Abstract
Antabuse®, generic name disulfiram, has been extensively used in daily clinical practice to treat alcohol abuse. In vivo and in vitro experiments have demonstrated that disulfiram was capable of inhibiting tumor cell proliferation; clinical studies have indicated that the administration of this drug was associated with favorable survival, whilst in vitro experiments have elucidated the anticancer mechanism of disulfiram. In addition, radiation and cancer biology studies have shown that disulfiram can protect normal cells and sensitize tumor cells during radiotherapy. This review aims at describing the antitumor activity of disulfiram in both preclinical studies and clinical trials, whilst focusing on the advances of this drug in radiation and cancer biology, and the promise of repurposing it as a novel sensitizer to, and protector against, radiation on the incoming clinical studies.
Keywords: disulfiram, cancer biology, radiotherapy, overview
Introduction
Cancer is a major public health problem. It is one of the leading causes of death across the world.1 Factors such as ageing, infections, tobacco, diet, obesity, alcohol abuse, air pollution, diet, epigenetic and genetic alterations are all known to contribute to carcinogenesis in humans.2,3 Primary cancer may be treated with radical surgery, radiotherapy, systemic chemotherapy, targeted therapy, hormone replacement therapy and/or immunotherapy.4–6 There has been significant progress with all of these treatment options, but a high tumor-related morbidity and mortality still exists. Therefore, an acceleration in the development of anticancer drug is still required.
Developing new medicines is a costly and time-consuming process, with high failure rates. Because of this, investigators have shifted their focus to pre-existing drugs that may have secondary anticancer activity.7 Antabuse, generic name disulfiram (DSF), is one of these drugs. Disulfiram has been approved to treat alcoholism for more than sixty years, only recently has it emerged as a candidate for drug repurposing in anticancer therapy. Several investigators have described the antitumor efficacy of disulfiram both in vivo and in vitro. Disulfiram also appears to be well tolerated and cause minimal side effects.8–10
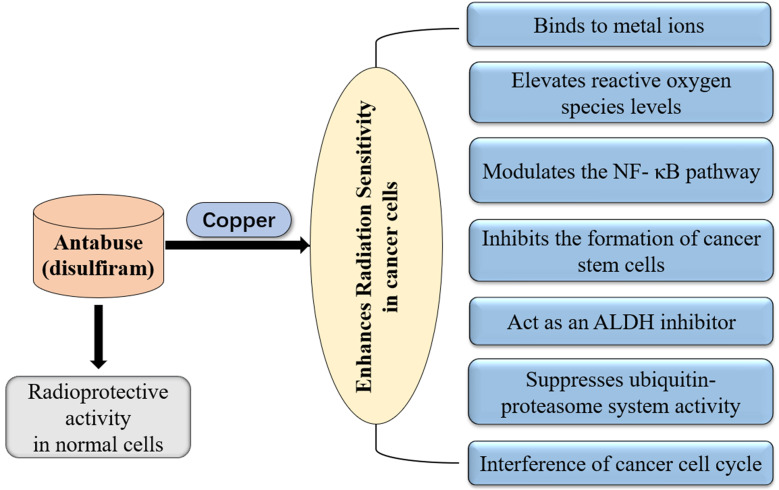
Recently, a Danish-Czech-US research group retrospectively analyzed the Danish nationwide demographic and health registries. More than 3000 patients diagnosed with cancer took disulfiram between 2000 and 2013. Compared with the patients who stopped taking the drug, the cancer mortality was 34% lower in those who stayed on disulfiram. Further investigation revealed that disulfiram chelates copper and converts into bis (diethyldithiocarbamate)-copper complex. The complex selectively targets NPL4-dependent segregase, a component of the ubiquitin proteasome system that is altered in tumor cells, thereby leading to increased cell death.11 The cellular mechanisms of action for disulfiram on cancer cells have been well elucidated. During the past few decades, many studies have demonstrated that disulfiram is metabolized to diethyldithiocarbamate. Diethyldithiocarbamate has antioxidant effects on cells, thereby serving as protection against radiation in normal tissues. Not only did disulfiram protect normal cells from radiation, it also appeared to enhance the sensitivity of cancer cells towards radiation.12–16 This review discusses and elaborates the unique role of disulfiram in cancer radiotherapy (Figure 1).
Figure 1.
Mechanisms of disulfiram in radiation protection and disulfiram-copper complex enhance the efficacy of radiotherapy in tumor cells.
Radioprotective Activity of Disulfiram
The radioprotective effects of disulfiram have been thoroughly investigated. Stromme et al described that disulfiram was metabolized to diethyldithiocarbamate (DTC) in mice, and that these animals were well protected against ionizing radiation.17 Other studies have shown that radiation exposure produces free radicals, which are highly reactive. Disulfiram is a potent antioxidant that protects deoxyribose against damage in normal cells.12,18 In the L-929 mouse fibroblast cell lines, disulfiram increased radiation sensitivity in tumor cells while reducing radiation toxicity in normal tissues. This observation was concentration dependent. Furthermore, disulfiram’s radiation modifier effect was achieved through its major metabolite DTC.19 Although the molecular mechanism of disulfiram in normal tissue remains largely unknown, these observations generated enthusiasm for the idea that disulfiram could protect against radiation.
One of the observations was made in response to gamma radiation. Following exposure to gamma radiation, radiation induced damage in normal cells lead to a decreased quantity of the supercoiled form of plasmid pBR322 DNA (deoxyribonucleic acid). Despite being irradiated at a dose of 300Gy, the plasmid DNA assumed complete protection in the presence of disulfiram; when the radiation dose was increased to 600 Gy, a near-linear increase of the membrane lipids peroxidation was detected and the administration of disulfiram was capable of reducing the damage. In mice models, whole body irradiation was delivered after the administration of disulfiram. Cellular DNA damage and membrane lipids peroxidation reduced in the liver of mice treated with disulfiram.12
Based on these studies, it appears that disulfiram protects DNA from radiation induced damage in normal tissues and warrants further investigation as a radioprotector.
Disulfiram Enhances Radiation Sensitivity of Tumor Cells
In the past few decades, much progress has been made in radiotherapy. However, there is still a high demand for improved therapies as local-regional recurrence rates remain significant.20,21 The main problem is that it is difficult to deliver a high dose of radiation to a tumor whilst limiting the dose to normal surrounding tissues. To get around this problem, radiobiologists have tried to find ideal radiation sensitizers to improve local tumor control rates.
Disulfiram is capable of enhancing the radiation sensitivity of cancer cells in a variety of ways.12–16 And one of the unique ways is through forming a complex with metal ions. A lack of metal ions decreases disulfiram’s antitumor activity.22 Meanwhile, in human cancer patients, an elevated copper level has been detected in both the tumor tissues and the serum. Further investigations showed that copper is involved in the biological process of tumorigenesis and metastasis.11,23 This makes disulfiram an ideal antitumor drug. In a previous study, disulfiram’s anticancer effect was shown to be copper-dependent. Disulfiram-copper complex inhibited breast cancer cell proliferation, but not in normal cells.24 In addition, a synergistic interaction was found between copper-complexed disulfiram and radiotherapy; in human SK-N-BE neuroblastoma and UVW/noradrenaline transporter glioma cells, the efficacy of both external beam radiotherapy and targeted radionuclide therapy were enhanced by disulfiram in a copper-dependent manner. High concentrations of disulfiram induced oxidative stress, which was associated with tumor cell death; however, disulfiram’s anticancer activity in low concentrations was copper-dependent.13 In addition, novel copper-based nanoparticle has been developed, this compound could be significantly activated by radiotherapy and was efficient in inhibiting human breast cancer cells (MCF-7) proliferation.25 Disulfiram combined with copper-based nanoparticles were demonstrated to enhance the efficacy of radiotherapy in esophageal cancer.26 Although there is little evidence, to date, in clinical applications, this unique property makes disulfiram essential to radiation oncology.
Disulfiram Causes Reactive Oxygen Species (ROS) Levels Alteration
Superoxide radicals play a crucial role in radiation-induced cell death, whereas cancer cells contain a low level of superoxide dismutase.27,28 Disulfiram and one of its metabolites DTC have been demonstrated to increase oxidative stress in tumor cells.29 Consequently, there are some studies focusing on the dynamic change in intracellular ROS levels in response to combined radiotherapy and disulfiram treatment. In Chinese hamster cell models, DTC, a copper chelating agent, was administered during the course of radiotherapy. Final results suggested that DTC inhibited the enzyme superoxide dismutase, promoting superoxide radical mediated toxicity. Eventually, the tumor cell radiation sensitivity was enhanced.30 In inflammatory breast cancer cellular models, a redox adaptive response means the cancer cells evade ROS-mediated death. The disulfiram and copper complex have emerged as a redox modulator that enhances radiation sensitivity by inducing oxidative stress mediated apoptosis in tumor cells. Importantly, no significant in vitro toxicity was observed in normal cells.22 The combination of disulfiram and copper enhanced both radiation sensitivity and chemotherapy sensitivity via upregulating ROS levels in head and neck squamous cell carcinoma.31 Park et al treated head and neck squamous cell carcinoma with disulfiram and copper complex, an increased ROS formation was observed and the complex significantly induced autophagic cell death.32 In glioblastoma, disulfiram acted as a novel ferroptosis inducer which was effective in triggering lysosomal membrane permeabilization and producing ROS, and finally improved the efficacy of radiotherapy.33 Disulfiram is capable of regulating intracellular antioxidative defense systems, but is harmless to normal tissues; thereby, serving as a novel and promising radiosensitizing agent for the treatment of cancer.
Disulfiram Targets Cancer Stem Cells (CSCs)
Previous studies have indicated that CSCs contribute to the inherent resistance of tumor cells to radiotherapy.34 Targeting CSCs is, therefore, a promising approach to kill off a tumor’s regenerative capacity following radiotherapy. In vivo and in vitro studies of pancreatic ductal adenocarcinoma implied that standard chemoradiation regimes accelerated the generation of CSCs, defined as Aldehyde dehydrogenase (ALDH) overexpressing tumor cells.14 ALDH belongs to an enzyme super family that catalyzes the oxidation aldehydes.35 Over the past few decades, several studies have shown that ALDH1 is a marker of CSCs and tumor initiating cells.36,37 As a result, ALDH could be an important target in anticancer therapy. Disulfiram treats alcoholism through its ability to inhibit the enzymatic activity of ALDH.38 Recent studies have suggested that disulfiram combined with radiotherapy are efficacious at suppressing CSCs. Choi et al reported that disulfiram can penetrate the blood brain barrier and accumulate in the brain. The accumulation of disulfiram in the brain induces apoptosis and, by inhibiting ALDH, decreases proliferation of the brain tumor initiating cells.39
The NF- κB Pathway
Several studies have demonstrated a role for NF- κB signaling in CSC biology. The administration of disulfiram-copper complexes in combination with irradiation, in vitro, inhibited CSCs via the NF-κB stemness gene pathway. The same study also used a breast cancer xenograft mouse model to show that tumor growth and metastasis were inhibited and apoptosis was induced by disulfiram, when combined with radiotherapy.40 Disulfiram-copper complex combined with chemoradiotherapy was found to be effective in inhibiting CSCs and tumor cells via downregulation of the NF-κB pathway; with an efficacy of 42% compared with 30% for the standard chemoradiotherapy regime.14
Disulfiram Act as an ALDH Inhibitor
Other cellular mechanisms of disulfiram in combination with radiotherapy, in CSCs, have been observed. CSCs of atypical teratoid/rhabdoid tumor express ALDH. Disulfiram, as an irreversible ALDH blocker, induces apoptosis in irradiated tumor cells, whilst increasing cell cycle arrest and autophagy by reducing DNA double strand break repair. In atypical teratoid/rhabdoid tumor mouse model, disulfiram combined with radiotherapy inhibited tumor growth and produced a survival benefit.15 Several mechanisms by which disulfiram targets CSCs have been shown. This gives further support to the idea that disulfiram may be an important radiation sensitizer in antitumor therapy, particularly in brain cancers.
The Inhibition of Ubiquitin-Proteasome System (UPS)
The UPS is involved in many biological processes, including cellular protein catabolism, signal transduction, cell cycle progression, apoptosis, chromosome maintenance.41 In recent years, several studies have indicated that UPS participates in the modulation of radiation induced responses in cancer.42,43 As previously mentioned, disulfiram is known to alter the intrinsic radiation sensitivity of cancer cells. In human radiation resistant cell lines, disulfiram combined with copper was an effective proteasome blocker, suppressing the activation of the NF-κB pathway and enhancing the effect of radiation treatments on killing cancer cells.40 Disulfiram-copper complex impaired DNA repair pathways in a glioblastoma cell line by inhibiting proteasome activity and acting as a radiation sensitizing agent.44 Previous studies described radiation induced protein burden in tumor cells.45,46 Disulfiram-copper was able to inhibit proteasome activity and misfolded protein accumulation in the tumor cells. The complexes have been shown to act, via its effect on proteasome function, as a radiosensitizer both in vitro and in vivo. Therefore, the combined strategy increased the protein burden and results in cell apoptosis.
The Interference of Cancer Cell Cycle
The radiation sensitivity of cancer cells varies in different phases of the cell cycle. Tesson et al reported that disulfiram, in the presence of copper supplement, could be considered as a cell cycle specific cellular radiation sensitizer. The disulfiram-copper complex works by inhibiting the proteasome activity. Only 0.3 μM of this complex was required to achieve the maximum anticancer effect of radiotherapy on the neuroblastoma cell line SK-N-BE and the glioma cell line UVW.16 Hassani et al demonstrated disulfiram copper complex caused the disturbance of the ROS balance and induced G0/G1 cell cycle arrest in acute myeloid leukaemia cell lines.47 These results, together, justify the evaluation of disulfiram as an adjuvant agent in patients receiving radiotherapy.
Future Perspective
The evidence of disulfiram’s radiation sensitizing activity and antitumor effects is accumulating. Laboratory studies have also revealed that disulfiram has an anti-angiogenic effect and causes epigenetic modifications.48,49 However, investigations in the context of radiation biology are extremely rare. There is still some disagreement over the conclusions of some of the in vitro and in vivo studies and the underlying mechanism of disulfiram’s radiosensitization effect remains to be fully elucidated. There are nine ongoing clinical trials focusing on the application of disulfiram as an anticancer agent worldwide. Only one of these clinical trials is studying the disulfiram-copper complex combined with radiotherapy (Table 1).
Table 1.
Ongoing Cancer Clinical Trials Using DSF in the Treatment of Cancer
| NCT Number | Year | Center | Tumor Type | Phase | Interventions | Recruitment |
|---|---|---|---|---|---|---|
| NCT0190716 | 2013 | Washington University School of Medicine, United States; | •Glioblastoma | Early Phase I | Temozolomide + DSF/Copper gluconate | Active, not recruiting |
| NCT02715609 | 2016 | •Washington University School of Medicine, United States; •University of Calgary, Canada; |
•Glioblastoma Multiforme | •Phase I •Phase II |
Surgery + Radiation + Temozolomide + DSF/Copper Gluconate | Recruiting |
| NCT0332334 | 2017 | •University Hospital Olomouc, •The Institute of Molecular and Translational Medicine, Czech Republic; |
•Breast Neoplasm Female •Metastatic Breast Cancer |
Phase II | DSF | Recruiting |
| NCT0267897 | 2017 | •St.Olavs University Hospital, Norway; •Sahlgrenska University Hospital, •Ryhov County Hospital, Sweden •Linköping University Hospital, •Lund University Hospital, •Karolinska University Hospital, •Uppsala University Hospital, •Örebro University Hospital, Sweden; |
•Recurrent Glioma •Recurrent Glioblastoma |
•Phase II •Phase III |
DSF/Copper + Alkylating Agents | Recruiting |
| NCT02963051 | 2017 | Duke University Medical Center, United States; | Prostate Cancer | Phase I | DSF/Copper gluconate | Recruiting |
| NCT0177791 | 2017 | •Olympion Medical Center, •University of; Ioannina, Greece; •University of Eastern Finland; •University of Ulm, German; |
Glioblastoma Multiforme | Phase II | Temozolomide and DSF/Copper | Not yet recruiting |
| NCT0303413 | 2017 | •Beaumont Hospital, •Washington University School of Medicine, •John Theurer Cancer Center, •Lenox Hill Hospital, •University of Cincinnati, •Vanderbilt Ingram Cancer Center, •Baylor University Medical Center, •Huntsman Cancer Institute, United States; |
Recurrent Glioblastoma | Phase II | DSF/Copper | Recruiting |
| NCT0315177 | 2017 | Sahlgrenska University Hospital, Sweden; | Glioblastoma | Phase I | DSF versus Metformin | Not yet recruiting |
| NCT0336365 | 2018 | Aurora St. Luke’s Medical Center, United States; | Glioblastoma/Glioblastoma Multiforme | Phase II | DSF/Copper gluconate + Temozolomide |
Not yet recruiting |
The lack of large-scale, prospective, observational studies is limiting the application of disulfiram as a radiosensitizer and radioprotector in clinical practice. Despite this, our knowledge of disulfiram and its molecular mechanisms, by which it regulates normal cells and cancerous cell response to ion irradiation, has continued to grow. This provides an excellent opportunity to develop a therapy that may decrease the local failure rates and toxicity of radiotherapy.
Conclusion
Finding a new use for an approved drug is appealing. Disulfiram is an old, inexpensive and tolerable drug to treat alcohol abuse, which has now been shown to sensitize cancer cells to radiotherapy. The studies reviewed here show that the anticancer activity of disulfiram, along with its copper dependence, has been well elucidated. While the radioprotective effects of disulfiram in normal cells and radiosensitizing activity in tumor cells still require a full investigation, the utility of this drug as a potential radiosensitizer is well recognized. One benefit of disulfiram being an approved drug, is that it has already passed safety testing. This, together with the results of the studies reviewed here, reaffirm the need for a clinical evaluation of disulfiram as a pre-existing drug that may increase the efficacy and safety of radiotherapy.
Funding Statement
This work was supported by the Key Research Project of Henan Provincial Education Department (519/32211041).
Abbreviations
ALDH, aldehyde dehydrogenase; Cu, copper; CSC, cancer stem cells; DSF, disulfiram; DTC, diethyldithiocarbamate; DNA, deoxyribonucleic acid; NF-κB, nuclear factor kappa B; NPL4, Nuclear protein localization protein 4 homolog; ROS, reactive oxygen species; UPS, ubiquitin-proteasome system.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54. doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 3.You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu D, Wang S, Bindeman W. Clinical applications of PD-L1 bioassays for cancer immunotherapy. J Hematol Oncol. 2017;10:110. doi: 10.1186/s13045-017-0479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo H, Cui Y, Song H, Mao R, Gao Q, Ge H. Should stereotactic body radiotherapy doses be adjusted according to tumor size in early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Future Oncol. 2019;15:3071–3079. doi: 10.2217/fon-2019-0240 [DOI] [PubMed] [Google Scholar]
- 6.Li J, Yuan Y, Yang F, et al. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J Hematol Oncol. 2019;12:16. doi: 10.1186/s13045-019-0702-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins FS. Mining for therapeutic gold. Nat Rev Drug Discov. 2011;10:397. doi: 10.1038/nrd3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iljin K, Ketola K, Vainio P, et al. High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin Cancer Res. 2009;15:6070–6078. doi: 10.1158/1078-0432.ccr-09-1035 [DOI] [PubMed] [Google Scholar]
- 9.Cvek B. Nonprofit drugs as the salvation of the world’s healthcare systems: the case of Antabuse (disulfiram). Drug Discov Today. 2012;17:409–412. doi: 10.1016/j.drudis.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 10.Dufour P, Lang JM, Giron C, et al. Sodium dithiocarb as adjuvant immunotherapy for high risk breast cancer: a randomized study. Biotherapy. 1993;6:9–12. doi: 10.1007/BF01877380 [DOI] [PubMed] [Google Scholar]
- 11.Skrott Z, Mistrik M, Andersen KK, et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature. 2017;552:194–199. doi: 10.1038/nature25016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi NM, Gopalaswamy UV, Nair C. Radiation protection by disulfiram: protection of membrane and DNA in vitro and in vivo against gamma-radiation. J Radiat Res. 2003;44:255–259. doi: 10.1269/jrr.44.255 [DOI] [PubMed] [Google Scholar]
- 13.Rae C, Tesson M, Babich JW, Boyd M, Sorensen A, Mairs RJ. The role of copper in disulfiram-induced toxicity and radiosensitization of cancer cells. J Nucl Med. 2013;54:953–960. doi: 10.2967/jnumed.112.113324 [DOI] [PubMed] [Google Scholar]
- 14.Cong J, Wang Y, Zhang X, et al. A novel chemoradiation targeting stem and nonstem pancreatic cancer cells by repurposing disulfiram. Cancer Lett. 2017;409:9–19. doi: 10.1016/j.canlet.2017.08.028 [DOI] [PubMed] [Google Scholar]
- 15.Lee YE, Choi SA, Kwack PA, et al. Repositioning disulfiram as a radiosensitizer against atypical teratoid/rhabdoid tumor. Neuro Oncol. 2017;19:1079–1087. doi: 10.1093/neuonc/now300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesson M, Anselmi G, Bell C, Mairs R. Cell cycle specific radiosensitisation by the disulfiram and copper complex. Oncotarget. 2017;8:65900–65916. doi: 10.18632/oncotarget.19539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stromme JH, Eldjarn L. Distribution and chemical forms of diethyldithiocarbamate and tetraethylthiuram disulphide (disculfiram) in mice in relation to radioprotection. Biochem Pharmacol. 1966;15:287–297. doi: 10.1016/0006-2952(66)90300-5 [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Cai H, Koizumi Y, et al. Effects of lipid composition on the transcorneal penetration of liposomes containing disulfiram, a potential anti-cataract agent, in the rabbit. Biol Pharm Bull. 2000;23:327–333. doi: 10.1248/bpb.23.327 [DOI] [PubMed] [Google Scholar]
- 19.Taylor RD, Maners AW, Salari H, Baker M, Walker EM Jr. Disulfiram as a radiation modifier. Ann Clin Lab Sci. 1986;16:443–449. [PubMed] [Google Scholar]
- 20.Wang HH, Cui YL, Zaorsky NG, et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 2016;375:349–359. doi: 10.1016/j.canlet.2016.02.033 [DOI] [PubMed] [Google Scholar]
- 21.Luo H, Ge H, Cui Y, et al. Systemic inflammation biomarkers predict survival in patients of early stage non-small cell lung cancer treated with stereotactic ablative radiotherapy - a single center experience. J Cancer. 2018;9:182–188. doi: 10.7150/jca.21703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allensworth JL, Evans MK, Bertucci F, et al. Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol Oncol. 2015;9:1155–1168. doi: 10.1016/j.molonc.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt SM, Frezza M, Dou QP. New applications of old metal-binding drugs in the treatment of human cancer. Front Biosci (Schol Ed). 2012;4:375–391. doi: 10.2741/s274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.can-06-2126 [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Zou X, Chen W. A new X-ray activated nanoparticle photosensitizer for cancer treatment. J Biomed Nanotechnol. 2014;10:1501–1508. doi: 10.1166/jbn.2014.1954 [DOI] [PubMed] [Google Scholar]
- 26.Chang Y, Wu F, Pandey NK, et al. Combination of disulfiram and copper–cysteamine nanoparticles for an enhanced antitumor effect on esophageal cancer. ACS Appl Bio Mater. 2020;3:7147–7157. doi: 10.1021/acsabm.0c00949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell S, McMillan TJ. DNA damage and repair following treatment with ionizing radiation. Radiother Oncol. 1990;19:95–108. doi: 10.1016/0167-8140(90)90123-e [DOI] [PubMed] [Google Scholar]
- 28.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruning A, Kast RE. Oxidizing to death: disulfiram for cancer cell killing. Cell Cycle. 2014;13:1513–1514. doi: 10.4161/cc.28959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin PS, Kwock L, Butterfield CE. Diethyldithiocarbamate enhancement of radiation and hyperthermic effects on Chinese hamster cells in vitro. Radiat Res. 1979;77:501–511. doi: 10.2307/3575161 [DOI] [PubMed] [Google Scholar]
- 31.Yao W, Qian X, Ochsenreither S, et al. Disulfiram acts as a potent radio-chemo sensitizer in head and neck squamous cell carcinoma cell lines and transplanted xenografts. Cells. 2021:10. doi: 10.3390/cells10030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YM, Go YY, Shin SH, Cho JG, Woo JS, Song JJ. Anti-cancer effects of disulfiram in head and neck squamous cell carcinoma via autophagic cell death. PLoS One. 2018;13:e0203069. doi: 10.1371/journal.pone.0203069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu C, Zhang X, Huang B, et al. Disulfiram, a ferroptosis inducer, triggers lysosomal membrane permeabilization by up-regulating ROS in Glioblastoma. Onco Targets Ther. 2020;13:10631–10640. doi: 10.2147/OTT.S272312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R’s of radiobiology revisited. Stem Cells. 2010;28:639–648. doi: 10.1002/stem.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425255.4.6.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.can-08-4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi SA, Lee JY, Phi JH, et al. Identification of brain tumour initiating cells using the stem cell marker aldehyde dehydrogenase. Eur J Cancer. 2014;50:137–149. doi: 10.1016/j.ejca.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 38.Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. The status of disulfiram: a half of a century later. J Clin Psychopharmacol. 2006;26:290–302. doi: 10.1097/01.jcp.0000222512.25649.08 [DOI] [PubMed] [Google Scholar]
- 39.Choi SA, Choi JW, Wang KC, et al. Disulfiram modulates stemness and metabolism of brain tumor initiating cells in atypical teratoid/rhabdoid tumors. Neuro Oncol. 2015;17:810–821. doi: 10.1093/neuonc/nou305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Li W, Patel SS, et al. Blocking the formation of radiation-induced breast cancer stem cells. Oncotarget. 2014;5:3743–3755. doi: 10.18632/oncotarget.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J Biosci. 2006;31:137–155. doi: 10.1007/BF02705243 [DOI] [PubMed] [Google Scholar]
- 42.McBride WH, Iwamoto KS, Syljuasen R, Pervan M, Pajonk F. The role of the ubiquitin/proteasome system in cellular responses to radiation. Oncogene. 2003;22:5755–5773. doi: 10.1038/sj.onc.1206676 [DOI] [PubMed] [Google Scholar]
- 43.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lun X, Wells JC, Grinshtein N, et al. Disulfiram when combined with copper enhances the therapeutic effects of temozolomide for the treatment of glioblastoma. Clin Cancer Res. 2016;22:3860–3875. doi: 10.1158/1078-0432.ccr-15-1798 [DOI] [PubMed] [Google Scholar]
- 45.Riha R, Gupta-Saraf P, Bhanja P, Badkul S, Saha S. Stressed out - therapeutic implications of ER stress related cancer research. Oncomedicine. 2017;2:156–167. doi: 10.7150/oncm.22477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koizumi M, Tanjung NG, Chen A, et al. Administration of salubrinal enhances radiation-induced cell death of SW1353 chondrosarcoma cells. Anticancer Res. 2012;32:3667–3673. [PubMed] [Google Scholar]
- 47.Hassani S, Ghaffari P, Chahardouli B, et al. Disulfiram/copper causes ROS levels alteration, cell cycle inhibition, and apoptosis in acute myeloid leukaemia cell lines with modulation in the expression of related genes. Biomed Pharmacother. 2018;99:561–569. doi: 10.1016/j.biopha.2018.01.109 [DOI] [PubMed] [Google Scholar]
- 48.Paranjpe A, Zhang R, Ali-Osman F, Bobustuc GC, Srivenugopal KS. Disulfiram is a direct and potent inhibitor of human O6-methylguanine-DNA methyltransferase (MGMT) in brain tumor cells and mouse brain and markedly increases the alkylating DNA damage. Carcinogenesis. 2014;35:692–702. doi: 10.1093/carcin/bgt366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Fu SY, Wang LH, et al. Copper improves the anti-angiogenic activity of disulfiram through the EGFR/Src/VEGF pathway in gliomas. Cancer Lett. 2015;369:86–96. doi: 10.1016/j.canlet.2015.07.029 [DOI] [PubMed] [Google Scholar]