable nanomaterials and applications in vaccines. Vaccine. 2020;38:1096–1104. doi: 10.1016/j.vaccine.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Bashiri S., Koirala P., Toth I., Skwarczynski M. Carbohydrate immune adjuvants in subunit vaccines. Pharmaceutics. 2020;12:1–33. doi: 10.3390/pharmaceutics12100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soares E., Jesus S., Borges O. Oral hepatitis B vaccine: chitosan or glucan based delivery systems for efficient HBsAg immunization following subcutaneous priming. Int. J. Pharm. 2018;535:261–271. doi: 10.1016/j.ijpharm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Vuong C.N., Kuttappan V.A., Faulkner O.B., Berghman L.R., Wolfenden A.D., Tellez-Isaias G., Jonas M., Kapczynski D.R., Hargis B.M., Bielke L.R. Comparison of oil emulsion, mannosylated chitosan, and Bacillus vector adjuvants for vaccination against influenza in chickens. J. Appl. Poult. Res. 2020;29:653–664. doi: 10.1016/j.japr.2020.04.003. [DOI] [Google Scholar]
- 13.Chen X., Han W., Wang G., Zhao X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020;164:331–343. doi: 10.1016/j.ijbiomac.2020.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wibowo D., Jorritsma S.H.T., Gonzaga Z.J., Evert B., Chen S., Rehm B.H.A. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials. 2021;268:120597. doi: 10.1016/j.biomaterials.2020.120597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosafer J., Sabbaghi A.H., Badiee A., Dehghan S., Tafaghodi M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 2019;14:216–221. doi: 10.1016/j.ajps.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M., Yang T., Jia J., Lu T., Wang H., Yan X., Wang L., Yu L., Zhao Y. Fabrication and characterization of DDAB/PLA-alginate composite microcapsules as single-shot vaccine. RSC Adv. 2018;8:13612–13624. doi: 10.1039/c8ra00013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raza Z.A., Taqi M., Tariq M.R. Antibacterial agents applied as antivirals in textile-based PPE: a narrative review. J. Text. Inst. 2021;0:1–13. doi: 10.1080/00405000.2021.1889166. [DOI] [Google Scholar]
- 18.Zhou X., Jiang X., Qu M., Aninwene G.E., Jucaud V., Moon J.J., Gu Z., Sun W., Khademhosseini A. Engineering antiviral vaccines. ACS Nano. 2020;14:12370–12389. doi: 10.1021/acsnano.0c06109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallakpour S., Azadi E., Hussain C.M. Chitosan/carbon nanotube hybrids: recent progress and achievements for industrial applications. New J. Chem. 2021:3756–3777. doi: 10.1039/d0nj06035f. [DOI] [Google Scholar]
- 20.Sharma N., Modak C., Singh P.K., Kumar R., Khatri D., Singh S.B. Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: a plausible molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021 doi: 10.1016/j.ijbiomac.2021.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallakpour S., Azadi E., Hussain C.M. Protection, disinfection, and immunization for healthcare during the COVID-19 pandemic: role of natural and synthetic macromolecules. Sci. Total Environ. 2021;776:145989. doi: 10.1016/j.scitotenv.2021.145989. [DOI] [Google Scholar]
- 22.Mallakpour S., Okhovat M. Hydroxyapatite mineralization of chitosan-tragacanth blend/ZnO/Ag nanocomposite films with enhanced antibacterial activity. Int. J. Biol. Macromol. 2021;175:330–340. doi: 10.1016/j.ijbiomac.2021.01.210. [DOI] [PubMed] [Google Scholar]
- 23.Wei W., Behloul N., Wang W., Baha S., Liu Z., Shi R., Meng J. Chitosan nanoparticles loaded with truncated ORF2 protein as an oral vaccine candidate against hepatitis E. Macromol. Biosci. 2021;2000375:1–10. doi: 10.1002/mabi.202000375. [DOI] [PubMed] [Google Scholar]
- 24.Namasivayam S. Karthick Raja, Nishanth A.N., Nivedh A.B.R.S.K., Syed N.H., R R.S. Hepatitis B-surface antigen (HBsAg) vaccine fabricated chitosan-polyethylene glycol nanocomposite (HBsAg-CS-PEG- NC) preparation, immunogenicity, controlled release pattern, biocompatibility or non-target toxicity. Int. J. Biol. Macromol. 2020;144:978–994. doi: 10.1016/j.ijbiomac.2019.09.175. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed S.H., Arafa A.S., Mady W.H., Fahmy H.A., Omer L.M., Morsi R.E. Preparation and immunological evaluation of inactivated avian influenza virus vaccine encapsulated in chitosan nanoparticles. Biologicals. 2018;51:46–53. doi: 10.1016/j.biologicals.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Dulce Bento S.J., Borges O., Lebre T.G. Filipa. Chitosan plus compound 48/80: formulation and preliminary evaluation as a hepatitis B vaccine adjuvant. Pharmaceutics. 2019;11:72. doi: 10.1016/j.imlet.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saraf S., Jain S., Sahoo R.N., Mallick S. Lipopolysaccharide derived alginate coated hepatitis B antigen loaded chitosan nanoparticles for oral mucosal immunization. Int. J. Biol. Macromol. 2020;154:466–476. doi: 10.1016/j.ijbiomac.2020.03.124. [DOI] [PubMed] [Google Scholar]
- 28.Tatlow D., Tatlow C., Tatlow S., Tatlow S. A novel concept for treatment and vaccination against Covid-19 with an inhaled chitosan-coated DNA vaccine encoding a secreted spike protein portion. Clin. Exp. Pharmacol. Physiol. 2020;47:1874–1878. doi: 10.1111/1440-1681.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrabi M., Dounighi N.M., Rezayat S.M. Novel approach to improve vaccine immunogenicity: Mannosylated chitosan nanoparticles loaded with recombinant hepatitis B antigen as a targeted vaccine delivery system. J. Drug Delivery Sci. Technol. 2018;44:19–26. doi: 10.1111/1440-1681.13393. [DOI] [Google Scholar]
- 30.Yue Yang P.L., Xing Ronge, Liu Song, Qin Yukun, Li Kecheng, Yu Huahua. Chitosan, hydroxypropyltrimethyl ammonium chloride chitosan and sulfated chitosan nanoparticles as adjuvants for inactivated Newcastle disease vaccine. Carbohydr. Polym. 2019;44:19–26. doi: 10.1016/j.jddst.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Renu S., Renukaradhya G.J. Chitosan nanoparticle based mucosal vaccines delivered against infectious diseases of poultry and pigs. Front. Bioeng. Biotechnol. 2020;8:558349. doi: 10.3389/fbioe.2020.558349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallakpour S., Behranvand V., Mallakpour F. Adsorptive performance of alginate/carbon nanotube-carbon dot-magnesium fluorohydroxyapatite hydrogel for methylene blue-contaminated water. J. Environ. Chem. Eng. 2021;9:105170. doi: 10.1016/j.jece.2021.105170. [DOI] [Google Scholar]
- 33.Hans N., Malik A., Naik S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: mini review. Bioresour. Technol. Rep. 2021;13:100623. doi: 10.1016/j.biteb.2020.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AbdelAllah N.H., Gaber Y., Rashed M.E., Azmy A.F., Abou-Taleb H.A., AbdelGhani S. Alginate-coated chitosan nanoparticles act as effective adjuvant for hepatitis A vaccine in mice. Int. J. Biol. Macromol. 2020;152:904–912. doi: 10.1016/j.ijbiomac.2020.02.287. [DOI] [PubMed] [Google Scholar]
- 35.Abdelallah N.H., Abdeltawab N.F., Boseila A.A., Amin M.A. Chitosan and sodium alginate combinations are alternative, efficient, and safe natural adjuvant systems for hepatitis B vaccine in mouse model. Evid. Based Complement. Alternat. Med. 2016;2016:7659684. doi: 10.1155/2016/7659684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocha C.E.V., Silva M.F., Guedes A.C.B., Carvalho T.P., Eckstein C., Ribeiro N.Q., Santos D.A., Melo M.M., Araújo M.S.S., Martins-Filho O.A., Santos R.L., Paixão T.A. Alginate-chitosan microcapsules improve vaccine potential of gamma-irradiated Listeria monocytogenes against listeriosis in murine model. Int. J. Biol. Macromol. 2021;176:567–577. doi: 10.1016/j.ijbiomac.2021.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Dehghan S., Kheiri M.T., Abnous K., Eskandari M., Tafaghodi M. Preparation, characterization and immunological evaluation of alginate nanoparticles loaded with whole inactivated influenza virus: dry powder formulation for nasal immunization in rabbits. Microb. Pathog. 2018;115:74–85. doi: 10.1016/j.micpath.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Borges O., Tavares J., de Sousa A., Borchard G., Junginger H.E., Cordeiro-da-Silva A. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. Eur. J. Pharm. Sci. 2007;32:278–290. doi: 10.1016/j.ejps.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Boesteanu A.C., Babu N.S., Wheatley M., Papazoglou E.S., Katsikis P.D. Biopolymer encapsulated live influenza virus as a universal CD8+ T cell vaccine against influenza virus. Vaccine. 2010;29:314–322. doi: 10.1016/j.vaccine.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanishita S., Ukawa M., Tomono T., Yoshida Y., Tsujioka T., Miyata K., Tobita E., Uto T., Baba M., Sakuma S. Cross-protective abilities of hyaluronic acid modified with tetraglycine-l-octaarginine as a mucosal adjuvant against infection with heterologous influenza viruses. Bioconjug. Chem. 2019;30:3028–3037. doi: 10.1021/acs.bioconjchem.9b00644. [DOI] [PubMed] [Google Scholar]
- 41.Ukawa M., Tanishita S., Yagi H., Yoshida Y., Tomono T., Shigeno K., Tobita E., Uto T., Baba M., Sakuma S. Biodegradable hyaluronic acid modified with tetraglycine-l-octaarginine as a safe adjuvant for mucosal vaccination. Mol. Pharm. 2019;16:1105–1118. doi: 10.1021/acs.molpharmaceut.8b01110. [DOI] [PubMed] [Google Scholar]
- 42.Choi I.J., Kang A., Ahn M.H., Jun H., Baek S.K., Park J.H., Na W., Choi S.O. Insertion-responsive microneedles for rapid intradermal delivery of canine influenza vaccine. J. Control. Release. 2018;286:460–466. doi: 10.1016/j.jconrel.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Schuch R.A., Oliveira T.L., Collares T.F., Monte L.G., Inda G.R., Dellagostin O.A., Vendruscolo C.T., Angelita Da Silveira M., Hartwig D.D. The use of xanthan gum as vaccine adjuvant: an evaluation of Immunostimulatory potential in BALB/c mice and cytotoxicity in vitro. Biomed. Res. Int. 2017;2017:3925024. doi: 10.1155/2017/3925024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silveira M.M., Conceiçáo F.R., Mendonga M., Moreira G.M. Schmidt Garcia, da Cunha C.E. Pouey, Rizzi C., Hartwig D.D., da Silveira Moreira A., Vendrusculo C.T., Moreira A.N. Biopolymer xanthan: a new adjuvant for DNA Vaccines. Braz. Arch. Biol. Technol. 2020;63:1–7. doi: 10.1590/1678-4324-2020190090. [DOI] [Google Scholar]
- 45.Takahashi C., Kozawa M. The effect of partially hydrolyzed guar gum on preventing influenza infection. Clin. Nutr. ESPEN. 2021;42:148–152. doi: 10.1016/j.clnesp.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Adeniran G.A., Jarikre T.A., Ola O.O., Adigun O., Odeniyi M.A., Emikpe B.O. Clinical and pathological responses of broilers to ocular vaccination using plant gum delivery and challenge with infectious bursal disease virus. Comp. Clin. Pathol. 2020;29:721–727. doi: 10.1007/s00580-020-03122-y. [DOI] [Google Scholar]
- 47.Mumin F.I., Emikpe B.O., Odeniyi M.A. Evaluation of mucoadhesive property and the effect of Boswellia carteri gum on intranasal vaccination against small ruminant morbillivirus infection (PPR) J. Immunoass. Immunochem. 2020;41:311–321. doi: 10.1080/15321819.2020.1734935. [DOI] [PubMed] [Google Scholar]
- 48.Oyebanji V.O., Jarikre T.A., Jagun-Jubril A., Adeniran G.A.A., Emikpe B.O. Haematological changes associated with Newcastle disease vaccination in chickens using gums from Cedrela odorata and Khaya senegalensis as delivery agents. Niger. J. Physiol. Sci. 2020;35:167–171. [PubMed] [Google Scholar]
- 49.Soares E., Groothuismink Z.M.A., Boonstra A., Borges O. Glucan particles are a powerful adjuvant for the HBsAg, favoring antiviral immunity. Mol. Pharm. 2019;16:1971–1981. doi: 10.1021/acs.molpharmaceut.8b01322. [DOI] [PubMed] [Google Scholar]
- 50.Jin J.W., Tang S.Q., Rong M.Z., Zhang M.Q. Synergistic effect of dual targeting vaccine adjuvant with aminated β-glucan and CpG-oligodeoxynucleotides for both humoral and cellular immune responses. Acta Biomater. 2018;78:211–223. doi: 10.1016/j.actbio.2018.08.002. [DOI] [PubMed] [Google Scholar]