Abstract
The cold-tolerant yeast Saccharomyces cerevisiae is industrially useful for lager fermentation, high-quality wine, and frozen dough production. S. cerevisiae Cheongdo is a recent isolate from frozen peach samples which has a good fermentation performance at low temperatures and desirable flavor profiles. Here, phenotype microarray was used to investigate industrial potentials of S. cerevisiae Cheongdo using 192 carbon sources. Compared to commercial wine yeast S. cerevisiae EC1118, Cheongdo showed significantly different growth rates on 34 substrates. The principal component analysis of the results highlighted that the better growth of Cheongdo on galactose than on EC1118 was the most significant difference between the two strains. The intact GAL4 gene and the galactose fermentation performance at a low temperatures suggested that S. cerevisiae Cheongdo is a promising host for industrial fermentation rich in galactose, such as lactose and agarose.
Keywords: phenotype microarray, yeast, cold tolerance, galactose, GAL4
1. Introduction
Phenotype microarray is an automated high-throughput technique for the phenotypic profiling of microbial isolates or communities [1,2]. Specifically, cellular growth on microplates is measured with different substrates and stress conditions. Phenotype microarray uses preconfigured sets of microplates, which makes it different from other growth-monitoring microplate readers, such as Bioscreen C [3]. Continuous growth curves from phenotype microarrays can be used to calculate the overall fitness of microbial cells, such as lag time, growth rate, and maximum density [4]. Phenotype microarray is an essential tool for microbial phenomics, in which phenotypic profiling is described on a genome-wide scale using a knockout library [5,6,7]. Yeast phenomics integrated with other omics approaches enables determining yeast phenotypic diversity and accurate phenotype prediction, including complex traits [8,9,10,11].
The industrial yeast Saccharomyces cerevisiae has a high level of genetic and phenotypic diversity, driven by long-term adaptation and evolution for different applications [12,13]. For example, wine industry strains are highly resistant to grape antioxidants and antimicrobial additives [14]. In contrast, the strains used in the bioethanol industry have a high ethanol productivity [14]. Hence, to isolate new yeast strains for industrial applications, it is important to establish a method for rapid screening and selecting phenotypes that are superior to existing industrial strains.
Among various industrially attractive phenotypes of S. cerevisiae, cold tolerance is one of the most essential traits for the beverage and food industry [15]. Wine and beer fermentation at a low temperature improves flavor by preventing spoilage microorganisms [16,17,18]. Moreover, cold-tolerant strains are required to produce unfermented frozen dough with viable cells [19]. Transcriptomics and quantitative trait loci (QTL) analysis suggested that cold tolerance is a complex trait that is difficult to engineer genetically [20,21]. As in the case of the most common lager yeast S. pastorianus (a hybrid of ale-type S. cerevisiae and cold-tolerant S. eubayanus), cold-tolerant S. cerevisiae can be achieved by prolonged adaptation to cold stress, which might accompany hybridization with other cold-tolerant wild yeast species [22]. Therefore, cold-tolerant isolates of S. cerevisiae could have different origins and fermentation phenotypes [23,24,25].
S. cerevisiae Cheongdo (KACC 93277P) is a cold-tolerant strain isolated from frozen peach samples [16]. In this study, the possibility of the industrial use of S. cerevisiae Cheongdo was investigated using a phenotype microarray with 192 carbon sources. The results suggested that the Cheongdo strain has a high potential for several industrial applications.
2. Materials and Methods
2.1. Strains and Fermentation
The cold-tolerant yeast S. cerevisiae Cheongdo [16] was compared to a commercial wine yeast S. cerevisiae EC1118 (LALVIN, Montreal, QC, Canada). Cells were precultured in 5 mL of YPD (1% yeast extract, 2% peptone, and 2% glucose). Flask fermentation was performed in 125 mL Erlenmeyer flasks containing 20 mL of YPD or YPG (1% yeast extract, 2% peptone, and 2% galactose) with an initial cell density of 0.01 or 0.1 OD600 (optical density at 600 nm). Flasks were incubated at 10 °C or 30 °C and at 130× rpm.
2.2. Phenotype Microarray
The Biolog PM01 and PM02 microplates were purchased from Biolog (Hayward, CA, USA). The strains were incubated overnight in 5 mL of YPD medium, then washed with distilled water. Afterwards, cells were suspended in IFY-0 base (Biolog 72231) at an initial cell density of 0.01 OD600. One hundred microliters of the cell suspension were inoculated into each microplate’s well; then, microplates were incubated in an OmniLog reader (Biolog). Each experiment was performed in duplicate. Each well contained a tetrazolium dye to detect color changes (colorless to red) due to cell respiration (NADH production). The color changes were automatically recorded every 15 min using a charge-coupled device camera and converted into OmniLog units [13,26]. The results were analyzed by Student’s t-test and principal component analysis (PCA) using R studio’s OPM (https://cran.r-project.org/src/contrib/Archive/opm/, accessed on 29 April 2021) and ggplot2 (https://cran.r-project.org/src/contrib/Archive/ggplot2/, accessed on 29 April 2021) [4,27].
2.3. DNA Sequencing
The genomic DNA was purified by Exgene Cell SV Kit (GeneAll, Seoul, Korea). The 2.6 kb GAL4 gene (NCBI Gene ID: 855828) was amplified by PCR using Q5 polymerase and the Kim1012 (5′-GATGCACAGTAGAAGTGAAC-3′) and Kim1013 (5′-CATCTCCAGATTGTGTCTAC-3′) primers, and the PCR product was purified by Exgene PCR SV Kit (GeneAll, Seoul, Korea). Sanger sequencing of the gene was performed by Cosmogenetech (Seoul, Korea) using Kim1012, Kim1013, Kim1037 (5′-GTTGTAATAATTGTGCGGTC-3′), and Kim1038 (5′-CACTCACCGACGCTAATGAT-3′) primers.
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
Glucose, galactose, and ethanol were analyzed by HPLC (Agilent Technologies, 1260 series, Karlsruhe, Germany) equipped with a Rezex-ROA Organic Acid H+ (8%) (150 mm × 4.6 mm) column (Phenomenex Inc., Torrance, CA, USA). Columns were eluted with 0.005 N H2SO4 at 50 °C, and the flow rate was set at 0.6 mL/min.
3. Results
3.1. Phenotype Microarray Analysis
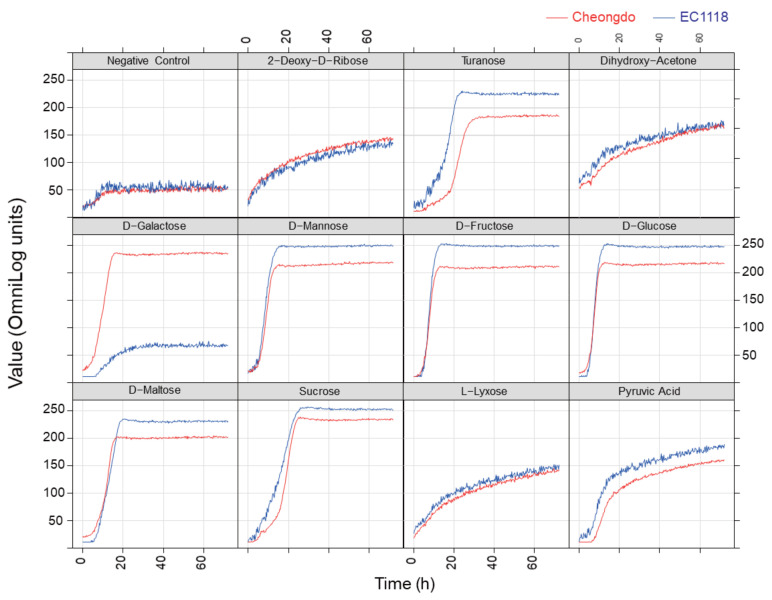
The growth patterns of S. cerevisiae Cheongdo and EC1118 strains on the phenotype microarray with 192 carbon sources were monitored for 72 h, and some representative growth patterns (12 carbon sources) are shown in Figure 1. The growth values (y-axis) were presented as OmniLog units, representing the degree of respiration. Each growth curve showed different maximum values and rates. In particular, conventional carbon sources, such as d-mannose, d-fructose, d-glucose, d-maltose, and sucrose, supported an efficient growth of both Cheongdo and EC1118 strains. Some carbon sources, such as 2-deoxy-d-ribose, dihydroxy-acetone, l-lyxose, and pyruvic acid, yielded lower cell growth than that observed with conventional sugars for both strains. The negative control (without carbon source) was considered as the baseline respiration signal (no growth).
Figure 1.
Representative growth patterns of S. cerevisiae Cheongdo (red) and EC1118 (blue) strains on Biolog PM01 and PM02 microplates with 192 carbon sources for 72 h at 30 °C. OmniLog units represent a measurement of dye reduction: i.e., a measurement of microbial respiration.
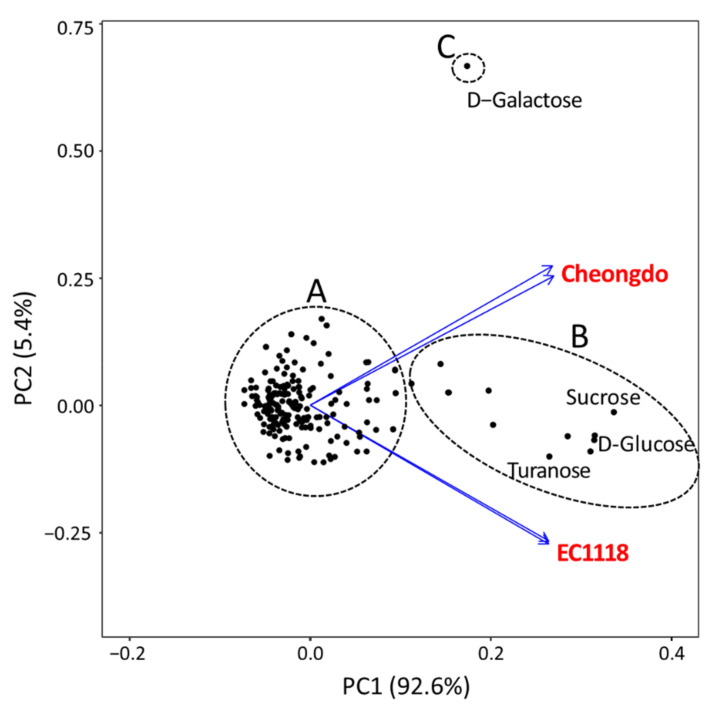
To better understand the phenotypic differences between Cheongdo and EC1118, the PCA of the maximum growth values (OmniLog units) on 192 carbon sources was performed. The results are presented as a biplot (Figure 2). The loadings (strains) were clearly separated, and the scores (carbon sources) were divided into three groups (A, B, and C). Group A represented carbon sources that did not support much growth for both strains. Group B represented carbon sources that showed different maximum growth values between the two strains, such as sucrose, d-glucose, and turanose. Lastly, group C represented galactose, which yielded the most significant difference between the two strains.
Figure 2.
Biplot of variable loadings (represented by vectors) and scores (marked by points) from principal component analysis (PCA) of the maximum growth values (OmniLog units) of S. cerevisiae Chengdu and EC1118 strains on 192 carbon sources for 72 h at 30 °C.
The detailed phenotypic differences between Cheongdo and EC1118 were compared based on the calculated growth rates (OmniLog units/h at 15 h). Table 1 shows the 34 carbon sources that yielded significantly different growth rates between the two strains (p < 0.05). Again, galactose resulted in the greatest difference between their growth rates; Cheongdo showed 5.6 times higher growth rates on galactose than on EC1118. EC1118 barely grew on galactose, similarly to the negative control (no carbon source). Meanwhile, the other 31 carbon sources, except for d-serine and γ-Cyclodextrin, supported higher growth rates for EC1118 than for Cheongdo strains. Although the growth on the 33 carbon sources was statistically significant, the values were close to those of the negative control, suggesting that these carbon sources cannot support the growth of both Cheongdo and EC1118 strains.
Table 1.
Significantly different growth rates 1 of S. cerevisiae Cheongdo and EC1118 strains on different carbon sources.
| Carbon Source 2 | Cheongdo | EC1118 | Difference | p-Value 3 |
|---|---|---|---|---|
| Negative Control | 3.23 ± 0.14 | 2.77 ± 1.56 | 0.47 | 0.74 |
| d-Galactose | 15.47 ± 0.85 | 2.73 ± 0.94 | 12.73 | 0.01 |
| l-Pyroglutamic Acid | 1.43 ± 0.33 | 4.83 ± 0.24 | 3.40 | 0.01 |
| 3-Hydroxy-2-Butanone | 2.93 ± 0.19 | 5.53 ± 0.28 | 2.60 | 0.01 |
| N-Acetyl-l-Glutamic Acid | 1.33 ± 0.19 | 3.73 ± 0.28 | 2.40 | 0.02 |
| 2,3-Butanedione | 1.87 ± 0.19 | 4.27 ± 0.38 | 2.40 | 0.03 |
| Butyric Acid | 2.30 ± 0.14 | 4.57 ± 0.33 | 2.27 | 0.04 |
| Palatinose | 1.97 ± 0.24 | 4.00 ± 0.19 | 2.03 | 0.01 |
| d,l-Carnitine | 1.33 ± 0.09 | 3.33 ± 0.19 | 2.00 | 0.02 |
| Putrescine | 1.03 ± 0.33 | 2.83 ± 0.33 | 1.80 | 0.03 |
| 3-0-β-d-Galactopyranosyl-d-Arabinose | 1.30 ± 0.14 | 3.07 ± 0.19 | 1.77 | 0.01 |
| l-Phenylalanine | 1.20 ± 0.19 | 2.97 ± 0.24 | 1.77 | 0.02 |
| d-Serine | 2.67 ± 0.00 | 1.00 ± 0.09 | 1.67 | 0.03 |
| β-Hydroxy Butyric Acid | 1.20 ± 0.09 | 2.73 ± 0.19 | 1.53 | 0.02 |
| l-Lysine | 1.43 ± 0.05 | 2.90 ± 0.05 | 1.47 | 0.00 |
| β-Methyl-d-Xyloside | 2.07 ± 0.09 | 3.53 ± 0.00 | 1.47 | 0.03 |
| Glycine | 0.80 ± 0.00 | 2.23 ± 0.05 | 1.43 | 0.01 |
| l-Alaninamide | 1.27 ± 0.19 | 2.67 ± 0.28 | 1.40 | 0.04 |
| l-Glutamic Acid | 3.43 ± 0.24 | 4.80 ± 0.19 | 1.37 | 0.03 |
| l-Arginine | 1.37 ± 0.24 | 2.73 ± 0.19 | 1.37 | 0.03 |
| d-Ribono-1,4-Lactone | 0.77 ± 0.05 | 2.10 ± 0.14 | 1.33 | 0.03 |
| d-Tartaric Acid | 1.63 ± 0.05 | 2.90 ± 0.05 | 1.27 | 0.00 |
| Hydroxy-l-Proline | 1.53 ± 0.09 | 2.77 ± 0.14 | 1.23 | 0.01 |
| Sorbic Acid | 1.27 ± 0.09 | 2.47 ± 0.19 | 1.20 | 0.03 |
| γ-Cyclodextrin | 3.03 ± 0.24 | 1.87 ± 0.19 | 1.17 | 0.04 |
| N-Acetyl-d-Glucosaminitol | 0.83 ± 0.14 | 1.97 ± 0.24 | 1.13 | 0.04 |
| 3-Methyl Glucose | 2.27 ± 0.09 | 3.30 ± 0.05 | 1.03 | 0.02 |
| l-Homoserine | 0.80 ± 0.09 | 1.83 ± 0.05 | 1.03 | 0.02 |
| δ-Amino Valeric Acid | 1.23 ± 0.05 | 2.23 ± 0.05 | 1.00 | 0.00 |
| d-Fucose | 2.23 ± 0.05 | 3.20 ± 0.00 | 0.97 | 0.02 |
| Sebacic Acid | 1.27 ± 0.09 | 2.20 ± 0.09 | 0.93 | 0.01 |
| l-Methionine | 1.43 ± 0.05 | 2.30 ± 0.05 | 0.87 | 0.00 |
| Citraconic Acid | 1.93 ± 0.19 | 2.77 ± 0.14 | 0.83 | 0.04 |
| γ-Amino Butyric Acid | 1.33 ± 0.00 | 1.97 ± 0.05 | 0.63 | 0.03 |
| l-Sorbose | 1.60 ± 0.09 | 2.20 ± 0.09 | 0.60 | 0.02 |
1 The average growth rate was calculated as OmniLog units/h at 15 h. 2 In descending order of growth differences. 3 p < 0.05.
3.2. Validation of Phenotype Microarray Results by Flask Fermentation
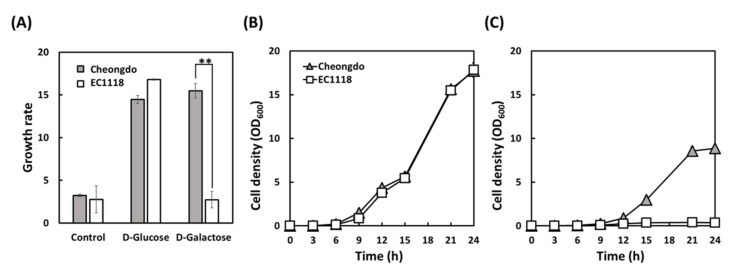
To validate the growth results on the Biolog microplates of phenotype microarray, S. cerevisiae Cheongdo and EC1118 strains were tested by flask fermentation. As highlighted in Figure 3A, the growth on galactose was significantly different between the two strains (p < 0.01) on microplates, while the growth on d-glucose was not. The results obtained by flask fermentation were in accordance with the microplate results. The growth curves in glucose using flasks overlapped for both strains, suggesting that there was no difference. However, a growth difference in galactose was observed between the two strains when using flasks (Figure 3B,C); EC1118 was unable to grow on galactose, whereas Cheongdo showed significantly increased growth. For the Cheongdo strain, a difference in the growth rate between glucose and galactose was observed with flask fermentation, which was not observed with microplates. We speculated that the different culture conditions in flasks (microaerobic) and microplates (no agitation) are associated with different growth patterns.
Figure 3.
Comparison of Biolog microplates (A) and Erlenmeyer flasks (B,C) for the growth of S. cerevisiae Cheongdo and EC1118 strains using glucose (A,B) and galactose (A,C) as a sole carbon source at 30 °C. (A) The average growth rate (OmniLog units/h) at 15 h on microplates. (B,C) Growth profiles (OD600, optical density at 600 nm). Control does not contain any carbon source. Two asterisks (**) indicate a significant difference at p < 0.01. An initial cell density was adjusted to 0.01 OD600 for both microplates and flasks.
3.3. Molecular Mechanism of the Difference in Galactose Fermentation
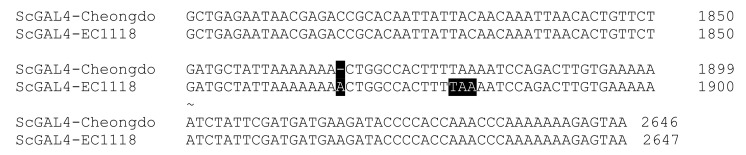
The galactose-negative phenotype of S. cerevisiae EC1118 and various industrial strains was previously reported, and the truncation of Gal4p, encoding a transcriptional activator of the GAL regulon, is responsible for the phenotype [28]. The Sanger sequencing of the GAL4 gene confirmed an insertional mutation and early termination of the GAL4 gene in EC1118 (Figure 4). However, Cheongdo had an intact GAL4 gene, which is identical to that of the laboratory S288C strain, although S288C cannot metabolize galactose because of mutations in other metabolic genes [28]. The genotype of the GAL4 gene supported the galactose-positive phenotype of S. cerevisiae Cheongdo, which was different from other industrial strains, including EC1118.
Figure 4.
Nucleotide sequence alignment of the GAL4 gene of S. cerevisiae Cheongdo (wild-type) and EC1118 (1867 insertion A) resulted in the frameshift and an early termination at TAA (highlighted in black; truncated Gal4p from 881 to 626 amino acids).
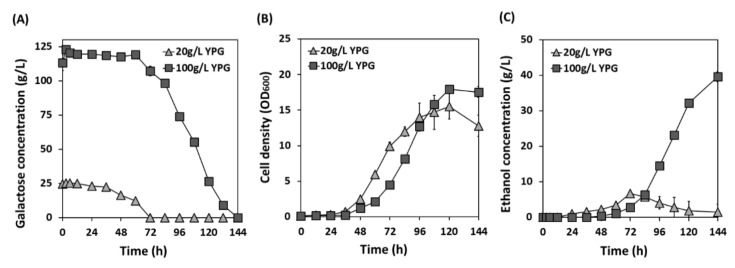
3.4. Galactose Fermentation at a Low Temperature
Our previous study reported that S. cerevisiae Cheongdo has an excellent glucose fermentation capability at 10°C [16]. This study also confirmed that Cheongdo was able to produce a significantly higher level of ethanol (0.60 g ethanol/L·h) than EC1118 (0.52 g ethanol/L·h) using 200 g/L of glucose at 10°C (Table 2). To test if the Cheongdo strain can ferment galactose at such a low temperature, 20 g/L and 100 g/L galactose were fermented at 10°C. As shown in Figure 5, the complete consumption of 20 g/L galactose took 72 h, and 6.8 g/L ethanol was produced (0.09 g ethanol/L·h, Table 2). At 100 g/L galactose, complete consumption was achieved in 144 h, and ethanol production and productivity were improved to 42.1 g/L and 0.29 g ethanol/L·h, respectively. Galactose fermentation at a low temperature slowed down the fermentation rate of Cheongdo by six times until the consumption of 100 g/L galactose was complete (from 24 h at 30 °C to 144 h at 10 °C). However, the ethanol titers (42.1–42.2 g/L) were not significantly affected by the fermentation temperature. From these results, it was concluded that the cold-tolerant phenotype of Cheongdo is applicable to both glucose and galactose fermentation.
Table 2.
Comparison of ethanol productivity (g/L-h) of cold-tolerant S. cerevisiae Cheongdo under various conditions
| Carbon Source 1 | 10 °C 2 | 30 °C 3 | ||
|---|---|---|---|---|
| Cheongdo | EC1118 | Cheongdo | EC1118 | |
| d-Glucose 20 g/L | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.66 ± 0.06 | 0.55 ± 0.02 |
| d-Glucose 200 g/L | 0.60 ± 0.00 * | 0.52 ± 0.00 | 3.33 ± 0.01 | 3.38 ± 0.03 |
| d-Galactose 20 g/L | 0.09 ± 0.01 | No growth | 0.59 ± 0.01 | No growth |
| d-Galactose 100 g/L | 0.29 ± 0.00 | No growth | 1.76 ± 0.17 | No growth |
* Significant difference between Cheongdo and EC1118 under the same conditions (p < 0.05). 1 Fermentation was performed at 130× rpm with an initial cell density of 0.1 OD600. 2 The average ethanol productivity at 10 °C was calculated at 72 h and 144 h for 20 g/L and 200 (100) g/L sugars, respectively. 3 The average ethanol productivity at 30 °C was calculated at 12 h and 24 h for 20 g/L and 200 (100) g/L sugars, respectively.
Figure 5.
Galactose fermentation capability of cold-tolerant S. cerevisiae Cheongdo at 10 °C. Galactose concentration (A), cell density (B), and ethanol concentration (C) were compared using galactose (20 g/L, 100 g/L) as a sole carbon source. Fermentation was performed at 10 °C and 130× rpm with an initial cell density of 0.1 OD600.
4. Discussion
In the present study, phenotype microarray was successfully applied to screen a substrate range and growth patterns of S. cerevisiae Cheongdo. Comparative analysis against a well-known industrial strain EC1118 confirmed that the growth of Cheongdo on conventional carbon sources, such as glucose and sucrose, is comparable to the growth of the industrial strain (i.e., no significant difference was found). Moreover, the superior galactose-fermenting phenotype of Cheongdo was easily identified via comparative phenotype microarray, which was also found to be industrially attractive.
Several industrial and laboratory strains of S. cerevisiae have the galactose-negative (Gal−) phenotype [28]. The Gal− phenotype of two representative wine yeast strains, EC1118 and LalvinQA23, can be explained by mutations in the GAL4 gene, which is a transcription factor required for GAL gene activation (GAL1/2/7/10) [29]. EC1118 and LalvinQA23 have different truncated GAL4 alleles, and are 626 amino acids (1867 insertion A), and 465 amino acids (1354 insertion A) in length, respectively, instead of the 881 amino acids of the wild-type sequence. The expression of the wild-type GAL4 gene in these strains recovered galactose metabolism, which confirmed that the truncated GAL4 alleles are responsible for their Gal− phenotype. Meanwhile, a representative laboratory strain S288C has the wild-type GAL4 gene, but it has a Gal− phenotype due to the nonsynonymous single-nucleotide polymorphisms in the GAL genes (GAL1/2/10) [30]. Although the Gal− phenotype is wildly spread among S. cerevisiae strains, S. cerevisiae Cheongdo is capable of fermenting galactose (20−100 g/L) even at a low temperature (10 °C), with ethanol productivities (0.09−0.29 g/L-h) comparable to those of glucose (0.10−0.60 g/L-h). To the best of our knowledge, this is the first report describing galactose fermentation by S. cerevisiae at 10 °C.
Limited information is available on the ethanol fermentation capabilities of galactose-fermenting (Gal+) S. cerevisiae strains. Representative laboratory strains, BY4743 and CEN.PK, showed 0.13–0.29 g/L-h ethanol productivities using 40–45 g/L galactose [31,32]. Their adaptive laboratory evolution improved ethanol productivities up to 0.68 g/L-h [33]. S. cerevisiae KL17 isolated from soil recorded the highest ethanol productivity (0.63–2.07 g/L-h) using 20–100 g/L galactose under highly aerobic conditions (200× rpm) with an initial cell density of 0.2–0.5 OD600 [34]. Given that conditions in the present study were less aerobic (130 rpm) with an lower initial cell density (0.1 OD600), the ethanol productivity of the Cheongdo strain (0.59–1.76 g/L-h) can be considered similar to that of the LK17 strain. Again, these results are limited to 30 °C fermentation, because there is no report of galactose fermentation at a lower temperature.
Several studies have reported metabolic engineering approaches to improve the galactose fermentation of S. cerevisiae [32,33,35]. The deletion of GAL gene repressors (GAL6, GAL80, and MIG1) improved the ethanol production from galactose, even though there were some growth defects [35]. An overexpression library led to the identification of truncated TUP1 as an overexpression target to activate GAL genes [32]. Recently, adaptive evolution successfully improved galactose fermentation, and their mechanisms were partially explained by a mutation in the GAL80 gene [33]. A milk-adapted natural isolate of S. cerevisiae showed the derepression of glucose on galactose, which was partially explained by mutations in the upstream repressing sequence (URS) sites of Gal genes, resulting in the lack of Mig1p-mediated repression [36]. Cheese strains of S. cerevisiae showed highly divergent alleles of high-affinity galactose transporter GAL2, which suggests that galactose uptake may limit galactose metabolism the most [13]. Meanwhile, the Cheongdo strain showed a similar ethanol productivity on both 20 g/L glucose and 20 g/L galactose, regardless of the fermentation temperature (Table 2). This efficient galactose fermentation capacity suggests that the GAL gene repressors of the Cheongdo strain may not function, which should be confirmed in a follow-up study.
The cold tolerance and galactose fermentation capacity of S. cerevisiae are phenotypes of particular interest in different foods and industrial fermentations. For coffee and cocoa bean fermentation, using a starter culture of S. cerevisiae was recently proposed for improved and consistent fermented products [37,38]. This is because the structural carbohydrates of coffee and cocoa beans are high in galactose [39,40], and their fermentation can be affected by the galactose fermentation capacity of S. cerevisiae. Moreover, galactose-rich food wastes, such as spent coffee grounds and cheese whey, can be transformed into value-added products by S. cerevisiae fermentation [41,42,43,44]. Lastly, red algae hydrolysates rich in galactose (approximately 20% of dry matter) [45] are promising alternative biomass for bioethanol and chemical production by S. cerevisiae [46,47]. Thus, S. cerevisiae Cheongdo has industrial potential that can be used for galactose-containing substrate fermentations, especially when low fermentation temperatures are preferred.
Author Contributions
Author Contributions: Conceptualization, K.-M.J. and S.R.K.; methodology, K.-M.J. and S.R.K.; software, J.P.; investigation, J.P., S.-H.J., and J.J.; data curation, J.P., S.-H.J., and J.J.; writing—original draft preparation, J.P.; writing—review and editing, K.-M.J., S.H.L., and S.R.K.; visualization, J.P. and J.J.; supervision, S.H.L. and S.R.K.; funding acquisition, K.-M.J. and S.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Rural Development Administration, Republic of Korea (PJ016164). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2019R1F1A1062633).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bochner B.R., Gadzinski P., Panomitros E. Phenotype MicroArrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 2001;11:1246–1255. doi: 10.1101/gr.186501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochner B.R. New technologies to assess genotype–phenotype relationships. Nat. Rev. Genet. 2003;4:309–314. doi: 10.1038/nrg1046. [DOI] [PubMed] [Google Scholar]
- 3.Warringer J., Blomberg A. Automated screening in environmental arrays allows analysis of quantitative phenotypic profiles in Saccharomyces cerevisiae. Yeast. 2003;20:53–67. doi: 10.1002/yea.931. [DOI] [PubMed] [Google Scholar]
- 4.Vaas L.A.I., Sikorski J., Hofner B., Fiebig A., Buddruhs N., Klenk H.P., Goker M. opm: An R package for analysing OmniLog((R)) phenotype microarray data. Bioinformatics. 2013;29:1823–1824. doi: 10.1093/bioinformatics/btt291. [DOI] [PubMed] [Google Scholar]
- 5.Warringer J., Ericson E., Fernandez L., Nerman O., Blomberg A. High-resolution yeast phenomics resolves different physiological features in the saline response. Proc. Natl. Acad. Sci. USA. 2003;100:15724–15729. doi: 10.1073/pnas.2435976100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Ricaud L., Warringer J., Ericson E., Pylvänäinen I., Kemp G.J.L., Nerman O., Blomberg A. PROPHECY—A database for high-resolution phenomics. Nucleic Acids Res. 2005;33:D369–D373. doi: 10.1093/nar/gki126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baryshnikova A., Costanzo M., Kim Y., Ding H., Koh J., Toufighi K., Youn J.-Y., Ou J., San Luis B.-J., Bandyopadhyay S., et al. Quantitative analysis of fitness and genetic interactions in yeast on a genome scale. Nat. Methods. 2010;7:1017–1024. doi: 10.1038/nmeth.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skelly D.A., Merrihew G.E., Riffle M., Connelly C.F., Kerr E.O., Johansson M., Jaschob D., Graczyk B., Shulman N.J., Wakefield J., et al. Integrative phenomics reveals insight into the structure of phenotypic diversity in budding yeast. Genome Res. 2013;23:1496–1504. doi: 10.1101/gr.155762.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Märtens K., Hallin J., Warringer J., Liti G., Parts L. Predicting quantitative traits from genome and phenome with near perfect accuracy. Nat. Commun. 2016;7:11512. doi: 10.1038/ncomms11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang K., Bergdahl B., Machado D., Dato L., Han T.L., Li J., Villas-Boas S., Herrgard M.J., Forster J., Panagiotou G. Linking genetic, metabolic, and phenotypic diversity among Saccharomyces cerevisiae strains using multi-omics associations. Gigascience. 2019;8:giz015. doi: 10.1093/gigascience/giz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyma K.E., Saerens S.M., Verstrepen K.J., Fay J.C. Divergence in wine characteristics produced by wild and domesticated strains of Saccharomyces cerevisiae. FEMS Yeast Res. 2011;11:540–551. doi: 10.1111/j.1567-1364.2011.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peter J., De Chiara M., Friedrich A., Yue J.X., Pflieger D., Bergstrom A., Sigwalt A., Barre B., Freel K., Llored A., et al. Genome evolution across 1011 Saccharomyces cerevisiae isolates. Nature. 2018;556:339. doi: 10.1038/s41586-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legras J.L., Galeote V., Bigey F., Camarasa C., Marsit S., Nidelet T., Sanchez I., Couloux A., Guy J., Franco-Duarte R., et al. Adaptation of S. cerevisiae to Fermented Food Environments Reveals Remarkable Genome Plasticity and the Footprints of Domestication. Mol. Biol. Evol. 2018;35:1712–1727. doi: 10.1093/molbev/msy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parapouli M., Vasileiadis A., Afendra A.-S., Hatziloukas E. Saccharomyces cerevisiae and its industrial applications. Aims Microbiol. 2020;6:1–31. doi: 10.3934/microbiol.2020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lengeler K.B., Stovicek V., Fennessy R.T., Katz M., Förster J. Never Change a Brewing Yeast? Why Not, There Are Plenty to Choose From. Front. Genet. 2020;11:582789. doi: 10.3389/fgene.2020.582789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung K.-M., Lee Y., Kim J.-w., Seol J., Jung Y.H., Kim S.R. Low-temperature alcoholic fermentation for the production of high quality vinegar using peach. KSBB J. 2018;33:95–103. doi: 10.7841/ksbbj.2018.33.2.95. [DOI] [Google Scholar]
- 17.Jeong D., Park H., Bae E.-Y., Seol M.-K., Ju Y.-B., Kim J.-S., Jang B.-K., Akhmadjon S., Kim B.-O., Cho Y.-J., et al. Source of contamination, detection techniques, and control methods of spoilage yeast in fermented foods. KSBB J. 2020;35:23–33. doi: 10.7841/ksbbj.2020.35.1.23. [DOI] [Google Scholar]
- 18.Liszkowska W., Berlowska J. Yeast Fermentation at Low Temperatures: Adaptation to Changing Environmental Conditions and Formation of Volatile Compounds. Molecules. 2021;26:1035. doi: 10.3390/molecules26041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magalhães F., Calton A., Heiniö R.-L., Gibson B. Frozen-dough baking potential of psychrotolerant Saccharomyces species and derived hybrids. Food Microbiol. 2021;94:103640. doi: 10.1016/j.fm.2020.103640. [DOI] [PubMed] [Google Scholar]
- 20.Aguilera J., Randez-Gil F., Prieto J.A. Cold response in Saccharomyces cerevisiae: New functions for old mechanisms. Fems Microbiol. Rev. 2007;31:327–341. doi: 10.1111/j.1574-6976.2007.00066.x. [DOI] [PubMed] [Google Scholar]
- 21.Feng L., Jia H., Qin Y., Song Y., Tao S., Liu Y. Rapid Identification of Major QTLS Associated With Near- Freezing Temperature Tolerance in Saccharomyces cerevisiae. Front. Microbiol. 2018;9:2110. doi: 10.3389/fmicb.2018.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libkind D., Hittinger C.T., Valério E., Gonçalves C., Dover J., Johnston M., Gonçalves P., Sampaio J.P. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA. 2011;108:14539–14544. doi: 10.1073/pnas.1105430108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Tovar G., Minebois R., Barrio E., Querol A., Pérez-Torrado R. Aroma production and fermentation performance of S. cerevisiae × S. kudriavzevii natural hybrids under cold oenological conditions. Int. J. Food Microbiol. 2019;297:51–59. doi: 10.1016/j.ijfoodmicro.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Kopsahelis N., Nisiotou A., Kourkoutas Y., Panas P., Nychas G.J.E., Kanellaki M. Molecular characterization and molasses fermentation performance of a wild yeast strain operating in an extremely wide temperature range. Bioresour. Technol. 2009;100:4854–4862. doi: 10.1016/j.biortech.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Paget C.M., Schwartz J.-M., Delneri D. Environmental systems biology of cold-tolerant phenotype in Saccharomyces species adapted to grow at different temperatures. Mol. Ecol. 2014;23:5241–5257. doi: 10.1111/mec.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bou Zeidan M., Zara G., Viti C., Decorosi F., Mannazzu I., Budroni M., Giovannetti L., Zara S. l-histidine inhibits biofilm formation and FLO11-associated phenotypes in Saccharomyces cerevisiae flor yeasts. PLoS ONE. 2014;9:e112141. doi: 10.1371/journal.pone.0112141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borglin S., Joyner D., DeAngelis K.M., Khudyakov J., D’haeseleer P., Joachimiak M.P., Hazen T. Application of phenotypic microarrays to environmental microbiology. Curr. Opin. Biotechnol. 2012;23:41–48. doi: 10.1016/j.copbio.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Dulermo R., Legras J.L., Brunel F., Devillers H., Sarilar V., Neuveglise C., Nguyen H.V. Truncation of Gal4p explains the inactivation of the GAL/MEL regulon in both Saccharomyces bayanus and some Saccharomyces cerevisiae wine strains. FEMS Yeast Res. 2016;16:fow070. doi: 10.1093/femsyr/fow070. [DOI] [PubMed] [Google Scholar]
- 29.Zhou P., Xu N., Yang Z., Du Y., Yue C., Ye L. Directed evolution of the transcription factor Gal4 for development of an improved transcriptional regulation system in Saccharomyces cerevisiae. Enzym. Microb. Technol. 2020;142:109675. doi: 10.1016/j.enzmictec.2020.109675. [DOI] [PubMed] [Google Scholar]
- 30.Otero J.M., Vongsangnak W., Asadollahi M.A., Olivares-Hernandes R., Maury J., Farinelli L., Barlocher L., Osterås M., Schalk M., Clark A., et al. Whole genome sequencing of Saccharomyces cerevisiae: From genotype to phenotype for improved metabolic engineering applications. BMC Genom. 2010;11:723. doi: 10.1186/1471-2164-11-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goshima T., Tsuji M., Inoue H., Yano S., Hoshino T., Matsushika A. Bioethanol Production from Lignocellulosic Biomass by a Novel Kluyveromyces marxianus Strain. Biosci. Biotechnol. Biochem. 2013;77:1505–1510. doi: 10.1271/bbb.130173. [DOI] [PubMed] [Google Scholar]
- 32.Lee K.-S., Hong M.-E., Jung S.-C., Ha S.-J., Yu B.J., Koo H.M., Park S.M., Seo J.-H., Kweon D.-H., Park J.C., et al. Improved galactose fermentation of Saccharomyces cerevisiae through inverse metabolic engineering. Biotechnol. Bioeng. 2011;108:621–631. doi: 10.1002/bit.22988. [DOI] [PubMed] [Google Scholar]
- 33.Quarterman J., Skerker J.M., Feng X., Liu I.Y., Zhao H., Arkin A.P., Jin Y.-S. Rapid and efficient galactose fermentation by engineered Saccharomyces cerevisiae. J. Biotechnol. 2016;229:13–21. doi: 10.1016/j.jbiotec.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.H., Ryu J., Huh I.Y., Hong S.-K., Kang H.A., Chang Y.K. Ethanol production from galactose by a newly isolated Saccharomyces cerevisiae KL17. Bioprocess. Biosyst. Eng. 2014;37:1871–1878. doi: 10.1007/s00449-014-1161-1. [DOI] [PubMed] [Google Scholar]
- 35.Ostergaard S., Olsson L., Johnston M., Nielsen J. Increasing galactose consumption by Saccharomyces cerevisiae through metabolic engineering of the GAL gene regulatory network. Nat. Biotechnol. 2000;18:1283–1286. doi: 10.1038/82400. [DOI] [PubMed] [Google Scholar]
- 36.Duan S.-F., Shi J.-Y., Yin Q., Zhang R.-P., Han P.-J., Wang Q.-M., Bai F.-Y. Reverse Evolution of a Classic Gene Network in Yeast Offers a Competitive Advantage. Curr. Biol. 2019;29:1126–1136.e1125. doi: 10.1016/j.cub.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Kouamé C., Loiseau G., Grabulos J., Boulanger R., Mestres C. Development of a model for the alcoholic fermentation of cocoa beans by a Saccharomyces cerevisiae strain. Int. J. Food Microbiol. 2021;337:108917. doi: 10.1016/j.ijfoodmicro.2020.108917. [DOI] [PubMed] [Google Scholar]
- 38.Bressani A.P.P., Martinez S.J., Evangelista S.R., Dias D.R., Schwan R.F. Characteristics of fermented coffee inoculated with yeast starter cultures using different inoculation methods. LWT. 2018;92:212–219. doi: 10.1016/j.lwt.2018.02.029. [DOI] [Google Scholar]
- 39.Barišić V., Kopjar M., Jozinović A., Flanjak I., Ačkar Đ., Miličević B., Šubarić D., Jokić S., Babić J. The Chemistry behind Chocolate Production. Molecules. 2019;24:3163. doi: 10.3390/molecules24173163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redgwell R.J., Curti D., Rogers J., Nicolas P., Fischer M. Changes to the galactose/mannose ratio in galactomannans during coffee bean (Coffea arabica L.) development: Implications for in vivo modification of galactomannan synthesis. Planta. 2003;217:316–326. doi: 10.1007/s00425-003-1003-x. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.-w., Jang J.H., Yeo H.J., Seol J., Kim S.R., Jung Y.H. Lactic Acid Production from a Whole Slurry of Acid-Pretreated Spent Coffee Grounds by Engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019;189:206–216. doi: 10.1007/s12010-019-03000-6. [DOI] [PubMed] [Google Scholar]
- 42.Mussatto S.I., Machado E.M.S., Carneiro L.M., Teixeira J.A. Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy. 2012;92:763–768. doi: 10.1016/j.apenergy.2011.08.020. [DOI] [Google Scholar]
- 43.Domingues L., Guimarães P.M.R., Oliveira C. Metabolic engineering of Saccharomyces cerevisiae for lactose/whey fermentation. Bioeng Bugs. 2010;1:164–171. doi: 10.4161/bbug.1.3.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner T.L., Kim E., Hwang C., Zhang G.-C., Liu J.-J., Jin Y.-S. Short communication: Conversion of lactose and whey into lactic acid by engineered yeast. J. Dairy Sci. 2017;100:124–128. doi: 10.3168/jds.2016-11784. [DOI] [PubMed] [Google Scholar]
- 45.Kim S.R., Ha S.-J., Wei N., Oh E.J., Jin Y.-S. Simultaneous co-fermentation of mixed sugars: A promising strategy for producing cellulosic ethanol. Trends Biotechnol. 2012;30:274–282. doi: 10.1016/j.tibtech.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Kim D.H., Liu J.J., Lee J.W., Pelton J.G., Yun E.J., Yu S., Jin Y.S., Kim K.H. Biological upgrading of 3,6-anhydro-l-galactose from agarose to a new platform chemical. Green Chem. 2020;22:1776–1785. doi: 10.1039/C9GC04265B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H.-J., Kim S.-J., Yoon J.-J., Kim K.H., Seo J.-H., Park Y.-C. Evolutionary engineering of Saccharomyces cerevisiae for efficient conversion of red algal biosugars to bioethanol. Bioresour. Technol. 2015;191:445–451. doi: 10.1016/j.biortech.2015.03.057. [DOI] [PubMed] [Google Scholar]