Abstract
Local damage (e.g., burning) induces a variation potential (VP), which is an important electrical signal in higher plants. A VP propagates into undamaged parts of the plant and influences numerous physiological processes, including photosynthesis. Rapidly increasing plant tolerance to stressors is likely to be a result of the physiological changes. Thus, developing methods of revealing VP-induced physiological changes can be used for the remote sensing of plant systemic responses to local damage. Previously, we showed that burning-induced VP influenced a photochemical reflectance index in pea leaves, but the influence of the electrical signals on other reflectance indices was not investigated. In this study, we performed a complex analysis of the influence of VP induction by local burning on difference reflectance indices based on 400–700 nm wavelengths in leaves of pea seedlings. Heat maps of the significance of local burning-induced changes in the reflectance indices and their correlations with photosynthetic parameters were constructed. Large spectral regions with significant changes in these indices after VP induction were revealed. Most changes were strongly correlated to photosynthetic parameters. Some indices, which can be potentially effective for revealing local burning-induced photosynthetic changes, are separately shown. Our results show that difference reflectance indices based on 400–700 nm wavelengths can potentially be used for the remote sensing of plant systemic responses induced by local damages and subsequent propagation of VPs.
Keywords: variation potential, reflectance indices, photosynthetic response, remote sensing, pea leaves
1. Introduction
Local actions of stressors on plants require systemic adaptive responses based on the generation and propagation of long-distance stress signals. Electrical signals (ESs), including action potential, system potential, and variation potential (VP) [1,2,3,4,5,6,7,8,9], play an important role in the induction of physiological changes in non-irritated parts of plants. It is known that an action potential is a self-propagating depolarization electrical signal [1,2,3,6,10] induced by non-damaging stimuli and is caused by both transient activation of Ca2+, K+, and anion channels, and inactivation of H+-ATPase in the plasma membrane. The system potential is a weakly investigated hyperpolarization signal [8,11,12] caused by transient activation of H+-ATPase and, possibly, changes in activity K+ channels.
A VP is a long-distance signal in higher plants induced by local damage [2,4,6,13], which is formed by long-term depolarization and short-term “AP-like” spikes. The generation of a VP is mainly based on transient inactivation of H+-ATPase, induced by Ca2+ influx through Ca2 channels [2,13], but anion and outward K+ channels can participate in the generation of AP-like spikes [4]. The mechanisms of VP propagation are still being discussed. The first hypothesis [13,14,15,16] supposes that a VP is a local electrical response induced by a hydraulic wave, which propagates through a plant from the damaged zone and activates mechanosensitive Ca2+ channels. The second hypothesis [4,17] supposes that the VP is the local electrical response induced by the propagation of a specific “wound substance” from the damaged zone and the activation of ligand-dependent Ca2+ channels. Additionally, there are hypotheses of VP propagation based on combinations of hydraulic and chemical signals [18,19,20,21].
ESs induced by local stimuli can strongly influence physiological processes in non-irritated parts of plants [2,3,5,7,8]. It is known that ESs increase the expression of defense genes [22,23,24,25], respiration [26,27,28], ATP content in leaves [28], production of phytohormones [9,25,29,30,31,32], etc. In contrast, other physiological processes (e.g., phloem loading [33], phloem mass flow [34,35], and plant growth [36]) can be suppressed after propagation of ESs. Photosynthesis is an important target of ESs [5]. It is known that ESs decrease photosynthetic CO2 assimilation, quantum yield of photosystem I, quantum yield of photosystem II (Y(II)), and mesophyll CO2 conductance [23,27,37,38,39,40,41,42,43]. Non-photochemical quenching of chlorophyll fluorescence (NPQ), cyclic electron flow, and photosynthetic light absorption can be stimulated by ESs [27,37,39,43,44,45]. Increasing plant tolerance to stressors (including tolerance of photosynthetic machinery) is likely the result of physiological changes [46,47,48,49,50,51,52,53].
It can be expected that monitoring of electrical activity and ES-induced physiological changes is a potential tool for revealing the actions of stressors on plants. Investigations of plant electrical activity show that (i) the total electrical activity of plants (“electrome”) can be strongly dependent on abiotic and biotic factors [53,54,55,56,57], and (ii) analysis of the electrical activity can be used for the classification of stressors that act on plants [58,59,60,61,62]. However, direct measurements of electrical activity cannot be used for the remote sensing of ES-induced systemic responses because electrodes would need to be connected to the plant. An alternative approach can be based on revealing relationships between ES-induced physiological changes and changes in plant reflectance. Previously, we showed that ESs induce changes in broadband reflectance indices [63,64] and decrease some narrowband indices, including the water index [64], which shows the water content in leaves, and the photochemical reflectance index (PRI) [65]. The PRI is strongly related to photosynthetic processes [66,67,68,69,70,71,72,73,74], including Y(II) and NPQ [75,76,77]. Its fast changes (seconds and minutes) are caused by the acidification of a chloroplast lumen through transitions in the xanthophyll cycle [66,68,78] and a change in light scattering at 530–546 nm [68,77], which is likely to be caused by chloroplast shrinkage [75,77]. It is known that ESs are accompanied by pH decreases in the cytoplasm, stroma, and lumen [38,39,79]; the luminal pH decrease is likely to be a mechanism of the ES’s influence on the PRI.
However, using only the current narrowband reflectance indices (RIs) can limit the search for new RIs that are sensitive to the ES-induced physiological changes, because the reflected light at most wavelengths is not analyzed in standard RIs. The limitation can be eliminated by using heat maps showing (i) the correlation coefficients of physiological parameters to all possible RIs calculated on the basis of measured spectra [80,81,82] or (ii) the significance of changes in these RIs under the action of stressors [83]. Considering these approaches, it can be expected that a similar analysis of RIs in the 400–700 nm spectral range, which is related to photosynthetic processes, could reveal new RIs that are sensitive to ES-induced photosynthetic changes.
The aim of the current work was to perform a complex analysis of the influence of local burning, which is a typical inductor of VP (a key ES in higher plants [5,8]), on difference reflectance indices based on 400–700 nm wavelengths in leaves of pea seedlings. The analysis was based on spectra and photosynthetic parameters, which were preliminarily measured in our work [65] devoted to VP influence on the PRI. The spectra were used for the calculation of all probable RIs, and their changes after VP induction and correlations with photosynthetic parameters were investigated.
2. Data Analysis
2.1. General Scheme of Data Analysis
We used spectral and photosynthetic data from [65] in our analysis. Only data taken from the second leaf were analyzed, because the local burning-induced electrical signals and photosynthetic and PRI changes in the fourth leaf [65] were weak. All spectra of reflected light and photosynthetic parameters measured in the second leaves were analyzed as a single experimental group (i.e., similar experimental groups from [65] were combined into one group). This group included 13 repetitions.
Figure 1 shows the scheme of the data analysis. Seven time points were used, including two points before VP induction (15 and 5 min before) and five points after the induction (5, 15, 25, 35, and 45 min after). We previously showed that RIs calculated on the basis of 400–700 nm wavelengths can have non-normal distributions [83]. Thus, we used non-parametric statistics in our analysis. Medians of the investigated values were used as the non-parametric analog of averaged values.
Figure 1.
General scheme of analysis of spectra of the reflected light and photosynthetic parameters (the quantum yield of photosystem II, Y(II), and non-photochemical quenching of chlorophyll fluorescence, NPQ) measured in the second pea leaf before and after induction of variation potential (VP) by local burning of the first leaf.
The values of the investigated parameters 5 min before VP induction were used as control values for each plant. We calculated the absolute values of the investigated parameters (RIs, Y(II), and NPQ) and their changes (differences between current and control values of the parameters in each plant, ΔRIs, ΔY(II), and ΔNPQ). Significant differences between values were calculated using the non-parametric Mann–Whitney U test. Relationships between reflectance indices and photosynthetic parameters were estimated on the basis of Pearson’s correlation coefficients. Medians, which were separately calculated on the basis of the absolute values of the parameters or their changes for each time point, were used for the calculation (n = 7).
2.2. Calculation of Difference Reflectance Indices and Construction of Heat Maps
The calculation of RIs (or ΔRIs) and the construction of heat maps were based on several programs, which were developed using the Python 3.8 programming language. They solved the following tasks:
(i) Calculation of all possible RIs on the basis of Equation (1):
| (1) |
where I(R1) and I(R2) are the intensities of the reflected light from a leaf at R1 and R2 wavelengths, respectively; IC(R1) and IC(R2) are the intensities of the reflected light from a white reflectance standard at R1 and R2 wavelengths (in accordance with [76,84]), respectively. To increase accuracy, averaged intensities of reflected light (3 nm range) were used. RIs were not calculated at R2 ≥ R1. Changes in RIs (ΔRIs) were calculated according to Equation (2):
| (2) |
where RITP is the RI at a specific time point, and RIC is the control RI equal to the RI at 5 min before VP induction.
(ii) Calculation of the significance (p) of differences between experimental and control values of RIs (or ΔRIs) on the basis of the non-parametric Mann–Whitney U test and estimation of the directions of the differences. Two-dimensional data arrays (significance and directions of changes for each RI as a function of R1 and R2) were used for the construction of heat maps.
(iii) Calculation of the medians of RIs (or ΔRIs) at each time point and Pearson’s correlation coefficients of these medians to similar medians of Y(II) and NPQ. Two-dimensional data arrays (correlation coefficients for each RI as a function of R1 and R2) were used for the construction of heat maps.
3. Results
3.1. Local Burning-Induced Changes in Difference Reflectance Indices
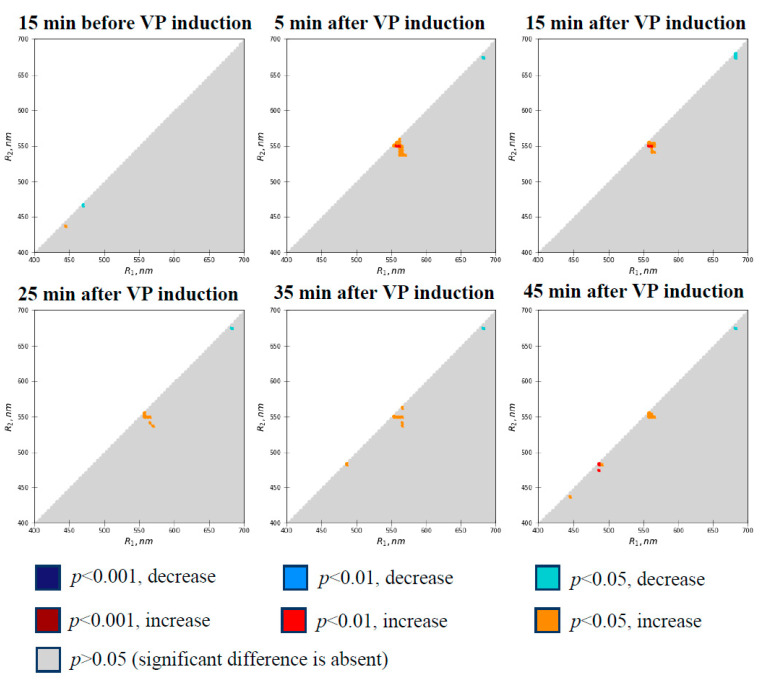
Figure 2 shows the heat maps of the significance and directions of differences between the absolute values of RIs at different time points and the control values. It is shown that differences were absent in the RIs before VP induction. Induction of a VP by local burning caused a transient increase in RIs (mostly at 5 and 15 min after heating) in a small spectral range (R1 was about 550–570 nm; R2 was about 535–560 nm). The increase in RIs approximately corresponded to the decrease in the PRI, which was shown in [65]. The opposite direction of RI changes is related to the opposite order of R1 and R2, because the PRI based on R1 = 531 nm and R2 = 570 nm [65] corresponds to the RI based on R1 = 570 nm and R2 = 531 nm in Figure 2. It is interesting that there were extremely small areas (pixel level) showing significant changes in RIs in other spectral ranges (e.g., RIs based on R1 equal to about 680 nm and R2 equal to about 675 nm; the measured reflected light at the wavelengths can additionally include chlorophyll fluorescence).
Figure 2.
Heat maps of significance and directions of changes in absolute values of RIs in the second pea leaf at different time intervals before and after induction of variation potential (n = 13). RIs were calculated based on Equation (1). Burning of the first leaf was used for VP induction. The Mann–Whitney U test was used for p-value calculations. The absolute values of RIs were compared to the values of RIs at 5 min before VP induction.
Previously, we showed that changes in RIs (e.g., PRI or broadband reflectance indices) were more sensitive to short-term actions of stressors [63,64,65,76,83,85] than their absolute values, because using ΔRIs excluded the individual variability of the initial values of RIs and decreased the standard errors of the measured values. The effect was also observed in works by other authors (e.g., [74,86] for PRI). Thus, we analyzed the significance of the changes in ΔRIs in a further analysis and constructed heat maps of the significance of changes in ΔRIs (Figure 3).
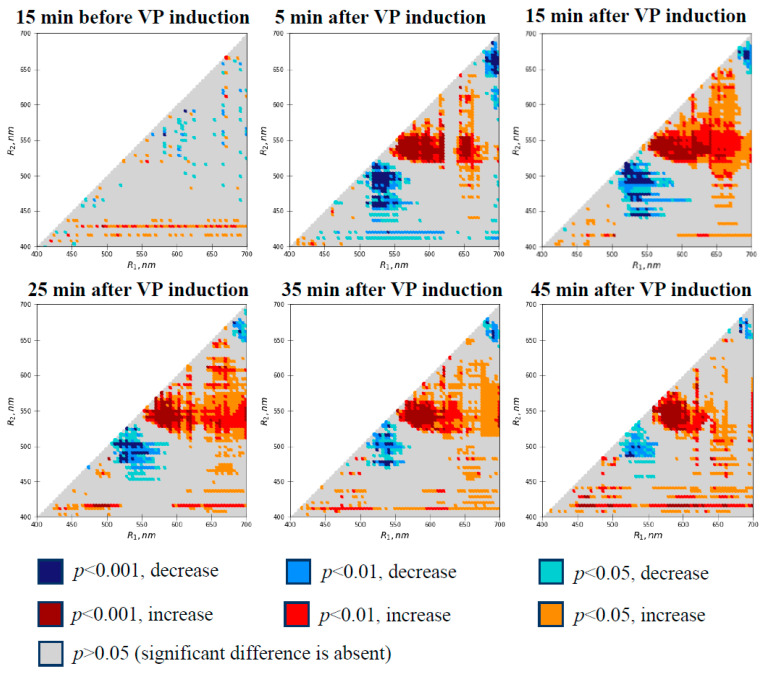
Figure 3.
Heat maps of significance and directions of changes in ΔRIs in the second pea leaf at different time intervals before and after induction of variation potential (n = 13). RIs were calculated based on Equation (1). Burning of the first leaf was used for VP induction. Each ΔRI was calculated as RITP–RIC. RIC is the control RI at 5 min before the VP induction. RITP is the difference in the reflectance index at a specific time point before or after VP induction. The Mann–Whitney U test was used for p-value calculations. The ΔRIs were compared to zero values (absence of changes). Using ΔRIs minimized the influence of individual plant differences on the local burning-induced changes in RIs.
There were only separate pixels on the first heat map showing a significant difference in ΔRIs at 15 min before VP induction (Figure 3). The localizations of the pixels were rather chaotic, excluding a few pixel lines in the lower part of the figure. It is also important to note that RIs with highly significant differences (p < 0.001) were practically absent in the variants (about 0.1% of the total quantity of RIs). Considering these results, we supposed that the changes in RIs were mainly related to revealing false changes, which were caused by stochastic differences in the spectra measured at different time intervals and the large quantity of simultaneously analyzed RIs. The false changes could be the result of cooperative effects of moderate stochastic differences in light measurements at both wavelengths (separate pixels) or high stochastic differences at a single wavelength (line of pixels).
VP induction by local burning strongly influenced ΔRIs (Figure 3). There were several large spectral regions in the heat maps with significant positive changes in ΔRIs (e.g., R1 was about 540–625 nm and R2 was about 520–560 nm), in addition to negative changes (e.g., R1 was about 510–560 nm and R2 was about 450–520 nm). The area of the spectral regions was dependent on the duration after VP induction. For example, ΔRIs with highly significant changes (p < 0.001) accounted for 8–9% of the total quantity of ΔRIs at 5 and 15 min after burning and for approximately 4–5% at 35 and 45 min.
The results show that the induction of VP by local burning caused changes in a large number of ΔRIs and weakly influenced the absolute values of RIs. The changes could be related to local burning-induced photosynthetic changes in pea leaves; as a result, an analysis of the relations of RIs and ΔRIs to photosynthetic parameters was the next task of investigation.
3.2. Relations of Local Burning-Induced Changes in Difference Reflectance Indices to Changes in Photosynthetic Parameters
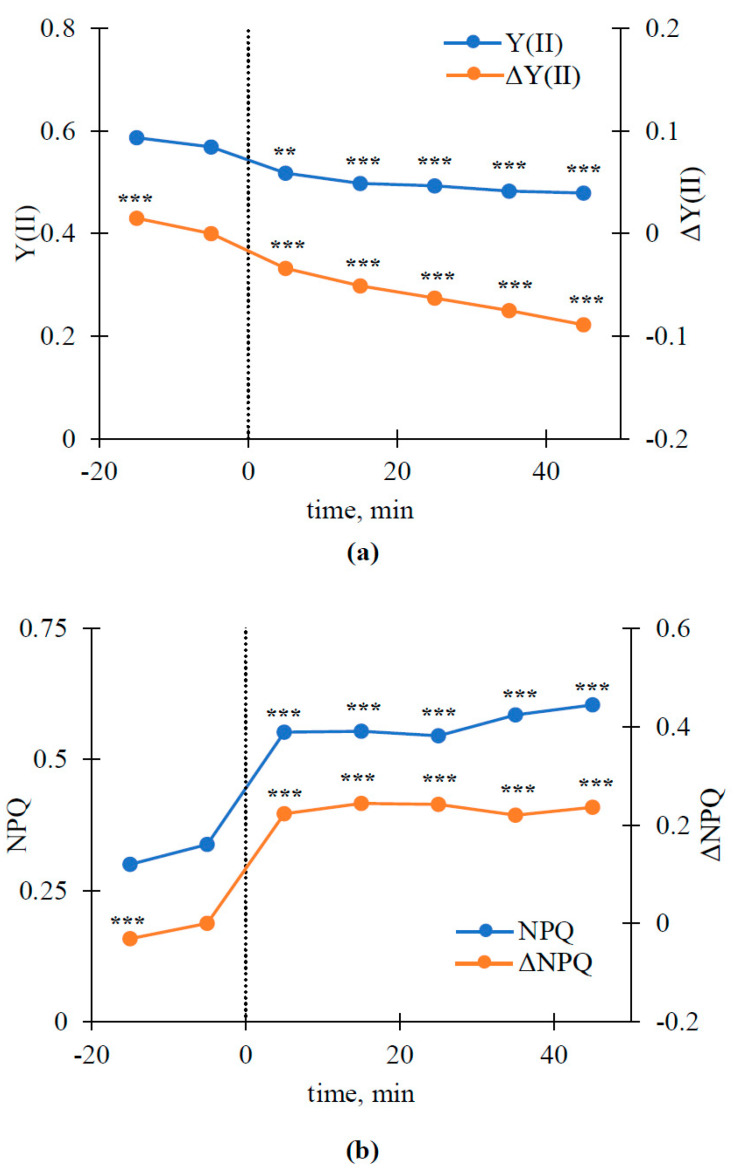
Figure 4 shows the absolute values of Y(II) and NPQ and changes in the parameters (ΔY(II) and ΔNPQ) before and after the induction of variation potential by burning the leaflet of the first pea leaf. The photosynthetic parameters were calculated on the basis of all photosynthetic responses in the second pea leaf, which were shown in previous work [65].
Figure 4.
Dynamics of Y(II) and ΔY(II) (a) and NPQ and ΔNPQ (b) before and after induction of variation potential in the second leaf (n = 13). Burning the first leaf was used for VP induction (the burning is marked by the time point zero and dotted line). ΔY(II) and ΔNPQ were calculated as Y(II)TP-Y(II)C and NPQTP-NPQC, respectively. Y(II)C is the control Y(II) at 5 min before VP induction. Y(II)TP is Y(II) at a specific time point before or after VP induction. NPQC is the control NPQ at 5 min before VP induction. NPQTP is NPQ at a specific time point before or after VP induction. The Mann–Whitney U test was used for p-value calculations. Y(II) and NPQ were compared to control values. ΔY(II) and ΔNPQ were compared to zero values (absence of changes). Using ΔY(II) and ΔNPQ minimized the influence of individual plant differences on local burning-induced changes in the parameters. **, p < 0.01 and ***, p < 0.001.
It was shown that the VP induction by local burning caused a fast decrease in the absolute value of Y(II) (Figure 4a) and increased the absolute value of NPQ (Figure 4b); both changes were significant. The result was in a good agreement with numerous works (e.g., see the review in [5]) devoted to investigating the influence of ESs on photosynthetic processes. The analysis of ΔY(II) and ΔNPQ showed similar changes in the parameters after the VP induction. However, small significant changes in ΔY(II) (decrease) and ΔNPQ (increase) were also observed before the induction of the variation potential. The last result showed slow changes in the investigated photosynthetic parameters (especially, ΔY(II)) before burning, which was in accordance with several works (e.g., [39,40,48,49,87]). The changes were related to the slow photosynthetic response (tens of minutes) induced by illumination with high intensity (probably by the photosynthetic state transition and (or) photodamage).
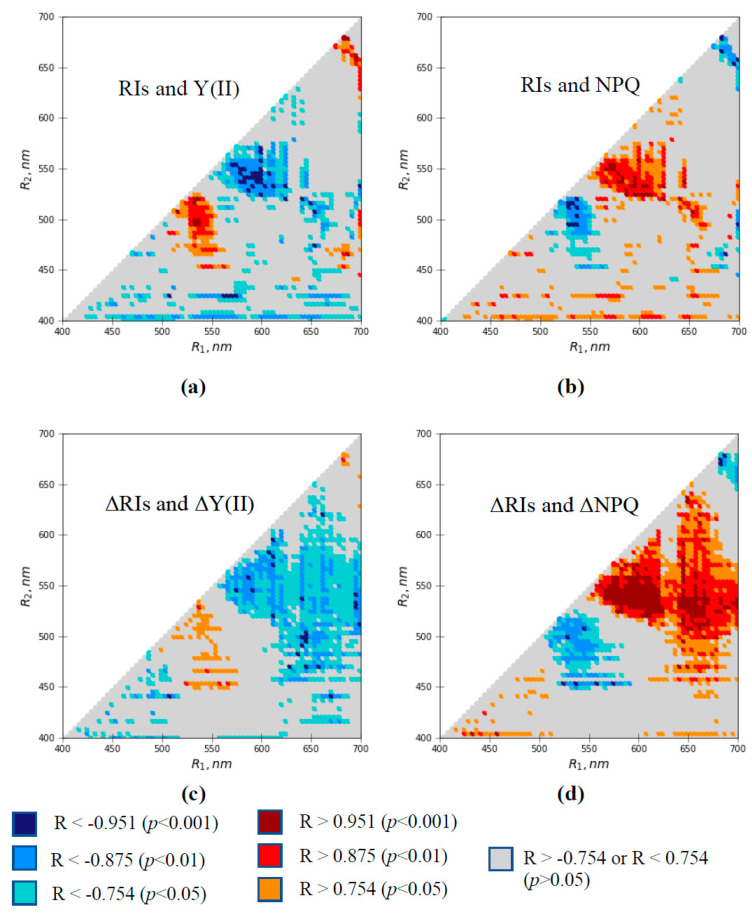
Figure 5 shows heat maps of Pearson’s correlation coefficients of RIs with Y(II) and NPQ and the correlation of ΔRIs with ΔY(II) and ΔNPQ. Only significant correlation coefficients are shown in the figure.
Figure 5.
Heat maps of linear correlation coefficients (R) between RIs and Y(II) (a), RIs and NPQ (b), ΔRIs and ΔY(II) (c), and ΔRIs and ΔY(II) (d). Pearson’s correlation coefficients were calculated based on medians of investigated parameters. The medians were calculated for each time point (n = 7). Only significant correlation coefficients are shown in the figure.
It was shown that both RIs and ΔRIs were strongly correlated to photosynthetic parameters in large spectral regions. In particular, modules of correlation coefficients could be more than 0.95 in several spectral regions (e.g., R1 is about 595–620 nm and R2 is about 525–540 nm in Figure 5d). The relations of RIs and ΔRIs to photosynthetic parameters could be positive and negative in different spectral regions. Relationships of reflectance indices to the quantum yield of photosystem II and non-photochemical quenching were mainly opposite, which is in good agreement with the opposing direction of the changes in Y(II) and NPQ (Figure 4).
Areas of the spectral regions with a significant correlation of ΔRIs with ΔY(II) and ΔNPQ were larger than the areas with a significant correlation of RIs with Y(II) and NPQ. The results support the conclusion that the sensitivity of ΔRIs to photosynthetic parameters is higher than the sensitivity of RIs.
Spectral regions with a significant correlation of RIs and ΔRIs with photosynthetic parameters (e.g., R1 was about 550–620 nm and R2 was about 500–575 nm, or R1 was about 510–560 nm and R2 was about 460–520 nm; Figure 5d) were similar to the spectral regions with significant changes in ΔRIs after the induction of VP (Figure 3). This showed that photosynthetic changes were likely to be the causes of the revealed changes in RIs, which were observed after the induction of the VP in pea leaves.
3.3. Dynamics of Local Burning-Induced Changes in Some Revealed Reflectance Indices
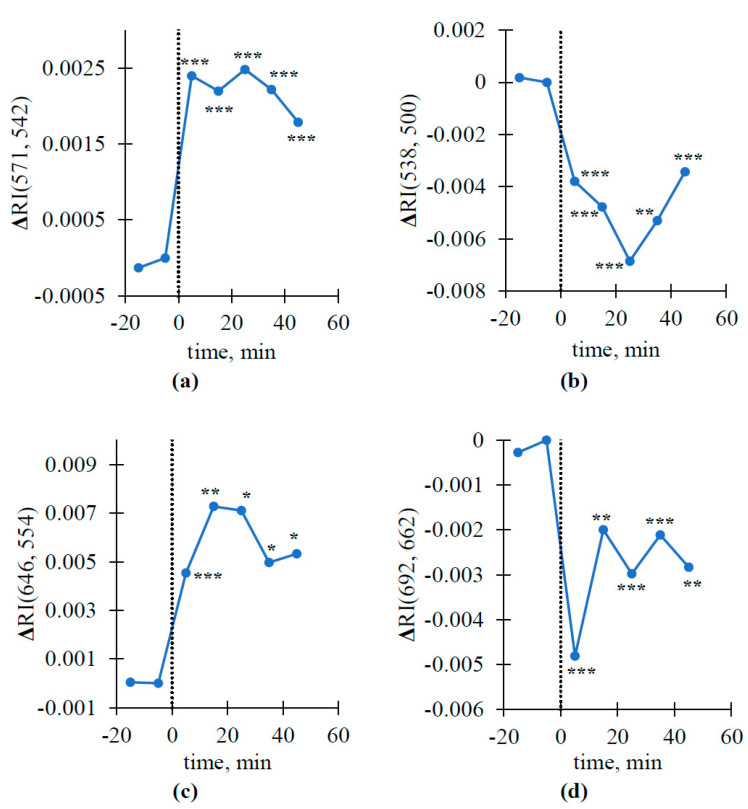
Furthermore, we analyzed the dynamics of local burning-induced changes in some reflectance indices, which were included in spectral areas with significant changes (Figure 6). Only ΔRIs were analyzed because changes in the absolute values of RIs were weak. The dynamics of burning-induced changes in ΔRI(571, 542) (R1 was 571 nm and R2 was 542 nm), ΔRI(538, 500) (R1 was 538 nm and R2 was 500 nm), ΔRI(646, 554) (R1 was 646 nm and R2 was 554 nm), and ΔRI(692, 662) (R1 was 692 nm and R2 was 662 nm), which were selected on the basis of different spectral ranges in Figure 3, were investigated.
Figure 6.
Dynamics of ΔRI(571, 542) (a), ΔRI(538, 500) (b), ΔRI(646, 554) (c), and ΔRI(692, 662) (d) before and after induction of variation potential in the second leaf (n = 13). Burning of the first leaf was used for VP induction (the burning is marked by the time point zero and the dotted line). The Mann–Whitney U test was used for p-value calculations; parameters were compared to zero values (absence of changes). *, p < 0.05, **, p < 0.01, and ***, p < 0.001.
It was shown that all investigated ΔRIs were strongly changed after VP induction. The magnitudes of the changes varied from approximately 0.0025 for ΔRI(571, 542) to 0.007–0.008 for ΔRI(538, 505) and ΔRI(646, 554), similar to the magnitudes of VP-induced changes in PRI [65]. The dynamics of the changes in different ΔRIs also differed: the minimum values of ΔRI(538, 500) and ΔRI(692, 662) were observed at 25 and 5 min after VP induction, respectively. The maximum value of ΔRI(646, 554) was observed at 15 min after burning. ΔRI(571, 542) reached maximal values at 5 min after the induction of variation potential, which were weakly changed after that.
4. Discussion
Damage-induced VP is a key electrical signal in higher plants influencing numerous physiological processes and, probably, participating in the induction of the systemic adaptive response [2,5,7,8]. Photosynthetic processes are an important target of VP [2,3,5,7,8,27,28,30,31,37,38,39,40,41,42,43,44,45,48]. Photosynthetic inactivation can be initiated for minutes after VP induction; both dark and light reactions are changed, modifications in photosynthetic processes participate in increasing tolerance of photosynthetic machinery, etc. The VP’s influence on photosynthetic processes is based on the pH increase in the apoplast and the pH decrease in the cytoplasm, stroma, and lumen [5,8,38,39,79].
It is known that photosynthetic processes are strongly related to plant optical properties, including fast changes in leaf reflectance (e.g., reflectance in the green spectral range [66,68,75,76,77,78,88,89,90], which is caused by acidification of the lumen of chloroplasts). Thus, it seems highly probable that VP-induced photosynthetic changes should be accompanied by changes in leaf reflectance, which can potentially be used for their remote sensing.
Our previous results showed that the local burning-induced VP causes changes in broadband reflectance indices and the narrowband water index [63,64], which are related to the water content in leaves, and decrease in the narrowband PRI [65], which is correlated to the quantum yield of photosystems and non-photochemical quenching. Potentially, the VP can induce changes in other difference reflectance indices because the changes are observed under direct actions of stressors (e.g., heating [83]); however, the problem requires an additional complex analysis, such as the analyses in works [80,81,82,83]. In the present work, we performed an analysis based on spectral and photosynthetic data, which were obtained from our previous work [65]. We calculated all possible RIs and ΔRIs based on the 400–700 nm spectral range, revealed their changes caused by local burning (the typical inductor of VP [4]), and estimated the relation of the RIs and ΔRIs to the quantum yield of photosystem II and non-photochemical quenching.
The complex analysis shows that the induction of VP by local burning causes significant changes in a large quantity of ΔRIs (Figure 3); in contrast, the changes in RIs were weak (Figure 2). This is in good agreement with results showing that fast changes in PRI (e.g., light-induced [74,76,77,85,86] or VP-induced [65] changes) are more effective estimators of photosynthetic parameters than absolute values of the index. The effect is based on the elimination of individual variability in reflectance spectra related to long-term changes in physiological processes (e.g., content of chlorophylls and carotenoids [71,74]). It is highly likely that a similar mechanism could also decrease errors in our analysis, which is supported by the increased sensitivity of ΔRIs in comparison to the absolute values of RIs in the complex analysis of the spectra of reflected light after short-term heating of plants [83].
Many spectral regions with significant changes in ΔRIs are based in the green and yellow spectral ranges (at least one of two R values is in the 500–600 nm range; Figure 3 and Figure 6); high Pearson’s correlation coefficients were also observed in this spectral range (Figure 5). The changes in reflectance indices can be explained by transitions in the xanthophyll cycle because they are sensitive to the photosynthetic decrease in pH in the lumen [88,91,92] and modify reflectance in the 510–560 nm range [66,78]. However, the maximum reflectance was observed at 525–535 nm [66,68,78]. This means that additional mechanisms of change in reflectance in the green and yellow spectral ranges are probable.
Potentially, the mechanisms could be related to light scattering, with maximum values at 530–546 nm, which is dependent on the pH in the lumen (probably through the induction of chloroplast shrinkage [77]) and can influence reflectance [68,75,77,79]. Previously, we showed [79] that light scattering can be stimulated by a VP in peas, and the dynamics of VP-induced changes in light scattering (see, e.g., Figure 7a in [79]) seem to be approximately similar to the dynamics of changes in ΔRI (646, 554) (Figure 6c). It is also known that light scattering is strongly related to NPQ [93,94]; i.e., it should show fast changes in photosynthetic processes.
Another potential mechanism of changes in reflectance can be related to an electrochromic shift in pigment absorbance with maximum values at 515–520 nm [94,95]. The electrochromic shift is caused by electrical potential across thylakoid membranes in chloroplasts [94,95]; this means that this parameter can be also related to photosynthesis. Our earlier results [79] showed that a VP decreases the electrochromic shift in pea leaves, but the magnitude of the decrease is small.
All potential mechanisms (transitions in the xanthophyll cycle, light scattering, and the electrochromic shift) induce changes in ΔRIs as a result of photosynthetic processes, including lumen acidification and formation of electrical potential across thylakoid membranes [92,93,94,95]. The correlations between photosynthetic parameters and changes in ΔRIs (Figure 5) support this mechanism.
However, changes in RIs (Figure 3 and Figure 6d) and correlations (Figure 5) were also observed in red spectral regions (R1 was about 680–700 nm and R2 was about 645–675 nm). It should be noted that we cannot divide reflected light itself and fluorescence in the measured reflected light. The maximum photosystem II fluorescence is known to be observed at 685 nm, and chlorophyll absorbance is shown in the blue and red spectral ranges [88]. As a result, we hypothesize that negative changes in ΔRIs based in the red spectral region (see, e.g., Figure 3) show a VP-induced decrease in chlorophyll fluorescence, which is included in I(R1), in comparison to I(R2), which is mainly dependent on chlorophyll light absorption. The hypothesis is in good agreement with works [27,37,39,43,44,45] showing that the non-photochemical quenching of chlorophyll fluorescence can be strongly stimulated by electrical signals [27,37,39,43,44,45]. The strong negative correlation between RIs (or ΔRIs) and NPQ (or ΔNPQ) in the red spectral range also supports the hypothesis about the participation of the decrease in fluorescence intensity in the VP-induced decrease in RIs based in this spectral region. It should be noted that the VP-induced NPQ increase is also caused by acidification of the chloroplast lumen. The mechanism can contribute to relations between RIs based in the red and green–yellow spectral regions.
Thus, our analysis shows that local burning-induced VP causes changes in a large quantity of ΔRIs, which are mainly related to changes in reflectance in the green and yellow spectral ranges; however, ΔRIs can also be significantly changed in the red spectral range. In the future, these results can provide the basis for the development of new indices for the remote sensing of ES-induced photosynthetic changes in plant leaves. In contrast, absolute values of RIs are weakly changed; i.e., they seem to be weakly effective for revealing the changes induced by VP in higher plants.
5. Materials and Methods
We used the spectra of reflected light and values of photosynthetic parameters (Y(II) and NPQ), which were measured in our earlier work [65]. Details of the experimental procedure were described in the work [65].
Briefly, pea seedlings (Pisum sativum L., 14–21 days old) were hydroponically cultivated in a Binder KBW 240 plant growth chamber (Binder GmbH, Tuttlingen, Germany).
VP was induced by burning the leaflet in the first leaf of the pea seedling (flame, 3–4 s, approximately 1 cm2). The leaflet was burned after 75 min of adaptation of the seedling in the system for measurements of photosynthetic and reflectance parameters.
Photosynthetic and reflectance parameters, which were measured in the second leaves of the pea seedlings, were used in the analysis.
A Pulse-Amplitude-Modulation (PAM) fluorometer Dual-PAM-100 (Heinz Walz GmbH, Effeltrich, Germany) was used for photosynthetic measurements (Y(II) and NPQ). The photosynthetic measurements were initiated after 15 min dark adaptation before turning on the actinic light.
A compact wide-range spectrometer S100 (SOLAR Laser Systems, Minsk, Belarus) and a fiber optics cable were used for measurement of reflected light. The measurements of the reflected light were initiated after 30 min illumination by the actinic light (30 min before VP induction). A white card (QPcard 101 Calibration Card v3, Argraph Corp., Carlstadt, NJ, USA) was used as the reflectance standard for calibration of the reflected light.
A halogen lamp (Osram Decostar, 3000 K, 20 W, 12 V, Germany) was used as the source of the white actinic light. The distance from the lamp to the investigated leaf was approximately 15 cm; the intensity of the leaf illumination by the actinic light was approximately 630 µmol m−2 s−1. The duration of illumination before VP induction was 60 min.
Author Contributions
Conceptualization, E.S. and V.S.; methodology, E.S.; software, E.S.; formal analysis, E.S., L.Y., E.G., and A.R.; investigation, L.Y., E.G., A.R., and V.V.; resources, V.V. and V.S.; writing—original draft preparation, E.S. and V.S.; writing—review and editing, V.S.; supervision, V.S.; project administration, V.S.; funding acquisition, V.S. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The development of programs for the complex analysis of reflectance indices and the construction of heat maps of significance changes in RIs and ΔRIs was funded by the Russian Foundation for Basic Research, project number 20-016-00234 A. The analysis of local burning-induced photosynthetic changes, the construction of heat maps of correlation coefficients between reflectance indices and photosynthetic parameters, and the analysis of the dynamics of separate ΔRIs were funded by the Russian Science Foundation, grant number 17-76-20032.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Trebacz K., Dziubinska H., Krol E. Electrical signals in long-distance communication in plants. In: Baluška F., Mancuso S., Volkmann D., editors. Communication in Plants. Neuronal Aspects of Plant Life. Springer; Berlin/Heidelberg, Germany: New York, NY, USA: 2006. pp. 277–290. [Google Scholar]
- 2.Fromm J., Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007;30:249–257. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 3.Gallé A., Lautner S., Flexas J., Fromm J. Environmental stimuli and physiological responses: The current view on electrical signaling. Environ. Exp. Bot. 2015;114:15–21. doi: 10.1016/j.envexpbot.2014.06.013. [DOI] [Google Scholar]
- 4.Vodeneev V., Akinchits E., Sukhov V. Variation potential in higher plants: Mechanisms of generation and propagation. Plant Signal. Behav. 2015;10:e1057365. doi: 10.1080/15592324.2015.1057365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukhov V. Electrical signals as mechanism of photosynthesis regulation in plants. Photosynth. Res. 2016;130:373–387. doi: 10.1007/s11120-016-0270-x. [DOI] [PubMed] [Google Scholar]
- 6.Sukhova E., Akinchits E., Sukhov V. Mathematical models of electrical activity in plants. J. Membr. Biol. 2017;250:407–423. doi: 10.1007/s00232-017-9969-7. [DOI] [PubMed] [Google Scholar]
- 7.Szechyńska-Hebda M., Lewandowska M., Karpiński S. Electrical signaling, photosynthesis and systemic acquired acclimation. Front. Physiol. 2017;8:684. doi: 10.3389/fphys.2017.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukhov V., Sukhova E., Vodeneev V. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Progr. Biophys. Mol. Biol. 2019;146:63–84. doi: 10.1016/j.pbiomolbio.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Farmer E.E., Gao Y.Q., Lenzoni G., Wolfender J.L., Wu Q. Wound- and mechanostimulated electrical signals control hormone responses. New Phytol. 2020;227:1037–1050. doi: 10.1111/nph.16646. [DOI] [PubMed] [Google Scholar]
- 10.Felle H.H., Zimmermann M.R. Systemic signalling in barley through action potentials. Planta. 2007;226:203–214. doi: 10.1007/s00425-006-0458-y. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann M.R., Maischak H., Mithöfer A., Boland W., Felle H.H. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol. 2009;149:1593–1600. doi: 10.1104/pp.108.133884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann M.R., Mithöfer A., Will T., Felle H.H., Furch A.C. Herbivore-triggered electrophysiological reactions: Candidates for systemic signals in higher plants and the challenge of their identification. Plant Physiol. 2016;170:2407–2419. doi: 10.1104/pp.15.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stahlberg R., Cleland R.E., van Volkenburgh E. Slow wave potentials—A propagating electrical signal unique to higher plants. In: Baluška F., Mancuso S., Volkmann D., editors. Communication in Plants. Neuronal Aspects of Plant Life. Springer; Berlin/Heidelberg, Germany: New York, NY, USA: 2006. pp. 291–308. [Google Scholar]
- 14.Stahlberg R., Cosgrove D.J. The propagation of slow wave potentials in pea epicotyls. Plant Physiol. 1997;113:209–217. doi: 10.1104/pp.113.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancuso S. Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Aust. J. Plant Physiol. 1999;26:55–61. doi: 10.1071/PP98098. [DOI] [Google Scholar]
- 16.Sukhova E., Akinchits E., Gudkov S.V., Pishchalnikov R.Y., Vodeneev V., Sukhov V. A theoretical analysis of relations between pressure changes along xylem vessels and propagation of variation potential in higher plants. Plants. 2021;10:372. doi: 10.3390/plants10020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyota M., Spencer D., Sawai-Toyota S., Jiaqi W., Zhang T., Koo A.J., Howe G.A., Gilroy S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science. 2018;361:1112–1115. doi: 10.1126/science.aat7744. [DOI] [PubMed] [Google Scholar]
- 18.Malone M. Wound-induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol. 1994;128:49–56. doi: 10.1111/j.1469-8137.1994.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 19.Evans M.J., Morris R.J. Chemical agents transported by xylem mass flow propagate variation potentials. Plant J. 2017;91:1029–1037. doi: 10.1111/tpj.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vodeneev V., Mudrilov M., Akinchits E., Balalaeva I., Sukhov V. Parameters of electrical signals and photosynthetic responses induced by them in pea seedlings depend on the nature of stimulus. Funct. Plant Biol. 2018;45:160–170. doi: 10.1071/FP16342. [DOI] [PubMed] [Google Scholar]
- 21.Blyth M.G., Morris R.J. Shear-enhanced dispersion of a wound substance as a candidate mechanism for variation potential transmission. Front. Plant Sci. 2019;10:1393. doi: 10.3389/fpls.2019.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wildon D.C., Thain J.F., Minchin P.E.H., Gubb I.R., Reilly A.J., Skipper Y.D., Doherty H.M., O’Donnell P.J., Bowles D. Electrical signalling and systemic proteinase inhibitor Induction in the wounded plant. Nature. 1992;360:62–65. doi: 10.1038/360062a0. [DOI] [Google Scholar]
- 23.Peña-Cortés H., Fisahn J., Willmitzer L. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc. Natl. Acad. Sci. USA. 1995;92:4106–4113. doi: 10.1073/pnas.92.10.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanković B., Davies E. Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. FEBS Lett. 1996;390:275–279. doi: 10.1016/0014-5793(96)00672-2. [DOI] [PubMed] [Google Scholar]
- 25.Mousavi S.A., Chauvin A., Pascaud F., Kellenberger S., Farmer E.E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature. 2013;500:422–426. doi: 10.1038/nature12478. [DOI] [PubMed] [Google Scholar]
- 26.Filek M., Kościelniak J. The effect of wounding the roots by high temperature on the respiration rate of the shoot and propagation of electric signal in horse bean seedlings (Vicia faba L. minor) Plant Sci. 1997;123:39–46. doi: 10.1016/S0168-9452(96)04567-0. [DOI] [Google Scholar]
- 27.Pavlovič A., Slováková L., Pandolfi C., Mancuso S. On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis) J. Exp. Bot. 2011;62:1991–2000. doi: 10.1093/jxb/erq404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surova L., Sherstneva O., Vodeneev V., Katicheva L., Semina M., Sukhov V. Variation potential-induced photosynthetic and respiratory changes increase ATP content in pea leaves. J. Plant Physiol. 2016;202:57–64. doi: 10.1016/j.jplph.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Lautner S., Stummer M., Matyssek R., Fromm J., Grams T.E.E. Involvement of respiratory processes in the transient knockout of net CO2 uptake in Mimosa pudica upon heat stimulation. Plant Cell Environ. 2014;37:254–260. doi: 10.1111/pce.12150. [DOI] [PubMed] [Google Scholar]
- 30.Hlavácková V., Krchnák P., Naus J., Novák O., Spundová M., Strnad M. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta. 2006;225:235–244. doi: 10.1007/s00425-006-0325-x. [DOI] [PubMed] [Google Scholar]
- 31.Krausko M., Perutka Z., Šebela M., Šamajová O., Šamaj J., Novák O., Pavlovič A. The role of electrical and jasmonate signalling in the recognition of captured prey in the carnivorous sundew plant Drosera capensis. New Phytol. 2017;213:1818–1835. doi: 10.1111/nph.14352. [DOI] [PubMed] [Google Scholar]
- 32.Pavlovič A., Mithöfer A. Jasmonate signalling in carnivorous plants: Copycat of plant defence mechanisms. J. Exp. Bot. 2019;70:3379–3389. doi: 10.1093/jxb/erz188. [DOI] [PubMed] [Google Scholar]
- 33.Fromm J. Control of phloem unloading by action potentials in Mimosa. Physiol. Plant. 1991;83:529–533. doi: 10.1111/j.1399-3054.1991.tb00130.x. [DOI] [Google Scholar]
- 34.Furch A.C., van Bel A.J., Fricker M.D., Felle H.H., Fuchs M., Hafke J.B. Sieve element Ca2+ channels as relay stations between remote stimuli and sieve tube occlusion in Vicia faba. Plant Cell. 2009;21:2118–2132. doi: 10.1105/tpc.108.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furch A.C., Zimmermann M.R., Will T., Hafke J.B., van Bel A.J. Remote-controlled stop of phloem mass flow by biphasic occlusion in Cucurbita maxima. J. Exp. Bot. 2010;61:3697–3708. doi: 10.1093/jxb/erq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiina T., Tazawa M. Action potential in Luffa cylindrica and its effects on elongation growth. Plant Cell Physiol. 1986;27:1081–1089. [Google Scholar]
- 37.Krupenina N.A., Bulychev A.A. Action potential in a plant cell lowers the light requirement for non-photochemical energy-dependent quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 2007;1767:781–788. doi: 10.1016/j.bbabio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Grams T.E., Lautner S., Felle H.H., Matyssek R., Fromm J. Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ. 2009;32:319–326. doi: 10.1111/j.1365-3040.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 39.Sukhov V., Sherstneva O., Surova L., Katicheva L., Vodeneev V. Proton cellular influx as a probable mechanism of variation potential influence on photosynthesis in pea. Plant Cell Environ. 2014;37:2532–2541. doi: 10.1111/pce.12321. [DOI] [PubMed] [Google Scholar]
- 40.Gallé A., Lautner S., Flexas J., Ribas-Carbo M., Hanson D., Roesgen J., Fromm J. Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ. 2013;36:542–552. doi: 10.1111/j.1365-3040.2012.02594.x. [DOI] [PubMed] [Google Scholar]
- 41.Vuralhan-Eckert J., Lautner S., Fromm J. Effect of simultaneously induced environmental stimuli on electrical signalling and gas exchange in maize plants. J. Plant Physiol. 2018;223:32–36. doi: 10.1016/j.jplph.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Yudina L., Sukhova E., Sherstneva O., Grinberg M., Ladeynova M., Vodeneev V., Sukhov V. Exogenous abscisic acid can influence photosynthetic processes in peas through a decrease in activity of H+-ATPase in the plasma membrane. Biology. 2020;9:324. doi: 10.3390/biology9100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yudina L., Sherstneva O., Sukhova E., Grinberg M., Mysyagin S., Vodeneev V., Sukhov V. Inactivation of H+-ATPase participates in the influence of variation potential on photosynthesis and respiration in peas. Plants. 2020;9:1585. doi: 10.3390/plants9111585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sukhov V., Surova L., Sherstneva O., Katicheva L., Vodeneev V. Variation potential influence on photosynthetic cyclic electron flow in pea. Front. Plant Sci. 2015;5:766. doi: 10.3389/fpls.2014.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sukhova E., Mudrilov M., Vodeneev V., Sukhov V. Influence of the variation potential on photosynthetic flows of light energy and electrons in pea. Photosynth. Res. 2018;136:215–228. doi: 10.1007/s11120-017-0460-1. [DOI] [PubMed] [Google Scholar]
- 46.Retivin V.G., Opritov V.A., Fedulina S.B. Generation of action potential induces preadaptation of Cucurbita pepo L. stem tissues to freezing injury. Russ. J. Plant Physiol. 1997;44:432–442. [Google Scholar]
- 47.Retivin V.G., Opritov V.A., Lobov S.A., Tarakanov S.A., Khudyakov V.A. Changes in the resistance of photosynthesizing cotyledon cells of pumpkin seedlings to cooling and heating, as induced by the stimulation of the root system with KCl solution. Russ. J. Plant Physiol. 1999;46:689–696. [Google Scholar]
- 48.Sukhov V., Surova L., Sherstneva O., Vodeneev V. Influence of variation potential on resistance of the photosynthetic machinery to heating in pea. Physiol. Plant. 2014;152:773–783. doi: 10.1111/ppl.12208. [DOI] [PubMed] [Google Scholar]
- 49.Sukhov V., Surova L., Sherstneva O., Bushueva A., Vodeneev V. Variation potential induces decreased PSI damage and increased PSII damage under high external temperatures in pea. Funct. Plant Biol. 2015;42:727–736. doi: 10.1071/FP15052. [DOI] [PubMed] [Google Scholar]
- 50.Surova L., Sherstneva O., Vodeneev V., Sukhov V. Variation potential propagation decreases heat-related damage of pea photosystem I by 2 different pathways. Plant Sign. Behav. 2016;11:e1145334. doi: 10.1080/15592324.2016.1145334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sukhov V., Gaspirovich V., Mysyagin S., Vodeneev V. High-temperature tolerance of photosynthesis can be linked to local electrical responses in leaves of pea. Front. Physiol. 2017;8:763. doi: 10.3389/fphys.2017.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki N., Miller G., Salazar C., Mondal H.A., Shulaev E., Cortes D.F., Shuman J.L., Luo X., Shah J., Schlauch K., et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell. 2013;25:3553–3569. doi: 10.1105/tpc.113.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Souza G.M., Ferreira A.S., Saraiva G.F., Toledo G.R. Plant “electrome” can be pushed toward a self-organized critical state by external cues: Evidences from a study with soybean seedlings subject to different environmental conditions. Plant Signal Behav. 2017;12:e1290040. doi: 10.1080/15592324.2017.1290040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saraiva G.F.R., Ferreira A.S., Souza G.M. Osmotic stress decreases complexity underlying the electrophysiological dynamic in soybean. Plant Biol. 2017;19:702–708. doi: 10.1111/plb.12576. [DOI] [PubMed] [Google Scholar]
- 55.Debono M.W., Souza G.M. Plants as electromic plastic interfaces: A mesological approach. Prog. Biophys. Mol. Biol. 2019;146:123–133. doi: 10.1016/j.pbiomolbio.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Simmi F.Z., Dallagnol L.J., Ferreira A.S., Pereira D.R., Souza G.M. Electrome alterations in a plant-pathogen system: Toward early diagnosis. Bioelectrochemistry. 2020;133:107493. doi: 10.1016/j.bioelechem.2020.107493. [DOI] [PubMed] [Google Scholar]
- 57.Parise A.G., Reissig G.N., Basso L.F., Senko L.G.S., Oliveira T.F.C., de Toledo G.R.A., Ferreira A.S., Souza G.M. Detection of different hosts from a distance alters the behaviour and bioelectrical activity of Cuscuta racemosa. Front. Plant Sci. 2021;12:594195. doi: 10.3389/fpls.2021.594195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chatterjee S.K., Ghosh S., Das S., Manzella V., Vitaletti A., Masi E., Santopolo L., Mancuso S., Maharatna K. Forward and inverse modelling approaches for prediction of light stimulus from electrophysiological response in plants. Measurement. 2014;53:101–116. doi: 10.1016/j.measurement.2014.03.040. [DOI] [Google Scholar]
- 59.Chatterjee S.K., Das S., Maharatna K., Masi E., Santopolo L., Mancuso S., Vitaletti A. Exploring strategies for classification of external stimuli using statistical features of the plant electrical response. J. R. Soc. Interface. 2015;12:20141225. doi: 10.1098/rsif.2014.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y., Zhao D.-J., Wang Z.-Y., Wang Z.-Y., Tang G., Huang L. Plant electrical signal classification based on waveform similarity. Algorithms. 2016;9:70. doi: 10.3390/a9040070. [DOI] [Google Scholar]
- 61.Chatterjee S.K., Malik O., Gupta S. Chemical sensing employing plant electrical signal response-classification of stimuli using curve fitting coefficients as features. Biosensors. 2018;8:83. doi: 10.3390/bios8030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin X.-H., Wang Z.-Y., Yao J.-P., Zhou Q., Zhao P.-F., Wang Z.-Y., Huang L. Using a one-dimensional convolutional neural network with a conditional generative adversarial network to classify plant electrical signals. Comp. Electron. Agric. 2020;174:105464. doi: 10.1016/j.compag.2020.105464. [DOI] [Google Scholar]
- 63.Sukhova E., Yudina L., Akinchits E., Vodeneev V., Sukhov V. Influence of electrical signals on pea leaf reflectance in the 400-800-nm range. Plant Signal. Behav. 2019;14:1610301. doi: 10.1080/15592324.2019.1610301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sukhova E., Yudina L., Gromova E., Nerush V., Vodeneev V., Sukhov V. Burning-induced electrical signals influence broadband reflectance indices and water index in pea leaves. Plant Signal. Behav. 2020;15:1737786. doi: 10.1080/15592324.2020.1737786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sukhov V., Sukhova E., Gromova E., Surova L., Nerush V., Vodeneev V. The electrical signal-induced systemic photosynthetic response is accompanied by changes in the photochemical reflectance index in pea. Func. Plant Biol. 2019;46:328–338. doi: 10.1071/FP18224. [DOI] [PubMed] [Google Scholar]
- 66.Gamon J.A., Peñuelas J., Field C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992;41:35–44. doi: 10.1016/0034-4257(92)90059-S. [DOI] [Google Scholar]
- 67.Filella I., Amaro T., Araus J.L., Peñuelas J. Relationship between photosynthetic radiation-use efficiency of barley canopies and the photochemical reflectance index (PRI) Physiol. Plant. 1996;96:211–216. doi: 10.1111/j.1399-3054.1996.tb00204.x. [DOI] [Google Scholar]
- 68.Gamon J.A., Serrano L., Surfus J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia. 1997;112:492–501. doi: 10.1007/s004420050337. [DOI] [PubMed] [Google Scholar]
- 69.Garbulsky M.F., Peñuelas J., Gamon J., Inoue Y., Filella I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies. A review and meta-analysis. Remote Sens. Environ. 2011;115:281–297. doi: 10.1016/j.rse.2010.08.023. [DOI] [Google Scholar]
- 70.Peñuelas J., Garbulsky M.F., Filella I. Photochemical reflectance index (PRI) and remote sensing of plant CO₂ uptake. New Phytol. 2011;191:596–599. doi: 10.1111/j.1469-8137.2011.03791.x. [DOI] [PubMed] [Google Scholar]
- 71.Porcar-Castell A., Garcia-Plazaola J.I., Nichol C.J., Kolari P., Olascoaga B., Kuusinen N., Fernández-Marín B., Pulkkinen M., Juurola E., Nikinmaa E. Physiology of the seasonal relationship between the photochemical reflectance index and photosynthetic light use efficiency. Oecologia. 2012;170:313–323. doi: 10.1007/s00442-012-2317-9. [DOI] [PubMed] [Google Scholar]
- 72.Zhang C., Filella I., Garbulsky M.F., Peñuelas J. Affecting factors and recent improvements of the photochemical reflectance index (PRI) for remotely sensing foliar, canopy and ecosystemic radiation-use efficiencies. Remote Sens. 2016;8:677. doi: 10.3390/rs8090677. [DOI] [Google Scholar]
- 73.Sukhova E., Sukhov V. Connection of the photochemical reflectance index (PRI) with the photosystem II quantum yield and nonphotochemical quenching can be dependent on variations of photosynthetic parameters among investigated plants: A meta-analysis. Remote Sens. 2018;10:771. doi: 10.3390/rs10050771. [DOI] [Google Scholar]
- 74.Kováč D., Veselá B., Klem K., Večeřová K., Kmecová Z.M., Peñuelas J., Urban O. Correction of PRI for carotenoid pigment pools improves photosynthesis estimation across different irradiance and temperature conditions. Remote Sens. Environ. 2020;244:111834. doi: 10.1016/j.rse.2020.111834. [DOI] [Google Scholar]
- 75.Evain S., Flexas J., Moya I. A new instrument for passive remote sensing: 2. Measurement of leaf and canopy reflectance changes at 531 nm and their relationship with photosynthesis and chlorophyll fluorescence. Remote Sens. Environ. 2004;91:175–185. doi: 10.1016/j.rse.2004.03.012. [DOI] [Google Scholar]
- 76.Sukhova E., Sukhov V. Analysis of light-induced changes in the photochemical reflectance index (PRI) in leaves of pea, wheat, and pumpkin using pulses of green-yellow measuring light. Remote Sens. 2019;11:810. doi: 10.3390/rs11070810. [DOI] [Google Scholar]
- 77.Sukhova E., Sukhov V. Relation of photochemical reflectance indices based on different wavelengths to the parameters of light reactions in photosystems I and II in pea plants. Remote Sens. 2020;12:1312. doi: 10.3390/rs12081312. [DOI] [Google Scholar]
- 78.Van Wittenberghe S., Laparra V., García-Plazaola J.I., Fernández-Marín B., Porcar-Castell A., Moreno J. Combined dynamics of the 500-600 nm leaf absorption and chlorophyll fluorescence changes in vivo: Evidence for the multifunctional energy quenching role of xanthophylls. Biochim. Biophys. Acta Bioenergy. 2021;1862:148351. doi: 10.1016/j.bbabio.2020.148351. [DOI] [PubMed] [Google Scholar]
- 79.Sukhov V., Surova L., Morozova E., Sherstneva O., Vodeneev V. Changes in H+-ATP synthase activity, proton electrochemical gradient, and pH in pea chloroplast can be connected with variation potential. Front Plant Sci. 2016;7:1092. doi: 10.3389/fpls.2016.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balzarolo M., Peñuelas J., Filella I., Portillo-Estrada M., Ceulemans R. Assessing ecosystem isoprene emissions by hyperspectral remote sensing. Remote Sens. 2018;10:1086. doi: 10.3390/rs10071086. [DOI] [Google Scholar]
- 81.Sytar O., Brücková K., Kovár M., Živčák M., Hemmerich I., Brestič M. Nondestructive detection and biochemical quantification of buckwheat leaves using visible (VIS) and near-infrared (NIR) hyperspectral reflectance imaging. J. Centr. Eur. Agric. 2017;18:864–878. doi: 10.5513/JCEA01/18.4.1978. [DOI] [Google Scholar]
- 82.Sun H., Feng M., Xiao L., Yang W., Wang C., Jia X., Zhao Y., Zhao C., Muhammad S.K., Li D. Assessment of plant water status in winter wheat (Triticum aestivum L.) based on canopy spectral indices. PLoS ONE. 2019;14:e0216890. doi: 10.1371/journal.pone.0216890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sukhova E., Yudina L., Gromova E., Ryabkova A., Kior D., Sukhov V. Complex analysis of the efficiency of difference reflectance indices on the basis of 400–700 nm wavelengths for revealing the influences of water shortage and heating on plant seedlings. Remote Sens. 2021;13:962. doi: 10.3390/rs13050962. [DOI] [Google Scholar]
- 84.Ibaraki Y., Dutta Gupta S. Nondestructive evaluation of the photosynthetic properties of micropropagated plantlets by imaging photochemical reflectance index under low light intensity. In Vitro Cell. Dev. Biol. Plant. 2010;46:530–536. doi: 10.1007/s11627-010-9296-5. [DOI] [Google Scholar]
- 85.Yudina L., Sukhova E., Gromova E., Nerush V., Vodeneev V., Sukhov V. A light-induced decrease in the photochemical reflectance index (PRI) can be used to estimate the energy-dependent component of non-photochemical quenching under heat stress and soil drought in pea, wheat, and pumpkin. Photosynth. Res. 2020;146:175–187. doi: 10.1007/s11120-020-00718-x. [DOI] [PubMed] [Google Scholar]
- 86.Kováč D., Veselovská P., Klem K., Večeřová K., Ač A., Peñuelas J., Urban O. Potential of photochemical reflectance index for indicating photochemistry and light use efficiency in leaves of European beech and Norway spruce trees. Remote Sens. 2018;10:1202. doi: 10.3390/rs10081202. [DOI] [Google Scholar]
- 87.Białasek M., Górecka M., Mittler R., Karpiński S. Evidence for the Involvement of electrical, calcium and ROS signaling in the systemic regulation of non-photochemical quenching and photosynthesis. Plant Cell Physiol. 2017;58:207–215. doi: 10.1093/pcp/pcw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Porcar-Castell A., Tyystjärvi E., Atherton J., van der Tol C., Flexas J., Pfündel E.E., Moreno J., Frankenberg C., Berry J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014;65:4065–4095. doi: 10.1093/jxb/eru191. [DOI] [PubMed] [Google Scholar]
- 89.Kohzuma K., Hikosaka K. Physiological validation of photochemical reflectance index (PRI) as a photosynthetic parameter using Arabidopsis thaliana mutants. Biochem. Biophys. Res. Commun. 2018;498:52–57. doi: 10.1016/j.bbrc.2018.02.192. [DOI] [PubMed] [Google Scholar]
- 90.Murakami K., Ibaraki Y. Time course of the photochemical reflectance index during photosynthetic induction: Its relationship with the photochemical yield of photosystem II. Physiol. Plant. 2019;165:524–536. doi: 10.1111/ppl.12745. [DOI] [PubMed] [Google Scholar]
- 91.Müller P., Li X.P., Niyogi K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruban A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016;170:1903–1916. doi: 10.1104/pp.15.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruban A.V., Pascal A.A., Robert B., Horton P. Activation of zeaxanthin is an obligatory event in the regulation of photosynthetic light harvesting. J. Biol. Chem. 2002;277:7785–7789. doi: 10.1074/jbc.M110693200. [DOI] [PubMed] [Google Scholar]
- 94.Schreiber U., Klughammer C. New accessory for the DUAL-PAM-100: The P515/535 module and examples of its application. PAM Appl. Notes. 2008;1:1–10. [Google Scholar]
- 95.Klughammer C., Siebke K., Schreiber U. Continuous ECS-indicated recording of the proton-motive charge flux in leaves. Photosynth Res. 2013;117:471–487. doi: 10.1007/s11120-013-9884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.