Abstract
Philadelphia-like (Ph-like) acute lymphoblastic leukemia (ALL) is a subgroup of B-cell precursor ALL which by gene expression analysis clusters with Philadelphia-positive ALL although lacking the pathognomonic BCR-ABL1 oncoprotein. Its prevalence increases with age and similar to BCR-ABL1-positive ALL, Ph-like ALL is characterized by IKZF1 or other B-lymphoid transcription factor gene deletions and by poor outcome to conventional therapeutic approaches. Genetic alterations are highly heterogenous across patients and include gene fusions, sequence mutations, DNA copy number changes and cryptic rearrangements. These lesions drive constitutively active cytokine receptor and kinase signaling pathways which deregulate ABL1 or JAK signaling and more rarely other kinase-driven pathways. The presence of activated kinase alterations and cytokine receptors has led to the incorporation of targeted therapy to the chemotherapy backbone which has improved treatment outcome for this high-risk subtype. More recently, retrospective studies have shown the efficacy of immunotherapies including both antibody drug-conjugates and chimeric antigen receptor T cell therapy and as they are not dependent on a specific genetic alteration, it is likely their use will increase in prospective clinical trials. This review summarizes the genomic landscape, clinical features, diagnostic assays, and novel therapeutic approaches for patients with Ph-like ALL.
Keywords: acute lymphoblastic leukemia (ALL), BCR-ABL1–like ALL, Ph-like ALL, cryptic rearrangements, kinase signaling, targeted therapy, immunotherapy
1. Introduction
Acute lymphoblastic leukemia (ALL) comprises over twenty different subtypes defined by distinct constellations of somatic genetic alterations that converge on distinct gene expression profiles [1]. These include subtypes defined by recurring chromosomal abnormalities including aneuploidy, chromosomal rearrangements and/or known gene fusions; subtypes defined by cryptic rearrangements not identifiable by conventional approaches or by single point mutations; or novel subtypes that “phenocopy” established subtypes, with similar gene expression profile but different founding alteration [1,2,3,4]. One such subtype is Philadelphia chromosome-like (Ph-like) or BCR-ABL1-like ALL that is characterized by a gene expression signature similar to that of Philadelphia chromosome-positive ALL (Ph+ ALL), but lacking the pathognomonic BCR-ABL1 oncoprotein of Ph + ALL [5,6]. Similar to patients with Ph+ ALL, patients with Ph-like ALL often exhibit adverse clinical features and poor outcome [4,7,8] and frequently harbor alterations of IKZF1 or other B-lymphoid transcription factor genes [6]. Multiple and heterogenous genetic alterations in kinases and cytokine receptors drive constitutively active kinase signaling which is amenable to targeted treatment with tyrosine kinase inhibitor (TKI) therapy [7,8]. Incidence of Ph-like ALL ranges from 10–15% in children [9,10] to 20% in older adults [11,12], with a peak (25–30%) in adolescent and young adult (AYA) population [4,13]. Due to the associated adverse prognosis and potential responsiveness of these patients to TKIs, the 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia recognized Ph-like ALL as a provisional entity [14]. This review describes the current state of art in Ph-like ALL, highlighting the genomic landscape, clinical features, diagnostic assays, and therapeutic implications.
2. Clinical Features of Ph-Like ALL
Prevalence and Outcome
The prevalence of Ph-like ALL increases with age. In children it accounts for up to 10% in National Cancer Institute (NCI) standard-risk B-ALL (SR; children age 1 to less than 10 years and white blood cell (WBC) count ≤ 50,000/μL) and up to 15% in NCI high-risk B-ALL (HR; age 10 to 16 years and/or WBC count ≥ 50,000/μL), and confers poor outcome [4,11,12]. In NCI HR childhood protocols from the Children’s Oncology Group (COG), the 5-year event-free survival was 58% compared to 84% for non-Ph-like ALL [4,15]. The prevalence of Ph-like ALL increases to approximately 20% in adolescents aged 16–20 years, reaches its peak (25–30%) in young adults aged 21–39 years and stabilizes at 20–24% in patients aged 40 to 86 years in the Unites States [4,10,11,12,13]. However, the prevalence of Ph-like in adults older than 40 years of age may vary according to the screened risk groups, the race and ethnicity of the patients studied, and the methodologies used to define Ph-like. For example, among adult patients with newly diagnosed B-ALL and gene expression profiling who received frontline chemotherapy at MD Anderson Cancer Center, 33.1% were Ph-like, of which 68% were of Hispanic ethnicity [12]. These patients had significantly worse overall survival and event-free survival compared with non-Ph-like B-ALL with a 5-year survival of 23% (vs. 59% for non Ph-like B-ALL) [12]. In European cohorts the prevalence of Ph-like ALL is lower with 17% of patients aged 16 to 71 years (of those 29% were patients aged 16–20 years; 43% were patients aged 21–39 and 29% patients aged 40–71 years) in a study from the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) [16]. Ph-like patients had a high rate of non-response to first-line treatment and a high relapse rate compared to other B-ALL subtypes. However, Ph-like patients who underwent allogeneic stem cell transplant (ASCT) had a lower cumulative incidence of relapse at 5 years compared with patients without a donor. Only one relapse was seen among five patients who underwent ASCT [16]. In the report from the German Multicenter Study Group for Adult ALL trials (GMALL), GMALL 06/99 e 07/03, prevalence of Ph-like ALL was ~20% in adolescents and young adults but it decreased to under 10% in adults aged 40–84 years. This study reported 100% complete remissions even for Ph-like ALL, although with a short duration, lower number of molecular remissions and a higher rate of early relapses, confirming the negative prognostic impact on survival of the Ph-like ALL subtype in intensively treated older adults [17]. A similar prevalence (~20%) and poor clinical outcome were observed in patients enrolled in different GIMEMA (Gruppo Italiano Malattie EMatologiche dell’Adulto) clinical trials [18], where patients with Ph-like ALL had a lower rate of complete remissions and a worse event free survival compared with non Ph-like ALL cases [18,19].
Overall, regardless of age and study cohort almost all studies on patients with Ph-like ALL report adverse clinical features and inferior outcomes. Ph-like ALL cases tend to present with high WBC counts, are more commonly male, with a male to-female ratio of ∼2:1, and are associated with elevated minimal residual disease (MRD) levels, a high rate of treatment failure, and poor overall survival compared to non Ph-like ALL patients [4,10,11,12,13,15,16,17,20]. The only exception so far is from St. Jude Total XV, where the adverse prognosis of pediatric Ph-like ALL was improved by effective risk-directed therapy based primarily on MRD levels during and at the end of remission induction therapy [9]. Notably, in contrast to other studies that selectively examined high-risk B-ALL and did not apply MRD measurement for risk-directed therapy, this study included all patients with newly diagnosed ALL and used MRD level to direct intensity of therapy. Moreover, this study had a lower frequency of CRLF2 (27.5%) and IKZF1 (27%) alterations in Ph-like ALL than in prior studies from the COG [3,6,17]. The improved outcome from St. Jude Total XV contrasts with the Australian and New Zealand Children’s Haematology/Oncology Group (ANZCHOG) ALL8 clinical trial [21], where despite a risk-adjusted treatment approach, a high rate of disease recurrence was reported among children who were retrospectively diagnosed with Ph-like ALL.
3. Genomic Features of Ph-Like ALL
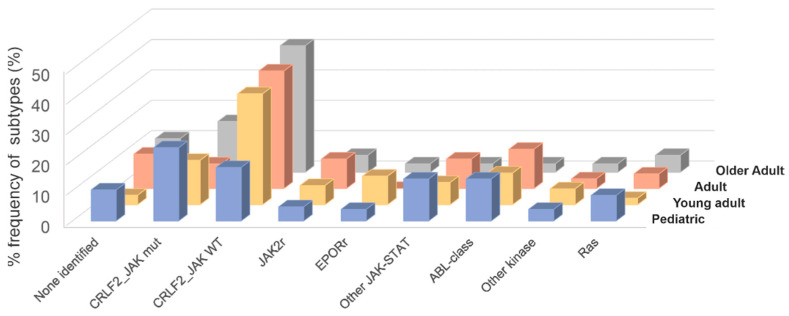
A wide spectrum of genetic alterations (>60), including translocations, cryptic rearrangements, sequence mutations and copy number changes have been described in Ph-like ALL, with slight differences in prevalence across age (Figure 1). These alterations drive constitutively active kinase or cytokine receptor signaling, many of which have been shown to be druggable with a variety of kinase inhibitors. The most commonly mutated pathways are the ABL and JAK-STAT pathways with multiple rearrangements and lesions that converge on downstream ABL/JAK-STAT signaling. Founder alterations may be grouped into three types: (i) JAK/STAT alterations including mutations activating cytokine receptors (e.g., CRLF2 and IL7R); gene fusions hijacking cytokine receptor expression (e.g., IGH-CRLF2 and P2RY8–CRLF2) [22,23,24,25]; gene fusions and/or mutations activating kinases (e.g., JAK1, JAK2, JAK3, TYK2); and rearrangements hijacking and truncating cytokine receptor expression (e.g., cryptic EPOR rearrangements) [24]; (ii) fusions involving ABL-class genes (ABL1, ABL2, CSF1R, LYN, PDGFRA, PDGFRB); (iii) less common fusions (FLT3, FGFR1, NTRK3, PTK2B) [7] whose number is growing with increasing sequencing studies of different cohorts (Figure 2).
Figure 1.
Frequency of Ph-like ALL subtypes according to each age group. Combined prevalence of Ph-like ALL subtypes in childhood (age 1 to 15 years), adolescent (age 16 to 20 years) and young adults (age 21 to 39 years), adults (age 40 to 59 years) and older adults (age > 60 years). Genomic subtypes include CRFL2-rearranged JAK mutant (mut), CRFL2-rearranged JAK wild-type (WT), JAK2 rearrangements (JAK2r), EPOR rearrangements (EPORr), Other JAK-STAT alterations, ABL1-class fusions (ABL1, ABL2, CSF1R, LYN, PDGFRA and PDGFRB), Ras mutations (KRAS, NRAS, NF1, PTPN11, BRAF and CBL), all other kinase lesions (FLT3, FGFR1, NTRK3) and unknown alterations.
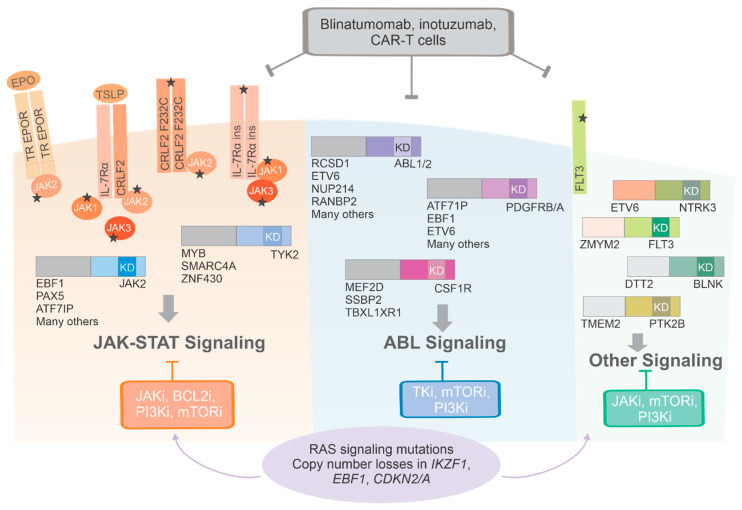
Figure 2.
Schematic representation of main genomic alterations in Ph-like ALL. Constellations of sequence mutations, cryptic rearrangements, chimeric gene fusions and copy number changes drive constitutive cytokine receptor and kinase signaling which is amenable to inhibition by a variety of tyrosine kinase inhibitors. The majority of alterations converge on two pathways that activate JAK- family member signaling or ABL-signaling. Immunotherapeutic agents are not dependent on specific genetic alterations and represent promising approaches that warrant further investigation in larger trials. Abbreviations: TR: truncated; JAKi, JAK inhibitors; BCL2i, BCL2 inhibitors; PI3Ki, phosphoinositide 3-kinase inhibitor; mTORi, mTOR inhibitors; TKi, tyrosine kinase inhibitors. The star sign indicates the occurrence of a mutation.
3.1. JAK/STAT Alterations
Approximately 50% of patients with Ph-like ALL harbor rearrangements of the cytokine receptor- like factor 2 (CRLF2) gene, located on the pseudoautosomal region 1 (PAR1) of chromosomes Xp22 and Yp11 [4,12,15,24]. In normal conditions CRLF2 dimerizes with the α- subunit of interleukin- 7 receptor (IL7RA) to form a heterodimeric thymic stromal lymphopoietin receptor (TSLPR) which actives downstream JAK2/STAT5 and thePI3K/AKT/mTOR pathways [26,27,28] and is implicated in early B-cell development [29]. CRLF2 deregulation results from three main mechanisms: (1) a cryptic rearrangement that juxtaposes CRLF2 to the immunoglobulin heavy chain locus (IGH); (2) a focal deletion in the pseudoautosomal region of the sex chromosomes resulting in P2Y receptor family member 8 (P2RY8)-CRLF2 fusion that positions CRLF2 under the control of the P2RY8 promoter; (3) and less frequently by an activating CRLF2 point mutation, F232C [13,17,22,23,25,30,31,32,33]. Rearrangements of CRLF2 account for 24% of pediatric patients with NCI SR Ph-like ALL [9], 55% of children with HR disease [10] and 50% to 60% of adolescent and adult patients with Ph-like ALL cases [4,11,12,13,17,18]. P2RY8-CRLF2 fusions occur more commonly in younger children and in patients with Down syndrome (DS) ALL [22,25], while IGH-CRLF2 fusions are detected more frequently in older patients and patients of Hispanic ethnicity [34]. In a genome-wide association study of CRLF2-rearranged ALL, the inherited GATA3 variant rs3824662 was associated with CRLF2 rearrangement, JAK mutation, IKZF1 deletion, variation in GATA3 expression and increased risk of relapse [35]. This variant is markedly more common in patients of Hispanic ethnicity (~40%) or Native American (~50%) genetic ancestry, while is it detected in only 14% of Europeans [35,36]. The point mutation changing phenylalanine 232 to cysteine in CRLF2 has been identified in 9% of DS-ALL patients [25] and 21% of adult B-ALL patients [23]. In in vitro assays, the expression of CRLF2 F232C in the absence of co-expression of mutant JAK2 promotes JAK2 signaling activation and cell transformation [23,25,37]. CRLF2 rearrangement and overexpression is associated with worse outcome compared to cases with lack of CRLF2 alterations [15,34,38,39]. However, the poor prognostic impact of CRLF2 overexpression is overcome by BCR-ABL1–like signature and IKZF1 deletion in the Dutch Childhood Oncology Group trials and German Cooperative ALL trials [37]. In about half of CRLF2-rearranged pediatric Ph-like ALL cases, concomitant JAK1 and JAK2 (most commonly in the pseudokinase domain at R683) mutations occur. In adults, the frequency of JAK mutations in patients with CRLF2 rearrangement is lower, with a ratio of 1:4 with JAK wild type [4,12,15,23]. In JAK1 the most common mutation is represented by V658F which is the homolog of JAK2 V617F, hotspot in myeloproliferative neoplasms. Other alterations leading to JAK/STAT activation target IL7RA, SH2B3, IL2RB, and TYK2 genes. Collectively these alterations are approximately two-fold higher in children (14%) compared to adolescents (5.0%), and adults (7.3%) [4,12,15]. IL7RA mutations occur in exon 6 and are mainly in-frame insertion/deletions in the juxtamembrane-transmembrane domain or, rarely, a serine-to-cysteine substitution at amino acid 185 in the extracellular domain [38]. Independent of CRLF2 rearrangements, JAK-STAT signaling activation can result from JAK2 (~7%) or erythropoietin receptor (EPOR, 5%) -rearrangements.
Over 20 different JAK2 gene fusion partners have been reported (most commonly EBF1, ETV6, PAX5, and BCR), making JAK2 the most promiscuous gene in Ph-like ALL. All fusions preserve the JAK2 kinase domain and result in STAT5 activation and growth factor independence, making cells expressing these fusions amenable to JAK2 inhibitors.
Common EPOR rearrangements involve juxtaposition or less frequently translocation of the EPOR gene in proximity of a strong enhancer, such as that of the immunoglobulin heavy (IGH) or kappa (IGK) loci, that drives its expression. Less frequent rearrangements involve insertion of EPOR into the upstream region of LAIR1 or the THADA loci [4,39]. All these rearrangements clip off the C-terminal cytoplasmic tail, thus preserving the proximal tyrosine requested for activation and removing almost all tyrosine sites required for shutting off the receptor signaling and down-regulate and internalize the receptor. This leads to transformation in in vivo models and sensitivity to a variety of different JAK2 inhibitors in in vitro and in vivo models. While IGH-EPOR fusion due the translocation t(14;19)(q32;p13) can be detected by fluorescence in situ hybridization (FISH) [40], the other EPOR rearrangements are cryptic and challenging to detect without using next-generation sequencing (NGS) technologies. The prevalence of EPOR rearrangements has a peak in young adults (9%) compared to children and adolescents (5% and 3%, respectively). They are rarely detected in adults (1%) [13,39]. JAK2 and EPOR rearrangements are associated with the poorest outcome compared with the other molecular Ph-like subtypes [12,13].
3.2. Fusions Involving ABL-Class Genes
The ABL-class gene fusions include rearrangements of ABL proto-oncogene 1 (ABL1; e.g., to RCSD1, NUP214, LSM14A, ETV6, RANBP2, CENPC, FOXP1, SFPQ, SNX1, SNX2, SPTNA1, ZMIZ1, NUP153), ABL proto-oncogene 2 (ABL2; e.g., to RCSD2, PAG1, ZC3HAV1), colony-stimulating factor 1 receptor (CSF1R; e.g., to SSBP2, MEF2D, TBL1XR1), platelet-derived growth factor receptor beta (PDGFRB; e.g., to EBF1, ETV6, ATF7IP, SNX29, SSBP2, TNIP1, ZEB2, ZMYND8, NUMA1) and LYN (GATAD2A-LYN, NCOR1-LYN [41]), with multiple partner genes, with ABL1 and PDGFRB being the most common. The prevalence of these rearrangements is 17% in children, 9% in adolescents, 10% young adults and 9% older adults [4,12,14,15]. Patients with ABL-class fusions respond poorly to chemotherapy regimens, and the EBF1-PDGFRB fusion in particular is associated with induction failure [42,43,44]. All fusions preserve the tyrosine kinase of the ABL-class gene and promote constitutive kinase signaling that confers the ability to survive and grow independently of cytokine in vitro [45]. Imatinib, the dual ABL1/SRC inhibitor dasatinib or other TKIs inhibit the downstream signaling induced by each of these chimeric fusion proteins [4,46,47] and are currently used in clinical trials. The best and first example is provided by the inhibition of EBF1-PDGFRB fusion by imatinib [44,46,47,48,49]. The emergence of kinase domain point mutations may represent a potential mechanism of relapse in EBF1-PDGFRB or other kinase driven-subtypes in Ph-like ALL. Recently, the T681I gatekeeper mutation has been demonstrated to be the most common resistant mutation in EBF1-PDGFRB Ph-like ALL to both imatinib and dasatinib in in vitro screens and it was associated with a trend towards increased risk of relapse in patients harboring T681I subclones at diagnosis compared to T681I-negative patients [50].
3.3. Other Kinase Fusions and Genetic Aberrations
Around 5% of Ph-like ALL cases harbor gene fusions or mutations involving NTRK3, BLNK, DGKH, PTK2B, FLT3, FGFR1, TYK2 and SH2B3. Among those, one percent of cases harbor the fusion between ETV6 and NTRK3 encoding a member of the tropomyosin receptor tyrosine kinase (TRK) family [51]. This fusion is not unique of Ph-like ALL since it has been identified in a range of hematological malignancies, such as acute myeloid leukemia [52], infantile sarcoma [53,54] and solid tumors [55,56,57,58]. In preclinical models, ETV6-NTRK3 has been shown to promote the development of an aggressive B-ALL and to be exquisitely sensitive to the TRK inhibitors larotrectinib (LOXO-101) or PLX7486 (Plexxikon) in both patient derived xenograft models and in B-ALL patients with ETV6-NTRK3 [55,59,60]. Recently, a clinical response to larotrectinib has been reported in an adult Ph-like ALL with cryptic ETV6-NTRK3 rearrangement and NRASGly12Asp mutation. The patient failed to respond to multiagent chemotherapy and relapse after investigational CD19-directed chimeric antigen receptor T-cell therapy with a clone positive for ETV6-NTRK3 but not anymore for the NRASGly12Asp mutation. The relapsed leukemia progressed with further chemo- and immunotherapy but showed substantial leukemic cytoreduction using the TRK inhibitor larotrectinib [61]. Fusions of the B Cell Linker Protein (BLNK) or SLP65 gene to DNTT (also known as TDT) have been also described [13,62]. BLNK encodes a cytoplasmic adapter protein important for B-cell development and function by activating BCR downstream signaling [63], while DNTT encodes a encodes a template-independent DNA polymerase that catalyzes the addition of deoxynucleotides and that is highly expressed in normal and malignant pre-B and pre-T lymphocytes during early differentiation [64].
In addition to gene fusions, RAS pathway activating mutations or deletions (KRAS, NRAS, NF1, PTPN11) and copy number aberrations in genes involved in B-cell development (IKZF1, PAX5, EBF1, and ETV6) and cell cycle regulators (CDKN2A/B, TP53, BTG1, and RB1) are recurrent. Deletions in IKZF1 occur in around 27% of pediatric cases and in approximately 70% of high-risk pediatric patients with ALL [4]. As in BCR-ABL positive ALL [6,59], IKZF1 deletions confer a poor prognostic outcome [20]. IKZF1 deletions are significantly more common in patients carrying kinase or cytokine receptor rearrangement (IGH-CRLF2) than a sequence mutation [4,13], especially in Hispanic/Latino (H/L) children with B-ALL (29% in H/L compared to 15% of non-Hispanic Whites) where both IGH-CRLF2 translocation and IKZF1 deletion provide a strong biological rationale for the higher death-rate H/L experience due to B-ALL [60].
4. Diagnosis of Ph-Like ALL
As Ph-like ALL patients respond poorly to conventional chemotherapy, it has become increasingly important to diagnose these HR patients at presentation for improved therapeutic intervention. However, the heterogeneous genomic landscape and often cytogenetically cryptic alterations identified in Ph-like ALL creates a challenge for rapid and accurate diagnosis. Furthermore, there is no clear consensus on the definition of Ph-like ALL or the diagnostic methodologies used to identify these patients, and this has created confusion within the field [65]. Comprehensive clinical NGS, including whole transcriptome sequencing (RNA-seq), is the best approach to identify Ph-like ALL patients with targetable kinase alterations. Until these methodologies become widely available in the clinical setting, a tiered screening approach using routine diagnostics, including flow cytometry and fluorescence in situ hybridization (FISH), is still effective for swift identification of Ph-like ALL (Figure 3), as discussed below.
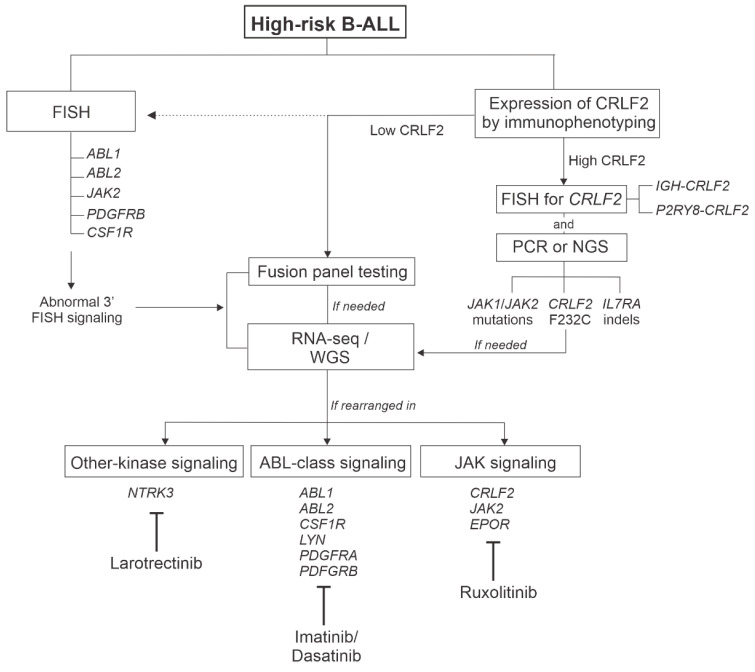
Figure 3.
Flowchart of the current diagnostic approaches used to identify Ph-like ALL samples and summary of treatment options according to genetic lesions. Dotted line represents alternative approaches. Abbreviations: FISH, fluorescence in situ hybridization; PCR, polymerase chain reaction; NGS, next-generation sequencing; RNA-seq, whole transcriptome sequencing; WGS, whole genome sequencing.
The original definition of Ph-like ALL was based on microarray gene expression profiling that identified cases with a similar gene expression signature to Ph+ ALL but that lacked the BCR-ABL1 fusion [5,6]. Subsequent studies identified the diverse array of additional kinase-activating alterations that comprise this subtype, and that are potentially targetable with TKIs [4,12,15]. Whilst gene expression profiling can be used as an initial screening tool to flag potential Ph-like ALL patients [18,66], as clinical studies have evolved over the last decade it has become increasingly clear that identification of the underlying therapeutically targetable rearrangement itself is more important than determining the Ph-like gene expression signature.
Conventional cytogenetic analysis and FISH studies are routinely performed in the diagnostic work-up of newly diagnosed ALL patients. Although karyotypic analysis can identify major chromosomal rearrangements such as the Philadelphia chromosome (t(9;22) resulting in BCR-ABL1), the majority of Ph-like ALL rearrangements are cytogenetically cryptic. However, cytogenetics and FISH can be used to rule out known molecular subgroups that are usually mutually exclusive from Ph-like ALL (e.g., aneuploidy, ETV6-RUNX1, BCR-ABL1, KMT2A-rearranged, TCF3-PBX1). In addition, clinical break-apart FISH probes have been designed for the major 3′ kinase genes rearranged in Ph-like ALL, including ABL1, ABL2, CRLF2, JAK2 and PDGFRB (which can also detect rearrangement of CSF1R). These assays can be performed with rapid turn-around and usually within a few days after the initial ALL diagnosis. Although FISH does not specify the 5′ partner gene of the kinase fusion, an abnormal result can be used to flag cases for downstream molecular analysis, and importantly, to facilitate entry onto clinical trials and early therapeutic intervention. Flow cytometric immunophenotyping for increased surface expression of TSLPR (encoded by CRLF2) is a highly cost-effective and predictive assay for detecting rearrangement of CRLF2 (both IGH-CRLF2 and P2RY8-CRLF2) and CRLF2 F232C missense mutation in primary ALL blasts [28], and is becoming routinely incorporated into diagnostic panels. A positive staining for TSLPR should be followed up with FISH to confirm the specific CRLF2 rearrangement. In CRLF2-rearranged cases, DNA-based polymerase chain reaction (PCR) assays can be used to determine the presence of JAK1, JAK2 and IL7R sequence mutations, if desired.
Flow cytometric staining for TSLPR and FISH for break-apart kinase genes are used primarily as a rapid screening tool to identify cases that are eligible for therapeutic intervention, and that require further molecular characterization to determine the specific kinase rearrangement. This can be achieved using capture-based targeted sequencing platforms or clinical NGS, both of which provide comprehensive information on the chimeric fusion gene present. The FoundationOne Heme panel is a targeted combined RNA- and DNA-based capture that detects common fusions and mutations in over 400 cancer-related genes, including those identified in Ph-like ALL [67]. Many institutional laboratories have adopted the Archer FusionPlex Heme panel as it has become more widely available, is cost effective, and does not require comprehensive bioinformatic analyses. This is an anchored multiplex PCR-based assay that targets 87 genes identified in hematological malignancies, and importantly, can identify both known and novel 5′ partner genes [68]. Clinical RNA-seq is considered the gold standard to identify most kinase rearrangements in Ph-like ALL. This approach is also considered to be the slowest, most expensive and most intensive with regard to bioinformatic analyses. However, clinical NGS will likely replace targeted platforms as it becomes faster and more widely implemented. For example, the current frontline ALL treatment protocol at St Jude Children’s Research Hospital, Total XVII (NCT03117751), incorporates established morphologic, immunophenotyping and molecular genetic assays with comprehensive NGS diagnostics of both tumor and germline samples (whole-genome, whole exome and RNA-seq) performed in a CAP/CLIA-accredited laboratory as the clinical standard of care for all consented patients. Rapid RNA-seq reports rearrangements by day 15 of remission induction. Tumor and germline sequence information are also interrogated for copy-number variations, structural variations, single-nucleotide variants and insertion/deletions, which are reported by days 28–42 of remission induction [69].
5. Treatment Strategies for Ph-like ALL
5.1. Tyrosine Kinase Inhibitors
Whilst improvements in risk stratification and chemotherapy intensification has traditionally been an effective strategy for improving the outcomes of ALL, it is likely that this approach is reaching a point where the risk of toxicities outweighs the benefit of improved survival. For example, whilst the outcomes of adolescents and young adults (AYAs) were significantly improved with the pediatric-inspired CALGB 10403 regimen, the presence of Ph-like ALL remained an independent poor prognostic factor [70]. Therefore, new therapeutic strategies are required for these HR patients. The presence of activated kinase alterations and cytokine receptors in the majority of patients with Ph-like ALL has led to significant interest in incorporating TKIs in the clinical management of this subgroup, fueled by the success of adding TKIs to chemotherapy in patients with Ph+ ALL [71,72,73,74,75]. The two common subgroups of rearrangements in Ph-like ALL are ABL-class fusions (including ABL1, ABL2, CSF1R, LYN, PDGFRA and PDGFRB) and those activating JAK-STAT signaling (CRLF2, JAK2 and EPOR) (Figure 3). For ABL-class fusions, preclinical studies have demonstrated encouraging anti-leukemic activity of TKIs in vitro and in patient-derived xenograft models [4,45]. Furthermore, anecdotal reports and case series have shown promising activity of either imatinib or dasatinib in patients with ABL-class fusions, either in the context of slow responders, as salvage for relapsed/refractory disease or as preemptive maintenance therapy post allogenic hematopoietic cell transplant in patients with persistent MRD [44,50,51,76,77]. Among 21 slow responders with an ABL-class fusion treated on UKALL2011, 13 were identified prospectively and treated with imatinib. The four-year relapse/refractory rate for the TKI group was 0% compared to 62.5% for the control group, with event-free survival rates of 83.9% vs. 37.5%, respectively [78]. Superior outcome was also observed in ABL-class fusion patients treated with TKI (imatinib or dasatinib) compared to chemotherapy alone on the AIEOP-BFM or FRALLE/GRAALL protocols [79,80]. Several large nonrandomized trials are currently ongoing to determine whether up-front addition of dasatinib to chemotherapy in children and AYAs decreases relapse risk and improves overall survival in Ph-like ABL-class fusion patients, including COG AALL1131 (NCT01406756) and SJCRH Total XVII (NCT03117751). Furthermore, the international AALL1631/EsPhALL2017 trial testing imatinib was amended to include patients with ABL-class Ph-like ALL (NCT03007147), and another international phase 3 trial for children with ABL-class Ph-like and Ph+ ALL is in development. A phase 1/2 international trial, AALL1922 (NCT04501614), will test the safety and efficacy of the third generation ABL1-inhibitor, ponatinib, in children and AYAs (1–21 years) with relapsed/refractory Ph+ ALL and Ph-like ABL-class ALL.
Preclinical studies have shown variable activity of JAK inhibitors (most commonly, ruxolitinib) in JAK-STAT activating ALL [39,81], and more recent studies demonstrate the need to inhibit multiple pathways in CRLF2-rearranged ALL, including PI3K and “BCR-like” signaling, to facilitate complete eradication of leukemic blasts [82,83]. Whilst several case reports have shown clinical activity for ruxolitinib in combination with chemotherapy in JAK2-rearranged ALL [84], convincing evidence of single agent activity in this setting is largely missing. The COG is running the largest trial testing the safety and efficacy of ruxolitinib in children and AYAs with newly diagnosed Ph-like ALL and CRLF2, JAK2 or EPOR rearrangements via the single-arm phase 2 trial AALL1521 (NCT02723994). Phase 1 of this trial demonstrated the safety of combining ruxolitinib with chemotherapy [76], whilst phase 2 is currently assessing the efficacy of ruxolitinib at a dose of 50 mg/m2 administered in cycles of 14 days-on followed by 14 days-off. The University of Chicago is also recruiting for a phase 1 trial studying ruxolitinib in combination with the CALGB 10403 chemotherapy regimen (NCT00558519) [70] specifically in AYA patients (18–39 years of age) with JAK-STAT activating alterations.
5.2. Novel Therapies
Whilst TKI plus chemotherapy trials of Ph-like ALL are the logical first step to improve therapeutic outcome in these patients, the next generation of Ph-like ALL trials will likely incorporate novel immunotherapies, which have been remarkably effective in the treatment of relapsed/refractory B-ALL in children and adults irrespective of high-risk genetics or response to prior chemotherapies. This new treatment paradigm of combining targeted and immune therapies is supported by the promising results of the D-ALBA trial assessing blinatumomab plus dasatinib in newly diagnosed adults with Ph+ ALL [77]. Although published studies of immune therapies have not directly examined their efficacy in Ph-like ALL, retrospective studies provide some insight into their activity that warrant further investigation in larger trials. In a case series of 42 adults with relapsed/refractory B-ALL treated at City of Hope with the CD3/CD19 bispecific antibody, blinatumomab, 23 were found to have Ph-like ALL with an encouraging CR/CRi rate of 75% for CRLF2-rearranged and 57% for non-CRLF2 patients compared to 33% for patients with non Ph-like ALL [85]. In another small series of patients treated at MDACC with the CD22 antibody drug-conjugate, inotuzumab, the response rate was comparable for Ph-like and non-Ph-like patients, suggesting this agent might at least abrogate the high rates of treatment failure observed with chemotherapy in Ph-like ALL [86]. Ongoing randomized frontline trials investigating the benefit of adding either blinatumomab (E1910 study; NCT02003222) or inotuzumab (A041501 Alliance study; NCT03150693) to chemotherapy regimens in adults with newly diagnosed Ph-negative ALL will provide valuable insights into the use of these novel therapies in Ph-like ALL. Chimeric antigen receptor (CAR) T cell therapy targeting CD19 has also shown remarkable results in relapsed/refractory B-ALL in children and adults, and whilst no studies to date have formally assessed outcomes in Ph-like ALL, it is likely that a significant number of patients enrolled on these trials were Ph-like, from which the response rate can be determined. Of the 4 children with relapsed/refractory Ph-like ALL treated with CD19 Car T cell therapy at Seattle Children’s Hospital, all responded and achieved MRD-negative CR. Notably, one patient received dasatinib post-CAR T therapy and two patients received HCT, indicating a combined therapeutic approach is required for the effective elimination of leukemic blasts in Ph-like ALL [87]. Analysis of larger CAR T cell trials will inform the benefit of this therapy in the treatment of Ph-like ALL.
6. Conclusions
The advent of integrated transcriptome and genomic studies have shed light on the genetic landscape of Ph-like ALL contributing to the identification of a wide range of targetable lesions previously not recognizable by standard diagnostics. Due to the complex heterogeneity of genetic alterations, diagnosis is challenging by conventional cytogenetic and/or molecular approaches and requires the use of comprehensive assays for detection of gene fusions, cryptic rearrangements, sequence mutations and copy number changes. Several commercial assays, mostly capture-based, have been developed and they are widely used in many institutional laboratories. The identification of specific genetic lesions is crucial for guiding targeted therapeutic intervention as the incorporation of tyrosine kinase inhibitors in the clinical management of these patients have led to significant improved outcomes. Lastly, the use of immunotherapeutic agents, such as blinatumomab, inotuzumab, and CAR-T cells, represents a promising alternative approach which is irrespective of a specific genetic alteration or response to prior chemotherapies.
Author Contributions
I.I. and K.G.R. wrote the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
I.I. received honoraria from Amgen.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gu Z., Churchman M.L., Roberts K.G., Moore I., Zhou X., Nakitandwe J., Hagiwara K., Pelletier S., Gingras S., Berns H., et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat. Genet. 2019;51:296–307. doi: 10.1038/s41588-018-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J.-F., Dai Y.-T., Lilljebjörn H., Shen S.-H., Cui B.-W., Bai L., Liu Y.-F., Qian M.-X., Kubota Y., Kiyoi H., et al. Transcriptional landscape of B cell precursor acute lymphoblastic leukemia based on an international study of 1,223 cases. Proc. Natl. Acad. Sci. USA. 2018;115:E11711–E11720. doi: 10.1073/pnas.1814397115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts K.G., Morin R.D., Zhang J., Hirst M., Zhao Y., Su X., Chen S.-C., Payne-Turner D., Churchman M.L., Harvey R.C., et al. Genetic Alterations Activating Kinase and Cytokine Receptor Signaling in High-Risk Acute Lymphoblastic Leukemia. Cancer Cell. 2012;22:153–166. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts K.G., Li Y., Payne-Turner D., Harvey R.C., Yang Y.-L., Pei D., McCastlain K., Ding L., Lu C., Song G., et al. Targetable Kinase-Activating Lesions in Ph-like Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2014;371:1005–1015. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Den Boer M.L., van Slegtenhorst M., De Menezes R.X., Cheok M.H., Buijs-Gladdines J.G., Peters S.T., Van Zutven L.J., Beverloo H.B., Van der Spek P.J., Escherich G., et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: A genome-wide classification study. Lancet Oncol. 2009;10:125–134. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullighan C.G., Su X., Zhang J., Radtke I., Phillips L.A., Miller C.B., Ma J., Liu W., Cheng C., Schulman B.A., et al. Deletion ofIKZF1and Prognosis in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts K.G. The biology of Philadelphia chromosome-like ALL. Best Pr. Res. Clin. Haematol. 2017;30:212–221. doi: 10.1016/j.beha.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Chiaretti S., Messina M., Foà R. BCR/ABL1-like acute lymphoblastic leukemia: How to diagnose and treat? Cancer. 2019;125:194–204. doi: 10.1002/cncr.31848. [DOI] [PubMed] [Google Scholar]
- 9.Roberts K.G., Reshmi S.C., Harvey R.C., Chen I.-M., Patel K., Stonerock E., Jenkins H., Dai Y., Valentine M., Gu Z., et al. Genomic and outcome analyses of Ph-like ALL in NCI standard-risk patients: A report from the Children’s Oncology Group. Blood. 2018;132:815–824. doi: 10.1182/blood-2018-04-841676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reshmi S.C., Harvey R.C., Roberts K.G., Stonerock E., Smith A., Jenkins H., Chen I.-M., Valentine M., Liu Y., Li Y., et al. Targetable kinase gene fusions in high-risk B-ALL: A study from the Children’s Oncology Group. Blood. 2017;129:3352–3361. doi: 10.1182/blood-2016-12-758979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tasian S.K., Hurtz C., Wertheim G.B., Bailey N.G., Lim M.S., Harvey R.C., Chen I.-M., Willman C.L., Astles R., Zebrowski A., et al. High incidence of Philadelphia chromosome-like acute lymphoblastic leukemia in older adults with B-ALL. Leukemia. 2017;31:981–984. doi: 10.1038/leu.2016.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain N., Roberts K.G., Jabbour E., Patel K., Eterovic A.K., Chen K., Zweidler-McKay P., Lu X., Fawcett G., Wang S.A., et al. Ph-like acute lymphoblastic leukemia: A high-risk subtype in adults. Blood. 2017;129:572–581. doi: 10.1182/blood-2016-07-726588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts K.G., Gu Z., Payne-Turner D., McCastlain K., Harvey R.C., Chen I.-M., Pei D., Iacobucci I., Valentine M., Pounds S.B., et al. High Frequency and Poor Outcome of Philadelphia Chromosome–Like Acute Lymphoblastic Leukemia in Adults. J. Clin. Oncol. 2017;35:394–401. doi: 10.1200/JCO.2016.69.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 15.Loh M.L., Zhang J., Harvey R.C., Roberts K., Payne-Turner D., Kang H., Wu G., Chen X., Becksfort J., Edmonson M., et al. Tyrosine kinome sequencing of pediatric acute lymphoblastic leukemia: A report from the Children’s Oncology Group TARGET Project. Blood. 2013;121:485–488. doi: 10.1182/blood-2012-04-422691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boer J.M., Koenders J.E., van der Holt B., Exalto C., Sanders M.A., Cornelissen J.J., Valk P.J., den Boer M.L., Rijneveld A.W. Expression profiling of adult acute lymphoblastic leukemia identifies a BCR-ABL1-like subgroup characterized by high non-response and relapse rates. Haematologica. 2015;100:e261–e264. doi: 10.3324/haematol.2014.117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold T., Schneider S., Metzeler K.H., Neumann M., Hartmann L., Roberts K.G., Konstandin N.P., Greif P.A., Bräundl K., Ksienzyk B., et al. Adults with Philadelphia chromosome–like acute lymphoblastic leukemia frequently have IGH-CRLF2 and JAK2 mutations, persistence of minimal residual disease and poor prognosis. Haematologica. 2016;102:130–138. doi: 10.3324/haematol.2015.136366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiaretti S., Messina M., Grammatico S., Piciocchi A., Fedullo A.L., Di Giacomo F., Peragine N., Gianfelici V., Lauretti A., Bareja R., et al. Rapid identification ofBCR/ABL1-like acute lymphoblastic leukaemia patients using a predictive statistical model based on quantitative real time-polymerase chain reaction: Clinical, prognostic and therapeutic implications. Br. J. Haematologica. 2018;181:642–652. doi: 10.1111/bjh.15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiaretti S., Messina M., Della Starza I., Piciocchi A., Cafforio L., Cavalli M., Taherinasab A., Ansuinelli M., Elia L., Petroni G.A., et al. Philadelphia-like acute lymphoblastic leukemia is associated with minimal residual disease persistence and poor outcome. First report of the minimal residual disease-oriented GIMEMA LAL1913. Haematologica. 2020 doi: 10.3324/haematol.2020.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts K.G., Pei D., Campana D., Payne-Turner D., Li Y., Cheng C., Sandlund J.T., Jeha S., Easton J., Becksfort J., et al. Outcomes of Children with BCR-ABL1–Like Acute Lymphoblastic Leukemia Treated with Risk-Directed Therapy Based on the Levels of Minimal Residual Disease. J. Clin. Oncol. 2014;32:3012–3020. doi: 10.1200/JCO.2014.55.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heatley S.L., Sadras T., Kok C.H., Nievergall E., Quek K., Dang P., McClure B., Venn N., Moore S., Suttle J., et al. High prevalence of relapse in children with Philadelphia-like acute lymphoblastic leukemia despite risk-adapted treatment. Haematologica. 2017;102:e490–e493. doi: 10.3324/haematol.2016.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullighan C.G., Collins-Underwood J.R., Phillips L.A., Loudin M.L., Liu W., Zhang J., Ma J., Coustan-Smith E., Harvey R.C., Willman C.L., et al. Rearrangement of CRLF2 in B-progenitor– and Down syndrome–associated acute lymphoblastic leukemia. Nat. Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoda A., Yoda Y., Chiaretti S., Bar-Natan M., Mani K., Rodig S.J., West N., Xiao Y., Brown J.R., Mitsiades C., et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA. 2010;107:252–257. doi: 10.1073/pnas.0911726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell L.J., Jones L., Enshaei A., Tonin S., Ryan S.L., Eswaran J., Nakjang S., Papaemmanuil E., Tubio J.M.C., Fielding A.K., et al. Characterisation of the genomic landscape ofCRLF2-rearranged acute lymphoblastic leukemia. Genes Chromosom. Cancer. 2017;56:363–372. doi: 10.1002/gcc.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hertzberg L., Vendramini E., Ganmore I., Cazzaniga G., Schmitz M., Chalker J., Shiloh R., Iacobucci I., Shochat C., Zeligson S., et al. Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: A report from the International BFM Study Group. Blood. 2010;115:1006–1017. doi: 10.1182/blood-2009-08-235408. [DOI] [PubMed] [Google Scholar]
- 26.Isaksen D.E., Baumann H., Trobridge P.A., Farr A.G., Levin S.D., Ziegler S.F. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J. Immunol. 1999;163:5971–5977. [PubMed] [Google Scholar]
- 27.Wohlmann A., Sebastian K., Borowski A., Krause S., Friedrich K. Signal transduction by the atopy-associated human thymic stromal lymphopoietin (TSLP) receptor depends on Janus kinase function. Biol. Chem. 2010;391:181–186. doi: 10.1515/bc.2010.029. [DOI] [PubMed] [Google Scholar]
- 28.Tasian S.K., Doral M.Y., Borowitz M.J., Wood B.L., Chen I.-M., Harvey R.C., Gastier-Foster J.M., Willman C.L., Hunger S.P., Mullighan C.G., et al. Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood. 2012;120:833–842. doi: 10.1182/blood-2011-12-389932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheeren F.A., Van Lent A.U., Nagasawa M., Weijer K., Spits H., Legrand N., Blom B. Thymic stromal lymphopoietin induces early human B-cell proliferation and differentiation. Eur. J. Immunol. 2010;40:955–965. doi: 10.1002/eji.200939419. [DOI] [PubMed] [Google Scholar]
- 30.Russell L.J., Enshaei A., Jones L., Erhorn A., Masic D., Bentley H., Laczko K.S., Fielding A.K., Goldstone A.H., Goulden N., et al. IGH@ Translocations Are Prevalent in Teenagers and Young Adults with Acute Lymphoblastic Leukemia and Are Associated with a Poor Outcome. J. Clin. Oncol. 2014;32:1453–1462. doi: 10.1200/JCO.2013.51.3242. [DOI] [PubMed] [Google Scholar]
- 31.Schmäh J., Fedders B., Panzer-Grümayer R., Fischer S., Zimmermann M., Dagdan E., Bens S., Schewe D., Moericke A., Alten J., et al. Molecular characterization of acute lymphoblastic leukemia with high CRLF2 gene expression in childhood. Pediatr. Blood Cancer. 2017;64:e26539. doi: 10.1002/pbc.26539. [DOI] [PubMed] [Google Scholar]
- 32.Konoplev S., Lu X., Konopleva M., Jain N., Ouyang J., Goswami M., Roberts K.G., Valentine M., Mullighan C.G., Bueso-Ramos C., et al. CRLF2-Positive B-Cell Acute Lymphoblastic Leukemia in Adult Patients: A Single-Institution Experience. Am. J. Clin. Pathol. 2017;147:357–363. doi: 10.1093/ajcp/aqx005. [DOI] [PubMed] [Google Scholar]
- 33.Imamura T., Kiyokawa N., Kato M., Imai C., Okamoto Y., Yano M., Ohki K., Yamashita Y., Kodama Y., Saito A., et al. Characterization of pediatric Philadelphia-negative B-cell precursor acute lymphoblastic leukemia with kinase fusions in Japan. Blood Cancer J. 2016;6:e419. doi: 10.1038/bcj.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey R.C., Mullighan C.G., Chen I.-M., Wharton W., Mikhail F.M., Carroll A.J., Kang H., Liu W., Dobbin K.K., Smith M.A., et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115:5312–5321. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Andreu V., Roberts K.G., Harvey R.C., Yang W., Cheng C., Pei D., Xu H., Gastierfoster J.M., E S., Lim J.Y.-S., et al. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat. Genet. 2013;45:1494–1498. doi: 10.1038/ng.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giddings B.M., Whitehead T.P., Metayer C., Miller M.D. Childhood leukemia incidence in California: High and rising in the Hispanic population. Cancer. 2016;122:2867–2875. doi: 10.1002/cncr.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Veer A., Waanders E., Pieters R., Willemse M.E., Van Reijmersdal S.V., Russell L.J., Harrison C.J., Evans W.E., van der Velden V.H., Hoogerbrugge P.M., et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122:2622–2629. doi: 10.1182/blood-2012-10-462358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shochat C., Tal N., Gryshkova V., Birger Y., Bandapalli O.R., Cazzaniga G., Gershman N., Kulozik A.E., Biondi A., Mansour M.R., et al. Novel activating mutations lacking cysteine in type I cytokine receptors in acute lymphoblastic leukemia. Blood. 2014;124:106–110. doi: 10.1182/blood-2013-10-529685. [DOI] [PubMed] [Google Scholar]
- 39.Iacobucci I., Li Y., Roberts K.G., Dobson S.M., Kim J.C., Payne-Turner D., Harvey R.C., Valentine M., McCastlain K., Easton J., et al. Truncating Erythropoietin Receptor Rearrangements in Acute Lymphoblastic Leukemia. Cancer Cell. 2016;29:186–200. doi: 10.1016/j.ccell.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell L.J., De Castro D.G., Griffiths M., Telford N., Bernard O., Panzer-Grümayer R., Heidenreich O., Moorman A.V., Harrison C.J. A novel translocation, t(14;19)(q32;p13), involving IGH@ and the cytokine receptor for erythropoietin. Leukemia. 2008;23:614–617. doi: 10.1038/leu.2008.250. [DOI] [PubMed] [Google Scholar]
- 41.Dai H.-P., Yin J., Li Z., Yang C.-X., Cao T., Chen P., Zong Y.-H., Zhu M.-Q., Zhu X.-M., Xiao S., et al. Rapid Molecular Response to Dasatinib in a Pediatric Relapsed Acute Lymphoblastic Leukemia with NCOR1-LYN Fusion. Front. Oncol. 2020;10:359. doi: 10.3389/fonc.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Den Boer M.L., Cario G., Moorman A.V., Boer J.M., de Groot-Kruseman H.A., Fiocco M., Escherich G., Imamura T., Yeoh A., Sutton R., et al. Outcomes of paediatric patients with B-cell acute lymphocytic leukaemia with ABL-class fusion in the pre-tyrosine-kinase inhibitor era: A multicentre, retrospective, cohort study. Lancet Haematol. 2021;8:e55–e66. doi: 10.1016/S2352-3026(20)30353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwab C.J., Roberts K.G., Boer J.M., Gohring G., Steinemann D., Vora A., Macartney C., Hough R.E., Thorn Z., Dillon R., et al. SSBP2-CSF1R is a Recurrent Fusion in B-lineage Acute Lymphoblastic Leukaemia with Diverse Genetic Presentation and Variable Outcome. Blood. 2020;137:1835–1838. doi: 10.1182/blood.2020008536. [DOI] [PubMed] [Google Scholar]
- 44.Schwab C., Ryan S.L., Chilton L., Elliott A., Murray J., Richardson S., Wragg C., Moppett J., Cummins M., Tunstall O., et al. EBF1-PDGFRB fusion in pediatric B-cell precursor acute lymphoblastic leukemia (BCP-ALL): Genetic profile and clinical implications. Blood. 2016;127:2214–2218. doi: 10.1182/blood-2015-09-670166. [DOI] [PubMed] [Google Scholar]
- 45.Roberts K.G., Yang Y.-L., Payne-Turner D., Lin W., Files J.K., Dickerson K., Gu Z., Taunton J., Janke L.J., Chen T., et al. Oncogenic role and therapeutic targeting of ABL-class and JAK-STAT activating kinase alterations in Ph-like ALL. Blood Adv. 2017;1:1657–1671. doi: 10.1182/bloodadvances.2017011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horiuchi M., Yoshida M., Yamasaki K., Sakagami R., Aoyama T., Tatsumi N., Tsutsumi M., Nakaya Y., Fuseya H., Yoshimura T., et al. Effective treatment with imatinib for acute B-lymphoblastic leukaemia with EBF1-PDGFRB fusion. Ann. Hematol. 2020;100:1329–1331. doi: 10.1007/s00277-020-04332-8. [DOI] [PubMed] [Google Scholar]
- 47.Fazio F., Barberi W., Cazzaniga G., Fazio G., Messina M., Della Starza I., De Propris M.S., Mancini F., Mohamed S., Del Giudice I., et al. Efficacy of imatinib and chemotherapy in a pediatric patient with Philadelphia-like acute lymphoblastic leukemia with Ebf1-Pdgfrb fusion transcript. Leuk. Lymphoma. 2020;61:469–472. doi: 10.1080/10428194.2019.1668938. [DOI] [PubMed] [Google Scholar]
- 48.Lengline E., Beldjord K., Dombret H., Soulier J., Boissel N., Clappier E. Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion. Haematologica. 2013;98:e146–e148. doi: 10.3324/haematol.2013.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weston B.W., Hayden M.A., Roberts K.G., Bowyer S., Hsu J., Fedoriw G., Rao K.W., Mullighan C.G. Tyrosine Kinase Inhibitor Therapy Induces Remission in a Patient with Refractory EBF1-PDGFRB–Positive Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2013;31:e413–e416. doi: 10.1200/JCO.2012.47.6770. [DOI] [PubMed] [Google Scholar]
- 50.Tran T.H., Nguyen J.V., Stecula A., Akutagawa J., Moorman A.V., Braun B.S., Sali A., Mullighan C.G., Shah N.P., Dai Y., et al. The EBF1-PDGFRB T681I mutation is highly resistant to imatinib and dasatinib in vitro and detectable in clinical samples prior to treatment. Haematologica. 2021 doi: 10.3324/haematol.2020.261354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts K.G., Janke L.J., Zhao Y., Seth A., Ma J., Finkelstein D., Smith S., Ebata K., Tuch B.B., Hunger S.P., et al. ETV6-NTRK3 induces aggressive acute lymphoblastic leukemia highly sensitive to selective TRK inhibition. Blood. 2018;132:861–865. doi: 10.1182/blood-2018-05-849554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kralik J.M., Kranewitter W., Boesmueller H., Marschon R., Tschurtschenthaler G., Rumpold H., Wiesinger K., Erdel M., Petzer A.L., Webersinke G. Characterization of a newly identified ETV6-NTRK3 fusion transcript in acute myeloid leukemia. Diagn. Pathol. 2011;6:19. doi: 10.1186/1746-1596-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang J., Park J.W., Won J.-K., Bae J.M., Koh J., Yim J., Yun H., Kim S.-K., Choi J.Y., Kang H.J., et al. Clinicopathological findings of pediatric NTRK fusion mesenchymal tumors. Diagn. Pathol. 2020;15:1–11. doi: 10.1186/s13000-020-01031-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knezevich S.R., McFadden D.E., Tao W., Lim J.F., Sorensen P.H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 1998;18:184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 55.Leeman-Neill R.J., Bs L.M.K., Liu P., Brenner A.V., Leeman-Neill R.J., Bogdanova T.I., Evdokimova V.N., Hatch M., Zurnadzy L.Y., Nikiforova M.N., et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120:799–807. doi: 10.1002/cncr.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brenca M., Rossi S., Polano M., Gasparotto D., Zanatta L., Racanelli D., Valori L., Lamon S., Tos A.P.D., Maestro R. Transcriptome sequencing identifiesETV6-NTRK3as a gene fusion involved in GIST. J. Pathol. 2016;238:543–549. doi: 10.1002/path.4677. [DOI] [PubMed] [Google Scholar]
- 57.Otsubo R., Mussazhanova Z., Akazawa Y., Sato A., Matsuda K., Matsumoto M., Yano H., Matsuse M., Mitsutake N., Ando T., et al. Sporadic pediatric papillary thyroid carcinoma harboring the ETV6/NTRK3 fusion oncogene in a 7-year-old Japanese girl: A case report and review of literature. J. Pediatr. Endocrinol. Metab. 2018;31:461–467. doi: 10.1515/jpem-2017-0292. [DOI] [PubMed] [Google Scholar]
- 58.Tognon C., Knezevich S.R., Huntsman D., Roskelley C.D., Melnyk N., Mathers J.A., Becker L., Carneiro F., MacPherson N., Horsman D., et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 59.Martinelli G., Iacobucci I., Storlazzi C.T., Vignetti M., Paoloni F., Cilloni D., Soverini S., Vitale A., Chiaretti S., Cimino G., et al. IKZF1 (Ikaros) Deletions in BCR-ABL1–Positive Acute Lymphoblastic Leukemia Are Associated with Short Disease-Free Survival and High Rate of Cumulative Incidence of Relapse: A GIMEMA AL WP Report. J. Clin. Oncol. 2009;27:5202–5207. doi: 10.1200/JCO.2008.21.6408. [DOI] [PubMed] [Google Scholar]
- 60.Raca G., Abdel-Azim H., Yue F., Broach J., Payne J.L., Reeves M.E., Gowda C., Schramm J., Desai D., Dovat E., et al. Increased Incidence of IKZF1 deletions and IGH-CRLF2 translocations in B-ALL of Hispanic/Latino children—a novel health disparity. Leukemia. 2021:1–4. doi: 10.1038/s41375-021-01133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nardi V., Ku N., Frigault M.J., Dubuc A.M., Tsai H.K., Amrein P.C., Hobbs G.S., Brunner A.M., Narayan R., Burke M.E., et al. Clinical response to larotrectinib in adult Philadelphia chromosome-like ALL with cryptic ETV6-NTRK3 rearrangement. Blood Adv. 2020;4:106–111. doi: 10.1182/bloodadvances.2019000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu Z., Churchman M., Roberts K., Li Y., Liu Y., Harvey R.C., McCastlain K., Reshmi S.C., Payne-Turner D., Iacobucci I., et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat. Commun. 2016;7:13331. doi: 10.1038/ncomms13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan J.E.-L., Wong S.-C., Gan S.K.-E., Xu S., Lam K.-P. The Adaptor Protein BLNK Is Required for B Cell Antigen Receptor-induced Activation of Nuclear Factor-κB and Cell Cycle Entry and Survival of B Lymphocytes. J. Biol. Chem. 2001;276:20055–20063. doi: 10.1074/jbc.M010800200. [DOI] [PubMed] [Google Scholar]
- 64.Benedict C.L., Gilfillan S., Thai T.H., Kearney J.F. Terminal deoxynucleotidyl transferase and repertoire development. Immunol. Rev. 2000;175:150–157. doi: 10.1111/j.1600-065X.2000.imr017518.x. [DOI] [PubMed] [Google Scholar]
- 65.Boer J.M., Marchante J.R., Evans W.E., Horstmann M.A., Escherich G., Pieters R., Den Boer M.L. BCR-ABL1-like cases in pediatric acute lymphoblastic leukemia: A comparison between DCOG/Erasmus MC and COG/St. Jude signatures. Haematologica. 2015;100:e354–e357. doi: 10.3324/haematol.2015.124941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harvey R.C., Kang H., Roberts K.G. Development and Validation Of a Highly Sensitive and Specific Gene Expression Classifier To Prospectively Screen and Identify B-Precursor Acute Lymphoblastic Leukemia (ALL) Patients with a Philadelphia Chromosome-Like (“Ph-like” or “BCR-ABL1-Like”) Signature For Therapeutic Targeting and Clinical Intervention. Blood. 2013;122:826. [Google Scholar]
- 67.He J., Abdel-Wahab O., Nahas M.K., Wang K., Rampal R.K., Intlekofer A.M., Patel J., Krivstov A., Frampton G.M., Young L.E., et al. Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood. 2016;127:3004–3014. doi: 10.1182/blood-2015-08-664649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Z., Liebers M., Zhelyazkova B., Cao Y., Panditi D., Lynch K.D., Chen J., Robinson H.E., Shim H.S., Chmielecki J., et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat. Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 69.Inaba H., Azzato E.M., Mullighan C.G. Integration of Next-Generation Sequencing to Treat Acute Lymphoblastic Leukemia with Targetable Lesions: The St. Jude Children’s Research Hospital Approach. Front. Pediatr. 2017;5:258. doi: 10.3389/fped.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stock W., Luger S.M., Advani A.S., Yin J., Harvey R.C., Mullighan C.G., Willman C.L., Fulton N., Laumann K.M., Malnassy G., et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: Results of CALGB 10403. Blood. 2019;133:1548–1559. doi: 10.1182/blood-2018-10-881961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biondi A., Schrappe M., De Lorenzo P., Castor A., Lucchini G., Gandemer V., Pieters R., Stary J., Escherich G., Campbell M., et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): A randomised, open-label, intergroup study. Lancet Oncol. 2012;13:936–945. doi: 10.1016/S1470-2045(12)70377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fielding A.K., Rowe J.M., Buck G., Foroni L., Gerrard G., Litzow M.R., Lazarus H., Luger S.M., Marks D.I., McMillan A.K., et al. UKALLXII/ECOG2993: Addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123:843–850. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foà R., Vitale A., Vignetti M., Meloni G., Guarini A., De Propris M.S., Elia L., Paoloni F., Fazi P., Cimino G., et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood. 2011;118:6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 74.Schultz K.R., Carroll A., Heerema N.A., Bowman W.P., Aledo A., Slayton W.B., Sather H., Devidas M., Zheng H.W., Davies S.M., et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014;28:1467–1471. doi: 10.1038/leu.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slayton W.B., Schultz K.R., Kairalla J.A., Devidas M., Mi X., Pulsipher M.A., Chang B.H., Mullighan C., Iacobucci I., Silverman L.B., et al. Dasatinib Plus Intensive Chemotherapy in Children, Adolescents, and Young Adults With Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia: Results of Children’s Oncology Group Trial AALL0622. J. Clin. Oncol. 2018;36:2306–2314. doi: 10.1200/JCO.2017.76.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tasian S.K., Assad A., Hunter D.S., Du Y., Loh M.L. A Phase 2 Study of Ruxolitinib with Chemotherapy in Children with Philadelphia Chromosome-like Acute Lymphoblastic Leukemia (INCB18424-269/AALL1521): Dose-Finding Results from the Part 1 Safety Phase. Blood. 2018;132:555. doi: 10.1182/blood-2018-99-110221. [DOI] [Google Scholar]
- 77.Foà R., Bassan R., Vitale A., Elia L., Piciocchi A., Puzzolo M.-C., Canichella M., Viero P., Ferrara F., Lunghi M., et al. Dasatinib–Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020;383:1613–1623. doi: 10.1056/NEJMoa2016272. [DOI] [PubMed] [Google Scholar]
- 78.Moorman A.V., Schwab C., Winterman E., Hancock J., Castleton A., Cummins M., Gibson B., Goulden N., Kearns P., James B., et al. Adjuvant tyrosine kinase inhibitor therapy improves outcome for children and adolescents with acute lymphoblastic leukaemia who have an ABL-class fusion. Br. J. Haematol. 2020;191:844–851. doi: 10.1111/bjh.17093. [DOI] [PubMed] [Google Scholar]
- 79.Cario G., Leoni V., Conter V., Attarbaschi A., Zaliova M., Sramkova L., Cazzaniga G., Fazio G., Sutton R., Elitzur S., et al. Relapses and treatment-related events contributed equally to poor prognosis in children with ABL-class fusion positive B-cell acute lymphoblastic leukemia treated according to AIEOP-BFM protocols. Haematologica. 2019;105:1887–1894. doi: 10.3324/haematol.2019.231720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanasi I., Ba I., Sirvent N., Braun T., Cuccuini W., Ballerini P., Duployez N., Tanguy-Schmidt A., Tamburini J., Maury S., et al. Efficacy of tyrosine kinase inhibitors in Ph-like acute lymphoblastic leukemia harboring ABL-class rearrangements. Blood. 2019;134:1351–1355. doi: 10.1182/blood.2019001244. [DOI] [PubMed] [Google Scholar]
- 81.Maude S.L., Tasian S.K., Vincent T., Hall J.W., Sheen C., Roberts K.G., Seif A.E., Barrett D.M., Chen I.-M., Collins J.R., et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. 2012;120:3510–3518. doi: 10.1182/blood-2012-03-415448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hurtz C., Wertheim G.B., Loftus J.P., Blumenthal D., Lehman A., Li Y., Bagashev A., Manning B., Cummins K.D., Burkhardt J.K., et al. Oncogene-independent BCR-like signaling adaptation confers drug resistance in Ph-like ALL. J. Clin. Investig. 2020;130:3637–3653. doi: 10.1172/JCI134424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tasian S.K., Teachey D.T., Li Y., Shen F., Harvey R.C., Chen I.-M., Ryan T., Vincent T.L., Willman C.L., Perl A.E., et al. Potent efficacy of combined PI3K/mTOR and JAK or ABL inhibition in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. 2017;129:177–187. doi: 10.1182/blood-2016-05-707653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding Y.Y., Stern J.W., Jubelirer T.F., Wertheim G.B., Lin F., Chang F., Gu Z., Mullighan C.G., Li Y., Harvey R.C., et al. Clinical efficacy of ruxolitinib and chemotherapy in a child with Philadelphia chromosome-like acute lymphoblastic leukemia with GOLGA5-JAK2 fusion and induction failure. Haematologica. 2018;103:e427–e431. doi: 10.3324/haematol.2018.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y., Aldoss I., Qu C., Crawford J.C., Gu Z., Allen E.K., Zamora A.E., Alexander T.B., Wang J., Goto H., et al. Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL. Blood. 2021;137:471–484. doi: 10.1182/blood.2020006287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jabbour E., Roberts K.G., Sasaki K., Zhao M.Y., Qu C., Gu Z., Jain N., Patel M.K.P., Ravandi F., Short N.J., et al. Inotuzumab Ozogamicin (Ino) May Overcome the Impact of Philadelphia Chromosome (Ph)-like Phenotype in Adult Patients (pts) with Relapsed/Refractory (R/R) Acute Lymphoblastic Leukemia (ALL) Blood. 2019;134:1641. doi: 10.1182/blood-2019-126940. [DOI] [Google Scholar]
- 87.Gardner R.A., Finney O., Annesley C., Brakke H., Summers C., Leger K., Bleakley M., Brown C., Mgebroff S., Kelly-Spratt K.S., et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.