Abstract
Naringenin and naringin are a class of hydrophobic polyphenol compounds and both have several biological activities containing antioxidant, anti-inflammatory and anti-tumor properties. Nevertheless, they have low water solubility and bioavailability, which limits their biological activity. In this study, an easy pH-driven method was applied to load naringenin or naringin into nanoliposomes based on the gradual reduction in their water solubility after the pH changed to acidity. Thus, the naringenin or naringin can be embedded into the hydrophobic region within nanoliposomes from the aqueous phase. A series of naringenin/naringin-loaded nanoliposomes with different pH values, lecithin contents and feeding naringenin/naringin concentrations were prepared by microfluidization and a pH-driven method. The naringin-loaded nanoliposome contained some free naringin due to its higher water solubility at lower pH values and had a relatively low encapsulation efficiency. However, the naringenin-loaded nanoliposomes were predominantly nanometric (44.95–104.4 nm), negatively charged (−14.1 to −19.3 mV) and exhibited relatively high encapsulation efficiency (EE = 95.34% for 0.75 mg/mL naringenin within 1% w/v lecithin). Additionally, the naringenin-loaded nanoliposomes still maintained good stability during 31 days of storage at 4 °C. This study may help to develop novel food-grade colloidal delivery systems and apply them to introducing naringenin or other lipophilic polyphenols into foods, supplements or drugs.
Keywords: naringenin, naringin, nanoliposomes, pH-driven, stability
1. Introduction
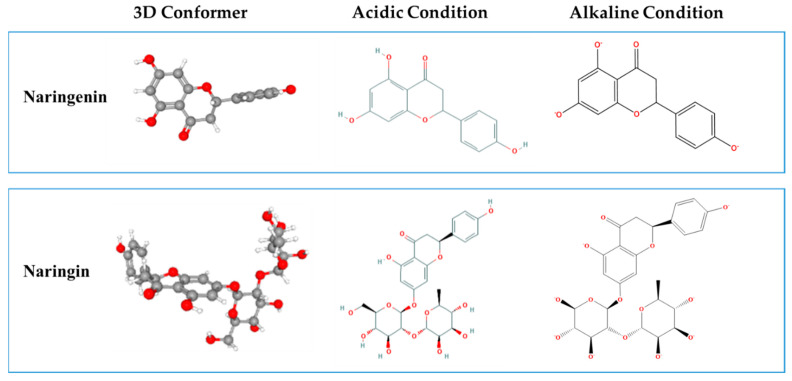
Naringenin (5,7,4′-trihydroxyflavanone) and naringin (5,7,4′-trihydroxyflavone 7-rhamno-glucoside) are flavones abundantly existent in fruits, including grapefruit, tomato skin and oranges [1]. Naringin can be formed by naringenin at the position of the 7th carbon atom with neohesperidose (Figure 1), which promotes its solubility in water. Their health-promoting features have been explored in vivo and in vitro models, such as antioxidant activity [2,3], anti-inflammatory activity [4,5], anticancer activity [6,7], anti-diabetic activity [8] and protective effects on the central nervous system [9] and the cardiovascular system [10]. However, naringenin and naringin are hydrophobic and low-soluble polyphenols with low water solubility of 38 and 500 µg/mL at room temperature, respectively [11,12], and taste bitter, which greatly limits their application in clinical and functional foods. To overcome these limits, the solubility and bioavailability of naringenin and naringin have been improved by encapsulation in various kinds of carefully designed colloidal delivery systems, including cyclodextrins complexation [12], nanovehicles [13,14], liposomal [15] and ternary complex particles [16].
Figure 1.
Three dimensions conformer of naringenin and naringin and their hydroxyl groups are non-ionized in acidic condition and negatively charged in alkaline condition.
The pH-driven method is a new method for constructing a delivery system, which has received extensive attention due to its easy-to-handle, cost-saving, energy-saving and organic solvent-free properties during operation. The process of this method includes dissolving the bioactivator under strongly alkaline conditions and then adjusting the pH to neutral or acidic after blending with the carrier system. Hydrophobic polyphenols like curcumin have poor solubility in neutral acidity, while they can be dissolved due to deprotonation at alkaline pH. According to this property of some polyphenols, Pan et al. firstly loaded curcumin into casein nanoparticles by the pH-driven method [17]. Subsequently, a series of studies on the colloidal delivery systems based on the pH-driven method began to emerge, especially for curcumin encapsulation. The successful preparation of a series of colloidal carrier systems loaded with curcumin enhanced its water solubility and bioavailability, which proved the feasibility of preparing the delivery system with the pH-driven method [18,19,20,21,22,23,24,25]. However, the pH-driven method has rarely been applied for the encapsulation of other hydrophobic polyphenols. A potential disadvantage of this method is that the encapsulation of polyphenols needs to be carried out under a highly alkaline conditions, which could facilitate the chemical degradation of polyphenols [26]. Peng and co-workers have demonstrated that the pH-driven method can be used to load certain kinds of lipophilic polyphenols such as curcumin and resveratrol but cannot encapsulate quercetin because the degradation rate of quercetin was extremely fast at alkaline pH values [26].
Liposomes are microcosmic phospholipid vesicles with a bilayered membrane structure. The hydrophilic segments of the phospholipids are located on the inside and outside surface of liposomes, while the hydrophobic tail forming lipid bilayers are separated from aqueous phase [27]. Nanoliposome is a nanometric versions of liposome, which can load bioactive substances with different lipophilicities into the various parts of liposomes, such as phospholipid bilayers, hydrophilic core or bilayer interface [28]. Therefore, nanoliposomes are an extremely promising encapsulation technology in the field of nutraceutical products. The superiority of nanoliposome technology is embodied in the protection of sensitive bioactivators, storage stability, excellent loading capacity, improved bioavailability and sustained-release ability [29]. The utilization of high-pressure homogenizer to prepare liposomes can overcome the traditional liposome preparation method that uses detergents or organic solvents that are either undesirable or not allowed ingredients in foods [25].
Naringenin and naringin, with hydrogen donor count as 3 and 8, respectively, whose hydroxyl groups are non-ionized under acidic conditions (Figure 1). When the pH changes to alkaline, they are easy to be negatively charged due to the deprotonation of the hydroxyl group, which extremely enhances their hydrophilic ability. We hypothesized that naringenin and naringin lose their charges due to the protonation of the same group under neutral and acidic conditions, which would reduce their water solubility. Therefore, when the naringenin/naringin alkaline solution and nanoliposome solutions are mixed, the pH is adjusted to an acidic condition, which causes the naringenin or naringin to be embedded into the hydrophobic region within nanoliposomes. In this study, we first determined the stability of naringenin and naringin under alkaline conditions and the change in solubility of naringenin and naringin in the transition from pH 12.0 to neutral or acidic were also measured, which ensured that they can be encapsulated into liposomes by the pH-driven method. Subsequently, nanoliposomes with different concentrations of naringenin or naringin and different contents of lecithin were prepared by microfluidazition and pH-driven methods, and their encapsulation efficiency and maximum loading capacity were determined. The structures of bioactivator-loaded nanoliposomes, particle size and ζ-potentials of nanoliposomes were determined to understand the bioactivator-loaded procedure using the pH-driven method. Finally, the stability of the naringenin-loaded nanoliposomes was studied. This study may lead to develop novel food-grade colloidal delivery systems and apply to introducing naringenin and naringin into foods, supplements, or drugs.
2. Materials and Methods
2.1. Chemicals
Naringenin (97%) and naringin (95%) powder were purchased from Macklin (Shanghai, China); lecithin from soybean (>70%) and dimethyl sulfoxide (DMSO) were obtained from Aladdin (Shanghai, China). Hydrochloric acid, sodium hydroxide, sodium dihydrogen phosphate, ethanol and other chemicals were all of analytical purity.
2.2. Stability of Naringenin and Naringin in Alkaline Condition
Sodium hydroxide solution (6.0 M) was added to 50 mM sodium dihydrogen phosphate solution to prepare a range of buffers with a pH value ranging from 7.0 to 12.0. Then 0.25 mL of naringenin or naringin ethanol solution (2.0 mg/mL) was mixed with buffer solutions (9.75 mL) of different pH values, respectively. These samples with different pH values were stirred using a magnetic stirrer at ambient temperature. At predetermined every 10 min (ended of 60 min), 0.3 mL of mixture was taken out and diluted with phosphate buffer solution (50 mM, pH 7.0) for the determination of naringenin and naringin content with a UV-vis spectrometer (U-T6A, Yipu Instrument Manufacturing Co., Ltd., Shanghai, China) at 288 and 282 nm, respectively.
2.3. Effect of pH Shift on the Solubility of Naringenin and Naringin
Naringenin was dissolved in NaH2PO4 buffer (50 mM, pH 12.0) at 5.0 mg/mL, then the pH values of the solutions were adjusted to 7.0–2.0 with 6.0 M hydrochloric acid. After storage for 24 h at ambient temperature, the suspension was centrifuged, and the supernatant was diluted with DMSO to an appropriate concentration for UV-vis measurement. The determination process for naringin was similar to that described above.
2.4. Narigenin-Loaded and Naringin-Loaded Nanoliposome Preparation
Nanoliposomes were produced by the high-pressure homogenization using a microfluidizer. Soybean lecithin (1.2% and 2.5%, w/v) was suspended in sodium dihydrogen phosphate solution (5 mM, pH 6.0) and stirred for 4 h to ensure complete hydration. Then the soybean lecithin suspension was passed through the microfluidizer 3 times under a working pressure of 121.0 MPa to fabricate nanoliposomes. The nanoliposome solutions were placed in 4 °C before using.
Naringenin was encapsulated into the nanoliposomes by the pH-driven method. Briefly, a range of alkaline naringenin solutions with various naringenin concentration were fabricated by dissolving naringenin in 0.08 M NaOH solution. The alkaline naringenin solutions and nanoliposome solutions were blended in various proportions, and then the mixed systems were rapidly adjusted to pH 7.0, 6.0 and 5.0 by 1.0 M HCl. The final lecithin content within nanoliposome solutions has two levels of 1.0% and 2.0% (w/v). Meanwhile, the final naringenin concentrations were 0.75, 1.00, 1.25, or 1.50 mg/mL with 1.0% (w/v) lecithin and the naringenin concentrations were 2.00 or 3.00 mg/mL with 2.0% (w/v) lecithin, respectively. All the naringenin-loaded nanoliposome samples were equilibrated at room temperature for 24 h.
A series of naringin-loaded nanoliposome (the final naringin concentrations were 1.00, 1.50, or 2.00 mg/mL with 1.0% (w/v) lecithin and 3.00 mg/mL with 2.0% (w/v) lecithin, respectively) were prepared only at the final pH of 6.0 according to the above method.
2.5. Encapsulation Efficiency and Loading Capacity of Naringenin and Naringin
The encapsulation efficiency (EE) and loading capacity (LC) were measured by a centrifugation method. Briefly, the bioactivator-loaded nanoliposome suspension was centrifuged at 11,000× g for 15 min at 4 °C to remove any unencapsulated bioactivator precipitate. Then, the bioactivator-loaded nanoliposome suspensions were centrifuged with ultrafiltration centrifuge tubes (MWCO: 10 kDa) at 11,000× g for 30 min and the subnatant was used to determine the concentration of free bioactivator. The concentration of bioactivator in the supernatant for first centrifugation and the subnatant for second centrifugation were measured using a UV-vis spectrophotometer. The EE and LC of the bioactivator-loaded nanoliposomes without free bioactivator were calculated according to the following expressions:
| (1) |
| (2) |
where is the mass of bioactivator in the nanoliposome solution, is the mass of free bioactivator in the nanoliposome solution, is the initial mass of bioactivator in the system, and is the total mass of the bioactivator-loaded nanoliposomes (bioactivator + lecithin).
2.6. Naringenin-Loaded and Naringin-Loaded Nanoliposomes Characterization
A combined dynamic light scattering (DLS)-electrophoresis instrument (ZETASIZER PRO, Malvern Instruments, Worcestershire, U.K.) was used to determine the mean particle size, polydispersity index (PDI) and ζ-potentials of naringenin-loaded and naringin-loaded nanoliposomes at 25 °C. The refractive indexes of the nanoliposomes and the dispersion phase water were set to 1.45 and 1.33, respectively. The nanoliposome suspension was diluted 20 times with buffer solution before measuring to avoid multiple scattering interferences.
2.7. Storage Stability
The effect of storage conditions (e.g., temperature and time) on the stability of nanoliposome suspensions were determined to assess their long-term physicochemical stability. The samples (1.0% w/v lecithin) with naringenin concentrations of 1.00 and 1.25 mg/mL were placed in 4 °C, 25 °C and 37 °C within 31 days. During storage, their encapsulation efficiency, mean particle size and ζ-potentials were recorded according to the same procedure described above at different time intervals.
2.8. Atomic Force Microscopy
Atomic force microscopy (AFM) was used for obtaining the microstructure of the blank nanoliposomes and naringenin-loaded nanoliposomes with a concentration of 1.0 and 1.25 mg/mL naringenin before and after 31 days of storage at 4 °C. A droplet of nanoliposome suspension was placed on to a newly cleaved silicon substrate and air-dried for 30 min, and then picture of the naringenin-loaded nanoliposomes were recorded through AFM (C300, Nanosurf, Liestal, Switzerland). AFM images were recorded in tapping mode with a scan time of 3 s and scan points of 512 to obtain sufficient data for statistical analysis.
2.9. Statistical Analysis
At least three freshly prepared samples were measured, and the results were expressed as mean ± standard deviation. Analysis of variance (ANOVA) was used to determine significance at the p < 0.05 level using Tukey’s HSD test.
3. Results and Discussion
3.1. Stability of Naringenin and Naringin in Alkaline Conditions
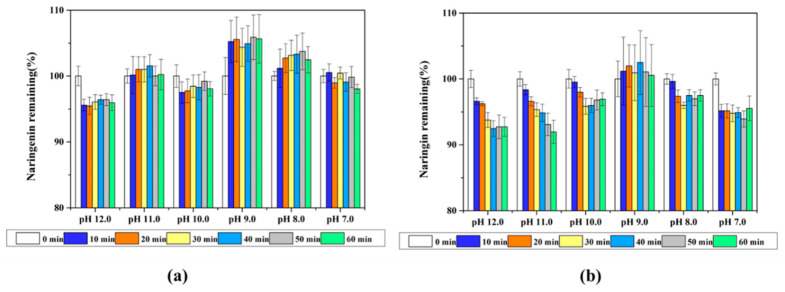
Naringenin molecule contains three hydroxyl groups that can be used as hydrogen donors and their pKa values are 7.86, 9.20 and 9.79 (obtained from chemicalize.com), respectively. The hydroxyl groups could be gradually ionized around and above their pKa value, leading to an increase in the negative charge of the naringenin and its hydrophilicity. Since naringenin was dissolved under alkaline conditions, it is necessary to investigate its stability under alkaline conditions. The chemical stability of naringenin at various pH conditions (from 12.0 to 7.0) was evaluated through measuring the change in the concentration remaining versus time (Figure 2a). The degradation rate of naringenin was about 5% when stored for 10 min at pH 12.0 and the degradation did not increase as the time was extended to 60 min. Ionized hydroxyl groups (phenate ion) of the polyphenols at high pH values are particularly susceptible to oxidation causing the degradation of polyphenols [26]. With the decrease of pH from 11.0 to 7.0, naringenin was relatively stable at this pH range and rarely degraded within 60 min.
Figure 2.
The change of naringenin (a) and naringin (b) remaining in different alkaline solutions over time.
Compared with naringenin, naringin molecule contains eight hydroxyl groups that can be used as hydrogen donors, and its solubility in alkaline solutions is higher than naringenin. The change of naringin remaining in alkaline solutions over time is shown in Figure 2b. The degradation rate of naringin was about 8% when stored for 60 min at pH 12.0 and 11.0, suggesting that naringin was relatively stable in alkaline solutions within a short time.
3.2. Solubility of Naringenin and Naringin with pH-Shift
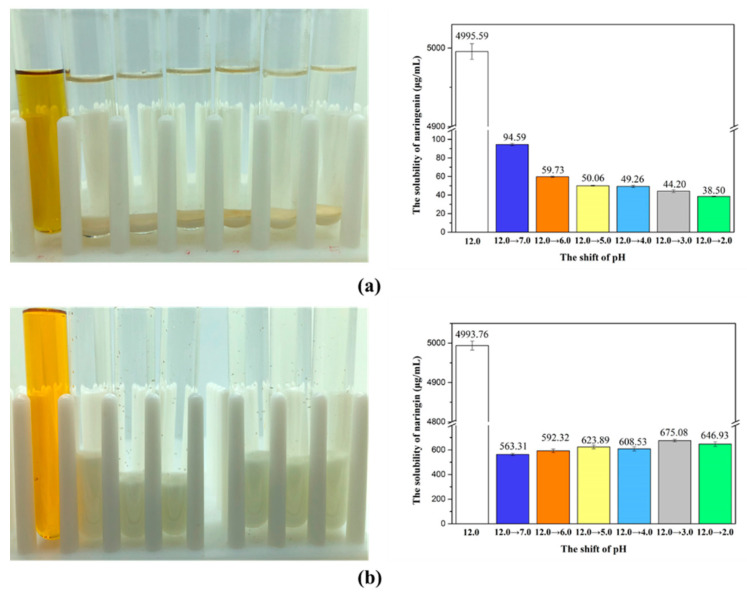
In order that naringenin can be moved from the aqueous phase into the hydrophobic domain of the nanoliposome instead of simply blending in the nanoliposome solution, we determined the solubility of naringenin after the pH-shift. Naringenin with a concentration of 5.00 mg/mL could quickly dissolved in a pH 12.0 phosphate buffer solution with a color of orange-yellow (Figure 3a). After adjusting the pH to neutral and acidic, naringenin could quickly restore electrical neutrality, resulting in reduced solubility and precipitated from solution. The solubility of naringenin decreased as the solution of pH-shift range increased. Similar to other hydrophobic polyphenols, such as curcumin, quercetin and resveratrol, the hydroxyl groups on their molecules became electronegative in a strongly alkaline solution which increased their water solubility, but then their water solubility decreased due to the molecules regaining neutrality when the pH was adjusted to neutral [26]. When the naringenin solution changed the pH from 12.0 to 7.0, the solubility of naringenin decreased from 5.00 mg/mL to 94.59 µg/mL, indicating that about 98% of naringenin had been precipitated.
Figure 3.
The image and solubility of naringenin (a) and naringin (b) dissolved at pH 12.0 solution (5.00 mg/mL) and then these samples were adjusted from pH 12.0 to 7.0, 6.0, 5.0, 4.0, 3.0 and 2.0 (left to right), which caused the bioactivators to restore electrical neutrality and reduce solubility.
Unlike naringenin, when naringin was dissolved in a NaH2PO4 buffer of pH 12.0 and then the pH was adjusted to acidity, naringin did not immediately precipitate out but some naringin slowly precipitated over time. However, even after standing for 24 h, the solubility of naringin in the pH 6.0 buffer still reached 592.32 µg/mL, which was about ten times that of naringenin (Figure 3b). Additionally, the solubility of naringin did not decrease as the transition pH decreases (Figure 3b). This result means that when using the pH-driven method to prepare naringin-loaded nanoliposomes, some free naringin may be blended in the nanoliposomes solution.
3.3. Encapsulation Efficiency (EE) and Loading Capacity (LC) of Naringenin and Naringin in Nanoliposomes
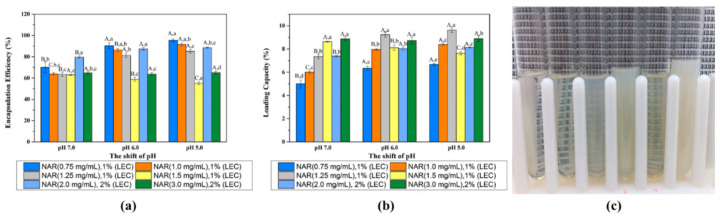
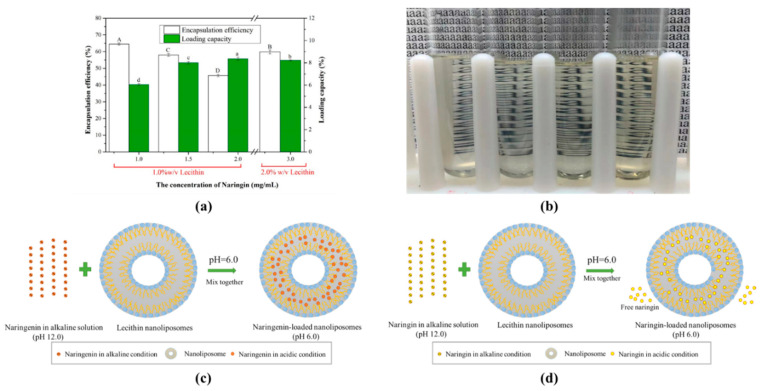
The EE and LC of nanoliposomes fabricated with different naringenin and lecithin concentrations at pH 7.0, 6.0 and 5.0 were determined, respectively (Figure 4). When the concentration of naringenin was 0.75 mg/mL within 1.0% w/v lecithin, the EE of naringenin in the nanoliposomes increased as the shift of pH went down during preparation, being 70.12%, 90.30% and 95.34% for pH 7.0, 6.0 and 5.0, respectively. The same trend appeared at naringenin concentration of 1.00 and 1.25 mg/mL, and the EE of 1.00 mg/mL nanoliposome were 63.89%, 86.49% and 91.47%, while 1.25 mg/mL nanoliposome were 63.47%, 81.39% and 85.20% for pH 7.0, 6.0 and 5.0, respectively. This is may be because more naringenin emerged into the hydrophobic cavity of the liposome as the decreased solubility of naringenin from alkaline solution (pH 12.0) to weakly acidic solution, so the EE of naringenin-loaded nanoliposomes increased [26]. However, when the naringenin concentration reached 1.50 mg/mL, the EE decreased as the pH decreased. When the feeding level of naringenin raised, the EE of nanoliposome gradually decreased, and the nanoliposome solution gradually changed from transparent to cloudy (Figure 4c). The maximum load of naringenin in the nanoliposome solutions (1.0% w/v lecithin + 1.25 mg/L naringenin) was 1.02 mg/mL at pH 6.0. When the concentration of naringenin attained 1.50 mg/mL, the maximum LC and EE began to decrease significantly, which means that nanoliposome may began to aggregate and easily formed naringenin crystals to initiate precipitation. Therefore, we tried to increase the amount of lecithin to load more naringenin. When the lecithin content reached 2.0% (w/v) and the concentration of naringenin was 2.00 mg/mL, the amount of naringenin loaded in nanoliposomes was 1.77 mg at pH 6.0, which was about twice than 1.00 mg/mL with 1.0% (w/v) lecithin, and their EE were close as 88.56% and 86.49%, respectively. Wang and co-workers prepared naringenin-loaded liposomes by thin-film hydration method, and the EE of the naringenin-loaded liposomes (4.0 mg/mL, 6% w/v lecithin) was 72.2% [13]. Compared with the thin-film hydration, the pH-driven is a simple and effective method without the use of high temperature or organic solvents, and also has a higher EE [24,25,26].
Figure 4.
Encapsulation efficiency (a) and loading capacity (b) of naringenin-loaded nanoliposomes with pH shift to 7.0, 6.0 and 5.0; The image (c) of naringenin-loaded nanoliposome solutions with naringenin concentrations of 0.75, 1.00, 1.25 and 1.50 mg/mL with 1% (w/v) lecithin, while naringenin concentrations of 2.00 and 3.00 mg/mL with 2% (w/v) lecithin from left to right at pH 6.0. Samples denoted with different letters (A–C) and (a–d) were significantly different (p < 0.05) when compared between different pH regions (same naringenin level) and different naringenin levels (same pH region), respectively.
LC was also used to evaluate the efficiency of loading naringenin with lecithin. The maximum loading capacity appeared as a naringenin concentration of 1.25 mg/mL were 9.23% and 9.63% for pH 6.0 and 5.0, while it was 8.63% at 1.50 mg/mL naringenin concentration for pH 7.0. The maximum loading capacity of nanoliposomes at pH 6.0 and 5.0 was close (without significant difference) when enough feeding naringenin was provided, which suggesting that the LC of the nanoliposome itself would not be affected in these pHs. When the nanoliposomes were prepared at pH 6.0, the loading capacity increased from 6.34% to 9.23% with the increase of naringenin concentration from 0.75 to 1.25 mg/mL and then dropped to 8.11% for 1.50 mg/mL naringenin.
The EE and LC of nanoliposomes fabricated with different naringin and lecithin concentrations at pH 6.0 were determined, respectively (Figure 5). After removing the free naringin by centrifugation and ultrafiltration, the encapsulation efficiency of naringin-loaded nanoliposomes was between 45.67%–64.54%. Moreover, the free naringin in the naringin-loaded nanoliposome solutions was between 17.78%–39.60%, which was contribute to the low encapsulation efficiency. The loading capacity of naringin-loaded nanoliposomes were 6.06%, 8.01%, 8.37% and 8.24% at naringin concentration of 1.0, 1.5, 2.0 and 3.0 mg/mL, respectively. Compared with naringenin-loaded nanoliposomes, the naringin-loaded nanoliposome solution did not become cloudy as the concentration of naringin increased (Figure 5b). Additionally, the maximum encapsulation efficiency and loading capacity of the naringin-loaded nanoliposomes were lower, which may attribute to its higher molecular weight and solubility in pH 6.0.
Figure 5.
Encapsulation efficiency and loading capacity (a) of naringin-loaded nanoliposomes with pH shift to 6.0; The image (b) of naringin-loaded nanoliposome solutions with naringin concentrations of 1.0, 1.5, and 2.0 mg/mL with 1% (w/v) lecithin, while naringin concentrations of 3.0 mg/mL with 2% (w/v) lecithin from left to right at pH 6.0. Schematic mechanism of the formation of naringenin-loaded (c) and naringin-loaded (d) nanoliposomes based on pH-driven method. Samples denoted with different letters (A–D) and (a–d) were significantly different (p < 0.05) in EE and LC when compared between different naringin levels, respectively.
Above all, these results suggested that naringenin can be encapsulated into nanoliposomes by an easy pH-driven method according to the reduction in its water-solubility after the pH changed to acidity, which causes the naringenin can be moved from the aqueous solution into the lipid bilayers within nanoliposomes (Figure 5c). However, the naringin-loaded nanoliposomes contained some free naringin in solutions due to its higher water-solubility at acidic conditions (Figure 5d).
3.4. Characterization of Naringenin-Loaded and Naringin-Loaded Nanoliposomes
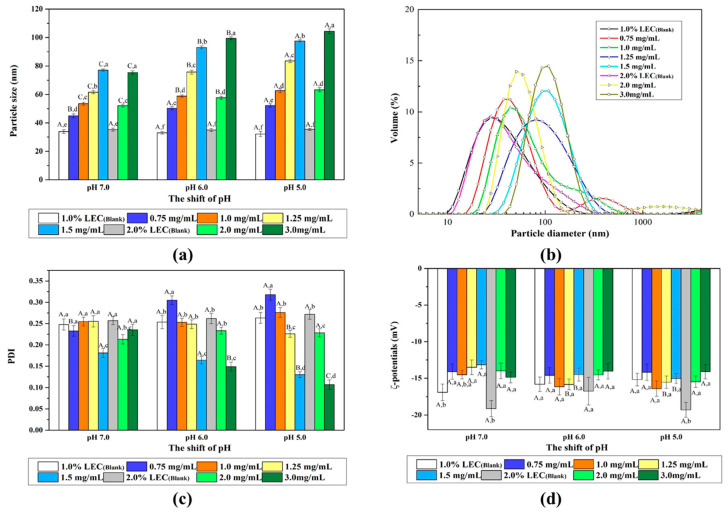
The mean particle size, PDI and ζ-potential changes of blank nanoliposomes and naringenin-loaded nanoliposomes were determined using DLS, as showed in Figure 6. The particle size of the blank nanoliposomes after passing through the microfluidizer consists of 1.0% or 2.0% w/v lecithin were about 33 and 35 nm, respectively. The results showed that the influence of different pH values and lecithin concentration on mean particle size changes of blank nanoliposome seems to be negligible (Figure 6a). When the naringenin was loaded into nanoliposomes, the particle size of nanoliposomes increased significantly. For the shift of pH, all the mean particle size of the naringenin-loaded nanoliposomes increased as the pH decreased. When the lecithin content in the system was 1.0% (w/v), the average diameters of nanoliposomes increased as the concentration of naringenin increased, which was related to the increased encapsulation efficiency and suggested that the larger particle diameter while the more naringenin loaded into nanoliposomes. The average diameters of the nanoliposomes with naringenin concentration of 1.00 mg/mL were 53.47, 58.85 and 62.63 nm for pH 7.0, 6.0 and 5.0, respectively. While the naringenin concentration was 1.25 mg/mL, the average diameters of the nanoliposomes were 61.56, 75.71 and 83.48 nm for pH 7.0, 6.0 and 5.0, respectively. For the 2.0% (w/v) lecithin concentration, the average diameters of nanoliposomes increased similarly as the shift of pH decreased and the concentration of naringenin increased. Particularly, the average diameters of nanoliposomes with a naringenin concentration of 2.00 mg/mL were 52.20, 57.68 and 63.32 nm for pH 7.0, 6.0 and 5.0, and with a naringenin concentration of 3.00 mg/mL were 75.44, 99.40 and 104.40 nm for pH 7.0, 6.0 and 5.0, respectively. The PDI of all the nanoliposome samples was relatively small (PDI < 0.3) apart from the 0.75 mg/mL naringenin-loaded nanoliposomes, suggesting that their particle size distributions were relatively narrow (Figure 6b,c). Compared with the blank nanoliposome, the PDI of the naringenin-loaded nanoliposomes with a naringenin concentration of 0.75 mg/mL increased, indicating that part of the unloaded and loaded nanoliposomes form a fairly wide particle size distribution (Figure 6c). When the feeding concentration of naringenin increased, the PDI of nanoliposomes decreased slightly. In particular, for the concentrations of 1.50 and 3.00 mg/mL naringenin, the PDI of nanoliposomes dropped significantly, from 0.262 (blank nanoliposome) to 0.164 and 0.149, respectively. The reason may be that the hydrophobicity of nanoliposomes increased after loading naringenin, which inhibited the aggregation of blank nanoliposomes, and the number of blank liposomes should be very small under this condition [22,30]. The ζ-potentials of those samples were negative and ranged from –14.01 to −19.30 mV (Figure 6d), and the pH and concentration of naringenin didn’t seem to cause significant changes in the ζ-potentials of nanoliposomes.
Figure 6.
Particle size (a), particle size distribution (b) at pH 6.0, polydispersity index (c, PDI) and ζ-potentials (d) of naringenin-loaded nanoliposomes with pH shift to 7.0, 6.0 and 5.0. Samples denoted with different letters (A–C) and (a–f) were significantly different (p < 0.05) when compared between different pH regions (same naringenin level) and different naringenin levels (same pH region), respectively.
Different from the nanoliposomes loaded with naringenin, the particle size, PDI and ζ-potentials of the naringin-loaded nanoliposomes did not change significantly after loading with naringin (Table 1). Unlike naringenin, naringin may has weak hydrophobicity and electrostatic interaction with nanoliposomes without forming a larger particle size [30].
Table 1.
The mean particle size, PDI and ζ-potentials of naringin-loaded nanoliposomes.
| Naringin Concentration (mg/mL) | Mean Particle Size (nm) | PDI | ζ-Potentials (mV) |
|---|---|---|---|
| Blank nanoliposomes | 34.93 ± 0.98 a | 0.262 ± 0.012 b | −16.74 ± 1.87 a |
| 1.0 (1% w/v lecithin) | 34.03 ± 0.69 a | 0.230 ± 0.010 a | −12.94 ± 1.31 a |
| 1.5 (1% w/v lecithin) | 34.25 ± 0.72 a | 0.237 ± 0.010 a,b | −16.16 ± 1.60 a |
| 2.0 (1% w/v lecithin) | 35.09 ± 0.86 a | 0.240 ± 0.012 a,b | −13.90 ± 1.07 a |
| 3.0 (2% w/v lecithin) | 35.71 ± 0.49 a | 0.260 ± 0.011 b | −16.97 ± 1.48 a |
Samples denoted with letters (a,b) were significantly different (p < 0.05) when compared between different naringin levels.
3.5. Stability of Naringenin-Loaded Nanoliposomes
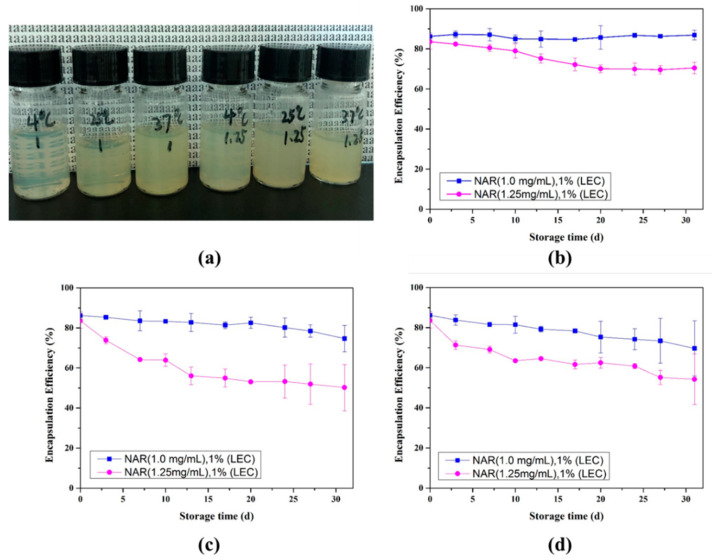
Liposomes need to have good storage stability in order to become a commercial product, which means that they must remain integrity during the entire life cycle of the product. We first investigated the change of the encapsulation efficiency of 1.00 and 1.25 mg/mL naringenin loaded nanoliposomes at 4 °C, 25 °C and 37 °C (Figure 7b–d). At 4 °C, the encapsulation efficiency of 1.00 mg/mL naringenin-loaded nanoliposomes solution remained constant at about 86% after one month of storage, while the nanoliposome solution with a concentration of 1.25 mg/mL dropped from 83.58% to 70.48%. At 25 °C, the encapsulation efficiency of all the nanoliposome solutions decreased, to 74.67% and 50.24%, respectively. At 37 °C, the encapsulation efficiency of 1.00 mg/mL naringenin loaded nanoliposomes solution decreased from 86.23% to 69.69%, and the 1.25 mg/mL naringenin loaded nanoliposomes solution decreased from 83.58% to 54.26%. With the increase of storage temperature, the nanoliposome solution finally changed from milky white to yellow at higher temperature (Figure 7a), indicating that the reduction of encapsulation efficiency is related to the precipitation of naringenin. These results suggest that at high temperatures, nanoliposomes loaded with more naringenin are more hydrophobicity and likely to accelerate aggregation, oxidation, leakage and other chemical reactions during storage, which cause the precipitation of naringenin crystals and the reduction in encapsulation efficiency [22]. Similar to previous research results, conventional liposomes without modification are thermodynamically unstable systems that tend to aggregate, fuse, degrade, or hydrolyze, causing leakage of loaded compounds [31,32,33]. However, the combination of synthetic polymers or biopolymer with liposomes can easily improve the stability and site-specific targeting of liposomes through surface modification [33,34,35].
Figure 7.
The image (a) of naringenin loaded nanoliposome solutions with naringenin concentrations of 1.00 and 1.25 mg/mL with 1% (w/v) lecithin after 31 days storage at different temperatures; Changes of encapsulation efficiency at 4 °C (b), 25 °C (c) and 37 °C (d).
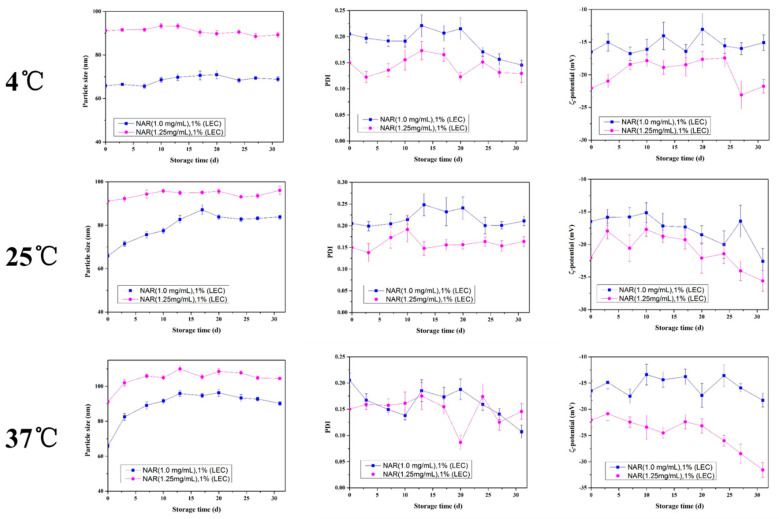
The storage stability of the nanoliposomes were also determined by recording changes in their mean particle size, PDI and ζ-potentials (Figure 8). There was little change in the mean particle size, PDI and ζ-potentials of the 1.00 and 1.25 mg/mL naringenin loaded nanoliposomes solutions when they were placed in 4 °C within 31 days. At 25 °C, the mean particle size of 1.00 mg/mL naringenin loaded nanoliposomes increased from 65.88 nm to 87.14 nm at 17-day, then slightly decreased to 83.83 nm at day 31. Similarly, the 1.25 mg/mL naringenin loaded nanoliposomes increased from 91.15 nm to 95.80 nm at 10-day, then slightly decreased to 93.55 nm at day 28. The change of the particle size of nanoliposomes with different naringenin concentration at 37 °C also followed the trend of first increasing and then decreasing. These results indicate that storing naringenin-loaded nanoliposomes at high temperature is prone to aggregation in the early stage, and then the particle size decreased in the later stage due to oxidation and leakage of the nanoliposomes [19]. In the early stage of storage, the aggregation of nanoliposomes may cause little increase the PDI, while the PDI of the later supersaturated nanoliposomes may decrease due to the precipitation of part of naringenin. Similar to Homayoonfal et al.’s study of the stability of anthocyanin compound loaded nanoliposomes at 37 °C, there were no significant difference in the parameters of PDI and ζ-potential after one month of storage, but the particle diameter and encapsulation efficiency significantly increased and decreased, respectively [36]. Although the instability mechanism is not yet clear, it may be because of the aggregation and leakage of nanoliposomes and chemical degradation of naringenin in nanoliposomes solutions, which results in color changes or precipitation at high store temperature [20,37,38].
Figure 8.
Changes of the naringenin-loaded nanoliposomes in mean particle size, PDI and ζ-potential during storage at 4, 25, and 37 °C for 31 days.
3.6. Micosturcture of Naringenin-Loaded Nanoliposomes
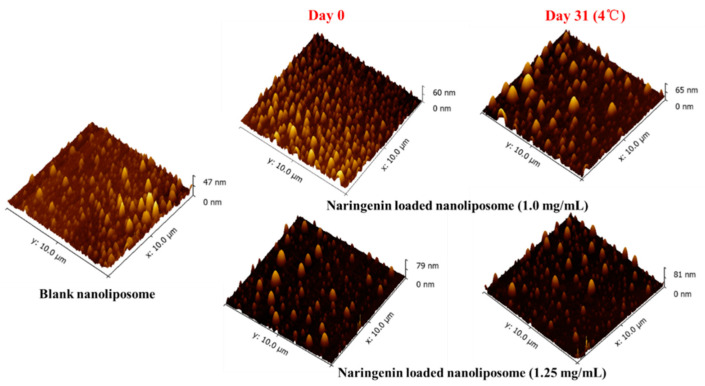
The microstructure of the blank nanoliposomes and naringenin loaded nanoliposomes were obtained using AFM. Figure 9 illustrates representative 3D pictures of the blank and nanoliposomes with different naringenin concentration obtained before and after storage at 4 °C for 31 days. Even the nanoliposomes after storage for 31 days, all the nanoliposomes displayed as smooth sphericities that were uniformly distributed throughout the whole pictures, and the particle sizes were similar with those measured by laser granulometry method.
Figure 9.
Atomic forces microscopy 3D images of blank nanoliposomes and naringenin-loaded nanoliposomes with a concentration of 1.00 and 1.25 mg/mL after 0 and 31 days of storage at 4 °C.
4. Conclusions
In conclusion, we have proved that naringenin could be encapsulated into nanoliposomes by an easy pH-driven method according to the reduction in its water-solubility after the pH changed to acidity, which causes the naringenin can be moved from the aqueous solution into the lipid bilayers within nanoliposomes, while the naringin-loaded nanoliposomes contained some free naringin due to its higher water-solubility at lower pH values. Experiments showed that the naringenin-loaded nanoliposomes were predominantly nanometric, negatively charged and exhibited relatively high encapsulation efficiency. Moreover, the naringenin-loaded nanoliposomes still maintain good stability during storage at 4 °C for 31 days, while they were unstable at high store temperatures. In general, an easy method for manufacturing naringenin-loaded nanoliposomes has been constructed that may help to develop novel food-grade colloidal delivery systems and apply to introducing naringenin or other lipophilic polyphenols into foods, supplements, or drugs.
Acknowledgments
All authors are thankful to their representative universities/institutes for the support and services used in this study.
Author Contributions
Conceptualization, M.C. and R.L.; methodology, R.L. and W.Z.; software, Y.G. and Y.Z.; validation, M.C., W.Z. and R.L.; formal analysis, R.L. and L.L.; investigation, M.C. and R.L.; resources, Y.G. and L.L.; data curation, Y.C. and W.Z.; writing—M.C. and R.L.; writing—review and editing, R.L. and W.Z.; visualization, Y.G., Y.C. and Y.Z.; supervision, W.Z. and J.L.; project administration, R.L. and J.L.; funding acquisition, R.L. and W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 32001689), the Natural Science Foundation of Guangdong Province of China (grant number 2021A1515010901), the Natural Science Foundation of Hainan Province of China (grant number 320QN325), the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (grant number 1630122017016 and 1630122021006), Innovative Research Team Construction Project for Modern Agricultural Industry Common Key Technologies of Guangdong Province (grant number 2020KJ116 and 2020KJ117), and the ‘Guangdong Special Support Plan’ Science and Technology Innovation Young Talents Project (2019TQ05N133).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raeisib S., Chavoshia H., Mohammadic M., Ghorbanid M., Sabzichie M., Ramezani F. Naringenin-loaded nano-structured lipid carrier fortifies oxaliplatin-dependent apoptosis in HT-29 cell line. Process Biochem. 2019;83:168–175. doi: 10.1016/j.procbio.2019.05.013. [DOI] [Google Scholar]
- 2.Rao K., Imran M., Jabri T., Ali I., Perveen S., Shafiullah, Ahmed S., Shah M.R. Gum tragacanth stabilized green gold nanoparticles as cargos for naringin loading: A morphological investigation through afm. Carbohyd. Polym. 2017;174:243. doi: 10.1016/j.carbpol.2017.06.071. [DOI] [PubMed] [Google Scholar]
- 3.Hermenean A., Ardelean A., Stan M., Herman H., Mihali C.V., Costache M., Dinischiotu A. Protective effects of naringenin on carbon tetrachloride-induced acute nephrotoxicity in mouse kidney. Chem. Biol. Interact. 2013;205:138–147. doi: 10.1016/j.cbi.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Bodet C., La V.D., Epifano F., Grenier D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J. Periodontal Res. 2008;43:400–407. doi: 10.1111/j.1600-0765.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 5.Fouad A.A., Albuali W.H., Jresat I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology. 2016;97:224–232. doi: 10.1159/000444262. [DOI] [PubMed] [Google Scholar]
- 6.Yen H.R., Liu C.J., Yeh C.C. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem. Biol. Interact. 2015;235:1–9. doi: 10.1016/j.cbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi T., Vanamala J., Taddeo S.S., Davidson L.A., Murphy M.E., Patil B.S., Wang N., Carroll R.J., Chapkin R.S., Lupton J.R., et al. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp. Biol. Med. 2010;235:710–717. doi: 10.1258/ebm.2010.009359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin C.Y., Ni C.C., Yin M.C., Lii C.K. Flavonoids protect pancreatic beta-cells from cytokines mediated apoptosis through the activation of PI3-kinase pathway. Cytokine. 2012;59:65–71. doi: 10.1016/j.cyto.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Al-Dosari D.I., Ahmed M.M., Al-Rejaie S.S., Alhomida A.S., Ola M.S. Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients. 2017;9:1161. doi: 10.3390/nu9101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zbarsky V., Datla K.P., Parkar S., Rai D.K., Aruoma O.I., Dexter D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 11.Lucas-Abellán C., Pérez-Abril M., Castillo J., Serrano A., Mercader M.T., Fortea M.I., Gabaldón J.A., Núñez-Delicado E. Effect of temperature, pH, β- and HP-β-cds on the solubility and stability of flavanones: Naringenin and hesperetin. LWT Food Sci.Technol. 2019;108:233–239. doi: 10.1016/j.lwt.2019.03.059. [DOI] [Google Scholar]
- 12.Uchiyama H., Kadota K., Nakanishi A., Tandia M., Tozuka Y. A simple blending with α-glycosylated naringin produces enhanced solubility and absorption of pranlukast hemihydrate. Int. J. Pharmaceut. 2019;567:118490. doi: 10.1016/j.ijpharm.2019.118490. [DOI] [PubMed] [Google Scholar]
- 13.Shpigelman A., Shoham Y., Israeli-Lev G., Livney Y.D. β-Lactoglobulin–naringenin complexes: Nano-vehicles for the delivery of a hydrophobic nutraceutical. Food Hydrocoll. 2014;40:214–224. doi: 10.1016/j.foodhyd.2014.02.023. [DOI] [Google Scholar]
- 14.Budel R.G., Silva D.A.D., Moreira M.P., Dalcin A.J.F., Boeck C.R. Toxicological evaluation of naringin-loaded nanocapsules in vitro and in vivo. Colloids Surf. B Biointerfaces. 2020;188:110754. doi: 10.1016/j.colsurfb.2019.110754. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y.W., Wang S.C., Firempong C.K., Zhang H.Y., Wang M.M., Zhang Y., Zhu Y., Yu J.N., Xu X.M. Enhanced Solubility and Bioavailability of Naringenin via Liposomal Nanoformulation: Preparation and In Vitro and In Vivo Evaluations. AAPS PharmSciTech. 2017;18:586–594. doi: 10.1208/s12249-016-0537-8. [DOI] [PubMed] [Google Scholar]
- 16.Yin X., Fu X., Cheng H., Wusigale, Liang L. α-Tocopherol and naringenin in whey protein isolate particles: Partition, antioxidant activity, stability and bioaccessibility. Food Hydrocoll. 2020;106:105895. doi: 10.1016/j.foodhyd.2020.105895. [DOI] [Google Scholar]
- 17.Pan K., Luo Y., Gan Y., Baek S.J., Zhong Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter. 2014;10:6820–6830. doi: 10.1039/C4SM00239C. [DOI] [PubMed] [Google Scholar]
- 18.Guo Q., Bayram I., Zhang W., Su J., Shu X., Yuan F., Mao L., Gao Y. Fabrication and characterization of curcumin-loaded pea protein isolate-surfactant complexes at neutral pH. Food Hydrocoll. 2021;111:106214. doi: 10.1016/j.foodhyd.2020.106214. [DOI] [Google Scholar]
- 19.Mcclements D.J., Peng S., Li Z., Zou L., Liu W., Liu C. Enhancement of curcumin bioavailability by encapsulation in sophorolipid-coated nanoparticles: An in Vitro and in Vivo Study. J. Agric. Food Chem. 2018;66:1488–1497. doi: 10.1021/acs.jafc.7b05478. [DOI] [PubMed] [Google Scholar]
- 20.Peng S., Li Z., Zou L., Liu W., Liu C., Mcclements D.J. Improving curcumin solubility and bioavailability by encapsulation in saponin-coated curcumin nanoparticles prepared using a simple pH-driven loading method. Food Funct. 2018;9:1829–1839. doi: 10.1039/C7FO01814B. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y., Chen S., Liao W., Zhang L., Liu J., Gao Y. Formation, physicochemical stability, and redispersibility of curcumin-loaded rhamnolipid nanoparticles using the pH-driven method. J. Agric. Food Chem. 2020;68:7103–7111. doi: 10.1021/acs.jafc.0c01326. [DOI] [PubMed] [Google Scholar]
- 22.Zhan X., Dai L., Zhang L., Gao Y. Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocoll. 2020;106:105839. doi: 10.1016/j.foodhyd.2020.105839. [DOI] [Google Scholar]
- 23.Zheng B., Peng S., Zhang X., Mcclements D.J. Impact of delivery system type on curcumin bioaccessibility: Comparison of curcumin-loaded nanoemulsions with commercial curcumin supplements. J. Agric. Food Chem. 2018;66:10816–10826. doi: 10.1021/acs.jafc.8b03174. [DOI] [PubMed] [Google Scholar]
- 24.Cheng C., Peng S., Li Z., Zou L., Liu W., Liu C. Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process. RSC Adv. 2017;7:25978–25986. doi: 10.1039/C7RA02861J. [DOI] [Google Scholar]
- 25.Peng S., Zou L., Liu C., Mcclements D.J. Fabrication and characterization of curcumin-loaded liposomes formed from sunflower lecithin: Impact of composition and environmental stress. J. Agric. Food Chem. 2018;66:12421–12430. doi: 10.1021/acs.jafc.8b04136. [DOI] [PubMed] [Google Scholar]
- 26.Peng S., Zou L., Zhou W., Liu W., Mcclements D.J. Encapsulation of lipophilic polyphenols into nanoliposomes using pH-driven method: Advantages and disadvantages. J. Agric. Food Chem. 2019;67:7506–7511. doi: 10.1021/acs.jafc.9b01602. [DOI] [PubMed] [Google Scholar]
- 27.Su C., Liu Y., He Y., Gu J. Analytical methods for investigating in vivo fate of nanoliposomes: A review. J. Pharm. Anal. 2018;8:219–225. doi: 10.1016/j.jpha.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozafari M.R. Nanoliposomes: Preparation and analysis. Methods Mol. Biol. 2010;60:29–50. doi: 10.1007/978-1-60327-360-2_2. [DOI] [PubMed] [Google Scholar]
- 29.Khorasani S., Danaei M., Mozafari M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018;79:106–115. doi: 10.1016/j.tifs.2018.07.009. [DOI] [Google Scholar]
- 30.Chen Y., Zhao Z., Xia G., Xue F., Zhang Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7, 8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2019;146:179–192. doi: 10.1016/j.ijbiomac.2019.12.251. [DOI] [PubMed] [Google Scholar]
- 31.Peng S., Zou L., Liu W., Li Z., Hu X., Chen X., Liu C. Hybrid liposomes composed of amphiphilic chitosan and phospholipid: Preparation, stability and bioavailability as a carrier for curcumin. Carbohydr. Polym. 2017;156:322–332. doi: 10.1016/j.carbpol.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Chang C., Zhai J., Yang Y., Yu H. Ascorbyl palmitate effects on the stability of curcumin-loaded soybean phosphatidylcholine liposomes. Food Biosci. 2021;41:100923. doi: 10.1016/j.fbio.2021.100923. [DOI] [Google Scholar]
- 33.Tan C., Wang J., Sun B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021;48:107727. doi: 10.1016/j.biotechadv.2021.107727. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Xie X., Chen H., Hou X., He Y., Shen J., Shi J., Feng N. Advances in next-generation lipid-polymer hybrid nanocarriers with emphasis on polymer-modified functional liposomes and cell-based-biomimetic nanocarriers for active ingredients and fractions from Chinese medicine delivery. Nanomed. Nanotechnol. Biol. Med. 2020;29:102237. doi: 10.1016/j.nano.2020.102237. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee A., Waters A.K., Kalyan P., Achrol A.S., Kesari S., Yenugonda V.M. Lipid-polymer hybrid nanoparticles as a nextgeneration drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019;14:1937–1952. doi: 10.2147/IJN.S198353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homayoonfal M., Mousavi S.M., Kiani H., Askari G., Desobry S., Arab-Tehrany E. Encapsulation of berberis vulgaris anthocyanins into nanoliposome composed of rapeseed lecithin: A comprehensive study on physicochemical characteristics and biocompatibility. Foods. 2021;10:492. doi: 10.3390/foods10030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R., Dai T., Tan Y., Fu G., Wan Y., Liu C., Mcclements D.J. Fabrication of pea protein-tannic acid complexes: Impact on formation, stability, and digestion of flaxseed oil emulsions. Food Chem. 2020;310:125828. doi: 10.1016/j.foodchem.2019.125828. [DOI] [PubMed] [Google Scholar]
- 38.Li R., Dai T., Zhou W., Fu G., Wan Y., McClements D.J., Li J. Impact of pH, ferrous ions, and tannic acid on lipid oxidation in plant-based emulsions containing saponin-coated flaxseed oil droplets. Food Res. Int. 2020;136:109618. doi: 10.1016/j.foodres.2020.109618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.