Abstract
Bacterial leaf blight, which is caused by Xanthomonas axonopodis pv. allii, annually causes significant yield losses to Welsh onion in many producing countries, including Vietnam. In this study, we isolated and characterized lytic phages Φ16, Φ17A and Φ31, specific to X. axonopodis pv. allii and belonging to a new phage species and genus within the Autographiviridae, from four provinces in the Mekong Delta of Vietnam. Moreover, we evaluated their efficacy for the biocontrol of leaf blight in greenhouse and field conditions. When applying the three highly related phages individually or as a three-phage cocktail at 108 PFU/mL in greenhouse conditions, our results show that treatment with Φ31 alone provides higher disease prevention than the two other phages or the phage cocktail. Furthermore, we compared phage concentrations from 105 to 108 and showed optimal disease control at 107 and 108 PFU/mL. Finally, under field conditions, both phage Φ31 alone and the phage cocktail treatments suppressed disease symptoms, which was comparable to the chemical bactericide oxolinic acid (Starner). Phage treatment also significantly improved yield, showing the potential of phage as a biocontrol strategy for managing leaf blight in Welsh onion.
Keywords: bacterial leaf blight, bacteriophages, Welsh onion, Xanthomonas axonopodis pv. allii, biocontrol
1. Introduction
Welsh onion (Allium fistulosum L.) is an important vegetable crop in Vietnam and other Asian countries, such as China and India, which are by far the largest onion producers in the world. This vegetable is mainly grown for its green leaves, which are widely used in Asian cuisine. Welsh onion, which is intensively cultivated throughout the year, is unfortunately also prone to bacterial infections. Bacterial leaf blight, caused by Xanthomonas axonopodis pv. allii (Xaa), is distributed worldwide and is one of the most important diseases for several onion species. Epidemic outbreaks have already been reported in several Allium spp. [1]. The bacterium infects all stages of the plant. Primary inoculum commonly comes from infected seeds, seedlings and plant debris [2,3]. The bacterium penetrates through the leaf stomata and disease rapidly progresses during periods of high humidity. Consequently, during wet and rainy seasons, the leaves rapidly collapse and the plant eventually dies [4]. Therefore, crop yields are significantly reduced, which has been reported for many growing areas worldwide [1,5,6,7].
To manage this bacterial disease, farmers mainly rely on chemical bactericides such as copper compounds and antibiotics, which can have negative effects on beneficial microbial communities and promote the development of resistant strains. In addition, overuse of antibiotics in agriculture would promote the transmission of antibiotic resistance genes from plant bacterial pathogens to human pathogens [8,9,10]. Moreover, chemical residues gradually build up in the environment and the food chain, which is undesirable [11,12].
Currently, biocontrol with bacteriophages is considered a promising alternative strategy for bacterial disease management [13,14]. The application of phages has been shown to be successful in controlling both soilborne [15,16] and airborne plant diseases [17,18,19]. For instance, Lang et al. [20] showed that biweekly phage applications could reduce disease severity symptoms of bacterial leaf blight on onion equal to or better than weekly applications of copper hydroxide plus mancozeb.
This study aims at controlling bacterial leaf blight on Welsh onion using phage biocontrol in Vietnam. We screened phages isolated from Xaa infected onion leaves and we selected three promising ones based on host range and plaque/halo size. These were then characterized by whole genome sequencing. The selected lytic phages were evaluated for their potential of controlling X. axonopodis pv. allii both in vitro and in greenhouse and field conditions.
2. Results
2.1. Isolation of Bacteriophages of Xanthomonas axonopodis pv. allii, Causing Leaf Blight on Welsh Onion in the Mekong Delta in Vietnam
In total, twelve Xaa strains and ten specific phage isolates were isolated from infected leaf samples collected from five provinces in the Mekong Delta of Vietnam: Vinh Long, An Giang, Can Tho, Bac Lieu and Soc Trang (Table 1). All twelve Xaa strains were able to cause disease during an in planta pathogenicity test.
Table 1.
Different Xanthomonas axonopodis pv. allii strains and phages isolated from infected Welsh onion leaves collected from different provinces in the Mekong Delta of Vietnam.
| Location (Mekong Delta, Vietnam) |
Host Isolate | Bacteriophage Isolate |
|---|---|---|
| Binh Tan-Vinh Long | XaaVL02 | |
| Chau Phu-An Giang | XaaAG04 | |
| Binh Tan-Vinh Long | XaaVL05 | Φ5A, Φ5B |
| Binh Tan-Vinh Long | XaaVL06 | Φ6 |
| Binh Tan-Vinh Long | XaaVL07 | Φ7A, Φ7B |
| O Mon-Can Tho | XaaCT10 | |
| Hiep Thanh-Bac Lieu | XaaBL11 | |
| Vinh Trach Đong-Bac Lieu | XaaBL12 | |
| Hiep Thanh-Bac Lieu | XaaBL13 | Φ14 |
| Hiep Thanh-Bac Lieu | XaaBL14 | Φ16 |
| Vinh Trach Đong-Bac Lieu | XaaBL17 | Φ17A, Φ17B |
| Vinh Chau-Soc Trang | XaaST22 | Φ31 |
Next, a host range analysis shows that the number of Xaa strains that are susceptible to each of the ten phage isolates ranges from four to twelve and clearly depends on the phage isolate (Table 2). Five phages (Φ6, Φ16, Φ17A, Φ17B and Φ31) expressed a broad host range with at least 9 out of 12 tested Xaa being susceptible to infection. Phage Φ31 even shows activity against all tested Xaa strains.
Table 2.
Evaluation of the host spectrum of ten Xaa phage isolates against twelve Xaa strains. Susceptibility of the Xaa strain to a phage is indicated with a ‘+’, while resistance is indicated with a ‘−‘. The bottom row indicates the total amount of strains susceptible to a certain phage.
| Xaa Strain | Xaa Phage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Φ5A | Φ5B | Φ6 | Φ7A | Φ7B | Φ14 | Φ16 | Φ17A | Φ17B | Φ31 | |
| XaaVL02 | + | + | − | + | + | − | + | + | + | + |
| XaaAG04 | − | − | + | − | − | − | + | + | − | + |
| XaaVL05 | − | − | + | + | + | − | + | + | + | + |
| XaaVL06 | − | − | + | − | − | + | + | + | + | + |
| XaaVL07 | − | − | + | + | + | + | + | + | + | + |
| XaaCT10 | + | + | + | − | − | − | + | + | + | + |
| XaaBL11 | + | + | + | + | + | + | + | + | + | + |
| XaaBL12 | − | − | − | + | + | + | − | + | + | + |
| XaaBL13 | + | + | + | − | − | − | + | + | + | + |
| XaaBL14 | + | + | − | + | + | − | + | − | − | + |
| XaaBL17 | − | − | + | − | − | + | + | + | + | + |
| XaaST22 | − | − | + | + | + | + | + | + | − | + |
| Total | 5 | 5 | 9 | 7 | 7 | 6 | 11 | 11 | 9 | 12 |
Of the twelve Xaa strains, XaaBL11 appears to be the most susceptible to phage infection as all tested phages were able to infect this strain. Moreover, this strain is causing severe symptoms in an in planta pathogenicity test. Therefore, this bacterium was selected as an amplification strain for phage propagation and for further experiments both in vitro and in vivo.
For the five phages with the broadest host range (Φ6, Φ16, Φ17A, Φ17B and Φ31), the plaque/halo diameter was monitored for 72 h. After 24 h incubation, phage Φ31 displayed the largest halo diameter (6.5 mm), compared to the other four phages. Φ16, Φ17A and Φ6 produced a halo diameter of 5.9 mm, 5.2 mm and 4.9 mm, respectively, all significantly bigger than the one for Φ17B (3.9 mm) (Table 3).
Table 3.
Halo sizes of five phages on a lawn of Xaa strain XaaBL11, 24, 48 and 72 h after plating.
| Phage Isolate | Location of Isolation | Halo Diameter (mm) 1 | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| Φ6 | Binh Tan-Vinh Long | 4.9 b | 5.9 c | 8.7 b |
| Φ16 | Vinh Trach Dong-Bac Lieu | 5.9 a | 11.2 a | 12.7 a |
| Φ17A | Hiep Thanh-Bac Lieu | 5.2 b | 7.2 b | 10.3 b |
| Φ17B | Hiep Thanh-Bac Lieu | 3.9 c | 6.0 c | 6.6 c |
| Φ31 | Vinh Chau-Soc Trang | 6.5 a | 11.5 a | 12.3 a |
1 Means followed by a different letter (a–c) in the same column do differ significantly (Tukey’s test; p-value < 0.05).
After incubation for 48 h, two phages, Φ31 and Φ16, showed the largest halo diameters measuring 11.5 mm and 11.2 mm, respectively. The other three phages showed diameters between 5.9 and 7.2 mm. Finally, 72 h after plating, Φ16 and Φ31 still displayed the biggest halo diameters (12.7 mm and 12.3 mm, respectively), followed by Φ17A (10.3 mm) and phage Φ6 and Φ17B (Appendix A Figure A1). These data show that the halos around the plaques, produced by the different phages in collection, increase over time, hinting at exopolysaccharides (EPS)-degrading properties associated with the phage tail.
2.2. Phage Characterization by Transmission Electron Microscopy and Whole-Genome Sequencing
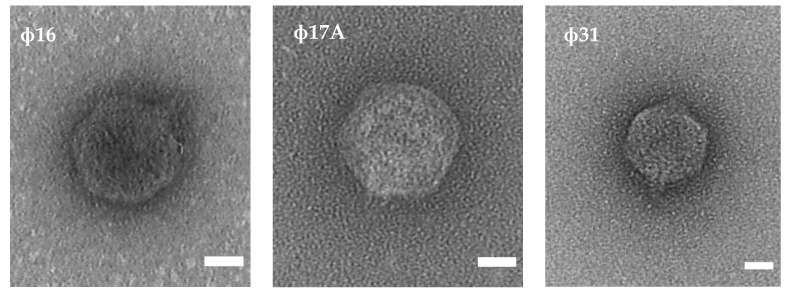
The three phages showing the biggest halo zones (Φ16, Φ17A and Φ31) were selected for further characterization. Based on transmission electron microscopy (TEM), all three phages were podoviruses, characterized by an icosahedral head and a short tail (Figure 1).
Figure 1.
TEM analysis shows the morphology of the three selected phages. All three phage virions contain an icosahedral head and a short tail, typically for the podoviruses. The white scale bar represents 20 nm.
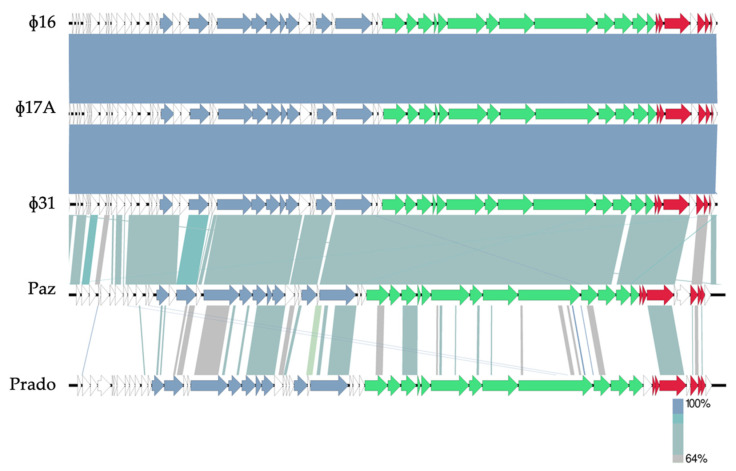
Subsequently, the genomes of the phages were sequenced, assembled and annotated (Figure 2). Based on a BLASTn analysis, phages Φ16, Φ17A and Φ31 have an 81% nucleotide similarity to Xylella phage Paz [21]. Furthermore, a Viptree proteome analysis shows that the three selected Xaa phages from the current collection can be classified within the family of Autographiviridae and are related to the Pradovirus genus. However, as there is only 9% nucleotide similarity between Φ16, Φ17A and Φ31 and Xylella phage Prado, they represent a new phage species [22] within the same new phage genus as phage Paz.
Figure 2.
Genome maps of Φ16, Φ17A and Φ31 and comparison between the genomes and related phages Paz and Prado using a BLASTn analysis. The selected phages follow a modular genome organization, typical for lytic phages of the Autographiviridae: first an early transcribed region with a lot of ORFan genes, followed by the DNA metabolism region, the structural protein region and the lysis cassette. Arrows represent the different coding sequences: in white—encoding hypothetical proteins, blue—encoding DNA-associated proteins, green—encoding structural proteins and red—encoding the lysis cassette (adapted from EasyFig).
Based on phage sequencing and the Autographiviridae genome architecture, there are no genes suggesting a temperate lifestyle for these phages. Moreover, the phage genomes do not encode known proteins associated with virulence or antibiotic resistance. Based on the amino acid sequence of the large terminase subunit, phages Φ16, Φ17A and Φ31 have short direct terminal repeat (DTR) sequences (250 bp) at the end of the sequence and hence a phage T7-like packaging strategy [23]. It is therefore unlikely that these phages will be able to transduce genetic material due to packaging artifacts. In conclusion, these phages are suitable candidates for phage biocontrol.
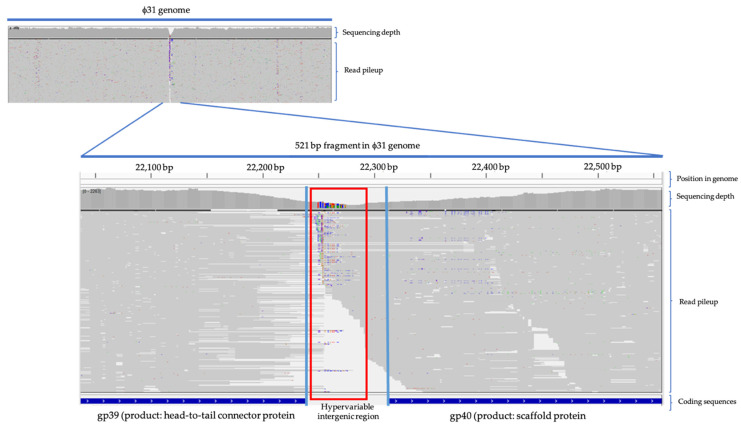
The genomic differences between Φ16, Φ17A and Φ31 are minimal. A variant calling between Φ16 and Φ17A shows a point mutation in Φ17A (A2858 to C2858 in 96% of the sequencing reads, with an e-value of 1.36 × 10−114), which results in a loss of the start codon of Φ16 gp12. In the case of Φ31, a hypervariable region was discovered while mapping the reads of Φ31 on the reference genome of Φ16. This region is located between gp39 (gene encoding the head-to-tail connector protein) and gp40 (gene encoding the scaffold protein). Here, a drop in the read pileup suggests a deletion event of the intergenic region between the two genes. The remaining reads show an accumulation of mutations in this specific intergenic region (Appendix A Figure A2).
2.3. Efficacy of Phages against Bacterial Leaf Blight on Welsh Onion in Greenhouse Conditions
The three selected lytic Xaa phages were evaluated for their biocontrol potential in a greenhouse experiment. Table 4 shows the percentage of infected leaf tissue for the following treatments of Welsh onion plants, which were all infected with Xaa: a monophage treatment, a phage cocktail treatment, no treatment and a commercial bactericide treatment. Nine days after inoculation (dai), a significant difference can be distinguished between each treatment, compared to the non-treated, infected control (67.5% infected leaf area). Similar reductions were obtained for phages Φ16 and Φ17A and the cocktail of the three phages (between 37.2 and 43.3% infected leaf area), which is still significantly higher compared to the diseased area in the Φ31 monophage treated plants (26.6% infected leaf area). The performance of the phages compared to the chemical bactericide was lower, as the area of diseased tissue was 18.3% when the plants were treated with oxolonic acid (Starner).
Table 4.
Percentage of leaf area infection caused by X. axonopodis pv. allii (XaaBL11) after different treatments in greenhouse conditions.
| Treatment | Percentage of Infected Leaf Area (%) 1 | ||
|---|---|---|---|
| 5 dai 2 | 7 dai 2 | 9 dai 2 | |
| Φ16 | 20.2 b | 33.2 b | 37.2 b |
| Φ17A | 22.6 b | 38.9 b | 39.8 b |
| Φ31 | 11.4 c | 22.4 c | 26.6 c |
| Three-phage cocktail | 26.3 ab | 40.3 b | 43.3 b |
| Starner | 7.7 c | 15.7 c | 18.3 d |
| Bacteria only | 30.5 a | 51.2 a | 67.5 a |
1 Means followed by a different letter (a–c) in the same column do differ significantly (Duncan’s test; p-value < 0.05). 2 Days after inoculation with XaaBL11.
Disease protection attributed to monophage treatment was the lowest for phage Φ17A (39.8% infected leaf area versus 67.5% for the untreated object) and the highest for phage Φ31 (26.6% infected tissue) at nine days after inoculation. In addition, the phage cocktail protected less compared to the individual phages (43.3% infected tissue). These results correspond to the phage concentrations on the leaf surface (Table 5), since the Φ31 titer on the leaf surface was significantly higher compared to the other individual phages and the phage cocktail 72 h after inoculation. Representative plants from the various treatments in the greenhouse trial are visually shown in Figure 3.
Table 5.
Bacteriophage density (pfu/g leaf) on Welsh onion phyllosphere at various timepoints after pathogen inoculation.
| Treatment | Density of Bacteriophage on Leaf Surface (pfu/g leaf) 1 | ||
|---|---|---|---|
| 0 hai 2 | 48 hai 2 | 72 hai 2 | |
| Φ16 | 3.53 b | 8.55 a | 7.35 bc |
| Φ17A | 4.04 ab | 8.42 a | 7.89 b |
| Φ31 | 3.97 b | 8.73 a | 9.03 a |
| Three-phage cocktail | 4.15 a | 7.60 b | 6.97 c |
1 Means followed by a different letter (a–c) in the same column do differ significantly (Duncan’s test; p-value < 0.05). 2 Hours after inoculation with XaaBL11.
Figure 3.
Development of leaf blight caused by X. axonopodis pv. allii on Welsh onion in greenhouse conditions. Five different treatments were tested for their efficacy to control leaf blight symptoms nine days after infection: (A) Oxolonic acid (Starner), (B) Φ 31, (C) Φ 16, (D) Φ 17A, (E) three-phage cocktail (Φ 16, Φ 17A and Φ 31) and (F) Control (bacteria only).
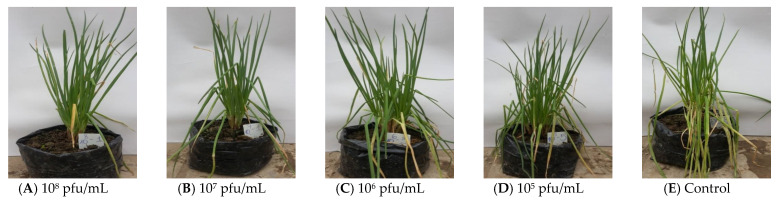
The optimal phage Φ31 concentration needed to treat the Welsh onion plants to achieve maximum disease control was determined by testing phage concentrations ranging from 105 to 108 pfu/mL (Table 6). A concentration as low as 105 pfu/mL significantly reduced the percentage of symptomatic leaf tissue compared to the control. However, phage applications at 107–108 pfu/mL appeared to be optimal as there was an equal, yet lower percentage of infected leaf area measured compared to a treatment with 105 or 106 pfu/mL. Appendix A Figure A3 provides an indication of the symptoms visible on the onion plants.
Table 6.
Percentage of leaf area infection caused by X. axonopodis pv. allii (XaaBL11) in different phage titer treatments of phage Φ31 in greenhouse conditions.
| Treatments | Percentage of Leaf Area Infection (%) 1 | ||
|---|---|---|---|
| 4 dai 2 | 6 dai 2 | 8 dai 2 | |
| 105 pfu/mL | 8.2 b | 21.4 b | 43.1 b |
| 106 pfu/mL | 6.1 bc | 16.6 bc | 34.8 c |
| 107 pfu/mL | 3.3 cd | 9.4 cd | 20.3 d |
| 108 pfu/mL | 0.6 d | 4.9 d | 18.4 d |
| Control | 15.9 a | 34.1 a | 60.0 a |
1 Means followed by a different letter (a–d) in the same column do differ significantly (Duncan’s test; p-value < 0.05). 2 Days after inoculation with XaaBL11.
2.4. Efficacy of Bacteriophage in Controlling Bacterial Leaf Blight in Field Conditions
Phage Φ31 and the phage cocktail were tested for their efficacy in a field trial where the plants were artificially inoculated (Table 7). The disease developed rapidly in the different test plants (four days after inoculation). At three different time points, all treatments, i.e., phage Φ31, three-phage cocktail at titer 108 pfu/mL and Starner, had a disease index and area under disease progress curve (AUDPC) significantly lower than the control. At four days after inoculation, phage Φ31, the phage cocktail and Starner displayed equal disease protection. Nine days after inoculation, Φ31 proved to be significantly better in disease reduction than the phage cocktail with a disease index of 13.1% and 17.8%, respectively. However, 15 days after inoculation, both phage treatments performed equally in disease reduction.
Table 7.
Efficacy of phage and oxolinic acid treatments on the control of bacterial leaf blight of rice caused by X. axonopodis pv. allii (XaaBL11) and on yield under field conditions.
| Treatments | Disease Index 1 | AUDPC 3 | Actual Yield (kg/25 m2) | Commercial Yield (kg/25 m2) | ||
|---|---|---|---|---|---|---|
| 4 dai 2 | 9 dai 2 | 15 dai 2 | ||||
| Control | 18.3 a | 23.2 a | 28.2 a | 288.8 a | 31.4 b | 26.3 b |
| Φ31 | 11.5 b | 13.1 c | 18.2 bc | 178.8 b | 34.0 ab | 30.9 a |
| Three-phage cocktail | 13.1 b | 17.8 b | 22.1 b | 216.6 b | 33.9 ab | 29.0 ab |
| Starner | 13.9 b | 14.6 bc | 15.9 c | 189.2 b | 34.8 a | 31.3 a |
| p-value 4 | 0.0087 | 0.0030 | 0.0013 | 0.0001 | 0.0238 | 0.0027 |
1 Means followed by the same letter ((a–c) in the same column do not differ statistically among themselves by a Tukey’s test (p < 0.05). 2 Days after inoculation with XaaBL11. 3 Area under disease progress curve. 4 Probability that there are no differences in treatment means according to an analysis of variance.
The plants treated with the bactericide and Φ31 still showed higher disease control based on AUDPC at the end of the experiment, with a disease index significantly lower compared to the phage cocktail (Figure 4). This shows that the phage treatment performs equally well as a commercially available bactericide treatment. With regard to actual yield, only plants treated with Starner had a significantly higher yield. However, with regard to the commercial yield levels, both the phage Φ31 treatment and Starner provided significantly higher yields than the control.
Figure 4.
Bacterial leaf blight infection caused by Xanthomonas axonopodis pv. allii in field conditions. Two different treatments are being shown: (A) bacteria only treatment and (B) bacteria and phage Φ31 treatment.
3. Discussion
3.1. Isolation of Promising Bacteriophages for Biocontrol of Xanthomonas axonopodis pv. allii
In this study, we isolated ten Xaa-specific phages displaying different host ranges. Our results indicate that phages are commonly present on infected onion leaves, since seven out of twelve samples contained phages. This ratio is higher compared to other systems such as the walnut—X. arboricola pv. juglandis pathosystem where 26 phages were isolated from 126 samples [24]. The isolated phages showed different host ranges when screened on all the Xaa strains. Good candidates for phage biocontrol preferably do not have a too narrow host range to maximize the chance that they can also lyse unknown Xaa strains in field conditions [25]. Alternatively, phage cocktails can be applied to further increase this host range. Therefore, out of five promising phages with the widest host range, the three phages (Φ16, Φ17A and Φ31) with the largest halo diameter and broadest host range were selected for further investigation. Interestingly, the three phages having the biggest halos around the plaques also have the broadest host range, which suggests a correlation between halo size and host range characteristics and should be investigated further, since it could be useful information in the in vitro selection of promising phages for biological control.
For the different phages in the collection, we observed halos around the plaques, increasing over time. This can indicate an EPS-degrading capacity by the bacteriophage through the presence of a polysaccharide depolymerase on the phage particles [26,27]. Olszak et al. [28] demonstrated that this capacity is related to the efficacy of the phage in reducing the pathogenicity of the bacterial pathogen, which also should be investigated for the selected phages in future research.
Genome sequencing confirmed the three selected phages Φ16, Φ17A and Φ31 are very closely related podoviruses, belonging to the Autographiviridae family, and that they have a lytic lifestyle. Strictly lytic phages are preferred for phage therapy and biocontrol to ensure that horizontal gene transfer between bacteria is limited [14,15,29]. Although the phages are almost identical at the genome level, they showed some minor differences in the host range. The observed SNPs responsible for these differences could be matched to a hypervariable region upstream of gp40 (gene encoding the scaffold protein) in Φ31. In general, these scaffolding proteins function as assemblers of the mature viral particle. Literature shows that these viral proteins have also obtained alternative functions such as immune evasion and receptor recognition [30]. As such, we can hypothesize that indeed changes in transcription or translation of this scaffolding protein are related to the differences in observed host range.
3.2. Efficacy of Phages against Bacterial Leaf Blight on Welsh Onion in Greenhouse and Field Conditions
Both greenhouse and field trials were performed to test the efficacy of the isolated Xaa phages in practice. These trials show that indeed both a single phage Φ31 and a phage cocktail consisting of three phages (Φ16, Φ17A and Φ31) are capable of reducing the progression of the disease. Yet, a treatment based on a single phage Φ31 showed higher disease protection compared to the phage cocktail in both conditions. This could be related to differences observed in the ability of the different phages in lysing the host bacterium XaaBL11 in vitro and/or to the fact that the Φ31 concentration in the cocktail is only one-third compared to the monophage treatment. Furthermore, the result could also indicate that these three phages compete for the same receptor and outcompete each other when a cell is infected with different viruses at the same time [31,32]. In this case, a cocktail would also not perform as well. Since the phages are almost identical at the genome level, it is indeed plausible that they compete during coinfection and hence reduce access by the most effective phage Φ31.
In addition, high disease protection of phage Φ31 could also be related to higher multiplication rate on the leaf surface, similar to the observation by Balogh et al. [33] in controlling bacterial leaf spot on tomato caused by Xanthomonas perforans. In our experiments, the plants were artificially infected with only one strain of Xaa, which could lead to a higher efficacy of disease control by an individual phage than a phage cocktail. Since, under natural field conditions, plants could be infected with different strains of Xaa, this result should be further investigated since bacteria could more rapidly develop resistance when only one phage is applied [34]. Under natural conditions, it could, therefore, still be useful to apply a cocktail of phages, preferentially having significant differences in infection mechanism. In addition, a cocktail could be less affected by environmental factors, since not every phage is equally sensitive to external conditions [35].
In our greenhouse trials, a phage titer of 105 pfu/mL was shown to already lead to disease reduction. However, higher phage titers resulted in higher levels of disease protection, with the optimal phage titer being 107 or 108 pfu/mL. This is in line with previous observations by Balogh et al. [33] that showed disease control between 106 and 108 pfu/mL, while 104 pfu/mL was ineffective in controlling bacterial leaf spot on tomato caused by Xanthomonas perforans.
In our field trials, four applications of single phage or phage cocktail at 108 pfu/mL once before pathogen inoculation and three additional applications (3, 8 and 13 days after infection) reduced the disease index between 35.5% and 43.5% for single phage treatments and between 21.6% and 28.4% for the cocktail treatments. A similar leaf blight disease reduction from 26% to 50% was recorded by Lang et al. [20] when a phage cocktail was applied biweekly at 108 pfu/mL.
Both the study by Lang et al. [20] and Jones et al. [13] indicate that the maximum disease reduction on Allium spp. lies around 50%, which could be related to pathogen aggressiveness on these plants in high humidity conditions. Therefore, management of this disease by a combination of phage biocontrol with other control methods could be considered, e.g., with plant activators or antagonistic microorganisms as suggested by Lang et al. [20] and Jones et al. [13], to further improve the level of disease control.
4. Materials and Methods
4.1. Host Bacterium Isolation and Phage Manipulations
Infected leaf blight samples were collected from different provinces in the Mekong Delta and were used for isolation of Xaa. After surface sterilization, the infected leaves were inspected for bacterial oozing under a microscope. Next, one drop of suspension containing bacterial ooze was plated on King’s B agar medium and streaked for individual colonies and then incubated for 48 h at 25 °C. Single colonies were picked up, then tested for their pathogenicity by spraying bacterial suspensions (OD600nm = 0.3; corresponding to 3 × 108 CFU/mL) on Welsh onion and scoring symptom development and used as a representative host for further experiments.
For phage isolation, the infected onion leaves were chopped and crushed using mortar and pestle. The homogenized leaves were mixed with an equal volume of water and subsequently centrifuged at 6000 rpm. The supernatant was transferred and treated with chloroform at a concentration of 3–5% and incubated for 5 min before another round of centrifugation (6000 rpm, 5 min). Phages were visualized by mixing 100 µL of this supernatant with 10 mL of 0.8% King’s B soft agar containing a bacterial suspension (the bacterial strain isolated from the same leaf sample) and pouring it on an agar plate. After 24 h incubation, individual plaques were picked up with a sterile toothpick and streaked on a fresh bacterial lawn with a cotton swab. A single plaque was harvested in water as a phage suspension and stored at 4 °C.
Phages were routinely amplified by cotton swab streaking of this phage stock on fresh soft King’s B agar plates containing host bacterium. After 24 h incubation at 25 °C, water was added for harvesting the phages, after excluding the remaining bacterial cells by centrifugation and chloroform treatment.
The host range of the different phages was tested by spotting 5 µL of phage suspension on a bacterial lawn containing the test strain. In short, these bacterial lawns of each test strain were prepared by adding 100 µL of bacterial suspension at an OD600nm of 0.3 to 10 mL of King’s B soft agar (0.8%). Plates were incubated for 24 h at room temperature. The lysis zone was recorded for all strains to determine the hot range.
Promising phages with broad host ranges were selected for further experiments. Plaque formation for the different phages was compared by plating 5 × 102 pfu/mL in triplicate (one plate per replicate). The diameter of the halos around the plaques was recorded 24, 48 and 72 h after infection (incubation at room temperature in darkness).
4.2. Phage Characterization
The virion morphology was determined by TEM analysis. The phage suspension was first allowed to adsorb for 3 min on carbon and formvar-coated copper–palladium grids, which were then rinsed several times with water. Next, the grids were negatively stained with aqueous 0.5% uranyl acetate and the excess fluid was removed with filter paper. Observations and photographs were made with a Philips CM10 transmission electron microscope (TEM) (Eindhoven, The Netherlands), operating at 80 kV. Micrograph films were developed and digitally acquired at high resolution with a D800 Nikon camera. Finally, the images were trimmed and adjusted for brightness and contrast using the Fiji software [36].
Next, to analyze the genome of these phages, 1010 pfu/mL phage suspensions were used for DNA phenol-chloroform extraction [37]. A sequencing library was then obtained using the Illumina Nextera flex kit and sequenced on an Illumina MiniSeq. The reads were assembled and annotated with RAST [38] using the PATRIC platform [39]. Phage sequences were compared to homologous phage sequences on NCBI using BLASTn [40]. Protein sequences were manually verified using BLASTp and Artemis [41] was used to polish the genbank files. Genome maps were drawn using EasyFig [42]. The comparison between the phage genomes was performed by mapping the reads on the reference genome using Bowtie2 [43] and variants were called using iVar [44]. The data were visualized using an integrated genome viewer [45].
4.3. Evaluation of the Efficacy of Phage Treatment in Greenhouse Conditions
The first greenhouse experiment was used to compare the efficacy of different phage treatments for controlling bacterial leaf blight on Welsh onion. The experiment was a completely randomized design with six treatments (three monophage treatments, one treatment with a cocktail of three phages (containing 1/3rd of each phage), a control treatment without phage application and a treatment with oxolinic acid). Each treatment included five replicates, each in a different pot.
Thirty-day-old Welsh onion plants were used for experiments. The phage suspension (108 PFU/mL) of each treatment was sprayed over the leaves (25 mL/pot). After 2 h, plants were inoculated with a phage-susceptible Xaa strain (XaaBL11, OD600nm of 0.3) on the leaf surface using a hand sprayer (again 25 mL/pot). The pots were covered with plastic bags for 24 h in darkness, at 25 °C and 100% humidity in a growth chamber. After 24 h, the plastic bags were removed, and the plants were grown in greenhouse conditions. The percentage of infected leaf area was recorded at several time points until the control treatment was almost fully infected. In addition, the bacteriophage density on the leaf surface of differently treated plants was determined at 0, 48 and 72 h after pathogen inoculation (three leaves per plant; three plants per treatment).
In the second greenhouse experiment, the optimal phage titer was determined using the same experimental setup. Pots were arranged completely randomly for five treatment conditions (four different phage titers, i.e., 105, 106, 107 and 108 pfu/mL and one control treatment without phage application).
4.4. Evaluation of the Efficacy of Phage Biocontrol in Field Conditions
A field trial was conducted in a 500 m2 Welsh onion field in the An Giang province. This experiment was set up as a completely randomized block design, in which four treatments were evaluated: (1) control treatment without the application of phage or chemicals, (2) phage Φ31, (3) a cocktail of three phages (Φ16, Φ17 and Φ31) and (4) bactericide (oxolinic acid) treatment. There were four replicates per treatment and the treatments were applied 30 days after planting the onion seedlings.
Phages were applied (phage Φ31 or the three-phage cocktail) by spraying a phage suspension (108 pfu/mL) at 1 L/25 m2 one hour before pathogen inoculation and at 3, 8 and 13 days after pathogen inoculation (dai). Bactericide treatment consisted of Starner 20 WP (20 g oxolinic acid/16 L water) at 1 L/25 m2, which was first applied when the percentage infection was around 5–10% (3 dai) and which was repeated at 8 and 13 dai. Pathogen inoculation was done by spraying XaaBL11 (OD600nm = 0.15, corresponding to 108 CFU/mL; 1 L/25 m2) on the leaf surface 30 days after planting. The disease index was recorded at 5, 9 and 15 dai. The actual yield (the whole plant) and commercial yield (without infected leaves) were recorded as well.
4.5. Statistical Analyses
All statistical analyses were carried out in MSTAT-C (Statistical software developed by the Crop and Soil Science Department of Michigan State University, USA). First, a Shapiro–Wilk test was used to test for normality of the experimental data with or without transformation by taking the square root of each datapoint. Next, Bartlett’s test was run to determine the equality of variances. Finally, means were separated pairwise using Duncan’s or Tukey’s range test, resulting in a significance level letter report.
5. Conclusions
During this research, ten bacteriophages were isolated from twelve bacterial blight-infected onion leaf samples and three promising phages Φ16, Φ17A and Φ31 were selected based on their host range and plaque/halo diameter. The three podoviruses are lytic phages based on whole-genome sequencing and form a new phage species. Phage Φ31 shows higher disease reduction compared to phage Φ16, Φ17A and phage cocktail in greenhouse conditions, and the optimal phage titer for disease control lies at 107 and 108 pfu/mL as these concentrations performed equally well. During field trials, phage Φ31 reduced disease symptoms equally compared to the bactericide Starner and provided a significant increase in crop yield.
Appendix A
Figure A1.
Plaque morphology of five phage isolates at 72 h after culturing on X. axonopodis pv. allii XaaBL11 strain.
Figure A2.
Read pileup (in grey) of Φ31 with Φ16 as reference. Blue lines at the bottom represent the end and beginning of gp39 and gp40, respectively. The red box indicates the hypervariable region in the intergenic region. There is a clear drop in het read pileup sequencing depth suggesting a deletion event. The remaining reads (each grey line) show an accumulation of mutations (colored dots in the reads) in this specific region.
Figure A3.
Development of leaf blight caused by X. axonopodis pv. allii on onion after different phage titer treatments (A–E) in greenhouse conditions.
Author Contributions
Conceptualization: N.T.T.N., K.K. and J.B.J.; methodology: N.T.T.N., T.N.T., N.L.K.N., N.P.H., D.T.K.T., N.H.K.-P., D.H., M.V. and J.W.; formal analysis: N.T.T.N., D.H., M.V. and J.W.; writing—original draft preparation: N.T.T.N., D.H. and J.W.; writing—review and editing, N.T.T.N., D.H., R.L., K.K., J.W. and J.B.J. All authors have read and agreed to the published version of the manuscript.
Funding
The research at Can Tho University was funded by JICA TC1 (grant ODA-TC2016-02) for a training course on phage morphology in the Kyoto Institute of Technology in Japan. The part of the research at KU Leuven was supported by the ‘Vlaams Agentschap Innoveren en Ondernemen’ (VLAIO) agriculture programme (LA) grant IWT.150914. DH holds a predoctoral scholarship from the ‘Fonds voor Wetenschappelijk Onderzoek Vlaanderen’ (FWO) strategic basic research grant 1S02520N.
Data Availability Statement
The genome data of this novel phage species were submitted to NCBI Genbank and are available through accession number MT951568.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Picard Y., Roumagnac P., Legrand D., Humeau L., Robène-Soustrade I., Chiroleu F., Gagnevin L., Pruvost O. Polyphasic characterization of Xanthomonas axonopodis pv. allii associated with outbreaks of bacterial blight on three Allium species in the Mascarene archipelago. Phytopathology. 2008;98:919–925. doi: 10.1094/PHYTO-98-8-0919. [DOI] [PubMed] [Google Scholar]
- 2.Gent D.H., Lang J.M., Bartolo M.E., Schwartz H.F. Inoculum Sources and Survival of Xanthomonas axonopodis pv. allii in Colorado. Plant Dis. 2005;89:507–514. doi: 10.1094/PD-89-0507. [DOI] [PubMed] [Google Scholar]
- 3.Humeau L., Roumagnac P., Picard Y., Robène-Soustrade I., Chiroleu F., Gagnevin L., Pruvost O. Quantitative and Molecular Epidemiology of Bacterial Blight of Onion in Seed Production Fields. Phytopathology. 2006;96:1345–1354. doi: 10.1094/PHYTO-96-1345. [DOI] [PubMed] [Google Scholar]
- 4.Black L., Conn K., Gabor B., Kao J., Lutton J. Onion Disease Guide. Seminis Vegetable Seeds; St. Louis, MO, USA: 2012. p. 7. [Google Scholar]
- 5.Schwartz H.F., Otto K. First Report of a Leaf Blight of Onion Caused by Xanthomonas campestris in Colorado. Plant Dis. 2000;84:922. doi: 10.1094/PDIS.2000.84.8.922D. [DOI] [PubMed] [Google Scholar]
- 6.Nunez J.J., Gilbertson R.L., Meng X., Davis R.M. First Report of Xanthomonas Leaf Blight of Onion in California. Plant Dis. 2002;86:330. doi: 10.1094/PDIS.2002.86.3.330B. [DOI] [PubMed] [Google Scholar]
- 7.Roumagnac P., Pruvost O., Chiroleu F., Hughes G. Spatial and Temporal Analyses of Bacterial Blight of Onion Caused by Xanthomonas axonopodis pv. allii. Phytopathology. 2004;94:138–146. doi: 10.1094/PHYTO.2004.94.2.138. [DOI] [PubMed] [Google Scholar]
- 8.Abbasi P.A., Khabbaz S.E., Weselowski B., Zhang L. Occurrence of copper-resistant strains and a shift in Xanthomonas spp. causing tomato bacterial spot in Ontario. Can. J. Microbiol. 2015;61:753–761. doi: 10.1139/cjm-2015-0228. [DOI] [PubMed] [Google Scholar]
- 9.Cooksey D.A., Azad H.R., Cha J.S., Lim C.K. Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Appl. Environ. Microbiol. 1990;56:431–435. doi: 10.1128/AEM.56.2.431-435.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundin G.W., Wang N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018;56:161–180. doi: 10.1146/annurev-phyto-080417-045946. [DOI] [PubMed] [Google Scholar]
- 11.Ter Kuile B.H., Kraupner N., Brul S. The risk of low concentrations of antibiotics in agriculture for resistance in human health care. FEMS Microbiol. Lett. 2016;363:fnw210. doi: 10.1093/femsle/fnw210. [DOI] [PubMed] [Google Scholar]
- 12.Thanner S., Drissner D., Walsh F. Antimicrobial Resistance in Agriculture. MBio. 2016;7:e02227-e15. doi: 10.1128/mBio.02227-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones J.B., Jackson L.E., Balogh B., Obradovic A., Iriarte F.B., Momol M.T. Bacteriophages for plant disease control. Annu. Rev. Phytopathol. 2007;45:245–262. doi: 10.1146/annurev.phyto.45.062806.094411. [DOI] [PubMed] [Google Scholar]
- 14.Holtappels D., Fortuna K., Lavigne R., Wagemans J. The future of phage biocontrol in integrated plant protection for sustainable crop production. Curr. Opin. Biotechnol. 2021;68:60–71. doi: 10.1016/j.copbio.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara A., Fujisawa M., Hamasaki R., Kawasaki T., Fujie M., Yamada T. Biocontrol of Ralstonia solanacearum by treatment with lytic bacteriophages. Appl. Environ. Microbiol. 2011;77:4155–4162. doi: 10.1128/AEM.02847-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Wei Z., Yang K., Wang J., Jousset A., Xu Y., Shen Q., Friman V.-P. Phage combination therapies for bacterial wilt disease in tomato. Nat. Biotechnol. 2019;37:1513–1520. doi: 10.1038/s41587-019-0328-3. [DOI] [PubMed] [Google Scholar]
- 17.Balogh B., Canteros B.I., Stall R.E., Jones J.B. Control of Citrus Canker and Citrus Bacterial Spot with Bacteriophages. Plant Dis. 2008;92:1048–1052. doi: 10.1094/PDIS-92-7-1048. [DOI] [PubMed] [Google Scholar]
- 18.Chae J.-C., Hung N.B., Yu S.-M., Lee H.K., Lee Y.H. Diversity of bacteriophages infecting Xanthomonas oryzae pv. oryzae in paddy fields and its potential to control bacterial leaf blight of rice. J. Microbiol. Biotechnol. 2014;24:740–747. doi: 10.4014/jmb.1402.02013. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim Y.E., Saleh A.A., Al-Saleh M.A. Management of Asiatic Citrus Canker Under Field Conditions in Saudi Arabia Using Bacteriophages and Acibenzolar-S-Methyl. Plant Dis. 2017;101:761–765. doi: 10.1094/PDIS-08-16-1213-RE. [DOI] [PubMed] [Google Scholar]
- 20.Lang J.M., Gent D.H., Schwartz H.F. Management of Xanthomonas Leaf Blight of Onion with Bacteriophages and a Plant Activator. Plant Dis. 2007;91:871–878. doi: 10.1094/PDIS-91-7-0871. [DOI] [PubMed] [Google Scholar]
- 21.Ahern S.J., Das M., Bhowmick T.S., Young R., Gonzalez C.F. Characterization of novel virulent broad-host-range phages of Xylella fastidiosa and Xanthomonas. J. Bacteriol. 2014;196:459–471. doi: 10.1128/JB.01080-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adriaenssens E.M., Rodney Brister J. How to Name and Classify Your Phage: An Informal Guide. Viruses. 2017;9:70. doi: 10.3390/v9040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merrill B.D., Ward A.T., Grose J.H., Hope S. Software-based analysis of bacteriophage genomes, physical ends, and packaging strategies. BMC Genom. 2016;17:1–16. doi: 10.1186/s12864-016-3018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero-Suarez S., Jordan B., Heinemann J.A. Isolation and characterization of bacteriophages infecting Xanthomonas arboricola pv. juglandis, the causal agent of walnut blight disease. World J. Microbiol. Biotechnol. 2012;28:1917–1927. doi: 10.1007/s11274-011-0992-z. [DOI] [PubMed] [Google Scholar]
- 25.Dong Z., Xing S., Liu J., Tang X., Ruan L., Sun M., Tong Y., Peng D. Isolation and characterization of a novel phage Xoo-sp2 that infects Xanthomonas oryzae pv. oryzae. J. Gen. Virol. 2018;99:1453–1462. doi: 10.1099/jgv.0.001133. [DOI] [PubMed] [Google Scholar]
- 26.Cornelissen A., Ceyssens P.-J.J., T’Syen J., Van Praet H., Noben J.-P.P., Shaburova O.V., Krylov V.N., Volckaert G., Lavigne R. The T7-Related Pseudomonas putida Phage phi15 Displays Virion-Associated Biofilm Degradation Properties. PLoS ONE. 2011;6:e18597. doi: 10.1371/journal.pone.0018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornelissen A., Ceyssens P.J., Krylov V.N., Noben J.P., Volckaert G., Lavigne R. Identification of EPS-degrading activity within the tail spikes of the novel Pseudomonas putida phage AF. Virology. 2012;434:251–256. doi: 10.1016/j.virol.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Olszak T., Shneider M.M., Latka A., Maciejewska B., Browning C., Sycheva L.V., Cornelissen A., Danis-Wlodarczyk K., Senchenkova S.N., Shashkov A.S., et al. The O-specific polysaccharide lyase from the phage LKA1 tailspike reduces Pseudomonas virulence. Sci. Rep. 2017;7:16302. doi: 10.1038/s41598-017-16411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada T. Filamentous phages of Ralstonia solanacearum: Double-edged swords for pathogenic bacteria. Front. Microbiol. 2013;4:325. doi: 10.3389/fmicb.2013.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prevelige P.E., Fane B.A. Building the Machines: Scaffolding Protein Functions During Bacteriophage Morphogenesis. In: Rossmann M.G., Rao V.B., editors. Viral Molecular Machines. Springer; Boston, MA, USA: 2012. pp. 325–350. [DOI] [PubMed] [Google Scholar]
- 31.Guo Q., Chen B., Tu Y., Du S., Chen X. Prophage LambdaSo uses replication interference to suppress reproduction of coexisting temperate phage MuSo2 in Shewanella oneidensis MR-1. Environ. Microbiol. 2019;21:2079–2094. doi: 10.1111/1462-2920.14592. [DOI] [PubMed] [Google Scholar]
- 32.Refardt D. Within-host competition determines reproductive success of temperate bacteriophages. ISME J. 2011;5:1451–1460. doi: 10.1038/ismej.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balogh B., Nga N.T.T., Jones J.B. Relative level of bacteriophage multiplication in vitro or in phyllosphere may not predict in planta efficacy for controlling bacterial leaf spot on tomato caused by Xanthomonas perforans. Front. Microbiol. 2018;9:1–10. doi: 10.3389/fmicb.2018.02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y., Shen W., Zhong Q., Chen Q., He X., Baker J.L., Xiong K., Jin X., Wang J., Hu F., et al. Development of a Bacteriophage Cocktail to Constrain the Emergence of Phage-Resistant Pseudomonas aeruginosa. Front. Microbiol. 2020;11:327. doi: 10.3389/fmicb.2020.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kering K.K., Kibii B.J., Wei H. Biocontrol of phytobacteria with bacteriophage cocktails. Pest Manag. Sci. 2019;75:1775–1781. doi: 10.1002/ps.5324. [DOI] [PubMed] [Google Scholar]
- 36.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J., Russell D.W. Molecular Cloning. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY, USA: 2001. [Google Scholar]
- 38.Brettin T., Davis J.J., Disz T., Edwards R.A., Gerdes S., Olsen G.J., Olson R., Overbeek R., Parrello B., Pusch G.D., et al. RASTtk: A modular and extensible implementation of the RAST algorithm for annotating batches of genomes. Sci. Rep. 2015;5:8365. doi: 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wattam A.R., Davis J.J., Assaf R., Boisvert S., Brettin T., Bun C., Conrad N., Dietrich E.M., Disz T., Gabbard J.L., et al. Improvements to PATRIC, the all-bacterial bioinformatics database and analysis resource center. Nucleic Acids Res. 2016;45:D535–D542. doi: 10.1093/nar/gkw1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 41.Carver T., Harris S.R., Berriman M., Parkhill J., Mcquillan J.A. Artemis: An integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics. 2012;28:464–469. doi: 10.1093/bioinformatics/btr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan M.J., Petty N.K., Beatson S.A. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grubaugh N.D., Gangavarapu K., Quick J., Matteson N.L., De Jesus J.G., Main B.J., Tan A.L., Paul L.M., Brackney D.E., Grewal S., et al. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 2019;20:8. doi: 10.1186/s13059-018-1618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genome data of this novel phage species were submitted to NCBI Genbank and are available through accession number MT951568.