Abstract
Background & aims
ESPEN guidelines advocate that energy needs of critically ill patients with COVID 19 should be assessed using indirect calorimetry, if safely available. This study described energy needs of intubated patients with COVID-19 and explores whether neuromuscular blockade administration (NMBAs) is associated with altered energy expenditure.
Methods
Resting energy expenditure (REE) and respiratory exchange rate (RER) evaluated among critically ill intubated COVID-19 patients until 28th day of intensive care unit stay (ICU–S) by indirect calorimetry. Paralysed patients were defined as those with drug induced paralysis using cicatracurium, for at least 3 days during their ICU-S.
Results
34 adult COVID 19 patients (59.8% male, 35.2% obese) requiring mechanical ventilation were assessed prospectively. REE measurements suggest a gradual increase of energy needs post 3rd day of ICU-S in both patients without obesity (non ob) ((from 17.8 kcal/kgr up to 29.3 kcal/kgr actual body weight (AcBW) during 28th day of ICU-S, p = 0.011)) and patients with obesity (ob) ((from 18.1 kcal/kgr up to 30.1 kcal/kgr adjusted body weight (AjBW) during 28th day of ICU-S, p = 0.021)). NMBAs use was accompanied by a significant drop in REE, especially during first 7 days of hospitalization, both in non ob (22.9 vs 17.9 kcal/kgr AcBW, p = 0.014) and ob patients (22.5 vs 19.5 kcal/kgr ABW, p = 0.027).
Conclusion
We identified the energy needs of COVID-19 intubated patients and highlighted a significant increase beyond the 1st week in the ICU. Administration of NMBAs should be considered, as it may impact resting energy expenditure.
Keywords: COVID 19, Resting energy expenditure, Neuromuscular blockade
Abbreviations: AcBW, actual body weight; AjBW, Adjusted Body Weight; APACHE, Acute Physiology and Chronic Health Evaluation; BMI, Body Mass Index; Fi02, fraction of inspired oxygen; ICU, intensive care unit; NMBAs, Neuromuscular blocking agents; NUTRIC SCORE, Nutrition Risk in the Critically Il; REE, Resting Energy Expenditure
1. Introduction
COVID 19 has affected the lives of millions of people worldwide since it was declared as a pandemic by the World Health Organization on March 11, 2020, and represent a major cause of morbidity and mortality. At a time when the scientific community is trying to provide a safe vaccine that effectively addresses the problem, the number of people being treated in intensive care units (ICU) is still very high.
The provision of nutritional support for critically ill patients with COVID-19 is a subject of intense debate and there is a lack of data -especially in energy needs assessment. Published guidelines from International organizations propose various equations to determine energy needs [1]. Recently published data from a US cohort [2] demonstrate a progressively hyper metabolism post 7th day of ICU stay among patients with SARS-CoV-2, probably due to the systemic inflammatory response that results from increased systemic cytokine production and contributes to the pathophysiology of severe COVID-19 [3]. In this regard, an attempt was made to measure energy expenditure in a series of patients with confirmed COVID-19 receiving prolonged mechanical ventilation and determine the effect of administration of a frequently used modality - neuromuscular blocking agents (NMBAs) - on energy expenditure.
2. Materials & methods
2.1. Study population
This prospective observational study included data from 34 adult patients with confirmed COVID-19 requiring mechanical ventilation, was approved by the Evangelismos General Hospital Ethics Committee (12/15-09-2020). Demographic data collected including age, sex, acute physiology and chronic health evaluation (APACHE) II score while nutritional parameters noted included route of feeding during the first 48 h on ICU admission, BMI and nutrition risk in the critically ill (NUTRIC Score).
Resting energy expenditure (REE) and respiratory exchange rate (RER) evaluated on the 3rd, 7th, 14th, 21st and 28th day of hospitalization by indirect calorimetry (Q-NRG, COSMED, EU) [4]. Prior to testing, patients were confirmed to be in stable condition with only steady-state measures for ≥20 min considered valid. Body weight was determined by electronic weighing beds (Narang Medical Llimited, HF 1043), while body height was measured by a trained nurse using a measuring tape with the patient in supine position to the accuracy of 0.1 cm. For calculations, actual body weight (AcBW) was used for patients without obesity (non ob) (BMI <30 kgr/m2) and adjusted body weight (AjBW) for patients with obesity (ob) (BMI≥ 30 kgr/m2) [AjBW = 0.4 (Total Body Weight – Ideal Body Weight) + Ideal Body Weight, whereas ideal body weight was defined as the weight corresponding to an ideal body mass index of 22 kg/m2] [5]. All patients were on a fraction of inspired oxygen ≤60% and did not require positive end-expiratory pressure (PEEP) higher than 14 mm cmH2O. To ensure the accuracy of indirect calorimetry measurements practical instructions of ESPEN for the safe application of indirect calorimetry were followed [6]. Paralysed patients (use of NMBAs) were defined those with pharmacologically induced paralysis with cisatracurium to control body temperature to 36.0 °C, for at least 3 days during their ICU stay. At the time of the study, all patients were sedated with either midazolam or propofol.
2.2. Statistical analysis
Data normality was assessed using the Shapiro Wilk test. REE and RER were expressed as median values (interquartile range, IQR). Mann Whitney U test was used to for comparison of REE and RER values between obese and non obese patients and paralysed and non paralysed patients, at certain time points (days). Kruskal Wallis test was used to compare repeated recordings of REE values. A p value of <0.05 was considered significant.
3. Results
A total of 34 COVID-19 critically ill intubated patients (59.8% male, 35.2% obese) had indirect calorimetry (IC) measurements (Table 1 ). Of these, 75.3% received enteral feeding during first 48 h on admission, while NMBAs was used in 76.4% of the patients and 28-day mortality was 31.2%. NMBAs administration was more prevalent (77.8% of patients that were given cisatracurium) during first week of ICU stay.
Table 1.
Patients Baseline characteristics (n = 34).
| Age (y) | 65.9 ± 17.9 |
| Sex, male (n) % | 20 (58.8) |
| Subjects at any time point participating in the study, n (%) | |
| 1st week of ICU stay | 34 (100) |
| 2nd week of ICU stay | 30 (88.2) |
| 3rd week of ICU stay | 27 (79.4) |
| 4th week of ICU stay | 22 (64.7) |
| Nutritional data | |
| BMI on admission, (kgr/m2), (n) % | |
| Normal (18.5–24.9) | 13 (38.5%) |
| Overweight (25–29.9) | 9 (26.4%) |
| Obese (≥30) | 12 (35.2%) |
| Sepsis, (n) % | 23 (68.2%) |
| NUTRIC Score at admission, n (%) | |
| Low risk (0–4 points) | 15 (44.1%) |
| High Risk (5–9 points) | 29 (55.9%) |
| aFeeding route, (n) % | |
| Enteral | 25 (73.5% ( |
| Parenteral | 17 (20.7%) |
| Enteral + Parenteral | 2 (5.8%) |
| Clinical Data | |
| Ventilator days (30-day study period only) (mean ± sd) | 21.4 ± 7.9 |
| In Hospital Mortality, n (%) | 13 (38.1%) |
| 28 day Mortality, n (%) | 10 (29.4%) |
| ICU length of stay (days) (median, IQR) | 16 (8–38) |
| Hospital length of stay (days) (median, IQR) | 23 (8–45) |
| APACHE II score on admission, (median, IQR) | 18.9 (11–26.2) |
| Use of prone positioning, n (%) | 3 (8.8) |
| Use of paralysis with NMBAs (cicatracurium), n (%) | 26 (76.4%) |
| PEEP (cmH2O) (mean ± SD) | 9.9 ± 4.9 |
| Fi02 (%) (median IQR) | 55.4 (36.2–75.3) |
| Serum albumin g/L (mean ± SD) | 2.86 ± 0.80 |
Values represent median (IQR) or means (+SD) or number of subjects (n, %).
Abbreviations: BMI; body mass index, PEEP; positive end expiratory pressure, NUTRIC SCORE; Nutrition Risk in the Critically Ill, Fi02; fraction of inspired oxygen, REE; resting energy expenditure, APACHE; Acute Physiology and Chronic Health Evaluation.
Feeding route during the first 48 h on ICU admission.
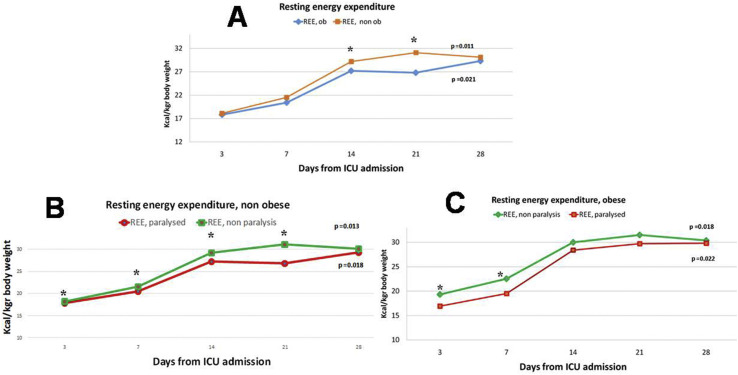
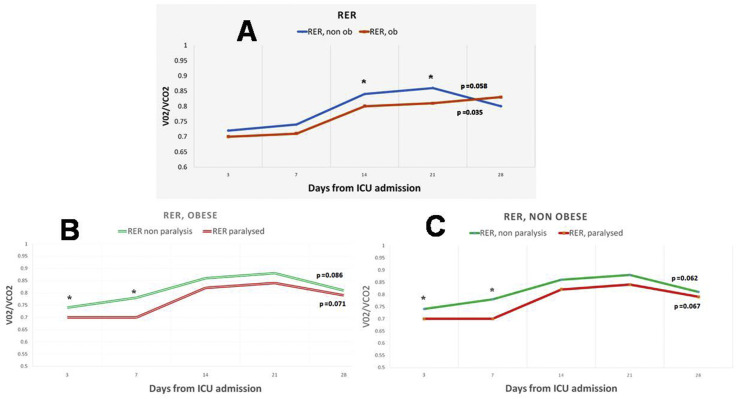
Typically, feeding was started on the second day, with 34.3% of patients on the first day, 40.7% patients on the second day, and 25% patients on the third day. 71.3% of patients on enteral feeding received adequate nutrition (80–110% of nutritional requirements), 82.7% of those receiving parenteral feeding, and 78.5% of those receiving enteral and supplemental parenteral nutrition (p = 0.092). In total, 172 indirect calorimetry measurements were taken. REE measurements suggest a gradual increase of median energy needs after 3rd day of ICU admission in both patients without obesity (non ob) (from 17.8 kcal/kgr up to 29.3 kcal/kgr AcBW during 28th day of hospitalization, p = 0.011) and patients with obesity (ob) (from 18.1 kcal/kgr up to 30.1 kcal/kgr AjBW during 28th day of hospitalization, p = 0.021, Fig. 1 ), a fact that has been observed by similar studies in critically ill patients. REE and RER values were significantly different between non ob and ob patients during 14th and 21st day of ICU stay (29.4 vs 27.2 and 31.1 vs 26.8 kcal/kgr, 0.84 vs 0.79 and 0.86 vs 0.81, all p < 0.05, Fig. 1, Fig. 2 ). Median energy expenditure values were significantly lower in patients receiving NMBAs compared to those that did not (2444 vs 2120 kcal/day, p = 0.023). Eventually, patients receiving NMBAs didn't found any evidence of survival benefit but experienced a tendency for fewer ventilator days (18.2 ± 6.5 vs 22.3 ± 7.2, p = 0.092) and ICU stay (15.2 vs 17.9, P = 0.071), compared to those that didn't receive.
Fig. 1.
Daily resting energy expenditure values (REE) (median values) over time, measured by indirect calorimetry. A. REE over time in intubated COVID-19 patients without obesity (non ob) and with obesity (ob). B. REE over time in intubated COVID-19 patients without obesity according to NRMBAs administration. C. REE over time in intubated COVID -9 patients with obesity according to NRMBAs administration. Body weight: actual body weight for non ob (BMI<30 kgr/m2) and adjusted body weight for ob (BMI≥ 30 kgr/m2). ∗ Denotes statistical significant different between groups at <0.05 level, p = p value for Kruskal Wallis test, that was conducted to examine differences regarding REE measurements between different times (days). Abbreviations: ob, COVID-19 patients with obesity (BMI≥ 30 kgr/m2); non ob; COVID-19 patients without obesity (BMI< 30 kgr/m2); paralysed, administration of NMBAs.
Fig. 2.
Daily respiratory exchange ratio (RER) (median) over time, measured by indirect calorimetry. A. RER over time in intubated COVID-19 patients without obesity (non ob) and with obesity (ob). B. RRE over time in intubated COVID-19 patients without obesity according to NRMBAs administration. C. REE over time in intubated COVID -9 patients with obesity according to NRMBAs administration. ∗ Denotes statistical significant different between groups at <0.05 level, p = p value for Kruskal Wallis test, that was conducted to examine differences regarding RER measurements between different times (days). Abbreviations: ob, COVID-19 patients with obesity (BMI≥ 30 kgr/m2); non ob; COVID-19 patients without obesity (BMI< 30 kgr/m2); paralysed, administration of NMBAs; VO2, volume of oxygen consumed per minute; VCO2, volume of carbon dioxide consumed per minute.
NMBAs use was accompanied by a significant drop in REE, especially during the first 7 days of ICU stay, both in non ob (22.9 vs 17.9 kcal/kgr AcBW, p = 0.014) and ob patients (22.5 vs 19.5 kcal/kgr ABW, p = 0.027), while the effect seems to last longer for non ob patients (up to 21 days, see Fig. 1). RER values were also significantly different between non ob and ob patients using NMBAs during their first 7 days of ICU stay (0.78 vs 0.71 and 0.77 vs 0.72, all p < 0.05). No association was found between BMI, REE, route of feeding and NMBAs use and major outcomes (in hospital and 28-day mortality, days of mechanical ventilation and ICU length of stay).
4. Discussion
In this prospective study we determined the energy needs of 34 critically ill intubated patients with confirmed COVID-19. Although there are data available from large cohorts about energy needs of adult ICU patients [7], with regard to COVID-19 patients, research findings are sparse. Current study adds to that of the LEEP-COVID study [3], which described an increase in energy needs up to 3 weeks of hospitalization in an ICU COVID-19 population. The main differences between 2 studies is that our sample size was larger (34 vs 22 patients), followed for a longer period after ICU admission (up to 28 days vs 3 weeks) and had higher rates of NMBAs use. In addition, another study among 7 patients from the US using a different measuring device [8] reported also an extreme hypermetabolic status in patients with SARS-CoV-2 infection, but results were presented in terms of total energy expenditure (kcal) instead of measures of body weight (per kgr), so it is quite difficult to document differences. Based on the data of current study, we emphasize prevention of overfeeding among critically ill COVID-19 patients, especially during their first week of ICU stay.
Our findings support that provision of NMBAs during hospitalization affect significantly total energy expenditure, especially during first 7 days following ICU admission. In contrast to data from Whittle et al. [3], where paralysis use was not accompanied by a significantly drop in energy expenditure, present study revealed that NMBAs administration have a profound effect, apparently due to a higher percentage of people using paralysis. Published data on the role of NMBAs in measuring energy expenditure in certain ICU populations (such as severe head injury patients) [9,10] indicate that metabolic rate is reduced by almost 20%. It is important to note that our results indicate a relative 22% reduction up to 3 weeks after ICU admission in COVID-19 patients without obesity, while patients with obesity exert a smaller degree of reduction (up to 12%). Reduced REE appear also to last longer in non ob patients compared to ob, up to 3 weeks following ICU admission. The underlying mechanism of this association is unknown, but NMBAs are well known blockers of skeletal muscle activity. Individuals without obesity tend to have a higher absolute fat free mass/fat mass proportion compared to those with obesity, so it is likely that the greater observed effect of NMBAs in COVID-19 intubated patients without obesity could be due to differences in body composition, and hence increased muscle mass. NMBAs administration was also associated with lower RER values in both obese and non-obese individuals, suggesting that their use may be associated with increased use of fatty acids as an energy source.
In conclusion, our findings demonstrate that energy needs of COVID-19 intubated patients show a significant increase after the 1st week and have a tendency to stabilize after the 3rd week of ICU stay. Importantly, these findings provide that NMBAs administration should be considered by clinicians in the nutritional care process, as it may have a significant impact on energy expenditure.
Statement of authorship
Dimitrios Karayiannis and Zafeiria Mastora contributed equally to this work. Anastasia Kotanidou supervised the entire project, and Zafeiria Mastora designed the study. Aikaterini Maragkouti, Theodoros Mikropoulos, Aikaterini Sarri and Zafeiria Mastora collected the data. Dimitrios Karayiannis conducted the data analyses, drafted the manuscript and Zafeiria Mastora revised it. All the authors critically reviewed the article and approved the final manuscript.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Union of Greek Shipowners for donating Cosmed Q NRG indirect calorimeter to the 1st Department of Critical Care Unit, Evangelismos General Hospital Athens.
References
- 1.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittle J., Molinger J., MacLeod D., Haines K., Wischmeyer P.E. Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care. 2020;24:581. doi: 10.1186/s13054-020-03286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 4.Oshima T., Delsoglio M., Dupertuis Y.M., Singer P., De Waele E., Veraar C., et al. The clinical evaluation of the new indirect calorimeter developed by the ICALIC project. Clin Nutr. 2020;39:3105–3111. doi: 10.1016/j.clnu.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Singer P., Pichard C., De Waele E. Practical guidance for the use of indirect calorimetry during COVID 19 pandemic. Clin Nutr Exp. 2020;33:18–23. doi: 10.1016/j.yclnex.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zusman O., Theilla M., Cohen J., Kagan I., Bendavid I., Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. 2016;20:367. doi: 10.1186/s13054-016-1538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu P.J., Cassiere H., DeRosa S., Bocchieri K., Yar S., Hartman A. Hypermetabolism and coronavirus disease 2019. JPEN - J Parenter Enter Nutr. 2020;44:1234–1236. doi: 10.1002/jpen.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osuka A., Uno T., Nakanishi J., Hinokiyama H., Takahashi Y., Matsuoka T. Energy expenditure in patients with severe head injury: controlled normothermia with sedation and neuromuscular blockade. J Crit Care. 2013;28 doi: 10.1016/j.jcrc.2012.05.012. 218.e9-13. [DOI] [PubMed] [Google Scholar]
- 10.McCall M., Jeejeebhoy K., Pencharz P., Moulton R. Effect of neuromuscular blockade on energy expenditure in patients with severe head injury. JPEN - J Parenter Enter Nutr. 2003;27:27–35. doi: 10.1177/014860710302700127. [DOI] [PubMed] [Google Scholar]