Abstract
High-affinity guests have been reported for the macrocyclic host cucurbit[7]uril (CB[7]), enabling widespread applications, but hindering CB[7] materials from being returned to their guest-free state for reuse. Here, we present polyhedral boron clusters (carboranes) as strongly-binding, yet easily removable, guests for CB[7]. Aided by a Pd-catalyzed coupling of an azide anion, we prepared boron-functionalized 9-amino-ortho-carborane that binds to CB[7] with a Ka≈1010 M−1. Upon basic treatment, ortho-carborane readily undergos deboronation to yield anionic nido-carborane, a poor guest for CB[7], facilitating recovery of guest-free CB[7]. We showcase the utility of the modified ortho-carborane guest by recycling a CB[7]-functionalized resin. With this report, we introduce stimuli-responsive decomplexation as an additional consideration in the design of high-affinity host-guest complexes.
Graphical Abstract
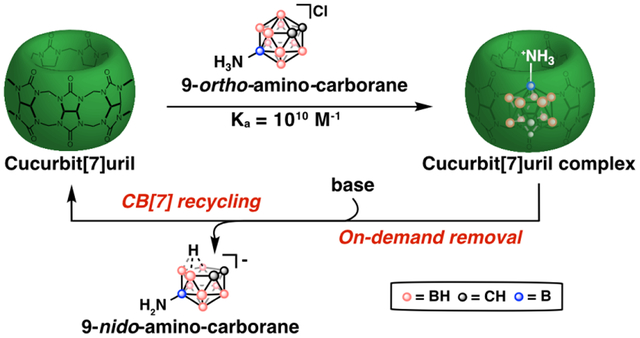
Molecular recognition is ubiquitous in nature with the biological processes relying on non-covalent interactions between biomolecules.1,2 Chemists have been inspired to develop host-guest complexes with comparable degrees of specificity for applications in functional materials,3 sensors,4 biological assays,5 and therapeutics.6–9 Early molecular recognition work involved crown ether, cyclophane, and cyclodextrin hosts that exhibit modest binding affinities (Ka≤105 M−1).10 Recently, focus has been on the development of high-affinity (Ka≥109 M−1) host-guest complexes that rival the binding affinities of Nature’s best molecular recognition systems: antibody-antigens and (strept)avidin-biotin.11 High binding affinity complexes facilitate the expansion of host-guest chemistry to applications in dilute, complex settings and decrease the need to exploit multivalency.12 However, large Ka values also lead to difficulties in readily dissociating the pair, limiting the reversibility and flexibility of the system (Figure 1A).13 Here we present “decomplexation”, the ability to remove a guest on demand, as an important feature in the design of high-affinity host-guest pairs (Figure 1B).
Figure 1.
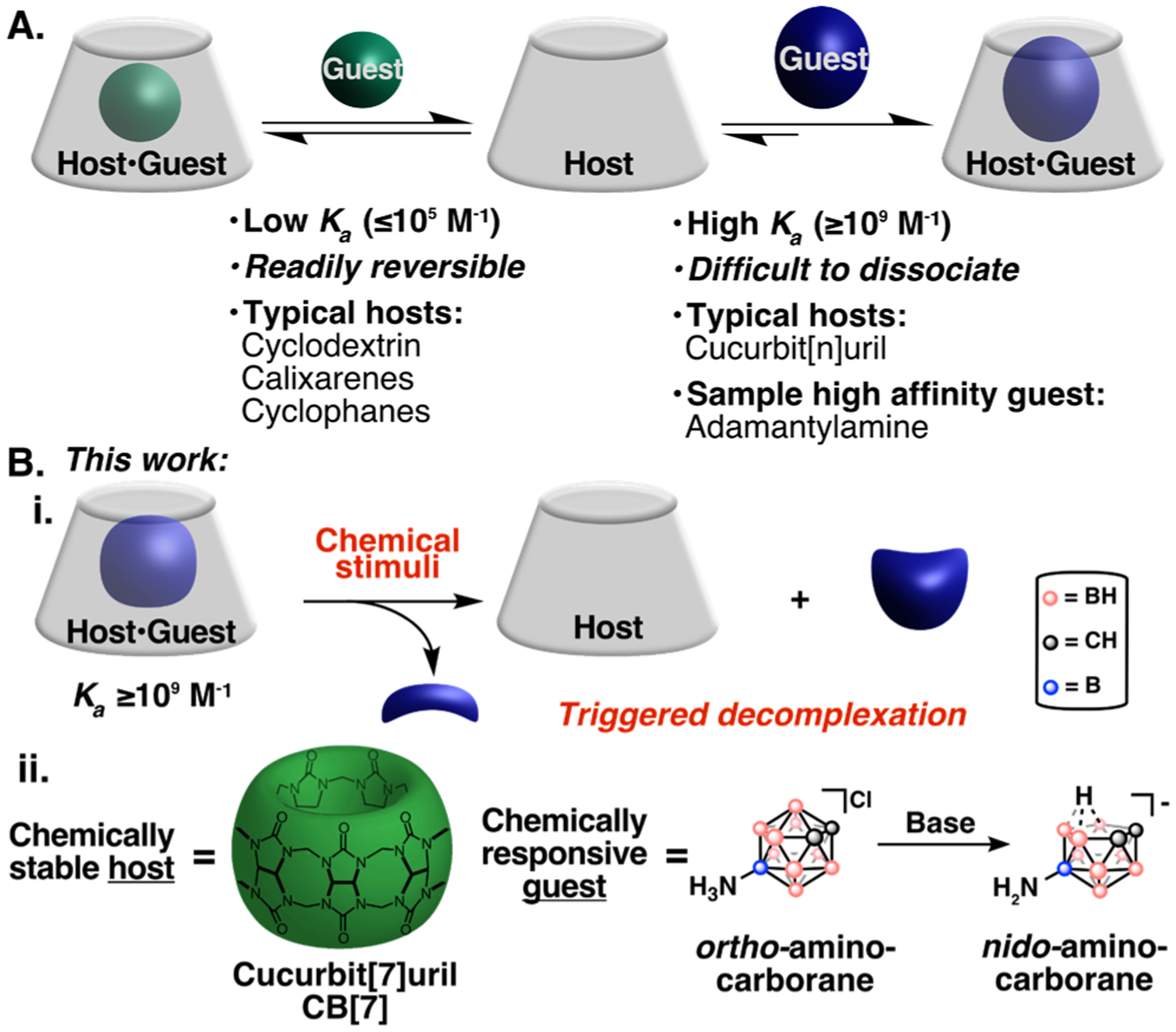
(A) Schematized synthetic host-guest pairs with low (green) and high (blue) binding affinities. (B) (i) This work presents triggered decomplexation as an advantageous property for high-affinity host-guest complexes, (ii) which we demonstrate with a cucurbit[7]uril (CB[7]) and 9-amino-ortho-carborane host-guest pair that undergoes decomplexation with base.
Cucurbiturils, cyclic oligomers of glycoluril linked by methylene units, have become the host of choice for high-affinity complexes.14,15 The heptamer, cucurbit[7]uril (CB[7]) displays the largest binding affinities, with the highest reported Ka being 1017 M−1 for complexation with 1,6-N-trimethylammonium diamantane.16 Other notable high-affinity guests for CB[7] are adamantylamine (Ka=1012 M−1) and derivatives (Ka=104–17 M−1)16,17 and functionalized ferrocenes (Ka=109–15 M−1).15 These guests all display excellent size and shape complementarity with the cavity and alignment of cationic functionality with the carbonyl rings.18
These high binding affinities have led to the exploration of the CB[7] scaffold as a biotin-(strept)avidin mimic.19–23 CB[7] has also found use in small molecule separation,24 dynamically crosslinked polymers,3,25 surface patterning26,27 and sensor development.28 In most of these applications, CB[7] is immobilized on solid-supports and the high binding affinity of guests with CB[7] render these materials one-time use. If compounds captured by the immobilized CB[7] need to be released, a higher affinity guest is introduced to displace the captured material, leaving complexed CB[7]. The difficulty in returning surfaces and devices containing CB[7] to their initial guest-free state limits the use of CB[7] to highly specialized applications where cost and scale are not significant factors.
To enable recycling of CB[7]-containing materials, we designed a high-affinity guest for CB[7] that upon chemical treatment could be transformed into a weak guest for easy removal. This guest could either be employed as the primary guest in the experiment or be used to displace an application-specific guest and then undergo decomplexation to allow regeneration of the CB[7] material. Notable previous efforts to reduce binding affinity and facilitate guest removal have used electrochemical or pH-dependent switching; however, complete removal from the cavity requires salt treatment or organic solvent (see SI Note 1).29–34 High-affinity guest removal from CB[7] has only been achieved via excessive salt treatment.23,35
In our search for guests to fit the criteria of both high binding affinity to CB[7] and the ability of triggered decomplexation, we looked to guests with shape complementarity to the cavity of CB[7] that could be readily transformed into a fragment that is poorly encapsulated by CB[7].17,36–38 Icosahedral carboranes (C2B10H12) appeared primed to meet these requirements. These 3D aromatic clusters39–41 bear a close topological similarity to adamantane42,43 and one of the three isomers, ortho-carborane, has previously been employed as a guest for CB[7], although no Ka was reported.44 Importantly, carboranes undergo Lewis base-mediated B–H vertex removal (termed “deboronation”) to generate the nido-7,8-C2B9H11 anion (Figure 1B).45–47 Ortho- and meta-carboranes have significantly different rates of deboronation, and distinct dipole moments.42,48 We set out to explore the binding affinities of derivatives of these two carborane isomers with CB[7] and their potential for removal upon deboronation to create a reliable recycling system for CB[7].
The electronic non-uniformity of carboranes is widely recognized, resulting in different electronic influences on bound substituents depending on the cage vertex.49 Given the similar inductive electronic effects of B-bound substituents on carboranes compared to bulky alkyl groups, we targeted N-substitution at the B9 vertices of the carboranes to most closely mimic the electronic environment of adamantylamine. Despite a variety of methods to functionalize ortho- and meta-carborane,50–54 the synthetic methodology developed for the amination of 9-Br-meta-carborane55 is incompatible with 9-Br-ortho-carborane (1) due to basic conditions leading to deboronation. Alternative routes to nitrogen-substituted ortho-carboranes were similarly unsuccessful.56
Thus, a new synthetic route (Figure 2A) to furnish the B9 aminated ortho-carborane target was necessary. Treatment of 1 with NaN3 under Pd-catalyzed cross-coupling conditions afforded the desired 9-N3-ortho-carborane (2)49 in 67% yield. This is a rare example of Pd-catalyzed cross-coupling of an azide anion.57 Staudinger reduction and hydrolysis with concentrated HCl gave the desired hydrochloride salt 4, confirmed by crystallography (Figure 2E). To probe the effect of a permanent positive charge on the guest, trimethylammonium derivatives 5 and 7 were prepared (Figure 2B,C). We synthesized the analogous hydrochloride salt 9-NH3-meta-carborane (8) by treating the corresponding amine with gaseous HCl (Figure 2D) to aid in binding affinity investigations.
Figure 2.
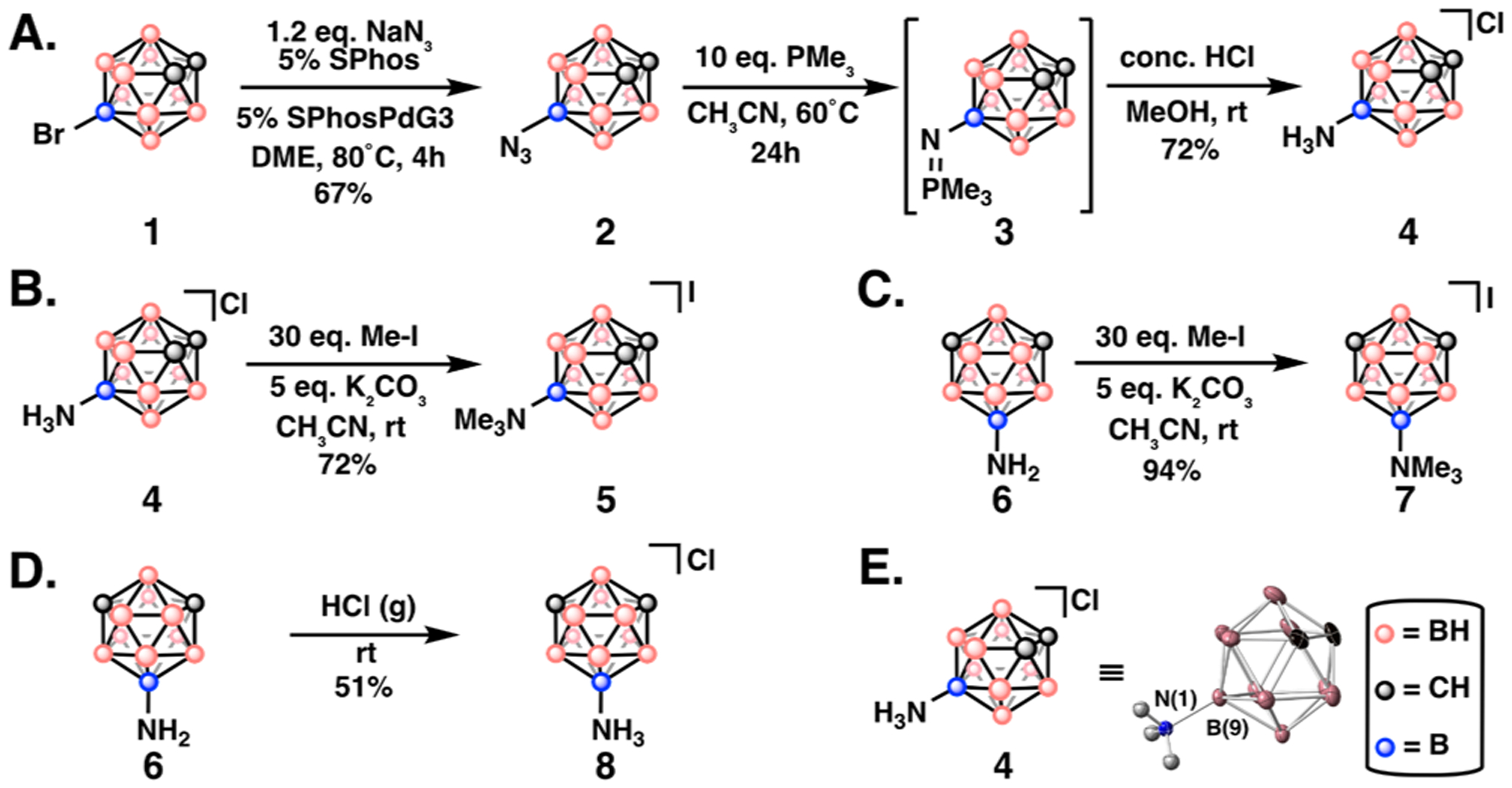
(A) Synthetic route to 9-amino-ortho-carborane (4) through 9-azido-ortho-carborane (2). (B, C) Treatment of 4 and 6 with Me-I affords trimethylated derivatives 5,7. (D) Formation of salt 8. (E) Single crystal X-ray diffraction of 4 (chloride counterion and cage-based hydrogens removed for clarity).
The aqueous solubility of the 9-amino- and 9-ammonium-carboranes (4, 5, 7, 8) allowed binding affinities to be determined by 1H-NMR spectroscopy in acetate buffer (Figure 3, Figures S1–S4). Competition experiments against (trimethylsilyl)methylamine were performed.17 Notably, the binding affinities are lower than those observed with adamantylamine, despite the structural analogy between carborane and adamantane.58 Carboranes’ greater inherent net dipole could be altering the positioning in the CB[7] hydrophobic pocket. We hypothesize that in 8 the net dipole allows for better positioning in the CB[7] cavity than its isomer 4. Similarly, trimethylation affects the Ka differently for ortho- and 9-meta-aminocarboranes. The decreased Ka for 7 compared to 5 could result from competing alignment of the trimethylammonium group with the CB[7] portals and carborane net dipole within CB[7].16,17,59 Overall, at Ka=109–11 M−1 we have established carboranes as a new class of high-affinity guests for CB[7].
Figure 3.
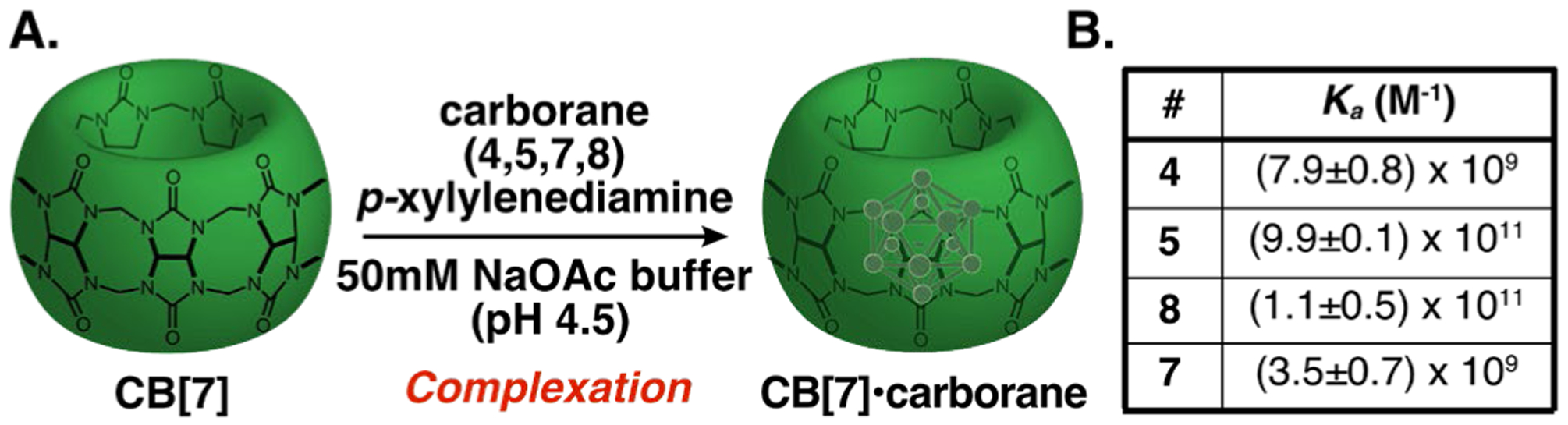
(A) Association of carborane with CB[7]. (B) Binding affinities of water-soluble carborane derivatives (4,5,7,8) (1 eq.) determined by 1H NMR spectroscopy competition experiments with (trimethylsilyl)methylamine (1.5 eq.) in 50mM NaOAc buffer.17
Next, we investigated the ability for carborane guests to be removed from CB[7] on demand. Initially, we employed unfunctionalized ortho-and meta-carborane, which have orthogonal decomplexation properties (Figure 4A). We characterized the CB[7]•carborane complexes in trifluoroacetic acid (TFA), due to limited water solubility of carboranes (Figure S5–S6).72 We found that a stable complex between CB[7] and ortho- and meta-carborane (35–55 mM, 1.2 eq) readily formed upon sonication and could be purified by washing with organic solvent (Figure S7). Carborane is clearly seen in the aqueous solution of host-guest complex via 11B-NMR spectroscopy when both ortho- (9) and meta-carborane (10) are introduced (Figure S8).
Figure 4:
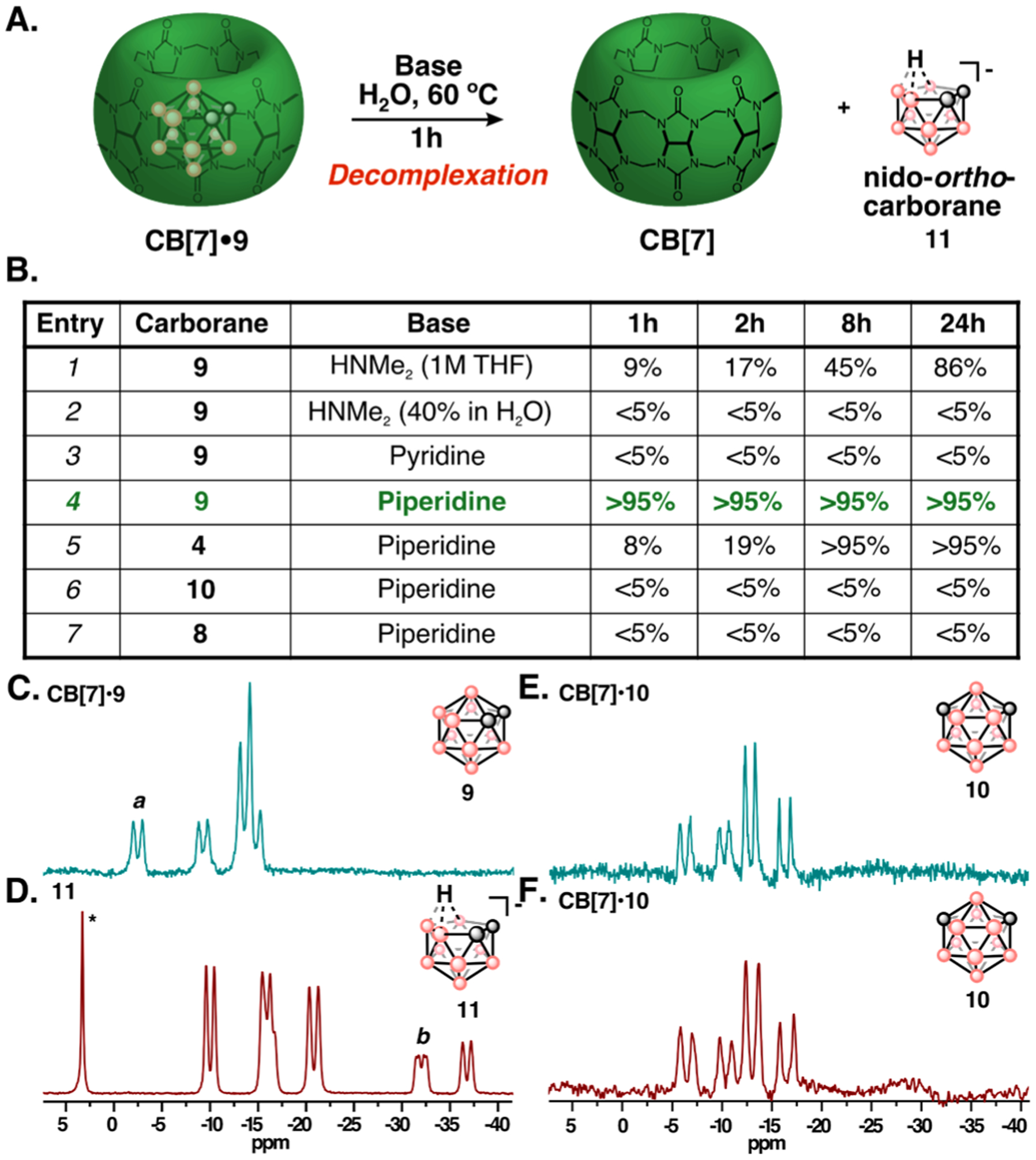
(A) Decomplexation of the CB[7]•9 complex through deboronation of ortho-carborane (9) with base to yield nido-ortho-carborane (11) and free CB[7]. (B) Conditions screened to evaluate the decomplexation of CB[7]•carborane complexes. CB[7]•carborane and base (5 equiv.) were combined in H2O, stirred at 60 °C, and monitored by 11B-NMR spectroscopy. Generation of 11 was calculated by relative integration of baseline corrected 11B-NMR spectra (Figure S9–S13, Table S1) (C-F) 11B-NMR spectra taken before (C) and after (D) 1h of subjecting CB[7]•9 complex (C,D) or CB[7]•10 complex (E,F) to 20% piperidine/H2O (v/v) at 60 °C. a, b denote the peaks used for relative integration measurements. *denotes borate side-product known to form during deboronation of 9.
After screening a small family of Lewis bases known to deboronate ortho-carborane (9) (Figure 4B, Figure S9–S13, Table S1), we established 20% aqueous piperidine at 60 °C as optimal decomplexation conditions for CB[7]•9 and CB[7]•4. Interestingly, when CB[7] was not present, other Lewis acids deboronated 4 at similar rates to piperidine (Figure S14), suggesting that a piperidine CB[7] interaction may enhance deboronation. We hypothesize that an electrostatic attraction of piperidine with the carbonyl portal improves the solubility of CB[7]•9 (or CB[7]•4), facilitating deboronation (see SI Note 2, Figure S15).
As predicted, when subjecting the meta-carborane complex (CB[7]•10) to similar conditions, no transformation is apparent for 10 in the presence or absence of CB[7] (Figure 4B, entry 6, Figure 4C–F, Figure S16–S18). These results are consistent with the diminished electrophilicity of the boron vertices adjacent to carbon vertices in meta-carborane (10) versus ortho-carborane (9) and demonstrate the potential for orthogonal chemical behavior of sterically identical guest molecules encapsulated by CB[7].
To showcase the utility of the on demand decomplexation offered by the ortho-carborane guests, we used 9 to isolate CB[7]-OH (Figure 5A). CB[7]-OH is an important intermediate for the creation of CB[7] conjugates, materials, and devices. CB[7] can be readily monohydroxylated by treatment of CB[7]•12 with persulfate salts; however, we found efficient removal of 12 from the CB[7]-OH cavity to be difficult (Scheme S1–S2).35 Gratifyingly, 12 could be displaced with 9 in less than 30 min in a H2O/TFA mixture (Figure 5B,C, blue). Upon TFA removal, treatment with piperidine for 1 h followed by a dichloromethane wash provided guest free CB[7]-OH (Figure 5B,C, red). In our hands, this is the fastest and highest yielding procedure to isolate guest-free CB[7]-OH. The decomplexation method was also used to prepare guest-free CB[7]-N3 (Figure S19).60,61
Figure 5:
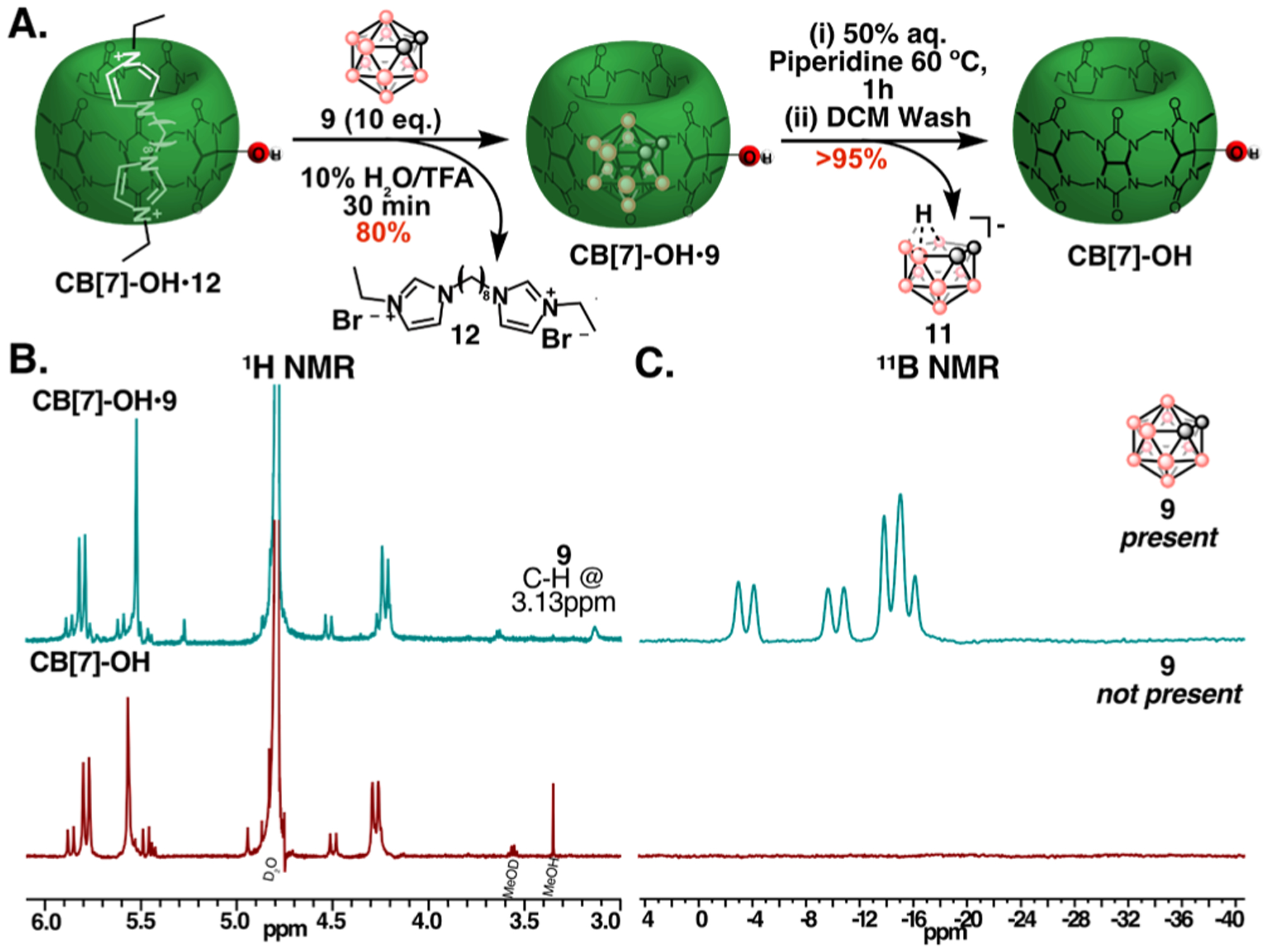
(A) Isolation of guest-free CB[7]-OH by guest exchange with 9 and subsequent decomplexation with piperidine. (B,C) Displacement of 12 by ortho-carborane (9) and formation of CB[7]-OH•9 (top, blue) and decomplexation and removal of (11) to form a guest-free CB[7]-OH cavity (bottom, red) observed by 1H-NMR (B) and 11B-NMR spectroscopy (C).
To demonstrate the ability to recycle CB[7]-constructs via decomplexation of ortho-carborane guests, we conjugated CB[7]-N360,62 to bicyclononyne-functionalized Wang resin (Wang-BCN, Figure 6A, Scheme S3) using copper-free click chemistry and confirmed successful immobilization using fluorescein adamantylamine conjugate 13 (Ka=108 M−1, Figure S20–S21). The resulting Wang-CB[7] resin was added to Jurkat lysate (2 mg/mL) containing 13 to selectively isolate the fluorescent guest from a complex mixture (Figure 6B, Step A). After washing away cell lysate, displacement with 4 rapidly releases 13 (Figure 6B, Step B). Finally, treatment with piperidine deboronates 4 to produce 14 and regenerate Wang-CB[7] (Figure 6B, Step C).73
Figure 6:
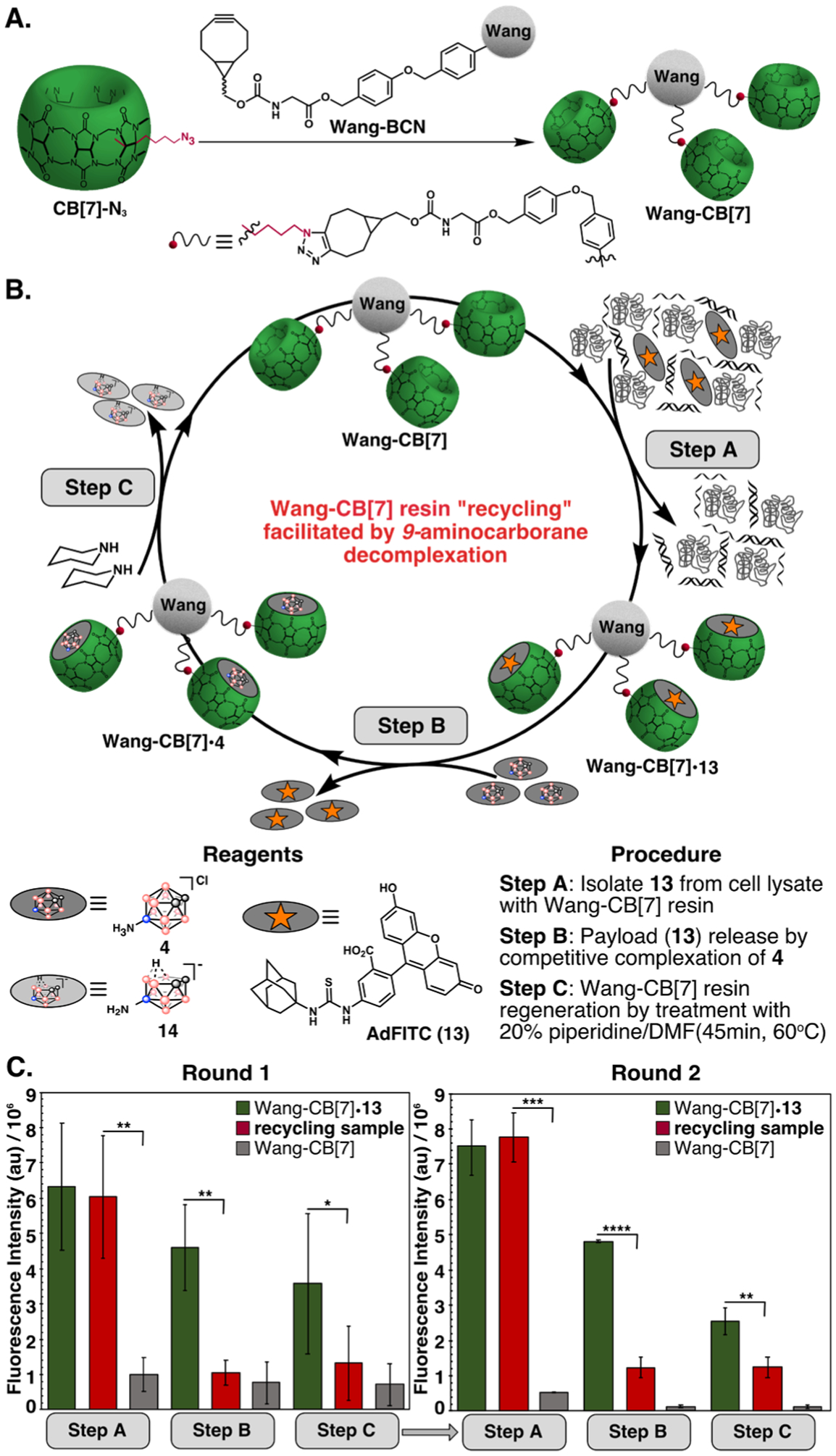
(A) Attachment of CB[7]-N3 to bicyclononyne (BCN)-functionalized Wang resin to produce Wang-CB[7]. (B) Wang-CB[7] was used to isolate payload, 13, from Jurkat lysate in 50% DMF/H2O (Step A), and was regenerated by complexation of 4 (Step B) followed by decomplexation (Step C). (C) Fluorescence of Wang-CB[7] throughout the recycling sequence (red) compared to fluorescent Wang-CB[7]•13 (green, samples not incubated with 4 in Step B) and non-fluorescent Wang-CB[7] (gray, samples not incubated with 13 or 4 in Steps A and B). Error bars represent standard deviation of three replicate samples. *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001.
The success of each step was monitored through the fluorescence of Wang-CB[7] (red) and compared to fluorescent Wang-CB[7]•13, where displacement by 4 was omitted (green) and non-fluorescent Wang-CB[7], where addition of 13 and 4 were omitted (gray) (Figure 6C). The cycle was repeated twice, at which point the signal became too low due to significant loss of resin in the washing steps (Figure S22–S23). We expect this limitation can be overcome by using a more water-compatible solid support. Recycling of the resin represents a novel method of reusing precious CB[7]-constructs that can be applied for payload isolation in biological and materials applications. Work toward even milder conditions for decomplexation is underway.
In summary, we present carboranes as high-affinity (Ka≈1010 M−1) binders for CB[7], which may be removed on demand through deboronation chemistry. We designed 9-aminocarborane guests to mimic the size and charge of adamantylamine and took advantage of the differential reactivity of carborane isomers to prepare guests that were (ortho) and were not (meta) readily deboronated. We utilized this scaffold to efficiently prepare guest-free CB[7]-OH and showcase the opportunity to “recycle” CB[7]-constructs that can be employed in biological assays and materials applications. We envision this work will overcome limitations of traditional biotin-(strept)avidin systems and enable CB[7] sensors and technologies. Finally, this work highlights how unique stimuli-responsive features of boron clusters can aid in the development of new hybrid chemical systems.63–71
Supplementary Material
ACKNOWLEDGMENT
We thank M. Miller for helpful discussion and R. Day, E. Cosco, and D. Estabrook for critical reading of the manuscript.
Funding Sources
This work was supported by NIGMS grants to E.M.S. (1DP2GM13268) and A.M.S. (R35GM124746). R.M.D. is a National Defense Science and Engineering Graduate Fellow and S.H. is an Undergraduate Research Scholars Program awardee.
Footnotes
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website. Figures S1–S23, Table S1 and additional experimental details including synthetic and experimental procedures, NMR and IR spectra, fluorescence spectra and X-ray crystallographic data (PDF)
REFERENCES
- (1).Lodish H; Berk A; Zipursky SL; Matsudaira P; Baltimore D; Darnell J Molecular Cell Biology 4th Edition; W. H. Freeman, 2000. [Google Scholar]
- (2).Frieden E Non-Covalent Interactions: Key to Biological Flexibility and Specificity. J. Chem. Educ 1975, 52, 754–761. [DOI] [PubMed] [Google Scholar]
- (3).Appel EA; del Barrio J; Loh XJ; Scherman OA Supramolecular Polymeric Hydrogels. Chem. Soc. Rev 2012, 41, 6195–6214. [DOI] [PubMed] [Google Scholar]
- (4).Mako TL; Racicot JM; Levine M Supramolecular Luminescent Sensors. Chem. Rev 2019, 119, 322–477. [DOI] [PubMed] [Google Scholar]
- (5).Agasti SS; Liong M; Tassa C; Chung HJ; Shaw SY; Lee H; Weissleder R Supramolecular Host-Guest Interaction for Labeling and Detection of Cellular Biomarkers. Angew. Chemie Int. Ed 2012, 51, 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Cui H; Xu B Supramolecular Medicine. Chem. Soc. Rev 2017, 46, 6430–6432. [DOI] [PubMed] [Google Scholar]
- (7).Webber MJ; Appel EA; Vinciguerra B; Cortinas AB; Thapa LS; Jhunjhunwala S; Isaacs L; Langer R; Anderson DG Supramolecular PEGylation of Biopharmaceuticals. Proc. Natl. Acad. Sci. U. S. A 2016, 113, 14189–14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Webber MJ; Langer R Drug Delivery by Supramolecular Design. Chem. Soc. Rev 2017, 46, 6600–6620. [DOI] [PubMed] [Google Scholar]
- (9).Wang L; Li L; Fan Y; Wang H Host-Guest Supramolecular Nanosystems for Cancer Diagnostics and Therapeutics. Adv. Mater 2013, 25, 3888–3898. [DOI] [PubMed] [Google Scholar]
- (10).Oshovsky G V; Reinhoudt, D. N.; Verboom, W. Supramolecular Chemistry in Water. Angew. Chem. Int. Ed 2007, 46, 2366–2393. [DOI] [PubMed] [Google Scholar]
- (11).Houk KN; Leach AG; Kim SP; Zhang X Binding Affinities of Host–Guest, Protein–Ligand, and Protein–Transition-State Complexes. Angew. Chemie Int. Ed 2003, 42, 4872–4897. [DOI] [PubMed] [Google Scholar]
- (12).Fasting C; Schalley CA; Weber M; Seitz O; Hecht S; Koksch B; Dernedde J; Graf C; Knapp E-W; Haag R Multivalency as a Chemical Organization and Action Principle. Angew. Chemie Int. Ed 2012, 51, 10472–10498. [DOI] [PubMed] [Google Scholar]
- (13).Liu W; Samanta SK; Smith BD; Isaacs L; Norquest KA; Smith BD; Smith BD; Pei Z; Hooley RJ; Selvapalam N; Ryu S; Kim K; Gilson MK; Kim K; Inoue Y Synthetic Mimics of Biotin/(Strept)Avidin. Chem. Soc. Rev 2017, 46, 2391–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Masson E; Ling X; Joseph R; Kyeremeh-Mensah L; Lu X Cucurbituril Chemistry: A Tale of Supramolecular Success. RSC Adv 2012, 2, 1213–1247. [Google Scholar]
- (15).Shetty D; Khedkar JK; Park KM; Kim K Can We Beat the Biotin–Avidin Pair?: Cucurbit[7]Uril-Based Ultrahigh Affinity Host–Guest Complexes and Their Applications. Chem. Soc. Rev 2015, 44, 8747–8761. [DOI] [PubMed] [Google Scholar]
- (16).Cao L; Šekutor M; Zavalij PY; Mlinarić-Majerski K; Glaser R; Isaacs L Cucurbit[7]Uril⋅Guest Pair with an Attomolar Dissociation Constant. Angew. Chemie Int. Ed 2014, 53, 988–993. [DOI] [PubMed] [Google Scholar]
- (17).Liu S; Ruspic C; Mukhopadhyay P; Chakrabarti S; Zavalij PY; Isaacs L The Cucurbit[ n ]Uril Family: Prime Components for Self-Sorting Systems. J. Am. Chem. Soc 2005, 127, 15959–15967. [DOI] [PubMed] [Google Scholar]
- (18).Moghaddam S; Yang C; Rekharsky M; Ko YH; Kim K; Inoue Y; Gilson MK New Ultrahigh Affinity Host−Guest Complexes of Cucurbit[7]Uril with Bicyclo[2.2.2]Octane and Adamantane Guests: Thermodynamic Analysis and Evaluation of M2 Affinity Calculations. J. Am. Chem. Soc 2011, 133, 3570–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lee D-W; Park KM; Banerjee M; Ha SH; Lee T; Suh K; Paul S; Jung H; Kim J; Selvapalam N; Ryu SH; Kim K Supramolecular Fishing for Plasma Membrane Proteins Using an Ultrastable Synthetic Host–Guest Binding Pair. Nat. Chem 2011, 3, 154–159. [DOI] [PubMed] [Google Scholar]
- (20).Hwang I; Baek K; Jung M; Kim Y; Kyeng MP; Lee DW; Selvapalam N; Kim K Noncovalent Immobilization of Proteins on a Solid Surface by Cucurbit[7]Uril-Ferrocenemethylammonium Pair, a Potential Replacement of Biotin-Avidin Pair. J. Am. Chem. Soc 2007, 129, 4170–4171. [DOI] [PubMed] [Google Scholar]
- (21).Kim KL; Sung G; Sim J; Murray J; Li M; Lee A; Shrinidhi A; Park KM; Kim K Supramolecular Latching System Based on Ultrastable Synthetic Binding Pairs as Versatile Tools for Protein Imaging. Nat. Commun 2018, 9, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Li W; Bockus AT; Vinciguerra B; Isaacs L; Urbach AR Predictive Recognition of Native Proteins by Cucurbit[7]Uril in a Complex Mixture. Chem. Commun 2016, 52, 8537–8540. [DOI] [PubMed] [Google Scholar]
- (23).An J; Kim S; Shrinidhi A; Kim J; Banna H; Sung G; Park KM; Kim K Purification of Protein Therapeutics via High-Affinity Supramolecular Host–Guest Interactions. Nat. Biomed. Eng 2020, 1–9. [DOI] [PubMed] [Google Scholar]
- (24).Mandadapu V; Day AI; Ghanem A Cucurbituril: Chiral Applications. Chirality 2014, 26, 712–723. [DOI] [PubMed] [Google Scholar]
- (25).Chen H; Chen Y; Wu H; Xu J-F; Sun Z; Zhang X Supramolecular Polymeric Chemotherapy Based on Cucurbit[7]Uril-PEG Copolymer. Biomaterials 2018, 178, 697–705. [DOI] [PubMed] [Google Scholar]
- (26).Young JF; Nguyen HD; Yang L; Huskens J; Jonkheijm P; Brunsveld L Strong and Reversible Monovalent Supramolecular Protein Immobilization. ChemBioChem 2010, 11, 180–183. [DOI] [PubMed] [Google Scholar]
- (27).Neirynck P; Brinkmann J; An Q; van der Schaft DWJ; Milroy L-G; Jonkheijm P; Brunsveld L Supramolecular Control of Cell Adhesion via Ferrocene–Cucurbit[7]Uril Host–Guest Binding on Gold Surfaces. Chem. Commun 2013, 49, 3679–3681. [DOI] [PubMed] [Google Scholar]
- (28).Lee D-W; Park KM; Gong B; Shetty D; Khedkar JK; Baek K; Kim J; Ryu SH; Kim K A Simple Modular Aptasensor Platform Utilizing Cucurbit[7]Uril and a Ferrocene Derivative as an Ultrastable Supramolecular Linker. Chem. Commun 2015, 51, 3098–3101. [DOI] [PubMed] [Google Scholar]
- (29).Cui L; Gadde S; Li W; Kaifer AE Electrochemistry of the Inclusion Complexes Formed between the Cucurbit [7]Uril Host and Several Cationic and Neutral Ferrocene Derivatives. Langmuir 2009, 25, 13763–13769. [DOI] [PubMed] [Google Scholar]
- (30).Li W; Kaifer AE Combining Proton and Electron Transfer to Modulate the Stability of Cucurbit[7]Uril Complexes. Langmuir 2012, 28, 15075–15079. [DOI] [PubMed] [Google Scholar]
- (31).Sindelar Vladimir; Silvi Serena; Parker Samantha E.; Sobransingh David; Kaifer AE Proton and Electron Transfer Controlof the Position of Cucurbit[n]Uril Wheelsin Pseudorotaxanes. Adv. Funct. Mater 2007, 17, 694–701. [Google Scholar]
- (32).Wang W; Kaifer AE Transfer of Cationic Cucurbit[7]Uril Inclusion Complexes from Water to Non-Aqueous Solvents. Supramol. Chem 2010, 22, 710–716. [Google Scholar]
- (33).Vázquez J; Romero MA; Dsouza RN; Pischel U Phototriggered Release of Amine from a Cucurbituril Macrocycle. Chem. Commun 2016, 52, 6245–6248. [DOI] [PubMed] [Google Scholar]
- (34).Ong W; Kaifer AE Salt Effects on the Apparent Stability of the Cucurbit[7]Uril-Methyl Viologen Inclusion Complex. J. Org. Chem 2004, 69, 1383–1385. [DOI] [PubMed] [Google Scholar]
- (35).Jiao D; Zhao N; Scherman OAA “Green” Method for Isolation of Cucurbit[7]Uril via a Solid State Metathesis Reaction. Chem. Commun 2010, 46, 2007. [DOI] [PubMed] [Google Scholar]
- (36).Márquez C; Hudgins RR; Nau WM Mechanism of Host−Guest Complexation by Cucurbituril. J. Am. Chem. SOC 2004, 126, 5806–5816. [DOI] [PubMed] [Google Scholar]
- (37).Miskolczy Z; Megyesi M; Biczók L; Prabodh A; Biedermann F Kinetics and Mechanism of Cation‐Induced Guest Release from Cucurbit[7]Uril. Chem. – A Eur. J 2020, chem.201905633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Mock WL; Shih NY Structure and Selectivity in Host-Guest Complexes of Cucurbituril. J. Org. Chem 1986, 51, 4440–4446. [Google Scholar]
- (39).von Ragué Schleyer P; Najafian K Stability and Three-Dimensional Aromaticity of Closo-Monocarbaborane Anions, CBn-1Hn- and Closo-Dicarboranes, C2Bn-2Hn. Inorg. Chem 1998, 37, 3454–3470. [DOI] [PubMed] [Google Scholar]
- (40).Chen Z; King RB Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures. Chem. Rev 2005, 105, 3613–3642. [DOI] [PubMed] [Google Scholar]
- (41).King RB Three-Dimensional Aromaticity in Polyhedral Boranes and Related Molecules. Chem. Rev 2001, 101, 1119–1152. [DOI] [PubMed] [Google Scholar]
- (42).Scholz M; Hey-Hawkins E Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev 2011, 111, 7035–7062. [DOI] [PubMed] [Google Scholar]
- (43).Plesek J Potential Applications of the Boron Cluster Compounds. Chem. Rev 1992, 92, 269–278. [Google Scholar]
- (44).Blanch RJ; Sleeman AJ; White TJ; Arnold AP; Day AI Cucurbit[7]Uril and o-Carborane Self-Assemble to Form a Molecular Ball Bearing. Nano Lett 2002, 2, 147–149. [Google Scholar]
- (45).Wiesboeck RA; Hawthorne MF Dicarbaundecaborane(13) and Derivatives. J. Am. Chem. Soc 1964, 86, 1642–1643. [Google Scholar]
- (46).Hawthorne MF; Young DC; Garrett PM; Owen DA; Schwerin SG; Tebbe FN; Wegner PA Preparation and Characterization of the (3)-1,2- and (3)-1,7-Dicarbadodecahydroundecaborate(-1) Ions. J. Am. Chem. Soc 1968, 90, 862–868. [Google Scholar]
- (47).Plešek J; Heřmánek S; Štíbr B; Waksman L; Sneddon LG Potassium Dodecahydro-7, 8-Dicarba- Nido -Undecaborate(1-), k[7, 8-c 2 b 9 h 12 ], Intermediates, Stock Solution, and Anhydrous Salt. Inorg. Synth 2007, 22, 231–234. [Google Scholar]
- (48).Fox MA; Goeta AE; Hughes AK; Johnson AL Crystal and Molecular Structures of the Nido-Carborane Anions, 7,9- and 2,9-C2B9H12-. J. Chem. Soc. Dalt. Trans 2002, 11, 2132–2141. [Google Scholar]
- (49).Spokoyny AM; Machan CW; Clingerman DJ; Rosen MS; Wiester MJ; Kennedy RD; Stern CL; Sarjeant AA; Mirkin CA A Coordination Chemistry Dichotomy for Icosahedral Carborane-Based Ligands. Nat. Chem 2011, 3, 590–596. [DOI] [PubMed] [Google Scholar]
- (50).Herzog A; Maderna A; Harakas GN; Knobler CB; Hawthorne MF A Camouflagednido-Carborane Anion: Facile Synthesis of Octa-B-Methyl-1,2-Dicarba-Closo-Dodecaborane(12) and Its Deboration Reaction. Chem. - A Eur. J 1999, 5, 1212–1217. [Google Scholar]
- (51).Dziedzic RM; Axtell JC; Rheingold AL; Spokoyny AM Off-Cycle Processes in Pd-Catalyzed Cross-Coupling of Carboranes. Org. Process Res. Dev 2019, 23, 1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Zakharkin LL; Ol’shevskaya VA; Vorontsov EV; Petrovsky PV Synthesis of Mono-, Di-, Tri-, and Tetraethyl-o-Carboranes by Electrophilic Alkylation Ofo-Carborane with Ethyl Bromide in the Presence of AICl3 and Their Transformations. Russ. Chem. Bull 1996, 45, 2614–2622. [Google Scholar]
- (53).Andrews JS; J Z; Jones MJ 9-Iodo-o-Carborane. Inorg. Chem 1985, 24, 3715–3716. [Google Scholar]
- (54).Sieckhaus JF; Semenuk NS; Knowles TA; Schroeder H Icosahedral Carboranes. XIII Halogenation of p-Carborane. Inorg. Chem 1969, 8, 2452–2457. [Google Scholar]
- (55).Dziedzic RM; Saleh LMA; Axtell JC; Martin JL; Stevens SL; Royappa AT; Rheingold AL; Spokoyny AM B–N, B–O, and B–CN Bond Formation via Palladium-Catalyzed Cross-Coupling of B-Bromo-Carboranes. J. Am. Chem. Soc 2016, 138, 9081–9084. [DOI] [PubMed] [Google Scholar]
- (56).Sevryugina Y; Julius RL; Hawthorne MF Novel Approach to Aminocarboranes by Mild Amidation of Selected Iodo-Carboranes. Inorg. Chem 2010, 49, 10627–10634. [DOI] [PubMed] [Google Scholar]
- (57).Grushin VV; Tolstaya TP; Lisichkina IN 9-o-Carboranyl Azide. Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.) 1982, 31, 2329. [Google Scholar]
- (58).Dziedzic RM; Axtell JC; Rheingold AL; Spokoyny AM Off-Cycle Processes in Pd-Catalyzed Cross-Coupling of Carboranes. Org. Process Res. Dev 2019, 23, 1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Cao L; Isaacs L Absolute and Relative Binding Affinity of Cucurbit[7]Uril towards a Series of Cationic Guests Supramolecular Chemistry, 2014; 26, 251–258. [Google Scholar]
- (60).Bockus AT; Smith LC; Grice AG; Ali OA; Young CC; Mobley W; Leek A; Roberts JL; Vinciguerra B; Isaacs L; Urbach AR Cucurbit[7]Uril–Tetramethylrhodamine Conjugate for Direct Sensing and Cellular Imaging. J. Am. Chem. Soc 2016, 138, 16549–16552. [DOI] [PubMed] [Google Scholar]
- (61).Zhou X; Su X; Pathak P; Vik R; Vinciguerra B; Isaacs L; Jayawickramarajah J Host–Guest Tethered DNA Transducer: ATP Fueled Release of a Protein Inhibitor from Cucurbit[7]Uril. J. Am. Chem. Soc 2017, 139, 13916–13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Vinciguerra B; Cao L; Cannon JR; Zavalij PY; Fenselau C; Isaacs L Synthesis and Self-Assembly Processes of Monofunctionalized Cucurbit[7]Uril. J. Am. Chem. Soc 2012, 134, 13133–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Mu X; Axtell JC; Bernier NA; Kirlikovali KO; Jung D; Umanzor A; Qian K; Chen X; Bay KL; Kirollos M; Rheingold AL; Houk KN; Spokoyny AM Sterically Unprotected Nucleophilic Boron Cluster Reagents. Chem 2019, 5, 2461–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Keener M; Hunt C; Carroll TG; Kampel V; Dobrovetsky R; Hayton TW; Ménard G Redox-Switchable Carboranes for Uranium Capture and Release. Nature 2020, 577, 652–655. [DOI] [PubMed] [Google Scholar]
- (65).Fuentes I; Pujols J; Viñas C; Ventura S; Teixidor F Dual Binding Mode of Metallacarborane Produces a Robust Shield on Proteins. Chem. – A Eur. J 2019, 25, 12820–12829. [DOI] [PubMed] [Google Scholar]
- (66).Fisher SP; McArthur SG; Tej V; Lee SE; Chan AL; Banda I; Gregory A; Berkley K; Tsay C; Rheingold AL; Guisado-Barrios G; Lavallo V Strongly Coordinating Ligands To Form Weakly Coordinating Yet Functional Organometallic Anions. J. Am. Chem. Soc 2020, 142, 251–256. [DOI] [PubMed] [Google Scholar]
- (67).Tao G; Duan Z; Mathey F Zwitterionic Nido -Carborane-Fused Phospholes. Org. Lett 2019, 21, 2273–2276. [DOI] [PubMed] [Google Scholar]
- (68).Zhang Y; Yang L; Wang L; Duttwyler S; Xing H A Microporous Metal‐Organic Framework Supramolecularly Assembled from a Cu II Dodecaborate Cluster Complex for Selective Gas Separation. Angew. Chemie Int. Ed 2019, 58, 8145–8150. [DOI] [PubMed] [Google Scholar]
- (69).Yan H; Yang F; Pan D; Lin Y; Hohman JN; Solis-Ibarra D; Li FH; Dahl JEP; Carlson RMK; Tkachenko BA; Fokin AA; Schreiner PR; Galli G; Mao WL; Shen Z-X; Melosh NA Sterically Controlled Mechanochemistry under Hydrostatic Pressure. Nature 2018, 554, 505–510. [DOI] [PubMed] [Google Scholar]
- (70).Van Nghia N; Oh J; Sujith S; Jung J; Lee MH Tuning the Photophysical Properties of Carboranyl Luminophores by Closo - to Nido -Carborane Conversion and Application to OFF–ON Fluoride Sensing. Dalt. Trans 2018, 47, 17441–17449. [DOI] [PubMed] [Google Scholar]
- (71).Wang H; Zhang J; Lee HK; Xie Z Borylene Insertion into Cage B–H Bond: A Route to Electron-Precise B–B Single Bond. J. Am. Chem. Soc 2018, 140, 3888–3891. [DOI] [PubMed] [Google Scholar]
- (72).Measurements in TFA or H2O showed no significant chemical shift changes by IR, 11B-NMR or 1H-NMR spectroscopy precluding a Ka determination of unfunctionalized carboranes (including nido-carborane) with CB[7].
- (73).The experiment was performed in 50% DMF/H2O to keep the Wang resin swollen and conjugated CB[7] accessible for binding and decomplexation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


