Abstract
Background
Public trust is central to the collection of genomic and health data and the sustainability of genomic research. To merit trust, those involved in collecting and sharing data need to demonstrate they are trustworthy. However, it is unclear what measures are most likely to demonstrate this.
Methods
We analyse the ‘Your DNA, Your Say’ online survey of public perspectives on genomic data sharing including responses from 36,268 individuals across 22 low-, middle- and high-income countries, gathered in 15 languages. We examine how participants perceived the relative value of measures to demonstrate the trustworthiness of those using donated DNA and/or medical information. We examine between-country variation and present a consolidated ranking of measures.
Results
Providing transparent information about who will benefit from data access was the most important measure to increase trust, endorsed by more than 50% of participants across 20 of 22 countries. It was followed by the option to withdraw data and transparency about who is using data and why. Variation was found for the importance of measures, notably information about sanctions for misuse of data—endorsed by 5% in India but almost 60% in Japan. A clustering analysis suggests alignment between some countries in the assessment of specific measures, such as the UK and Canada, Spain and Mexico and Portugal and Brazil. China and Russia are less closely aligned with other countries in terms of the value of the measures presented.
Conclusions
Our findings highlight the importance of transparency about data use and about the goals and potential benefits associated with data sharing, including to whom such benefits accrue. They show that members of the public value knowing what benefits accrue from the use of data. The study highlights the importance of locally sensitive measures to increase trust as genomic data sharing continues globally.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13073-021-00903-0.
Background
The future of genomic medicine relies on the ability of researchers and clinicians to access large quantities of genomic and health data. The support of patients and the public for the collection and use of data is central to the success and sustainability of genomic research [1]. However, public willingness to share data and trust in the bodies responsible for the collection and sharing of genomic data varies between countries and between actors involved in the genomic data ecosystem [2, 3]. Trust in the for-profit research sector and governments, for example, is commonly lower than that in non-profit and clinical organisations [4–7]. In this paper, we present findings on public views of measures that may increase trust by ensuring or demonstrating the trustworthiness of the organisations, institutions and individuals working with genomic datasets.
The shift in focus from trust to trustworthiness is an important one, recognised in a growing body of work on genomic medicine [2, 8]. Trust involves a relationship between two actors with an expectation of an outcome [9]. Discussion of trust often places the emphasis on the one placing trust, whether a patient or a member of the public. However, these individuals are placing trust that another actor—for example a clinician, researcher or company—is motivated to act to pursue a particular goal [10]. It is here that trustworthiness is critical. If these actors are not trustworthy, trust is neither merited nor meaningful. Trust misplaced in this way has the potential to harm both those who place their trust and those who betray it, for example through long-term impact on reputation or the loss of future research opportunities.
An emphasis on trustworthiness moves the focus away from the public to those involved in collecting and using data and presents an opportunity for the latter to act to exhibit qualities that demonstrate that they are worthy of trust [11]. The meaning of trustworthiness in practice, however, remains unclear, including the activities or measures that show that those collecting and using data are worthy of trust [2, 12–14]. A number of features exhibited by ‘trustworthy’ systems for genomic data have been suggested. These include the importance of establishing shared values and common goals and motives between researchers and participants or members of the public involved in research. This may include demonstrating a focus on the common good and the equitable distribution of risks and benefits [15]. It may also mean supporting research ethics measures such as informed consent with robust governance that is responsive to stakeholders, respectful, transparent, sustainable, audited and regularly assessed, potentially combined with legal protections [1, 16–19]. Further, work to embed research in relation to local values and goals may be particularly important in addressing potential distrust arising from historical discrimination in healthcare and research [10, 16].
This prior work identifies practices that are already in place in at least some large genomic data initiatives and those to which they and other initiatives might aspire—some that might be new, others that may involve refinement of existing activities. To date, however, discussions of the trustworthiness of genomic and health data initiatives and their relationship with public trust have rarely enabled comparisons between contexts, particularly between countries [3, 5, 20]. This makes it difficult to consider how transferable measures to establish trustworthiness might be and how they are differently valued in different research contexts. Such an international perspective is critical given the need for international data sharing and has the potential to support the development of policies for the sharing of genomic data through initiatives like the Global Alliance for Genomics and Health (GA4GH) [21].
We provide an international perspective through an analysis of the ‘Your DNA, Your Say’ online survey, a study of public perspectives on genomic data sharing that draws on responses from 36,268 individuals across 22 low-, middle- and high-income countries, gathered in 15 languages. We have previously reported on variations in trust within and between these countries, and the relationship between trust and willingness to donate DNA and health information [6, 22]. Here we focus in detail on responses to a question asking participants, who would consider donating DNA or medical information, which measures would help them trust those with whom their data may be shared. Our aim with this question is to draw out how current and potential practices contribute to demonstrating the trustworthiness of data users. We analyse how participants perceived the relative value of these measures, how this varied across the 22 countries of the study, and provide a consolidated ranking of the measures. We then examine similarities and differences between countries, clustering countries that share similar perspectives on the value of these measures. This allows us to consider the implications of our findings for data sharing policies and their applicability across social, cultural and regulatory contexts.
Methods
Sample
Via the international network of researchers affiliated with the GA4GH, the research team invited social science, genetic counselling, bioethics and policy collaborators around the world to participate in conducting the ‘Your DNA, Your Say’ project, either supporting recruitment into the project and/or translating the survey. For all countries except Japan, Pakistan and India (see below), data were collected using a cross-sectional online survey with participants recruited via market research company Dynata. We aimed to recruit a sample that was as representative as possible of each country’s population with regard to gender, age and education level. To this end, participant characteristics were monitored during recruitment to proactively include individuals from under-represented population subgroups. Sociodemographic characteristics of participants from each country are shown in Additional file 1: Table S1 and Additional file 1: Figures S1-S5.
In Japan, participants were recruited through a survey research company (Cross Marketing) using the same approach. In Pakistan and India, recruitment was conducted by market research companies (Foresight and Maction, respectively), and methods were varied to account for lower Internet access. In Pakistan, participants completed the questionnaire on a tablet at a central location rather than at home. In India, participants completed the questionnaire on tablets provided by field researchers. Completed surveys were gathered from Argentina, Australia, Belgium, Brazil, Canada, China, Egypt, France, Germany, India, Italy, Japan, Mexico, Pakistan, Poland, Portugal, Russia, Spain, Sweden, Switzerland, UK and the USA. Participants were paid a small financial reward (<£1) for participating, and due to the nature of the recruitment, there are no details on the non-response rate. The study methodology, design, recruitment strategy, limitations and process of data collection are described separately [23].
Measures
Our online survey can be accessed from www.YourDNAYourSay.org. It contains 29 questions; background information about the landscape of genomic research and data sharing is provided via nine films that sit within the survey (see Fig. 1); no prior knowledge about genomics is required to participate.
Fig. 1.
The appearance of the online Your DNA, Your Say survey (Arabic version). The Your DNA, Your Say questionnaire was presented in 15 languages. Background information on genomic research and data sharing was provided by nine films
In this paper, we analyse a question that asked participants what information would help them to trust the people asking them to donate DNA information and/or medical information. As shown in Table 1, this question allowed for a structured response, with participants selecting from a range of measures suggested in existing work on the ethics and governance of genomic data platforms. These measures include practices already in place in some, if not many, genomic data initiatives, and those that are more aspirational [24].
Table 1.
YDYS survey question relating to measures that would help people to trust recipients of donated DNA/medical information
| Q: What information would help you to trust the people asking you to donate DNA information and/or medical information? (choose all that apply) |
• Transparent information about WHO will benefit from the data access • Transparent information about HOW others will benefit personally, professionally and commercially from the data access • A website that clearly explains the pros and cons of data access • The option to opt out of having your information accessed by other researchers • The option to withdraw your information in the future • Biographies and photos of the sorts of researchers who would access the data • Knowing exactly who is using your information, and for what purpose • The ability to access your own DNA information and/or medical information • Being able to communicate directly with gatekeepers of my DNA information and/or medical information • Details about the sanctions applicable if my data is misused by others • Other, please provide: • I would not donate my DNA information and/or medical information |
One feature of relevance to this question is how we presented information about motives associated with data donation and collection; in the survey films and text, we articulated how different actors may obtain and be motivated by disparate multiple and divergent benefits from the use of donated data. When asking whether participants would be willing to donate their DNA and medical information to medical doctors for use in making a diagnosis in another patient, they were told: ‘Whilst there might be benefits to patients from this work, medical doctors might benefit too. For example, through getting more diagnoses for patients and therefore being better at their jobs or getting scientific publications’. When asking whether participants would be willing to donate their DNA and medical information for use by non-profit researchers doing research, for example on how DNA links to disease, they were told: ‘There might be benefits to society from this work. But also, individual researchers and organisations might benefit too. For example, individual researchers could advance their career and organisations bring in new funding’. Then finally, when asking whether participants would be willing to donate their DNA and medical information for use by for-profit researchers doing research, for example, developing new medicines, they were told: ‘There might be benefits to society from this work. But also, individual researchers and organisations might benefit too. For example, individual researchers might advance their career and companies make a profit’. Thus, we were explicit in giving examples of how access and use of data might be motivated by, and lead to, the accrual of personal and organisational, as well as societal and clinical benefit.
We treat countries as the unit of analysis rather than individuals, deriving country-level variables from data collected at the individual level. We use participant-provided information on country of residence and on which measures would help support trust. We focus on those who said that they would potentially be willing to donate DNA or medical information (n=29,814); participants who were not willing to donate were excluded from further analyses. The breakdown of refusals by country is shown in Additional file 1: Table S2 (range 4% (India) to 33% (Japan); mean 16.6%).
Analysis
Country-level responses
We converted individual responses to this question into a country-level ranked list of these measures from most to least important. This ranking is the variable of interest. We also calculated a ‘global’ ranked list based on overall responses. This was compared with a consensus-generated list derived as part of the analysis (described below).
Consolidated ranking across countries
We used the top-k approach [25] to identify common top-ranking responses across countries and to generate a consolidated consensus rank for the measures. We calculated the consensus ranking for the measures using three algorithms (Borda, Markov Chain – majority rule, and the Order Explicit Algorithm) using the TopKLists package [26].
Correlation of responses between countries
The rank of the different measures was calculated for each country. We examined pair-wise correlations between all countries, estimated using Kendall’s tau-b coefficient, which permits estimation of correlations between non-parametric data with ties. In total, there are 231 unique pair-wise correlations; we used a Bonferroni correction to guide interpretation of significance of correlations. We used the Superheat package to visualise the pair-wise correlations and generate a cluster dendrogram illustrating similarities between countries [27].
Results
The percentage of participants endorsing each measure to increase trust in the recipients of donated data is shown for the overall sample and by country in Table 2 and Fig. 2. Measures are ordered by ranking in the overall sample.
Table 2.
Percentage of participants endorsing each measure proposed to help to trust recipients of donated DNA/medical information, overall and by country
| Measure | Overall | Argentina | Australia | Belgium | Brazil | Canada | China | Egypt | France | Germany | India | Italy | Japan | Mexico | Pakistan | Poland | Portugal | Russia | Spain | Sweden | Switzerland | UK | USA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transparent information about WHO will benefit from the data access | 61 | 74 | 68 | 58 | 69 | 63 | 32 | 75 | 59 | 57 | 61 | 66 | 55 | 68 | 61 | 63 | 73 | 47 | 68 | 66 | 70 | 67 | 62 |
| The option to withdraw your information in the future | 54 | 58 | 67 | 56 | 50 | 62 | 39 | 31 | 57 | 50 | 46 | 48 | 55 | 60 | 51 | 58 | 59 | 49 | 58 | 53 | 62 | 61 | 51 |
| Knowing exactly who is using your information, and for what purpose | 53 | 56 | 61 | 61 | 52 | 56 | 46 | 45 | 53 | 63 | 40 | 45 | 56 | 57 | 47 | 52 | 60 | 62 | 52 | 53 | 63 | 53 | 45 |
| Transparent information about HOW others will benefit personally, professionally and commercially from the data access | 49 | 57 | 62 | 35 | 51 | 54 | 38 | 52 | 41 | 37 | 56 | 52 | 42 | 59 | 45 | 41 | 61 | 36 | 55 | 54 | 42 | 55 | 53 |
| The option to opt out of having your information accessed by other researchers | 45 | 33 | 60 | 44 | 45 | 54 | 51 | 34 | 45 | 30 | 42 | 34 | 37 | 38 | 44 | 52 | 51 | 37 | 37 | 25 | 49 | 53 | 44 |
| Details about the sanctions applicable if my data is misused by others | 40 | 48 | 49 | 37 | 36 | 42 | 28 | 44 | 36 | 43 | 5 | 29 | 58 | 45 | 22 | 38 | 49 | 50 | 37 | 36 | 42 | 41 | 29 |
| The ability to access your own DNA and/or medical information | 38 | 40 | 53 | 51 | 24 | 47 | 31 | 28 | 43 | 37 | 27 | 30 | 28 | 46 | 27 | 45 | 43 | 52 | 40 | 43 | 48 | 42 | 41 |
| A website that clearly explains the pros and cons of data access | 37 | 38 | 49 | 32 | 35 | 40 | 38 | 38 | 28 | 29 | 56 | 29 | 36 | 35 | 42 | 31 | 27 | 28 | 29 | 30 | 33 | 46 | 49 |
| Being able to communicate directly with gatekeepers of my DNA and/or medical information | 27 | 30 | 37 | 29 | 30 | 32 | 24 | 32 | 28 | 28 | 13 | 16 | 17 | 30 | 29 | 28 | 35 | 36 | 29 | 22 | 28 | 29 | 26 |
| Biographies and photos of the sorts of researchers who would access the data | 21 | 21 | 17 | 14 | 21 | 20 | 34 | 28 | 19 | 15 | 31 | 13 | 24 | 28 | 23 | 17 | 16 | 20 | 19 | 14 | 13 | 15 | 23 |
| Other | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 2 | 1 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
Fig. 2.
Measures to help trust recipients of donated DNA/medical information. Percentage of participants endorsing each measure proposed to help to trust recipients of donated DNA/medical information, overall and by country
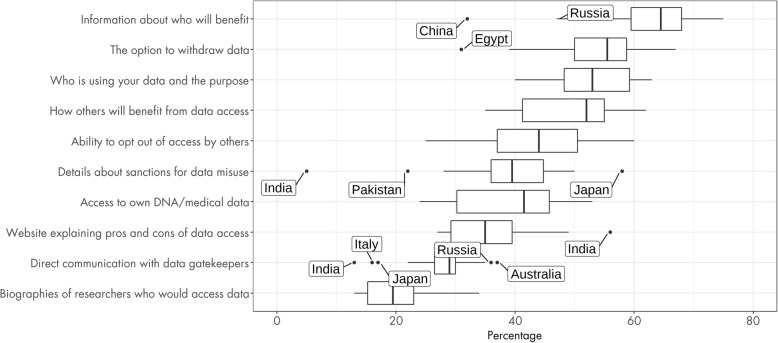
The figure and table illustrate that there is substantial variability between countries in terms of the ranking of the different measures. This is shown further in the boxplots in Fig. 3.
Fig. 3.
Percentage of participants endorsing each measure by country. Boxplots show the percentage endorsing each measure per country, showing the variability associated with each option. Outliers are labelled
For all but two countries, provision of transparent information about who will benefit from data access was endorsed by a majority (i.e. > 50%) of participants, including more than 70% of respondents in Egypt, Argentina, Portugal and Switzerland, although China was an extreme outlier with only 32% of respondents endorsing this. Overall, the option to withdraw information in the future was the second most endorsed measure across the sample, with the greatest number of respondents endorsing this option in Australia, Canada, Switzerland and the UK, but only 31% in Egypt.
Endorsement was most variable for details about sanctions for misuse of data, ranging from 5% in India to almost 60% in Japan, where it was the most chosen option. Direct interaction with gatekeepers was a divisive measure with many outliers both above (Australia, Russia, Portugal) and below (Italy, Japan, India) the average endorsement.
Countries varied in the number of measures that were endorsed by a majority of respondents. While five or more measures were endorsed by more than 50% of respondents in the UK, Portugal, Canada and Australia, only one—the ability to opt out of having information access by other researchers—was endorsed by an equivalent proportion of respondents in China.
Consolidated ranking
The consensus results from the top-k approach (Table 3) confirm the rank order obtained from the overall ranking based on percentage endorsement (from most to least important). The only exception is that the ability to access one’s own DNA/or medical information is ranked one place above details about sanctions (as opposed to one rank lower based on percentage endorsement).
Table 3.
Consolidated ranking of measures to increase trust based on the top-k approach
|
1. Transparent information about WHO will benefit from the data access 2. The option to withdraw your information in the future 3. Knowing exactly who is using your information, and for what purpose 4. Transparent information about HOW others will benefit personally, professionally and commercially from the data access 5. The option to opt out of having your information accessed by other researchers 6. The ability to access your own DNA and/or medical information 7. Details about the sanctions applicable if my data is misused by others 8. A website that clearly explains the pros and cons of data access 9. Being able to communicate directly with gatekeepers of my DNA and/or medical information 10. Biographies and photos of the sorts of researchers who would access the data |
Correlation of responses between countries
The pair-wise correlation estimates for all country pairs are shown in Fig. 4. Pairwise correlations are shown in Table S3 for all estimates greater than or equal to 0.9. The two strongest correlations, with p values below the Bonferroni-corrected threshold of 0.0002, were between Mexico and Spain, and France and Poland (both at 0.98). The lowest correlation (data not shown) was between Russia and India, with an estimate of − 0.16.
Fig. 4.
Heatmap of pair-wise correlation estimates. The strength of the pairwise correlation is indicated by the colour of the square, from dark blue to yellow. The dendrogram indicates clustering among countries where responses were closely aligned
Overall, the heatmap and dendrogram in Fig. 4 show groups of countries for which the rankings of these measures were very similar and that form specific clades. Similarity is not necessarily related to geographical proximity, as for example in the similarity of Spain and Mexico or the UK and Australia. Some countries, notably China and Russia and to some extent India, Egypt, and Japan have consistently lower correlations with most other countries and are linked only at higher levels of the dendrogram—with China and Russia forming a separate, distinct clade from the other 19 countries in the sample.
Discussion
Transparency about aspirations and access
Across countries, measures to demonstrate the trustworthiness of the actors asking for and sharing genomic and health data should focus on transparency about the potential benefits of research, to whom such benefits will accrue and how this will happen. That is, it is not just about describing the general promise and potential of research—such as a benefit to future patients—but also providing an outline of how these benefits will be realised through research. While existing work has suggested the importance of a clear common goal in building trusting relationships with potential data donors, our findings are the first to emphasise the global importance of being clear in this message [16, 17].
It is also important to note, however, that transparency is not limited to outlining expected societal benefits (e.g. finding treatment to a disease) and how they are to be realised. A recurrent feature of research on public trust has been the low levels of trust associated with for-profit or private sector actors, particularly in relation to financial profits for shareholders [5–7]. Yet the benefits motivating the use of research or clinical access to large datasets are not only pecuniary. A further step towards demonstrating trustworthiness, and ‘respectful interactions’ [17] around data would be for potential data users to reflect on and acknowledge their multiple interests in and motivations for collecting and using health data, and to be transparent about these. Such transparency may enable data donors to more clearly understand what motivates the use of data, and thus make a more confident assessment of a data users’ motivation and the extent to which it reflects a desirable or common goal [10, 15].
A further role of transparency relates to the responsibility of those collecting and using genomic data to provide clear and accessible information on who is using data and for what purposes [28]. Our work suggests that such transparency is indeed an important feature of trustworthy data stewardship across the 22 countries studied. It also draws attention to the potential importance of familiarity, not just with genetics—as we have highlighted elsewhere—but with the individuals or organisations responsible for genomic data [22, 29].
Our findings suggest, however, that the value of openness does not necessarily extend to individuals’ ability to access their own DNA and health data. Such direct access as a reciprocation of individual contributions was comparatively less important to participants as a measure to increase trust. Thus, while access to data may be valued by some participants in light of personal interest and perceived personal utility, communicating the potential for such access is not necessarily likely to demonstrate the trustworthiness of actors using of genomic data.
Data rights and regulation
While the measures discussed above concentrate on the goals and roles of genomic research and transparency about access to data, the high ranking of withdrawal and opt outs from data use highlights the importance and value of demonstrating adherence to research ethics frameworks, including informed consent. The right to withdraw, for example, is protected and reinforced throughout clinical research ethics guidance as well as data protection law (e.g. General Data Protection Regulation), but presents specific challenges in the case of long-term genomics research [1, 30]. Our findings suggest the value of efforts to reinforce and protect this right, even as data are de-identified and shared, making it clear to data donors that this right exists, but also to be open about its limits and the difficulties associated with tracking shared de-identified data.
The comparatively lesser importance attributed to sanctions for data misuse suggests that regulation and enforcement measures to prevent misuse and exploitation may be important to prevent a loss of trust but make a smaller contribution to demonstrating trustworthiness [14]. Respondents across all countries were less likely to value being able to communicate directly with the gatekeepers of genomic and health data collections, or to be able to access websites about data sharing or see profiles of researchers. The first is interesting, given the importance placed by policy-makers on identifying those responsible for data in regulatory interventions such as European Union’s General Data Protection Regulation (GDPR). The latter suggests that, a website might be of informational value to some, but that efforts to build trust may need more proactive engagement with data donors. This concords with work that suggests details about individual data users may have little value to data donors without knowledge of why that individual is trustworthy, returning discussion to the measures outlined above [31].
Cross-country consistency and variation
While the overall picture provided by both the consolidated ranking and the individual country rankings grouped in Figs. 2 and 3 are consistent, our data show variation between countries in their views of measures to enhance trust. The option of communicating with gatekeepers, for example, was selected by a far higher proportion of respondents in Australia, Portugal and Russia than in Japan, Italy or India. Variation is also seen among countries that share, to an extent, legal and decision-making frameworks relevant to genomic data, such as member states of the European Union—although all ten European countries in the sample fall within the same broad clade of 17 countries in Fig. 4, each often has greatest similarity with countries outside the bloc. At a European level, these findings may contribute to the exploration of national variation in the implementation of regulation and legal safeguards derived from the GDPR [32].
Further patterns of consistency and divergence can be seen in the detail of the dendrogram. Some results appear intuitive while others are more unexpected. For example, the clade in which the UK and Australia cluster may be anticipated given their (partly) shared histories, and similar governance and social healthcare systems. The connection between France and Switzerland might also be expected, particularly among Francophone respondents. Other results are less expected —it is interesting that the USA is not as closely associated with some countries as might be anticipated given geographic proximity and/or shared histories, such as Canada or the UK. The clade that brings together India and Egypt with Japan, Russia and China is also suggestive of interesting directions for further investigation.
As a whole, the patterns of consistency and variation shown here provide nuance to discussions of trustworthiness and present challenges to those developing standards and governance models to facilitate the international sharing of genomic and health data. Most significantly, they suggest that while it is important to work to establish codes of conduct and demonstrate shared values and goals between researchers and data donors, conclusions from such work can only be tentatively extended across national settings. They further point to the need for detailed comparative work, including qualitative studies, to understand how and why trustworthiness can be demonstrated by the individuals and institutions using genomic and health data.
Limitations
The limitations of the study and design have been published separately [24]. As an exploratory cross-sectional online survey, the study is limited in that it captures intended behaviour at a single time point. Three particular limitations are important to note. Firstly, our analysis is limited to those who would be willing to consider donating their DNA or health information for research. While this includes the majority of the sample, it cannot tell us which measures to increase trust may be more valued by those who definitely will not donate. Second, although the survey was translated and back-translated, nuances of language and culture may affect how participants interpret the options presented. Finally, measures deemed as important in this study, while important and likely necessary, are unlikely to be sufficient on their own to assure potential donors of DNA and health data of the trustworthiness of actors involved in collecting, using and sharing data.
Conclusions
The analysis of responses across the 22 countries included in the Your DNA, Your Say survey suggests practical findings related to demonstrating the trustworthiness of genomic data initiatives, and directions for further research to explore global public perspectives in more detail. They show the importance of research ethics principles related to the right to withdraw, but suggest that legal and regulatory controls may be more important in preventing the loss of trust than in rebuilding it. While highlighting significant variation between countries, they emphasise the importance of transparency about data uses, but particularly about the goals of data collection, and the potential benefits for patients and society, and for data users themselves.
Supplementary Information
Additional file 1. Additional tables and figures related to the Your DNA, Your Say sample and responses in pdf format.
Acknowledgements
This work was supported by Wellcome grant [206194] to Society and Ethics Research, Connecting Science, Wellcome Genome Campus. We would also like to acknowledge the input of Noémie Aubert Bonn, Universiteit Hasselt, Belgium. In memory of Professor Christine Critchley.
Authors’ contributions
RM conceived and drafted the paper and interpreted the data. AM conceived and designed the study and substantively revised the work. HH and EN co-designed the survey and study concepts and revised the work. KM, JP and BL analysed and interpreted the data. PB, JS and CS created the Your DNA, Your Say research platform. All other authors were involved in the acquisition or analysis of data and revised the work. All authors approved the submitted version
Funding
This work was supported by Wellcome Trust grant [206194] to Society and Ethics Research, Connecting Science, Wellcome Genome Campus.
Availability of data and materials
The full dataset is published at https://societyandethicsresearch.wellcomeconnectingscience.org/project/your-dna-your-say and available, without restriction, for anyone to access, download and analyse.
Declarations
Ethics approval and consent to participate
The online survey is fully anonymous. Participants are informed that their consent is given when they choose to click off the landing page and start answering the questions. On the landing page, the purpose of the project is explained as well as what participation involves, participants have a choice at any stage within the survey, to stop answering the questions and withdraw. The online project is physically based at the Wellcome Genome Campus with all data collected and stored in encrypted files at the Wellcome Sanger Institute in Cambridge. As part of the conditions of research delivery at this research institution, the project passed ethical review by the Human Materials and Data Management Committee of the Wellcome Sanger Institute (Registration Number: 16/029) as well as legal review to ensure that it was compliant with ethical and legal standards for participant involvement, data collection and storage. This ethics approval was sufficient to cover recruitment into the online survey for most of the collaborators attached to the project; with the exception of Australia, whereby the University of Tasmania required an additional local IRB process to be completed plus their own separate consent form adding onto the landing page of the survey for Australian participants only. The study was approved by the Tasmanian Social Sciences Human Research Ethics Committee on the 5th of July 2017, reference number H0016682. This research conformed to the Declaration of Helsinki.
Consent for publication
Not applicable
Competing interests
KM, DM, QH and SA declare no competing interests associated with their affiliations. The remaining authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaye J. The tension between data sharing and the protection of privacy in genomics research. Annu Rev Genomics Hum Genet. 2012;13(1):415–431. doi: 10.1146/annurev-genom-082410-101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton EW, Halverson CM, Sathe NA, Malin BA. A systematic literature review of individuals’ perspectives on privacy and genetic information in the United States. PLoS One. 2018;13(10). 10.1371/journal.pone.0204417. [DOI] [PMC free article] [PubMed]
- 3.Ghafur S, Dael JV, Leis M, Darzi A, Sheikh A. Public perceptions on data sharing: key insights from the UK and the USA. Lancet Digit Health. 2020;0(0). 10.1016/S2589-7500(20)30161-8. [DOI] [PMC free article] [PubMed]
- 4.Critchley C, Nicol D, Otlowski M. The impact of commercialisation and genetic data sharing arrangements on public trust and the intention to participate in biobank research. Public Health Genomics. 2015;18(3):160–172. doi: 10.1159/000375441. [DOI] [PubMed] [Google Scholar]
- 5.Gaskell G, Gottweis H, Starkbaum J, Gerber MM, Broerse J, Gottweis U, Hobbs A, Helén I, Paschou M, Snell K, Soulier A. Publics and biobanks: Pan-European diversity and the challenge of responsible innovation. Eur J Hum Genet. 2013;21(1):14–20. doi: 10.1038/ejhg.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milne R, Morley KI, Howard H, et al. Trust in genomic data sharing among members of the general public in the UK, USA, Canada and Australia. Hum Genet. 2019;138(11):1237–46. 10.1007/s00439-019-02062-0. [DOI] [PMC free article] [PubMed]
- 7.Voigt TH, Holtz V, Niemiec E, Howard HC, Middleton A, Prainsack B. Willingness to donate genomic and other medical data: results from Germany. Eur J Hum Genet. 2020;28(8):1000–1009. doi: 10.1038/s41431-020-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dheensa S, Samuel G, Lucassen AM, Farsides B. Towards a national genomics medicine service: the challenges facing clinical-research hybrid practices and the case of the 100 000 genomes project. J Med Ethics. 2018;44(6):397–403. doi: 10.1136/medethics-2017-104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardin R. Trust and Trustworthiness. New York: Russell Sage Foundation; 2002.
- 10.Baier A. Trust and Antitrust. Ethics. 1986;96(2):231–260. doi: 10.1086/292745. [DOI] [Google Scholar]
- 11.O’Doherty KC, Burgess MM, Edwards K, Gallagher RP, Hawkins AK, Kaye J, McCaffrey V, Winickoff DE. From consent to institutions: Designing adaptive governance for genomic biobanks. Soc Sci Med. 2011;73(3):367–374. doi: 10.1016/j.socscimed.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Nicol D, Critchley C, McWhirter R, Whitton T. Understanding public reactions to commercialization of biobanks and use of biobank resources. Soc Sci Med. 2016;162:79–87. doi: 10.1016/J.SOCSCIMED.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Burke W, Beskow LM, Trinidad SB, Fullerton SM, Brelsford K. Informed consent in translational genomics: insufficient without trustworthy governance. J Law Med Ethics. 2018;46(1):79–86. doi: 10.1177/1073110518766023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheehan M, Friesen P, Balmer A, Cheeks C, Davidson S, Devereux J, et al. Trust, trustworthiness and sharing patient data for research. J Med Ethics. 2020:medethics-2019-106048. 10.1136/medethics-2019-106048. [DOI] [PubMed]
- 15.Deverka PA, Gilmore D, Richmond J, Smith Z, Mangrum R, Koenig BA, Cook-Deegan R, Villanueva AG, Majumder MA, McGuire AL. Hopeful and concerned: public input on building a trustworthy medical information commons. J Law Med Ethics. 2019;47(1):70–87. doi: 10.1177/1073110519840486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft SA, Cho MK, Gillespie K, Halley M, Varsava N, Ormond KE, Luft HS, Wilfond BS, Lee SSJ. Beyond consent: building trusting relationships with diverse populations in precision medicine research. Am J Bioeth. 2018;18(4):3–20. doi: 10.1080/15265161.2018.1431322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xafis V, Schaefer GO, Labude MK, Brassington I, Ballantyne A, Lim HY, Lipworth W, Lysaght T, Stewart C, Sun S, Laurie GT, Tai ES. An ethics framework for big data in health and research. ABR. 2019;11(3):227–254. doi: 10.1007/s41649-019-00099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicol D, Eckstein L, Bentzen HB, Borry P, Burgess M, Burke W, Chalmers D, Cho M, Dove E, Fullerton S, Ida R, Kato K, Kaye J, Koenig B, Manson S, McGrail K, Meslin E, O'Doherty K, Prainsack B, Shabani M, Tabor H, Thorogood A, de Vries J. Consent insufficient for data release. Science. 2019;364(6439):445–446. doi: 10.1126/science.aax0892. [DOI] [PubMed] [Google Scholar]
- 19.Dixon-Woods M, Ashcroft RE. Regulation and the social licence for medical research. Med Health Care Philos. 2008;11(4):381–391. doi: 10.1007/s11019-008-9152-0. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh Z, Hoeyer K. “That is why I have trust”: unpacking what ‘trust’ means to participants in international genetic research in Pakistan and Denmark. Med Health Care Philos. 2018;21(2):169–179. doi: 10.1007/s11019-017-9795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stark Z, Dolman L, Manolio TA, Ozenberger B, Hill SL, Caulfied MJ, Levy Y, Glazer D, Wilson J, Lawler M, Boughtwood T, Braithwaite J, Goodhand P, Birney E, North KN. Integrating genomics into healthcare: a global responsibility. Am J Hum Genet. 2019;104(1):13–20. doi: 10.1016/j.ajhg.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton A, Milne, R, Almarri M, Anwer S, Atutornu J, et al. Global public perceptions of genomic data sharing: what shapes the willingness to donate DNA and health data? Am J Hum Genet. 2020;107(4):743–52. 10.1016/j.ajhg.2020.08.023. [DOI] [PMC free article] [PubMed]
- 23.Middleton A, Your DNA. Your Say. New Bioeth. 2017;23(1):74–80. doi: 10.1080/20502877.2017.1314890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middleton A, Niemiec E, Prainsack B, Bobe J, Farley L, Steed C, Smith J, Bevan P, Bonhomme N, Kleiderman E, Thorogood A, Schickhardt C, Garattini C, Vears D, Littler K, Banner N, Scott E, Kovalevskaya NV, Levin E, Morley KI, Howard HC. ‘Your DNA, Your Say’: global survey gathering attitudes toward genomics: design, delivery and methods. Personalized Med. 2018;15(4):311–318. doi: 10.2217/pme-2018-0032. [DOI] [PubMed] [Google Scholar]
- 25.Hall P, Schimek MG. Moderate-deviation-based inference for random degeneration in paired rank lists. J Am Stat Assoc. 2012;107(498):661–672. doi: 10.1080/01621459.2012.682539. [DOI] [Google Scholar]
- 26.Schimek MG, Budinská E, Kugler KG, Švendová V, Ding J, Lin S. TopKLists: a comprehensive R package for statistical inference, stochastic aggregation, and visualization of multiple omics ranked lists. Stat Appl Genet Mol Biol. 2015;14(3):311–316. doi: 10.1515/sagmb-2014-0093. [DOI] [PubMed] [Google Scholar]
- 27.Barter RL, Yu B. Superheat: An R package for creating beautiful and extendable heatmaps for visualizing complex data. J Comput Graphical Stat. 2018;27(4):910–922. doi: 10.1080/10618600.2018.1473780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrd JB, Greene AC, Prasad DV, Jiang X, Greene CS. Responsible, practical genomic data sharing that accelerates research. Nat Rev Genet. 2020;(10):1–15. 10.1038/s41576-020-0257-5. [DOI] [PMC free article] [PubMed]
- 29.Passmore SR, Jamison AM, Hancock GR, Abdelwadoud M, Mullins CD, Rogers TB, Thomas SB. “I’m a Little More Trusting”: components of trustworthiness in the decision to participate in genomics research for African Americans. PHG. 2019;22(5-6):215–226. doi: 10.1159/000505271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staunton C, Slokenberga S, Mascalzoni D. The GDPR and the research exemption: considerations on the necessary safeguards for research biobanks. Eur J Hum Genet. 2019;27(8):1159–1167. doi: 10.1038/s41431-019-0386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ipsos MORI. The One-Way Mirror: Public Attitudes to Commercial Access to Health Data. London: The Wellcome Trust; 2016.
- 32.Molnár-Gábor F, Korbel JO. Genomic data sharing in Europe is stumbling—Could a code of conduct prevent its fall? EMBO Mol Med. 2020;12(3):e11421. doi: 10.15252/emmm.201911421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional tables and figures related to the Your DNA, Your Say sample and responses in pdf format.
Data Availability Statement
The full dataset is published at https://societyandethicsresearch.wellcomeconnectingscience.org/project/your-dna-your-say and available, without restriction, for anyone to access, download and analyse.