Abstract
Objectives
One of the most significant features of poor prognosis in COVID-19 is pulmonary fibrosis. Nintedanib is a new antifibrotic agent that interferes with processes of pulmonary fibrosis. This study aimed to investigate the efficacy and safety of nintedanib in COVID-19.
Methods
This was an interventional study in which adult patients with COVID-19 requiring mechanical ventilation were consecutively enrolled. The primary endpoint was 28-day mortality after the initiation of mechanical ventilation. The secondary endpoints were length of mechanical ventilation, volume of lung injury, and the incidence of gastrointestinal adverse events and acute liver failure.
Results
Thirty patients with COVID-19 underwent nintedanib therapy. We included 30 patients not receiving nintedanib as the historical control group. There were no significant differences in 28-day mortality between the groups (23.3% vs 20%, P = 0.834). Lengths of mechanical ventilation were significantly shorter in the nintedanib group (P = 0.046). Computed tomography volumetry showed that the percentages of high-attenuation areas were significantly lower in the nintedanib group at liberation from mechanical ventilation (38.7% vs 25.7%, P = 0.027). There were no significant differences in the adverse events.
Conclusions
The administration of nintedanib may offer potential benefits for minimizing lung injury in COVID-19.
Keywords: COVID-19, Respiratory distress syndrome, Pulmonary fibrosis, Nintedanib, Respiration, Artificial, Intensive care units
Introduction
SARS-CoV-2, a novel RNA coronavirus, was identified in early January 2020 as the cause of a severe pneumonia in Wuhan, China. After causing the deaths of thousands of patients in China, SARS-CoV-2 infection (COVID-19) has rapidly spread worldwide (Zhu et al., 2020). As of February 15, 2021, more than 108.0 million confirmed infections, with approximately 2.4 million deaths, were reported from 223 countries and territories around the world (WHO Coronavirus Disease Dashboard, 2021).
COVID-19 mainly affects the respiratory system, with some patients rapidly progressing to severe acute respiratory distress syndrome (ARDS), which has appeared to be associated with increased risk of death (Burki, 2020). The mortality rate among patients with COVID-19-induced ARDS requiring mechanical ventilation has actually ranged widely between 35.7% to 94% and is considerably higher than that of COVID-19 patients without ARDS (Auld et al., 2020, Wu et al., 2020, Sinha et al., 2020). One of the most significant features of poor prognosis in COVID-19-induced ARDS is the development of pulmonary fibrosis. Pulmonary fibrosis is a pathological consequence of interstitial lung diseases that is characterized by unsuccessful reconstruction of the damaged alveolar epithelium, persistence of fibroblasts and excessive deposition of collagen (Sime and O’Reilly, 2001). Histopathologically, 3 phases are recognized during the evolution of lung injury in ARDS: exudative, proliferative, and fibrotic. In the proliferative phase, type II alveolar cells proliferate with epithelial cell regeneration and remodeling, together with proliferation of fibroblasts. Subsequently, the third fibrotic phase involving collagen deposition in alveolar, vascular, and interstitial beds progresses in some patients, leading to irreversible development of cystic changes and limited functional recovery (Tomashefski Jr, 2000). A number of clinical, radiological and histopathological studies have suggested that the progression of pulmonary fibrosis commonly complicating severe COVID-19 patients can potentially worsen survival and functional outcomes (Pan et al., 2020, Schwensen et al., 2020, Tian et al., 2020, Zhou et al., 2020). In this context, several antifibrotic agents are expected to be beneficial treatments to prevent the progression of pulmonary fibrosis in severe COVID-19-induced ARDS.
Nintedanib is an intracellular inhibitor of tyrosine kinases that is reported in several preclinical studies to interfere with processes of active fibrosis such as proliferation, migration and differentiation of fibroblasts (Wollin et al., 2014, Wollin et al., 2015, Redente et al., 2018). Recently, several large clinical trials showed that treatment with nintedanib reduced the rate of decline in forced vital capacity in patients with idiopathic pulmonary fibrosis (IPF) and systemic sclerosis-associated interstitial lung disease (Richeldi et al., 2011, Richeldi et al., 2014, Distler et al., 2019).
Herein, we hypothesized that treatment with nintedanib could improve the survival and functional outcomes in severe COVID-19-induced ARDS by inhibiting the progression of pulmonary fibrosis, and we conducted an interventional study using a historical control to investigate the efficacy and safety of nintedanib in patients with COVID-19-induced ARDS.
Methods
Study design and setting
We undertook a single-center interventional study using a historical control conducted in the intensive care unit (ICU) of a tertiary care hospital in Japan. A data monitoring committee provided independent assessment of the safety, scientific validity and integrity of the trial. Also, an independent investigator who was unaware of the group assignments reviewed medical documentation to adjudicate the primary cause of all deaths and all adverse events. This trial was conducted in accordance with the principles of the Declaration of Helsinki and the Japanese Clinical Trials Act (Act No. 16 of April 14, 2017).
The use of nintedanib (OFEV®) for patients with COVID-19 is not yet approved by the Japanese Ministry of Health, Labor and Welfare. The trial protocol was approved by the Institutional Review Board for Clinical Research of Osaka General Medical Center as a specified clinical trial (IRB No. S202004004) and is available at the Japanese Registry of Clinical Trials (jRCTs051200036). Before study inclusion, written informed consent was obtained from all patients who received nintedanib.
Participants
Patients admitted to the ICU with the diagnosis of COVID-19 were consecutively recruited from August to October 2020 and were eligible for the nintedanib group if they were 20 years of age or older and required mechanical ventilation.
The exclusion criteria included a history of IPF or chronic liver failure (Child Pugh B or C), breastfeeding, pregnancy, a history or proven risk factors of thrombosis, proven risk factors of bleeding, unavailable to use an enteral tube, limitation of sustained life care, and post-cardiopulmonary arrest resuscitation status at the time of diagnosis of COVID-19. As the historical control group, we consecutively enrolled from March to July 2020 an equal number of the most recent consecutive adult patients with COVID-19 who required mechanical ventilation and did not meet the exclusion criteria.
Sample size
Because there were no previous data on the survival benefit of nintedanib for patients with COVID-19, we could not calculate a sample size. However, previous studies suggested that 15 to 30 participants per group are required to ensure statistical validity of the results in a pilot study (Hertzog, 2008, Viechtbauer et al., 2015). Therefore, we decided to include a total of 60 participants with 30 participants per group.
Diagnosis of COVID-19
The diagnosis of COVID-19 was performed according to World Health Organization interim guidance (World Health Organization, 2021) and confirmed by RNA detection of the SARS-CoV-2 in a clinical laboratory of the Osaka Institute of Public Health. We defined the first day of mechanical ventilation as “day 1” both in the nintedanib and control groups.
Treatment
Nintedanib therapy consisted of 150 mg of nintedanib administered via nasogastric tube twice daily from day 1 to liberation from mechanical ventilation within 28 days. We also used favipiravir, a new antiviral agent, for both the nintedanib and historical control groups unless contraindications were present. Mechanical ventilation for all study patients was typically conducted according to The National Institutes of Health treatment guidelines for COVID-19-related ARDS (The National Institutes of Health, 2021). Other supportive care during mechanical ventilation was given per institutional policies and the discretion of the treating physicians.
Data collection and outcomes
Patients were followed up until hospital discharge or death. Patient information collected included demographic characteristics, pre-existing comorbidities (assessed by Charlson comorbidity index), laboratory tests, severity scores and therapeutic interventions. Severity of illness was evaluated according to the PaO2/FiO2 (P/F) ratio, the Sequential Organ Failure Assessment (SOFA) score, and the Acute Physiology and Chronic Health Evaluation (APACHE) II score. The APACHE II and SOFA scores were evaluated at day 1, and the P/F ratio was evaluated every day during mechanical ventilation. The primary endpoint was 28-day mortality after the initiation of mechanical ventilation. The secondary endpoints were 1) length of mechanical ventilation, 2) time-related alteration in the P/F ratio over 14 days during mechanical ventilation, 3) computed tomographic (CT) quantitative evaluation for lung injury in surviving patients, and 4) the incidence of gastrointestinal adverse events and acute liver failure within 28 days after the initiation of mechanical ventilation. The length of mechanical ventilation was evaluated by Kaplan-Meier methods and the number of ventilator-free days within 28 days.
Quantitative CT volumetry of the high-attenuation areas, including both ground-glass opacities and consolidation, was conducted at the time of initiation of and liberation from mechanical ventilation (Supplementary Figure S1). The segmentation of the high-attenuation areas was conducted by 2 physicians blinded from the patient data and treatment allocation (K.M. and Y.M.). Volumes of high-attenuation areas were calculated by another investigator blinded from the patient data and treatment allocation (N.O.) using a digital image analysis application (fcuro-label, fcuro Co., Ltd., Osaka, Japan).
Gastrointestinal adverse complications were defined as the following 3 events: 1) mild events including occurrence of vomiting and diarrhea (more than 4 times per day), 2) moderate events including mild to moderate gastrointestinal bleeding or total parenteral nutrition requirements, and 3) severe events requiring surgical or endoscopic intervention (e.g., intestinal perforation and massive gastrointestinal bleeding). The incidence of acute liver failure was defined as the elevation in serum aspartate aminotransferase or alanine aminotransferase to levels of 1.5 (mild event), 5 (moderate event), or 10 times (severe event) above the upper limit of normal range.
Statistical analysis
Descriptive statistics are calculated as medians (interquartile range) or proportions (numbers), as appropriate. Univariate differences between groups were assessed using the Mann-Whitney U test or chi-square test, as appropriate. Missing values were not imputed in any of the regression models. To assess the time-related alteration in the P/F ratio, we fitted a multilevel mixed-effects regression model with fixed effects assigned to patient categorization (the control or the nintedanib groups), time points during mechanical ventilation and two-way interaction term for these independent variables, and random effects assigned to individual identification numbers. Kaplan–Meier curves were constructed to evaluate 28-day mortality and length of mechanical ventilation. A log-rank test was conducted for 28-day mortality, and the Gehan–Breslow–Wilcoxon test was conducted for the length of mechanical ventilation to compare the Kaplan–Meier curves between 2 groups. All statistical inferences were performed with a two-sided P at the 5% significance level. Because of the underpowered nature of the interaction analysis, we used a two-sided significance level of 20% with statistical inferences for the interaction analyses (Ramos et al., 2008). All statistical analyses were conducted using STATA Data Analysis and Statistical Software version 15.0 (StataCorp, College Station, TX).
Results
Study population
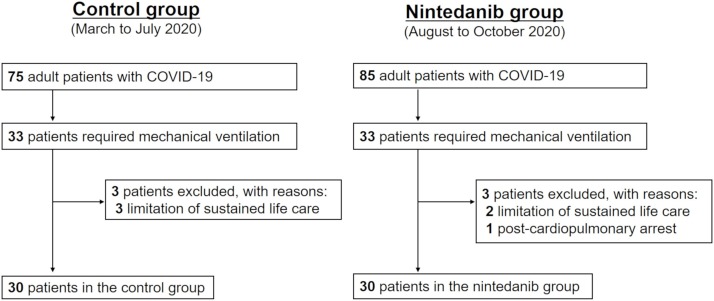
The patient flow diagram is shown in Figure 1 . From August to October 2020, 33 consecutive patients were admitted to the ICUs with the diagnosis of COVID-19 and underwent mechanical ventilation. After excluding 3 patients who met the exclusion criteria, 30 patients were enrolled in this study and underwent the nintedanib therapy (nintedanib group). We also included the 30 most recent consecutive adult patients with COVID-19 who required mechanical ventilation and did not meet the exclusion criteria from March to Jury 2020 as the control group.
Figure 1.
Patient flow diagram. COVID-19 = coronavirus disease 2019.
Baseline characteristics, laboratory tests and illness severity scores obtained on day 1 in the 2 groups are shown in Table 1 . Age, sex and body mass index were similar between the 2 groups. Although the nintedanib group had a significantly higher rate of chronic respiratory disease, rates of other preexisting comorbidities were not significantly different, and the Charlson comorbidity index was similar between the groups. Similarly, there were no significant differences between the groups in physiological and laboratory parameters on day 1, including Glasgow Coma Scale score, body temperature, P/F ratio, white blood cell and platelet counts, and C-reactive protein, creatinine and total bilirubin levels.
Table 1.
Baseline characteristics, severity and therapeutic interventions.
| Control | Nintedanib | P Value | |
|---|---|---|---|
| N = 30 | N = 30 | ||
| Age, years | 70 (62–77) | 75 (66–80) | 0.109 |
| Sex, male | 18 (60%) | 23 (76.7%) | 0.165 |
| BMI (kg/m2) | 23.2 (20.9–25.5) | 24.6 (20.3–27.9) | 0.756 |
| Pre-existing conditions | |||
| Hypertension | 16 (53.3%) | 19 (63.3%) | 0.432 |
| Chronic respiratory disease | 4 (13.3%) | 12 (40%) | 0.020 |
| Chronic heart failure | 2 (6.7%) | 7 (23.3%) | 0.071 |
| Chronic kidney disease | 6 (20%) | 5 (16.7%) | 0.739 |
| Diabetes mellitus | 13 (43.3%) | 12 (40%) | 0.793 |
| Cancer | 1 (3.3%) | 4 (13.3%) | 0.161 |
| Charlson comorbidity index | 2 (0–3) | 2 (1–4) | 0.143 |
| Findings at time of intubation | |||
| Glasgow Coma Scale score | 13 (6–15) | 11 (3–15) | 0.789 |
| Body temperature (°C) | 37.4 (36.8–37.7) | 37.1 (36.4–37.6) | 0.176 |
| PaO2/FiO2 ratio | 181 (120–242) | 178 (121–255) | 0.487 |
| White blood cell count (103/μL) | 7.1 (5.6–9) | 7 (3.7–11.9) | 0.756 |
| Platelet count (103/μL) | 171 (131–256) | 165 (125–209) | 0.217 |
| C-reactive protein (mg/dL) | 11.8 (7.8–18.2) | 10.1 (6.7–15.8) | 0.174 |
| Creatinine (mg/dL) | 0.9 (0.6–1.1) | 0.9 (0.8–1.2) | 0.243 |
| Bilirubin (mg/dL) | 0.6 (0.5–0.8) | 0.6 (0.4–0.9) | 0.917 |
| APACHE II score | 17 (11–21) | 17 (13–24) | 0.358 |
| SOFA score | 7 (4–8) | 7 (5–10) | 0.480 |
| ISTH overt-DIC score | 0 (0–2) | 0 (0–2) | 0.963 |
| Therapeutic interventions | |||
| Corticosteroids | 26 (86.7%) | 29 (96.7%) | 0.161 |
| Favipiravir | 27 (90%) | 23 (76.7%) | 0.166 |
| V-V ECMO | 4 (13.3%) | 3 (10%) | 0.688 |
| Prone position | 12 (40%) | 14 (46.7%) | 0.602 |
| Unfractionated heparin | 24 (80%) | 28 (93.3%) | 0.129 |
BMI, body mass index; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; ISTH, International Society on Thrombosis and Hemostasis; DIC, disseminated intravascular coagulation; V-V ECMO, veno-venous extracorporeal membrane oxygenation.
There was no significant difference between the 2 groups in therapeutic interventions, such as the use of corticosteroids, favipiravir, veno-venous extracorporeal membrane oxygenation, prone position and low-dose unfractionated heparin for prophylaxis against venous thromboembolism.
Effect of treatment on survival and lung injury
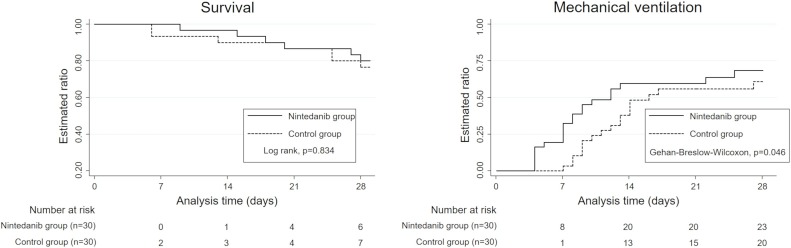
The survival curves and lengths of mechanical ventilation are shown in Figure 2 . The 28-day mortality rate was 23.3% (7 of 30 patients) in the control group versus 20% (6 of 30 patients) in the nintedanib group. The difference between the 2 groups was not significant (P = 0.834, log-rank test). However, the lengths of mechanical ventilation were significantly shorter in the nintedanib group (P = 0.046, Gehan–Breslow–Wilcoxon test). Furthermore, the median number of ventilator-free days within 28 days was significantly longer in the nintedanib group (12 vs 17 days, P = 0.038, Table 2 ).
Figure 2.
Kaplan–Meier curves for evaluation of 28-day mortality and length of mechanical ventilation. The solid black lines represent patients in the nintedanib group, and the dotted black lines represent patients in the control group. Log rank test was conducted for 28-day mortality, and the Gehan–Breslow–Wilcoxon test was conducted for the length of mechanical ventilation to compare the curves between the groups.
Table 2.
Secondary endpoints.
| Control | Nintedanib | P Value | |
|---|---|---|---|
| N = 30 | N = 30 | ||
| Ventilator-free days within 28 days | 12 (0–17) | 17 (0–21) | 0.038 |
| Percentage of high-attenuation areas on CT | |||
| Induction of mechanical ventilation (%) | 29.1 (20.3–33.1) | 30.8 (20.9–46.7) | 0.651 |
| Liberation from mechanical ventilation (%) | 38.7 (20.9–45.6) | 25.7 (9.4–34.6) | 0.027 |
| Gastrointestinal adverse events | |||
| Mild events | 27 (90%) | 25 (83.3%) | 0.448 |
| Moderate events | 6 (20%) | 9 (30%) | 0.371 |
| Severe events | 3 (10%) | 2 (6.7%) | 0.640 |
| Acute liver failure | |||
| Mild | 20 (66.7%) | 24 (80%) | 0.243 |
| Moderate | 6 (20%) | 11 (36.7%) | 0.158 |
| Severe | 2 (6.7%) | 2 (6.7%) | 1.00 |
CT, computed tomography.
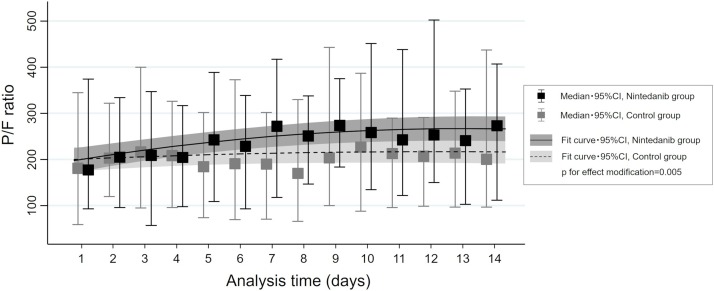
A multilevel mixed-effects regression model showed that time-related alterations in P/F ratio within 14 days from the initiation of mechanical ventilation were significantly different between the 2 groups (P for interaction = 0.005), and the nintedanib group had relatively higher P/F ratios as time advanced compared with those in the control group (Figure 3 ).
Figure 3.
Time series alterations in PaO2/FiO2 (PF) ratio in the nintedanib and control groups. Regression line with 95% confidence interval (CI) in each group estimated by multilevel mixed-effects regression model with a two-way interaction term between the group and time series. The solid black lines represent patients in the nintedanib group, and the dotted black lines represent patients in the control group. Median and 95% CI of the PF ratio at each time point are also indicated by box and whisker plots.
CT volumetry showed that the percentages of high-attenuation areas were not different between the 2 groups at initiation of mechanical ventilation (29.1% vs 30.8%, P = 0.651, Table 2). However, they were significantly lower in the nintedanib group at liberation from mechanical ventilation (38.7% vs 25.7%, P = 0.027, Table 2).
Adverse events
The incidence of adverse events in the 2 groups is summarized in Table 2. There were no significant differences in the incidence of mild, moderate and severe gastrointestinal adverse events between the 2 groups. The incidence of mild and moderate acute liver failure was slightly higher in the nintedanib group, but the differences were not statistically significant (P = 0.243 and 0.158, respectively). The incidence of severe acute liver failure was not different between the 2 groups.
Discussion
As the worldwide deaths from COVID-19 continue to rise, a substantial therapy to suppress lung injury and improve patient survival is strongly required. In the present study, we investigated the efficacy and safety of nintedanib, a new antifibrotic agent for IPF, in severe COVID-19 patients.
Effect of nintedanib on lung injury
Pulmonary fibrosis is a life-threatening pathological consequence of acute and chronic interstitial lung diseases that is characterized by unsuccessful reconstruction of the injured alveolar epithelium, persistence of fibroblasts and excessive deposition of collagen (Sime and O’Reilly, 2001). Recently, a number of studies reported that pulmonary fibrosis was also occurring as a sequela of acute lung injury induced by COVID-19 (Pan et al., 2020, Schwensen et al., 2020, Tian et al., 2020, Zhou et al., 2020). Besides, in COVID-19, serum levels of pro-inflammatory and pro-fibrotic mediators such as monocyte-1 chemoattractant protein, transforming growth factor 1, tumor necrosis factor α, fibroblast growth factor, platelet-derived growth factor, interleukin-1b and interleukin-6 were reported to be highly increased to the same levels as those in IPF (Nile et al., 2020, Xiong et al., 2020, Yuki et al., 2020). The similar cytokine profiles in IPF and COVID-19 suggested analogous mechanisms of pulmonary fibrosis in these diseases; therefore, nintedanib, which was shown to be useful in the treatment of IPF, could potentially be beneficial for severe COVID-19 patients (Lechowicz et al., 2020).
In the present study, the nintedanib group had a significantly higher P/F ratio, shorter lengths of mechanical ventilation and lower volume of high-attenuation areas on CT images. These findings clearly suggested that the administration of nintedanib could ameliorate the lung injury induced by COVID-19, potentially by modulating the progression of pulmonary fibrosis. Furthermore, the findings of CT volumetry suggested that nintedanib could minimize the long-term sequelae of COVID-19 on respiratory function, which have become an increasingly recognized and troubling health problem all over the world (WHO Coronavirus Disease Dashboard, 2021).
Effect of nintedanib on mortality
Despite the favorable effects of nintedanib on respiratory function, the 28-day mortality rate was not significantly different between the 2 groups. Possible explanations for these conflicting results might be attributable to the supposition that attenuating pulmonary fibrosis with nintedanib might not contribute to reducing acute-phase deaths because pulmonary fibrosis is a late pathological finding associated with late death. In a cohort study of 159 autopsies of patients with ARDS, pulmonary fibrosis had developed in 4% of the patients with a disease duration of less than 1 week, 24% of those with a disease duration of between 1 and 3 weeks, and 61% of those with a disease duration of greater than 3 weeks (Thille et al., 2013). The results of the present study suggested that pulmonary fibrosis could develop early in the course of ARDS but could also be largely associated with patient survival in a later phase. Further clinical trials focusing on long-term mortality need to be performed.
Safety of nintedanib
Major side effects of nintedanib were reported to include liver problems, diarrhea, nausea, vomiting, heart attack, stroke, bleeding problems and tears (perforation) in the stomach or intestinal wall (Safety and Side Effects of OFEV, 2021). The present study thus recorded gastrointestinal and liver dysfunctions as possible adverse events that could be increased by the administration of nintedanib. However, there were no significant differences between the 2 groups in the incidence of mild, moderate and severe gastrointestinal and liver adverse events.
In a previous study of patients with IPF, the rates of diarrhea, the most frequent adverse event, were significantly higher in the nintedanib versus non-nintedanib group (61.5% vs 18.6%) (Richeldi et al., 2011). The reason for the divergent findings between the previous and present studies might be attributable to the difference in disease severity and baseline risk of adverse events. In other words, gastrointestinal adverse events, including diarrhea, commonly complicate ICU patients requiring mechanical ventilation, and thus an additional harming effect from the administration of nintedanib might be concealed.
Limitations
We acknowledge several limitations regarding the present study design. First, this study is not a randomized controlled trial, and we compared the nintedanib group with a historical control group. Therefore, multiple unmeasured confounding variables might account for the outcome differences observed. Second, the number of patients included in this study was small, which might lead to higher variability of the study results and potential undercoverage bias. Third, the single-center design potentially caused a sampling bias in terms of generalizability, i.e., only one specific population with COVID-19 might be included in this study. To resolve these imbalances, further large-scale multicenter randomized trials are needed to thoroughly evaluate the effects of nintedanib on the treatment of patients with severe COVID-19.
Conclusion
In the present study, the administration of nintedanib, a new antifibrotic agent, to patients with severe COVID-19 undergoing mechanical ventilation was associated with a higher P/F ratio, shorter length of mechanical ventilation time and lower volume of high-attenuation areas on CT imaging. The use of nintedanib may offer potential benefits for minimizing the lung injury induced by COVID-19.
Ethics approval and consent to participate
This trial was conducted in accordance with the principles of the Declaration of Helsinki and the Japanese Clinical Trials Act (Act No. 16 of April 14, 2017). The trial protocol was approved by the Institutional Review Board for Clinical Research of Osaka General Medical center as a specified clinical trial (IRB No. S202004004) and is available at the Japanese Registry of Clinical Trials (jRCTs051200036). Before study entry, written informed consent was obtained from all patients who received nintedanib.
Consent for publication
Informed consent for publication was obtained from all the participants.
Availability of data and materials
The statistical codes and full dataset are available from the corresponding author.
Competing interests
The authors declare that they have no competing interests.
Funding
This clinical study was supported by institutional funding for research projects in Osaka General Medical Center.
Authors’ contributions
Y. Umemura conceived and designed this study; contributed to acquisition, analysis and interpretation of the data; and was responsible for drafting, editing and submission of the manuscript. Y. Mitsuyama and K. Minami contributed to the study design and conducted the segmentation of the high-attenuation areas for CT volumetry. N. Okada contributed to the study design and calculated the volumes of high-attenuation areas. K. Yamakawa contributed to the study design; acquisition, analysis and interpretation of the data; and drafting of the manuscript. K. Nochioka had a significant influence on the study design. T. Nishida, A. Watanabe and S. Fujimi contributed to the interpretation of the data and critical appraisal of the manuscript. All of the authors reviewed, discussed, and approved the final manuscript.
Acknowledgements
The authors thank all the emergency medical service personnel, nurses, and physicians who have confronted the COVID-19 outbreak and the patients who contributed to this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.05.055.
Appendix A. Supplementary data
The following is Supplementary data to this article:
Example of segmentation of the high-attenuation areas in quantitative computed tomography volumetry.
References
- Auld S.C., Caridi-Scheible M., Blum J.M. Emory COVID-19 Quality and Clinical Research Collaborative: ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48:e799–e804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T.K. Coronavirus in China. Lancet Respir Med. 2020;8:238. doi: 10.1016/S2213-2600(20)30056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler O., Highland K.B., Gahlemann M. Nintedanib for systemic sclerosis–associated interstitial lung disease. N Engl J Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- Hertzog M.A. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- Lechowicz K., Drożdżal S., Machaj F. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med. 2020;9:1917. doi: 10.3390/jcm9061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile S.H., Nile A., Qiu J. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Guan H., Zhou S. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L.F., Shintani A., Ikizler T.A. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008;19:593–599. doi: 10.1681/ASN.2007030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redente E.F., Aguilar M.A., Black B.P. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2018;314:L998–L1009. doi: 10.1152/ajplung.00304.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L., Costabel U., Selman M. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- Richeldi L., du Bois R.M., Raghu G. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- Safety and Side Effects of OFEV. Available at: https://www.ofev.com/taking-ofev/side-effects. [Accessed 25 February 2021].
- Schwensen H.F., Borreschmidt L.K., Storgaard M. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J Clin Pathol. 2020;(July) doi: 10.1136/jclinpath-2020-206879. jclinpath-2020-206879. [DOI] [PubMed] [Google Scholar]
- Sime P.J., O’Reilly K.M. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99:308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- Sinha P., Calfee C.S., Cherian S. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med. 2020;8:1209–1218. doi: 10.1016/S2213-2600(20)30366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institutes of Health . 2021. COVID-19 treatment guidelines. Available at: https://www.nih.gov/coronavirus. [Accessed 15 February 2021] [PubMed] [Google Scholar]
- Thille A.W., Esteban A., Fernández-Segoviano P. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir Med. 2013;1:395–401. doi: 10.1016/S2213-2600(13)70053-5. [DOI] [PubMed] [Google Scholar]
- Tian S., Xiong Y., Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashefski J.F., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W., Smits L., Kotz D. A simple formula for the calculation of sample size in pilot studies. J Clin Epidemiol. 2015;68:1375–1379. doi: 10.1016/j.jclinepi.2015.04.014. [DOI] [PubMed] [Google Scholar]
- WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/. [Accessed 15 February 2021].
- Wollin L., Maillet I., Quesniaux V. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- Wollin L., Wex E., Pautsch A. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2021. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. [Accessed 15 February 2021] [Google Scholar]
- Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Liu Y., Cao L. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Wang Y., Zhu T. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example of segmentation of the high-attenuation areas in quantitative computed tomography volumetry.
Data Availability Statement
The statistical codes and full dataset are available from the corresponding author.