Abstract
The current trend worldwide is searching plant extracts towards prevention of neurodegenerative disorders. This study aimed to investigate the neuroprotective effect of Alpinia galanga leaves (ALE), Alpinia galanga rhizomes (ARE), Vitis vinifera seeds (VSE), Moringa oleifera leaves (MLE), Panax ginseng leaves (PLE) and Panax ginseng rhizomes (PRE) ethanolic extracts on human neuroblastoma (SHSY5Y) cells. The 1-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging of VSE and MLE were 81% and 58%, respectively. Ferric-reducing antioxidant power (FRAP) of ALE and MLE (33.57 ± 0.20 and 26.76 ± 0.30 μmol Fe(ΙΙ)/g dry wt., respectively) were higher than for the other extracts. Liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (LC-QTOF/MS) revealed MLE active compounds. Intracellular study by nitro-blue tetrazolium (NBT) test showed that MLE and VSE had high O2− scavenging (0.83 ± 0.09 vs. 0.98 ± 0.08 mg/mL, respectively). MLE had the highest ROS scavenging followed by PRE (0.71 ± 0.08 vs. 0.83 ± 0.08 mg/mL, respectively), by 2,7-dichlorodihydrofluorescein diacetate (DCFHDA) assay. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity and neuroprotection tests on SHSY5Y showed that PRE had a better neuroprotective effect but higher cytotoxicity compared to MLE (viable cells 51% vs. 44%, IC50 1.92 ± 0.04 vs. 2.7 ± 0.2 mg/mL, respectively). In conclusion, among the studied plants, MLE has potential for developing as a neuroprotective agent.
Keywords: Moringa oleifera, ROS, H2O2, oxidative stress, superoxide anion, SHSY5Y neuroblastoma cell line
1. Introduction
Reactive oxygen species (ROS) are a group of oxygen-containing molecules that are highly reactive due to the presence of unpaired electrons; ROS mainly include hydroxyl radical (OH•), superoxide anion (O2−), and hydrogen peroxide (H2O2). There are many factors that affect ROS production in cells. The key producer of ROS is environmental stress, and ROS are a common byproduct of the normal metabolic pathway of oxygen molecules. Increased ROS levels either outside or inside the cells result in significant damage to all biological macromolecules, namely lipids, proteins, and nucleic acids. Cells naturally generate ROS such as O2− and H2O2 through one-electron reduction of oxygen to O2− catalyzed by an NADPH or NADH oxidase utilizing NADPH or NADH as electron donors. Part of the O2− is converted to H2O2 via spontaneous or enzyme-facilitated dismutase; this reaction is called oxidative burst. The H2O2 causes significant physiological and pathological effects due to its being highly diffusible and its ability to cross the plasma membrane [1]. Oxidative stress, achieved by the accumulation of ROS, is suggested to be an initiator of neurodegenerative disease.
The brain is an organ that is prone to producing ROS (such as O2−, H2O2, and OH•) due to its requiring a large quantity of oxygen to maintain its normal function. Furthermore, neuronal cells are more sensitive to oxidative stress than the cells in other types of tissue. Therefore, the inhibition of oxidative stress is considered to be a strategy to prevent neuronal progressive disease [2]. Oxidative stress leads to apoptotic neuronal cell death by initiating mitochondrial dysfunction, which causes neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease; so far, no potent drugs that fully prevent neuronal cell death in neurodegenerative diseases are available [3]. The qualified preservation process of neuronal structure and function is called neuroprotection; its aim is to inhibit or delay disease progression and nerve cell damage by preventing or reducing the loss of neurons [1]. In fact, dietary polyphenols have been shown to display powerful neuroprotective effects as antioxidants. These dietary polyphenols are interesting due to their role in suppressing the oxidation of proteins, lipid peroxidation, and the generation of ROS in diverse in vitro and in vivo models of neurological disorders [4].
Many of the therapeutic strategies that focus on the prevention of the ROS formation mediated by antioxidants seem to have an impact on delaying the disease’s progression. Numerous synthetic antioxidants have been established to be potent radical scavengers, but they are also mutagenic and cause cell damage. Therefore, much attention has recently been paid to antioxidants from natural origins with neuroprotective effects. Various plant species in different regions of the world have been found to be rich sources of several bioactive compounds with potential health benefits. Phenolic compounds, which mainly exist in vegetables, fruits, and dry fruits, are natural antioxidants and are the biggest group of potent antioxidants [5]. Phenolic compounds are classified as “chain breaking antioxidants” due to their ability to chelate transition metal ions, thereby inhibiting oxidative chain reactions in cells [6]. They have been reported to quench free radicals by donating a hydrogen atom and/or an electron to free radicals.
Many plants rich in phenolic compounds are consumed almost daily by Asian people in their diet; selecting and investigating the antioxidant effects of the most commonly used plants will provide a source of natural, reliable, cheap, and safe neuroprotection compounds for everyone. The traditional use of Moringa oleifera is reported including for anti-coagulation for snake bites, induction of breast milk production, and hair care products by delivering nutrients to the hair follicles [7]. Alpinia galanga is commonly used as a medicinal plant to treat various diseases such as chest pain, rheumatic pains, fever, kidney disease, burning of the liver, and dyspepsia. The seed of A. galanga is used for cleaning the mouth, stimulating digestive power, and inducing appetite. Its rhizome is usually used as a spice in Asian communities and consider a rich source of essential oil [8]. In various countries, Vitis vinifera are often found in wine, juice, and raisins as well as rootstocks [9]. Panax ginseng is popular for aging therapy, psychiatric complications, and remedy disorders of the digestive system [10]. In this study, M. oleifera leaves, leaves and rhizomes of A. galanga, V. vinifera seeds, and leaves and rhizomes of P. ginseng were selected for investigation of their antioxidant capabilities and neuroprotective antioxidant activities on a neuroblastoma cell line (SHSY5Y).
SHSY5Y cell line is a subline of the SK-N-SH cell line, established in 1970 from a bone marrow biopsy of a neuroblastoma tumor of a four-year-old female in the metastatic stage that underwent three rounds of clonal selection [11]. In addition, the active compounds contained in M. oleifera leaves extract (MLE) related to antioxidant capacity were elucidated by liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (LC–QTOF/MS). This is the first report on the investigation of M. oleifera cultivated in Thailand in both antioxidant actions (capacity and activity) as well as providing profile analysis of valuable phenolic compounds, particularly flavonoids. This information could pave the way for further study of their antioxidant activities and effects as a single compound on neuroprotective agents that exhibit significance for mental health protection, particularly those for neurodegenerative disorders induced by oxidative stress.
2. Materials and Methods
2.1. Plant Materials
Leaves and rhizomes of the selected plant species A. galanga, and P. ginseng and leaves of M. oleifera were obtained from local gardens in Khon Kaen, Thailand in October 2017, whereas V. vinifera seeds were provided by Visootha (Nikki) Lohinavy of GranMonte Asoke Valley Winery from a winemaking factory in Pak Chong, Nakorn Rachasima, Thailand. Plant materials were cleaned, dried at 65 °C using a hot air oven (Model FD240, Binder, Frankfurt, Germany), and crushed using a Philips mill (Model 600W, Eindhoven, The Netherlands). The ground materials were kept in screwcap containers at room temperature away from the sun until the beginning of the extraction process.
2.2. Preparation of Plant Extracts
All plant samples were subjected to extraction using ethanol with a ratio of 1:4 and stirring with a magnetic stirrer for 8 h, then were filtrated using Whatman No. 1 filter paper (Camlab, Cambridge, UK). V. vinifera seeds [12,13], M. oleifera leaves [14,15], and P. ginseng leaves and rhizomes [16,17] were extracted with 70% ethanol, whereas A. galanga leaves and rhizomes were extracted with 95% ethanol [18,19]. Each sample was concentrated using a rotary evaporator (Model Heidolgh VV2000, Heidolph Instruments Gmbh, Schwabach, Germany). The A. galanga rhizomes extract (ARE) consisted of two layers, with a pale yellow upper layer (PYL) with oily characteristics and a brown lower layer (BL). A. galanga leaves extract (ALE), P. ginseng leaves and rhizomes extracts (PLE and PRE), V. vinifera seeds extract (VSE) and M. oleifera leaves extract (MLE) were obtained in powder form while ARE (both BL and PYL) was in semi-solid form due to the presence of essential oil. Each extract was kept separately in screwcap vials at 4 °C.
2.3. Determination of Antioxidant Capacity
2.3.1. Scavenging of the 1,1-Diphenyl-1-Picrylhydrazyl Radical (DPPH)
The estimation of the free DPPH radical system was performed as described in [5]. The reaction was prepared by adding 2.94 mL of 0.1 mM DPPH in methanol into 60 µL of each plant extract, with a final concentration of 200 µg/mL compared to methanol as negative control and gallic acid as positive control (with 95% DPPH scavenging). The reaction mixture was incubated in a dark place for 30 min, then vigorously shaken for 20 s. The decrease in absorbance at 516 nm was recorded using a UV Probe-1800 spectrophotometer (Shimadzu, Kyoto, Japan). The percentage of DPPH radical scavenging was calculated using the following equation:
| % DPPH scavenging = [(Ab0 − Ab1/Ab0)] × 100 | (1) |
where Ab0 is the absorbance of the control (no extract) and Ab1 is the absorbance of the test extract.
2.3.2. Ferric-Reducing Antioxidant Power (FRAP)
The FRAP assay was achieved according to [20] with some modifications. The FRAP solution was prepared by mixing 25 mL of 300 mM acetate buffer, pH 3, with 2.5 mL of 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) in 40 mM HCl and 2.5 mL of 20 mM of Iron(III) chloride hexahydrate (FeCl3. 6H2O). Distilled water was used as a negative control while Trolox was used as a positive control (with 0.03 ± 0.1 mg/mL for ferric-reducing power). The reaction was started by adding 100 µL of crude extract into 2 mL of the FRAP working solution and kept for 30 min in dark conditions. The absorption of the ferrous tripyridyltriazine complex was recorded at 593 nm. The standard curve was obtained by various concentrations of ferrous sulfate ranging from 5 to 100 µM/mL. The results of FRAP were expressed in μmol Fe (ΙΙ)/g dry weight.
2.4. Neuroblastoma Cell Cultures (SHSY5Y)
The SHSY5Y cell line (human neuroblastoma) (The American Type Culture Collection (ATCC®), CRL2266™, Manassas, VA, USA) was obtained from the Institute of Biotechnology and Genetic Engineering, Chulalongkorn University, Bangkok, Thailand. The medium DMEM/F-12 containing Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F-12 Nutrient Mixture at a ratio of 1:1 was used in the SHSY5Y culture. Cells were grown in DMEM/F-12 medium containing 1 mM sodium pyruvate, 0.1 mM nonessential amino acid, 1.5 g/L sodium bicarbonate, 100 U/mL penicillin, and 100 µg/mL streptomycin supplemented with 10% heat-activated fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA) and incubated at 37 °C in an incubator with 85% humidified atmosphere containing 5% CO2.
2.4.1. Culturing and Harvesting of the SHSY5Y Cells
SHSY5Y cells in a culture flask were trypsinized and re-suspended in the medium, and 100 µL of 2 × 104 cells/well were plated in 96-well culture plates. After 24 h, cells were used for further investigations.
2.4.2. Cytotoxicity of Plant Extracts on the SHSY5Y Cell Line
The cellular viability assay described in [21,22] was used to investigate cytotoxicity on the SHSY5Y cell line. Incubated cells as described in the previous step were exposed to 100 µL of crude extracts with concentrations ranging from 0.25 to 4 mg/mL. The negative control did not contain an extract. Cells were incubated in 5% CO2 atmosphere at 37 °C for 24 h. Then, the medium was removed, 100 µL of 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in Dulbecco’s phosphate buffer saline (DPBS) was added, and cells were incubated for an additional 4 h. Finally, MTT solution was gently removed and 100 µL of dimethyl sulfoxide (DMSO) was added to dissolve crystal formazan. Triton X-100 was used as positive control (considered as 100% cell death). The plate was shaken for 1 min, and the absorbance of formazan in each well was measured in a microplate reader at 570 nm. The percentage of cytotoxicity compared with the negative control (untreated cells, considered as 100% cells viability) was calculated according to the following equation:
| Cytotoxicity % = (Ab. of control cells − Ab. of treated cells)/(Ab. of control cells) × 100 | (2) |
The plot of percent cytotoxicity versus sample concentration was used to calculate the extract concentration that killed 50% of the cells (IC50).
2.4.3. Neuroprotection of Plant Extracts on the SHSY5Y Cell Line
H2O2 was used to induce oxidative stress in this study. SHSY5Y cells were treated with H2O2 in the range of 100–500 μM and incubated for 24 h, and 100 μL of 250 μM H2O2, which inhibited 70%–80% cell viability, was selected to examine the neuroprotective effects of plant extracts. To assess the dose-dependent neuroprotective effects of the plant extracts against oxidative-stress-induced neuron cells, cells were pretreated with each tested plant extract (IC50 was selected based on MTT assay) for 6 h and then exposed to 250 μM H2O2 for 1 h. The cytotoxic effect was calculated as a percentage of viability when compared with untreated cells (negative control considered as 100% of viability) and cells treated with 250 μM H2O2 for 1 h (positive control considered as 100% of cell death) [4]. After each assay, cell viability was measured using the MTT assay as described in Section 2.4.2.
2.5. Determination of Intracellular O2− and ROS inside the SHSY5Y Cell Line
2.5.1. Determination of O2− by Nitro-Blue Tetrazolium (NBT) Reduction Test
The reduction of NBT to insoluble blue formazan was used as a probe for O2− generation inside the viable cells according to the method in [23] with slight modification. Supernatants of SHSY5Y cells described in Section 2.4.1 were eliminated and replaced by a medium with different concentrations (0.25, 0.5, 1, 2, and 4 mg/mL) of tested plant extracts, then 15 µL of 1% NBT solution was added. Untreated cells (negative control: considered as moderate absorbance) and cells treated with 200 μM H2O2 for 1 h (positive control: considered as massive absorbance). Following 3 h incubation, the supernatants were removed, and the formation of formazan product was solubilized in 100 µL DMSO. After mixing the contents in each well, the absorption of colored formazan solution was read at 620 nm using a Varioskan LUX multimode microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
2.5.2. Determination of Intracellular ROS by DCFHDA Assay
To investigate the formation of ROS inside SHSY5Y cell line with and without plant extracts, 2,7-dichlorodihydrofluorescein diacetate (DCFHDA) was used [24]. Briefly, SHSY5Y cells were incubated with various concentrations of plant extract (0.25, 0.5, 1, 2, and 4 mg/mL) to induce the antioxidant system in cells using bioactive compounds. After 3 h of incubation, cells were washed with Dulbecco’s phosphate buffer saline (DPBS) and then incubated for 60 min in 10 μM DCFHDA (Molecular Probes, Invitrogen, Basel, Switzerland) in complete medium at 37 °C and 5% CO2. After the washing step with DPBS, the cells were treated with 200 µM of H2O2 for 2 h to undergo intracellular oxidation. Untreated cells were negative control (considered as moderate absorbance) and cells treated with 200 μM H2O2 for 1 h were positive control (considered as massive absorbance). Fluorescence intensity was measured using the Varioskan LUX multimode microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) at an excitation wavelength of 485 nm and an emission wavelength of 528 nm.
SHSY5Y cells were incubated separately with different volumes of DMSO (solvent used for solubilizing plant extracts) to check the viability of cells.
2.6. Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometer (LC–QTOF/MS) Analysis
A MLE sample with a concentration of 101.23 mg/mL in 50% methanol was prepared, ultrasonicated for 20 min, and centrifuged at 10,000 rpm at 4 °C for 10 min. The supernatant was filtrated with 0.2 µm nylon membrane and injected into liquid chromatography coupled with a quadrupole time-of-flight mass spectrometer (LC–QTOF/MS) (1290 Infinity II LC-6545 Quadrupole-TOF, Agilent Technologies, Santa Carla, CA, USA). The liquid chromatographic system consisted of a binary pump and an online vacuum degasser connected to a Dual AJS ESI source mass spectrometer (Agilent Technologies, Santa Carla, CA, USA). Full-scan mode was used from m/z 100 to 1700. Zorbax Eclipse Plus column (Agilent Technologies, Santa Carla, CA, USA) (C18 2.1 × 150 mm, 1.8 µm) was used for the analysis. Formic acid 0.1% in distilled water (solvent A) and 100% acetonitrile (solvent B) were used as the mobile phase-gradient elution as follows: 98% A, 0–2 min; 90% A, 2–25 min; 85% A, 25–40 min; 80% A, 40–48 min; 75% A, 48–68 min; 70% A, 68–80 min; 50% A, 80–85 min; 0% A, 85–90 min; 98% A, 90–100 min. Peaks were detected at wavelengths of 254 and 280 nm. The MS spectra were acquired in both positive and negative ion modes, auto MS/MS. The mass fragmentations were identified using the spectrum database for organic compounds in the METLIN database (a cost-free reachable web-based data source, has been developed to facilitate in a wide range of metabolite research and to assist natural products identification through mass analysis).
2.7. Statistical Analysis
All investigations were conducted in this study in triplicate (n = 3). Data were reported as mean ± SD values. Analysis of variant (ANOVA) was used to evaluate the differences among the treatments by Duncan’s multiple range tests (DMRTs) with p value of 0.05 using SPSS Statistics Base version 19 program for Windows (IBM Corp, Chicago, IL, USA).
3. Results and Discussion
In the present study, the antioxidant capacities and neuroprotective activities of the four selected Asian plants—M. oleifera, A. galanga, V. vinifera, and P. ginseng—were investigated. Different parts of these plants were extracted with 70% and 95% ethanol, which is a polar solvent. Therefore, the compounds in the plant extracts were those with high polarity. The extracts were assayed for their antioxidant capacity by FRAP and DPPH methods. The DPPH assay is achieved based on both hydrogen atom transfer (HAT) and single electron transfer (SET) mechanisms [25]. VSE gave the highest activity in quenching DPPH radicals, followed by MLE, with 81% and 58%, respectively (Table 1). The ability of VSE and MLE extracted with 70% ethanolic solvent in scavenging DPPH radicals agreed with the results obtained by [26,27,28], who reported that catechin and epicatechin are the most abundant compounds among phenolic components in VSE. Luteolin seems to play a role in the antioxidant capacity of MLE [29]. Table 1 also shows that ALE gave the highest activity of FRAP, followed by MLE (33.57 and 26.76 μmol Fe (ΙΙ)/g dry wt., respectively). VSE showed moderate ferric reducing activity, with 19.45 μmol Fe (ΙΙ)/g dry wt. PRE, PLE, and ARE (PYL) had low FRAP activity, which implied that these have the lowest electron donation ability. The FRAP activity of ALE may be explained by the presence of the galango flavonoid, which was reported in ethanolic extract of the galanga plant. Previous study reported that galango flavonoids are effective in free radicals scavenging as well as metal chelating activity [30]. Meanwhile, dried MLE provide a rich source of polyphenolic compounds such as phenolic acids and flavonoids; both subgroups are characterized as highly effective antioxidant molecules [31]. Although the ranking of the tested extracts’ capacities varied in both (DPPH and FRAP) tests, MLE holds on as the second most active performer.
Table 1.
Cellular viability and antioxidant capacity and activity of selected plant extracts estimated by various tests (1,1-diphenyl-1-picryl hydrazyl (DPPH), ferric-reducing antioxidant power (FRAP), nitro-blue tetrazolium (NBT), intracellular reactive oxygen species (ROS) level by 2,7-dichlorodihydrofluorescein diacetate (DCFHDA), and neuroprotection activity).
| Plant Extract | DPPH (Inhibition %) |
FRAP (μmol/g) | Cytotoxicity by MTT Assay (IC50 mg/mL) | NBT Reduction Test (IC50 mg/mL) | Intracellular ROS Levels by DCFHDA (IC50 mg/mL) | Neuroprotection (% Cell Viability) |
|---|---|---|---|---|---|---|
| MLE | 58 | 26.76 ± 0.3 e | 2.7 ± 0.2 a | 0.83 ± 0.09 c | 0.71 ± 0.08 c | 44 |
| VSE | 81 | 9.45 ± 0.08 d | 2.43 ± 0.1 b | 0.98 ± 0.08 c | 1.24 ± 0.06 a | 38 |
| PLE | 0.05 | 7.46 ± 0.2 a | 1.96 ± 0.02 c | 1.65 ± 0.04 a | 1.34 ± 0.06 a | 34 |
| PRE | 2 | 10.05 ± 0.08 c | 1.92 ± 0.04 c | 1.22 ± 0.06 b | 0.83 ± 0.08 c | 51 |
| ALE | 41 | 33.57 ± 0.2 g | 2.53 ± 0.1 b | 1.24 ± 0.05 b | 2.48 ± 0.03 d | 32 |
| ARE (PYL) | 0.01 | 5.62 ± 0.02 b | 0.34 ± 0.02 d | 1.62 ± 0.1 a | 0.96 ± 0.1 b | 40 |
| ARE (BL) | 31 | 24.19 ± 0.1 f | ND | ND | ND | ND |
The data are expressed as mean ± SD (n = 3) while for DPPH and neuroprotection results expressed in cell viability percentage, (μmol/g): μmol of Fe (ΙΙ)/g of dry weight plant extract). ND (not determined): extract excluded from viable system (SHSY5Y) experiments due to high cytotoxicity. a, b, c, d, e, f, g are significant differences with p-value (<0.05). MLE: M. oleifera leaves extract; VSE: V. Vinifera seeds extract; PLE: P. ginseng leaves extract; PRE: P. ginseng rhizomes extract; ALE: A. galanga leaves extract; ARE (PYL): A. galanga rhizomes extract (pale yellow layer); ARE (BL): A. galanga rhizomes extract (brown layer).
According to its high antioxidant capacity in scavenging DPPH radicals and FRAP activity, MLE was selected for further investigation of its phytochemical contents by LC–QTOF/MS analysis. The results in Table 2 reveal that MLE contained barbatoflavan, isorhamnetin, quercetin derivatives, kaempferol derivative, caffeoylquinic acid, coumaroyltrifolin, naringenin, pheophytin, and vitexin. These compounds have the ability to scavenge DPPH and have reducing power (FRAP) by improving hydrogen-atom-transfer (HAT) and electron-donation-based pathways.
Table 2.
List of compounds identified in MLE by LC–QTOF/MS analysis.
| Compound | Molecular Formula | Retention Time (min) | Candidate Mass | Effect | Reference |
|---|---|---|---|---|---|
| Barbatoflavan | C24H28O13 | 33.051 | 525.15 | -Established scavenging properties toward the DPPH radicals | [41] |
| Isorhamnetin | C16H12O7 | 83.499 | 315.05 | -Scavenging DPPH free radical | [42] |
| Quercetin | C15H10O7 | 48.551 | 303.04 | -Scavenging O2− and DPPH radicals | [43] |
| Quercetin 3-(6″-malonylglucoside)-7-rhamnoside | C30H32O19 | 39.686 | 697.16 | -Scavenging O2− and DPPH radicals | [43] |
| Quercetin 3-O-(6-O-malonyl-β-d-glucoside) | C24H22O15 | 51.250 | 549.08 | -Scavenging O2− and DPPH radicals | [43] |
| Quercetin 3-methyl ether | C16H12O7 | 53.451 | 317.06 | -Free radical scavenging -Inhibitory effect on O2− generation |
[44,45] |
| Kaempferol 3-[6‴-p-coumarylglucosyl-(1→2)-rhamnoside] | C36H36O17 | 61.466 | 741.20 | -Improves the potency of the hydrogen-atom-transfer (HAT)-based pathways | [46] |
| 4,5-Di-O-caffeoylquinic acid | C25H24O12 | 69.978 | 515.11 | -Ability to protect cells by conducting electron transfer (ET), H+ transfer, and Fe2+ chelation | [47] |
| 6″-O-p-Coumaroyltrifolin | C30H26O13 | 72.336 | 593.12 | -Improving the ET- and HAT-based pathways -Boosts Fe2+ -Chelating ability |
[48] |
| (±)-Naringenin | C15H12O5 | 78.072 | 271.06 | -Electron-donating substituents via weakening the bond dissociation enthalpy (BDE) | [49,50] |
| Pheophytin | C55H74N4O5 | 92.966 | 869.55 | -Fe(II) chelator -Suppresses lipid peroxidation -The conjugated double bonds presented in the porphyrin ring can act as electron transfer that will stabilize a radical compound |
[51,52] |
| Vitexin 4′-O-galactoside | C27H30O15 | 40.024 | 593.15 | -Electron donor that may act as a good radical scavenger -Suppresses O2− generation by promoting superoxide dismutase |
[53] |
| Luteolin 7-methyl glucuronide | C22H20O12 | 41.903 | 475.09 | -Suppresses O2− generation | [54] |
| Quercetin 7-(6″-acetylglucoside) | C23H22O13 | 51.213 | 515.10 | -Influence on O2− -Reduction in radicals -Decreases intracellular hydrogen peroxide accumulation |
[55] |
| Isorhamnetin 3-(6″-acetylglucoside) | C24H24O13 | 58.203 | 519.11 | -Free radical scavenger -Inhibits O2− production -Suppresses lipid peroxidation by preventing the conversion of hydrogen peroxide into hydroxyl radical by the Haber–Weiss reaction |
[55] |
| Saponarin (apigenin-6-C-glucosyl-7-O-glucoside) |
C27H30O15 | 37.047 | 593.14 | -O2− scavenging activity -Neutralizes hazardous free radicals |
[56] |
| Apigenin | C15H10O5 | 79.602 | 269.04 | -Scavenges O2− by reversed decreasing of superoxide dismutase | [57] |
| Quercetin 3-galactoside | C21H20O12 | 48.637 | 713.15 | -Scavenges ROS | [58] |
| Kaempferol | C15H10O6 | 35.262 | 287.05 | -Efficiently prevents ROS generation | [59] |
| Luteolin | C15H10O6 | 80.827 | 285.04 | -Scavenges O2− -Inhibits H2O2 production and scavenges H2O2 -Reduction in ROS production |
[60] |
| Luteolin 7-(6′′′-acetyl allosyl-(1→2)-glucoside) | C29H32O17 | 37.044 | 651.16 | -Ability to scavenge ROS | [26] |
| l-Ascorbic acid-2-glucoside (AA2G) | C12H18O11 | 3.129 | 337.08 | -Directly interacts and scavenges free radicals -Suppresses H2O2, which induces oxidative stress |
[61] |
| Diosmetin | C16H12O6 | 63.793 | 299.05 | -Slight decrease in ROS | [62] |
| Esculetin | C9H6O4 | 21.757 | 177.01 | -Attenuates ROS production to approximately 40% | [63] |
| Apigenin 7-rhamnosyl-(1→2)-galacturonide | C27H28O15 | 56.514 | 591.13 | -Supports a powerful antioxidant activity | [64] |
| Cartormin | C27H29NO13 | 45.007 | 574.15 | -Increases the total antioxidative activity | [65] |
| Kaempferol 4′-glucoside | C21H20O11 | 52.776 | 447.09 | -Supports a powerful antioxidant activity | [66] |
| Eriodictyol | C15H12O6 | 54.307 | 259.06 | -Protects cells against oxidative-stress-induced cell damage | [67] |
| Hesperetin | C16H14O6 | 79.820 | 301.07 | -Supports antioxidant activity | [49] |
| Hydroxy tyrosol 1-O-glucoside (HT) | C14H20O8 | 13.423 | 315.10 | -Scavenges the free radicals -Scavenges O2− by promoting superoxide dismutase activity |
[68] |
| Gallic acid | C7H6O5 | 49.582 | 169.01 | -Protects cells from free-radical-induced cell damage | [69] |
All of the studied plants are well known in the daily life of Asian people. After comparison of their properties, the results showed that MLE had better biological activities than any others. It showed the unique antioxidant characteristic inside and outside viable systems. As the main objective of this study was screening the most powerful plant used in the daily Asian diet, MLE was therefore selected to analyze its composition.
It is known that antioxidant compounds in plants have benefits for the prevention or treatment of various disease conditions, including neurodegenerative diseases. Moreover, polyphenolic compounds possess numerous biological activities such as anti-inflammation and enzyme inhibition activities, gene expression, and signal transductions that play a significant role in health. In the present study, the evaluation of neuroprotective effect achieved on the human neuroblastoma (SHSY5Y) cell line was performed. This neuronal cell line was selected for the following reasons: (a) this cell line is acquired from a human origin, a source from which primary neurons are difficult to obtain (b) cells have been proposed as a useful model for studying the effect of free radicals formation and scavenging in the human neurons as they possess many biochemical and functional properties of neurons (c) the cell population is reasonably homogenous, which hypothetically translates into reproducibility and (d) these cells are oncogenic and their proliferation to yield a sufficient quantity for investigations can be accomplished.
Before investigation of their neuroprotection activities, all of the studied plant extracts were tested for cytotoxicity on SHSY5Y cells by MTT assay. The results of 50% inhibition concentration (IC50) of the extracts are summarized in Table 1. MLE, ALE, and VSE showed the lowest cytotoxicity, with IC50 values of 2.7 ± 0.2, 2.53 ± 0.1, and 2.43 ± 0.1 mg/mL, respectively, whereas ARE (PYL) showed the most cytotoxic effect to SHSY5Y cells with an IC50 of 0.34 ± 0.02 mg/mL. The data of ARE (BL) showed high toxicity on the neuroblastoma cell line, even in low concentrations. After testing the cytotoxicity of each extract and optimizing the lethal concentration of H2O2 on SHSY5Y cell line, the extracts were investigated for their neuroprotection activity in the presence of H2O2. It was found that PRE provided the greatest amount of neuroprotection, with 51% of viable cells, followed by MLE and ARE (PYL), with 44% and 40% of viable cells, respectively. Several studies reported that ginsenosides in PRE can support brain function, prevent neuroinflammation and oxidative stress, and inhibit or weaken various neurodegenerative disorders such as Alzheimer’s disease, Huntington’s disease (HD), and traumatic brain injury [32]. MLE can improve the memory of Alzheimer’s disease patients by significantly increasing superoxide dismutase (SOD) and catalase which are the antioxidant enzymes, and also by reducing lipid peroxidase levels. These antioxidant activities may improve cognitive functions [33]. ARE acts as a suppressor of lipid peroxidation in the brain by preserving the activities of endogenous antioxidant enzymes, including SOD, catalase, and other antioxidant molecules such as glutathione, which could reduce oxidative stress. Aqueous and alcoholic extract of ARE was shown to be a powerful scavenger of ROS that encouraged the progression of neurodegenerative disorders [34].
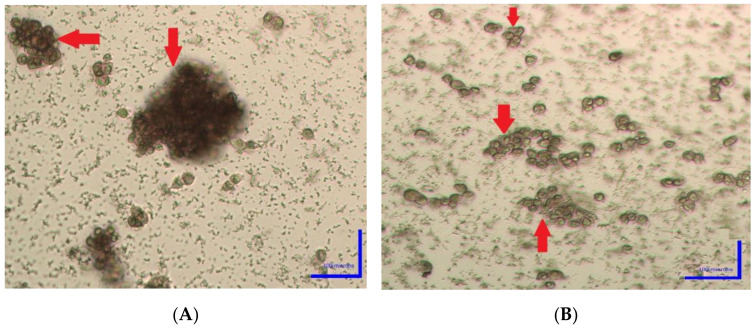
Using the NBT test, we assayed different plant extracts for their capacity to protect the SHSY5Y cell line from O2− radicals; the results are summarized in Table 1. MLE showed the best NBT reduction with the lowest IC50 concentration (0.83 ± 0.09 mg/mL), followed by VSE (0.98 ± 0.08 mg/mL). ALE and PRE showed less reduction activity—1.24 ± 0.05 and 1.22 ± 0.06 mg/mL, respectively. ARE (PYL) and PLE showed the lowest O2− radical scavenging activities—1.62 ± 0.1 and 1.65 ± 0.04 mg/mL, respectively. Figure 1A,B show the difference between control cells; SHSY5Y cells without MLE (dark colored with high O2−) and treated cells with MLE (light colored with low O2−).
Figure 1.
Nitro-blue tetrazolium (NBT) reduction test: (A) control cells (SHSY5Y cells without MLE); (B) SHSY5Y cells treated with MLE.
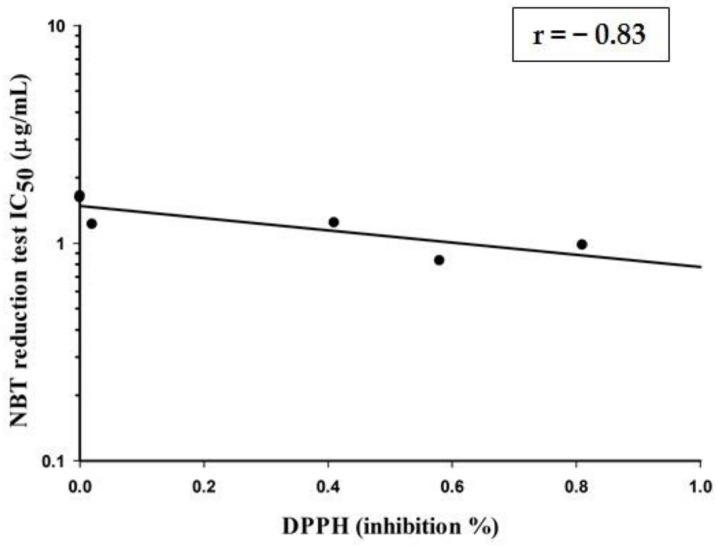
Correlations between the free radical scavenging effect of tested extracts (MLE, VSE, PLE, PRE, ARE (PYL) and ALE) outside the cells by DPPH and FRAP tests and inside the viable cells by scavenging O2− and intracellular ROS were determined using Pearson coefficient correlation. The strongest and best correlation was found between DPPH and IC50 of NBT reduction activity with a value of r = −0.83, p < 0.05; the results of the correlation established that extracts that possess higher quenching DPPH values are able to scavenge O2− with lower IC50 in the NBT test, as shown in Figure 2. The other correlations showed non-significant correlation (p-value > 0.05).
Figure 2.
The correlation between the NBT reduction test and DPPH inhibition activity of plant extracts (r = − 0.83, p < 0.05).
To detect the capacity of the obtained plant extracts to scavenge intracellular levels of ROS in SHSY5Y cells, DCFHDA was applied as a fluorogenic substrate. This substrate was converted to highly fluorescent DCFH by ROS and was monitored by the spectrophotometer. Table 1 shows the concentrations of extracts that inhibit 50% of intracellular ROS levels by the DCFHDA assay. MLE, PRE, and ARE (PYL), with IC50 values of 0.71 ± 0.08, 0.83 ± 0.08, and 0.96 ± 0.1 mg/mL, respectively, exhibited the best ROS scavenging activity. VSE (1.24 ± 0.06 mg/mL) and PLE (1.34 ± 0.06 mg/mL) showed less effectiveness in decreasing intracellular ROS levels, whereas ALE (2.48 ± 0.03 mg/mL) showed the lowest antioxidant activity in SHSY5Y cells. Moreover, SHSY5Y cells treated with DMSO showed no effect on the cells. The unique activity of MLE might be due to the presence of potent compounds that are commonly known to have antioxidant efficiency, such as quercetin derivatives, isorhamnetin glucoside, kaempferol, luteolin and its derivatives, ascorbic acid derivative, diosmetin, apigenin and its derivatives (saponarin), hydroxytyrosol 1-O-glucoside (HT), and esculetin, as shown in Table 2. Ginsenoside, the main active ingredient in PRE, could protect SHSY5Y from reactive oxygen species by the Bcl-2-associated X protein (BAX) pathway [35]. Coumarins, sesqiterpenes, and volatile oil, including eugenol and its derivatives in ARE, could protect ROS in 4T1 breast cancer cells and NIH-3T3 fibroblast cells [36].
From the data mentioned above, this extract ranked as first or second place in all experiments; the revealed data established that ethanolic MLE extract is pharmacologically more active than other tested extracts, which might be because of the synergistic effects of various potent components present in the whole extract.
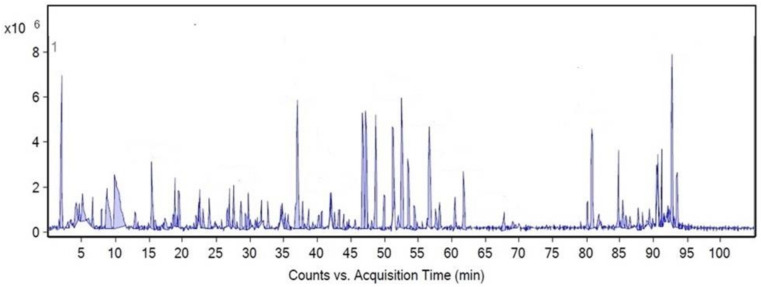
Taken together, MLE, grown in Thailand, is interesting as a potential neuroprotective agent with further applications due to its low cytotoxicity and high antioxidant activities. LC–QTOF/MS analysis (Figure 3 and Table 2) of MLE in the present study, confirmed by the fragmentation MS spectra (see the Supplementary Materials Figure S1), showed that the extract was significantly rich in antioxidant ingredients (31 compounds) compared with constituents found in moringa leaves cultivated in China and India (11 compounds that were also present in our extract) [37], whereas the phytochemicals reported in a moringa extract from Malaysia include multiflorin-B and derivatives of apigenin, quercetin, and kaempferol [38]. This means that the moringa plant cultivated in Thailand ranks as one of the best sources of potent antioxidant compounds. The difference in phytochemical constituents might be due to the plants’ adaptation to the climatic environment and soil factors such as soil type, pH, and soil nutrients [39]. Therefore, concentrations of these constituents are usually associated with factors such as the environment and growing areas [40]. In Table 2, MLE is shown to contain eriodictyol and gallic acid, which play a role in scavenging H2O2 and preventing cell damage by oxidative stress. It also contained various effective phenolic compounds that support a powerful antioxidant activity, including apigenin 7-rhamnosyl-(1→2)-galacturonide, cartormin, kaempferol 4′-glucoside, hesperetin, and isorhamnetin 3-(6″-acetylglucoside).
Figure 3.
Liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (LC–QTOF/MS) chromatogram of MLE.
MLE requires more attention, as it plays an important and unique biological role as an ecofriendly and non-cytotoxic antioxidant agent compared to other tested extracts in all tests.
4. Conclusions
A variety of phytochemicals have been reported to prevent the risk of numerous diseases, including neurodegenerative diseases. In Asian countries, many researchers are seeking bioactive compounds from edible plants or herbs for the prevention or treatment of neurodegenerative diseases. A. galanga, V. vinifera, M. oleifera, and P. ginseng cultivated in Thailand were selected in the present study.
MLE showed high antioxidant activities (DPPH and FRAP assays) with low cytotoxicity to SHSY5Y cell lines compared to the other studied plants. Its mechanism of action for neuroprotective effect on SHSY5Y cells is probably due to its high level of polyphenolic and other antioxidant compounds, and it possesses the ability to scavenge free radicals or activate the cellular antioxidant system. LC–QTOF/MS analysis confirmed that MLE consists of phenolic compounds, which are a good source of antioxidants. The determination of pure bioactive compounds involved in neurodegeneration contained in MLE should be the subject of further investigation.
Acknowledgments
The authors would like to thank the Institute of Biotechnology and Genetic Engineering (Chulalongkorn University, Thailand) for providing the SHSY5Y cell line.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10050889/s1, Figure S1: Mass spectrum of antioxidant components of MLE.
Author Contributions
F.J.H. accomplished the majority of the research and wrote the draft; S.V., P.B. and K.V. contributed to the data analysis; K.V. planned and supervised the study; P.B. read and edited the final version of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fermentation Research Center for Value Added Agricultural Products (FerVAAP) Grant Number 05-2561 and the National Research Council of Thailand (NRCT), Khon Kaen University, Thailand Grant Number 6200026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NirmalaDevi D., Venkataramana M., Chandranayaka S., Ramesha A., Jameel N.M., Srinivas C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 2014;34:973–985. doi: 10.1007/s10571-014-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng C., Luo T., Zhang S., Liu K., Zhang Y., Luo Y., Ge P. Lycopene protects human SH-SY5Y neuroblastoma cells against hydrogen peroxide-induced death via inhibition of oxidative stress and mitochondria-associated apoptotic pathways. Mol. Med. Rep. 2016;13:4205–4214. doi: 10.3892/mmr.2016.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhuna K., Dhuna V., Bhatia G., Singh J., Kamboj S.S. Cytoprotective effect of methanolic extract of Nardostachys jatamansi against hydrogen peroxide induced oxidative damage in C6 glioma cells. Acta Biochim. Pol. 2013;60 doi: 10.18388/abp.2013_1946. [DOI] [PubMed] [Google Scholar]
- 4.González-Sarrías A., Núñez-Sánchez M.Á., Tomás-Barberán F.A., Espín J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017;65:752–758. doi: 10.1021/acs.jafc.6b04538. [DOI] [PubMed] [Google Scholar]
- 5.Dawidowicz A.L., Olszowy M., Jóźwik-Dolęba M. Importance of solvent association in the estimation of antioxidant properties of phenolic compounds by DPPH method. J. Food Sci. Technol. 2014;52:4523–4529. doi: 10.1007/s13197-014-1451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortés-Castell E., Veciana-Galindo C., Torró-Montell L., Palazón-Bru A., Sirvent-Segura E., Gil-Guillén V., Rizo-Baeza M. Protection by polyphenol extract from olive stones against apoptosis produced by oxidative stress in human neuroblastoma cells. Nutr. Hosp. 2016;33:118–122. doi: 10.20960/nh.39. [DOI] [PubMed] [Google Scholar]
- 7.Matic I., Guidi A., Kenzo M., Mattei M., Galgani A. Investigation of medicinal plants traditionally used as dietary supplements: A review on Moringa oleifera. J. Public Health Afr. 2018;9:841. doi: 10.4081/jphia.2018.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chouni A., Paul S. Review on phytochemical and pharmacological potential of Alpinia galanga. Pharm. J. 2018;10:9–15. doi: 10.5530/pj.2018.1.2. [DOI] [Google Scholar]
- 9.García-Lomillo J., González-SanJosé M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food Saf. 2017;16:3–22. doi: 10.1111/1541-4337.12238. [DOI] [PubMed] [Google Scholar]
- 10.Kim J.K., Kim J.K., Tabassum N., Uddin R., Park S.U. Ginseng: A miracle sources of herbal and pharmacological uses. Orient. Pharm. Exp. Med. 2016;16:243–250. doi: 10.1007/s13596-016-0246-6. [DOI] [Google Scholar]
- 11.Xicoy H., Wieringa B., Martens G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017;12:1–11. doi: 10.1186/s13024-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vayupharp B., Laksanalamai V. Recovery of antioxidants from grape seeds and its application in fried food. J. Food Process. Technol. 2012;3:1–6. doi: 10.4172/2157-7110.1000152. [DOI] [Google Scholar]
- 13.Li H., Wang X., Li P., Li Y., Wang H. Comparative study of antioxidant activity of grape (Vitis vinifera) seed powder assessed by different methods. J. Food Drug Anal. 2008;16:12. doi: 10.38212/2224-6614.2321. [DOI] [Google Scholar]
- 14.Torres-Castillo J.A., Sinagawa-García S.R., Martínez-Ávila G.C.G., López-Flores A.B., Sánchez-González E.I., Aguirre-Arzola V.E., Gutiérrez-Díez A. Moringa oleifera: Phytochemical detection, antioxidants, enzymes and antifugal properties. Int. J. Exp. Bot. 2013;82:193–202. [Google Scholar]
- 15.Hashim F.J., Mathkor T.H., Hussein H., Shawkat M.S. GC-MS and FTIR analysis of Moringa oleifera leaves different extracts and evaluation of their antioxidant activity. Asian J. Microbiol. Biotechnol. Environ. Sci. 2017;19:861–871. [Google Scholar]
- 16.Shin S., Lee J.-A., Son D., Park D., Jung E. Anti-skin-aging activity of a standardized extract from Panax ginseng leaves in vitro and in human volunteer. Cosmetics. 2017;4:18. doi: 10.3390/cosmetics4020018. [DOI] [Google Scholar]
- 17.Hwang E., Park S.-Y., Yin C.S., Kim H.-T., Kim Y.M., Yi T.H. Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J. Ginseng Res. 2017;41:69–77. doi: 10.1016/j.jgr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo C.-Y., Liu P.-L., Lin L.-C., Chen Y.-T., Hseu Y.-C., Wen Z.-H., Wang H.-M. Antimelanoma and antityrosinase from Alpinia galangal constituents. Sci. World J. 2013;2013:1–5. doi: 10.1155/2013/186505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh D., McGhie T.K., Zhang J., Adaim A., Skinner M. Effects of anthocyanins and other phenolics of boysenberry and blackcurrant as inhibitors of oxidative stress and damage to cellular DNA in SH-SY5Y and HL-60 cells. J. Sci. Food Agric. 2006;86:678–686. doi: 10.1002/jsfa.2409. [DOI] [Google Scholar]
- 20.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Hashim F.J., Shawkat M.S., Al-Jewari H. Cytotoxicity of curcumin against leukemic cell lines via apoptosis activity. Curr. Res. J. Biol. Sci. 2012;4:60–64. [Google Scholar]
- 23.Muñoz M., Cedeño R., Rodríguez J., Van Der Knaap W.P., Mialhe E., Bachère E. Measurement of reactive oxygen intermediate production in haemocytes of the penaeid shrimp, Penaeus vannamei. Aquaculture. 2000;191:89–107. doi: 10.1016/S0044-8486(00)00420-8. [DOI] [Google Scholar]
- 24.Roesslein M., Hirsch C., Kaiser J.-P., Krug H.F., Wick P. Comparability of in vitro tests for bioactive nanoparticles: A common assay to detect reactive oxygen species as an example. Int. J. Mol. Sci. 2013;14:24320–24337. doi: 10.3390/ijms141224320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang N., Kitts D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules. 2014;19:19180–19208. doi: 10.3390/molecules191119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 27.Lin D., Xiao M., Zhao J., Li Z., Xing B., Li X., Kong M., Li L., Zhang Q., Liu Y., et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21:1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Z.F., Zhang H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants. 2017;6:71. doi: 10.3390/antiox6030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leopoldini M., Russo N., Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;125:288–306. doi: 10.1016/j.foodchem.2010.08.012. [DOI] [Google Scholar]
- 30.Malik T., Pandey D.K., Roy P., Okram A. Evaluation of phytochemicals, antioxidant, antibacterial and antidiabetic potential of Alpinia galanga and Eryngium foetidum Plants of Manipur (India) Pharm. J. 2016;8:459–464. doi: 10.5530/pj.2016.5.8. [DOI] [Google Scholar]
- 31.Vergara-Jimenez M., AlMatrafi M.M., Fernandez M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants. 2017;6:91. doi: 10.3390/antiox6040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X., Li N., Pu Y., Zhang T., Wang B. Neuroprotective effects of ginseng phytochemicals: Recent perspectives. Molecules. 2019;24:2939. doi: 10.3390/molecules24162939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranira G., Rimi H., Kaushik R., Debajani G. Effect of Moringa oleifera in experimental model of Alzheimer’s disease: Role of antioxidant. Ann. Neuscosci. 2005;12:33–36. doi: 10.5214/ans.0972.7531.2005.12030. [DOI] [Google Scholar]
- 34.Mundugaru R., Sivanesan S., Udaykumar P., Vidyadhara D.J., Prabhu S.N., Ravishankar B. Neuroprotective functions of Alpinia galanga in forebrain ischemia induced neuronal damage and oxidative insults in rat hippocampus. Indian J. Pharm. Educ. Res. 2018;52:s77–s85. doi: 10.5530/ijper.52.4s.79. [DOI] [Google Scholar]
- 35.Zhang Y., Zhang Z., Wang H., Cai N., Zhou S., Zhao Y., Chen X., Zheng S., Si Q., Zhang W. Neuroprotective effect of ginsenoside Rg1 prevents cognitive impairment induced by isoflurane anesthesia in aged rats via antioxidant, anti-inflammatory and anti-apoptotic effects mediated by the PI3K/AKT/GSK-3β pathway. Mol. Med. Rep. 2016;14:2778–2784. doi: 10.3892/mmr.2016.5556. [DOI] [PubMed] [Google Scholar]
- 36.Ahlina F.N., Nugraheni N., Salsabila I.A., Haryanti S., Da’i M., Meiyanto E. Revealing the reversal effect of galangal (Alpinia galanga L.) extract against oxidative stress in metastatic breast cancer cells and normal fibroblast cells intended as a co-chemotherapeutic and anti-ageing agent. Asian Pac. J. Cancer Prev. 2020;21:107–117. doi: 10.31557/APJCP.2020.21.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin H., Zhu H., Tan J., Wang H., Wang Z., Li P., Zhao C., Liu J. Comparative analysis of chemical constituents of Moringa oleifera leaves from China and India by ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Molecules. 2019;24:942. doi: 10.3390/molecules24050942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karthivashan G., Fard M.T., Arulselvan P., Abas F., Fakurazi S. Identification of bioactive candidate compounds responsible for oxidative challenge from hydro-ethanolic extract of Moringa oleifera leaves. J. Food Sci. 2013;78:C1368–C1375. doi: 10.1111/1750-3841.12233. [DOI] [PubMed] [Google Scholar]
- 39.Wright R.J., Lee K.S., Hyacinth H.I., Hibbert J.M., Reid M.E., Wheatley A.O., Asemota H.N. An investigation of the antioxidant capacity in extracts from Moringa oleifera plants grown in Jamaica. Plants. 2017;6:48. doi: 10.3390/plants6040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sulastri E., Zubair M.S., Anas N.I., Abidin S., Hardani R., Yulianti R., Aliyah Total phenolic, total flavonoid, quercetin content and antioxidant activity of standardized extract of Moringa oleifera leaf from regions with different elevation. Pharm. J. 2018;10:s104–s108. doi: 10.5530/pj.2018.6s.20. [DOI] [Google Scholar]
- 41.Hussain S.A., Hameed A., Nazir Y., Naz T., Wu Y., Suleria H.A.R., Song Y. Microencapsulation and the characterization of poly herbal formulation (PHF) rich in natural polyphenolic compounds. Nutrients. 2018;10:843. doi: 10.3390/nu10070843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuo A., Yu Y., Li J., Xu B., Yu X., Qiu Y., Cao S. Study on the relation of structure and antioxidant activity of isorhamnetin, quercetin, phloretin, silybin and phloretin isonicotinyl hydrazone. Free Radic. Antioxid. 2011;1:39–47. doi: 10.5530/ax.2011.4.7. [DOI] [Google Scholar]
- 43.Panat N.A., Amrute B.K., Bhattu S., Haram S.K., Sharma G.K., Ghaskadbi S.S. Antioxidant profiling of C3 quercetin glycosides: Quercitrin, quercetin 3-β-d-glucoside and quercetin 3-O-(6″-O-malonyl)-β-D glucoside in cell free environment. Free Radic. Antiox. 2015;5:90–100. doi: 10.5530/fra.2015.2.7. [DOI] [Google Scholar]
- 44.Li J., Mottamal M., Li H., Liu K., Zhu F., Cho Y.-Y., Sosa C.P., Zhou K., Bowden G.T., Bode A.M., et al. Quercetin-3-methyl ether suppresses proliferation of mouse epidermal JB6 P+ cells by targeting ERKs. Carcinogenesis. 2011;33:459–465. doi: 10.1093/carcin/bgr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei B.-L., Lu C.-M., Tsao L.-T., Wang J.-P., Lin C.-N. In vitro anti-inflammatory effects of quercetin 3-O-methyl ether and other constituents from Rhamnuss species. Planta Medica. 2001;67:745–747. doi: 10.1055/s-2001-18339. [DOI] [PubMed] [Google Scholar]
- 46.Pierre Luhata L., Gaston Luhata W. Tiliroside: Biosynthesis, bioactivity and structure activity relationship (SAR)-a review. J. Phytopharmacol. 2017;6:343–348. [Google Scholar]
- 47.Li X., Li K., Xie H., Xie Y., Li Y., Zhao X., Jiang X., Chen D. Antioxidant and cytoprotective effects of the Di-O-caffeoylquinic acid family: The mechanism, structure–activity relationship, and conformational effect. Molecules. 2018;23:222. doi: 10.3390/molecules23010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X., Tian Y., Wang T., Lin Q., Feng X., Jiang Q., Liu Y., Chen D. Role of the p-coumaroyl moiety in the antioxidant and cytoprotective effects of flavonoid glycosides: Comparison of astragalin and tiliroside. Molecules. 2017;22:1165. doi: 10.3390/molecules22071165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D.-S., Lim S.-B. Semi-continuous subcritical water extraction of flavonoids from Citrus unshiu Peel: Their antioxidant and enzyme inhibitory activities. Antioxidants. 2020;9:360. doi: 10.3390/antiox9050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y.-Z., Deng G., Guo R., Chen D.-F., Fu Z.-M. DFT Studies on the antioxidant activity of naringenin and its derivatives: Effects of the substituents at C3. Int. J. Mol. Sci. 2019;20:1450. doi: 10.3390/ijms20061450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu C.-Y., Chao P.-Y., Hu S.-P., Yang C.-M. The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr. Sci. 2013;4:1–8. doi: 10.4236/fns.2013.48A001. [DOI] [Google Scholar]
- 52.Kusmita L., Puspitaningrum I., Limantara L. Identification, isolation and antioxidant activity of pheophytin from green tea (Camellia sinensis (L.) Kuntze) Procedia Chem. 2015;14:232–238. doi: 10.1016/j.proche.2015.03.033. [DOI] [Google Scholar]
- 53.Babaei F., Moafizad A., Darvishvand Z., Mirzababaei M., Hosseinzadeh H., Nassiri-Asl M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020;8:2569–2580. doi: 10.1002/fsn3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nugroho A., Choi J.S., Park H.-J. Analysis of flavonoid composition of Korean herbs in the family of compositae and their utilization for health. Nat. Prod. Sci. 2016;22:1–12. doi: 10.20307/nps.2016.22.1.1. [DOI] [Google Scholar]
- 55.Zielińska M., Kostrzewa A., Ignatowicz E., Budzianowski J. The flavonoids, quercetin and isorhamnetin 3-O-acylglucosides diminish neutrophil oxidative metabolism and lipid peroxidation. Acta Biochim. Pol. 2001;48:183–189. doi: 10.18388/abp.2001_5125. [DOI] [PubMed] [Google Scholar]
- 56.Simeonova R., Vitcheva V., Kondeva-Burdina M., Krasteva I., Manov V., Mitcheva M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats. BioMed Res. Int. 2013;2013:1–10. doi: 10.1155/2013/757126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A., Lucarini M., Santini A., Souto E.B., Novellino E., et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019;20:1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y., Wang D., Yang L., Zhou D., Zhang J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE. 2014;9:e105725. doi: 10.1371/journal.pone.0105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imran M., Salehi B., Sharifi-Rad J., Gondal T.A., Saeed F., Imran A., Shahbaz M., Fokou P.V.T., Arshad M.U., Khan H., et al. Kaempferol: A key emphasis to its anticancer potential. Molecules. 2019;24:2277. doi: 10.3390/molecules24122277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seelinger G., Merfort I., Schempp C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica. 2008;74:1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 61.Wang S.-F., Liu X., Ding M.-Y., Ma S., Zhao J., Wang Y., Li S. 2-O-β-d-glucopyranosyl-l-ascorbic acid, a novel vitamin C derivative from Lycium barbarum, prevents oxidative stress. Redox Biol. 2019;24:101173. doi: 10.1016/j.redox.2019.101173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poór M., Veres B., Jakus P.B., Antus C., Montskó G., Zrínyi Z., Vladimir-Knežević S., Petrik J., Kőszegi T. Flavonoid diosmetin increases ATP levels in kidney cells and relieves ATP depleting effect of ochratoxin A. J. Photochem. Photobiol. B Biol. 2014;132:1–9. doi: 10.1016/j.jphotobiol.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Kim Y., Lee J. Esculetin inhibits adipogenesis and increases antioxidant activity during adipocyte differentiation in 3T3-L1 Cells. Prev. Nutr. Food Sci. 2017;22:118–123. doi: 10.3746/pnf.2017.22.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamayose C.I., Romoff P., Toyama D.O., Gaeta H.H., Costa C.R.C., Belchor M.N., Ortolan B.D., Velozo L.S.M., Kaplan M.A.C., Ferreira M.J.P., et al. Non-clinical studies for evaluation of 8-C-rhamnosyl apigenin purified from Peperomia obtusifolia against acute edema. Int. J. Mol. Sci. 2017;18:1972. doi: 10.3390/ijms18091972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yue S., Tang Y., Li S., Duan J.-A. Chemical and biological properties of quinochalcone C-glycosides from the florets of Carthamus tinctorius. Molecules. 2013;18:15220–15254. doi: 10.3390/molecules181215220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Tang C., Zhang H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J. Food Drug Anal. 2015;23:310–317. doi: 10.1016/j.jfda.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee S.E., Yang H., Son G.W., Park H.R., Park C.-S., Jin Y.-H., Park Y.S. Eriodictyol protects endothelial cells against oxidative stress-induced cell death through modulating ERK/Nrf2/ARE-dependent heme oxygenase-1 expression. Int. J. Mol. Sci. 2015;16:14526–14539. doi: 10.3390/ijms160714526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colica C., Di Renzo L., Trombetta D., Smeriglio A., Bernardini S., Cioccoloni G., De Miranda R.C., Gualtieri P., Salimei P.S., De Lorenzo A. Antioxidant effects of a hydroxytyrosol-based pharmaceutical formulation on body composition, metabolic state, and gene expression: A randomized double-blinded, placebo-controlled crossover trial. Oxidative Med. Cell. Longev. 2017;2017:1–14. doi: 10.1155/2017/2473495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao J., Hu J., Hu D., Yang X. A role of gallic acid in oxidative damage diseases: A comprehensive review. Nat. Prod. Commun. 2019;14:1–9. doi: 10.1177/1934578X19874174. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.