Abstract
Aim: Proprotein convertase subtilisin/kexin type 9 (PCSK9) has been identified as an important regulator of low-density lipoprotein (LDL) receptor processing. Evolocumab and alirocumab are PCSK9 inhibitors; however, little is known about the association between PCSK9 levels and lipid profiles in a general population. Because PCSK9 inhibitors have LDL-C lowering effects, we investigated whether there is a positive correlation between serum PCSK9 levels and LDL-C or lipoprotein(a) [Lp(a)].
Methods: In Uku town, 674 residents (mean age; 69.2 ± 8.3 years) received health check-ups. The participants underwent a physical examination and blood tests, including PCSK9 and Lp(a). Serum PCSK9 and Lp(a) were measured by ELISA and Latex methods, respectively. HOMA-IR was calculated by fasting plasma glucose × insulin levels/405.
Results: The mean (range) of PCSK9 and Lp(a) were 211.2 (49–601) ng/mL and 60 (1–107) mg/dL, respectively. Because of a skewed distribution, the log-transformed values were used. With univariate linear regression analysis, PCSK9 levels were associated with Lp(a) (p = 0.028), triglycerides (p < 0.001), and HOMA-IR (p < 0.001), but not with LDL-C (p = 0.138) levels. Multiple stepwise regression analysis revealed that serum PCSK9 levels were independently associated with triglycerides (p < 0.001), Lp(a) (p = 0.033) and HOMA-IR (p = 0.041).
Conclusions: PCSK-9 is independently associated with triglycerides, Lp(a) levels, and HOMA-IR, but not LDL-C, in a relatively large general population sample.
Keywords: Proprotein convertase subtilisin/kexin type 9 (PCSK9), Insulin resistance, Lipoprotein(a), Epidemiology
See editorial vol. 28: 317–318
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9), a member of the proprotein convertase family, is produced mainly by the liver and released directly into the circulation, playing a major role in regulating blood levels of low-density lipoprotein cholesterol (LDL-C). It binds with LDL-receptor (LDL-R) on hepatocytes, preventing their recycling and promoting their degradation through the endosomal/lysosomal pathway1).
Data from clinical, in vivo, in vitro, and animal studies have associated PCSK9 with triglycerides2), triglycerides-rich lipoproteins3), high-density lipoprotein cholesterol (HDL-C)4), insulin, and insulin resistance5, 6). Studies on the relationship between PCSK9 and the components of metabolic syndrome have been reported; however, the results are inconsistent7, 8).
Although evidence has been also presented in epidemiological studies2, 9), lipoprotein(a) [Lp(a)]) has been highlighted as a novel risk factor for coronary artery disease (CAD) rather than PCSK910). The positive association between PCSK9 and Lp(a) was reported in relatively small studies11, 12); however, these studies enrolled patients, but not the general population. Although PCSK9 inhibitors have been already approved as evolocumab and alirocumab13–17), little is known about the association between serum PCSK9 levels and lipid profiles, including Lp(a), in a general population. Therefore, in the present study, we examined the association between serum PCSK9 levels and lipid profiles, including Lp(a) levels and insulin resistance, in a general population.
Methods
Study Population
The present study enrolled 674 subjects (296 males and 378 females: aged over 40 years), who received a population-based health examination in Uku town, a fishing community in southwestern Japan, between 2017 and 2019. This town is an isolated island in Sasebo city, located in Nagasaki prefecture, and the total population is about 2,100. While the subjects' demographic backgrounds are similar to other Nagasaki prefecture residents, their diets and lifestyles differ slightly. Because this community is a typical island of fishermen, fish and shellfish are their main sources of protein. A detailed content of the recent survey in the same district was described previously 18, 19).
Data Collection
Medical histories, including the presence of heart diseases (angina pectoris, myocardial infarction, and arrhythmia), as well as other diseases, such as valvular diseases and stroke (cerebral hemorrhage, ischemic stroke, and subarachnoid hemorrhage), and medication data of lipid-lowering drugs were obtained. Height and weight were measured, and body mass index (BMI) was calculated as weight (kilograms) divided by the square of height (square meters) as an index of the presence or absence of obesity. Waist circumference was measured at the level of the umbilicus in a standing position. Blood pressure (BP) was measured twice with the subjects in the sitting (first) and supine (second) position. The subjects were advised to avoid vigorous physical activity and smoking for at least 30 minutes before BP measurements. The second BP, with the fifth phase diastolic pressure, was used for analysis. Blood was drawn from the antecubital vein for lipid profiles (total cholesterol, LDL-C, HDL-C, triglycerides, and lipoprotein(a) [Lp(a)]), liver functions (aspartate transaminase [AST], alanine transaminase [ALT], and γ-glutamyl transferase [γ-GTP]), creatinine, uric acid, fasting plasma glucose (FPG), insulin, and glycated hemoglobin A1c [HbA1c (NGSP)] in the morning after 12-hour fasting. Fasting blood samples were centrifuged within one hour of collection. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease study equation, modified with a Japanese coefficient20). HOMA-IR was calculated from FPG and insulin levels [FPG (mg/dl)×insulin (µU/ml)/405] as a marker of insulin resistance21), which was defined as HOMA ≥ 1.73, according to the diagnostic criteria used in Japan22–24). Serum Lp(a) was measured by latex immunoassay (LIA) method25). PCSK9 was measured by ELISA26). Intra- and interassay coefficients of variation of PCSK9 in the commercially available laboratory (SRL inc. Laboratory, Fukuoka, Japan) were 7.6% and 7.8%, respectively.
Statistical Analysis
Because of skewed distributions, the natural logarithmic transformation was performed for Lp(a), insulin, HOMA index, and triglycerides. Mean values, and upper and lower 95% confidence limits, were exponentiated and presented as geometric mean ± standard deviation (SD), where the SD was approximated as the difference of the exponentiated confidence limits divided by 3.92, the number of SD in a 95% confidence interval for normally distributed data. Chi-square tests were used to evaluate categorical parameters. Uni- and multiple linear regression analyses, adjusted for age and sex, were used. Using some significant factors from multivariate linear regression analysis, adjusted for age and sex, we performed the multiple stepwise regression analysis to determine the strength and independence of serum PCSK9 levels. We eventually re-analyzed data to examine the association between PCSK9 and parameters for participants, with or without medication for dyslipidemia, using multiple linear regression analysis.
P-values < 0.05 were considered statistically significant. All statistical analyses were performed using the SAS system (Release 9.4, SAS Institute, Cary, NC, USA).
Ethical Considerations
The mayor and the welfare section of Uku town and the Ethical Committee of Kurume University (No. 2284) approved this study. All participants gave informed consent.
Results
The 674 subjects' characteristics are presented in Table 1. There was no significant gender difference (p = 0.789) in serum PCSK9 levels. Fig. 1 shows scatter gram of PCSK9 with age by gender. PCSK9 was significantly and inversely correlated with age in both genders. Also, a scatter gram of PCSK9 indicated significant correlation with Lp(a) (Fig. 2A) and triglycerides (Fig. 2B), but not with LDL-C (Fig. 2C). Table 2 presents the results of uni- and multivariate analyses, adjusted for age and sex, for correlates of PCSK9 levels. With univariate linear regression analysis, PCSK9 levels were associated with BMI (p = 0.004), waist circumference (p = 0.005), systolic BP (p = 0.013), Lp(a) (p = 0.028), triglycerides (p < 0.001), HOMA-IR (p < 0.001), hemoglobin A1c (p < 0.001), hs-CRP (p = 0.005), and medication for dyslipidemia (p < 0.001), but not with LDL-C (p = 0.138). Multivariate linear regression analysis, after adjustment for age, sex, and medication for dyslipidemia, showed that the significances of these factors, except for BMI, remained. Multiple stepwise regression analysis revealed that serum PCSK9 levels were independently associated with medication for dyslipidemia (p < 0.001), triglycerides (p < 0.001), age (p < 0.001; inversely), γ-GTP (p = 0.003), systolic BP (p = 0.007), Lp(a) (p = 0.033), hemoglobin A1c (p = 0.033), and HOMA-IR (p = 0.041) (Table 3).
Table 1. Demographics of study subjects.
| Total (N = 674) | Male (N = 296) | Female (N = 378) | p-value | |
|---|---|---|---|---|
| Age, years | 69.2 ± 8.3 | 69.5 ± 7.8 | 68.9 ± 8.8 | 0.334 |
| Body mass index, kg/m2 | 23.7 ± 3.6 | 24.1 ± 3.3 | 23.4 ± 3.8 | 0.015 |
| Waist circumference, cm | 85.0 ± 9.8 | 86 ± 9.7 | 84.2 ± 9.9 | 0.015 |
| Systolic blood pressure, mmHg | 138.1 ± 18.8 | 138.2 ± 17.9 | 138 ± 19.5 | 0.862 |
| Diastolic blood pressure, mmHg | 76.4 ± 10.4 | 78.1 ± 10.7 | 75.2 ± 10.1 | < 0.001 |
| Aspartate transaminase, U/L | 24.9 ± 11.7 | 26.5 ± 15.3 | 23.7 ± 7.5 | 0.004 |
| Alanine transaminase, U/L | 20.2 ± 10.8 | 22.4 ± 12.4 | 18.5 ± 8.9 | < 0.001 |
| γ-glutamyl transferase, U/L | 37.6 ± 58.0 | 50.6 ± 79.7 | 27.4 ± 28 | < 0.001 |
| Estimated GFR, ml/min/1.73 m2 | 70.2 ± 16.6 | 71.1 ± 16.7 | 69.5 ± 16.5 | 0.220 |
| Triglycerides†, mg/dL (range) | 85.7 (28–860) | 91.3 (29–860) | 81.6 (28–550) | 0.003 |
| Total cholesterol, mg/dL | 197.1 ± 34.4 | 186.4 ± 31.5 | 205.6 ± 34.2 | < 0.001 |
| HDL-cholesterol, mg/dL | 65.9 ± 17.4 | 61.6 ± 18.6 | 69.4 ± 15.6 | < 0.001 |
| LDL-cholesterol, mg/dL | 112.2 ± 29.4 | 104.4 ± 26.4 | 118.4 ± 30.2 | < 0.001 |
| Lipoprotein(a)†, mg/dl (range) | 6.0 (1–107) | 4.8 (1–75) | 7.2 (1–107) | < 0.001 |
| PCSK9†, ng/mL (range) | 211.2 (49–601) | 212 (96–601) | 210.6 (49–520) | 0.789 |
| HOMA-IR (range) | 0.95 (0.19–19.01) | 1.10 (0.19–19.01) | 0.90 (0.2–5.77) | < 0.001 |
| Hemoglobin A1c, % (NGSP) | 5.7 ± 0.4 | 5.7 ± 0.5 | 5.7 ± 0.4 | 0.217 |
| Hs-CRP†, mg/dL (range) | 0.04 (0–6.45) | 0.06 (0–6.45) | 0.04 (0–1.16) | < 0.001 |
| Alcohol intake, %yes | 316 (46.9) | 215 (72.6) | 101 (26.7) | < 0.001 |
| Current smoking, %yes | 63 (9.3) | 58 (19.6) | 5 (1.3) | < 0.001 |
| History of hypertension, %yes | 422 (62.6) | 201 (67.9) | 221 (58.5) | 0.012 |
| History of diabetes, %yes | 80 (11.9) | 47 (15.9) | 33 (8.7) | 0.006 |
| History of dyslipidemia, %yes | 342 (50.6) | 124 (41.9) | 218 (57.7) | < 0.001 |
| Medication for dyslipidemia, %yes | 251 (37.2) | 99 (33.4) | 152 (40.2) | 0.076 |
| History of heart disease, %yes | 139 (20.6) | 74 (25.0) | 65 (17.2) | 0.015 |
| History of stroke, %yes | 31 (4.6) | 14 (4.7) | 17 (4.5) | 0.004 |
The variable was represented in the original scale after analysis using log (natural) transformed values.
Fig. 1.
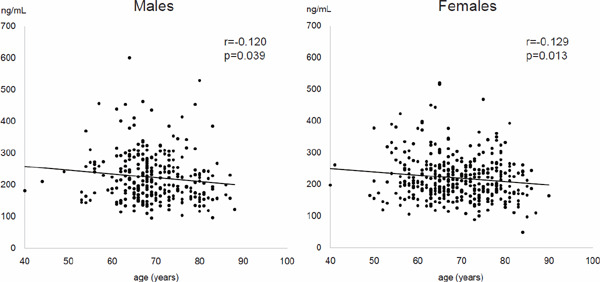
Scatter gram of PCSK9 with age by gender
Fig. 2.
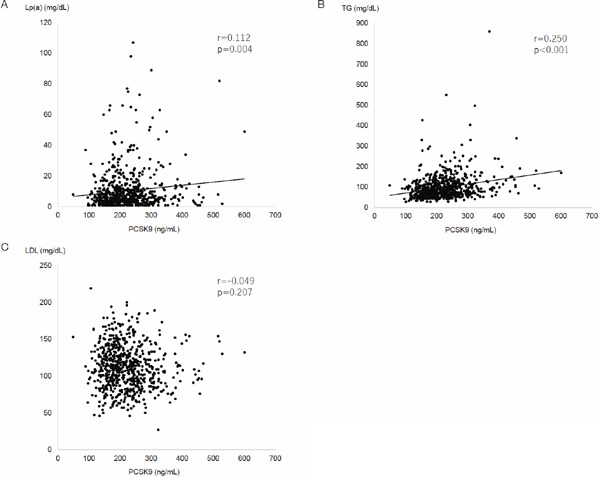
Scatter gram between PCSK9 and Lp(a) (A), triglycerides (B), and LDL-C (C)
Table 2. Univariable and multivariable linear regression analyses for correlates of serum PCSK9 level.
| Univariate |
Adjusted for age, sex, and medication for dyslipidemia |
|||||
|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | |
| Age | −0.005 | 0.001 | < 0.001 | |||
| Sex (Male= 0, Female= 1) | −0.007 | 0.024 | 0.789 | |||
| Body mass index | 0.001 | 0.003 | 0.004 | 0.006 | 0.003 | 0.085 |
| Waist circumference | 0.003 | 0.001 | 0.005 | 0.002 | 0.001 | 0.042 |
| Systolic blood pressure | 0.002 | 0.001 | 0.013 | 0.002 | 0.001 | 0.001 |
| Diastolic blood pressure | 0.002 | 0.001 | 0.077 | |||
| Aspartate transaminase | 0.003 | 0.001 | 0.004 | 0.003 | 0.001 | 0.002 |
| Alanine transaminase | 0.004 | 0.001 | 0.001 | 0.003 | 0.001 | 0.012 |
| γ-glutamyl transferase | 0.001 | 0.0002 | < 0.001 | 0.001 | 0.001 | < 0.001 |
| Estimated GFR | 0.001 | 0.001 | 0.247 | |||
| Triglycerides† | 0.180 | 0.024 | < 0.001 | 0.136 | 0.024 | < 0.001 |
| Total cholesterol | −0.0001 | 0.0003 | 0.830 | |||
| HDL-cholesterol | 0.0002 | 0.001 | 0.776 | |||
| LDL-cholesterol | −0.001 | 0.0004 | 0.138 | |||
| Lipoprotein(a)† | 0.026 | 0.012 | 0.028 | 0.021 | 0.011 | 0.039 |
| HOMA-IR† | 0.095 | 0.017 | < 0.001 | 0.072 | 0.017 | < 0.001 |
| Hemoglobin A1c (NGSP) | 0.105 | 0.027 | < 0.001 | 0.081 | 0.026 | 0.002 |
| Hs-CRP | 0.027 | 0.010 | 0.005 | 0.024 | 0.009 | 0.008 |
| Alcohol intake | 0.043 | 0.024 | 0.077 | |||
| Current smoking | −0.006 | 0.041 | 0.891 | |||
| Medication for dyslipidemia | 0.214 | 0.023 | < 0.001 | |||
The variable was represented in the original scale after analysis using log (natural) transformed values.
Table 3. Multivariable stepwise linear regression analysis between serum PCSK9 level and parameters.
| β | SE | p-value | |
|---|---|---|---|
| Medication for dyslipidemia | 0.170 | 0.023 | < 0.001 |
| Triglycerides† | 0.094 | 0.026 | < 0.001 |
| Age | −0.005 | 0.001 | < 0.001 |
| γ-glutamyl transferase | 0.001 | 0.001 | 0.003 |
| Systolic blood pressure | 0.002 | 0.001 | 0.007 |
| Lipoprotein(a)† | 0.023 | 0.011 | 0.033 |
| Hemoglobin A1c | 0.057 | 0.027 | 0.033 |
| HOMA-IR† | 0.037 | 0.018 | 0.041 |
R2 = 0.203
The variable was represented in the original scale after analysis using log (natural) transformed values.
We further analyzed the data to examine the association between PCSK9 and parameters for participants without medication for dyslipidemia using multiple linear regression analysis. Supplementary Table 1 demonstrated univariable and multivariable linear regression analyses for correlates of serum PCSK9 level without lipid-lowering medication subjects (n = 422). With multivariate analysis adjusted for age and sex, PCSK9 levels were associated with systolic BP (p = 0.006), AST (p < 0.001), ALT (p < 0.001), γ-GTP (p < 0.001), triglycerides (p < 0.001), total cholesterol (p = 0.003), HOMA-IR (p = 0.007), and HbA1c (p = 0.011). Supplementary Table 2 showed univariable and multivariable linear regression analyses for correlates of serum PCSK9 level with lipid-lowering medication subjects (n = 252). With multivariate analysis adjusted for age and sex, PCSK9 levels were associated with γ-GTP (p = 0.002), triglycerides (p = 0.003), HDL-C (p = 0.043), hs-CRP (p = 0.028), and alcohol intake (p = 0.029).
Supplementary Table 1. Univariable and multivariable linear regression analyses for correlates of serum PCSK9 level without lipid-lowering medication subjects (n = 422).
| Univariate |
Adjusted for age and sex |
|||||
|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | |
| Age | −0.006 | 0.002 | < 0.001 | |||
| Sex (Male = 0, Female = 1) | −0.051 | 0.027 | 0.065 | |||
| Body mass index | 0.004 | 0.004 | 0.283 | |||
| Waist circumference | 0.002 | 0.001 | 0.321 | |||
| Systolic blood pressure | 0.002 | 0.001 | 0.040 | 0.002 | 0.001 | 0.006 |
| Diastolic blood pressure | 0.002 | 0.001 | 0.047 | 0.001 | 0.001 | 0.357 |
| Aspartate transaminase | 0.004 | 0.001 | < 0.001 | 0.004 | 0.001 | < 0.001 |
| Alanine transaminase | 0.005 | 0.001 | < 0.001 | 0.004 | 0.001 | < 0.001 |
| γ-glutamyl transferase | 0.001 | 0.0002 | < 0.001 | 0.001 | 0.0002 | < 0.001 |
| Estimated GFR | 0.002 | 0.011 | 0.005 | 0.001 | 0.001 | 0.190 |
| Triglycerides† | 0.150 | 0.027 | < 0.001 | 0.143 | 0.027 | < 0.001 |
| Total cholesterol | 0.001 | 0.0004 | 0.014 | 0.001 | 0.0004 | 0.003 |
| HDL-cholesterol | 0.0002 | 0.001 | 0.807 | |||
| LDL-cholesterol | 0.001 | 0.0004 | 0.117 | |||
| Lipoprotein(a)† | 0.002 | 0.014 | 0.908 | |||
| HOMA-IR† | 0.060 | 0.020 | 0.003 | 0.054 | 0.020 | 0.007 |
| Hs-CRP† | 0.017 | 0.010 | 0.094 | |||
| Hemoglobin A1c (NGSP) | 0.071 | 0.031 | 0.021 | 0.076 | 0.030 | 0.011 |
| Alcohol intake | 0.055 | 0.027 | 0.043 | 0.022 | 0.031 | 0.475 |
| Current smoking | 0.052 | 0.044 | 0.234 | |||
The variable was represented in the original scale after analysis using log (natural) transformed values
Supplementary Table 2. Univariable and multivariable linear regression analyses for correlates of serum PCSK9 level with lipidlowering medication subjects (n = 252).
| Univariate |
Adjusted for age and sex |
|||||
|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | |
| Age | −0.007 | 0.002 | 0.002 | |||
| Sex (Male = 0, Female = 1) | 0.019 | 0.038 | 0.614 | |||
| Body mass index | 0.006 | 0.005 | 0.270 | |||
| Waist circumference | 0.001 | 0.002 | 0.724 | |||
| Systolic blood pressure | 0.001 | 0.001 | 0.964 | |||
| Diastolic blood pressure | 0.001 | 0.002 | 0.752 | |||
| Aspartate transaminase | −0.001 | 0.003 | 0.807 | |||
| Alanine transaminase | 0.001 | 0.002 | 0.944 | |||
| γ-glutamyl transferase | 0.002 | 0.001 | 0.005 | 0.002 | 0.001 | 0.002 |
| Estimated GFR | 0.001 | 0.001 | 0.661 | |||
| Triglycerides† | 0.119 | 0.041 | 0.004 | 0.123 | 0.041 | 0.003 |
| Total cholesterol | 0.001 | 0.001 | 0.082 | |||
| HDL-cholesterol | 0.003 | 0.001 | 0.023 | 0.002 | 0.061 | 0.043 |
| LDL-cholesterol | 0.001 | 0.001 | 0.809 | |||
| Lipoprotein(a)† | 0.018 | 0.018 | 0.307 | |||
| HOMA-IR† | 0.049 | 0.027 | 0.074 | |||
| Hs-CRP† | 0.034 | 0.016 | 0.040 | 0.037 | 0.017 | 0.028 |
| Hemoglobin A1c (NGSP) | 0.037 | 0.046 | 0.430 | |||
| Alcohol intake | 0.082 | 0.037 | 0.028 | 0.090 | 0.041 | 0.029 |
| Current smoking | −0.023 | 0.075 | 0.762 | |||
The variable was represented in the original scale after analysis using log (natural) transformed values
Discussion
In the present study, we demonstrated that serum PCSK9 was independently associated with insulin resistance, triglyceride, and Lp(a), but not with LDL-C, in a general population.
1. Associations between PCSK9 Levels and Triglycerides/Insulin Resistance
Several investigations previously revealed the relationship between serum PCSK9 and metabolic syndrome or insulin resistance1–3). Hasan H, et al.1) correlated PCSK9 with some components of metabolic syndrome and central obesity. Baass A, et al.2) also associated PCSK9 with age, sex, and multiple metabolic markers, including HOMA-IR, both of which enrolled a small number of young females subjects1) and youth2). In Japan, Furuhashi M, et al.9) reported that PCSK9 was independently correlated with metabolic factors, such as triglycerides and HOMA-IR, in 265 participants in a general population. Consistently, our data, obtained from a relatively large number of participants (n = 674), demonstrated that PCSK9 levels were independently associated with age, triglycerides, and HOMA-IR.
A strong positive association of PCSK9 with triglycerides was also observed in a previous study1), suggesting that high PCSK9 levels regulate lipid profiles in a general population with normal nutritional status. This association may explain an increased risk of atherosclerosis development in such individuals, who seem to be normal but are “metabolically unhealthy.”
In a clinical study, the highest insulin resistance tertile, as evaluated by the HOMA-IR, presented significantly higher mean PCSK9 levels compared to the lowest tertile group27), which demonstrated insulin's importance in PCSK9 regulation. Although the clinical study subjects were limited to postmenopausal obese women, our study, from the largest general population, consistently indicated that serum PCSK9 levels were positively and independently correlated with insulin resistance and triglycerides.
One of the mechanisms by which serum PCSK9 levels are correlated to triglycerides and HOMA-IR is that insulin upregulates LDL-R transcription in the liver, and LDL-R endocytosis is a major clearance pathway of plasma PCSK9. Thus, increased hepatocyte surface LDL-R would promote increased PCSK9 clearance27).
2. Associations between PCSK9 and LDL-C
The present study was unable to demonstrate a significant association between PCSK9 and LDL-C, as consistently observed in the manuscript of Hasan H, et al.1) PCSK9's role in regulating LDL-C through LDL-R, and association of lower levels of PCSK9 with reduced CAD risk, have been well documented28). No association between PCSK9 and LDL-C may be attributed, in part, to the presence of intra- and extracellular routes for PCSK9's interaction with LDL-R, which may influence PCSK9's secretion rate into the circulation29). One more explanation may be the influence of dyslipidemia medication. Although a significant association between PCSK9 and LDL-C was not demonstrated, a weak tendency was found in the group with no lipid-lowering medication in the present study.
3. Associations between PCSK9 and Lp(a)
Serum Lp(a) has been highlighted as a novel risk factor for CAD10). The positive association between PCSK9 and Lp(a) was reported in relatively small studies11, 12, 30). Serum PCSK9 was associated with Lp(a) in type 2 diabetic patients in a study in Tunisia11), and serum levels of PCSK9 were positively correlated to serum levels of Lp(a) in patients with CAD in Japan30). However, a report from Han Chinese found no association between PCSK9 with Lp(a) levels in subjects with or without type 2 diabetes12). The comparative analysis in different ethnic groups indicates marked inter-racial differences in the distribution of Lp(a) concentrations31, 32). Therefore, the significant association between PCSK9 and Lp(a) remains controversial. Furthermore, statin treatment may increase Lp(a) levels in subjects with a low molecular weight Lp(a) phenotype33). When we divided subjects into two groups (without and with lipid-lowering medication), Lp(a)'s significance disappeared, probably because the number of each group became smaller.
4. Associations between PCSK9 and systolic blood pressure/γ-glutamyl transferase
In the final multiple linear regression analysis, systolic BP and γ-GTP were also independently associated with PCSK9. Because previous studies investigated the relationship between PCSK9 polymorphism and alcohol consumption34, 35), alcohol consumption may modify the PCSK9 E670G polymorphism's effects on serum total cholesterol and LDL-C levels34). Of several single nucleotide polymorphisms (SNPs), the PCSK9 rs505151 genotype in drinkers was associated with higher BP levels rather than other genotypes. These findings indicate that several lipid-related SNPs may be associated with BP regulation and involved in hypertension development in the population study35).
As for the association between PCSK9 and γ-GTP, Ruscica M, et al.36) suggested that circulating PCSK9 increases with hepatic fat accumulation and correlates with the severity of steatosis, independent of metabolic confounders and liver damage. Recently, Paquette M, et al.37) reported that circulating PCSK9 levels were strongly associated with all circulating liver biomarkers, as well as the presence of hepatic steatosis. These associations were independent of insulin resistance status, although the strength of the association between PCSK9 and hepatic steatosis decreased when corrected for HOMA-IR, BMI, and alcohol use. Since insulin resistance is a hallmark of metabolic syndrome, the association between PCSK9 and γ-GTP may reflect metabolic syndrome.
5. Strengths and Limitations
The strengths of the current study were the community- based cohort study design and its relatively large sample size. Our study results may be helpful for clinical studies38) in terms of PCSK9 distribution or association between PCSK9 and triglycerides, Lp(a), or statin use. It may be suggestive as an indicator of PCSK9 inhibitor use.
There are several limitations in our study. First, the study design was cross-sectional. Thus, nothing conclusive about the association of PCSK9 and Lp(a) can be stated. Second, we used only a single measurement to evaluate the PCSK9 and Lp(a). Third, it remains unclear whether the conclusions of a Japanese population-based study can be generalized to other ethnic populations with different genetic backgrounds.
Conclusions
We demonstrated that PCSK9 levels were significantly and independently associated with triglycerides, Lp(a), and HOMA-IR, but not LDL-C, suggesting that the measuring serum PCSK9 levels may detect the early stage of atherosclerosis.
Acknowledgements
We express our gratitude to elected officials and residents of the Uku town, and the team of physicians in the Department of Internal Medicine, Division of Cardio-vascular Medicine, Kurume University School of Medicine, for their help in carrying out the health examinations.
Notice of Grant Support
This study was supported in part by the Kimura Memorial Heart Foundation, Fukuoka; and by Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology (Grant No. 19K19447).
References
- 1). Hasan H, Attlee A, Raigangar V, Madkour M, Awadallah S: Proprotein convertase subtilisin/kexin type 9 (PCSK9) and metabolic syndrome components among young adult females. Diabetes Metab Syndr, 2017; 11 (Suppl 1): S337-S341 [DOI] [PubMed] [Google Scholar]
- 2). Baass A, Dubuc G, Tremblay M, Delvin EE, O'Loughlin J, Levy E, Davignon J, Lambert M: Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin Chem, 2009; 55: 1637-1645 [DOI] [PubMed] [Google Scholar]
- 3). Chan DC, Pang J, McQuillan BM, Hung J, Beilby JP, Barrett PH, Watts GF: Plasma proprotein convertase subtilisin kexin type 9 as a predictor of carotid atherosclerosis in asymptomatic adults. Heart Lung Circ, 2016; 25: 520-525 [DOI] [PubMed] [Google Scholar]
- 4). Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH; Dallas Heart Study Investigators: The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol, 2004; 93: 1473-1480 [DOI] [PubMed] [Google Scholar]
- 5). Miao J, Manthena PV, Haas ME, Ling AV, Shin DJ, Graham MJ, Crooke RM, Liu J, Biddinger SB: Role of insulin in the regulation of proprotein convertase subtilisin/kexin type 9. Arterioscler Thromb Vasc Biol, 2015; 35: 1589-1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux AL, Grefhorst A, Staels B, Krempf M: Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem, 2006; 281: 6211-6218 [DOI] [PubMed] [Google Scholar]
- 7). Girona J, Ibarretxe D, Plana N, Guaita-Esteruelas S, Amigo N, Heras M, Masana L: Circulating PCSK9 levels and CETP plasma activity are independently associated in patients with metabolic diseases. Cardiovasc Diabetol, 2016; 15: 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Ibarretxe D, Girona J, Plana N, Cabré A, Ferré R, Amigó N, Guaita S, Mallol R, Heras M, Masana L: Circulating PCSK9 in patients with type 2 diabetes and related metabolic disorders. Clin Investig Arterioscler, 2016; 28: 71-78 [DOI] [PubMed] [Google Scholar]
- 9). Furuhashi M, Omori A, Matsumoto M, Kataoka Y, Tanaka M, Moniwa N, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T: Independent link between levels of proprotein convertase subtilisin/kexin type 9 and FABP4 in a general population without medication. Am J Cardiol, 2016; 118: 198-203 [DOI] [PubMed] [Google Scholar]
- 10). Kassner U, Schlabs T, Rosada A, Steinhagen-Thiessen E: Lipoprotein(a)--An independent causal risk factor for cardiovascular disease and current therapeutic options. Atherosclerosis (Suppl), 2015; 18: 263-267 [DOI] [PubMed] [Google Scholar]
- 11). Nekaies Y, Baudin B, Kelbousi S, Sakly M, Attia N: Plasma proprotein convertase subtilisin/kexin type 9 is associated with Lp(a) in type 2 diabetic patients. J Diabetes Complications, 2015; 29: 1165-1170 [DOI] [PubMed] [Google Scholar]
- 12). Yang SH, Li S, Zhang Y, Xu RX, Zhu CG, Guo YL, Wu NQ, Qing P, Gao Y, Cui CJ, Dong Q, Sun J, Li JJ: Analysis of the association between plasma PCSK9 and Lp(a) in Han Chinese. J Endocrinol Invest, 2016; 39: 875-883 [DOI] [PubMed] [Google Scholar]
- 13). Koba S, Inoue I, Cyrille M, Lu C, Inomata H, Shimauchi J, Kajinami K: Evolocumab vs. ezetimibe in statin-intolerant hyperlipidemic Japanese patients: phase 3 GAUSS-4 trial. J Atheroscler Thromb, 2020; 27: 471-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Teramoto T, Usami M, Takagi Y, Baccara-Dinet MT; ODYSSEY Japan Investigators: Efficacy and safety of alirocumab in Japanese patients with diabetes mellitus: Posthoc subanalysis of ODYSSEY Japan. J Atheroscler Thromb, 2019; 26: 282-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins-Domingo K: Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA, 2016; 316: 743-753 [DOI] [PubMed] [Google Scholar]
- 16). Pećin I, Hartgers ML, Hovingh GK, Dent R, Reiner Ž: Prevention of cardiovascular disease in patients with familial hypercholesterolaemia: The role of PCSK9 inhibitors. Eur J Prev Cardiol, 2017; 24: 1383-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Guedeney P, Giustino G, Sorrentino S, Claessen BE, Camaj A, Kalkman DN, Vogel B, Sartori S, De Rosa S, Baber U, Indolfi C, Montalescot G, Dangas GD, Rosenson RS, Pocock SJ, Mehran R: Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J, 2019 (in press) [DOI] [PubMed] [Google Scholar]
- 18). Obuchi A, Adachi H, Enomoto M, Fukami A, Kumagai E, Nakamura S, Yoshimura A, Nohara Y, Nakao E, Umeki Y, Fukumoto Y, Imaizumi T: High plasma fetuin-A levels are associated with metabolic syndrome among males but not females in a Japanese general population. Diabetes Res Clin Pract, 2014; 106: 128-135 [DOI] [PubMed] [Google Scholar]
- 19). Fukami A, Adachi H, Hirai Y, Enomoto M, Otsuka M, Kumagai E, Nakamura S, Yoshimura A, Obuchi A, Nohara Y, Nakao E, Hori K, Fukumoto Y: Association of serum eicosapentaenoic acid to arachidonic acid ratio with microalbuminuria in a population of community-dwelling Japanese. Atherosclerosis, 2015; 239: 577-582 [DOI] [PubMed] [Google Scholar]
- 20). Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 21). Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985; 28: 412-419 [DOI] [PubMed] [Google Scholar]
- 22). Ura N, Saitoh S, Shimamoto K: Clinical diagnosis of metabolic syndrome 1. Metabolic syndrome and insulin resistance. Intern Med, 2007; 46: 1283-1284 [DOI] [PubMed] [Google Scholar]
- 23). Ohnishi H, Saitoh S, Takagi S, Ohata J, Takeuchi H, Isobe N, Katoh N, Chiba Y, Fujiwara T, Akasaka H, Shimamoto K: Incidence of insulin resistance in obese subjects in a rural Japanese population: The Tanno and Sobetsu study. Diabetes Obes Metab, 2005; 7: 83-87 [DOI] [PubMed] [Google Scholar]
- 24). Akasaka H, Katsuya T, Saitoh S, Sugimoto K, FU Y, Takagi S, Ohnishi H, Rakugi H, Ura N, Shimanoto K, Ogihara T: Effects of angiotensin II type 1 receptor gene polymorphisms on insulin resistance in a Japanese general population: The Tanno-Sobetsu Study. Hypertens Res, 2006; 26: 961-967 [DOI] [PubMed] [Google Scholar]
- 25). Abe A, Yoshimura Y, Sekine T, Maeda S, Yamashita S, Noma A: Fully mechanized latex immunoassay for serum lipoprotein (a). Clin Chim Acta, 1994; 225: 105-113 [DOI] [PubMed] [Google Scholar]
- 26). Dubuc G, Tremblay M, Paré G, Jacques H, Hamelin J, Benjannet S, Boulet L, Genest J, Bernier L, Seidah NG, Davignon J: A new method for measurement of total plasma PCSK9: clinical applications. J Lipid Res, 2010; 51: 140-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Awan Z, Dubuc G, Faraj M, Dufour R, Seidah NG, Davignon J, Rabasa-Lhoret R, Baass A: The effect of insulin on circulating PCSK9 in postmenopausal obese women. Clin Biochem, 2014; 47: 1033-1039 [DOI] [PubMed] [Google Scholar]
- 28). Leren TP: Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin Genet, 2004; 65: 419-422 [DOI] [PubMed] [Google Scholar]
- 29). Awan Z, Seidah NG, MacFadyen JG, Benjannet S, Chasman DI, Ridker PM, Genest J: Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial. Clin Chem, 2012; 58: 183-189 [DOI] [PubMed] [Google Scholar]
- 30). Nozue T, Hattori H, Ogawa K, Kujiraoka T, Iwasaki T, Hirano T, Michishita I: Correlation between serum levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) and atherogenic lipoproteins in patients with coronary artery disease. Lipids Health Dis, 2016; 15: 165-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Gaw A, Boerwinkle E, Cohen JC, Hobbs HH: Comparative analysis of the apo(a) gene, apo(a) glycoprotein, and plasma concentrations of Lp (a) in three ethnic groups. Evidence for no common “null” allele at the apo(a) locus. J Clin Invest, 1994; 93: 2526-2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Sandholzer C, Hallman DM, Saha N, Sigurdsson G, Lackner C, Császár A, Boerwinkle E, Utermann G: Effects of the apolipoprotein(a) size polymorphism on the lipoprotein(a) concentration in 7 ethnic groups. Hum Genet, 1991; 86: 607-614 [DOI] [PubMed] [Google Scholar]
- 33). Yahya R, Berk K, Verhoeven A, Bos S, van der Zee L, Touw J, Erhart G, Kronenberg F, Timman R, Sijbrands E, Roeters van Lennep J, Mulder M: Statin treatment increases lipoprotein(a) levels in subjects with low molecular weight apolipoprotein(a) phenotype. Atherosclerosis, 2019; 289: 201-205 [DOI] [PubMed] [Google Scholar]
- 34). Aung LH, Yin RX, Wu DF, Cao XL, Hu XJ, Miao L: Proprotein convertase subtilisin/kexin type 9 gene E670G polymorphism interacts with alcohol consumption to modulate serum lipid levels. Int J Med Sci, 2013; 10: 124-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Yin RX, Aung LH, Long XJ, Yan TT, Cao XL, Huang F, Wu JZ, Yang DZ, Lin WX, Pan SL: Interactions of several genetic polymorphisms and alcohol consumption on blood pressure levels. Biofactors, 2015; 41: 339-351 [DOI] [PubMed] [Google Scholar]
- 36). Ruscica M, Ferri N, Macchi C, Meroni M, Lanti C, Ricci C, Maggioni M, Fracanzani AL, Badiali S, Fargion S, Magni P, Valenti L, Dongiovanni P: Liver fat accumulation is associated with circulating PCSK9. Ann Med, 2016; 48: 384-391 [DOI] [PubMed] [Google Scholar]
- 37). Paquette M, Gauthier D, Chamberland A, Prat A, De Lucia Rolfe E, Rasmussen JJ, Kaduka L, Seidah NG, Bernard S, Christensen DL, Baass A: Circulating PCSK9 is associated with liver biomarkers and hepatic steatosis. Clin Biochem, 2020; 77: 20-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Leander K, Mälarstig A, Van't Hooft FM, Hyde C, Hellénius ML, Troutt JS, Konrad RJ, Öhrvik J, Hamsten A, de Faire U: Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. Circulation, 2016; 133: 1230-1239 [DOI] [PubMed] [Google Scholar]


