Abstract
Background
Institutional-level disparities in non-small cell lung cancer (NSCLC) survival may be driven by reversible differences in care-delivery processes. We quantified the impact of differences in readily identifiable quality metrics on long-term survival disparities in resected NSCLC.
Research Question
How do reversible differences in oncologic quality of care contribute to institutional-level disparities in early-stage NSCLC survival?
Study Design and Methods
We retrospectively analyzed patients in the National Cancer Data Base who underwent NSCLC resection from 2004 through 2015 within institutions categorized as Community, Comprehensive Community, Integrated Network, Academic, and National Cancer Institute (NCI)-Designated Cancer Programs. We estimated percentages and adjusted ORs for six potentially avoidable poor-quality markers: incomplete resection, nonexamination of lymph nodes, nonanatomic resection, non-evidence-based use of adjuvant chemotherapy, non-evidence-based use of adjuvant radiation therapy, and 60-day postoperative mortality. By sequentially eliminating patients with poor-quality markers and calculating adjusted hazard ratios, we quantified their overall survival impact.
Results
Of 169,775 patients, 7%, 46%, 10%, 24%, and 12% underwent surgery at Community, Comprehensive Community, Integrated Network, Academic, and NCI-Designated Cancer Programs, with 5-year overall survival rates of 52%, 56%, 58%, 60% and 66%, respectively. After the sequential elimination process, using NCI-Designated Cancer Centers as a reference, the adjusted hazard ratio for 5-year overall survival changed from 1.47 (95% CI, 1.41-1.53), 1.29 (95% CI, 1.25-1.33), 1.18 (95% CI, 1.14-1.23), and 1.20 (95% CI, 1.16-1.24) for Community, Comprehensive Community, Integrated Networks, and Academic Cancer Programs to 1.35 (95% CI, 1.28-1.42), 1.22 (95% CI, 1.17-1.26), 1.16 (95% CI, 1.11-1.22), and 1.17 (95% CI, 1.12-1.21), respectively (P < .001 for all comparisons with NCI-designated programs). Differences in quality of surgical resection and postoperative care accounted for 11% to 26% of the interinstitutional survival disparities.
Interpretation
Targeting six readily identified poor-quality markers narrowed, but did not eliminate, institutional survival disparities. The greatest impact was in community programs. Residual factors driving persistent institution-level long-term NSCLC survival disparities must be characterized to eliminate them.
Key Words: health-care disparities, lung cancer surgery, non-small cell lung cancer, quality improvement
Abbreviations: CoC, American College of Surgeons Commission on Cancer; HR, hazard ratio; NCI, National Cancer Institute; NSCLC, non-small cell lung cancer; OS, overall survival
FOR EDITORIAL COMMENT, SEE PAGE 1330
Aggregate lung cancer survival improved only slightly from 12% in the 1970s to 18% in 2017 in the United States.1 This relatively poor progress is attributable to multiple factors, including the predominance of patients with advanced disease,2 a paucity of organized screening and early detection programs,3,4 slow progress in managing patients with advanced disease,5 and significant racial, socioeconomic, and geographic disparities in access to high-quality care.6,7 The advent of low-dose CT screening,8, 9, 10 immunotherapy,11, 12, 13, 14 and molecularly targeted therapy15,16 promises to alleviate some of these factors. However, the care and outcome disparity problem continues unabated.6,17, 18, 19
Examination of institution-level outcome disparities in early-stage non-small cell lung cancer (NSCLC) has focused mainly on postoperative mortality: the risk of failing to survive the cancer operation.20, 21, 22 However, most lung cancer patients who undergo surgery survive the operation. The predominant danger they face is the risk of not surviving the cancer.23, 24, 25 In addition to differences in perioperative management and mortality risk, differences in the oncologic quality of care may play a role in the disparate outcomes between patients treated at different institutions. Examples of such differences in oncologic quality include the completeness of resection,26,27 thoroughness of lymph node examination,28, 29, 30 use of anatomic resection,31,32 and evidence-based use of adjuvant therapy for high-risk patients.33, 34, 35, 36, 37 In theory, eliminating institutional-level survival disparities requires dissemination of best practices across institutions and providers of various characteristics and eliminating disparities in the processes of care.38,39 To quantify the degree to which institutional differences in NSCLC survival are driven by differences in quality of care, we compared the survival improvement after sequentially eliminating prespecified survival-impactful metrics of poor-quality care.
Methods
Study Population
We analyzed the National Cancer Data Base, a hospital-based oncology outcomes database jointly sponsored by the American College of Surgeons and the American Cancer Society, which captures approximately 70% of newly diagnosed cancer cases in the United States from more than 1500 American College of Surgeons Commission on Cancer (CoC)-accredited facilities.40 This project was exempted from full review by the institutional review board of the Morehouse School of Medicine, Atlanta, GA.
We identified patients 18 to 90 years of age with a first diagnosis of American Joint Committee on Cancer (7th edition) clinical stages I to IIIA NSCLC. Patient selection was based on International Classification of Disease for Oncology, 3rd edition, site codes C34.0 through C34.9. Patients were diagnosed from 2004 through 2015 and underwent cancer-directed surgery in a CoC-accredited facility (e-Fig 1). We included patients with missing clinical stage who underwent curative-intent surgery for pathologic stages I to IV (M1a), on the assumption that most had a clinical stage of disease suggestive of resectability. We excluded patients with unknown dates of diagnosis, last contact, surgery, or adjuvant chemotherapy or radiation. We also excluded those with unknown census region of residence, those with unknown node examination status, and recipients of preoperative chemotherapy or radiation therapy. Finally, we excluded patients with government-sponsored insurance other than Medicaid or Medicare, more than one surgical procedure, or surgery more than 6 months from the date of diagnosis (e-Fig 1). The institutions where patients underwent surgery were categorized by the CoC as Community, Comprehensive Community, Integrated Network, Academic, and National Cancer Institute (NCI)-Designated Cancer Programs.40
Metrics of Poor Quality
Based on existing evidence, we evaluated the following seven adverse quality measures: postoperative mortality at 30, 60, 90, and 120 days41, 42, 43; resection with positive margins26,27; nonanatomic resection44; resection without lymph node examination (pNX)45,46; resection for stage I and II NSCLC with fewer than 10 examined lymph nodes47; and non-evidence-based use of adjuvant chemotherapy36,48 or radiation.33 We defined evidence-based adjuvant chemotherapy as use in patients with pathologic stages IB (T2N0M0 tumors of more than 4 cm), IIA, to IIIB,34, 35, 36,48 and we defined any use of adjuvant chemotherapy outside of these stage groups as non-evidence based. We defined evidence-based postoperative radiation therapy as use in patients with pathologic stage N2 or N3 disease only, and we defined any use outside of these pathologic nodal stage groups as non-evidence based.33,49,50 Given the inclusion of incomplete resection as an independent adverse quality measure, we limited the indications for adjuvant therapy to complete resections.
Examination of Institutional Differences
We first quantified crude and adjusted aggregate survival differences stratified by stage, tumor size, nodal status, and number of nodes examined among patients treated within different institutional categories using a multivariate model with patient-level factors. Then we calculated percentages and adjusted ORs of quality metrics in each CoC institutional category, using the NCI-Designated Programs as the reference group. Next, we tested each individual potential poor-quality metric (except postoperative mortality) in the entire population in univariate and multivariate fashion to ascertain their independent association with survival in the cohort. We then sequentially and hierarchically eliminated patients with specific poor-quality metrics in descending order of hazard ratio (HR) while comparing the adjusted survival of patients remaining in each institutional category. Finally, we eliminated patients who died within 60 days of surgery. Our objective was to compare the interinstitutional survival disparity in the residual cohort of patients.
To examine the impact of the thoroughness of pathologic nodal staging, we compared the survival of patients with different numbers of lymph nodes examined across institutional categories, stratified by pathologic nodal stage.47,51 For a snapshot of the thoroughness of pathologic staging, we compared clinical and pathologic tumor (T) and node (N) upstaging and downstaging rates within each CoC institutional category. Control variables included age at diagnosis, race or ethnicity, sex, year of diagnosis, insurance, geographic region of the facility, comorbidity score, and clinical stage.
We used descriptive analyses to summarize patient sociodemographic, clinical, and treatment characteristics by institutional category with comparisons using χ 2 tests, with a significance level of 0.05. Our primary outcome of interest was 5-year overall survival (OS). We used the Kaplan-Meier method to estimate unadjusted 5-year aggregate and stratified OS probabilities by stage, tumor size, nodal stage, and number of nodes examined for each institutional category and used log-rank tests to compare survival between institutional categories. We estimated 5-year aggregate OS probabilities for each institutional category after sequentially eliminating patients with poor-quality metrics. Follow-up time was from date of diagnosis until December 31, 2015, last contact date, or death (whichever occurred first). We examined survival using the 2004 through 2014 cohort, enabling at least 1 year of follow-up. In sensitivity analysis, we limited the cohort to 2004 through 2010, enabling a minimum of 5 years of follow-up. We conducted additional analysis limited to the cohort with clinical stage I to IIIA disease. Because results were similar, we report only on the larger cohort.
Univariate and multivariate Cox proportional hazards models, adjusting for patient sociodemographic characteristics and comorbidity, were conducted to estimate 5-year risk of all-cause mortality and were stratified by stage, tumor size, nodal stage, and number of nodes examined comparing patients treated at each institutional category with those treated at NCI-Designated programs. We also generated HRs to estimate 5-year risk of all-cause mortality for patients treated within each institutional category compared with those treated at NCI-Designated programs after sequentially and hierarchically eliminating patients with poor-quality variables. We used multivariable logistic regression analyses to predict the adjusted OR of each poor-quality metric for patients treated within each institutional category compared with those treated at NCI-Designated programs. Because results essentially were similar, we only report adjusted data. We used multivariate Cox proportional hazards models to estimate the HRs to determine the relative survival impact of each poor-quality metric. In sensitivity analyses, we used 90-day mortality as a quality measure and included area-level income and tumor grade in the multivariate model. All statistical analyses were performed using SAS version 9.4 software (SAS Institute).
Results
From 2004 through 2015, 169,775 eligible patients underwent resection for NSCLC: 46% at Comprehensive Community Cancer Programs, 24% at Academic Cancer Programs, 12% at NCI-Designated Programs, 10% at Integrated Network Programs, and 7% at Community Cancer Programs. Relevant patient sociodemographic, clinical, and treatment characteristics used for multivariate analyses are presented in e-Table 1.
Comparative Institution-Stratified Survival
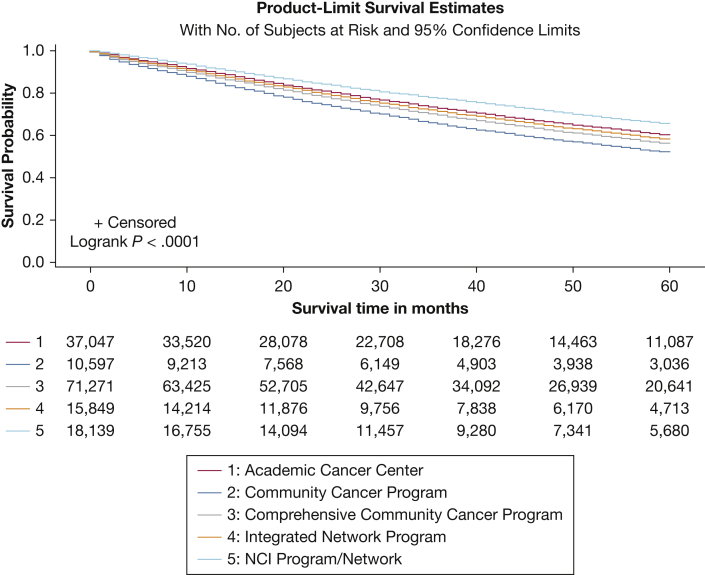
Significant differences were found in crude 5-year OS between patients treated at each institutional category in the general hierarchy, in order from best to worst: NCI-Designated, Academic, Integrated Network, Comprehensive Community, and Community Cancer Programs (Table 1, Fig 1). Adjusting for patient-level factors, treatment at Community, Comprehensive Community, Integrated Network, and Academic Programs was associated with a 47%, 29%, 18%, and 20% higher risk of death compared with treatment at NCI-Designated Programs (Table 2). Stratification according to tumor size, aggregate stage, and pathologic nodal status (Tables 1, 2) or number of lymph nodes examined (e-Table 2) yielded similar results.
Table 1.
Aggregate and Stage-Stratified Crude 5-Year Overall Survival Probabilities of Non-Small Cell Lung Cancer Patients Who Underwent Surgery According to Facility Type, Adjusted for Patient-Level Variables
| Variable | Community Cancer Program (n = 10,597) | Comprehensive Community Cancer Program (n = 71,271) | Integrated Network (n = 15,849) | Academic Cancer Program (n = 37,047) | NCI Program/Network (n = 18,139) |
|---|---|---|---|---|---|
| No. of deaths | 4,345 | 25,975 | 5,484 | 12,073 | 4,964 |
| 5-yr overall survival | 0.52 (0.51-0.53) | 0.56 (0.56-0.57) | 0.58 (0.57-0.59) | 0.60 (0.59-0.61) | 0.66 (0.65-0.66) |
| By tumor size, cm | |||||
| ≤ 3 (n = 97,143) | 0.57 (0.56-0.59) | 0.62 (0.61-0.63) | 0.64 (0.63-0.65) | 0.66 (0.65-0.67) | 0.73 (0.72-0.73) |
| > 3-5 (n = 36,238) | 0.47 (0.44-0.49) | 0.50 (0.49-0.51) | 0.51 (0.49-0.52) | 0.52 (0.51-0.53) | 0.57 (0.55-0.59) |
| > 5-7 (n = 11,802) | 0.39 (0.35-0.42) | 0.42 (0.41-0.44) | 0.48 (0.45-0.51) | 0.46 (0.44-0.48) | 0.49 (0.45-0.52) |
| > 7 (n = 7,171) | 0.31 (0.26-0.35) | 0.34 (0.32-0.36) | 0.32 (0.29-0.36) | 0.40 (0.38-0.43) | 0.42 (0.38-0.45) |
| By clinical stage | |||||
| I (n = 91,309) | 0.58 (0.57-0.59) | 0.62 (0.62-0.63) | 0.64 (0.63-0.65) | 0.67 (0.0.66-0.68) | 0.71 (0.70-0.72) |
| II (n = 15,296) | 0.36 (0.33-0.40) | 0.43 (0.41-0.44) | 0.46 (0.43-0.49) | 0.47 (0.45-0.49) | 0.52 (0.49-0.55) |
| IIIA (n= 6,634) | 0.28 (0.23-0.33) | 0.34 (0.32-0.36) | 0.34 (0.29-0.39) | 0.37 (0.34-0.40) | 0.42 (0.38-0.46) |
| Unknown (n = 39,664) | 0.49 (0.47-0.51) | 0.50 (0.50-0.51) | 0.53 (0.52-0.55) | 0.54 (0.53-0.55) | 0.62 (0.0.60-0.63) |
| By pathologic stage | |||||
| I (n = 102,674) | 0.60 (0.58-0.61) | 0.63 (0.63-0.64) | 0.65 (0.64-0.66) | 0.67 (0.67-0.68) | 0.73 (0.72-0.74) |
| II (n = 26,837) | 0.37 (0.35-0.40) | 0.42 (0.41-0.43) | 0.46 (0.44-0.48) | 0.47 (0.45-0.48) | 0.53 (0.51-0.55) |
| III (14,128) | 0.27 (0.24-0.31) | 0.32 (0.31-0.33) | 0.34 (0.31-0.37) | 0.34 (0.32-0.36) | 0.41 (0.38-0.44) |
| IV (n = 1,734) | 0.23 (0.16-0.31) | 0.23 (0.20-0.26) | 0.27 (0.20-0.34) | 0.29 (0.25-0.34) | 0.34 (0.27-0.40) |
| Unknown (n = 7,530) | 0.48 (0.44-0.52) | 0.54 (0.0.52-0.56) | 0.56 (0.51-0.60) | 0.57 (0.54-0.60) | 0.60 (0.56-0.64) |
| By pathologic nodal status | |||||
| pN0 (n = 115,337) | 0.59 (0.57-0.60) | 0.62 (0.62-0.63) | 0.64 (0.63-0.65) | 0.66 (0.65-0.67) | 0.71 (0.70-0.72) |
| pN1 (n = 19,405) | 0.32 (0.29-0.34) | 0.38 (0.37-0.39) | 0.41 (0.38-0.43) | 0.42 (0.0.41-0.44) | 0.50 (0.47-0.52) |
| pN2 (n = 10,915) | 0.26 (0.22-0.0.30) | 0.30 (0.29-0.32) | 0.32 (0.29-0.36) | 0.31 (0.29-0.33) | 0.39 (0.36-0.42) |
| pN3 (n = 69) | 0.15 (0.01-0.45) | 0.36 (0.16-0.56) | 0.17 (0.008-0.52) | 0.38 (0.15-0.61) | 0.32 (0.05-0.65) |
| pNX (n = 7,177) | 0.45 (0.41-0.49) | 0.49 (0.47-0.51) | 0.53 (0.49-0.58) | 0.53 (0.50-0.55) | 0.57 (0.52-0.62) |
Data are presented as No. or overall survival probability (95% CI). NCI = National Cancer Institute; pN0 = pathologic node-negative; pNX = resection without lymph node examination. P < .0001 for all survival comparisons.
Figure 1.
Line graph showing 5-year overall survival probabilities for patients who underwent primary surgical resection for non-small cell lung cancer in the National Cancer Data Base from 2004 through 2015, categorized by the Commission-on-Cancer institutional category where the surgery was performed. NCI = National Cancer Institute.
Table 2.
Adjusted Comparative 5-Year Aggregate and Stage-Stratified Survival Probabilities of Non-Small Cell Lung Cancer Patients Who Underwent Primary Surgical Resection, According to Institution Typea
| Variable | Community Cancer Program (n = 10,597) | Comprehensive Community Cancer Program (n = 71,271) | Integrated Network (n = 15,849) | Academic Cancer Program (n = 37,047) | NCI Program/Network (n = 18,139) |
|---|---|---|---|---|---|
| 5-year overall survival | 1.47 (1.41-1.53)b | 1.29 (1.25-1.33)b | 1.18 (1.14-1.23)b | 1.20 (1.16-1.24)b | 1 |
| By tumor size, cm | |||||
| ≤ 3 | 1.64 (1.55-1.73)b | 1.40 (1.34-1.46)b | 1.26 (1.19-1.33)b | 1.28 (1.22-1.34)b | 1 |
| 3-5 | 1.29 (1.20-1.40)b | 1.17 (1.11-1.24)b | 1.11 (1.04-1.20)c | 1.15 (1.09-1.23)b | 1 |
| > 5-7 | 1.35 (1.20-1.53)b | 1.20 (1.10-1.32)b | 1.02 (0.90-1.14) | 1.11 (1.00-1.22)d | 1 |
| > 7 | 1.44 (1.24-1.67)b | 1.21 (1.09-1.34)b | 1.27 (1.11-1.45)b | 1.07 (0.95-1.19) | 1 |
| By clinical stage | |||||
| I | 1.47 (1.39-1.56)b | 1.27 (1.21-1.32)b | 1.17 (1.11-1.24)b | 1.16 (1.11-1.21)b | 1 |
| II | 1.50 (1.35-1.68)b | 1.24 (1.14-1.35)b | 1.12 (1.01-1.25)d | 1.10 (1.00-1.20) | 1 |
| IIIA | 1.48 (1.28-1.72)b | 1.23 (1.10-1.38)b | 1.14 (0.98-1.33) | 1.18 (1.04-1.33)c | 1 |
| Unknown | 1.42 (1.31-1.53)b | 1.34 (1.26-1.42)b | 1.21 (1.13-1.30)b | 1.25 (1.18-1.33)b | 1 |
| By pathologic stage | |||||
| I | 1.54 (1.46-1.63)b | 1.33 (1.28-1.39)b | 1.22 (1.16-1.29)b | 1.24 (1.18-1.30)b | 1 |
| II | 1.46 (1.34-1.59)b | 1.28 (1.20-1.36)b | 1.14 (1.05-1.24)c | 1.15 (1.08-1.24)b | 1 |
| III | 1.38 (1.24-1.54)b | 1.23 (1.14-1.33)b | 1.11 (1.01-1.23) | 1.18 (1.09-1.28)d | 1 |
| IV | 1.46 (1.11-1.92)c | 1.54 (1.27-1.87)b | 1.24 (0.97-1.59) | 1.22 (0.99-1.50) | 1 |
| Unknown | 1.27 (1.07-1.51)c | 1.09 (0.95-1.25) | 1.00 (0.83-1.20) | 1.04 (0.90-1.21) | 1 |
| By pathologic nodal status | |||||
| pN0 | 1.48 (1.41-1.56)b | 1.29 (1.24-1.34)b | 1.19 (1.13-1.25)b | 1.21 (1.16-1.26)b | 1 |
| pN1 | 1.52 (1.38-1.67)b | 1.30 (1.21-1.40)b | 1.17 (1.07-1.28)b | 1.17 (1.08-1.26)b | 1 |
| pN2 | 1.41 (1.25-1.59)b | 1.26 (1.16-1.37)b | 1.16 (1.04-1.29)c | 1.23 (1.12-1.34)b | 1 |
| pNX | 1.33 (1.11-1.61)c | 1.17 (0.99-1.37) | 0.96 (0.79-1.18) | 1.09 (0.92-1.30) | 1 |
Data are presented as No. or overall survival probability (95% CI), unless otherwise indicated. NCI = National Cancer Institute; pN0 = pathologic node-negative; pNX = resection without lymph node examination.
Adjusted hazard ratios, accounting for age at diagnosis, race or ethnicity, sex, year of diagnosis, insurance, geographic region of the facility, and comorbidity score.
P < .001.
P < .01.
P < .05.
Impact of Poor-Quality Metrics
Evidence-based, survival-impactful NSCLC quality care metrics varied significantly among CoC categories. Postoperative mortality rates at 30, 60, 90, and 120 days differed significantly in sequential order, as follows: Community, Comprehensive Community, Integrated Network, Academic, and NCI-Designated Programs (Table 3). We selected the 60-day mortality rate as the preferred elimination interval because it enabled accounting for late mortality events directly related to surgery while being sufficiently early to minimize confounding by adjuvant therapy-related causes of mortality.
Table 3.
Percentage and Adjusted ORs Predicting the Odds of Potential Poor-Quality Metrics by Facility Typea
| Variable | Community Cancer Program (n = 10,597) | Comprehensive Community Cancer Program (n = 71,271) | Integrated Network Program (n = 15,849) | Academic Cancer Program (n = 37,047) | NCI Program (n = 18,139) |
|---|---|---|---|---|---|
| Postoperative mortality | |||||
| 30 d | 3.6 | 2.7 | 2.3 | 2.2 | 1.4 |
| OR (95% CI) | 2.36 (2.00-2.78) | 1.78 (1.55-2.03) | 1.46 (1.23-1.72) | 1.61 (1.40-1.87) | 1 |
| 60 d | 5.2 | 4.0 | 3.4 | 3.2 | 2.0 |
| OR (95% CI) | 2.35 (2.05-2.69) | 1.76 (1.57-1.97) | 1.47 (1.28-1.69) | 1.60 (1.42-1.80) | 1 |
| 90 d | 6.3 | 4.9 | 4.1 | 4.0 | 2.6 |
| OR (95% CI) | 2.24 (1.98-2.53) | 1.70 (1.54-1.88) | 1.39 (1.23-1.57) | 1.54 (1.38-1.71) | 1 |
| 120 d | 7.2 | 5.8 | 4.9 | 4.6 | 3.0 |
| OR (95% CI) | 2.18 (1.95-2.45) | 1.71 (1.56-1.88) | 1.41 (1.26-1.58) | 1.53 (1.38-1.69) | 1 |
| Positive margin | 6.12 | 5.15 | 4.72 | 4.44 | 3.57 |
| OR (95% CI) | 1.65 (1.48-1.85) | 1.41 (1.29-1.54) | 1.26 (1.13-1.41) | 1.23 (1.12-1.35) | 1 |
| Wedge resection | 14.00 | 12.13 | 10.87 | 13.19 | 12.53 |
| OR (95% CI) | 1.19 (1.10-1.27) | 1.01 (0.96-1.06) | 0.93 (0.87-0.99) | 1.07 (1.01-1.13) | 1 |
| pNX rate | 7.29 | 5.18 | 3.65 | 4.45 | 2.67 |
| OR (95% CI) | 2.96 (2.63-3.33) | 2.10 (1.90-2.32) | 1.53 (1.35-1.74) | 1.69 (1.52-1.87) | 1 |
| < 10 lymph nodes resectedb | 73.26 | 67.56 | 61.29 | 59.62 | 47.68 |
| OR (95% CI) | 2.97 (2.80-3.15) | 2.28 (2.19-2.36) | 1.67 (1.59-1.75) | 1.60 (1.53-1.66) | 1 |
| Non-evidence-based use of adjuvant radiation therapyc | 4.45 | 2.91 | 2.65 | 2.58 | 1.77 |
| OR (95% CI) | 2.49 (2.13-2.92) | 1.70 (1.49-1.93) | 1.49 (1.27-1.75) | 1.42 (1.24-1.63) | 1 |
| Non-evidence-based use of adjuvant chemotherapyd | 9.80 | 8.33 | 8.21 | 7.64 | 6.99 |
| OR (95% CI) | 1.44 (1.30-1.61) | 1.30 (1.20-1.41) | 1.22 (1.10-1.34) | 1.08 (0.99-1.18) | 1 |
Data are presented as percentage unless otherwise indicated. NCI = National Cancer Institute. P < .0001 for all comparisons.
Adjusted for age at diagnosis, race or ethnicity, sex, year of diagnosis, insurance, geographic region of the facility, comorbidity score, and clinical stage.
Among patients with pathologic stage I-II, including patients with 0 lymph nodes, excluding patients with unknown number of nodes.
Defined as use in patients with pathologic N0 and N1 and negative resection margins.
Defined as use in patients with pathologic stage I with tumor size ≤ 4 cm and negative resection margins.
Resections with positive margins showed a 5-year OS of 32%, compared with 59% for those with negative margins, and showed an adjusted HR (aHR) of 2.03 (95% CI, 1.96-2.09; P < .0001). This was the most survival-impact quality metric (Table 4). The ORs for resection with positive margins were 1.65 (95% CI, 1.48-1.85), 1.41 (95% CI, 1.29-1.54), 1.26 (95% CI, 1.13-1.41), and 1.23 (95% CI, 1.12-1.35) in the above-noted institutional order (Table 3). Wedge resections were associated with a 5-year survival of 53%, compared with anatomic resections, which had a 5-year survival of 59% (Table 4). Wedge resection was associated with an aHR of 1.20 (95% CI, 1.17-1.23; P < .0001). The ORs for use of nonanatomic resection were 1.19 (95% CI, 1.10-1.27), 1.01 (95% CI, 0.96-1.06), 0.93 (95% CI, 0.87-0.99), and 1.07 (95% CI, 1.01-1.13), respectively (Table 3).
Table 4.
Survival Probabilities With Adjusted Hazard Ratios in Those Who Had Potential Poor-Quality Metricsa
| Variable | 5-Year OS | Unadjusted HRb | Adjusted HRb |
|---|---|---|---|
| Positive margin (n = 152,903) | 0.32 (0.31-0.33) vs 0.59 (0.59-0.60) | 2.38 (2.31-2.46) | 2.03 (1.96-2.09) |
| Wedge resection (n = 152,903) | 0.53 (0.52-0.53) vs 0.59 (0.58-0.59) | 1.17 (1.14-1.20) | 1.20 (1.17-1.23) |
| pNX (n = 122,514) | 0.50 (0.49-0.52) vs 0.64 (0.64-0.64), pN0 | 1.54 (1.48-1.60) | 1.48 (1.43-1.54) |
| < 10-lymph node resection among pathologic stage I-IIc (n = 120,693) | 0.61 (0.60-0.61) vs 0.63 (0.62-0.63) | 1.06 (1.04-1.09) | 1.07 (1.05-1.09) |
| Non-evidence-based use of adjuvant radiation therapyd (n = 129,412) | 0.41 (0.39-0.42) vs 0.62 (0.62-0.63) | 1.97 (1.88-2.06) | 1.70 (1.62-1.78) |
| Non-evidence-based use of adjuvant chemotherapye (n = 101,561) | 0.64 (0.63-0.66) vs 0.66 (0.65-0.66) | 1.05 (1.01-1.09)f | 1.11 (1.07-1.16) |
Data are presented as OS (95% CI) or HR (95% CI). HR = hazard ratio; OS = overall survival; pN0 = pathologic node-negative; pNX = resection without lymph node examination.
Hazard ratios adjusted for age at diagnosis, race or ethnicity, sex, year of diagnosis, insurance, geographic region of the facility, comorbidity score, and clinical stage.
P < .0001 unless otherwise stated.
Among patients with pathologic stage I-II, including patients with 0 lymph nodes, excluding patients with unknown number of nodes.
Patients with pathologic N0 and N1 and negative resection margins.
Patients with pathological stage I with tumor size ≤ 4 cm and negative resection margins.
P = .0238.
Patients who underwent resection without lymph node examination showed a 5-year OS of 50%, compared with 64% for patients with pathologic node-negative, with an aHR of 1.48 (95% CI, 1.43-1.54; P < .0001). The ORs for resection without lymph node examination by institutional category were 2.96 (95% CI, 2.63-3.33), 2.10 (95% CI, 1.90-2.32), 1.53 (95% CI, 1.35-1.74), and 1.69 (95% CI, 1.52-1.87), in the above-mentioned order. For patients with pathologic stage I and II disease, examination of fewer than 10 lymph nodes was associated with a 5-year survival of 61%, compared with 63% for those with 10 or more nodes examined, with an aHR of 1.07 (95% CI, 1.05-1.09; P < .0001). The proportion of patients with examination of fewer than 10 lymph nodes ranged from 73% at Community to 48% at NCI-Designated Programs (Tables 3, 4).
In patients with complete resection, the non-evidence-based use of adjuvant radiation was associated with a 5-year OS of 41%, compared with 62% in patients who did not have such inappropriate use of radiation therapy, with an aHR of 1.70 (95% CI, 1.62-1.78; P < .0001) (Table 4). This practice varied significantly among institutions, with an OR of 2.49 (95% CI, 2.13-2.92), 1.70 (95% CI, 1.49-1.93), 1.49 (95% CI, 1.27-1.75), and 1.42 (95% CI, 1.24-1.63) in the same sequential institutional order (Table 3). The non-evidence-based use of adjuvant chemotherapy was associated with a 5-year OS of 64% vs 66% for nonuse, with an aHR of 1.11 (95% CI, 1.07-1.16; P < .0001). The ORs for non-evidence-based use of adjuvant chemotherapy were 1.44 (95% CI, 1.30-1.61), 1.30 (95% CI, 1.20-1.41), 1.22 (95% CI, 1.10-1.34), and 1.08 (95% CI, 0.99-1.18) (Tables 3, 4).
In descending order of magnitude of the aHR, the sequential elimination hierarchy was: resections with positive margins, non-evidence-based use of radiation therapy, nonexamination of lymph nodes, nonanatomic resection, and non-evidence-based use of adjuvant chemotherapy. Finally, we eliminated deaths within 60 days. Although we determined the magnitude of its contribution, we excluded examination of 10 or more lymph nodes from the sequential elimination model because it is measured less reliably and the CoC has dropped it as a quality benchmark. The sequential elimination process improved survival statistics with each step in all institutional categories, except for the non-evidence-based use of adjuvant chemotherapy, which was associated with a numerically lower survival in the residual population in all institutional categories (Table 5). Survival improved from 52% to 58% (absolute, 6%) in Community, 56% to 61% (absolute, 5%) in Comprehensive Community, 58% to 62% (absolute, 4%) in Integrated Networks, 60% to 64% (absolute, 4%) in Academic, and 66 to 69% (absolute, 3%) in NCI Programs. Eliminating the combination of 6 of the 7 metrics was associated with improved aggregate 5-year OS to the baseline level of the next one or two institutional categories, except for Academic Cancer Centers.
Table 5.
Comparative Impact of Potential Poor-Quality Metrics on Survival Outcome After Sequential Elimination by Facility Type
| Variable | Community Cancer Program (n = 10,597) | Comprehensive Community Cancer Program (n = 71,271) | Integrated Network Program (n = 15,849) | Academic Cancer Program (n = 37,047) | NCI Program/Network (n = 18,139) |
|---|---|---|---|---|---|
| No. of deaths | 4,345 | 25,975 | 5,484 | 12,073 | 4,964 |
| Survival rates | |||||
| Overall 5-yr survival | 0.5190 (0.5081-0.5297) | 0.5608 (0.5565-0.5650) | 0.5797 (0.5707-0.5886) | 0.5994 (0.5936-0.6052) | 0.6552 (0.6470-0.6633) |
| Sequential elimination | |||||
| Margin positive patients (n = 145,544) | 0.5345 (0.5233-0.5455) | 0.5749 (0.5706-0.5792) | 0.5918 (0.5826-0.6008) | 0.6114 (0.6055-0.6174) | 0.6647 (0.6564-0.6729) |
| Non-evidence-based radiation therapy (n = 141,962) | 0.5438 (0.5324-0.5551) | 0.5799 (0.5755-0.5842) | 0.5958 (0.5865-0.6050) | 0.6156 (0.6096-0.6216) | 0.6682 (0.6598-0.6765) |
| pNX (n = 135,531)a | 0.5484 (0.5366-0.5601) | 0.5832 (0.5787-0.5877) | 0.5970 (0.5875-0.6063) | 0.6186 (0.6124-0.6247) | 0.6702 (0.6617-0.6786) |
| Non-anatomic resection (n = 123,623) | 0.5537 (0.5413-0.5658) | 0.5867 (0.5820-0.5913) | 0.5987 (0.58889-0.6084) | 0.6206 (0.6141-0.6269) | 0.6745 (0.6656-0.6832) |
| Non-evidence-based chemotherapy (n = 115,994)b | 0.5452 (0.5323-0.5580) | 0.5828 (0.5779-0.5876) | 0.5947 (0.5844-0.6047) | 0.6167 (0.6100-0.6233) | 0.6728 (0.6636-0.6819) |
| Death within 60 d of surgery (n = 111,571) | 0.5781 (0.5647-0.5912) | 0.6090 (0.6040-0.6139) | 0.6169 (0.6066-0.6271) | 0.6386 (0.6318-0.6453) | 0.6870 (0.6776-0.6961) |
| Adjusted HR (95% CI)c | |||||
| Overall 5-yr HRc | 1.47 (1.41-1.53) | 1.29 (1.25-1.33) | 1.18 (1.14-1.23) | 1.20 (1.16-1.24) | 1 |
| Sequential eliminationc | |||||
| Margin positive patientsc | 1.45 (1.39-1.51) | 1.27 (1.23-1.31) | 1.17 (1.13-1.22) | 1.19 (1.15-1.24) | 1 |
| Non-evidence-based radiation therapya,c | 1.42 (1.36-1.49) | 1.26 (1.22-1.30) | 1.17 (1.12-1.22) | 1.19 (1.15-1.23) | 1 |
| pNXc | 1.42 (1.36-1.48) | 1.26 (1.22-1.30) | 1.18 (1.13-1.23) | 1.19 (1.15-1.24) | 1 |
| Nonanatomic resectionc | 1.42 (1.35-1.48) | 1.26 (1.22-1.31) | 1.18 (1.13-1.24) | 1.20 (1.15-1.24) | 1 |
| Non-evidence-based chemotherapyb,c | 1.44 (1.37-1.51) | 1.26 (1.22-1.31) | 1.19 (1.13-1.24) | 1.20 (1.16-1.25) | 1 |
| Death within 60 d of surgeryc | 1.35 (1.28-1.42) | 1.22 (1.17-1.26) | 1.16 (1.11-1.22) | 1.17 (1.12-1.21) | 1 |
HR = hazard ratio; NCI = National Cancer Institute; OS = overall survival; pNX = resection without lymph node examination.
aDefined as use in patients with pathologic N0 and N1 and negative resection margins.
bDefined as use in patients with pathologic stage I with tumor size ≤ 4 cm and negative resection margins; P < .001 for all interinstitutional comparisons.
cHazard ratios adjusted for age at diagnosis, race or ethnicity, sex, year of diagnosis, insurance, geographic region of the facility, comorbidity score, and clinical stage.
With reference to NCI Programs, after the sequential elimination process, the difference in overall 5-year aHR narrowed, but was not eliminated: from 1.47 to 1.35 in Community, 1.29 to 1.22 in Comprehensive Community, 1.18 to 1.16 in Integrated Networks, 1.20 to 1.17 in Academic Programs (Table 5). That is, markers of poor-quality care considered in this analysis explained 11% to 26% of the mortality disparity between patients treated in NCI-Designated programs and other categories of CoC-accredited facilities. The pattern of survival differences remained the same when analysis was restricted to the cohort from 2004 through 2010 (e-Table 3). The proportion of patients eliminated for quality deficit ranged from 32% in Community to 23% in NCI-Designated Programs (e-Table 4). The proportion of patients with combinations of poor-quality metrics was similarly hierarchical, being highest in Community and lowest in NCI Programs. Sensitivity analysis including patient income and tumor grade in the multivariate model, and 90-day postoperative mortality in the sequential elimination model did not change the results significantly (e-Table 5).
Stratifying the cohorts of patients within each institutional category by the number of lymph nodes examined, as a surrogate for the thoroughness (and, presumably, accuracy), of lymph node examination did not disrupt the hierarchy of survival disparity within homogenous groups of patients (e-Table 2). However, the thoroughness of pathologic staging, as indicated by shifts between clinical and pathologic stage, also generally followed the same directional hierarchy, with T-category changes even more frequent than N-category changes. Stage shifts were most common in NCI-Designated programs and least common in Community Cancer Programs (e-Table 6).
Discussion
We found a remarkable directional consistency in the pattern of differences in metrics of poor-quality surgical and postsurgical care of patients with potentially curable NSCLC across CoC institutional categories, even after adjusting for differences in patient characteristics. The differences with the greatest impact on survival were those in rates of incomplete resection, non-evidence-based use of adjuvant radiation therapy, nonexamination of lymph nodes, and postoperative mortality. Also striking and directional differences were found in the thoroughness of nodal examination, exemplified by the rate of examination of at least 10 lymph nodes in patients with stage I or II resections, and the pathologic nodal upstaging rate.
Eliminating patients with six of the seven poor-quality metrics had the general effect of sequentially moving each group of institutions approximately to the baseline level of the next one or two categories, suggesting the value of quality improvement interventions designed to target them. The impact was greatest in Community Programs, which leapt up two categories, and was least in Academic Cancer Centers, which failed to reach the baseline level of NCI-Designated programs. Hypothetically equalizing quality of care by eliminating these six poor-quality metrics eliminated 11% (Integrated Network Programs) to 25% (Community Programs) of the survival disparity between NCI-Designated institutions and others, which would be a major population-level achievement. However, this means that 75% to 89% of the interinstitutional survival disparities remained. This large residual interinstitutional survival disparity suggests the existence of significant residual drivers of survival differences beyond those included in our model. Such unmeasured drivers of residual outcome differences may include factors such as the selection of patients for surgery, evaluation and preparation for surgery, the rates of appropriate (as opposed to inappropriate) use of adjuvant therapy, the quality of team interaction, and exposure to clinical trials.52 Nevertheless, we uncovered several potential directions on which to focus quality improvement efforts. Eliminating incomplete resections, nonexamination of lymph nodes, and the inappropriate use of adjuvant radiation for stage I and II patients would benefit most institutions.
Better understanding of events and processes within high-performing and low-performing institutions is necessary if we are to raise quality and to improve population-level outcomes. Outcome differences linked to structural institutional differences are less amenable to corrective intervention because institutional structure change is relatively difficult. For example, 85% of patients undergo lung cancer surgery at CoC-accredited and non-CoC-accredited community-level institutions in the United States.53 Eliminating care at such institutions is fraught with political, ethical, and logistical challenges; will reduce access to care; and may impair outcomes in tertiary care institutions by overburdening them. In the absence of the full understanding of the core drivers of good outcomes in high-functioning institutions, regionalizing care may fail to improve survival.54 High volume does not guarantee high-quality practice; for example, unexpectedly high rates of incomplete resections occur in some high-volume institutions.27
Although most amenable to measurement and corrective intervention, care-delivery process changes are only useful to the degree that they promote desired outcomes. As soon as a strong link is established between processes and desired outcomes, closer examination of the provider-level and organizational-level factors driving process differences is necessary to deepen understanding of the relationships among structure, processes, and outcomes. Discovery of the key processes that impact survival and linkage with their human and organizational drivers is necessary before sustainable knowledge transfer from high-functioning to low-functioning institutions is possible. Achieving this goal will require mixed-methods studies comparing high-performing and low-performing institutions. Structural categorization alone is insufficient because performance within each structural category is neither uniform nor guaranteed.27
Study Limitations
First, although large, the National Cancer Data Base is not a population-based dataset. However, demographic and clinical characteristics of patients and treatment patterns between the National Cancer Data Base and the population-based Surveillance, Epidemiology and End-Results dataset are remarkably similar.55 Second, our retrospective cohort approach precludes causal inference. Third, because we included patients with unspecified clinical stage who underwent resection, our inclusion criteria may have been too broad. We used these broad criteria to reflect the true nature of preoperative decision-making and expanded to include pathologic N3 and M1a disease, on the assumption that these probably were detected during surgery. However, analysis limited to clinical stage I through IIIA provided similar results. Fourth, rather than attempting to discover the full range of quality markers impacting survival, we used prespecified evidence-based quality metrics. Furthermore, we included 60-day mortality in the model designed primarily to evaluate disparity in long-term survival. We justify this because failure to survive the operation eliminates the opportunity for long-term survival. Disparity in short-term postoperative survival also needs corrective intervention. Fifth, institutional differences in patient risk mix may have overwhelmed our statistical adjustments for them. Finally, we have not included institutional case volume in our multivariate models because volume differences are embedded within the CoC structural categorization, and the volume-to-outcome relationship has been cast in doubt by recent efforts to regionalize cancer care.54,56, 57, 58
Interpretation
These limitations notwithstanding, institutional variation in long-term survival among patients undergoing curative-intent surgery for lung cancer is an understudied problem that needs greater attention. Most care is delivered at nonacademic institutions with poorer outcomes, raising uncomfortable questions about the real-world application of clinical trial evidence, because outcomes at the institutions from which patients are recruited differ significantly from institutions where the results from such trials most often are applied. We show striking directional differences in quality of surgical resection and postoperative care for NSCLC among institutions of different structural characteristics, adjustment for which explained up to 26% of interinstitutional long-term NSCLC survival disparities. Further characterization of human and organizational factors driving performance differences between high-achieving and low-achieving institutions is needed to eliminate persistent disparities in long-term outcomes of surgical NSCLC care.
Acknowledgments
Author contributions: R. U. O. is the guarantor of this body of work and takes responsibility for all content presented including data and analysis. H. M. S. had full access to the National Cancer Data Base data and takes responsibility for the integrity of the data used for this study. R. U. O., H. M. S., and A. J. had full access to the data used for this study and take responsibility for the integrity and accuracy of the data analysis. R. U. O., H. M. S., C. C. L., and A. J. contributed substantially to the study design, interpretation of the data analysis, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. U. O. discloses stock ownership in Eli Lilly, Gilead Sciences, and Pfizer; consulting or advisory roles with the American Cancer Society, the Association of Community Cancer Centers, AstraZeneca, Eli Lilly, Genentech/Roche, and Triptych Health Partners; patents for a lung cancer specimen kit; and is the founder Oncobox Device, Inc. H. M. S. and C. C. L. (at the time of the study) and A. J. are employed by the American Cancer Society, which receives grants from private and corporate foundations, including foundations associated with companies in the health sector for research outside of the submitted work. The authors’ salary, however, is solely funded through American Cancer Society funds.
Role of sponsors: None of the sponsors had any impact on the analysis, interpretation or preparation of this manuscript.
Other contributions: The data used in the study are derived from a limited data set of the National Cancer Data Base. The authors acknowledge the efforts of the American College of Surgeons, the Commission on Cancer, and the American Cancer Society in the creation of the National Cancer Data Base. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the authors. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its board of governors, or its methodology committee.
Additional information: The e-Tables and e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported in part by a Patient-Centered Outcomes Research Institute award [Grant IH-1304-6147] and the National Institutes of Health [Grant R01CA172253 to R. U. O.]. H. M. S. and A. J. were supported intramurally by the American Cancer Society.
Supplementary Data
References
- 1.Mokdad A.H., Dwyer-Lindgren L., Fitzmaurice C. Trends and patterns of disparities in cancer mortality among US counties, 1980-2014. JAMA. 2017;317:388–406. doi: 10.1001/jama.2016.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Mulshine J.L., Sullivan D.C. Clinical practice. Lung cancer screening. N Engl J Med. 2005;352:2714–2720. doi: 10.1056/NEJMcp042630. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3:1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiller J.H., Harrington D., Belani C.P. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Virgo K.S., Little A.G., Fedewa S.A., Chen A.Y., Flanders W.D., Ward E.M. Safety-net burden hospitals and likelihood of curative-intent surgery for non-small cell lung cancer. J Am Coll Surg. 2011;213(5):633–643. doi: 10.1016/j.jamcollsurg.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Sineshaw H.M., Sahar L., Osarogiagbon R.U., Flanders W.D., Yabroff K.R., Jemal A. County-level variations in receipt of surgery for early-stage non-small cell lung cancer in the United States. Chest. 2020;157(1):212–222. doi: 10.1016/j.chest.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Early Lung Cancer Action Program Investigators. Henschke C.I., Yankelevitz D.F. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763-1771. Erratum in: N Engl J Med. 2008;358:1875. N Engl J Med. 2008;358:1862. N Engl J Med. 2008;359:877. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 9.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Koning H.J., van der Aalst C.M., de Jong P.A. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garon E.B., Rizvi N.A., Hui R. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 13.Rittmeyer A., Barlesi F., Waterkamp D. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonia S.J., Villegas A., Daniel D. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 15.Kris M.G., Johnson B.E., Berry L.D. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J.J., Cardarella S., Lydon C.A. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol. 2016;11:556–565. doi: 10.1016/j.jtho.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lathan C.S., Neville B.A., Earle C.C. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 18.Atkins G.T., Kim T., Munson J. Residence in rural areas of the United States and lung cancer mortality. Ann Am Thorac Soc. 2017;14:403–411. doi: 10.1513/AnnalsATS.201606-469OC. [DOI] [PubMed] [Google Scholar]
- 19.Ray M.A., Faris N.R., Derrick A., Smeltzer M.P., Osarogiagbon R.U. Rurality, stage-stratified use of treatment modalities, and survival of non-small cell lung cancer. Chest. 2020;158:787–796. doi: 10.1016/j.chest.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bach P.B., Cramer L.D., Schrag D., Downey R.J., Gelfand S.E., Begg C.B. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 21.Birkmeyer J.D., Siewers A.E., Finlayson E.V. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 22.Birkmeyer J.D., Stukel T.A., Siewers A.E., Goodney P.P., Wennberg D.E., Lucas F.L. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 23.Kelsey C.R., Marks L.B., Hollis D. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer. 2009;115:5218–5227. doi: 10.1002/cncr.24625. [DOI] [PubMed] [Google Scholar]
- 24.Bilimoria K.Y., Bentrem D.J., Feinglass J.M. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. 2008;26:4626–4633. doi: 10.1200/JCO.2007.15.6356. [DOI] [PubMed] [Google Scholar]
- 25.Osarogiagbon R.U., D’Amico T.A. Improving lung cancer outcomes by improving the quality of surgical care. Transl Lung Cancer Res. 2015;4:424–431. doi: 10.3978/j.issn.2218-6751.2015.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osarogiagbon R.U., Lin C.C., Smeltzer M.P., Jemal A. Prevalence, prognostic implications, and survival modulators of incompletely resected non-small cell lung cancer in the U.S. National Cancer Data Base. J Thorac Oncol. 2016;11:e5–e16. doi: 10.1016/j.jtho.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin C.C., Smeltzer M.P., Jemal A., Osarogiagbon R.U. Risk-adjusted margin positivity rate as a surgical quality metric for non-small cell lung cancer. Ann Thorac Surg. 2017;104:1161–1170. doi: 10.1016/j.athoracsur.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osarogiagbon R.U., Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol. 2012;7:1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 29.Osarogiagbon R.U., Decker P.A., Ballman K., Wigle D., Allen M.S., Darling G.E. Survival implications of variation in the thoroughness of pathologic lymph node examination in American College of Surgeons Oncology Group Z0030 (Alliance) Ann Thorac Surg. 2016;102:363–369. doi: 10.1016/j.athoracsur.2016.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osarogiagbon R.U., Lee Y.S., Faris N.R., Ray M.A., Ojeabulu P.O., Smeltzer M.P. Invasive mediastinal staging for resected non-small cell lung cancer in a population-based cohort. J Thorac Cardiovasc Surg. 2019;158(4):1220–1229. doi: 10.1016/j.jtcvs.2019.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rami-Porta R., Tsuboi M. Sublobar resection for lung cancer. Eur Respir J. 2009;33(2):426–435. doi: 10.1183/09031936.00099808. [DOI] [PubMed] [Google Scholar]
- 32.Kent M., Landreneau R., Mandrekar S. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. Ann Thorac Surg. 2013;96:1747–1754. doi: 10.1016/j.athoracsur.2013.05.104. discussion 1754-1755. [DOI] [PubMed] [Google Scholar]
- 33.Burdett S., Rydzewska L., Tierney J. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2016;10:CD002142. doi: 10.1002/14651858.CD002142.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arriagada R., Bergman B., Dunant A. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 35.Winton T., Livingston R., Johnson D. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 36.Pignon J.P., Tribodet H., Scagliotti G.V. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 37.Rajaram R., Paruch J.L., Mohanty S. Patterns and predictors of chemotherapy use for resected non-small cell lung cancer. Ann Thorac Surg. 2016;101:533–540. doi: 10.1016/j.athoracsur.2015.08.077. [DOI] [PubMed] [Google Scholar]
- 38.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 39.Birkmeyer J.D., Dimick J.B., Birkmeyer N.J. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg. 2004;198:626–632. doi: 10.1016/j.jamcollsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 40.American College of Surgeons National Cancer Database. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/ncdb
- 41.Bryant A.S., Rudemiller K., Cerfolio R.J. The 30- versus 90-day operative mortality after pulmonary resection. Ann Thorac Surg. 2010;89:1717–1722. doi: 10.1016/j.athoracsur.2010.01.069. discussion 1722-1723. [DOI] [PubMed] [Google Scholar]
- 42.Pezzi C.M., Mallin K., Mendez A.S., Greer Gay E., Putnam J.B., Jr. Ninety-day mortality after resection for lung cancer is nearly double 30-day mortality. J Thorac Cardiovasc Surg. 2014;148:2269–2277. doi: 10.1016/j.jtcvs.2014.07.077. [DOI] [PubMed] [Google Scholar]
- 43.D’Amico T.A. Defining and improving postoperative care. J Thorac Cardiovasc Surg. 2014;148(5):1792–1793. doi: 10.1016/j.jtcvs.2014.09.095. [DOI] [PubMed] [Google Scholar]
- 44.Donington J.S. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: analysis from the National Cancer Database. J Thorac Oncol. 2015;10:1513–1514. doi: 10.1097/JTO.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 45.Osarogiagbon R.U., Allen J.W., Farooq A., Berry A., Spencer D., O’Brien T. Outcome of surgical resection for pathologic N0 and Nx non-small cell lung cancer. J Thorac Oncol. 2010;5:191–196. doi: 10.1097/JTO.0b013e3181c8cc32. [DOI] [PubMed] [Google Scholar]
- 46.Osarogiagbon R.U., Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96:1178–1189. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Ludwig M.S., Goodman M., Miller D.L., Johnstone P.A. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128(3):1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 48.Kris M.G., Gaspar L.E., Chaft J.E. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline update. J Clin Oncol. 2017;35:2960–2974. doi: 10.1200/JCO.2017.72.4401. [DOI] [PubMed] [Google Scholar]
- 49.Lally B.E., Zelterman D., Colasanto J.M., Haffty B.G., Detterbeck F.C., Wilson L.D. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 50.Smeltzer M.P., Lin C.C., Kong F.S., Jemal A., Osarogiagbon R.U. Survival impact of postoperative therapy modalities according to margin status in non-small cell lung cancer patients in the United States. J Thorac Cardiovasc Surg. 2017;154:661–672. doi: 10.1016/j.jtcvs.2017.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osarogiagbon R.U., Ogbata O., Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg. 2014;97:385–393. doi: 10.1016/j.athoracsur.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unger J.M., Moseley A., Symington B. Geographic distribution and survival outcomes of rural patients with cancer treated in clinical trials. JAMA Network Open. 2018;1(4) doi: 10.1001/jamanetworkopen.2018.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bilimoria K.Y., Bentrem D.J., Stewart A.K., Winchester D.P., Ko C.Y. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–4181. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
- 54.Bendzsak A.M., Baxter N.N., Darling G.E., Austin P.C., Urbach D.R. Regionalization and outcomes of lung cancer surgery in Ontario, Canada. J Clin Oncol. 2017;35(24):2772–2780. doi: 10.1200/JCO.2016.69.8076. [DOI] [PubMed] [Google Scholar]
- 55.Lerro C.C., Robbins A.S., Phillips J.L., Stewart A.K. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759–1765. doi: 10.1245/s10434-013-2901-1. [DOI] [PubMed] [Google Scholar]
- 56.Gupta V., Bubis L., Kidane B. Readmission rates following esophageal cancer resection are similar at regionalized and non-regionalized centers: a population-based cohort study. J Thorac Cardiovasc Surg. 2019;158(3):934–942. doi: 10.1016/j.jtcvs.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 57.Farjah F., Grau-Sepulveda M.V., Gaissert H. The volume pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38(30):3518–3527. doi: 10.1200/JCO.20.00329. [DOI] [PubMed] [Google Scholar]
- 58.Osarogiagbon R.U. Volume-based care regionalization: pitfalls and challenges. J Clin Oncol. 2020;38(30):3465–3467. doi: 10.1200/JCO.20.02269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.