Abstract
Aim: We aimed to investigate the relationship of trimethylamine N-oxide (TMAO) concentrations with ischemic stroke in a large-scale case-control study conducted among the hospital-based general population.
Methods: We recruited 953 case-control sex- and age-matched pairs, and cases were confined to first acute ischemic stroke in this study. Fasting plasma TMAO was measured using high-performance liquid chromatography–tandem mass spectroscopy. Conditional logistic regression analysis was conducted to calculate odds ratios (OR) for the association of plasma TMAO with ischemic stroke.
Results: We found that plasma TMAO concentrations in patients with ischemic stroke were significantly higher than that in the control group (median: 2.85 µmol/L vs. 2.33 µmol/L, P < 0.001). In multivariable conditional logistic regression models, higher plasma TMAO concentrations were associated with increased odds of ischemic stroke [fully adjusted OR for highest vs. lowest TMAO quartile: 1.81; 95% confidence interval (CI): 1.27, 2.59; P for trend < 0.001]. The multivariable-adjusted OR for ischemic stroke per 1 µmol/L increment of plasma TMAO was 1.05 (95% CI: 1.02, 1.08). Additionally, the positive association also persisted in subgroups stratified by age, sex, body mass index, smoking status, alcohol habits, history of diabetes, and history of hypertension.
Conclusions: This study suggested a positive association between plasma TMAO and ischemic stroke. Further studies are required to explore the role of plasma TMAO concentrations in predicting stroke risk.
Keywords: Trimethylamine N-oxide, Case–control study, Ischemic stroke
See editorial vol. 28: 314–316
Introduction
Stroke represents a leading cause of mortality and disability worldwide1). Despite stable incidence rates and declining mortality rates over the past decade, the global burden of stroke is increasing, with an estimated 80.1 million prevalent cases of stroke (84.4% were ischemic stroke) and 13.7 million new stroke cases in 2016 2). Beyond traditional risk factors, including age, cigarette smoking, excessive alcohol use, physical inactivity, diabetes, hypertension, and dyslipidemia3, 4), there is substantial interest in identifying novel modifiable risk factors to inform the primary prevention of stroke.
Recently, the interplay between dietary composition, intestinal bacteria, and microbiota-dependent metabolites has been intensively investigated5). Specifically, trimethylamine N-oxide (TMAO), a gut floradependent metabolite of choline, was identified as a promoter of atherosclerosis and as a novel risk factor for the development of cardiovascular diseases6–8). Dietary choline, phosphatidylcholine, and L-carnitine are converted by intestinal bacteria into trimethylamine, which is absorbed and subsequently oxidized to TMAO by hepatic flavin-containing monooxygenases9).
Elevated TMAO concentrations enhance platelet hyperreactivity and thrombosis, induce endothelial dysfunction, and affect lipid metabolism and inflammation8, 10), suggesting the importance of this molecule in the cardiovascular system. In numerous studies, it has been shown that blood TMAO concentrations are positively associated with long-term mortality risk in individuals with atherosclerosis, heart failure, and chronic kidney disease8, 11, 12). A subsequent meta-analysis including 17 clinical studies demonstrated a positive dose-dependent association between plasma TMAO concentrations and increased cardiovascular risk and mortality13). However, most studies on TMAO and cardiovascular risk were conducted in the United States and Europe, where diet, ethnicity, and patterns of gut microbiome composition are different from that of Asian countries. By far, few researches have been done to directly evaluate the relationship of TMAO with stroke, and the results are inconsistent. One nested case–control study of a hypertensive Chinese population demonstrated that higher TMAO concentrations were associated with an increased risk of first stroke14). Nevertheless, this study was confined exclusively to patients with hypertension and did not specifically focus on ischemic stroke, which accounts for the majority of stroke cases2, 15). Conversely, another study of 322 patients revealed that plasma TMAO concentrations in patients with stroke or transient ischemic attack were significantly lower, rather than higher, compared with asymptomatic controls16). Herein, the purpose of this study was to investigate the association of plasma TMAO with first ischemic stroke in a large-scale case–control study conducted among the hospital-based general population.
Methods
Study Population and Data Collection
This was a hospital-based case–control study involving 953 ischemic stroke cases and 953 control subjects. The flowchart of participant recruitment and case–control selection is shown in Supplemental Fig. 1. From October 2012 to June 2017, patients with first acute ischemic stroke who were admitted to People's Hospital of Shenzhen, Guangdong, China, were consecutively recruited. Concomitantly, control subjects were recruited from the general population who attended an annual health examination at the hospital physical examination center. Controls were free of diagnosis of stroke and were 1:1 matched with cases for sex and age (± 5 years). According to the WHO criteria, the definition of stroke was clinical and based on the sudden onset of neurologic deficit lasting longer than 24 h or leading to death, with no apparent cause other than that of vascular origin17). Ischemic stroke was further confirmed by the results of full neurologic examination, computed head tomography, and/or magnetic resonance imaging, according to the International Classification of Disease (9th revision, codes 430–438). The inclusion criteria of cases and controls in this study were as follows: age ≥ 35 years, body mass index (BMI) < 40 kg/m2, Chinese Han ethnicity, and no history of a diagnosis of cerebrovascular disease. Subjects with myocardial infarction, heart failure, malignant tumor, other systemic diseases, or who are using antibiotics within 3 months were also excluded from the study. The study was reviewed and approved by the Ethics Committee of Shenzhen Center for Disease Control and Prevention, and written informed consents were obtained from all involved participants.
Supplemental Fig. 1.
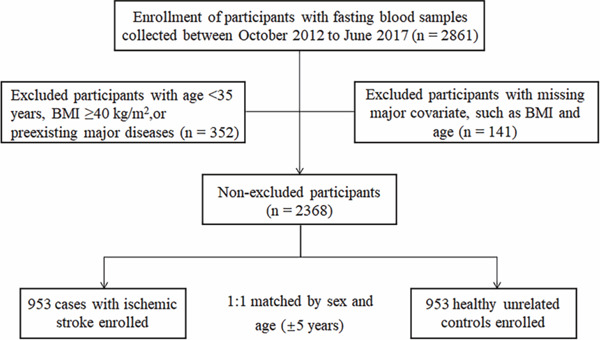
Flowcharts of the participant recruitment and case–control selection
Data on basic demographics, lifestyle, and health-related information were collected using standardized questionnaires. Interviewers were required to conduct face-to-face surveys with all participants involved, and proxy respondents were used for patients who were unable to communicate adequately. Current smoking (≥ 1 cigarette/day) and current drinking (drink alcoholic beverages ≥ 1 time/week) were defined by the participants' self-report. Body weight and height were measured through a standardized protocol, and BMI was calculated by dividing weight in kg to the square of height in meters.
Laboratory Assay
The fasting blood samples were drawn from all subjects on the first morning after admission, and plasma aliquots were immediately centrifuged and stored at −80°C until analysis. Routine biochemical measurements such as fasting plasma glucose, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-cholesterol), and high-density lipoprotein cholesterol (HDL-cholesterol) were performed using automated enzymatic procedure.
Plasma TMAO concentrations were quantified by stable isotope dilution high-performance liquid chromatography–tandem mass spectroscopy (LC-MS/MS; AB Sciex Q-Trap 4500; Applied Biosystems) as previously described18). Briefly, plasma sample (50 µL) was combined with 20 µL of a 500 µg/L d9-(trimethyl)-labeled internal standard in acetonitrile, and then the sample was extracted with 200 µL acetonitrile by vortex for 1 min. The supernatant was collected after centrifugation at 20,238 g at 4 °C for 5 min, and the procedure was repeated once. The combined supernatant was evaporated to dryness under vacuum at 45°C and was reconstituted with 50 µL solvent (acetonitrile/water, 1:1, vol:vol). The sample was vortexed for 1 min and then centrifuged at 20,238 g at 4°C for 5 min. Finally, 5 µL of the clear supernatant was analyzed with LC-MS/MS. For quality check, pooled plasma reference samples were inserted every 15 samples as a quality control. The intra- and interassay coefficients of variation for the quality control samples were both less than 10%.
Statistical Analysis
Continuous covariates were summarized as mean (standard deviation) or median (interquartile ranges) and compared between groups using Student's t test or the Mann–Whitney U test when appropriate. Categorical covariates were summarized as number (%) and compared with chi-squared test. Conditional multivariate logistic regression models were performed to estimate the odds ratios and 95% confidence interval for ischemic stroke for each quartile of plasma TMAO (based on the distribution of controls). P-values for trend were tested by treating the median value of each quartile as a continuous variable in the conditional logistic regression models. The fully adjusted model included age, sex, BMI, smoking status, alcohol habits, history of diabetes and hypertension, triglycerides, LDL-cholesterol, and HDL-cholesterol. We explored a potential nonlinear relationship between plasma TMAO and ischemic stroke by fitting restricted cubic splines with four knots (placed at the 20th, 40th, 60th, and 80th percentiles of plasma TMAO concentrations) to logistic regression models, excluding the values outside the 95th percentiles, to make the graph more stable. We further carried out subgroup analyses and interaction tests by age (< 65, ≥ 65 years), sex, BMI (< 24, ≥ 24 kg/m2)19), smoking status, alcohol habits, history of diabetes, and history of hypertension. Our analyses used all participants for whom the major variables were available. All statistical analyses were done with Stata/SE 12.0 software (Stata- Corp LP).
Results
Table 1 summarizes the descriptive characteristics of the participants included in our study. Compared to controls, plasma TMAO concentrations were significantly higher in patients with ischemic stroke [median: 2.85 µmol/L (interquartile range: 1.74, 4.59) vs. 2.33 µmol/L (interquartile range: 1.53, 3.83), P < 0.001]. Additionally, ischemic stroke cases had higher concentrations of fasting glucose and triglycerides, lower HDL-cholesterol concentrations, and higher rates of diabetes and hypertension and were more likely to be current smokers than controls.
Table 1. Baseline characteristics of study participants.
| Parameters | Cases (n = 953) | Controls (n = 953) | P-value |
|---|---|---|---|
| Age (years) | 63.14 (9.26) | 63.32 (8.47) | 0.650 |
| Male, n (%) | 544 (57.08) | 544 (57.08) | 1.000 |
| BMI (kg/m2) | 23.68 (2.98) | 23.84 (2.86) | 0.224 |
| Fasting glucose (mmol/L) | 6.43 (2.54) | 5.92 (1.43) | < 0.001 |
| Triglycerides (mmol/L) | 1.35 (1.02–1.89) | 1.27 (0.89–1.86) | 0.001 |
| Total cholesterol (mmol/L) | 4.96 (4.19–5.71) | 5.35 (4.58–6.11) | < 0.001 |
| HDL-cholesterol (mmol/L) | 1.07 (0.92–1.26) | 1.39 (1.16–1.62) | < 0.001 |
| LDL-cholesterol (mmol/L) | 2.89 (2.34–3.53) | 2.99 (2.39–3.68) | 0.037 |
| Hypertension, n (%) | 650 (68.21%) | 376 (39.45%) | < 0.001 |
| Type 2 diabetes, n (%) | 272 (28.54%) | 132 (13.85%) | < 0.001 |
| Current smoking, n (%) | 163 (17.10%) | 117 (12.28%) | 0.003 |
| Current drinking, n (%) | 63 (6.61%) | 113 (11.86%) | < 0.001 |
| Plasma TMAO (µmol/L) | 2.85 (1.74–4.59) | 2.33 (1.53–3.83) | < 0.001 |
Data are expressed as n (%) for categorical data, mean (standard deviation) for parametrically distributed data, or median (interquartile range) for nonparametrically distributed data. BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TMAO, trimethylamine N-oxide.
As shown in Table 2, a significantly positive association between plasma TMAO concentrations and ischemic stroke was observed in this study. After adjustment for age, sex, BMI, smoking status, alcohol habits, history of diabetes, and history of hypertension, the ORs (95% CIs) of ischemic stroke from the lowest to highest quartiles were 1 (referent), 1.13 (0.83, 1.55), 1.77 (1.31, 2.38), and 1.80 (1.34, 2.43), respectively (P for trend < 0.001). The multivariableadjusted OR for ischemic stroke per 1 µmol/L increment of plasma TMAO was 1.05 (95% CI: 1.02, 1.08). Further adjustment for triglycerides, LDL-cholesterol, and HDL-cholesterol concentrations did not substantially alter the above results.
Table 2. Association between plasma TMAO concentrations and ischemic stroke.
| Variable | Quartile of plasma TMAO concentrations, µmol/L |
Per 1 µmol/L increment of plasma TMAO | P-trend | |||
|---|---|---|---|---|---|---|
| Q1 (referent): ≤ 1.53 |
Q2: > 1.53–2.33 |
Q3: > 2.33–3.83 |
Q4: > 3.83 |
|||
| Cases/control, n/n | 169/239 | 198/235 | 266/239 | 320/240 | ||
| Crude OR (95% CI) | 1 | 1.16 (0.88, 1.53) | 1.54 (1.18, 2.00) | 1.91 (1.47, 2.50) | 1.06 (1.03, 1.09) | < 0.001 |
| Adjusted OR1 (95% CI) | 1 | 1.13 (0.83, 1.55) | 1.77 (1.31, 2.38) | 1.80 (1.34, 2.43) | 1.05 (1.02, 1.08) | < 0.001 |
| Adjusted OR2 (95% CI) | 1 | 0.94 (0.65, 1.37) | 1.64 (1.16, 2.33) | 1.81 (1.27, 2.59) | 1.05 (1.01, 1.09) | < 0.001 |
Model 1, adjusted for age, sex, BMI, smoking status, alcohol habit, history of hypertension, and history of diabetes
Model 2, additionally adjusted for triglycerides, LDL-cholesterol, and HDL-cholesterol. CI, confidence interval; OR, odd ratio; Q, quartile; TMAO, trimethylamine N-oxide.
The restricted cubic spline regression model revealed a nonlinear positive association between plasma TMAO and the odds of ischemic stroke, with a steeper increment at less than 2.46 µmol/L of plasma TMAO (Fig. 1). Subsequently, we further performed subgroup analyses stratified according to age (< 65, ≥ 65 years), sex, BMI (< 24, ≥ 24 kg/m2), smoking status, alcohol habits, history of diabetes, and history of hypertension. The positive association between plasma TMAO concentrations and ischemic stroke also persisted across different subgroups, and none of the tests for interaction between subgroups was statistically significant (Table 3).
Fig. 1.
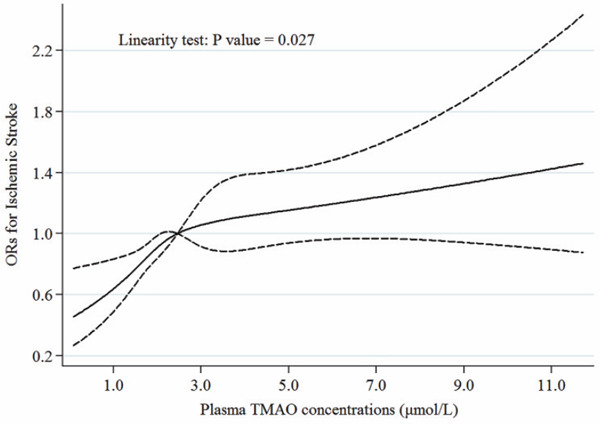
Association of plasma trimethylamine N-oxide (TMAO) concentrations with ischemic stroke
The restricted cubic spline regression was conducted with the use of four knots (placed at the 20th, 40th, 60th, and 80th percentiles of plasma TMAO concentrations) and adjusted for age, sex, BMI, smoking status, alcohol habit, history of hypertension, and history of diabetes. Solid lines are ORs, and dashed lines are 95% CI.
Table 3. Stratified analyses of ischemic stroke and plasma TMAO concentrations by age, sex, BMI, smoking status, alcohol habit, history of diabetes, and history of hypertension.
| Groups | Quartile of plasma TMAO concentrations, µmol/L |
P for interaction | |||
|---|---|---|---|---|---|
| Q1 (referent): ≤ 1.53 | Q2: > 1.53–2.33 | Q3: > 2.33–3.83 | Q4: > 3.83 | ||
| Sex | 0.501 | ||||
| Male (n = 1,088) | 1 | 1.06 (0.72, 1.56) | 1.74 (1.20, 2.54) | 1.55 (1.08, 2.24) | |
| Female (n = 818) | 1 | 1.23 (0.79, 1.91) | 1.37 (0.90, 2.10) | 1.73 (1.14, 2.65) | |
| Age | 0.399 | ||||
| < 65 y (n = 1,047) | 1 | 1.21 (0.82, 1.78) | 1.43 (0.98, 2.09) | 1.44 (0.99, 2.09) | |
| ≥ 65 y (n = 859) | 1 | 1.05 (0.67, 1.65) | 1.77 (1.15, 2.72) | 1.86 (1.23, 2.83) | |
| BMI | 0.715 | ||||
| < 24 (n = 1,039) | 1 | 1.32 (0.90, 1.94) | 1.64 (1.12, 2.40) | 1.76 (1.22, 2.55) | |
| ≥ 24 (n = 867) | 1 | 0.92 (0.58, 1.44) | 1.44 (0.94, 2.20) | 1.46 (0.96, 2.23) | |
| Current smoking | 0.159 | ||||
| Yes (n = 280) | 1 | 0.56 (0.25, 1.26) | 1.50 (0.71, 3.16) | 1.28 (0.64, 2.56) | |
| No (n = 1,626) | 1 | 1.27 (0.92, 1.74) | 1.61 (1.18, 2.19) | 1.73 (1.28, 2.35) | |
| Current drinking | 0.545 | ||||
| Yes (n = 176) | 1 | 0.69 (0.21, 2.28) | 1.80 (0.69, 4.73) | 1.49 (0.56, 3.99) | |
| No (n = 1,730) | 1 | 1.19 (0.88, 1.61) | 1.58 (1.17, 2.12) | 1.68 (1.26, 2.24) | |
| History of diabetes | 0.794 | ||||
| Yes (n = 404) | 1 | 1.50 (0.74, 3.04) | 2.15 (1.06, 4.37) | 1.98 (1.03, 3.83) | |
| No (n = 1,502) | 1 | 1.07 (0.78, 1.47) | 1.48 (1.09, 2.01) | 1.54 (1.13, 2.08) | |
| History of hypertension | 0.607 | ||||
| Yes (n = 1,026) | 1 | 1.29 (0.86, 1.93) | 1.73 (1.17, 2.56) | 1.95 (1.34, 2.84) | |
| No (n = 880) | 1 | 0.98 (0.64, 1.49) | 1.41 (0.95, 2.11) | 1.34 (0.89, 2.01) | |
Data are presented as OR (95% CIs). The multivariate model was adjusted for age, sex, BMI, smoking status, alcohol habit, history of hypertension, and history of diabetes. Q, quartile; TMAO, trimethylamine N-oxide.
Discussion
In this large-scale case–control study conducted among a hospital-based population, we demonstrated that elevated concentrations of plasma TMAO were associated, in a dose-dependent manner, with increased odds of first ischemic stroke. Following full multivariate adjustment, plasma TMAO concentrations remained positively associated with the odds of ischemic stroke. Furthermore, the association of plasma TMAO with the odds of ischemic stroke was also consistent across examined subgroups. Collectively, our findings suggested a positive association between plasma TMAO concentrations and ischemic stroke.
As a gut microbial-generated metabolite, TMAO was first identified and then shown to predict risk for cardiovascular disease in an independent large clinical cohort by Wang et al. 6). Since then, numerous cohort studies showed that an increasing number of adverse outcomes are associated with elevated TMAO concentrations7, 8, 11, 12). Likewise, a systematic review of 19 prospective studies found that high concentrations of TMAO and TMAO precursors were positively associated with the risk of major adverse cardiovascular events and all-cause mortality independently of traditional cardiovascular risk factors20). Our findings are suggestive of a positive association between plasma TMAO and ischemic stroke, which is consistent with prior observations of cardiovascular events. To the best of our knowledge, few studies have directly examined TMAO concentrations and the risk of stroke. Nie et al. conducted a case-control study among hypertensive patients and observed that higher TMAO concentrations were associated with increased risk of first stroke14). Another study from Rexidamu et al. demonstrated that the serum concentrations of TMAO in stroke were significantly higher than in healthy volunteers21). Compared with these studies, our analysis here has greater precision because of much larger sample sizes and has further explored the potential nonlinear relationship between plasma TMAO and ischemic stroke. Interestingly, in another case–control study of patients with large-artery atherosclerotic ischemic stroke or transient ischemic attack, a significant decrease, rather than an increase, in TMAO concentrations in the patients was observed as compared to the asymptomatic group16). Considering that either the preexisting stroke or the treatment may reduce TMAO concentrations and the inclusion of patients and controls was not well balanced, this result should be interpreted with caution.
As previously mentioned, the median of plasma TMAO in the current study was 2.33 µmol/L, which is comparable to other Chinese populations [2.3 (1.4–3.7) µmol/L in subjects from the CSPPT14), 2.18 (1.34–3.90) µmol/L in patients with STEMI22) and 1.77 (1.09–2.80) in diabetic patients23)]. However, plasma TMAO concentrations in the above populations are significantly lower than that in Western countries [4.4 µmol/L in the United States24), 5.6 µmol/L in the United Kingdom25), 3.2 µmol/L in the Netherlands26), and 20.4 µmol/L in Canada27)]. These discrepancies could be attributed to lower consumption of red meat and fat in China than in Western countries28, 29). Recent studies reported that increased TMAO concentrations were dependent on consumption of dietary phosphatidylcholine and L-carnitine, which are commonly found in Western diet such as red meat and full fat dairy products7, 8). Other than diet, ethnic diversity of gut microbiota in populations may also contribute to this difference30). In particular, associations appeared to be stronger among the participants with hypertension or diabetes, although such differences did not attain statistical significance. It is possible to speculate that the effects of dietary differences influencing TMAO concentrations might be more apparent in patients with hypertension or diabetes. Previous evidences showed that higher concentrations of circulating TMAO were associated with increased risk of type 2 diabetes and hypertension31, 32), both of which are risk factors for stroke. Whether hypertension or diabetes accounted for the association of TMAO with ischemic stroke should be further investigated.
Though the mechanisms through which TMAO promotes atherosclerosis are not fully elucidated, several adverse aspects of TMAO have been proposed, including potential interactions with cholesterol metabolism, pro-inflammatory pathways, platelet activation, and subsequent thrombosis. TMAO can modulate cholesterol and sterol metabolism that would, at least partly, contribute to the development of cardiovascular diseases6, 8). TMAO is also known to function as a switch to activate pro-inflammatory cascades, causing arterial damage that allows cholesterol to enter the arterial walls and subsequent plaque formation33). Notably, the TMAO-generating enzyme flavin mono-oxygenase 3 (FMO3) is identified as a key regulator of lipid cholesterol and inflammation34), and perturbation of FMO3 expression has profound effects on glucose and lipid metabolism and atherosclerosis35). Finally, studies conducted on animal models and humans suggest that high concentrations of TMAO contribute directly to platelet hyperreactivity and enhance thrombosis risk10), and the inhibition of gut microbial trimethylamine and TMAO production reduce thrombosis potential36).
Our study has several strengths. In the present study, only patients with first acute ischemic stroke were included to avoid possible dietary changes before stroke onset, which may confound the association of plasma TMAO with ischemic stroke risk. Additionally, the influence of several potential confounders and traditional risk factors for stroke was carefully assessed to minimize the possible residual confounding. Several limitations also warrant consideration. Firstly, blood samples were collected at the time of diagnosis, and a single measurement in our study may not capture the long-term concentrations of TMAO. Secondly, some clinical conditions (including diabetes, hypertension, and dyslipidemia) might lead to dietary changes over time before the onset of stroke, which could diminish the ability to examine the relationship between plasma TMAO and stroke risk. However, such bias seemed to attenuate the association as patients with such symptoms probably limit intake of phosphatidylcholinerich foods, since these foods are typically high in fat and cholesterol37). Thirdly, TMAO status can be modulated by diet and the composition of gut microbiota, but neither was able to be measured in this study, limiting the ability to investigate their potential roles in the effect of TMAO on ischemic stroke. Finally, the case-control nature of this study did not allow us to infer any causal relationship between TMAO and ischemic stroke.
Conclusions
The findings of this hospital-based case–control study demonstrated a positive association between plasma TMAO concentrations and ischemic stroke, which may contribute to knowledge regarding the prevention of ischemic stroke. Further longitudinal studies are required to explore the role of plasma TMAO levels in predicting stroke risk.
Acknowledgments
The chief acknowledgment is to the staff based in the involved hospital and the Shenzhen Center for Disease Control and Prevention for assisting with the fieldwork. We also thank all the participants of this study for their cooperation.
Author Contributions
Taoping Sun, Jinquan Cheng, and Liegang Liu designed the research. Yanwei Zhang, Suli Huang, Ying Wen, and Liangkai Chen performed the data collection. Taoping Sun, Jiawei Yin, Xiaobo Peng, Li Zhou, and Benfeng Cao conducted the experiments. Taoping Sun and Liegang Liu analyzed the data and wrote the manuscript. Xiaoqin Li, Wei Yang, and Aijun Tan supervised and provided critical comments on the manuscript. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This study was supported by the China Postdoctoral Science Foundation (2019M662647), the National Natural Science Foundation of China (81903312), and Sanming Project of Medicine in Shenzhen (SZSM201511007).
References
- 1). Feigin VL, Mensah GA, Norrving B, Murray CJ and Roth GA: Atlas of the Global Burden of Stroke (1990–2013): The GBD 2013 Study. Neuroepidemiology, 2015; 45: 230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 2019; 18: 439-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X and Yusuf S: Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet, 2010; 376: 112-123 [DOI] [PubMed] [Google Scholar]
- 4). Hankey GJ: Stroke. Lancet, 2017; 389: 641-654 [DOI] [PubMed] [Google Scholar]
- 5). Tang WH and Hazen SL: The contributory role of gut microbiota in cardiovascular disease. J Clin Invest, 2014; 124: 4204-4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ and Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 2011; 472: 57-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y and Hazen SL: Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med, 2013; 368: 1575-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ and Hazen SL: Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med, 2013; 19: 576-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL and Lusis AJ: Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab, 2013; 17: 49-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ and Shih DM: Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen- Activated Protein Kinase and Nuclear Factor-kappaB. J Am Heart Assoc, 2016; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y and Hazen SL: Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine- N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol, 2014; 64: 1908-1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS and Hazen SL: Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res, 2015; 116: 448-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G and Perrino C: Gut microbe-generated metabolite trimethylamine- N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J, 2017; 38: 2948-2956 [DOI] [PubMed] [Google Scholar]
- 14). Nie J, Xie L, Zhao BX, Li Y, Qiu B, Zhu F, Li GF, He M, Wang Y, Wang B, Liu S, Zhang H, Guo H, Cai Y, Huo Y, Hou FF, Xu X and Qin X: Serum Trimethylamine N-Oxide Concentration Is Positively Associated With First Stroke in Hypertensive Patients. Stroke, 2018; 49: 2021-2028 [DOI] [PubMed] [Google Scholar]
- 15). Kim AS, Cahill E and Cheng NT: Global Stroke Belt: Geographic Variation in Stroke Burden Worldwide. Stroke, 2015; 46: 3564-3570 [DOI] [PubMed] [Google Scholar]
- 16). Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L, Pan SY and Zhou HW: Dysbiosis of Gut Microbiota With Reduced Trimethylamine-NOxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J Am Heart Assoc, 2015; 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Hatano S: Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ, 1976; 54: 541-553 [PMC free article] [PubMed] [Google Scholar]
- 18). Li P, Zhong C, Li S, Sun T, Huang H, Chen X, Zhu Y, Hu X, Peng X, Zhang X, Bao W, Shan Z, Cheng J, Hu FB, Yang N and Liu L: Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am J Clin Nutr, 2018; 108: 603-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Zhou BF: Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci, 2002; 15: 83-96 [PubMed] [Google Scholar]
- 20). Heianza Y, Ma W, Manson JE, Rexrode KM and Qi L: Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J Am Heart Assoc, 2017; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Rexidamu M, Li H, Jin H and Huang J: Serum levels of Trimethylamine-N-oxide in patients with ischemic stroke. Biosci Rep, 2019; 39: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Sheng Z, Tan Y, Liu C, Zhou P, Li J, Zhou J, Chen R, Chen Y, Song L, Zhao H and Yan H: Relation of Circulating Trimethylamine N-Oxide With Coronary Atherosclerotic Burden in Patients With ST-segment Elevation Myocardial Infarction. The American journal of cardiology, 2019; 123: 894-898 [DOI] [PubMed] [Google Scholar]
- 23). Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, Yang W, Yang X, Yao P, Cheng J, Hu FB and Liu L: Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr, 2017; 106: 888-894 [DOI] [PubMed] [Google Scholar]
- 24). Tang WH, Wang Z, Li XS, Fan Y, Li DS, Wu Y and Hazen SL: Increased Trimethylamine N-Oxide Portends High Mortality Risk Independent of Glycemic Control in Patients with Type 2 Diabetes Mellitus. Clin Chem, 2017; 63: 297-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Suzuki T, Heaney LM, Bhandari SS, Jones DJ and Ng LL: Trimethylamine N-oxide and prognosis in acute heart failure. Heart, 2016; 102: 841-848 [DOI] [PubMed] [Google Scholar]
- 26). Gruppen EG, Garcia E, Connelly MA, Jeyarajah EJ, Otvos JD, Bakker SJL and Dullaart RPF: TMAO is Associated with Mortality: Impact of Modestly Impaired Renal Function. Scientific reports, 2017; 7: 13781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Kim RB, Morse BL, Djurdjev O, Tang M, Muirhead N, Barrett B, Holmes DT, Madore F, Clase CM, Rigatto C and Levin A: Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney international, 2016; 89: 1144-1152 [DOI] [PubMed] [Google Scholar]
- 28). Daniel CR, Cross AJ, Koebnick C and Sinha R: Trends in meat consumption in the USA. Public Health Nutr, 2011; 14: 575-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Wang ZH, Zhai FY, Wang HJ, Zhang JG, Du WW, Su C, Zhang J, Jiang HR and Zhang B: Secular trends in meat and seafood consumption patterns among Chinese adults, 1991–2011. European journal of clinical nutrition, 2015; 69: 227-233 [DOI] [PubMed] [Google Scholar]
- 30). Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, Tremaroli V, Bakker GJ, Attaye I, Pinto-Sietsma SJ, van Raalte DH, Snijder MB, Nicolaou M, Peters R, Zwinderman AH, Backhed F and Nieuwdorp M: Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med, 2018; 24: 1526-1531 [DOI] [PubMed] [Google Scholar]
- 31). Zhuang R, Ge X, Han L, Yu P, Gong X, Meng Q, Zhang Y, Fan H, Zheng L, Liu Z and Zhou X: Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes Rev, 2019; 20: 883-894 [DOI] [PubMed] [Google Scholar]
- 32). Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu G, Xi X, Zhou X and Fan H: The Gut Microbial Metabolite Trimethylamine N-Oxide and Hypertension Risk: A Systematic Review and Dose-Response Meta-analysis. Adv Nutr, 2020; 11: 66-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Nam HS: Gut Microbiota and Ischemic Stroke: The Role of Trimethylamine N-Oxide. J Stroke, 2019; 21: 151-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE and Brown JM: The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell reports, 2015; 10: 326-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL and Lusis AJ: Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res, 2015; 56: 22-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, Barrington WT, Russell MW, Reed JM, Duzan A, Lang JM, Fu X, Li L, Myers AJ, Rachakonda S, DiDonato JA, Brown JM, Gogonea V, Lusis AJ, Garcia-Garcia JC and Hazen SL: Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med, 2018; 24: 1407-1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM, Zeisel SH, Dacosta KA and Mar M-H: USDA Database for the Choline Content of Common Foods, Release Two, Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA, 2008 [Google Scholar]


