Abstract
Uterine fibroids represent the most common benign tumors of the uterus. They are considered a typical fibrotic disorder. In fact, the extracellular matrix (ECM) proteins—above all, collagen 1A1, fibronectin and versican—are upregulated in this pathology. The uterine fibroids etiology has not yet been clarified, and this represents an important matter about their resolution. A model has been proposed according to which the formation of an altered ECM could be the result of an excessive wound healing, in turn driven by a dysregulated inflammation process. A lot of molecules act in the complex inflammatory response. Macrophages have a great flexibility since they can assume different phenotypes leading to the tissue repair process. The dysregulation of macrophage proliferation, accumulation and infiltration could lead to an uncontrolled tissue repair and to the consequent pathological fibrosis. In addition, molecules such as monocyte chemoattractant protein-1 (MCP-1), granulocyte macrophage-colony-stimulating factor (GM-CSF), transforming growth factor-beta (TGF-β), activin A and tumor necrosis factor-alfa (TNF-α) were demonstrated to play an important role in the macrophage action within the uncontrolled tissue repair that contributes to the pathological fibrosis that represents a typical feature of the uterine fibroids.
Keywords: uterine fibroids, ECM, inflammatory process, tissue repair, macrophages, pathological fibrosis
1. Uterine Leiomyoma: A Typical Fibrotic Pathology
Uterine leiomyomas (leiomyomas, myomas, uterine fibroids, fibroids) are the most common benign tumors of the uterus. The perimetrium constitutes the more external layer of the uterus; it equals the peritoneum and is surrounded by a thin connective tissue layer. The perimetrium resembles a typical serosa/adventitia layer. The endometrium constitutes the more internal layer. It is formed by a simple columnar epithelium and contains numerous tubular glands. In addition, a cell-dense connective tissue layer can be individuated at the level of this structure. Finally, a transition to squamous non-keratinized epithelium at the portio (squamocolumnar junction) can be appreciated. Functionally, the endometrium can be divided into two sublayers: the so-called stratum basale, which represents the basal layer, and the so-called stratum functionale, which is the real functional layer. The endometrium resembles a typical mucosa layer. Finally, the myometrium constitutes the intermediate layer between the perimetrium and the endometrium and represents the muscularis structure of the uterus. The uterine musculature shows properly the typical characteristics of the smooth muscle tissue. More precisely, the myometrium is composed of three smooth muscle layers: the subvascular layer, which is quite thin; the vascular layer, which is rather strong and well-perfused; and the supravascular layer, which is composed of a complex of crossing muscle fibers. The subvascular layer is mainly involved in the separation of the endometrium during the menstrual cycle. The vascular layer runs around the uterus and, in doing this, it forms a kind of net for the perfusion of the tissue. It plays a major role during labor within the complex mechanism that regulates the uterine contractions during the partum [1]. The supravascular, with its muscle fibers, stabilizes the uterine wall [2,3]. The cells of the myometrium can transform themselves into uterine leiomyoma cells. So, the uterine leiomyoma is a pathology that involves, in detail, the myometrium. The uterine fibroids incidence in reproductive age women is approximately 60%, and if we consider black women, this percentage reaches 80% [4]. The symptomatology of uterine fibroids is very heavy. One of the most relevant clinical symptoms is prolonged or heavy menstrual bleeding. In addition, the irregular and excessive bleeding often experienced by the women affected by uterine leiomyomas, a lot of times, leads to anemia. Other symptoms of the uterine fibroids are represented by pelvic pain or pressure, pain at the level of the back of the legs, a pressure sensation at the level of the lower part of abdomen, bowel and bladder dysfunctions and pain during sexual intercourse.
In addition to all these physical ailments, uterine leiomyomas may also impact the pregnancy outcome. Depending on their position, size and number, uterine leiomyomas can be a cause of infertility and recurrent miscarriage [5,6,7,8,9]. Although uterine leiomyomas are not malignant tumors, they can cause significant morbidity. Thus, this pathology represents one of the most important public health problems worldwide [10]. This fact becomes also more relevant if we bear in mind that, at the moment, no long-term medical treatments are available for fibroids resolution [11].
Considering the role played by estrogens and progesterone in the leiomyoma growth [11,12], for the treatment of uterine fibroids, the U.S. Food and Drug Administration (FDA) approved leuprolide acetate, which is a gonadotropin-releasing hormone analog. However, these kinds of molecules, in particular in young women, can provoke several side effects, above all, a hypogonadal state; this is the reason why the duration of therapy is currently limited. Uterine leiomyomas usually start to grow again after breaking off the treatment [13,14]. Nevertheless, it was demonstrated that leuprolide acetate can be effectively used in order to decrease the volume of the uterine fibroids with improved fibroid-related symptoms. [15,16,17]. Of the treatments that have been studied up to now, the focus has above all been on those belonging to two categories: antiprogestin and selective progesterone receptor modulators (SPRMs). Thus, clinical trial results suggested mifepristone, which is an antiprogestinic molecule [18], and asoprisnil [19] and telapristone acetate (CDB-4124) [20], both belonging to the SPRMs category, as candidate therapeutic drugs against uterine fibroids (https://clinicaltrials.gov accessed on 7 April 2021). In particular, 17a-acetoxy-11b-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione, also referred to as CDB-2914 and ulipristal acetate (UPA) [21,22,23,24], is an SPRM molecule, and it is very interesting to study because of the high affinity that it has shown in binding progesterone receptor isoforms A and B [25,26].
Currently, in the international literature, there is a debate about the usefulness and safety of the use of UPA [27].
A few years ago, we demonstrated that UPA can exert a downregulation effect at the level of the mRNA of activin A, a pro-fibrotic factor for leiomyoma. The UPA causes a similar impairing effect also on follistatin (FST), activin receptor type II (ActRIIB) and activin receptor-like kinase 4 (ALK4) mRNAs [28]. All these molecules together represent the activin pathway and these results consider activin A and its receptors as UPA targets and at the same time reinforce the validity of UPA as a treatment for uterine fibroids.
In 2012, the European Medicines Agency (EMA) approved the clinical use of UPA 5 mg, sold under the trade name Esmya (or generic medicines), but limited it to a three-month period and pre-surgery. However, in 2018, other limitations occurred since cases of severe liver toxicity had been reported. Following cases of liver damage that even required transplantation, in November 2020, the EMA recommended limiting the prescription of UPA 5 mg (Esmya or generic medicines) as much as possible. So, currently, Esmya and generic medicines containing UPA 5 mg are only allowed to treat uterine fibroids in premenopausal women for whom surgical procedures (including uterine fibroid embolisation) are not appropriate or have not worked. On the other hand, these medicines must not be used for controlling the symptoms of uterine fibroids in the pre-surgical phase. Besides, it had already been demonstrated that in the women that had been pre-surgically treated with UPA, the myomas appeared softer and showed less clear cleavage planes. So, the result was that it was less easy to enucleate if compared to the enucleation modalities of the myomas belonging to women not pre-surgically treated with UPA [29]. In addition to all this, after the patients stop taking the UPA, leiomyomas revert [30,31]. Nowadays, hysterectomy remains the definitive treatment against uterine fibroids. In fact, at the moment, uterine leiomyomas represent the most common indication for hysterectomy in the world. However, it represents itself an additional problem concerning uterine fibroids and also the less invasive myomectomy leads to a serious postoperative morbidity [32,33]. Hysterectomy exerts also a very significant economic impact on the healthcare system all over the world, reaching an amount almost equal to $2.2 billion/year for the United States of America alone [34].
According to their anatomical location, uterine fibroids can be classified into three different types: submucosal fibroids, intramural fibroids and subserosal fibroids [35].
Uterine fibroids present themselves as solid, rounded masses, with an inhomogeneous eco-structure [36].
From a histological point of view, uterine fibroids can be classified into different types: usual leiomyoma, cellular leiomyoma that shows increased cellularity [37], lipoleiomyoma that exhibits adipocytes [38], apoplectic leiomyoma that shows stellate zones of recent hemorrhage [39] and rare, bizarre leiomyoma [40,41]. Among them, the usual leiomyoma is the most common histological variant with an incidence equal to approximately 94% and it is what is commonly referred to as a leiomyoma unless otherwise specified.
Usual leiomyomas are the ones considered a fibrotic disorder [42,43].
The leiomyomas were described as typical fibrotic tissues because they exhibit the upregulation of the extracellular matrix (ECM) proteins—above all, collagen 1A1, fibronectin and versican [44]. In particular, numerous authors showed that the uterine fibroids contain approximately 50% more ECM than the corresponding myometrium [45,46,47,48,49]. In addition, the ECM was suggested to represent a reservoir for growth factors, cytokines, chemokines, angiogenic and inflammatory response mediators, and proteases [43,50,51,52,53], which are all molecules thought to be involved in the initiation and development of the uterine fibroids [11].
In this regard, a very important matter about uterine fibroids is that their etiopathogenesis has not yet been clarified [53].
Nowadays, some major risk factors associated with the uterine leiomyomas are known and, among them, the following are the most important ones: early menarche, nulliparity, age (meaning late reproductive years), polycystic ovary syndrome, diabetes, hypertension, obesity, and heredity [10,50,54]. In addition to this, since black women have a high incidence rate of uterine leiomyomas [4], ethnicity may also be considered as a potential risk factor for this pathology.
Of the most important factors involved in the pathogenesis of uterine leiomyoma, in the literature, it has been reported that chromosomal abnormalities, both at the level of alterations of karyotypic character and at the level of alterations of cytogenetic character, are present in about 50% of leiomyomas [55,56,57]. In addition, in the leiomyomas, the chromosomes 2, 3, 6, 7, 8, 10, 11, 12, 13, 14 and 22 were demonstrated to present genetic alterations with the genes MED12, HMGA2, HMGA1, FH, BHD, TSC2, PCOLCE, ORC5L, and LHFPL3 supposed to be mutated in some way [50,58,59,60,61,62,63,64,65,66]. Mutations at the level of these genes and, in particular, MED12 mutation, FH inactivation and HMGA2 overexpression, as well as COL4A6-COL4A5 deletion were confirmed also by studies based on the modern high-throughput sequencing techniques [67].
Furthermore, as well as genetic factors, molecules and cellular events belonging to typical epigenetic pathways, such as several microRNAs, DNA methylation and histone modification, have also been described to be involved in leiomyomas [68,69,70,71]. In particular, uterine leiomyomas have been shown to present a dysregulation about a lot of different microRNAs and, among them also let7, miR-21, miR-93, miR-106b, and miR-200 and their predicted target genes. In addition, the same type of dysregulation has not been found in the healthy myometrium [68,72,73,74,75,76,77,78]. In addition, other potential gene-markers for the uterine leiomyoma can be provided through the use of gene set enrichment analysis [79].
Moreover, it has been clearly highlighted that estrogens and progesterone, the most important female hormones, as well as their correspondent receptors, exert a very relevant effect on uterine leiomyoma growth, and it was shown that, in doing this, the action of these molecules undergoes the mediation of other molecules such as growth factors, cytokines, and chemokines [11,80]. Sometimes, in the postmenopausal period, women need hormone replacement treatment (HRT) based on estrogens and progesterone in order to cope with some of the typical menopausal symptoms. So, also in postmenopausal women affected by uterine leiomyoma, estrogens and progesterone due to HRT can exert an important effect on uterine leiomyoma growth. For this reason, the use of these hormones should be limited [81].
Epidermal growth factor (EGF), heparin-binding epidermal growth factor (HB-EGF), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), transforming growth factor-alfa (TGF-α), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), acidic fibroblast growth factor (acidic-FGF), basic fibroblast growth factor (basic-FGF), activin and myostatin are the most important growth factors that mediate the estrogens and progesterone action within the uterine leiomyoma physiology [54,80,82,83,84]. In addition to this, interleukin (IL)-1, IL-6, IL-11, IL-13, IL-15, tumor necrosis factor-alfa (TNF-α), granulocyte macrophage-colony-stimulating factor (GM-CSF) and erythropoietin (EPO) are all cytokines that interact with estrogens and progesterone, playing an important role in uterine leiomyoma growth [85,86,87,88]. Additionally, chemokines, with their receptors and in particular, macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated on activation normal T cell expression and presumably secreted (RANTES), Eotaxin, Eotaxin-2, IL-8, chemokine CC-motif receptor (CCR) 1, CCR3, CCR5, C-X-C chemokine receptor (CXCR) 1, CXCR2 and monocyte chemoattractant protein-1 (MCP-1) stimulate the uterine leiomyoma growth after the interaction with estrogens and progesterone [88,89].
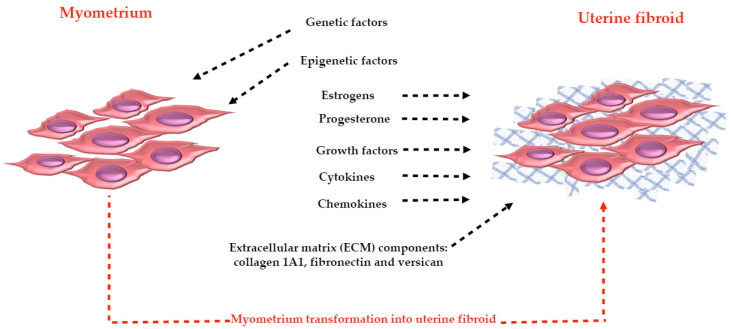
So, not only were growth factors [54,80,82], cytokines [11], chemokines [89], inflammatory response mediators [90], proteases [43,91,92,93] and the ECM, in particular as a reservoir of these molecules [43,50,51,52], shown to represent important actors in the establishment and in the growth of uterine fibroids [11], but also genetic alterations [50,55,64,94,95] and epigenetic mechanisms [69,70] as well as estrogens [96,97] and progesterone [97,98,99,100,101,102,103,104] can be considered as promoters of fibroid growth (Figure 1).
Figure 1.
Illustration of the promoters of fibroid growth. The blue net represents the typical extracellular matrix (ECM) proteins: collagen 1A1, fibronectin and versican. The abundant ECM in uterine fibroids (approximately 50% more than the corresponding myometrium) was suggested to represent a reservoir for the other promoters of fibroid growth.
So far, we have discussed the anatomical environment and the histological features of the uterine fibroids as well as their incidence, their heavy symptoms, the available treatments and those still under study, the risk factors and also what is known about their pathogenesis. In this review, we will continue the discussion, thoroughly summarizing the role of the inflammatory process in uterine fibroid development and growth with particular regard towards the importance of the macrophages and the immune response in the uterine fibroids, trying to contribute to shed light on their etiopathogenesis.
The inflammatory process seems to have a noteworthy role in the establishment of the uterine fibroids [105]. In fact, we have just mentioned that the leiomyomas were described as typical fibrotic tissues [44] with a great deal of ECM [45,46,47,48].
In general, the fibrotic response arises from the recruitment of inflammatory cells such as monocytes and macrophages by means of inflammatory signals into the site of injury in every tissue and the consequent activation of fibroblasts that start producing collagen [106].
These fibroblasts are usually activated by inflammatory signals and they differentiate into myofibroblasts. They head the ECM turnover [107], leading to tissue homeostasis restoration [108,109]. A dysregulation in the myofibroblasts action can generate pathological fibrosis [106]. In fertile women, transient inflammation is a physiological and important process for the correct achievement of menstruation, ovulation, and parturition. An altered response can produce chronic inflammation in the uterus, ultimately leading to dysregulated tissue repair [90]. In particular, about leiomyoma development and growth, Leppert and her group suggested a model according to which, after a tissue injury, an abnormal response to tissue repair could occur, leading to disordered healing [42]. In a leiomyoma, smooth muscle cells, as well as fibroblasts or stem cells, can gain a myofibroblastic phenotype. In a dysregulated process, after myofibroblast transformation, the myofibroblasts cannot undergo apoptosis with the consequent formation of an altered ECM [30], which is a distinctive trait of the leiomyomas [44,45,46,47,48]. About this, it was noticed that fibroids exhibit a remarkable similarity to keloids, especially because of the disordered appearance of ECM and dysregulation of many genes in the ECM. In fact, microarray experiments have shown that fibroids possess gene features that resemble keloids [42]. So, fibroids could represent a disorder of wound healing and could arise in response to dysregulated extracellular signals as well as keloids [42]. Additionally, myomectomy and caesarean section, which have already been demonstrated to be causes of uterine rupture, may themselves represent a kind of damage followed by a wound healing response. In women showing disordered extracellular signals because of these alterations, a fibroid may develop [110].
2. The Role of Macrophages in Tissue Repair and Fibrosis in Several Organs
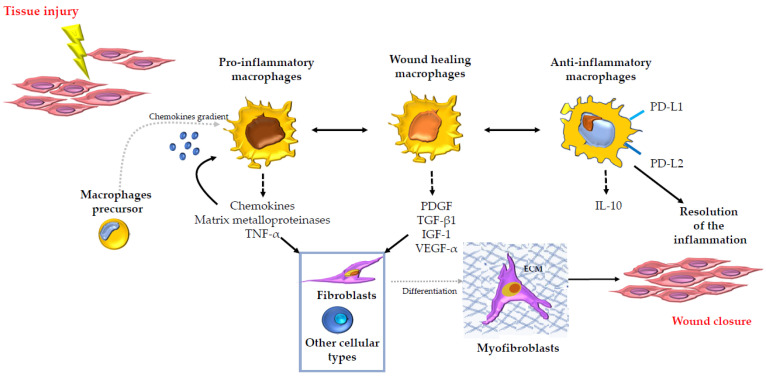
Although a lot of different cellular types such as fibroblasts, epithelial cells, endothelial cells, stem cells, neutrophils, innate lymphoid cells (ILCs), NK cells, B cells and T cells join to the complex inflammatory response that leads to tissue repair [109], macrophages develop a key regulatory role in every stage that characterizes the tissue repair and fibrosis [111]. This capability could be due to the macrophages’ highly flexible programming [112]. In fact, within the injured tissue, the macrophages can be found in several different phenotypic states, and this flexibility allows them to perform many functions beginning from the promotion and resolution of inflammation, including the removal of apoptotic cells, up to the support of cell proliferation following injury [113]. After tissue injury, through chemokine gradients and some different adhesion molecules, a lot of inflammatory monocytes and macrophage precursors are recalled from the bone marrow to the injured site. These recruited cells outnumber the resident tissue macrophages [114,115]. At this point, the release in the local tissue microenvironment of cytokines and growth factors represents the signal for the proliferation of both the recruited and resident macrophages [116,117]. In addition, in response to these signals, the macrophages also change their aspect in order to develop their functions [116,117]. In this way, macrophages assume the phenotype that could be called “pro-inflammatory macrophages” and so they can lead the initial phase of the response to injury since they represent an important source of chemokines, matrix metalloproteinases and other inflammatory mediators such as TNF-α [111]. The inflammatory process in response to injury goes on because of the macrophages’ high flexibility [112]. In fact, they assume the phenotype that could be called "wound healing macrophages", which are specialized in the production and consequent secretion of several growth factors such as PDGF, transforming growth factor-beta 1 (TGF-β1), insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor-alfa (VEGF-α) [118,119,120,121,122]. These molecules stimulate cell proliferation and angiogenesis [118,119,120,121,122]. In addition, under the effect of the soluble mediators produced by the wound healing macrophages, local and recruited tissue fibroblasts are induced to differentiate into myofibroblasts that drive the wound contraction and closure especially through the synthesis of extracellular matrix components [123] such as collagen 1A1, fibronectin and versican. Wound healing macrophages develop their regulatory role [111] also towards neighboring parenchymal and stromal cells’ proliferation and expansion, and they can recruit additional stem cell and local progenitor cell populations in order to make them join to tissue repair in case of severe injury. At this point, the macrophages again change their aspect, gaining another phenotype, which can be called "anti-inflammatory macrophages" [124]. Anti-inflammatory macrophages act in response to several inhibitory mediators such as IL-10 and in turn they release a wide range of anti-inflammatory mediators such as IL-10 and TGF-β1 and show as cell surface receptors the proteins programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), which represent the principal molecules involved in the immune system suppression and in the resolution of the inflammation [125,126,127,128] (Figure 2). Therefore, wound healing is a process that must be tightly regulated, otherwise it may lead to the formation of chronic wounds that in turn may facilitate the development of pathological fibrosis [129]. The macrophages, with their great flexibility that allows them to adopt different phenotypes [112,113], could play a unique, important and critical role at each stage of the wound healing, from the initiation and maintenance up to the resolution of the tissue repair process. Different studies have highlighted the macrophages’ great flexibility. In the literature, this plasticity is often reported as the M1/M2 dichotomy of macrophages. It describes the different macrophage subtypes that are involved in the tissue repair process. The M1/M2 dichotomy describes the macrophage subsets showing the M1 subtypes expressing higher levels of several pro-inflammatory cytokines, such as TNF-α and interleukin-1 beta (IL-1β) and the M2 subtypes expressing increased levels of anti-inflammatory cytokines, such as IL-10 and TGF-β [130,131,132]. Even if this is a widespread nomenclature, it is now thought that the M1/M2 dichotomy is not sufficient at all to describe the several different phenotypes and functions of macrophages in vivo [133], also because both M1 and M2 markers can often be expressed at the same time [134]. In addition to this, studies about tissue repair in skeletal muscle showed that in vivo macrophage activation signaling pathways do not correspond to in vitro M1/M2 ones. Among them, for example, we can mention the signal transducer and activator of transcription 1 (STAT1)/interferon gamma (IFN-γ) receptor [135], canonical M2 markers induced by IL-4 [135], the transducer and activator of transcription 6 (STAT6) in IL-4 signaling [136], the IL-4/IL-13 signaling [137], and last but not least, hypoxia-inducible factors (HIFs) in M1/M2 gene expression [138] and in macrophage accumulation [139] pathways. Therefore, it can be affirmed the M1/M2 macrophage dichotomy was conceived by studying macrophages in culture and it is not suitable in order to describe macrophages in vivo straightforward [140]. The most noteworthy concept we have to focus on is that both definitions of M1/M2 macrophages and pro-inflammatory/wound healing/anti-inflammatory macrophages agree with the fact that macrophages have a great flexibility so that they can assume several different phenotypes [112,113], and this capability may enable them to lead the tissue repair process. Indeed, something dysregulated such as macrophage proliferation, accumulation and infiltration, within the reported macrophage action could lead to uncontrolled repair tissue and to the consequent pathological fibrosis. Several studies have been carried out in order to characterize the macrophages’ behavior within the initiation, maintenance and resolution of the tightly regulated wound healing response in different organs.
Figure 2.
Illustration of the role of the macrophages and their highly flexible programming in tissue repair and fibrosis in several organs. Macrophages, because of their high flexibility, can play a key regulatory role in every stage that characterizes the tissue repair and fibrosis from the promotion to the resolution of the inflammation leading to the wound closure. The figure shows the principal events and principal molecules: chemokines, Matrix metalloproteinases, tumor necrosis factor-alfa (TNF-α), platelet-derived growth factor (PDGF), transforming growth factor-beta 1 (TGF-β1), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor-alfa (VEGF-α), programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), interleukin-10 (IL-10) involved in the process, highlighting the different phenotypic states that the macrophages can assume in the process. The blue net represents the extracellular matrix (ECM) that is produced by myofibroblasts after that fibroblasts or other cellular types differentiated into them.
3. Macrophages in Uterine Fibroids
As it has been mentioned before, inflammation plays an important role in the pathophysiology of the uterine leiomyoma [105], which was defined as a typical fibrotic tissue [42,43].
Several studies have highlighted the involvement and importance of the macrophages in the inflammation and consequent fibrosis that are typical features of leiomyoma tissue [42,43,44,45,46,47,48,89,90,105,106].
Through the use of the glycosylated transmembrane glycoprotein antigen (CD68) that belongs to a family of lysosomal granules [141] as a marker of mature and activated macrophages, Miura et al. studied the macrophages’ infiltration in different types of uterine leiomyomas. They demonstrated the myoma nodules and the autologous endometrium of the submucosal myomas (SMM) and intramural myomas (IMM) show a higher level of macrophage infiltration compared to the corresponding tissues of the subserosal myomas (SSM) or to the eutopic endometrium belonging to women without uterine myomas used as a control [142]. In addition to this, the authors showed a similar pattern also for the MCP-1 concentration. Moreover, MCP-1 concentration was shown to be positively correlated with the macrophage infiltration in SMM and IMM myoma nodules and endometrium [142]. So, the overproduction of MCP-1, which is one of the most important chemokines involved in the monocytes’/macrophages’ migration and infiltration [143], may represent the cause of the macrophages’ infiltration in women with SMM and IMM, and this accumulation of inflammatory macrophages could lead to a negative effect on reproductive outcomes in women with SMM or IMM [142]. Anyway, the increased infiltration and accumulation of macrophages within some subtypes of fibroid tissue may represent proof of the macrophages’ importance within leiomyoma pathology.
In support of all this, Khan and colleagues demonstrated that endometria belonging to women with uterine fibroids undergoing gonadotrophin-releasing hormone agonist (GnRHa) therapy exhibited decreased values of macrophage infiltration and MCP-1 levels when compared to corresponding values of macrophage infiltration and MCP-1 levels in endometria belonging to women with uterine fibroids that had not undergone GnRHa therapy [144].
On the other hand, a previous study conducted by Sozen highlighted that the myometrium of the women with uterine fibroids taking GnRHa and in particular the endothelial cells of blood vessels in myometrial tissues surrounding the leiomyoma show higher MCP-1 levels than the myometrium of the women with uterine fibroids not taking GnRHa [145]. This difference was not accompanied by a significant difference in the number of tissue macrophages between women who had undergone GnRHa therapy and women who had not undergone GnRHa therapy [145]. In this study, Sozen and colleagues expected to detect a macrophage infiltration increase following the MCP-1 increase because of the GnRHa use, but these results were disproved [145]. It is known that the uterus after GnRHa exposition shows a reduced arterial blood flow [146,147] and this may impair the macrophage accumulation, representing the explanation for why a macrophage infiltration increase does not accompany the MCP-1 increase in the myometrium of the women with uterine fibroids taking GnRHa [145].
In addition, estrogens and progesterone, which are recognized to be important promoters of the leiomyoma growth [96,97,98,99,100,101,102,103,104], impair MCP-1 expression [89].
Therefore, the discrepancy between the results obtained by Khan [144] and the results obtained by Sozen [145] may be due to the use of different tissue types or to the difference in tissue specificity and number of analyzed samples. The most important point to focus on is that within the complex network of molecules that are involved in the leiomyomas’ development and growth, MCP-1 can also carry out an important role, taking part in the regulation of the macrophage infiltration. The MCP-1 regulatory action on macrophage infiltration may in turn be important for the development of the uterine fibroids. In addition to this, the cited studies about GnRHa, which is known to be commonly used for the treatment of uterine myomas, testify in any case that macrophages represent an aspect to be taken into consideration for the treatment and further clarification of the etiopathogenesis of uterine fibroids.
In addition, Kitaya and Yasuo have provided further evidence of the involvement and importance of the macrophages in the pathology of uterine fibroids. They analyzed the leukocyte density and composition in the human cycling endometrium in women affected by uterine fibroids. By immunohistochemical analysis, the authors compared endometrium with neighboring nodules with autologous endometrium without neighboring nodules and with allogeneic endometrium belonging to women without uterine fibroids. In particular, the macrophage (CD68 positive cells) density is significantly higher in the endometrium close to the leiomyoma nodules compared to the autologous endometrium far from the leiomyoma, as well as compared to the allogenic endometrium of women without uterine fibroids in the mid-to-late secretory phase [148]. The authors reported also that the endometrium far from the leiomyoma nodules had more macrophages than the endometrium of women without uterine fibroids in the proliferative and late secretory phase [148].
In addition, according to the results obtained by Miura et al. [142], the authors highlighted that the macrophage density is significantly higher in SMM than in IMM and SSM [148].
In addition, Kitaya and Yasuo showed that the whole stromal pan-leukocyte density is altered in the endometrium containing neighboring nodules of the women affected by uterine fibroids. Above all, they highlighted that the increased stromal pan-leukocyte density in endometrium with neighboring nodules during the proliferative phase is largely due to the increased macrophage density [148]. These findings testify once again that macrophages represent a very important aspect within the pathology of uterine fibroids.
Another aspect that is important to highlight about the involvement of the macrophages in uterine fibroids is the GM-CSF expression in leiomyoma and in myometrium. In fact, this cytokine represents the most important growth factor for macrophage proliferation, differentiation and functional activation [149].
In addition to this, GM-CSF has been demonstrated to determine the fibrotic reaction in several tissues [150,151,152,153,154]. In particular, thinking about the association between the overexpression of TGF-β and the establishment of tissue fibrosis through the stimulation of the conversion of fibroblasts into myofibroblasts in various sites throughout the body [155,156,157], GM-CSF has been shown to be involved in a fibrotic process that includes the accumulation of α smooth muscle actin-rich myofibroblasts through a mechanism involving TGF-β expression [150,151,152,153,154,157]. In addition, bearing in mind that it was demonstrated that TGF-β synthesis and release are increased in uterine fibroids [158], all of this makes GM-CSF one of the most important cytokines that may be able to play a key role in the initiation and maintenance of uterine leiomyoma, which is a typical fibrotic disorder [44].
In addition, since GM-CSF is considered to be the most important growth factor for macrophage proliferation, differentiation and functional activation [149], we could think that GM-CSF action and macrophage infiltration as they have been previously described could be interconnected within the development of the uterine fibroid pathology.
Considering the relationship between macrophages and uterine fibroids development, it is relevant that TGF-β is involved in tissue fibrosis in several sites throughout the body [155,156], is overexpressed in leiomyomas [158], and at the same time is the most important growth factor produced by macrophages [159]. In addition, TGF-β contributes to myofibroblast transformation [159], which represents another important aspect leading to the development of uterine fibroids [42,53].
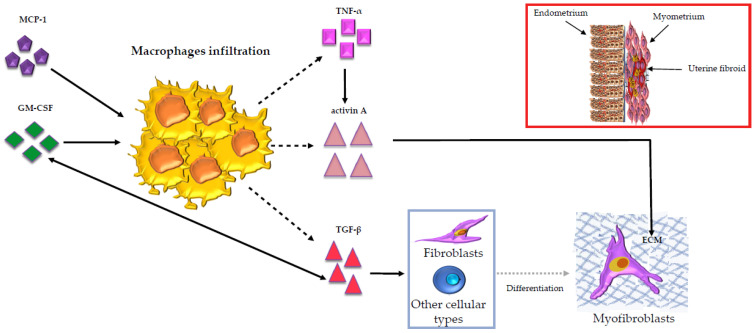
It is very important to highlight that macrophages secrete not only TGF-β, which plays a key role in the progression of the fibrosis [159], but also produce activin A, an immunoregulator belonging to the TGF-β family [160]. Our group showed that, in primary leiomyoma cells, activin A acts as a pro-fibrotic factor leading to the expression of ECM proteins [161] that are upregulated in leiomyoma [44]. In addition to this, we later demonstrated in leiomyoma that activin A mRNA expression is upregulated by TNF-α [53] according to the literature, where the same effect is reported also in human bone marrow stromal cells and monocytes, human bone marrow stromal cell lines, cultured fibroblasts and keratinocytes [162,163,164,165]. The most remarkable aspect about activin A upregulation by TNF-α is that TNF-α is also mainly produced by macrophages [166] (Figure 3).
Figure 3.
Illustration of the macrophages’ (yellow in the figure) role in uterine fibroids. Monocyte chemoattractant protein-1 (MCP-1) takes part in the regulation of the macrophages’ infiltration. The granulocyte macrophage-colony-stimulating factor (GM-CSF) is considered the most important growth factor for macrophage proliferation. GM-CSF can establish regulatory interactions with the transforming growth factor-beta (TGF-β), which was shown to be the most important growth factor secreted by macrophages. In uterine fibroids, TGF-β is overexpressed and it contributes to myofibroblast differentiation. Macrophages also secrete activin A, an immuno-regulator belonging to the TGF-β family. Activin A develops a pro-fibrotic action leading to the expression of the extracellular matrix (ECM) proteins (represented by the blue net in the figure), which are overexpressed in uterine fibroids. Activin A mRNA expression in uterine fibroids is upregulated by the tumor necrosis factor-alfa (TNF-α), an inflammatory mediator mainly produced by macrophages. On the right, above the image that portrays the myofibroblasts, the uterine fibroids (red in the figure) with the myometrium (pink in the figure), the endometrium (brown in the figure), the macrophages (yellow in the figure) and the overexpressed ECM proteins (blue net in the figure) are represented. The blood vessels within endometrium are also represented (red lines in the figure).
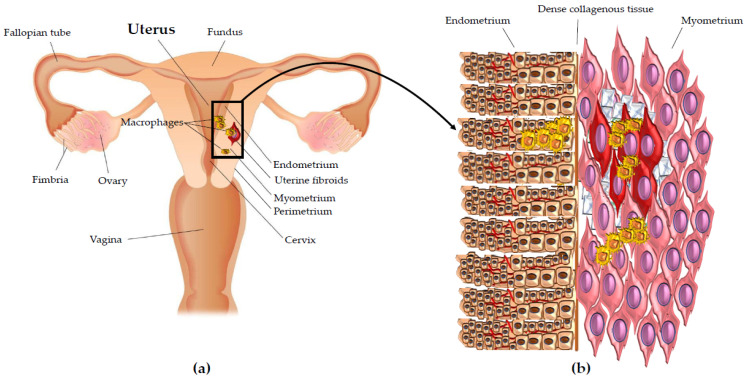
Studying leiomyomas, we found, according to the other results reported in this paper, that macrophage infiltration inside the leiomyoma is significantly higher compared with autologous myometrium more than 1.5 cm from the leiomyoma [53]. More precisely, by CD68 staining, our group found that macrophages predominantly localize inside leiomyoma and in the myometrium tissue next to leiomyoma. On the contrary, autologous distant myometrium showed low levels of CD68-positive macrophages [53] (Figure 4a,b). So, these findings highlight unequivocally the importance of inflammation, and above all, the key role of the macrophages in the development and growth of uterine fibroids.
Figure 4.
Macrophages in uterine fibroids. (a) Illustration of uterus showing the macrophage density in uterine fibroids pathology. (b) Enlargement of the detail showing the macrophage density in uterine fibroids pathology. Macrophages (yellow in the figure) predominantly localize inside uterine fibroids (red in the figure) and in the myometrium tissue (pink in the figure) next to them. Autologous distant myometrium shows low levels of macrophage infiltration. The macrophage density is higher also in the endometrium (brown in the figure) next to uterine fibroids than in the autologous endometrium far from uterine fibroid nodules. The extracellular matrix (ECM) around and within the uterine fibroids is also represented (blue net in the figure). The blood vessels within endometrium are also represented (red lines in the figure).
Since these results were obtained by studying different histotypes of leiomyomas, we could add that the reported macrophage localization is valid for both cellular and usual leiomyomas with the cellular leiomyoma showing higher levels of CD68-positive macrophages compared with usual leiomyoma [53].
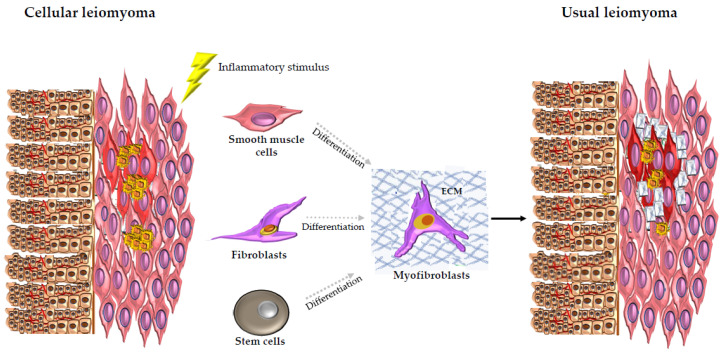
So, our group proposed a possible phase mechanism for the leiomyoma development. According to this mechanism, cellular leiomyoma histotype, as also suggested by Dixon et al. [54], could be considered as the first step in the tumoral transformation. In fact, cellular leiomyomas show low levels of the typical ECM proteins. On the other hand, our group noticed that cellular leiomyomas, in addition to higher levels of CD68 positive macrophages, also have an increased number of leukocytes and mast cells that are other types of inflammatory cells [53]. This aspect could represent a response to an inflammatory stimulus. As a result, these first step cells of the cellular leiomyoma undergo myofibroblast differentiation with the consequent upregulation of the typical ECM proteins [53]. In fact, usual leiomyomas that could be considered as the late-phase tumor show a larger amount of ECM proteins than we observed [53] (Figure 5).
Figure 5.
Illustration of the possible phase mechanism of leiomyoma development proposed by our group. Cellular leiomyoma is considered as the first step in the tumoral transformation. In fact, cellular leiomyoma shows higher levels of macrophage (yellow in the figure) infiltration and an increased number of inflammatory cells. This aspect could represent a response to an inflammatory stimulus that leads some cellular leiomyoma cells to myofibroblast differentiation with the consequent upregulation of the typical extracellular matrix (ECM) proteins. In fact, usual leiomyoma shows a larger amount of ECM proteins and low levels of macrophage infiltration. So, usual leiomyoma could be considered as the late-phase tumor. The blue net represents the typical ECM proteins: collagen 1A1, fibronectin and versican. The red color represents the uterine fibroids (light red for cellular leiomyoma histotype and dark red for usual leiomyoma histotype). The pink color represents the myometrium; the brown color represents the endometrium. The blood vessels within endometrium are also represented (red lines in the figure).
So, the data published by our group provide additional proof of the involvement of inflammation and the importance of the macrophages’ action in the pathophysiology of uterine fibroids.
4. Conclusions
About uterine fibroids, whose etiopathogenesis has not yet understood at all, a deregulated inflammatory process leading to an exaggerated tissue repair may explain the abundant ECM, a typical feature of the uterine fibroids that, just because of this characteristic, is considered a typical fibrotic tissue. In particular, a key role in this complex network can be played by the macrophages when a deregulation in their action happens. In fact, the macrophages are important tissue repair actors by means of their highly flexible programming and their consequent plasticity. So, during the inflammation process and the consequent wound healing that the inflammatory mechanisms lead to, macrophages have to proliferate and infiltrate within the damaged tissue; then, they assume different phenotypes and produce molecules that start, drive and finally stop the tissue repair up to the wound closure. So, there are a lot of critical checkpoints that need to be tightly regulated. In the uterine fibroids, sufficient proof of deregulated macrophage action was provided. In fact, increased infiltration and accumulation of macrophages within some subtypes of fibroid tissue were demonstrated. In addition to this, the importance of cytokines and chemokines such as GM-CSF and MCP-1 for the proliferation and infiltration of the macrophages in the uterine fibroids was shown. Furthermore, their expression in leiomyomas has been altered. All this, in turn, has an impact on the molecules that are secreted by macrophages. Among these molecules, the inflammation mediator TNF-α and the growth factors activin A and TGF-β can be considered the most important ones because they are known to be involved in the fibrosis that characterizes the uterine fibroids. In addition, these molecules, secreted by macrophages, were demonstrated to be interconnected with each other and with the GM-CSF. In this way, they establish in the uterine fibroids a complex network that, because of a dysregulation, at one or more levels, may explain the mechanisms that occur from an excessive wound healing driven by the inflammatory process to the fibrosis.
Better understanding the process leading to the increased infiltration and accumulation of macrophages in leiomyomas and the molecules involved within the consequent exaggerated tissue repair that arises from it, may represent proof of the macrophages’ importance for the leiomyoma pathology. All this can also contribute to shed light on uterine fibroids etiology.
In turn, better understanding the uterine fibroids’ etiology may represent the starting point to identify possible new therapy targets. This could improve the quality of life of the women affected by uterine fibroids.
Last but not least, a therapy against this pathology could also bring about better outcomes for pregnant women affected by this pathology.
Author Contributions
All authors participated in the writing, read and accepted the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McEvoy A., Sabir S. Physiology, Pregnancy Contractions. StatPearls; Treasure Island, FL, USA: 2021. [PubMed] [Google Scholar]
- 2.Valente R., Malesani M.G. Dizionario Medico. Larousse; Paris, France: 1984. p. 928. [Google Scholar]
- 3.Gentile F. Enciclopedia Italiana. Volume 16. Grolier; New Delhi, India: 1987. p. 271. [Google Scholar]
- 4.Day Baird D., Dunson D.B., Hill M.C., Cousins D., Schectman J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 5.Stewart E.A. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 6.Buttram V.C., Jr., Reiter R.C. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil. Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 7.Eldar-Geva T., Meagher S., Healy D.L., MacLachlan V., Breheny S., Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil. Steril. 1998;70:687–691. doi: 10.1016/S0015-0282(98)00265-9. [DOI] [PubMed] [Google Scholar]
- 8.Evans P., Brunsell S. Uterine fibroid tumors: Diagnosis and treatment. Am. Fam. Physician. 2007;75:1503–1508. [PubMed] [Google Scholar]
- 9.Marsh E.E., Bulun S.E. Steroid hormones and leiomyomas. Obstet. Gynecol. Clin. N. Am. 2006;33:59–67. doi: 10.1016/j.ogc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008;22:571–588. doi: 10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Islam M.S., Protic O., Stortoni P., Grechi G., Lamanna P., Petraglia F., Castellucci M., Ciarmela P. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil. Steril. 2013;100:178–193. doi: 10.1016/j.fertnstert.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Ciarmela P., Islam M.S., Reis F.M., Gray P.C., Bloise E., Petraglia F., Vale W., Castellucci M. Growth factors and myometrium: Biological effects in uterine fibroid and possible clinical implications. Hum. Reprod. Update. 2011;17:772–790. doi: 10.1093/humupd/dmr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lethaby A., Puscasiu L., Vollenhoven B. Preoperative medical therapy before surgery for uterine fibroids. Cochrane Database Syst. Rev. 2017;11:CD000547. doi: 10.1002/14651858.CD000547.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angioni S., D’Alterio M.N., Daniilidis A. Highlights on Medical Treatment of Uterine Fibroids. Curr. Pharm. Des. 2021 doi: 10.2174/1381612826666210101152820. [DOI] [PubMed] [Google Scholar]
- 15.Friedman A.J., Hoffman D.I., Comite F., Browneller R.W., Miller J.D. Treatment of leiomyomata uteri with leuprolide acetate depot: A double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet. Gynecol. 1991;77:720–725. [PubMed] [Google Scholar]
- 16.Schlaff W.D., Zerhouni E.A., Huth J.A., Chen J., Damewood M.D., Rock J.A. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet. Gynecol. 1989;74:856–862. [PubMed] [Google Scholar]
- 17.Stovall T.G., Muneyyirci-Delale O., Summitt R.L., Jr., Scialli A.R. GnRH agonist and iron versus placebo and iron in the anemic patient before surgery for leiomyomas: A randomized controlled trial. Leuprolide Acetate Study Group. Obstet. Gynecol. 1995;86:65–71. doi: 10.1016/0029-7844(95)00102-W. [DOI] [PubMed] [Google Scholar]
- 18.Carbonell J.L., Acosta R., Perez Y., Garces R., Sanchez C., Tomasi G. Treatment of Uterine Myoma with 2.5 or 5 mg Mifepristone Daily during 3 Months with 9 Months Posttreatment Followup: Randomized Clinical Trial. ISRN Obstret. Gynecol. 2013;2013:649030. doi: 10.1155/2013/649030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chwalisz K., Larsen L., Mattia-Goldberg C., Edmonds A., Elger W., Winkel C.A. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil. Steril. 2007;87:1399–1412. doi: 10.1016/j.fertnstert.2006.11.094. [DOI] [PubMed] [Google Scholar]
- 20.Wiehle R.D., Goldberg J., Brodniewicz T., Jarus-Dziedzic K., Jabiry-Zieniewicz Z. Effects of a new progesterone receptor modulator, CDB-4124, on fibroid size and uterine bleeding. Obstet. Gynecol. 2008;3:17–20. [Google Scholar]
- 21.Levens E.D., Potlog-Nahari C., Armstrong A.Y., Wesley R., Premkumar A., Blithe D.L., Blocker W., Nieman L.K. CDB-2914 for uterine leiomyomata treatment: A randomized controlled trial. Obstret. Gynecol. 2008;111:1129–1136. doi: 10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieman L.K., Blocker W., Nansel T., Mahoney S., Reynolds J., Blithe D., Wesley R., Armstrong A. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: A randomized, double-blind, placebo-controlled, phase IIb study. Fertil. Steril. 2011;95:767–772.e2. doi: 10.1016/j.fertnstert.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnez J., Tatarchuk T.F., Bouchard P., Puscasiu L., Zakharenko N.F., Ivanova T., Ugocsai G., Mara M., Jilla M.P., Bestel E. Ulipristal acetate versus placebo for fibroid treatment before surgery. N. Engl. J. Med. 2012;366:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 24.Donnez J., Tomaszewski J., Vázquez F., Bouchard P., Lemieszczuk B., Baró F., Nouri K., Selvaggi L., Sodowski K., Bestel E. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N. Engl. J. Med. 2012;366:421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- 25.Blithe D.L., Nieman L.K., Blye R.P., Stratton P., Passaro M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68:1013–1017. doi: 10.1016/S0039-128X(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 26.Attardi B.J., Burgenson J., Hild S.A., Reel J.R., Blye R.P. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: In vitro comparison to mifepristone and CDB-2914. Mol. Cell. Endocrinol. 2002;188:111–123. doi: 10.1016/S0303-7207(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 27.Del Forno S., Degli Esposti E., Salucci P., Leonardi D., Iodice R., Arena A., Raimondo D., Paradisi R., Seracchioli R. Liver function, tolerability and satisfaction during treatment with ulipristal acetate in women with fibroids: A single center experience. Gynecol. Endocrinol. 2020;36:445–447. doi: 10.1080/09513590.2019.1680626. [DOI] [PubMed] [Google Scholar]
- 28.Ciarmela P., Carrarelli P., Islam M.S., Janjusevic M., Zupi E., Tosti C., Castellucci M., Petraglia F. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod. Sci. 2014;21:1120–1125. doi: 10.1177/1933719114542019. [DOI] [PubMed] [Google Scholar]
- 29.Frasca C., Arena A., Degli Esposti E., Raimondo D., Del Forno S., Moro E., Zanello M., Mabrouk M., Seracchioli R. First Impressions Can Be Deceiving: Surgical Outcomes of Laparoscopic Myomectomy in Patients Pretreated with Ulipristal Acetate. J. Minim. Invasive Gynecol. 2020;27:633–638. doi: 10.1016/j.jmig.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Friedman A.J., Rein M.S., Harrison-Atlas D., Garfield J.M., Doubilet P.M. A randomized, placebo-controlled, double-blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertil. Steril. 1989;52:728–733. doi: 10.1016/S0015-0282(16)61022-1. [DOI] [PubMed] [Google Scholar]
- 31.Islam M.S., Protic O., Giannubilo S.R., Toti P., Tranquilli A.L., Petraglia F., Castellucci M., Ciarmela P. Uterine leiomyoma: Available medical treatments and new possible therapeutic options. J. Clin. Endocrinol. Metab. 2013;98:921–934. doi: 10.1210/jc.2012-3237. [DOI] [PubMed] [Google Scholar]
- 32.Wallach E.E., Vlahos N.F. Uterine myomas: An overview of development, clinical features, and management. Obstret. Gynecol. 2004;104:393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- 33.Kim T., Purdy M.P., Kendall-Rauchfuss L., Habermann E.B., Bews K.A., Glasgow A.E., Khan Z. Myomectomy associated blood transfusion risk and morbidity after surgery. Fertil. Steril. 2020;114:175–184. doi: 10.1016/j.fertnstert.2020.02.110. [DOI] [PubMed] [Google Scholar]
- 34.Flynn M., Jamison M., Datta S., Myers E. Health care resource use for uterine fibroid tumors in the United States. Am. J. Obstet. Gynecol. 2006;195:955–964. doi: 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Zepiridis L.I., Grimbizis G.F., Tarlatzis B.C. Infertility and uterine fibroids. Best Pract. Res. Clin. Obstret. Gynaecol. 2016;34:66–73. doi: 10.1016/j.bpobgyn.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Kurman R., Ellenson L., Ronnett B. Blaustein’s Pathology of the Female Genital Tract. Int. J. Gynecol. Pathol. 2016 doi: 10.1007/1978-1001-4614-3165-1007. [DOI] [Google Scholar]
- 37.Rosai J., Ackerman L. Rosai and Ackerman’s Surgical Pathology. 10th ed. Elsevier; Amsterdam, The Netherlands: 2012. pp. 1508–1513. [Google Scholar]
- 38.Avritscher R., Iyer R.B., Ro J., Whitman G. Lipoleiomyoma of the uterus. AJR Am. J. Roentgenol. 2001;177:856. doi: 10.2214/ajr.177.4.1770856. [DOI] [PubMed] [Google Scholar]
- 39.Myles J.L., Hart W.R. Apoplectic leiomyomas of the uterus. A clinicopathologic study of five distinctive hemorrhagic leiomyomas associated with oral contraceptive usage. Am. J. Surg. Pathol. 1985;9:798–805. doi: 10.1097/00000478-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Ciarmela P., Islam M.S., Lamanna P., Tranquilli A.L., Castellucci M. Healthy and pathological changes of myometrium: Pregnant myometrium, uterine fibroids and leiomyosarcoma. Rev. Argent. Anatomía Clínica. 2012;4:7–13. doi: 10.31051/1852.8023.v4.n1.13945. [DOI] [Google Scholar]
- 41.Toledo G., Oliva E. Smooth muscle tumors of the uterus: A practical approach. Arch. Pathol. Lab. Med. 2008;132:595–605. doi: 10.5858/2008-132-595-SMTOTU. [DOI] [PubMed] [Google Scholar]
- 42.Leppert P.C., Catherino W.H., Segars J.H. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am. J. Obstret. Gynecol. 2006;195:415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malik M., Norian J., McCarthy-Keith D., Britten J., Catherino W.H. Why leiomyomas are called fibroids: The central role of extracellular matrix in symptomatic women. Semin. Reprod. Med. 2010;28:169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- 44.Gelse K., Pöschl E., Aigner T. Collagens-structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Fujita M. Histological and biochemical studies of collagen in human uterine leiomyomas. Hokkaido J. Med. Sci. 1985;60:602–615. [PubMed] [Google Scholar]
- 46.Arici A., Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil. Steril. 2000;73:1006–1011. doi: 10.1016/S0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 47.Norian J.M., Malik M., Parker C.Y., Joseph D., Leppert P.C., Segars J.H., Catherino W.H. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod. Sci. 2009;16:1153–1164. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart E.A., Friedman A.J., Peck K., Nowak R.A. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J. Clin. Endocrinol. Metab. 1994;79:900–906. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- 49.Giuliani A., Greco S., Pacile S., Zannotti A., Delli Carpini G., Tromba G., Giannubilo S.R., Ciavattini A., Ciarmela P. Advanced 3D Imaging of Uterine Leiomyoma’s Morphology by Propagation-based Phase-Contrast Microtomography. Sci. Rep. 2019;9:10580. doi: 10.1038/s41598-019-47048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker C.L., Stewart E.A. Uterine fibroids: The elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 51.Hulboy D.L., Rudolph L.A., Matrisian L.M. Matrix metalloproteinases as mediators of reproductive function. Mol. Hum. Reprod. 1997;3:27–45. doi: 10.1093/molehr/3.1.27. [DOI] [PubMed] [Google Scholar]
- 52.Ohara N. Sex steroidal modulation of collagen metabolism in uterine leiomyomas. Clin. Exp. Obstet. Gynecol. 2009;36:10. [PubMed] [Google Scholar]
- 53.Protic O., Toti P., Islam M.S., Occhini R., Giannubilo S.R., Catherino W.H., Cinti S., Petraglia F., Ciavattini A., Castellucci M., et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016;364:415–427. doi: 10.1007/s00441-015-2324-3. [DOI] [PubMed] [Google Scholar]
- 54.Flake G.P., Andersen J., Dixon D. Etiology and pathogenesis of uterine leiomyomas: A review. Environ. Health Perspect. 2003;111:1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiechle-Schwarz M., Sreekantaiah C., Berger C.S., Pedron S., Medchill M.T., Surti U., Sandberg A.A. Nonrandom cytogenetic changes in leiomyomas of the female genitourinary tract. A report of 35 cases. Cancer Genet. Cytogenet. 1991;53:125–136. doi: 10.1016/0165-4608(91)90124-D. [DOI] [PubMed] [Google Scholar]
- 56.Rein M.S., Friedman A.J., Barbieri R.L., Pavelka K., Fletcher J.A., Morton C.C. Cytogenetic abnormalities in uterine leiomyomata. Obstet. Gynecol. 1991;77:923–926. [PubMed] [Google Scholar]
- 57.Meloni A.M., Surti U., Contento A.M., Davare J., Sandberg A.A. Uterine leiomyomas: Cytogenetic and histologic profile. Obstet. Gynecol. 1992;80:209–217. [PubMed] [Google Scholar]
- 58.Cha P.C., Takahashi A., Hosono N., Low S.K., Kamatani N., Kubo M., Nakamura Y. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat. Genet. 2011;43:447–450. doi: 10.1038/ng.805. [DOI] [PubMed] [Google Scholar]
- 59.Ligon A.H., Scott I.C., Takahara K., Greenspan D.S., Morton C.C. PCOLCE deletion and expression analyses in uterine leiomyomata. Cancer Genet. Cytogenet. 2002;137:133–137. doi: 10.1016/S0165-4608(02)00547-2. [DOI] [PubMed] [Google Scholar]
- 60.Ptacek T., Song C., Walker C.L., Sell S.M. Physical mapping of distinct 7q22 deletions in uterine leiomyoma and analysis of a recently annotated 7q22 candidate gene. Cancer Genet. Cytogenet. 2007;174:116–120. doi: 10.1016/j.cancergencyto.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 61.Velagaleti G.V., Tonk V.S., Hakim N.M., Wang X., Zhang H., Erickson-Johnson M.R., Medeiros F., Oliveira A.M. Fusion of HMGA2 to COG5 in uterine leiomyoma. Cancer Genet. Cytogenet. 2011;202:11–16. doi: 10.1016/j.cancergencyto.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Mäkinen N., Mehine M., Tolvanen J., Kaasinen E., Li Y., Lehtonen H.J., Gentile M., Yan J., Enge M., Taipale M. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334:252–255. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 63.Nezhad M.H., Drieschner N., Helms S., Meyer A., Tadayyon M., Klemke M., Belge G., Bartnitzke S., Burchardt K., Frantzen C. 6p21 rearrangements in uterine leiomyomas targeting HMGA1. Cancer Genet. Cytogenet. 2010;203:247–252. doi: 10.1016/j.cancergencyto.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Sandberg A.A. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: Leiomyoma. Cancer Genet. Cytogenet. 2005;158:1–26. doi: 10.1016/j.cancergencyto.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 65.El-Gharib M.N., Elsobky E.S. Cytogenetic aberrations and the development of uterine leiomyomata. J. Obstet. Gynaecol. Res. 2010;36:101–107. doi: 10.1111/j.1447-0756.2009.01099.x. [DOI] [PubMed] [Google Scholar]
- 66.Mehine M., Kaasinen E., Makinen N., Katainen R., Kampjarvi K., Pitkanen E., Heinonen H.R., Butzow R., Kilpivaara O., Kuosmanen A., et al. Characterization of uterine leiomyomas by whole-genome sequencing. N. Engl. J. Med. 2013;369:43–53. doi: 10.1056/NEJMoa1302736. [DOI] [PubMed] [Google Scholar]
- 67.Mehine M., Makinen N., Heinonen H.R., Aaltonen L.A., Vahteristo P. Genomics of uterine leiomyomas: Insights from high-throughput sequencing. Fertil. Steril. 2014;102:621–629. doi: 10.1016/j.fertnstert.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 68.Marsh E.E., Lin Z., Yin P., Milad M., Chakravarti D., Bulun S.E. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil. Steril. 2008;89:1771–1776. doi: 10.1016/j.fertnstert.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navarro A., Yin P., Monsivais D., Lin S.M., Du P., Wei J.J., Bulun S.E. Genome-Wide DNA Methylation Indicates Silencing of Tumor Suppressor Genes in Uterine Leiomyoma. PLoS ONE. 2012;7:e33284. doi: 10.1371/journal.pone.0033284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei L.H., Torng P.L., Hsiao S.M., Jeng Y.M., Chen M.W., Chen C.A. Histone Deacetylase 6 Regulates Estrogen Receptor α in Uterine Leiomyoma. Reprod. Sci. 2011;18:755–762. doi: 10.1177/1933719111398147. [DOI] [PubMed] [Google Scholar]
- 71.Yang Q., Mas A., Diamond M.P., Al-Hendy A. The Mechanism and Function of Epigenetics in Uterine Leiomyoma Development. Reprod. Sci. 2016;23:163–175. doi: 10.1177/1933719115584449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang T., Zhang X., Obijuru L., Laser J., Aris V., Lee P., Mittal K., Soteropoulos P., Wei J.J. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 73.Liu J., Matsuo H., Xu Q., Chen W., Wang J., Maruo T. Concentration-dependent effects of a selective estrogen receptor modulator raloxifene on proliferation and apoptosis in human uterine leiomyoma cells cultured in vitro. Hum. Reprod. 2007;22:1253–1259. doi: 10.1093/humrep/del515. [DOI] [PubMed] [Google Scholar]
- 74.Georgieva B., Milev I., Minkov I., Dimitrova I., Bradford A.P., Baev V. Characterization of the uterine leiomyoma microRNAome by deep sequencing. Genomics. 2012;93:275–281. doi: 10.1016/j.ygeno.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Ciebiera M., Wlodarczyk M., Zgliczynski S., Lozinski T., Walczak K., Czekierdowski A. The Role of miRNA and Related Pathways in Pathophysiology of Uterine Fibroids-From Bench to Bedside. Int. J. Mol. Sci. 2020;21:3016. doi: 10.3390/ijms21083016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nothnick W.B. Non-coding RNAs in Uterine Development, Function and Disease. Adv. Exp. Med. Biol. 2016;886:171–189. doi: 10.1007/978-94-017-7417-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baranov V.S., Osinovskaya N.S., Yarmolinskaya M.I. Pathogenomics of Uterine Fibroids Development. Int. J. Mol. Sci. 2019;20:6151. doi: 10.3390/ijms20246151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McWilliams M.M., Chennathukuzhi V.M. Recent Advances in Uterine Fibroid Etiology. Semin. Reprod. Med. 2017;35:181–189. doi: 10.1055/s-0037-1599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mallik S., Maulik U. MiRNA-TF-gene network analysis through ranking of biomolecules for multi-informative uterine leiomyoma dataset. J. Biomed. Inf. 2015;57:308–319. doi: 10.1016/j.jbi.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 80.Ciarmela P., Bloise E., Gray P.C., Carrarelli P., Islam M.S., De Pascalis F., Severi F.M., Vale W., Castellucci M., Petraglia F. Activin-A and myostatin response and steroid regulation in human myometrium: Disruption of their signalling in uterine fibroid. J. Clin. Endocrinol. Metab. 2011;96:755–765. doi: 10.1210/jc.2010-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moro E., Degli Esposti E., Borghese G., Manzara F., Zanello M., Raimondo D., Gava G., Arena A., Casadio P., Meriggiola M.C., et al. The Impact of Hormonal Replacement Treatment in Postmenopausal Women with Uterine Fibroids: A State-of-the-Art Review of the Literature. Medicina (Kaunas) 2019;55:549. doi: 10.3390/medicina55090549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sozen I., Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil. Steril. 2002;78:1–12. doi: 10.1016/S0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- 83.Ciarmela P., Wiater E., Vale W. Activin-A in myometrium: Characterization of the actions on myometrial cells. Endocrinology. 2008;149:2506–2516. doi: 10.1210/en.2007-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ciarmela P., Wiater E., Smith S.M., Vale W. Presence, actions, and regulation of myostatin in rat uterus and myometrial cells. Endocrinology. 2009;150:906–914. doi: 10.1210/en.2008-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hatthachote P., Gillespie J.I. Complex interactions between sex steroids and cytokines in the human pregnant myometrium: Evidence for an autocrine signaling system at term. Endocrinology. 1999;140:2533–2540. doi: 10.1210/endo.140.6.6785. [DOI] [PubMed] [Google Scholar]
- 86.Litovkin K.V., Domenyuk V.P., Bubnov V.V., Zaporozhan V.N. Interleukin-6-174G/C polymorphism in breast cancer and uterine leiomyoma patients: A population-based case control study. Exp. Oncol. 2007;29:295–298. [PubMed] [Google Scholar]
- 87.Kurachi O., Matsuo H., Samoto T., Maruo T. Tumor necrosis factor-α expression in human uterine leiomyoma and its down-regulation by progesterone. J. Clin. Endocrinol. Metab. 2001;86:2275–2280. doi: 10.1210/jc.86.5.2275. [DOI] [PubMed] [Google Scholar]
- 88.Syssoev K.A., Kulagina N.V., Chukhlovin A.B., Morozova E.B., Totolian A.A. Expression of mRNA for chemokines and chemokine receptors in tissues of the myometrium and uterine leiomyoma. Bull. Exp. Biol. Med. 2008;145:84–89. doi: 10.1007/s10517-008-0038-1. [DOI] [PubMed] [Google Scholar]
- 89.Sozen I., Olive D.L., Arici A. Expression and hormonal regulation of monocyte chemotactic protein-1 in myometrium and leiomyomata. Fertil. Steril. 1998;69:1095–1102. doi: 10.1016/S0015-0282(98)00072-7. [DOI] [PubMed] [Google Scholar]
- 90.Nair S., Al-Hendy A. Adipocytes enhance the proliferation of human leiomyoma cells via TNF-α proinflammatory cytokine. Reprod. Sci. 2011;18:1186–1192. doi: 10.1177/1933719111408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bodner-Adler B., Bodner K., Kimberger O., Czerwenka K., Leodolter S., Mayerhofer K. Expression of matrix metalloproteinases in patients with uterine smooth muscle tumors: An immunohistochemical analysis of MMP-1 and MMP-2 protein expression in leiomyoma, uterine smooth muscle tumor of uncertain malignant potential, and leiomyosarcoma. J. Soc. Gynecol. Investig. 2004;11:182–186. doi: 10.1016/j.jsgi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 92.Wolanska M., Sobolewski K., Bańkowski E., Jaworski S. Matrix metalloproteinases of human leiomyoma in various stages of tumor growth. Gynecol. Obstet. Investig. 2004;58:14–18. doi: 10.1159/000077177. [DOI] [PubMed] [Google Scholar]
- 93.Bogusiewicz M., Stryjecka-Zimmer M., Postawski K., Jakimiuk A.J., Rechberger T. Activity of matrix metalloproteinase-2 and-9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol. Endocrinol. 2007;23:541–546. doi: 10.1080/09513590701557416. [DOI] [PubMed] [Google Scholar]
- 94.Walker C.L., Hunter D., Everitt J.I. Uterine leiomyoma in the Eker rat: A unique model for important diseases of women. Genes Chromosomes Cancer. 2003;38:349–356. doi: 10.1002/gcc.10281. [DOI] [PubMed] [Google Scholar]
- 95.Cook J.D., Walker C.L. The Eker rat: Establishing a genetic paradigm linking renal cell carcinoma and uterine leiomyoma. Curr. Mol. Med. 2004;4:813–824. doi: 10.2174/1566524043359656. [DOI] [PubMed] [Google Scholar]
- 96.Andersen J., Barbieri R.L. Abnormal gene expression in uterine leiomyomas. J. Soc. Gynecol. Investig. 1995;2:663–672. doi: 10.1177/107155769500200501. [DOI] [PubMed] [Google Scholar]
- 97.Maruo T., Ohara N., Wang J., Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update. 2004;10:207–220. doi: 10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- 98.Sadan O., Van Iddekinge B., Van Gelderen C.J., Savage N., Becker P.J., Van Der Walt L.A., Robinson M. Oestrogen and progesterone receptor concentrations in leiomyoma and normal myometrium. Ann. Clin. Biochem. 1987;24:263–267. doi: 10.1177/000456328702400304. [DOI] [PubMed] [Google Scholar]
- 99.Kim J.J., Sefton E.C. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol. Cell. Endocrinol. 2011;358:223–231. doi: 10.1016/j.mce.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brandon D.D., Bethea C.L., Strawn E.Y., Novy M.J., Burry K.A., Harrington M.S., Erickson T.E., Warner C., Keenan E.J., Clinton G.M. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am. J. Obstet. Gynecol. 1993;169:78–85. doi: 10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- 101.Marelli G., Codegoni A.M., Bizzi A. Estrogen and progesterone receptors in leiomyomas and normal uterine tissues during reproductive life. Acta Eur. Fertil. 1989;20:19–22. [PubMed] [Google Scholar]
- 102.Viville B., Charnock-Jones D.S., Sharkey A.M., Wetzka B., Smith S.K. Distribution of the A and B forms of the progesterone receptor messenger ribonucleic acid and protein in uterine leiomyomata and adjacent myometrium. Hum. Reprod. 1997;12:815–822. doi: 10.1093/humrep/12.4.815. [DOI] [PubMed] [Google Scholar]
- 103.Ying Z., Weiyuan Z. Dual actions of progesterone on uterine leiomyoma correlate with the ratio of progesterone receptor A:B. Gynecol. Endocrinol. 2009;25:520–523. doi: 10.1080/09513590902972117. [DOI] [PubMed] [Google Scholar]
- 104.Fujimoto J., Hirose R., Ichigo S., Sakaguchi H., Li Y., Tamaya T. Expression of progesterone receptor form A and B mRNAs in uterine leiomyoma. Tumor Biol. 1998;19:126–131. doi: 10.1159/000029983. [DOI] [PubMed] [Google Scholar]
- 105.Protic O., Islam M.S., Greco S., Giannubilo S.R., Lamanna P., Petraglia F., Ciavattini A., Castellucci M., Hinz B., Ciarmela P. Activin A in Inflammation, Tissue Repair, and Fibrosis: Possible Role as Inflammatory and Fibrotic Mediator of Uterine Fibroid Development and Growth. Semin. Reprod. Med. 2017;35:499–509. doi: 10.1055/s-0037-1607265. [DOI] [PubMed] [Google Scholar]
- 106.Kisseleva T., Brenner D.A. Mechanisms of fibrogenesis. Exp. Biol. Med. 2008;233:109–122. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 107.Hinz B., Phan S.H., Thannickal V.J., Prunotto M., Desmouliere A., Varga J., De Wever O., Mareel M., Gabbiani G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hinz B. Formation and function of the myofibroblast during tissue repair. J. Investig. Derm. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 109.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kacperczyk J., Bartnik P., Romejko-Wolniewicz E., Dobrowolska-Redo A. Postmyomectomic Uterine Rupture Despite Cesarean Section. Anticancer Res. 2016;36:1011–1013. [PubMed] [Google Scholar]
- 111.Wynn T.A., Barron L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lech M., Anders H.J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 114.Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat. Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Galli S.J., Borregaard N., Wynn T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jenkins S.J., Ruckerl D., Cook P.C., Jones L.H., Finkelman F.D., van Rooijen N., MacDonald A.S., Allen J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jenkins S.J., Ruckerl D., Thomas G.D., Hewitson J.P., Duncan S., Brombacher F., Maizels R.M., Hume D.A., Allen J.E. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J. Exp. Med. 2013;210:2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berse B., Brown L.F., Van de Water L., Dvorak H.F., Senger D.R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol. Biol. Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chujo S., Shirasaki F., Kondo-Miyazaki M., Ikawa Y., Takehara K. Role of connective tissue growth factor and its interaction with basic fibroblast growth factor and macrophage chemoattractant protein-1 in skin fibrosis. J. Cell Physiol. 2009;220:189–195. doi: 10.1002/jcp.21750. [DOI] [PubMed] [Google Scholar]
- 120.Rappolee D.A., Mark D., Banda M.J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: Analysis by mRNA phenotyping. Science. 1988;241:708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- 121.Shimokado K., Raines E.W., Madtes D.K., Barrett T.B., Benditt E.P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985;43:277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- 122.Willenborg S., Lucas T., van Loo G., Knipper J.A., Krieg T., Haase I., Brachvogel B., Hammerschmidt M., Nagy A., Ferrara N., et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 123.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramachandran P., Iredale J.P., Fallowfield J.A. Resolution of liver fibrosis: Basic mechanisms and clinical relevance. Semin. Liver Dis. 2015;35:119–131. doi: 10.1055/s-0035-1550057. [DOI] [PubMed] [Google Scholar]
- 125.Khalil N., Bereznay O., Sporn M., Greenberg A.H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J. Exp. Med. 1989;170:727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Said E.A., Dupuy F.P., Trautmann L., Zhang Y., Shi Y., El-Far M., Hill B.J., Noto A., Ancuta P., Peretz Y., et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shouval D.S., Biswas A., Goettel J.A., McCann K., Conaway E., Redhu N.S., Mascanfroni I.D., Al Adham Z., Lavoie S., Ibourk M., et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zigmond E., Bernshtein B., Friedlander G., Walker C.R., Yona S., Kim K.W., Brenner O., Krauthgamer R., Varol C., Muller W., et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 129.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tidball J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017;17:165–178. doi: 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R.K., Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saini J., McPhee J.S., Al-Dabbagh S., Stewart C.E., Al-Shanti N. Regenerative function of immune system: Modulation of muscle stem cells. Ageing Res. Rev. 2016;27:67–76. doi: 10.1016/j.arr.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 133.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stables M.J., Shah S., Camon E.B., Lovering R.C., Newson J., Bystrom J., Farrow S., Gilroy D.W. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–e208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Varga T., Mounier R., Horvath A., Cuvellier S., Dumont F., Poliska S., Ardjoune H., Juban G., Nagy L., Chazaud B. Highly Dynamic Transcriptional Signature of Distinct Macrophage Subsets during Sterile Inflammation, Resolution, and Tissue Repair. J. Immunol. 2016;196:4771–4782. doi: 10.4049/jimmunol.1502490. [DOI] [PubMed] [Google Scholar]
- 136.Varga T., Mounier R., Patsalos A., Gogolak P., Peloquin M., Horvath A., Pap A., Daniel B., Nagy G., Pintye E., et al. Macrophage PPARgamma, a Lipid Activated Transcription Factor Controls the Growth Factor GDF3 and Skeletal Muscle Regeneration. Immunity. 2016;45:1038–1051. doi: 10.1016/j.immuni.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heredia J.E., Mukundan L., Chen F.M., Mueller A.A., Deo R.C., Locksley R.M., Rando T.A., Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Takeda N., O’Dea E.L., Doedens A., Kim J.W., Weidemann A., Stockmann C., Asagiri M., Simon M.C., Hoffmann A., Johnson R.S. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gondin J., Theret M., Duhamel G., Pegan K., Mathieu J.R., Peyssonnaux C., Cuvellier S., Latroche C., Chazaud B., Bendahan D., et al. Myeloid HIFs are dispensable for resolution of inflammation during skeletal muscle regeneration. J. Immunol. 2015;194:3389–3399. doi: 10.4049/jimmunol.1401420. [DOI] [PubMed] [Google Scholar]
- 140.Oishi Y., Manabe I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018;30:511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 141.Holness C.L., Simmons D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. doi: 10.1182/blood.V81.6.1607.1607. [DOI] [PubMed] [Google Scholar]
- 142.Miura S., Khan K.N., Kitajima M., Hiraki K., Moriyama S., Masuzaki H., Samejima T., Fujishita A., Ishimaru T. Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas. Hum. Reprod. 2006;21:2545–2554. doi: 10.1093/humrep/del205. [DOI] [PubMed] [Google Scholar]
- 143.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khan K.N., Kitajima M., Hiraki K., Fujishita A., Sekine I., Ishimaru T., Masuzaki H. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum. Reprod. 2010;25:642–653. doi: 10.1093/humrep/dep437. [DOI] [PubMed] [Google Scholar]
- 145.Sozen I., Senturk L.M., Arici A. Effect of gonadotropin-releasing hormone agonists on monocyte chemotactic protein-1 production and macrophage infiltration in leiomyomatous uterus. Fertil. Steril. 2001;76:792–796. doi: 10.1016/S0015-0282(01)02378-0. [DOI] [PubMed] [Google Scholar]
- 146.Aleem F.A., Predanic M. The hemodynamic effect of GnRH agonist therapy on uterine leiomyoma vascularity: A prospective study using transvaginal color Doppler sonography. Gynecol. Endocrinol. 1995;9:253–258. doi: 10.3109/09513599509160454. [DOI] [PubMed] [Google Scholar]
- 147.Matta W.H., Stabile I., Shaw R.W., Campbell S. Doppler assessment of uterine blood flow changes in patients with fibroids receiving the gonadotropin-releasing hormone agonist Buserelin. Fertil. Steril. 1988;49:1083–1085. doi: 10.1016/S0015-0282(16)59966-X. [DOI] [PubMed] [Google Scholar]
- 148.Kitaya K., Yasuo T. Leukocyte density and composition in human cycling endometrium with uterine fibroids. Hum. Immunol. 2010;71:158–163. doi: 10.1016/j.humimm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 149.Rasko J.E., Ben H. Granulocyte-macrophage colony stimulating factor. In: Thomson A., editor. The Cytokine Handbook. Academic Press; London, UK: 1994. pp. 343–369. [Google Scholar]
- 150.Andreutti D., Gabbiani G., Neuville P. Early granulocyte-macrophage colony-stimulating factor expression by alveolar inflammatory cells during bleomycin-induced rat lung fibrosis. Lab. Investig. 1998;78:1493–1502. [PubMed] [Google Scholar]
- 151.Rubbia-Brandt L., Sappino A.P., Gabbiani G. Locally applied GM-CSF induces the accumulation of alpha-smooth muscle actin containing myofibroblasts. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1991;60:73–82. doi: 10.1007/BF02899530. [DOI] [PubMed] [Google Scholar]
- 152.Xing Z., Gauldie J., Tremblay G.M., Hewlett B.R., Addison C. Intradermal transgenic expression of granulocyte-macrophage colony-stimulating factor induces neutrophilia, epidermal hyperplasia, Langerhans’ cell/macrophage accumulation, and dermal fibrosis. Lab. Investig. A J. Tech. Methods Pathol. 1997;77:615. [PubMed] [Google Scholar]
- 153.Xing Z., Tremblay G.M., Sime P.J., Gauldie J. Overexpression of granulocyte-macrophage colony-stimulating factor induces pulmonary granulation tissue formation and fibrosis by induction of transforming growth factor-beta 1 and myofibroblast accumulation. Am. J. Pathol. 1997;150:59–66. [PMC free article] [PubMed] [Google Scholar]
- 154.Xing Z., Ohkawara Y., Jordana M., Graham F., Gauldie J. Transfer of granulocyte-macrophage colony-stimulating factor gene to rat lung induces eosinophilia, monocytosis, and fibrotic reactions. J. Clin. Investig. 1996;97:1102–1110. doi: 10.1172/JCI118503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Border W.A., Noble N.A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 156.Roberts A.B. Transforming growth factor-beta: Activity and efficacy in animal models of wound healing. Wound Repair Regen. 1995;3:408–418. doi: 10.1046/j.1524-475X.1995.30405.x. [DOI] [PubMed] [Google Scholar]
- 157.Vyalov S., Desmouliere A., Gabbiani G. GM-CSF-induced granulation tissue formation: Relationships between macrophage and myofibroblast accumulation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993;63:231–239. doi: 10.1007/BF02899267. [DOI] [PubMed] [Google Scholar]
- 158.Ciebiera M., Wlodarczyk M., Wrzosek M., Meczekalski B., Nowicka G., Lukaszuk K., Ciebiera M., Slabuszewska-Jozwiak A., Jakiel G. Role of Transforming Growth Factor beta in Uterine Fibroid Biology. Int. J. Mol. Sci. 2017;18:2435. doi: 10.3390/ijms18112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fallowfield J.A., Mizuno M., Kendall T.J., Constandinou C.M., Benyon R.C., Duffield J.S., Iredale J.P. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 160.Sierra-Filardi E., Puig-Kroger A., Blanco F.J., Nieto C., Bragado R., Palomero M.I., Bernabeu C., Vega M.A., Corbi A.L. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011;117:5092–5101. doi: 10.1182/blood-2010-09-306993. [DOI] [PubMed] [Google Scholar]
- 161.Islam M.S., Catherino W.H., Protic O., Janjusevic M., Gray P.C., Giannubilo S.R., Ciavattini A., Lamanna P., Tranquilli A.L., Petraglia F., et al. Role of activin-A and myostatin and their signaling pathway in human myometrial and leiomyoma cell function. J. Clin. Endocrinol. Metab. 2014;99:E775–E785. doi: 10.1210/jc.2013-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hubner G., Werner S. Serum growth factors and proinflammatory cytokines are potent inducers of activin expression in cultured fibroblasts and keratinocytes. Exp. Cell Res. 1996;228:106–113. doi: 10.1006/excr.1996.0305. [DOI] [PubMed] [Google Scholar]
- 163.Shao L., Frigon N.L., Jr., Sehy D.W., Yu A.L., Lofgren J., Schwall R., Yu J. Regulation of production of activin A in human marrow stromal cells and monocytes. Exp. Hematol. 1992;20:1235–1242. [PubMed] [Google Scholar]
- 164.Shao L.E., Frigon N.L., Jr., Yu A., Palyash J., Yu J. Contrasting effects of inflammatory cytokines and glucocorticoids on the production of activin A in human marrow stromal cells and their implications. Cytokine. 1998;10:227–235. doi: 10.1006/cyto.1997.0282. [DOI] [PubMed] [Google Scholar]
- 165.Takahashi S., Uchimaru K., Harigaya K., Asano S., Yamashita T. Tumor necrosis factor and interleukin-1 induce activin A gene expression in a human bone marrow stromal cell line. Biochem. Biophys. Res. Commun. 1992;188:310–317. doi: 10.1016/0006-291X(92)92386-C. [DOI] [PubMed] [Google Scholar]
- 166.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.